- 1Department of Pharmacy Practice, Manipal College of Pharmaceutical Sciences, Manipal Academy of High Education, Manipal, Karnataka, India
- 2Manipal Centre for Infectious Diseases, Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, Karnataka, India
- 3NGSM Institute of Pharmaceutical Sciences, NITTE (Deemed to be University), Mangalore, Karnataka, India
- 4Department of Translational Medicine, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India
- 5Department of Infectious Diseases, Kasturba Medical College and Hospital, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India
- 6Department of General & Laparoscopic Surgery, Aster Al Raffah Hospital, Sohar, Oman
- 7Department of Laboratory Medicine, Rajendra Institute of Medical Sciences, Ranchi, Jharkhand, India
Background: Diabetic foot ulcers (DFU) are a major complication of diabetes mellitus (DM). Nutrient deficiencies are among the major risk factors in DFU development and healing. In this context, we aimed to investigate the possible association between micronutrient status and risk of DFU.
Methods: A systematic review (Prospero registration: CRD42021259817) of articles, published in PubMed, Web of Science, Scopus, CINAHL Complete, and Embase, that measured the status of micronutrients in DFU patients was performed.
Results: Thirty-seven studies were considered, of which thirty were included for meta-analysis. These studies reported levels of 11 micronutrients: vitamins B9, B12, C, D, E, calcium, magnesium, iron, selenium, copper, and zinc. DFU, compared to healthy controls (HC) had significantly lower vitamin D (MD: -10.82 14 ng/ml, 95% CI: -20.47, -1.16), magnesium (MD: -0.45 mg/dL, 95% CI: -0.78, -0.12) and selenium (MD: -0.33 µmol/L, 95% CI: -0.34, -0.32) levels. DFU, compared to DM patients without DFU, had significantly lower vitamin D (MD: -5.41 ng/ml, 95% CI: -8.06, -2.76), and magnesium (MD: -0.20 mg/dL, 95% CI: -0.25, -0.15) levels. The overall analysis showed lower levels of vitamin D [15.55ng/ml (95% CI:13.44, 17.65)], vitamin C [4.99µmol/L (95% CI:3.16, 6.83)], magnesium [1.53mg/dL (95% CI:1.28, 1.78)] and selenium [0.54µmol/L (95% CI:0.45, 0.64)].
Conclusion: This review provides evidence that micronutrient levels significantly differ in DFU patients, suggesting an association between micronutrient status and risk of DFU. Therefore, routine monitoring and supplementations are warranted in DFU patients. We suggest that personalized nutrition therapy may be considered in the DFU management guidelines.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=259817, identifier CRD42021259817.
1 Introduction
Chronic wound infections pose a significant health concern, especially diabetic foot ulcers (DFU) with maximum severity. It is estimated that foot ulcer complications account for 24.4% of healthcare costs among diabetics (1). The rising prevalence of diabetes projects DFU as a growing health concern that accounts for maximum non-traumatic amputation globally. Prevalence of DFU among diabetics has risen from 15 - 25% to 19 - 35% (2). The global prevalence of DFU is 6.3%, higher in males and type 2 diabetes mellitus (DM) than in females and type 1 DM (3). A recent study reported the one-, two -, and five-year survival rates in DFU patients as 81%, 69%, and 29%, indicating the robust association with mortality (4). Foot ulcers are less likely to heal in diabetics because of disorders in the intrinsic wound-healing process, such as compromised collagen cross-linking, altered functioning of matrix metalloproteinases, and immunological reasons (5). Management strategies include patient education, wound dressings, debridement, adequate offloading, blood sugar control, infection management, revascularisation, and advanced therapies (6, 7).
Nutrient deficiencies are among the major risk factors in DFU development and healing. Nutrient deficiencies modify the physiological responses to infection by diminishing the immune response, predisposing the skin to become thin and flaky, thereby developing a wound. The deficiencies also decrease subcutaneous fat at pressure points, together exacerbating the vulnerability to pressure wounds. Nutrient deficiencies also reduce the collagen synthesis required for wound healing and promote immobility due to diminished energy reserves (8). Malnutrition adversely affects the complex wound-healing process.
Hyperglycaemia and glucose-lowering drugs alter nutrient absorption in DM patients, resulting in nutritional deficiencies (9). Oxidative stress from glucose metabolism in DM depletes the natural antioxidant reserves of vitamins A, C, and E (9). Persistent hyperglycaemia and open wounds push the body into a catabolic state. As a result of insulin deprivation, negative nitrogen balance develops from gluconeogenesis from protein breakdown. Altered nutritional status and systemic deficiencies impair fibroblast, protein, and collagen synthesis (5).
Micronutrients affect wound healing comprehensively, via antioxidant and anti-inflammatory action, collagen stabilization, cell growth regulation, and differentiation. A closer monitoring of micronutrient status in DFU is warranted, as nutrient status is an easily modifiable factor as compared to non-modifiable factors such as age, DM duration, metabolic factors, and micro-, and macro-vascular disorders. The focus of this study was to systematically review the literature and provide the nature of nutritional deficiencies in DFU patients as compared to DM and non-diabetic healthy controls (HC). This would help identify the primary micronutrient deficiencies in DFU patients and initiate supplementations accordingly. Therefore, we have collated and analysed multiple data related to micronutrient status in patients with DFU, DM, and healthy controls (HC).
2 Methods
This systematic review appraises the association between micronutrient status and the risk of DFU. We have followed the preferred reporting items for systematic review and meta-analysis (PRISMA) 2020 guidelines and developed the research question using the PECOS format: The original research articles (study design) among DFU patients (participants), micronutrient status (exposure) as a risk for foot ulcers (outcome) compared to the control groups (comparator). The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), identification number CRD42021259817 (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=259817).
2.1 Search strategy
Initial search was performed in July 2021 and updated on 21st October 2021. We systematically searched and identified relevant studies from the following databases: PubMed, Web of Science, Scopus, CINAHL Complete, and Embase. The references cited by the included articles were examined to identify more articles. We used the following search terms: ‘micronutrient*’, ‘nutrient*’, ‘nutritional status’, ‘trace element*’, ‘vitamin*’, ‘provitamin*’, ‘mineral’, ‘diabetic foot ulcer*’, ‘DFU’, ‘diabetic foot infection*’, ‘diabetic foot osteomyelitis’, ‘diabetic foot’, ‘diabetic feet’ combined using ‘AND’ and ‘OR’, without restrictions on date of publication and language.
2.2 Eligibility and study selection
The study titles and abstracts were initially screened, and full texts were examined for potential eligibility. We included studies published in English and all original research studies (RCTs and observational studies) that measured micronutrient status in DFU without date restrictions. Only baseline data regarding the demographics and micronutrient levels in DFU patients were retrieved from RCTs. We excluded animal studies, editorials, case reports, case series, abstract-only papers, conference proceedings, and publications that did not measure micronutrient levels. After the initial search, all references were downloaded to Endnote X9.3.3 software. Further, SJK and RB independently assessed the title and abstracts to check for eligibility based on inclusion and exclusion criteria. Disagreements were resolved by SSM.
2.3 Data extraction and quality assessment
Data from the included studies were extracted into a pre-framed data extraction sheet. The following variables were extracted: author name(s), year of publication, place of study, study design, patient demographic characteristics, number of patients in cases/control, sample size, DFU classification, and micronutrient assessed and status of micronutrient. SJK performed primary data extraction, which was cross-checked for accuracy by TB and RB. Disagreements were resolved by discussion/consultation with SSM. For RCTs, only the baseline micronutrient levels were extracted.
We used Cochrane risk-of-bias tool to assess the quality of RCTs, the Newcastle-Ottawa Scale (NOS) for observational studies (e.g., case-control and cohort studies), and Joanna Briggs Institute (JBI) critical appraisal checklist for cross-sectional studies. SJK and TB independently performed the quality assessment, and disagreements between reviewers were settled through consensus/discussion with SSM.
2.4 Statistical analysis
From extracted data, we developed a narrative synthesis structured around micronutrient status, findings are presented in tabular form. We employed RevMan 5.4.1 software to perform meta-analysis of selected studies with quantitative estimation.
All data were systematically collected and converted to standard units to maintain uniformity of data using conversion tools (10). We used the statistics toolkit (STATTOOLS) developed by The Department of Obstetrics and Gynaecology of the Chinese University of Hong Kong (11) to combine the mean and standard deviation (SD), where cases or controls were categorized into multiple groups. The formula was used to convert SD to standard error (SE) and vice versa as per Cochrane guideline (12).
Studies reporting vitamin E were excluded from the meta-analysis because we could not convert multiple units of measurement into a standardized uniform unit. Similarly, zinc values from Momen-Heravi et al. study were excluded from the meta-analysis (13). Unit mismatches could be due to the differences in analytical methods. We excluded vitamin D levels reported by Qasim et al. from the review because it had the lowest score in quality assessment (14). We also excluded vitamin D levels reported by Greenhagen et al. from meta-analysis because SD values were not mentioned (15).
The I2 statistic was used to identify the heterogeneity among studies. A random-effects meta-analyses model was conducted because there was significant heterogeneity (I2>50%; P<0.01) in all the analyses performed. Subgroup analysis was carried out based on the geographical location, but not age and gender because of insufficient data.
2.5 Publication bias and sensitivity analysis
The publication bias was assessed using funnel plots. Based on the risk assessment scores, sensitivity analysis was performed to ensure the robustness of the data.
