- 1Institute of Health Data Management, Huanghuai University, Zhumadian, He’nan, China
- 2Affiliated Hospital of Huanghuai University, Zhumadian Central Hospital, Zhumadian, He’nan, China
- 3Jiyuan Center for Disease Control and Prevention, Jiyuan, He’nan, China
- 4Department of Cancer Prevention and Control, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, China
Background: Chinese visceral adiposity index (CVAI) is a reliable indicator of visceral obesity, but little is known about the association of CVAI with comorbidity of hypertension (HTN) and diabetes mellitus (DM). This study aimed to explore the associations of CVAI with HTN-DM comorbidity, HTN or DM, HTN, and DM in elderly people and evaluate the mediating role of insulin resistance in the associations.
Methods: A total of 3,316 Chinese participants aged ≥60 years were included in this cross-sectional study. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Restricted cubic splines were applied to explore the dose–response associations. Mediation analyses were used to assess the mediating effect of triglyceride-glucose (TyG) index in the associations.
Results: The prevalence rate of HTN-DM comorbidity, HTN or DM, HTN, and DM was 13.78%, 72.26%, 67.16%, and 18.88%, respectively. Linear associations between CVAI and HTN-DM comorbidity, HTN or DM, HTN, and DM were found, and ORs (95%CIs) were 1.45 (1.30–1.61), 1.39 (1.28–1.52), 1.36 (1.25–1.48), and 1.28 (1.16–1.41) for per SD increase in CVAI. Compared with quartile 1 of CVAI, the risk of HTN-DM comorbidity, HTN or DM, HTN, and DM increased 190%, 125%, 112%, and 96% for quartile 4. In addition, we found TyG index playing a key role in the associations of CVAI with HTN-DM comorbidity, HTN or DM, and DM.
Conclusion: CVAI is linearly and positively correlated with HTN-DM comorbidity, HTN or DM, HTN, and DM. The potential mechanism is insulin resistance largely mediating the associations.
Introduction
Hypertension (HTN) and diabetes mellitus (DM) are two important risk factors for cardiovascular disease and premature death worldwide (1, 2). With the increasing prevalence of HTN and DM and intensification of aging, HTN-DM comorbidity has become a serious public health problem, especially in Chinese population (3–5). Data from China Kadoorie Biobank (CKB) showed that the prevalence rate of HTN-DM comorbidity increased by 162% (2.72% vs. 7.12%) during 8 years of follow-up (6). China Chronic Disease and Risk Factor Surveillance (CCDRFS) in 2018 including 134,950 participants aged ≥45 years showed that the total prevalence rate of HTN, DM, and HTN-DM comorbidity was 46.0%, 19.5%, and 12.3%; the corresponding prevalence rate was 59.2%, 25.0%, and 17.7% for participants aged 65–75 years and 67.6%, 26.7%, and 20.6% for participants aged ≥75 years (7). Because of the high prevalence and low control level, identifying risk factors, early detection, and subsequent intervention in HTN-DM comorbidity is urgently needed.
Chinese visceral adiposity index (CVAI), like visceral adiposity index (VAI) for western population, was established to estimate visceral adipose tissue (VAT) for Chinese population and can predict metabolic disorders well (8, 9). Previous studies have reported positive associations of CVAI with HTN (10, 11), DM (12, 13), and cardiovascular disease (14, 15), which mainly focus on single disease. The effect of CVAI on HTN-DM comorbidity was unknown, and the dose–response association was unclear. In addition, a recent study including 59,429 described that both VAT and visceral to total adipose tissue (VAT/TAT) ratio increased with increasing age (16). Therefore, it is essential to assess the association between CVAI and HTN-DM comorbidity in elderly people. Considering insulin resistance as the common mechanism of HTN and DM (17, 18), evaluating the mediating role of insulin resistance in the associations would benefit understanding the internal mechanism the association between CVAI and HTN-DM comorbidity.
Hence, this study aimed to explore the dose–response association of CVAI with HTN-DM comorbidity, HTN or DM, HTN, and DM in elderly people and test whether the associations are mediated by insulin resistance.
Methods
Study population
During 2019–2020, a total of 5,068 participants aged ≥60 years were selected from 10 community health service centers. After excluding participants with unknown status of HTN and DM (n= 453), missing data for age, body mass index (BMI), waist circumference (WC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) levels (n= 1,449), 3,166 individuals were included to explore the associations of CVAI and HTN-DM comorbidity, HTN or DM, HTN, and DM.
Data collection
Data on demographic characteristics (age, gender, marital status, and educational level), lifestyle factors (smoking, drinking, dietary habits, and physical activity), and medical history (anti-hypertensive medication, lipid lowering, and antidiabetic history) were collected by uniformly trained medical staff. Height, weight, WC, and blood pressure (BP) were measured, and all instruments were calibrated before use every time. Fasting blood was collected for fasting plasma glucose (FPG), TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and HDL-C. Quality control was conducted by establishing a detailed study protocol, using standardized questionnaire, training medical staff uniformly, calibrating instrument before use, and inputting information doubly.
