- 1Reproductive Medical Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Department of Obstetrics and Gynecology, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Shenzhen Research Institute, The Chinese University of Hong Kong, Shenzhen, China
- 4Maternal-Fetal Medicine Institute, Shenzhen Baoan Women’s and Children’s Hospital, Shenzhen University, Shenzhen, China
- 5The Fertility Preservation Research Center, Department of Obstetrics and Gynecology, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
Survival rates for children and adolescents diagnosed with malignancy have been steadily increasing due to advances in oncology treatments. These treatments can have a toxic effect on the gonads. Currently, oocyte and sperm cryopreservation are recognized as well-established and successful strategies for fertility preservation for pubertal patients, while the use of gonadotropin-releasing hormone agonists for ovarian protection is controversial. For prepubertal girls, ovarian tissue cryopreservation is the sole option. However, the endocrinological and reproductive outcomes after ovarian tissue transplantation are highly heterogeneous. On the other hand, immature testicular tissue cryopreservation remains the only alternative for prepubertal boys, yet it is still experimental. Although there are several published guidelines for navigating fertility preservation for pediatric and adolescent patients as well as transgender populations, it is still restricted in clinical practice. This review aims to discuss the indications and clinical outcomes of fertility preservation. We also discuss the probably effective and efficient workflow to facilitate fertility preservation.
Introduction
Long-term survival for children and adolescents diagnosed with malignancy has steadily increased and exceeded 80% over the past decade (1–3). As these cancer survivors reach adulthood, a substantial proportion of them experience infertility associated with previous gonadotoxic chemotherapy and/or radiotherapy (4, 5). In addition to oncology treatment, other non-oncological conditions and related therapy may raise fertility problems, including nephrotic syndrome (6), Turner Syndrome (7) and systemic lupus erythematosus (8). Currently, fertility issues have been increasingly recognized as a major concern for those newly diagnosed patients and their families (9, 10). Failing to achieve parenthood raises tremendous psychosocial stress on patients and their families and impairs their well-being. While scientists have established a range of methods for fertility preservation, including embryo cryopreservation, gamete cryopreservation, and gonad tissue cryopreservation (11), the clinical practice is not as satisfactory as expected (12, 13). This review aims to discuss the indications, methods, and clinical outcomes of fertility preservation in the pediatric and adolescent populations. The potential effective and efficient workflow to facilitate fertility preservation is discussed as well.
Indications for fertility preservation in the pediatric and adolescent populations
Oncological causes
The incidence of pediatric and adolescent cancers is estimated to range between 50 to 200 cases per million children per year (14, 15) and more than 80% of these cancers are now potentially curable with current treatments (1, 2). Chemotherapy and radiotherapy in cancer treatments can lead to temporary, long-term and permanent gonadal toxicity, making fertility impairment another issue that distresses cancer survivors and their families (16). Alkylating agents are highly gonadotoxic and are associated with premature ovarian insufficiency (17, 18) and oligo- or azoospermia (19) depending on agent and dose (17, 20).
In females, these treatments substantially accelerate the activation and atresia of primordial follicles, leading to premature ovarian insufficiency (POI) and permanent amenorrhea (18, 21–24). Depending on agents and regimes, Impact on fertility may be broadly classified in low (<20%), medium (20-80%), or high (>80%) (25). A 13-fold increased chance of developing premature menopause was observed in a childhood cancer survivor study (26). Ovarian radiation, on the other hand, can cause 50% of follicle depletion at the dosage of 2 Gy and 60% chances of ovarian insufficiency at 2.5-5 Gy (25). Besides, long-term follow up of pediatric patients also demonstrated significant decline in anti-Müllerian hormone (AMH) after cancer therapy (23, 27), suggesting fertility losses and future fertility problems.
Spermatogenesis is particularly sensitive to chemotherapy and radiotherapy (28). Long-term follow up observed 25% and 28% of adult survivors of childhood cancer suffered from azoospermia and oligospermia after chemotherapy with cyclophosphamide equivalent dose less than 4000 mg/m² (29). In some regimes, several agents were administrated together, when cyclophosphamide is given > 7500 m g/m², almost all patients developed permanent azoospermia (16). Similarly, exposure to radiation causes germ cell loss in a dose-dependent manner (30), with immature spermatogonia the most radiosensitive, followed by spermatocyte and spermatid (28). Radiation at 0.1 Gy can result in morphological and quantitative changes to spermatogonia, increasing the dosage leads to spermatocyte and spermatid reduction (31). The threshold of radiation dose leading to permanent azoospermia remains unclear. But Castillo et al. found that all boys with acute lymphoblastic leukemia receiving testis radiotherapy at dose over 12 Gy developed azoospermia (19). A more recent study suggests that testicular radiation > 6 Gy may lead to permanent infertility (31).
Non-oncological causes
Younger patients affected by certain non-oncological medical conditions which require gonadotoxic treatments are potential candidates for fertility preservation as well (17, 32, 33). For example, gonadotoxic alkylating agents are widely used for diseases including nephrotic syndrome (6), systemic lupus erythematosus (34, 35), refractory idiopathic thrombocytopenic purpura (36). Also, a range of hematopoietic disorders, including thalassemia major, sickle cell anemia, aplastic anemia and myeloproliferative diseases, may be treated with hematopoietic stem cell transplant, which preconditions alkylating chemotherapy with or without radiotherapy (37, 38). In addition, some diseases can affect patients’ fertility at an early age, including Turner syndrome (7, 39), Klinefelter’s syndrome (40), fragile X syndrome (33), endometriosis (41), and gonad injury (42). Transgender populations receiving gender-affirming treatments may also require fertility preservation (43). Some of the most common non-oncological conditions which may require consideration of fertility preservation is presented in Table 1.
Potential risks for fertility impairment vary depending on patients’ age, gender, body mass index, medical condition, and subsequent treatment scheme. A comprehensive and individual assessment is essential to determine the appropriate timing and methods for fertility preservation (46). Previous guidelines have extensively discussed the fertility risk assessment of specific agents or therapy regimes (25, 47–51), which have provided useful guidance to current practice.
Available options for fertility preservation
Females
Oocyte cryopreservation
Embryo cryopreservation, as a long-established fertility preservation method, can guarantee the best outcomes for fertility preservation. However, oocyte cryopreservation is preferred since most adolescents are unlikely to have a permanent partner and using donor sperm is less desired and poses ethical issues (11, 17). Since 2012, oocyte cryopreservation is no longer considered an experimental method for fertility preservation (52). However, outcomes in adolescents are less clear.
Controlled ovarian stimulation is the most effective strategy to obtain mature oocytes (53). However, conducting ovarian stimulation on a basis of diagnosed disease requires modifications to conventional protocols to address potential restrictions, including limited time allowed, and temporary exposure to high estradiol levels (54). Advances in ovarian stimulation have allowed fertility specialists to finish ovarian stimulation and oocyte retrieval within two weeks (55, 56). In urgent situations, the gonadotrophin-releasing hormone (GnRH) antagonist protocol is considered optimal for its short time and safety. Meanwhile, random and double stimulation are feasible alternatives (32, 57). In non-urgent situations, on the other hand, both GnRH antagonist protocol and long protocol are appropriate (32). Anti-estrogenic agents may be added to abolish estradiol reproduction in estradiol-sensitive diseases (53, 54). In addition, cryopreservation of in vitro maturated oocytes may be a feasible strategy when present with time constraints (58), which eliminates potential estrogen elevation and minimizes delay in treatment. Immature oocytes can be obtained at the time of ovarian tissue cryopreservation or oophorectomy as well (59). Various cryopreservation methods have been developed to freeze oocytes. If patients survive the original diseases and desire pregnancy in the future, these oocytes can be thawed and used for assisted reproductive techniques (Figure 1A). Recent advances in cryoprotectants, cryopreservation techniques (vitrification), and fertilization with intracytoplasmic sperm injection (ICSI) have significantly improved the clinical efficacy of cryopreserved oocytes (60–62). A series of studies which investigated the efficacy of different cryopreservation protocols concluded that vitrification outperforms slow freezing (63). The survival rate of vitrified oocytes ranges between 73.6% and 92.7%, significantly higher than that of slow freezing (58.0%-72.3%) (63–70). Vitrification is also superior regarding other outcomes like fertilization, implantation, clinical pregnancy, and live birth (63, 65, 71).
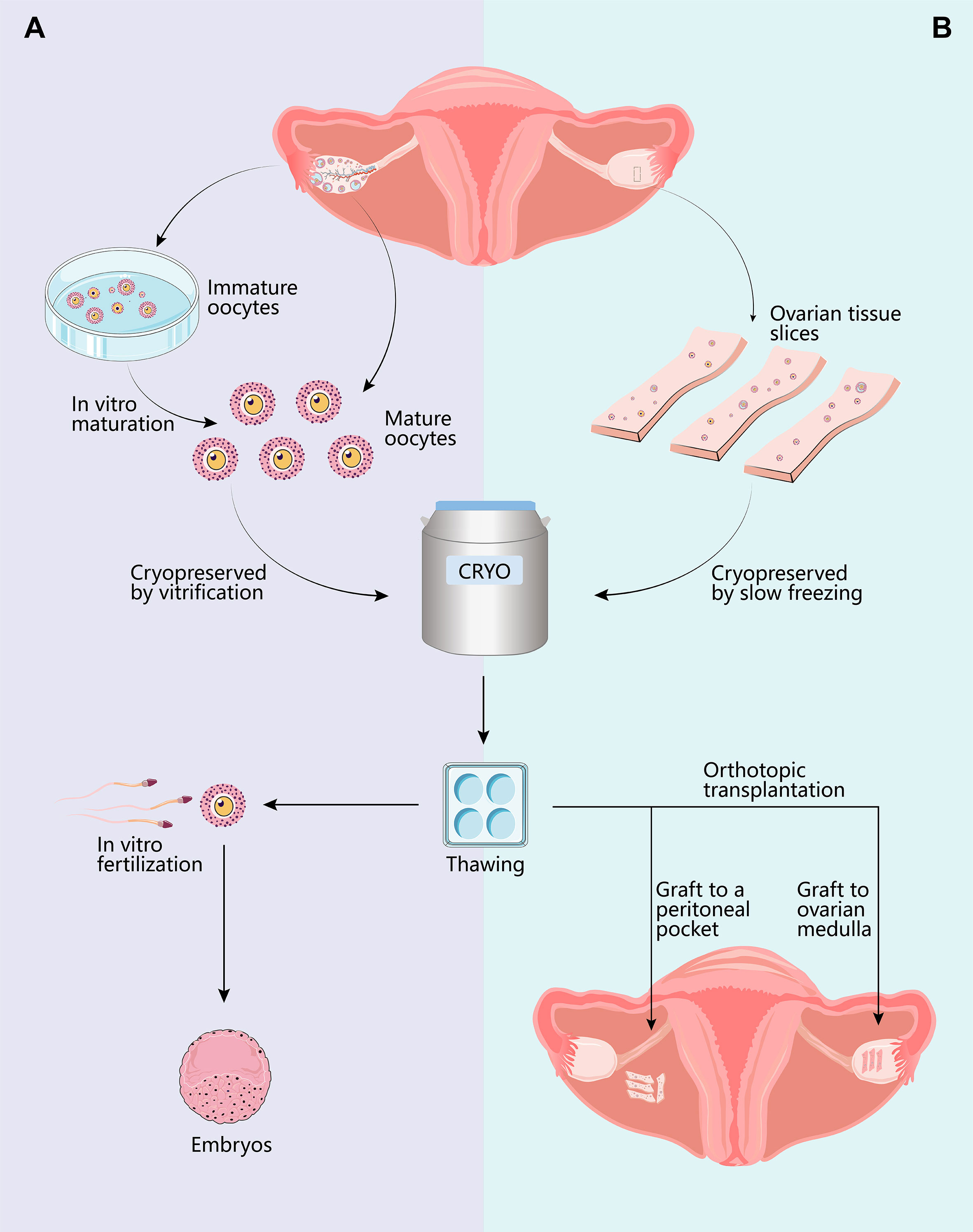
Figure 1 Options for female fertility preservation. For pubertal patients, mature oocyte cryopreservation is the optimal strategy. Controlled ovarian hyperstimulation and oocyte retrieval can be completed within two weeks if the treatments can be delayed. Another method requiring less time for ovarian stimulation is cryopreservation of in vitro matured immature cumulus-oocyte-complex (COCs). Additionally, immature COCs can be obtained while harvesting ovarian tissue for cryopreservation. Thawed oocytes are utilized for in vitro fertilization with intracellular sperm injection (A), resulting in live birth rates per transfer varying between 39% and 52%. If the patient is prepubertal or requires immediate treatments, ovarian tissue cryopreservation remains the only option. The ovarian cortex is surgically removed, dissected, and cryopreserved. While vitrification is ideal for the cryopreservation of oocytes, slow-freezing is currently preferred for the preservation of ovarian tissue. Thawed ovarian slices may be transplanted either orthotopically or heterotopically (B). Transplantation to orthotopic sites (broad ligament and ovarian medulla) provides the chance for spontaneous conception, whereas transplanting to heterotopic sites necessitates assisted reproductive techniques. The overall live birth rates after OTT range from 18.2% to 43.3%.
GnRH-agonist protection
The clinical efficacy of gonadotrophin-releasing hormone agonists (GnRH-a) during chemotherapy is controversial (54) and current recommendations regarding its use remain conflicting (11, 32, 53). Some meta-analyses evaluated the protective effect of GnRH-a during chemotherapy in premenopausal patients with breast cancer or lymphoma. Lower risks of chemotherapy-induced POI/amenorrhea and a higher number of spontaneous pregnancies after GnRH-a withdrawal were observed in the study group (72–75). However, the evidence is relatively weak due to heterogeneous populations, varying chemotherapy regimens and study endpoints (72, 76, 77). More importantly, these studies were conducted in adult subjects with an already established HPO axis. Relevant studies among adolescent patients with cancer are scarce (78, 79). A prospective study found GnRH-a administration during chemotherapy protected ovarian function and preserved fertility in adolescent patients (78). Another retrospective study drew a similar conclusion, with normal ovarian function maintained at the clinical, laboratory, and ultrasonic levels in 27/36 patients after GnRH-a co-administration (79). Overall, well-designed, large, prospective, randomized, controlled trials are essential to determine the protective effect of GnRH-a in children and adolescent patients (80).
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation (OTC) is perhaps the sole option for fertility preservation in prepubertal children and post-pubertal adolescents who cannot delay the start of chemotherapy (11, 17, 32, 53, 81). Roughly 50% of the cortex from one ovary is surgically removed, dissected, and cryopreserved for future use (82, 83). The ovarian tissue is cryopreserved by either slow freezing (84–86) or vitrification (87). When a patient intends to restore ovarian function and/or fertility, the cryopreserved tissue can be thawed and replaced (Figure 1B).
