- Radiation, Chemical and Environmental Hazards (RCE), Department of Toxicology, UK Health Security Agency (UKHSA), Harwell Science and Innovation Campus, Chilton, United Kingdom
The most prevalent liver disease in humans is non-alcoholic fatty liver disease, characterised by excessive hepatic fat accumulation, or steatosis. The western diet and a sedentary lifestyle are considered to be major influences, but chemical exposure may also play a role. Suspected environmental chemicals of concern include pesticides, plasticizers, metals, and perfluorinated compounds. Here we present a detailed literature analysis of chemicals that may (or may not) be implicated in lipid accumulation in the liver, to provide a basis for developing and optimizing human steatosis-relevant in vitro test methods. Independently collated and reviewed reference and proficiency chemicals are needed to assist in the test method development where an assay is intended to ultimately be taken forward for OECD Test Guideline development purposes. The selection criteria and considerations required for acceptance of proficiency chemical selection for OECD Test Guideline development. (i.e., structural diversity, range of activity including negatives, relevant chemical sectors, global restrictions, etc.) is described herein. Of 160 chemicals initially screened for inclusion, 36 were prioritized for detailed review. Based on the selection criteria and a weight-of-evidence basis, 18 chemicals (9 steatosis inducers, 9 negatives), including some environmental chemicals of concern, were ranked as high priority chemicals to assist in vitro human steatosis test method optimisation and proficiency testing, and inform potential subsequent test method (pre-)validation.
1 Introduction
The global increase in metabolic disorders is not only due to diet, lifestyle and genetic factors; that environmental factors also play a role is being increasingly acknowledged. Exposure to endocrine disrupting chemicals (EDCs) which disrupt metabolic functions – chemicals collectively referred to as ‘metabolic disrupting chemicals’ (MDCs) – is an environmental risk factor of concern that requires investigation to support public health protection via regulatory and policy action.
Within European chemical regulations, criteria to identify EDCs have been proposed that require information on a chemicals’ endocrine mode of action (MoA) and related adverse effects relevant for human health (1). This involves the screening and testing of EDCs according to the EU Test Methods Regulation, which mainly incorporates internationally accepted test methods developed under the Organisation for Economic Cooperation and Development (OECD). Currently, test methods to identify EDCs are based upon well-studied endocrine pathways in the oestrogen, androgen, steroidogenesis, and thyroid systems, although the need for additional endocrine modality test methods, including metabolic disruption, was recognised by OECD member countries a decade ago (2). The need for test development in the field of metabolic disorders has also been highlighted in expert surveys on identification of gaps in available test methods for EDC evaluation (3, 4) and in work on temporal aspects of EDCs (5). Currently, introduction and definition of hazard classes for the classification, labeling, and packaging of EDCs is being discussed in the EU1, particularly in relation to the established oestrogen, androgen, steroidogenesis and thyroid modalities, but for other endocrine modalities, such as MDCs, the potential test method tools are insufficiently characterized for regulatory purposes as yet.
MDCs can be endogenous, natural and anthropogenic chemicals that have the ability to promote metabolic changes that can ultimately result in obesity, diabetes and non-alcoholic fatty liver disease in humans (6). Whilst there are no standardised test methods adopted as regulatory chemical hazard assessment tools as yet, work is underway internationally, including as part of the EU-funded Horizon 2020 GOLIATH project (7) (https://beatinggoliath.eu/; https://cordis.europa.eu/project/id/825489). GOLIATH is developing and pre-validating in vitro test methods for chemical hazard testing in relation to metabolic disruption, including steatosis as a key event (KE).
Non-alcoholic fatty liver disease (NAFLD) is ‘characterised by excessive hepatic fat accumulation, defined by the presence of steatosis in >5% of hepatocytes’ (8). It is the most prevalent liver disease in humans and linked to sedentary lifestyle, western diet, but also exposure to chemicals. It can progress from (reversible) steatosis to steatohepatitis, fibrosis, or cirrhosis and cancer (Figure 1) (9).
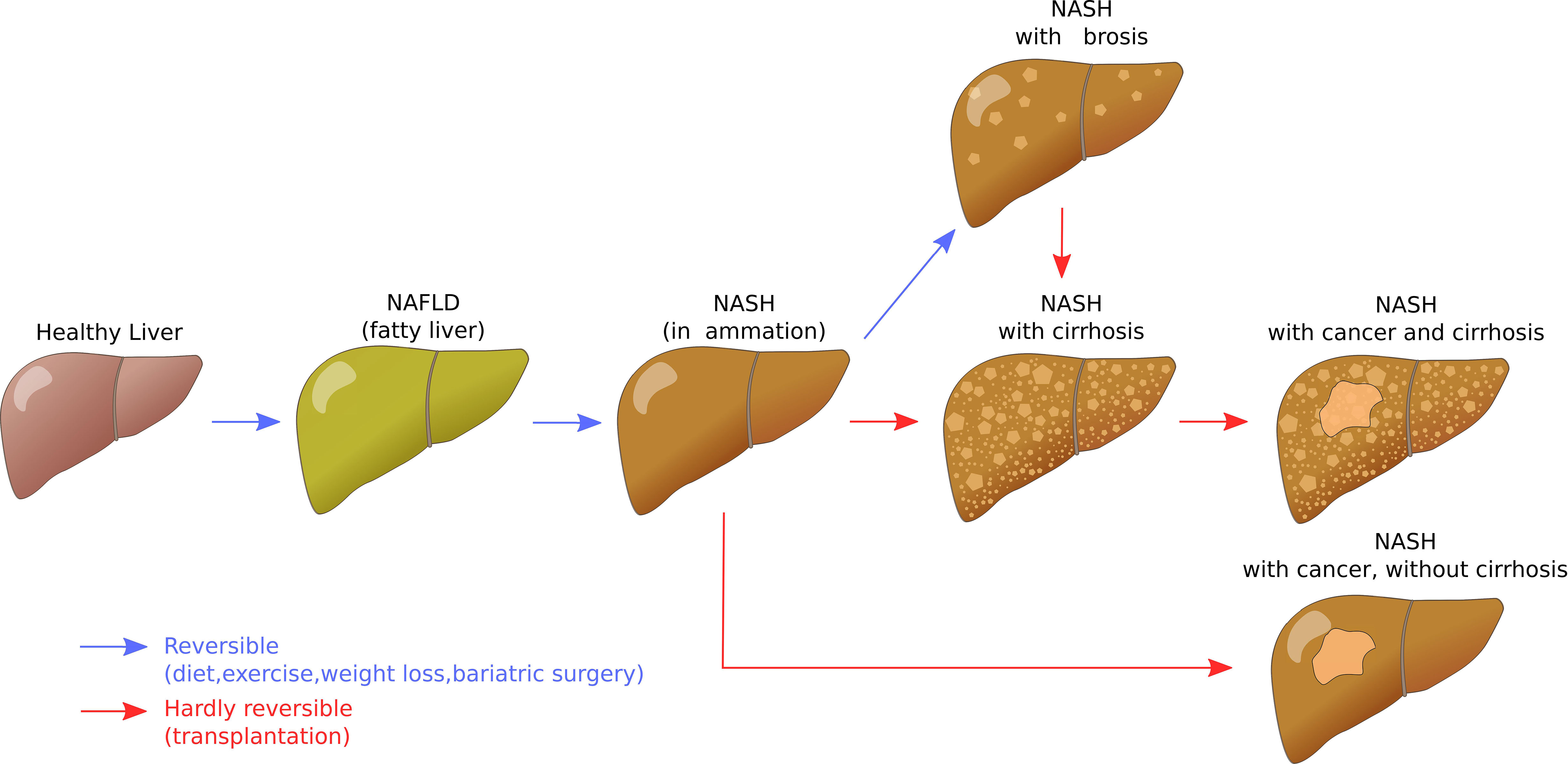
Figure 1 Liver disease progression for non-alcoholic fatty liver disease. Author: Signimu Figure version from 7 October 2019, 20:06, accessed on 4 January 2023. https://web.archive.org/web/20230104123719/; https://commons.wikimedia.org/wiki/File:NAFLD_liver_progression.svg (Wikimedia Commons, CC BY-SA 3.0).
Hepatic steatosis is well-known in the fields of pharmacology, medicine, and nutrition with respect to the development of NAFLD, but the significance of chemical perturbation leading to steatosis and down-stream adverse events, in response to xenobiotics is less well understood, and has been identified as a key gap in the safety assessment of chemicals at international and European levels (2, 7). The fact that it is a disease characterised by the ‘presence of steatosis in >5% of hepatocytes’, means that the occurrence and progression is measurable in cells such as hepatocytes, and in vitro tests can be developed on this basis. Appropriate tests need to be developed and shown to be relevant, reliable, and reproducible, before they can reasonably be expected to be utilised in Integrated Approaches to Testing and Assessment (IATA), and ultimately be suitable tool kits to be incorporated into chemical legislation.