Smoking was defined as smoking ≥100 cigarettes in their lifetime and drinking as drinking alcohol ≥12 times during the last year. Ideal diet was regarded as good mixture of meat and vegetables. Ideal physical activity was defined as more than 30 min of light and/or more than 20 min of moderate physical activity and/or more than 10 min of heavy physical activity per day for more than 5 years. BMI was calculated as weight (kg) divided by height (m) squared. Triglyceride-glucose (TyG) index, a reliable indicator assessing insulin resistance, was calculated as Ln (TG × 88.55 × FPG × 18/2) (both TG and FPG units in mmol/L) (19). CVAIs were calculated as follows (8):
Outcomes assessment
HTN was defined as systolic BP (SBP) ≥140 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg and/or use of antihypertensive medication (20). DM was defined as FPG ≥7.0 mmol/L and/or current treatment with anti-diabetes medication according to the Chinese guidelines for T2DM (21). HTN-DM comorbidity was defined as participant having both HTN and DM. Participants with HTN or DM were having at least one of HTN or DM.
Statistical analyses
Continuous variables were described as median (interquartile range) and categorical variables as number (percentage). Wilcoxon two-sample test or chi-square test was used to test differences between men and women. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of HTN-DM comorbidity, HTN or DM, HTN, and DM for per standard deviation (SD) increase and quartiles of CVAI. Model 1 was adjusted for age and gender; model 2 was adjusted for model 1 plus educational level, marital status, dietary, smoking, drinking, physical activity, and family history of HTN or DM; and model 3 was further adjusted for model 2 and TC, cardiovascular disease, and cancer. Restricted cubic splines (RCS) were used to explore the dose–response association of CVAI with the outcomes. Subgroup analyses were conducted to identify the consistency of the results across gender (men vs. women) and age (<75 years vs. ≥75 years) and to explore possible interaction by multiplicative model. Sensitivity analyses were conducted to test the robustness of the results by excluding participants with cardiovascular disease or cancer.
