- 1Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Alzheimer’s Disease and Related Disorders Center, Shanghai Jiao Tong University, Shanghai, China
Background: It is well known that schizophrenia is associated with sex differences. However, no study has explored the sex differences in obesity and cognitive function in elderly Chinese patients with schizophrenia.
Objective: This study aimed to compare sex differences in obesity and cognitive function in elderly Chinese individuals with schizophrenia.
Methods: A total of 304 elderly patients with schizophrenia and 130 sex- and age-matched healthy controls from the community were recruited. Demographic, clinical, and lipid parameters were collected for all subjects. The Montreal Cognitive Assessment (MoCA) was used to assess the global cognitive functions of the participants, while the Positive and Negative Syndrome Scale (PANSS) was used to assess psychopathological symptoms in patients with schizophrenia.
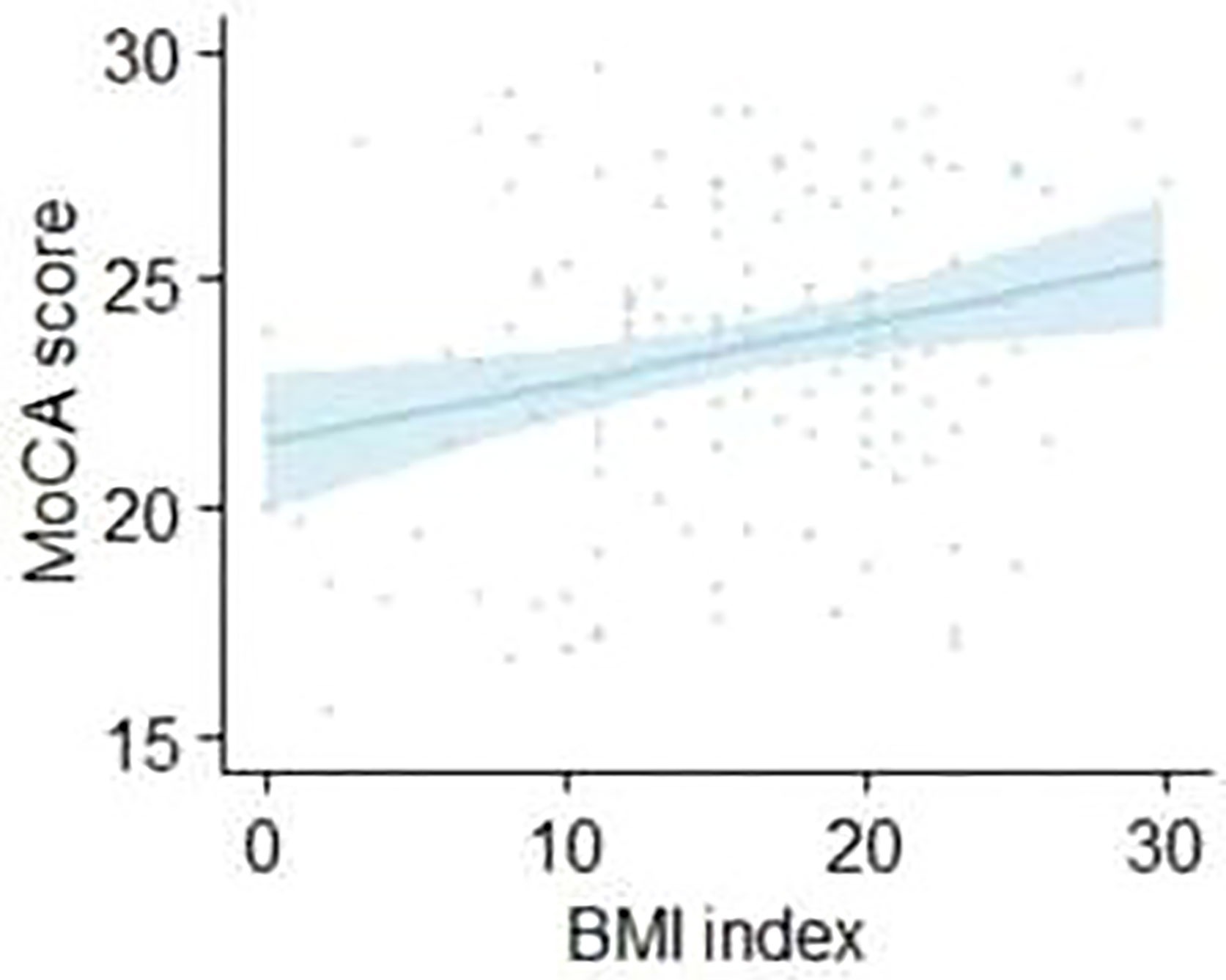
Results: Of the patients with schizophrenia, the prevalence of obesity in men and women was 11.7% (19/163) and 21.3% (30/141), respectively. The score (14.51 ± 6.504) of MOCA in elderly male patients with schizophrenia was significantly higher than that (11.40 ± 6.822) in female patients. There was a positive correlation between the MOCA scores and body mass index (BMI) (r=0.206, p=0.018) in male elderly patients with schizophrenia. Conversely, the MOCA scores of female elderly patients with schizophrenia did not correlate with BMI (p>0.05). However, we found no sex differences in obesity and cognition among control older adults.
Conclusions: Our findings suggest that there are significant sex differences in obesity and cognitive function in elderly Chinese patients with schizophrenia.
Introduction
Sex differences have been reported in schizophrenia (1). Previous studies have shown that the incidence rates (cases per 100,000 per year) of schizophrenia in men are 1.4 higher than in women (2). Moreover, men with schizophrenia typically present with more negative symptoms (e.g. Flattened affect, social withdrawal) (3), and they are more likely to have fewer years of education, are less productive at work, and have more impaired social functioning than women (4). In contrast, women tend to have a later onset (on average, women develop the disease three to four years later than men, with a second peak around menopause (5)) and fewer symptoms. Additionally, since women are less likely to suffer from social drift, substance abuse, and law infringement, they often have a better course and outcome (6).
