- 1Non-Communicable Diseases Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
- 2Department of Diagnostic Medicine & Pathobiology, College of Veterinary Medicine, Kansas State University, Manhattan, KS, United States
- 3Leishmaniasis Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
- 4Department of Epidemiology, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran
- 5Faculty of Medicine, Birjand University of Medical Science, Birjand, Iran
- 6Department of Pediatrics, School of Medicine, Zabol University of Medical Sciences, Zabol, Iran
- 7Pediatric Gastroenterology and Hepatology Research Center, Zabol University of Medical Sciences, Zabol, Iran
- 8Department of Radiation Oncology, Edward Hines Jr Veterans Administration (VA) Hospital and Loyola University Chicago Stritch School of Medicine, Chicago, IL, United States
- 9Student Research Committee, Sabzevar University of Medical Sciences, Sabzevar, Iran
- 10Department of Public Health, School of Health, Torbat Heydariyeh University of Medical Sciences, Torbat Heydariyeh, Iran
Background: Data on the efficacy and safety of COVID-19 vaccines in patients with malignancy are immature. In this paper, we assessed the literature involving the use of COVID-19 vaccines in cancer patients and reported the seroconversion rates as the main outcome and severity of COVID-19 infection and side effects following COVID-19 vaccination as the secondary outcomes.
Methods: A systematic review with meta-analysis was performed. Searches were conducted in electronic websites, databases, and journals, including Scopus, PubMed, Embase, and Web of Science from January 01, 2019, to November 30, 2021. Studies reporting data on the safety and efficacy of COVID vaccine in cancer patients using any human samples were included. The risk of bias was assessed using the NEWCASTLE-OTTAWA scale in the included studies.
Results: A total of 724 articles were identified from databases, out of which 201 articles were duplicates and were discarded. Subsequently, 454 articles were excluded through initial screening of the titles and abstracts. Moreover, 41 studies did not report the precise seroconversion rate either based on the type of cancer or after injection of a second dose of COVID vaccine. Finally, 28 articles met all the inclusion criteria and were included in this systematic review. The overall seroconversion rates after receiving a second dose of COVID-19 vaccine, based on type of cancer were 88% (95% CI, 81%-92%) and 70% (95% CI, 60%-79%) in patients with solid tumors and hematologic malignancies, respectively.
Conclusion: Overall, we conclude that vaccination against COVID-19 in patients with active malignancies using activated and inactivated vaccines is a safe and tolerable procedure that is also accompanied by a high efficacy.
1 Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected many cancer patients worldwide. Besides the impact on delivery of cancer care due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, studies have shown that these patients have a higher risk of severe illness from COVID-19 and exhibit poorer outcomes following COVID-19 treatments (1–3).
COVID-19 vaccination is presently the only available and valid option to overcome the pandemic considering the lack of effective treatment and the emerging coronavirus variants, even in patients with malignancies suffering from compromised immune responses (4–7). After introducing the first COVID-19 vaccines, patients with cancer have been prioritized for vaccination. Subsequently, numerous cohort and cross-sectional articles reporting the safety and the efficacy of the COVID-19 vaccines have been published. Due to the absence of a clinical trial and various scales to report the efficacy of vaccination, data on the efficacy and safety of COVID-19 vaccines in patients with malignancies are immature. Cancer patients were excluded from many of the clinical trials of COVID-19 vaccination. In this paper, we assessed the literature involving the use of COVID-19 vaccines in cancer patients and reported the seroconversion rates as the main outcome, with severity of COVID-19 infection and side effects following COVID-19 vaccination as the secondary outcomes.