3 Results
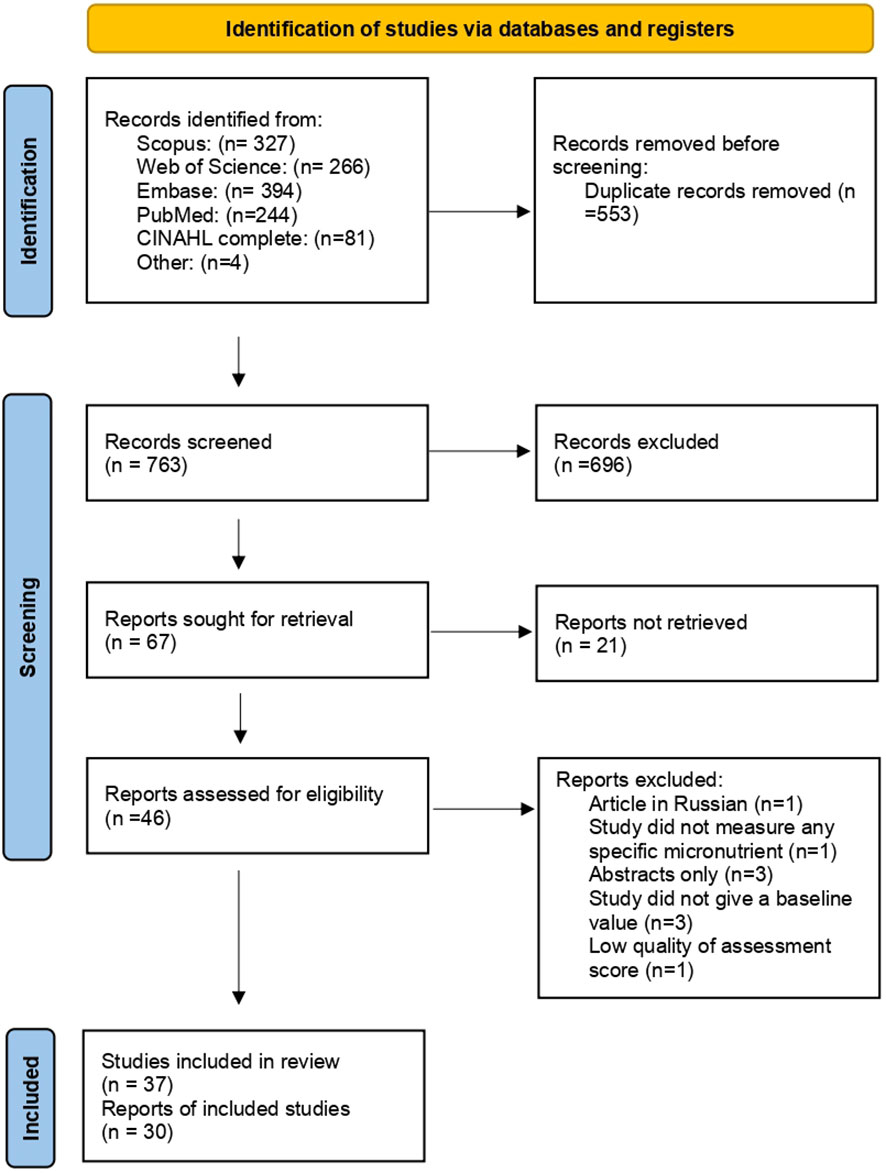
We identified 1312 records from the databases listed. We identified four more relevant studies by manually searching literature references. We removed 553 duplicate records. The remaining 763 were screened based on title and abstract, of which 67 were selected for retrieval. Finally, a total of 46 articles were assessed for eligibility based on criteria, of which 9 were excluded as some were abstract only (n=3), baseline micronutrient levels were not reported (n=3), a specific micronutrient assessment was not made (n=1), low-quality assessment score (n=1), and an article was not in English. 37 were included in the review and 30 for meta-analysis. Figure 1: The PRISMA flow chart of study selection.
3.1 Study characteristics
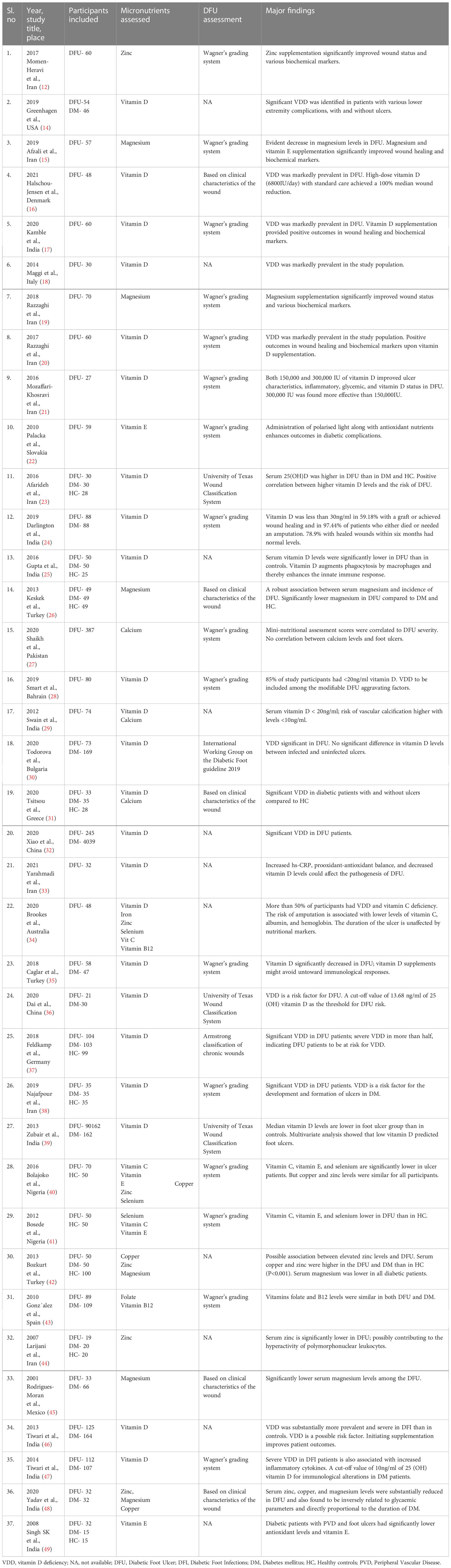
A total of 37 articles were retrieved after a systematic literature search. Nine were RCTs (13, 16–23), and 28 were observational studies (15, 24–50) (12 cross-sectional, seven cohort, and nine case-control studies).
Nine (24.32%) each were reported from India (18, 25, 26, 30, 40, 47–50), and Iran (13, 16, 20–22, 24, 34, 39, 45), three (8.10%) from Turkey (27, 36, 43), two (5.40%) each from China (33, 37) and Nigeria (41, 42), and one (2.70%) each from Italy (19), Bulgaria (31), Greece (32), Pakistan (28), Bahrain (29), USA (15), Germany (38), Australia (35), Spain (44), Mexico (46), Denmark (17), and Slovakia (23). Number of DFU patients (men and women) ranged from 19 to 387. Multiple classification systems were used for DFU assessment such as University of Texas Wound Classification System, Wagner’s grading system, International Working Group on the Diabetic Foot (IWGDF) guideline 2019, and Armstrong classification of chronic wounds, and some were based on the clinical characteristics of the wound. These studies reported levels of 11 nutrients: vitamins B9, B12, C, D, E, calcium, magnesium, iron, selenium, copper, and zinc. Table 1 provides the study characteristics.
3.2 Quality assessment
We employed the Cochrane risk-of-bias tool to assess the quality of RCTs. Case-control and cohort studies were assessed using the NOS. The overall NOS scores for the cohort and case-control studies were 5 to 7, and 6 to 8, respectively, indicating moderate quality. We used JBI checklist for cross-sectional studies. The highest and lowest scores were 8, and 2. Qasim et al. (lowest score) was excluded (14). Table 2 lists the Quality assessment scores of all included studies.
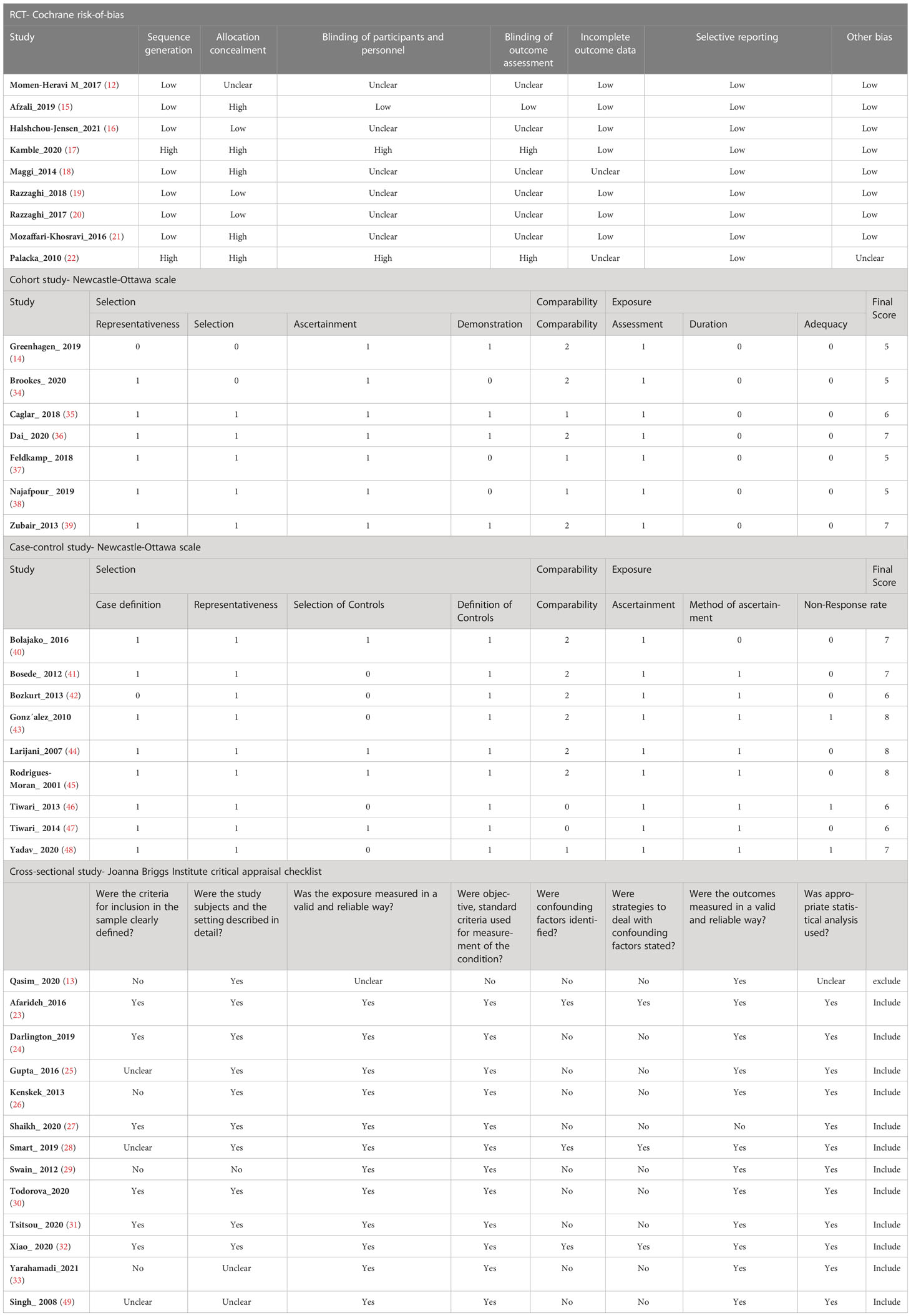
Table 2 Risk of bias assessment using Cochrane risk-of-bias tool for RCTs and Newcastle-Ottawa scale for observational studies, and Joanna Briggs Institute (JBI) critical appraisal checklist for cross-sectional studies.