Mediation analysis was used to explore the mediating effect of TyG index in the associations between CVAI and HTN-DM comorbidity, HTN or DM, HTN, and DM by the PROCESS procedure in SAS v.9.4 (22). Mediated effect values and 95% CI were evaluated by a bias-corrected non-parametric percentile bootstrap method with 5,000 random sampling times. Statistical analyses were carried out in SAS V.9.4 (SAS Inst., Cary, NC) and R V.4.2.2 (23). A two-sided p-value <0.05 was considered statistically significant.
Results
Characteristics of study participants
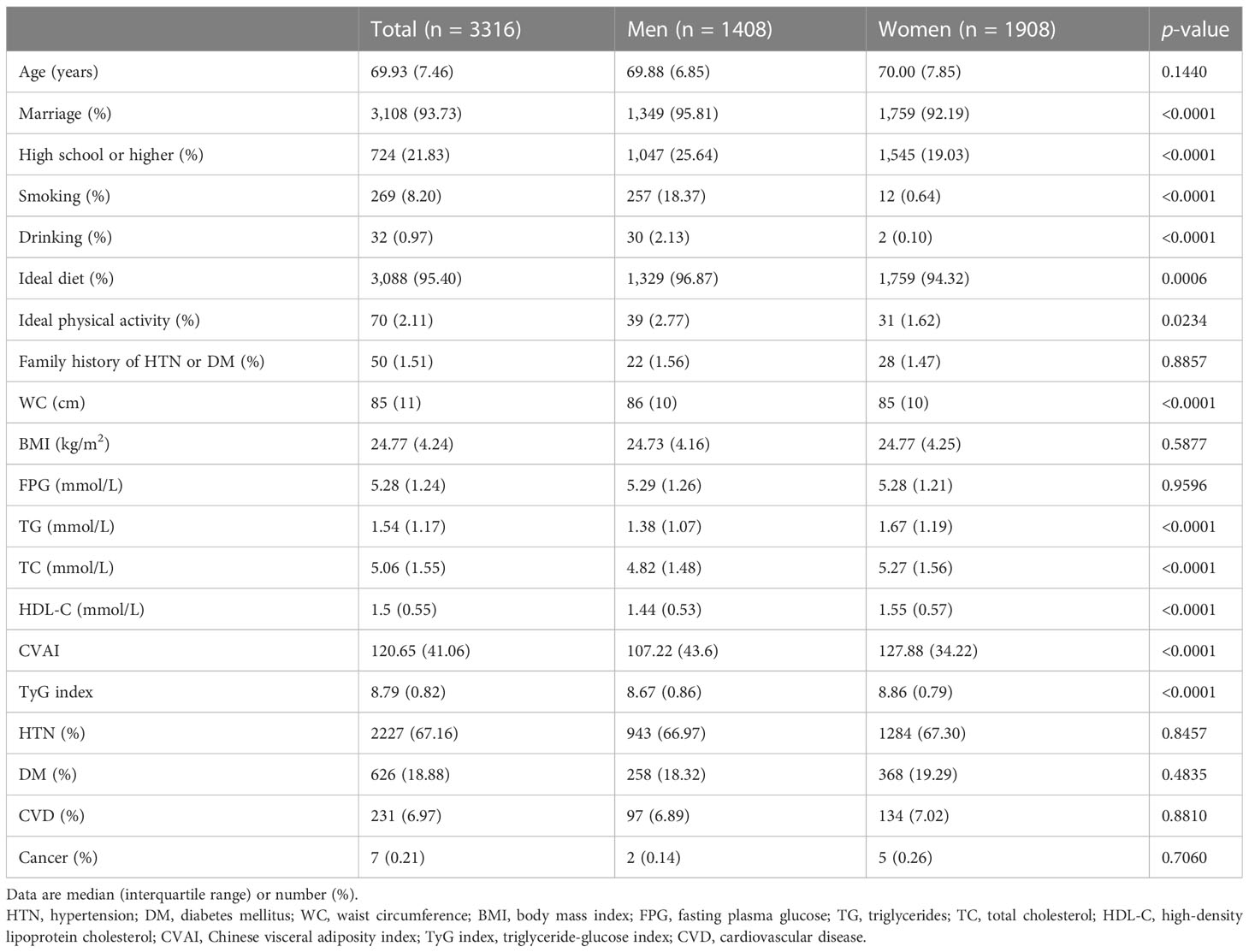
The characteristics of the 3,316 study participants are shown in Table 1. Median age was 69.93 (7.46) years, and 42.46% were men. Compared with women, men were more likely to have higher proportion of marriage, higher education level, smoking, drinking, ideal diet and physical activity, higher level of WC, CVAI, TyG index, and lower level of TG, TC, HDL-C (all p <0.05). Among those participants, 457 (13.78%) had HTN-DM comorbidity, 2,396 (72.26%) had HTN or DM, 2,227 (67.16%) had HTN, and 626 (18.88%) had DM.
Dose–response associations of CVAI with HTN-DM comorbidity
RCS curve showed linear associations between CVAI and HTN-DM comorbidity, HTN or DM, HTN, and DM, with p-values of 0.2672, 0.3480, 0.1983, and 0.9019, respectively (Figure 1). After adjusting potential confounders, per SD increase in CVAI was associated 45%, 39%, 36%, and 28% increased risk of HTN-DM comorbidity, HTN or DM, HTN, and DM (Figure 2), and the corresponding ORs (95% CIs) were 1.45 (1.30–1.61), 1.39 (1.28–1.52), 1.36 (1.25–1.48), and 1.28 (1.16–1.41). The positive associations persisted on further excluding participants with cardiovascular disease and cancer (Supplementary Table S1).
