- 1Department of Obstetrics and Gynecology, National Clinical Research Center for Obstetric & Gynecologic Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 2Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 3Reproductive Medical Center, Haidian Maternal and Child Health Hospital, Beijing, China
- 4Reproductive Medicine Center, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 5Department of Obstetrics and Gynecology, Shuangliu Maternal and Child Health Hospital, Chengdu, China
- 6Department of Reproductive Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang, China
Objective: To assess the effect of antiandrogenic pretreatment using combined oral contraceptives (COCs) before ovulation induction in infertile patients with polycystic ovary syndrome (PCOS) with hyperandrogenism.
Design: Prospective, randomized open-labeled cohort study
Setting: Multicenter
Patients: PCOS patients with hyperandrogenism and requiring infertility treatments
Interventions: Randomization to direct ovulation induction of letrozole (letrozole group) or ethinylestradiol/cyproterone acetate (EE/CPA) for 3 months and subsequent letrozole-induced ovulation (EE/CPA+ letrozole group). The maximum number of ovulation induction cycle was three to four.
Main Outcome Measures: Ovulation rate, conception rate, ongoing pregnancy rate, and live birth rate were the main outcomes of the study.
Results: There were no significant differences in the cumulative ovulation, conception, ongoing pregnancy, and live birth rates between the letrozole and EE/CPA+ letrozole groups (cumulative ovulation: 206/254 [81.10%] vs. 169/205 [82.44%], risk ratio [RR]= 1.09 [0.68,1.76], P=0.713; conception: 44/90 [48.89%] vs. 42/76 [55.26%], RR= 1.29 [0.70,2.38], P=0.413; ongoing pregnancy: 33/90 [36.67%] vs. 33/76 [43.42%], RR=1.33 [0.71,2.47], P=0.376; and live birth: 32/90 [35.56%] vs. 31/76 [40.79%], RR=1.25 [0.67, 2.34], P=0.489).
Conclusions: The results of this study showed that COC pretreatment was not superior to direct letrozole-induced ovulation therapy in improving ovulation and pregnancy results in women with PCOS. There is no benefit to perform antiandrogenic therapy before ovulation induction in patients with PCOS in clinical practice.
Clinical Trial Registration: www.clinicaltrials.gov, identifier ChiCTR1900022839
Introduction
Polycystic ovary syndrome (PCOS) is the most prevalent endocrine disorder affecting approximately 6–15% of women of reproductive age (1). Reproduction problems, including anovulatory infertility and increased adverse pregnancy events, affect 40% of patients with PCOS (2). Hence, one of the priorities in the treatment of PCOS is to improve the fertility of patients, and tremendous endeavor has been made by clinicians in this regard.
Hyperandrogenism is a prominent clinical hallmark of PCOS, which plays a detrimental role in female fecundity and may be a treatment target for conquering the reproduction problems (1). Hyperandrogenism exerts a deleterious impact on granulosa cell activity and follicular growth, leading to follicle dysmaturity and subsequent sterility (3, 4). Previous studies have also revealed that hyperandrogenism is associated with an increased risk of preterm delivery and preeclampsia in patients with PCOS (5). Consequently, antiandrogenic therapy may help enhance pregnancy outcomes in patients with PCOS with androgen excess in the long run.
Pretreatment approaches before ovulation induction has been investigated by previous studies to improve pregnancy outcomes for the patients with PCOS, including lifestyle modification (6) and insulin sensitizing agents (7, 8). Given the potential role of antiandrogenic therapy for infertile PCOS women, antiandrogenic pretreatment might also assist in enhancing fertility for the patients.
However, controversy exists among researchers whether antiandrogenic therapy with combined oral contraceptives (COCs), such as ethinylestradiol/cyproterone acetate (EE/CPA), should be performed before starting ovulation induction. One study suggested that COC pretreatment could increase implantation and pregnancy rates (9). In contrast, another study demonstrated that no improvement in pregnancy outcomes could be verified after the implementation of COC pretreatment (10). Additionally, the time needed to produce a benefit from antiandrogenic therapy may reduce the woman’s remaining reproductive window, depleting both patience and valuable fecundity, since female age continues to be the most important predictor of infertility treatment failure (11). Due to the lack of high-quality evidence regarding antiandrogenic pretreatment, well-designed clinical studies are needed to address this clinical dilemma.
Therefore, we designed this multicentered prospective clinical study to evaluate whether delayed infertility therapy for the antiandrogenic pretreatment using EE/CPA has advantages over immediate therapy in PCOS patients with hyperandrogenism. Additionally, the effect of antiandrogenic pretreatment on patients with different clinical characteristics was assessed. This study aimed to assist clinicians in recommending possible treatment strategies for infertile PCOS patients with hyperandrogenism.
Materials and Methods
Study Design and Ethics Approval
This was a multicentered prospective randomized controlled trial (RCT) study, conducted in six centers from the south, west, north, and central regions of China according to the Consolidated Standards of Reporting Trials (CONSORT) (12). This study was registered in the Chinese Registry of Clinical Trials at www.clinicaltrials.gov (registration number: ChiCTR1900022839) and was approved by the Institutional Review Board (IRB) of Peking Union Medical College Hospital (No: JS-1796). All procedures involving human subjects were conducted in accordance with the institutional ethical standards of the research center and the 1964 Declaration of Helsinki and its subsequent modifications or equivalent ethical standards. Written informed consent was obtained from all participants who were provided with a clear explanation of the trial by the assistant of each center.
Data Management and Randomization
The Peking Union Medical College Hospital served as the coordination center for the trial, which was in charge of all data input, management, and analysis. The first author and corresponding author are responsible for the correctness and completeness of data. No commercial support was provided for this study.
We utilized an online third-party data management system to perform data collection and randomization. A computer-based random number generator was applied to perform block randomization in a 1:1 ratio, with stratification according to the participating centers. A random order was kept for 6–16 repeats. The research assistants conducted randomization of the patients after recruitment. Clinicians who recruited the patients were blinded of the participants’ assignments. However, the assignment results could not be blinded to the assistants and patients due to the differences in the treatment method and duration between the two treatment groups. The data analysts who performed the final statistical analysis were blinded to the clinical information of the patients.
Study Population
Eligible participants were recruited between May 2019 and December 2020. All subjects were diagnosed with PCOS according to the diagnostic criteria proposed by the Rotterdam consensus as follows: (1) clinical or chemical hyperandrogenism, presented by hirsutism, acne, or androgenic alopecia (13); (2) oligomenorrhea, defined as menstrual cycle length > 35 days with < 8 menstrual cycles per year or amenorrhea, defined as no menstrual bleeding for 6 months or longer (14); and/or (3) polycystic ovaries, characterized as having more than 12 antral follicles with a diameter of 10 mm or single ovarian volume > 10 cm3; (4) exclusion of other causes of ovulation dysfunction and hyperandrogenism, including prolactin diseases, adrenal hyperplasia, and Cushing’s syndrome. Additional inclusion criteria were as follows: (1) age, 18–38 years old; (2) BMI, 18.5–28 kg/m2; (3) normal uterus and at least one patent fallopian tube, verified by hysterosalpingography, sonohysterography, or intrauterine pregnancy history in the past 3 years; (4) a male partner with a minimum sperm concentration of 14 million per milliliter, as defined by the World Health Organization (15); (5) a commitment by the couples to engage in regular intercourse with the intention of pregnancy throughout the study; and (6) no contraindications or allergic reactions of COCs and letrozole.
We excluded patients who were using any form of COCs, steroid hormones, antiandrogen therapies, or insulin sensitizers within 3 months; patients complicated with abnormal hepatic or renal functions; and patients with cardiovascular diseases, blood or immune diseases, malignancy, or mental disorders.