In 1994, Gosden et al. successfully restored ovarian function to several castrated sheep using frozen-thawed ovarian slices (88). Symbolically, this has resulted in several lambs and maintained long-term ovarian function for up to 2 years (89). After that, studies of cryopreserved human ovarian tissue reported normal follicular morphology after thawing (90), follicular survival (91), and growth of follicles to antral stages (92) when replaced to immunodeficient mice. Thereafter, follicular growth (93, 94), ovarian endocrine function restoration (94), and in vitro embryo formation (95) were reported after being transplanted to humans. The first live birth after autologous ovarian tissue transplantation (OTT) using cryopreserved ovarian tissue in humans was documented in 2004 (96) and the second in 2005 (97). Since the milestone event, ovarian tissue cryopreservation and transplantation is gaining increasing attention in the field of fertility preservation. The cumulation of success has recently made it an accepted technique for fertility preservation (53).
Multiple transplantation strategies have been developed. The frozen ovarian tissue can be replaced either orthotopically (17) and/or heterotopically (98). Orthotopic sites include the remaining ovary (96) and peritoneal pockets created on the broad ligament (99). The orthotopic graft provides the possibility of spontaneous pregnancy because of the proximity to the fallopian tube (98, 100–103). Notably, the first live birth was conceived spontaneously without either ovarian stimulation or in vitro fertilization (IVF) (96). In some studies, more pregnancies and live births were obtained naturally (99, 101, 102, 104). Many women have been reported to conceive and deliver more than once (104), with 3 cases delivering three times (105, 106) and one case conceiving four times (107). Heterotopic sites include subcutaneous areas in the forearm (98), abdomen wall (95), chest wall (100), breast (108), rectus muscle (108), and subperitoneal tissue (109), where a favorable environment for follicular development such as optimal temperature, paracrine factors, and blood supply may not be provided (100, 108). Thus, the procedure is adopted less frequently (110). Heterotopic autografting eliminates the possibility of conceiving naturally but not with the use of assisted reproductive techniques (ART). Clinical pregnancy (110) and live birth (111) following this procedure have been reported recently, partly removing its controversies. Meanwhile, the procedure also offers several potential advantages (100, 112), including, (1) less invasive surgery; (2) easier follicular monitoring and oocyte retrieval for IVF; (3) easier monitoring for cancer recurrence and removal of the graft, if necessary; and (4) more cost-effective options in case of repetitive transplantations. For some females who wish to restore ovarian function but do not desire pregnancy (98, 99), these advantages probably make heterotopic autograft a potentially preferred option.
Survival of grafted tissue and ovarian follicles depends on several factors, including the timing and location of transplantation, surgical techniques, and most importantly, the levels of revascularization soon after the procedure (113). Studies suggest that it takes 48 hours to revascularize after OTT in rodents (114, 115) but it may take up to 5 days in humans (115). In addition, research shows most follicles die before complete revascularization, with more than 70% of primordial follicles failing to survive the procedure in both humans (116) and sheep (89). There are challenges to further improving the survival of the graft and clinical outcomes (54, 113). On the other hand, cryopreserved ovarian tissue can be transplanted repeatedly in case of replantation failure (98). In a review including 318 women and 369 OTTs worldwide, Gellert et al. found that the average amount of transplanted tissue at the first OTT accounted for 46%, with 37% and 38% of the total amount of cryopreserved tissue being transplanted for the second and the third time, respectively (102). It seems a feasible strategy to extend the duration of ovarian function by repeating grafting procedures.
One of the leading concerns over the autograft of cryopreserved ovarian tissue is the risk of reintroducing malignant cells among malignancy survivors (100), which is considered high in hematological malignancies like leukemia and Burkitt lymphoma, and moderate in the case of Ewing sarcoma, advanced breast cancer, colon cancer, cervical adenocarcinoma (54, 112). In a recent systematic review, metastases were repeatedly detected in ovarian tissue obtained from patients with leukemia, but it was less common in other malignancies (117). Several methods have been applied to detect possible malignancy contamination before transplantation, such as histology (118), immunohistochemistry (119), and polymerase chain reaction (if specific markers are available) (117, 120). It has been proposed that ovarian tissue might be first xenografted to immunodeficient mice to assess the risk before grafted to humans (121). The recurrence rate after ovarian tissue graft in several large cohorts ranges between 3.9% and 7.0% (98, 99, 102), with a study comparing the relapse rate with those who did not accept transplantation and demonstrating similar recurrence rate (7%, 3/41 vs. 7%, 48/691) (99). None of these malignancy relapses was deemed related to OTT but dependent on the primary disease (98, 102), which has been endorsed by multiple studies (98, 102, 103, 107, 111, 122–126). Nonetheless, further studies are warranted to determine the safety of autograft of ovarian tissue among malignancy survivors (100, 102, 119, 121).
Males
Sperm cryopreservation
Sperm cryopreservation with masturbation is the easiest and most reliable method for fertility preservation for pubertal boys (11, 20, 33, 127, 128). Penile vibro-stimulation, as a noninvasive method, can be an alternative when having difficulties with masturbation (20). However, considering the invasiveness, electro-ejaculation and testicular sperm extraction (TESE) should be conducted only after weighing the benefits and harms (20, 129).
Cryopreservation of immature testicular tissue
Cryopreservation of immature testicular tissue (ITT) is the only fertility preservation option for prepubertal boys as spermatogenesis is absent (11). Small pieces of immature testicular tissue are surgically removed for cryopreservation. Yet still experimental, it is stressed that the procedure is provided exclusively for research purposes under ethical approval or novel technologies governance (20, 33, 76, 130). According to a survey, at least 1033 prepubertal boys aged between 3 months and 18 years have received the procedure (131). Multiple surveys reveal parents are willing to embrace the experimental technique (132–135), in hope that future advances in reproductive techniques will allow fertility restoration by the time their children have grown up (136). To date, however, comprehensive progress is still needed to make testicular tissue cryopreservation clinically applicable.
Testicular stem cells (TSC) can be stored in immature testicular tissue or a cell suspension. Detailed procedures of both strategies, including sample preparation, storage containers, cryoprotection, and cooling and warming process, have been elaborated elsewhere (137). Different cryopreservation strategies, including slow freezing and vitrification, have been attempted in human and animal models, leading to conflicting results (138–140). But slow freezing remains the most popular option for testicular tissue cryopreservation (130, 131), with both controlled (141–143) and uncontrolled (138) slow-freezing protocols under use.
The overall process of immature testicular tissue cryopreservation and fertility restoration procedures have been vividly described in a recent review (144). Potential methods for fertility restoration include autologous graft of immature testicular tissue (145), injection of testicular stem cells into the testis (146, 147), and in vitro maturation of TSCs (148, 149). The main advantage of ITT graft is the preservation of TSCs within their original niche (130). The maintenance of cell interaction and paracrine are preferable for tissue maturation, stem cell self-renewal, and differentiation (50, 150, 151). However, several male pediatric cancers, including testicular cancer, leukemia, and lymphoma, are prone to metastasize to the testes (152), significantly increasing the risks of malignancy relapse after autograft (33, 142). In vitro maturation of TSCs and reinjection of a TSC suspension free of malignant cells into testes, by contrast, can avoid the risks of cancer reoccurrence (50). But the original supporting conditions for in vivo spermatogenesis are absent.
Clinical outcomes after fertility preservation
Oocyte cryopreservation
For female patients, embryo preservation, if available, is the best method for female fertility preservation. However, lack of a permanent partner and ethical concerns to use of donor sperm make oocyte cryopreservation adopted far more frequently in post-pubertal adolescent patients (17). Poorer outcomes are seen compared to embryo cryopreservation due to oocyte degeneration after thawing (53), which is even greater when it comes to immature oocyte cryopreservation (153–155).
The clinical outcomes using cryopreserved mature oocytes have been steadily improving as freezing/thawing techniques evolve and ICSI is used for fertilization (63). Some randomized control trials compared the clinical outcomes between vitrified oocytes and fresh oocytes, which confirmed the non-inferiority of vitrified oocytes to fresh oocytes in terms of fertilization rate, embryo development, implantation rate, clinical pregnancy rate, and live birth rate (62, 156), with similar conclusions drawn in other publications (61, 66–69, 157). The fertilization rates of oocytes with ICSI after thawing based on a large sample size ranged between 70.0% and 81.6% (61, 67, 158). The implantation rates fluctuated around 40% per embryo transferred (62, 67, 68). Notably, some studies found that the implantation rate using autologous vitrified oocytes was significantly lower than that of donor oocytes (63, 157). Current data suggests the clinical pregnancy rates per transfer can be as high as 50.7% to 62.6% (61, 63, 157, 159) whereas live birth rates per transfer range between 39% and 52% (61, 67, 157). A study found poor success rates among cancer patients than those who pursue elective oocyte preservation, but no statistically significant differences were observed after correction for age and controlled ovarian stimulation protocols (159). Current data collectively suggest that oocyte cryopreservation is an effective method for female fertility preservation. However, the efficiency of frozen/thawed oocytes remains unknown, which is vital for appropriate consultation regarding the number of oocytes to freeze to obtain at least a live birth in the future (61). Preliminary investigations revealed the overall percentage of warmed mature oocytes resulting in a live birth ranged between 4.2% and 10.8% (9.3 to 23.8 vitrified/thawed oocytes can lead to a live birth) (61, 63, 66). Besides, most previous studies were based on adult women aged >30 years, concluding that advanced age was negatively correlated with reproductive outcomes (61, 160, 161) and warranting more studies to counsel adolescent patients on the ideal number of oocytes needed to achieve a live birth (162).
While evidence indicates that advanced paternal age is less associated with increased rates of human embryonic aneuploidy (163, 164), it is well known that maternal age is highly correlated with oocyte/embryo aneuploidy (165) and it is one of the strongest predictors of IVF success (166). Interestingly, a recent study revealed that aneuploidy is also common among very young women (167). Gruhn et al. investigated the oocyte aneuploidy rates in women aged 9 to 43 years and found that oocytes aneuploidy rates from young women aged under 20 were significantly higher than those from women in their 20s and early 30s, which exhibited a U-shape curve. In comparison to women in their 20s to early 30s, younger women are also reported to experience higher rates of embryonic aneuploidy (165) and miscarriage (168), which deserves attention when providing counselling to post-pubertal adolescent patients on the clinical outcomes of oocyte cryopreservation.
Oocyte cryopreservation is an effective method for female fertility preservation. However, the relationship between long-term freezing and clinical efficacy, or offspring safety requires ongoing study (169). A multicenter study assessed the outcomes of oocytes cryopreserved for up to 48 months, no apparent differences in post-thawing oocyte survival, fertilization, cleavage, implantation, and live birth were observed when compared with those preserved for shorter periods (170). A more recent study reported a woman whose oocyte was frozen for 14 years and resulted in a healthy baby after fertilization with ICSI (171). On the other hand, congenital malformations were reported at rates ranging from 0.005% to 5.6% in several large cohorts (172, 173), which is close to the incidence in the USA national birth record in 2019 (3%) (169). Nonetheless, long-term follow-up of these children based on large cohorts is still needed.
Ovarian tissue cryopreservation
Currently, ovarian tissue cryopreservation is already considered an accepted technique for female fertility preservation given its success in restoring ovarian function and fertility (53). Recent studies demonstrated that reimplantation of ovarian tissue in the pelvic cavity resulted in the restoration of ovarian function in 85% to 95% of adult recipients (101, 103, 113, 174), as evidenced by the return of menstruation (98, 113, 175) or pregnancy (102, 113). Researchers also examined the serum hormone profiles before and after ovarian tissue transplantations, demonstrating a gradual decline in both follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels and return to premenopausal levels 4 to 5 months after transplantation, which was accompanied by the resumption of menstrual cycles and the disappearance of menopausal symptoms (45, 99, 176, 177). However, restoration of ovarian endocrine function may not be reflected by AMH, which was almost undetectable in most cases (176, 178, 179), indicating a limited follicular population in the graft. According to Diaz-Garcia et al., the mean intervals between ovarian tissue transplantation and ovarian function resumption was 94.3 days (103), with most reported cases ranging between 3 and 6.5 months (98, 99, 104, 108, 123, 124, 176, 178, 180–182). The time frame of ovarian function resumption is consistent with that of folliculogenesis (183).
Duration of ovarian function after grafting can depend on the quantity of primordial follicles at the time of transplant and proportion that survive the grafting process (99). The mean duration is approximately 4 to 5 years in humans (174). However, in a study including 41 young women (aged 32.9 on average at the time of OTT), more than half of the transplantations resulted in a functional life span between 1 and 4 years, with some cases lasting for more than 10 years while several cases lasting less than one year (99). The longest duration of restored ovarian function recorded to date is 13.5 years by repeating the transplantation procedure (98). The heterogeneity indicates the necessity of improving and standardizing procedures for ovarian tissue cryopreservation and transplantation.
Since the first live birth report after ovarian tissue autografting in 2004 (96) and the second in 2005 (97), the number of pregnancies and live birth have continued to climb steadily, showing an exponential trend (82). Live births after autografting of ovarian tissue cryopreserved before (184, 185) and after (186, 187) menarche have been reported recently. The number of live births after ovarian tissue transplantation was estimated to exceed 200 in 2020 (188). However, the total number of transplantations worldwide (the denominator) is unknown, leading to the unavailability of accurate pregnancy rates and live birth rates. In an early study based on five centers worldwide including 111 patients, the pregnancy rate and live birth rate were 29% and 21%, respectively (105). These figures were subsequently confirmed by several case series and pooled analyses with larger sample sizes (98, 99, 101–104, 113, 182), yielding a clinical pregnancy rate between 27.3% and 65.6%, and a live birth rate between 18.2% and 43.3%, respectively. Nonetheless, these results have been confounded by both patient factors (i.e. age at OTC/OTT, exposure to gonadotoxic therapy, the number and size of ovarian slices replaced, and residual ovarian function, etc.) and technical factors (i.e. surgical techniques, application of proangiogenic agents, the assistance of artificial reproductive techniques, etc.) (100).
Currently, most studies focus on adult subjects with little attention to ovarian tissue cryopreservation and transplantation from tissue taken from prepubertal children and post-pubertal adolescents. There are several large cohorts of young girls reporting ovarian tissue cryopreservation, but the return-to-use rates are extremely low (45, 189, 190), leaving limited data to evaluate the endocrinological and reproductive function after ovarian tissue replantation in this population. Table 2 includes some of the current reports on ovarian tissue transplantation that were cryopreserved at the age of ≤ 20 years. In 2012, there were two cases of ovarian tissue transplantation in pre-pubertal girls to induce puberty (191, 192), resulting in gonadotropins decline and estradiol secretion. Although ovarian activity ceased about 2 years after the grafting, both patients established normal menstrual cycles and secondary sex characteristics shortly after the grafting, demonstrating proof of concept in inducing puberty. As the amount of tissue required for pregnancy and parenthood is unknown in any individual, concerns regarding use of OTT for pubertal induction remain (198, 199).