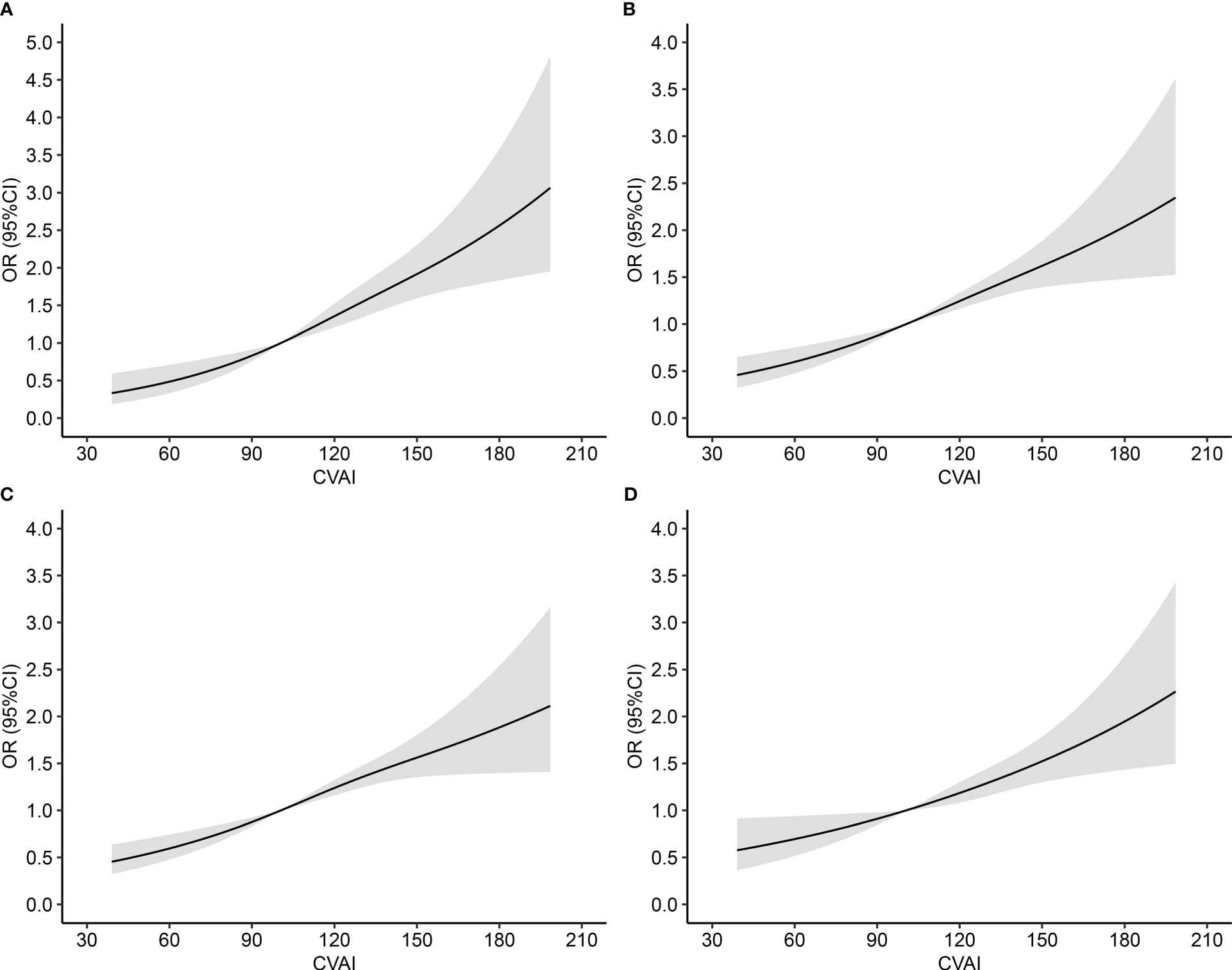
Figure 1 Dose–response association between Chinese visceral adiposity index and risk of comorbidity of hypertension and diabetes mellitus. OR, odds ratio; CI, confidence interval; CVAI, Chinese visceral adiposity index; HTN, Hypertension; DM, diabetes mellitus. (A) HTN-DM comorbidity; (B) HTN or DM; (C) HTN; (D) DM. Adjusted for age, gender, educational level, marital status, dietary, smoking, drinking, physical activity, family history of HTN or DM, total cholesterol, cardiovascular disease, and cancer.
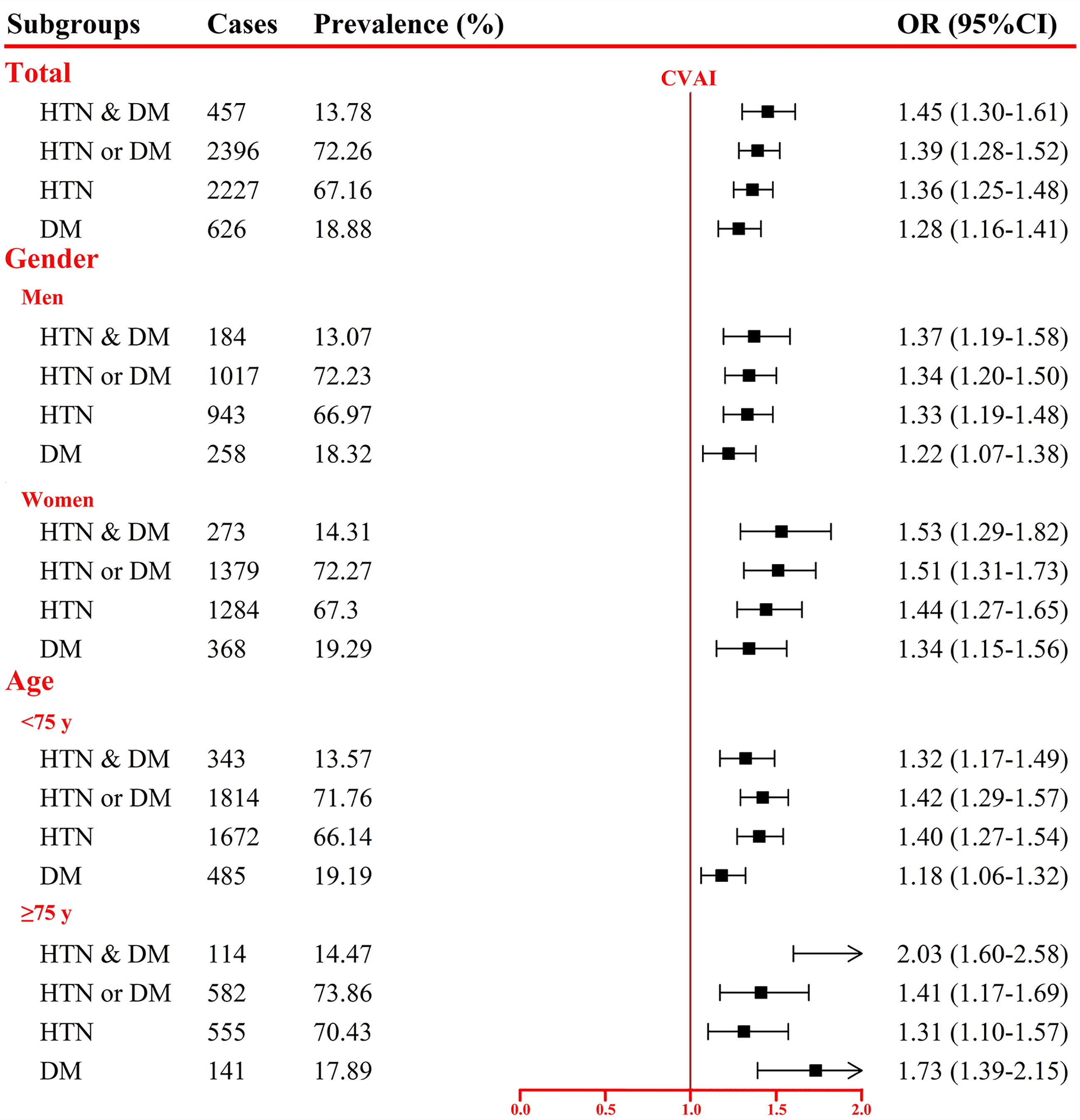
Figure 2 Association between per SD increase in Chinese visceral adiposity index and comorbidity of hypertension and diabetes mellitus. OR, odds ratio; CI, confidence interval; HTN, hypertension; DM, diabetes mellitus. Adjusted for age, gender, educational level, marital status, dietary, smoking, drinking, physical activity, family history of HTN or DM, total cholesterol, cardiovascular disease, and cancer.
In addition, we also assessed the associations stratified by gender and age (<75 vs. ≥75 years; Figure 2). Risk of HTN-DM comorbidity, HTN or DM, HTN, and DM associated with per SD increment in CVAI increased 37%, 34%, 33%, and 22% for men and 53%, 51%, 44%, and 34% for women. Participants aged ≥75 years had higher risk of HTN-DM comorbidity (103% vs. 32%) and DM (73% vs. 18%) than those aged <75 years (pinteraction <0.05). Per SD increase in CVAI was associated with 42% and 40% increased risk of HTN or DM and HTN for participants aged <75 years and 41% and 31% for those aged ≥75 years. Sensitivity analyses showed the similar results (Supplementary Table S1).
Risk of HTN-DM comorbidity by quartiles of CVAI