3.3 Meta-analysis
Micronutrient levels of DFU patients were compared against those with DM [Figure 2A] and HC [Figure 2B] and are reported in mean differences (MD). Figure 3 presents the summary results of micronutrient levels in DFU patients.
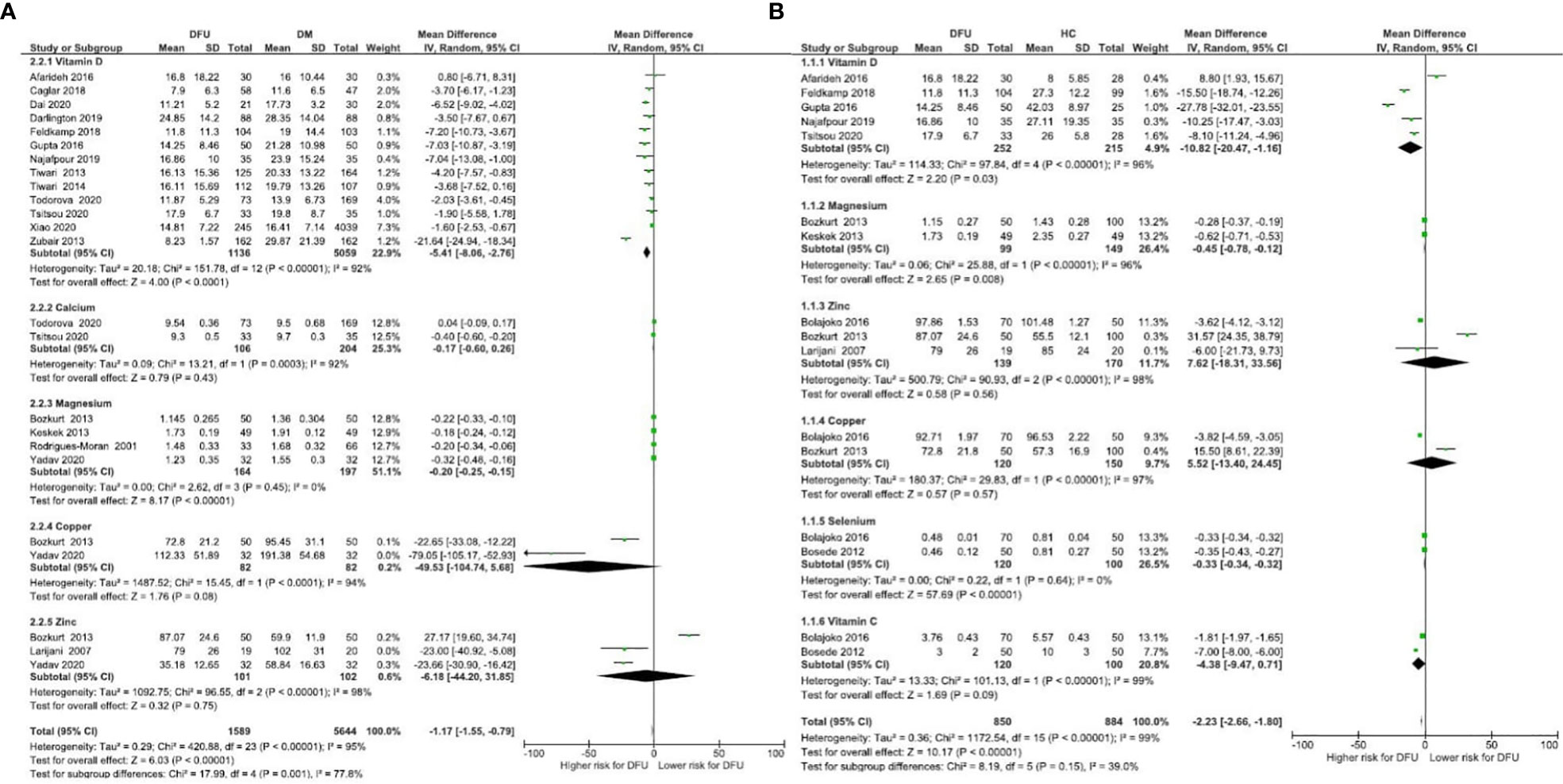
Figure 2 Forest plot of pooled mean difference of micronutrient status in DFU patients compared to DM and HC (A) Micronutrient levels of DFU patients were compared against those with DM; (B) Micronutrient levels of DFU patients were compared against those with HC.
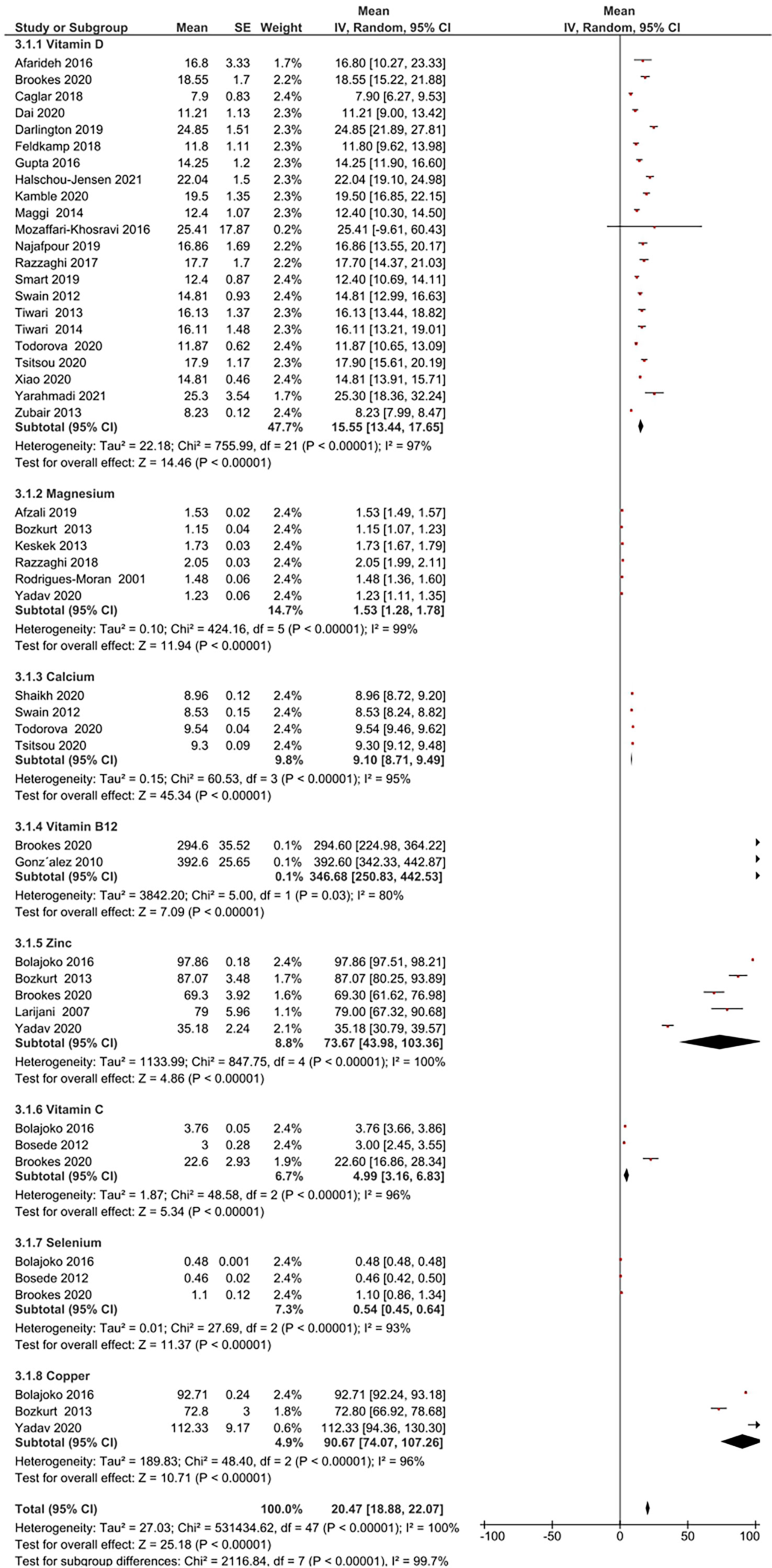
Figure 3 Summary of micronutrient levels in DFU patients. Forest plot of mean micronutrient status in DFU patients.