Table 2 Current information about ovarian tissue cryopreservation and transplantation in prepubertal children and post-pubertal adolescents.
Another subject of study is the relationship between follicular density/quantity and the longevity of restored ovarian function. Several studies observed an association between younger age at the time of ovarian tissue cryopreservation and preferable clinical outcomes including the longevity of graft survival (200) and live birth rates (102, 103, 113, 196), though challenged by another study (99). The association may be, at least partly, explained by the greater number of follicles residing in the tissue upon harvest in the younger population. In fact, the primordial follicle pool in cryopreserved ovarian tissue retrieved from prepuberty adolescents is significantly larger than those from older patients (189, 201). However, in the report by Ernst et al. (191) and Poirot et al. (192), the ovarian tissue was cryopreserved at the age of 9 and 10 years, respectively. After the graft, however, the endocrinological function was maintained for merely 19 months and 2 years, respectively, much shorter than the average duration reported in adult subjects. These discrepancies mean much optimization is still needed to maximize the clinical outcomes.
Notably, some patients may undergo chemotherapy before ovarian tissue cryopreservation. The latest studies have demonstrated similar resumption in ovarian function and pregnancy rates per woman in patients who received low gonadotoxic risk chemotherapy compared to those who were chemo-naïve (98, 104, 182). Importantly, ovarian harvest in those who have achieved complete remission following chemotherapy may reduce the chance of malignant contamination among patients with leukemia (117–119). However, chemotherapy containing alkylating agents does adversely impact the clinical outcomes including both the pregnancy rate and live birth rate (98).
Sperm and testicular tissue cryopreservation
Sperm cryopreservation is the most successful method for male fertility preservation. While some studies suggested reduced sperm viability and motility after thawing (202, 203), cryopreserved sperm from patients with a previous malignancy has comparable potential to obtain a clinical pregnancy as ART evolves, especially with the use of ICSI for fertilization (204, 205).
The documented clinical pregnancy rates using thawed sperm collected before cancer therapy ranges from 18% to 57% (204, 206–210). Meanwhile, a higher success rate is observed in ICSI programs, followed by IVF, with intrauterine insemination (IUI) being the least successful (206–208, 210). According to an early study, it took a median of 3 cycles to get pregnant in ICSI, whereas 8 cycles were required in IVF (211). Similar pregnancy rates have been observed compared with non-cancer control or fresh sperm (205, 208). A large cohort involving 272 males with cancers reported a live birth rate of 62.1% per patient, comparable to the non-cancer infertile population (205). Specifically, the outcomes of couples using testicular sperm do not differ between fresh and frozen-thawed sperm among patients with Klinefelter syndrome (212, 213), obstructive azoospermia (214), and non-obstructive azoospermia (215, 216). But the cumulative live birth rate was lower than that of ejaculated sperm (216, 217).
Sperm can be stored for decades under ultra-low temperatures. The longest duration of sperm cryopreservation to date is 28 years, which successfully resulted in a healthy live birth with IUI (218). Unfortunately, it remains unknown whether the freezing-thawing process poses an adverse impact on the long-term development of children born with cryopreserved sperm.
For those patients who had their testicular tissue cryopreserved, the fertility restoration strategy includes autologous grafting of immature testicular tissue (145), injection of TSCs into the testis (146, 147) and in vitro maturation of TSCs (148, 149) and these options have been extensively discussed in a recent review (130). Due to its experimental nature, clinical outcomes on testicular tissue transplantation in human subjects are still unavailable regardless of great achievements obtained using animal models (20, 33, 50).
Transplantation of immature testicular tissue appears to be one of the most promising methods for male fertility preservation. Since the live birth of mice and rabbits after fresh and cryopreserved immature testicular tissue transplantation in 2002 (219). Achievements have been made on various animal models, with promise toward clinical use. In summary, functional spermatogenesis after testicular tissue graft has been made possible in mice (220), ferret (221), sheep (222), pigs (223), collared peccary (149), bison (224), buffalo (225), Coturnix japonica (226), and in non-human primates including marmoset (227), cynomolgus monkey (228), and rhesus macaques (145, 229). In some species, offspring using graft-derived sperm with ICSI have been reported (145, 223, 226, 228), proving its potential in fertility preservation and restoration in prepubertal males. The anatomy and physiology of the testis in non-human primates resemble humans the most and make them perfect preclinical models for ITT transplantation research (130). Most recently, fresh and cryopreserved testicular tissues from prepubertal rhesus macaques were autologously transplanted under the back skin and scrotal skin after castration. Surprisingly, all grafts survived, grew, and restored testosterone reproduction as well as endogenous spermatogenesis. A healthy female baby was produced with graft-originated sperm (145). This study marks the biggest milestone for testicular tissue cryopreservation and auto-transplantation toward clinical translation.
Reinjection of TSCs was first introduced in 1994 (146, 147). TSCs isolated from immature mice were injected into the testes of infertile hosts and successfully colonized the seminiferous tubules. The host mice restored natural spermatogenesis and produced offspring using sperm from donor tissue. From that onwards, the method has been proven successful in multiple animals. Offspring were obtained by either natural mating or assisted reproductive techniques after fertility recovery in mice (230, 231), rats (232, 233), goats (234), sheep (235), chicken (236), and zebrafish (237). However, the overall success using primate models is limited. In vivo spermatogenesis was recovered (229, 238, 239), and in some cases, embryo formation was documented (238), but no offspring have been reported yet.
In vitro maturation of TSCs was also established on mice models which successfully resulted in offspring using round spermatids with ICSI (230). Researches focusing on rats (240), pigs (241), calf (242), and buffalo (243) has successfully induced post-miotic cells (haploid germ cells), and some studies progressed to the formation of preimplantation blastocysts (244). But healthy offspring was reported in mice exclusively (130). IVM of TSCs in non-human primates (245, 246) and humans (247–249) has led to similar results. Only post-meiotic cells were documented. Overall, this technique is still in its infancy.
Transgender population
Transgender individuals represent a special population who recognize internal gender as different from biological gender. The latest statistics estimated that there are 150,000 young and 1.4 million adult transgender women (transwomen, MtF) or transgender men (transmen, FtM) in the United States (250). In addition, there is a trend towards presentation at younger ages (251). To alleviate gender dysphoria, many of them choose gender-affirming therapy including gender-affirming hormone therapy (GAHT) and gender-affirming surgery (GAS) (43), rendering temporary subfertility or permanent sterility (252). Accordingly, gender-affirming therapy, both hormonal and surgical, is one of the indications for fertility preservation (43, 253).
A variety of studies suggest transgender individuals have a strong desire for parenthood. 62% to 82% of transgender individuals want to have children, biological or adopted (254–256). But the desire to have children declines throughout the GAHT process (257). Meanwhile, nearly half of the transgender adolescents noted that their desire to have their biological children may change when they grow up (258) whereas a proportion of them regretted not undergoing fertility preservation (259, 260). A recent survey revealed that almost all (94.6%, 387/409) transgender respondents agreed that fertility preservation should be offered to all transgender individuals (261).
Several scientific societies have issued guidelines navigating health care to the transgender population, recommending that all transgender individuals should receive a consultation about potential fertility risks of gender-affirming treatments and preservation options before transition (43, 253, 262). However, no guideline specifies the optimal time to initiate discussion and counseling, leading to some situations where patients have their first discussion about fertility preservation after the initiation of gender-affirming therapy (263, 264). Inadequate and belated information provision puts patients in a dilemma between fertility preservation and discontinuation/delay of gender-affirming therapy. Considering most transgender persons are reluctant to postpone or suspend gender-affirming therapy (254, 261), it appears even more important to start consultation as earlier as possible.
Fertility preservation for transmen
Oocyte cryopreservation, embryo preservation, and ovarian tissue cryopreservation are established methods for fertility preservation for transmen. While ovarian stimulation and the accompanying unpleasant experience of estradiol elevation, vaginal examination and oocyte retrieval are unavoidable for oocyte cryopreservation (265, 266), ovarian tissue can be obtained at the time of gender-affirming surgery. For transmen who have not started GAHT, ovarian stimulation protocols are the same as those used for infertility (251). GnRH antagonist protocol can be considered for its efficacy in oocyte yield (32), and the addition of letrozole can reduce estradiol levels and related symptoms (267).
Some transmen may have already started testosterone treatment before ovarian stimulation. It was previously deemed that testosterone induced polycystic ovary syndrome (PCOS) (268). But recent studies demonstrated testosterone exposure for more than a year did not disturb ovarian follicle distribution (269, 270). Some studies suggest temporary discontinuation of hormonal therapy before ovarian stimulation (251, 265). While some small studies demonstrated no differences in oocyte yield between transmen with continuous hormonal therapy and ciswomen (271–273).
In vitro maturation of immature oocytes from oophorectomy can be another source of oocytes (274). However, a recent study demonstrated the low feasibility of this strategy for fertility preservation (275). Almost 2,000 cumulus-oocyte-complex (COCs) were collected at the time of oophorectomy and merely 23.8% of them matured after in vitro culture. Of the 151 out of 208 mature oocytes that survived vitrification/thawing, 139 oocytes were fertilized with ICSI, leading to 48 normal fertilizations (34.5%) and 4 transferable blastocysts. Collectively, given the poor maturation rate (28% to 36%) and utilization efficacy after IVM (59, 276), as well as lower pregnancy rates and higher pregnancy loss rates (277–279), IVM should not be used as the only method for fertility preservation in transmen (280).
For prepuberty transmen and those who are unwilling to accept ovarian stimulation, ovarian tissue cryopreservation is the sole option for fertility preservation (253). Ovarian tissue can be obtained after oophorectomy without testosterone discontinuation (280). Auto-transplantation of cryopreserved ovarian tissue has resulted in more than 200 live birth (188). But there is no report of ovarian tissue transplantation in transmen.
Fertility preservation for transwomen
Sperm cryopreservation is a reliable option for fertility preservation among transwomen (253). Sperm may be obtained by either masturbation, assisted ejaculation, or TESE (33, 129). Cryopreserved sperm could be used for IUI, alternatively, IVF/ICSI using oocytes from a donor or cisgender female partner (251). Cumulative live birth rate using frozen/thawed sperm before anticancer treatment can be as high as 62.1% with ART (205), but these results may not apply to the transgender population because gender-affirming therapy (280–282) and some behavioral factors (283, 284) pose reversible or irreversible threats on semen quality.
For prepubertal transgender girls, testicular tissue cryopreservation remains the only option for fertility preservation (251). Some transgender girls may have received puberty suppression and estrogen supplementation at different pubertal stages (43), leading to decreased testosterone levels. The deficiency of intratesticular testosterone results in severe spermatogenesis dysfunction (280). The effect of testosterone suppression on spermatogenesis in adults have been extensively investigated in cisgender male contraceptive research and is reversible after cessation of suppression therapy (285). But the effect on pubertal transgender girls remains partly unanswered. de Nie et al. investigated the histology of testes using orchiectomy samples under testosterone suppression and/or estrogen exposure (286). They found only immature germ cells (spermatocytes and spermatogonia) present in the seminiferous tubules when medical intervention started at Tanner stage 2-3, with additional mature sperm observed in 57% of subjects who initiated medical treatment at Tanner stage 4 or later. These findings indicate the potential of testicular tissue cryopreservation for fertility after the initiation of puberty suppression and estrogen therapy. However, testicular tissue cryopreservation is currently experimental (131). Future use depends on advances in IVM of testicular stem cells since the in vivo microenvironment for spermatogenesis is unobtainable after orchiectomy.
Fertility preservation program
Current challenges
Several scientific societies have issued clinical practice guidelines for fertility preservation in pediatric and adolescent cancer populations (11, 17, 32, 50, 76), but surveys indicate limited knowledge of guidelines and poor compliance with recommendations by medical professionals (12, 13, 287, 288). According to a survey in the United States, only 46% and 12% of oncologists routinely refer male and female pubertal patients to fertility preservation services before cancer treatment, respectively (289). Similar research among adolescent and young adult cancer survivors reveals that 80% and 68% of male patients can recall being offered information about potential fertility impairment and referral to fertility preservation service, but the figures for female patients are only 48% and 14%, respectively (290). Evidence suggests that most patients and their parents are dissatisfied with the content of information that healthcare professionals provided concerning fertility risk and available options to preserve it (17). Younger patients and their parents are concerned about fertility issues, but they find it difficult to extend discussions with their physicians (291–293). A major barrier hindering preferable fertility preservation practice is the lack of a structured and coordinated fertility preservation program (12, 13). Meanwhile, some well-organized fertility preservation programs have been proven very successful (294–297).
Education for health professionals
Clinicians’ knowledge and attitudes toward fertility preservation significantly influences fertility preservation practice (12, 13, 288, 298). Physicians may be restrained by their limited understanding of the gonadotoxic nature of chemotherapy or radiotherapy, potential risks regarding future family planning patients may face, fertility preservation possibilities, and the highly time-sensitive nature of this intervention (12, 288, 299). While some physicians have realized the sensitivity and importance of fertility issues, oncologists tend to provide treatments that maximize the chance of survival and regard fertility issues as a non-priority (12, 76). However, based on the increasing proportion of children and adolescents who survive malignancies (1–3), it is crucial to focus on patients’ quality of life (294, 300) and incorporate fertility preservation into cancer care (76, 301). Accomplishing the objectives should start with educating healthcare professionals (12, 76, 295), possibly including pediatric oncologists, radiation oncologists, gynecologists, urologists, hematologists, surgeons, nurses (11, 32, 127, 302). The clinical team is supposed to have sound knowledge about infertility risk assessment and fertility preservation consultation (11, 12, 17, 32, 76) as fertility risk assessment of specific agents or therapy regimes has been extensively discussed in previous guidelines (25, 47–50). Some of the most common gonadotoxic agents are presented in Table 3. To better facilitate fertility preservation, it is suggested to be incorporated into general education of oncology (76, 294, 302). According to a recent study, a simple fertility training program can considerably increase oncologists’ knowledge of infertility risk assessment and fertility preservation strategies (303).
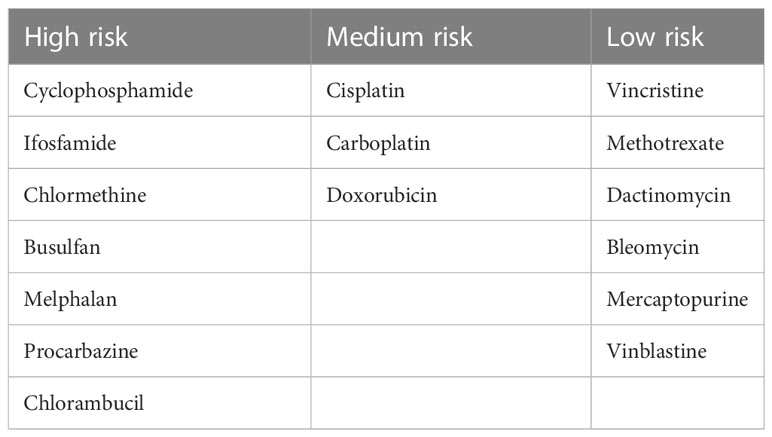
Table 3 Estimated risk of chemotherapy for gonadal function (46).
