- 1Research Institute for Microbial Diseases, Osaka University, Suita, Japan
- 2PRESTO, Japan Science and Technology Agency, Kawaguchi, Japan
- 3The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
- 4CREST, Japan Science and Technology Agency, Kawaguchi, Japan
The physiological roles of proteolysis are not limited to degrading unnecessary proteins. Proteolysis plays pivotal roles in various biological processes through cleaving peptide bonds to activate and inactivate proteins including enzymes, transcription factors, and receptors. As a wide range of cellular processes is regulated by proteolysis, abnormalities or dysregulation of such proteolytic processes therefore often cause diseases. Recent genetic studies have clarified the inclusion of proteases and protease inhibitors in various reproductive processes such as development of gonads, generation and activation of gametes, and physical interaction between gametes in various species including yeast, animals, and plants. Such studies not only clarify proteolysis-related factors but the biological processes regulated by proteolysis for successful reproduction. Here the physiological roles of proteases and proteolysis in reproduction will be reviewed based on findings using gene-modified organisms.
Introduction
Although a simple peptide bond between two amino acids in water at room temperature has a half-life of several years (1), the hydrolysis of a peptide bond is significantly accelerated under the presence of proteases. As well as mediating non-specific protein hydrolysis, proteases also act as processing enzymes that perform highly selective, limited, and efficient cleavage of specific substrates. As many biological processes are influenced by this irreversible post-translational protein modification, dysregulation of the expression and/or function of proteases underlie many human pathological processes and have therefore been an intensely studied class of targets for drug discovery.
By searching Saccharomyces cerevisiae, Drosophila melanogaster, and Caenorhabditis elegans genome databases with a gene ontology term “peptidase activity” (GO:0008233), 51, 506, and 448 genes encoding proteases, respectively, can be identified (2–4). In the mouse and human genome, 628 and 553 protease genes exist, respectively (5). In Arabidopsis thaliana, 723 protease genes were reported (6). Based on catalytic mechanisms, proteases can be divided into five classes: cysteine proteases, serine proteases, metalloproteases, threonine proteases, and aspartic proteases. After activation of the amide, cysteine, serine, and threonine proteases utilize the namesake residue to attack the amide carbonyl group, whereas metalloproteases and aspartic proteases use an activated water molecule as a nucleophile. As proteases bind their substrates between the substrate side chains and well-defined substrate-binding pockets within the active site, they have their own preference for substrate amino acid sequence proximal to the cleavage site (7). There are some enzymatically inactive pseudoproteases encoded in the mammalian genome in which the amino acid residues indispensable for catalytic activity are substituted. As proteases are potentially toxic, their activities are strictly regulated as such by pH, specific ion concentrations, posttranslational modifications, and spatiotemporal expression of protease inhibitors.
The contribution of proteases depends on their intracellular or extracellular localization where they act on substrate proteins. The ubiquitin-proteasome system (UPS) is a complex but sophisticated intracellular proteolytic system in eukaryotes; this complex system degrades unneeded or damaged proteins by proteolysis. When target proteins are post-translationally labeled with ubiquitin, a protein of 76-amino acid residues exhibiting high sequence conservation among eukaryotes, they will be recognized and degraded by the proteasome.
Proteolytic processing events are fundamental in reproductive processes including gametogenesis, fertilization, and embryonic development. Recent advances in generating gene-modified animals have identified many proteases and their regulators associated with reproduction in various species including yeast, invertebrates, vertebrates, and plants. In the following sections the physiological importance of proteolysis in reproduction will be overviewed based on findings obtained by gene-modified organism studies. Proteolysis-related genes essential in reproduction identified by gene-modified animal studies are listed in Table 1. Few proteins are known to be proteolytically processed under certain reproductive situations. They are, however, not included in this review as the physiological roles of such processing in reproduction are not fully clarified at present.
Unicellular Organisms
Saccharomyces cerevisiae
S. cerevisiae, Baker’s yeast, is a model diploid unicellular organism. S. cerevisiae can stably exist as either a diploid or a haploid. When stressed, S. cerevisiae can undergo meiosis to produce four haploid spores. Haploid cells are capable of fusing with other haploid cells of the opposite mating type (an ‘a’ cell can only mate with an ‘α’ cell, and vice versa) to produce a stable diploid cell. a and α cells produce mating peptide pheromones a-factor and α-factor, respectively. Ste24p and Axl1p encoded by ste24 and alx1, respectively, are metalloendopeptidases that process precursor peptide to produce mature mating a-factor pheromone (8, 9).
Multicellular Organisms I: Invertebrates
The body of multicellular organisms consists of two types of cells with different lineages, i.e., germ cells and somatic cells. Germ cells produce gametes for fertilization, whereas somatic cells develop reproductive organs to support gametogenesis and fertilization by germ cells. Therefore, dysfunction of proteolysis in either cell lineage can result in fertility defects.
Nematodes
Caenorhabditis elegans is androdioecious; i.e., it has two sexes, hermaphrodite and male, whereas Ascaris suum is dioecious, being either male or female. They develop two U-shaped gonads in which gametes are generated and fertilization occurs. Several proteases and inhibitors have been identified to regulate nematode reproductive processes.
Oogenesis and fertilization are affected when cpi-2a, encoding a cystatin-like cysteine protease inhibitor, is mutated (10). Nullification of dss-1 encoding a 26S proteasome subunit provokes sterility because of deficient oogenesis (14). Knockdown of puromycin-sensitive aminopeptidase encoded by pam-1 causes delayed oocyte maturation and subfertility (17). Deletion of dpf-3 encoding a serine protease causes sterility because of impaired spermatogenesis (15). gon-1 encoding a disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif (ADAMTS) is necessary for morphogenesis of U-shaped gonads (11, 12). A mutant worm lacking timp-1 encoding a tissue inhibitor of metalloproteinase also shows deficient gonadal development (13). A double mutant in which sup-17 and adm-4, encoding nematode orthologs of mammalian membrane metalloproteases ADAM10 and ADAM17, respectively, are sterile because of aberrant spermathecal function (16).
Unlike mammalian flagellated sperm, nematode sperm are amoeboid cells. For successful fertilization, sperm must be activated prior to contacting an oocyte in both C. elegans and A. suum. This sperm activation is called spermiogenesis through which round immobile spermatids transform into motile, fertilization-competent spermatozoa. Mechanistically, spermiogenesis occurs by sensing extracellular signals and can be reproduced in vitro by exposing spermatids to proteases such as Pronase and proteinase K. A trypsin-like secreted protease encoded by try-5 is expressed in the vas deferens and triggers activation of spermatids (18). swm-1 encodes a secreted protein with a trypsin inhibitor-like domain, and swm-1 mutant males are infertile because of ectopic premature activation of sperm (19). Like in C. elegans, activation of spermatozoa by exposure to extrinsic protease in vitro can also be seen in several insect species (132, 133). spe-4 encoding a presenilin, an aspartyl protease with intramembrane proteolytic activity prevents spermatid activation because spe-4 mutant males progress directly to functional spermatozoa without the need for an activation signal (134).
gcna-1 encodes nuclear metalloprotease. gcna-1 deletion causes genomic instability decreasing fertility in later generations (20). T12E12.6 encodes intracellular metalloprotease whereas zmp-2 encodes secreted metalloproteases. Knockdown of either of them results in reduced offspring production (17, 21).
Insects
The reproductive system of Drosophila melanogaster is more complex compared with nematodes; it is composed of gonads, genital ducts, and accessory structures. Several proteases have been implicated in D. melanogaster spermatogenesis. In the D. melanogaster genome, there are five genes paralogous to S. cerevisiae ste24 encoding a type I prenyl protease. Deletion of three tandemly arrayed ste24 paralogs results in male fertility defects manifesting late in spermatogenesis (22).
All Drosophila spermatid nuclei descended from a primary spermatocyte remain connected to each other via an extensive network of cytoplasmic bridges. Spermatids should therefore be physically dissociated from each other by a process referred as individualization and a ubiquitin-proteasome system regulates this process. Males in which Prosalpha6T encoding a testis-specific proteasome core particle subunit was ablated are sterile because of defects in sperm individualization and nuclear maturation (23). Duba encodes a deubiquitylating enzyme and Duba null mutants are male sterile and display defects in spermatid individualization (24). The non-apoptotic function of caspases also contributes to individualization. DARK is a Drosophila homolog of mammalian caspase activator Apaf-1, whereas DRONC and DREDD are Drosophila apical caspases. Flies deficient in DARK or expressing a dominant-negative version of DRONC failed individualization (25, 135). Dredd-null flies also often show individualization defects (25).
In D. melanogaster sperm, mitochondrial derivatives run along the entire flagellum to provide structural rigidity for flagellar movement. Two mitochondrial derivatives (i.e., major and minor) differentiate and major one accumulates paracrystalline material by the end of spermatogenesis. S-Lap1-8, Sperm-Leucylaminopeptidase (S-Lap) family members are constituents of paracrystalline material. S-Lap mutants possess defects in paracrystalline material accumulation and abnormal structure of the elongated major mitochondrial derivatives and male sterility (28). Htra2 encodes a mitochondrial serine protease. In one Htra2-null mutant line males are infertile because sperm are completely immotile (26), whereas spermatogenesis is defective in another Htra2 mutant line (27).
Seminal fluid produced in the accessory gland includes proteases and protease inhibitors and is thought to contribute to fertilization in a post-mating manner. Seminase is a trypsin-like protease encoded by Sems and included in seminal fluid. When females mated with Sems knockdown males, they laid significantly fewer eggs (29). In cricket, Allonemobius socius, an ejaculate serine protease encoded by ejac-sp is expressed in male reproductive accessory glands. RNAi knockdown of ejac-sp resulted in a significant reduction of the male’s ability to induce a female to lay eggs (38). Nep4, a drosophila ortholog of mammalian Mmel1, encodes a metalloprotease expressed in male gonads (136). Nep4 mutant males are infertile; mutant sperm are quickly discarded by females (30). When Dcp-1 encoding a cysteine protease was ablated in their germline, the resulting females were infertile because of defective oogenesis (31).
Several proteases also of concern in Drosophila reproduction include maternal haploid or mh encodes the Drosophila homolog of SPRTN, a conserved metalloprotease essential for resolving DNA–protein cross-linked products. Paternal chromatids of mh mutants are unable to separate in the anaphase of the first embryonic mitosis and form a chromatin bridge. As a consequence, haploid nuclei of maternal origin rapidly separate from the damaged paternal chromosomes and haploid embryos develop but become lethal in a maternal effect manner (32, 33, 137). Ance encodes a putative homologue of mammalian angiotensin-converting enzyme (ACE). Compound heterozygote for two different Ance lethal alleles exhibit male sterility (34), but the molecular details are unknown. RNAi knockdown of Slfc encoding a secreted serine protease causes male infertility (35). When a membrane serine protease encoded by ome was mutated, males became subfertile (36). RNAi knockdown of a secreted metalloprotease encoded by Mmp2 caused female subfertility because ovulation was blocked (37).
Several pest control attempts target reproduction-associated proteases. In pests Spodoptera litura and Plutella xylostella, targeted inactivation of serine protease genes Osp and Ser2, respectively, resulted in female and male infertility as also observed in silkworm moth Bombyx mori (39, 40). In other pests Hyphantria cunea, and Bactrocera dorsalis, RNAi knockdown of Hcser2, and Bdcp-1 encoding serine protease and cysteine protease, respectively, also resulted in infertility (41, 42). Thus, proteases are potential targets for pest population control.
Multicellular Organisms II: Vertebrates
Findings in vertebrates were obtained by genetic studies in rodents, fish, and human patients. Genes disrupted in these species include those encoding proteases, protease inhibitors, and non-catalytically active pseudo-proteases. Proteolysis-related factors are included in various aspects of male and female reproductive processes such as gamete production, gamete maturation, fertilization, post-fertilization events, and mating behavior.
UPS in Gamete Production
For the fine-tuning of cellular processes, intracellular proteins are timely degraded by UPS. The proteasome localizes in the nucleus and cytoplasm where it degrades ubiquitylated proteins. Spermatoproteasome, a testis-specific proteasome, is one of the three tissue-specific proteasomes identified together with the immunoproteasome and the thymoproteasome in mammals (138). Deletion of Psma8, which encodes a testis-specific 20S proteasome component, leads to spermatogenesis arrest at the spermatocyte stage (43). Psme3 encodes REGγ, a proteasome activator. Psme3-null males are subfertile with decreased sperm number and motility (44). This is probable because REGγ regulates p53-mediated transcription of Plzf, a transcription factor necessary for spermatogonial stem cell self-renewal and proliferation (139). Psme4 encodes PA200 proteasome activator. Psme4-null males have reduced fertility due to defects in meiotic spermatocytes and post-meiotic spermatids (45). Psme3;Psme4 double KO males were infertile; mutant sperm appeared morphologically normal but exhibited remarkable defects in motility and decreased proteasome activity (46).
