- 1Renal Division, Department of Medicine, Heilongjiang Academy of Chinese Medicine Sciences, Harbin, China
- 2Shunyi Hospital, Beijing Hospital of Traditional Chinese Medicine, Beijing, China
Diabetic nephropathy (DN) is a major microvascular complication of diabetes and a common cause of chronic kidney disease. There is currently a lack of effective treatments for DN, and the prognosis for patients remains poor. Hirudin, one of the primary active components derived from leeches, demonstrates anti-coagulant, anti-fibrotic, anti-thrombotic, and anti-inflammatory properties, exhibiting significant protective effects on the kidneys. In recent years, there has been a surge of interest in studying the potential benefits of hirudin, especially in its role in the management of DN. This article delves into the mechanisms by which hirudin contributes to the treatment of DN and its clinical efficacy.
1 Introduction
DN stands as the most prominent microvascular complication of diabetes, and it’s frequently the leading cause of death among diabetic patients. Globally, DN is the primary cause of end-stage renal disease (ESRD) (1). In China, with the increasing incidence of diabetes, the prevalence of DN is also on the rise (2).
Leeches have long been utilized in traditional Chinese medicine, demonstrating significant therapeutic efficacy in treating renal-related disorders. Modern pharmacological studies have identified that the principal component extracted from leeches is hirudin. This polypeptide, composed of 65-66 amino acid residues, serves as a natural thrombin inhibitor, showcasing properties like anticoagulation, antifibrotic, antithrombotic, and anti-inflammatory effects (3).
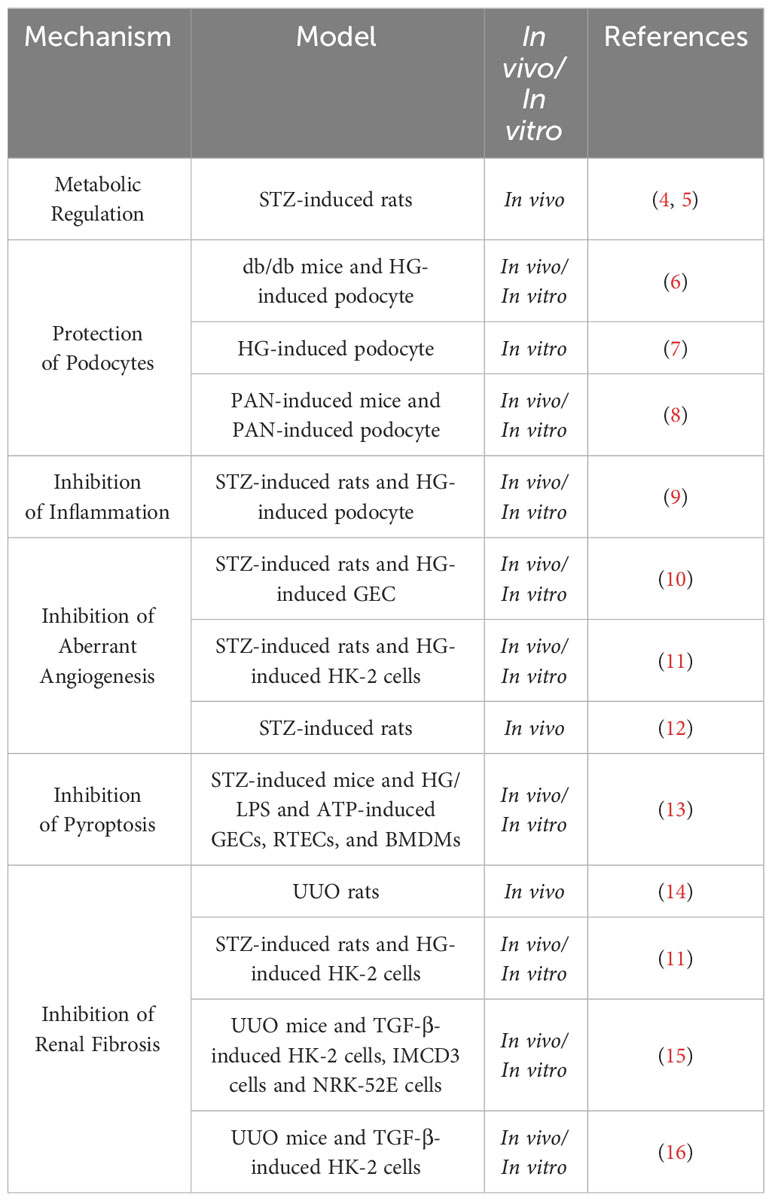
The mechanisms by which hirudin exerts its protective role on the kidneys are detailed in Table 1 and Figure 1.