Informed consent
Discussion about potential fertility risks and methods to preserve it must begin at the time of diagnosis (mostly at the time of cancer diagnosis) (11, 76). It may be necessary to assume that every young patient diagnosed with a disease that requires gonadotoxic therapy is concerned with fertility issues (127). One approach to promote fertility preservation practice would be to make it the clinicians’ (pediatricians and oncologists in particular) regular responsibility to evaluate potential fertility risks and initiate discussions about fertility preservation including options, benefits, risks, and costs with patients (53, 294). It is proposed that the clinical team assign a knowledgeable team member to offer a comprehensive and in-depth consultation, providing detailed information concerning potential risks of fertility loss with proposed treatment regimes, current options for fertility preservation, possible risks of delaying treatment, the overall prognosis of cancer, and location of local or regional fertility preservation service, and be ready to answer questions that patients are interested (294).
A formal and separate discussion about fertility issues is advocated to ensure patients’ full comprehension of potential fertility risks and fertility preservation options (136, 294). Importantly, considering the sensitive nature of the age group and topics, patients should be offered a chance to speak freely without the presence of their parents (136, 294). In addition, institutions can design printed or online education materials for interested patients and the ordinary public to facilitate information provision (32). Notably, both formal conversation and supplementary materials should be organized in lay languages and avoid professional terms to ensure that recipients can comprehend the information (127, 294). The provision of relevant medical information should be documented in the patient’s medical record and patients who decide to seek fertility preservation must provide written informed consent (32, 294).
Referral
The establishment of a standardized intra-institutional or inter-institutional referral pathway between the clinical team and fertility preservation team is strongly suggested (32, 53). In addition, a coordinator between both teams would play a crucial role in navigating the fertility preservation process (32, 127). This institutional arrangement has several advantages.
Firstly, it facilitates fertility preservation consultation (32, 294, 304). Concerns by oncologists about lack of efficacy can hinder referral of patients to fertility preservation services (12), thus, collaboration with fertility specialists allows the chance to eliminate their doubts and update them on the latest fertility preservation technologies (76). Furthermore, fertility preservation specialists can contribute to fertility education provided to oncologists as described above.
Secondly, it facilitates referral (32, 294). Another barrier hindering oncologists’ raising fertility discussion is that they are not aware of local or regional fertility services (12, 302). A direct link between the clinical team and the fertility preservation team would greatly facilitate referral. For instance, a male patient who wishes to bank sperm can make first contact with the sperm bank (294, 296, 304). This would not only save patients time and costs upon the life-changing event (cancer) but also reduce their psychological stress.
Thirdly, it enables optimal decision-making (76). The fertility team is proficient in fertility preservation techniques. But they must cooperate with the clinical team to decide whether it is medically feasible to perform fertility preservation procedures and determine the optimal timing and strategy (53, 76, 304).
Finally, it reduces repetitive work and improve efficiency. As described above, a fertility specialist can engage in both patient counseling and proposing a feasible fertility preservation plan with the clinical team, saving their time and energy. A fertility specialist can focus on other affairs concerning fertility preservation, such as logistics of the procedure, cryopreservation of relevant materials, and future use. Considering the highly time-sensitive nature of fertility preservation, any improvement in efficiency would benefit patients significantly.
Effectiveness and efficiency
Based on previous literature, there are two identified key factors in establishing an effective and efficient fertility preservation program, namely sensitive faculties (294, 297, 304, 305) and a rapid referral pathway (294, 295, 304). Those patients who require fertility preservation do not generally visit a reproductive clinic. Instead, most of these patients are identified in a pediatric or an oncological clinic. The optimal strategy would be educating pediatricians, oncologists, and relevant nurses to raise their sensitivity to fertility issues (32). More importantly, they should be familiar with common gonadotoxic therapy so that they can assess fertility risks and decide whether to refer a patient or not and fertility preservation can be moved forward quickly. Moreover, a rapid referral approach would substantially increase patients’ awareness and accessibility to fertility preservation services. Patients and their families tend to feel overwhelmed upon the diagnosis, sparing them limited time and energy to access information concerning fertility risks and ways to preserve it (294). As mentioned previously, even some medical professionals lack the necessary knowledge about the potential fertility risks of chemotherapy and radiotherapy and available local or regional fertility preservation services (12, 13, 302). It is reasonable to assume most patients and their families are ignorant of these risks and relevant services. A rapid referral pathway significantly reduces the time and costs patients need to access fertility preservation services. The role of the fertility preservation team is relatively dependent on referral since patients do not generally visit them. However, once engaged, they can work with the clinical team and propose an optimal fertility preservation scheme as soon as possible. Given the urgency of both cancer therapy and fertility preservation, any delay in the process would potentially harm patients’ interests.
Future directions
Although different communities have made great achievements in fertility preservation, there are still some restrictions in clinical practice. For instance, unlike embryo or gamete cryopreservation, transplantation of cryopreserved ovarian tissue generally leads to variable outcomes between patients and institutions, warranting optimization and standardization of this technique. Currently, the greatest challenge is the massive follicle atresia after transplantation, with more than 70% of primordial follicles failing to survive (116). Future studies should focus on promoting follicular survival by enhancing revascularization and reducing ischemia-reperfusion injury soon after the transplantation (54). Potential strategies include transplanting ovarian tissue with biocompatible decellularized extracellular matrix scaffold (306), and the utilization of antioxidants, anti-apoptotic agents (307, 308), or proangiogenic factors (309, 310). In addition, artificial ovary (311) and complete in vitro development of follicles (312) are potential strategies for female fertility restoration. Recent advances in testicular tissue autograft from prepubertal rhesus macaques are encouraging (145), highlighting its promise in male fertility restoration but more similar studies are required to move it towards open clinical trials. Meanwhile, additional efforts are required to address the risks of malignancy reintroducing and investigate the long-term health of children born through fertility preservation programs. Finally, disciplines must collaborate to set up an effective and efficient fertility preservation program that enhances awareness among medical professionals and patients and removes the barriers to fertility preservation services.
Conclusions
In conclusion, fertility preservation is an increasingly important issue in pediatric and adolescent healthcare as most malignant diseases are curable with contemporary means. Mature gamete cryopreservation is the most reliable and successful strategy for fertility preservation when embryo freezing is not practicable. In addition, ovarian tissue cryopreservation is an effective option for female fertility preservation though there is room for improvement in its efficacy. Autograft of immature testicular tissue is currently the most promising method for prepubertal patients, but additional efforts are required to gather data on animal models and in clinical trials.
Author contributions
LC, ZD and XC: Substantial contribution to the conception and design of the work. LC: Participation in the acquisition of literature. LC and XC: Manuscript drafting and revision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Shenzhen Key Medical Discipline Construction Fund (Grant No. SZXK028), Shenzhen Science and Technology Program (Grant No. JCYJ20210324141403009, RCYX20210609104608036) and Natural Science Funding of China (Grant No. 82201851).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mertens AC, Yong J, Dietz AC, Kreiter E, Yasui Y, Bleyer A, et al. Conditional survival in pediatric malignancies: analysis of data from the childhood cancer survivor study and the surveillance, epidemiology, and end results program. Cancer (2015) 121(7):1108–17. doi: 10.1002/cncr.29170
2. Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the united states: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev (2015) 24(4):653–63. doi: 10.1158/1055-9965.EPI-14-1418
3. Hudson MM, Link MP, Simone JV. Milestones in the curability of pediatric cancers. J Clin Oncol (2014) 32(23):2391–7. doi: 10.1200/JCO.2014.55.6571
4. Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update (2019) 25(6):673–93. doi: 10.1093/humupd/dmz027
5. Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol (2015) 3(7):556–67. doi: 10.1016/S2213-8587(15)00039-X
6. Fernández-Juárez G, Rojas-Rivera J, Logt AV, Justino J, Sevillano A, Caravaca-Fontán F, et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int (2021) 99(4):986–98. doi: 10.1016/j.kint.2020.10.014
7. Oktay K, Bedoschi G, Berkowitz K, Bronson R, Kashani B, McGovern P, et al. Fertility preservation in women with turner syndrome: a comprehensive review and practical guidelines. J Pediatr Adolesc Gynecol (2016) 29(5):409–16. doi: 10.1016/j.jpag.2015.10.011
8. Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis (2017) 76(3):476–85. doi: 10.1136/annrheumdis-2016-209770
9. Stein DM, Victorson DE, Choy JT, Waimey KE, Pearman TP, Smith K, et al. Fertility preservation preferences and perspectives among adult Male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol (2014) 3(2):75–82. doi: 10.1089/jayao.2014.0007
10. Nahata L, Caltabellotta NM, Yeager ND, Lehmann V, Whiteside SL, O’Brien SH, et al. Fertility perspectives and priorities among male adolescents and young adults in cancer survivorship. Pediatr Blood Cancer (2018) 65(7):e27019. doi: 10.1002/pbc.27019
11. Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol (2018) 36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914
12. Covelli A, Facey M, Kennedy E, Brezden-Masley C, Gupta AA, Greenblatt E, et al. Clinicians’ perspectives on barriers to discussing infertility and fertility preservation with young women with cancer. JAMA Netw Open (2019) 2(11):e1914511. doi: 10.1001/jamanetworkopen.2019.14511
13. van den Berg M, Baysal Ö, Nelen W, Braat DDM, Beerendonk CCM, Hermens R. Professionals’ barriers in female oncofertility care and strategies for improvement. Hum Reprod (2019) 34(6):1074–82. doi: 10.1093/humrep/dez062
14. Magrath I, Steliarova-Foucher E, Epelman S, Ribeiro RC, Harif M, Li CK, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol (2013) 14(3):e104–16. doi: 10.1016/S1470-2045(13)70008-1
15. Ni X, Li Z, Li X, Zhang X, Bai G, Liu Y, et al. Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: a cross-sectional study. Lancet (2022) 400(10357):1020–32. doi: 10.1016/S0140-6736(22)01541-0
16. Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril (2013) 100(5):1180–6. doi: 10.1016/j.fertnstert.2013.08.010
17. Mulder RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC, et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE consortium and the international late effects of childhood cancer guideline harmonization group. Lancet Oncol (2021) 22(2):e45–56. doi: 10.1016/S1470-2045(20)30594-5
18. van Dorp W, Mulder RL, Kremer LC, Hudson MM, van den Heuvel-Eibrink MM, van den Berg MH, et al. Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: a report from the international late effects of childhood cancer guideline harmonization group in collaboration with the PanCareSurFup consortium. J Clin Oncol (2016) 34(28):3440–50. doi: 10.1200/JCO.2015.64.3288
19. Castillo LA, Craft AW, Kernahan J, Evans RG, Aynsley-Green A. Gonadal function after 12-gy testicular irradiation in childhood acute lymphoblastic leukaemia. Med Pediatr Oncol (1990) 18(3):185–9. doi: 10.1002/mpo.2950180304
20. Mulder RL, Font-Gonzalez A, Green DM, Loeffen EAH, Hudson MM, Loonen J, et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE consortium and the international late effects of childhood cancer guideline harmonization group. Lancet Oncol (2021) 22(2):e57–67. doi: 10.1016/S1470-2045(20)30582-9
21. Thomas-Teinturier C, Allodji RS, Svetlova E, Frey MA, Oberlin O, Millischer AE, et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod (2015) 30(6):1437–46. doi: 10.1093/humrep/dev060
22. van den Berg MH, Overbeek A, Lambalk CB, Kaspers GJL, Bresters D, van den Heuvel-Eibrink MM, et al. Long-term effects of childhood cancer treatment on hormonal and ultrasound markers of ovarian reserve. Hum Reprod (2018) 33(8):1474–88. doi: 10.1093/humrep/dey229
23. Anderson RA, Cameron D, Clatot F, Demeestere I, Lambertini M, Nelson SM, et al. Anti-müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. Hum Reprod Update (2022) 28(3):417–34. doi: 10.1093/humupd/dmac004
24. Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol (2009) 27(16):2677–85. doi: 10.1200/JCO.2008.20.1541
25. Schüring AN, Fehm T, Behringer K, Goeckenjan M, Wimberger P, Henes M, et al. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. part I: indications for fertility preservation. Arch Gynecol Obstet (2018) 297(1):241–55. doi: 10.1007/s00404-017-4594-3
26. Green DM, Sklar CA, Boice JD Jr., Mulvihill JJ, Whitton JA, Stovall M, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the childhood cancer survivor study. J Clin Oncol (2009) 27(14):2374–81. doi: 10.1200/JCO.2008.21.1839
27. Charpentier AM, Chong AL, Gingras-Hill G, Ahmed S, Cigsar C, Gupta AA, et al. Anti-müllerian hormone screening to assess ovarian reserve among female survivors of childhood cancer. J Cancer Surviv (2014) 8(4):548–54. doi: 10.1007/s11764-014-0364-4
28. Wang K, Tepper JE. Radiation therapy-associated toxicity: etiology, management, and prevention. CA Cancer J Clin (2021) 71(5):437–54. doi: 10.3322/caac.21689
29. Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude lifetime cohort study. Lancet Oncol (2014) 15(11):1215–23. doi: 10.1016/S1470-2045(14)70408-5
30. Okada K, Fujisawa M. Recovery of spermatogenesis following cancer treatment with cytotoxic chemotherapy and radiotherapy. World J Mens Health (2019) 37(2):166–74. doi: 10.5534/wjmh.180043
31. Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr (2005) (34):12–7. doi: 10.1093/jncimonographs/lgi003
32. Anderson RA, Amant F, Braat D, D’Angelo A, Chuva de Sousa Lopes SM, Demeestere I, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open (2020) 2020(4):hoaa052. doi: 10.1093/hropen/hoaa052
33. Martinez F. Update on fertility preservation from the Barcelona international society for fertility preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril (2017) 108(3):407–15.e11. doi: 10.1016/j.fertnstert.2017.05.024
34. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update on the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis (2021) 80(1):14–25. doi: 10.1136/annrheumdis-2020-218272
35. Ono M, Matsumoto K, Boku N, Fujii N, Tsuchida Y, Furui T, et al. Indications for fertility preservation not included in the 2017 Japan society of clinical oncology guideline for fertility preservation in pediatric, adolescent, and young adult patients treated with gonadal toxicity, including benign diseases. Int J Clin Oncol (2022) 27(2):301–9. doi: 10.1007/s10147-021-02082-9
36. Wang J, Wang B, Sun Z, Xue K. Therapeutic effects of rituximab combined with cyclophosphamide on refractory idiopathic thrombocytopenic purpura. Exp Ther Med (2019) 17(3):2137–42. doi: 10.3892/etm.2019.7196
37. Yasmin E, Balachandren N, Davies MC, Jones GL, Lane S, Mathur R, et al. Fertility preservation for medical reasons in girls and women: British fertility society policy and practice guideline. Hum Fertil (Camb) (2018) 21(1):3–26. doi: 10.1080/14647273.2017.1422297
38. Joshi S, Savani BN, Chow EJ, Gilleece MH, Halter J, Jacobsohn DA, et al. Clinical guide to fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transpl (2014) 49(4):477–84. doi: 10.1038/bmt.2013.211
39. Oktay K, Rodriguez-Wallberg KA, Sahin G. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with turner syndrome mosaicism and impending premature ovarian failure. Fertil Steril (2010) 94(2):753.e15–9. doi: 10.1016/j.fertnstert.2010.01.044
40. De Sanctis V, Ciccone S. Fertility preservation in adolescents with klinefelter’s syndrome. Pediatr Endocrinol Rev (2010) 8 Suppl 1:178–81.