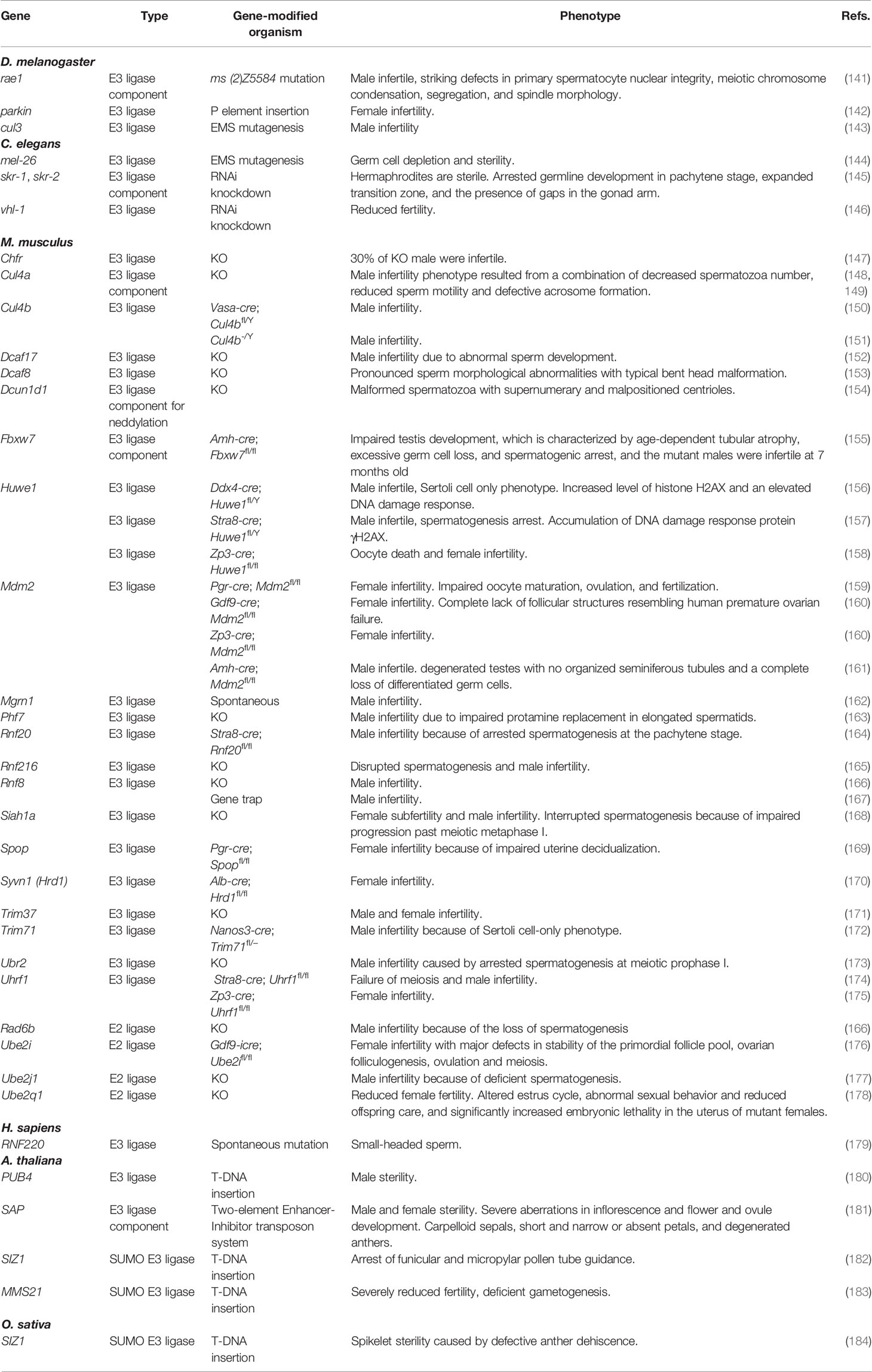
Proteasome target proteins are ubiquitylated by E3 ubiquitin ligases which transfer the ubiquityl group from E2 ligase to the target protein. There are ∼600 E3 ligases encoded in the mammalian genome (140). The ubiquitin ligases, which are not proteases but included in ubiquitin-proteasome system-mediated protein degradation, indispensable for mammalian reproduction are listed in Table 2. Here only Huwe1 is mentioned as how E3 ligases function in reproductive processes. Huwe1 ubiquitylates histone H2AX, which is phosphorylated in response to DNA damage and is essential to the efficient recognition and repair of DNA double-strand breaks. Germline-specific Huwe1 ablation increased histone H2AX level, elevated DNA damage response, and caused Sertoli cell only phenotype. Thus Huwe1 likely regulates the response to spontaneous DNA damage by UPS-mediated H2AX degradation to maintain cell survival (156).
Cullin-RING E3 ubiquitin ligases are known to be reversibly neddylated, i.e., conjugated with NEDD8, a ubiquitin-like protein. By conjugation with NEDD8, cullin-RING E3 ligases increase their stability and ligase activity. The constitutive photomorphogenic-9 signalosome (CSN) deneddylates cullin-RING E3 ligases by cleaving the isopeptide bond of neddylated lysine to regulate the cellular ubiquitylation status. COPS5 is the fifth component of the CSN and abundant in mouse testis (185). Cops5-null males were infertile because of significant reduction of sperm number caused by premeiotic apoptosis of germ cells (47).
Ubiquitylated proteins can be deubiquitylated by deubiquitylating enzymes such as ubiquitin-specific proteases (USPs), cysteine endopeptidases encoded by Usp genes, thereby expression levels and activity of target proteins are regulated. USP1 deubiquitylates FANCD2 which is included in the repair of DNA crosslinks. Usp1 null males were infertile and the seminiferous tubules were markedly atrophic and mostly devoid of spermatogenic cells in the mutant testis. Usp2-null males possessed severely reduced fertility and the mutant sperm were defective in sperm motility and egg fertilizing ability in vitro (48). Germ cell-specific ablation of Usp9x using Vasa-cre possessed spermatogenic cell apoptosis at the early spermatocyte stage and resulted in complete infertility (49). Usp26 is an X-linked gene exclusively expressed in testis (186). Usp26 -null males are subfertile because of reduced number of haploid cells in testis (50, 51). Usp1-null female mice showed reduced fertility probably because of a reduced number of oocytes in ovaries (52). Thus, UPS is critically important for germ cell production in both sexes.
Non-Proteasomal Intracellular and Extracellular Proteolysis Factors in Sperm Production
Intracellular and extracellular proteolysis factors critically function in spermatogenesis. Cleavage of specific peptide bonds also contributes to spermatogenesis. Apaf1 encodes a caspase activator, and Apaf1-null males are infertile because of degeneration of spermatogonia, which results in the absence of sperm (53). Agbl5 encodes an intracellular metalloprotease. Agbl5-null males are infertile because of defective spermatogenesis (54, 55). A cytosolic carboxypeptidase 1, another metalloprotease encoded by Agtpbp1 deglutamylates polyglutamylated proteins. Agtpbp1 mutant mice known as Purkinje cell degeneration (pcd) possess male infertility (109–112) because of defective spermatogenesis (110). A germ cell nuclear antigen encoded by Gcna contains a metalloprotease domain. Gcna-null males are nearly devoid of sperm and infertile (56). In human, GCNA spontaneous mutations were identified in spermatogenic failure patients (124, 125).
Separin, a caspase-like cysteine protease encoded by Espl1, plays a central role in chromosome segregation by cleaving the SCC1/RAD21 subunit of the cohesin complex (187–189). A point mutation in Espl1 which substitutes inhibitory phosphorylation site Ser1121 to Ala depletes spermatogonia because of chromosome misalignment during proliferation of the postmigratory primordial germ cells and following mitotic arrest, aneuploidy, and cell death (105). Threonine aspartase 1 (TASP1) is an intracellular endopeptidase that cleaves after distinct aspartate residues of the conserved IXQL(V)D/G motif (190). TASP1 cleaves general transcription factor TFIIAα−β to enable testis-specific transcription; Tasp1-null male mice were unable to activate spermatogenic gene activation, which lead to the release of immature germ cells and infertility (57). A serine protease ClpP is located in the mitochondrial matrix and participates in mitochondrial protein quality control by degrading misfolded or damaged proteins. In Clpp-null mutants spermatogenesis was disrupted by the spermatid stage (114). Tysnd1 encodes a serine protease that processes peroxisomal leader peptides. Tysnd1-null mutant males possess globozoospermia and their spermatozoa lack the acrosomal cap (58). Spink2 encodes a Kazal-type serine protease inhibitor abundantly expressed in testis and epididymis (191). Spink2-null males had azoospermia, and a homozygous splice mutation of SPINK2 was found in infertile men (59). Ablation of Serpina5 encoding another serine protease inhibitor also results in an abnormality in sperm production in the testis (60).
Puromycin-sensitive aminopeptidase encoded by Npepps is also an intracellular protease. It appears to contribute indirectly to spermatogenesis. Npepps-null testes and seminal vesicles were significantly reduced in weight, spermatogenesis was impaired, and copulatory behavior was lacking. It is suggested that the defects in the testes likely arises from dysfunction of Sertoli cells, whereas the lack of copulatory behavior results from defects in the brain (115).
A null mutation of Adamts2 encoding secreted metalloproteinase caused male infertility (61). Decreased spermatogenesis was observed but copulatory behavior and/or copulatory plug formation may also be impaired because a copulatory plug was never observed (61).
Proteolysis Factors Associated With Sperm Function
Acrosomal Function
The acrosome is a Golgi-derived sperm head organelle in which many digestive enzymes such as proteases and hyaluronidases are included to penetrate egg surroundings. Acrosin is a serine protease and a major component of the acrosome. Although acrosin-deficient male mice are fertile (62, 63), disruption of hamster acrosin resulted in complete male infertility (120). In vitro, mutant hamster spermatozoa attached to the zona pellucida, but failed to penetrate it (120), suggesting that acrosomal function can be attributed to specific factors in a species-specific manner.
Proprotein convertases convert inactive precursor proteins into their mature and active forms. PCSK4 is a member of proprotein convertases expressed on the sperm surface overlying the acrosome (64). Pcsk4-null males showed impaired fertility (64, 65) and mutant sperm exhibited accelerated capacitation, precocious acrosome reaction, reduced binding to egg zona pellucida (64). Acrosome formation during spermatogenesis was also abnormal (192).
Sperm Maturation
A group of genes encoding proteases, enzymatically inactive pseudoproteases, and protease inhibitors is apparently associated with the same physiological function, i.e., maturation of sperm conferring abilities to migrate into female oviduct and bind with zona pellucida. Ablation of Tmprss12 (66), Prss55 (67, 68), Tryx5 (69), Prss37 (70), Ace (71), Adam1a (72), Adam2 (73), Adam3 (74, 75), and Adam6 (76) results in deficient sperm migration into the oviduct and binding to the zona pellucida of eggs. Among them, Adam1a, Adam2, Adam3, Adam6, and Prss37 encode catalytically inactive pseudoproteases. A disintegrin and metallopeptidase domain (ADAM) 3, a catalytically inactive transmembrane pseudoprotease appears to be central to a molecular mechanism that governs sperm migratory and adhesion abilities, because ADAM3 expression is a prerequisite for sperm to acquire these abilities (193).
ADAM3 is expressed as a precursor and the processed into mature form as spermatozoa mature in epididymis (194). Similarly, enzymatically inactive pseudoproteases ADAM2 and ADAM6 are processed during sperm maturation in epididymis (195, 196). Therefore, they are rather substrates for other proteases. Ablation of ADAM2 or ADAM6 also results in significant decrease or loss of ADAM3 from epididymal sperm (74, 76) indicating the involvement of both ADAM2 and ADAM6 in ADAM3 expression. PRSS37 supports ADAM3 precursor translocation to the sperm cell surface by collaborating with PDILT, a testis-specific protein disulfide isomerase indispensable for ADAM3 surface expression (197, 198). TMPRSS12, PRSS55, and TRYX5, all of which are serine proteases and retain catalytic triad residues, are necessary for the production or stable localization of processed ADAM3 on the cell surface of epididymal spermatozoa (66–69), although it remains uncertain whether these proteases directly cleave ADAM3.
Cystatins are secreted cysteine proteinase inhibitors. Cystatin genes Cst8, 9, 11, 12, 13, dc1, dc2, and l1 are clustered on mouse chromosome 2 and expressed in both testis and epididymis. Their simultaneous ablation resulted in the loss of ADAM3 from epididymal sperm and deficient sperm migration into the oviduct (77), implying the importance of regulated proteolysis in sperm maturation. Ovochymase 2 (OVCH2) is a chymotrypsin-like serine protease. OVCH2 is specifically expressed in the caput epididymis under the regulation of lumicrine signaling, in which testis-derived secreted protein NELL2 transiting through the luminal space acts on the epididymal epithelium by binding to its receptor ROS1 tyrosine kinase to differentiate (78). Ablation of Ovch2 results in abnormal sperm ADAM3 processing and deficient sperm migration into the oviduct (78). Thus, regulated proteolysis on or outside spermatozoa apparently modulates sperm maturation.
