- Department of Endocrinology and Metabolism, Shanghai Tenth People’s Hospital, School of Medicine, Tongji University, Shanghai, China
Epidemiological studies suggest associations between diabetes mellitus and some cancers. The risk of a number of cancers appears to be increased in diabetes mellitus. On the other hand, some cancer and cancer therapies could lead to diabetes mellitus. Genetic factors, obesity, inflammation, oxidative stress, hyperglycemia, hyperinsulinemia, cancer therapies, insulin and some oral hypoglycemic drugs appear to play a role in the crosstalk between diabetes mellitus and cancers. This review summarized the associations between various types of diabetes and cancers and updated available evidence of underlying mechanisms between diabetes and cancers.
Introduction
The link between diabetes and cancer has been proposed for more than 100 years (1). The risk of cancers appears to be increased in both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) (2). Cancer was also reported to be the second most common cause of death for people with T1DM (3). On the other hand, approximately 8%-18% of patients with cancer have diabetes (4). Further, previous studies have suggested that diabetes is associated with increased risk of cancer mortality (5, 6). However, the underlying mechanisms between various types of diabetes and cancers have not yet been summarized. This review summarizes the associations between various types of diabetes and cancers, and updated available evidence underlying mechanisms between diabetes and cancers.
Incidence and Mortality of Cancers in Patients With Diabetes Mellitus
T1DM
A five-country study of cancers in patients with T1DM has reported that T1DM was correlated with the risk of several common cancers. For non-sex-specific cancers, the estimated hazard ratio (HR) and 95% confidence intervals (CIs) for overall cancer were 1.15 (1.11, 1.19) among men and 1.17 (1.13, 1.22) among women when compared to the general population (7). Cancer incidence of liver, pancreas, kidney, esophagus, stomach, lung, thyroid, squamous cell carcinoma, and leukaemia significantly increased for both sexes with T1DM (7–10). Incidence of non-Hodgkin’s lymphoma and colon cancer significantly increased for men (7); while incidence of the ovary, esophagus, endometrium, vulva and vagina, and thyroid cancer significantly increased for women (7, 11, 12). On the contrary, incidences of prostate cancer and testis cancer significantly decreased in men with T1DM in comparison with the general population (7, 13). Women with T1DM had significantly lower risk for breast cancer, melanoma, and Hodgkin’s lymphoma (7, 10). Previous cohort studies also reported an overall increased standardized mortality ratio for cancers among patients with T1DM compared with the general population (14).
Several studies generated inconsistent findings. Some early studies found no significant associations between T1DM and a range of site-specific cancers (15). Previous large cohort studies conducted in the UK suggested that neither the risk of urinary bladder cancer nor mortality from urinary bladder cancer was increased in patients with T1DM or T2DM (11, 16). This is in line with the results found in other study undertaken in Sweden (9, 17). Similarly, some studies found no significant association between the risk of breast cancer and T1DM in women (11, 17). In addition, cohort studies undertaken in the UK (11, 18) and the USA (19) reported that no significantly increased all-cause cancer mortality was found in patients with T1DM when compared to the general population. But, there was evidence of heterogeneity in risk of some cancers by country, and T1DM duration (7). Thus, study population selection (e.g., ethnicity, age range, and gender), study design, publication bias, other demographic and socioeconomic factors should be considered when interpreting these results.
T2DM
A comprehensive meta-analysis has concluded that the presence of T2DM is associated with approximately 10% increase of the risk to develop cancer (5). Previous studies have provided substantial evidence of associations between T2DM and risks of cancer in hepatocellular, biliary tract, gallbladder, pancreas, gastrointestinal, kidney, bladder, lung, thyroid, breast, ovarian, endometrial, oral, leukemia, glioma, and melanoma (5, 20–25). Among them, the highest risks has been demonstrated for colorectal cancer (26), hepatocellular cancer (27), or pancreatic cancer (28).
On the contrary, some cancers showed a null or decreased risk in diabetic patients in some studies, including brain, buccal cavity, esophageal, lung, breast, urinary bladder, and laryngeal cancer (20). It is worth noting that numerous studies that were conducted in Americans and Europeans indicated a reduced risk of prostate cancer in patients with T2DM (29, 30). Moreover, the protective effect was more evident for patients with more than 10 years T2DM duration (13). Indeed, men with diabetes had lower levels of testosterone (31) than those without, and testosterone has been demonstrated to be associated with an elevated risk of prostate cancer (32). Additionally, studies with genome-wide association analyses indicated that HNF1B gene variants would not only drive haplotype-carrying people to diabetes, but also protect them from prostate cancer (33). However, studies in Asians reported contradicting results, and large meta-analyses suggested that there was a positive association between T2DM and prostate cancer in Asians (30, 34).
Previous large meta-analyses have estimated that diabetes is associated with 25%-41% increased risk of mortality from any cancer (35, 36). In a prospective cohort conducted in US adults, diabetes was related to increases in any cancer mortality of 7% in men and 11% in women, respectively (29). In an analysis of 19 Asian cohorts followed for up to 21 years, T2DM was related to a 26% increase in the risk of cancer mortality (6). Significant positive associations between T2DM and mortality from cancers were observed for the cancers of stomach, colorectum, oral cavity, gallbladder, bile duct, liver, pancreas, ovary, endometrium, breast, thyroid, prostate, lung, kidney, bladder, and lymphoma (6, 22, 37). Controversially, some studies reported a null association between T2DM and the risk of death from cancers of the lung, bladder, stomach, cervix, esophagus, as well as leukaemia (6, 38), suggesting that the role of diabetes in these site cancer needs further clarification.
Type 3c Diabetes (T3cDM) or Pancreoprivic Diabetes
Type 3c diabetes (T3cDM) or pancreoprivic diabetes is caused by various diseases of the exocrine pancreas (39). The diverse causes of T3cDM include pancreatic carcinoma, acute and chronic pancreatitis, cystic fibrosis, trauma or pancreatectomy, fibrocalculous pancreatopathy, hemochromatosis, idiopathic forms, and rare genetic disorders. Pendharkar et al. showed that the prevalence of diabetes in individuals with exocrine pancreas diseases was approximately 0.11% (40). Ewald N et al. reported that approximately 9.2% of patients with diabetes were identified as T3cDM (41).
A comprehensive meta-analysis showed that the relative risk of pancreatic cancer was negatively associated with the diabetes duration, with the highest risk of pancreatic cancer found among patients whose diabetic history within less than 1 year (42). It indicates that diabetes may have resulted from undiagnosed pancreatic cancer (43). Indeed, T3cDM occurs in up to 30% of patients with pancreatic cancer (44). On the other hand, successful treatment of pancreatic cancer could improve hyperglycemia for patients with T3cDM due to pancreatic cancer (45). Additionally, the risk of pancreatic cancer has been increased 10- to 20-fold in patients with chronic pancreatitis, which is the most common cause of T3cDM; this risk has been increased 33-fold in patients with the combination of chronic pancreatitis and diabetes mellitus (46). A previous study estimated that approximately 10% T3cDM patients had pancreatic cancer (41).
Animal studies found the presence of hyperinsulinemia (47) and insulin secretory impairments (48) in pancreatic cancer models. Indeed, euglycemic glucose clamp studies demonstrated that both the insulin sensitivity and beta-cell function were markedly impaired in patients with pancreatic cancer (45, 49). T3cDM secondary to pancreatic cancer seems to be related to the mediators released by cancer (50). Adrenomedullin was identified as one of the key mediators for beta-cell toxicity in a cell-line study of pancreatic cancer (51). Further, a clinical study reported that the levels of adrenomedullin are higher in patients with pancreatic cancer-induced diabetes in comparison to general population (52). In addition, the upregulation of connexin and S100A8/A9 in pancreatic tissues could attenuate the glucose utilization (53, 54). Furthermore, interleukin-1β and tumor necrosis factor (TNF)-α are found abundant in a tumor microenvironment in diabetes due to pancreatic cancer (55), which somehow explains the impaired beta-cell function observed in patients with pancreatic cancer (56).
Cancer Treatment and Diabetes
Chemotherapy
Most chemotherapeutic agents result in the cell cycle or cellular DNA damage and thus leading to apoptosis disproportionately in rapidly dividing cells. A number of studies reported that patients who received chemotherapy such as Tegafur-uracil (UFT) (57), paclitaxel (58), or interferon alpha (59) had developed fulminant T1DM or autoimmune-mediated T1DM. Mouse studies indicated that interferon alpha causes autoimmune diabetes by promoting the maturation of conventional dendritic cells and the activation of B cells. Further, interferon alpha could directly damage pancreatic beta cell functions by inducing cytokines and enhancing their susceptibility to invasion by diabetogenic T cells (60). Diabetes is also a rare complication of UFT use. UFT could cause fulminant T1DM through immune suppression or an immunological reaction, and the effects of thymidine phosphorylase (57).