After adjusting for the potential confounders (age, gender, educational level, marital status, dietary, smoking, drinking, physical activity, family history of HTN or DM, TC, cardiovascular disease, and cancer), compared with participants in quartile 1 (Table 2), the ORs (95% CIs) in quartiles 2–4 of CVAI were 1.57 (1.13–2.19), 1.97 (1.41–2.75), and 2.90 (2.11–4.00) for HTN-DM comorbidity; 1.32 (1.07–1.64), 1.58 (1.26–1.99), and 2.25 (1.77–2.86) for HTN or DM; 1.41 (1.14–1.74), 1.64 (1.31–2.04), and 2.12 (1.69–2.66) for HTN; and 1.16 (0.88–1.52), 1.38 (1.04–1.82), and 1.96 (1.50–2.56) for DM, respectively. With increasing CVAI quartile, risk of the outcomes increased substantially. Results of sensitivity analyses showed consistent associations (Supplementary Table S2).

Table 2 Risk for comorbidity of hypertension and diabetes mellitus by quartiles of Chinese visceral adiposity index.
Mediating effects of TyG index on association of CVAI with HTN-DM comorbidity
Results of the mediation analysis for TyG index is shown in Table 3. After accounting for potential confounders, CVAI was found positively associated with TyG index (path a), and TyG index associated with HTN-DM comorbidity (path b; OR, 3.18; 95%CI, 2.63–3.83). The total (path c) and indirect (path ab#) effects were statistically significant, and direct (path c’) effect was marginally significant, with adjusted OR (95%CI) of 1.45 (1.30–1.62), 1.32 (1.25–1.40), and 1.13 (1.00–1.27), respectively. TyG index largely mediated the association between CVAI and HTN-DM comorbidity.

Table 3 Mediating effects of TyG-mediated effects of Chinese visceral adiposity index and risk of comorbidity of hypertension and diabetes mellitus.
For HTN or DM, the OR (95%CI) for total (path c), direct (path c’), and indirect (path ab#) effects were 1.39 (1.27–1.52), 1.29 (1.17–1.41), and 1.08 (1.04–1.12), which indicated that TyG index partly mediated the association between CVAI and HTN or DM. For DM, significant total (path c), indirect (path ab#) effects, and insignificant direct (path c’) effect suggested that TyG index completely mediated the association. However, we failed to observe the mediating effect for TyG index on the association of CVAI with HTN.