Intervention
At baseline, participants were randomly assigned at a 1:1 ratio to the following two treatment groups: Group 1(letrozole group), immediate ovulation induction using letrozole; and Group 2 (EE/CPA+ letrozole group), antiandrogenic pretreatment using Diane-35 (EE/CPA) for 3 months and subsequent ovulation induction using letrozole. Letrozole was started on the third day of the menstrual cycle and continued for 5 days. A maximum of three or four cycles of ovulation induction was provided to the patients. In both treatment groups, the dose of letrozole was increased in subsequent menstrual cycles, up to a maximum daily dose of 7.5mg, if the patients showed nonresponse to the drug, defined as monophasic basal body temperature, or a midluteal progesterone level of less than 3 ng/mL, or consequent ultrasound scanning revealed no dominant follicle expulsion (performed by a proficient sonographer) (16). At least two to four times of intercourse per week were required for participating couples, which were also recorded by the patients accordingly (17).
Clinical Measurements
Demographic and Anthropometric Data of Participants
We collected demographic data, including age, education, occupation, and medical history of each participant, and anthropometric measurements, including body mass index (BMI) and waist-to-hip ratio (WHR). BMI was calculated according to the formula: BMI = weight (kg)/height (m)2. The WHR was determined by calculating the ratio of the standing waist circumference to hip circumference. Normal weight and overweight were classified according to the criteria of the World Health Organization, as BMI of 18.5–23.9 kg/m2, 24–28 kg/m2, respectively (18, 19).
Clinical Assessments of PCOS
Menstruation and clinical hyperandrogenism of the patients were evaluated by a senior physician in each center. We utilized the modified Ferriman–Gallwey (m-FG) scores to evaluate the density of terminal hair at nine different body sites, including the upper lip, chin, chest, upper back, lower back, upper abdomen, lower abdomen, arm and thigh. And hirsutism was identified with scores ≥ 8 (20). The severity of acne was assessed by counting the number of lesions and their spread on the face, back, and chest, classified as mild, moderate, moderate to severe, and severe (21).
Biochemical Measurements
Fasting blood tests for hepatic and renal functions, complete blood count, and blood coagulation functions were conducted as the safety parameters for recruitment at baseline. Plasma glucose, total cholesterol, low-density lipoprotein cholesterol (LDL-cholesterol), and serum insulin were measured after recruitment. The standard 75-g oral glucose tolerance test (OGTT) was also implemented for the patients (22). Insulin resistance (IR) was quantified by the homeostasis model assessment for IR (HOMA-IR) as follows: HOMA-IR = fasting serum insulin (U/L) × fasting plasma glucose (mmol/L)/22.5 (23). IR was defined as HOMA-IR ≥ 2.63 (24).
Assessment of Endocrine Parameters
Biochemical tests were performed 3–5 days following spontaneous menstruation to determine the levels of serum triiodothyronine (T3), luteinizing hormone (LH), follicle-stimulating hormone (FSH), total testosterone, sex hormone binding globulin (SHBG), and sulfated dehydroepiandrosterone (DHEAS). Free androgen index (FAI) was calculated according to the formula: FAI = total testosterone (nmol/L)/serum SHBG (nmol/L) × 100 (25). The intra-assay variations of employed testing techniques were 1.6–6.3%, and inter-assay variations 5.8–9.6%.
Outcomes
Cumulative ovulation, conception, ongoing clinical pregnancy, and live birth rates were considered as the primary outcomes of the study. Secondary outcomes included ovulation rate per treatment cycle, conception rate per treatment cycle, average cycles taken to ovulation, letrozole dosage for ovulation, average cycles taken to pregnancy, and letrozole dosage for pregnancy. Ovulation was defined as a biphasic basal body temperature, or a midluteal progesterone level of more than 3 ng/mL, or dominant follicle expulsion under consequent ultrasound scanning (26). Conception was determined as a positive result of human chorionic gonadotropin (hCG) with the values > 10 mIU/mL following ovulation induction (27). Ongoing clinical pregnancy was defined as a clinical pregnancy that continued for 12 weeks of gestation (28). The definition of live birth was an infant born alive after 28 weeks of gestation. Preterm delivery was defined as birth occurring prior to 37 weeks of gestation (29).
Sample Size Calculation
We estimated that live birth rate of the immediate ovulation induction group (letrozole group) and the COC pretreatment group (EE/CPA+ letrozole group) was 20% and 40%, respectively. In this study, we aimed to detect the difference in live birth proportion between the two groups with 80% power and 5% two-sided significance. We calculated that a sample size of 81 per treatment group was needed using Pearson’s chi-square test. And we expanded to 115 per group to account for a 30% dropout rate.
The formulation for calculating sample size:
n, sample size; p1, live birth rate of letrozole group; p2, live birth rate of EE/CPA+ letrozole group; , mean of p1 and p2; , mean of (1-p1) and (1-p2); With an α of 0.05 and a two-sided Z, Zα = 1.96; with a β of 0.8, Zβ = 0.84.
Statistical Analysis
Continuous variables were expressed as mean with standard deviation (SD) when normally distributed, or as median with interquartile range if not. Frequency and percentage values were calculated to express categorical variables. The Student’s t-test was used to compare continuous variables. The Chi-squared test was used to compare categorical variables.
A log-binomial regression model was used to compare the letrozole and EE/CPA+ letrozole groups for binary outcomes (e.g., ovulation, conception, ongoing pregnancy and live birth). Due to the prospective nature of our research, the log-binomial model allowed us to estimate the risk ratio (RR) rather than the odds ratio (OR). We categorized the patients into two groups according to their age(<30, ≥30), BMI(18.5–23.9, 24–28), and HOMA-IR(<2.63,≥2.63), and three groups based on the FAI tertile, to evaluate the effect of COC pretreatment on PCOS patients with different clinical characteristics.
We utilized Kaplan–Meier curves to describe the effect of the two treatment methods on the conception rates based on the time from randomization to conception. And we depicted the Kaplan–Meier curves according to age, BMI, HOMA-IR, and FAI stratification.
All data were analyzed using R (http://www.r-project.org) and Empower Stats 2.2 (X&Y Solutions), and a p value < 0.05 was deemed statistically significant.
Adverse Events
Serious adverse events were defined as immediately life-threatening events, permanent disability, necessitated or extended inpatient hospitalization, intentional or accidental overdoses.