2 Methods
This study is the first meta-data analysis to assess the safety and efficacy of COVID vaccine in cancer patients based on available studies published within the last 2 years. This study was conducted under the Guideline of Preferred Reporting Items Systematic Meta-Analyses Checklist (PRISMA) (8). At the beginning of this systematic review, all articles extracted from various databases were transferred to Endnote software.
2.1 Search Strategy
The online search was conducted by two separate authors (S.A.J. and F.A.) from electronic websites, databases, and journals, including Scopus, PubMed, Embase, and Web of Science from January 01, 2019, to November 30, 2021. Additionally, we manually screened references or citations of each article. The keywords used as a search term in this database were: [cancer patients [title] OR solid tumors [title] OR hematological malignancies [title] OR malignancy [title] OR chemotherapy [title] OR radiotherapy [title] OR immunotherapy [title] OR targeted therapy [title]] AND [COVID-19 [title] OR SARS-CoV-2 [title] OR COVID-19 vaccines [title] OR RNA vaccines [title] OR adenovirus-based vaccines [title] OR protein-based vaccines [title] OR inactivated coronavirus vaccines [title] OR seroconversion [title] OR side effects [title]]. After the primary search based on “search terms”, we removed duplicate studies.
2.2 Inclusion Criteria
The eligibility criteria for inclusion in this study were the following: (a) Randomized controlled trial (RCT) or Cohort Study design and (b) studies on cancer patients.
2.3 Exclusion Criteria
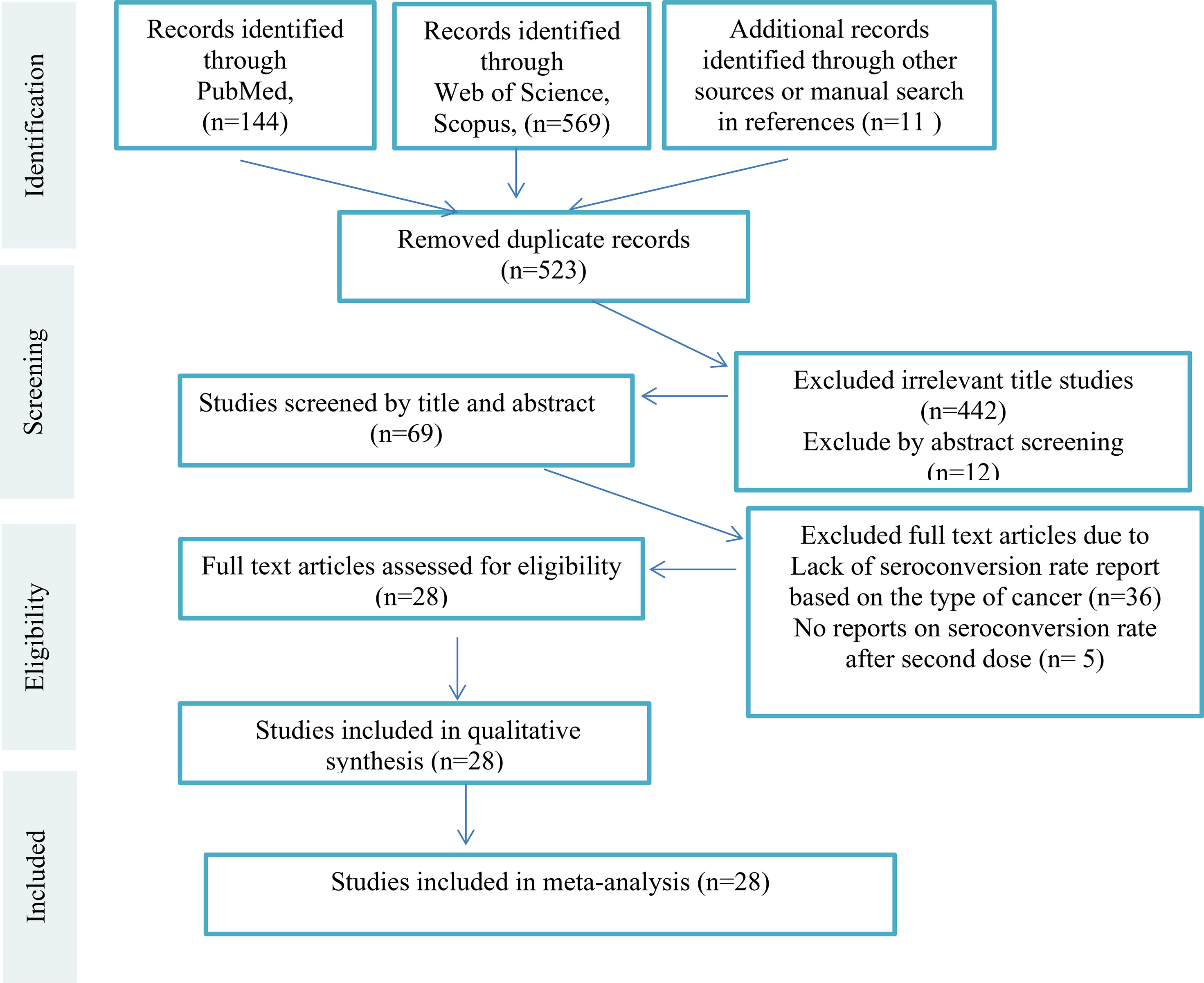
We excluded studies that were: (a) Animal studies (b) Evaluating non-cancer patients or healthy people (c) Reviews and book chapters, and (d) Articles written in any languages other than English. Editorials, commentary papers, and presentation reports could be included if they reported the seroconversion rates and prevalence of side effects rather than the author(s)’ opinion only. Then all remaining studies were screened by titles and abstract and irrelevant studies were excluded. Subsequently, full text versions of the remaining articles were assessed for eligibility independently by two authors (S.A.J. and F.A.). The third researcher (K.A.) evaluated the accuracy of selection of studies independently in all steps. All steps of study selection (PRISMA Flow Diagram) are shown in Figure 1 [Supplementary Material (Data Sheet 1)].
2.4 Quality Assessment
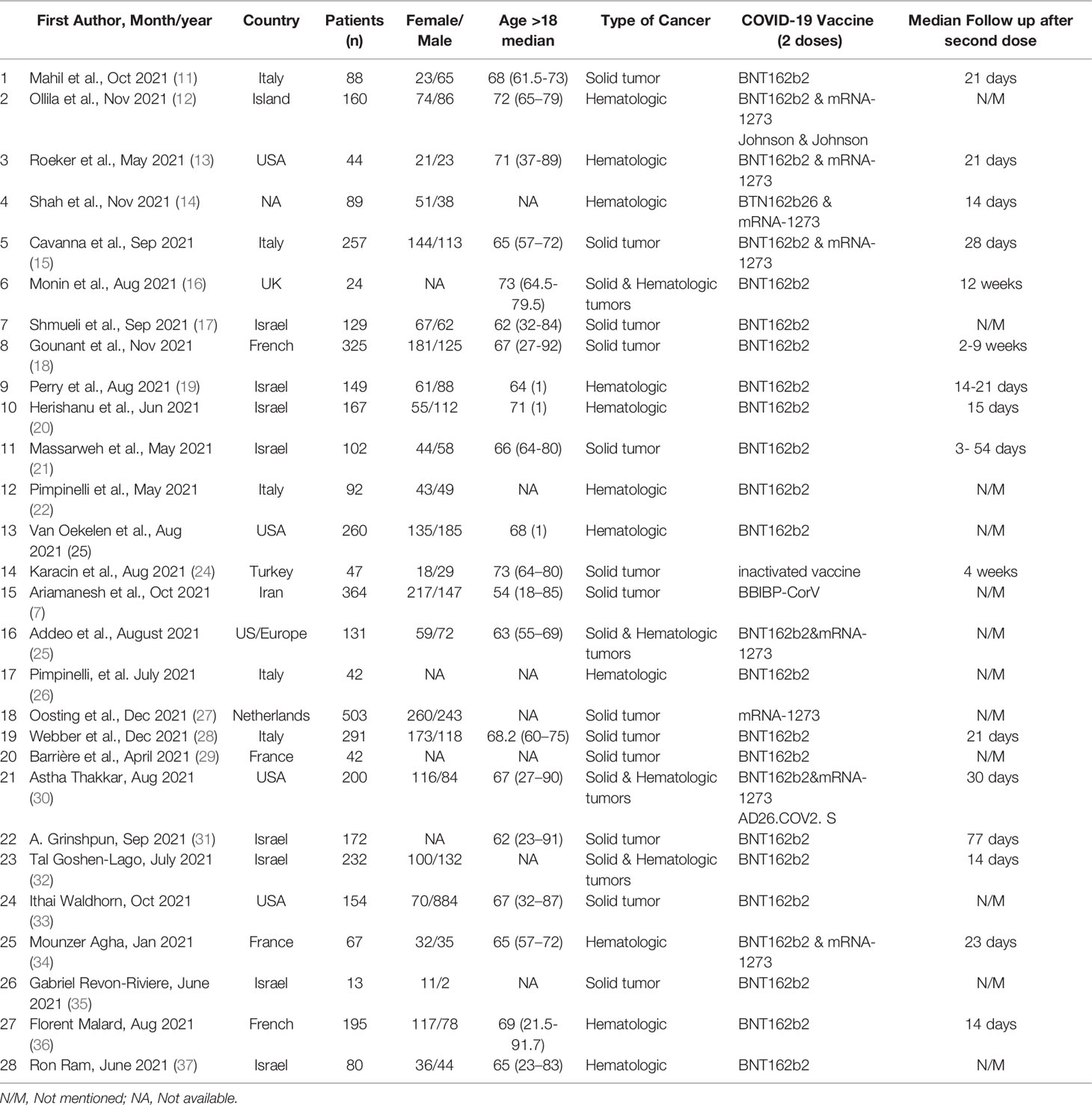
The quality of eligible studies was evaluated using the NEWCASTLE-OTTAWA scale (9). This scale is a good measure of evaluation of the possible risk of bias in individual studies and quality assessment of cohort studies. Each study could be awarded 3 or more “stars” by S.A.J. and F.A. who read each article and completed the checklists separately. Stars were awarded in the representative selection sample, comparability between groups and completeness. Finally unbiased studies entered the meta-analysis (10). Provided that the results were discordant, the third author (K.A.) completed the checklist and the best matching results were selected (Table 1).
2.5 Data Extraction
The main variables in the present study include study design, authors’ name, publication months/year, type of the cancers and treatments administered, the stage of disease, type of COVID-19 vaccine (messenger RNA vaccines, adenovirus-based vaccines, protein-based vaccines and inactivated vaccines), number of doses, follow-up duration, clinical and serological outcomes, and side-effects. The data were extracted by S.A.J., F.D and F.A for each study. The fourth researcher (K.A.) evaluated the accuracy of selection studies independently in all steps.