Glucocorticoid
Glucocorticoids are a commonly used treatment for cancers of blood system (61). Additionally, they are used to treat cancer pain, chemotherapy-induced side-effects such as nausea and vomiting, and cancer-related cachexia (62, 63). Furthermore, they have an ancillary role in treatment of inflammatory complications of cancer therapy and autoimmune conditions of immunomodulatory therapies (64). Steroid-induced diabetes mellitus has been recognized as a complication of glucocorticoid use for over 50 years (65). A previous study found that patients with previously well controlled T1DM treatment with 60 mg prednisone daily for 3 days led to deterioration of glycemic control despite average 70% increase of insulin dosage (66). It is likely that glucocorticoid administration causes hyperglycemic states or diabetes mellitus by impairing pancreatic beta-cell functions and insulin sensitivity (67). An in vitro study observed impaired insulin secretion of prednisone-treated INS-1E cells in response to a glucose challenge. On the contrary, this phenomenon was reversed in the presence of prednisone with the glucocorticoid receptor antagonist, RU486 (68). Glucocorticoids could induce insulin resistance through several mechanisms. For example, glucocorticoids increase the levels of serum fatty acids by regulating the expression of PEPCK gene in adipose tissue and liver and controlling glyceroneogenesis. It is well known that an increase in fatty acids interferes with glucose utilization and results in insulin resistance (69). Moreover, glucocorticoids decrease insulin sensitivity by directly interfering with components of the insulin signaling cascade, such as glycogen synthase kinase-3, glycogen synthase and GLUT4 translocation (67, 70).
Targeted Cancer Therapies
Targeted cancer therapies attempt to treat cancer by targeting the changed cellular pathways that drive unregulated growth. This treatment can somehow impair insulin sensitivity since some altered cellular pathways are linked to the actions of insulin. For instance, the anti–insulin like growth factor 1 receptor (IGF-IR) inhibition has been long proposed as a treatment strategy of various cancers (71, 72). A phase I dose escalation study of the Anti-IGF-IR monoclonal antibody CP-751,871 in patients with refractory solid tumors reported that the treatment with CP-751,871 increased serum glucose levels (73). It is likely that the levels of growth hormone (GH) increase after IGF-1 blockade, thereby leading to an increase in insulin resistance (74). In addition, mammalian target of rapamycin (mTOR) inhibitors have been used for multiple types of cancer such as breast cancer and renal cell carcinoma. Data from clinical trials suggested that a treatment with mTOR inhibitors was associated with a high incidence of hyperglycemia and new-onset diabetes, ranging from 13% to 50% (75). The mechanisms responsible for hyperglycemia with mTOR inhibitors are likely due to the combination of impaired insulin secretion and insulin resistance (76, 77). Hyperinsulinemia and hyperglycemia were also seen after administrations target the proteins in the same pathway, including PI3 kinase and Akt in mice (78).
Cancer Immunotherapy
Cancer immunotherapies, including immune checkpoint inhibitors, adoptive cell therapy, oncolytic viruses, and cancer vaccines, manipulate the immune system to recognize and attack cancer cells. These therapies have the potential to lead to toxicity profiles for endocrine system. For instance, insulin-dependent diabetes has been reported in patients treated with anti-programmed cell death protein 1 (PD-1) or anti-programmed cell death ligand-1 (PDL-1) antibodies (79). The prevalence of diabetes was estimated at 0.4%-0.9% in this population (80–82). Animal studies also indicated that anti-PD-1 or anti-PDL-1 antibody injection triggered onset of diabetes in mice (83, 84). But, to date, the exact mechanism is poorly known. Histologic analysis of the pancreas found massive destructive insulitis in mice receiving anti-PD-1 or anti-PD-L1 antibodies.
Mechanisms Underlying the Association Between Diabetes Mellitus and Cancers
Genetic Background
Genetic factors have been identified as contributing to the associations between diabetes and some cancers. For instance, individuals who have a family history of pancreatic cancer often have a higher risk of developing pancreatic cancer (85). Indeed, several studies reported that the glucose-raising allele of MADD rs11039149, MTNR1B rs1387153, FTO rs8050136 per allele, glucokinase regulator rs780094 of T2DM were positively associated with the risk of pancreatic cancer (86, 87).
Common Risk Factors
Obesity
It is well known that most patients with prediabetes or T2DM have overweight or obesity (39). A large cohort study which included 900,000 individuals with an over 16-year duration of follow-up reported that severe obesity was associated with a significantly increased mortality from cancers of the liver, pancreas, colon and rectum, kidney, non-Hodgkins lymphoma, esophagus, and multiple myeloma. The greatest influences were observed in cancers of liver, colon and rectum, and pancreas (88). Additionally, a lower incidence of obesity-related cancers (89) and a significant reduction of cancer-related medical care (90) were found in bariatric surgery patients when compared with morbidly obese controls.
Obesity may act as an important confounder or an effect modifier in the relationship between T2DM and cancer (4). Obesity was associated with increased risk of cancers probably by mechanisms that involve cellular proliferation, inflammation, and hormonal balance (91), which have also been supposed for the relationship between T2DM and cancer. Taking pancreas for example, Butler et al. studied the effects of obesity and diabetes mellitus on pancreatic ductal pathology and found that the replication of pancreatic duct was increased ten-fold in specimens obtained from obese nondiabetics compared with lean nondiabetics, and duct epithelial replication was increased four-fold in lean diabetics in comparison with lean nondiabetics. These results suggest the independent effects of diabetes and obesity on the risk of the development of pancreatic exocrine neoplasia (92).
Inflammation and Oxidative Stress
Inflammation is a key element in the link between diabetes mellitus and cancer (93). T2DM is associated with insulin secretory defects related to inflammation (39). Chronic inflammation, which is characterized by high levels of oxidative stress and reactive oxygen species (ROS), activation of pro-inflammatory pathways, and abnormal adipokine production, may establish a micro-environment thereby promote tumor cell growth, enhance metastasis, increase angiogenesis and impair the function of natural killer cells and macrophages (94).
Oxidative stress plays an important role in the crosstalk between cancer and diabetes. Hyperglycemia could increase superoxide production (95). Furthermore, insulin could stimulate reactive oxygen species (ROS) production (96). It has been confirmed that oxidative stress has a strong influence on a number of genes expression and signal transduction pathways that have an important role in tumorigenesis (97). ROS have been demonstrated to interfere with cell proliferation and apoptosis by activating cytokine-dependent activation of nuclear factor (NF)-кB pathways (98). NF-кB was demonstrated to be hyperactivated in colorectal cancer (99), breast, blood neoplasms, and pancreas cell lines (97, 100).
Hyperglycemia
Epidemiological data have shown that hyperglycemia is related to higher risk of colorectal, liver, gastric, lung and pancreatic cancer (101, 102). The phenomenon termed “the Warburg effect” partly explains why hyperglycemia favors tumorigenesis (103). Normally, cells differentiates rely on mitochondrial oxidative phosphorylation to provide the energy to cellular processes, while cancer cells tend to use a less efficient glycolytic pathway for proliferation (103, 104). Cancer cells therefore require increased glucose uptake to generate sufficient energy hence meet their proliferation needs (105). The cancer predisposition associated with diabetes may result from imbalance of signal transduction pathways that manage the utilization of nutrient and fuels (106).
Hyperglycemia stimulates the production of advanced glycation end products (AGEs). AGEs often interact with their specific receptor, RAGE, activate NF-кB and generate ROS in cells, thereby accelerating oxidative stress that leads to increased proinflammatory signaling (107). Activation of the AGEs pathway has been demonstrated to promote tumor transformation of epithelial cells (108). Clinical tests also confirmed a positive association between the AGE/RAGE interaction and risk of gastric cancer (109), pancreatic cancer (110), and melanoma (111). In addition, Han et al. reported that hyperglycemia stimulates proliferation of pancreatic cancer cell via the induction of epithelial growth factor (EGF) expression and transactivation of the EGF receptor (112). Furthermore, hyperglycemia has been supposed to damage the lung structure, which is the basis for lung cancer (113). Moreover, hyperglycemia is responsible for DNA damage, which is the first stage of tumorigenesis (114).
Hyperinsulinemia
Several epidemiological studies have shown that hyperinsulinemia is associated with an increased risk for several cancers, including cancers of the endometrium, ovarian, breast, colon, pancreas, and kidney (115, 116). Indeed, both in vitro and in vivo studies demonstrated that insulin and insulin receptor (IR) played a key role in cancer biology (117). In hyperinsulinemic states, the hepatic IGF-1 production increased due to the upregulation of the growth hormone receptor (GHR) and augment of GHR signaling (118). Epidemiological studies and meta-analyses suggested that higher IGF-1 levels were correlated with an increased risk of colorectal, lung, premenopausal breast and prostate cancer (119). Animal studies confirmed that IGF-1 administration increased the cancer cells proliferation and their capacity to spread in secondary sites. On the contrary, knock-out of the Igf-1 gene inhibited growth of the tumor (120). In addition, IGF-2 overexpression has been also associated with colon cancer development in mouse models (121). Insulin, IGF-1 and IGF-2 could activate the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway, thereby promoting the development of cancers (122).
