- 1Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 2Department of Endocrinology, Wuxi Hospital of Traditional Chinese Medicine, Wuxi, China
- 3Department of Endocrinology, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, China
- 4Department of Endocrinology, Huai’an Second People’s Hospital and The Affiliated Huai’an Hospital of Xuzhou Medical University, Huai’an, China
- 5Department of Endocrinology, The Affiliated Suqian First People’s Hospital of Nanjing Medical University, Suqian, China
Aim: To explore the chronic effects of metformin on testosterone levels in men with type 2 diabetes mellitus (T2DM).
Methods: This is a secondary analysis of a real-world study evaluating the efficacy and safety of premixed insulin treatment in patients with T2DM via 3-month intermittent flash glucose monitoring. Male patients aged 18-60 who were using metformin during the 3-month study period were included as the metformin group. The control group included males without metformin therapy by propensity score matching analysis with age as a covariate. Testosterone levels were measured at baseline and after 3-month treatment.
Results: After 3-month treatment, the control group had higher levels of total testosterone, free and bioavailable testosterone than those at baseline (P<0.05). Compared with the control group, the change of total (-0.82 ± 0.59 vs. 0.99 ± 0.59 nmol/L) and bioavailable (-0.13 ± 0.16 vs. 0.36 ± 0.16 nmol/L) testosterone levels in the metformin group significantly decreased (P=0.036 and 0.029, respectively). In Glycated Albumin (GA) improved subgroup, the TT, FT, and Bio-T levels in the control subgroup were higher than their baseline levels (P < 0.05). Compared with the metformin subgroup, TT level in the control subgroup also increased significantly (P=0.044). In GA unimproved subgroup, the change of TT level in the metformin subgroup was significantly lower than that in the control subgroup (P=0.040).
Conclusion: In men with T2DM, 3-month metformin therapy can reduce testosterone levels, and counteract the testosterone elevation that accompanied with the improvement of blood glucose.
Clinical Trial Registration: https://www.clinicaltrials.gov/ct2/show/NCT04847219?term=04847219&draw=2&rank=1.
Introduction
Type 2 diabetes mellitus (T2DM) is a common metabolic disease characterized by hyperglycemia and insulin resistance, which can affect the normal function of the whole body, lead to cardiomyopathy, atherosclerosis, nephropathy and peripheral neuropathy, and increase the risk of neurodegenerative and other endocrine diseases (1, 2). It not only affects the quality of life and survival of patients, but also brings substantial physiological and psychological burden to patients (3).
Testosterone, the main androgen in the male reproductive process, is responsible for the development of secondary sexual characteristics, sexual desire, and erectile function (4). Normal testosterone level in men is essential to maintain bone mineral density, muscle growth, brain nervous system and cognitive health (5, 6). It is reported that low testosterone is widespread in men with metabolic syndrome such as T2DM and obesity. About one-third of T2DM men have hypogonadism (7, 8). The reason may be due to long-term hyperglycemia, resulting in metabolic imbalance, inflammation, and oxidative stress (9). There is increasing evidence that men with low testosterone levels have lower survival rates and higher all-cause, cardiovascular, cancer, and respiratory mortality rates than men with high or normal testosterone levels (10, 11). Therefore, we should pay attention to the change of testosterone level in patients with T2DM. Metformin is a first-line drug widely used in the treatment of T2DM. It is a stable, low molecular weight hydrophilic compound, which can reach a variety of tissues, including muscle, liver, pancreas, adipose tissue, hypothalamus, pituitary, and gonad (12). In addition to the treatment of T2DM, metformin also plays some other beneficial effects. Recent data have demonstrated the advantageous effects of metformin in cancer, cardiovascular disease, and polycystic ovary syndrome (13, 14).
Previous study found that patients with T2DM had significantly lower testosterone levels after one month of metformin treatment (15). In the previous study, patients experienced rapid normalization of blood glucose using insulin therapy within 5 days and followed with a short term of metformin therapy for one month. Therefore, to exclude the influence of the rapid change of blood glucose before metformin therapy and further explore the chronic effect of metformin treatment on testosterone levels in male patients with T2DM, this study observed testosterone levels in male patients with T2DM who had stable insulin therapy for at least 2 months, and prolonged metformin treatment for another 3 months.