2.6 Statistical Analysis
We used the reported overall point estimation of seroconversion rate after more than 2 weeks of the injection of COVID-19 vaccine (second dose), as a measure of immunogenicity of COVID 19 vaccine. We extracted seroconversion rate and confidence intervals (CIs) for studies that provided them separately, then we reported overall seroconversion rate and confidence intervals (CIs) by use of the assumptions of a random-effects model (which considers both within-study and between-study variations) as a measure of immunogenicity of COVID-19 vaccine in cancer patients. The rate of seroconversion estimation from random effects models were used, which incorporates between-study variability, since the tests for heterogeneity were statistically significant in all analyses. Statistical heterogeneity was evaluated with Cochran’s Q test and quantified by the I 2 statistic (38). To assess sources of heterogeneity, we conducted a subgroup analysis. Publication bias was assessed by visual inspection of funnel plot (Figure 2) (39). Statistical analyses were carried out with Comprehensive Meta-Analysis software, Version 3. A value of p<0.05 was considered statistically significant. All statistical tests were two-sided.
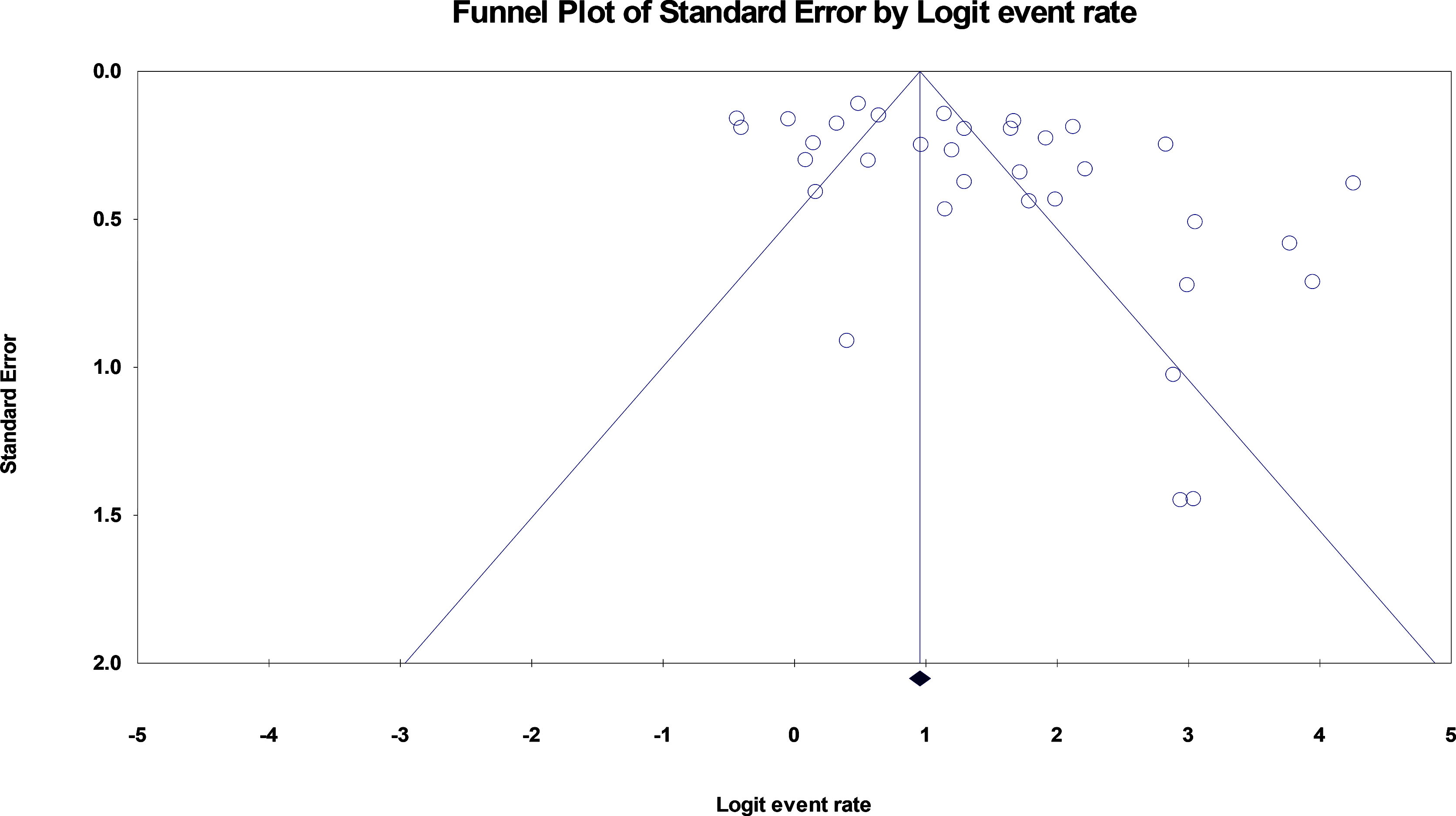
Figure 2 funnel plot of standard error by logit event rate. The Egger’s test was used, SE 3.7 (95% CI, 1.03, 6.37), t-valu82, df 32, P = 0.008 indicating a strong publication bias across the articles.
3 Results
The study flowchart (Figure 1) summarizes the searching process. A total of 724 articles were identified from databases, including Scopus, PubMed, Embase, Web of Science, and ScienceDirect out of which 201 articles were duplicates and discarded. Of the remaining 523 articles, 454 articles were unrelated and excluded by screening title and abstract, 69 full text articles were assessed for eligibility, 41 studies did not have an exact report of seroconversion rate based on type of cancer or did not report seroconversion rate after injection of the second dose of COVID-19 vaccine. Among the 28 remaining studies, 4 were letters and 24 were original studies. Finally, 28 articles met all the inclusion criteria and were included in this systematic review (Table 1). Table 2 summarizes the available clinical data (number of patients, disease characteristics, treatment details and outcomes) from the 28 studies that used COVID-19 vaccines in cancer patients. The clinical data evaluated includes 24 cohort studies (17 prospective studies, 11 retrospective studies).
The overall point estimation of seroconversion after more than 2 weeks beyond the second dose of COVID-19 vaccine injection was 81% (95% CI, 75%-86%) (Random effect), although heterogeneity between studies was significant (I2 = 93%, P heterogeneity<0.001) (Figure 3). In subgroup meta-analyses conducted by the type of cancer, the overall seroconversion rate after receiving the second dose of COVID-19 vaccine-based type of cancer were 88% (95% CI 81%-92%) and 70% (95% CI, 60%-79%) in patients with solid tumor and hematologic malignancy, respectively. Heterogeneity between subgroups categorizing the types of cancer was also significant (I2 = 93.9%, P heterogeneity<0.001 and I2 = 91.5%, P heterogeneity<0.001, respectively) (Figure 4).