Discussion
The current study first found a linear association between CVAI and HTN-DM comorbidity, and per SD increase was associated with 45% increased risk. Linear associations of CVAI with HTN or DM, HTN, and DM were also reported, and the risk increased 39%, 36%, and 28% for per SD increment. Compared with quartile 1 of CVAI, the risk of HTN-DM comorbidity, HTN or DM, HTN, and DM increased 190%, 125%, 112%, and 96% for quartile 4. In addition, we also found TyG index largely mediating the association between CVAI and HTN-DM comorbidity, partly mediating the association for HTN or DM, and completely mediating the association for DM. Our results provide additional epidemiological evidence for preventing comorbidity or multimorbidity of metabolic disease.
With the improvement of medical care, increasing prevalence of chronic disease, and intensification of aging, comorbidity or multimorbidity has become common (7, 24). In addition, comorbidity or multimorbidity would become more common when treating chronic disease without tackling excess adiposity (25, 26). Consistent with our study, positive associations of CVAI with HTN and DM were reported based on Chinese and Japanese population (10, 12, 27). Different from the above study, we focused on elderly people and explored the association between CVAI and HTN-DM comorbidity and HTN or DM. We found that CVAI was linearly associated with HTN-DM comorbidity, and age difference (<75 vs. ≥75 years) was detected in the association (ORs, 1.32 vs. 2.03). The different effects across age groups may be due to the difference in fat distribution and accumulation, aging process, and other underlying mechanisms (16, 28, 29).
Our mediation analyses showed TyG index playing a key role in the association of CVAI with HTN-DM comorbidity. Similar to our results, a study by Dong et al. also reported TyG index mediating the association between BMI/WC and HTN-DM comorbidity (30). In addition, consistent with our findings for HTN or DM, recent studies found that the BMI-HTN or BMI-DM association was medicated by TyG index (31, 32). Our results also suggested a positive association between CVAI and insulin resistance (TyG index), which was consistent with other studies (33, 34). A recent review described that features induced by obesity including hyperinsulinemia, activation of the sympathetic nervous system, chronic inflammation, and changes in adipokines were potential mechanisms for HTN-DM comorbidity (35). Despite of those pathophysiological bases, some genetic predisposition for obesity may also increase risk of HTN-DM comorbidity (36, 37).
These findings have certain public health implications. Due to the accumulation and heterogeneity of HTN and DM, comorbidity presents a difficult prevention target if approaching these diseases separately (38, 39). Obesity is an important, modifiable, and economical target for disease prevention (39, 40). Considering a different health effect for different quantity and distribution of body fat in different age stages, CVAI, a reliable indicator of VAT, may be a suitable index of obesity to evaluate its health effect (41–43). Because Asian populations have more VAT accumulation at lower BMI values as compared with Western populations, VAI developed in Western population may not reflect AVT well in Chinese adults and has been proven in previous studies (10, 12, 44, 45). Additionally, CVAI is established based on age, BMI, WC, TG, and HDL-C, which are feasible for measuring in routine clinical practice. The measurement of CVAI will benefit identifying high risk of HTN-DM comorbidity, and maintaining healthy lifestyles to reduce CVAI will help to prevent incident HTN-DM comorbidity (46, 47).
Despite its valuable findings and potential public health implications, some limitations should be noted. First, the cross-sectional design cannot provide causal association between CVAI and HTN-DM comorbidity. Second, although we adjusted for various covariates, other confounders, such as environmental pollution and psychological factors, may also affect the association. Third, the study participants were Chinese elderly people, so our findings require validation in other Asian populations. Guidelines focusing on visceral adiposity for different populations should also be developed based on native evidence. Finally, although we conducted the research with strict accordance to the study protocol, potential measurement bias may exist due to the differences of medical staff, measuring environment, and so on.
Conclusions
Our results showed linear associations of CVAI with HTN-DM comorbidity, HTN or DM, HTN, and DM in Chinese elderly population. TyG index played an important role in the CVAI comorbidity of HTN-DN association. Our findings needed to be validated in prospective cohort studies and clinical trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Huanghuai University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YR substantially contributed to the design and drafting of the study and the analysis and interpretation of the data. LC, RQ, and MH revised it critically for important intellectual content. All authors were involved in collecting data and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 82103935) and Henan Province Science and Technology Project (grant no. 232102310497).