Exogenous Insulin and Insulin Analog Therapy
There is some evidence that patients with insulin therapy have a higher incidence of cancers when compared to patients with no insulin use (123), including cancers in colorectum, breast, pancreas, liver, kidney, stomach and respiratory system (124, 125). A retrospective study showed that patients treated with insulin agents such as human insulin, aspart, lispro and glargine exhibited a dose-dependent increased risk of cancer development (126). An animal study showed that insulin administration increased colonic epithelial tissue proliferation, thereby promoted colon cancer growth (127). The possible mutagenic effects of insulin or insulin analog and increased levels of IGF-1 might be the potential biological plausibility for the increase risks of cancers (128, 129). It should be kept in mind that insulin analogs may have a metabolic action and a mitogenic action altered from that of human insulin (130). Further, compared to insulin, the mitogenic pathways may be more activated when using long-acting analogues (131). However, some previous large randomized controlled trial study (132), cohort study (133), and systematic review (134) concluded that insulin (analog) treatment does not impact the risk of cancer overall and some site-specific cancers.
Oral Hypoglycemic Drugs
Metformin
Numerous clinical studies and meta-analyses have demonstrated that diabetes exposure to metformin was associated with a significantly decreased cancer incidence and mortality (135–137). Moreover, the addition of metformin ameliorates the increased risk of cancer in patient therapy with sulfonylurea or insulin (138). Studies in animal models and in cancer cell lines in vitro complemented these results that metformin could inhibit development of cancer (139). The potential mechanism is that metformin may inhibits the mTOR in an adenosine monophosphate (AMP)-activated protein kinase (AMPK)-dependent manner, concomitant reduces insulin levels, and increases insulin sensitivity (139, 140). Metformin could also inhibit tumorigenesis by modulating several other targets such as STAT3, TP, p53, etc. (140). In addition, metformin has been demonstrated to enhance the activity of several cancer drugs such as platinum compounds (140). Recently, the METAL (METformin in Advanced Lung cancer) study provided evidence that metformin plus erlotinib in second-line treatment of patients with stage IV NSCLC prolonged median progression-free survival (141). Moreover, Morgillo et al. demonstrated that metformin increases the antitumor activity of MEK inhibitors in human LKB1-wild-type non-small cell lung cancer cell (NSCLC) lines by reducing the NF-kB (p65)-mediated transcription of MMP-2 and MMP-9 and through downregulation of GLI1 (142).
Glucagon-Like Peptide-1 Receptor Agonist and Dipeptidyl Peptidase-IV Inhibitor
Incretin-based therapy, including dipeptidyl peptidase-IV (DPP-IV) inhibitor and glucagon-like peptide-1(GLP-1) receptor agonist is increasingly used in T2DM. Elashoff et al. reported that the use of DDP-IV inhibitor sitagliptin or the GLP1 analog exenatide was associated with a significant increase in the incidence of pancreatic cancer (143). Indeed, Matveyenko et al. observed that sitagliptin induced replication and apoptosis of beta-cell, pancreatic ductal metaplasia, and a four-fold increase in duct cell proliferation, suggesting that sitagliptin is the risk factor for the development of pancreatic cancer (144). Furthermore, animal studies showed that exendin-4, the GLP-1 analog, increased duct cell replication and the development of dysplastic pancreatic intraepithelial neoplasia lesions (145). In addition, liraglutide, a GLP-1 receptor agonist, was associated with increased risk of thyroid c-cell focal hyperplasia, indicating an increased risk of medullary cell thyroid cancer (146). However, a meta-analysis suggested that there is no exact evidence that the risk of pancreatic cancer in patients on incretin-based therapies is significantly higher than that in patients on other therapies (147, 148).
Sodium-Glucose Linked Transporter 2 (SGLT 2) Inhibitors
A meta-analysis suggested that the risk of bladder cancer might be increased in patients with SGLT2 inhibitors, especially with empagliflozin (149). However, this association was not confirmed by other authors (150, 151). Scafoglio C et al. even suggested that SGLT2 inhibitors may be useful for cancer therapy (152), as SGLT2 inhibitor was associated to increased tumor necrosis and hence induced tumor shrinkage (152). Indeed, canagliflozin was demonstrated to inhibit cancer growth by inhibiting the complex I of the mitochondrial respiratory chain (153).
Sulfonylureas (SUs)
Previous studies have indicated that patients treated with sulfonylureas therapy have an increased incidence of cancer and risk of cancer mortality (154, 155), particularly in pancreatic (138) and breast cancer (156). However, some randomized controlled trials showed no statistically significant difference in the risk of cancer between the use of SUs and other treatments (157).
Thiazolidinediones (TZDs)
TZDs have potent insulin-sensitizing activity used to improve lipid and glucose metabolism through the activation of peroxisome proliferator-activated receptors (PPARs) (158). In 2005, the PROactive Study firstly proposed the positive association of bladder cancer with pioglitazone use in patients with T2DM (159). However, pioglitazone bladder cancer concerns have been largely attenuated by recent evidence (160). Lv et al. demonstrated that the activation of PPARγ induced cell cycle G2 arrest and inhibition of bladder cancer cells proliferation by inhibiting the PI3K-Akt pathway in vitro (161). Additionally, PPAR-γ activation has been found to inhibit the growth of other tumor cells such as colon, breast and lung cancer cell lines through induction of apoptosis (162, 163). Ciaramella et al. investigated the anti-tumor effects of pioglitazone in NSCLC cell lines and found that pioglitazone reduced proliferative and invasive abilities and induced apoptosis of NSCLC cells by inhibiting MAPK/AKT cascade as well as on the TGFβ/SMADs system (164). Indeed, Mazzone et al. indicated that the TZDs treatment was associated with a lower risk of developing lung cancer in patients with diabetes (165). A meta-analysis also suggested that TZDs were associated with a significantly lower risk of colorectal and breast cancer (166, 167). In addition to anti-proliferative effects, TZDs can also enhance cytotoxic effects of some anticancer therapies such as cisplatin and oxaliplatin by increasing the expression of apoptosis-inducing factor (AIF) and suppressing survivin (168).
Conclusion
There is a complicated association between diabetes mellitus and cancers. In summary, the risk of a number of cancers and cancer mortality is increased in T1DM and T2DM. On the other hand, some kinds of cancer and cancer therapies are associated with the increased risk of diabetes mellitus. Additionally, genetic factors, obesity, inflammation, oxidative stress, hyperglycemia, hyperinsulinemia, cancer therapies, insulin and some oral hypoglycemic drugs appear to play a role in the crosstalk between diabetes mellitus and cancers (Figure 1). Thus, we suggest that cancer screening should be conducted in patients with diabetes, and precautions for diabetes should be taken in patients suffering from cancer. Further researches are merited to explore on the associations between these different diseases.
