- 1Department of Endocrinology, The Second Affiliated Hospital of Shandong First Medical University, Taian, Shandong, China
- 2Department of Operating Room, The Second Affiliated Hospital of Shandong First Medical University, Taian, Shandong, China
- 3Department of Case Room, The Second Affiliated Hospital of Shandong First Medical University, Taian, Shandong, China
Background & aims: Existing evidence on the possible effects of probiotics on obese or overweight adolescents has not been fully established. Therefore, the aim of this study was to explore the effects of probiotic supplementation on anthropometric indices, inflammatory markers and metabolic indices in obese or overweight adolescents.
Methods: The literature up to March 2023 related to probiotic intervention in obese or overweight adolescents was searched and screened from multiple databases, including the CNKI(China national knowledge infrastructure), CBM(Chinese biomedical literature database), PubMed, EmBase, and Cochrane library databases. All randomized controlled trials using probiotic supplements in obese or overweight adolescents were included in this systematic review and meta-analysis.
Results: A total of 8 studies that met the inclusion criteria were included in this study. There were 201 cases in the experimental group (probiotic treatment) and 190 cases in the control group. Compared to the control group, probiotic intervention in adolescents resulted in a decrease in body mass index, fasting blood glucose and C-reactive protein with WMD(Weighted mean difference) and 95% CI of -2.53 (-4.8 to -0.26) kg/m2, -0.80 (-1.13 to -0.47) mol/L and -0.24 (-0.43 to -0.05) mg/L, respectively. No significant changes were found in weight, waist circumference, waist-to-hip ratio, insulin, Homeostatic Model Assessment of insulin resistance, interleukin 6, tumor necrosis factor alpha and so on; however, an unfavorable elevated effect in total cholesterol, triglycerides, and low-density lipoproteins was detected with WMD and 95% CI of 0.06 (0.02 to 0.09) mmol/L, 0.18 (0.14 to 0.21) mmol/L, and 0.19 (0.18 to 0.20) mmol/L, respectively.
Conclusion: According to our results, probiotic supplementation was beneficial in managing metabolic indicators such as fasting blood glucose, body mass index and inflammation-related C-reactive protein in overweight or obese adolescents. Further large scale studies are warranted to confirm present findings and to identify the effects and mechanisms to provide more precise evidence for clinical intervention.
Systematic review registration: doi: 10.37766/inplasy2024.1.0081, identifier INPLASY202410081.
1 Introduction
The prevalence of overweight and obesity in adolescents has risen dramatically in recent decades and has become one of the most important public health problems (1, 2). Adolescence is a unique transition period accompanied by significant physiological and psychological changes. Obesity-related comorbidities may negatively impact adolescent growth and developmental trajectories, while the rising prevalence of obesity in adolescents is associated with an increase in adult-onset diseases (e.g., type 2 diabetes mellitus, hypertension, nonalcoholic fatty liver disease, obstructive sleep apnea, cancer, and dyslipidemia) (3, 4). As the importance of gut flora is further demonstrated, an increasing number of studies are focusing on the correlation between gut microbiota and obesity (5).
The gut microbiota is the most complex ecosystem in nature, possessing large bacterial populations in the gut (6). A significant correlation between obesity and the specific composition of the gut microbiota has been demonstrated (5).
The gut microbiota is not stationary, and short-term changes can occur through diet and lifestyle changes (7). Studies have shown that the microbial imbalances associated with obesity can be re-established with probiotics and a balanced dietary regimen (8). Currently, the number of studies on the effects of probiotics on obesity is small and mainly based on animal models. The effects of probiotics on human host metabolism, particularly on obesity, have been controversial because of the paucity of research data, inconsistent findings and lack of long-term follow-up results, as well as a serious lack of consistency in terms of strain type, sample size, dosage parameters, treatment duration, and mode of administration, which has hampered comparative analyses between studies and made their safety and efficacy for the treatment of obesity an ongoing controversy. Several studies have shown that probiotics not only lead to weight loss and improved obesity but also have a positive effect on metabolic parameters such as blood glucose, systemic inflammation and energy intake (9). Despite the existing data suggesting that probiotics have significant therapeutic potential in obesity, there are still many hurdles to overcome before probiotic therapy can be recognized in the medical practice of adolescent obesity, and there is controversy and uncertainty regarding the use of probiotics in adolescent obese patients (10).
New medical studies are being published continuously and clinicians are faced with increasingly large amounts of new information, to the point where it has become nearly impossible for clinicians to read and evaluate all available data in a medical field. In addition, the study results are not consistently reproducible and the results of individual studies are often insufficient to provide confident answers (11). Consequently, clinical decision making is particularly difficult when the published data are conflicting or the sample size is too small to be reliable (11). Evidence-based medicine is the best available evidence in the medical literature (12). Moreover, the best evidence in evidence-based medicine is from meta-analyses, which provide a less biased, more precise estimate on a clinical issue (13). Meta-analyses is a statistical technique for combining the results from different studies on the same topic (14), and is becoming popular for resolving discrepancies in clinical research. As such, a meta-analysis is an objective, quantitative synthesis of research findings (14) that increases the statistical strength and precision for estimating effects by combining the results of previous studies and, thus, overcoming the problem of small sample sizes and inadequate statistical strength (15). A meta-analysis can explore the sources of heterogeneity, and identify subgroups associated with the factor of interest, potentially providing new insights for future studies (15).
The purpose of this study is to further clarify whether probiotics can be recommended for the treatment of overweight or obesity in adolescents and to provide more scientific and evidence-based medical evidence for clinical interventions for the disease.
2 Methods
2.1 Literature retrieval strategy
The PubMed, Embase, Cochrane Library, CNKI, Wanfang and CBM databases were searched to obtain relevant randomized controlled trials published up to March 2023. We used the keywords and subjects both in Chinese and English as follows: “adolescent or adolescent or minors or teen or teenager”; “obesity or overweight”; “probiotics or probiotic or synbiotics”.
2.2 Selection criteria
The inclusion criteria were as follows: ①The study participants were obese or overweight adolescents; ②The intervention was probiotic treatment; ③Posttreatment means and standard deviations were directly available in the literature, or posttreatment-related means and standard deviations could be obtained by formula calculation; ④The type of study was a randomized controlled trial.
The exclusion criteria were as follows: ①The study participants were not adolescents; ②Noncontrolled trials, animal experiments or in vitro experiments; ③Literature with incomplete data or where the original data statistics could not be extracted; ④Conference papers, abstracts, reviews or meta-analyses; ⑤Secondary obesity with a clear etiology; ⑥For duplicate reports or multiple publications targeting the same subject, the literature with the most recent publications and the most complete data was selected.