Acknowledgments
The investigators are grateful to the dedicated participants and all research staff of the study, and the Young Key Teacher Funding Program of Huanghuai University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1187381/full#supplementary-material
References
1. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet (2020) 396(10258):1223–49. doi: 10.1016/S0140-6736(20)30752-2
2. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet (2018) 392(10159):1736–88. doi: 10.1016/S0140-6736(18)32203-7
3. Zhang M, Shi Y, Zhou B, Huang Z, Zhao Z, Li C, et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004-18: findings from six rounds of a national survey. Bmj (2023) 380:e071952. doi: 10.1136/bmj-2022-071952
4. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. Jama (2021) 326(24):2498–506. doi: 10.1001/jama.2021.22208
5. Kuan V, Denaxas S, Patalay P, Nitsch D, Mathur R, Gonzalez-Izquierdo A, et al. Identifying and visualising multimorbidity and comorbidity patterns in patients in the English national health service: a population-based study. Lancet Digit Health (2023) 5(1):e16–27. doi: 10.1016/S2589-7500(22)00187-X
6. Sun ZJ, Fan JN, Yu CQ, Guo Y, Bian Z, Pei P, et al. [Prevalence, patterns and long-term changes of multimorbidity in adults from 10 regions of China]. Zhonghua Liu Xing Bing Xue Za Zhi (2021) 42(5):755–62. doi: 10.3760/cma.j.cn112338-20200305-00259
7. Yu N, Zhang M, Zhang X, Zhao Z, Li C, Huang Z, et al. [Study on the status and influencing factors of comorbidity of hypertension, diabetes, and dyslipidemia among middle-aged and elderly Chinese adults]. Zhonghua Liu Xing Bing Xue Za Zhi (2023) 44(2):196–204. doi: 10.3760/cma.j.cn112338-20220523-00451
8. Xia MF, Chen Y, Lin HD, Ma H, Li X, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep (2016) 6:38214. doi: 10.1038/srep38214
9. Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care (2010) 33(4):920–2. doi: 10.2337/dc09-1825
10. Han M, Qie R, Li Q, Liu L, Huang S, Wu X, et al. Chinese Visceral adiposity index, a novel indicator of visceral obesity for assessing the risk of incident hypertension in a prospective cohort study. Br J Nutr (2021) 126(4):612–20. doi: 10.1017/S0007114520004298
11. Li B, Wang J, Zhou X, Liu Y, Wang W, Gao Z, et al. Chinese Visceral adiposity index is more closely associated with hypertension and prehypertension than traditional adiposity indices in Chinese population: results from the REACTION study. Front Endocrinol (Lausanne) (2022) 13:921997. doi: 10.3389/fendo.2022.921997
12. Han M, Qin P, Li Q, Qie R, Liu L, Zhao Y, et al. Chinese Visceral adiposity index: a reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev (2021) 37(2):e3370. doi: 10.1002/dmrr.3370
13. Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: a prospective cohort study. Diabetes Metab Res Rev (2018) 34(7):e3048. doi: 10.1002/dmrr.3048
14. Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, et al. Association between abdominal obesity indices and risk of cardiovascular events in Chinese populations with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol (2022) 21(1):225. doi: 10.1186/s12933-022-01670-x
15. Xie Y, Zhang Y, Qin P, Ping Z, Wang C, Peng X, et al. The association between Chinese visceral adipose index and coronary heart disease: a cohort study in China. Nutr Metab Cardiovasc Dis (2022) 32(3):550–9. doi: 10.1016/j.numecd.2021.10.020
16. Zeng Q, Wang L, Dong S, Zha X, Ran L, Li Y, et al. CT-derived abdominal adiposity: distributions and better predictive ability than BMI in a nationwide study of 59,429 adults in China. Metabolism (2021) 115:154456. doi: 10.1016/j.metabol.2020.154456
17. Quesada O, Claggett B, Rodriguez F, Cai J, Moncrieft AE, Garcia K, et al. Associations of insulin resistance with systolic and diastolic blood pressure: a study from the HCHS/SOL. Hypertension (2021) 78(3):716–25. doi: 10.1161/HYPERTENSIONAHA.120.16905
18. Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes? Cell Metab (2022) 34(1):11–20. doi: 10.1016/j.cmet.2021.12.012
19. Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol (2021) 20(1):76. doi: 10.1186/s12933-021-01268-9
20. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension (2003) 42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2
21. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev (2019) 35(6):e3158. doi: 10.1002/dmrr.3158
22. Hayes AF. Introduction to mediation, moderation and conditional process analysis: a regression-based approach. New York: The Guilford Press (2013).
23. Core Team R. *R: a language and environment for statistical computing. Vienna, Austria: * R Foundation for Statistical Computing (2020).