Methods
Study Design and Participants
Our study is a secondary analysis of a premix insulin study (ClinicalTrials.gov NCT 04847219, Supplementary file), which was conducted in the outpatient department of endocrinology of five hospitals in Jiangsu Province from October 2019 to April 2021. The study was approved by ethics committee of Nanjing First Hospital. All operations were in accordance with the ethical standards of the hospital and the 1964 Helsinki Declaration revised in 2013. All participants obtained informed consent.
The inclusion criteria were as follows: 1) patients aged >18 years and diagnosed with T2DM according to World Health Organization 1999 diagnostic criteria of T2DM; 2) patients using subcutaneous injection with premix insulin Bid/Tid, single drug and/or combination of oral hypoglycemic drugs, the treatment regimen was stable for more than 2 months; 3) subjects were willing to undergo Flash Glucose Mornitoring (FGM) examination;
The exclusion criteria were as follows: 1) patients treated with GLP-1 agonist or systemic hormone therapy in recent 3 months; 2) patients with insulin allergy or FGM intolerance; 3) impaired liver and renal function, ALT 2.5 times higher than the upper limit of normal value; serum creatinine was 1.3 times higher than the upper limit of normal; 4) patients with acute metabolic diabetic complications, infection, stress or any other apparent condition as determined by the investigator (e.g., severe heart and lung disease, endocrine disease, neurological disease, tumor disease, other pancreatic disease, history of mental illness).
All eligible subjects received intermittent FGM once a month for 3 months. Doctors adjusted the hypoglycemia treatment, and diabetes specialist nurses provided educations about insulin injection techniques, self-management of diet and exercise according to the FGM data every month.
Based on this study, we selected male patients aged 18-60 who were treated with metformin for three months during the premixed insulin study as the metformin group (n=40). The mean dose of metformin (Merck Serono) was 1500 mg daily. The control group was selected from the remaining male patients who were not treated with metformin through propensity score matching adjusted for age (n=40).
Data such as age, weight, insulin dose and oral hypoglycemic drugs were collected at baseline and end point of study. Biochemical parameters such as fast C-peptide and fast insulin were measured by routine laboratory methods at baseline and end point of the study. Glycated hemoglobin (HbA1c) was determined by high performance liquid chromatography (Bio-Rad, Diastat HbA1c analyzer). Glycated Albumin (GA) was determined by peroxidase method (Jiuqiang biological kit). Total testosterone (TT) and sex hormone binding globulin (SHBG) were measured by chemiluminescence (Unicel of Beckman Coulter, USA™ DXI 800 automatic analyzer). Free testosterone (FT) and bioavailable testosterone (Bio-T) were calculated (16). The change of testosterone levels was the main observation index of this study.
Statistical Analysis
Spss23.0 software (SPSS, IL, USA) was used for statistical analysis. The data of normal distribution are expressed as average ± standard error and nonnormal distribution data are represented by median in the quartile range. Enumeration data was analyzed by chi-square test (the proportion of hypoglycemic drugs and percentage of subjects with TT<12nmol/L). The data before and after treatment were analyzed by Student paired t-test or Wilcoxon test. Covariance analysis was used to analyze the differences in testosterone levels between groups, and insulin dose was used as a covariate. The significance level was 5%.
Results
Baseline Characteristics
A total of 40 male patients were enrolled in the metformin group, and another 40 males who did not use metformin were matched as controls. Finally, 80 people were included in the analysis of this study. According to International Society for Sexual Medicine, International Society for Sexual Medicine (ISSM) guidelines, men with TT < 12nmo/L have a lack of testosterone levels (17). Low serum testosterone usually indicates hypogonadism. All characteristics were not significantly different between the two groups at baseline (P all >0.05, Table 1).