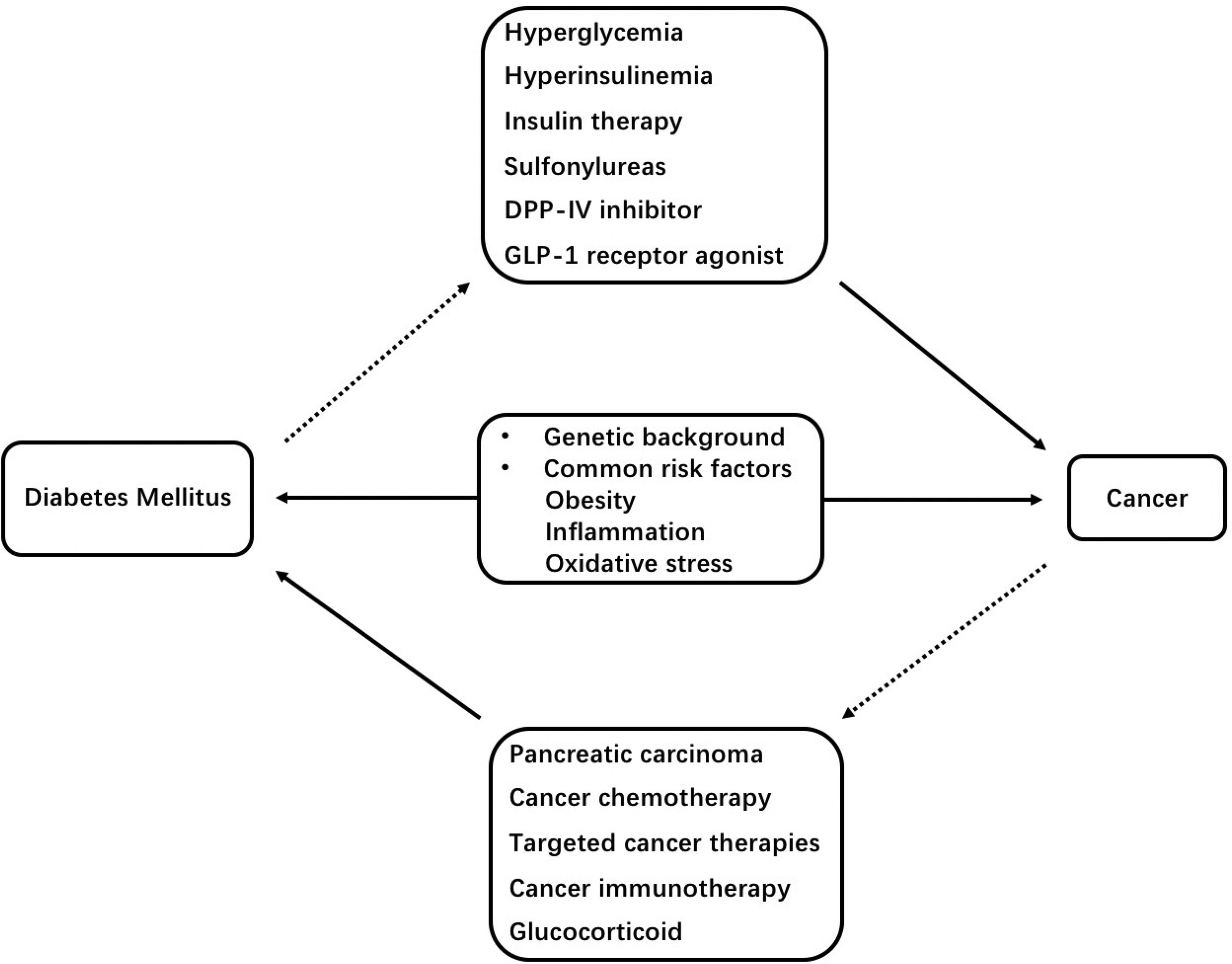
Figure 1 Schematic representation of mechanisms underlying the association between diabetes mellitus and cancers. DPP-IV, dipeptidyl peptidase-IV; GLP-1, glucagon-like peptide-1.
Author Contributions
Both authors have met the requirements for authorship. BZ and SQ summarized and edited the manuscript. Both authors have read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (No.2018YFC1314100, SQ), the National Natural Science Foundation of China (82100903, BZ), and the Shanghai Sailing Program (21YF1435200, BZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge Professor Li Ming Wen (School of Public Health, University of Sydney, Australia) for their kind support for editing and proofreading this manuscript.
References
1. Greenwood M, Wood F. The Relation Between the Cancer and Diabetes Death-Rates. J Hyg (Lond) (1914) 14:83–118. doi: 10.1017/s0022172400005702
2. Cignarelli A, Genchi VA, Caruso I, Natalicchio A, Perrini S, Laviola L, et al. Diabetes and Cancer: Pathophysiological Fundamentals of a ‘Dangerous Affair’. Diabetes Res Clin Pract (2018) 143:378–88. doi: 10.1016/j.diabres.2018.04.002
3. Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated Life Expectancy in a Scottish Cohort With Type 1 Diabetes, 2008-2010. JAMA (2015) 313:37–44. doi: 10.1001/jama.2014.16425
4. Suh S, Kim KW. Diabetes and Cancer: Cancer Should Be Screened in Routine Diabetes Assessment. Diabetes Metab J (2019) 43:733–43. doi: 10.4093/dmj.2019.0177
5. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 Diabetes and Cancer: Umbrella Review of Meta-Analyses of Observational Studies. BMJ (2015) 350:g7607. doi: 10.1136/bmj.g7607
6. Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, et al. Association Between Type 2 Diabetes and Risk of Cancer Mortality: A Pooled Analysis of Over 771,000 Individuals in the Asia Cohort Consortium. Diabetologia (2017) 60:1022–32. doi: 10.1007/s00125-017-4229-z
7. Carstensen B, Read SH, Friis S, Sund R, Keskimaki I, Svensson AM, et al. Cancer Incidence in Persons With Type 1 Diabetes: A Five-Country Study of 9,000 Cancers in Type 1 Diabetic Individuals. Diabetologia (2016) 59:980–8. doi: 10.1007/s00125-016-3884-9
8. Stevens RJ, Roddam AW, Beral V. Pancreatic Cancer in Type 1 and Young-Onset Diabetes: Systematic Review and Meta-Analysis. Br J Cancer (2007) 96:507–9. doi: 10.1038/sj.bjc.6603571
9. Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K. Cancer Risk Among Patients Hospitalized for Type 1 Diabetes Mellitus: A Population-Based Cohort Study in Sweden. Diabetes Med (2010) 27:791–7. doi: 10.1111/j.1464-5491.2010.03011.x
10. Sona MF, Myung SK, Park K, Jargalsaikhan G. Type 1 Diabetes Mellitus and Risk of Cancer: A Meta-Analysis of Observational Studies. Jpn J Clin Oncol (2018) 48:426–33. doi: 10.1093/jjco/hyy047
11. Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, et al. Cancer Incidence and Mortality in Patients With Insulin-Treated Diabetes: A UK Cohort Study. Br J Cancer (2005) 92:2070–5. doi: 10.1038/sj.bjc.6602611
12. Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes Mellitus and Risk of Endometrial Cancer: A Meta-Analysis. Diabetologia (2007) 50:1365–74. doi: 10.1007/s00125-007-0681-5
13. Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer Risk Among People With Type 1 and Type 2 Diabetes: Disentangling True Associations, Detection Bias, and Reverse Causation. Diabetes Care (2015) 38:264–70. doi: 10.2337/dc14-1996
14. Dawson SI, Willis J, Florkowski CM, Scott RS. Cause-Specific Mortality in Insulin-Treated Diabetic Patients: A 20-Year Follow-Up. Diabetes Res Clin Pract (2008) 80:16–23. doi: 10.1016/j.diabres.2007.10.034
15. Green A, Jensen OM. Frequency of Cancer Among Insulin-Treated Diabetic Patients in Denmark. Diabetologia (1985) 28:128–30. doi: 10.1007/BF00273858
16. Goossens ME, Zeegers MP, Bazelier MT, De Bruin ML, Buntinx F, de Vries F. Risk of Bladder Cancer in Patients With Diabetes: A Retrospective Cohort Study. BMJ Open (2015) 5:e007470. doi: 10.1136/bmjopen-2014-007470
17. Zendehdel K, Nyren O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer Incidence in Patients With Type 1 Diabetes Mellitus: A Population-Based Cohort Study in Sweden. J Natl Cancer Inst (2003) 95:1797–800. doi: 10.1093/jnci/djg105
18. Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-Cause Mortality Rates in Patients With Type 1 Diabetes Mellitus Compared With a non-Diabetic Population From the UK General Practice Research Database, 1992-1999. Diabetologia (2006) 49:660–6. doi: 10.1007/s00125-005-0120-4
19. Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-Specific Mortality Trends in a Large Population-Based Cohort With Long-Standing Childhood-Onset Type 1 Diabetes. Diabetes (2010) 59:3216–22. doi: 10.2337/db10-0862
20. Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of Cancer in a Large Cohort of U.S. Veterans With Diabetes. Int J Cancer (2011) 128:635–43. doi: 10.1002/ijc.25362
21. Yu WS, Lee CY, Park SY, Suh JW, Narm KS, Kim DJ, et al. Prognostic Factors for Resected non-Small Cell Lung Cancer in Patients With Type 2 Diabetes Mellitus. J Surg Oncol (2018) 117:985–93. doi: 10.1002/jso.24989
22. Gong Y, Wei B, Yu L, Pan W. Type 2 Diabetes Mellitus and Risk of Oral Cancer and Precancerous Lesions: A Meta-Analysis of Observational Studies. Oral Oncol (2015) 51:332–40. doi: 10.1016/j.oraloncology.2015.01.003
23. Li H, Qian J. Association of Diabetes Mellitus With Thyroid Cancer Risk: A Meta-Analysis of Cohort Studies. Med (Baltimore) (2017) 96:e8230. doi: 10.1097/MD.0000000000008230
24. Zhao L, Zheng Z, Huang P. Diabetes Mellitus and the Risk of Glioma: A Meta-Analysis. Oncotarget (2016) 7:4483–9. doi: 10.18632/oncotarget.6605
25. Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and Breast Cancer Risk: A Meta-Analysis. Br J Cancer (2012) 107:1608–17. doi: 10.1038/bjc.2012.414
26. Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes Mellitus and Incidence and Mortality of Colorectal Cancer: A Systematic Review and Meta-Analysis of Cohort Studies. Eur J Epidemiol (2011) 26:863–76. doi: 10.1007/s10654-011-9617-y
27. Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, et al. Increased Risk of Hepatocellular Carcinoma in Patients With Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies. Int J Cancer (2012) 130:1639–48. doi: 10.1002/ijc.26165
28. Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II Diabetes and Pancreatic Cancer: A Meta-Analysis of 36 Studies. Br J Cancer (2005) 92:2076–83. doi: 10.1038/sj.bjc.6602619
29. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and Cause-Specific Mortality in a Prospective Cohort of One Million U.S. adults. Diabetes Care (2012) 35:1835–44. doi: 10.2337/dc12-0002
30. Jian Gang P, Mo L, Lu Y, Runqi L, Xing Z. Diabetes Mellitus and the Risk of Prostate Cancer: An Update and Cumulative Meta-Analysis. Endocr Res (2015) 40:54–61. doi: 10.3109/07435800.2014.934961
31. Tsilidis KK, Allen NE, Appleby PN, Rohrmann S, Nothlings U, Arriola L, et al. Diabetes Mellitus and Risk of Prostate Cancer in the European Prospective Investigation Into Cancer and Nutrition. Int J Cancer (2015) 136:372–81. doi: 10.1002/ijc.28989
32. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective Study of Sex Hormone Levels and Risk of Prostate Cancer. J Natl Cancer Inst (1996) 88:1118–26. doi: 10.1093/jnci/88.16.1118
33. Frayling TM, Colhoun H, Florez JC. A Genetic Link Between Type 2 Diabetes and Prostate Cancer. Diabetologia (2008) 51:1757–60. doi: 10.1007/s00125-008-1114-9
34. Long XJ, Lin S, Sun YN, Zheng ZF. Diabetes Mellitus and Prostate Cancer Risk in Asian Countries: A Meta-Analysis. Asian Pac J Cancer Prev (2012) 13:4097–100. doi: 10.7314/apjcp.2012.13.8.4097
35. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes Mellitus, Fasting Glucose, and Risk of Cause-Specific Death. N Engl J Med (2011) 364:829–41. doi: 10.1056/NEJMoa1008862
36. Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-Term All-Cause Mortality in Cancer Patients With Preexisting Diabetes Mellitus: A Systematic Review and Meta-Analysis. JAMA (2008) 300:2754–64. doi: 10.1001/jama.2008.824
37. Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, et al. Insulin-Like Growth Factors, Their Binding Proteins, and Prostate Cancer Risk: Analysis of Individual Patient Data From 12 Prospective Studies. Ann Intern Med (2008) 149:461–71. doi: 10.7326/0003-4819-149-7-200810070-00006 W483-468.