24. Skou ST, Mair FS, Fortin M, Guthrie B, Nunes BP, Miranda JJ, et al. Multimorbidity. Nat Rev Dis Primers (2022) 8(1):48. doi: 10.1038/s41572-022-00376-4
25. Sattar N, McMurray JJV, McInnes IB, Aroda VR, Lean MEJ. Treating chronic diseases without tackling excess adiposity promotes multimorbidity. Lancet Diabetes Endocrinol (2023) 11(1):58–62. doi: 10.1016/S2213-8587(22)00317-5
26. Kivimäki M, Strandberg T, Pentti J, Nyberg ST, Frank P, Jokela M, et al. Body-mass index and risk of obesity-related complex multimorbidity: an observational multicohort study. Lancet Diabetes Endocrinol (2022) 10(4):253–63. doi: 10.1016/S2213-8587(22)00033-X
27. Shang L, Li R, Zhao Y, Sun H, Tang B, Hou Y, et al. Association between Chinese visceral adiposity index and incident type 2 diabetes mellitus in Japanese adults. Diabetes Metab Syndr Obes (2021) 14:3743–51. doi: 10.2147/DMSO.S322935
28. Ponti F, Santoro A, Mercatelli D, Gasperini C, Conte M, Martucci M, et al. Aging and imaging assessment of body composition: from fat to facts. Front Endocrinol (Lausanne) (2019) 10:861. doi: 10.3389/fendo.2019.00861
29. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell (2023) 186(2):243–78. doi: 10.1016/j.cell.2022.11.001
30. Dong J, Liu YH, Lu YK, Hu LK, Chen N, Ma LL, et al. Association between surrogate indicators of insulin resistance and risk of type 2 diabetes combined with hypertension among Chinese adults: two independent cohort studies. Nutr Metab (Lond) (2022) 19(1):85. doi: 10.1186/s12986-022-00720-1
31. Zhao Y, Yang X, Wu Y, Huang H, Hu F, Zhang M, et al. Association of triglyceride-glucose index and its 6-year change with risk of hypertension: a prospective cohort study. Nutr Metab Cardiovasc Dis (2023) 33(3):568–76. doi: 10.1016/j.numecd.2022.12.001
32. Low S, Khoo KCJ, Irwan B, Sum CF, Subramaniam T, Lim SC, et al. The role of triglyceride glucose index in development of type 2 diabetes mellitus. Diabetes Res Clin Pract (2018) 143:43–9. doi: 10.1016/j.diabres.2018.06.006
33. Jiang K, Luan H, Pu X, Wang M, Yin J, Gong R. Association between visceral adiposity index and insulin resistance: a cross-sectional study based on US adults. Front Endocrinol (Lausanne) (2022) 13:921067. doi: 10.3389/fendo.2022.921067
34. Sun K, Lin D, Feng Q, Li F, Qi Y, Feng W, et al. Assessment of adiposity distribution and its association with diabetes and insulin resistance: a population-based study. Diabetol Metab Syndr (2019) 11:51. doi: 10.1186/s13098-019-0450-x
35. Usui I. Common metabolic features of hypertension and type 2 diabetes. Hypertens Res (2023). doi: 10.1038/s41440-023-01233-x
36. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi , et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature (2015) 518(7538):187–96. doi: 10.1038/nature14132
37. Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature (2017) 541(7635):81–6. doi: 10.1038/nature20784
38. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet (2012) 380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2
39. Lee JK, McCutcheon LRM, Fazel MT, Cooley JH, Slack MK. Assessment of interprofessional collaborative practices and outcomes in adults with diabetes and hypertension in primary care: a systematic review and meta-analysis. JAMA Netw Open (2021) 4(2):e2036725. doi: 10.1001/jamanetworkopen.2020.36725
40. Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol (2021) 9(7):446–61. doi: 10.1016/S2213-8587(21)00118-2
41. Chen P, Hou X, Hu G, Wei L, Jiao L, Wang H, et al. Abdominal subcutaneous adipose tissue: a favorable adipose depot for diabetes? Cardiovasc Diabetol (2018) 17(1):93. doi: 10.1186/s12933-018-0734-8
42. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the framingham heart Study. Circulation (2007) 116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355
43. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega F, et al. An overview and update on obesity and the obesity paradox in cardiovascular Diseases. Prog Cardiovasc Dis (2018) 61(2):142–50. doi: 10.1016/j.pcad.2018.07.003
44. Nazare JA, Smith JD, Borel AL, Haffner S, Balkau B, Ross R, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic Risk/Intra-abdominal adiposity. Am J Clin Nutr (2012) 96(4):714–26. doi: 10.3945/ajcn.112.035758
45. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev (2002) 3(3):141–6. doi: 10.1046/j.1467-789X.2002.00065.x
46. Pan XF, Wang L, Pan. Epidemiology A. And determinants of obesity in China. Lancet Diabetes Endocrinol (2021) 9(6):373–92. doi: 10.1016/S2213-8587(21)00045-0
Keywords: Chinese visceral adiposity index, comorbidity, mediation analysis, elder people, insulin resistance
Citation: Ren Y, Cheng L, Qie R, Han M, Kong L, Yan W, Li Z, Li Y and Lei Y (2023) Dose–response association of Chinese visceral adiposity index with comorbidity of hypertension and diabetes mellitus among elderly people. Front. Endocrinol. 14:1187381. doi: 10.3389/fendo.2023.1187381
Received: 16 March 2023; Accepted: 27 April 2023;
Published: 12 May 2023.
Edited by:
Richard Daniel Rainbow, University of Liverpool, United KingdomReviewed by:
Herbert F. Jelinek, Khalifa University, United Arab EmiratesAyman Jaaouani, Iuliu Hatieganu University of Medicine and Pharmacy, Romania
Dafeng Liu, Public Health and Clinical Center of Chengdu, China
Copyright © 2023 Ren, Cheng, Qie, Han, Kong, Yan, Li, Li and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongcheng Ren, cmVueW9uZ2NoZW5nQGh1YW5naHVhaS5lZHUuY24=
 Yongcheng Ren
Yongcheng Ren Lulu Cheng2
Lulu Cheng2