- 1Reproductive Medical Center, Zhengzhou University People’s Hospital, Zhengzhou, China
- 2Reproductive Medical Center, Henan Provincial People’s Hospital, Zhengzhou, China
- 3Reproductive Medical Center, Henan University People’s Hospital, Zhengzhou, China
Backgrounds: The present study was designed to establish and validate a prediction model for high ovarian response (HOR) in the GnRH antagonist protocol.
Methods: In this retrospective study, the data of 4160 cycles were analyzed following the in vitro fertilization (IVF) at our reproductive medical center from June 2018 to May 2022. The cycles were divided into a training cohort (n=3121) and a validation cohort (n=1039) using a random sampling method. Univariate and multivariate logistic regression analyses were used to screen out the risk factors for HOR, and the nomogram was established based on the regression coefficient of the relevant variables. The area under the receiver operating characteristic curve (AUC), the calibration curve, and the decision curve analysis were used to evaluate the performance of the prediction model.
Results: Multivariate logistic regression analysis revealed that age, body mass index (BMI), follicle-stimulating hormone (FSH), antral follicle count (AFC), and anti-mullerian hormone (AMH) were independent risk factors for HOR (all P< 0.05). The prediction model for HOR was constructed based on these factors. The AUC of the training cohort was 0.884 (95% CI: 0.869–0.899), and the AUC of the validation cohort was 0.884 (95% CI:0.863–0.905).
Conclusion: The prediction model can predict the probability of high ovarian response prior to IVF treatment, enabling clinicians to better predict the risk of HOR and guide treatment strategies.
1 Introduction
Infertility is a major global health problem that threatens female reproductive health. Assisted reproductive technology (ART) is considered one of the most effective treatments for infertility (1). Ovarian hyperstimulation syndrome (OHSS) is a common and severe iatrogenic complication of in vitro fertilization (IVF) in which clinical symptoms are ovarian enlargement, abnormal capillary permeability, excessive estradiol, ascites, and pleural effusion (2). The incidence of mild OHSS is 20%–33%, and the incidence of moderate to severe OHSS is 3%–8% (3). In addition to causing miscarriage, prolonged time to pregnancy, and pregnancy complications, severe OHSS can even cause acute renal insufficiency, acute respiratory distress, venous thrombosis, and even death of patients, which brings both economic and psychological burdens to patients (4). The main risk factor for OHSS is high ovarian response (HOR) which is defined as an abnormal sensitivity to exogenous gonadotropin, resulting in multiple follicle recruitment, development, and steroid abnormalities (5, 6).
GnRH antagonist protocol is gaining popularity among fertility centers worldwide because of its simplified procedures, reduced drug dosages, shorter treatment cycles, safety, and efficacy (7). Several studies have shown that the GnRH antagonist protocol is beneficial for HOR patients, as it can significantly reduce the risk of OHSS (8, 9). However, OHSS can’t be avoided completely, so it is essential to predict HOR to reduced it whenever possible.
Age, anti-mullerian hormone (AMH), antral follicle count (AFC), and basal sex hormones are commonly used to predict ovarian response (10), but most studies predict the risk of HOR with only one or two factors. To our knowledge, only a small amount of literatures construct the multi-variable models without the calibration curve and the decision curve analysis. In our study, we identified the independent risk factors affecting HOR and constructed a nomogram to predict the occurrence of HOR based on the results of the multivariate logistic regression. In addition, we drew the calibration curve and the decision curve to assure the accuracy and utility of the model. We aimed to create a practical algorithm to assist clinicians in implementing personalized reproductive strategies.
2 Materials and methods
2.1 Patient population and study design
This study was a single-center retrospective observational study of women who underwent IVF at the Reproductive Medicine Center of Henan Provincial People’s Hospital from June 2018 to May 2022. Enrolled as subjects of the study were patients with the GnRH antagonist protocol. The exclusion criteria were as follows: 1) cycles with missingness or outliers; 2) endocrine disorders such as abnormal thyroid function, diabetes and hyperprolactinemia; 3) tuberculosis of the reproductive system, pituitary tumors and other systemic diseases; 4) women who received preimplantation genetic testing, and those with chromosomal abnormalities; 5) canceled oocyte retrieval cycles; 6) <4 oocytes retrieved. Cycles were divided into the high ovarian response group (>15 oocytes retrieved) and the normal ovarian response group (4–15 oocytes retrieved) (11–13). The study was approved by the Ethics Committee of Reproductive Medicine of Henan Provincial People’s Hospital with the number SYSZ-LL-2021091501.
2.2 Ovarian stimulation
All patients adhered to the GnRH antagonist protocol. In this protocol, ovarian stimulation was initiated from day 2 or 3 of menstruation with the appropriate amount of gonadotropin at a dose of 75–300 IU/day until the hCG trigger day. During the stimulation process, the gonadotropin dose was adjusted according to follicular development, as determined by ultrasound and serum hormone levels, up to 300 IU/day. A daily dose of 0.25 mg GnRH antagonist was initiated when a dominant follicle reached a mean diameter of 12–14 mm or when the blood luteinizing hormone (LH) levels exhibited a significant upward trend until the day of hCG injection.
If there were three follicles of ≥ 16 mm diameter, two follicles of ≥ 17 mm diameter, or one follicle of ≥18 mm diameter, 5,000–10,000 IU of hCG was injected. Approximately 36–38 hours after the trigger, ultrasound-guided vaginal follicular aspiration was performed for oocyte retrieval.