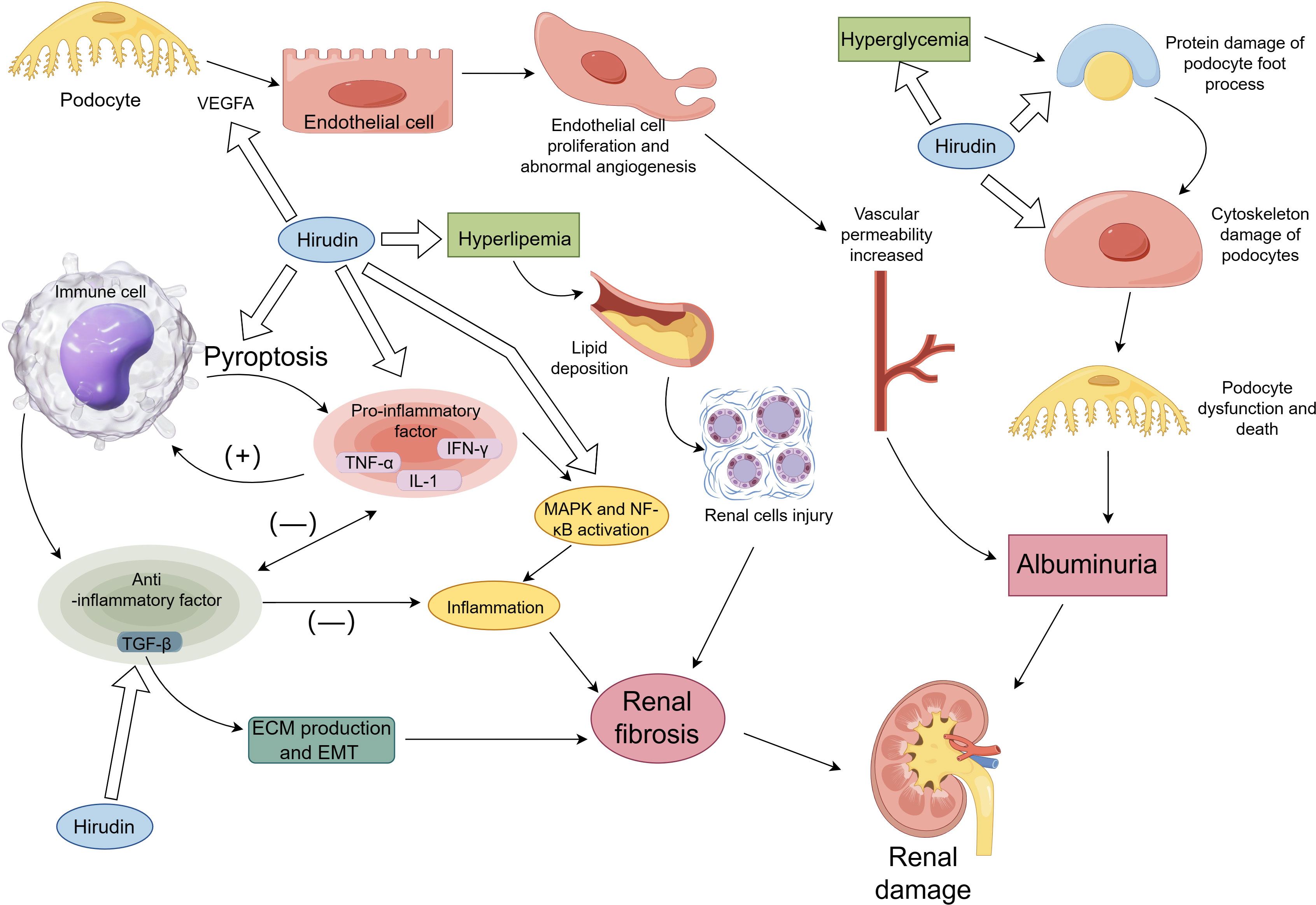
Figure 1 The mechanism of hirudin in DN. Figure 1 briefly describes the mechanisms involved in renal damage and shows the renal protective effects of hirudin, including modulation of hyperglycemia and hyperlipidemia, inhibition of TGF-β-mediated renal fibrosis, inhibition of inflammation, pyroptosis, VEGF-mediated aberrant angiogenesis and protection of podocytes.