2.3 Quality assessment
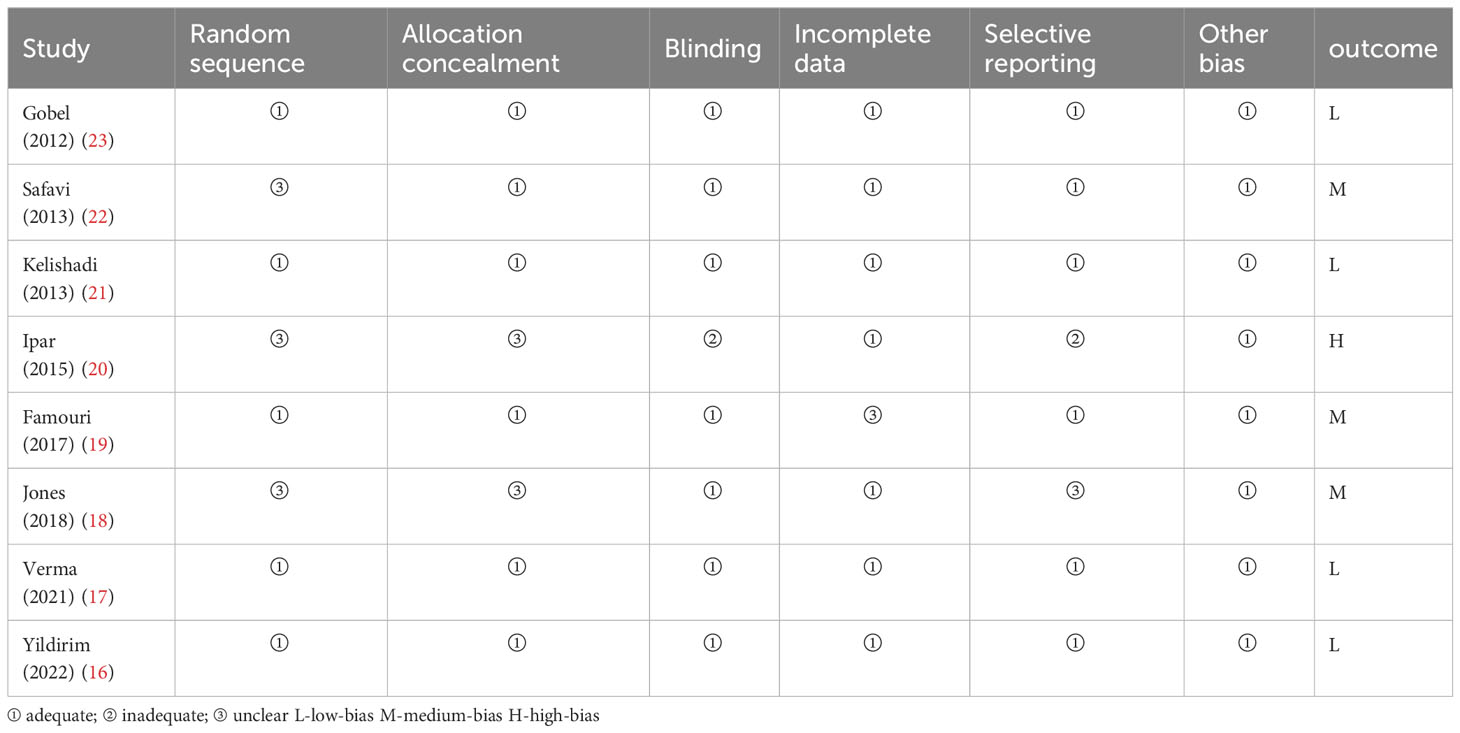
All included studies were randomized controlled studies; therefore, the Cochrane Risk Assessment Tool was used to assess the quality of the included studies. The scale consists of six dimensions (1): random sequence generation (2); allocation concealment (3); blinding (4); incomplete outcome data (5); selective reporting; and (6) other bias. If all of the above criteria are “adequate”, there is a low likelihood of bias; if one of the criteria is “unclear”, there is a moderate likelihood of bias; if one of the criteria is “inadequate” or “not used”, there is a high likelihood of bias.
2.4 Data extraction and management
Relevant data were extracted by 2 independent investigators through joint discussion to determine final inclusion, with a third investigator assisting in resolution if necessary. The relevant data were extracted, including author, year and country of publication, target population, number and mean age of participants, intervention duration and type. Additionally, the mean and standard deviation (SD) of the effect indicators of concern at the end of the intervention were extracted.
For each parameter, we used the mean and SD of the postintervention values for the probiotics and control groups, as the study population was randomly grouped for inclusion in the literature after reviewing the references, and as the inclusion literature was clearly stated, we considered that there was no difference between the initial data for the experimental and control groups.
2.5 Statistical analysis
R 4.1.1 software is used to calculate the merger effect. Heterogeneity between studies was assessed using the Q test and the I2 test. If P>0.05 and I2<50%, a fixed effects model was used; if P<0.05 and I2≥ 50%, indicating greater heterogeneity, sources of heterogeneity were then explored through sensitivity and subgroup analyses. Finally, publication bias was assessed by Begg’s and Egger’s tests. The WMD or SMD(Standardized mean difference) and 95% CI of the individual studies combined were recorded, and forest plots were used to characterize the results of the individual studies.