2.3 Statistical analysis
Categorical variables included the type of infertility. Continuous variables included age, body mass index (BMI), duration of infertility, LH (luteinizing hormone), estradiol (E2), progesterone (P), follicle-stimulating hormone (FSH), antral follicle count (AFC), and anti-mullerian hormone (AMH). AFC was measured using transvaginal ultrasonography on day 2 or 3 of the menstrual cycle. FSH, LH, E2, and P were detected in the blood on day 2 or 3 of the menstrual cycle.
Normally distributed variables are presented as mean ± standard deviation (SD), and a t-test was used to compare groups. Non-normally distributed variables are presented as median (interquartile range), and the Mann-Whitney test was used for comparison. Categorical variables are presented as frequencies and percentages, and the chi-squared test was used for comparison.
Cycles with complete records were divided into training and validation cohorts using a 3:1 random sampling method. Univariate logistic regression analysis was performed in the training cohort to determine the risk factors for the occurrence of HOR. Variables with P< 0.05 in the univariate analysis and variables reported to be associated with HOR were included in the multivariate analysis. Variables were selected using stepwise regression and fitted to a more parsimonious model. A nomogram was established according to the regression coefficient of the relevant variables. The multicollinearity among the factors was assessed using the variance inflation factor (VIF).
The prediction performance was verified through ROC curves, and an area under the ROC curve (AUC) > 0.8 indicates good compliance with the prediction model (14). Calibration curves were used to assess the performance of the prediction model. Decision curve analysis was also performed to assess the clinical applicability of the model. Statistical analysis was performed using software packages R (http://www.R-project.org, The R Foundation) and Empower (R) (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA). A two-tailed P value< 0.05 was considered statistically significant.
3 Results
3.1 Description of the study population
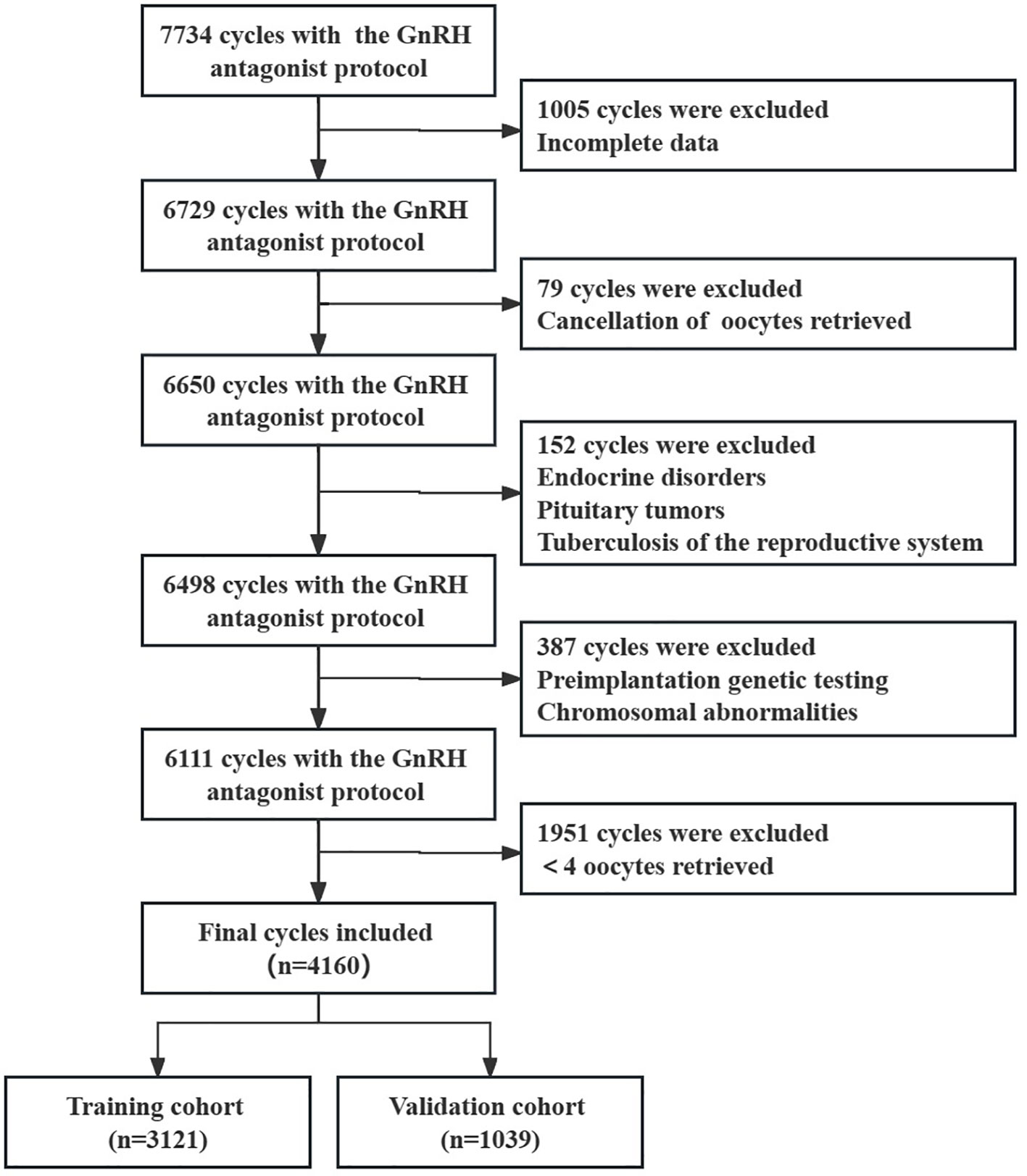
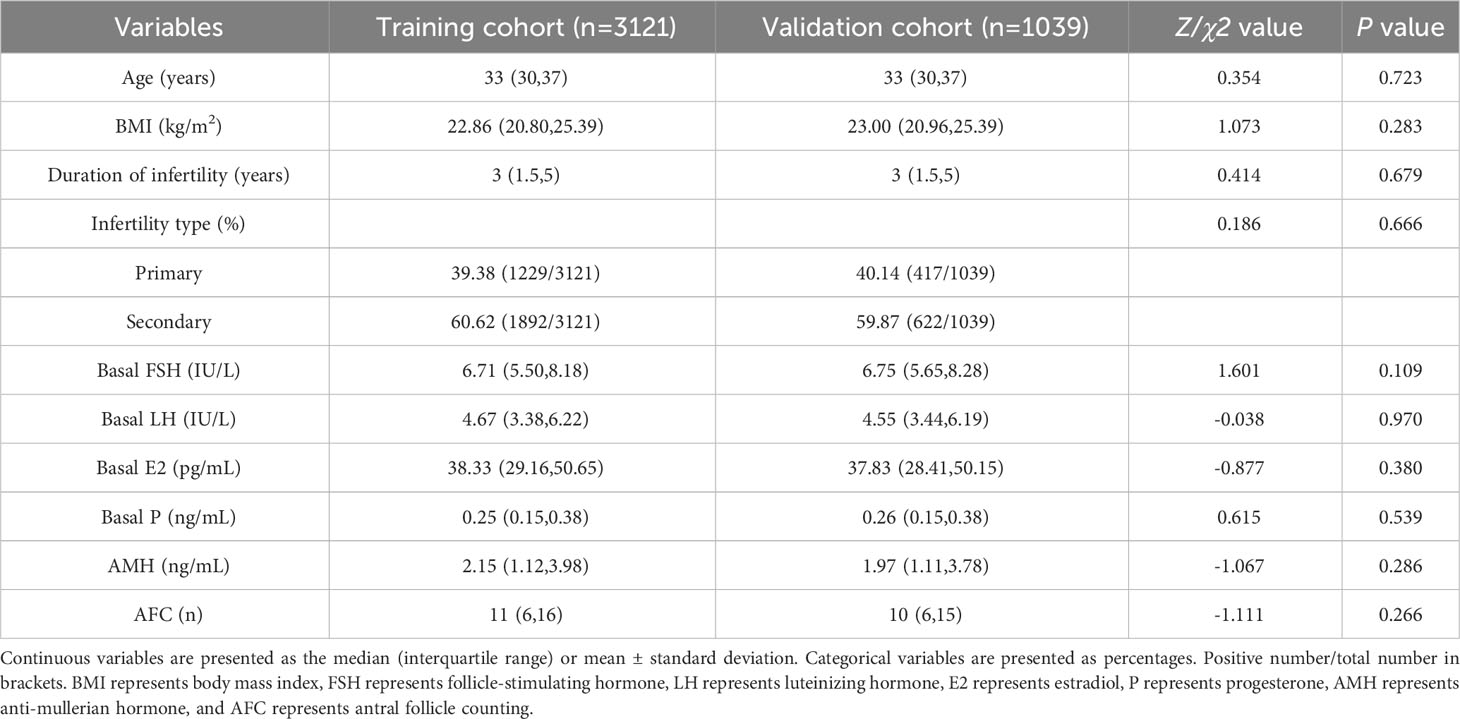
Based on the inclusion and exclusion criteria, 4160 cycles were enrolled, with 3121 cases in the training cohort and 1039 cases in the validation cohort (Figure 1). The baseline characteristics of the two groups are shown in Table 1. There were no significant differences between the items in two groups (all P > 0.05).
3.1.1 Construction of the model
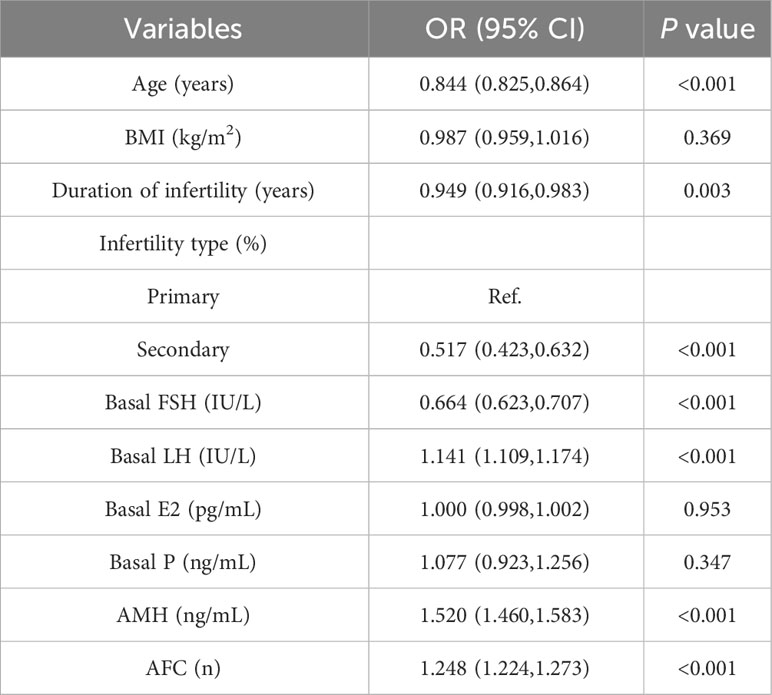
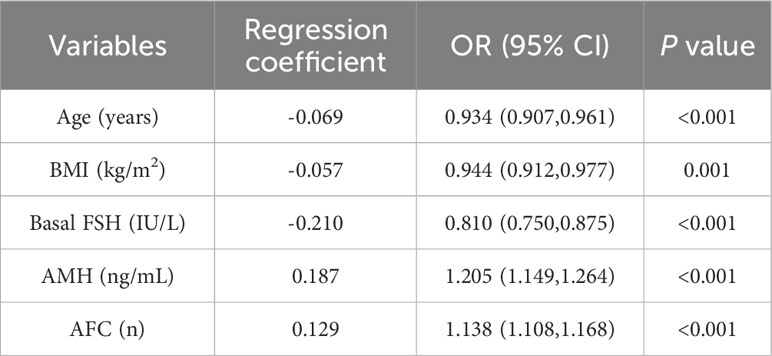