2 Study on the mechanism of hirudin on DN
The processes implicated in renal harm in DN and the associated processes of safeguarding renal function by hirudin are depicted in Figure 1. This section presents a comprehensive account of the hirudin experimental investigation to alleviate renal harm in Figure 1, addressing several factors, such as metabolic regulation, protection of podocytes, inhibition of inflammation, inhibition of aberrant angiogenesis, inhibition of pyroptosis and inhibition of renal fibrosis.
2.1 Metabolic regulation
Persistent hyperglycemia is a primary cause of DN. Prolonged poor glycemic control further aggravates the condition, making blood sugar regulation an essential approach to slow down DN’s progression. Hirudin has been shown to reduce blood sugar levels and glycated hemoglobin in rat models of DN induced by a high-fat and high-sugar diet combined with Streptozotocin (STZ). Furthermore, it offers protective effects on renal function (4).
Dyslipidemia is a crucial factor in the microvascular complications seen in diabetic patients. Lipid disorders can adversely affect the fibrinolytic system, leading to a heightened viscosity in blood, thereby increasing the formation of micro-thrombi. This, in turn, intensifies the ischemic and hypoxic conditions in the renal tissues (17). Furthermore, lipid accumulation predominantly takes place in the renal tubules, which has been associated with tubulointerstitial fibrosis. Such accumulations have deleterious effects on glomerular cells and podocytes (18). Managing lipid levels can reduce the risk of these complications. Hirudin has demonstrated its capability to lower total cholesterol, high-density lipoprotein, and other lipid metabolism markers, as well as hemorheological parameters in DN rat models induced by a high-fat and high-sugar diet combined with STZ (5).
Figure 1 shows that hyperlipidemia and hyperglycemia lead to renal damage and that hirudin protects the kidneys by regulating lipid and glucose metabolism.
2.2 Protection of podocytes
A hallmark of DN is the development of proteinuria, a result of podocyte injury. The slit diaphragm protein of podocytes governs the permeability of the glomeruli. Damage or loss of the slit diaphragm leads to a restructuring of the podocyte cytoskeleton (19, 20). In the early stages of DN, the podocyte foot processes vanish, which is closely related to the cellular cytoskeletal remodeling (21). The apical surface of the podocyte is covered with a negatively charged polysaccharide-protein complex, including podocalyxin and glomerular epithelial protein 1 (GLEPP1) (22, 23). This complex is a vital component of the glomerular charge barrier. Damage to this charge barrier can also result in proteinuria. Hirudin safeguards the podocytes of db/db mice, maintaining the cellular cytoskeleton of podocytes. In podocyte induced by high glucose, hirudin inhibits the activity of RhoA, preserving the slit diaphragm proteins nephrin and podocin of foot processes (6). Another study indicated that hirudin increases the expression of podocalyxin and GLEPP1 in the apical region of podocytes under high glucose conditions, thus shielding podocytes and maintaining the integrity of the glomerular filtration barrier’s structure and function (7). In puromycin aminonucleoside (PAN) mouse models and PAN-induced podocyte models, hirudin protects the kidneys and prevents proteinuria by suppressing the transmission of the p38 MAPK signaling pathway, reducing endoplasmic reticulum stress in podocytes, and attenuating the damage to the cytoskeletal proteins of podocytes by PAN (8).
As shown in Figure 1, hirudin protects the kidney by inhibiting protein and cytoskeletal damage in podocytes.
2.3 Inhibition of inflammation
Inflammatory reactions play a pivotal role in the progression of DN. Pro-inflammatory cytokines, especially TNF-α, IL-1, and IL-6, are of particular importance. The presence of IL6 mRNA has been confirmed in renal biopsy specimens of DN patients, specifically in the glomeruli and interstitium (24). Levels of IL-6 in serum and urinary have emerged as potential markers for DN (25). They directly reflect the renal tissue inflammation status in DN (26). The inflammatory response results in the production of anti-inflammatory factors, predominantly including TGF-β (27). The anti-inflammatory effects of TGF-β and its pro-fibrotic effects are demonstrated in Figure 1. Upon tissue injury and concomitant inflammation, TGF-β is expressed in significant quantities, thereby restraining the inflammatory response and averting further tissue damage. However, excessive TGF-β expression leads to augmented ECM synthesis and consequent fibrosis in the tissue (28). Although TGF-β has the ability to inhibit the synthesis of inflammatory cytokines, there have been limited studies assessing its potential as an anti-inflammatory mediator for therapy (29). In the STZ induced diabetic rat model, both urinary and renal tissue expressions of TNF were found to be elevated (30). In HG-induced podocytes, hirudin can reduce the expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and the activation of p38 and NF-κB. In STZ-induced DN rat models, hirudin lowers renal indicators such as serum creatinine (Scr) and blood urea nitrogen (BUN) by reducing macrophage infiltration and inhibiting the activation of p38 and NF-κB (9). Another study found that hirudin modulates the NF-κB signaling pathway. By suppressing the activation of NF-κB, hirudin mitigates the inflammatory response, thereby improving renal fibrosis (14).