3 Result
3.1 Literature screening process and results
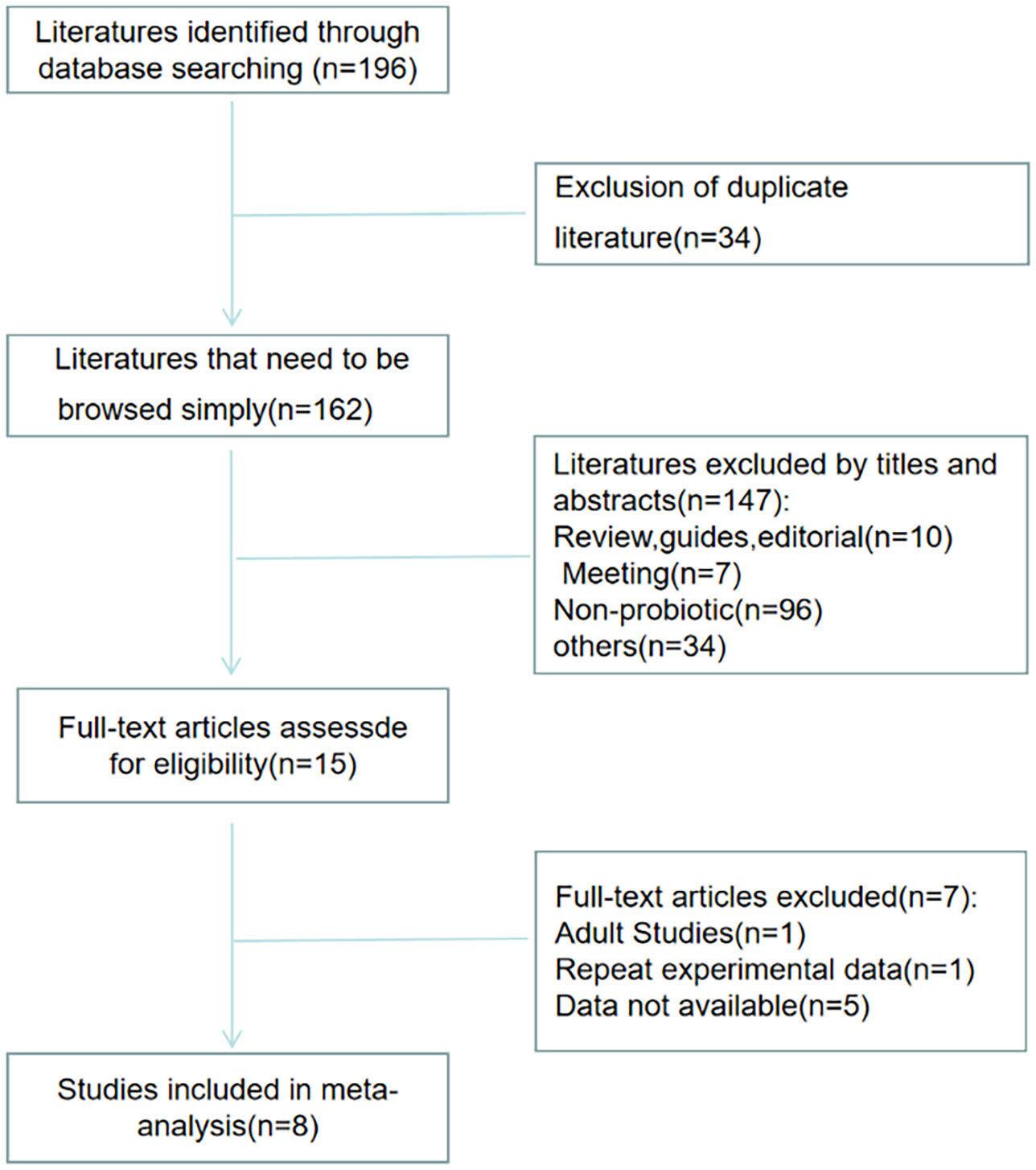
Figure 1 shows the selection process of the studies. There were 196 studies relevant to the search strategy, of which 34 were duplicates. A further 147 of these articles were excluded by browsing the titles and abstracts. After evaluating the full texts of the remaining studies, another 7 articles were excluded. Finally, 8 articles containing 201 cases and 190 controls were included in this meta-analysis (16–23).
3.2 Quality evaluation results
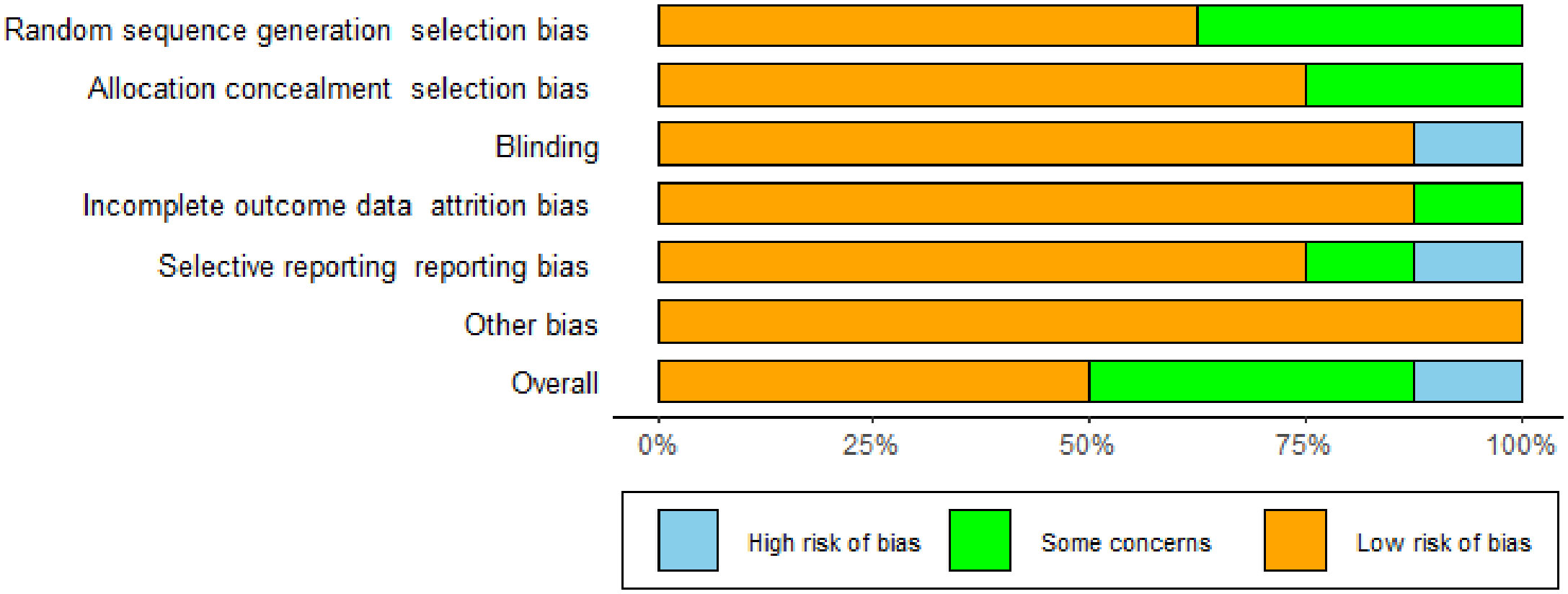
The studies we included were all randomized controlled studies, so the risk of bias evaluation was performed using the Cochrane Risk Assessment Tool, which was used to assess the quality of eight publications. The randomized allocation of participants was mentioned in all included trials. However, only five trials described randomized sequence generation methods (16, 17, 19, 21, 23), and six trials reported allocation concealment (16, 17, 19, 21–23). With the exception of Ipar’s study (20), which used an open-label design, the remaining seven studies had a low risk of blinding bias among participants, researchers, and outcome assessors. Based on incomplete outcome data and selective outcome reporting, most studies showed a low/unspecified risk of bias, and no other sources of bias were identified. Based on the above assessment, among the eight papers we included in the study, there were four low-bias papers, three medium-bias papers, and one high-bias paper. The risk of bias assessment is detailed in Table 1 and Figure 2.