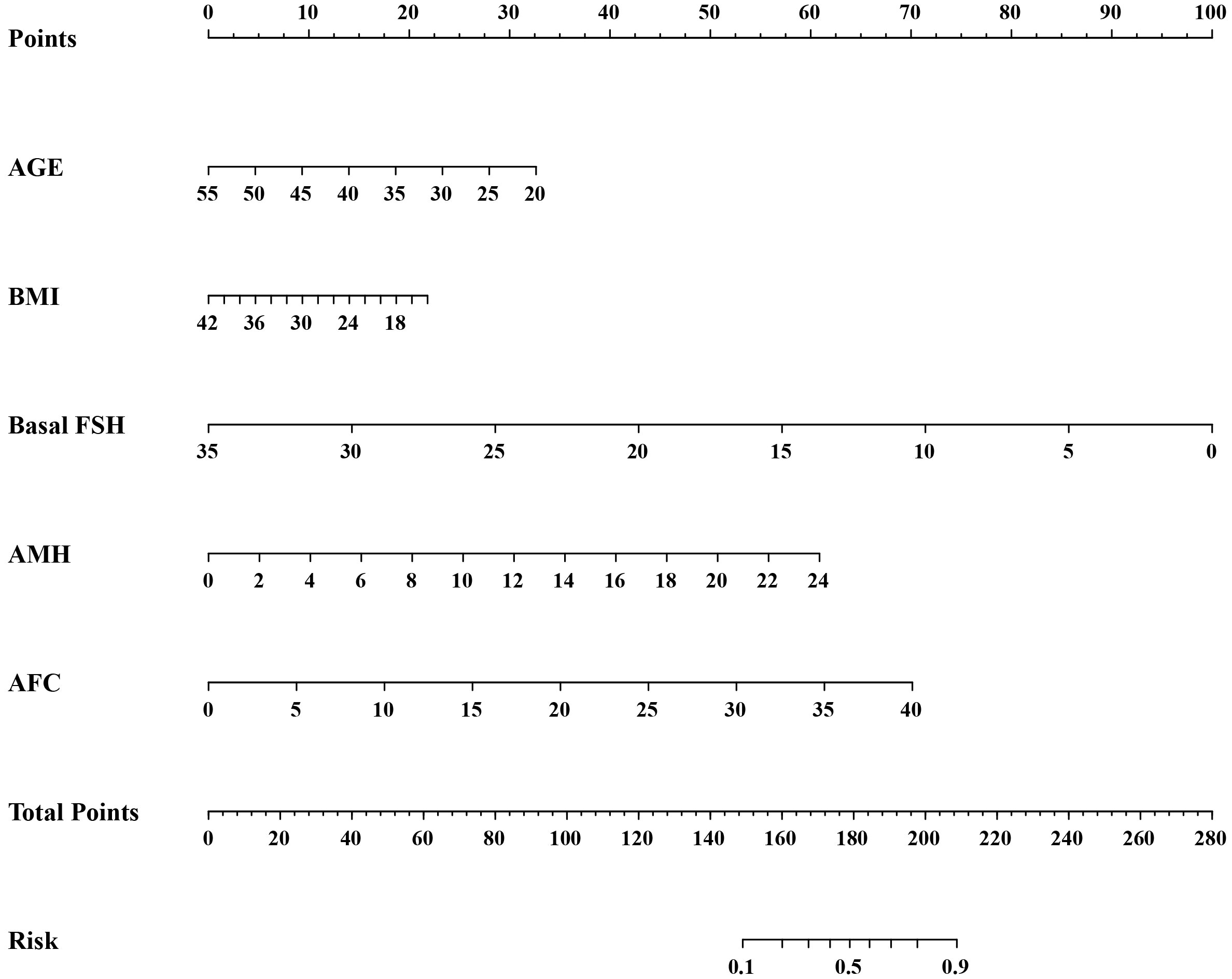
Logistic regression analysis was used to find out the variables that included in the model. The univariate logistic regression analysis of HOR in the training cohort is listed in Table 2. The result suggested age, duration of infertility, type of infertility, FSH, LH, AMH, and AFC were all associated with HOR (all P< 0.05). Although BMI wasn’t the risk factor for HOR in our study, serval studies showed it is related to ovarian response (15). Age, duration of infertility, type of infertility, FSH, LH, AMH, AFC, and BMI were included in the multivariate analysis eventually. The multivariate logistic regression analysis revealed that AMH (OR = 1.205, 95% CI: 1.149–1.264, P<0.001), AFC (OR = 1.138, 95% CI: 1.108–1.168, P<0.001) were both risk factors for the occurrence of HOR, and age (OR = 0.934, 95% CI: 0.907–0.961, P<0.001), BMI (OR = 0.944, 95% CI: 0.912–0.977, P=0.001), FSH (OR = 0.810, 95% CI: 0.750–0.875, P<0.001) were all protective factors for HOR (Table 3). Based on the results of the multivariate logistic regression analysis, the nomogram model (Figure 2) was successfully established: logit (P) = 0.375-0.069 Age-0.057 BMI-0.210 FSH+0.187 AMH+0.129 AFC. The scores of all indicators were added to obtain the total score which corresponds to the risk of HOR.