NL1 encoded by Mmel1 is a zinc metallopeptidase expressed in testis. NL1 is expressed as a type II transmembrane protein but released as a soluble form. Mmel1-null mice show normal spermatogenesis but reduced egg fertilization, suggesting the role of NL1 in sperm maturation (79). It remains, however, uncertain whether NL1 is included in ADAM3-mediated sperm maturation. Testisin encoded by Prss21 is a GPI-anchored serine protease. Prss21 KO males are subfertile because mutant spermatozoa possessed decreased motility, angulated and curled tails, and fragile necks (80). In another Prss21 mutant line in vitro sperm binding to egg zona pellucida, acrosome reaction, and fertility were decreased (81).
Other Proteolytic Factors Associated With Male Reproduction
Several cell surface and extracellular proteases and inhibitors seem to regulate male fertility in more indirect manners. Adamts16 homozygous mutant rat males resulted in cryptorchidism and male sterility (121). The mutant testis undescended during development because of the failure of gubernacular migration (122). γ-glutamyltranspeptidase 1 (GGT1) is a type II transmembrane protein which cleaves γ-glutamyl bond of extracellular glutathione (γ-Glu-Cys-Gly), glutathione conjugates, and other γ-glutamyl compounds. The resulting cysteinyl-glycine is further cleaved by dipeptidase into free amino acids. Ggt1-null males are infertile because of decreased epididymal sperm number and failure in copulatory plug formation (117). Although Ggt1-null testis was small, spermatogenesis inside seminiferous tubules appeared normal and seminal vesicles were hypoplastic. As N-acetylcysteine-fed mutant mice were fertile, the observed infertility is a consequence of cysteine deficiency (117),. Carboxypeptidase E (CPE) is a metallo-carboxypeptidase and functions as a prohormone processing exopeptidase. Cpefat/fat males are infertile and deficient in Pro-gonadotropin-releasing hormone processing in the hypothalamus (82). ADAM24 is a metalloproteinase localized on the mature sperm surface. Adam24-null males are subfertile and polyspermic fertilization increased in vitro and in vivo, suggesting a physiological role of ADAM24 for prevention of polyspermy (83). ADAM7 is a membrane-anchored protein with a catalytically-inactive metalloproteinase domain abundantly expressed in the epididymis (199). Adam7 ablation resulted in a modest reduction of male fertility; impaired epididymal morphology and integrity may affect sperm maturation (84).
Cystatin C encoded by Cst3 is a cysteine protease inhibitor abundantly expressed in testis and epididymis. Substitution of Leu68 to Gln is an amyloid-forming mutation found in a hereditary form of cystatin C amyloid angiopathy. Heterozygous male mice were infertile and increased levels of amyloid was observed in the epididymal fluid (85). Nonpathological function of amyloid during epididymal sperm maturation is also suggested (200).
Immp2l encodes an inner mitochondrial membrane peptidase 2-like. Immp2l-null homozygous males were severely subfertile because of erectile dysfunction (118). Tumor necrosis factor-α (TNFα) converting enzyme encoded by Adam17 is involved in the proteolytic release of the ectodomain of diverse cell surface proteins. Conditional ablation of Adam17 with Sox9-cre severely impaired male fertility but the details are uncertain (119).
Serpine2 encodes protease nexin-1, a serine protease inhibitor expressed in seminal fluid. Serpine2-null males possessed reduced fertility because of impaired semen coagulation and copulatory plug formation (86).
Proteolytic Factors in Ovary and Follicle Development
Both intracellular and extracellular proteolytic factors are included in ovary and follicle development. Conditional ablation of separase under the control of Zp3-cre hindered extrusion of the first polar body and caused female sterility (106). Introduction of a Ser1121 to Ala deregulatory mutation into separase led to primordial germ cell apoptosis during embryonic oogenesis (107). Ablation of cytosolic carboxypeptidase 1 encoded by Agtpbp1 results in female subfertility because secondary follicles poorly develop into antral follicles (113). Oocyte-specific ablation of nuclear cysteine protease separase causes female infertility because mutant oocytes are able neither to extrude polar bodies in meiosis I nor to resolve chiasmata (106).
A deregulatory mutation into separin encoded by Espl1 at early embryonic period caused primordial germ cell depletion by apoptosis during embryonic oogenesis, which led to female infertility (105, 107). The introduction of the same mutation at later oocyte development by using Zp3-cre also resulted in female infertility but because of failure in preimplantation development (108).
Matriptase encoded by Tmprss6 is a type II transmembrane serine protease which functions in iron homeostasis by cleaving cell surface proteins associated with iron absorption. Tmprss6-null females possessed marked retardation in ovarian maturation (87), probably because of severe decrease in plasma iron levels. The defective ovarian follicle development and female infertility can be mimicked by a low iron diet (201).
The inter-α-trypsin inhibitor (IαI) family are abundantly found in body fluids including blood plasma and urine and possess inhibitory activity for serine proteases. They are composed of bikunin, a proteoglycan with a single chondroitin sulfate chain, and heavy chains covalently bound to chondroitin sulfate chain of bikunin. IαI family members are able to transfer their heavy chains from IαI to hyaluronan in the presence of tumor necrosis factor-stimulated gene-6. This reaction results in the modified hyaluronan covalently linked heavy chain and is necessary for hyaluronan-rich cumulus matrix expansion. When the bikunin-coding region was deleted from Ambp gene, the resulting homozygous females ovulate oocytes deficient in hyaluronan-rich cumulus matrix expansion, leading to female infertility (88, 89).
γ-secretase is an endoprotease complex that catalyzes the intramembrane cleavage of integral membrane proteins. Psen1 encodes presenillin-1, a catalytic subunit of γ-secretase. Female mice homozygous with a Leu166 to Pro mutation, an aggressive mutation found in familial Alzheimer’s disease patients, are infertile and their ovaries consisted largely of stromal elements with primordial follicles near the cortex (90).
ADAMTS1 is a secreted metalloproteinase expressed in the granulosa cell layer of mature follicles in the ovary (91). Adamts1-null females possessed lower numbers of mature follicles in the ovary and a thick and convoluted uterus (92). In another mutant mouse line, ovulation in null females was impaired because mature oocytes remained trapped in ovarian follicles (91). In zebrafish, adamts9-null females possess ovarian malformation and are unable to ovulate (123).
Lonp encodes a mitochondrial serine protease. Oocyte-specific Lonp ablation by Gdf9-cre or Zp3-cre; Lonp1fl/fl results in female infertility because of impaired follicular development, progressive oocyte death, ovarian reserve loss (93). Furin encodes a transmembrane serine protease localized in Golgi appratus, endosome, plasma membrane; it is necessary for mature protein release by cleaving at RX(K/R)R consensus motif. Conditional ablation of Furin by Gdf9-cre or Zp3-cre; Furinfl/fl result in female infertility because of the arrested oogenesis at early secondary follicles (94). Pappa encodes an extracellular metalloprotease. Pappa KO females decreased their litter size and ovulatory capacity, probably because of decreased bioavailability of ovarian insulin-like growth factor (95).
Loss of GGT1 causes infertility in not only males but females. In the Ggt1-null females, antral follicles and corpora lutea were absent and follicles degenerated due to the reduced intracellular cysteine levels (117).
Mitochondrial proteases also affect ovarian follicle development. Ablation of Clpp encoding mitochondrial matrix ClpP protease caused relatively small ovaries in which follicular differentiation was impaired probably because of the reduction of the granulosa cell layers (114). When the inner mitochondrial membrane peptidase 2-like encoded by Immp2l was ablated, the resulting mutant females were deficient in folliculogenesis and ovulation and infertile, probably because of low availability of nitric oxide caused by mitochondrial dysfunction (118).
Proteolytic Factors in Post-Fertilization Events of Female Reproduction
Several proteolysis-associated secreted proteins contribute to post-fertilization events including the hardening of the egg-surrounding zona pellucida. Ovastacin encoded by Astl is a secreted metalloendopeptidase deposited in cortical granules of oocytes. Ovastatin is secreted into the extracellular space in response to egg activation triggered by fertilization. In Astl-null eggs, ZP2 cleavage necessary for zona pellucida hardening and the postfertilization block to polyspermy did not occur after fertilization (96). Fetuin is a cystatin family protease inhibitor abundantly expressed in blood plasma. Fetuin-B prevents premature ZP hardening probably by inhibiting ovastacin derived from spontaneous cortical granule release, as fetuin-B inhibited ovastacin protease activity in vitro and Fetub-deficient oocytes undergo premature zona pellucida hardening (97).
Antithrombin encoded by Serpinc1 inhibits thrombin and some other coagulation factors by binding heparin and heparan sulfate. When an Arg48 to Cys mutation, which corresponds to human thrombosis mutation, was introduced into mice, the resulting homozygous females had decreased their litter size, probably because thrombosis occurred in placenta (98).
Adam10 encodes a membrane metalloprotease. Conditional ablation of vascular Adam10 by Tie2-Cre; Adam10fl/fl causes impaired decidualization and female subfertility (99). Adamts18 encodes a member of secreted metalloprotease ADAMTS. Adamts18-null females suffer from vaginal obstruction, due to either a dorsoventral vaginal septum or imperforate vagina and infertility or subfertility (100).
Other Proteolytic Factors in Female Reproduction
Several proteolysis-associated factors regulate female reproduction in a more indirect manner. Npepps-null females lacking a puromycin-sensitive aminopeptidase impairs corpus luteum formation and are infertile, probably because of disruption of the hypothalamic-pituitary axis (116). Plasmin is a secreted serine protease generated from plasminogen through activation by tissue-type or urokinase-type plasminogen activators. The fertility of plasmin-deficient Plg-null female mice appeared to be compromised (101, 102). It seems not to be the consequence of the impaired proteolytic process essential for ovulation, as plasminogen-deficient mice had normal ovulation efficiency (202). Timp1 encodes a tissue inhibitor of metalloproteinases 1, an inhibitor for matrix metalloproteinases. Timp1 mutation reduced the reproductive lifespan of female but not male mice (103). When Pcsk2 encoding neuroendocrine convertase 2 was ablated, the number of consecutive litters from mutant female mice was small and Pcsk2-null female mice sometimes gave birth to dead pups (104) for uncertain reason. Conditional ablation of TNFα converting enzyme by Sox9-cre; Adam17fl/fl resulted in female infertility but details are uncertain (119).
Fertility-Associated Proteases in Plants
Several aspartic proteases are associated with pollen development and function. In Arabidopsis thaliana, A36 and A39 are GPI-anchored putative aspartic proteases predominantly expressed in pollen and the pollen tube. In a36; a39 double mutant, pollen grains underwent apoptosis-like programmed cell death and the pollen tube compromised micropylar guidance (126). UND encodes a secreted aspartic protease UNDEAD, and its silencing using small interfering RNA caused premature tapetal and pollen programmed cell death (128).
In Oryza sativa, OsAP65 encodes an aspartic protease localized in the pre-vacuolar compartment. T-DNA-inserted OsAP65 mutant alleles could not be transmitted through the male gamete; the mutant pollen matured normally, but did not germinate or elongate, indicating its essentiality in pollen germination and tube growth (131). PCS1 encodes an aspartic protease and its loss-of-function mutation caused degenerated male and female gametophytes (127).
A cysteine protease also contributes to pollen development; when a papain-like vacuolar cysteine protease encoded by CEP1 was ablated, the resulting mutants are male subfertile because of aborted tapetal programmed cell death and decreased pollen fertility with abnormal pollen exine (129).
Some aspect of A. thaliana reproduction includes Small Ubiquitin-related Modifier (SUMO). SPF1 and SPF2 are cysteine proteases and function in desumoylation of sumoylated proteins. spf1; spf2 double mutants exhibit severe abnormalities in microgametogenesis, megagametogenesis, and embryo development (130). There are SUMO-E3 ligases involved in gametophyte development (182, 183) in A. thaliana and in anther dehiscence in O. sativa (184).
Conclusion and Perspective
By a comprehensive survey, it has been demonstrated that proteolysis regulates reproduction in various species including yeast, insects, nematodes, vertebrates, and plants. Regulation of reproduction by proteolysis already exist in unicellular yeast. In multicellular organisms, proteolysis regulates the formation and function of gametes derived from germ cells as well as the development and function of reproductive organs by somatic cells, thereby securing successful reproduction. In these cell lineages, both limited proteolysis and degrative proteolysis by ubiquitin-proteasome system play critical roles.