Cognitive impairment is another prominent clinical feature of schizophrenia (7). Patients with schizophrenia often show cognitive deficits across several domains, including attention, memory, learning, executive functioning, and cognitive processing speed (8). They have been linked to poor functional outcomes and long-term disability (9). Sex differences in cognition are also found in patients with schizophrenia; for example, men often show an advantage in visuospatial abilities, whereas women show an advantage in verbal fluency, visual scanning, verbal memory, and fine motor skills (10). Moreover, executive functioning may have a lesser impact on female patients’ symptoms and function profiles with schizophrenia spectrum than on male patients (11). Moreover, men with schizophrenia tend to have more significant overall cognitive deficits (12). However, studies have shown no differences in cognitive performance between men and women with schizophrenia (13). Therefore, the conclusions of relevant studies are not consistent, and we speculate that they may be related to the inclusion criteria, assessment tools, and the effects of drugs.
Obesity is another critical issue in patients with schizophrenia (14) due to sedentary lifestyles, side effects of antipsychotics, and poor dietary choices resulting from cognitive deficits (15). Obesity is linked to greater morbidity, mortality, and decreased life expectancy and lower quality of life (16–18). The connection between obesity and worse cognitive performance has been demonstrated in non-psychiatric samples (19–21). Some studies have shown that obesity can also increase cognitive impairment in individuals with schizophrenia. For example, Lindenmayer et al. (22) reported a significant association between waist circumference and domains of attention/vigilance and processing speed, suggesting that obesity might lead to cognitive impairment. Spangaro et al. (23) found that elevated body mass index (BMI) might contribute to white matter (WM) disruption in schizophrenia by hampering structural connectivity in critical cortico-limbic networks. However, some studies show that cognition is not associated with obesity (24, 25). Therefore, pertinent research conclusions are inconsistent.
Over the past few decades, the interest in differences between men and women with schizophrenia has expanded to obesity and neurocognitive function (26). For example, Fang Yang et al. found that there were sex differences in obesity, BMI, and brain-derived neurotrophic factor (BDNF) levels, and BMI was only negatively correlated with BDNF in female patients (27). Xiao-E Lang et al. found sex differences in white matter dis-connectivity and its relationship to psychopathological symptoms in an early course of schizophrenia onset (4). Moreover, Xinxin Huang et al. pointed out that miR-195 was associated with cognitive impairment in female schizophrenia patients, and it may be involved in the underlying mechanism of sex differences in cognitive impairment in schizophrenia (28). Understanding these sex differences can help better understand the pathogenesis of schizophrenia and give more targeted treatment to patients of different genders. Genes have important influences on obesity and cognition, and both obesity and cognitive levels will change with age (29, 30). However, to the best of our knowledge, there is no research on sex differences in obesity and cognitive function in elderly Chinese patients with schizophrenia. This is therefore the first (cross-sectional) study to explore the effects of sex on obesity and cognitive function in elderly patients with schizophrenia.
Materials and Methods
Sample Size Calculation Basis
Previous studies suggested that the prevalence of obesity in chronic schizophrenia was 16.4% (31), and the prevalence of obesity in male and female patients with schizophrenia was 31.82% and 15.83% (32), respectively. Therefore, at least 30/0.32 = 93.75 for males and 30/0.16 = 187.5 for females were required for estimation based on the minimum sample size of 30 for each group, and a total of at least 282 subjects were included. Finally, 304 elderly patients with schizophrenia were included.
Participants
Inclusion and Exclusion Criteria
Based on the previous sample size calculation, a total of 304 elderly patients (men/women=163/141) with schizophrenia were recruited from the Shanghai Mental Health Center. All participants were required to meet the following requirements: 1) age ≥ 60 years; 2) diagnosed with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5): Two (or more) of the following: 1) delusions; 2) hallucinations; 3) disorganized speech; 4) grossly disorganized or catatonic behavior; 5) Negative symptoms, i.e., affective flattening, alogia, or avolition; and each present for a significant portion of time during a 1-month period (or less if successfully treated); 3) without severe medical conditions, such as cancer and infections; and 4) willingness to cooperate. Subjects with schizoaffective disorder, depressive or bipolar disorder with psychotic features, cancer, dementia, cardiovascular disease, organic brain disease, sex hormone use history or with some relevant diseases of sex hormone disorder were ruled out. All the eligible participants’ information, including general demographic information (age, education, sex), daily life habit information (smoking, drinking, drinking tea, physical exercise, and hobbies), and currently prescribed medicines (clozapine, olanzapine, quetiapine, risperidone, aripiprazole), was collected using standardized questionnaires. Additional information was gathered from medical records and collateral resources. A complete physical examination, medical history, and laboratory tests were also performed for each subject. Detailed inclusion criteria and information collection process were introduced in our previous studies (33).
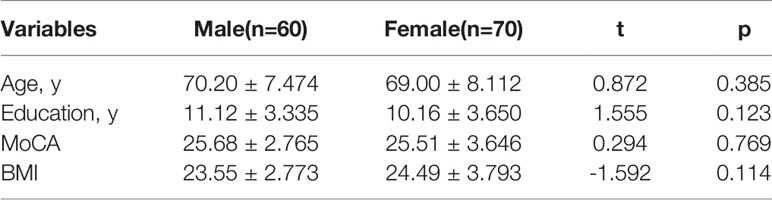
Moreover, 130 healthy controls (60 males and 70 females) were also recruited by advertisements at the local community, who were matched for age and sex with patients with schizophrenia (they had a mean education of 10.60 ± 3.528 years and an average age of 69.55 ± 7.817 years). All healthy controls were interviewed by trained investigators overseen by a research psychiatrist. None had any personal or family history, nor any psychiatric evaluation for clinical mental illness.
Ethical
The Research Ethical Committee of the Affiliated Mental Health Center of Shanghai Jiaotong University School of Medicine approved the study protocol. Written informed consent was obtained from all the participants before the study. All research procedures were carried out per the principles of the Declaration of Helsinki.
Measurement of Body Mass Index (BMI), Fasting Blood Glucose, and Lipid Profile
BMI was determined by dividing weight by height squared (kg/m2). Based on the Chinese Working Group on Obesity in China (WGOC) criteria (34), participants with BMI≥28 were classified as obese and those with BMI < 28 as non-obese. Peripheral blood samples were collected between 7 and 9 am after an overnight shift. The values of serum fasting blood glucose, triglyceride, cholesterol, low-density lipoprotein, and high-density lipoprotein were obtained using the hexokinase method on an auto-analyser (Dimension Xpand plus).