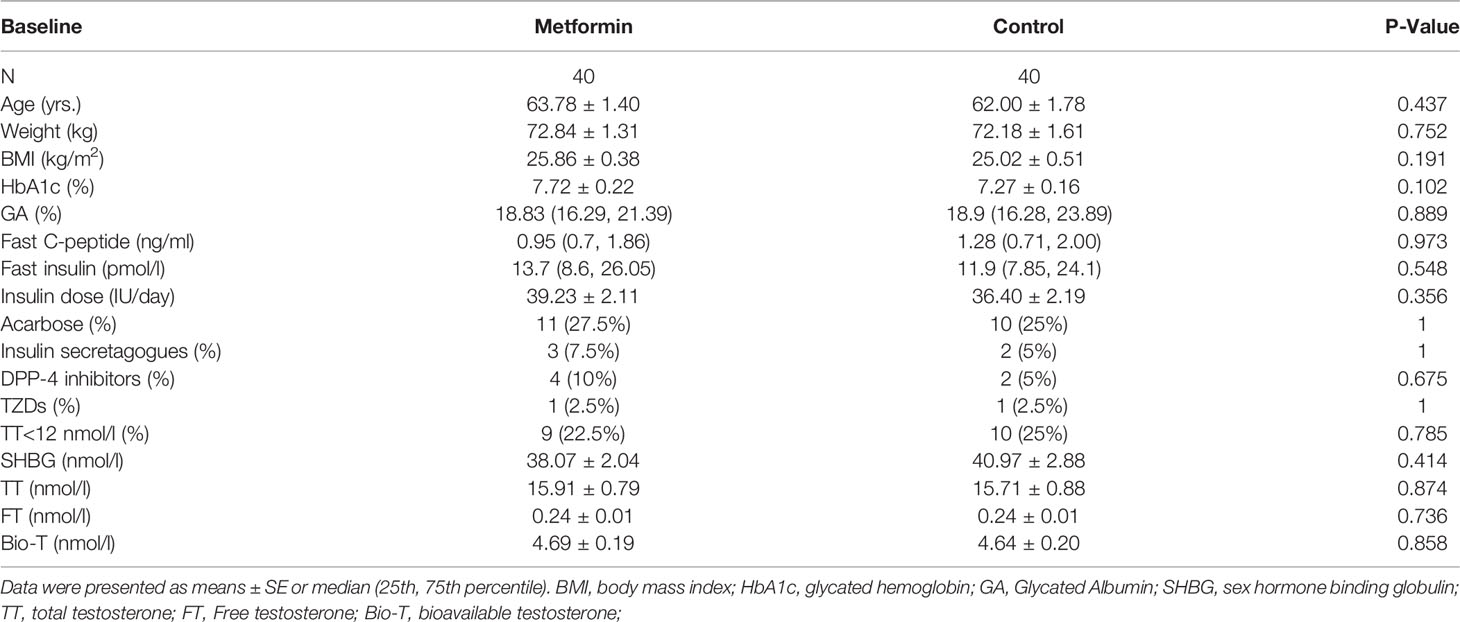
The changes of Testosterone Levels in the Two Groups
TT, FT, and Bio-T levels significantly increased after 3-month in the control group. (15.71 ± 0.88 vs. 16.79 ± 1.01 nmol/L, 0.25 ± 0.02 vs. 0.27 ± 0.02 nmol/L, 4.89 ± 0.23 vs. 5.21 ± 0.24 nmol/L, P all<0.05, Figures 1A–C), but the change was lost in metformin group (P all >0.05).
Figure 1 (A–C) TT, FT and Bio-T levels at baseline and endpoint in metformin and control group. Data are mean ± SE.
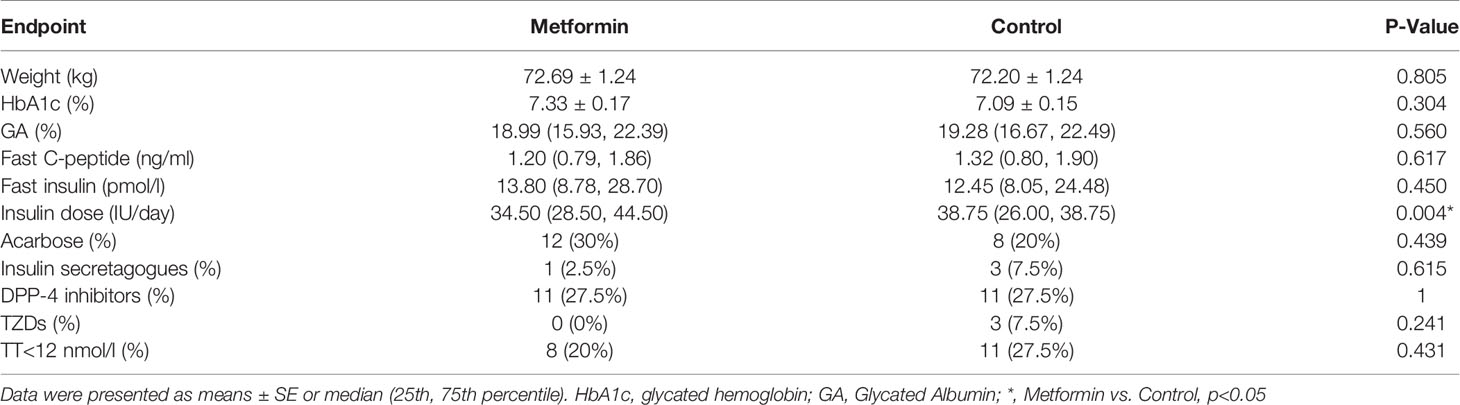
The change of testosterone levels = TT level after treatment -TT level before treatment. Patients in metformin group caused superior reductions in TT and Bio-T versus the control group (estimated treatment difference: -1.81 95%CI [-3.507, -0.112], -0.49 95%CI [-0.929, -0.051], P=0.037 and 0.029, respectively, Figures 2A, C). There was no significant difference found in the change of FT between two groups. (Figure 2B)
Figure 2 (A–C) The changes of TT, FT and Bio-T levels between metformin and control group before and after treatment. Data are mean ± SE.