38. Karlin NJ, Amin SB, Buras MR, Kosiorek HE, Verona PM, Cook CB. Patient Outcomes From Lung Cancer and Diabetes Mellitus: A Matched Case-Control Study. Future Sci OA (2018) 4:FSO248. doi: 10.4155/fsoa-2017-0081
39. American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44:S15–33. doi: 10.2337/dc21-S002
40. Pendharkar SA, Mathew J, Petrov MS. Age- and Sex-Specific Prevalence of Diabetes Associated With Diseases of the Exocrine Pancreas: A Population-Based Study. Dig Liver Dis (2017) 49:540–4. doi: 10.1016/j.dld.2016.12.010
41. Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of Diabetes Mellitus Secondary to Pancreatic Diseases (Type 3c). Diabetes Metab Res Rev (2012) 28:338–42. doi: 10.1002/dmrr.2260
42. Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, et al. Diabetes Mellitus and Risk of Pancreatic Cancer: A Meta-Analysis of Cohort Studies. Eur J Cancer (2011) 47:1928–37. doi: 10.1016/j.ejca.2011.03.003
43. Gullo L, Pezzilli R, Morselli-Labate AM. Italian Pancreatic Cancer Study GDiabetes and the Risk of Pancreatic Cancer. N Engl J Med (1994) 331:81–4. doi: 10.1056/NEJM199407143310203
44. Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early Diagnosis and Treatment of Pancreatic Dysplasia in Patients With a Family History of Pancreatic Cancer. Ann Intern Med (1999) 131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003
45. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and Clinical Profile of Pancreatic Cancer-Associated Diabetes Mellitus. Gastroenterology (2008) 134:981–7. doi: 10.1053/j.gastro.2008.01.039
46. Cui Y, Andersen DK. Pancreatogenic Diabetes: Special Considerations for Management. Pancreatology (2011) 11:279–94. doi: 10.1159/000329188
47. Liu J, Knezetic JA, Strommer L, Permert J, Larsson J, Adrian TE. The Intracellular Mechanism of Insulin Resistance in Pancreatic Cancer Patients. J Clin Endocrinol Metab (2000) 85:1232–8. doi: 10.1210/jcem.85.3.6400
48. Permert J, Herrington M, Kazakoff K, Pour PM, Adrian TE. Early Changes in Islet Hormone Secretion in the Hamster Pancreatic Cancer Model. Teratog Carcinog Mutagen (2001) 21:59–67. doi: 10.1002/1520-6866(2001)21:1<59::AID-TCM6>3.0.CO;2-V
49. Chari ST, Zapiach M, Yadav D, Rizza RA. Beta-Cell Function and Insulin Resistance Evaluated by HOMA in Pancreatic Cancer Subjects With Varying Degrees of Glucose Intolerance. Pancreatology (2005) 5:229–33. doi: 10.1159/000085276
50. Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New Insights Into Pancreatic Cancer-Induced Paraneoplastic Diabetes. Nat Rev Gastroenterol Hepatol (2013) 10:423–33. doi: 10.1038/nrgastro.2013.49
51. Sekine N, Takano K, Kimata-Hayashi N, Kadowaki T, Fujita T. Adrenomedullin Inhibits Insulin Exocytosis via Pertussis Toxin-Sensitive G Protein-Coupled Mechanism. Am J Physiol Endocrinol Metab (2006) 291:E9–14. doi: 10.1152/ajpendo.00213.2005
52. Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, et al. Adrenomedullin is Up-Regulated in Patients With Pancreatic Cancer and Causes Insulin Resistance in Beta Cells and Mice. Gastroenterology (2012) 143:1510–7.e1511. doi: 10.1053/j.gastro.2012.08.044
53. Pfeffer F, Koczan D, Adam U, Benz S, von Dobschuetz E, Prall F, et al. Expression of Connexin26 in Islets of Langerhans is Associated With Impaired Glucose Tolerance in Patients With Pancreatic Adenocarcinoma. Pancreas (2004) 29:284–90. doi: 10.1097/00006676-200411000-00007
54. Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in Inflammation and Cancer. Biochem Pharmacol (2006) 72:1622–31. doi: 10.1016/j.bcp.2006.05.017
55. Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and Cancer Progression: The Emerging Role of Interleukin-1 Receptor Antagonist as a Novel Therapeutic Agent in Cancer Treatment. J Transl Med (2006) 4:48. doi: 10.1186/1479-5876-4-48
56. Andersson AK, Flodstrom M, Sandler S. Cytokine-Induced Inhibition of Insulin Release From Mouse Pancreatic Beta-Cells Deficient in Inducible Nitric Oxide Synthase. Biochem Biophys Res Commun (2001) 281:396–403. doi: 10.1006/bbrc.2001.4361
57. Adachi J, Mimura M, Gotyo N, Watanabe T. The Development of Fulminant Type 1 Diabetes During Chemotherapy for Rectal Cancer. Intern Med (2015) 54:819–22. doi: 10.2169/internalmedicine.54.3413
58. Yanase K, Nakamura Y, Yasui T, Kakimoto MOY. Fulminant Type 1 Diabetes Mellitus Onset During Postoperative Chemotherapy for Breast Cancer. Tonyobyo (J Jpn Diabetes Soc) (2010) 53:107–11.
59. Uto H, Matsuoka H, Murata M, Okamoto T, Miyata Y, Hori T, et al. A Case of Chronic Hepatitis C Developing Insulin-Dependent Diabetes Mellitus Associated With Various Autoantibodies During Interferon Therapy. Diabetes Res Clin Pract (2000) 49:101–6. doi: 10.1016/s0168-8227(00)00143-1
60. Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, et al. Crosstalk Between Neutrophils, B-1a Cells and Plasmacytoid Dendritic Cells Initiates Autoimmune Diabetes. Nat Med (2013) 19:65–73. doi: 10.1038/nm.3042
61. Heidari N, Miller AV, Hicks MA, Marking CB, Harada H. Glucocorticoid-Mediated BIM Induction and Apoptosis are Regulated by Runx2 and C-Jun in Leukemia Cells. Cell Death Dis (2012) 3:e349. doi: 10.1038/cddis.2012.89
62. Mustian KM, Darling TV, Janelsins MC, Jean-Pierre P, Roscoe JA, Morrow GR. Chemotherapy-Induced Nausea and Vomiting. US Oncol (2008) 4:19–23. doi: 10.17925/ohr.2008.04.1.19
63. Paulsen O, Aass N, Kaasa S, Dale O. Do Corticosteroids Provide Analgesic Effects in Cancer Patients? A Systematic Literature Review. J Pain Symptom Manage (2013) 46:96–105. doi: 10.1016/j.jpainsymman.2012.06.019
64. Jamil MO, Mineishi S. State-Of-the-Art Acute and Chronic GVHD Treatment. Int J Hematol (2015) 101:452–66. doi: 10.1007/s12185-015-1785-1
65. Conn JW, Fajans SS. Influence of Adrenal Cortical Steroids on Carbohydrate Metabolism in Man. Metabolism (1956) 5:114–27.