2.4 Inhibition of aberrant angiogenesis
A hallmark of DN is its aberrant angiogenesis, with vascular endothelial growth factor (VEGF) acting as the main mediator of this abnormal vascular growth. Changes in local VEGF concentrations and distributions within renal tissues are closely related to proteinuria onset and the severity of renal lesions in patients. In DN mouse models induced by STZ, overexpression of VEGF in podocyte vessels has been found to accelerate the progression of diabetic nephropathy (31). A significant correlation exists between circulating VEGF-A and serum Hypoxia inducible factor-1α(HIF-1α) levels, related to the pathogenesis of DN (32). Prolonged overexpression of HIF-1α may eventually promote organ fibrosis (33). Research indicates that the HIF-1α/VEGF pathway plays a role in the regulation of the extracellular matrix (ECM) (34). Studies show that hirudin can inhibit the migration of glomerular endothelial cells induced by high glucose (HG) and reduce the expression of angiogenesis-related proteins by suppressing the RhoA/p38/NF-kB pathway. In STZ-induced DN rat models, hirudin inhibited the expression of angiogenesis-related proteins VEGF and thrombomodulin-1, alleviating renal damage in rats (10). Hirudin significantly enhanced the activity of HG-induced HK-2 cells, reducing cell ECM expression by modulating the HIF-1α/VEGF pathway. In STZ-induced DN rats, hirudin reduced ECM deposition by adjusting the HIF-1α/VEGF pathway, thereby improving kidney function (11). Hirudin also managed to suppress the expression of VEGF and transforming growth factor β (TGF-β) in STZ-induced DN rats, offering renal protection (12). As shown in Figure 1, hirudin attenuated renal injury by inhibiting the VEGF pathway.
2.5 Inhibition of pyroptosis
Pyroptosis is an inflammatory form of programmed cell death, marked by the creation of pores in the plasma membrane due to gasdermin D (GSDMD) stimulation, resulting in cellular swelling, release of cellular contents (including pro-inflammatory factors like IL-1β and IL-18), and ultimately leading to cell death through the activation of inflammatory vesicles such as NLRP3 (35). Recent findings have underscored a significant linkage between pyroptosis and the pathogenesis of DN (36). Gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy (37). Research suggests that in glomerular endothelial cells and renal tubular epithelial cells induced by HG, as well as macrophages induced by lipopolysaccharide and Adenosine 5’-triphosphate, hirudin can reduce the expression of Gsdmd, thus inhibiting cell pyroptosis. In DN mouse models induced by STZ, hirudin moderates renal injury by regulating Irf2, subsequently inhibiting the expression of Gsdmd, IL-1β, and IL-18 (13). As shown in Figure 1, cellular pyroptosis can lead to the release of pro-inflammatory factors. Hirudin inhibits pyroptosis and reduces the release of pro-inflammatory factors.
2.6 Inhibition of renal fibrosis
Hirudin inhibits ECM expression in STZ-induced DN rats and HG-induced HK-2 cells by modulating the HIF-1α/VEGF pathway (11). In STZ-induced DN rats, hirudin inhibited the expression of TGF-β (12). The degree of tubulointerstitial fibrosis is closely related to the progression of DN. The TGF-β/Smad signaling and inflammatory responses play significant roles in fibrosis. In unilateral ureteral obstruction (UUO) rats model, hirudin significantly reduces ECM accumulation induced by UUO by modulating the expression of fibronectin, collagen III, and α-smooth muscle actin. It attenuates the inflammatory response by suppressing the NF-κB signaling, while concurrently inhibiting the TGF-β/Smad signaling and PAR1 to alleviate renal fibrosis (14). Hirudin can decrease the expression of inflammatory cytokines such as IL-1β, IL-6, and TNF-α in renal cells induced by TGF-β and diminish Epithelial–mesenchymal transition (EMT) and renal cell apoptosis. Moreover, hirudin suppresses the expression of inflammatory factors, fibrotic proteins, and ECM in UUO mice, thus counteracting renal fibrosis (15). In the HK-2 cells and renal tissues of UUO mice, hirudin weakens the upregulation of PAR1, S1PR2, and S1PR3 mediated by TGF-β and downregulates the S1P/S1PR1/S2PR1 signaling-mediated PAR3, diminishing EMT, fibrosis, and MCP-1 expression in HK-2 cells induced by TGF-β (16). The fibre-promoting effects of TGF-β are shown in Figure 1 and hirudin inhibits expression of TGF-β.