3.2 Validation of the model
The AUC was calculated to verify the accuracy of the nomogram in Figure 3. The AUC of the training cohort was 0.884 (95% CI: 0.869–0.899), and the AUC of the validation cohort was 0.884 (95% CI: 0.863–0.905). The calibration plot revealed good predictive accuracy between actual and predicted probability in Figure 3. Furthermore, the decision curve analysis demonstrated that the prediction model was the higher line on the decision curve, indicating that the prediction model leads to a higher net benefit and greater clinical utility (Figure 4). In the training cohort, the curve demonstrated that if a patient’s threshold probability falls between 1 and 78%, utilizing the nomogram for predicting HOR offers greater benefits compared to using either the treat-all-patients or the treat-none scheme.
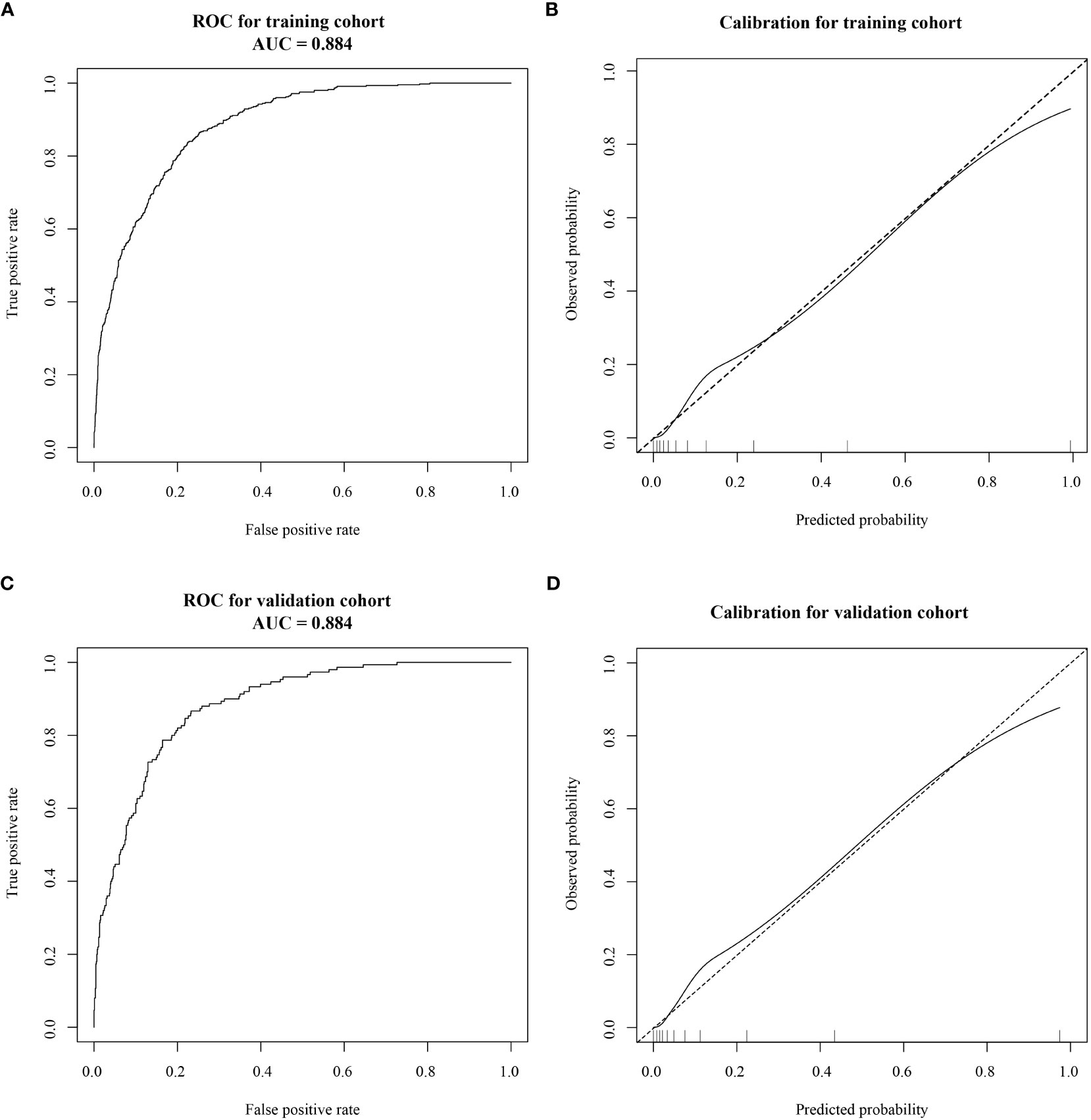
Figure 3 Receiver operating characteristic (ROC) curves and calibration plots of the training and validation cohorts. (A) The area under the ROC curve (AUC) of the training cohort was 0.884 (95% CI: 0.869–0.899). (B) Calibration curve for the training cohort. (C) The AUC of the validation cohort was 0.884 (95% CI: 0.863–0.905). (D) Calibration curve for the validation cohort. Calibration curves were used to evaluate the calibration of the model. The horizontal axis is the predicted probability provided by this model, and the vertical axis is the observed incidence of pregnancy failure. The ideal line with a 45° slope represents a perfect prediction (the predicted probability equals the observed probability).