Assessment of chemical hazards towards the endpoint of steatosis, and informing upon the adverse human health endpoint of NAFLD for chemical regulatory purposes, are currently based mainly upon rodent in vivo liver histochemistry and blood biomarkers for liver function. These parameters are reported when conducting OECD in vivo Test Guidelines (TGs) for acute, sub-chronic, and chronic studies, as for example under the European Union’s Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH, (10)), and Plant Protection Product legislation (11). However, as steatosis is of high interest to nutrition research and the pharmacological industry, many potential candidate in vitro models that inform upon both molecular mechanisms and biomarkers, and also on the more apical lipid accumulation in hepatocytes relevant towards human hepatic steatosis, are being developed. They have also been mapped to a varying extent in (preliminary) Adverse Outcome Pathway models (12–14) and are also indicated in Figure 2.
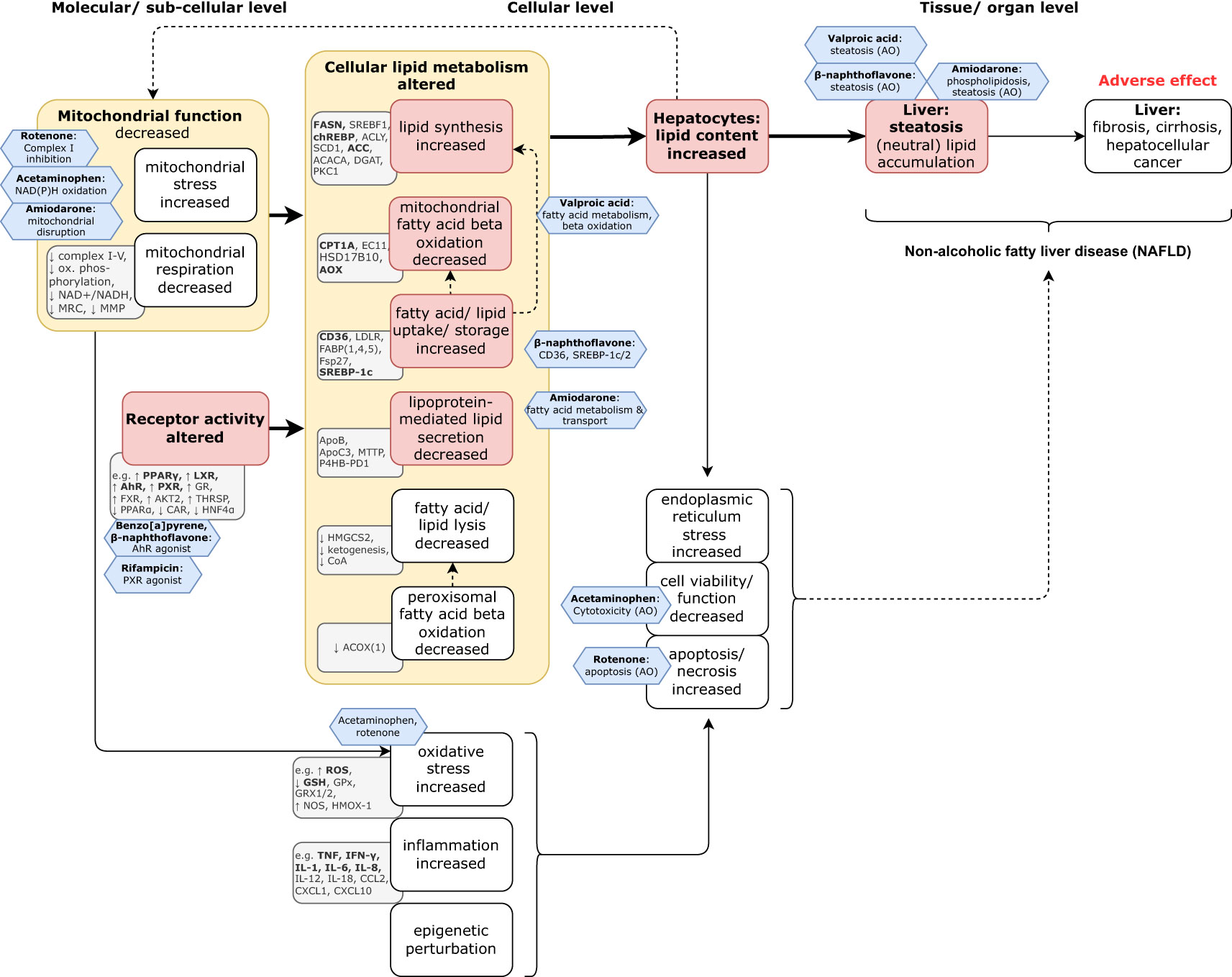
Figure 2 Model of the natural history of the progression towards steatosis. Steatosis is the first adaptive response to liver stress, and first indicator of NAFLD, but is (potentially) reversible. However, if left untreated, it can progress to more severe and progressively less reversible (maladaptive) disease states of fibrosis, cirrhosis, and ultimately, hepatocellular cancer. Key: On the basis of the mechanistic literature reviewed herein (including the Supplementary Table 1), the grey rectangles identify indicative biomarkers (unless otherwise indicated, the upwards or downwards arrows indicate the trend of the biomarker that corresponds to that of the associated key event); the red boxes indicate pivotal key events and the yellow boxes specify the Mode of Action. The blue hexagons capture the prototypical chemical effects/ stressors (modified from (15, 16), and with data from Supplementary Table 2).
For regulatory purposes, test methods need to demonstrate that they are able to address the intended chemical applicability domain and to classify chemicals correctly (17). To date, safety assessment of chemicals in the European Union still heavily relies on animal testing. However, substantial efforts over the last 20 years to develop, validate, and demonstrate regulatory acceptability of New Approach Methodologies (NAMs) are evident (18, 19) in an international consensus-driven approach to reduce and replace animal testing, whilst increasing human health protection relevance when assessing chemical hazard (20–23). In vitro test method tools can provide mechanistic understanding of apical adverse outcomes earlier, thereby contributing to avoiding redundant in vivo studies (19).
The objectives of the study presented here are to a) propose a list of reference and proficiency chemicals to facilitate the development, refinement, and (pre-)validation of human-health relevant steatosis in vitro test methods, and b) to prepare a chemical evidence basis for the integration of suitable steatosis in vitro test method(s), as part of a battery of suitable tests for an IATA for metabolic disruption (7, 24, 25).
Here we present a detailed analysis of chemicals, including putative negatives, as a basis for developing and optimizing human steatosis-relevant in vitro test methods with a measurable endpoint of hepatic lipid accumulation, for regulatory applications.
2 Methods
Critical considerations for the selection of reference and proficiency chemicals for test method development and validation were reported in detail earlier (17). Briefly, the chemicals selected need to be structurally diverse and representative of the chemical universe that is intended to be tested, for the respective endpoint. This includes chemicals from different industrial sectors, such as pharmaceuticals, industrial manufacturing chemicals, food additive and packaging materials, industrial by-products and contaminants, pesticides, but also (endogenous) metabolites, and naturally occurring chemicals. Chemicals subject to international agreements limiting their use and transport (e.g., the Stockholm Convention on Persistent Organic Pollutants) and undefined chemical mixtures, including isomeric mixtures, were avoided where possible, such that one can have increased confidence that the effects being reported are due to the specific chemical being investigated, and are not confounded by the interactions of other/additional chemical(s). Chemicals of limited availability or excessive cost (e.g., experimental pharmaceutical chemicals) were not prioritised in the selection, despite such chemicals often exerting specific molecular effects that are of scientific interest and can target e.g., specific nuclear receptors and signalling pathways. This is because for the intended OECD test method guideline purposes, chemicals need to be reasonably available globally in the foreseeable future, for ease of access by TG end-users. Finally, proficiency chemicals proposed for method optimisation and (pre-)validation testing should cover a range of activity (weak, moderate, strong activity), including chemicals that are negative on the respective endpoint (i.e., lipid accumulation in hepatocytes, steatosis). Ideally, the share of negative chemicals should be 25-50%, in order to reliably inform as to whether a test method can discriminate between positive and negative chemicals, and ultimately be suitable to predict the chemical activity and ideally potency in relation to the target endpoint.