Results
Characteristics of the Patients
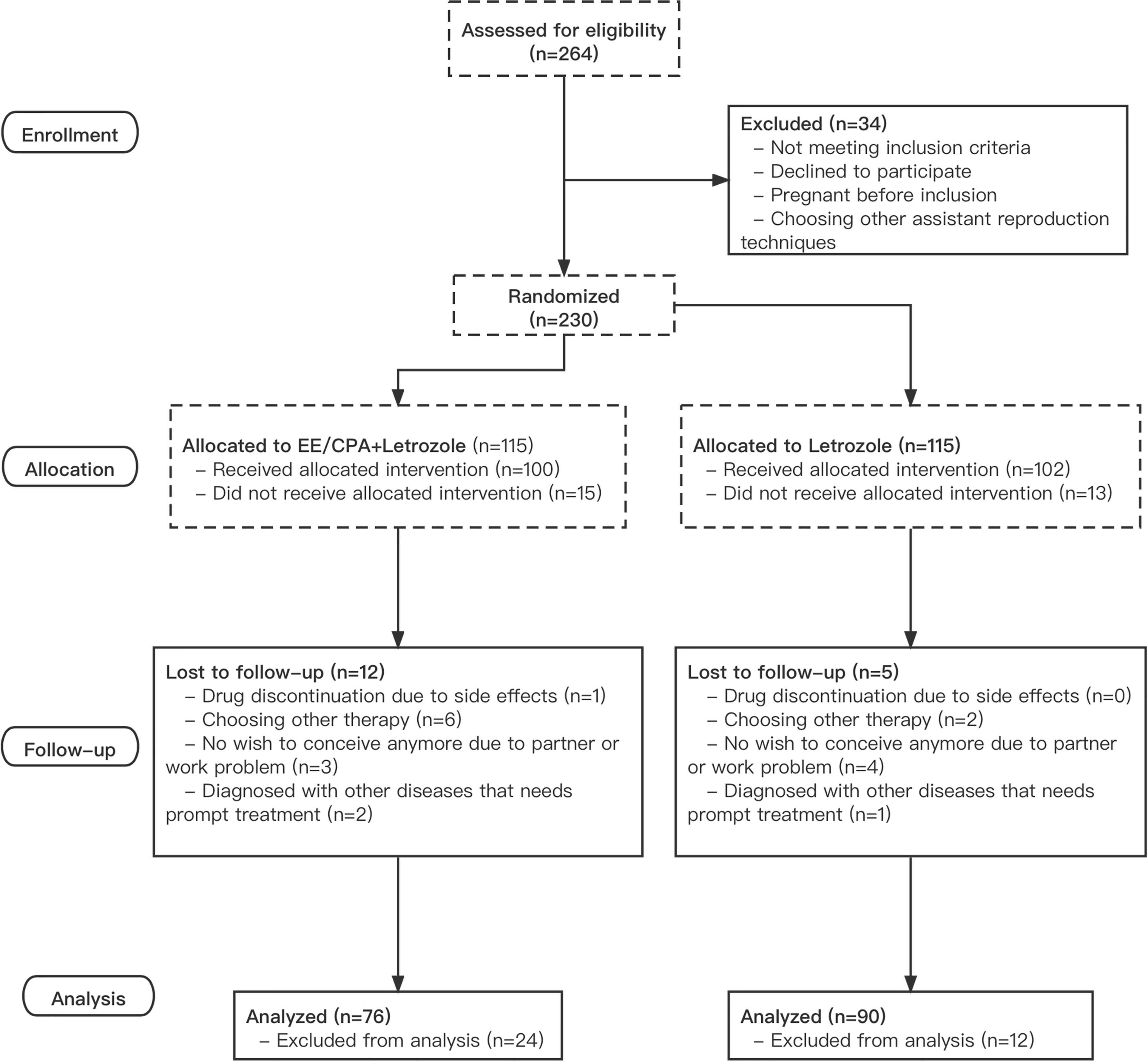
A total of 230 patients with PCOS were randomly allocated to one of the two treatment groups, with 115 patients each in the letrozole and EE/CPA+ letrozole groups. The last patient recruited in the trial completed the last ovulation cycle on December 20, 2020, and the final delivery occurred on September 20, 2021. A total of 39 of 115 patients in the EE/CPA+ letrozole group (33.9%) and 29 of 115 in the letrozole group (21.7%) dropped out or removed from further analysis. As a result, a total of 76 patients in the EE/CPA+ letrozole group and 90 patients in the letrozole group were included in the final analysis (Figure 1).
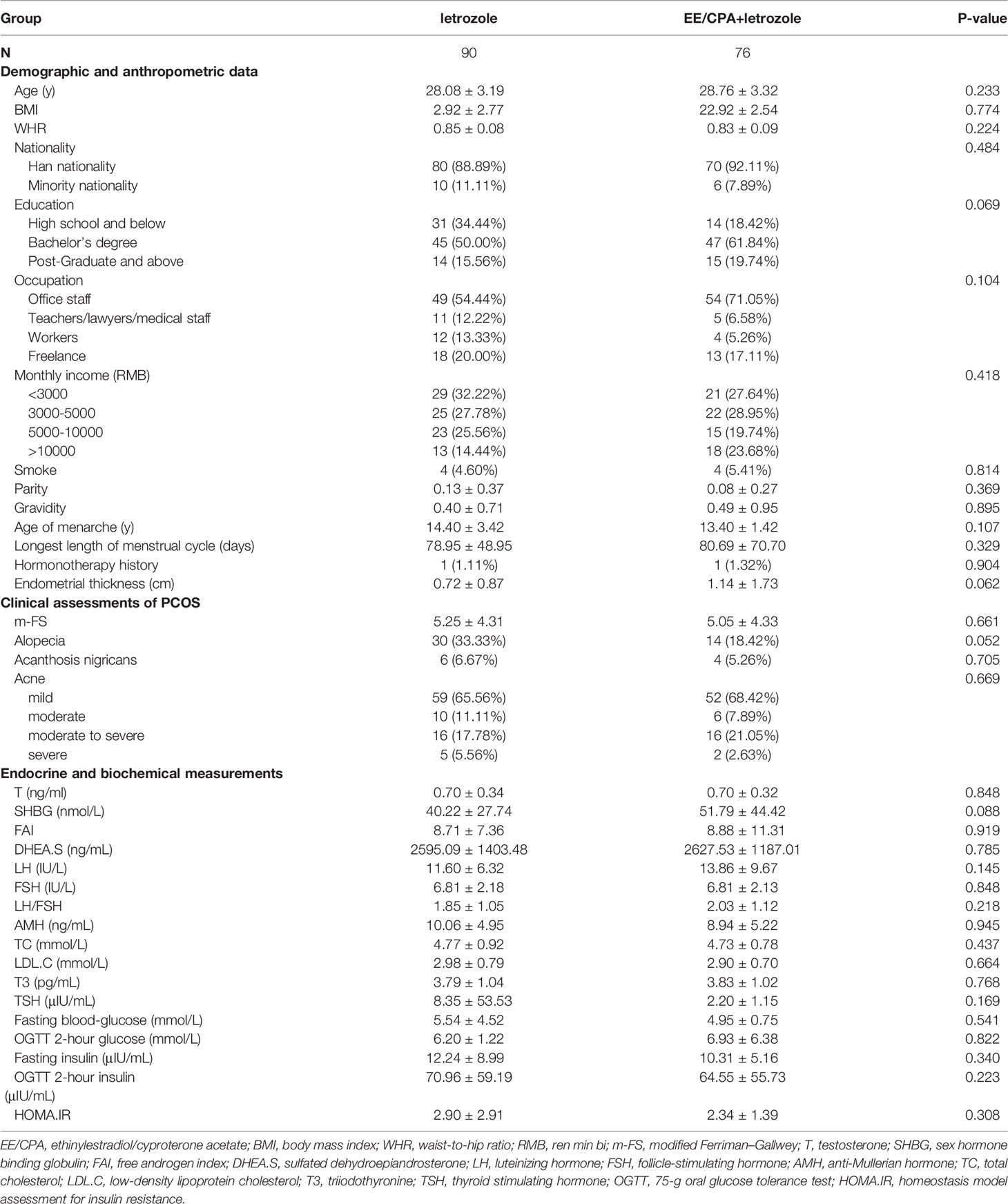
The clinical and demographic characteristics of the patients are listed in Table 1, and the two groups were well matched at baseline. Approximately half of the recruited patients in the two groups had a bachelor’s degree, and 40% of them had an income of > 5000RMB per month. Overall, the two groups had similar results in glucose, lipid, thyroid hormone, and sex hormone profiles at baseline (fasting glucose, fasting insulin, total cholesterol, LDL-C, T3, TSH, FSH, LH, total testosterone, SHBG, FAI, and AMH, P>0.05).