3 Study of leeches and herbal prescriptions
In a model of STZ-induced DN in rats with a high-fat diet, leech lyophilised powder reduced serum levels of MDA, TNF-α, IL-1β, and MCP-1, and restored SOD activity. This was achieved primarily through inhibiting the expression of proteins associated with the JAK2/STAT1/STAT3 pathway (38). In a model of STZ-induced DN in rats receiving a diet high in sugar and fat, Chinese herbal granules (containing leeches) were found to increase the expression of podocyte α-actinin-4, Synaptopodin protein, and cleavage proteins podocin and CD2AP, while also reducing levels of 24-hour urinary protein (24h-Upro) in DN rats (39, 40). Patients with DN exhibit albuminuria, initially presenting as microalbuminuria and progressing into massive proteinuria and renal decompensation (41). As albuminuria is present throughout the course of DN and is both a consequence and contributor to renal injury, it is crucial to control albuminuria as a means of delaying DN progression to ESRD. Several clinical observations have shown the effectiveness of leeches and their active components in decreasing albuminuria. For example, Maixuekang capsules (containing hirudin) in combination with telmisartan have been shown to reduce the 24h-Upro in patients with DN (42). Hirudin capsules combined with ginkgolide have been found effective in reducing Scr, BUN, and 24h-Upro levels in DN patients (43). Yiqi Huoxue Buxue kidney formula, which contains hirudin, effectively lowers Scr, BUN, and microalbuminuria levels and notably improves clinical symptoms (44). Qihi Jiangtang capsules can regulate the expression of microcirculatory-related factors like nitric oxide and endothelin-1, and decrease BUN, Scr, and 24h-Upro levels (45). Shuxietong and Naoxuekang, both containing hirudin, can lower MALB in DN patients, improve coagulation function, and show no severe complications like gastrointestinal bleeding (46). No adverse reactions, including liver dysfunction and others, were observed in patients with chronic kidney disease who consumed large amounts of leech powder (9-12 g/d) (47). Similarly, patients with DN who were treated with leech medication did not experience any adverse reactions such as liver dysfunction (43). After DN progresses to ESRD, haemodialysis is the main treatment for ESRD. The recombinant hirudin structure is distinct from hirudin and is commonly used for anticoagulant therapy (48). Recombinant hirudin prevents thrombosis in experimental haemodialysis and is suitable for use as an anticoagulant in this extracorporeal circulation (49). Recombinant hirudin prevents thrombosis in ESRD patients during haemodialysis (50). And the studies have shown that anticoagulation with recombinant hirudin in critically ill patients on continuous hemodialysis can be performed (51).
4 Conclusion and perspectives
The pathogenesis of DN is intricate, involving a multitude of mechanisms. There is limited research on hirudin’s ability to regulate oxidative stress, inflammation, and pyroptosis. Specific mechanisms regarding its influence on glucose and lipid metabolism remain understudied. Given the evident clinical efficacy of hirudin, exploring how to develop drugs that offer better therapeutic effects for patients is a question that researchers should pursue in the future.
Author contributions
FT: Writing – original draft. XY: Writing – original draft. FY: Writing – original draft. YC: Writing – original draft. WZ: Writing – original draft. PL: Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82274489).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet (2013) 382(9888):260–72. doi: 10.1016/S0140-6736(13)60687-X
2. Peng YQ, Zheng Y, Chen YS, Lin TF, Lin L, Zhong ZH, et al. Clinical features of chronic kidney disease with different etiology at middle and advanced stage and the analysis of its potential significance. Heilongjiang Med J (2011) 35(2):90–3. doi: 10.3969/j.issn.1004-5775.2011.02.004
3. Xie JM, Duan Z, Zhou XM, Yuan C, Su ZH. Functional research progress of hirudin. J Hubei Polytechnic Univ (2020) 36(03):53–7. doi: 10.3969/j.issn.2095-4565.2020.03.010
4. Cheng GH, Wang S, Li SJ, Chen H, Wang CA, Niu ML. Protective effect of hirudin on renal injury in diabetic nephropathy rats. J Pract Traditional Chin Med (2023) 39(04):635–7.