Cognitive Assessment and Psychiatric Symptom Assessment
The Montreal Cognitive Assessment (MoCA) (35) and the Positive and Negative Syndrome Scale (PANSS) (36) were used to assess the overall cognitive function and the severity of psychotic symptoms of the participants, respectively. Previous studies have suggested that MoCA is a validated, clinician friendly, brief instrument for screening cognitive deficits in schizophrenia (37, 38). Moreover, we also utilized the Geriatric Depression Scale (GDS) (39) to exclude depression, and a GDS score of 10 or more was considered depressed. In our current study, no one was excluded because of depression.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or median (p25, p75) and categorical variables as frequencies (%). First, a single-sample Kolmogorov-Smirnov test was used to check whether the data was normal. Next, an independent sample t-test was used to compare the normal data between the male and female groups. The Mann-Whitney U test was used to compare data with a non-normal distribution. The Chi-square test was used to analyze the categorical variables between the two groups. The MoCA was analyzed using a 2 × 2 ANOVA representing the factors of obesity and gender (male vs. female). In the MoCA comparisons, age and education level were used as covariables in the multivariate analysis of covariance (MANCOVA). They were also included in the MoCA total score and its seven cognitive domains of dependency measures to examine significant diagnostic differences. The independent predictors were group, gender, and group×gender interaction. In the post hoc comparisons, a multiple testing correction was also performed. After controlling for several potential confounding factors, such as age, education level, and clinical variables, partial correlation analysis was used to explore the relationship between MOCA and BMI. Two-tailed tests were performed at a significance level of P<0.05, and Bonferroni correction was used to correct p-values for multiple comparisons. All the statistical analysis was performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Prevalence of Obesity in Men and Women in Patients With Schizophrenia and Controls
Of the patients with schizophrenia, the prevalence of obesity in men and women was 11.7% (19/163) and 21.3% (30/141), respectively. The Chi-square test results showed a statistically significant difference (p=0.028) in the prevalence of obesity in men and women. In control older adults, the prevalence of obesity was 6.7%(4/60) in men and 15.7%(11/70) in women. However, chi-square results showed no difference (p=0.168) between the two groups.
Sample Characteristics
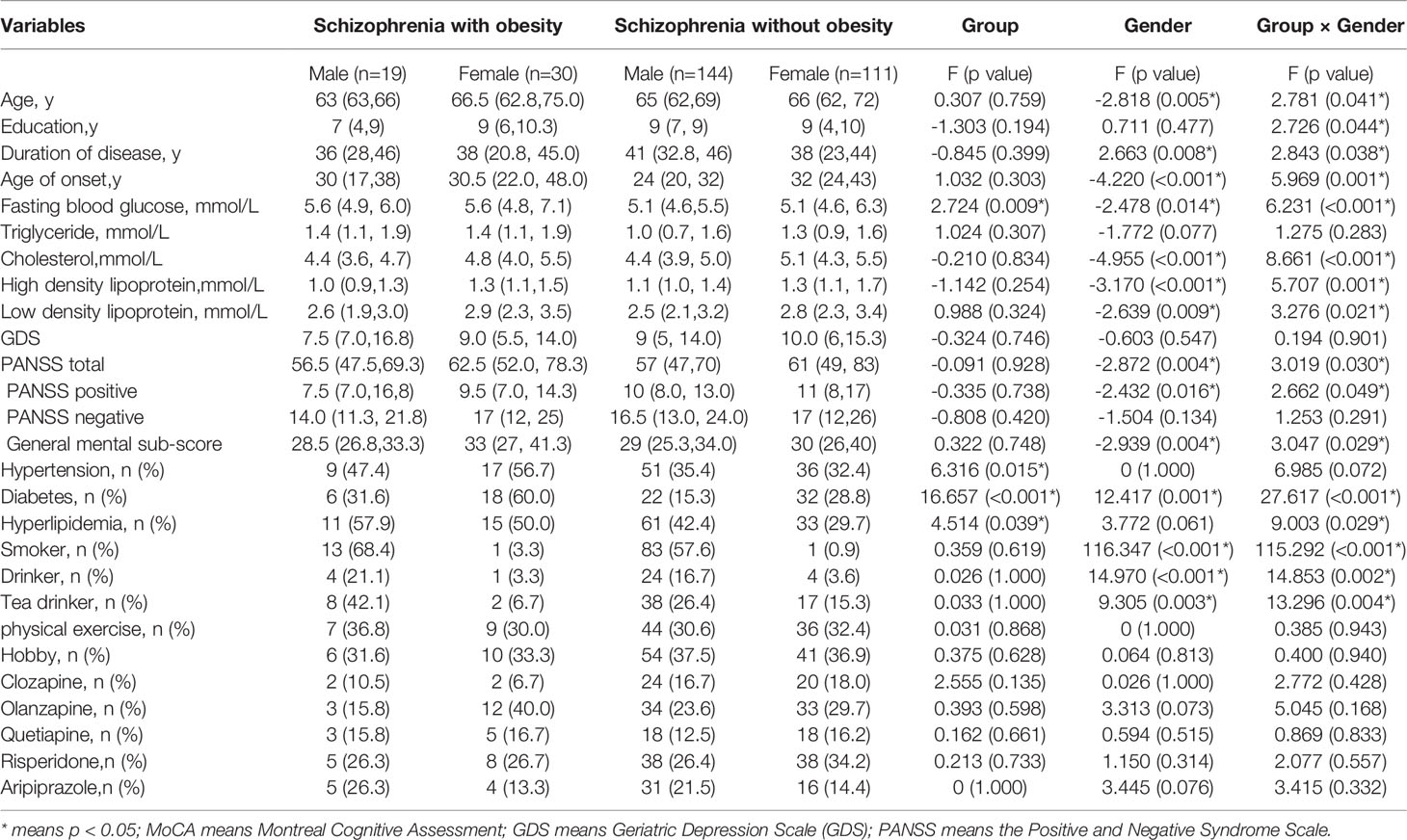
Demographic and clinical characteristics of schizophrenia are summarized in Table 1. There were significant gender differences (p<0.05) in age, duration of disease, age of onset, fasting blood glucose, cholesterol, high density lipoprotein, low density lipoprotein, PANSS total, PANSS positive symptoms, PANSS general psychopathology, diabetes, smoker, drinker, and tea drinker. However, there were no significant gender differences (p>0.05) in education, triglyceride, GDS, PANSS negative, hypertension, hyperlipidemia, physical exercise, hobby, clozapine, olanzapine, quetiapine, risperidone and Aripiprazole. There was a significant interaction between obesity and gender in age, education, duration of disease, age of onset, fasting blood glucose, cholesterol, high density lipoprotein, low density lipoprotein, PANSS total, PANSS positive symptoms, PANSS general psychopathology, diabetes, smoker, drinker, and tea drinker (p < 0.05). Moreover, there were no significant gender differences (p>0.05) in age, education, BMI and MoCA in control older adults. Table 2 shows the results.