Primary and Secondary Outcomes
As shown in Table 2, there were no significant differences in the conception (44/90 [48.89%] vs. 42/76 [55.26%], RR= 1.29 [0.70,2.38], P=0.713), ongoing pregnancy (33/90 [36.67%] vs. 33/76 [43.42%], RR=1.33 [0.71,2.47], P=0.376), and live birth rates (32/90 [35.56%] vs. 31/76 [40.79%], RR=1.25 [0.67, 2.34], P=0.489) between the letrozole and EE/CPA+ letrozole groups. Additionally, no statistical difference in the cumulative ovulation rate was observed between the letrozole and EE/CPA+ letrozole groups (206/254 [81.10%] vs. 169/205 [82.44%], RR=1.09 [0.68,1.76], P=0.713).
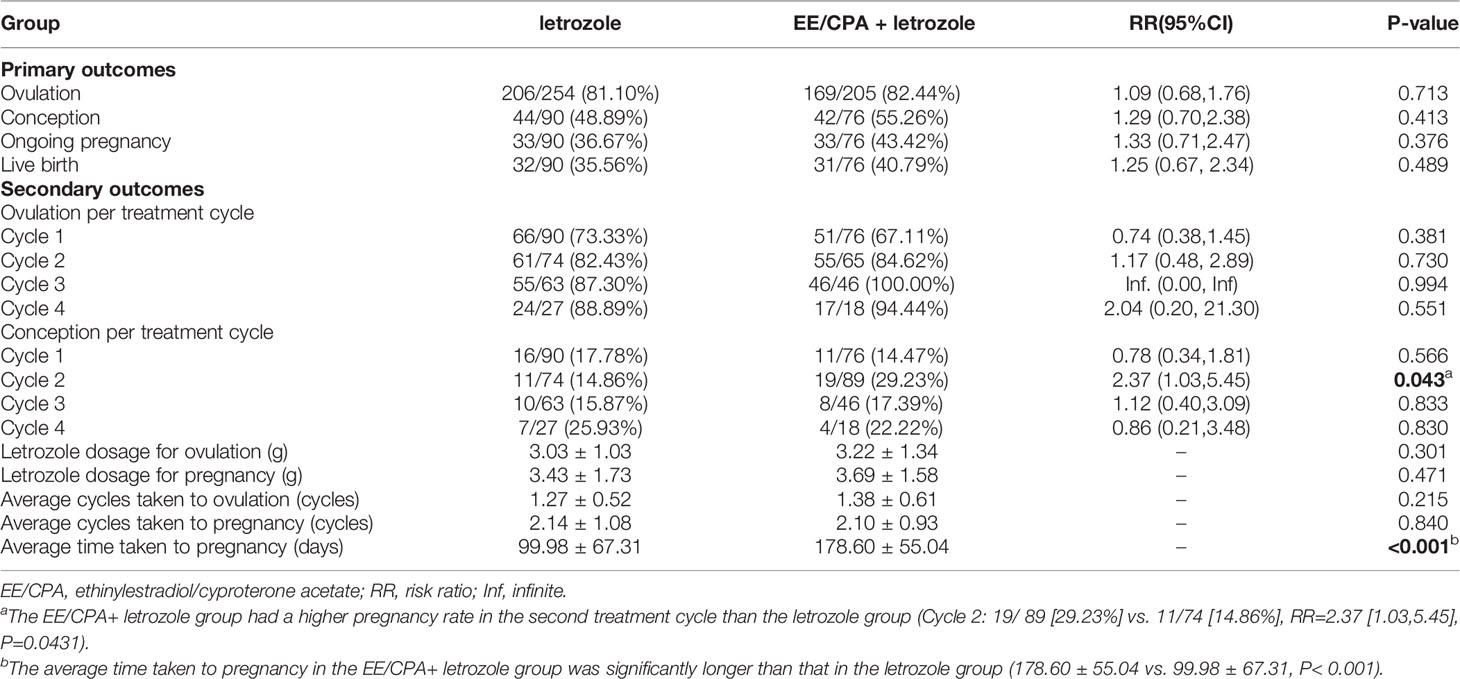
Table 2 Primary outcomes and secondary outcomes of patients of EE/CPA + letrozole and letrozole groups.
The EE/CPA+ letrozole group had a higher pregnancy rate in the second treatment cycle than the letrozole group (Cycle 2: 19/89 [29.23%] vs. 11/74 [14.86%], RR=2.37 [1.03,5.45], P=0.0431).However, the letrozole and EE/CPA+ letrozole groups showed no statistical differences in the secondary outcomes, including ovulation rate per treatment cycle (Cycle 1: 66/90 [73.33%] vs. 51/76[67.11%], P=0.381; Cycle 2: 61/74 [82.43%] vs. 55/65[84.62%], P=0.730; Cycle 3: 55/63 [87.30%] vs. 46/46 [100.00%], P=0.994; Cycle 4: 24/27 [88.89%] vs. 17/18 [94.44%], P=0.551), conception rate per treatment cycle (Cycle 1: 16/90 [17.78%] vs. 11/76 [14.47%], P=0.566; Cycle 3: 10/63 [15.87%] vs. 8/46 [17.39%], P=0.833; Cycle 4: 7/27 [25.93%] vs. 4/18 [22.22%], P=0.830), average cycles taken to ovulation (1.38 ± 0.61 vs. 1.27 ± 0.52, P=0.215), letrozole dosage for ovulation (3.03 ± 1.03 vs. 3.22 ± 1.34, P=0.301), average cycles taken to pregnancy (2.14 ± 1.08 vs. 2.10 ± 0.93, P=0.84), and letrozole dosage for pregnancy (3.43 ± 1.73 vs. 3.69 ± 1.58, P=0.471). To note, the average time taken to pregnancy in the EE/CPA+ letrozole group was significantly longer than that in the letrozole group (178.60 ± 55.04 vs. 99.98 ± 67.31, P< 0.001), as a result of extended 3 months of antiandrogen pretreatment in the EE/CPA+ letrozole group.
We also analyzed the effect of COC pretreatment based on age, BMI, HOMA-IR, FAI stratification. No significant differences in ovulation rate per cycle, cumulative ovulation, conception, ongoing pregnancy, and live birth rate were observed between the two treatment groups according to age (Table 3), BMI (Table 4), HOMA-IR (Table 5), and FAI stratification (Table 6).

Table 3 Primary outcomes and secondary outcomes of patients of EE/CPA + letrozole and letrozole groups based on age stratification.