3.3.1 Vitamin B
Gonzalez et al. estimated folic acid and vitamin B12 levels among DM (n= 109) and DFU (n= 89) patients (44). Serum folic acid (24.9 ± 11.51 vs 25.8 ± 16.6 nmol/L, P = 0.67), and vitamin B12 (392.6 ± 242 vs 453.9 ± 290.8 pmol/L, P = 0.15) were similar in both groups. Brookes et al. reported vitamin B12 in DFU (n= 39) patients with a mean 294.6 ± 221.8 pmol/L (35). The pooled vitamin B12 level in DFU (n= 128) patients was 346.68 pmol/L, 95% CI: 250.83, 442.53; P<00001; I2 = 80%.
3.3.2 Vitamin C
Two studies compared vitamin C in DFU (n=120) and HC (n= 100) patients (41, 42). Combined results showed no significant difference in vitamin C levels between the two groups (MD: -4.38 µmol/L, 95% CI: -9.47, 0.71; P= 0.09; I2 = 99%). A total of three studies measured vitamin C in patients with DFU (35, 41, 42). The mean vitamin C level in DFU (n= 166) patients was 4.99 µmol/L, 95% CI: 3.16, 6.83; P<00001; I2 = 96%.
3.3.3 Vitamin D
Thirteen studies compared vitamin D levels in DFU (n= 1136) and DM (n= 5059) patients (24–26, 31–33, 36–40, 47, 48). Combined results showed significantly lower vitamin D levels in DFU patients (MD: -5.41 ng/ml, 95% CI: -8.06, -2.76; P<0001; I2 = 92%). Combined results of five studies in DFU (n= 252) and HC (n= 215) (24, 26, 32, 38, 39); show significantly lower vitamin D levels in DFU (MD: -10.82 14 ng/ml, 95% CI: -20.47, -1.16; P=0.03; I2 = 96%). From 22 studies that measured vitamin D in patients with DFU (n= 1433) (17–19, 21, 22, 24–26, 29–40, 47, 48), mean levels in patients were 15.55ng/ml, 95% CI: 13.44, 17.65; P<00001; I2 = 97%. Greenhagen et al. reported 18.7ng/ml of vitamin D in 54 DFU patients compared to 23.6 ng/ml in DM (n= 46) patients without ulcers (15).
3.3.4 Vitamin E
Four studies estimated Vitamin E. Singh et al. measured vitamin E levels in DFU (n= 32) patients, DM (n= 15), and HC (n= 15) (50). Vitamin E levels were substantially lower in DFU, compared to DM (5.04 ± 1.76 vs. 9.10 ± 2.83 ng/L, P<0.001) and HC (10.68 ± 2.58ng/L). Bolajoko et al. found lower vitamin E levels in DFU (n= 120) vs DM (n= 50) 19.57 ± 1.01 vs 25.57 ± 0.27 µmol/L, P= 0.0001 (41). A study by Bosede et al. demonstrated no significant difference in vitamin E between DFU (n= 50) and HC (n=50) (0.05 ± 0.02 vs. 0.06 ± 0.005 mmol/L) (42). Palacka et al. assessed multiple baseline metabolic parameters in DFU patients, among which vitamin E was 18.48 ± 7.62 mmol/L (23).
3.3.5 Calcium
Two studies compared calcium levels in DFU (n= 106) and DM (n= 204) patients (31, 32). The combined results showed similar calcium levels in both groups (MD: -0.17 mg/dL, 95% CI: -0.60, 0.26; P=0.43; I2 = 92%). A total of four studies measured calcium in DFU (n=567) (28, 30–32), with mean levels of 9.10 mg/dL, 95% CI: 8.71, 9.49; P<00001; I2 = 95%.
3.3.6 Magnesium
Combined results from 4 studies comparing magnesium levels in DFU (n= 164) and DM (n= 197) patients (27, 43, 46, 49); showed lower magnesium levels in DFU (MD: -0.20 mg/dL, 95% CI: -0.25, -0.15; P<00001; I2 = 0%). Combined results of two other comparison studies in DFU (n= 99) and HC (n= 149) patients (27, 43); showed lower magnesium levels in DFU patients (MD: -0.45 mg/dL, 95% CI: -0.78, -0.12; P=0.008; I2 = 96%). From total of six studies (16, 20, 27, 43, 46, 49], pooled magnesium level was 1.53mg/dL, 95% CI: 1.28, 1.78; P<00001; I2 = 99% in DFU (n= 291).
3.3.7 Iron
Only one study reported Iron levels. A retrospective analysis by Brookes et al. reported mean iron levels of 8.4 ± 5.9 µmol/L in 29 DFU patients (35).
3.3.8 Selenium
Combined results of two studies comparing selenium in DFU (n=120) and HC (n=100) (41, 42); showed significant difference in selenium levels between both groups (MD: -0.33 µmol/L, 95% CI: -0.34, -0.32; P< 0.00001; I2 = 0%). A total of three studies measuring selenium in DFU (n=123) (35, 41, 42), reported mean levels of 0.54 µmol/L, 95% CI: 0.45, 0.64; P<00001; I2 = 93%.
3.3.9 Copper
Combined results of two studies comparing copper levels in DFU (n=82) and DM (n=82) (43, 49) showed similar copper levels in both groups (MD: -49.53 μg/dL, 95% CI: -104.74, 5.68; P= 0.08; I2 = 94%). Combined results of two studies comparing copper levels in DFU (n= 120) and HC (n= 150) (41, 43); showed similar levels in both groups (MD: 5.52 μg/dL, 95% CI: -13.40, 24.45; P=0.57; I2 = 97%). Three studies measuring copper in DFU (n= 152) (41, 43, 49), reported mean levels of 90.67 μg/dL, 95% CI: 74.07, 107.26; P<00001; I2 = 96%.
3.3.10 Zinc
Combined results of three studies comparing zinc levels in DFU (n=101) and DM (n= 102) patients (43, 45, 49) showed similar levels in both groups (MD: -6.18 μg/dL, 95% CI: -44.20, 31.85; P=0.75; I2 = 98%). Combined results of three studies comparing zinc levels in DFU (n= 139) and HC (n=170) (41, 43, 45); showed similar levels in both groups (MD: 7.62 μg/dL, 95% CI: -18.31, 33.56; P= 0.56; I2 = 98%). A total of five studies measuring zinc in patients with DFU (n= 180) (35, 41, 43, 45, 49) reported overall level of 73.67 μg/dL, 95% CI: 43.98, 103.36; P<00001; I2 = 100%. One RCT by Momen-Heravi et al. on the effect of zinc supplements in DFU patients reported the baseline zinc level as 77 ± 9.60 mg/dL (13).
3.4 Subgroup analysis, sensitivity analysis, and publication bias
Due to insufficient data, subgroup analysis (based on geographic location) was conducted only for vitamin D, zinc, and calcium. The mean vitamin D levels [(Figure 4A] were not significantly different across Middle East, Europe, and Asia/Pacific regions (P=0.96). Mean zinc levels [(Figure 4B] significantly differed between Middle East, Asia/Pacific, and African regions (P<0.0001). The mean calcium levels [(Figure 4C] differed significantly between Europe and Asia/Pacific regions (P=0.006).
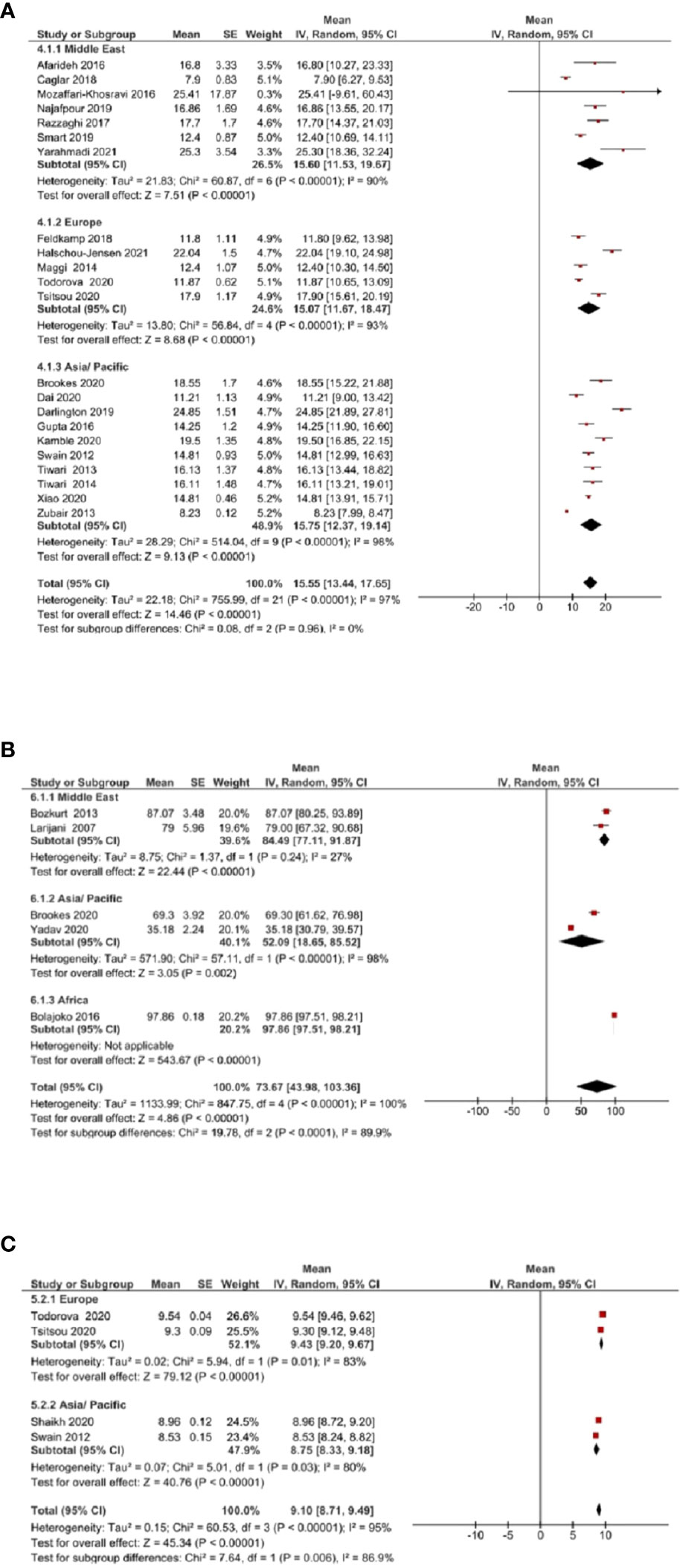
Figure 4 Subgroup analysis based on geographic location was assessed for vitamin D, zinc, and calcium. (A) Mean vitamin D levels across Middle East, Europe, and Asia/Pacific regions. (B) Mean zinc levels across Middle East, Asia/Pacific, and African regions. (C) Mean calcium levels across Europe and Asia/Pacific regions.