2.1 Data sources and critical evaluation
A schematic workflow on the identification and prioritisation of chemicals for detailed steatosis-specific literature review is depicted in Figure 3. To retrieve relevant publications, the Scopus database (https://www.scopus.com/) was initially queried to obtain publications relevant towards (human) hepatic steatosis (see Supplementary Material 1, “Broad Search”). The retrieved publications were screened based on title and abstract to identify chemicals that were tested in relation to hepatic steatosis in reverse chronological order (i.e., starting from the most recent publications), with application of the above-mentioned inclusion/exclusion criteria (e.g., exclusion of studies reporting on experiments with undefined chemical mixtures/undefined extracts, co-exposure experiments, etc.). Upon identification of 160 chemicals (data not shown), this selection underwent expert review to identify focus chemicals for chemical-specific database queries in Scopus; 36 chemicals were identified for chemical-specific searches.
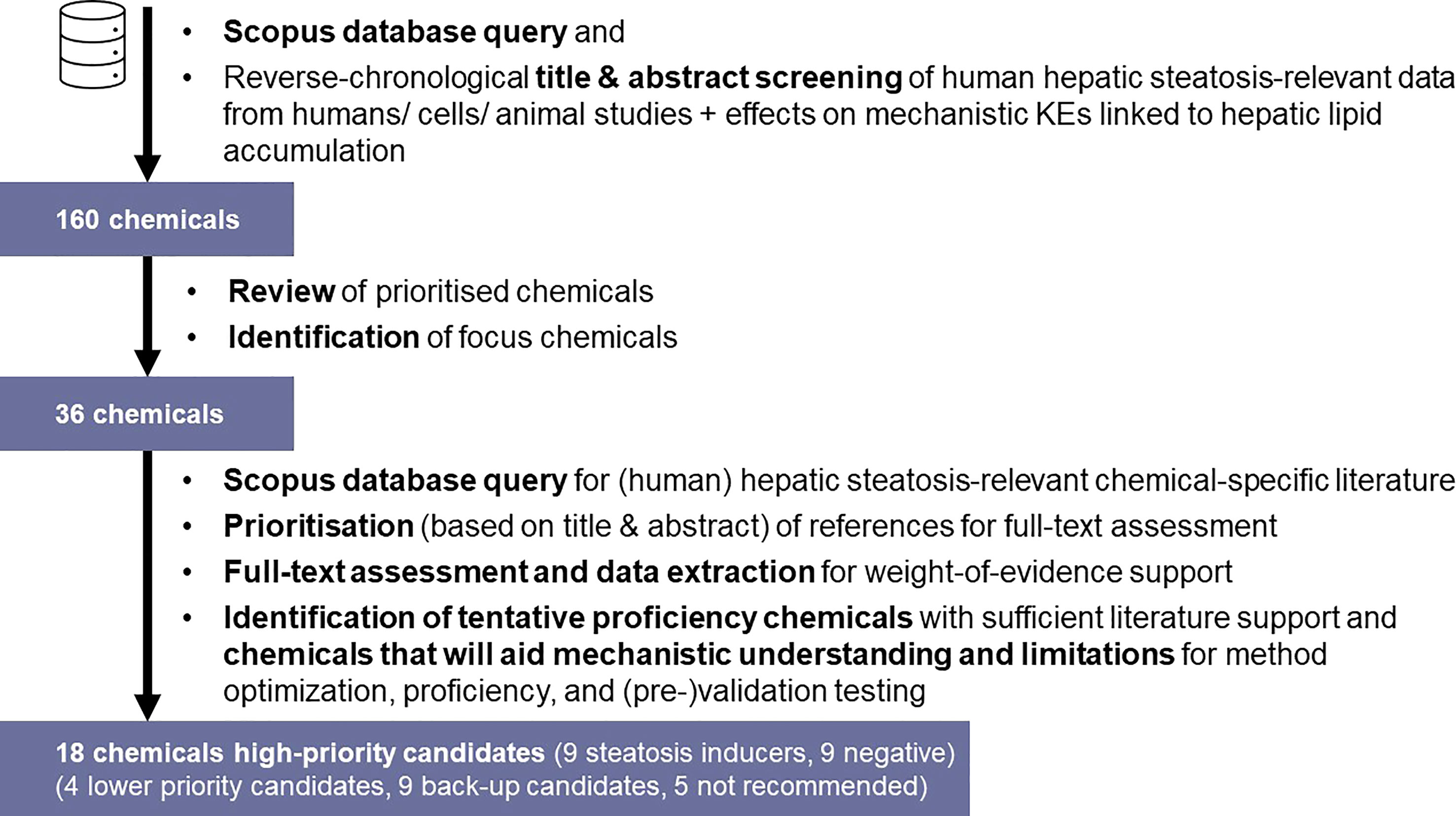
Figure 3 Tiered search strategy for the identification and prioritisation of preliminary proposed proficiency chemicals for in vitro hepatic steatosis/lipid accumulation test method optimisation, proficiency, and (pre-)validation testing.
For chemical-specific sub-searches (see Supplementary Material 1, “Chemical-specific sub-searches”), the initial search strings were complemented with chemical-specific identifiers (such as chemical name, CAS number, IUPAC-compliant chemical name, name of formulation (if applicable)). If the search returned unfeasibly many hits (> 1000 per chemical) to conduct the review, the search was refined to include articles only in English, focused on original research, but including systematic reviews and Open Access articles (including hybrid/green Open Access modalities) as far as possible; this is denoted in the chemical-specific search strings provided in Annex 1, where applicable. In addition, WHO/FAO Joint Meeting on Pesticide Residues (JMPR) monographs and EFSA pesticide summaries were checked for any relevant metabolic disruption and lipid dysregulation information for the pesticides considered here, and EMA and US FDA evaluations were consulted for specified pharmaceuticals.
The chemical-specific searches were filtered for relevance towards (human) steatosis/hepatic lipid accumulation; prioritisation of references for full-text assessment was based on title and abstract screening.
Highest priority was given to human-health relevant publications (human in vivo epidemiological studies, clinical trials, adverse event case reports, meta-analyses, genome/ transcriptome/ metabolome-wide association studies, and in vitro studies with human liver-relevant models, such as primary human hepatocytes, HepaRG, or HepG2 cells). However, where only epidemiological studies with no defined exposure scenario (i.e., quantification and duration/timing of exposure) were available, the utility for conclusion on chemical hazard assessment was considered limited and utilised as supportive, rather than primary evidence. Mechanistic information from in chemico and in silico studies was considered for weight-of-evidence support, followed by rodent in vivo and in vitro studies, and studies with other well-characterised species frequently used for chemical hazard risk assessment, such as Danio rerio (zebrafish) or Xenopus laevis (African clawfrog).
The literature searches and search iterations were conducted between 6-8th January 2021, and 15th February 2022 (details are listed in Annex 1 for each chemical and database query).
Where available, we utilised recommendations for chemical testing related to hepatotoxicity, including steatosis, developed previously in the EU funded projects SEURAT-1 (16) and LIINTOP (15). Due to lower confidence in the predictive capacity of some high throughput data sources such as ToxCast/Tox21 projects for nuclear receptor transactivation assays and triglyceride lipid accumulation in murine pre-adipocytes (3T3-L1) (26, 27), screening of this database was not considered reliable for the chemical selection, and we did not have the resources to conduct adequate manual data cleaning, curation and repeat testing, as for example reported by (28).
Full-text assessment of prioritised references and data-extraction was conducted by two team members and tabulated during October 2021-April 2022; each reviewing the others’ results, and an additional third team member reviewed for scientific correctness and consistency (April-July 2022).
Where feasible, preliminary expected potency activity profiles for the chemicals with respect to human steatosis induction were postulated on the basis of the literature evaluation for each (prioritised) chemical. Chemicals where the evidence basis was considered supported by a reasonable number of independent literature reports were proposed as tentative proficiency chemicals.
The focus of the chemical review was to address the induction or increase of hepatic steatosis/ hepatic lipid accumulation, and not a decrease in lipid accumulation. It is noted that some chemicals, particularly pharmaceuticals and essential nutrients included in the list of proposed proficiency chemicals are reported to decrease steatosis/ hepatic lipid content in vivo and/or in vitro (Table 1 and Supplementary Material 2). Reduction of lipid accumulation in the liver is an important area for therapeutic drug development (12). Human biomarkers have been identified, and there are many animal models (including genetically modified) that have been developed for studying disease mechanisms and in relation to drug discovery of hepatic lipid-lowering steatosis drugs. Additionally, there are in vitro steatosis models including with fatty acid pre-loading, or animal models on high fat diets (59–61). Data from these assays are included as part of the weight-of-evidence evaluation in Supplementary Material 2. All chemicals that do either not induce steatosis, or reduce steatosis were categorised as “negative”.