66. Bevier WC, Zisser HC, Jovanovic L, Finan DA, Palerm CC, Seborg DE, et al. Use of Continuous Glucose Monitoring to Estimate Insulin Requirements in Patients With Type 1 Diabetes Mellitus During a Short Course of Prednisone. J Diabetes Sci Technol (2008) 2:578–83. doi: 10.1177/193229680800200408
67. van Raalte DH, Ouwens DM, Diamant M. Novel Insights Into Glucocorticoid-Mediated Diabetogenic Effects: Towards Expansion of Therapeutic Options? Eur J Clin Invest (2009) 39:81–93. doi: 10.1111/j.1365-2362.2008.02067.x
68. Linssen MM, van Raalte DH, Toonen EJ, Alkema W, van der Zon GC, Dokter WH, et al. Prednisolone-Induced Beta Cell Dysfunction is Associated With Impaired Endoplasmic Reticulum Homeostasis in INS-1E Cells. Cell Signal (2011) 23:1708–15. doi: 10.1016/j.cellsig.2011.06.002
69. Franckhauser S, Antras-Ferry J, Robin P, Robin D, Granner DK, Forest C. Expression of the Phosphoenolpyruvate Carboxykinase Gene in 3T3-F442A Adipose Cells: Opposite Effects of Dexamethasone and Isoprenaline on Transcription. Biochem J (1995) 305(Pt 1):65–71. doi: 10.1042/bj3050065
70. Weinstein SP, Wilson CM, Pritsker A, Cushman SW. Dexamethasone Inhibits Insulin-Stimulated Recruitment of GLUT4 to the Cell Surface in Rat Skeletal Muscle. Metabolism (1998) 47:3–6. doi: 10.1016/s0026-0495(98)90184-6
71. Arteaga CL, Kitten LJ, Coronado EB, Jacobs S, Kull FC Jr., Allred DC, et al. Blockade of the Type I Somatomedin Receptor Inhibits Growth of Human Breast Cancer Cells in Athymic Mice. J Clin Invest (1989) 84:1418–23. doi: 10.1172/JCI114315
72. Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, et al. Phase I, Pharmacokinetic and Pharmacodynamic Study of the Anti-Insulinlike Growth Factor Type 1 Receptor Monoclonal Antibody CP-751,871 in Patients With Multiple Myeloma. J Clin Oncol (2008) 26:3196–203. doi: 10.1200/JCO.2007.15.9319
73. Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, et al. Phase I Dose Escalation Study of the Anti Insulin-Like Growth Factor-I Receptor Monoclonal Antibody CP-751,871 in Patients With Refractory Solid Tumors. Clin Cancer Res (2007) 13:5834–40. doi: 10.1158/1078-0432.CCR-07-1118
74. del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, et al. Growth Hormone Regulation of P85alpha Expression and Phosphoinositide 3-Kinase Activity in Adipose Tissue: Mechanism for Growth Hormone-Mediated Insulin Resistance. Diabetes (2007) 56:1638–46. doi: 10.2337/db06-0299
75. Verges B, Cariou B. mTOR Inhibitors and Diabetes. Diabetes Res Clin Pract (2015) 110:101–8. doi: 10.1016/j.diabres.2015.09.014
76. Kleinert M, Sylow L, Fazakerley DJ, Krycer JR, Thomas KC, Oxboll AJ, et al. Acute mTOR Inhibition Induces Insulin Resistance and Alters Substrate Utilization In Vivo. Mol Metab (2014) 3:630–41. doi: 10.1016/j.molmet.2014.06.004
77. Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, et al. mTOR Inhibition by Rapamycin Prevents Beta-Cell Adaptation to Hyperglycemia and Exacerbates the Metabolic State in Type 2 Diabetes. Diabetes (2008) 57:945–57. doi: 10.2337/db07-0922
78. Crouthamel MC, Kahana JA, Korenchuk S, Zhang SY, Sundaresan G, Eberwein DJ, et al. Mechanism and Management of AKT Inhibitor-Induced Hyperglycemia. Clin Cancer Res (2009) 15:217–25. doi: 10.1158/1078-0432.CCR-08-1253
79. Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, et al. Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care (2015) 38:e137–8. doi: 10.2337/dc15-0889
80. Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, Gastrointestinal, Hepatic, Endocrine, and Renal Side-Effects of Anti-PD-1 Therapy. Eur J Cancer (2016) 60:190–209. doi: 10.1016/j.ejca.2016.02.025
81. Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The Spectrum, Incidence, Kinetics and Management of Endocrinopathies With Immune Checkpoint Inhibitors for Metastatic Melanoma. Eur J Endocrinol (2018) 178:173–80. doi: 10.1530/EJE-17-0810
82. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes (2018) 67:1471–80. doi: 10.2337/dbi18-0002
83. Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The Programmed Death-1 (PD-1) Pathway Regulates Autoimmune Diabetes in Nonobese Diabetic (NOD) Mice. J Exp Med (2003) 198:63–9. doi: 10.1084/jem.20022125
84. Guleria I, Gubbels Bupp M, Dada S, Fife B, Tang Q, Ansari MJ, et al. Mechanisms of PDL1-Mediated Regulation of Autoimmune Diabetes. Clin Immunol (2007) 125:16–25. doi: 10.1016/j.clim.2007.05.013
85. Klein AP. Genetic Susceptibility to Pancreatic Cancer. Mol Carcinog (2012) 51:14–24. doi: 10.1002/mc.20855
86. Pierce BL, Austin MA, Ahsan H. Association Study of Type 2 Diabetes Genetic Susceptibility Variants and Risk of Pancreatic Cancer: An Analysis of PanScan-I Data. Cancer Causes Control (2011) 22:877–83. doi: 10.1007/s10552-011-9760-5
87. Prizment AE, Gross M, Rasmussen-Torvik L, Peacock JM, Anderson KE. Genes Related to Diabetes may be Associated With Pancreatic Cancer in a Population-Based Case-Control Study in Minnesota. Pancreas (2012) 41:50–3. doi: 10.1097/MPA.0b013e3182247625
88. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality From Cancer in a Prospectively Studied Cohort of U.S. Adults. N Engl J Med (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
89. Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, et al. Cancer Incidence and Mortality After Gastric Bypass Surgery. Obes (Silver Spring) (2009) 17:796–802. doi: 10.1038/oby.2008.610
90. Christou NV, Lieberman M, Sampalis F, Sampalis JS. Bariatric Surgery Reduces Cancer Risk in Morbidly Obese Patients. Surg Obes Relat Dis (2008) 4:691–5. doi: 10.1016/j.soard.2008.08.025
91. Byers T, Sedjo RL. Body Fatness as a Cause of Cancer: Epidemiologic Clues to Biologic Mechanisms. Endocr Relat Cancer (2015) 22:R125–134. doi: 10.1530/ERC-14-0580
92. Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC. Pancreatic Duct Replication is Increased With Obesity and Type 2 Diabetes in Humans. Diabetologia (2010) 53:21–6. doi: 10.1007/s00125-009-1556-8
93. Balkwill F, Mantovani A. Inflammation and Cancer: Back to Virchow? Lancet (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
94. Lin WW, Karin M. A Cytokine-Mediated Link Between Innate Immunity, Inflammation, and Cancer. J Clin Invest (2007) 117:1175–83. doi: 10.1172/JCI31537
95. Yu T, Robotham JL, Yoon Y. Increased Production of Reactive Oxygen Species in Hyperglycemic Conditions Requires Dynamic Change of Mitochondrial Morphology. Proc Natl Acad Sci USA (2006) 103:2653–8. doi: 10.1073/pnas.0511154103
96. Aggeli IK, Theofilatos D, Beis I, Gaitanaki C. Insulin-Induced Oxidative Stress Up-Regulates Heme Oxygenase-1 via Diverse Signaling Cascades in the C2 Skeletal Myoblast Cell Line. Endocrinology (2011) 152:1274–83. doi: 10.1210/en.2010-1319
97. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem Biol Interact (2006) 160:1–40. doi: 10.1016/j.cbi.2005.12.009
98. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int J Biochem Cell Biol (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
99. Fenton JI, Birmingham JM. Adipokine Regulation of Colon Cancer: Adiponectin Attenuates Interleukin-6-Induced Colon Carcinoma Cell Proliferation via STAT-3. Mol Carcinog (2010) 49:700–9. doi: 10.1002/mc.20644
100. Aggarwal BB. Nuclear Factor-Kappab: The Enemy Within. Cancer Cell (2004) 6:203–8. doi: 10.1016/j.ccr.2004.09.003
101. de Beer JC, Liebenberg L. Does Cancer Risk Increase With HbA1c, Independent of Diabetes? Br J Cancer (2014) 110:2361–8. doi: 10.1038/bjc.2014.150
102. Xu J, Ye Y, Wu H, Duerksen-Hughes P, Zhang H, Li P, et al. Association Between Markers of Glucose Metabolism and Risk of Colorectal Cancer. BMJ Open (2016) 6:e011430. doi: 10.1136/bmjopen-2016-011430
103. Warburg O. On the Origin of Cancer Cells. Science (1956) 123:309–14. doi: 10.1126/science.123.3191.309
104. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science (2009) 324:1029–33. doi: 10.1126/science.1160809
105. Devic S. Warburg Effect - a Consequence or the Cause of Carcinogenesis? J Cancer (2016) 7:817–22. doi: 10.7150/jca.14274
106. Pollak M. Insulin and Insulin-Like Growth Factor Signalling in Neoplasia. Nat Rev Cancer (2008) 8:915–28. doi: 10.1038/nrc2536
107. Abe R, Yamagishi S. AGE-RAGE System and Carcinogenesis. Curr Pharm Des (2008) 14:940–5. doi: 10.2174/138161208784139765
108. Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, et al. RAGE (Receptor for Advanced Glycation Endproducts)and Their Role in Cancer and Inflammation. J Transl Med (2009) 7:17. doi: 10.1186/1479-5876-7-17
109. Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, et al. Expression of Receptors for Advanced Glycation End-Products (RAGE) Is Closely Associated With the Invasive and Metastatic Activity of Gastric Cancer. J Pathol (2002) 196:163–70. doi: 10.1002/path.1031
110. Takada M, Hirata K, Ajiki T, Suzuki Y, Kuroda Y. Expression of Receptor for Advanced Glycation End Products (RAGE) and MMP-9 in Human Pancreatic Cancer Cells. Hepatogastroenterology (2004) 51:928–30.