The sensitivity analysis by removing two studies (Swain et al. and Yarahmadi et al.) (30, 34) with the lowest risk assessment scores, does not alter the original results (mean = 20.53, 95% CI: 18.90, 22.15). The result of the sensitivity analysis is depicted in Figure 5.
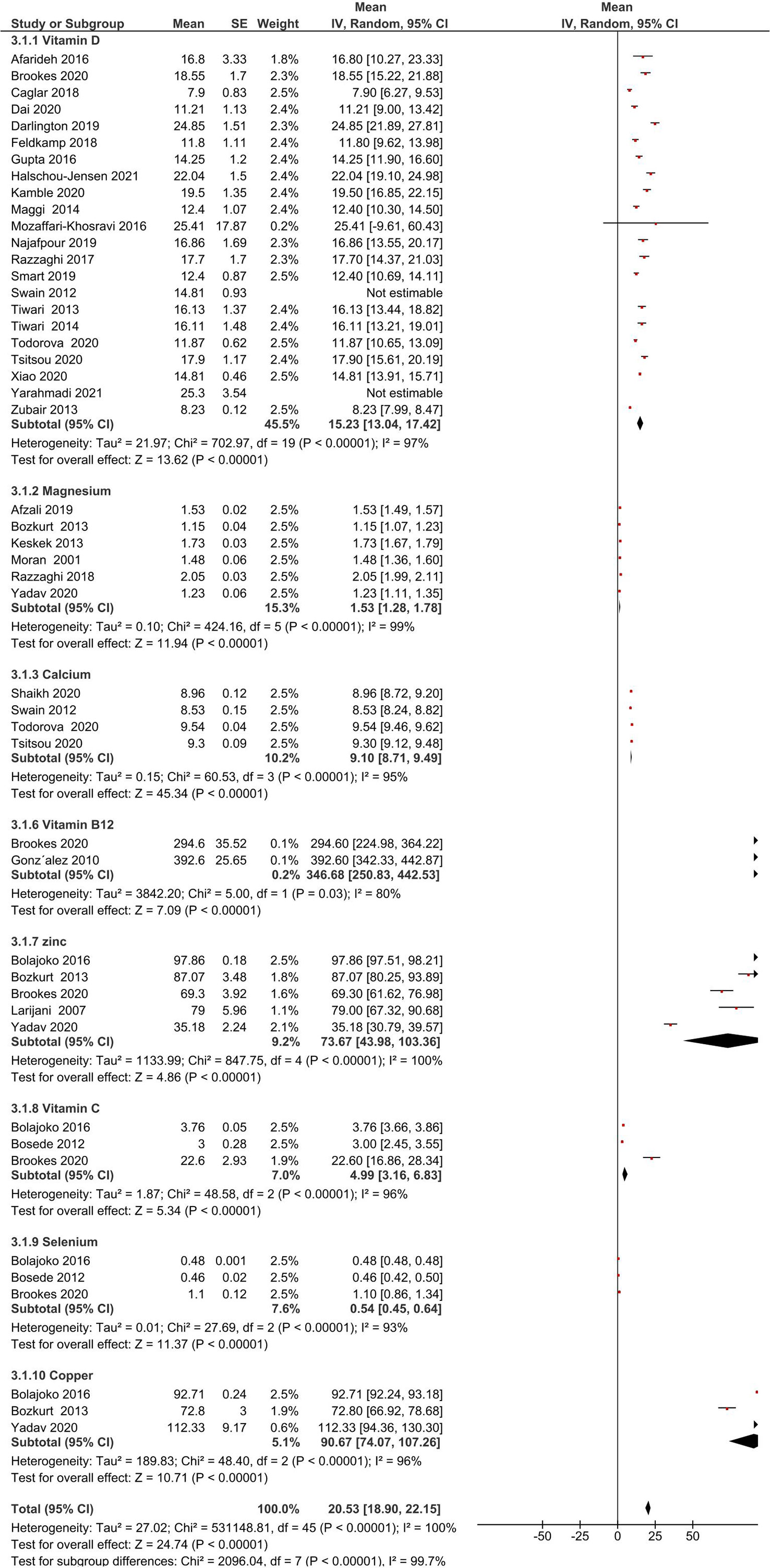
Figure 5 Forest plot for the sensitivity analysis. Sensitivity analysis was performed by eliminating results of two studies with the lowest risk assessment scores.
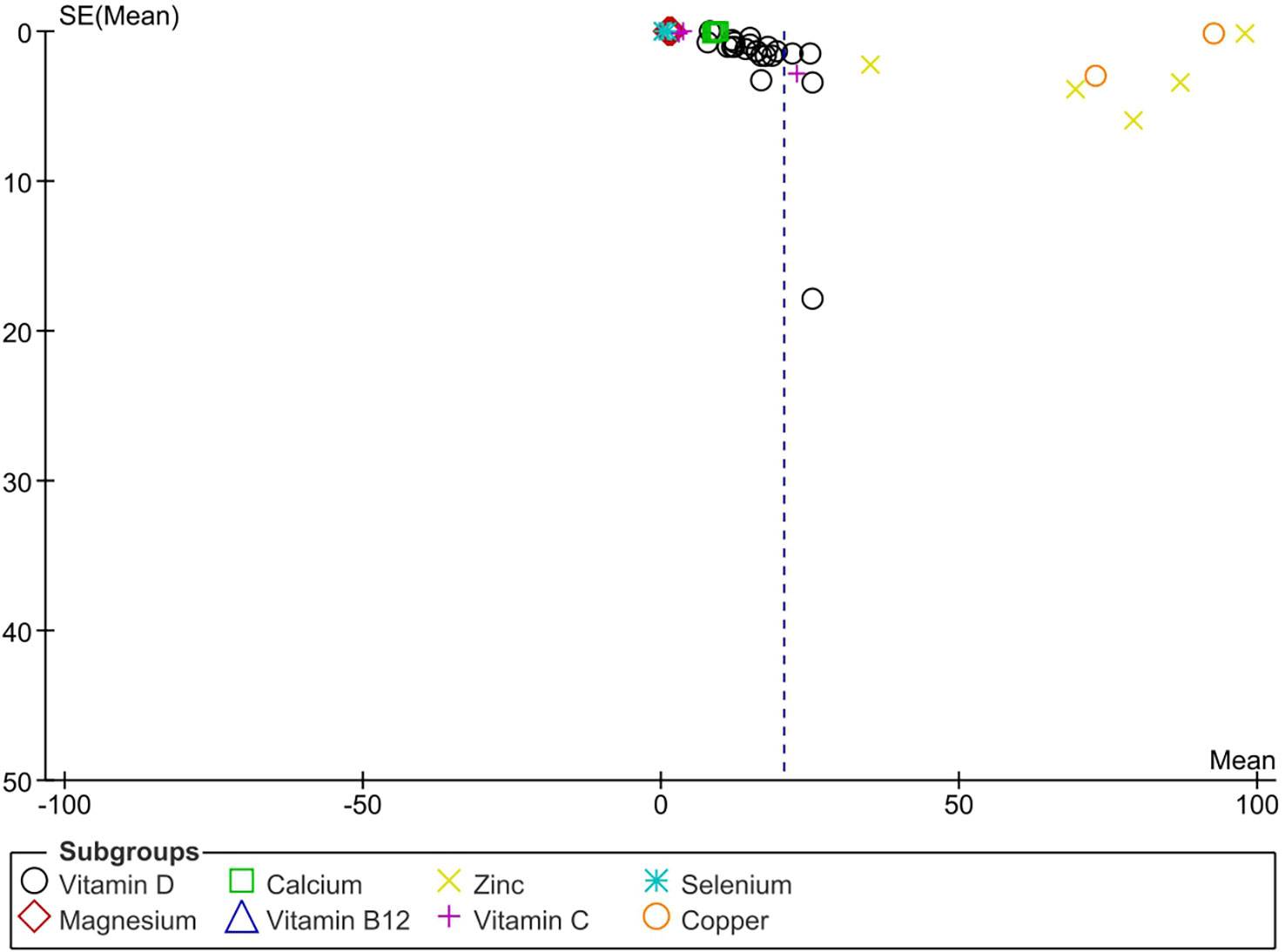
The apparent asymmetry in the funnel plot (Figure 6) suggests possible publication bias.
4 Discussion
Identifying and managing chronic wounds is a critical healthcare objective. DFU generally starts with minor injuries that go unnoticed because of diabetic neuropathy (altered sensitivity and nerve damage). Convergence of immunological, vascular, nutritional, glycaemic, and infectious conditions influences wound healing. The present meta-analysis has revealed significantly lower circulating levels of vitamin D, vitamin C, magnesium, and selenium among patients with DFU than in control groups. However, other micronutrients did not differ significantly between DFU patients and controls.