3.3 Characteristics of included studies
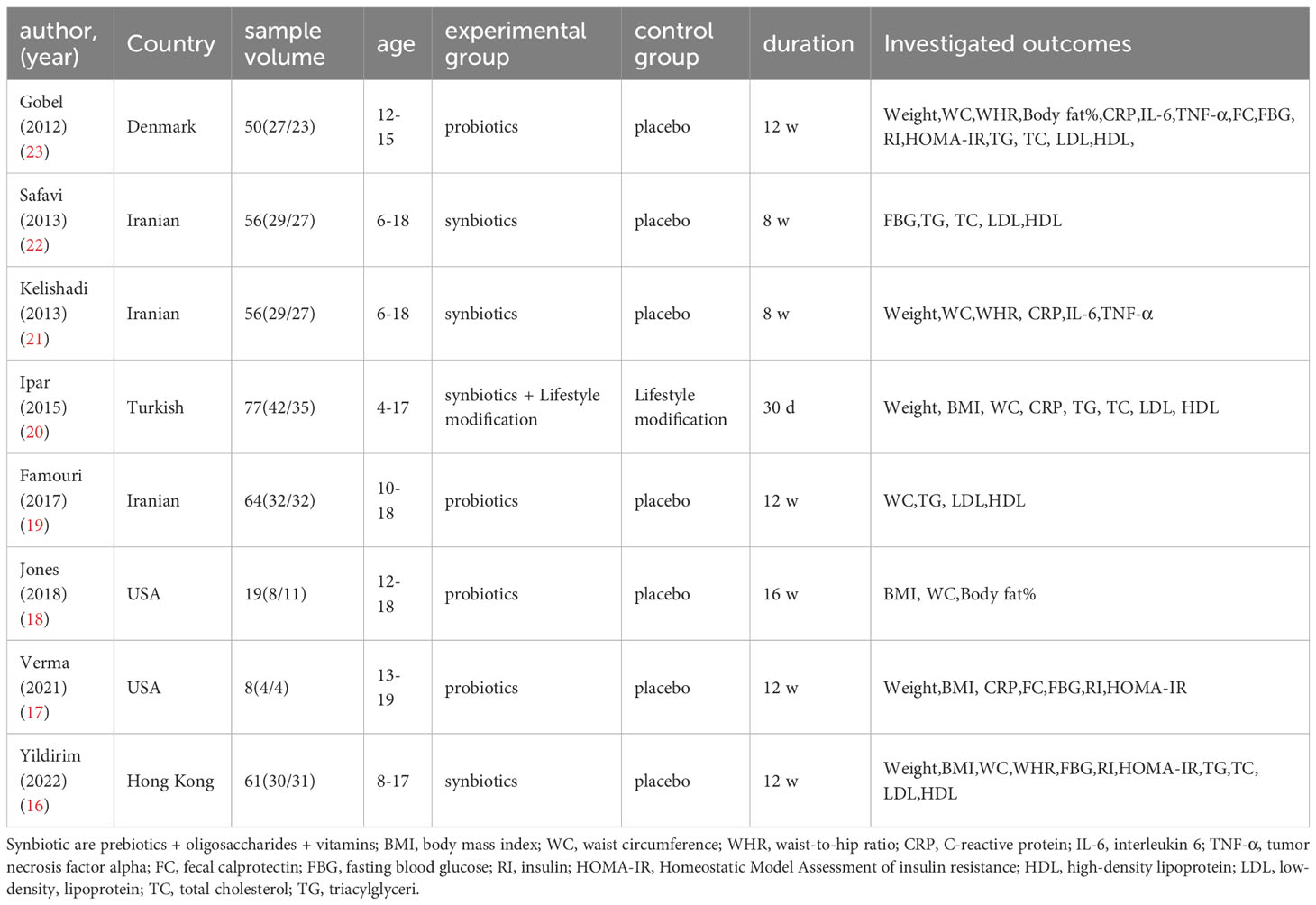
Details of the included studies are shown in Tables 2 and 3. We extracted the following data from the literature: author, year, country, age of study subjects, sample size, intervention, duration, mean and standard deviation of the investigated outcomes, where some articles did not provide standard deviation, but only sample size and standard error, which we obtained by Equation 1.
3.4 The effect of probiotics in obese or overweight adolescents
3.4.1 The effect of probiotics on anthropometric indices (weight, BMI, WC, WHR, body fat%)
The effect of probiotics on weight was analyzed by five studies (16, 17, 20, 21, 23), 132 in the experimental group and 120 in the control group. The meta-merge heterogeneity was significant (I2 = 68%, P=0.01), and the random effect model was applied to merge, as shown in Figure 3A. The WMD and 95% CI were 1.59 (-7.86, 4.68) kg, suggesting that the probiotic intervention did not have a significant effect on weight. To find the source of heterogeneity, sensitivity analysis was performed and found that heterogeneity was weakened by excluding the Kelishadi study (21) (Figure 3B). After excluding this study, the heterogeneity was I2 = 7%, P=0.36, and the fixed effect model was applied to merge the two studies, as shown in Figure 3C. The WMD and 95% CI were -4.35 (-9.53, 0.82) kg, respectively, indicating that the probiotic intervention had no significant effect on improving the weight of the obese adolescent. The results were consistent with the results before the exclusion of the Kelishadi study, suggesting that although heterogeneity was high, study sensitivity was low, and the results were more robust and trustworthy. Begg’s test (P = 0.3272) and Egger’s test (P = 0.064) suggested that there was no significant publication bias.