111. Abe R, Shimizu T, Sugawara H, Watanabe H, Nakamura H, Choei H, et al. Regulation of Human Melanoma Growth and Metastasis by AGE-AGE Receptor Interactions. J Invest Dermatol (2004) 122:461–7. doi: 10.1046/j.0022-202X.2004.22218.x
112. Han L, Ma Q, Li J, Liu H, Li W, Ma G, et al. High Glucose Promotes Pancreatic Cancer Cell Proliferation via the Induction of EGF Expression and Transactivation of EGFR. PloS One (2011) 6:e27074. doi: 10.1371/journal.pone.0027074
113. Lee G, Walser TC, Dubinett SM. Chronic Inflammation, Chronic Obstructive Pulmonary Disease, and Lung Cancer. Curr Opin Pulm Med (2009) 15:303–7. doi: 10.1097/MCP.0b013e32832c975a
114. Calle EE, Kaaks R. Overweight, Obesity and Cancer: Epidemiological Evidence and Proposed Mechanisms. Nat Rev Cancer (2004) 4:579–91. doi: 10.1038/nrc1408
115. Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, et al. Insulin, Glucose, Insulin Resistance, and Pancreatic Cancer in Male Smokers. JAMA (2005) 294:2872–8. doi: 10.1001/jama.294.22.2872
116. Friberg E, Mantzoros CS, Wolk A. Diabetes and Risk of Endometrial Cancer: A Population-Based Prospective Cohort Study. Cancer Epidemiol Biomarkers Prev (2007) 16:276–80. doi: 10.1158/1055-9965.EPI-06-0751
117. Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, et al. The Role of Insulin Receptors and IGF-I Receptors in Cancer and Other Diseases. Arch Physiol Biochem (2008) 114:23–37. doi: 10.1080/13813450801969715
118. Baxter RC, Bryson JM, Turtle JR. Somatogenic Receptors of Rat Liver: Regulation by Insulin. Endocrinology (1980) 107:1176–81. doi: 10.1210/endo-107-4-1176
119. Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y, et al. Phenotypes and Genotypes of Insulin-Like Growth Factor 1, IGF-Binding Protein-3 and Cancer Risk: Evidence From 96 Studies. Eur J Hum Genet (2009) 17:1668–75. doi: 10.1038/ejhg.2009.86
120. Yakar S, Leroith D, Brodt P. The Role of the Growth Hormone/Insulin-Like Growth Factor Axis in Tumor Growth and Progression: Lessons From Animal Models. Cytokine Growth Factor Rev (2005) 16:407–20. doi: 10.1016/j.cytogfr.2005.01.010
121. Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, et al. Loss of Imprinting of Igf2 Alters Intestinal Maturation and Tumorigenesis in Mice. Science (2005) 307:1976–8. doi: 10.1126/science.1108080
122. Gallagher EJ, LeRoith D. The Proliferating Role of Insulin and Insulin-Like Growth Factors in Cancer. Trends Endocrinol Metab (2010) 21:610–8. doi: 10.1016/j.tem.2010.06.007
123. Carstensen B, Witte DR, Friis S. Cancer Occurrence in Danish Diabetic Patients: Duration and Insulin Effects. Diabetologia (2012) 55:948–58. doi: 10.1007/s00125-011-2381-4
124. Karlstad O, Starup-Linde J, Vestergaard P, Hjellvik V, Bazelier MT, Schmidt MK, et al. Use of Insulin and Insulin Analogs and Risk of Cancer - Systematic Review and Meta-Analysis of Observational Studies. Curr Drug Saf (2013) 8:333–48. doi: 10.2174/15680266113136660067
125. Wu JW, Azoulay L, Majdan A, Boivin JF, Pollak M, Suissa S. Long-Term Use of Long-Acting Insulin Analogs and Breast Cancer Incidence in Women With Type 2 Diabetes. J Clin Oncol (2017) 35:3647–53. doi: 10.1200/JCO.2017.73.4491
126. Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, et al. Risk of Malignancies in Patients With Diabetes Treated With Human Insulin or Insulin Analogues: A Cohort Study. Diabetologia (2009) 52:1732–44. doi: 10.1007/s00125-009-1418-4
127. Tran TT, Naigamwalla D, Oprescu AI, Lam L, McKeown-Eyssen G, Bruce WR, et al. Hyperinsulinemia, But Not Other Factors Associated With Insulin Resistance, Acutely Enhances Colorectal Epithelial Proliferation In Vivo. Endocrinology (2006) 147:1830–7. doi: 10.1210/en.2005-1012
128. Kim WY, Jin Q, Oh SH, Kim ES, Yang YJ, Lee DH, et al. Elevated Epithelial Insulin-Like Growth Factor Expression is a Risk Factor for Lung Cancer Development. Cancer Res (2009) 69:7439–48. doi: 10.1158/0008-5472.CAN-08-3792
129. Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlations of Receptor Binding and Metabolic and Mitogenic Potencies of Insulin Analogs Designed for Clinical Use. Diabetes (2000) 49:999–1005. doi: 10.2337/diabetes.49.6.999
130. Varewijck AJ, Janssen JA. Insulin and its Analogues and Their Affinities for the IGF1 Receptor. Endocr Relat Cancer (2012) 19:F63–75. doi: 10.1530/ERC-12-0026
131. Sciacca L, Cassarino MF, Genua M, Pandini G, Le Moli R, Squatrito S, et al. Insulin Analogues Differently Activate Insulin Receptor Isoforms and Post-Receptor Signalling. Diabetologia (2010) 53:1743–53. doi: 10.1007/s00125-010-1760-6
132. Investigators OT, Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, et al. Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N Engl J Med (2012) 367:319–28. doi: 10.1056/NEJMoa1203858
133. But A, De Bruin ML, Bazelier MT, Hjellvik V, Andersen M, Auvinen A, et al. Cancer Risk Among Insulin Users: Comparing Analogues With Human Insulin in the CARING Five-Country Cohort Study. Diabetologia (2017) 60:1691–703. doi: 10.1007/s00125-017-4312-5
134. Bronsveld HK, ter Braak B, Karlstad O, Vestergaard P, Starup-Linde J, Bazelier MT, et al. Treatment With Insulin (Analogues) and Breast Cancer Risk in Diabetics; a Systematic Review and Meta-Analysis of In Vitro, Animal and Human Evidence. Breast Cancer Res (2015) 17:100. doi: 10.1186/s13058-015-0611-2
135. Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, et al. Cancer Risk Associated With Use of Metformin and Sulfonylurea in Type 2 Diabetes: A Meta-Analysis. Oncologist (2012) 17:813–22. doi: 10.1634/theoncologist.2011-0462
136. Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 Diabetes Increases and Metformin Reduces Total, Colorectal, Liver and Pancreatic Cancer Incidences in Taiwanese: A Representative Population Prospective Cohort Study of 800,000 Individuals. BMC Cancer (2011) 11:20. doi: 10.1186/1471-2407-11-20
137. Chaiteerakij R, Petersen GM, Bamlet WR, Chaffee KG, Zhen DB, Burch PA, et al. Metformin Use and Survival of Patients With Pancreatic Cancer: A Cautionary Lesson. J Clin Oncol (2016) 34:1898–904. doi: 10.1200/JCO.2015.63.3511
138. Currie CJ, Poole CD, Gale EA. The Influence of Glucose-Lowering Therapies on Cancer Risk in Type 2 Diabetes. Diabetologia (2009) 52:1766–77. doi: 10.1007/s00125-009-1440-6
139. Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing Metformin for the Prevention of Cancer and Cancer Recurrence. Diabetologia (2017) 60:1639–47. doi: 10.1007/s00125-017-4372-6
140. Morgillo F, Sasso FC, Della Corte CM, Festino L, Manzo A, Martinelli E, et al. Metformin in Lung Cancer: Rationale for a Combination Therapy. Expert Opin Investig Drugs (2013) 22:1401–9. doi: 10.1517/13543784.2013.828691