41. Elizur SE, Chian RC, Holzer HE, Gidoni Y, Tulandi T, Tan SL. Cryopreservation of oocytes in a young woman with severe and symptomatic endometriosis: a new indication for fertility preservation. Fertil Steril (2009) 91(1):293.e1–3. doi: 10.1016/j.fertnstert.2007.06.040
42. Stahl PJ, Stember DS, Hsiao W, Schlegel PN. Indications and strategies for fertility preservation in men. Clin Obstet Gynecol (2010) 53(4):815–27. doi: 10.1097/GRF.0b013e3181f980b3
43. Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-Dysphoric/Gender-Incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2017) 102(11):3869–903. doi: 10.1210/jc.2017-01658
44. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American College of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol (2020) 72(4):529–56. doi: 10.1002/art.41191
45. Jadoul P, Guilmain A, Squifflet J, Luyckx M, Votino R, Wyns C, et al. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod (2017) 32(5):1046–54. doi: 10.1093/humrep/dex040
46. Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol (2005) 6(4):209–18. doi: 10.1016/S1470-2045(05)70092-9
47. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO clinical practice guidelines(†). Ann Oncol (2020) 31(12):1664–78. doi: 10.1016/j.annonc.2020.09.006
48. Trottmann M, Becker AJ, Stadler T, Straub J, Soljanik I, Schlenker B, et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol (2007) 52(2):355–67. doi: 10.1016/j.eururo.2007.03.085
49. Lambertini M, Del Mastro L, Pescio MC, Andersen CY, Azim HA Jr., Peccatori FA, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med (2016) 14:1. doi: 10.1186/s12916-015-0545-7
50. Tournaye H, Dohle GR, Barratt CL. Fertility preservation in men with cancer. Lancet (2014) 384(9950):1295–301. doi: 10.1016/S0140-6736(14)60495-5
51. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol (2006) 24(18):2917–31. doi: 10.1200/JCO.2006.06.5888
52. Amato P, Brzyski R, Benward J, Stein A, Steinbock B, Wilder B, et al. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril (2013) 100(5):1224–31. doi: 10.1016/j.fertnstert.2013.08.041
53. Penzias A, Bendikson K, Falcone T, Gitlin S, Gracia C, Hansen K, et al. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril (2019) 112(6):1022–33. doi: 10.1016/j.fertnstert.2019.09.013
54. Fisch B, Abir R. Female fertility preservation: past, present and future. Reproduction (2018) 156(1):F11–f27. doi: 10.1530/REP-17-0483
55. Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful oocyte cryopreservation in reproductive-aged cancer survivors. Obstet Gynecol (2016) 127(3):474–80. doi: 10.1097/AOG.0000000000001248
56. Peddie VL, Maheshwari A. Successful controlled ovarian stimulation and vitrification of oocytes in an adolescent diagnosed with myelodysplastic/pre-malignant clone with monosomy 7. Hum Fertil (Camb) (2018) 21(1):39–44. doi: 10.1080/14647273.2017.1347288
57. Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI(†). Hum Reprod Open (2020) 2020(2):hoaa009. doi: 10.1093/hropen/hoaa009
58. Huang JY, Chian RC, Gilbert L, Fleiszer D, Holzer H, Dermitas E, et al. Retrieval of immature oocytes from unstimulated ovaries followed by in vitro maturation and vitrification: a novel strategy of fertility preservation for breast cancer patients. Am J Surg (2010) 200(1):177–83. doi: 10.1016/j.amjsurg.2009.04.004
59. Fasano G, Dechène J, Antonacci R, Biramane J, Vannin AS, Van Langendonckt A, et al. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod BioMed Online (2017) 34(6):575–82. doi: 10.1016/j.rbmo.2017.03.007
60. Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril (2016) 105(3):755–64.e8. doi: 10.1016/j.fertnstert.2015.11.027
61. Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril (2016) 105(2):459–66.e2. doi: 10.1016/j.fertnstert.2015.10.026
62. Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod (2010) 25(9):2239–46. doi: 10.1093/humrep/deq146
63. Nagy ZP, Anderson RE, Feinberg EC, Hayward B, Mahony MC. The human oocyte preservation experience (HOPE) registry: evaluation of cryopreservation techniques and oocyte source on outcomes. Reprod Biol Endocrinol (2017) 15(1):10. doi: 10.1186/s12958-017-0228-7
64. Sciorio R, Antonini E, Engl B. Live birth and clinical outcome of vitrification-warming donor oocyte programme: an experience of a single IVF unit. Zygote (2021) 29(5):410–6. doi: 10.1017/S0967199421000204
65. Jia QP, Sun WQ. PERSPECTIVE: cryopreservation of human oocytes and the ‘Carryover’ effect on early embryo development. Cryo Lett (2021) 42(3):120–8.
66. Seshadri S, Saab W, Exeter H, Drew E, Petrie A, Davies M, et al. Clinical outcomes of a vitrified donor oocyte programme: a single UK centre experience. Eur J Obstet Gynecol Reprod Biol (2018) 225:136–40. doi: 10.1016/j.ejogrb.2018.04.017
67. Papatheodorou A, Vanderzwalmen P, Panagiotidis Y, Petousis S, Gullo G, Kasapi E, et al. How does closed system vitrification of human oocytes affect the clinical outcome? a prospective, observational, cohort, noninferiority trial in an oocyte donation program. Fertil Steril (2016) 106(6):1348–55. doi: 10.1016/j.fertnstert.2016.07.1066
68. García JI, Noriega-Portella L, Noriega-Hoces L. Efficacy of oocyte vitrification combined with blastocyst stage transfer in an egg donation program. Hum Reprod (2011) 26(4):782–90. doi: 10.1093/humrep/der008
69. Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril (2010) 93(2):391–6. doi: 10.1016/j.fertnstert.2009.02.067
70. Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update (2017) 23(2):139–55. doi: 10.1093/humupd/dmw038
71. Glujovsky D, Riestra B, Sueldo C, Fiszbajn G, Repping S, Nodar F, et al. Vitrification versus slow freezing for women undergoing oocyte cryopreservation. Cochrane Database Syst Rev (2014) (9):Cd010047. doi: 10.1002/14651858.CD010047.pub2
72. Senra JC, Roque M, Talim MCT, Reis FM, Tavares RLC. Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound Obstet Gynecol (2018) 51(1):77–86. doi: 10.1002/uog.18934
73. Li ZY, Dong YL, Cao XZ, Ren SS, Zhang Z. Gonadotropin-releasing hormone agonists for ovarian protection during breast cancer chemotherapy: a systematic review and meta-analysis. Menopause (2022) 29(9):1093–100. doi: 10.1097/GME.0000000000002019
74. Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol (2018) 36(19):1981–90. doi: 10.1200/JCO.2018.78.0858
75. Lambertini M, Ceppi M, Poggio F, Peccatori FA, Azim HA Jr., Ugolini D, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol (2015) 26(12):2408–19. doi: 10.1093/annonc/mdv374
76. Daar J, Benward J, Collins L, Davis J, Francis L, Gates E, et al. Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril (2018) 110(3):380–6. doi: 10.1016/j.fertnstert.2018.05.034
77. Oktay K, Sönmezer M. Gonadotropin-releasing hormone analogs in fertility preservation-lack of biological basis? Nat Clin Pract Endocrinol Metab (2008) 4(9):488–9. doi: 10.1038/ncpendmet0892
78. Pereyra Pacheco B, Méndez Ribas JM, Milone G, Fernández I, Kvicala R, Mila T, et al. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: a preliminary report. Gynecol Oncol (2001) 81(3):391–7. doi: 10.1006/gyno.2001.6181
79. Meli M, Caruso-Nicoletti M, La Spina M, Nigro LL, Samperi P, D’Amico S, et al. Triptorelin for fertility preservation in adolescents treated with chemotherapy for cancer. J Pediatr Hematol Oncol (2018) 40(4):269–76. doi: 10.1097/MPH.0000000000001144
80. Roness H, Kalich-Philosoph L, Meirow D. Prevention of chemotherapy-induced ovarian damage: possible roles for hormonal and non-hormonal attenuating agents. Hum Reprod Update (2014) 20(5):759–74. doi: 10.1093/humupd/dmu019
81. Mertes H. Let’s not forget that many prepubertal girls do have other options besides ovarian tissue cryopreservation. Hum Reprod (2015) 30(9):2011–3. doi: 10.1093/humrep/dev176
82. Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med (2017) 377(17):1657–65. doi: 10.1056/NEJMra1614676
83. von Wolff M, Germeyer A, Liebenthron J, Korell M, Nawroth F. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part II: Fertil Preservation Techniques Arch Gynecol Obstet (2018) 297(1):257–67. doi: 10.1007/s00404-017-4595-2
84. Gudlevičienė Ž, Žilinskas K, Kundrotas G, Grubliauskaitė M, Baltriukienė D, Bukelskienė V. Slow-freezing cryopreservation ensures high ovarian tissue quality followed by In Vivo and In Vitro methods and is safe for fertility preservation. Medicina (Kaunas) (2020) 56(10). doi: 10.3390/medicina56100547
85. Fabbri R, Vicenti R, Macciocca M, Martino NA, Dell’Aquila ME, Pasquinelli G, et al. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum Reprod (2016) 31(8):1838–49. doi: 10.1093/humrep/dew134
86. Dalman A, Deheshkar Gooneh Farahani NS, Totonchi M, Pirjani R, Ebrahimi B, Rezazadeh Valojerdi M. Slow freezing versus vitrification technique for human ovarian tissue cryopreservation: an evaluation of histological changes, WNT signaling pathway and apoptotic genes expression. Cryobiology (2017) 79:29–36. doi: 10.1016/j.cryobiol.2017.09.007
87. Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep (2017) 7(1):8538. doi: 10.1038/s41598-017-09005-7
88. Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees c. Hum Reprod (1994) 9(4):597–603. doi: 10.1093/oxfordjournals.humrep.a138556
89. Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 c. Endocrinology (1999) 140(1):462–71. doi: 10.1210/endo.140.1.6453
90. Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, et al. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod (1996) 11(6):1268–72. doi: 10.1093/oxfordjournals.humrep.a019370
91. Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod (1996) 11(7):1487–91. doi: 10.1093/oxfordjournals.humrep.a019423
92. Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod (1998) 13(5):1133–8. doi: 10.1093/humrep/13.5.1133
93. Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med (2000) 342(25):1919. doi: 10.1056/NEJM200006223422516
94. Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. Jama (2001) 286(12):1490–3. doi: 10.1001/jama.286.12.1490
95. Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet (2004) 363(9412):837–40. doi: 10.1016/S0140-6736(04)15728-0
96. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet (2004) 364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X
97. Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med (2005) 353(3):318–21. doi: 10.1056/NEJMc055237
98. Dolmans MM, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, et al. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril (2021) 115(5):1102–15. doi: 10.1016/j.fertnstert.2021.03.008
99. Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod (2015) 30(12):2838–45. doi: 10.1093/humrep/dev230
100. Pfeifer S, Goldberg J, Lobo R, Pisarska M, Thomas M, Widra E, et al. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril (2014) 101(5):1237–43. doi: 10.1016/j.fertnstert.2014.02.052
101. Liebenthron J, Montag M, Reinsberg J, Köster M, Isachenko V, van der Ven K, et al. Overnight ovarian tissue transportation for centralized cryobanking: a feasible option. Reprod BioMed Online (2019) 38(5):740–9. doi: 10.1016/j.rbmo.2019.01.006
102. Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet (2018) 35(4):561–70. doi: 10.1007/s10815-018-1144-2
103. Diaz-Garcia C, Domingo J, Garcia-Velasco JA, Herraiz S, Mirabet V, Iniesta I, et al. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril (2018) 109(3):478–85.e2. doi: 10.1016/j.fertnstert.2017.11.018
104. Shapira M, Dolmans MM, Silber S, Meirow D. Evaluation of ovarian tissue transplantation: results from three clinical centers. Fertil Steril (2020) 114(2):388–97. doi: 10.1016/j.fertnstert.2020.03.037
105. Donnez J, Dolmans MM, Diaz C, Pellicer A. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril (2015) 104(5):1097–8. doi: 10.1016/j.fertnstert.2015.08.005
106. Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod BioMed Online (2012) 25(2):128–32. doi: 10.1016/j.rbmo.2012.03.014
107. Meirow D, Ra’anani H, Shapira M, Brenghausen M, Derech Chaim S, Aviel-Ronen S, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril (2016) 106(2):467–74. doi: 10.1016/j.fertnstert.2016.04.031
108. Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril (2004) 82(4):930–2. doi: 10.1016/j.fertnstert.2004.02.137
109. Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, et al. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod (2006) 21(8):2006–9. doi: 10.1093/humrep/del140
110. Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod (2013) 28(11):2996–9. doi: 10.1093/humrep/det360
111. Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod (2014) 29(8):1828. doi: 10.1093/humrep/deu119
112. Dolmans MM, Donnez J. Fertility preservation in women for medical and social reasons: oocytes vs ovarian tissue. Best Pract Res Clin Obstet Gynaecol (2021) 70:63–80. doi: 10.1016/j.bpobgyn.2020.06.011
113. Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci (2017) 24(8):1111–20. doi: 10.1177/1933719117702251
114. Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology (1994) 134(3):1146–54. doi: 10.1210/endo.134.3.8119153
115. Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril (2010) 93(5):1676–85. doi: 10.1016/j.fertnstert.2009.04.048
116. Gosden RG. Low temperature storage and grafting of human ovarian tissue. Mol Cell Endocrinol (2000) 163(1-2):125–9. doi: 10.1016/S0303-7207(99)00248-8
117. Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update (2013) 19(5):483–506. doi: 10.1093/humupd/dmt020
118. Shapira M, Raanani H, Barshack I, Amariglio N, Derech-Haim S, Marciano MN, et al. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril (2018) 109(1):48–53. doi: 10.1016/j.fertnstert.2017.09.001
119. Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sørensen SD, Rosendahl M, et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood (2012) 120(22):4311–6. doi: 10.1182/blood-2012-01-403022
120. Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril (2013) 99(6):1514–22. doi: 10.1016/j.fertnstert.2013.03.027
121. Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood (2010) 116(16):2908–14. doi: 10.1182/blood-2010-01-265751
122. Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, et al. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod BioMed Online (2011) 22(2):162–71. doi: 10.1016/j.rbmo.2010.10.015
123. Ernst EH, Offersen BV, Andersen CY, Ernst E. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue due to cancer recurrence. J Assist Reprod Genet (2013) 30(7):975–8. doi: 10.1007/s10815-013-0026-x
124. Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril (2015) 103(2):462–8. doi: 10.1016/j.fertnstert.2014.10.045
125. Yding Andersen C, Ernst E, Bærentzen S, Birkebæk NH, Clausen N. No malignancy detected in surplus ovarian tissue from a former Ewing sarcoma patient who experienced relapse four years after being grafted with frozen/thawed ovarian tissue. J Assist Reprod Genet (2014) 31(11):1567–8. doi: 10.1007/s10815-014-0357-2
126. Greve T, Wielenga VT, Grauslund M, Sørensen N, Christiansen DB, Rosendahl M, et al. Ovarian tissue cryopreserved for fertility preservation from patients with Ewing or other sarcomas appear to have no tumour cell contamination. Eur J Cancer (2013) 49(8):1932–8. doi: 10.1016/j.ejca.2013.01.032
127. Halpern JA, Hill R, Brannigan RE. Guideline based approach to male fertility preservation. Urol Oncol (2020) 38(1):31–5. doi: 10.1016/j.urolonc.2019.02.009
128. Fernbach A, Lockart B, Armus CL, Bashore LM, Levine J, Kroon L, et al. Evidence-based recommendations for fertility preservation options for inclusion in treatment protocols for pediatric and adolescent patients diagnosed with cancer. J Pediatr Oncol Nurs (2014) 31(4):211–22. doi: 10.1177/1043454214532025
129. Jensen CFS, Dong L, Gul M, Fode M, Hildorf S, Thorup J, et al. Fertility preservation in boys facing gonadotoxic cancer therapy. Nat Rev Urol (2022) 19(2):71–83. doi: 10.1038/s41585-021-00523-8
130. Wyns C, Kanbar M, Giudice MG, Poels J. Fertility preservation for prepubertal boys: lessons learned from the past and update on remaining challenges towards clinical translation. Hum Reprod Update (2021) 27(3):433–59. doi: 10.1093/humupd/dmaa050
131. Goossens E, Jahnukainen K, Mitchell RT, van Pelt A, Pennings G, Rives N, et al. Fertility preservation in boys: recent developments and new insights (†). Hum Reprod Open (2020) 2020(3):hoaa016. doi: 10.1093/hropen/hoaa016
132. van den Berg H, Repping S, van der Veen F. Parental desire and acceptability of spermatogonial stem cell cryopreservation in boys with cancer. Hum Reprod (2007) 22(2):594–7. doi: 10.1093/humrep/del375
133. Ginsberg JP, Li Y, Carlson CA, Gracia CR, Hobbie WL, Miller VA, et al. Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer (2014) 61(9):1673–8. doi: 10.1002/pbc.25078
134. Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, et al. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod (2010) 25(1):37–41. doi: 10.1093/humrep/dep371
135. Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, et al. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic university of louvain. Hum Reprod (2011) 26(4):737–47. doi: 10.1093/humrep/deq387
136. Katz DJ, Kolon TF, Feldman DR, Mulhall JP. Fertility preservation strategies for male patients with cancer. Nat Rev Urol (2013) 10(8):463–72. doi: 10.1038/nrurol.2013.145
137. Onofre J, Baert Y, Faes K, Goossens E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update (2016) 22(6):744–61. doi: 10.1093/humupd/dmw029
138. Baert Y, Van Saen D, Haentjens P, In’t Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod (2013) 28(7):1816–26. doi: 10.1093/humrep/det100
139. Dumont L, Chalmel F, Oblette A, Berby B, Rives A, Duchesne V, et al. Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue. Mol Hum Reprod (2017) 23(11):738–54. doi: 10.1093/molehr/gax054
140. Baert Y, Goossens E, van Saen D, Ning L, in’t Veld P, Tournaye H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril (2012) 97(5):1152–7.e1-2. doi: 10.1016/j.fertnstert.2012.02.010
141. Baert Y, Onofre J, Van Saen D, Goossens E. Cryopreservation of human testicular tissue by isopropyl-controlled slow freezing. Methods Mol Biol (2018) 1748:287–94. doi: 10.1007/978-1-4939-7698-0_20
142. Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: from research to clinic. Hum Reprod (2013) 28(4):897–907. doi: 10.1093/humrep/det039
143. Portela JMD, de Winter-Korver CM, van Daalen SKM, Meißner A, de Melker AA, Repping S, et al. Assessment of fresh and cryopreserved testicular tissues from (pre)pubertal boys during organ culture as a strategy for in vitro spermatogenesis. Hum Reprod (2019) 34(12):2443–55. doi: 10.1093/humrep/dez180
144. Kanbar M, Delwiche G, Wyns C. Fertility preservation for prepubertal boys: are we ready for autologous grafting of cryopreserved immature testicular tissue? Ann Endocrinol (Paris) (2022) 83(3):210–7. doi: 10.1016/j.ando.2022.04.006
145. Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science (2019) 363(6433):1314–9. doi: 10.1126/science.aav2914
146. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A (1994) 91(24):11298–302. doi: 10.1073/pnas.91.24.11298
147. Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A (1994) 91(24):11303–7. doi: 10.1073/pnas.91.24.11303
148. AbuMadighem A, Shuchat S, Lunenfeld E, Yossifon G, Huleihel M. Testis on a chip-a microfluidic three-dimensional culture system for the development of spermatogenesis in vitro. Biofabrication (2022) 14(3). doi: 10.1088/1758-5090/ac6126
149. Campos-Junior PH, Costa GM, Avelar GF, Lacerda SM, da Costa NN, Ohashi OM, et al. Derivation of sperm from xenografted testis cells and tissues of the peccary (Tayassu tajacu). Reproduction (2014) 147(3):291–9. doi: 10.1530/REP-13-0581
150. Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod (2015) 30(11):2463–75. doi: 10.1093/humrep/dev190
151. Kanbar M, de Michele F, Wyns C. ryostorage of testicular tissue and retransplantation of spermatogonial stem cells in the infertile male. Best Pract Res Clin Endocrinol Metab (2019) 33(1):103–15. doi: 10.1016/j.beem.2018.10.003
152. Akhtar M, Ali MA, Burgess A, Aur RJ. Fine-needle aspiration biopsy (FNAB) diagnosis of testicular involvement in acute lymphoblastic leukemia in children. Diagn Cytopathol (1991) 7(5):504–7. doi: 10.1002/dc.2840070512
153. Cao YX, Chian RC. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med (2009) 27(6):456–64. doi: 10.1055/s-0029-1241055
154. Khalili MA, Shahedi A, Ashourzadeh S, Nottola SA, Macchiarelli G, Palmerini MG. Vitrification of human immature oocytes before and after in vitro maturation: a review. J Assist Reprod Genet (2017) 34(11):1413–26. doi: 10.1007/s10815-017-1005-4
155. Lee JA, Barritt J, Moschini RM, Slifkin RE, Copperman AB. Optimizing human oocyte cryopreservation for fertility preservation patients: should we mature then freeze or freeze then mature? Fertil Steril (2013) 99(5):1356–62. doi: 10.1016/j.fertnstert.2012.11.042
156. Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod (2010) 25(1):66–73. doi: 10.1093/humrep/dep346
157. Crawford S, Boulet SL, Kawwass JF, Jamieson DJ, Kissin DM. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, united states, 2013. Fertil Steril (2017) 107(1):110–8. doi: 10.1016/j.fertnstert.2016.10.002
158. Solé M, Santaló J, Boada M, Clua E, Rodríguez I, Martínez F, et al. How does vitrification affect oocyte viability in oocyte donation cycles? a prospective study to compare outcomes achieved with fresh versus vitrified sibling oocytes. Hum Reprod (2013) 28(8):2087–92. doi: 10.1093/humrep/det242
159. Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod (2018) 33(12):2222–31. doi: 10.1093/humrep/dey321
160. Goldman RH, Racowsky C, Farland LV, Munné S, Ribustello L, Fox JH. Predicting the likelihood of live birth for elective oocyte cryopreservation: a counseling tool for physicians and patients. Hum Reprod (2017) 32(4):853–9. doi: 10.1093/humrep/dex008
161. Maslow BL, Guarnaccia MM, Ramirez L, Klein JU. Likelihood of achieving a 50%, 60%, or 70% estimated live birth rate threshold with 1 or 2 cycles of planned oocyte cryopreservation. J Assist Reprod Genet (2020) 37(7):1637–43. doi: 10.1007/s10815-020-01791-w
162. Penzias A, Azziz R, Bendikson K, Falcone T, Goldstein J, Hansen K, et al. Evidence-based outcomes after oocyte cryopreservation for donor oocyte in vitro fertilization and planned oocyte cryopreservation: a guideline. Fertil Steril (2021) 116(1):36–47. doi: 10.1016/j.fertnstert.2021.02.024
163. Dviri M, Madjunkova S, Koziarz A, Madjunkov M, Mashiach J, Nekolaichuk E, et al. Is there an association between paternal age and aneuploidy? evidence from young donor oocyte-derived embryos: a systematic review and individual patient data meta-analysis. Hum Reprod Update (2021) 27(3):486–500. doi: 10.1093/humupd/dmaa052
164. Dviri M, Madjunkova S, Koziarz A, Antes R, Abramov R, Mashiach J, et al. Is there a correlation between paternal age and aneuploidy rate? an analysis of 3,118 embryos derived from young egg donors. Fertil Steril (2020) 114(2):293–300. doi: 10.1016/j.fertnstert.2020.03.034
165. Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril (2014) 101(3):656–63.e1. doi: 10.1016/j.fertnstert.2013.11.004
166. Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PloS Med (2011) 8(1):e1000386. doi: 10.1371/journal.pmed.1000386
167. Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science (2019) 365(6460):1466–9. doi: 10.1126/science.aav7321
168. Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. Bmj (2019) 364:l869. doi: 10.1136/bmj.l869
169. Da Luz CM, Caetano MA, Berteli TS, Vireque AA, Navarro PA. The impact of oocyte vitrification on offspring: a systematic review. Reprod Sci (2022) 29(11):3222–34. doi: 10.1007/s43032-022-00868-4
170. Parmegiani L, Garello C, Granella F, Guidetti D, Bernardi S, Cognigni GE, et al. Long-term cryostorage does not adversely affect the outcome of oocyte thawing cycles. Reprod BioMed Online (2009) 19(3):374–9. doi: 10.1016/S1472-6483(10)60171-X
171. Urquiza MF, Carretero I, Cano Carabajal PR, Pasqualini RA, Felici MM, Pasqualini RS, et al. Successful live birth from oocytes after more than 14 years of cryopreservation. J Assist Reprod Genet (2014) 31(11):1553–5. doi: 10.1007/s10815-014-0318-9
172. Levi-Setti PE, Borini A, Patrizio P, Bolli S, Vigiliano V, De Luca R, et al. ART results with frozen oocytes: data from the Italian ART registry (2005-2013). J Assist Reprod Genet (2016) 33(1):123–8. doi: 10.1007/s10815-015-0629-5
173. Van Reckem M, Blockeel C, Bonduelle M, Buysse A, Roelants M, Verheyen G, et al. Health of 2-year-old children born after vitrified oocyte donation in comparison with peers born after fresh oocyte donation. Hum Reprod Open (2021) 2021(1):hoab002. doi: 10.1093/hropen/hoab013
174. Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet (2015) 32(8):1167–70. doi: 10.1007/s10815-015-0544-9
175. Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod (2005) 20(12):3539–46. doi: 10.1093/humrep/dei250
176. Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril (2011) 95(2):695–701. doi: 10.1016/j.fertnstert.2010.07.1080
177. Fabbri R, Pasquinelli G, Magnani V, Macciocca M, Vicenti R, Parazza I, et al. Autotransplantation of cryopreserved ovarian tissue in oncological patients: recovery of ovarian function. Future Oncol (2014) 10(4):549–61. doi: 10.2217/fon.13.234
178. Janse F, Donnez J, Anckaert E, de Jong FH, Fauser BC, Dolmans MM. Limited value of ovarian function markers following orthotopic transplantation of ovarian tissue after gonadotoxic treatment. J Clin Endocrinol Metab (2011) 96(4):1136–44. doi: 10.1210/jc.2010-2188
179. Greve T, Schmidt KT, Kristensen SG, Ernst E, Andersen CY. Evaluation of the ovarian reserve in women transplanted with frozen and thawed ovarian cortical tissue. Fertil Steril (2012) 97(6):1394–8.e1. doi: 10.1016/j.fertnstert.2012.02.036
180. Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet (2012) 29(6):489–93. doi: 10.1007/s10815-012-9757-3
181. Ruan X, Du J, Korell M, Kong W, Lu D, Jin F, et al. Case report of the first successful cryopreserved ovarian tissue retransplantation in China. Climacteric (2018) 21(6):613–6. doi: 10.1080/13697137.2018.1514005
182. Poirot C, Fortin A, Dhédin N, Brice P, Socié G, Lacorte JM, et al. Post-transplant outcome of ovarian tissue cryopreserved after chemotherapy in hematologic malignancies. Haematologica (2019) 104(8):e360–e3. doi: 10.3324/haematol.2018.211094
183. Hao X, Anastácio A, Liu K, Rodriguez-Wallberg KA. Ovarian follicle depletion induced by chemotherapy and the investigational stages of potential fertility-protective treatments-a review. Int J Mol Sci (2019) 20(19). doi: 10.3390/ijms20194720
184. Demeestere I, Simon P, Dedeken L, Moffa F, Tsépélidis S, Brachet C, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod (2015) 30(9):2107–9. doi: 10.1093/humrep/dev128
185. Matthews SJ, Picton H, Ernst E, Andersen CY. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol (2018) 70(4):432–5. doi: 10.23736/S0026-4784.18.04240-5
186. Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril (2012) 98(3):720–5. doi: 10.1016/j.fertnstert.2012.05.017
187. Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod (2011) 26(5):1097–103. doi: 10.1093/humrep/der063
188. Dolmans MM, Falcone T, Patrizio P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil Steril (2020) 114(2):279–80. doi: 10.1016/j.fertnstert.2020.04.050