The Changes of Testosterone Levels Stratified by Glycated Albumin
To explore the influence of blood glucose changes on the testosterone level of patients, we subdivided the patients into four groups according to changes in GA: GA improved (endpoint GA– baseline GA ≤ 0) metformin group (metformin-1 group, n=22); GA improved control group (control-1 group, n=22); GA unimproved (endpoint GA– baseline GA >0) group (metformin -2 group, n=18); GA unimproved control group (control-2 group, n=18). With 3-month treatment, the control-1 group had higher levels of TT, FT, and Bio-T than those at baseline (P all <0.05, Figures 3A–C). There was no statistically significant difference in TT, FT, and Bio-T levels among the other three groups before and after treatment (P all >0.05, Figures 3A–C). The change of TT level in the control group 1 was higher than that in the metformin group (P=0.044, Figure 3D), and the change in the metformin group 2 was significantly lower than that in the control group 2 (P=0.040, Figure 3D). As shown in Figures 3E, F, there was no difference between groups in the changes of FT and Bio-T.
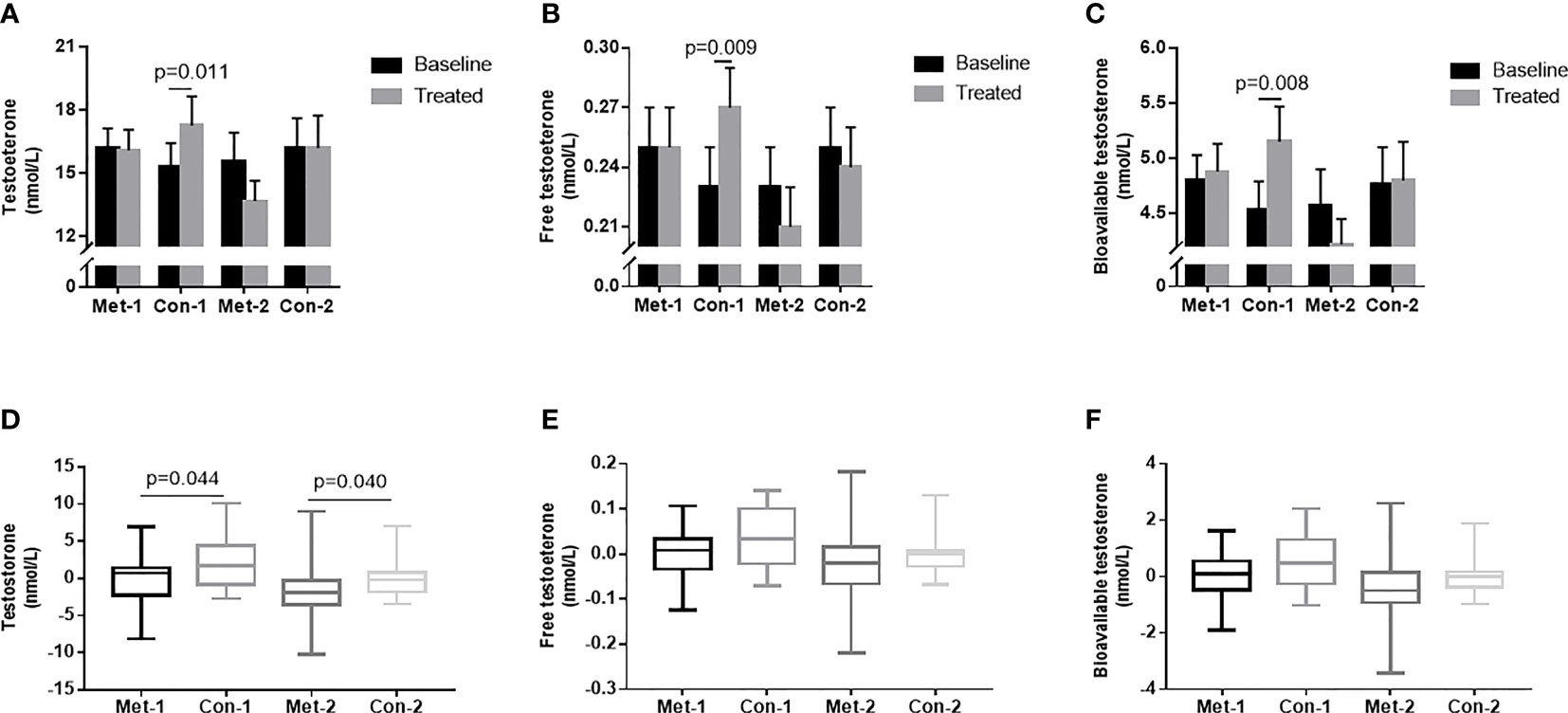
Figure 3 (A–C) TT, FT and Bio-T levels at baseline and endpoint in metformin and control groups under different stratification. (D–F) the changes of TT, FT and Bio-T levels between metformin and control group before and after treatment under different stratification. 1=△GA ≤ 0; 2 =△GA>0; Data are mean ± SE.
Endpoint Characteristics
At the endpoint, the insulin dose used in the metformin group was lower than that in the control group (P=0.004). There was no significant difference between the two groups in the parameters that could affect testosterone levels, such as body weight HbA1C, GA, fast C-peptide, fast insulin, TT<12nmol/L (%) and other oral drugs between the two groups (P all >0.05, Table 2).
Discussion
This real-word study reveals that 3-month metformin therapy can reduce testosterone levels, and counteract the testosterone elevation that accompanied with the improvement of blood glucose in men with T2DM.