One of intriguing paradigms emerging in this review is that many sperm surface and extracellular proteases, pseudoproteases, and inhibitors are included in the acquisition of mammalian sperm conferring abilities to migrate into the oviduct and to bind to the zona pellucida of eggs. As spermatozoa are transcriptionally and translationally silent, post-translational modification mechanisms such as proteolysis may largely contribute to sperm maturation.
Many compounds have been designed to inhibit the enzymatic activity of proteases. Clinically, there have been numerous successes including angiotensin-converting enzyme inhibitors for cardiovascular disorders (203), thrombin inhibitors for thromboembolism and bleeding disorders (204, 205), and HIV protease inhibitors in the treatment of HIV and AIDS (206), among others (207, 208). In addition, enzymatically active proteases could also be good druggable targets for contraceptives.
Genome editing techniques developed in recent years will identify fertility-associated proteolytic factors further. In addition to identifying novel factors, more intense studies on the molecular basis of proteolysis including the identification of substrates will clarify how proteolytic events govern reproduction. It will also clarify the physiological significance of molecular events governed by proteolysis in reproduction.
Author Contributions
DK and MI wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI grants (JP21H00231 to D.K. and JP21H05033 to MI), Japan Science and Technology Agency (21460710 to D.K. and 21467777 to MI), National Institutes of Health (R01HD088412 and P01HD087157 to MI), and the Bill & Melinda Gates Foundation (Grant INV-001902 to MI). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr Julio Castaneda for critical reading of this manuscript.
Abbreviations
ACE, angiotensin converting enzyme; ADAM, a disintegrin-like and metalloproteinase domain; ADAMTS, a disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif; CSN, constitutive photomorphogenic-9 signalosome; EMS, ethylmethane-sulfonate; GGT, glutamyltranspeptidase; IαI, inter-α-trypsin inhibitor; KI, knock-in; KO, knockout; OVCH2, ovochymase 2; S-Lap, sperm-Leucylaminopeptidase; SUMO, small ubiquitin-related modifier; TASP1, threonine aspartase 1; TMP,trimethylpsoralen; TNFα, tumor necrosis factor-α; UPS, ubiquitin-proteasome system; USP, ubiquitin-specific protease.
References
1. Kahne D, Still WC. Hydrolysis of a Peptide Bond in Neutral Water. J Am Chem Assoc (1988) 110(22):7529–34. doi: 10.1021/ja00230a041
2. Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, et al. Saccharomyces Genome Database: The Genomics Resource of Budding Yeast. Nucleic Acids Res (2012) 40(Database issue):D700–5. doi: 10.1093/nar/gkr1029
3. Larkin A, Marygold SJ, Antonazzo G, Attrill H, Dos Santos G, Garapati PV, et al. FlyBase: Updates to the Drosophila Melanogaster Knowledge Base. Nucleic Acids Res (2021) 49(D1):D899–907. doi: 10.1093/nar/gkaa1026
4. Davis P, Zarowiecki M, Arnaboldi V, Becerra A, Cain S, Chan J, et al. WormBase in 2022-Data, Processes, and Tools for Analyzing Caenorhabditis Elegans. Genetics (2022) 220(4):iyac003. doi: 10.1093/genetics/iyac003
5. Puente XS, Sanchez LM, Overall CM, López-Otin C. {H}uman and Mouse Proteases: A Comparative Genomic Approach. Nat Rev Genet (2003) 4(7):544–58. doi: 10.1038/nrg1111
6. García-Lorenzo M, Sjödin A, Jansson S, Funk C. Protease Gene Families in Populus and Arabidopsis. BMC Plant Biol (2006) 6:30. doi: 10.1186/1471-2229-6-30
7. Klein T, Eckhard U, Dufour A, Solis N, Overall CM. Proteolytic Cleavage - Mechanisms, Function, and “Omic” Approaches for a Near-Ubiquitous Posttranslational Modification. Chem Rev (2018) 118(3):1137–68. doi: 10.1021/acs.chemrev.7b00120
8. Fujimura-Kamada K, Nouvet FJ, Michaelis S. A Novel Membrane-Associated Metalloprotease, Ste24p, is Required for the First Step of NH2-Terminal Processing of the Yeast a-Factor Precursor. J Cell Biol (1997) 136(2):271–85. doi: 10.1083/jcb.136.2.271
9. Adames N, Blundell K, Ashby MN, Boone C. Role of Yeast Insulin-Degrading Enzyme Homologs in Propheromone Processing and Bud Site Selection. Sci (1995) 270(5235):464–7. doi: 10.1126/science.270.5235.464
10. Hashmi S, Zhang J, Oksov Y, Ji Q, Lustigman S. The Caenorhabditis Elegans CPI-2a Cystatin-Like Inhibitor has an Essential Regulatory Role During Oogenesis and Fertilization. J Biol Chem (2006) 281(38):28415–29. doi: 10.1074/jbc.M600254200
11. Blelloch R, Kimble J. Control of Organ Shape by a Secreted Metalloprotease in the Nematode Caenorhabditis Elegans. Nat (1999) 399(6736):586–90. doi: 10.1038/21196
12. Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J. The Gon-1 Gene is Required for Gonadal Morphogenesis in Caenorhabditis Elegans. Dev Biol (1999) 216(1):382–93. doi: 10.1006/dbio.1999.9491
13. Kubota Y, Nishiwaki K, Ito M, Sugimoto A. The Role of Tissue Inhibitors of Metalloproteinases in Organ Development and Regulation of ADAMTS Family Metalloproteinases in Caenorhabditis Elegans. Genetics (2019) 212(2):523–35. doi: 10.1534/genetics.119.301795
14. Pispa J, Palmén S, Holmberg CI, Jäntti JC. Elegans Dss-1 is Functionally Conserved and Required for Oogenesis and Larval Growth. BMC Dev Biol (2008) 8:51. doi: 10.1186/1471-213X-8-51
15. Gudipati RK, Braun K, Gypas F, Hess D, Schreier J, Carl SH, et al. Protease-Mediated Processing of Argonaute Proteins Controls Small RNA Association. Mol Cell (2021) 81(11):2388–2402.e8. doi: 10.1016/j.molcel.2021.03.029
16. Jarriault S, Greenwald I. Evidence for Functional Redundancy Between C. Elegans ADAM Proteins SUP-17/Kuzbanian and ADM-4/TACE. Dev Biol (2005) 287(1):1–10. doi: 10.1016/j.ydbio.2005.08.014
17. Althoff MJ, Flick K, Trzepacz C. Collaboration Within the M1 Aminopeptidase Family Promotes Reproductive Success in Caenorhabditis Elegans. Dev Genes Evol (2014) 224(3):137–46. doi: 10.1007/s00427-014-0470-3
18. Smith JR, Stanfield GM. TRY-5 is a Sperm-Activating Protease in Caenorhabditis Elegans Seminal Fluid. PloS Genet (2011) 7(11):e1002375. doi: 10.1371/journal.pgen.1002375
19. Stanfield GM, Villeneuve AM. Regulation of Sperm Activation by SWM-1 is Required for Reproductive Success of C Elegans Males. Curr Biol (2006) 16(3):252–63. doi: 10.1016/j.cub.2005.12.041
20. Bhargava V, Goldstein CD, Russell L, Xu L, Ahmed M, Li W, et al. GCNA Preserves Genome Integrity and Fertility Across Species. Dev Cell (2020) 52(1):38–52.e10. doi: 10.1016/j.devcel.2019.11.007
21. Altincicek B, Fischer M, Fischer M, Lüersen K, Boll M, Wenzel U, et al. Role of Matrix Metalloproteinase ZMP-2 in Pathogen Resistance and Development in Caenorhabditis Elegans. Dev Comp Immunol (2010) 34(11):1160–9. doi: 10.1016/j.dci.2010.06.010
22. Adolphsen K, Amell A, Havko N, Kevorkian S, Mears K, Neher H, et al. Type-I Prenyl Protease Function is Required in the Male Germline of Drosophila Melanogaster. G3 (Bethesda) (2012) 2(6):629–42. doi: 10.1534/g3.112.002188
23. Zhong L, Belote JM. The Testis-Specific Proteasome Subunit Prosalpha6T of D. Melanogaster is Required for Individualization and Nuclear Maturation During Spermatogenesis. Development (2007) 134(19):3517–25. doi: 10.1242/dev.004770
24. Koerver L, Melzer J, Roca EA, Teichert D, Glatter T, Arama E, et al. The De-Ubiquitylating Enzyme DUBA is Essential for Spermatogenesis in Drosophila. Cell Death Differ (2016) 23(12):2019–30. doi: 10.1038/cdd.2016.79
25. Huh JR, Vernooy SY, Yu H, Yan N, Shi Y, Guo M, et al. Multiple Apoptotic Caspase Cascades are Required in Nonapoptotic Roles for Drosophila Spermatid Individualization. PloS Biol (2004) 2(1):E15. doi: 10.1371/journal.pbio.0020015
26. Tain LS, Chowdhury RB, Tao RN, Plun-Favreau H, Moisoi N, Martins LM, et al. Drosophila HtrA2 is Dispensable for Apoptosis But Acts Downstream of PINK1 Independently From Parkin. Cell Death Differ (2009) 16(8):1118–25. doi: 10.1038/cdd.2009.23
27. Yun J, Cao JH, Dodson MW, Clark IE, Kapahi P, Chowdhury RB, et al. Loss-Of-Function Analysis Suggests That Omi/HtrA2 is Not an Essential Component of the PINK1/PARKIN Pathway In Vivo. J Neurosci (2008) 28(53):14500–10. doi: 10.1523/JNEUROSCI.5141-08.2008
28. Laurinyecz B, Vedelek V, Kovács AL, Szilasi K, Lipinszki Z, Slezák C, et al. Sperm-Leucylaminopeptidases are Required for Male Fertility as Structural Components of Mitochondrial Paracrystalline Material in Drosophila Melanogaster Sperm. PloS Genet (2019) 15(2):e1007987. doi: 10.1371/journal.pgen.1007987
29. LaFlamme BA, Ram KR, Wolfner MF. The Drosophila Melanogaster Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes. PloS Genet (2012) 8(1):e1002435. doi: 10.1371/journal.pgen.1002435
30. Ohsako T, Shirakami M, Oiwa K, Ibaraki K, Karr TL, Tomaru M, et al. The Drosophila Neprilysin 4 Gene Is Essential for Sperm Function Following Sperm Transfer to Females. Genes Genet Syst (2021) 96(4):177–86. doi: 10.1266/ggs.21-00024
31. McCall K, Steller H. Requirement for DCP-1 Caspase During Drosophila Oogenesis. Sci (1998) 279(5348):230–4. doi: 10.1126/science.279.5348.230