Gender Difference in Cognitive Performance in Schizophrenia and Controls With and Without Obesity
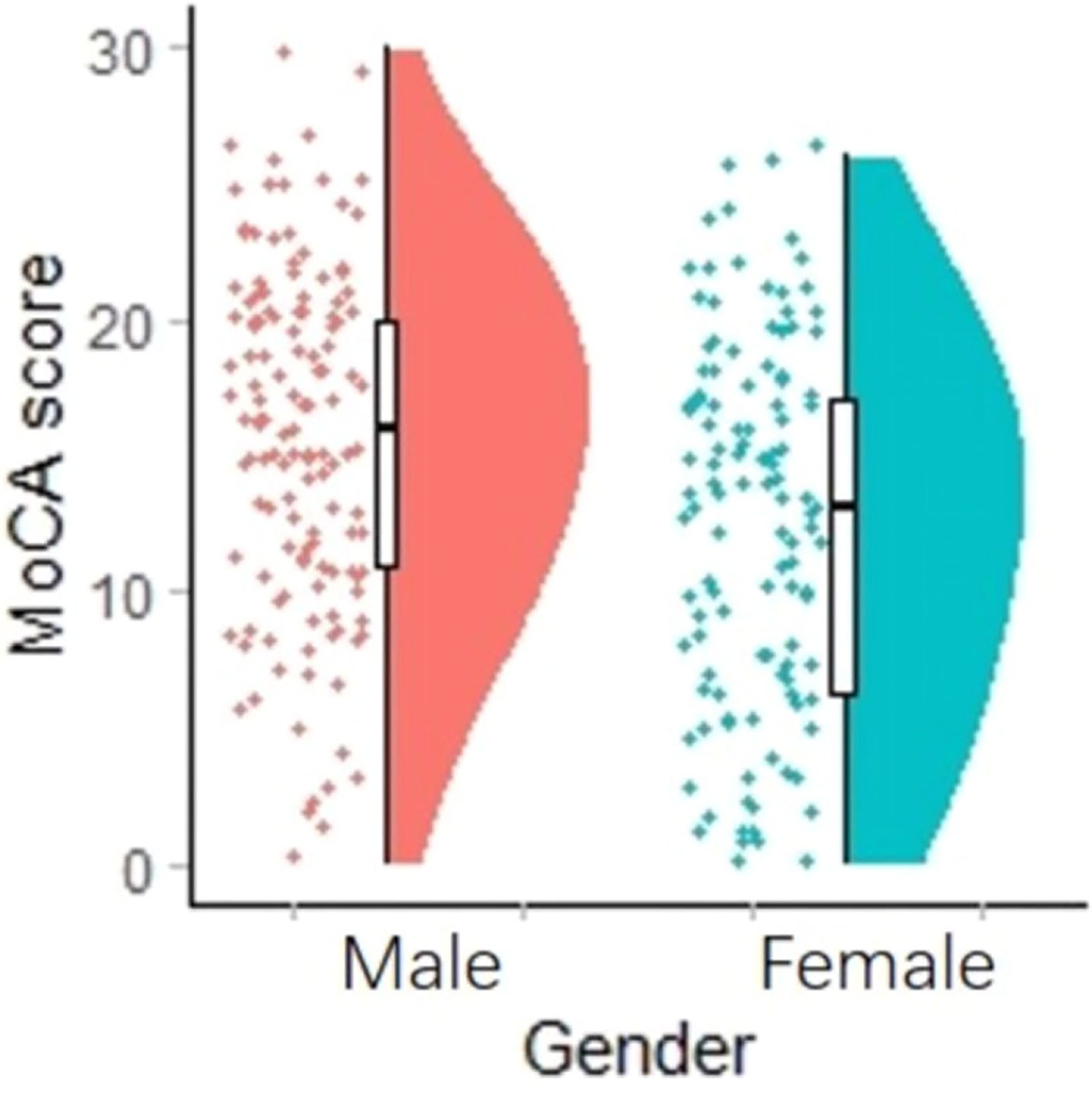
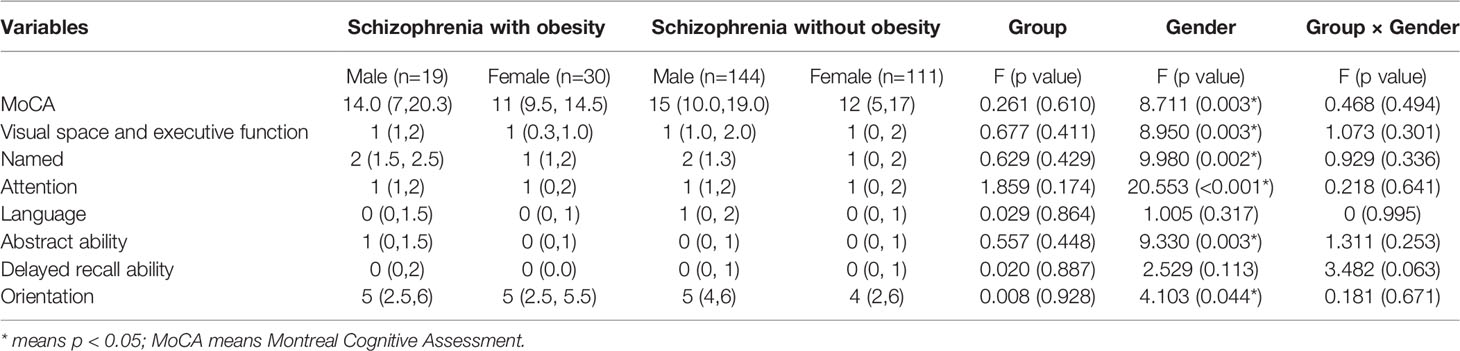
Multivariate analysis of covariance (MANCOVA) was used to investigate the effects of gender and obesity on cognitive functions. After controlling for age, education and PANSS, we found no statistically significant differences in MoCA scores and subtests between obesity and non-obesity groups, while male schizophrenics had higher MoCA scores, visual space and executive function, named, attention, abstract ability, and orientation than female schizophrenics (Figure 1); Multivariate analysis of covariance (MANCOVA) and post hoc comparisons showed that gender and obesity had no interaction effect on MoCA total score and other sub-test scores (when both sex and obesity were included, gender differences in MOCA scores and subtests disappeared). Table 3 shows the results. In control older adults, we found that there was no effect of gender or obesity on cognitive function.