In men, there is strong evidence that low testosterone levels are significantly associated with T2DM (18). The mechanism remains unclear. One reason may be the pro-inflammatory cytokines induced by hyperglycemia (19). Proinflammatory cytokines can inhibit the secretion of gonadotropin-releasing hormones, thereby reducing the level of circulating luteinizing hormone in vivo and in vitro (20), indicating that a pro-inflammatory state may contribute to the central inhibition of the male sex axis. Intensive insulin therapy can reduce the inflammatory response (21). Previous studies have also found that short-term intensive therapy for 5 days can significantly increase the level of testosterone (15). In the present study, improvement of glycemic control increased the testosterone level in a longer term of 3 months, which confirmed the effects of hyperglycemia on sexual hormones.
Animal studies have found that metformin can improve the testicular function and spermatogenesis of obese male mice induced by high-fat and high-cholesterol diet (22), and restore the gonadotropin and leptin systems in the testes of streptomycin-induced male rats (23). However, given the complex between glycemic control and hormone, these studies did not rule out the effect of blood glucose changes on it. Our previous study showed that metformin therapy can reduce testosterone levels in males with T2DM who had normalized blood control (15). This finding indicated that the use of metformin may be another reason of the high prevalence of low testosterone in males with T2DM. The present study prolonged the duration of metformin therapy, and the results were consistent with the previous study. Moreover, our stratification analysis with the change of GA indicated that the effects of metformin on testosterone levels existed regardless of the change of blood glucose. Combined with the results of the two studies, the effect of metformin on testosterone levels was independent of blood glucose.
M Faure et al. (24) found that metformin exposure in vitro may contribute to the decrease of cell proliferation and the change in secretory ability of testicular Sertoli cells. In vivo, metformin exposure negatively affected the germ cell population. Metformin exposure resulted in a decrease in testicular weight and sperm cell production in chickens, suggesting that taking metformin in drinking water for 3 weeks was sufficient to delay spermatogenesis. Sertoli cells are well known to regulate the synthesis and secretion of testosterone by Leydig cells (25). This may contribute to the reduction of testosterone by metformin. However, additional studies are needed to clarify the mechanisms that metformin reduces testosterone in male patients with T2DM.