32. Loppin B, Berger F, Couble P. Paternal Chromosome Incorporation Into the Zygote Nucleus is Controlled by Maternal Haploid in Drosophila. Dev Biol (2001) 231(2):383–96. doi: 10.1006/dbio.2000.0152
33. Delabaere L, Orsi GA, Sapey-Triomphe L, Horard B, Couble P, Loppin B. The Spartan Ortholog Maternal Haploid Is Required for Paternal Chromosome Integrity in the Drosophila Zygote. Curr Biol (2014) 24(19):2281–7. doi: 10.1016/j.cub.2014.08.010
34. Tatei K, Cai H, Ip YT, Levine M. Race: A Drosophila Homologue of the Angiotensin Converting Enzyme. Mech Dev (1995) 51(2–3):157–68. doi: 10.1016/0925-4773(95)00349-5
35. Chen S, Yang H, Krinsky BH, Zhang A, Long M. Roles of Young Serine-Endopeptidase Genes in Survival and Reproduction Revealed Rapid Evolution of Phenotypic Effects at Adult Stages. Fly (Austin) (2011) 5(4):345–51. doi: 10.4161/fly.5.4.17808
36. Chihara CJ, Song C, LaMonte G, Fetalvero K, Hinchman K, Phan H, et al. Identification and Partial Characterization of the Enzyme of Omega: One of Five Putative DPP IV Genes in Drosophila Melanogaster. J Insect Sci (2005) 5:26. doi: 10.1093/jis/5.1.26
37. Deady LD, Shen W, Mosure SA, Spradling AC, Sun J. Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in Drosophila. PloS Genet (2015) 11(2):e1004989. doi: 10.1371/journal.pgen.1004989
38. Marshall JL, Huestis DL, Hiromasa Y, Wheeler S, Oppert C, Marshall SA, et al. Identification, RNAi Knockdown, and Functional Analysis of an Ejaculate Protein That Mediates a Postmating, Prezygotic Phenotype in a Cricket. PloS One (2009) 4(10):e7537. doi: 10.1371/journal.pone.0007537
39. Xu X, Bi H, Wang Y, Li X, Xu J, Liu Z, et al. Disruption of the Ovarian Serine Protease (Osp) Gene Causes Female Sterility in Bombyx Mori and Spodoptera Litura. Pest Manag Sci (2020) 76(4):1245–55. doi: 10.1002/ps.5634
40. Xu X, Wang Y, Bi H, Xu J, Liu Z, Niu C, et al. Mutation of the Seminal Protease Gene, Serine Protease 2, Results in Male Sterility in Diverse Lepidopterans. Insect Biochem Mol Biol (2020) 116:103243. doi: 10.1016/j.ibmb.2019.103243
41. Li X, Liu Q, Bi H, Wang Y, Xu X, Sun W, et al. Piggybac-Based Transgenic RNAi of Serine Protease 2 Results in Male Sterility in Hyphantria Cunea. Insect Biochem Mol Biol (2022) 143:103726. doi: 10.1016/j.ibmb.2022.103726
42. Liu G, Lv Z, Wu Q, Zhou Z, Zhang G, Wan F, et al. The Bactrocera Dorsalis Caspase-1 Gene is Expressed Throughout Development and Required for Female Fertility. Pest Manag Sci (2020) 76(12):4104–11. doi: 10.1002/ps.5966
43. Zhang Q, Ji S-Y, Busayavalasa K, Shao J, Yu C. Meiosis I Progression in Spermatogenesis Requires a Type of Testis-Specific 20S Core Proteasome. Nat Commun (2019) 10(1):3387. doi: 10.1038/s41467-019-11346-y
44. Gao X, Chen H, Liu J, Shen S, Wang Q, Clement TM, et al. The Regγ-Proteasome Regulates Spermatogenesis Partially by P53-PLZF Signaling. Stem Cell Rep (2019) 13(3):559–71. doi: 10.1016/j.stemcr.2019.07.010
45. Khor B, Bredemeyer AL, Huang C-Y, Turnbull IR, Evans R, Maggi LB, et al. Proteasome Activator PA200 is Required for Normal Spermatogenesis. Mol Cell Biol (2006) 26(8):2999–3007. doi: 10.1128/MCB.26.8.2999-3007.2006
46. Huang L, Haratake K, Miyahara H, Chiba T. Proteasome Activators, PA28γ and PA200, Play Indispensable Roles in Male Fertility. Sci Rep (2016) 6:23171. doi: 10.1038/srep23171
47. Huang Q, Liu H, Zeng J, Li W, Zhang S, Zhang L, et al. COP9 Signalosome Complex Subunit 5, an IFT20 Binding Partner, is Essential to Maintain Male Germ Cell Survival and Acrosome Biogenesis†. Biol Reprod (2020) 102(1):233–47. doi: 10.1093/biolre/ioz154
48. Bedard N, Yang Y, Gregory M, Cyr DG, Suzuki J, Yu X, et al. Mice Lacking the USP2 Deubiquitinating Enzyme Have Severe Male Subfertility Associated With Defects in Fertilization and Sperm Motility. Biol Reprod (2011) 85(3):594–604. doi: 10.1095/biolreprod.110.088542
49. Kishi K, Uchida A, Takase HM, Suzuki H, Kurohmaru M, Tsunekawa N, et al. Spermatogonial Deubiquitinase USP9X Is Essential for Proper Spermatogenesis in Mice. Reproduction (2017) 154(2):135–43. doi: 10.1530/REP-17-0184
50. Tian H, Huo Y, Zhang J, Ding S, Wang Z, Li H, et al. Disruption of Ubiquitin Specific Protease 26 Gene Causes Male Subfertility Associated With Spermatogenesis Defects in Mice†. Biol Reprod (2019) 100(4):1118–28. doi: 10.1093/biolre/ioy258
51. Sakai K, Ito C, Wakabayashi M, Kanzaki S, Ito T, Takada S, et al. Usp26 Mutation in Mice Leads to Defective Spermatogenesis Depending on Genetic Background. Sci Rep (2019) 9(1):13757. doi: 10.1038/s41598-019-50318-6
52. Kim JM, Parmar K, Huang M, Weinstock DM, Ruit CA, Kutok JL, et al. Inactivation of Murine Usp1 Results in Genomic Instability and a Fanconi Anemia Phenotype. Dev Cell (2009) 16(2):314–20. doi: 10.1016/j.devcel.2009.01.001
53. Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-Deficient Mice Exhibit Male Infertility. Dev Biol (2000) 218(2):248–58. doi: 10.1006/dbio.1999.9585
54. Wu H-Y, Wei P, Morgan JI. Role of Cytosolic Carboxypeptidase 5 in Neuronal Survival and Spermatogenesis. Sci Rep (2017) 7:41428. doi: 10.1038/srep41428
55. Giordano T, Gadadhar S, Bodakuntla S, Straub J, Leboucher S, Martinez G, et al. Loss of the Deglutamylase CCP5 Perturbs Multiple Steps of Spermatogenesis and Leads to Male Infertility. J Cell Sci (2019) 132(3):jcs226951. doi: 10.1242/jcs.226951
56. Carmell MA, Dokshin GA, Skaletsky H, Hu Y-C, van Wolfswinkel JC, Igarashi KJ, et al. A Widely Employed Germ Cell Marker is an Ancient Disordered Protein With Reproductive Functions in Diverse Eukaryotes. Elife (2016) 5:e19993. doi: 10.7554/eLife.19993
57. Oyama T, Sasagawa S, Takeda S, Hess RA, Lieberman PM, Cheng EH, et al. Cleavage of TFIIA by Taspase1 Activates TRF2-Specified Mammalian Male Germ Cell Programs. Dev Cell (2013) 27(2):188–200. doi: 10.1016/j.devcel.2013.09.025
58. Mizuno Y, Ninomiya Y, Nakachi Y, Iseki M, Iwasa H, Akita M, et al. Tysnd1 Deficiency in Mice Interferes With the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility. PloS Genet (2013) 9(2):e1003286. doi: 10.1371/journal.pgen.1003286
59. Kherraf Z-E, Christou-Kent M, Karaouzene T, Amiri-Yekta A, Martinez G, Vargas AS, et al. SPINK2 Deficiency Causes Infertility by Inducing Sperm Defects in Heterozygotes and Azoospermia in Homozygotes. EMBO Mol Med (2017) 9(8):1132–49. doi: 10.15252/emmm.201607461
60. Uhrin P, Dewerchin M, Hilpert M, Chrenek P, Schöfer C, Zechmeister-Machhart M, et al. Disruption of the Protein C Inhibitor Gene Results in Impaired Spermatogenesis and Male Infertility. J Clin Invest (2000) 106(12):1531–9. doi: 10.1172/JCI10768
61. Li S-WW, Arita M, Fertala A, Bao Y, Kopen GC, Långsjö TK, et al. Transgenic Mice With Inactive Alleles for Procollagen N-Proteinase (ADAMTS-2) Develop Fragile Skin and Male Sterility. Biochem J (2001) 355(2):271–8. doi: 10.1042/bj3550271
62. Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm From Mice Carrying a Targeted Mutation of the Acrosin Gene can Penetrate the Oocyte Zona Pellucida and Effect Fertilization. J Biol Chem (1994) 269(50):31845–9. doi: 10.1016/S0021-9258(18)31772-1
63. Adham IM, Nayernia K, Engel W. Spermatozoa Lacking Acrosin Protein Show Delayed Fertilization. Mol Reprod Dev (1997) 46(3):370–6. doi: 10.1002/(SICI)1098-2795(199703)46:3<370::AID-MRD16>3.0.CO;2-2
64. Gyamera-Acheampong C, Tantibhedhyangkul J, Weerachatyanukul W, Tadros H, Xu H, van de Loo J-W, et al. Sperm From Mice Genetically Deficient for the PCSK4 Proteinase Exhibit Accelerated Capacitation, Precocious Acrosome Reaction, Reduced Binding to Egg Zona Pellucida, and Impaired Fertilizing Ability. Biol Reprod (2006) 74(4):666–73. doi: 10.1095/biolreprod.105.046821
65. Mbikay M, Tadros H, Ishida N, Lerner CP, De Lamirande E, Chen A, et al. Impaired Fertility in Mice Deficient for the Testicular Germ-Cell Protease PC4. Proc Natl Acad Sci USA (1997) 94(13):6842–6. doi: 10.1073/pnas.94.13.6842
66. Larasati T, Noda T, Fujihara Y, Shimada K, Tobita T, Yu Z, et al. Tmprss12 is Required for Sperm Motility and Uterotubal Junction Migration in Mice†. Biol Reprod (2020) 103(2):254–63. doi: 10.1093/biolre/ioaa060
67. Shang X, Shen C, Liu J, Tang L, Zhang H, Wang Y, et al. Serine Protease PRSS55 is Crucial for Male Mouse Fertility via Affecting Sperm Migration and Sperm-Egg Binding. Cell Mol Life Sci (2018) 75(23):4371–84. doi: 10.1007/s00018-018-2878-9
68. Kobayashi K, Endo T, Matsumura T, Lu Y, Yu Z, Matzuk MM, et al. Prss55 But Not Prss51 is Required for Male Fertility in Mice†. Biol Reprod (2020) 103(2):223–34. doi: 10.1093/biolre/ioaa041
69. Zhang H, Li Y, Cui K, Chen X, Shang C, Min W, et al. Male Fertility in Mus Musculus Requires the Activity of TRYX5 in Sperm Migration Into the Oviduct. J Cell Physiol (2020) 235(9):6058–72. doi: 10.1002/jcp.29534
70. Shen C, Kuang Y, Liu J, Feng J, Chen X, Wu W, et al. Prss37 is Required for Male Fertility in the Mouse. Biol Reprod (2013) 88(5):123, 1–11. doi: 10.1095/biolreprod.112.107086
71. Esther CR, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice Lacking Angiotensin-Converting Enzyme Have Low Blood Pressure, Renal Pathology, and Reduced Male Fertility. Lab Invest (1996) 74(5):953–65.