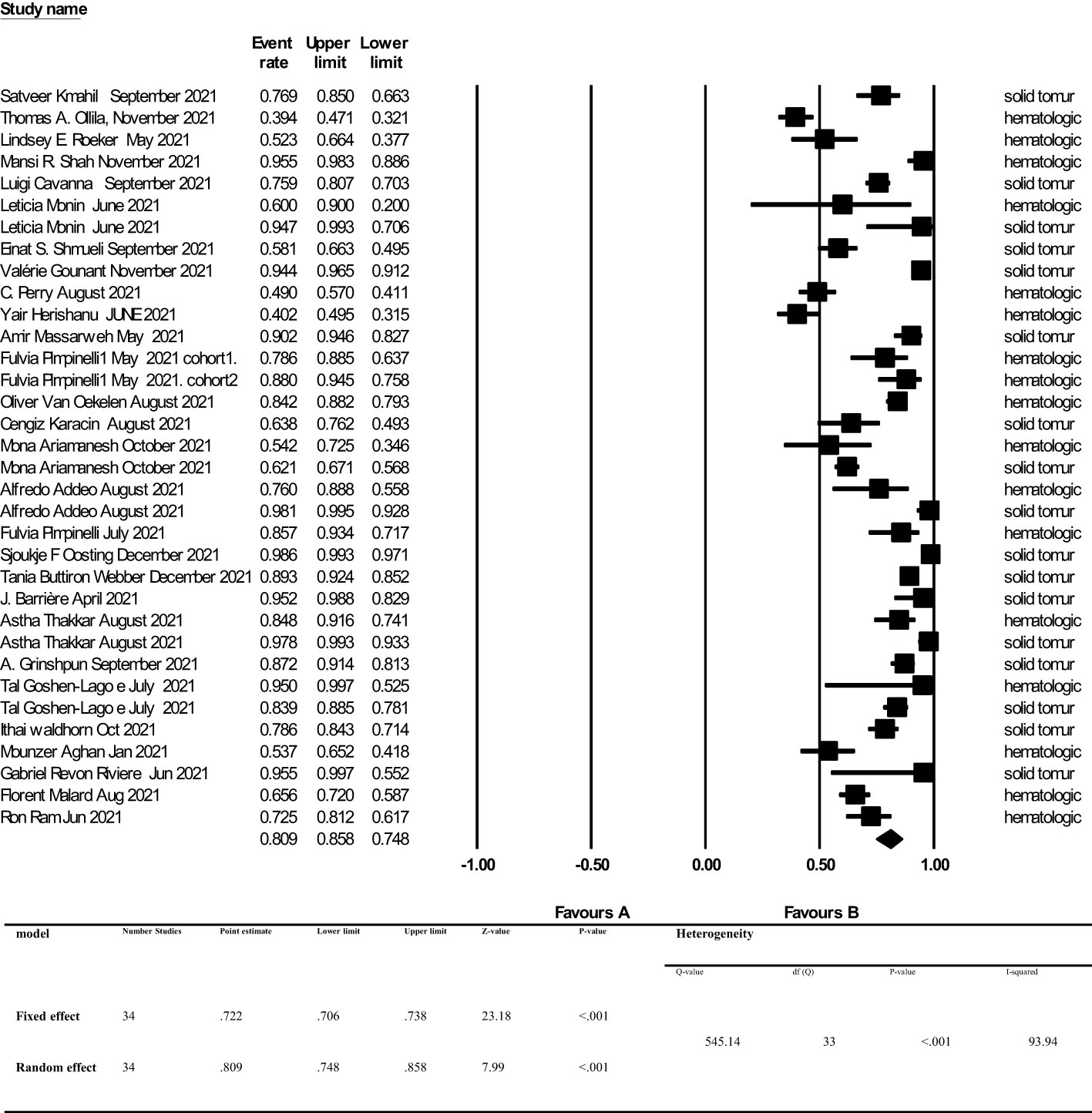
Figure 3 Seroconversion rate after more than 2 weeks of receiving second dose of COVID-19 vaccine in cancer patients. Addeo et al. (25), Agha et al. (34), Ariamanesh et al. (7), Barrière et al. (29), Cavanna et al. (15), Goshen-Lago et al. (32), Gounant et al. (18), Grinshpun et al. (31), Herishanu et al. (20), Karacin et al. (24), Mahil et al. (11), Malard et al. (36), Massarweh et al. (21), Monin et al. (16), Ollila et al. (12), Oosting et al. (27), Perry et al. (19), Pimpinelli et al. (22), Pimpinelli et al. (26), Ram et al. (37), Revon-Riviere et al. (35), Roeker et al. (13), Shah et al. (14), Shmueli et al. (17), Thakkar et al. (30), van Oekelen et al. (23), Waldhorn et al. (33), Webber et al. (28).
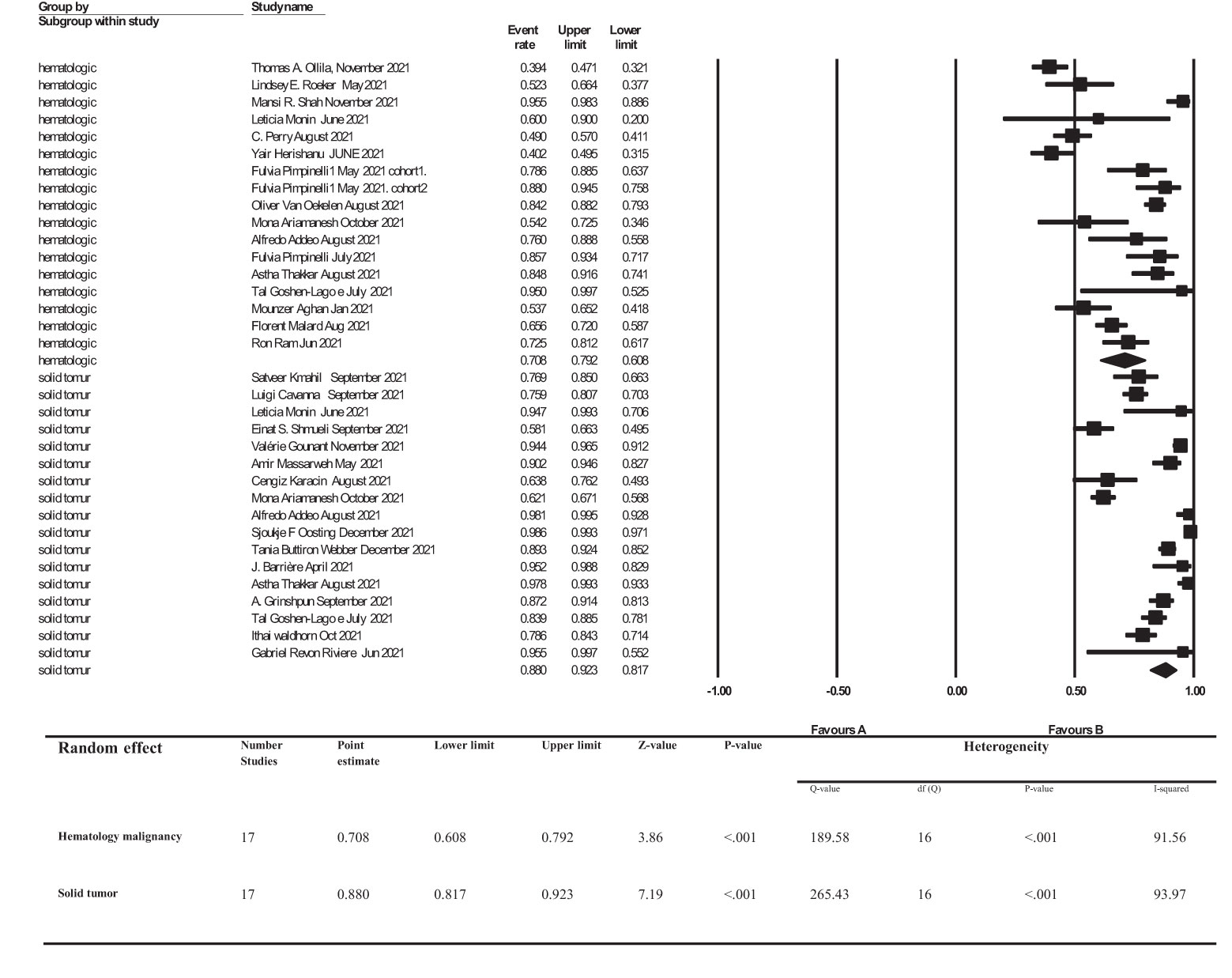
Figure 4 subgroup analysis of Seroconversion rate after more than 2 weeks of receiving second dose of COVID-19 vaccine in cancer patients. Addeo et al. (25), Agha et al. (34), Ariamanesh et al. (7), Barrière et al. (29), Cavanna et al. (15), Goshen-Lago et al. (32), Gounant et al. (18), Grinshpun et al. (31), Herishanu et al. (20), Karacin et al. (24), Mahil et al. (11), Malard et al. (36), Massarweh et al. (21), Monin et al. (16), Ollila et al. (12), Oosting et al. (27), Perry et al. (19), Pimpinelli et al. (22), Pimpinelli et al. (26), Ram et al. (37), Revon-Riviere et al. (35), Roeker et al. (13), Shah et al. (14), Shmueli et al. (17), Thakkar et al. (30), van Oekelen et al. (23), Waldhorn et al. (33), Webber et al. (28).