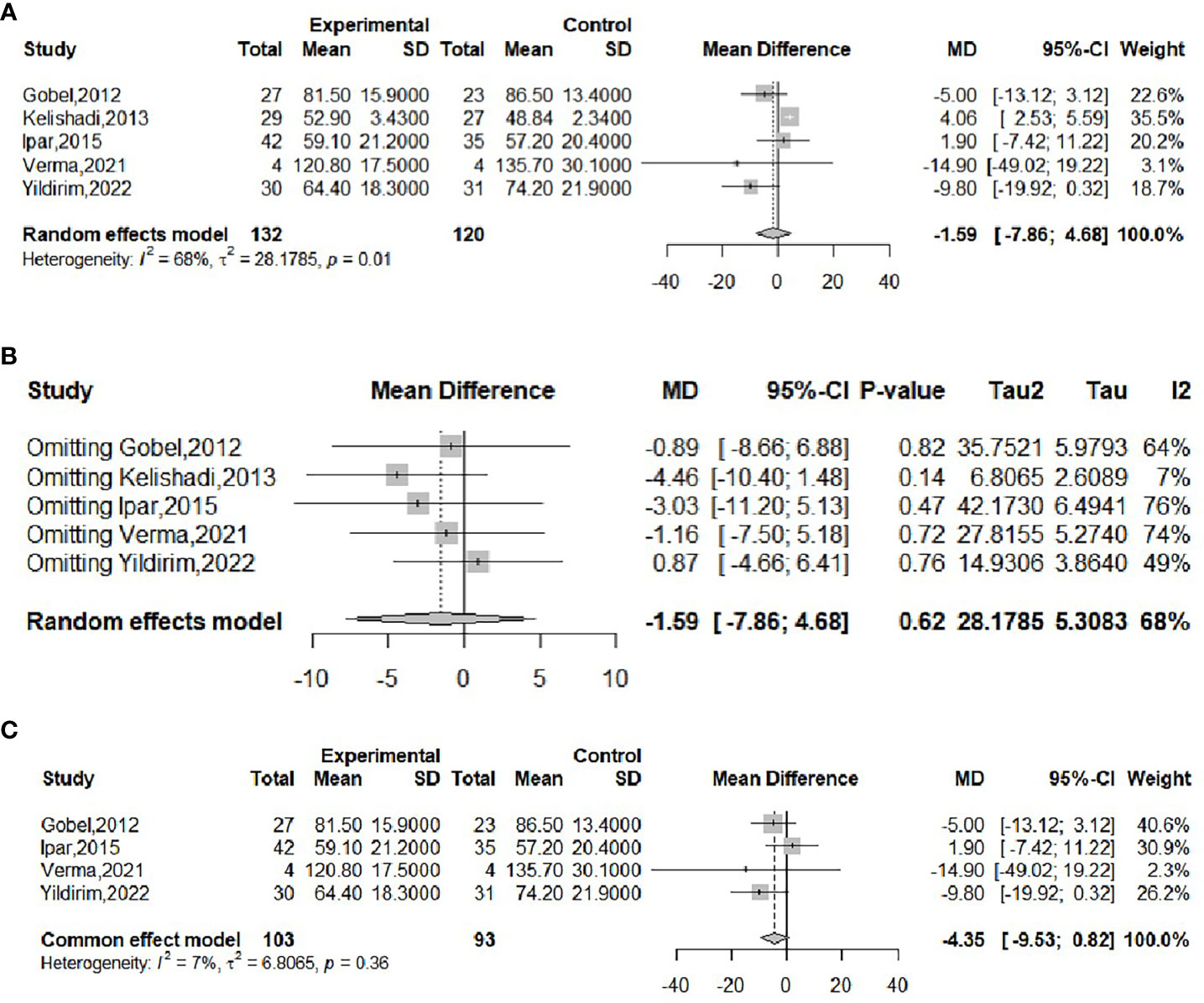
Figure 3 Forest plot of the effect of probiotic supplementation on weight (A). Sensitivity analysis of the effect of probiotic supplementation on weight (B). Forest plot of the effect of probiotic supplementation on weight (after elimination) (C).
The effect of probiotics on BMI was analyzed by four studies (16, 18, 20, 21), 84 in the experimental group and 81 in the control group. The meta-merge heterogeneity was significant (I2 = 65%, P=0.03), and the random effect model was applied to merge, as shown in Figure 4A. The WMD and 95% CI were -0.93 (-4.26,2.41) kg/m2, suggesting that probiotic intervention had no significant effect on BMI. To find the source of heterogeneity, sensitivity analysis was performed and found that the heterogeneity was weakened by excluding the Ipar study (20) (Figure 4B). After excluding this literature, the heterogeneity was I2 = 0%, P=0.87, which was combined by applying a fixed-effects model, as shown in Figure 4C, and the WMD and 95% CI were -2.53 (-4.8, -0.26) kg/m2, respectively, indicating that probiotic intervention was beneficial for the BMI of obese adolescents. This result is