Nutritional deficiencies impede normal stages of wound healing, a complex four-step process involving hemostasis, inflammation, proliferation, and tissue remodelling (51). Chronic wounds generally get stalled at the inflammatory phase stage due to the continuous recruitment of neutrophils to the healing site, producing various alterations at systemic and molecular levels. Malnutrition also prolongs the inflammatory phase by decreasing fibroblast proliferation, and collagen formation, in addition to altering its tensile strength and angiogenesis. Malnutrition can increase the risk for infection by reducing T-cell function, phagocytic activity, complement, and antibody levels (52). Nutrients can aid wound healing by minimizing free radicals (neutrophils can release reactive oxygen species) and oxidative stress parameters by balancing the oxidant-antioxidant defenses (53). The higher proportion of nutrient insufficiencies in DFU could disturb glycaemic control, which in turn delays wound healing (49).
Vitamin D is well known for its pleiotropy. Vitamin D deficiency (VDD) is associated with impaired beta-cell function, insulin resistance (54), and micro and macro-vascular complications of DM progression. A recent systematic review and meta-analysis of 1115 patients reported that severe VDD increased DFU risk by 3.2 times (55). Interestingly, Darlington et al. observed similar vitamin D levels between DM and DFU patients but with poor DFU outcomes (25). Pena et al. identified VDD to be dominantly prevalent (55.7%) among DFU patients (6). Dai et al. proposed vitamin D levels below 13.68 ng/ml as the threshold for DFU risk (37).
Vitamin D positively improves immunological, neurological, and vascular conditions associated with DFU. Vitamin D is also an immunomodulator that facilitates T and B cell activation by macrophages. Gupta B and Singh SK showed that macrophages treated with vitamin D3, in vitro, enhanced phagocytosis in DFU setting (26). Vitamin D inhibits T-helper cells-1 (Th1) that promote cell-mediated inflammatory response while stimulating Th2 cells that aid wound healing (56). Tiwari et al. suggest 10ng/ml of 25-hydroxy vitamin D [25 (OH)D] as the threshold for immunological alterations in DM. Reports suggest that VDD is associated with an increased release of inflammatory cytokines (TNF-α, IL-1β, IL-6) in DFU patients (48). Vitamin D induces the transcription of cathelicidin and defensins that aid in phagocytosis, thereby enhancing the antimicrobial innate immune system (57).
Asian DM patients with VDD are at 1.22 times greater risk for developing peripheral neuropathy than those with normal vitamin D levels (58). Basit et al. showed that 600,000 IU of vitamin D, over 20 weeks, offered significant pain relief in painful diabetic neuropathy (59). VDD may also be associated with increased sensitivity to pain (60). Swain et al. reported that nearly 52% of DFU patients with vascular calcification (VC) had severe VDD (30). Their subgroup analyses showed that the risk for VC was 2.4 times higher in patients with vitamin D levels < 10 ng/ml. Sugden et al. demonstrated that a single high dose of vitamin D supplementation can improve the flow-mediated vasodilation of the brachial artery by 2.3% (61).
Most studies have focused on the significant role of vitamin D in DFU compared to other nutrients. We need more clinical and molecular studies to explain the results. We identified four clinical trials that estimated 25 (OH) D levels and studied the effects of vitamin D supplementation on DFU outcomes. Kamble et al. and Razzaghi et al. investigated the effect of 60,000 IU and 50,000 IU of vitamin D, respectively, for 12 weeks, in DFU healing (18, 21) and reported that supplements improved wound healing and biochemical parameters. Halschou-Jensen et al. showed that two daily doses (170 µg and 20 µg) of vitamin D supplements in chronic DFU (17) delivered a median ulcer reduction of 100% (high dose) and 57% (low dose). Mozaffari-Khosravi et al. demonstrated that a single dose of 300,000 IU of vitamin D improved DFU outcomes compared to 150,000 IU (22).
Magnesium is an essential element with a pivotal role in human physiology, especially as a cofactor for enzymatic and metabolic pathways (62). Magnesium, essential for collagen formation and tissue development, is altered in DM (63). Hypomagnesemia in DM could result from enhanced renal excretion associated with insulin resistance, glycosuria, and hyperglycemia. Diabetic autonomic neuropathy alters intestinal absorption (27) and reduces dietary intake of magnesium. Improving insulin metabolism can potentially delay vascular complications in DFU. Magnesium plays a role in the formation of malonyl-COA and inhibits voltage-dependent calcium channels that facilitate insulin secretion (20). Hypomagnesemia has been associated with abnormal platelet activity and can induce a proinflammatory response that activates systemic inflammation (64). Hypomagnesemia has also been linked with neuronal damage and diabetic peripheral neuropathy in DM patients (65, 66). Further magnesium supplementation was found to promote peripheral nerve regeneration (67).
Yadav et al. observed an inverse relationship between DM duration and serum magnesium, copper, and zinc levels (49). Rodrigues-Moran et al. provided the first evidence for hypomagnesemia as a risk factor for DFU (OR: 2.9, 95% CI: 1.7-6.8; P = 0.01) (46). Interestingly, Moon et al. have reported that hypermagnesemia is a risk factor for amputation in hospitalized DFU patients (OR:2.480; P= 0.043), which could be attributed to the association between renal disorder and hypermagnesemia (68).
Two studies have investigated the role of magnesium supplementation in DFU patients. Razzaghi et al. found that 250 mg of magnesium for 12 weeks improved the ulcer area, glycaemic parameters, and other antioxidant and anti-inflammatory parameters (20). Afzali et al. showed that 250mg magnesium plus 400 IU vitamin E can improve ulcer area, glycaemic parameters, lipid profile, and other antioxidant and anti-inflammatory parameters (16). Coger et al. have suggested magnesium supplements during the late-inflammatory and mid-proliferative phases (69).
A population-based cohort study (25,639 participants; 8-12 years) demonstrated an inverse association between vitamin C levels and incidence of DM (70). Vitamin C is a strong antioxidant, a vital co-factor in several enzymatic reactions, and promotes anti-inflammatory and pro-resolution effects in macrophages, together alleviating pro-inflammatory responses (71). Vitamin C deficiency in DM has been established, and its impact on serum malondialdehyde suggests increased oxidative stress, aggravating micro- and macro-vascular complications in DM (72).
A meta-analysis of RCTs shows that vitamin C supplements significantly improved endothelial function in DM. Vitamin C is a direct antioxidant that scavenges reactive oxygen species and enhances the bioavailability of nitric oxide (NO) (73). In 2021, an RCT (n= 16) of vitamin C supplements showed benefits on foot ulcers (74). Inadequate vitamin C supplements can cause stagnation in the proliferative and maturation phases of wound healing, thereby prolonging wound healing time (71). Vitamin C facilitates the synthesis and cross-linking of collagen, enhancing vascular integrity and capillary bed strength (75). Pena et al. identified 73% of DFU patients with suboptimal levels of vitamin C (6). An RCT by Yarahmadi et al. showed that a combination of platelet-rich plasma, fibrin glue dressing, and vitamins E and C improved wound healing of DFU by alleviating oxidative stress (76).
Dixit et al. reported a significant difference between selenium levels in patients with chronic non-healing wounds and HC (77). An in vivo study on diabetic mice demonstrated an antioxidant role for selenium (restoring normal antioxidant status), and as an insulin mimetic in normalizing glucose levels. Selenium can also downregulate connexin expression, which promotes anti-inflammatory and anti-apoptotic signals, in addition to enhancing angiogenesis (78). Macrophages treated with selenium promote peroxisome proliferator-activated receptor (PPAR)-γ- dependent switch from M1 to M2 phenotype in the presence of IL-4 (79), suggesting selenium’s wound healing potential.
Currently, available evidence suggests that immune-endocrine effects and antioxidant properties of selenium benefit infections in DM (80). Although we did not identify any interventional studies on the effect of selenium in DFU, selenium levels were markedly different in DFU patients vis-a-vis HC and DM (35, 41, 42).
The strength of the current study: This is the first systematic review with meta-analysis comparing micronutrient status in DFU between HC and DM. The limitations are First: relatively small sample size in some studies. Second: most study designs were retrospective or cross-sectional, limiting the possibility of establishing a causal relationship between the micronutrients and DFU. Third: marked publication bias was observed. Fourth: cannot rule out the possibility of ecology and environment as confounders. Nevertheless, the existing challenge is to articulate the effect of these supplementations in the patient population as the number of well-designed RCT’s are few.
We have observed a significant association between DFU and vitamin D, vitamin C, magnesium, copper, and selenium levels. Although other micronutrients also influence multiple phases of wound healing, we did not observe a significant association. Nevertheless, we recommend assessing micronutrient levels in DFU patients and investigating their pathological correlation. Future investigations should address the effect of specific micronutrients in DFU management, molecular mechanisms of action of micronutrients, as well as nutrigenomic studies that reveal gene-nutrient interaction and its possible effects on DFU healing. Individual genetic variants could respond differently to micronutrients, and thus directly or indirectly influence the prevention and management of DFU. Nutrigenomic approaches would deliver a holistic and personalized approach to the management of DFU.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SK and SM formulated the research question and designed the study. SK, TB, RB, and SM were involved in carrying out the study, analyzing the data, and interpreting the findings. SK and SM wrote the manuscript. MU, MM, KS, GR, MR, and AK, critically evaluated the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
SK is grateful to Indian Council of Medical Research (ICMR), New Delhi for the award of Senior Research Fellowship [No. 3/1/3 (16)/Endo-fellowship/21-NCD-III]. The authors thank the Manipal Academy of Higher Education for providing support and resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sargen MR, Hoffstad O, Margolis DJ. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J Diabetes Complications (2013) 27:128–33. doi: 10.1016/j.jdiacomp.2012.09.003
2. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
3. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med (2017) 49:106–16. doi: 10.1080/07853890.2016.1231932
4. Brennan MB, Hess TM, Bartle B, Cooper JM, Kang J, Huang ES, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications (2017) 31:556–61. doi: 10.1016/j.jdiacomp.2016.11.020
5. Hobizal KB, Wukich DK. Diabetic foot infections: current concept review. Diabetes Foot Ankle (2012) 2012:3. doi: 10.3402/dfa.v3i0.18409
6. Pena G, Kuang B, Cowled P, Howell S, Dawson J, Philpot R, et al. Micronutrient status in diabetic patients with foot ulcers. Adv Wound Care New Rochelle (2019) 9:9–15. doi: 10.1089/wound.2019.0973
7. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes (2015) 6:37–53. doi: 10.4239/wjd.v6.i1.37
8. Ghaly P, Iliopoulos J, Ahmad M. The role of nutrition in wound healing: an overview. Br J Nurs (2021) 30:S38–42. doi: 10.12968/bjon.2021.30.5.S38
9. Valdés-Ramos R, Ana Laura G-L, Beatriz Elina M-C, Alejandra Donaji B-A. Vitamins and type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets (2015) 15:54–63. doi: 10.2174/1871530314666141111103217
10. . Available at: https://unitslab.com/ (Accessed 05 February 2022). unitslab.com.