189. Fabbri R, Vicenti R, Magnani V, Paradisi R, Lima M, De Meis L, et al. Ovarian tissue cryopreservation and transplantation: 20 years experience in Bologna university. Front Endocrinol (Lausanne) (2022) 13:1035109. doi: 10.3389/fendo.2022.1035109
190. Poirot C, Brugieres L, Yakouben K, Prades-Borio M, Marzouk F, de Lambert G, et al. Ovarian tissue cryopreservation for fertility preservation in 418 girls and adolescents up to 15 years of age facing highly gonadotoxic treatment. Twenty years of experience at a single center. Acta Obstet Gynecol Scand (2019) 98(5):630–7. doi: 10.1111/aogs.13616
191. Ernst E, Kjærsgaard M, Birkebæk NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer (2013) 49(4):911–4. doi: 10.1016/j.ejca.2012.09.028
192. Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet (2012) 379(9815):588. doi: 10.1016/S0140-6736(11)61781-9
193. Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med (2011) 43(6):437–50. doi: 10.3109/07853890.2010.546807
194. Póvoa A, Xavier P, Calejo L, Soares S, Sousa M, Silva J, et al. First transplantation of cryopreserved ovarian tissue in Portugal, stored for 10 years: an unexpected indication. Reprod BioMed Online (2016) 32(3):334–6. doi: 10.1016/j.rbmo.2015.12.002
195. Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril (2010) 93(7):2413.e15–9. doi: 10.1016/j.fertnstert.2009.12.022
196. Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krüssel J, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod (2016) 31(9):2031–41. doi: 10.1093/humrep/dew165
197. Callejo J, Salvador C, González-Nuñez S, Almeida L, Rodriguez L, Marqués L, et al. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res (2013) 6(1):33. doi: 10.1186/1757-2215-6-33
198. von Wolff M, Stute P, Flück C. Autologous transplantation of cryopreserved ovarian tissue to induce puberty-the endocrinologists’ view. Eur J Pediatr (2016) 175(12):2007–10. doi: 10.1007/s00431-016-2771-1
199. Anderson RA, Hindmarsh PC, Wallace WH. Induction of puberty by autograft of cryopreserved ovarian tissue in a patient previously treated for Ewing sarcoma. Eur J Cancer (2013) 49(13):2960–1. doi: 10.1016/j.ejca.2013.04.031
200. Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet (2016) 33(12):1595–603. doi: 10.1007/s10815-016-0814-1
201. Ruan X, Cheng J, Du J, Jin F, Gu M, Li Y, et al. Analysis of fertility preservation by ovarian tissue cryopreservation in pediatric children in China. Front Endocrinol (Lausanne) (2022) 13:930786. doi: 10.3389/fendo.2022.930786
202. Punyatanasakchai P, Sophonsritsuk A, Weerakiet S, Wansumrit S, Chompurat D. Comparison of cryopreserved human sperm in vapor and liquid phases of liquid nitrogen: effect on motility parameters, morphology, and sperm function. Fertil Steril (2008) 90(5):1978–82. doi: 10.1016/j.fertnstert.2007.09.066
203. Degl’Innocenti S, Filimberti E, Magini A, Krausz C, Lombardi G, Fino MG, et al. Semen cryopreservation for men banking for oligospermia, cancers, and other pathologies: prediction of post-thaw outcome using basal semen quality. Fertil Steril (2013) 100(6):1555–63.e1-3. doi: 10.1016/j.fertnstert.2013.08.005
204. Hourvitz A, Goldschlag DE, Davis OK, Gosden LV, Palermo GD, Rosenwaks Z. Intracytoplasmic sperm injection (ICSI) using cryopreserved sperm from men with malignant neoplasm yields high pregnancy rates. Fertil Steril (2008) 90(3):557–63. doi: 10.1016/j.fertnstert.2007.03.002
205. García A, Herrero MB, Holzer H, Tulandi T, Chan P. Assisted reproductive outcomes of male cancer survivors. J Cancer Surviv (2015) 9(2):208–14. doi: 10.1007/s11764-014-0398-7
206. van Casteren NJ, van Santbrink EJ, van Inzen W, Romijn JC, Dohle GR. Use rate and assisted reproduction technologies outcome of cryopreserved semen from 629 cancer patients. Fertil Steril (2008) 90(6):2245–50. doi: 10.1016/j.fertnstert.2007.10.055
207. Agarwal A, Ranganathan P, Kattal N, Pasqualotto F, Hallak J, Khayal S, et al. Fertility after cancer: a prospective review of assisted reproductive outcome with banked semen specimens. Fertil Steril (2004) 81(2):342–8. doi: 10.1016/j.fertnstert.2003.07.021
208. Schmidt KL, Larsen E, Bangsbøll S, Meinertz H, Carlsen E, Andersen AN. Assisted reproduction in male cancer survivors: fertility treatment and outcome in 67 couples. Hum Reprod (2004) 19(12):2806–10. doi: 10.1093/humrep/deh518
209. Crha I, Ventruba P, Zakova J, Huser M, Kubesova B, Hudecek R, et al. Survival and infertility treatment in male cancer patients after sperm banking. Fertil Steril (2009) 91(6):2344–8. doi: 10.1016/j.fertnstert.2008.03.053
210. Freour T, Mirallie S, Jean M, Barriere P. Sperm banking and assisted reproductive outcome in men with cancer: a 10 years’ experience. Int J Clin Oncol (2012) 17(6):598–603. doi: 10.1007/s10147-011-0330-3
211. Kelleher S, Wishart SM, Liu PY, Turner L, Di Pierro I, Conway AJ, et al. Long-term outcomes of elective human sperm cryostorage. Hum Reprod (2001) 16(12):2632–9. doi: 10.1093/humrep/16.12.2632
212. Corona G, Pizzocaro A, Lanfranco F, Garolla A, Pelliccione F, Vignozzi L, et al. Sperm recovery and ICSI outcomes in klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update (2017) 23(3):265–75. doi: 10.1093/humupd/dmx008
213. Vicdan K, Akarsu C, Sözen E, Buluç B, Vicdan A, Yılmaz Y, et al. Outcome of intracytoplasmic sperm injection using fresh and cryopreserved-thawed testıcular spermatozoa in 83 azoospermic men with klinefelter syndrome. J Obstet Gynaecol Res (2016) 42(11):1558–66. doi: 10.1111/jog.13090
214. Wu S, Zhao J, Wu Y, Hu Y, Fang L, Chen W. Comparison of pregnancy and neonatal outcomes of intracytoplasmic sperm injection performed with frozen versus fresh testicular sperm. Transl Androl Urol (2022) 11(4):472–9. doi: 10.21037/tau-22-125
215. Corona G, Minhas S, Giwercman A, Bettocchi C, Dinkelman-Smit M, Dohle G, et al. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: a systematic review and meta-analysis. Hum Reprod Update (2019) 25(6):733–57. doi: 10.1093/humupd/dmz028
216. Karacan M, Alwaeely F, Erkan S, Çebi Z, Berberoğlugil M, Batukan M, et al. Outcome of intracytoplasmic sperm injection cycles with fresh testicular spermatozoa obtained on the day of or the day before oocyte collection and with cryopreserved testicular sperm in patients with azoospermia. Fertil Steril (2013) 100(4):975–80. doi: 10.1016/j.fertnstert.2013.06.031
217. Aizer A, Dratviman-Storobinsky O, Noach-Hirsh M, Konopnicki S, Lazarovich A, Raviv G, et al. Testicular sperm retrieval: what should we expect from the fresh and subsequent cryopreserved sperm injection? Andrologia (2021) 53(1):e13849. doi: 10.1111/and.13849
218. Feldschuh J, Brassel J, Durso N, Levine A. Successful sperm storage for 28 years. Fertil Steril (2005) 84(4):1017. doi: 10.1016/j.fertnstert.2005.05.015
219. Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod (2002) 17(12):3039–45. doi: 10.1093/humrep/17.12.3039
220. Yildiz C, Mullen B, Jarvi K, McKerlie C, Lo KC. Effect of different cryoprotectant agents on spermatogenesis efficiency in cryopreserved and grafted neonatal mouse testicular tissue. Cryobiology (2013) 67(1):70–5. doi: 10.1016/j.cryobiol.2013.05.004
221. Gourdon JC, Travis AJ. Spermatogenesis in ferret testis xenografts: a new model. Comp Med (2011) 61(2):145–9.
222. Pukazhenthi BS, Nagashima J, Travis AJ, Costa GM, Escobar EN, França LR, et al. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PloS One (2015) 10(4):e0123957. doi: 10.1371/journal.pone.0123957
223. Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, et al. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction (2010) 139(2):331–5. doi: 10.1530/REP-09-0509
224. Abbasi S, Honaramooz A. Xenografting of testis tissue from bison calf donors into recipient mice as a strategy for salvaging genetic material. Theriogenology (2011) 76(4):607–14. doi: 10.1016/j.theriogenology.2011.03.011
225. Reddy N, Mahla RS, Thathi R, Suman SK, Jose J, Goel S. Gonadal status of male recipient mice influences germ cell development in immature buffalo testis tissue xenograft. Reproduction (2012) 143(1):59–69. doi: 10.1530/REP-11-0286
226. Liu J, Cheng KM, Silversides FG. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in Japanese quail (Coturnix japonica). Biol Reprod (2013) 88(5):124. doi: 10.1095/biolreprod.113.108951
227. Ntemou E, Kadam P, Van Saen D, Wistuba J, Mitchell RT, Schlatt S, et al. Complete spermatogenesis in intratesticular testis tissue xenotransplants from immature non-human primate. Hum Reprod (2019) 34(3):403–13. doi: 10.1093/humrep/dey373
228. Liu Z, Nie YH, Zhang CC, Cai YJ, Wang Y, Lu HP, et al. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res (2016) 26(1):139–42. doi: 10.1038/cr.2015.112
229. Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res (2012) 72(20):5174–8. doi: 10.1158/0008-5472.CAN-12-1317
230. Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro Production of functional sperm in cultured neonatal mouse testes. Nature (2011) 471(7339):504–7. doi: 10.1038/nature09850
231. Goossens E, De Rycke M, Haentjens P, Tournaye H. DNA Methylation patterns of spermatozoa and two generations of offspring obtained after murine spermatogonial stem cell transplantation. Hum Reprod (2009) 24(9):2255–63. doi: 10.1093/humrep/dep213
232. Kanatsu-Shinohara M, Kato M, Takehashi M, Morimoto H, Takashima S, Chuma S, et al. Production of transgenic rats via lentiviral transduction and xenogeneic transplantation of spermatogonial stem cells. Biol Reprod (2008) 79(6):1121–8. doi: 10.1095/biolreprod.108.071159
233. Richardson TE, Chapman KM, Tenenhaus Dann C, Hammer RE, Hamra FK. Sterile testis complementation with spermatogonial lines restores fertility to DAZL-deficient rats and maximizes donor germline transmission. PloS One (2009) 4(7):e6308. doi: 10.1371/journal.pone.0006308
234. Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod (2003) 69(4):1260–4. doi: 10.1095/biolreprod.103.018788
235. Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod (2009) 81(5):898–905. doi: 10.1095/biolreprod.109.078279
236. Trefil P, Micáková A, Mucksová J, Hejnar J, Poplstein M, Bakst MR, et al. Restoration of spermatogenesis and male fertility by transplantation of dispersed testicular cells in the chicken. Biol Reprod (2006) 75(4):575–81. doi: 10.1095/biolreprod.105.050278
237. Kawasaki T, Saito K, Sakai C, Shinya M, Sakai N. Production of zebrafish offspring from cultured spermatogonial stem cells. Genes Cells (2012) 17(4):316–25. doi: 10.1111/j.1365-2443.2012.01589.x
238. Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell (2012) 11(5):715–26. doi: 10.1016/j.stem.2012.07.017
239. Shetty G, Mitchell JM, Lam TNA, Wu Z, Zhang J, Hill L, et al. Donor spermatogenesis in de novo formed seminiferous tubules from transplanted testicular cells in rhesus monkey testis. Hum Reprod (2018) 33(12):2249–55. doi: 10.1093/humrep/dey316
240. Perrard MH, Sereni N, Schluth-Bolard C, Blondet A, SG DE, Plotton I, et al. Complete human and rat ex vivo spermatogenesis from fresh or frozen testicular tissue. Biol Reprod (2016) 95(4):89. doi: 10.1095/biolreprod.116.142802
241. Zhao HM, Yang H, Luo FH, Li MX, Zhang S, Yang XG, et al. Isolation, proliferation, and induction of bama mini-pig spermatogonial stem cells in vitro. Genet Mol Res (2016) 15(3). doi: 10.4238/gmr.15038602
242. Zheng P, Zhao XW, Zheng XM, Khalid A, Zhao Q, Zhang GX. In vitro Differentiation of sperm from male germline stem cell. Genet Mol Res (2015) 14(2):2964–9. doi: 10.4238/2015.April.10.5
243. Xie B, Qin Z, Huang B, Xie T, Yao H, Wei Y, et al. In vitro Culture and differentiation of buffalo (Bubalus bubalis) spermatogonia. Reprod Domest Anim (2010) 45(2):275–82. doi: 10.1111/j.1439-0531.2008.01281.x
244. Yu K, Zhang Y, Zhang BL, Wu HY, Jiang WQ, Wang ST, et al. In-vitro Differentiation of early pig spermatogenic cells to haploid germ cells. Mol Hum Reprod (2019) 25(9):507–18. doi: 10.1093/molehr/gaz043
245. Huleihel M, Nourashrafeddin S, Plant TM. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J Androl (2015) 17(6):972–80. doi: 10.4103/1008-682X.154994
246. Heckmann L, Langenstroth-Röwer D, Wistuba J, Portela JMD, van Pelt AMM, Redmann K, et al. The initial maturation status of marmoset testicular tissues has an impact on germ cell maintenance and somatic cell response in tissue fragment culture. Mol Hum Reprod (2020) 26(6):374–88. doi: 10.1093/molehr/gaaa024
247. Abofoul-Azab M, AbuMadighem A, Lunenfeld E, Kapelushnik J, Shi Q, Pinkas H, et al. Development of postmeiotic cells In Vitro from spermatogonial cells of prepubertal cancer patients. Stem Cells Dev (2018) 27(15):1007–20. doi: 10.1089/scd.2017.0301
248. de Michele F, Poels J, Vermeulen M, Ambroise J, Gruson D, Guiot Y, et al. Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front Physiol (2018) 9:1413. doi: 10.3389/fphys.2018.01413
249. Mohaqiq M, Movahedin M, Mazaheri Z, Amirjannati N. In vitro Transplantation of spermatogonial stem cells isolated from human frozen-thawed testis tissue can induce spermatogenesis under 3-dimensional tissue culture conditions. Biol Res (2019) 52(1):16. doi: 10.1186/s40659-019-0223-x
250. American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists’ Committee on Health Care for Underserved Women. Health care for transgender and gender diverse individuals: ACOG committee opinion, number 823. Obstet Gynecol (2021) 137(3):e75–88. doi: 10.1097/AOG.0000000000004294
251. Ainsworth AJ, Allyse M, Khan Z. Fertility preservation for transgender individuals: a review. Mayo Clin Proc (2020) 95(4):784–92. doi: 10.1016/j.mayocp.2019.10.040