4 Discussion
In this review and analysis, we assessed the literature involving the use of COVID-19 vaccines in cancer patients and reported the seroconversion rates, clinical COVID-19 infection rates, severity of COVID-19 infection and side effects following COVID-19 vaccination.
4.1 Efficacy of COVID-19 Vaccination
The overall seroconversion rate beyond 2 weeks after the second dose of a COVID-19 vaccine was 81%. However, we found that the seroconversion rate was significantly higher in patients with solid tumors compared to patients with hematologic malignancies [88% (95% CI, 81%-92%) vs 70% (95% CI, 60%-79%), respectively].
4.1.1 Factors Affecting the Seroconversion Rates After COVID-19 Vaccination in Patients With Cancer
We observed evidence of seroconversion and antibody production against the SARS-CoV-2 virus in about 80% of cancer patients who were vaccinated. Nevertheless, the intensity of humoral response depends on several factors including type of malignancy (solid tumors versus hematologic malignancies), type of cancer treatment administered, demographic characteristics of the patient and other underlying factors, which will be discussed in the following sections.
4.1.1.1 Type of Malignancy
In the studies which only investigated patients with solid tumors (n=17), authors in general reported a seroconversion rate of 0.817 to 0.923. Moreover, in the studies exclusively on hematologic malignancies (n=11) seroconversion rates were between 0.608 and 0.792. Finally, in studies performed by Monin et al. (16), Pimpinelli et al. (22, 26), Ariamanesh et al. (7), Addeo et al. (25) and Thakkar et al. (30) on patients with both solid tumors and hematologic malignancies, the seroconversion rate was significantly lower in patients with hematologic malignancies (60-81.7% vs 93.8-98%). A proper immune response to either vaccines or viral infections requires both cellular and humoral immunity. An adequate antibody response can, in turn, increase T lymphocyte activity (CD4+ T helper) and lead to higher stimulation of B lymphocytes to produce higher levels of antibodies (20, 40, 41). It has been shown that there is a correlation between CD4+ T helper cell response to viral spike protein and anti-SARS-CoV-2 IgG and IgA titer levels (42–46). In general, based on our findings, it can be concluded that seroconversion rates in patients with hematologic malignancies are lower than in patients with solid tumors. It has been previously shown that, compared to patients with solid tumors and healthy individuals, in patients with hematologic malignancies, T cell immune responses are reduced both after COVID-19 infection and post-vaccination, with CD4+ T cells playing a principal role in comparison to CD8+ (24, 32, 35–37, 40). Furthermore, patients with hematologic malignancies undergo somewhat more significant immunosuppression, mainly due to cancer-induced immune dysregulation or because they receive therapies that can provoke myelosuppression and lymphodepletion. These factors collectively are not only involved in a greater risk for infection and mortality, but also a poorer response to vaccination (47–51). Our findings, indicating differences in immune responses based on type of malignancy, are in agreement with previous publications investigating vaccination-based immune responses against seasonal influenza (52, 53).
4.1.1.2 Type of Treatment
In the studies performed by Cavanna et al., (15) (15 seroconversion to 22, 68.18%), Addeo et al., (25) (13 to 14, 93%) and Oosting et al., (27) (122 to 131, 93%) on cancer patients receiving only immunotherapy as their anticancer therapeutic regimen, seroconversion rate was 68.18-93%. Additionally, in patients only receiving chemotherapy investigated in the studies by Oosting et al., (27), (192 of 229, 84%), Webber et al., (28) (99 to 115, 86%) and Grinshpun et al., (31), (81.3%) seroconversion rates of 81.3-86% were reported. Although vaccinated patients receiving immunotherapy comprise a small portion of most of previous researches, it appears that in patients who underwent immunological manipulation, seroconversion rate is weakened. As an example, it has been previously reported that active treatment with B cell-depleting agents such as rituximab and obinutuzumab (anti-CD20), or plasma cell depleting-agents such as daratumumab (anti-CD38) can decrease antibody production following COVID-19 vaccination, especially in patients with lymphoid malignancies. This is mainly a consequence of peripheral B cell depletion and B cell signaling pathway disruption caused by anti-CD20 agents, and anti-CD38-induced reduction of normal plasma cells, followed by a decrease in polyclonal immunoglobulin levels which are vital for humoral immunity (18, 22, 47, 49, 54–57). Furthermore, impaired response to vaccination induced by anti-CD20 therapy in patients with cancer has been similarly reported in the case of influenza or Streptococcus pneumonia vaccines (52, 58).
4.1.1.3 Other Factors
In addition to the type of malignancy and anticancer treatment, patient’s demographic factors such as age and gender might also contribute to the rate of seroconversion in response to vaccination against COVID-19. Ma et al. have shown that age is the most prominent predicting factor with regards to the failure of anti-COVID-19 vaccine in cancer patients and negatively correlates with seroconversion rate in these individuals (59). These results are consistent with another study on BNT162b2 vaccine which shows a remarkable difference between the seroconversion rate of young, versus elderly cancer patients, with the titer levels also being significantly different (32).
Gender has been shown to be another contributing factor in seroconversion rate among patients with cancer. In a study on seroprevalence against COVID-19 in cancer patients, Javadinia et al. has shown that seropositivity against COVID-19 is greater in females, with higher rates in patients with breast cancer and gynecologic cancers (60). Female hormones are known to enhance the immune system, and sex steroids have been shown to correlate with different immune responses to COVID-19 (61, 62).
4.2 Safety of COVID-19 Vaccination
Studies have shown that COVID-19 vaccination in patients with malignancy is safe and tolerable regardless of their types. Both systemic and locoregional side-effects including fatigue, hypersensitivity reactions, fever, asthenia, vomiting, headaches, muscle and joint pain, chills, transient lymphadenopathy and injection site reactions (pain, erythema, itchiness, swelling, and redness) were reported. Although the various instruments and methods which were used for assessment of frequency, severity, and extend of the side-effects make it difficult to perform a meta-analysis investigation in this context, most of reported vaccines related side-effects were grade II, maximally (7, 11, 15, 17, 19, 20, 22, 24, 28, 32, 35–37).