11. Department of Obstetrics or Gynaecology. StatTools: Combine means and SDs into one group program . Available at: http://www.obg.cuhk.edu.hk/ResearchSupport/StatTools/CombineMeansSDs_Pgm.php (Accessed 05 February 2022).
12. Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect, in: Cochrane handbook for systematic reviews of interventions (2019). Available at: https://training.cochrane.org/handbook/current/chapter-06 (Accessed 02 January 2022).
13. Momen-Heravi M, Barahimi E, Razzaghi R, Bahmani F, Gilasi HR, Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Wound Repair Regen (2017) 25:512–20. doi: 10.1111/wrr.12537
14. Qasim SM, Ra’ad Al-dorri AZ, Mahmood Al-Izzi MH. The relationship between vitamin d deficiency and interleukins 8 and 10 in diabetes mellitus. SRP (2020) 11:149–55. doi: 10.31838/srp.2020.9.26
15. Greenhagen RM, Frykberg RG, Wukich DK. Serum vitamin d and diabetic foot complications. Diabetes Foot Ankle (2019) 10:1579631. doi: 10.1080/2000625X.2019.1579631
16. Afzali H, Jafari Kashi AH, Momen-Heravi M, Razzaghi R, Amirani E, Bahmani F, et al. The effects of magnesium and vitamin e co-supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Wound Repair Regen (2019) 27:277–84. doi: 10.1111/wrr.12701
17. Halschou-Jensen PM, Sauer J, Bouchelouche P, Fabrin J, Brorson S, Ohrt-Nissen S. Improved healing of diabetic foot ulcers after high-dose vitamin d: A randomized double-blinded clinical trial. Int J Low Extrem Wounds (2021), 1–9. doi: 10.1177/15347346211020268
18. Kamble A, Ambad RS, Padamwar M, Kakade A, Yeola M. To study the effect of oral vitamin d supplements on wound healing in patient with diabetic foot ulcer and its effect on lipid metabolism. Int J Res Pharm Sci (2020) 11:2701–6. doi: 10.26452/ijrps.v11i2.2290
19. Maggi S, Siviero P, Brocco E, Albertin M, Romanato G, Crepaldi G. Vitamin d deficiency, serum leptin and osteoprotegerin levels in older diabetic patients: An input to new research avenues. Acta Diabetol (2014) 51:461–9. doi: 10.1007/s00592-013-0540-4
20. Razzaghi R, Pidar F, Momen-Heravi M, Bahmani F, Akbari H, Asemi Z. Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Biol Tra Elem Res (2018) 181:207–15. doi: 10.1007/s12011-017-1056-5
21. Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, et al. The effects of vitamin d supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications (2017) 31:766–72. doi: 10.1016/j.jdiacomp.2016.06.017
22. Mozaffari-Khosravi H, Haratian-Arab M, Tavakkoli HM, Nadjarzadeh A. Comparative effect of two different doses of vitamin d on diabetic foot ulcer and inflammatory indices among the type 2 diabetic patients: a randomized clinical trial. Iran J Diabetes Obes (2016) 8:164–71.
23. Palacka P, Kucharska J, Murin J, Dostalova K, Okkelova A, Cizova M, et al. Complementary therapy in diabetic patients with chronic complications: A pilot study. Bratisl Lek Listy (2010) 111:205–11.
24. Afarideh M, Ghanbari P, Noshad S, Ghajar A, Nakhjavani M, Esteghamati A. Raised serum 25-hydroxyvitamin d levels in patients with active diabetic foot ulcers. Br J Nutr (2016) 115:1938–46. doi: 10.1017/S0007114516001094
25. Darlington CJD, Kumar SS, Jagdish S, Sridhar MG. Evaluation of serum vitamin d levels in diabetic foot infections: A cross-sectional study in a tertiary care center in south India. Iran J Med Sci (2019) 44:474–82. doi: 10.30476/ijms.2018.44951
26. Gupta B, Singh SK. Invitro study of role of vitamin d on macrophages dysfunction in patients with diabetic foot infection. Int J Adv Res (2016) 4:1633–7. doi: 10.21474/IJAR01/753
27. Keşkek SO, Kırım S, Karaca A, Saler T. Low serum magnesium levels and diabetic foot ulcers. Pak J Med Sci (2013) 29:1329–33. doi: 10.12669/pjms.296.3978
28. Shaikh IA, Masood N, Shaikh FA, Shaikh MA. Diabetic foot ulcers: Correlation of nutritional status of type 2 diabetic patients of Hyderabad sindh, Pakistan. TPMJ (2017) 24:707–12. doi: 10.17957/TPMJ/17.3869
29. Smart H, AlGhareeb AM, Smart SA. 25-hydroxyvitamin d deficiency: Impacting deep-wound infection and poor healing outcomes in patients with diabetes. Adv Skin Wound Care (2019) 32:321–8. doi: 10.1097/01.ASW.0000559614.90819.45
30. Swain J, Tiwari S, Pratyush D, Dwivedi A, Gupta B, Shukla RC, et al. Vascular calcification in diabetic foot and its association with calcium homeostasis. Indian J Endocrinol Metab (2012) 16, Suppl 2:S450–2. doi: 10.4103/2230-8210.104128
31. Todorova AS, Jude EB, Dimova RB, Chakarova NY, Serdarova MS, Grozeva GG, et al. Vitamin d status in a Bulgarian population with type 2 diabetes and diabetic foot ulcers. Int J Low Extrem Wounds (2022) 21:506–12. doi: 10.1177/1534734620965820
32. Tsitsou S, Dimosthenopoulos C, Eleftheriadou I, Andrianesis V, Tentolouris N. Evaluation of vitamin d levels in patients with diabetic foot ulcers. Int J Low Extrem Wounds (2023) 22:27–35. doi: 10.1177/1534734620984584
33. Xiao Y, Wei L, Xiong X, Yang M, Sun L. Association between vitamin d status and diabetic complications in patients with type 2 diabetes mellitus: A cross-sectional study in hunan China. Front Endocrinol (Lausanne) (2020) 11:564738. doi: 10.3389/fendo.2020.564738
34. Yarahmadi A, Mostafavi-Pour Z, Saeed MH, Azarpira N, Mousavian A, Bonakdaran S, et al. Association between serum vitamin d, hs-CRP, and prooxidant-antioxidant balance with anthropometric and biochemical parameters in patients with diabetic foot ulcers. Clin Diabetol (2021) 10:138–43. doi: 10.5603/DK.2020.0064
35. Brookes JDL, Jaya JS, Tran H, Vaska A, Werner-Gibbings K, D’Mello AC, et al. Broad-ranging nutritional deficiencies predict amputation in diabetic foot ulcers. Int J Low Extrem Wounds (2020) 19:27–33. doi: 10.1177/1534734619876779
36. Caglar S, Caglar A, Pilten S, Albay G, Beytemur O, Sari H. Osteoprotegerin and 25-hydroxy vitamin d levels in patients with diabetic foot. Eklem Hastalik Cerrahisi (2018) 29:170–5. doi: 10.5606/ehc.2018.60797
37. Dai J, Yu M, Chen H, Chai Y. Association between serum 25-OH-Vitamin d and diabetic foot ulcer in patients with type 2 diabetes. Front Nutr (2020) 7:109. doi: 10.3389/fnut.2020.00109
38. Feldkamp J, Jungheim K, Schott M, Jacobs B, Roden M. Severe vitamin D3 deficiency in the majority of patients with diabetic foot ulcers. Horm Metal Res (2018) 50:615–9. doi: 10.1055/a-0648-8178
39. Najafipour F, Aghamohammadza N, Zonouz NR, Houshyar J. Role of serum vitamin d level in progression of diabetic foot ulcer. JCDR (2019) 13:15–7. doi: 10.7860/JCDR/2019/39974.12689
40. Zubair M, Malik A, Meerza D, Ahmad J. 25-hydroxyvitamin d [25(OH)D] levels and diabetic foot ulcer: Is there any relationship? Diabetes (2013) 7:148–53. doi: 10.1016/j.dsx.2013.06.008
41. Bolajokol EB, Akinosui OM, Anetor JI, Mossanda KS. Micronutrient status and its effect on glycaemic indices in type 2 diabetics with foot ulcer in ibadan, Nigeria. Afr J Med Med Sci (2016) 45:83–90.
42. Bosede BE, Olubayo A, John A, Adesoji F, Aduragbemi A, Ayodele I, et al. Ameliorative role of antioxidant micronutrients: Selenium, vitamins c and e on oxidative stress and wound healing in type 2 diabetic patients with foot ulcer in ibadan. IIOAB J (2012) 3:1–5.