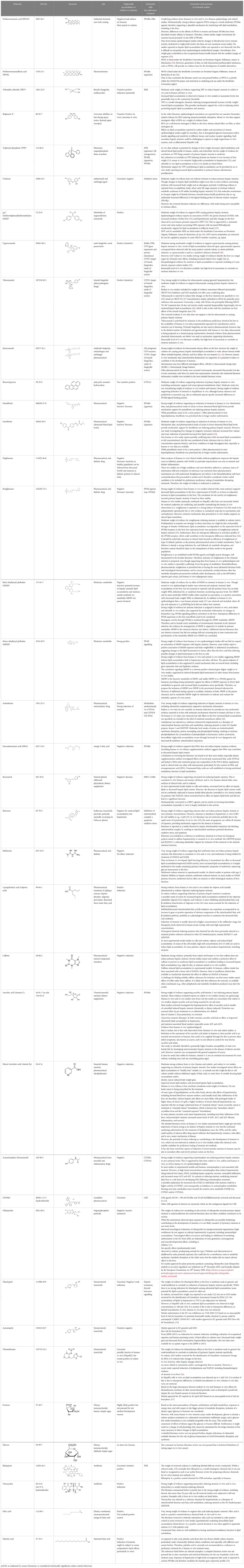
Table 1 Summary of the evaluation of chemicals to be proposed as preliminary reference and proficiency chemicals for (pre-)validation of in vitro human steatosis test method(s).
The development of in vitro models representative of a healthy lean individual (i.e., without additional fatty acid stimulation or fatty acid pre-loading) could be considered to be more technically challenging when attempting to capture therapeutic mechanisms leading to a decrease of lipid accumulation, such as very low basal lipid accumulation.
2.2 Chemical potency ranges
Whilst in vitro test methods or ‘NAMs’ were initially developed within the OECD Test Guideline Programme more for hazard classification and as prioritisation tools for subsequent in vivo testing, such that it was sufficient to only have negative or positive classifications, this is not the situation any longer. As we seek to be able to (gradually) replace in vivo testing with in vitro testing, the need for potency data is growing. The regulatory testing paradigm is now shifting towards IATAs and defined testing approaches, such that relevant in vitro test methods can ultimately be combined together in an appropriate integrated testing fashion.
Chemical potency can be used in the context of an AOP to inform as to whether an adverse effect at the molecular level leads to higher cellular and tissue effects, i.e., if it surpasses the “tipping point” leading from one key event to the next, or if physiological adaptation and compensatory mechanisms can prevent adversity at higher levels (62–66). In the context of an IATA, such potency information could be used to inform if or which higher-tier tests are needed to confidently conclude on chemical hazard characterization. For regulatory purposes, chemical potency information can be a first indicator to inform upon the derivation of a toxicological reference dose (RfD), and regulatory exposure limits such as the acceptable daily intake (ADI) (66).
While ideally chemicals of different potency (i.e., negative, weak/moderate/strong inducers) should be included in proficiency chemical lists, this was not robustly feasible for most chemicals that were evaluated. A critical limitation for such classification is the lack of a gold standard (in vitro) test method to conclude on such activity. For example, a chemical could be considered a weak/moderate/strong inducer both, based on the absolute magnitude of lipid accumulation achieved, in the tested concentration range, or based on the lowest observed effect concentration (LOEC). Probably, both criteria should inform upon the classification of a chemical, once a sufficiently robust test method is available. Further, the LOEC of a chemical can depend on the endpoint assessed (e.g., lipid accumulation vs. alteration of early molecular-level biomarkers), and therefore was variable across the studies included in the weight-of-evidence evaluation. Whilst not formally concluded upon in the summary tables, information on “active” concentration ranges is given in the relevant columns in Supplementary Material 2, where available in the respective study.
3 Results
A detailed table for all chemicals considered in full-text assessment, including the extracted evidence basis for the tentative assignment of steatosis activity profiles is listed in Supplementary Material 2, a summary of the weight-of-evidence assessment is provided in Table 1. Table 2 summarises the recommendations and prioritisation for in vitro human steatosis test method optimisation, proficiency, and (pre-)validation testing, based on the weight-of-evidence assessment, and inclusion/ prioritisation criteria outlined in the Methods section above, in line with OECD practice and guidance for in vitro test method development (67).
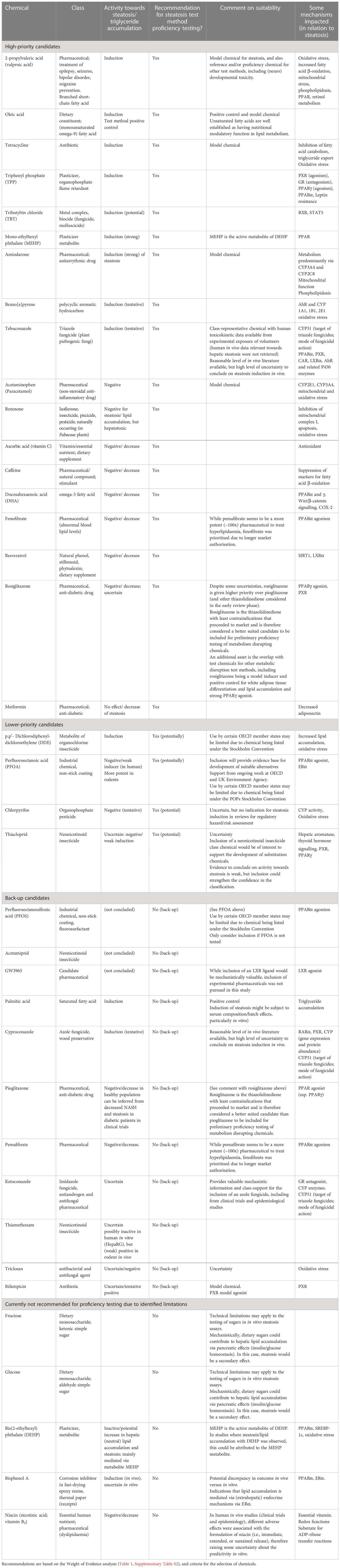
Table 2 Prioritisation summary of potentially suitable proficiency chemicals for in vitro steatosis test method optimisation, proficiency, and (pre-)validation testing.
Overall, 18 chemicals (9 steatosis inducing chemicals: amiodarone, benzo[a]pyrene, MEHP, oleic acid, TBT chloride, tebuconazole, tetracycline, TPP, valproic acid; 9 negative chemicals: acetaminophen, ascorbic acid, caffeine, DHA, fenofibrate, metformin, resveratrol, rosiglitazone, rotenone) are identified as high-priority tentative proficiency chemicals for in vitro human steatosis test method optimisation, proficiency, and (pre-)validation testing. Evidence supporting these chemicals was at least “moderate”, and the reviewed literature was consistent between in vivo and in vitro findings.
Four chemicals are listed as lower priority candidate chemicals, as they are subject to international restrictions for use and/or transport (DDE, PFOA) or the weight of evidence supporting the conclusion on their activity was weaker (chlorpyrifos, thiacloprid).
While per-/polyfluoroalkyl substances (PFAS), including PFOA and PFOS, have lower priority as proficiency chemicals, the very high persistence of these chemicals in the environment will result in continued exposure over generations. Despite the international efforts to restrict manufacture and release of PFAS, e.g., through inclusion in the Stockholm Convention on Persistent Organic Pollutants (30), sadly, it has recently been demonstrated that the planetary boundary for PFAS toxicity has already been exceeded and these hazardous chemicals are detected above tolerable limits in many environmental matrices, including surface water, rainwater, and ubiquitously in soils (68).
Nine chemicals that underwent detailed chemical-specific database searches were not prioritised for proficiency testing. This includes chemical classes where another chemical with similar structure and/or activity is included in the high(er) priority chemicals (cyproconazole, ketoconazole, pemafibrate, pioglitazone, rifampicin, thiamethoxam), that are proposed as potential replacement chemicals (acetamiprid, PFOS), or that activate pathways of scientific interest to strengthen mechanistic understanding of chemically-induced steatosis, but were excluded per the search criteria (e.g., Liver X Receptor activation by GW3965, an experimental pharmaceutical chemical). It should be noted that pioglitazone had particularly strong literature support and would have been a very good high-priority candidate chemical. However, the structurally similar thiazolidinedione pharmaceutical, rosiglitazone, is the most broadly studied representative of the pharmaceutical group. It also serves as a positive control in parallel test methods addressing key processes of metabolic disruption, such as PPARγ agonism and commitment of differentiating cells to the white adipose tissue fate and lipid accumulation in (pre-)adipocytes.