Although previous studies have found that testosterone levels are negatively correlated with insulin resistance (26, 27), testosterone replacement therapy can reduce insulin resistance (28). In this study, although the testosterone level of the metformin group decreased, the dose of insulin injection was reduced, and the blood glucose of the two groups were similar, indicating that the level of insulin resistance still improved. Therefore, the use of metformin needs to be considered in the relevant research on improving insulin resistance through testosterone replacement therapy, and the relationship between testosterone changes and insulin resistance in patients using metformin still needs to be further studied.
In fact, the importance of testosterone in men with T2DM is often ignored. In 2018, the American Diabetes Association added a recommendation in the standard of diabetes medical care to measure testosterone levels in men with symptoms of diabetes and hypogonadism (29). Many studies have shown that testosterone replacement therapy can prevent prediabetes or T2DM status in men with low testosterone (30–32). Furthermore, attention should be paid to the effect of drugs on testosterone levels in men with T2DM, which is the value of this study.
Some limitations of this study deserve comment. The study population is patients using premixed insulin. Whether the research results can be extended to the whole population needs further research. Although there is a control group, the effect of changes in the dose of insulin or other hypoglycemic drugs cannot be completely excluded in the real-world study.
In conclusion, our data indicate that the 3-month metformin treatment can reduce testosterone levels in men with T2DM. In the future, attention should be paid to the effect of drugs on male testosterone levels in the treatment of diabetes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of Nanjing First Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TC and YH performed, analyzed data and wrote the manuscript. BD, RY, BL, LCa, TJ, LJ, YW, HW, YZ, KH, LX, LCh, CC organized data. XX modified the manuscript. JM conceived, and directed the study. All authors contributed to the article and approved the submitted version.
Funding
This study was partly supported by the National Key R&D Program of China (No. 2018YFC1314103).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the members of Endocrinology department of Nanjing First Hospital for their support.
References
1. Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat Rev Endocrinol (2016) 12(10):616–22. doi: 10.1038/nrendo.2016.105
2. Gregg EW, Sattar N, Ali MK. The Changing Face of Diabetes Complications. Lancet Diabetes Endocrinol (2016) 4(6):537–47. doi: 10.1016/S2213-8587(16)30010-9
3. Renner S, Blutke A, Clauss S, Deeg CA, Kemter E, Merkus D, et al. Porcine Models for Studying Complications and Organ Crosstalk in Diabetes Mellitus. Cell Tissue Res (2020) 380(2):341–78. doi: 10.1007/s00441-019-03158-9
4. Corona G, Giorda CB, Cucinotta D, Guida P, Nada E, Gruppo di studio SUBITO-DE. Sexual Dysfunction at the Onset of Type 2 Diabetes: The Interplay of Depression, Hormonal and Cardiovascular Factors. J Sex Med (2014) 11(8):2065–73. doi: 10.1111/jsm.12601
5. Bain J. The Many Faces of Testosterone. Clin Interv Aging (2007) 2(4):567–76. doi: 10.2147/cia.s1417
6. Tyagi V, Scordo M, Yoon RS, Liporace FA, Greene LW. Revisiting the Role of Testosterone: Are We Missing Something. Rev Urol (2017) 19(1):16–24. doi: 10.3909/riu0716
7. Rastrelli G, Corona G, Tarocchi M, Mannucci E, Maggi M. How to Define Hypogonadism? Results From a Population of Men Consulting for Sexual Dysfunction. J Endocrinol Invest (2016) 39(4):473–84. doi: 10.1007/s40618-015-0425-1
8. Ghanim H, Dhindsa S, Batra M, Green K, Abuaysheh S, Kuhadiya ND, et al. Testosterone Increases the Expression and Phosphorylation of AMP Kinase α in Men With Hypogonadism and Type 2 Diabetes. J Clin Endocrinol Metab (2020) 105(4):1169–75. doi: 10.1210/clinem/dgz288
9. Derkach KV, Bakhtyukov AA, Bayunova LV, Zorina II, Shpakov AO. Normalization of Testicular Steroidogenesis and Spermatogenesis in Male Rats With Type 2 Diabetes Mellitus Under the Conditions of Metformin Therapy. Dokl Biol Sci (2020) 493(1):110–3. doi: 10.1134/S0012496620040031