Relationship Between MoCA and BMI in Schizophrenia and Controls
We then explored the relationship between BMI and cognitive functions in both sexes in patients with schizophrenia. Using partial correlation analysis (age, education, and PANSS were controlled), we found a positive correlation between the MOCA score and BMI (r=0.206, p=0.018) in male elderly patients with schizophrenia. Conversely, the MOCA score of female elderly patients with schizophrenia did not correlate with BMI (r=0.053, p=0.578). Figure 2 shows the results. As above, we also did not find a link between BMI and MOCA in control older adults in both sexes.
Discussion
This study revealed several interesting findings: 1) the prevalence of obesity in elderly Chinese men and women with schizophrenia was 11.7% and 21.3%, respectively; 2) the MOCA score in elderly male patients with schizophrenia was significantly higher than that in female patients, and there was no interaction between gender and BMI; 3) there was a positive correlation between MOCA scores and BMI (r=0.206, p=0.018) in male elderly patients with schizophrenia, while the scores of female elderly patients with schizophrenia did not correlate with BMI (p>0.05). However, this conclusion does not apply to general elderly people.
In the present study, we found that female patients had a higher prevalence of obesity than male patients (nearly double), consistent with previous studies (17, 40, 41). Moreover, compared to non-obese individuals, obese women had a higher proportion of hypertension, diabetes, and hyperlipidaemia, suggesting that they develop metabolic syndrome (42, 43). However, in our study, we did not find that obesity had any effect on mental symptoms or cognitive function among female patients with schizophrenia, inconsistent with other research conclusions (44, 45). The reason may be related to our failure to consider the effects of medication dose and menopause on antipsychotic medications whose metabolism is influenced by estrogen levels.
In addition, we found that aging men with schizophrenia had better overall cognitive function than women. The above conclusion is still valid after controlling for age, education, duration of disease, and other related variables. Zhang BH (46) reported that in schizophrenia with diabetes, men had significantly worse cognition than women in all cognitive domains. Contrastingly, in schizophrenia without diabetes, men showed worse performance in immediate and delayed memory than women. Zhang et al. (40) found that male patients with schizophrenia had more cognitive impairment than female patients in reasoning and problem solving, working memory, social cognition, verbal learning, processing speed, and visual learning. Zhang XY (8) found that male patients with chronic schizophrenia had significantly lower immediate memory and delayed memory scores than female patients. Therefore, our conclusions are the opposite. We noticed that the age of male patients (66.31 ± 5.895) was significantly lower than that of female patients (68.32 ± 7.252), which may explain the differences in these studies. Moreover, men with schizophrenia in our study had a higher rate of smoking, which might also be a protective factor for cognitive function (47, 48).The mechanism might be as follows: cholinergic neurotransmission plays an important role in cognition. It has been reported that patients with schizophrenia have significant impairment of cholinergic function. The high smoking schizophrenia comorbidities observed in schizophrenics might be an attempt to compensate for cholinergic dysfunction (49). Moreover, smoking may reduce the blood concentration of some antipsychotic drugs (50).
In addition, we found statistical differences in cognitive scores between men and women with schizophrenia, but did not find an interaction between obesity and gender. Men with schizophrenia, though, showed a better cognitive state, however, after taking into account both gender and obesity, we found that the male advantage in MOCA scores, visual space and executive function, named, attention, abstract ability, and orientation disappeared. In Benjamin AM’s study, they found that there was a sex-influenced association between genetic variation at the LYPLAL1 locus and obesity-related traits (51). In Meng-Qi Chen’ s study, they found that high BMI and high waist-to-hip ratio (WHpR) have synergistic interactions with hypertension on the risk of diabetes for females (52). Moreover, Yunker AG et al. also found that female individuals and those with obesity may be particularly sensitive to disparate neural responsivity elicited by sucralose compared with sucrose consumption (53). Therefore, the interaction between obesity and gender on cognitive function needs further validation with larger sample sizes.
Finally, we explored the relationship between BMI and cognitive functions in men and women. We found that MoCA was only positively correlated with BMI in men with schizophrenia, while there was no such association in women with schizophrenia. However, Rashid NA (54) found that the indirect effect of BMI on cognition through schizophrenia was present in both sexes. Conversely, the indirect effect of cognition on BMI through schizophrenia was only found in women. In addition, Hidese et al. (55) pointed out that BMI scores were significantly negatively correlated with the Brief Assessment of Cognition in Schizophrenia (BACS) test composite score, which was in line with other research conclusions (56–58). Therefore, our findings are inconsistent. These differences might be ascribed to age and sex mismatches. In addition to that, men tend to have an earlier onset of illness while females have lower negative symptom scores (59) and are more likely to be obese. So studying these two groups separately might allow us to shed light on the relationship between gender, obesity, schizophrenia and cognition (54).
We acknowledge that there are several limitations to our research. First, this was a cross-sectional study, and it was impossible to establish causality between sex and obesity or cognition; second, all the data were collected from chronic Chinese patients who had been taking antipsychotics for a long time, and the dose and specifics of antipsychotic treatment might impact the results; third, relevant variables were not addressed, such as controlling for dose of antipsychotics and separate investigation of pre and post-menopausal women.