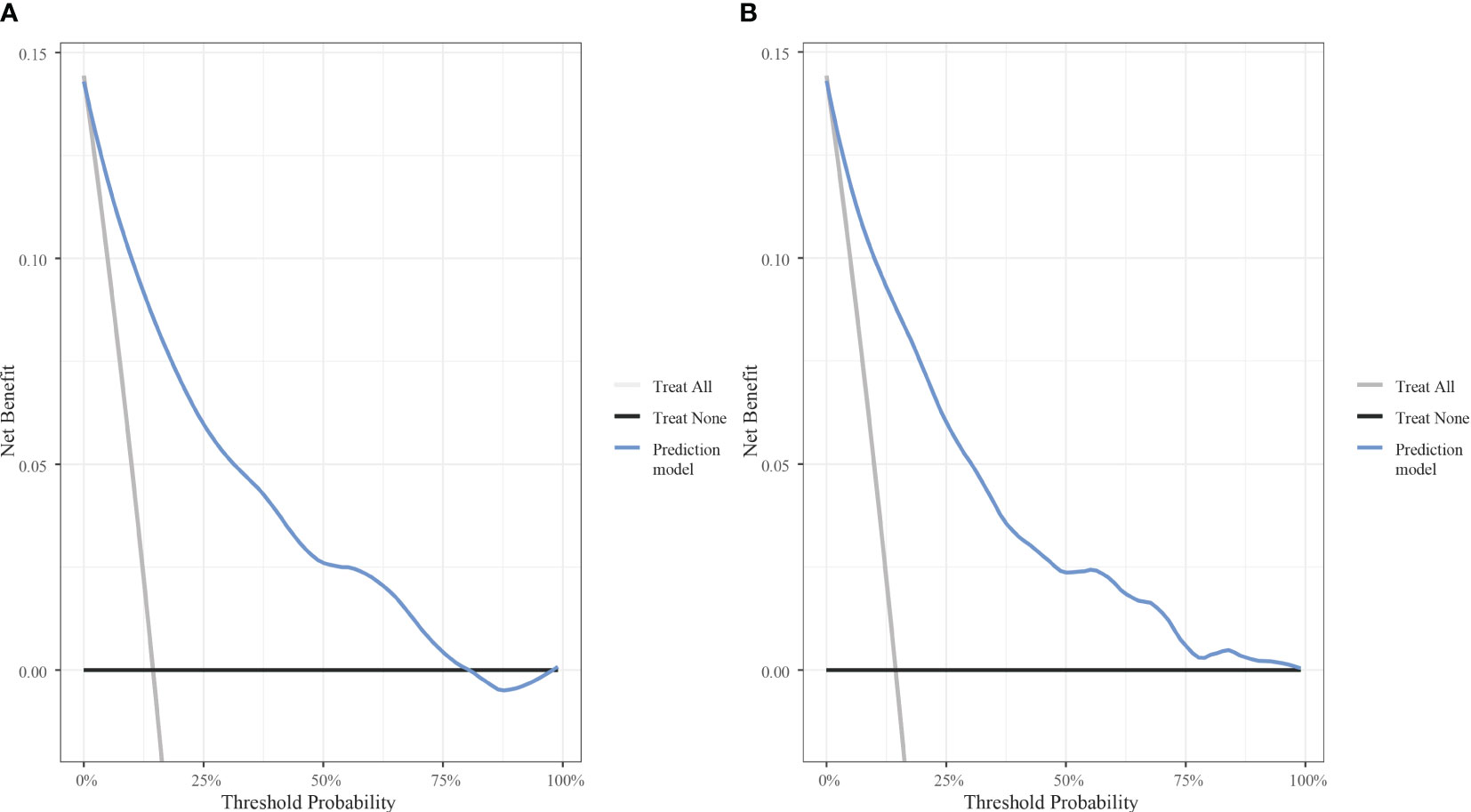
Figure 4 Decision curve analysis of the model with the net benefit as the vertical axis and the threshold probability as the horizontal axis. (A) Decision curve analysis for the training cohort. (B) Decision curve analysis for the validation cohort.
4 Discussion
Adopting individualized treatment strategies has been one of the most important topics in ART. The selection of an individualized controlled ovarian stimulation protocol is based on the ovarian response so that patients can obtain the appropriate number of follicles, embryos, and optimal pregnancy outcomes while avoiding severe adverse effects and complications, such as OHSS. Although the GnRH antagonist protocol significantly reduces the risk of OHSS, controlling and reducing it in HOR patients is a major challenge for clinicians. In our study, multivariate logistic regression analysis revealed that age, BMI, FSH, AMH, and AFC were independent risk factors for HOR in the GnRH antagonist protocol, which is consistent with the results of previous studies.
In previous studies, age and FSH often were used to assess ovarian response. Broer et al. conducted a meta-analysis showing that age was the strongest single predictor of high response (OR = 0.89, 95% CI: 0.85–0.93) (12). After the age of 35, the number of follicles decreases sharply, probably due to the incidence of chromosomal defects increases, and the sensitivity of granulosa cells to gonadotropin decreases. Some studies suggested that serum FSH increases significantly when ovarian reserve is severely declined and fluctuates significantly during the menstrual cycle; therefore, FSH has no optimal sensitivity or specificity in predicting ovarian response (16). Our data indicated that FSH was associated with ovarian response, but it shouldn’t be single-used as the predictor due to its defects.
AFC and AMH have recently become the most widely used markers for assessing ovarian reserve and ovarian response in ART. AFC is the most intuitive indicator of ovarian reserve function in patients, as it reflects the number of ovarian follicles that can be stimulated by exogenous gonadotropin in a given cycle. A review reported that AFC > 14 was a predictor of HOR (17). Another study demonstrated that the optimal threshold for predicting HOR was AFC > 16 or 18 (sensitivity: 82%, specificity: 80%) (10). However, AFC requires careful counting by skilled ultrasound operators. Of all the ovarian markers, AMH showed the best ability to predict ovarian response. AMH is mainly secreted by granulosa cells of the ovary and regulates folliculogenesis. Serum AMH reflects the number of antral follicles in the ovary, and its concentration is not affected by the menstrual cycle. Rong Li et al. demonstrated that the optimal AMH cut-off value predicting high ovarian response was 2.6 ng/mL (sensitivity: 81.28%, specificity: 59.51%) (18). Another study with 373 cycles suggested that an AMH cut-off value greater than 4.385 ng/mL may predict HOR, and the AUC was 0.845 (95% CI: 0.778–0.912) (19). Studies involving AMH have reported that the optimal threshold for predicting HOR is AMH > 3.18, 3.50, or 4.5 ng/mL (10, 20, 21). Although AMH is a reliable biomarker of ovarian response, there is no standard cut-off value because of differences in reagent kits and assays.