72. Nishimura H, Kim E, Nakanishi T, Baba T. Possible Function of the ADAM1a/ADAM2 Fertilin Complex in the Appearance of ADAM3 on the Sperm Surface. J Biol Chem (2004) 279(33):34957–62. doi: 10.1074/jbc.M314249200
73. Cho C, Bunch DOD, Faure JE, Goulding EH, Eddy EM, Primakoff P, et al. Fertilization Defects in Sperm From Mice Lacking Fertilin β. Sci (80-) (1998) 281(5384):1857–9. doi: 10.1126/science.281.5384.1857
74. Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P. Analysis of Loss of Adhesive Function in Sperm Lacking Cyritestin or Fertilin Beta. Dev Biol (2001) 233(1):204–13. doi: 10.1006/dbio.2001.0166
75. Yamaguchi R, Muro Y, Isotani A, Tokuhiro K, Takumi K, Adham I, et al. Disruption of ADAM3 Impairs the Migration of Sperm Into Oviduct in Mouse. Biol Reprod (2009) 81(1):142–6. doi: 10.1095/biolreprod.108.074021
76. Voronina VA, Harris FM, Schmahl J, Galligan C, Oristian D, Zamfirova R, et al. Deletion of Adam6 in Mus Musculus Leads to Male Subfertility and Deficits in Sperm Ascent Into the Oviduct. Biol Reprod (2019) 100(3):686–96. doi: 10.1093/biolre/ioy210
77. Fujihara Y, Noda T, Kobayashi K, Oji A, Kobayashi S, Matsumura T, et al. Identification of Multiple Male Reproductive Tractspecific Proteins That Regulate Sperm Migration Through the Oviduct in Mice. Proc Natl Acad Sci USA (2019) 116(37):18498–506. doi: 10.1073/pnas.1908736116
78. Kiyozumi D, Noda T, Yamaguchi R, Tobita T, Matsumura T, Shimada K, et al. NELL2-Mediated Lumicrine Signaling Through OVCH2 is Required for Male Fertility. Sci (80-) (2020) 368(6495):1132–5. doi: 10.1126/science.aay5134
79. Carpentier M, Guillemette C, Bailey JL, Boileau G, Jeannotte L, DesGroseillers L, et al. Reduced Fertility in Male Mice Deficient in the Zinc Metallopeptidase NL1. Mol Cell Biol (2004) 24(10):4428–37. doi: 10.1128/MCB.24.10.4428-4437.2004
80. Netzel-Arnett S, Bugge TH, Hess RA, Carnes K, Stringer BW, Scarman AL, et al. The Glycosylphosphatidylinositol-Anchored Serine Protease PRSS21 (Testisin) Imparts Murine Epididymal Sperm Cell Maturation and Fertilizing Ability. Biol Reprod (2009) 81(5):921–32. doi: 10.1095/biolreprod.109.076273
81. Yamashita M, Honda A, Ogura A, Kashiwabara S, Fukami K, Baba T. Reduced Fertility of Mouse Epididymal Sperm Lacking Prss21/Tesp5 is Rescued by Sperm Exposure to Uterine Microenvironment. Genes Cells (2008) 13(10):1001–13. doi: 10.1111/j.1365-2443.2008.01222.x
82. Srinivasan S, Bunch DO, Feng Y, Rodriguiz RM, Li M, Ravenell RL, et al. Deficits in Reproduction and Pro-Gonadotropin-Releasing Hormone Processing in Male Cpefat Mice. Endocrinol (2004) 145(4):2023–34. doi: 10.1210/en.2003-1442
83. Zhu G-Z, Gupta S, Myles DG, Primakoff P. Testase 1 (ADAM 24) a Sperm Surface Metalloprotease is Required for Normal Fertility in Mice. Mol Reprod Dev (2009) 76(11):1106–14. doi: 10.1002/mrd.21076
84. Choi H, Han C, Jin S, Kwon JT, Kim J, Jeong J, et al. Reduced Fertility and Altered Epididymal and Sperm Integrity in Mice Lacking Adam7. Biol Reprod (2015) 93(3):70. doi: 10.1095/biolreprod.115.130252
85. Whelly S, Serobian G, Borchardt C, Powell J, Johnson S, Hakansson K, et al. Fertility Defects in Mice Expressing the L68Q Variant of Human Cystatin C: A Role for Amyloid in Male Infertility. J Biol Chem (2014) 289(11):7718–29. doi: 10.1074/jbc.M113.515759
86. Murer V, Spetz JF, Hengst U, Altrogge LM, de Agostini A, Monard D. Male Fertility Defects in Mice Lacking the Serine Protease Inhibitor Protease Nexin-1. Proc Natl Acad Sci USA (2001) 98(6):3029–33. doi: 10.1073/pnas.051630698
87. Folgueras AR, de Lara FM, Pendás AM, Garabaya C, Rodríguez F, Astudillo A, et al. Membrane-Bound Serine Protease Matriptase-2 (Tmprss6) Is an Essential Regulator of Iron Homeostasis. Blood (2008) 112(6):2539–45. doi: 10.1182/blood-2008-04-149773
88. Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, et al. Impaired Fertility in Female Mice Lacking Urinary Trypsin Inhibitor. Biochem Biophys Res Commun (2001) 281(5):1154–60. doi: 10.1006/bbrc.2001.4475
89. Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, et al. Defect in SHAP-Hyaluronan Complex Causes Severe Female Infertility. A Study by Inactivation of the Bikunin Gene in Mice. J Biol Chem (2001) 276(11):7693–6. doi: 10.1074/jbc.C000899200
90. Vidal R, Sammeta N, Garringer HJ, Sambamurti K, Miravalle L, Lamb BT, et al. The Psen1-L166P-Knock-in Mutation Leads to Amyloid Deposition in Human Wild-Type Amyloid Precursor Protein YAC Transgenic Mice. FASEB J (2012) 26(7):2899–910. doi: 10.1096/fj.12-205542
91. Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, et al. Adamts-1 is Essential for the Development and Function of the Urogenital System. Biol Reprod (2004) 70(4):1096–105. doi: 10.1095/biolreprod.103.023911
92. Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, et al. ADAMTS-1: A Metalloproteinase-Disintegrin Essential for Normal Growth, Fertility, and Organ Morphology and Function. J Clin Invest (2000) 105(10):1345–52. doi: 10.1172/JCI8635
93. Sheng X, Liu C, Yan G, Li G, Liu J, Yang Y, et al. The Mitochondrial Protease LONP1 Maintains Oocyte Development and Survival by Suppressing Nuclear Translocation of AIFM1 in Mammals. EBioMedicine (2022) 75:103790. doi: 10.1016/j.ebiom.2021.103790
94. Meng T-G, Hu M-W, Ma X-S, Huang L, Liang Q-X, Yuan Y, et al. Oocyte-Specific Deletion of Furin Leads to Female Infertility by Causing Early Secondary Follicle Arrest in Mice. Cell Death Dis (2017) 8(6):e2846. doi: 10.1038/cddis.2017.231
95. Nyegaard M, Overgaard MT, Su Y-Q, Hamilton AE, Kwintkiewicz J, Hsieh M, et al. Lack of Functional Pregnancy-Associated Plasma Protein-A (PAPPA) Compromises Mouse Ovarian Steroidogenesis and Female Fertility. Biol Reprod (2010) 82(6):1129–38. doi: 10.1095/biolreprod.109.079517
96. Burkart AD, Xiong B, Baibakov B, Jiménez-Movilla M, Dean J. Ovastacin, a Cortical Granule Protease, Cleaves ZP2 in the Zona Pellucida to Prevent Polyspermy. J Cell Biol (2012) 197(1):37–44. doi: 10.1083/jcb.201112094
97. Dietzel E, Wessling J, Floehr J, Schäfer C, Ensslen S, Denecke B, et al. Fetuin-B, a Liver-Derived Plasma Protein is Essential for Fertilization. Dev Cell (2013) 25(1):106–12. doi: 10.1016/j.devcel.2013.03.001
98. Dewerchin M, Hérault J-P, Wallays G, Petitou M, Schaeffer P, Millet L, et al. Life-Threatening Thrombosis in Mice With Targeted Arg48-To-Cys Mutation of the Heparin-Binding Domain of Antithrombin. Circ Res (2003) 93(11):1120–6. doi: 10.1161/01.RES.0000103634.69868.4F
99. Lustgarten Guahmich N, Farber G, Shafiei S, McNally D, Redmond D, Kallinos E, et al. Endothelial Deletion of ADAM10, A Key Regulator of Notch Signaling, Causes Impaired Decidualization and Reduced Fertility in Female Mice. Angiogenesis (2020) 23(3):443–58. doi: 10.1007/s10456-020-09723-z
100. Ataca D, Caikovski M, Piersigilli A, Moulin A, Benarafa C, Earp SE, et al. Adamts18 Deletion Results in Distinct Developmental Defects and Provides a Model for Congenital Disorders of Lens, Lung, and Female Reproductive Tract Development. Biol Open (2016) 5(11):1585–94. doi: 10.1242/bio.019711
101. Ploplis VA, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow EF, et al. Effects of Disruption of the Plasminogen Gene on Thrombosis, Growth, and Health in Mice. Circulation (1995) 92(9):2585–93. doi: 10.1161/01.CIR.92.9.2585
102. Lund LR, Bjørn SF, Sternlicht MD, Nielsen BS, Solberg H, Usher PA, et al. Lactational Competence and Involution of the Mouse Mammary Gland Require Plasminogen. Development (2000) 127(20):4481–92. doi: 10.1242/dev.127.20.4481
103. Nothnick WB. Reduction in Reproductive Lifespan of Tissue Inhibitor of Metalloproteinase 1 (TIMP-1)-Deficient Female Mice. Reproduction (2001) 122(6):923–7. doi: 10.1530/rep.0.1220923
104. Furuta M, Yano H, Zhou A, Rouillé Y, Holst JJ, Carroll R, et al. Defective Prohormone Processing and Altered Pancreatic Islet Morphology in Mice Lacking Active SPC2. Proc Natl Acad Sci USA (1997) 2494(13):6646–51. doi: 10.1073/pnas.94.13.6646
105. Huang X, Andreu-Vieyra CV, York JP, Hatcher R, Lu T, Matzuk MM, et al. Inhibitory Phosphorylation of Separase is Essential for Genome Stability and Viability of Murine Embryonic Germ Cells. PloS Biol (2008) 6(1):e15. doi: 10.1371/journal.pbio.0060015
106. Kudo NR, Wassmann K, Anger M, Schuh M, Wirth KG, Xu H, et al. Resolution of Chiasmata in Oocytes Requires Separase-Mediated Proteolysis. Cell (2006) 126(1):135–46. doi: 10.1016/j.cell.2006.05.033
107. Xu J, Wang M, Gao X, Hu B, Du Y, Zhou J, et al. Separase Phosphosite Mutation Leads to Genome Instability and Primordial Germ Cell Depletion During Oogenesis. PloS One (2011) 6(4):e18763. doi: 10.1371/journal.pone.0018763
108. Huang X, Andreu-Vieyra CV, Wang M, Cooney AJ, Matzuk MM, Zhang P. Preimplantation Mouse Embryos Depend on Inhibitory Phosphorylation of Separase to Prevent Chromosome Missegregation. Mol Cell Biol (2009) 29(6):1498–505. doi: 10.1128/MCB.01778-08
109. Mullen RJ, Eicher EM, Sidman RL. Purkinje Cell Degeneration, a New Neurological Mutation in the Mouse. Proc Natl Acad Sci (1976) 73(1):208–12. doi: 10.1073/pnas.73.1.208
110. Krulewski TF, Neumann PE, Gordon JW. Insertional Mutation in a Transgenic Mouse Allelic With Purkinje Cell Degeneration. Proc Natl Acad Sci USA (1989) 86(10):3709–12. doi: 10.1073/pnas.86.10.3709
111. Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, et al. Purkinje Cell Degeneration (Pcd) Phenotypes Caused by Mutations in the Axotomy-Induced Gene, Nna1. Sci (2002) 295(5561):1904–6. doi: 10.1126/science.1068912
112. Chakrabarti L, Neal JT, Miles M, Martinez RA, Smith AC, Sopher BL, et al. The Purkinje Cell Degeneration 5J Mutation is a Single Amino Acid Insertion That Destabilizes Nna1 Protein. Mamm Genome (2006) 17(2):103–10. doi: 10.1007/s00335-005-0096-x
113. Song N, Kim N, Xiao R, Choi H, Chun H-I, Kang M-H, et al. Lack of Cytosolic Carboxypeptidase 1 Leads to Subfertility Due to the Reduced Number of Antral Follicles in Pcd3j-/- Females. PloS One (2015) 10(10):e0139557. doi: 10.1371/journal.pone.0139557
114. Gispert S, Parganlija D, Klinkenberg M, Dröse S, Wittig I, Mittelbronn M, et al. Loss of Mitochondrial Peptidase Clpp Leads to Infertility, Hearing Loss Plus Growth Retardation via Accumulation of CLPX, mtDNA and Inflammatory Factors. Hum Mol Genet (2013) 22(24):4871–87. doi: 10.1093/hmg/ddt338
115. Osada T, Watanabe G, Kondo S, Toyoda M, Sakaki Y, Takeuchi T. Male Reproductive Defects Caused by Puromycin-Sensitive Aminopeptidase Deficiency in Mice. Mol Endocrinol (2001) 15(6):960–71. doi: 10.1210/mend.15.6.0643