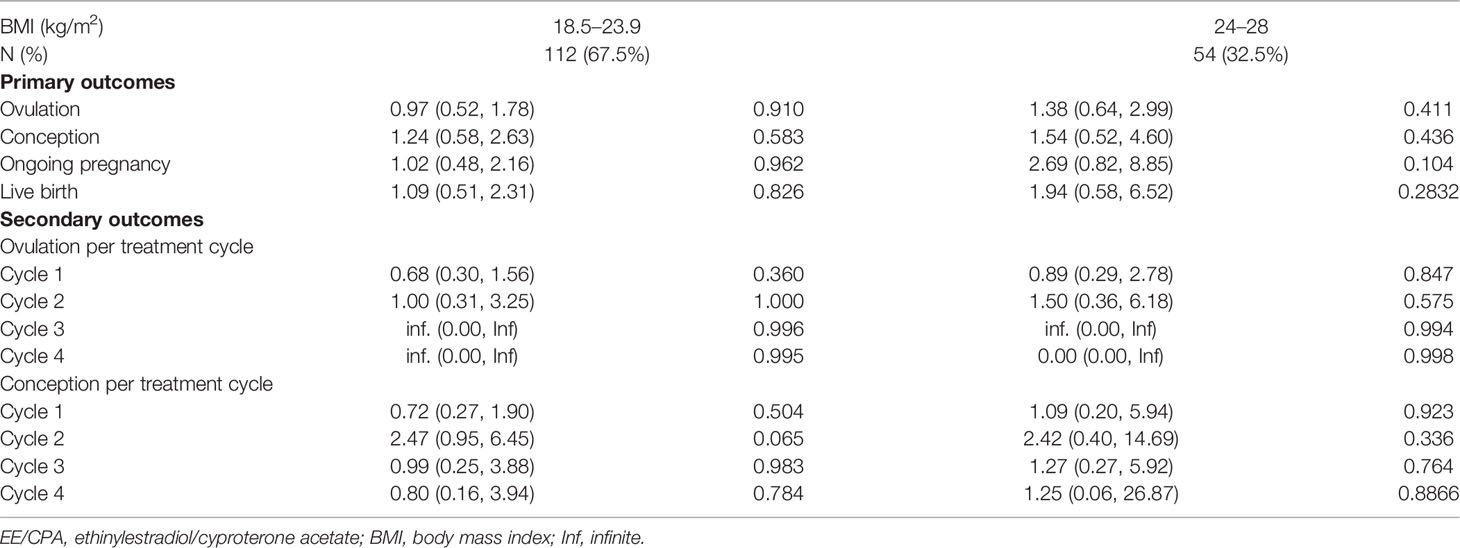
Table 4 Primary outcomes and secondary outcomes of patients of EE/CPA + letrozole and letrozole groups based on BMI stratification.
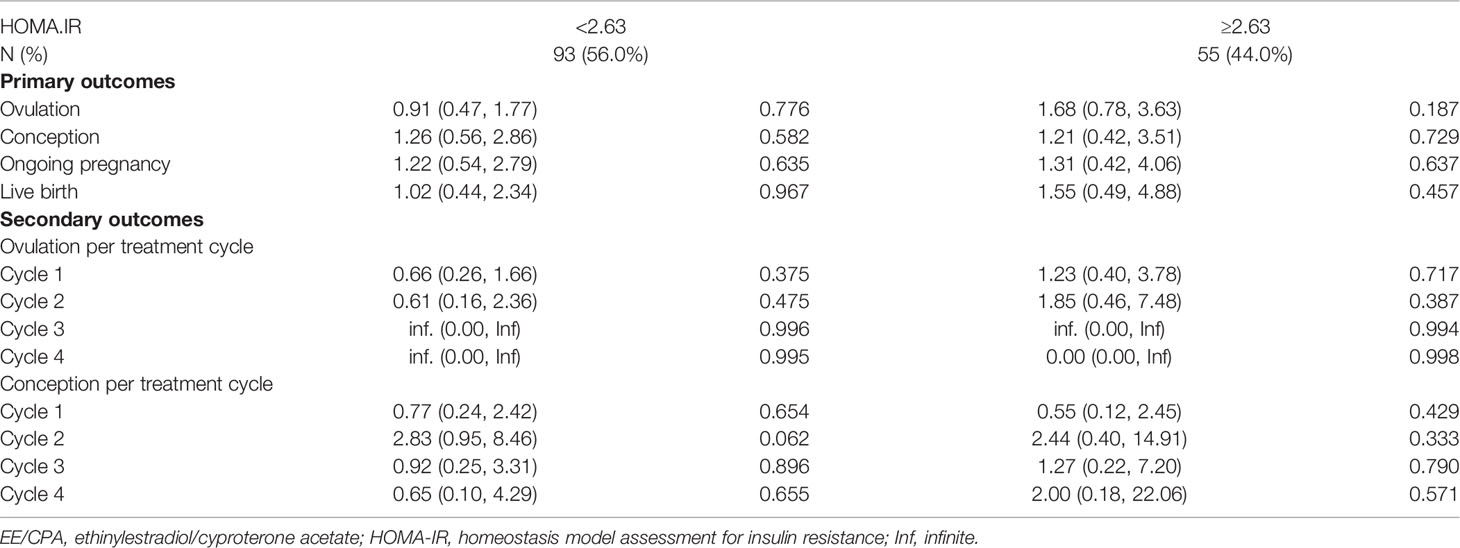
Table 5 Primary outcomes and secondary outcomes of patients of EE/CPA + letrozole and letrozole groups based on HOMA.IR stratification.
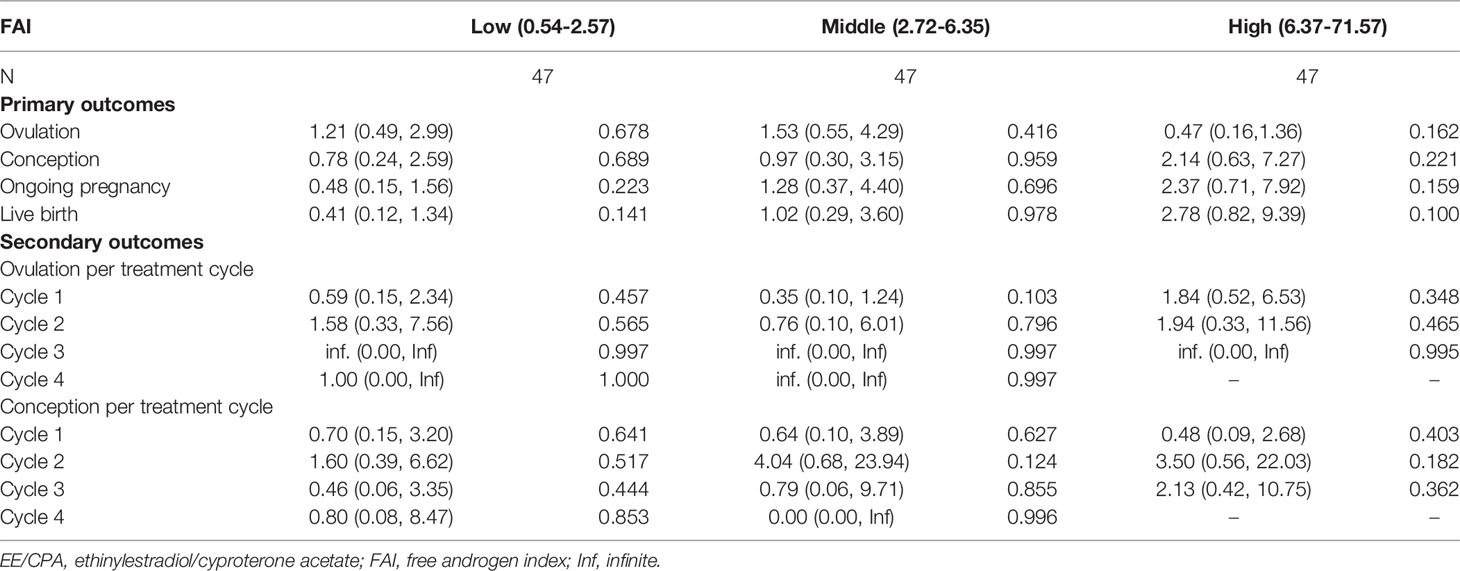
Table 6 Primary outcomes and secondary outcomes of patients of EE/CPA + letrozole and letrozole groups based on FAI stratification.