4.3 Limitations
This meta-analysis harbors a strong publication bias which we believe is due to the high verity of published articles’ methodologies and extent of factors affecting the immune response in this population [age, type of cancer (solid tumors and hematologic malignancies) or status of treatment (receiving chemotherapy or bone marrow transplant vs radiotherapy or follow-up)]. Moreover, not reporting the exact prevalence of PCR-confirmed COVID-19 infection and the its outcome is another important limitation of previous studies affecting the results of present review.
5 Conclusion
In general, we can conclude that vaccination against COVID-19 in patients dealing with malignancies is a safe and tolerable procedure, which is also accompanied by a high efficacy. Nevertheless, a number of factors such as type of malignancy, type of anti-cancer therapy administered, and demographic factors of the patient can influence the efficiency of anti-covid vaccination in these patients. There are a couple of suggestions to improve the efficiency of anti-covid vaccination in cancer patients, particularly patients with hematologic malignancies, and subsequent to receiving immunosuppressive anti-cancer therapies such as anti-CD20 or anti-CD38 therapies. These patients can take advantage of passive immunization using anti-COVID-19 antibodies. Moreover, booster doses or higher doses of some vaccines, or taking benefit from mixed vaccine types are suggested in these individuals. Additional studies are required to investigate the efficacy of the aforementioned approaches in these patients, especially in the individuals who were vaccinated in the course of active treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics Statement
The Ethic Committee of Sabzevar University of Medical Sciences approved the study protocol (ID IR.MEDSAB.REC.1400.148).
Author Contributions
Conceptualization; SJ and SF. Data curation; KA and FA. Funding acquisition; SJ. Investigation; FA and M-SM. Methodology; FA, FN, and FD. Project administration; SJ. Software; FA, SJ, FN, and FD. Supervision; SJ and JW. Validation; FA and HH. Visualization; SJ, FA. Roles/Writing original draft; FA, SJ, and KA. Writing - review & editing; FA, SJ, M-SM, and KA. All authors contributed to the article and approved the submitted version.
Funding
Sabzevar University of Medical Sciences (grant number 400237).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgements
Authors sincerely thank form Vasei Clinical Research Development Unit in Sabzevar University of Medical Sciences, for providing advice and guidance in conducting this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.860238/full#supplementary-material
References
1. Taghizadeh-Hesary F, Porouhan P, Soroosh D, PeyroShabany B, Shahidsales S, Keykhosravi B, et al. COVID-19 in Cancer and Non-Cancer Patients. Int J Cancer Manage (2021) 14(4):e110907. doi: 10.5812/ijcm.110907
2. Shahidsales S, Aledavood SA, Joudi M, Molaie F, Esmaily H, Javadinia SA. COVID-19 in Cancer Patients may be Presented by Atypical Symptoms and Higher Mortality Rate, a Case-Controlled Study From Iran. Cancer Rep (2021) 4(5) :e1378. doi: 10.1002/cnr2.1378
3. Mohseni Afshar Z, Hosseinzadeh R, Barary M, Ebrahimpour S, Alijanpour A, Sayad B, et al. Challenges Posed by COVID-19 in Cancer Patients: A Narrative Review. Cancer Med (2021) 11(4):1119–35. doi: 10.22541/au.163111096.65981177/v1
4. Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 Vaccine Up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. Lancet (2021) 398(10309):1407–16. doi: 10.1016/S0140-6736(21)02183-8
5. Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N Engl J Med (2021) 386(5):492–4. doi: 10.1101/2021.12.13.21267670
6. Poudel S, Ishak A, Perez-Fernandez J, Garcia E, León-Figueroa DA, Romaní L, et al. Highly Mutated SARS-CoV-2 Omicron Variant Sparks Significant Concern Among Global Experts – What is Known So Far? Travel Med Infect Dis (2022) 45:102234. doi: 10.1016/j.tmaid.2021.102234
7. Ariamanesh M, Porouhan P, PeyroShabany B, Fazilat-Panah D, Dehghani M, Nabavifard M, et al. Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients With Malignancy. Cancer Invest (2022) 40(1):26–34. doi: 10.1080/07357907.2021.1992420
8. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
9. Wells G, Shea B, O’Connell D, Peterson JE, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. (2000). Available at: http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf. (Access date March 29, 2022).
10. Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute (2011) p. 1–12.
11. Lasagna A, Agustoni F, Percivalle E, Borgetto S, Paulet A, Comolli G, et al. A Snapshot of the Immunogenicity, Efficacy and Safety of a Full Course of BNT162b2 Anti-SARS-CoV-2 Vaccine in Cancer Patients Treated With PD-1/PD-L1 Inhibitors: A Longitudinal Cohort Study. ESMO Open (2021) 6(5):100272. doi: 10.1016/j.esmoop.2021.100272
12. Ollila TA, Lu S, Masel R, Zayac A, Paiva K, Rogers RD, et al. Antibody Response to COVID-19 Vaccination in Adults With Hematologic Malignant Disease. JAMA Oncol (2021) 7(11):1714–6. doi: 10.1001/jamaoncol.2021.4381
13. Roeker LE, Knorr DA, Thompson MC, Nivar M, Lebowitz S, Peters N, et al. COVID-19 Vaccine Efficacy in Patients With Chronic Lymphocytic Leukemia. Leukemia (2021) 35(9):2703–5. doi: 10.1038/s41375-021-01270-w
14. Shah MR, Gabel A, Beers S, Salaru G, Lin Y, Cooper DL. COVID-19 Vaccine Responses in Patients With Plasma Cell Dyscrasias After Complete Vaccination. Clin Lymphoma Myeloma Leuk (2022) 11;S2152–2650(21)02422-8. doi: 10.1016/j.clml.2021.11.001
15. Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, et al. COVID-19 Vaccines in Adult Cancer Patients With Solid Tumours Undergoing Active Treatment: Seropositivity and Safety. A Prospective Observational Study in Italy. Eur J Cancer (2021) 157:441–9. doi: 10.1016/j.ejca.2021.08.035
16. Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, del Molino del Barrio I, Alaguthurai T, et al. Safety and Immunogenicity of One Versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients With Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol (2021) 22(6):765–78. doi: 10.1016/S1470-2045(21)00213-8