Conclusions
The prevalence of obesity in elderly women with schizophrenia is higher than that in men; 2) the cognitive function of elderly women with schizophrenia is worse than that of men; 3) In this study, BMI is positively correlated with cognitive scores only in the men. In summary, there are many factors leading to cognitive decline in elderly patients with schizophrenia, among which are body mass index and gender.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The Research Ethical Committee of the Affiliated Mental Health Center of Shanghai Jiaotong University School of Medicine approved the study protocol. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WL and SL contributed to the concept and design of the study. LY acquired the data. YF and SX analysed the data and drafted the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from “National Key R&D Program of China” 2018YFC2002302, the Clinical Research Centre Project of Shanghai Mental Health Center (CRC2017ZD02), Shanghai Clinical Research Center for Mental Health (19MC1911100); the Cultivation of Multidisciplinary Interdisciplinary Project in Shanghai Jiaotong University (YG2019QNA10), curriculum reform of the Medical College of Shanghai Jiaotong University, the Feixiang Program of Shanghai Mental Health Center (2020-FX-03), and Youth Scientific Research Project of Shanghai Municipal Commission of Health and Family Planning (20184Y0298).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MHX declared a shared affiliation with the author(s) HA to the handling editor at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MoCA, Montreal Cognitive Assessment; PANSS, Positive and Negative Syndrome Scale; SDs, Standard deviations; ICD-10, International Classification of Diseases 10 diagnostic standards; BMI, Body mass index; WGOC, Chinese Working Group on Obesity in China criteria; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale.
References
1. Aleman A, Kahn RS, Selten JP. Sex Differences in the Risk of Schizophrenia: Evidence From Meta-Analysis. Arch Gen Psychiatry (2003) 60(6):565–71. doi: 10.1001/archpsyc.60.6.565
2. McGrath J, Saha S, Chant D, Welham J. Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality. Epidemiol Rev (2008) 30:67–76. doi: 10.1093/epirev/mxn001
3. Chang WC, Hui CL, Tang JY, Wong GH, Lam MM, Chan SK, et al. Persistent Negative Symptoms in First-Episode Schizophrenia: A Prospective Three-Year Follow-Up Study. Schizophr Res (2011) 133(1-3):22–8. doi: 10.1016/j.schres.2011.09.006
4. Lang XE, Zhu D, Zhang G, Du X, Jia Q, Yin G, et al. Sex Difference in Association of Symptoms and White Matter Deficits in First-Episode and Drug-Naive Schizophrenia. Trans Psychiatry (2018) 8(1):281. doi: 10.1038/s41398-018-0346-9
5. Häfner H, an der Heiden W. Epidemiology of Schizophrenia. Can J Psychiatry Rev Can Psychiatrie (1997) 42(2):139–51. doi: 10.1177/070674379704200204
6. Cocchi A, Lora A, Meneghelli A, La Greca E, Pisano A, Cascio MT, et al. Sex Differences in First-Episode Psychosis and in People at Ultra-High Risk. Psychiatry Res (2014) 215(2):314–22. doi: 10.1016/j.psychres.2013.11.023
7. Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal Neuropsychological Follow-Up Study of Patients With First-Episode Schizophrenia. Am J Psychiatry (1999) 156(9):1336–41. doi: 10.1176/ajp.156.9.1336
8. Zhang XY, Chen DC, Tan YL, Tan SP, Wang ZR, Yang FD, et al. Gender Difference in Association of Cognition With BDNF in Chronic Schizophrenia. Psychoneuroendocrinology (2014) 48:136–46. doi: 10.1016/j.psyneuen.2014.06.004
9. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive Deficits and Functional Outcome in Schizophrenia: Are We Measuring the “Right Stuff”? Schizophr Bull (2000) 26(1):119–36. doi: 10.1093/oxfordjournals.schbul.a033430
10. Rubin LH, Carter CS, Drogos LL, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Effects of Sex, Menstrual Cycle Phase, and Endogenous Hormones on Cognition in Schizophrenia. Schizophr Res (2015) 166(1-3):269–75. doi: 10.1016/j.schres.2015.04.039
11. Karilampi U, Helldin L, Archer T. Cognition and Global Assessment of Functioning in Male and Female Outpatients With Schizophrenia Spectrum Disorders. J Nervous Ment Dis (2011) 199(7):445–8. doi: 10.1097/NMD.0b013e318221413e
12. Bangasser DA, Eck SR, Telenson AM, Salvatore M. Sex Differences in Stress Regulation of Arousal and Cognition. Physiol Behav (2018) 187:42–50. doi: 10.1016/j.physbeh.2017.09.025
13. Ayesa-Arriola R, Rodriguez-Sanchez JM, Gomez-Ruiz E, Roiz-Santiáñez R, Reeves LL, Crespo-Facorro B. No Sex Differences in Neuropsychological Performance in First Episode Psychosis Patients. Prog Neuropsychopharmacol Biol Psychiatry (2014) 48:149–54. doi: 10.1016/j.pnpbp.2013.09.009
14. Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll CU. Weight Gain and Obesity in Schizophrenia: Epidemiology, Pathobiology, and Management. Acta Psychiatrica Scand (2015) 132(2):97–108. doi: 10.1111/acps.12445