According to Kaplan-Meier analysis (Figure 2), the conception rate of letrozole group was higher than that of COC pretreatment group when considering the longer period from randomization to outcome event in EE/CPA+ letrozole group (P<0.0001), which was also observed in age subgroups (age<30: P=0.0083, age≥30: P=0.0071), BMI subgroups (BMI<24: P=0.0010, BMI≥24: P=0.0425), HOMA-IR subgroups (HOMA-IR<2.63: P=0.0061, HOMA-IR≥2.63: P=0.0224), and FAI subgroups (Middle FAI (2.72-6.35): P=0.0065, High FAI (6.37-71.57): P=0.0162).
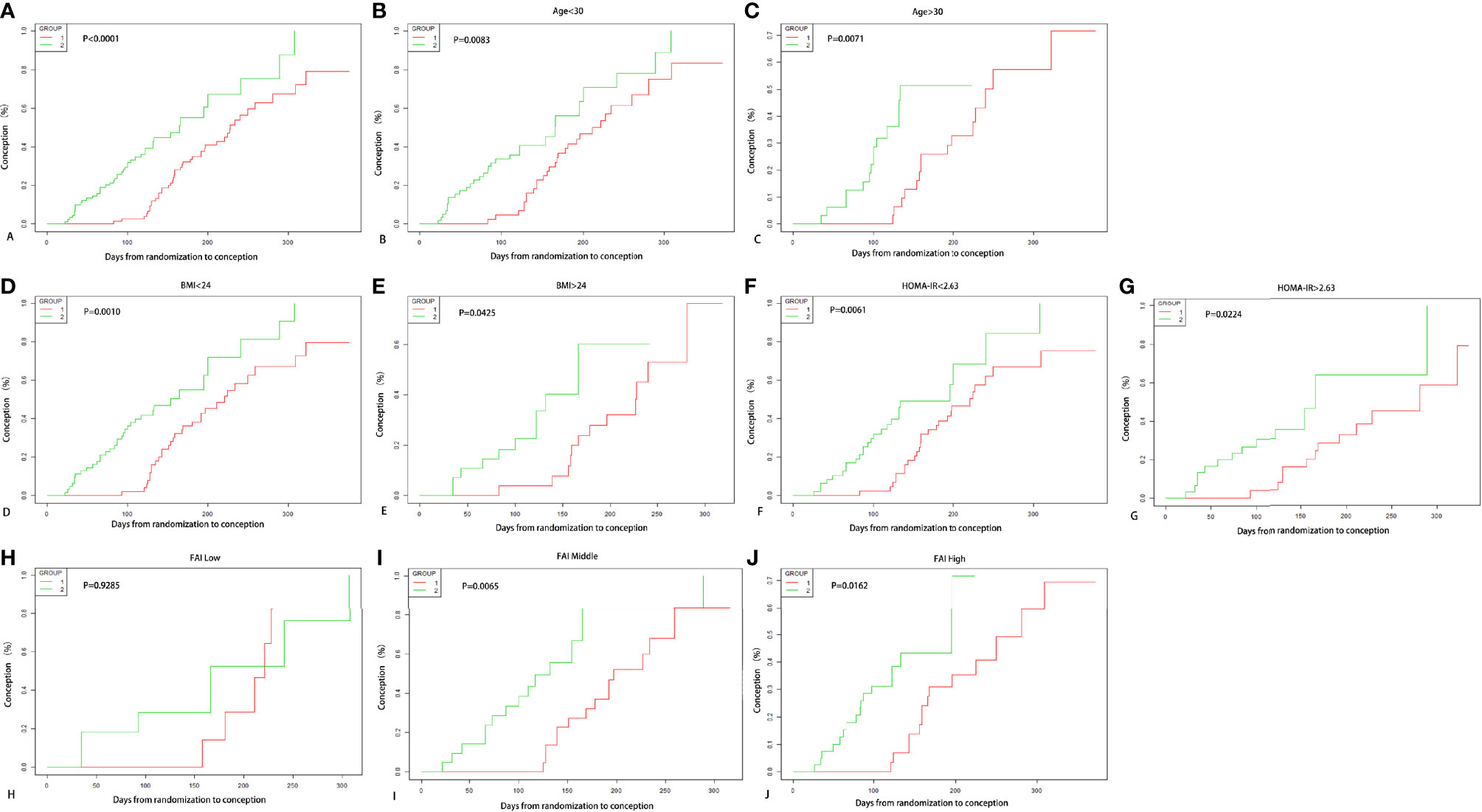
Figure 2 Kaplan-Meier Curves for conception. Conception rate varying with days from randomization to outcome of two treatment groups are shown in (A), and conception rate of two groups according to age subgrouping in (B, C), and according to BMI subgrouping in (D, E), and according to HOMA-IR subgrouping in (F, G), and according to FAI tertile subgrouping in (H–J).
Adverse Events
None of the patients in our study experienced serious adverse events. The adverse events reported in our study were transient and mild. During COC pretreatment in the EE/CPA+ letrozole group, one patient complained of diarrhea, two had headache, and eight had irregular vaginal bleeding. In addition, during the period of ovulation induction, there were two cases of nausea, one case of headache, and four cases of breast distending pain in the letrozole group. While in the EE/CPA+ letrozole group, three patients reported nausea, and two had breast distending pain. There were no significant differences in adverse events between the letrozole and EE/CPA+ letrozole groups (7/90 [7.7%] and 5/76 [6.5%], respectively, P>0.05).
There was one patient in EE/CPA+ letrozole group undergoing induced abortion in the second trimester due to twin-twin transfusion syndrome (TTTS). And multiple pregnancy rates in the letrozole and EE/CPA+ letrozole groups were 1.3% (1/76) and 1.1% (1/90), respectively. Gestational diabetes and pre-eclampsia were shown to be prevalent in 2.6% (2/76) and 3.33% (3/90) of the live deliveries in the two groups, respectively.
Discussion
In this randomized clinical trial, we investigated the effectiveness of COC antiandrogenic pretreatment prior to ovulation induction in PCOS patients with hyperandrogenism by comparing the pregnancy outcomes of the patients receiving COC pretreatment before ovulation induction and those immediately receiving letrozole treatment. The results showed no differences in cumulative ovulation, conception, ongoing pregnancy, and live birth rates between the two groups, indicating the equal effectiveness of both treatment strategies. Given the time and psychological costs of delaying treatment, prompt ovulation induction can be advised for those patients with conception plans.
As excessive androgen levels cause a series of harmful reproduction-related complications in women with PCOS, antiandrogenic therapy may also have the potential to be an auxiliary method in enhancing pregnancy outcomes. COCs are common therapeutic agents for reducing androgen expression in patients with PCOS who have no pregnancy intentions (30–32). However, their effect on improving pregnancy outcomes in patients with PCOS with anovulatory dysfunction remains uncertain. The COC pretreatment is also inhibited by the dilemma of initiate prompt ovulation induction or improving the endocrine environment prior to infertility therapy for patients with PCOS. On one hand, EE/CPA pretreatment can reduce androgen levels and improve endocrine and metabolic status in patients with PCOS, with potential benefits for pregnancy outcomes (33). On the other hand, postponing treatment may cause impatience and anxiety in patients, which might result in an adverse effect. As a result, the rationale for providing COC pretreatment and delaying immediate infertility therapy in clinical practice is in great demand for clinicians and patients. Yet, the effect of COC pretreatment prior to ovulation induction has seldom been discussed.