5. Cheng SH, Wang S, Chen H, Wang CA, Li SJ, Shi JB, et al. Effects of hirudin on lipid metabolism and hemorheology in rats with diabetic nephropathy. Clin J Chin Med (2022) 14(22):37–40. doi: 10.3969/j.issn.1674-7860.2022.22.009
6. Chen MS, Liu L, Xu CL, Shao LN, Ren Y, Ye XL. Effect and mechanism of hirudin in protecting podocyte cytoskeleton through rhoA signaling to improve diabetic nephropathy. J Liaoning Univ Traditional Chin Med (2022) 24(12):30–5. doi: 10.13194/j.issn.1673-842x.2022.12.006
7. Guo Q, Chen ZQ, Fang J, Xu J, Han FS, Li XR, et al. Effects of hirudin on the apical membrane domain protein in high glucose-cultured podocytes. China J Traditional Chin Med Pharm (2021) 36(05):2494–8.
8. Long C, Lin Q, Mo J, Xiao Y, Xie Y. Hirudin attenuates puromycin aminonucleoside-induced glomerular podocyte injury by inhibiting MAPK-mediated endoplasmic reticulum stress. Drug Dev Res (2022) 83(4):1047–56. doi: 10.1002/ddr.21932
9. Han J, Pang X, Zhang Y, Peng Z, Shi X, Xing Y. Hirudin protects against kidney damage in streptozotocin-induced diabetic nephropathy rats by inhibiting inflammation via P38 MAPK/NF-kappaB pathway. Drug Des Devel Ther (2020) 14:3223–34. doi: 10.2147/DDDT.S257613
10. Pang X, Zhang Y, Peng Z, Shi X, Han J, Xing Y. Hirudin reduces nephropathy microangiopathy in STZ-induced diabetes rats by inhibiting endothelial cell migration and angiogenesis. Life Sci (2020) 255:117779. doi: 10.1016/j.lfs.2020.117779
11. Pang X, Zhang Y, Shi X, Peng Z, Xing Y, Jiarui H. Hirudin reduces the expression of markers of the extracellular matrix in renal tubular epithelial cells in a rat model of diabetic kidney disease through the hypoxia-inducible factor-1alpha (HIF-1alpha)/vascular endothelial growth factor (VEGF) signaling pathway. Med Sci Monit (2020) 26:e921894. doi: 10.12659/MSM.921894
12. Wang H, Cui H, Lin L, Ji Y, Ni Q, Li J, et al. The effects of a hirudin/liposome complex on a diabetic nephropathy rat model. BMC Complement Altern Med (2019) 19(1):118. doi: 10.1186/s12906-019-2531-7
13. Han J, Zuo Z, Shi X, Zhang Y, Peng Z, Xing Y, et al. Hirudin ameliorates diabetic nephropathy by inhibiting Gsdmd-mediated pyroptosis. Cell Biol Toxicol (2023) 39(3):573–89. doi: 10.1007/s10565-021-09622-z
14. Yang K, Fan B, Zhao Q, Ji Y, Liu P, Gao S, et al. Hirudin ameliorates renal interstitial fibrosis via regulating TGF-beta1/smad and NF-kappaB signaling in UUO rat model. Evid Based Complement Alternat Med (2020) 2020:7291075. doi: 10.1155/2020/7291075
15. Xie Y, Lan F, Zhao J, Shi W. Hirudin improves renal interstitial fibrosis by reducing renal tubule injury and inflammation in unilateral ureteral obstruction (UUO) mice. Int Immunopharmacol (2020) 81:106249. doi: 10.1016/j.intimp.2020.106249
16. Lin Q, Long C, Wang Z, Wang R, Shi W, Qiu J, et al. Hirudin, a thrombin inhibitor, attenuates TGF-beta-induced fibrosis in renal proximal tubular epithelial cells by inhibition of protease-activated receptor 1 expression via S1P/S1PR2/S1PR3 signaling. Exp Ther Med (2022) 23(1):3. doi: 10.3892/etm.2021.10924
17. Lee M, Zhao H, Liu X, Liu D, Chen J, Li Z, et al. Protective effect of hydroxysafflor yellow A on nephropathy by attenuating oxidative stress and inhibiting apoptosis in induced type 2 diabetes in rat. Oxid Med Cell Longev (2020) 2020:7805393. doi: 10.1155/2020/7805393
18. Opazo-Rios L, Mas S, Marín-Royo G, Mezzano S, Gómez-Guerrero C, Moreno JA, et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci (2020) 21(7):2632. doi: 10.3390/ijms21072632
19. Garg P. A review of podocyte biology. Am J Nephrol (2018) 47(Suppl 1):3–13. doi: 10.1159/000481633
20. Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol (2020) 24(3):193–204. doi: 10.1007/s10157-020-01854-3
21. Ahmadian E, Eftekhari A, Atakishizada S, Valiyeva M, Ardalan M, Khalilov R, et al. Podocytopathy: The role of actin cytoskeleton. BioMed Pharmacother (2022) 156:113920. doi: 10.1016/j.biopha.2022.113920
22. Porras G, Ayuso MS, Gonzalez-Manchon C. Leukocyte-endothelial cell interaction is enhanced in podocalyxin-deficient mice. Int J Biochem Cell Biol (2018) 99:72–9. doi: 10.1016/j.biocel.2018.03.018
23. Wang R, St John PL, Kretzler M, Wiggins RC, Abrahamson DR. Molecular cloning, expression, and distribution of glomerular epithelial protein 1 in developing mouse kidney. Kidney Int (2000) 57(5):1847–59. doi: 10.1046/j.1523-1755.2000.00034.x
24. Suzuki D, Miyazaki M, Naka R, Koji T, Yagame M, Jinde K, et al. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes (1995) 44(10):1233–8. doi: 10.2337/diab.44.10.1233
25. Shelbaya S, Amer H, Seddik S, Allah AA, Sabry IM, Mohamed T, et al. Study of the role of interleukin-6 and highly sensitive C-reactive protein in diabetic nephropathy in type 1 diabetic patients. Eur Rev Med Pharmacol Sci (2012) 16(2):176–82.
26. Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int (2011) 79(4):464–70. doi: 10.1038/ki.2010.404
27. Sun JK, Zhang WH, Chen WX, Wang X, Mu XW. Effects of early enteral nutrition on Th17/Treg cells and IL-23/IL-17 in septic patients. World J Gastroenterol (2019) 25(22):2799–808. doi: 10.3748/wjg.v25.i22.2799
28. Xie Y, Ostriker AC, Jin Y, Hu H, Sizer AJ, Peng G, et al. LMO7 is a negative feedback regulator of transforming growth factor beta signaling and fibrosis. Circulation (2019) 139(5):679–93. doi: 10.1161/CIRCULATIONAHA.118.034615
29. Li W, Liu T, Wu L, Chen C, Jia Z, Bai X, et al. Blocking the function of inflammatory cytokines and mediators by using IL-10 and TGF-beta: a potential biological immunotherapy for intervertebral disc degeneration in a beagle model. Int J Mol Sci (2014) 15(10):17270–83. doi: 10.3390/ijms151017270
30. Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int (2003) 64(4):1208–13. doi: 10.1046/j.1523-1755.2003.00237.x
31. Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, Jimenez J, et al. Podocyte vascular endothelial growth factor (Vegf(1)(6)(4)) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia (2011) 54(5):1227–41. doi: 10.1007/s00125-010-2034-z
32. Shao Y, Lv C, Yuan Q, Wang Q. Levels of serum 25(OH)VD3, HIF-1alpha, VEGF, vWf, and IGF-1 and their correlation in type 2 diabetes patients with different urine albumin creatinine ratio. J Diabetes Res (2016) 2016:1925424. doi: 10.1155/2016/1925424
33. Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res (2016) 365(3):553–62. doi: 10.1007/s00441-016-2461-3
34. Ando A, Hashimoto N, Sakamoto K, Omote N, Miyazaki S, Nakahara Y, et al. Repressive role of stabilized hypoxia inducible factor 1alpha expression on transforming growth factor beta-induced extracellular matrix production in lung cancer cells. Cancer Sci (2019) 110(6):1959–73. doi: 10.1111/cas.14027
35. Al MA, Ara Mimi A, Wu Y, Zaeem M, Abdul Aziz M, Aktar Suchi S, et al. Pyroptosis in diabetic nephropathy. Clin Chim Acta (2021) 523:131–43. doi: 10.1016/j.cca.2021.09.003
36. Wu P, Shi J, Sun W, Zhang H. Identification and validation of a pyroptosis-related prognostic signature for thyroid cancer. Cancer Cell Int (2021) 21(1):523. doi: 10.1186/s12935-021-02231-0
37. Cheng Q, Pan J, Zhou ZL, Yin F, Xie HY, Chen PP, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin (2021) 42(6):954–63. doi: 10.1038/s41401-020-00525-z