On the basis of the weight-of-evidence evaluation (Table 1 and Supplementary Material 2, and references therein, chemicals not currently recommended for proficiency testing (5 candidates) include dietary sugars (fructose, glucose). These are technically difficult to test during longer-term cell culture, as glucose levels are maintained in cell culture medium to mimic blood glucose levels in healthy individuals and are critical for cell health and survival. Furthermore, alteration of (dietary) sugars in the cell culture medium might be more reflective of dietary-induced, rather than chemically-induced effects. DEHP is not prioritised due to its bioactive metabolite, MEHP, being selected as a high-priority chemical. As a dietary constituent and essential vitamin niacin was reviewed as a potentially negative candidate chemical. However, despite its use for medicinal purposes and as a dietary supplement since the 1950s, adverse effects including on the liver were reported depending upon on the formulation of the vitamin (i.e., immediate release (crystalline form), sustained, or extended release) and/or idiosyncratic reactions. Also, another vitamin (ascorbic acid) is already included as a high-priority chemical. For BPA the reviewed literature suggests mechanisms leading to steatosis that might be mediated by other organs/tissues (e.g., oestrogen receptor-dependent effects on the pancreas and subsequent alterations to glucose/insulin homeostasis), and a discrepancy between activity observed in vivo versus in vitro was noted. Whilst BPA led to adverse effects to the liver, including indications of increased lipid accumulation/ steatosis, the literature data from in vitro studies is variable.I It is notable that the in vitro models are not sufficient to reliably model the interactions between the endocrine pancreas, liver, and/or other organs or tissues. This remains an important limitation to explore further, but on the basis of the reviewed literature herein, BPA is not considered to be a suitable chemical for steatosis proficiency testing, as yet.
However, testing of lower priority and back-up candidate chemicals would be of high regulatory and scientific interest, and can contribute to increasing confidence in the results generated with a test method, once it has been sufficiently demonstrated that the respective method is capable of correctly identifying and distinguishing the predicted activity for the higher priority chemicals.
4 Discussion
NAFLD encompasses a spectrum of progressive (reversible) disease states, from benign steatosis to irreversible pathological cirrhosis, with an estimated worldwide prevalence of approx. 25% (69). Strikingly, this was recently also confirmed in a young British cohort (4021 participants, mean age 24 years), with suspected steatosis detected in 20.7 %, and 10.0 % showing findings of severe steatosis (70). While steatosis characterised by accumulation of lipids in hepatocytes, is benign and reversible, if unrecognised and/or untreated it can progress to steatohepatitis with inflammation, fibrosis, and possibly even to irreversible stages of hepatic cirrhosis and hepatocellular carcinoma. The high prevalence of NAFLD, including in the younger population, could result in a substantial public health burden in the next decades (9). Particularly chemical/drug-induced liver disease is a subject of substantial research with regard to its implications in cardiovascular disease and metabolic disruption.
Due to the clinical importance of NAFLD, including steatosis, previous efforts to provide chemicals with high confidence and scientifical consensus on their mechanism and involvement in NAFLD are supported and expanded upon in this study (15, 16). The chemical applicability domain was expanded to account for inclusion of negative/ non-inducing chemicals and address the specific regulatory needs of the OECD Test Guidelines Programme. Further, the chemicals prioritised and tentatively proposed in this study also address mechanisms relevant to chemical-induced metabolic disruption beyond or in addition to hepatotoxicity.
We acknowledge the extensive nutrition research on fatty acid metabolism (as summarised in e.g., 71), however this is outside the scope of this chemical selection evaluation for steatosis/ hepatic lipid accumulation. Independent of the nutritional implications of diet fatty acid composition and balance, the weight of evidence for oleic acid as a positive control chemical for hepatic lipid accumulation/ liver steatosis both, in vitro and in vivo is very high (see Supplementary Table 2).
The work described herein provides a basis upon which suitable test methods can be developed and optimised, with the intention of ultimately adopting appropriate test methods as TGs to be used for the hazard assessment of metabolic disruption, and in this case steatosis.
Within the GOLIATH project, in vitro test methods to determine the potential chemical hazard of chemicals towards different key events and endpoints of metabolism disruption, including hepatic steatosis, are being developed and optimised for (pre-)validation, with the aim to inform and develop integrated testing strategies for MDCs.
As such, while steatosis is an important key event in the manifestation of metabolism disruption, it is important to integrate results from other models representative of different tissues/organs in order to achieve the most accurate representation of the (human) in vivo situation by in vitro methods. E.g., based on the retrieved and reviewed literature, BPA seems to alter hepatic metabolism, and could be inferred to contribute to hepatic steatosis (72–74). However, this was not reliably reproduced in the in vitro models reviewed here. A possible explanation is, that in vivo a parallel metabolism disrupting action of BPA is on the endocrine pancreas, altering survival of, and subsequently insulin secretion by pancreatic β cells in an oestrogen signalling-related fashion (75, 76). The resulting changes in circulating blood insulin/glucose levels can affect (hepatic) lipid metabolism and lead to lipid accumulation in hepatocytes (steatosis). Indeed, diabetes is a risk factor for the development of steatosis (77, 78). However, such indirect steatogenic effects of BPA via modulation of pancreatic insulin secretion might be missed in a single-tissue hepatic model. It is therefore paramount to integrate information from different relevant in vitro test methods, for more accurate human health hazard and risk assessment. This can be achieved, for example by delineating the use and combination of test methods in the form of an IATA.
Indeed, it has been recently demonstrated how mechanistic events leading to steatosis can be included in a read-across IATA (13, 14). Using valproic acid as a prototypical steatosis-inducing chemical, the authors comprehensively map how molecular-level events such as nuclear receptor activation can be translated across levels of biological organisation and contribute or lead to microvesicular liver steatosis (Figure 1 in 14). Based upon such mechanistic understanding of the cellular events, a tiered testing strategy for hepatic steatosis is proposed, encompassing amongst others non-target transcriptomics, targeted molecular-level screening via reporter gene assays (PPARγ, PXR, AhR, GR, LXR, Nrf2, ESRE, SRXN1, BIP), mitochondrial stress, cellular-level triglyceride accumulation in human hepatocyte cell lines (HepG2, HepaRG, and primary human hepatocytes), and supporting lines of evidence arising from relevant non-human models (e.g., histopathological alterations of liver in zebrafish embryos). Finally, a probabilistic approach towards weighting the evidence and uncertainties (Dempster-Shafer Theory) is successfully employed in order to inform on the hazard potential of specific branched-chain carboxylic acids (14). Despite being driven by the key event “hepatic steatosis”, this IATA case study demonstrates how read-across approaches can be supplemented with in vitro NAM data in order to increase confidence in in silico findings, and finally contribute to reducing the use of animals for research purposes, replacing animal testing with human-relevant data thus increasing human relevance. Whilst a successful demonstration of applying a read-across IATA to determining the steatosis hazard of a branched chain carboxylic acid based on NAM data, this approach was not applicable to the chemical selection reported in this study. Read-across in silico methods depend on a data-rich training set of structurally similar chemicals, whilst to the contrary, the proficiency chemical set for test method development needs to demonstrate a broad structural diversity to cover different chemical applicability domains (see also methods section above). However, once sufficient reliable high-quality data have been generated on the proficiency chemical set and other chemicals, e.g., with a validated test method, it would be beneficial to supplement the proficiency chemicals with in silico information, and potentially to employ in silico methods to subsequently expand and/or refine the proficiency chemical set.
The availability of an independently selected, reviewed, and expert-endorsed set of reference and proficiency chemicals is a first critical step in the development and validation of regulatory accepted test methods including NAMs. This will be utilised in the development of appropriate test systems and models.
Until relatively recently, regulatory testing relied predominantly upon rodent (in vivo) models, but now with the paradigm shift to in vitro testing, the use of human cell-based models that are more reflective of human biology are being developed and adopted as regulatory decision-making tools. With a vast diversity of human cell lines available, two models are of particular interest and relevance for modelling human hepatic processes: primary human hepatocytes (PHH) and (differentiated) human HepaRG cells. While primary human hepatocytes can reflect the interindividual diversity of a population, including sex and ethnic backgrounds, such variability reduces the reproducibility needed to ensure regulatory confidence in the results. It is therefore differentiated HepaRG cells that have been prioritised for successful validation of a human hepatic metabolism in vitro test method at the level of the OECD (79, 80). Another frequently used human liver model are HepG2 cells. However, HepG2 cells have been reported to be misidentified: originally thought to be a hepatocellular carcinoma cell line, it was shown to be from an hepatoblastoma, which can have implications in the interpretation of biological (and therapeutic) processes (81).