15. Guo X, Zhang Z, Zhai J, Wu R, Liu F, Zhao J. The Relationship Between Obesity and Health-Related Quality of Life in Chinese Patients With Schizophrenia. Int J Psychiatry Clin Pract (2013) 17(1):16–20. doi: 10.3109/13651501.2012.745574
16. Laursen TM, Nordentoft M, Mortensen PB. Excess Early Mortality in Schizophrenia. Annu Rev Clin Psychol (2014) 10:425–48. doi: 10.1146/annurev-clinpsy-032813-153657
17. Subramaniam M, Lam M, Guo ME, He VY, Lee J, Verma S, et al. Body Mass Index, Obesity, and Psychopathology in Patients With Schizophrenia. J Clin Psychopharmacol (2014) 34(1):40–6. doi: 10.1097/JCP.0000000000000058
18. Nordentoft M, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, et al. Excess Mortality, Causes of Death and Life Expectancy in 270,770 Patients With Recent Onset of Mental Disorders in Denmark, Finland and Sweden. PloS One (2013) 8(1):e55176. doi: 10.1371/journal.pone.0055176
19. de Nijs J, Pet MA. Metabolic Syndrome in Schizophrenia Patients Associated With Poor Premorbid School Performance in Early Adolescence. Acta Psychiatrica Scand (2016) 133(4):289–97. doi: 10.1111/acps.12528
20. Yaffe K. Metabolic Syndrome and Cognitive Decline. Curr Alzheimer Res (2007) 4(2):123–6. doi: 10.2174/156720507780362191
21. Pedditzi E, Peters R, Beckett N. The Risk of Overweight/Obesity in Mid-Life and Late Life for the Development of Dementia: A Systematic Review and Meta-Analysis of Longitudinal Studies. Age Age (2016) 45(1):14–21. doi: 10.1093/ageing/afv151
22. Lindenmayer JP, Khan A, Kaushik S, Thanju A, Praveen R, Hoffman L, et al. Relationship Between Metabolic Syndrome and Cognition in Patients With Schizophrenia. Schizophr Res (2012) 142(1-3):171–6. doi: 10.1016/j.schres.2012.09.019
23. Spangaro M, Mazza E, Poletti S, Cavallaro R, Benedetti F. Obesity Influences White Matter Integrity in Schizophrenia. Psychoneuroendocrinology (2018) 97:135–42. doi: 10.1016/j.psyneuen.2018.07.017
24. Friedman JI, Wallenstein S, Moshier E, Parrella M, White L, Bowler S, et al. The Effects of Hypertension and Body Mass Index on Cognition in Schizophrenia. Am J Psychiatry (2010) 167(10):1232–9. doi: 10.1176/appi.ajp.2010.09091328
25. Takayanagi Y, Cascella NG, Sawa A, Eaton WW. Diabetes is Associated With Lower Global Cognitive Function in Schizophrenia. Schizophr Res (2012) 142(1-3):183–7. doi: 10.1016/j.schres.2012.08.034
26. Mendrek A, Mancini-Marïe A. Sex/gender Differences in the Brain and Cognition in Schizophrenia. Neurosci Biobehav Rev (2016) 67:57–78. doi: 10.1016/j.neubiorev.2015.10.013
27. Yang F, Wang K, Du X, Deng H, Wu HE, Yin G, et al. Sex Difference in the Association of Body Mass Index and BDNF Levels in Chinese Patients With Chronic Schizophrenia. Psychopharmacology (2019) 236(2):753–62. doi: 10.1007/s00213-018-5107-1
28. Huang X, Bao C, Lv Q, Zhao J, Wang Y, Lang X, et al. Sex Difference in Cognitive Impairment in Drug-Free Schizophrenia: Association With miR-195 Levels. Psychoneuroendocrinology (2020) 119:104748. doi: 10.1016/j.psyneuen.2020.104748
29. Wang Y, Beydoun MA. The Obesity Epidemic in the United States–gender, Age, Socioeconomic, Racial/Ethnic, and Geographic Characteristics: A Systematic Review and Meta-Regression Analysis. Epidemiol Rev (2007) 29:6–28. doi: 10.1093/epirev/mxm007
30. Isong IA, Rao SR, Bind MA, Avendaño M, Kawachi I, Richmond TK. Racial and Ethnic Disparities in Early Childhood Obesity. Pediatrics (2018) 141(1):865–72. doi: 10.1542/peds.2017-0865
31. Tian Y, Liu D, Wang D, Wang J, Xu H, Dai Q, et al. Obesity in Chinese Patients With Chronic Schizophrenia: Prevalence, Clinical Correlates and Relationship With Cognitive Deficits. Schizophr Res (2020) 215:270–6. doi: 10.1016/j.schres.2019.10.017
32. Li Q, Chen D, Liu T, Walss-Bass C, de Quevedo JL, Soares JC, et al. Sex Differences in Body Mass Index and Obesity in Chinese Patients With Chronic Schizophrenia. J Clin Psychopharmacol (2016) 36(6):643–8. doi: 10.1097/JCP.0000000000000594
33. Ban C, Zhang Q, Feng J, Li H, Qiu Q, Tian Y, et al. Low Prevalence of Lipid Metabolism Abnormalities in APOE Epsilon2-Genotype and Male Patients 60 Years or Older With Schizophrenia. BMC Psychiatry (2017) 17(1):399. doi: 10.1186/s12888-017-1530-9
34. Ji CY, Chen TJ. Empirical Changes in the Prevalence of Overweight and Obesity Among Chinese Students From 1985 to 2010 and Corresponding Preventive Strategies. Biomed Environ Sci: BES (2013) 26(1):1–12. doi: 10.5402/2013/898691
35. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J Am Geriatr Society (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x
36. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, Misuse, Drawbacks, and a New Alternative for Schizophrenia Research. Ann Clin Psychiatry: Off J Am Acad Clin Psychiatrists (2016) 28(2):125–31.