10. Jones TH. Testosterone Deficiency: A Risk Factor for Cardiovascular Disease. Trends Endocrinol Metab (2010) 21(8):496–503. doi: 10.1016/j.tem.2010.03.002
11. Kelly DM, Jones TH. Testosterone and Cardiovascular Risk in Men. Front Horm Res (2014) 43:1–20. doi: 10.1159/000360553
12. Faure M, Bertoldo MJ, Khoueiry R, Bongrani A, Brion F, Giulivi C, et al. Metformin in Reproductive Biology. Front Endocrinol (Lausanne) (2018) 9:675. doi: 10.3389/fendo.2018.00675
13. Ohara M, Yoshida-Komiya H, Ono-Okutsu M, Yamaguchi-Ito A, Takahashi T, Fujimori K. Metformin Reduces Androgen Receptor and Upregulates Homeobox A10 Expression in Uterine Endometrium in Women With Polycystic Ovary Syndrome. Reprod Biol Endocrinol (2021) 19(1):77. doi: 10.1186/s12958-021-00765-6
14. Apaijai N, Chinda K, Palee S, Chattipakorn S, Chattipakorn N. Combined Vildagliptin and Metformin Exert Better Cardioprotection Than Monotherapy Against Ischemia-Reperfusion Injury in Obese-Insulin Resistant Rats. PloS One (2014) 9(7):e102374. doi: 10.1371/journal.pone.0102374
15. Hu Y, Ding B, Shen Y, Yan RN, Li FF, Sun R, et al. Rapid Changes in Serum Testosterone in Men With Newly Diagnosed Type 2 Diabetes With Intensive Insulin and Metformin. Diabetes Care (2021) 44(4):1059–61. doi: 10.2337/dc20-1558
16. de Ronde W, van der Schouw YT, Pols HA, Gooren LJ, Muller M, Grobbee DE, et al. Calculation of Bioavailable and Free Testosterone in Men: A Comparison of 5 Published Algorithms. Clin Chem (2006) 52(9):1777–84. doi: 10.1373/clinchem.2005.063354
17. Dean JD, McMahon CG, Guay AT, Morgentaler A, Althof SE, Becher EF, et al. The International Society for Sexual Medicine’s Process of Care for the Assessment and Management of Testosterone Deficiency in Adult Men. J Sex Med (2015) 12:1660–86. doi: 10.1111/jsm.12952
18. Li SY, Zhao YL, Yang YF, Wang X, Nie M, Wu XY, et al. Metabolic Effects of Testosterone Replacement Therapy in Patients With Type 2 Diabetes Mellitus or Metabolic Syndrome: A Meta-Analysis. Int J Endocrinol (2020) 2020:4732021. doi: 10.1155/2020/4732021
19. Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, et al. Low Testosterone Levels are Common and Associated With Insulin Resistance in Men With Diabetes. J Clin Endocrinol Metab (2008) 93(5):1834–40. doi: 10.1210/jc.2007-2177
20. Watanobe H, Hayakawa Y. Hypothalamic Interleukin-1 Beta and Tumor Necrosis Factor-Alpha, But Not Interleukin-6, Mediate the Endotoxin-Induced Suppression of the Reproductive Axis in Rats. Endocrinology (2003) 144(11):4868–75. doi: 10.1210/en.2003-0644
21. Derosa G, Catena G, Scelsi L, D'Angelo A, Raddino R, Cosentino E, et al. Glyco-Metabolic Control, Inflammation Markers, and Cardiovascular Outcomes in Type 1 and Type 2 Diabetic Patients on Insulin Pump or Multiple Daily Injection (Italico Study). Diabetes Metab Res Rev (2020) 36(1):e3219. doi: 10.1002/dmrr.3219
22. Liu CY, Chang TC, Lin SH, Wu ST, Cha TL, Tsao CW. Metformin Ameliorates Testicular Function and Spermatogenesis in Male Mice With High-Fat and High-Cholesterol Diet-Induced Obesity. Nutrients (2020) 12(7):1932. doi: 10.3390/nu12071932
23. Derkach KV, Bakhtyukov AA, Romanova IV, Zorina II, Bayunova LV, Bondareva VM, et al. The Effect of Metformin Treatment on the Basal and Gonadotropin-Stimulated Steroidogenesis in Male Rats With Type 2 Diabetes Mellitus. Andrologia (2020) 52(11):e13816. doi: 10.1111/and.13816
24. Faure M, Guibert E, Alves S, Pain B, Ramé C, Dupont J, et al. The Insulin Sensitiser Metformin Regulates Chicken Sertoli and Germ Cell Populations. Reproduction (2016) 151(5):527–38. doi: 10.1530/REP-15-0565
25. Zhang Y, Wang S, Wang X, Liao S, Wu Y, Han C. Endogenously Produced FGF2 is Essential for the Survival and Proliferation of Cultured Mouse Spermatogonial Stem Cells. Cell Res (2012) 22(4):773–6. doi: 10.1038/cr.2012.17
26. Nieschlag E. Late-Onset Hypogonadism: A Concept Comes of Age. Andrology (2020) 8(6):1506–11. doi: 10.1111/andr.12719
27. Corona G, Maseroli E, Rastrelli G, Francomano D, Aversa A, Hackett GI, et al. Is Late-Onset Hypogonadotropic Hypogonadism a Specific Age-Dependent Disease, or Merely an Epiphenomenon Caused by Accumulating Disease-Burden. Minerva Endocrinol (2016) 41(2):196–210.