Additionally, it is being recognised that concentration-response data, that are often already recorded in non-animal alternative methods, but not yet utilised for regulatory purposes are key to leverage in vitro test methods beyond prioritisation and regulatory hazard identification (66). It was intended that the tentative proposed chemicals for human in vitro steatosis test method optimisation, proficiency, and (pre-)validation testing provided in this study cover chemicals with a range of activity (negative, low, moderate, high induction potential) towards steatosis. While good coverage and satisfactory weight-of-evidence of negative and generally positive chemicals was identified (Table 1), the intended potency range could not be covered in full with sufficient supporting evidence. This especially applies to chemicals that have low steatosis induction potential, or where steatosis was induced at concentrations close to (cyto-)toxicity. We recommend that initial method optimisation and proficiency testing should be based on the qualitative criterion distinguishing between steatosis (non-)inducing chemicals but testing and reporting on a range of concentrations. Once the above proposed chemicals (Table 1) are tested in a sufficiently developed and robust human in vitro steatosis test method, a (semi)quantitative secondary criterion for distinguishing between week/ moderate/ strong steatosis inducers could be added, ideally supported by indicative benchmark activity bands obtained with the respective test method.
It is acknowledged that the development of steatosis is a complex outcome, and that secondary effects e.g., due to signalling from other tissues such as the endocrine pancreas pose a challenge to in vitro hepatic models. This is true for most in vitro models, particularly those addressing more apical endpoints/ higher key events. However, the chemical selection proposed herein does not exclude other relevant endpoint-specific test methods.
In the future, developments in the field of multi-tissue in vitro methods, such as organ or human on a chip, may potentially help narrow the whole organism extrapolation gap between in vitro and in vivo test systems.
5 Conclusion
Here we have proposed a minimum set of 18 tentative preliminary proficiency chemicals for human in vitro steatosis test method optimisation, proficiency, and (pre-)validation testing. These chemicals have good and unequivocal support from publicly available literature which, together with a weight-of-evidence assessment with respect to their identified activity towards steatosis, showed a reasonable weight of evidence.
The provision of this set of tentative proficiency chemicals will aid the development, refinement, (pre-)validation, and acceptance of (human in vitro) models for steatosis, also in the context of the human health hazard and risk assessment of metabolism disrupting chemicals. Finally, this study will contribute to the forthcoming OECD detailed review paper on metabolism disrupting chemicals (OECD Test Guideline Programme workplan project 4.147).
Author contributions
Conceptualization: MJ, BK. Methodology: BK, MJ. Investigation and review: BK, MJ. Data Curation: BK. Writing – Original Draft: BK, MJ. Writing – Review & Editing: BK, MJ. Supervision: MJ. Project Administration: MJ. Funding Acquisition: MJ. All authors contributed to the article and approved the submitted version.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 825489 (“GOLIATH”). This output reflects only the authors’ views and the European Union’s Research Executive Agency and the European Commission are not responsible for any use that may be made of the information it contains.
Acknowledgments
We gratefully acknowledge Eugene Boshoff, who, whilst employed by UKHSA, contributed to the conduction of the literature review, and Timothy W Gant for internal review. The critical external review of the chemical selection for regulatory applicability conducted by Susan Laws (US EPA, retired) is gratefully acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1126880/full#supplementary-material
Footnotes
- ^ Impact Assessment available here: https://circabc.europa.eu/ui/group/a0b483a2-4c05-4058-addf-2a4de71b9a98/library/9d5b03e5-6a0b-4214-8ef4-622bd7f50ad6/details.
References
1. ECHA, EFSA, JRC, Andersson N, Arena M, Auteri D, et al. Guidance for the identification of endocrine disruptors in the context of regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J (2018) 16:E05311.
2. OECD. Detailed Review Paper on the state of science on novel in vitro and in vivo screening and testing methods and endpoints for evaluating endocrine disruptors. ENV/JM/MONO(2012)23. JT03325419, Paris: Organisation For Economic Co-Operation And Development, Environment Directorate (2012).
3. Bopp S, Nepelska M, Halder M, Munn S. Expert survey on identification of gaps in available test methods for evaluation of endocrine disruptors, EUR 28592 en. Luxembourg: Publications Office Of The European Union (2017).
4. EC DG ENV. European Commission, Directorate-General for Environment, Setting priorities for further development and validation of test methods and testing approaches for evaluating endocrine disruptors: Final report Vol. 2018. Publications Office (2018). doi: 10.2779/21828
5. EC DG ENV, Joas A, Bohn P, Geoffroy L, et al. Temporal aspects in the testing of chemicals for endocrine disrupting effects (In relation to human health and the environment) : Final report Vol. 2018. Publications Office (2018). doi: 10.2779/789059
6. Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol (2017) 68:3–33. doi: 10.1016/j.reprotox.2016.10.001
7. Legler J, Zalko D, Jourdan F, Jacobs M, Fromenty B, Balaguer P, et al. The GOLIATH project: Towards an internationally harmonised approach for testing metabolism disrupting compounds. Int J Of Mol Sci (2020) 21:3480. doi: 10.3390/ijms21103480
8. EASL, EASD, EASO. Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
9. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
10. EC. Regulation (EC) No 1907/2006 of the European parlament and of the council of 18 December 2006 concenrning the registration, evaluation, authorisation and restriction of chemicals (REACH), establishing a European chemicals agency, amending directive 1999/45/EC and repealing council regulation (EEC) No 793/93 and commission regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off J Of Eur Union (2006).
11. EC. Regulation (EC) No. 1107/2009 of the European parliament and of the council of 21 October 2009 concerning the placing of plant protection products on the market and repealing council directives 79/117/EEC and 91/414/EEC. Off J Of Eur Union (2021).
12. Willebrords J, Pereira IVA, Maes M, Crespo Yanguas S, Colle I, Van Den Bossche B, et al. Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research. Prog In Lipid Res (2015) 59:106–25. doi: 10.1016/j.plipres.2015.05.002
13. OECD. Case study on the use of integrated approaches to testing and assessment for predictivity of a 90 day repeated dose toxicity study (OECD 408) for 2-ethylbutyric acid using a read-across approach from other branched carboxylic acids. In: OECD Series on Testing and Assessment. Paris: OECD Publishing (2018).
14. Escher SE, Aguayo-Orozco A, Benfenati E, Bitsch A, Braunbeck T, Brotzmann K, et al. Integrate mechanistic evidence from new approach methodologies (NAMs) into a read-across assessment to characterise trends in shared mode of action. Toxicol In Vitro (2022) 79:105269. doi: 10.1016/j.tiv.2021.105269
15. Gómez-Lechón MJ, Tolosa L, Castell JV, Donato MT. Mechanism-based selection of compounds for the development of innovative In vitro approaches to hepatotoxicity studies in the LIINTOP project. Toxicol In Vitro (2010) 24:1879–89. doi: 10.1016/j.tiv.2010.07.018
16. Jennings P, Schwarz M, Landesmann B, Maggioni S, Goumenou M, Bower D, et al. Seurat-1 liver gold reference compounds: A mechanism-based review. Arch Of Toxicol (2014) 88:2099–133. doi: 10.1007/s00204-014-1410-8
17. Jacobs MN, Kubickova B, Boshoff E. Candidate proficiency test chemicals to address industrial chemical applicability domains for In vitro human cytochrome P450 enzyme induction. Front Toxicol (2022) 4:880818. doi: 10.3389/ftox.2022.880818
18. Knight DJ, Deluyker H, Chaudhry Q, Vidal J-M, De Boer A. A call for action on the development and implementation of new methodologies for safety assessment of chemical-based products in the EU – a short communication. Regul Toxicol And Pharmacol (2021) 119:104837. doi: 10.1016/j.yrtph.2020.104837
19. Zuang V, Dura A, Ahs Lopez E, Barroso J, Batista Leite S, Berggren E, et al. Non-animal methods in science and regulation. Luxembourg: Publications Office Of The European Union (2022).