141. Morgillo F, Fasano M, Della Corte CM, Sasso FC, Papaccio F, Viscardi G, et al. Results of the Safety Run-in Part of the METAL (METformin in Advanced Lung Cancer) Study: A Multicentre, Open-Label Phase I-II Study of Metformin With Erlotinib in Second-Line Therapy of Patients With Stage IV Non-Small-Cell Lung Cancer. ESMO Open (2017) 2:e000132. doi: 10.1136/esmoopen-2016-000132
142. Della Corte CM, Ciaramella V, Di Mauro C, Castellone MD, Papaccio F, Fasano M, et al. Metformin Increases Antitumor Activity of MEK Inhibitors Through GLI1 Downregulation in LKB1 Positive Human NSCLC Cancer Cells. Oncotarget (2016) 7:4265–78. doi: 10.18632/oncotarget.6559
143. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1-Based Therapies. Gastroenterology (2011) 141:150–6. doi: 10.1053/j.gastro.2011.02.018
144. Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, et al. Beneficial Endocrine But Adverse Exocrine Effects of Sitagliptin in the Human Islet Amyloid Polypeptide Transgenic Rat Model of Type 2 Diabetes: Interactions With Metformin. Diabetes (2009) 58:1604–15. doi: 10.2337/db09-0058
145. Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 Receptor Activation by Exendin-4 Induces Expansion of Pancreatic Duct Glands in Rats and Accelerates Formation of Dysplastic Lesions and Chronic Pancreatitis in the Kras(G12D) Mouse Model. Diabetes (2012) 61:1250–62. doi: 10.2337/db11-1109
146. Parks M, Rosebraugh C. Weighing Risks and Benefits of Liraglutide–the FDA’s Review of a New Antidiabetic Therapy. N Engl J Med (2010) 362:774–7. doi: 10.1056/NEJMp1001578
147. Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, et al. Safety Issues With Glucagon-Like Peptide-1 Receptor Agonists (Pancreatitis, Pancreatic Cancer and Cholelithiasis): Data From Randomized Controlled Trials. Diabetes Obes Metab (2017) 19:1233–41. doi: 10.1111/dom.12926
148. Zhang Z, Chen X, Lu P, Zhang J, Xu Y, He W, et al. Incretin-Based Agents in Type 2 Diabetic Patients at Cardiovascular Risk: Compare the Effect of GLP-1 Agonists and DPP-4 Inhibitors on Cardiovascular and Pancreatic Outcomes. Cardiovasc Diabetol (2017) 16:31. doi: 10.1186/s12933-017-0512-z
149. Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 Inhibitors and Risk of Cancer in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Diabetologia (2017) 60:1862–72. doi: 10.1007/s00125-017-4370-8
150. Shaikh AMY. SGLT2 Inhibitors and Cancer: Why Further Evidence is Required. Diabetologia (2017) 60:2536–7. doi: 10.1007/s00125-017-4434-9
151. Ptaszynska A, Cohen SM, Messing EM, Reilly TP, Johnsson E, Johnsson K. Assessing Bladder Cancer Risk in Type 2 Diabetes Clinical Trials: The Dapagliflozin Drug Development Program as a ‘Case Study’. Diabetes Ther (2015) 6:357–75. doi: 10.1007/s13300-015-0128-9
152. Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, et al. Functional Expression of Sodium-Glucose Transporters in Cancer. Proc Natl Acad Sci USA (2015) 112:E4111–9. doi: 10.1073/pnas.1511698112
153. Villani LA, Smith BK, Marcinko K, Ford RJ, Broadfield LA, Green AE, et al. The Diabetes Medication Canagliflozin Reduces Cancer Cell Proliferation by Inhibiting Mitochondrial Complex-I Supported Respiration. Mol Metab (2016) 5:1048–56. doi: 10.1016/j.molmet.2016.08.014
154. Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and Cancer: A Case-Control Study. Acta Diabetol (2009) 46:279–84. doi: 10.1007/s00592-008-0083-2
155. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased Cancer-Related Mortality for Patients With Type 2 Diabetes Who Use Sulfonylureas or Insulin. Diabetes Care (2006) 29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558
156. Chen L, Chubak J, Boudreau DM, Barlow WE, Weiss NS, Li CI. Diabetes Treatments and Risks of Adverse Breast Cancer Outcomes Among Early-Stage Breast Cancer Patients: A SEER-Medicare Analysis. Cancer Res (2017) 77:6033–41. doi: 10.1158/0008-5472.CAN-17-0687
157. Chen Y, Du L, Li L, Ma J, Geng X, Yao X, et al. Cancer Risk of Sulfonylureas in Patients With Type 2 Diabetes Mellitus: A Systematic Review. J Diabetes (2017) 9:482–94. doi: 10.1111/1753-0407.12435
158. Nanjan MJ, Mohammed M, Prashantha Kumar BR, Chandrasekar MJN. Thiazolidinediones as Antidiabetic Agents: A Critical Review. Bioorg Chem (2018) 77:548–67. doi: 10.1016/j.bioorg.2018.02.009
159. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary Prevention of Macrovascular Events in Patients With Type 2 Diabetes in the PROactive Study (PROspective Pioglitazone Clinical Trial In Macrovascular Events): A Randomised Controlled Trial. Lancet (2005) 366:1279–89. doi: 10.1016/S0140-6736(05)67528-9
160. Cornell S. Comparison of the Diabetes Guidelines From the ADA/EASD and the AACE/ACE. J Am Pharm Assoc (2013) (2017) 57:261–5. doi: 10.1016/j.japh.2016.11.005
161. Lv S, Wang W, Wang H, Zhu Y, Lei C. PPARgamma Activation Serves as Therapeutic Strategy Against Bladder Cancer via Inhibiting PI3K-Akt Signaling Pathway. BMC Cancer (2019) 19:204. doi: 10.1186/s12885-019-5426-6
162. Lamichane S, Dahal Lamichane B, Kwon SM. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int J Mol Sci (2018) 19:949. doi: 10.3390/ijms19040949
163. Ma Y, Wang B, Li L, Wang F, Xia X. The Administration of Peroxisome Proliferator-Activated Receptors Alpha/Gamma Agonist TZD18 Inhibits Cell Growth and Induces Apoptosis in Human Gastric Cancer Cell Lines. J Cancer Res Ther (2019) 15:120–5. doi: 10.4103/0973-1482.208753
164. Ciaramella V, Sasso FC, Di Liello R, Corte CMD, Barra G, Viscardi G, et al. Activity and Molecular Targets of Pioglitazone via Blockade of Proliferation, Invasiveness and Bioenergetics in Human NSCLC. J Exp Clin Cancer Res (2019) 38:178. doi: 10.1186/s13046-019-1176-1
165. Mazzone PJ, Rai H, Beukemann M, Xu M, Jain A, Sasidhar M. The Effect of Metformin and Thiazolidinedione Use on Lung Cancer in Diabetics. BMC Cancer (2012) 12:410. doi: 10.1186/1471-2407-12-410
166. Monami M, Dicembrini I, Mannucci E. Thiazolidinediones and Cancer: Results of a Meta-Analysis of Randomized Clinical Trials. Acta Diabetol (2014) 51:91–101. doi: 10.1007/s00592-013-0504-8
167. Wu L, Zhu J, Prokop LJ, Murad MH. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Sci Rep (2015) 5:10147. doi: 10.1038/srep10147
Keywords: cancers, mechanism, type 2 diabetes (T2D), type 1 diabetes, type 3C diabetes mellitus
Citation: Zhu B and Qu S (2022) The Relationship Between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 13:800995. doi: 10.3389/fendo.2022.800995
Received: 24 October 2021; Accepted: 12 January 2022;
Published: 11 February 2022.
Edited by:
Martin Heni, University of Tübingen, GermanyReviewed by:
Ferdinando Carlo Sasso, Università della Campania Luigi Vanvitelli, ItalyLin Liao, Shandong University, China
Copyright © 2022 Zhu and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shen Qu, cXVzaGVuY25AaG90bWFpbC5jb20=
 Bing Zhu
Bing Zhu Shen Qu
Shen Qu