116. Osada T, Watanabe G, Sakaki Y, Takeuchi T. Puromycin-Sensitive Aminopeptidase is Essential for the Maternal Recognition of Pregnancy in Mice. Mol Endocrinol (2001) 15(6):882–93. doi: 10.1210/mend.15.6.0644
117. Kumar TR, Wiseman AL, Kala G, Kala SV, Matzuk MM, Lieberman MW. Reproductive Defects in Gamma-Glutamyl Transpeptidase-Deficient Mice. Endocrinol (2000) 141(11):4270–7. doi: 10.1210/endo.141.11.7760
118. Lu B, Poirier C, Gaspar T, Gratzke C, Harrison W, Busija D, et al. A Mutation in the Inner Mitochondrial Membrane Peptidase 2-Like Gene (Immp2l) Affects Mitochondrial Function and Impairs Fertility in Mice. Biol Reprod (2008) 78(4):601–10. doi: 10.1095/biolreprod.107.065987
119. Horiuchi K, Kimura T, Miyamoto T, Miyamoto K, Akiyama H, Takaishi H, et al. Conditional Inactivation of TACE by a Sox9 Promoter Leads to Osteoporosis and Increased Granulopoiesis via Dysregulation of IL-17 and G-CSF. J Immunol (2009) 182(4):2093–101. doi: 10.4049/jimmunol.0802491
120. Hirose M, Honda A, Fulka H, Tamura-Nakano M, Matoba S, Tomishima T, et al. Acrosin is Essential for Sperm Penetration Through the Zona Pellucida in Hamsters. Proc Natl Acad Sci USA (2020) 117(5):2513–8. doi: 10.1073/pnas.1917595117
121. Abdul-Majeed S, Mell B, Nauli SM, Joe B. Cryptorchidism and Infertility in Rats With Targeted Disruption of the Adamts16 Locus. PloS One (2014) 9(7):e100967. doi: 10.1371/journal.pone.0100967
122. Sarila G, Bao T, Abeydeera SA, Li R, Mell B, Joe B, et al. Interplay Between Collagenase and Undescended Testes in Adamts16 Knockout Rats. J Pediatr Surg (2020) 55(9):1952–8. doi: 10.1016/j.jpedsurg.2019.12.019
123. Carter NJ, Roach ZA, Byrnes MM, Zhu Y. Adamts9 is Necessary for Ovarian Development in Zebrafish. Gen Comp Endocrinol (2019) 277(March):130–40. doi: 10.1016/j.ygcen.2019.04.003
124. Arafat M, Kleiman SE, AbuMadighem A, Zeadna A, Levitas E, Vardi IH, et al. Pathogenic Variations in Germ Cell Nuclear Acidic Peptidase (GCNA) are Associated With Human Male Infertility. Eur J Hum Genet (2021) 29(12):1781–8. doi: 10.1038/s41431-021-00946-2
125. Hardy JJ, Wyrwoll MJ, Mcfadden W, Malcher A, Rotte N, Pollock NC, et al. Variants in GCNA, X-Linked Germ-Cell Genome Integrity Gene, Identified in Men With Primary Spermatogenic Failure. Hum Genet (2021) 140(8):1169–82. doi: 10.1007/s00439-021-02287-y
126. Gao H, Zhang Y, Wang W, Zhao K, Liu C, Bai L, et al. Two Membrane-Anchored Aspartic Proteases Contribute to Pollen and Ovule Development. Plant Physiol (2017) 173(1):219–39. doi: 10.1104/pp.16.01719
127. Ge X, Dietrich C, Matsuno M, Li G, Berg H, Xia Y. An Arabidopsis Aspartic Protease Functions as an Anti-Cell-Death Component in Reproduction and Embryogenesis. EMBO Rep (2005) 6(3):282–8. doi: 10.1038/sj.embor.7400357
128. Phan HA, Iacuone S, Li SF, Parish RW. The MYB80 Transcription Factor is Required for Pollen Development and the Regulation of Tapetal Programmed Cell Death in Arabidopsis Thaliana. Plant Cell (2011) 23(6):2209–24. doi: 10.1105/tpc.110.082651
129. Zhang D, Liu D, Lv X, Wang Y, Xun Z, Liu Z, et al. The Cysteine Protease CEP1, a Key Executor Involved in Tapetal Programmed Cell Death, Regulates Pollen Development in Arabidopsis. Plant Cell (2014) 26(7):2939–61. doi: 10.1105/tpc.114.127282
130. Liu L, Jiang Y, Zhang X, Wang X, Wang Y, Han Y, et al. Two SUMO Proteases SUMO PROTEASE RELATED TO FERTILITY1 and 2 Are Required for Fertility in Arabidopsis. Plant Physiol (2017) 175(4):1703–19. doi: 10.1104/pp.17.00021
131. Huang J, Zhao X, Cheng K, Jiang Y, Ouyang Y, Xu C, et al. OsAP65, a Rice Aspartic Protease, is Essential for Male Fertility and Plays a Role in Pollen Germination and Pollen Tube Growth. J Exp Bot (2013) 64(11):3351–60. doi: 10.1093/jxb/ert173
132. Thaler CD, Miyata H, Haimo LT, Cardullo RA. Waveform Generation is Controlled by Phosphorylation and Swimming Direction Is Controlled by Ca2+ in Sperm From the Mosquito Culex Quinquefasciatus. Biol Reprod (2013) 89(6):135. doi: 10.1095/biolreprod.113.109488
133. Miyata H, Thaler CD, Haimo LT, Cardullo RA. Protease Activation and the Signal Transduction Pathway Regulating Motility in Sperm From the Water Strider Aquarius Remigis. Cytoskeleton (2012) 69(4):207–20. doi: 10.1002/cm.21012
134. Gosney R, Liau W-S, Lamunyon CW. A Novel Function for the Presenilin Family Member Spe-4: Inhibition of Spermatid Activation in Caenorhabditis Elegans. BMC Dev Biol (2008) 8:44. doi: 10.1186/1471-213X-8-44
135. Rodriguez A, Oliver H, Zou H, Chen P, Wang X, Abrams JM. Dark is a Drosophila Homologue of Apaf-1/CED-4 and Functions in an Evolutionarily Conserved Death Pathway. Nat Cell Biol (1999) 1(5):272–9. doi: 10.1038/12984
136. Sitnik JL, Francis C, Hens K, Huybrechts R, Wolfner MF, Callaerts P. Neprilysins: An Evolutionarily Conserved Family of Metalloproteases That Play Important Roles in Reproduction in Drosophila. Genetics (2014) 196(3):781–97. doi: 10.1534/genetics.113.160945
137. Tang X, Cao J, Zhang L, Huang Y, Zhang Q, Rong YS. Maternal Haploid, a Metalloprotease Enriched at the Largest Satellite Repeat and Essential for Genome Integrity in Drosophila Embryos. Genetics (2017) 206(4):1829–39. doi: 10.1534/genetics.117.200949
138. Qian M-X, Pang Y, Liu CH, Haratake K, Du B-Y, Ji D-Y, et al. Acetylation-Mediated Proteasomal Degradation of Core Histones During DNA Repair and Spermatogenesis. Cell (2013) 153(5):1012–24. doi: 10.1016/j.cell.2013.04.032
139. Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, et al. Essential Role of Plzf in Maintenance of Spermatogonial Stem Cells. Nat Genet (2004) 36(6):653–9. doi: 10.1038/ng1367
140. Jevtić P, Haakonsen DL, Rapé M. An E3 Ligase Guide to the Galaxy of Small-Molecule-Induced Protein Degradation. Cell Chem Biol (2021) 28(7):1000–13. doi: 10.1016/j.chembiol.2021.04.002
141. Volpi S, Bongiorni S, Fabbretti F, Wakimoto BT, Prantera G. Drosophila Rae1 is Required for Male Meiosis and Spermatogenesis. J Cell Sci (2013) 126(Pt 16):3541–51. doi: 10.1242/jcs.111328
142. Ottone C, Galasso A, Gemei M, Pisa V, Gigliotti S, Piccioni F, et al. Diminution of Eif4e Activity Suppresses Parkin Mutant Phenotypes. Gene (2011) 470(1–2):12–9. doi: 10.1016/j.gene.2010.09.003
143. Arama E, Bader M, Rieckhof GE, Steller H. A Ubiquitin Ligase Complex Regulates Caspase Activation During Sperm Differentiation in Drosophila. PloS Biol (2007) 5(10):e251. doi: 10.1371/journal.pbio.0050251
144. Luke-Glaser S, Pintard L, Tyers M, Peter M. The AAA-ATPase FIGL-1 Controls Mitotic Progression, and its Levels are Regulated by the CUL-3mel-26 E3 Ligase in the C. Elegans Germ Line. J Cell Sci (2007) 120(Pt 18):3179–87. doi: 10.1242/jcs.015883
145. Nayak S, Santiago FE, Jin H, Lin D, Schedl T, Kipreos ET. The Caenorhabditis Elegans Skp1-Related Gene Family: Diverse Functions in Cell Proliferation, Morphogenesis, and Meiosis. Curr Biol (2002) 12(4):277–87. doi: 10.1016/S0960-9822(02)00682-6
146. Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, et al. Proteasomal Regulation of the Hypoxic Response Modulates Aging in C. elegans Sci (2009) 324(5931):1196–8. doi: 10.1126/science.1173507
147. Lu L-Y, Yu X. CHFR is Important for the Survival of Male Premeiotic Germ Cells. Cell Cycle (2015) 14(21):3454–60. doi: 10.1080/15384101.2015.1093701
148. Yin Y, Lin C, Kim ST, Roig I, Chen H, Liu L, et al. The E3 Ubiquitin Ligase Cullin 4A Regulates Meiotic Progression in Mouse Spermatogenesis. Dev Biol (2011) 356(1):51–62. doi: 10.1016/j.ydbio.2011.05.661
149. Kopanja D, Roy N, Stoyanova T, Hess RA, Bagchi S, Raychaudhuri P. Cul4A is Essential for Spermatogenesis and Male Fertility. Dev Biol (2011) 352(2):278–87. doi: 10.1016/j.ydbio.2011.01.028
150. Yin Y, Liu L, Yang C, Lin C, Veith GM, Wang C, et al. Cell Autonomous and Nonautonomous Function of CUL4B in Mouse Spermatogenesis. J Biol Chem (2016) 291(13):6923–35. doi: 10.1074/jbc.M115.699660
151. Lin C-Y, Chen C-Y, Yu C-H, Yu I-S, Lin S-R, Wu J-T, et al. Human X-Linked Intellectual Disability Factor CUL4B Is Required for Post-Meiotic Sperm Development and Male Fertility. Sci Rep (2016) 6:20227. doi: 10.1038/srep20227
152. Ali A, Mistry BV, Ahmed HA, Abdulla R, Amer HA, Prince A, et al. Deletion of DDB1- and CUL4- Associated Factor-17 (Dcaf17) Gene Causes Spermatogenesis Defects and Male Infertility in Mice. Sci Rep (2018) 8(1):9202. doi: 10.1038/s41598-018-27379-0
153. Zhang X, Xia Z, Lv X, Li D, Liu M, Zhang R, et al. DDB1- and CUL4-Associated Factor 8 Plays a Critical Role in Spermatogenesis. Front Med (2021) 15(2):302–12. doi: 10.1007/s11684-021-0851-8
154. Huang G, Kaufman AJ, Ryan RJH, Romin Y, Huryn L, Bains S, et al. Mouse DCUN1D1 (SCCRO) is Required for Spermatogenetic Individualization. PloS One (2019) 14(1):e0209995. doi: 10.1371/journal.pone.0209995
155. Zhang H, Chen F, Dong H, Xie M, Zhang H, Chen Y, et al. Loss of Fbxw7 in Sertoli Cells Impairs Testis Development and Causes Infertility in Mice†. Biol Reprod (2020) 102(4):963–74. doi: 10.1093/biolre/ioz230
156. Fok KL, Bose R, Sheng K, Chang C-W, Katz-Egorov M, Culty M, et al. Huwe1 Regulates the Establishment and Maintenance of Spermatogonia by Suppressing DNA Damage Response. Endocrinol (2017) 158(11):4000–16. doi: 10.1210/en.2017-00396
157. Bose R, Sheng K, Moawad AR, Manku G, O’Flaherty C, Taketo T, et al. Ubiquitin Ligase Huwe1 Modulates Spermatogenesis by Regulating Spermatogonial Differentiation and Entry Into Meiosis. Sci Rep (2017) 7(1):17759. doi: 10.1038/s41598-017-17902-0
158. Eisa AA, Bang S, Crawford KJ, Murphy EM, Feng WW, Dey S, et al. X-Linked Huwe1 Is Essential for Oocyte Maturation and Preimplantation Embryo Development. iSci (2020) 23(9):101523. doi: 10.1016/j.isci.2020.101523
159. Haraguchi H, Hirota Y, Saito-Fujita T, Tanaka T, Shimizu-Hirota R, Harada M, et al. Mdm2-P53-SF1 Pathway in Ovarian Granulosa Cells Directs Ovulation and Fertilization by Conditioning Oocyte Quality. FASEB J (2019) 33(2):2610–20. doi: 10.1096/fj.201801401R
160. Zhang C-X, Zhang Q, Xie Y-Y, He X-Y, Xiang C, Hou X-S, et al. Mouse Double Minute 2 Actively Suppresses P53 Activity in Oocytes During Mouse Folliculogenesis. Am J Pathol (2017) 187(2):339–51. doi: 10.1016/j.ajpath.2016.09.023
161. Fouchécourt S, Livera G, Messiaen S, Fumel B, Parent A-S, Marine J-C, et al. Apoptosis of Sertoli Cells After Conditional Ablation of Murine Double Minute 2 (Mdm2) Gene is P53-Dependent and Results in Male Sterility. Cell Death Differ (2016) 23(3):521–30. doi: 10.1038/cdd.2015.120
162. Cheng D, Xiong C, Li J, Sui C, Wang S, Li H, et al. The Effect of Mahogunin Gene Mutant on Reproduction in Male Mice: A New Sight for Infertility? Andrologia (2014) 46(2):98–105. doi: 10.1111/and.12050