252. Barrett J. Fertility preservation for transgender individuals. Reprod Fertil (2022) 3(2):C11–c3. doi: 10.1530/RAF-21-0090
253. Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender and nonbinary persons: an ethics committee opinion. Fertil Steril (2021) 115(4):874–8. doi: 10.1016/j.fertnstert.2021.01.049
254. Chiniara LN, Viner C, Palmert M, Bonifacio H. Perspectives on fertility preservation and parenthood among transgender youth and their parents. Arch Dis Child (2019) 104(8):739–44. doi: 10.1136/archdischild-2018-316080
255. Mattelin E, Strandell A, Bryman I. Fertility preservation and fertility treatment in transgender adolescents and adults in a Swedish region, 2013-2018. Hum Reprod Open (2022) 2022(2):hoac008. doi: 10.1093/hropen/hoac008
256. Alpern S, Yaish I, Wagner-Kolasko G, Greenman Y, Sofer Y, Paltiel Lifshitz D, et al. Why fertility preservation rates of transgender men are much lower than those of transgender women. Reprod BioMed Online (2022) 44(5):943–50. doi: 10.1016/j.rbmo.2022.01.003
257. Auer MK, Fuss J, Nieder TO, Briken P, Biedermann SV, Stalla GK, et al. Desire to have children among transgender people in Germany: a cross-sectional multi-center study. J Sex Med (2018) 15(5):757–67. doi: 10.1016/j.jsxm.2018.03.083
258. Strang JF, Jarin J, Call D, Clark B, Wallace GL, Anthony LG, et al. Transgender youth fertility attitudes questionnaire: measure development in nonautistic and autistic transgender youth and their parents. J Adolesc Health (2018) 62(2):128–35. doi: 10.1016/j.jadohealth.2017.07.022
259. Wiepjes CM, Nota NM, de Blok CJM, Klaver M, de Vries ALC, Wensing-Kruger SA, et al. The Amsterdam cohort of gender dysphoria study (1972-2015): trends in prevalence, treatment, and regrets. J Sex Med (2018) 15(4):582–90. doi: 10.1016/j.jsxm.2018.01.016
260. Durcan E, Turan S, Bircan BE, Yaylamaz S, Okur I, Demir AN, et al. Fertility desire and motivation among individuals with gender dysphoria: a comparative study. J Sex Marital Ther (2022) 48(8):789–803. doi: 10.1080/0092623X.2022.2053617
261. Riggs DW, Bartholomaeus C. Fertility preservation decision making amongst Australian transgender and non-binary adults. Reprod Health (2018) 15(1):181. doi: 10.1186/s12978-018-0627-z
262. Committee opinion no. 685: care for transgender adolescents. Obstet Gynecol (2017) 129(1):e11–e6. doi: 10.1097/AOG.0000000000001861
263. Douglas CR, Phillips D, Sokalska A, Aghajanova L. Fertility preservation for transgender males: counseling and timing of treatment. Obstet Gynecol (2022) 139(6):1012–7. doi: 10.1097/AOG.0000000000004751
264. Komorowski AS, Fisher AR, Jungheim ES, Lewis CS, Omurtag KR. Fertility preservation discussions, referral and follow-up in male-to-female and female-to-male adolescent transgender patients. Hum Fertil (Camb) (2021), 1–5. doi: 10.1080/14647273.2021.2015804
265. Armuand G, Dhejne C, Olofsson JI, Rodriguez-Wallberg KA. Transgender men’s experiences of fertility preservation: a qualitative study. Hum Reprod (2017) 32(2):383–90. doi: 10.1093/humrep/dew323
266. Chen D, Kyweluk MA, Sajwani A, Gordon EJ, Johnson EK, Finlayson CA, et al. Factors affecting fertility decision-making among transgender adolescents and young adults. LGBT Health (2019) 6(3):107–15. doi: 10.1089/lgbt.2018.0250
267. Martin CE, Lewis C, Omurtag K. Successful oocyte cryopreservation using letrozole as an adjunct to stimulation in a transgender adolescent after GnRH agonist suppression. Fertil Steril (2021) 116(2):522–7. doi: 10.1016/j.fertnstert.2021.02.025
268. Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PG, et al. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab (1989) 69(1):151–7. doi: 10.1210/jcem-69-1-151
269. De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod BioMed Online (2017) 34(6):557–66. doi: 10.1016/j.rbmo.2017.03.008
270. Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, et al. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod (2013) 28(2):453–61. doi: 10.1093/humrep/des385
271. Amir H, Oren A, Klochendler Frishman E, Sapir O, Shufaro Y, Segev Becker A, et al. Oocyte retrieval outcomes among adolescent transgender males. J Assist Reprod Genet (2020) 37(7):1737–44. doi: 10.1007/s10815-020-01815-5
272. Adeleye AJ, Cedars MI, Smith J, Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet (2019) 36(10):2155–61. doi: 10.1007/s10815-019-01558-y
273. Leung A, Sakkas D, Pang S, Thornton K, Resetkova N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril (2019) 112(5):858–65. doi: 10.1016/j.fertnstert.2019.07.014
274. Lierman S, Tilleman K, Braeckmans K, Peynshaert K, Weyers S, T’Sjoen G, et al. Fertility preservation for trans men: frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. J Assist Reprod Genet (2017) 34(11):1449–56. doi: 10.1007/s10815-017-0976-5
275. Lierman S, Tolpe A, De Croo I, De Gheselle S, Defreyne J, Baetens M, et al. Low feasibility of in vitro matured oocytes originating from cumulus complexes found during ovarian tissue preparation at the moment of gender confirmation surgery and during testosterone treatment for fertility preservation in transgender men. Fertil Steril (2021) 116(4):1068–76. doi: 10.1016/j.fertnstert.2021.03.009
276. Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro Maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet (2015) 32(8):1221–31. doi: 10.1007/s10815-015-0528-9
277. Das M, Son WY, Buckett W, Tulandi T, Holzer H. In-vitro Maturation versus IVF with GnRH antagonist for women with polycystic ovary syndrome: treatment outcome and rates of ovarian hyperstimulation syndrome. Reprod BioMed Online (2014) 29(5):545–51. doi: 10.1016/j.rbmo.2014.07.019
278. Smitz JE, Thompson JG, Gilchrist RB. The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med (2011) 29(1):24–37. doi: 10.1055/s-0030-1268701
279. Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology. In vitro Maturation: a committee opinion. Fertil Steril (2021) 115(2):298–304. doi: 10.1016/j.fertnstert.2020.11.018
280. Mattawanon N, Spencer JB, Schirmer DA 3rd, Tangpricha V. Fertility preservation options in transgender people: a review. Rev Endocr Metab Disord (2018) 19(3):231–42. doi: 10.1007/s11154-018-9462-3
281. Adeleye AJ, Reid G, Kao CN, Mok-Lin E, Smith JF. Semen parameters among transgender women with a history of hormonal treatment. Urology (2019) 124:136–41. doi: 10.1016/j.urology.2018.10.005
282. Rodriguez-Wallberg KA, Häljestig J, Arver S, Johansson ALV, Lundberg FE. Sperm quality in transgender women before or after gender affirming hormone therapy-a prospective cohort study. Andrology (2021) 9(6):1773–80. doi: 10.1111/andr.12999
283. Amir H, Perl L, Barda S, Lantsberg D, Becker AS, Israeli G, et al. Adolescent transgender females present impaired semen quality that is suitable for intracytoplasmic sperm injection even before initiating gender-affirming hormone treatment. Reprod Sci (2022) 29(1):260–9. doi: 10.1007/s43032-021-00561-y
284. Marsh C, McCracken M, Gray M, Nangia A, Gay J, Roby KF. Low total motile sperm in transgender women seeking hormone therapy. J Assist Reprod Genet (2019) 36(8):1639–48. doi: 10.1007/s10815-019-01504-y
285. Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet (2006) 367(9520):1412–20. doi: 10.1016/S0140-6736(06)68614-5
286. de Nie I, Mulder CL, Meißner A, Schut Y, Holleman EM, van der Sluis WB, et al. Histological study on the influence of puberty suppression and hormonal treatment on developing germ cells in transgender women. Hum Reprod (2022) 37(2):297–308. doi: 10.1093/humrep/deab240
287. Campbell JE, Assanasen C, Robinson RD, Knudtson JF. Fertility preservation counseling for pediatric and adolescent cancer patients. J Adolesc Young Adult Oncol (2016) 5(1):58–63. doi: 10.1089/jayao.2015.0040
288. Tholeti P, Uppangala S, Bhat V, Udupa KS, Kumar V, Patted S, et al. Oncofertility: knowledge, attitudes, and barriers among Indian oncologists and gynecologists. J Adolesc Young Adult Oncol (2021) 10(1):71–7. doi: 10.1089/jayao.2020.0034
289. Köhler TS, Kondapalli LA, Shah A, Chan S, Woodruff TK, Brannigan RE. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet (2011) 28(3):269–77. doi: 10.1007/s10815-010-9504-6
290. Armuand GM, Rodriguez-Wallberg KA, Wettergren L, Ahlgren J, Enblad G, Höglund M, et al. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol (2012) 30(17):2147–53. doi: 10.1200/JCO.2011.40.6470
291. Armuand GM, Wettergren L, Rodriguez-Wallberg KA, Lampic C. Women more vulnerable than men when facing risk for treatment-induced infertility: a qualitative study of young adults newly diagnosed with cancer. Acta Oncol (2015) 54(2):243–52. doi: 10.3109/0284186X.2014.948573
292. Quinn GP, Vadaparampil ST. Fertility preservation and adolescent/young adult cancer patients: physician communication challenges. J Adolesc Health (2009) 44(4):394–400. doi: 10.1016/j.jadohealth.2008.08.014
293. Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, et al. Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod (2015) 30(9):2022–30. doi: 10.1093/humrep/dev161
294. Johnson RH, Kroon L. Optimizing fertility preservation practices for adolescent and young adult cancer patients. J Natl Compr Canc Netw (2013) 11(1):71–7. doi: 10.6004/jnccn.2013.0010
295. Behl S, Joshi VB, Hussein RS, Walker DL, Lampat KL, Krenik AG, et al. Consult and procedure incidence outcomes following establishment of a fertility preservation program for children with cancer. J Assist Reprod Genet (2021) 38(2):495–501. doi: 10.1007/s10815-020-02042-8
296. Lopategui DM, Ibrahim E, Aballa TC, Brackett NL, Yechieli R, Barredo JC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol (2018) 7(Suppl 3):S271–s5. doi: 10.21037/tau.2018.04.24
297. Sheth KR, Sharma V, Helfand BT, Cashy J, Smith K, Hedges JC, et al. Improved fertility preservation care for male patients with cancer after establishment of formalized oncofertility program. J Urol (2012) 187(3):979–86. doi: 10.1016/j.juro.2011.10.154
298. Frederick NN, Klosky JL, Meacham LR, Quinn GP, Kelvin JF, Cherven B, et al. Infrastructure of fertility preservation services for pediatric cancer patients: a report from the children’s oncology group. JCO Oncol Pract (2022) 18(3):e325–e33. doi: 10.1200/OP.21.00275
299. Robbins HL, Zahoor A, Jones K. Fertility preservation in young cancer patients–too little, too late? Support Care Cancer (2015) 23(12):3395–7. doi: 10.1007/s00520-015-2890-7
300. Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer (2012) 118(6):1710–7. doi: 10.1002/cncr.26459
301. Redig AJ, Brannigan R, Stryker SJ, Woodruff TK, Jeruss JS. Incorporating fertility preservation into the care of young oncology patients. Cancer (2011) 117(1):4–10. doi: 10.1002/cncr.25398
302. Lampic C, Wettergren L. Oncologists’ and pediatric oncologists’ perspectives and challenges for fertility preservation. Acta Obstet Gynecol Scand (2019) 98(5):598–603. doi: 10.1111/aogs.13551
303. Kaneva K, Erickson L, Rowell E, Badawy SM. Fertility preservation education for pediatric hematology-oncology fellows, faculty and advanced practice providers: a pilot study. Pediatr Hematol Oncol (2022) 39(1):68–73. doi: 10.1080/08880018.2021.1928348
304. Joshi VB, Behl S, Pittock ST, Arndt CAS, Zhao Y, Khan Z, et al. Establishment of a pediatric ovarian and testicular cryopreservation program for malignant and non-malignant conditions: the Mayo clinic experience. J Pediatr Adolesc Gynecol (2021) 34(5):673–80. doi: 10.1016/j.jpag.2021.04.006
305. Carlson CA, Kolon TF, Mattei P, Hobbie W, Gracia CR, Ogle S, et al. Developing a hospital-wide fertility preservation service for pediatric and young adult patients. J Adolesc Health (2017) 61(5):571–6. doi: 10.1016/j.jadohealth.2017.07.008
306. Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol (2016) 214(1):94.e1–9. doi: 10.1016/j.ajog.2015.10.001
307. Kim EJ, Lee HJ, Lee J, Youm HW, Lee JR, Suh CS, et al. The beneficial effects of polyethylene glycol-superoxide dismutase on ovarian tissue culture and transplantation. J Assist Reprod Genet (2015) 32(10):1561–9. doi: 10.1007/s10815-015-0537-8
308. Hemadi M, Shokri S, Pourmatroud E, Moramezi F, Khodadai A. Follicular dynamic and immunoreactions of the vitrified ovarian graft after host treatment with variable regimens of melatonin. Am J Reprod Immunol (2012) 67(5):401–12. doi: 10.1111/j.1600-0897.2011.01087.x
309. Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PloS One (2011) 6(4):e19475. doi: 10.1371/journal.pone.0019475
310. Mahmoodi M, Soleimani Mehranjani M, Shariatzadeh SM, Eimani H, Shahverdi A. Effects of erythropoietin on ischemia, follicular survival, and ovarian function in ovarian grafts. Reproduction (2014) 147(5):733–41. doi: 10.1530/REP-13-0379
311. Dolmans MM, Donnez J, Cacciottola L. Fertility preservation: the challenge of freezing and transplanting ovarian tissue. Trends Mol Med (2021) 27(8):777–91. doi: 10.1016/j.molmed.2020.11.003
Keywords: fertility preservation, oocyte cryopreservation, ovarian tissue cryopreservation, testicular tissue cryopreservation, pediatric
Citation: Chen L, Dong Z and Chen X (2023) Fertility preservation in pediatric healthcare: a review. Front. Endocrinol. 14:1147898. doi: 10.3389/fendo.2023.1147898
Received: 19 January 2023; Accepted: 12 April 2023;
Published: 03 May 2023.
Edited by:
Yasmin Jayasinghe, The University of Melbourne, AustraliaReviewed by:
Tianren Wang, The University of Hong Kong, ChinaSeok-Yeong Yu, University of Nebraska Medical Center, United States
Copyright © 2023 Chen, Dong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Chen, Y2hlbnhpYW95YW5AY3Voay5lZHUuaGs=; Y2hlbnhpYW95YW4xMjE0QHlhaG9vLmNvbQ==
 Lin Chen
Lin Chen Zirui Dong
Zirui Dong Xiaoyan Chen
Xiaoyan Chen