completely different from the results before the exclusion of the Ipar study, suggesting that the literature is highly sensitive and the results less robust. Begg’s test (P = 1) and Egger’s test (P = 0.4802) suggested that there was no significant publication bias.
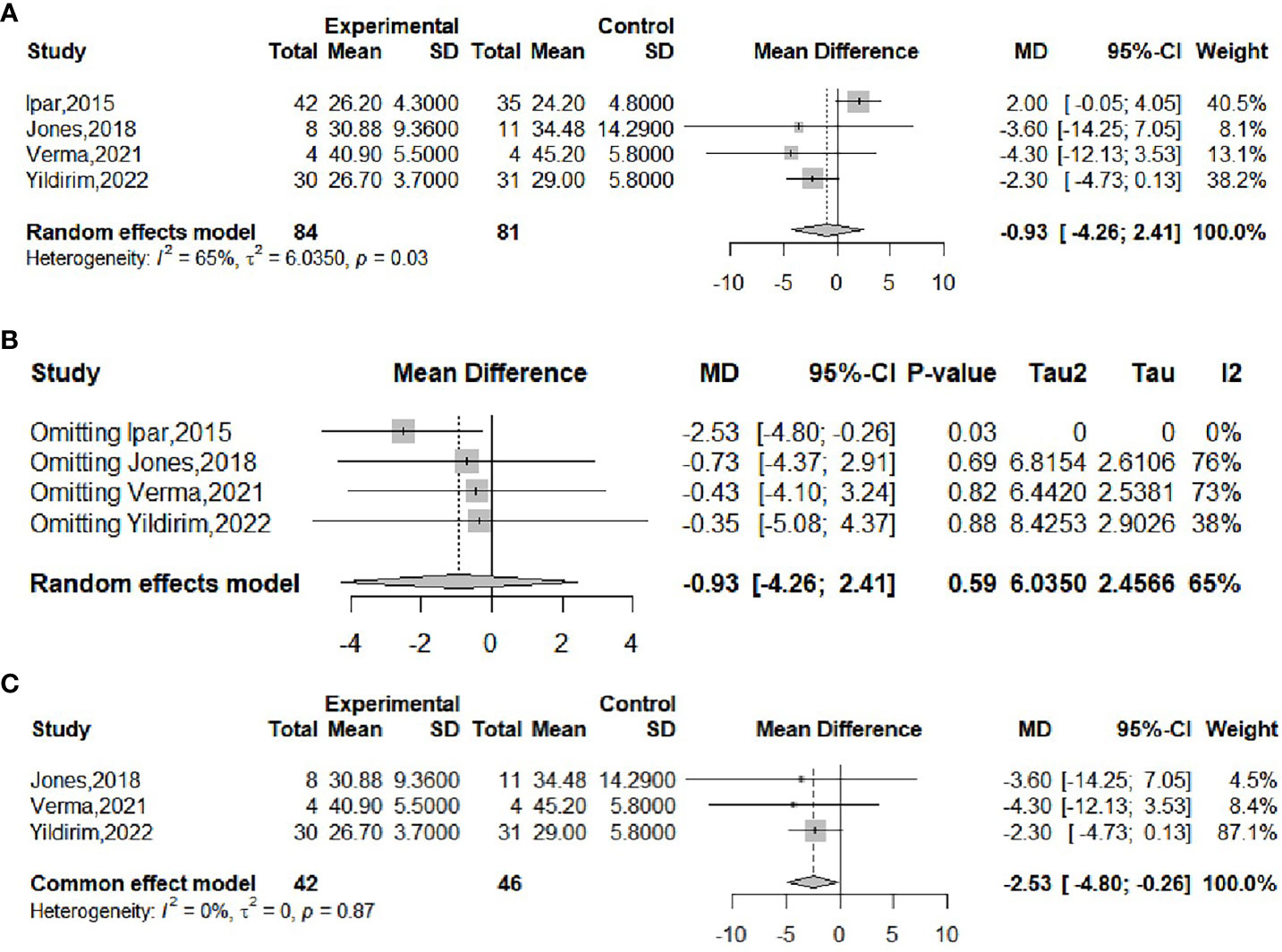
Figure 4 Forest plot of the effect of probiotic supplementation on BMI (A). Sensitivity analysis of the effect of probiotic supplementation on BMI (B). Forest plot of the effect of probiotic supplementation on BMI (after elimination) (C).
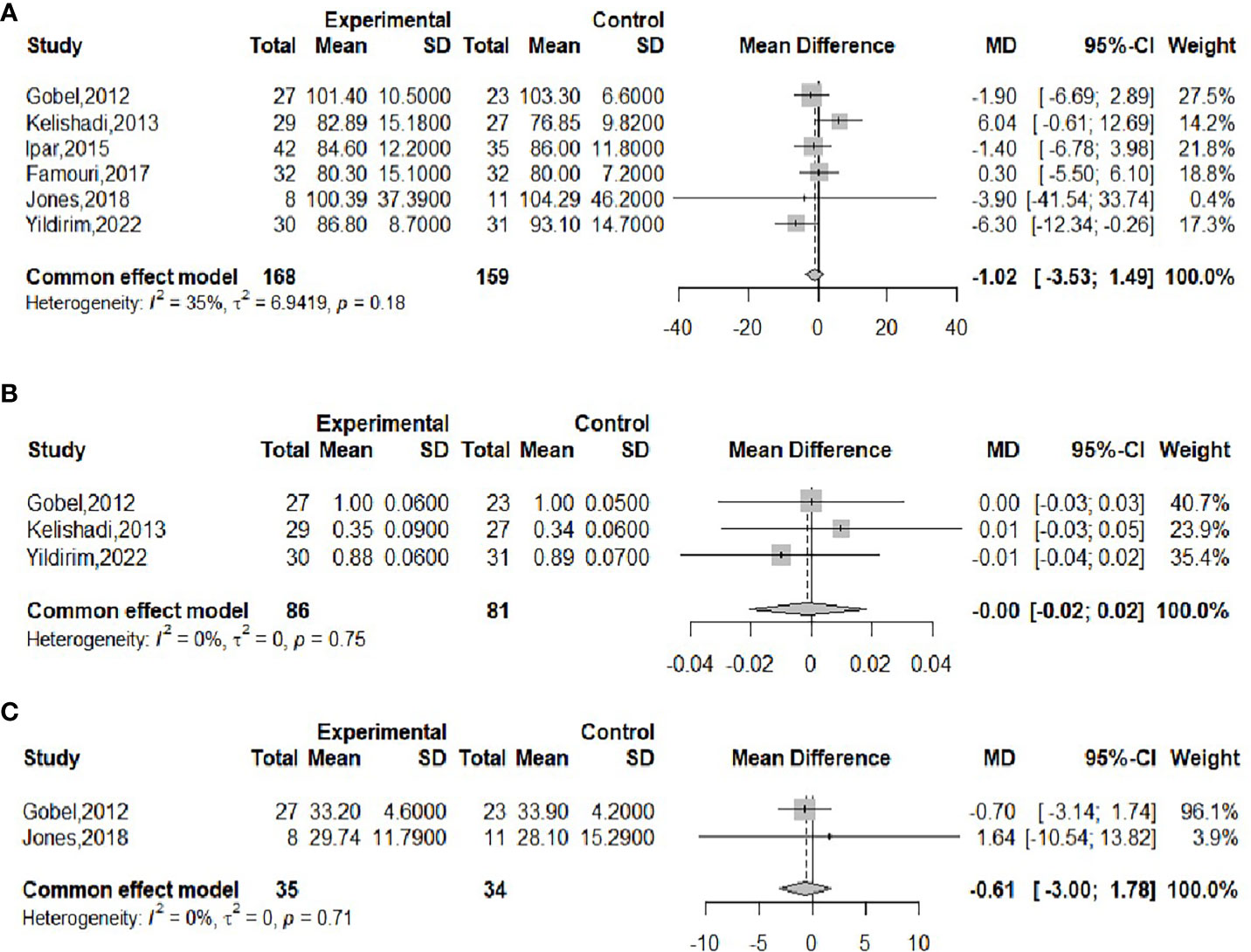
The effect of probiotics on WC was analyzed by six studies (16, 18–21, 23), including 168 cases in the experimental group and 159 cases in the control group. There was no significant heterogeneity after performing meta-merge (I2 = 35%, P=0.18), and the fixed effect model was applied for the merge, as shown in Figure 5A. The WMD and 95% CI were -1.02 (-3.53, 1.49) cm, respectively, which indicated that the probiotic intervention did not have a significant effect on WC in obese adolescents. Begg’s test (P = 0.3476) and Egger’s test (P = 0.9032) indicated no significant publication bias.