One single-centered prospective study investigated the role of COC pretreatment in patients with PCOS with clomiphene resistance, of which the findings could not be generalized to other populations (34). The first adequately controlled study to explore COC pretreatment before inducing ovulation in patients with PCOS was performed by Paloma et al. (9). In their study, the experimental group received one cycle of COC pretreatment, comprising of 20 mg of ethinyl estradiol (E2) and 75 mg of gestodene. All patients underwent controlled ovarian stimulation using a low-dose stet-up highly purified urine FSH (u-FSH) protocol. No significant improvement in pregnancy outcomes, including pregnancy and live birth rates, was identified in patients receiving COC pretreatment. Later in 2016, Lergo et al. combined two randomized concurrently conducted clinical trials and discovered no difference in ovulation and live birth rates between COC pretreatment and immediate clomiphene therapy. In this post hoc study, the sample size for the COC group was relatively small, with 47 patients enrolled (35). Our findings that the experimental and control groups had virtually equal ovulation and pregnancy rates are consistent with those earlier studies, indicating that androgen suppression by EE/CPA pretreatment offers minimal reproductive advantage for PCOS patients with hyperandrogenism.
Kaplan-Meier analysis of our study have shown that the conception rate of letrozole group was higher than that of COC pretreatment group. However, it is challenging to evaluate benefits of two groups, due to the life table analyses employed in the primary outcome (ovulation induction in EE/CPA+ letrozole group started later than that in the letrozole group) (36). Furthermore, because of the greater conception rate of immediate therapy compared to that during the intervention of the COC pretreatment, the Kaplan-Meier curve would likely be biased in favor of immediate treatment (letrozole group).
There are various possible explanations for the result that antiandrogenic pretreatment with EE/CPA presented no additional fertility benefits. Firstly, the progestin administration with COC pretreatment might be correlated with decreased conception rates, as reported in a secondary analysis of PCOSI data by Legro and colleagues (37). Progestin, on the one hand, has adverse effects on the endometrium directly, which may be detrimental to subsequent conception. On the other hand, progestin exposure may affect the hypothalamic-pituitary-ovarian axis that modifies the sex hormones in women, thus impacting later conception indirectly (38). Another possibility is that the benefit of androgen reduction on pregnancy outcomes is insufficient to compensate for the potential unfavorable effect caused by the “lost” time of three consecutive cycles of EE/CPA antiandrogenic therapy.
The prospective and multi-centered design constituted the main strength of the study. Before initiating recruitment, the investigators from all participating centers received standard training programs about the study protocol and blinding methods. A representative from the corresponding center supervises each center’s research processes and progress to guarantee that the study was conducted in strict compliance with the protocol enacted by experts. Compared with previous studies, the randomly controlled and multi-centered design of the study reinforced the conclusion and could serve as a reference in clinical practice.
This study has several limitations. First, we did not re-assess the androgen levels of the individuals after EE/CPA pretreatment for 3 months, considering the excellent antiandrogenic effect of EE/CPA in patients with PCOS and cost reduction in this study (39). Additionally, patients of the two groups received therapies with different time durations and fertility guidance. For those who had direct ovulation induction, routine intercourse was instructed in the cycles, which was not required for those who had COC pretreatment in the first 3 months. As a result, this study used an open-label design, in which patients and research assistants could not be blinded to the treatment groups. However, the doctors who were in charge of recruiting patients as well as the data analysts were blinded to the random allocation and specific grouping. Moreover, the high dropout rate constitutes another notable shortcoming of this research, especially in the COC pretreatment group. The dropout rate in our study was similar to that of other studies involving ovulation induction (40). We speculated that the high dropout rate could be related to the discouragement and anxiety of couples who have undergone long periods of preparation for pregnancy. The couples tended to resort to other assistant reproduction techniques after multiple failures. The strict anti-epidemic quarantine policy in China also contributed to treatment discontinuation and changes in pregnancy plans.
Conclusion
Our study showed that COC pretreatment was not superior to direct letrozole-induced ovulation therapy in improving ovulation and pregnancy outcomes in women with PCOS. Based on the results of this multicentered randomized study, there is no benefit to antiandrogenic pretreatment before ovulation induction in PCOS patients with hyperandrogenism. With the design and implementation process of this study, we believe that the results are representative and can be a reference for clinical practice.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Resman platform (http://www.medresman.org.cn).
Ethics Statement
The studies involving human participants were reviewed and approved by The Institutional Review Board (IRB) of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZC, JT, HW, BZ, JL, and GH collected and validated the patient data. ZC analyzed and interpreted the patient data. ZS managed the data and edited the manuscript and QY supervised the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Supported by the National Key Research and Development Program (2018YFC1002105) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (NO. 2020-PT320-003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are deeply grateful to all participants involved in this study and all the doctors and researchers who have contributed significantly to the study, and that all authors agree with the content of the manuscript.
References
1. Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic Ovarian Syndrome: Correlation Between Hyperandrogenism, Insulin Resistance and Obesity. Clin Chim Acta (2020) 502:214–21. doi: 10.1016/j.cca.2019.11.003
2. Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol (2018) 132:321–36. doi: 10.1097/AOG.0000000000002698
3. Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. Female Infertility, Infertility-Associated Diagnoses, and Comorbidities: A Review. J Assist Reprod Genet (2017) 34:167–77. doi: 10.1007/s10815-016-0836-8
4. Chatziaggelou A, Sakkas EG, Votino R, Papagianni M, Mastorakos G. Assisted Reproduction in Congenital Adrenal Hyperplasia. Front Endocrinol (Lausanne) (2019) 10:723. doi: 10.3389/fendo.2019.00723
5. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy Complications in Women With Polycystic Ovary Syndrome. Hum Reprod Update (2015) 21:575–92. doi: 10.1093/humupd/dmv029
6. Cooney LG, Milman LW, Hantsoo L, Kornfield S, Sammel MD, Allison KC, et al. Cognitive-Behavioral Therapy Improves Weight Loss and Quality of Life in Women With Polycystic Ovary Syndrome: A Pilot Randomized Clinical Trial. Fertil Steril (2018) 110:161–171.e1. doi: 10.1016/j.fertnstert.2018.03.028
7. Practice Committee of the American Society for Reproductive Medicine. Role of Metformin for Ovulation Induction in Infertile Patients With Polycystic Ovary Syndrome (PCOS): A Guideline. Fertil Steril (2017) 108:426–41. doi: 10.1016/j.fertnstert.2017.06.026
8. Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and Lifestyle Modification in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Hum Reprod Update (2015) 21:560–74. doi: 10.1093/humupd/dmv025
9. Palomba S, Falbo A, Orio F Jr., Russo T, Tolino A, Zullo F. Pretreatment With Oral Contraceptives in Infertile Anovulatory Patients With Polycystic Ovary Syndrome Who Receive Gonadotropins for Controlled Ovarian Stimulation. Fertil Steril (2008) 89:1838–42. doi: 10.1016/j.fertnstert.2007.05.035
10. Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized Controlled Trial of Preconception Interventions in Infertile Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2015) 100:4048–58. doi: 10.1210/jc.2015-2778
11. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations From the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil Steril (2018) 110:364–79. doi: 10.1016/j.fertnstert.2018.05.004
12. Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of Noninferiority and Equivalence Randomized Trials: Extension of the CONSORT 2010 Statement. Jama (2012) 308:2594–604. doi: 10.1001/jama.2012.87802
13. Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and Insulin Resistance: The Chief Culprit of Polycystic Ovary Syndrome. Life Sci (2019) 236:116940. doi: 10.1016/j.lfs.2019.116940
14. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: The Complete Task Force Report. Fertil Steril (2009) 91:456–88. doi: 10.1016/j.fertnstert.2008.06.035
15. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization Reference Values for Human Semen Characteristics. Hum Reprod Update (2010) 16:231–45. doi: 10.1093/humupd/dmp048
16. Amer SA, Smith J, Mahran A, Fox P, Fakis A. Double-Blind Randomized Controlled Trial of Letrozole Versus Clomiphene Citrate in Subfertile Women With Polycystic Ovarian Syndrome. Hum Reprod (2017) 32:1631–8. doi: 10.1093/humrep/dex227
17. Mejia RB, Summers KM, Kresowik JD, Van Voorhis BJ. A Randomized Controlled Trial of Combination Letrozole and Clomiphene Citrate or Letrozole Alone for Ovulation Induction in Women With Polycystic Ovary Syndrome. Fertil Steril (2019) 111:571–578.e1. doi: 10.1016/j.fertnstert.2018.11.030
18. Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. In: StatPearls, StatPearls Publishing Copyright © 2021. Treasure Island (FL: StatPearls Publishing LLC. (2021).