163. Wang X, Kang J-Y, Wei L, Yang X, Sun H, Yang S, et al. PHF7 is a Novel Histone H2A E3 Ligase Prior to Histone-to-Protamine Exchange During Spermiogenesis. Development (2019) 146(13):dev175547. doi: 10.1242/dev.175547
164. Xu Z, Song Z, Li G, Tu H, Liu W, Liu Y, et al. H2B Ubiquitination Regulates Meiotic Recombination by Promoting Chromatin Relaxation. Nucleic Acids Res (2016) 44(20):9681–97. doi: 10.1093/nar/gkw652
165. Melnick AF, Gao Y, Liu J, Ding D, Predom A, Kelly C, et al. RNF216 is Essential for Spermatogenesis and Male Fertility†. Biol Reprod (2019) 100(5):1132–4. doi: 10.1093/biolre/ioz006
166. Guo Y, Song Y, Guo Z, Hu M, Liu B, Duan H, et al. Function of RAD6B and RNF8 in Spermatogenesis. Cell Cycle (2018) 17(2):162–73. doi: 10.1080/15384101.2017.1361066
167. Lu L-Y, Wu J, Ye L, Gavrilina GB, Saunders TL, Yu X. RNF8-Dependent Histone Modifications Regulate Nucleosome Removal During Spermatogenesis. Dev Cell (2010) 18(3):371–84. doi: 10.1016/j.devcel.2010.01.010
168. Dickins RA, Frew IJ, House CM, O’Bryan MK, Holloway AJ, Haviv I, et al. The Ubiquitin Ligase Component Siah1a is Required for Completion of Meiosis I in Male Mice. Mol Cell Biol (2002) 22(7):2294–303. doi: 10.1128/MCB.22.7.2294-2303.2002
169. Hai L, Szwarc MM, He B, Lonard DM, Kommagani R, DeMayo FJ, et al. Uterine Function in the Mouse Requires Speckle-Type Poz Protein. Biol Reprod (2018) 98(6):856–69. doi: 10.1093/biolre/ioy060
170. Wei J, Chen L, Li F, Yuan Y, Wang Y, Xia W, et al. HRD1-ERAD Controls Production of the Hepatokine FGF21 Through CREBH Polyubiquitination. EMBO J (2018) 37(22):e98942. doi: 10.15252/embj.201898942
171. Kettunen KM, Karikoski R, Hämäläinen RH, Toivonen TT, Antonenkov VD, Kulesskaya N, et al. Trim37-Deficient Mice Recapitulate Several Features of the Multi-Organ Disorder Mulibrey Nanism. Biol Open (2016) 5(5):584–95. doi: 10.1242/bio.016246
172. Torres-Fernández LA, Emich J, Port Y, Mitschka S, Wöste M, Schneider S, et al. TRIM71 Deficiency Causes Germ Cell Loss During Mouse Embryogenesis and Is Associated With Human Male Infertility. Front Cell Dev Biol (2021) 9:658966. doi: 10.3389/fcell.2021.658966
173. Kwon YT, Xia Z, An JY, Tasaki T, Davydov IV, Seo JW, et al. Female Lethality and Apoptosis of Spermatocytes in Mice Lacking the UBR2 Ubiquitin Ligase of the N-End Rule Pathway. Mol Cell Biol (2003) 23(22):8255–71. doi: 10.1128/MCB.23.22.8255-8271.2003
174. Pan H, Jiang N, Sun S, Jiang H, Xu J, Jiang X, et al. UHRF1-Repressed 5’-Hydroxymethylcytosine is Essential for the Male Meiotic Prophase I. Cell Death Dis (2020) 11(2):142. doi: 10.1038/s41419-020-2333-3
175. Cao Y, Li M, Liu F, Ni X, Wang S, Zhang H, et al. Deletion of Maternal UHRF1 Severely Reduces Mouse Oocyte Quality and Causes Developmental Defects in Preimplantation Embryos. FASEB J (2019) 33(7):8294–305. doi: 10.1096/fj.201801696RRRR
176. Rodriguez A, Briley SM, Patton BK, Tripurani SK, Rajapakshe K, Coarfa C, et al. Loss of the E2 SUMO-Conjugating Enzyme Ube2i in Oocytes During Ovarian Folliculogenesis Causes Infertility in Mice. Development (2019) 146(23):dev176701. doi: 10.1242/dev.176701
177. Koenig P-A, Nicholls PK, Schmidt FI, Hagiwara M, Maruyama T, Frydman GH, et al. The E2 Ubiquitin-Conjugating Enzyme UBE2J1 is Required for Spermiogenesis in Mice. J Biol Chem (2014) 289(50):34490–502. doi: 10.1074/jbc.M114.604132
178. Grzmil P, Altmann ME, Adham IM, Engel U, Jarry H, Schweyer S, et al. Embryo Implantation Failure and Other Reproductive Defects in Ube2q1-Deficient Female Mice. Reproduction (2013) 145(1):45–56. doi: 10.1530/REP-12-0054
179. Jiang X, Wang X, Zhang X, Xiao Z, Zhang C, Liu X, et al. A Homozygous RNF220 Mutation Leads to Male Infertility With Small-Headed Sperm. Gene (2019) 688:13–8. doi: 10.1016/j.gene.2018.11.074
180. Wang H, Lu Y, Jiang T, Berg H, Li C, Xia Y. The Arabidopsis U-Box/ARM Repeat E3 Ligase AtPUB4 Influences Growth and Degeneration of Tapetal Cells, and its Mutation Leads to Conditional Male Sterility. Plant J (2013) 74(3):511–23. doi: 10.1111/tpj.12146
181. Byzova MV, Franken J, Aarts MG, de Almeida-Engler J, Engler G, Mariani C, et al. Arabidopsis STERILE APETALA, a Multifunctional Gene Regulating Inflorescence, Flower, and Ovule Development. Genes Dev (1999) 13(8):1002–14. doi: 10.1101/gad.13.8.1002
182. Ling Y, Zhang C, Chen T, Hao H, Liu P, Bressan RA, et al. Mutation in SUMO E3 Ligase, SIZ1, Disrupts the Mature Female Gametophyte in Arabidopsis. PloS One (2012) 7(1):e29470. doi: 10.1371/journal.pone.0029470
183. Liu M, Shi S, Zhang S, Xu P, Lai J, Liu Y, et al. SUMO E3 Ligase AtMMS21 is Required for Normal Meiosis and Gametophyte Development in Arabidopsis. BMC Plant Biol (2014) 14:153. doi: 10.1186/1471-2229-14-153
184. Thangasamy S, Guo C-L, Chuang M-H, Lai M-H, Chen J, Jauh G-Y. Rice SIZ1, a SUMO E3 Ligase, Controls Spikelet Fertility Through Regulation of Anther Dehiscence. New Phytol (2011) 189(3):869–82. doi: 10.1111/j.1469-8137.2010.03538.x
185. Cayli S, Ocakli S, Erdemir F, Tas U, Aslan H, Yener T, et al. Developmental Expression of P97/VCP (Valosin-Containing Protein) and Jab1/CSN5 in the Rat Testis and Epididymis. Reprod Biol Endocrinol (2011) 9:117. doi: 10.1186/1477-7827-9-117
186. Wang PJ, McCarrey JR, Yang F, Page DC. An Abundance of X-Linked Genes Expressed in Spermatogonia. Nat Genet (2001) 27(4):422–6. doi: 10.1038/86927
187. Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K. Cleavage of Cohesin by the CD Clan Protease Separin Triggers Anaphase in Yeast. Cell (2000) 103(3):375–86. doi: 10.1016/S0092-8674(00)00130-6
188. Buonomo SB, Clyne RK, Fuchs J, Loidl J, Uhlmann F, Nasmyth K. Disjunction of Homologous Chromosomes in Meiosis I Depends on Proteolytic Cleavage of the Meiotic Cohesin Rec8 by Separin. Cell (2000) 103(3):387–98. doi: 10.1016/S0092-8674(00)00131-8
189. Waizenegger IC, Hauf S, Meinke A, Peters JM. Two Distinct Pathways Remove Mammalian Cohesin From Chromosome Arms in Prophase and From Centromeres in Anaphase. Cell (2000) 103(3):399–410. doi: 10.1016/S0092-8674(00)00132-X
190. Chen DY, Lee Y, Van Tine BA, Searleman AC, Westergard TD, Liu H, et al. A Pharmacologic Inhibitor of the Protease Taspase1 Effectively Inhibits Breast and Brain Tumor Growth. Cancer Res (2012) 72(3):736–46. doi: 10.1158/0008-5472.CAN-11-2584
191. Lee B, Park I, Jin S, Choi H, Kwon JT, Kim J, et al. Impaired Spermatogenesis and Fertility in Mice Carrying a Mutation in the Spink2 Gene Expressed Predominantly in Testes. J Biol Chem (2011) 286(33):29108–17. doi: 10.1074/jbc.M111.244905
192. Tardif S, Guyonnet B, Cormier N, Cornwall GA. Alteration in the Processing of the ACRBP/sp32 Protein and Sperm Head/Acrosome Malformations in Proprotein Convertase 4 (PCSK4) Null Mice. Mol Hum Reprod (2012) 18(6):298–307. doi: 10.1093/molehr/gas009
193. Fujihara Y, Miyata H, Ikawa M. Factors Controlling Sperm Migration Through the Oviduct Revealed by Gene-Modified Mouse Models. Exp Anim (2018) 67(2):91–104. doi: 10.1538/expanim.17-0153
194. Linder B, Bammer S, Heinlein UA. Delayed Translation and Posttranslational Processing of Cyritestin, an Integral Transmembrane Protein of the Mouse Acrosome. Exp Cell Res (1995) 221(1):66–72. doi: 10.1006/excr.1995.1353
195. Blobel CP, Myles DG, Primakoff P, White JM. Proteolytic Processing of a Protein Involved in Sperm-Egg Fusion Correlates With Acquisition of Fertilization Competence. J Cell Biol (1990) 111(1):69–78. doi: 10.1083/jcb.111.1.69
196. Han C, Choi E, Park I, Lee B, Jin S, Kim DH, et al. Comprehensive Analysis of Reproductive ADAMs: Relationship of ADAM4 and ADAM6 With an ADAM Complex Required for Fertilization in Mice. Biol Reprod (2009) 80(5):1001–8. doi: 10.1095/biolreprod.108.073700
197. Tokuhiro K, Ikawa M, Benham AM, Okabe M. Protein Disulfide Isomerase Homolog PDILT is Required for Quality Control of Sperm Membrane Protein ADAM3 and Male Fertility. PNAS (2012) 109(10):3850–5. doi: 10.1073/pnas.1117963109
198. Xiong W, Shen C, Li C, Zhang X, Ge H, Tang L, et al. Dissecting the PRSS37 Interactome and Potential Mechanisms Leading to ADAM3 Loss in PRSS37-Null Sperm. J Cell Sci (2021) 134(10):jcs258426. doi: 10.1242/jcs.258426
199. Cornwall GA, Hsia N. ADAM7, A Member of the ADAM (a Disintegrin and Metalloprotease) Gene Family is Specifically Expressed in the Mouse Anterior Pituitary and Epididymis. Endocrinol (1997) 138(10):4262–72. doi: 10.1210/endo.138.10.5468
200. Whelly S, Johnson S, Powell J, Borchardt C, Hastert MC, Cornwall GA. Nonpathological Extracellular Amyloid is Present During Normal Epididymal Sperm Maturation. PloS One (2012) 7(5):e36394. doi: 10.1371/journal.pone.0036394
201. Tonai S, Kawabata A, Nakanishi T, Lee JY, Okamoto A, Shimada M, et al. Iron Deficiency Induces Female Infertile in Order to Failure of Follicular Development in Mice. J Reprod Dev (2020) 66(5):475–83. doi: 10.1262/jrd.2020-074
202. Ny A, Leonardsson G, Hägglund AC, Hägglöf P, Ploplis VA, Carmeliet P, et al. Ovulation in Plasminogen-Deficient Mice. Endocrinol (1999) 140(11):5030–5. doi: 10.1210/endo.140.11.7113
203. Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. (2000) 57(Suppl 1):S3–7. doi: 10.1093/ajhp/57.suppl_1.S3
204. Straub A, Roehrig S, Hillisch A. Oral, Direct Thrombin and Factor Xa Inhibitors: The Replacement for Warfarin, Leeches, and Pig Intestines? Angew Chem Int Ed Engl (2011) 50(20):4574–90. doi: 10.1002/anie.201004575
205. Nutescu EA, Wittkowsky AK. Direct Thrombin Inhibitors for Anticoagulation. Ann Pharmacother (2004) 38(1):99–109. doi: 10.1345/aph.1D066
206. Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 Protease Inhibitors. A Rev Clin JAMA (1997) 277(2):145–53. doi: 10.1001/jama.1997.03540260059037
207. Agbowuro AA, Huston WM, Gamble AB, Tyndall JDA. Proteases and Protease Inhibitors in Infectious Diseases. Med Res Rev (2018) 38(4):1295–331. doi: 10.1002/med.21475
Keywords: protease, fertilization, proteolysis, protease inhibitor, pseudoprotease, gene-modified animal models, ubiquitin-proteasome system, sperm maturation
Citation: Kiyozumi D and Ikawa M (2022) Proteolysis in Reproduction: Lessons From Gene-Modified Organism Studies. Front. Endocrinol. 13:876370. doi: 10.3389/fendo.2022.876370
Received: 15 February 2022; Accepted: 28 March 2022;
Published: 04 May 2022.
Edited by:
Erwin Goldberg, Northwestern University, United StatesReviewed by:
Toshinobu Tokumoto, Shizuoka University, JapanMartine Culty, University of Southern California, United States
Copyright © 2022 Kiyozumi and Ikawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daiji Kiyozumi, a2l5b3p1bWlAYmlrZW4ub3Nha2EtdS5hYy5qcA==; Masahito Ikawa, aWthd2FAYmlrZW4ub3Nha2EtdS5hYy5qcA==
 Daiji Kiyozumi
Daiji Kiyozumi Masahito Ikawa
Masahito Ikawa