High BMI is associated with impaired ovarian response and has a negative effect on IVF outcomes (22). Qiu et al. noted that high BMI in PCOS patients had a negative effect on ovarian response (23). In our study, low BMI is associated with the development of HOR. BMI ≥ 28 kg/m2 is defined as obesity in China. Obesity alters the function of the hypothalamic-pituitary axis, which is associated with high levels of insulin, androgen, and estrogen and is involved in the impairment of ovarian folliculogenesis. Although obese women receive higher doses of gonadotropin and longer stimulation periods than normal weight women, they may still have poor ovarian response because of decreased drug bioavailability (24). Chalumeau et al. developed a predictive model of ovarian response, including BMI and other factors, that could explain 60% of the variance in ovarian response to stimulation (25).
Recent studies have used multi-variable models which are more credible and stable. A meta-analysis with complete available data from 1023 patients revealed that FSH, AFC, and AMH could all be combined with age for HOR prediction, where age+AMH/AFC was more accurate compared with age alone for prediction, and age+FSH exhibited a smaller increase in accuracy. The combination of age+AFC+AMH (AUC=0.85) demonstrated good accuracy for predicting HOR (12). Tan et al. reported that AFC, AMH, and P levels on the human chorionic gonadotropin (HCG) day were identified as independent predictors of HOR. The nomogram was established with the data of 480 eligible patients (26). In our study, greater number of cycles were included and a combined prediction model with age, BMI, FSH, AMH, and AFC was developed with good performance. Simultaneously, we plotted a nomogram to visualize our model. The AUC value of the combined prediction model reached 0.884, indicating the excellent discrimination of the model, and the validation confirmed the accuracy and feasibility of the model.
In the present study, risk factors of HOR were investigated and a well-calibrated prediction model was successfully proposed in patients undergoing the GnRH antagonist protocol. Our model possesses the following strengths: The variables enrolled in the nomogram can be easily measured, making it convenient to use in clinical practice. Moreover, the model was validated to ensure its reproducibility in a wider population. It was not only the discrimination and calibration but also the utility that evaluated the performance of clinical prediction models in this study. The utility of models can be assessed by Decision curve analysis (DCA), which plots net benefit (NB) at a range of clinically reasonable risk thresholds. We constructed decision models for the groups respectively, of which the results demonstrated favourable net benefits. In IVF cycles, the use of the GnRH antagonist protocol, coasting protocol, low initial gonadotropin dose, aspirin, calcium, the GnRH agonist trigger, and the whole embryo freezing strategy should be considered for patients with HOR to reduce the likelihood of OHSS, venous thrombosis, and other hazards in patients (27).
Our study also has some limitations. Environmental exposure, genetic elements, and unhealthy lifestyle habits also affect HOR, which were not included in the study because of the limited data sources. In the future, prospective, large-scale, multi-center clinical studies should be conducted to develop a more systematic and comprehensive clinical prediction model.
5 Conclusion
In conclusion, we aimed at infertile women using GnRH antagonist protocol in ART and successfully developed a prediction model that enables us to predict HOR patients in a simple, effective and visual way. We hope the model can help clinicians select personalized treatments to improve ART outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Reproductive Medicine of Henan Provincial People’s Hospital with the number SYSZ-LL-2021091501. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YJ conceived study design. TW and CC performed the data collection and statistical analysis. JG edited the manuscript. CZ was responsible for providing data and guiding study. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC) (project number: U2004130).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Qiao J, Wang Y, Li X, Jiang F, Zhang Y, Ma J, et al. A Lancet Commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet (2021) 397:2497–536. doi: 10.1016/S0140-6736(20)32708-2
2. Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Systematic Rev (2017) (1). doi: 10.1002/14651858.CD012103.pub2
3. Blumenfeld Z. The ovarian hyperstimulation syndrome. Vitamins Hormones (2018) 107:423–51. doi: 10.1016/bs.vh.2018.01.018
4. Pfeifer S, Butts S, Dumesic D, Fossum G, Gracia C, La Barbera A, et al. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertility Sterility (2016) 106:1634–47. doi: 10.1016/j.fertnstert.2016.08.048
5. Schachter-Safrai N, Karavani G, Esh-Broder E, Levitas E, Wainstock T, Har-Vardi I, et al. High ovarian response to ovarian stimulation: effect on morphokinetic milestones and cycle outcomes. J Assist Reprod Genet (2021) 38:3083–90. doi: 10.1007/s10815-021-02323-w
6. Nardo LG, Bosch E, Lambalk CB, Gelbaya TA. Controlled ovarian hyperstimulation regimens: a review of the available evidence for clinical practice. Produced on behalf of the BFS Policy and Practice Committee. Hum Fertility (2013) 16:144–50. doi: 10.3109/14647273.2013.795385
7. Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod BioMedicine Online (2012) 24:6–22. doi: 10.1016/j.rbmo.2011.09.017
8. The ESHRE Guideline Group on Ovarian Stimulation, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open (2020) 2020):hoaa009. doi: 10.1093/hropen/hoaa009
9. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update (2017) 23:560–79. doi: 10.1093/humupd/dmx017
10. Broer SL, Dolleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJM. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update (2011) 17:46–54. doi: 10.1093/humupd/dmq034
11. Oudshoorn SC, Van Tilborg TC, Hamdine O, Torrance HL, Eijkemans MJC, Lentjes EGWM, et al. Ovarian response to controlled ovarian hyperstimulation: what does serum FSH say? Hum Reprod (2017) 32:1701–9. doi: 10.1093/humrep/dex222
12. Broer SL, Dólleman M, Van Disseldorp J, Broeze KA, Opmeer BC, Bossuyt PMM, et al. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertility Sterility (2013) 100:420–29.e7. doi: 10.1016/j.fertnstert.2013.04.024
13. Polyzos NP, Tournaye H, Guzman L, Camus M, Nelson SM. Predictors of ovarian response in women treated with corifollitropin alfa for in vitro fertilization/intracytoplasmic sperm injection. Fertility Sterility (2013) 100:430–7. doi: 10.1016/j.fertnstert.2013.04.029
14. Ballester M, Oppenheimer A, Mathieu d’Argent E, Touboul C, Antoine J-M, Coutant C, et al. Nomogram to predict pregnancy rate after ICSI-IVF cycle in patients with endometriosis. Hum Reprod (2012) 27:451–6. doi: 10.1093/humrep/der392
15. Hu D, Huang B, Xiong M, Yao J, Yang S, Wu R, et al. Impact of elevated body mass index on cumulative live birth rate and obstetric safety in women undergoing assisted reproductive technology. Sci Rep (2022) 12:18858. doi: 10.1038/s41598-022-23576-0
16. Hu L, Sun B, Ma Y, Li L, Wang F, Shi H, et al. The relationship between serum delta FSH level and ovarian response in IVF/ICSI cycles. Front Endocrinol (2020) 11:536100. doi: 10.3389/fendo.2020.536100
17. Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertility Sterility (2010) 94:389–400. doi: 10.1016/j.fertnstert.2010.03.028
18. Li R, Gong F, Zhu Y, Fang W, Yang J, Liu J, et al. Anti-Müllerian hormone for prediction of ovarian response in Chinese infertile women undergoing IVF/ICSI cycles: a prospective, multi-centre, observational study. Reprod BioMedicine Online (2016) 33:506–12. doi: 10.1016/j.rbmo.2016.07.003
19. Xu H, Zeng L, Yang R, Feng Y, Li R, Qiao J. Retrospective cohort study: AMH is the best ovarian reserve markers in predicting ovarian response but has unfavorable value in predicting clinical pregnancy in GnRH antagonist protocol. Arch Gynecol Obstet (2017) 295:763–70. doi: 10.1007/s00404-016-4274-8
20. Hamdine O, Eijkemans MJC, Lentjes EWG, Torrance HL, Macklon NS, Fauser BCJM, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod (2015) 30:170–8. doi: 10.1093/humrep/deu266
21. Ganidou MA, Kolibianakis EM, Venetis CA, Gerou S, Makedos GA, Klearchou N, et al. Is assessment of anti-mullerian hormone and/or antral follicle count useful in the prediction of ovarian response in expected normal responders treated with a fixed dose of recombinant FSH and GnRH antagonists? A prospective observational study. Gynecological Endocrinol (2014) 30:817–21. doi: 10.3109/09513590.2014.938624
22. Souter I, Baltagi LM, Kuleta D, Meeker JD, Petrozza JC. Women, weight, and fertility: The effect of body mass index on the outcome of superovulation/intrauterine insemination cycles. Fertility Sterility (2011) 95:1042–7. doi: 10.1016/j.fertnstert.2010.11.062
23. Qiu M, Tao Y, Kuang Y, Wang Y. Effect of body mass index on pregnancy outcomes with the freeze-all strategy in women with polycystic ovarian syndrome. Fertility Sterility (2019) 112:1172–9. doi: 10.1016/j.fertnstert.2019.08.009
24. Ozekinci M, Seven A, Olgan S, Sakinci M, Keskin U, Akar ME, et al. Does obesity have detrimental effects on IVF treatment outcomes? BMC Womens Health (2015) 15:61. doi: 10.1186/s12905-015-0223-0
25. Chalumeau C, Moreau J, Gatimel N, Cohade C, Lesourd F, Parinaud J, et al. Establishment and validation of a score to predict ovarian response to stimulation in IVF. Reprod BioMedicine Online (2018) 36:26–31. doi: 10.1016/j.rbmo.2017.09.011
26. Tan X, Xi H, Yang J, Wang W. Development and validation of prediction model for high ovarian response in in vitro fertilization-embryo transfer: A longitudinal study. Comput Math Methods Med (2021) 2021:7822119. doi: 10.1155/2021/7822119
Keywords: GnRH antagonist protocol, prediction model, high ovarian response, controlled ovarian stimulation, nomogram
Citation: Jiang Y, Cui C, Guo J, Wang T and Zhang C (2023) A prediction model for high ovarian response in the GnRH antagonist protocol. Front. Endocrinol. 14:1238092. doi: 10.3389/fendo.2023.1238092
Received: 10 June 2023; Accepted: 31 October 2023;
Published: 15 November 2023.
Edited by:
Naguib Bin Salleh, University of Malaya, MalaysiaReviewed by:
Akmal El-Mazny, Cairo University, EgyptJing-Yan Song, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China
Tsung-Hsien Lee, Chung Shan Medical University, Taiwan
Copyright © 2023 Jiang, Cui, Guo, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuilian Zhang, bHVja3l6Y2xAcXEuY29t
 Yilin Jiang
Yilin Jiang Chenchen Cui2
Chenchen Cui2 Jiayu Guo
Jiayu Guo Ting Wang
Ting Wang