20. Fentem J, Malcomber I, Maxwell G, Westmoreland C. Upholding the EU's commitment to 'Animal testing as a last resort' under reach requires a paradigm shift in how we assess chemical safety to close the gap between regulatory testing and modern safety science. Altern Lab Anim (2021) 49:122–32. doi: 10.1177/02611929211040824
21. Ball N, Bars R, Botham PA, Cuciureanu A, Cronin MTD, Doe JE, et al. A framework for chemical safety assessment incorporating new approach methodologies within reach. Arch Of Toxicol (2022) 96:743–66. doi: 10.1007/s00204-021-03215-9
22. Carmichael PL, Baltazar MT, Cable S, Cochrane S, Dent M, Li H, et al. Ready for regulatory use: NAMs and NGRA for chemical safety assurance. ALTEX (2022) 39:359–66. doi: 10.14573/altex.2204281
23. Westmoreland C, Bender HJ, Doe JE, Jacobs MN, Kass GEN, Madia F, et al. Use of new approach methodologies (NAMs) in regulatory decisions for chemical safety: Report from an EPAA deep dive workshop. Regul Toxicol And Pharmacol (2022) 135:105261. doi: 10.1016/j.yrtph.2022.105261
24. Audouze K, Sarigiannis D, Alonso-Magdalena P, Brochot C, Casas M, Vrijheid M, et al. Integrative strategy of testing systems for identification of endocrine disruptors inducing metabolic disorders-an introduction to the OBERON project. Int J Mol Sci (2020) 21(8):2988. doi: 10.3390/Ijms21082988
25. Küblbeck J, Vuorio T, Niskanen J, Fortino V, Braeuning A, Abass K, et al. The edcmet project: Metabolic effects of endocrine disruptors. Int J Mol Sci (2020) 21:3021. doi: 10.3390/Ijms21083021
26. Janesick AS, Dimastrogiovanni G, Vanek L, Boulos C, Chamorro-García R, Tang W, et al. On the utility of ToxCast™ and ToxPi as methods for identifying new obesogens. Environ Health Perspect (2016) 124:1214–26. doi: 10.1289/ehp.1510352
27. Foley B, Doheny DL, Black MB, Pendse SN, Wetmore BA, Clewell RA, et al. Editor's highlight: Screening ToxCast prioritized chemicals for PPARG function in a human adipose-derived stem cell model of adipogenesis. Toxicol Sci (2017) 155:85–100. doi: 10.1093/toxsci/kfw186
28. Filer DL, Hoffman K, Sargis RM, Trasande L, Kassotis CD. On the utility of ToxCast-based predictive models to evaluate potential metabolic disruption by environmental chemicals. Environ Health Perspect (2022) 130:57005. doi: 10.1289/EHP6779
29. Butenhoff JL, Gaylor DW, Moore JA, Olsen GW, Rodricks J, Mandel JH, et al. Characterization of risk for general population exposure to perfluorooctanoate. Regul Toxicol Pharmacol (2004) 39:363–80. doi: 10.1016/j.yrtph.2004.03.003
30. UNEP. Stockholm Convention on persistent organic pollutants (POPs) (2019 revised version in press). In: Texts and annexes (2019). Available at: http://chm.pops.int/theconvention/overview/textoftheconvention/tabid/2232/default.aspx.
31. ECHA, ANSES. Analysis of the most appropriate risk management option (RMOA). In: Justification for the selection of a candidate CoRAP substance. ANSES On Behalf Of FR-MSCA (2019).
32. Wang D, Yan S, Yan J, Teng M, Meng Z, Li R, et al. Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis. Environ pollut (2019) 246:630–8. doi: 10.1016/j.envpol.2018.12.053
33. Du Z, Zhang Y, Wang G, Peng J, Wang Z, Gao S. Tphp exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver. Sci Rep (2016) 6. doi: 10.1038/srep21827
34. Regnault C, Usal M, Veyrenc S, Couturier K, Batandier C, Bulteau AL, et al. Unexpected metabolic disorders induced by endocrine disruptors in xenopus tropicalis provide new lead for understanding amphibian decline. Proc Natl Acad Sci U.S.A. (2018) 115:E4416–25. doi: 10.1073/pnas.1721267115
35. La Merrill MA, Johnson CL, Smith MT, Kandula NR, Macherone A, Pennell KD, et al. Exposure to persistent organic pollutants (POPs) and their relationship to hepatic fat and insulin insensitivity among Asian Indian immigrants in the united states. Environ Sci And Technol (2019) 53:13906–18. doi: 10.1021/acs.est.9b03373
36. Takayama S, Sieber SM, Dalgard DW, Thorgeirsson UP, Adamson RH. Effects of long-term oral administration of DDT on nonhuman primates. J Cancer Res Clin Oncol (1999) 125:219–25. doi: 10.1007/s004320050266
37. Cano-Sancho G, Salmon AG, La Merrill MA. Association between exposure to p,p'-DDT and its metabolite p,p'-DDE with obesity: Integrated systematic review and meta-analysis. Environ Health Perspect (2017) 125:096002. doi: 10.1289/EHP527
38. Lichtenstein D, Mentz A, Schmidt FF, Luckert C, Buhrke T, Marx-Stoelting P, et al. Transcript and protein marker patterns for the identification of steatotic compounds in human HepaRG cells. Food And Chem Toxicol (2020) 145:111690. doi: 10.1016/j.fct.2020.111690
39. EFSA. Scientific opinion on risk assessment for a selected group of pesticides from the triazole group to test possible methodologies to assess cumulative effects from exposure through food from these pesticides on human health. EFSA panel on plant protection products and their residues. EFSA J (2009) 7:1167.
40. Schmidt F, Marx-Stoelting P, Haider W, Heise T, Kneuer C, Ladwig M, et al. Combination effects of azole fungicides in Male rats in a broad dose range. Toxicology (2016) 355-356:54–63. doi: 10.1016/j.tox.2016.05.018
41. US FDA. FDA drug safety communication: FDA limits usage of nizoral (Ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems. Available at: https://www.Fda.Gov/Drugs/Drug-Safety-And-Availability/Fda-Drug-Safety-Communication-Fda-Warns-Prescribing-Nizoral-Ketoconazole-Oral-Tablets-Unapproved (Accessed 05/01/2023).
42. EMA. European Medicines Agency recommends suspension of marketing authorisations for oral ketoconazole, in: Benefit of oral ketoconazole does not outweigh risk of liver injury in fungal infections (2013). Available at: https://Www.Ema.Europa.Eu/En/Documents/Press-Release/European-Medicines-Agency-Recommends-Suspension-Marketing-Authorisations-Oral-Ketoconazole_En.Pdf (Accessed 5/01/2023).
43. Oscarsson J, Önnerhag K, Risérus U, Sundén M, Johansson L, Jansson PA, et al. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J Of Clin Lipidol (2018) 12:1390–1403.E4. doi: 10.1016/j.jacl.2018.08.003
44. Franco ME, Fernandez-Luna MT, Ramirez AJ, Lavado R. Metabolomic-based assessment reveals dysregulation of lipid profiles in human liver cells exposed to environmental obesogens. Toxicol And Appl Pharmacol (2020) 398. doi: 10.1016/j.taap.2020.115009
45. Gao M, Ma Y, Alsaggar M, Liu D. Dual outcomes of rosiglitazone treatment on fatty liver. AAPS J (2016) 18:1023–31. doi: 10.1208/s12248-016-9919-9
46. Garoche C, Boulahtouf A, Grimaldi M, Chiavarina B, Toporova L, Den Broeder MJ, et al. Interspecies differences in activation of peroxisome proliferator-activated receptor γ by pharmaceutical and environmental chemicals. Environ Sci Technol (2021) 55:16489–501. doi: 10.1021/acs.est.1c04318
47. Satake S, Nakamura C, Minamide Y, Kudo S, Maeda H, Chihaya Y, et al. Effect of a Large dose of di (2-ethylhexyl) phthalate (DEHP) on hepatic peroxisome in cynomolgus monkeys (Macaca fascicularis). J Of Toxicol Pathol (2010) 23:75–83. doi: 10.1293/tox.23.75
48. US FDA. Cordarone (Amiodarone hcl) tablets, medicinal product print label. Available at: https://www.Accessdata.Fda.Gov/Drugsatfda_Docs/Label/2010/018972s042lbl.Pdf (Accessed 27/04 2022).