17. Shmueli ES, Itay A, Margalit O, Berger R, Halperin S, Jurkowicz M, et al. Efficacy and Safety of BNT162b2 Vaccination in Patients With Solid Cancer Receiving Anticancer Therapy – A Single Centre Prospective Study. Eur J Cancer (2021) 157:124–31. doi: 10.1016/j.ejca.2021.08.007
18. Gounant V, Ferré VM, Soussi G, Charpentier C, Flament H, Fidouh N, et al. Efficacy of Severe Acute Respiratory Syndrome Coronavirus-2 Vaccine in Patients With Thoracic Cancer: A Prospective Study Supporting a Third Dose in Patients With Minimal Serologic Response After Two Vaccine Doses. J Thorac Oncol (2021) 17(2):239–51. doi: 10.1101/2021.08.12.21261806
19. Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 Vaccine in Patients With B-Cell non-Hodgkin Lymphoma. Blood Adv (2021) 5(16):3053–61. doi: 10.1182/bloodadvances.2021005094
20. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 Vaccine in Patients With Chronic Lymphocytic Leukemia. Blood (2021) 137(23):3165–73. doi: 10.1182/blood.2021011568
21. Massarweh A, Eliakim-Raz N, Stemmer A, Levy-Barda A, Yust-Katz S, Zer A, et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol (2021) 7(8):1133–40. doi: 10.1001/jamaoncol.2021.2155
22. Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth-Week Immunogenicity and Safety of Anti-SARS-CoV-2 BNT162b2 Vaccine in Patients With Multiple Myeloma and Myeloproliferative Malignancies on Active Treatment: Preliminary Data From a Single Institution. J Hematol Oncol (2021) 14(1):81. doi: 10.1186/s13045-021-01090-6
23. van Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, et al. Highly Variable SARS-CoV-2 Spike Antibody Responses to Two Doses of COVID-19 RNA Vaccination in Patients With Multiple Myeloma. Cancer Cell (2021) 39(8):1028–30. doi: 10.1016/j.ccell.2021.06.014
24. Karacin C, Eren T, Zeynelgil E, Imamoglu GI, Altinbas M, Karadag I, et al. Immunogenicity and Safety of the CoronaVac Vaccine in Patients With Cancer Receiving Active Systemic Therapy. Future Oncol (2021) 17(33):4447–56. doi: 10.2217/fon-2021-0597
25. Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, di Marco M, et al. Immunogenicity of SARS-CoV-2 Messenger RNA Vaccines in Patients With Cancer. Cancer Cell (2021) 39(8):1091–8.e2. doi: 10.1016/j.ccell.2021.06.009
26. Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Lower Response to BNT162b2 Vaccine in Patients With Myelofibrosis Compared to Polycythemia Vera and Essential Thrombocythemia. J Hematol Oncol (2021) 14(1):119. doi: 10.1186/s13045-021-01130-1
27. Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans A-MC, et al. mRNA-1273 COVID-19 Vaccination in Patients Receiving Chemotherapy, Immunotherapy, or Chemoimmunotherapy for Solid Tumours: A Prospective, Multicentre, non-Inferiority Trial. Lancet Oncol (2021) 22(12):1681–91. doi: 10.1016/S1470-2045(21)00574-X
28. Buttiron Webber T, Provinciali N, Musso M, Ugolini M, Boitano M, Clavarezza M, et al. Predictors of Poor Seroconversion and Adverse Events to SARS-CoV-2 mRNA BNT162b2 Vaccine in Cancer Patients on Active Treatment. Eur J Cancer (2021) 159:105–12. doi: 10.1016/j.ejca.2021.09.030
29. Barrière J, Chamorey E, Adjtoutah Z, Castelnau O, Mahamat A, Marco S, et al. Impaired Immunogenicity of BNT162b2 Anti-SARS-CoV-2 Vaccine in Patients Treated for Solid Tumors. Ann Oncol (2021) 32(8):1053–5. doi: 10.1016/j.annonc.2021.04.019
30. Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion Rates Following COVID-19 Vaccination Among Patients With Cancer. Cancer Cell (2021) 39(8):1081–90.e2. doi: 10.1016/j.ccell.2021.06.002
31. Grinshpun A, Rottenberg Y, Ben-Dov IZ, Djian E, Wolf DG, Kadouri L. Serologic Response to COVID-19 Infection and/or Vaccine in Cancer Patients on Active Treatment. ESMO Open (2021) 6(6):100283. doi: 10.1016/j.esmoop.2021.100283
32. Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol (2021) 7(10):1507–13. doi: 10.1001/jamaoncol.2021.2675
33. Waldhorn I, Holland R, Goshen-Lago T, Shirman Y, Szwarcwort-Cohen M, Reiner-Benaim A, et al. Six-Month Efficacy and Toxicity Profile of BNT162b2 Vaccine in Cancer Patients With Solid Tumors. Cancer Discov (2021) 11(10):2430. doi: 10.1158/2159-8290.CD-21-1072
34. Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal Response to COVID-19 mRNA Vaccines in Hematologic Malignancies Patients. medRxiv (2021) 2021.04.06.21254949. doi: 10.1101/2021.04.06.21254949
35. Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, et al. The BNT162b2 mRNA COVID-19 Vaccine in Adolescents and Young Adults With Cancer: A Monocentric Experience. Eur J Cancer (2021), 154:30–4. doi: 10.1016/j.ejca.2021.06.002
36. Malard F, Gaugler B, Gozlan J, Bouquet L, Fofana D, Siblany L, et al. Weak Immunogenicity of SARS-CoV-2 Vaccine in Patients With Hematologic Malignancies. Blood Cancer J (2021) 11(8):142. doi: 10.1038/s41408-021-00534-z
37. Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 Vaccine in Patients After Allogeneic HCT or CD19-Based CART Therapy—A Single-Center Prospective Cohort Study. Transplant Cell Ther (2021) 27(9):788–94. doi: 10.1016/j.jtct.2021.06.024
38. Higgins JPT, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
39. Egger M, Smith GD, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315(7109):629. doi: 10.1136/bmj.315.7109.629
40. Alizadeh K, Alizadeh K. The Use of Dendritic Cells in Cancer Immunotherapy. J Cell Immunother (2017) 3:19–20. doi: 10.1016/j.jocit.2017.04.026
41. Ranjbar R, Behjatfar M, Teimouri A, Aghaie Fard A, Maniati M, Taheri-Anganeh M. Long non-Coding RNAs and Microorganism-Associated Cancers. Cell Biochem Funct (2021) 39(7):844–53. doi: 10.1002/cbf.3657
42. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans With COVID-19 Disease and Unexposed Individuals. Cell (2020) 181(7):1489–501.e15. doi: 10.1016/j.cell.2020.05.015
43. Shepherd STC, Fendler A, Au L, Byrne F, Wilkinson K, Wu M, et al. 1557o Adaptive Immunity to SARS-CoV-2 Infection and Vaccination in Cancer Patients: The CAPTURE Study. Ann Oncol (2021) 32:S1129. doi: 10.1016/j.annonc.2021.08.1550
44. Mairhofer M, Kausche L, Kaltenbrunner S, Ghanem R, Stegemann M, Klein K, et al. Humoral and Cellular Immune Responses in SARS-CoV-2 mRNA-Vaccinated Patients With Cancer. Cancer Cell (2021) 39(9):1171–2. doi: 10.1016/j.ccell.2021.08.001