19. Chen X, Cui J, Zhang Y, Peng W. The Association Between BMI and Health-Related Physical Fitness Among Chinese College Students: A Cross-Sectional Study. BMC Public Health (2020) 20:444. doi: 10.1186/s12889-020-08517-8
20. Lizneva D, Gavrilova-Jordan L, Walker W, Azziz R. Androgen Excess: Investigations and Management. Best Pract Res Clin Obstet Gynaecol (2016) 37:98–118. doi: 10.1016/j.bpobgyn.2016.05.003
21. Wang XL, Wang HW, Zhang LL, Guo MX, Huang Z. Topical ALA PDT for the Treatment of Severe Acne Vulgaris. Photodiagnosis Photodyn Ther (2010) 7:33–8. doi: 10.1016/j.pdpdt.2010.01.003
22. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care (2011) 34(Suppl 1):S62–9. doi: 10.2337/dc11-S062
23. Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the Appropriate Method for the Assessment of Insulin Resistance. BMC Med Res Methodol (2011) 11:158. doi: 10.1186/1471-2288-11-158
24. Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of Insulin Sensitivity/Resistance. Indian J Endocrinol Metab (2015) 19:160–4. doi: 10.4103/2230-8210.146874
25. Barth JH, Field HP, Yasmin E, Balen AH. Defining Hyperandrogenism in Polycystic Ovary Syndrome: Measurement of Testosterone and Androstenedione by Liquid Chromatography-Tandem Mass Spectrometry and Analysis by Receiver Operator Characteristic Plots. Eur J Endocrinol (2010) 162:611–5. doi: 10.1530/EJE-09-0741
26. Legro RS. Ovulation Induction in Polycystic Ovary Syndrome: Current Options. Best Pract Res Clin Obstet Gynaecol (2016) 37:152–9. doi: 10.1016/j.bpobgyn.2016.08.001
27. Wu XK, Wang YY, Liu JP, Liang RN, Xue HY, Ma HX, et al. Randomized Controlled Trial of Letrozole, Berberine, or a Combination for Infertility in the Polycystic Ovary Syndrome. Fertil Steril (2016) 106:757–765.e1. doi: 10.1016/j.fertnstert.2016.05.022
28. Bansal S, Goyal M, Sharma C, Shekhar S. Letrozole Versus Clomiphene Citrate for Ovulation Induction in Anovulatory Women With Polycystic Ovarian Syndrome: A Randomized Controlled Trial. Int J Gynaecol Obstet (2021) 152:345–50. doi: 10.1002/ijgo.13375
29. Hu S, Xu B, Long R, Jin L. The Effect of Polycystic Ovary Syndrome Without Hyperandrogenism on Pregnancy-Related Outcomes: A Retrospective Cohort Study. Bjog (2021) 128:1003–10. doi: 10.1111/1471-0528.16557
30. Hoeger KM, Dokras A, Piltonen T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J Clin Endocrinol Metab (2021) 106:e1071–83. doi: 10.1210/clinem/dgaa839
31. Dokras A. Noncontraceptive Use of Oral Combined Hormonal Contraceptives in Polycystic Ovary Syndrome-Risks Versus Benefits. Fertil Steril (2016) 106:1572–9. doi: 10.1016/j.fertnstert.2016.10.027
32. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet Gynecol (2018) 131:e157–71. doi: 10.1097/AOG.0000000000002656
33. Amiri M, Ramezani Tehrani F, Nahidi F, Kabir A, Azizi F, Carmina E. Effects of Oral Contraceptives on Metabolic Profile in Women With Polycystic Ovary Syndrome: A Meta-Analysis Comparing Products Containing Cyproterone Acetate With Third Generation Progestins. Metabolism (2017) 73:22–35. doi: 10.1016/j.metabol.2017.05.001
34. Branigan EF, Estes MA. A Randomized Clinical Trial of Treatment of Clomiphene Citrate-Resistant Anovulation With the Use of Oral Contraceptive Pill Suppression and Repeat Clomiphene Citrate Treatment. Am J Obstet Gynecol (2003) 188:1424–8; discussion 1429-30. doi: 10.1067/mob.2003.459
35. Legro RS, Dodson WC, Kunselman AR, Stetter CM, Kris-Etherton PM, Williams NI, et al. Benefit of Delayed Fertility Therapy With Preconception Weight Loss Over Immediate Therapy in Obese Women With PCOS. J Clin Endocrinol Metab (2016) 101:2658–66. doi: 10.1210/jc.2016-1659
36. Legro RS, Wu X, Barnhart KT, Farquhar C, Fauser BC, Mol B. Improving the Reporting of Clinical Trials of Infertility Treatments (IMPRINT): Modifying the CONSORT Statement†‡. Hum Reprod (2014) 29:2075–82. doi: 10.1093/humrep/deu218
37. Diamond MP, Kruger M, Santoro N, Zhang H, Casson P, Schlaff W, et al. Endometrial Shedding Effect on Conception and Live Birth in Women With Polycystic Ovary Syndrome. Obstet Gynecol (2012) 119:902–8. doi: 10.1097/AOG.0b013e31824da35c
38. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic Ovary Syndrome: Evidence for Reduced Sensitivity of the Gonadotropin-Releasing Hormone Pulse Generator to Inhibition by Estradiol and Progesterone. J Clin Endocrinol Metab (1998) 83:582–90. doi: 10.1210/jc.83.2.582
39. Amiri M, Kabir A, Nahidi F, Shekofteh M, Ramezani Tehrani F. Effects of Combined Oral Contraceptives on the Clinical and Biochemical Parameters of Hyperandrogenism in Patients With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Eur J Contracept Reprod Health Care (2018) 23:64–77. doi: 10.1080/13625187.2018.1435779
Keywords: combined oral contraceptives, hyperandrogenism, ovulation induction, polycystic ovary syndrome, pregnancy outcomes
Citation: Chen Z, Tan J, Wang H, Zheng B, Liu J, Hao G, Guo Z, Sun Z and Yu Q (2022) A Randomized Cohort Study: Is It Worth the Time to Receive Antiandrogenic Pretreatment Before Ovulation Induction for Women With Polycystic Ovary Syndrome? Front. Endocrinol. 13:813188. doi: 10.3389/fendo.2022.813188
Received: 11 November 2021; Accepted: 28 January 2022;
Published: 24 February 2022.
Edited by:
Tom Kelsey, University of St. Andrews, United KingdomReviewed by:
Mohd Ashraf Ganie, Sher-I-Kashmir Institute of Medical Sciences, IndiaHexia Xia, Fudan University, China
Duru Shah, Gynaecworld, India
Copyright © 2022 Chen, Tan, Wang, Zheng, Liu, Hao, Guo, Sun and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Yu, eXVxaTIwMDgwMDFAc2luYS5jb20=
 Zhiyan Chen
Zhiyan Chen Jichun Tan
Jichun Tan Huichun Wang3
Huichun Wang3 Guimin Hao
Guimin Hao Zaixin Guo
Zaixin Guo Qi Yu
Qi Yu