The effect of probiotics on WHR was analyzed by three studies (16, 21, 23), 86 in the experimental group and 81 in the control group. No significant heterogeneity was observed after performing meta-merge (I2 = 0%, P = 0.75), and the fixed-effects model was applied for merging, as shown in Figure 5B, with WMD and 95% CI 0.00 (-0.02,0.02), indicating that there was no significant effect of probiotic interventions on the WHR of the obese adolescents. Begg’s test (P = 0.6015) and Egger’s test (P = 0.5549) indicated no significant publication bias.
The effect of probiotics on body fat% was analyzed by two studies (18, 23), 35 in the experimental group and 34 in the control group. There was no significant heterogeneity after performing meta-merge (I2 = 0%, P=0.71), and the fixed effect model was applied for merging, as shown in Figure 5C, with WMD and 95% CI -0.61 (-3.00, 1.78)%, which indicated that probiotic interventions did not have a significant effect on the body fat % of the obese adolescents. Due to the small number of studies, no publication bias test was performed.
3.4.2 The effect of probiotics on inflammatory markers (CRP, IL-6, TNF-α, and FC)
The effect of probiotics on CRP was analyzed by four studies (17, 20, 21, 23), 101 in the experimental group and 89 in the control group. There was no significant heterogeneity after performing meta-merge (I2 = 0%, P = 0.77), and the fixed-effects model was applied for merging, as shown in Figure 6A, with WMD and 95% CI -0.80 (-1.13, -0.47) mg/L, which indicated that probiotic interventions could reduce CRP levels in obese adolescents. Begg’s test (P = 0.4969) and Egger’s test (P = 0.2276) indicated no significant publication bias.
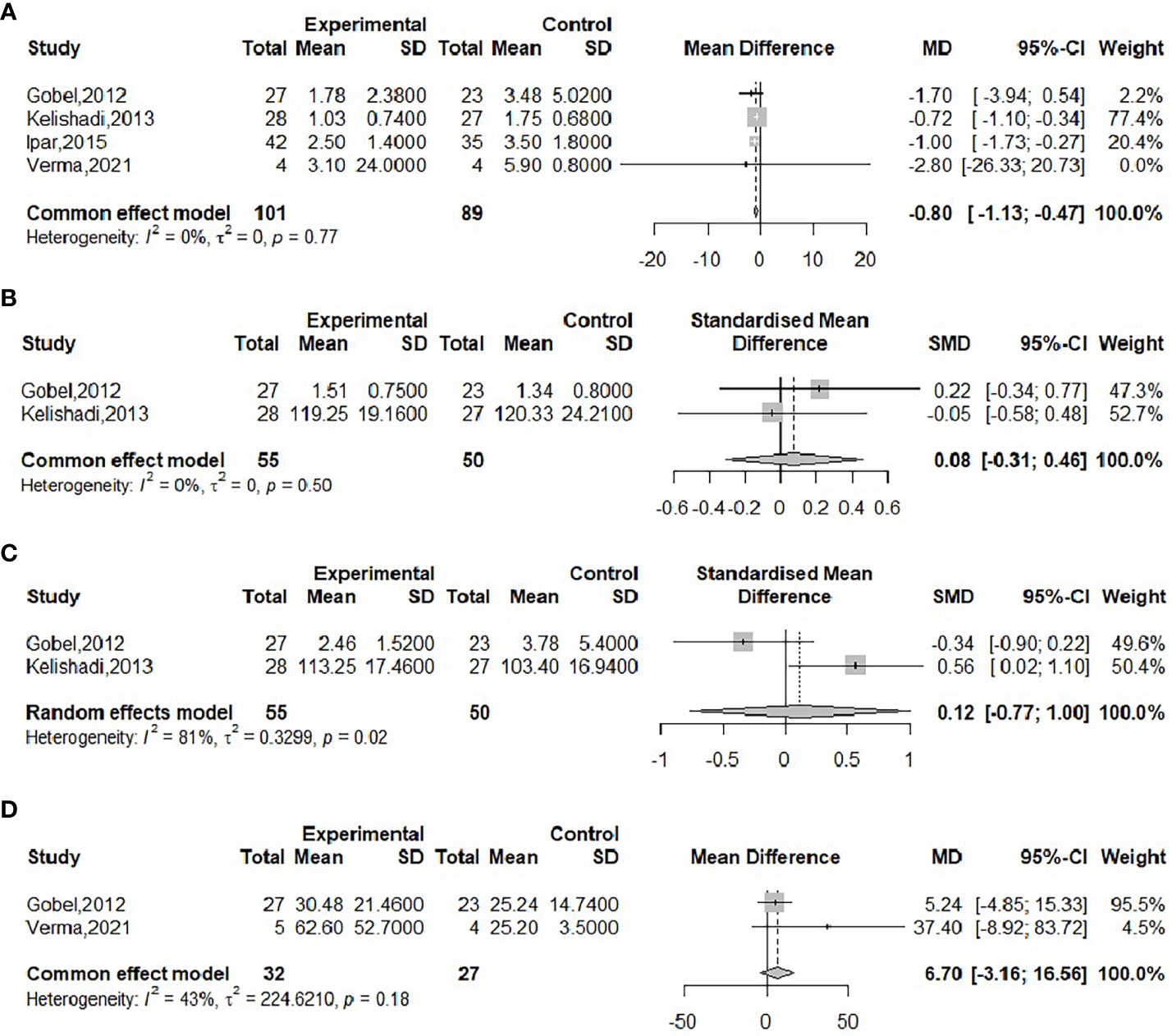
Figure 6 Forest plot of the effect of probiotic supplementation on CRP (A), IL-6 (B), TNF-α (C), FC (D).
The effect of probiotics on IL-6 in obese adolescents was analyzed by two studies (21, 23), 55 in the experimental group and 50 in the control group. There was no significant heterogeneity after performing meta-merge (I2 = 0%, P=0.50), applying the fixed effect model for merging, and because of its inconsistent units, effect sizes were merged with SMD, as shown in Figure 6B, with SMD and 95% CI 0.08 (-0.31, 0.46), respectively, indicating that probiotic intervention had no effect on IL-6. Due to the small number of included studies, a publication bias test was not performed.
The effect of probiotics on TNF-α in obese adolescents was analyzed by two studies (21, 23), 55 in the experimental group and 50 in the control group. The meta-merger heterogeneity was significant (I2 = 81%, P=0.02). Due to the small number of included studies, sensitivity analysis and subgroup analysis were not performed, so the random effect model was applied to merge, and because of its inconsistent units, the effect size was merged with SMD, as shown in Figure 6C. The SMD and the 95% CI were 0.12 (-0.77, 1.00), respectively, indicating that probiotic intervention had no effect on TNF-α. Due to the small number of included studies, a publication bias test was not performed.