45. Sahranavard M, Akhavan Rezayat A, Zamiri Bidary M, Omranzadeh A, Rohani F, Hamidi Farahani R, et al. Cardiac Complications in COVID-19: A Systematic Review and Meta-Analysis. Arch Iranian Med (2021) 24(2):152–63. doi: 10.34172/aim.2021.24
46. Chan L, Karimi N, Morovati S, Alizadeh K, Kakish JE, Vanderkamp S, et al. The Roles of Neutrophils in Cytokine Storms. Viruses (2021) 13(11):2318. doi: 10.3390/v13112318
47. Hafezi B, Chan L, Knapp JP, Karimi N, Alizadeh K, Mehrani Y, et al. Cytokine Storm Syndrome in SARS-CoV-2 Infections: A Functional Role of Mast Cells. Cells (2021) 10(7):1761. doi: 10.3390/cells10071761
48. Vafadar Moradi E, Teimouri A, Rezaee R, Morovatdar N, Foroughian M, Layegh P, et al. Increased Age, Neutrophil-to-Lymphocyte Ratio (NLR) and White Blood Cells Count are Associated With Higher COVID-19 Mortality. Am J Emergency Med (2021) 40:11–4. doi: 10.1016/j.ajem.2020.12.003
49. Barrière J, Re D, Peyrade F, Carles M. Current Perspectives for SARS-CoV-2 Vaccination Efficacy Improvement in Patients With Active Treatment Against Cancer. Eur J Cancer (2021) 154:66–72. doi: 10.1016/j.ejca.2021.06.008
50. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical Characteristics and Risk Factors Associated With COVID-19 Severity in Patients With Haematological Malignancies in Italy: A Retrospective, Multicentre, Cohort Study. Lancet Haematol (2020) 7(10):e737–45. doi: 10.1016/S2352-3026(20)30251-9
51. Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 Disease Severity in Patients With Cancer. Nat Med (2020) 26(8):1218–23. doi: 10.1038/s41591-020-0979-0
52. Chan L, Alizadeh K, Alizadeh K, Fazel F, Kakish JE, Karimi N, et al. Review of Influenza Virus Vaccines: The Qualitative Nature of Immune Responses to Infection and Vaccination Is a Critical Consideration. Vaccines (2021) 9(9):979. doi: 10.3390/vaccines9090979
53. Blanchette PS, Chung H, Pritchard KI, Earle CC, Campitelli MA, Buchan SA, et al. Influenza Vaccine Effectiveness Among Patients With Cancer: A Population-Based Study Using Health Administrative and Laboratory Testing Data From Ontario, Canada. J Clin Oncol (2019) 37(30):2795–804. doi: 10.1200/JCO.19.00354
54. Ghione P, Gu JJ, Attwood K, Torka P, Goel S, Sundaram S, et al. Impaired Humoral Responses to COVID-19 Vaccination in Patients With Lymphoma Receiving B-Cell–Directed Therapies. Blood (2021) 138(9):811–4. doi: 10.1182/blood.2021012443
55. Gounant V, Ferré VM, Soussi G, Charpentier C, Flament H, Fidouh N, et al. Efficacy of SARS-CoV-2 Vaccine in Thoracic Cancer Patients: A Prospective Study Supporting a Third Dose in Patients With Minimal Serologic Response After Two Vaccine Doses. medRxiv (2021) 2021.08.12.21261806. doi: 10.1101/2021.08.12.21261806
56. Desage A-L, Bouleftour W, Rivoirard R, Magne N, Collard O, Fournel P, et al. Vaccination and Immune Checkpoint Inhibitors. Am J Clin Oncol (2021) 44(3):109–13. doi: 10.1097/COC.0000000000000788
57. Jalali A, Karimialavijeh E, Babaniamansour P, Aliniagerdroudbari E, Babaniamansour S. Predicting the 30-Day Adverse Outcomes of Non-Critical New-Onset COVID-19 Patients in Emergency Departments Based on Their Lung CT Scan Findings; A Pilot Study for Derivation an Emergency Scoring Tool. Front Emergency Med (2021) 5(4):e40. doi: 10.18502/fem.v5i4.6691
58. Zagouri F, Terpos E, Fiste O, Liontos M, Briasoulis A, Katsiana I, et al. SARS-CoV-2 Neutralizing Antibodies After First Vaccination Dose in Breast Cancer Patients Receiving CDK4/6 Inhibitors. Breast (2021) 60:58–61. doi: 10.1016/j.breast.2021.08.017
59. Ma Y, Liu N, Wang Y, Zeng J, Hu Y-Y, Hao W, et al. Immune Checkpoint Blocking Impact and Nomogram Prediction of COVID-19 Inactivated Vaccine Seroconversion in Patients With Cancer: A Propensity-Score Matched Analysis. J ImmunoTher Cancer (2021) 9(11):e003712. doi: 10.1136/jitc-2021-003712
60. Javadinia SA, Ariamanesh M, Nabavifard M, Porouhan P, PeyroShabany B, Fazilat-Panah D, et al. Multicenter Study of Antibody Seroprevalence Against COVID-19 in Patients Presenting to Iranian Cancer Centers After One Year of the COVID-19 Pandemic. Cancer Invest (2021) 40(2):115–23. doi: 10.1080/07357907.2021.1995742.
61. Gadi N, Wu SC, Spihlman AP, Moulton VR. What’s Sex Got to Do With COVID-19? Gender-Based Differences in the Host Immune Response to Coronaviruses. Front Immunol (2020) 11.
62. Joudi M, Moradi Binabaj M, Porouhan P, PeyroShabany B, Tabasi M, Fazilat-Panah D, et al. A Cohort Study on the Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients With Breast Cancer; Does Trastuzumab Interfere With the Outcome? Front Endocrinol (Lausanne) (2022) 13:798975. doi: 10.3389/fendo.2022.798975
Keywords: COVID-19, SARS-CoV-2, COVID-19 vaccines, neoplasms, cancer, vaccine, seroconversion, meta-analysis
Citation: Javadinia SA, Alizadeh K, Mojadadi M-S, Nikbakht F, Dashti F, Joudi M, Harati H, Welsh JS, Farahmand SA and Attarian F (2022) COVID-19 Vaccination in Patients With Malignancy; A Systematic Review and Meta-Analysis of the Efficacy and Safety. Front. Endocrinol. 13:860238. doi: 10.3389/fendo.2022.860238
Received: 22 January 2022; Accepted: 25 February 2022;
Published: 02 May 2022.
Edited by:
Rong Li, Guilin Medical University, ChinaReviewed by:
Bhanu Prasad Venkatesulu, Henry Ford Hospital, United StatesKeng Po Lai, Guilin Medical University, China
Copyright © 2022 Javadinia, Alizadeh, Mojadadi, Nikbakht, Dashti, Joudi, Harati, Welsh, Farahmand and Attarian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed Alireza Javadinia, SmF2YWRpbmlhLkFsaXJlemFAZ21haWwuY29t; Fahimeh Attarian, YXR0YXJpYW41ODFAZ21haWwuY29t
†ORCID: Seyed Alireza Javadinia, orcid.org/0000-0003-2467-837X
Kimia Alizadeh, orcid.org/0000-0002-9096-7463
Mohammad-Shafi Mojadadi, orcid.org/0000-0002-8013-2059
Fateme Nikbakht, orcid.org/0000-0003-1761-0887
Farzaneh Dashti, orcid.org/0000-0002-2178-4428
Maryam Joudi, orcid.org/0000-0002-0468-1590
James S. Welsh, orcid.org/0000-0002-3255-6412
Seyed Amir Farahmand, orcid.org/0000-0001-5791-9614
Fahimeh Attarian, orcid.org/0000-0001-9752-0480
 Seyed Alireza Javadinia
Seyed Alireza Javadinia Kimia Alizadeh2†
Kimia Alizadeh2† Mohammad-Shafi Mojadadi
Mohammad-Shafi Mojadadi James S. Welsh
James S. Welsh Fahimeh Attarian
Fahimeh Attarian