28. Corona G, Rastrelli G, Vignozzi L, Barbonetti A, Sforza A, Mannucci E, et al. The Role of Testosterone Treatment in Patients With Metabolic Disorders. Expert Rev Clin Pharmacol (2021) 14(9):1091–103. doi: 10.1080/17512433.2021.1938548
29. Summary of Revisions: Standards of Medical Care in Diabetes-2018. Diabetes Care (2018) 41:S4–4S6. doi: 10.2337/dc18-Srev01
30. Haider KS, Haider A, Saad F, Doros G, Hanefeld M, Dhindsa S, et al. Remission of Type 2 Diabetes Following Long-Term Treatment With Injectable Testosterone Undecanoate in Patients With Hypogonadism and Type 2 Diabetes: 11-Year Data From a Real-World Registry Study. Diabetes Obes Metab (2020) 22:2055–68. doi: 10.1111/dom.14122
31. Yassin A, Haider A, Haider KS, Caliber M, Doros G, Saad F, et al. Testosterone Therapy in Men With Hypogonadism Prevents Progression From Prediabetes to Type 2 Diabetes: Eight-Year Data From a Registry Study. Diabetes Care (2019) 42:1104–11. doi: 10.2337/dc18-2388
32. Wittert G, Bracken K, Robledo KP, Grossmann M, Yeap BB, Handelsman DJ, et al. Testosterone Treatment to Prevent or Revert Type 2 Diabetes in Men Enrolled in a Lifestyle Programme (T4DM): A Randomised, Double-Blind, Placebo-Controlled, 2-Year, Phase 3b Trial. Lancet Diabetes Endocrinol (2021) 9:32–45. doi: 10.1016/S2213-8587(20)30367-3
Keywords: type 2 diabetic mellitus, metformin, testosterone levels, Glycated Albumin, blood glucose
Citation: Cai T, Hu Y, Ding B, Yan R, Liu B, Cai L, Jing T, Jiang L, Xie X, Wang Y, Wang H, Zhou Y, He K, Xu L, Chen L, Cheng C and Ma J (2021) Effect of Metformin on Testosterone Levels in Male Patients With Type 2 Diabetes Mellitus Treated With Insulin. Front. Endocrinol. 12:813067. doi: 10.3389/fendo.2021.813067
Received: 11 November 2021; Accepted: 08 December 2021;
Published: 24 December 2021.
Edited by:
Vito Angelo Giagulli, University of Bari Medical School, ItalyReviewed by:
Farid Saad, Bayer, GermanyVitarani D. A Ningrum, Islamic University of Indonesia, Indonesia
Copyright © 2021 Cai, Hu, Ding, Yan, Liu, Cai, Jing, Jiang, Xie, Wang, Wang, Zhou, He, Xu, Chen, Cheng and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Ma, bWFqaWFuaHVhMTk2NTAzQDEyNi5jb20=
†These authors have contributed equally to this work
 Tingting Cai
Tingting Cai Yun Hu1†
Yun Hu1† Bingli Liu
Bingli Liu Yuming Wang
Yuming Wang Yunting Zhou
Yunting Zhou Jianhua Ma
Jianhua Ma