49. Tolosa L, Gómez-Lechón MJ, Jiménez N, Hervás D, Jover R, Donato MT. Advantageous use of heparg cells for the screening and mechanistic study of drug-induced steatosis. Toxicol And Appl Pharmacol (2016) 302:1–9. doi: 10.1016/j.taap.2016.04.007
50. Isenberg JS, Klaunig JE. Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol Sci (2000) 53:340–51. doi: 10.1093/toxsci/53.2.340
51. EFSA. Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos. EFSA J (2019) 17:23.
52. Alarcan J, Waizenegger J, Solano MLM, Lichtenstein D, Luckert C, Peijnenburg A, et al. Hepatotoxicity of the pesticides imazalil, thiacloprid and clothianidin - individual and mixture effects in a 28-day study in female wistar rats. Food Chem Toxicol (2020) 140:111306. doi: 10.1016/j.fct.2020.111306
53. Nielsen E, Nørhede P, Boberg J, Krag Isling L, Kroghsbo S, Hadrup N, et al. Identification of cumulative assessment groups of pesticides. EFSA Supporting Publications (2012) 9:269e. doi: 10.2903/sp.efsa.2012.EN-269
54. EFSA. Peer review of the pesticide risk assessment of the active substance thiacloprid. EFSA J (2019) 17.
55. EFSA. Peer review of the pesticide risk assessment of the active substance acetamiprid. EFSA J (2016) 14.
56. Yang D, Zhang X, Yue L, Hu H, Wei X, Guo Q, et al. Thiamethoxam induces nonalcoholic fatty liver disease in mice via methionine metabolism disturb via nicotinamide n-methyltransferase overexpression. Chemosphere (2021) 273:129727. doi: 10.1016/j.chemosphere.2021.129727
57. EC. Commission implementing regulation (EU) 2018/785 of 29 May 2018 amending implementing regulation (EU) No 540/2011 as regards the conditions of approval of the active substance thiamethoxam (Text with EEA relevance.). Off J Of Eur Union (2018).
58. Allard J, Bucher S, Massart J, Ferron P-J, Le Guillou D, Loyant R, et al. Drug-induced hepatic steatosis in absence of severe mitochondrial dysfunction in heparg cells: Proof of multiple mechanism-based toxicity. Cell Biol Toxicol (2021) 37(2):151–75. doi: 10.1007/S10565-020-09537-1
59. Vinken M. Adverse outcome pathways and drug-induced liver injury testing. Chem Res In Toxicol (2015) 28:1391–7. doi: 10.1021/acs.chemrestox.5b00208
60. Abe N, Kato S, Tsuchida T, Sugimoto K, Saito R, Verschuren L, et al. Longitudinal characterization of diet-induced genetic murine models of non-alcoholic steatohepatitis with metabolic, histological, and transcriptomic hallmarks of human patients. Biol Open (2019) 8. doi: 10.1242/bio.041251
61. Fernandez-Checa JC, Bagnaninchi P, Ye H, Sancho-Bru P, Falcon-Perez JM, Royo F, et al. Advanced preclinical models for evaluation of drug-induced liver injury – consensus statement by the European drug-induced liver injury network [Pro-Euro-Dili-Net]. J Of Hepatol (2021) 75:935–59. doi: 10.1016/j.jhep.2021.06.021
62. Shah I, Setzer RW, Jack J, Houck KA, Judson RS, Knudsen TB, et al. Using ToxCast data to reconstruct dynamic cell state trajectories and estimate toxicological points of departure. Environ Health Perspect (2016) 124:910–9. doi: 10.1289/ehp.1409029
63. Middleton A, Cooper S, Cull T, Stark R, Adeleye Y, Boekelheide K, et al. Case studies in cellular stress: Defining Adversity/Adaptation tipping points. Appl In Vitro Toxicol (2017) 3:199–210. doi: 10.1089/aivt.2017.0003
64. Frank CL, Brown JP, Wallace K, Wambaugh JF, Shah I, Shafer TJ. Defining toxicological tipping points in neuronal network development. Toxicol And Appl Pharmacol (2018) 354:81–93. doi: 10.1016/j.taap.2018.01.017
65. Jacobs MN, Colacci A, Corvi R, Vaccari M, Aguila MC, Corvaro M, et al. Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch Of Toxicol (2020) 94:2899–923. doi: 10.1007/s00204-020-02784-5
66. Jacobs MN, Ezendam J, Hakkert B, Oelgeschlaeger M. Potential of concentration-response data to broaden regulatory application of In vitro test guidelines. ALTEX (2022) 39:315–21.
67. OECD. Guidance document on good in vitro method practices (GIVIMP). In: OECD Series on testing and assessment. Paris: OECD Publishing (2018). doi: 10.1787/9789264304796-En
68. Cousins IT, Johansson JH, Salter ME, Sha B, Scheringer M. Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (Pfas). Environ Sci Technol (2022) 56:11172–9. doi: 10.1021/acs.est.2c02765
69. Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther (2011) 34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x
70. Abeysekera KWM, Fernandes GS, Hammerton G, Portal AJ, Gordon FH, Heron J, et al. Prevalence of steatosis and fibrosis in young adults in the UK: A population-based study. Lancet Gastroenterol Hepatol (2020) 5:295–305. doi: 10.1016/S2468-1253(19)30419-4
71. Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J Of Acad Of Nutr And Dietetics (2014) 114:136–53. doi: 10.1016/j.jand.2013.11.001
72. ANSES. Opinion of the French agency for food, environmental and occupational health & safety (Anses) in response to the consultation of the European food safety authority on its draft opinion regarding the assessment of risks to human health related to dietary exposure to Bisphenol A. (2014). Available at: https://www.anses.fr/en/system/files/SUBSTANCES2014sa0033EN.pdf.
73. EFSA. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA panel on food contact materials, enzymes, flavourings, processing aids and panel on dietetic products, nutrition. EFSA J (2015) 13:3978. doi: 10.2903/j.efsa.2015.3978
74. Le Magueresse-Battistoni B, Multigner L, Beausoleil C, Rousselle C. Effects of bisphenol a on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol And Cell Endocrinol (2018) 475:74–91. doi: 10.1016/j.mce.2018.02.009
75. Babiloni-Chust I, Dos Santos RS, Medina-Gali RM, Perez-Serna AA, Encinar J-A, Martinez-Pinna J, et al. G Protein-coupled estrogen receptor activation by bisphenol-a disrupts the protection from apoptosis conferred by the estrogen receptors ERα and ERβ in pancreatic beta cells. Environ Int (2022) 164:107250. doi: 10.1016/j.envint.2022.107250
76. Dos Santos RS, Medina-Gali RM, Babiloni-Chust I, Marroqui L, Nadal A. In vitro assays to identify metabolism-disrupting chemicals with diabetogenic activity in a human pancreatic beta-cell model. Int J Of Mol Sci (2022) 23:5040. doi: 10.3390/ijms23095040
77. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology (2004) 126:460–8. doi: 10.1053/j.gastro.2003.10.065
78. Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: The Edinburgh type 2 diabetes study. Diabetes Care (2011) 34:1139–44. doi: 10.2337/dc10-2229
79. JRC. Multi-study validation trial for cytochrome P450 induction providing a reliable human metabolically competent standard model or method using the human cryopreserved primary hepatocytes and the human cryopreserved heparg® cell line. In: Validation project report. European Comission Joint Research Centre, Institute For Health And Consumer Protection, European Union Reference Laboratory For Alternatives To Animal Testing (EURL ECVAM) (2014). Available at: https://Tsar.Jrc.Ec.Europa.Eu/System/Files/Published/Cyp_Validation%20project%20report_Final%2020140314_0.Pdf.
80. Bernasconi C, Pelkonen O, Andersson TB, Strickland J, Wilk-Zasadna I, Asturiol D, et al. Validation of In vitro methods for human cytochrome P450 enzyme induction: Outcome of a multi-laboratory study. Toxicol In Vitro (2019) 60:212–28. doi: 10.1016/j.tiv.2019.05.019
Keywords: HepaRG, human hazard, lipid accumulation, triglyceride, drug-induced liver injury, validation, alternative method, new approach methodology
Citation: Kubickova B and Jacobs MN (2023) Development of a reference and proficiency chemical list for human steatosis endpoints in vitro. Front. Endocrinol. 14:1126880. doi: 10.3389/fendo.2023.1126880
Received: 18 December 2022; Accepted: 17 March 2023;
Published: 24 April 2023.
Edited by:
Anna-Maria Andersson, Rigshospitalet, DenmarkReviewed by:
Philipp Antczak, University Hospital of Cologne, GermanyPatrick Balaguer, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Copyright © 2023 Kubickova and Jacobs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miriam N. Jacobs, TWlyaWFtLkphY29ic0B1a2hzYS5nb3YudWs=
 Barbara Kubickova
Barbara Kubickova Miriam N. Jacobs
Miriam N. Jacobs