37. Fisekovic S, Memic A, Pasalic A. Correlation Between Moca and Mmse for the Assessment of Cognition in Schizophrenia. Acta Informatica medica: AIM: J Soc Med Inf Bosnia Herzegovina: Casopis Drustva za medicinsku informatiku BiH. (2012) 20(3):186–9. doi: 10.5455/aim.2012.20.186-189
38. Chen KW, Lee YC, Yu TY, Cheng LJ, Chao CY, Hsieh CL. Test-Retest Reliability and Convergent Validity of the Test of Nonverbal Intelligence-Fourth Edition in Patients With Schizophrenia. BMC Psychiatry (2021) 21(1):39. doi: 10.1186/s12888-021-03041-4
39. Lin X, Haralambous B, Pachana NA, Bryant C, LoGiudice D, Goh A, et al. Screening for Depression and Anxiety Among Older Chinese Immigrants Living in Western Countries: The Use of the Geriatric Depression Scale (GDS) and the Geriatric Anxiety Inventory (GAI). Asia-Pacific Psychiatry: Off J Pacific Rim Coll Psychiatrists (2016) 8(1):32–43. doi: 10.1111/appy.12191
40. Zhang B, Han M, Tan S, De Yang F, Tan Y, Jiang S, et al. Gender Differences Measured by the MATRICS Consensus Cognitive Battery in Chronic Schizophrenia Patients. Sci Rep (2017) 7(1):11821. doi: 10.1038/s41598-017-12027-w
41. Hui L, Ye M, Tang W, Zhang F, Liu J, Liu L, et al. Obesity Correlates With Fewer Symptoms in Schizophrenia Treated With Long-Term Clozapine: Gender Difference. Psychiatry Res (2015) 225(3):741–2. doi: 10.1016/j.psychres.2014.12.035
42. Hopkins JL, Hopkins PN, Brinton EA, Adams TD, Davidson LE, Nanjee MN, et al. Expression of Metabolic Syndrome in Women With Severe Obesity. Metab Syndrome Relat Disord (2017) 15(6):283–90. doi: 10.1089/met.2016.0116
43. Al-Amodi HS, Abdelbasit NA, Fatani SH, Babakr AT, Mukhtar MM. The Effect of Obesity and Components of Metabolic Syndrome on Leptin Levels in Saudi Women. Diabetes Metab Syndrome (2018) 12(3):357–64. doi: 10.1016/j.dsx.2017.12.030
44. Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender Differences in Schizophrenia and First-Episode Psychosis: A Comprehensive Literature Review. Schizophr Res Treat (2012) 2012:916198. doi: 10.1155/2012/916198
45. Shtasel DL, Gur RE, Gallacher F, Heimberg C, Gur RC. Gender Differences in the Clinical Expression of Schizophrenia. Schizophr Res (1992) 7(3):225–31. doi: 10.1016/0920-9964(92)90016-X
46. Zhang BH, Han M, Zhang XY, Hui L, Jiang SR, Yang FD, et al. Gender Differences in Cognitive Deficits in Schizophrenia With and Without Diabetes. Compr Psychiatry (2015) 63:1–9. doi: 10.1016/j.comppsych.2015.07.003
47. Beck AK, Baker AL, Todd J. Smoking in Schizophrenia: Cognitive Impact of Nicotine and Relationship to Smoking Motivators. Schizophr Res Cogn (2015) 2(1):26–32. doi: 10.1016/j.scog.2014.12.001
48. Kozak K, Lowe DJE, George TP. Effects of Tobacco Smoking Status on Verbal Learning and Memory in Patients With Schizophrenia and Non-Psychiatric Controls. Am J Addictions (2019) 28(6):503–11. doi: 10.1111/ajad.12903
49. D’Souza MS, Markou A. Schizophrenia and Tobacco Smoking Comorbidity: nAChR Agonists in the Treatment of Schizophrenia-Associated Cognitive Deficits. Neuropharmacology (2012) 62(3):1564–73. doi: 10.1016/j.neuropharm.2011.01.044
50. Kroon LA. Drug Interactions With Smoking. Am J Health System Pharmacy: AJHP: Off J Am Soc Health System Pharmacists (2007) 64(18):1917–21. doi: 10.2146/ajhp060414
51. Benjamin AM, Suchindran S, Pearce K, Rowell J, Lien LF, Guyton JR, et al. Gene by Sex Interaction for Measures of Obesity in the Framingham Heart Study. J Obes (2011) 2011:329038. doi: 10.1155/2011/329038
52. Chen MQ, Shi WR, Wang HY, Li Z, Guo XF, Sun YX. Interaction of General or Central Obesity and Hypertension on Diabetes: Sex-Specific Differences in a Rural Population in Northeast China. Diabetes Metab Syndrome Obes: Targets Ther (2021) 14:1061–72. doi: 10.2147/DMSO.S295960
53. Yunker AG, Alves JM, Luo S, Angelo B, DeFendis A, Pickering TA, et al. Obesity and Sex-Related Associations With Differential Effects of Sucralose vs Sucrose on Appetite and Reward Processing: A Randomized Crossover Trial. JAMA Netw Open (2021) 4(9):e2126313. doi: 10.1001/jamanetworkopen.2021.26313
54. Rashid NA, Lim J, Lam M, Chong SA, Keefe RS, Lee J. Unraveling the Relationship Between Obesity, Schizophrenia and Cognition. Schizophr Res (2013) 151(1-3):107–12. doi: 10.1016/j.schres.2013.09.020
55. Hidese S, Matsuo J, Ishida I, Hiraishi M, Teraishi T, Ota M, et al. Relationship of Handgrip Strength and Body Mass Index With Cognitive Function in Patients With Schizophrenia. Front Psychiatry (2018) 9:156. doi: 10.3389/fpsyt.2018.00156
56. Dye L, Boyle NB, Champ C, Lawton C. The Relationship Between Obesity and Cognitive Health and Decline. Proc Nutr Society (2017) 76(4):443–54. doi: 10.1017/S0029665117002014
57. Miller AA, Spencer SJ. Obesity and Neuroinflammation: A Pathway to Cognitive Impairment. Brain Behav Immunity (2014) 42:10–21. doi: 10.1016/j.bbi.2014.04.001
58. Solas M, Milagro FI, Ramirez MJ, Martinez JA. Inflammation and Gut-Brain Axis Link Obesity to Cognitive Dysfunction: Plausible Pharmacological Interventions. Curr Opin Pharmacol (2017) 37:87–92. doi: 10.1016/j.coph.2017.10.005
Keywords: elderly, obesity, schizophrenia, Chinese, cognitive impairment, sex difference
Citation: Li W, Lin S, Yue L, Fang Y and Xiao S (2022) Sex Differences in Obesity and Cognitive Function in Chinese Elderly Patients With Chronic Schizophrenia. Front. Endocrinol. 13:742474. doi: 10.3389/fendo.2022.742474
Received: 16 July 2021; Accepted: 01 March 2022;
Published: 01 April 2022.
Edited by:
Flavia Cicuttini, Monash University, AustraliaReviewed by:
Yuzhen Xu, Tongji University, ChinaMary V. Seeman, University of Toronto, Canada
Mei Hong Xiu, Peking University, China
Copyright © 2022 Li, Lin, Yue, Fang and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shifu Xiao, eGlhb3NoaWZ1QG1zbi5jb20=; Yuan Fang, ZmFuZ3l1YW5fODYxMUAxNjMuY29t
†These authors have contributed equally to this work
 Wei Li
Wei Li Sun Lin
Sun Lin Ling Yue
Ling Yue Yuan Fang
Yuan Fang Shifu Xiao
Shifu Xiao