38. Yang F, Li Y, Guo S, Pan Y, Yan C, Chen Z. Protective effects of hirudo lyophilized powder on renal injury in diabetic nephropathy rats. Chin Traditional Herbal Drugs (2021) 52(04):1020–5. doi: 10.7501/j.issn.0253-2670.2021.04.014
39. Fang J, Chen ZQ, Guo Q, Chen CY, Wang CN, Xie T, et al. Regulatory effect of chinese drugs for stasis removing and collaterals dredging on the expressions of podocin and CD2AP in podocyte slit diaphragm of diabetic nephropathy rats. Chin J Integrated Traditional Western Med (2016) 36(07):835–41. doi: 10.7661/CJIM.2016.07.0835
40. Chen ZQ, Fang J, Xu J, Zhang JH, Li LL. Effect of huayu tongluo traditional chinese medicine on skeleton protein in podocytes of rats with diabetic nephropathy. Natural Product Res Dev (2016) 28(10):1540–1544+1584. doi: 10.16333/j.1001-6880.2016.10.007
41. Zhao Q, Li ZS, Wang Y, Huang HP, Ao L, Huang ZM. Research progress on pathogenesis of diabetic nephropathy. J Guizhou Univ Traditional Chin Med (2022) 44(02):76–80. doi: 10.16588/j.cnki.issn2096-8426.2022.02.016
42. Chen YQ, Ji K. Effect of Maixuekang Capsule Combined with Telmisartan Tablets on Patients with Diabetic kidney disease. J Hebei North University(Natural Sci Edition) (2017) 33(12):13–15+18. doi: 10.3696/j.issn.1673-1492.2017.12.005
43. Li WY, Sun J, Gao ZH, Zhou SH, Wei JX, Li KM. Ginkgo diterpene lactone combined with leech capsule as adjuvant treatment of Clinical observation on diabetic nephropathy. Shandong Med J (2017) 57(28):48–50. doi: 10.3969/j.issn.1002-266X.2017.28.014
44. Li YY, Shu YQ. Therapeutic efficacy of treating early type 2 diabetic nephropathy with the Yiqi Huoxue Buxue kidney formula. Contemp Med Symposium (2019) 17(03):199–200. doi: 10.3969/j.issn.2095-7629.2019.03.149
45. Si LL, Xue Y. Effects of Qizhi Jiangtang capsules on microcirculation and renal function in patients with diabetic nephropathy. Chronic Pathematology J (2021) 22(04):588–589+592. doi: 10.16440/j.cnki.1674-8166.2021.04.033
46. Li Y, Cui L. Clinical study on hirudin in diabetic nephropathy with umalb as the main manifestations and hypertension kidney disease. Chin J Clin Rational Drug Use (2010) 3(22):6–7. doi: 10.3969/j.issn.1674-3296.2010.22.005
47. Zhan JH, Wang CR, Hu MR. Comparative study of 54 cases of chronic kidney disease treated with leeches. J Guizhou Univ Traditional Chin Med (2017) 39(02):61–63+97. doi: 10.16588/j.cnki.issn1002-1108.2017.02.016
48. Fischer KG. Hirudin in renal insufficiency. Semin Thromb Hemost (2002) 28(5):467–82. doi: 10.1055/s-2002-35288
49. Bucha E, Markwardt F, Nowak G. Hirudin in haemodialysis. Thromb Res (1990) 60(6):445–55. doi: 10.1016/0049-3848(90)90229-6
50. van Wyk V, Badenhorst PN, Luus HG, Kotzé HF. A comparison between the use of recombinant hirudin and heparin during hemodialysis. Kidney Int (1995) 48(4):1338–43. doi: 10.1038/ki.1995.419
Keywords: diabetic nephropathy, leech, hirudin, traditional herbal medicine, research progress
Citation: Tian F, Yi X, Yang F, Chen Y, Zhu W, Liu P and Li S (2024) Research progress on the treatment of diabetic nephropathy with leech and its active ingredients. Front. Endocrinol. 15:1296843. doi: 10.3389/fendo.2024.1296843
Received: 19 September 2023; Accepted: 08 January 2024;
Published: 26 January 2024.
Edited by:
Manisha Jignesh Oza, Dr. Bhanuben Nanavati College of Pharmacy, IndiaReviewed by:
Hemant Giri, Oklahoma Medical Research Foundation, United StatesCopyright © 2024 Tian, Yi, Yang, Chen, Zhu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Liu, ZHJsaXVwZW5nQHNpbmEuY24=; Shuju Li, bHNqMjAwNTEwMjlAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Feng Tian1†
Feng Tian1† Yao Chen
Yao Chen Wenhui Zhu
Wenhui Zhu Peng Liu
Peng Liu