43. Bozkurt F, Tekin R, Gulsun S, Satıcı O, Deveci O, Hosoglu S. The levels of copper, zinc and magnesium in type II diabetic patients complicated with foot infections. Int J Diabetes Dev Ctries (2013) 33:165–9. doi: 10.1007/s13410-013-0130-6
44. González R, Pedro T, Real JT, Martínez-Hervás S, Abellán MR, Lorente R, et al. Plasma homocysteine levels are associated with ulceration of the foot in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev (2010) 26:115–20. doi: 10.1002/dmrr.1061
45. Larijani B, Shooshtarizadeh P, Mosaffa N, Heshmat R. Polymorphonuclear leucocyte respiratory burst activity correlates with serum zinc level in type 2 diabetic patients with foot ulcers. Br J BioMed Sci (2007) 64:13–7. doi: 10.1080/09674845.2007.11732749
46. Rodríguez-Morán M, Guerrero-Romero F. Low serum magnesium levels and foot ulcers in subjects with type 2 diabetes. Arch Med Res (2001) 32:300–3. doi: 10.1016/S0188-4409(01)00298-3
47. Tiwari S, Pratyush DD, Gupta B, Dwivedi A, Chaudhary S, Rayicherla RK, et al. Prevalence and severity of vitamin d deficiency in patients with diabetic foot infection. Br J Nutr (2013) 109:99–102. doi: 10.1017/S0007114512000578
48. Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin d deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr (2014) 112:1938–43. doi: 10.1017/S0007114514003018
49. Yadav C, Srikantiah RM, Manjrekar P, Shenoy MT, Chaudhury D. Assessment of mineral pathophysiology in patients with diabetic foot ulcer. Biol Trace Elem Res (2020) 195:366–72. doi: 10.1007/s12011-019-01868-3
50. Singh S, Sahay R, Krishna A. Oxidative stress in diabetic foot ulcer. Diabetes Metab Syndr: Clin Res Rev (2008) 2:109–13. doi: 10.1016/j.dsx.2008.02.003
51. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res (2010) 89:219–29. doi: 10.1177/0022034509359125
52. Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract (2010) 25:61–8. doi: 10.1177/0884533609358997
53. Amini MR, Aalaa M, Nasli-Esfahani E, Atlasi R, Sanjari M, Namazi N. The effects of dietary/herbal supplements and the serum levels of micronutrients on the healing of diabetic foot ulcers in animal and human models: a systematic review. J Diabetes Metab Disord (2021) 20:973–88. doi: 10.1007/s40200-021-00793-4
54. Contreras-Bolívar V, García-Fontana B, García-Fontana C, Muñoz-Torres M. Mechanisms involved in the relationship between vitamin d and insulin resistance: impact on clinical practice. Nutrients (2021) 13:3491. doi: 10.3390/nu13103491
55. Dai J, Jiang C, Chen H, Chai Y. Vitamin d and diabetic foot ulcer: a systematic review and meta-analysis. Nutr Diabetes (2019) 9:1–6. doi: 10.1038/s41387-019-0078-9
56. Szymczak I, Pawliczak R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol (2016) 83:83–91. doi: 10.1111/sji.12403
57. Bishop E L, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin d and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR plus (2021) 5:e10405. doi: 10.1002/jbm4.10405
58. Qu G-B, Wang L-L, Tang X, Wu W, Sun Y-H. The association between vitamin d level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: An update systematic review and meta-analysis. J Clin Transl Endocrinol (2017) 9:25–31. doi: 10.1016/j.jcte.2017.04.001
59. Basit A, Basit KA, Fawwad A, Shaheen F, Fatima N, Petropoulos IN, et al. Vitamin d for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care (2016) 4:e000148. doi: 10.1136/bmjdrc-2015-000148
60. Alam U, Nelson AJ, Cuthbertson DJ, Malik RA. An update on vitamin d and b deficiency in the pathogenesis and treatment of diabetic neuropathy: a narrative review. Future Neurol (2018) 13:135–42. doi: 10.2217/fnl-2017-0034
61. Sugden J, Davies J, Witham M, Morris A, Struthers A. Vitamin d improves endothelial function in patients with type 2 diabetes mellitus and low vitamin d levels. Diabetes Med (2008) 25:320–5. doi: 10.1111/j.1464-5491.2007.02360.x
62. Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J (2012) 5, Suppl. 1:i3–14. doi: 10.1093/ndtplus/sfr163
63. Chaudhary DP, Sharma R, Bansal DD. Implications of magnesium deficiency in type 2 diabetes: a review. Biol Trace Elem Res (2010) 134:119–29. doi: 10.1007/s12011-009-8465-z
64. Liu M, Dudley SC Jr. Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxid (Basel) (2020) 9:907. doi: 10.3390/antiox9100907
65. Strom A, Strassburger K, Schmuck M, Shevalye H, Davidson E, Zivehe F, et al. Interaction between magnesium and methylglyoxal in diabetic polyneuropathy and neuronal models. Mol Metab (2021) 43:101114. doi: 10.1016/j.molmet.2020.101114
66. Jamali AA, Jamali GM, Tanwani BM, Jamali AA, Tanwani Y, Jamali NM. Association of hypomagnesemia in type 2 diabetic patients with and without peripheral neuropathy. J Diabetes Mellitus (2018) 8:27–42. doi: 10.4236/jdm.2018.82004
67. Zhang J, Zhang B, Zhang J, Lin W, Zhang S. Magnesium promotes the regeneration of the peripheral nerve. Front Cell Dev Biol (2021) 9:717854. doi: 10.3389/fcell.2021.717854
68. Moon K-C, Kim S-B, Han S-K, Jeong S-H, Dhong E-S.. Risk factors for major amputation in hospitalized diabetic patients with forefoot ulcers. Diabetes Res Clin Pract (2019) 158:107905. doi: 10.1016/j.diabres.2019.107905
69. Coger V, Million N, Rehbock C, Sures B, Nachev M, Barcikowski S, et al. Tissue concentrations of zinc, iron, copper, and magnesium during the phases of full thickness wound healing in a rodent model. Biol Trace Element Res (2019) 191:167–76. doi: 10.1007/s12011-018-1600-y
70. Harding A-H, Wareham NJ, Bingham SA, Khaw K, Luben R, Welch A, et al. Plasma vitamin c level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer–Norfolk prospective study. Arch Intern Med (2008) 168:1493–9. doi: 10.1001/archinte.168.14.1493
71. Mohammed BM, Fisher BJ, Kraskauskas D, Ward S, Wayne JS, Brophy DF, et al. Vitamin c promotes wound healing through novel pleiotropic mechanisms. Int Wound J (2016) 13:572–84. doi: 10.1111/iwj.12484
72. Devanandan P, Puvvada RC, Muthukumar VA. Association of vitamin c status in diabetes mellitus: prevalence and predictors of vitamin c deficiency. Future J Pharm Sci (2020) 6:1–5. doi: 10.1186/s43094-020-00040-2
73. Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin c on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis (2014) 235:9–20. doi: 10.1016/j.atherosclerosis.2014.04.004
74. Gunton JE, Girgis CM, Lau T, Vicaretti M, Begg L, Flood V. Vitamin c improves healing of foot ulcers: a randomised, double-blind, placebo-controlled trial. Br J Nutr (2021) 126:1451–8. doi: 10.1017/S0007114520003815
75. Moores J. Vitamin c: a wound healing perspective. Br J Community Nurs (2013) 18:S6–S11. doi: 10.12968/bjcn.2013.18.Sup12.S6
76. Yarahmadi A, Saeed Modaghegh M-H, Mostafavi-Pour Z, Azarpira N, Mousavian A, Bonakdaran S, et al. The effect of platelet-rich plasma-fibrin glue dressing in combination with oral vitamin e and c for treatment of non-healing diabetic foot ulcers: a randomized, double-blind, parallel-group, clinical trial. Expert Opin Biol Ther (2021) 21:687–96. doi: 10.1080/14712598.2021.1897100
77. Dixit R, Chaudhary NK, Mishra PK, Srivastava P, Bhartiya SK, Pratap A, et al. Study on blood serum levels of heavy and trace metals in chronic non-healing wounds. Int J Low Extrem Wounds (2022). doi: 10.1177/15347346221074161
78. Bajpai S, Mishra M, Kumar H, Tripathi K, Singh SK, Pandey HP, et al. Effect of selenium on connexin expression, angiogenesis, and antioxidant status in diabetic wound healing. Biol Trace Element Res (2011) 144:327–38. doi: 10.1007/s12011-011-9097-7
79. Nelson SM, Lei X, Prabhu KS. Selenium levels affect the IL-4–induced expression of alternative activation markers in murine macrophages. J Nutr (2011) 141:1754–61. doi: 10.3945/jn.111.141176
Keywords: diabetic foot ulcers, micronutrients, vitamins, minerals, risk
Citation: Kurian SJ, Baral T, Unnikrishnan MK, Benson R, Munisamy M, Saravu K, Rodrigues GS, Rao M, Kumar A and Miraj SS (2023) The association between micronutrient levels and diabetic foot ulcer: A systematic review with meta-analysis. Front. Endocrinol. 14:1152854. doi: 10.3389/fendo.2023.1152854
Received: 28 January 2023; Accepted: 14 March 2023;
Published: 29 March 2023.
Edited by:
Joanne Paton, University of Plymouth, United KingdomReviewed by:
Zulfiqarali Abbas, Muhimbili University of Health and Allied Sciences, TanzaniaPeter Fasching, Vienna Health Association, Austria
Copyright © 2023 Kurian, Baral, Unnikrishnan, Benson, Munisamy, Saravu, Rodrigues, Rao, Kumar and Miraj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sonal Sekhar Miraj, c29uYWwuc2VraGFyQG1hbmlwYWwuZWR1
 Shilia Jacob Kurian1,2
Shilia Jacob Kurian1,2 Ruby Benson
Ruby Benson Mahadev Rao
Mahadev Rao Amit Kumar
Amit Kumar Sonal Sekhar Miraj
Sonal Sekhar Miraj