The effect of probiotics on FC in obese adolescents was analyzed by two studies (17, 23), 32 in the experimental group and 27 in the control group. No significant heterogeneity was observed after performing meta-merging (I2 = 43%, P=0.18), and the fixed effect model was applied for merging, as shown in Figure 6D, with a WMD and 95% CI of 6.70 (-3.16, 16.56) mg/kg, which indicated that probiotic intervention had no effect on FC levels. The small number of included studies was not tested for publication bias.
3.4.3 The effect of probiotics on FBG, RI, and HOMA-IR
The effect of probiotics on FBG in obese adolescents was analyzed by four studies (16, 17, 22, 23), 90 in the experimental group and 85 in the control group. Significant heterogeneity (I2 = 64%, P=0.04) was observed when meta-merging was performed (Figure 7A), and a sensitivity analysis did not reveal a significant reduction in heterogeneity after excluding a particular piece of literature (Figure 7B), suggesting that the results of the included studies were relatively robust. To further look for sources of interstudy heterogeneity, subgroup analyses based on the type of intervention (probiotic or synbiotic) and duration of intervention (8 weeks/12 weeks) were performed. When grouped by intervention type (Figure 7C), two of the studies with intervention type of probiotic (17, 23) and two of the studies with intervention type of synbiotic (16, 22) were meta-merged separately, and there was no significant heterogeneity in either (I2 = 0), suggesting that the intervention type might be the source of heterogeneity, with WMD and 95% CI in the probiotic group of -0.24 (-0.43, 0.05) mmol/L and WMD and 95% CI in the synbiotic group of 0.03 (-0.03, 0.09) mmol/L, respectively. This suggests that probiotic treatment can reduce FBG levels, while synbiotic treatment has no effect on FBG. When grouped by intervention duration (Figure 7D), three studies (16, 17, 23) had an intervention duration of 12 weeks, one study (22) had an intervention duration of 8 weeks, and there was no significant attenuation of heterogeneity after the merger, suggesting that interstudy heterogeneity was not related to intervention duration. Begg’s test (P = 0.1742) and Egger’s test (P = 0.3993) indicated no significant publication bias.
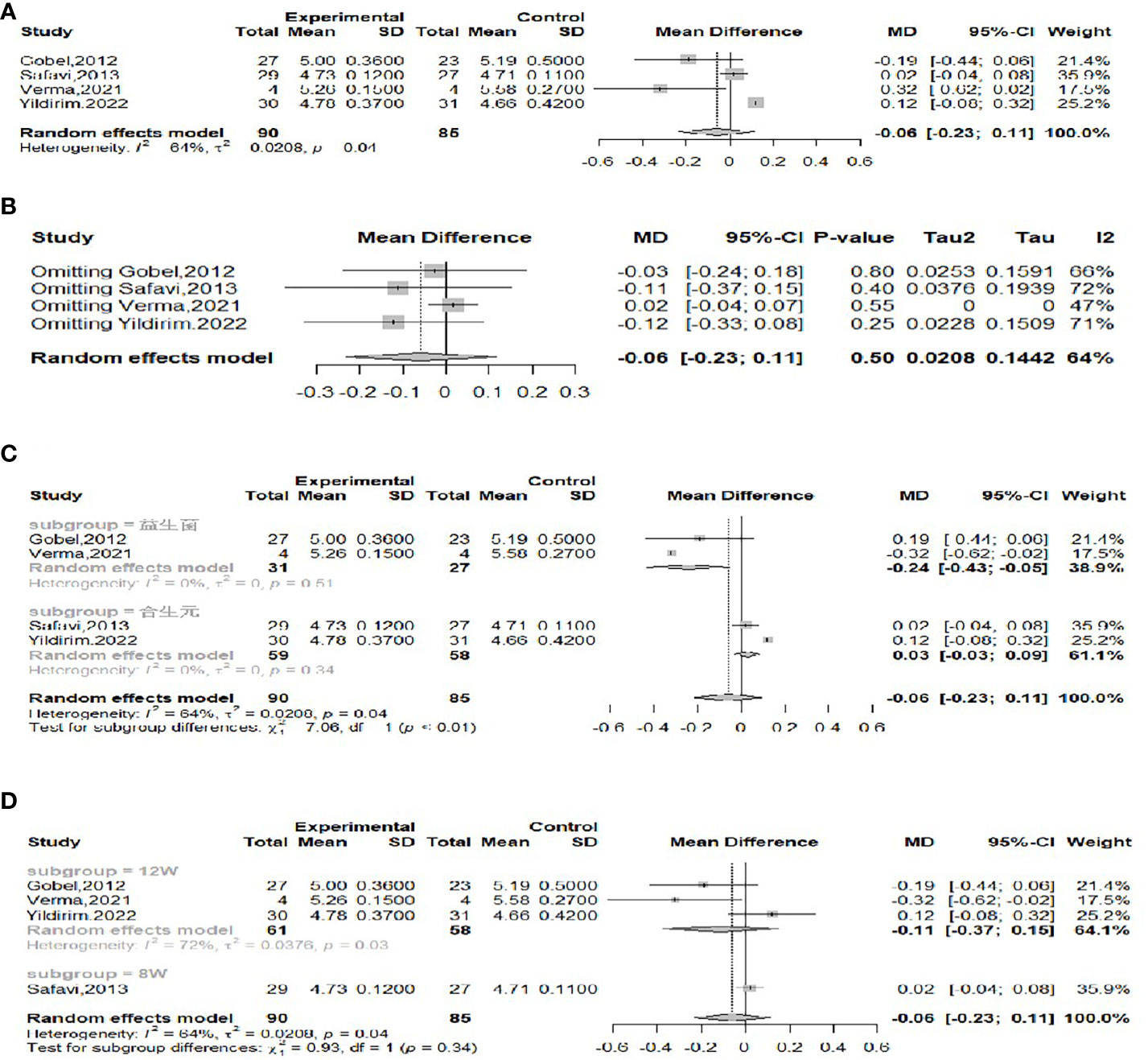
Figure 7 Forest plot of the effect of probiotic supplementation on FBG (A). Sensitivity analysis of the effect of probiotic supplementation on FBG (B). Subgroup analysis based on the type of intervention (probiotic/synbiotic) (C). Subgroup analysis based on duration of intervention (8weeks/12weeks) (D).
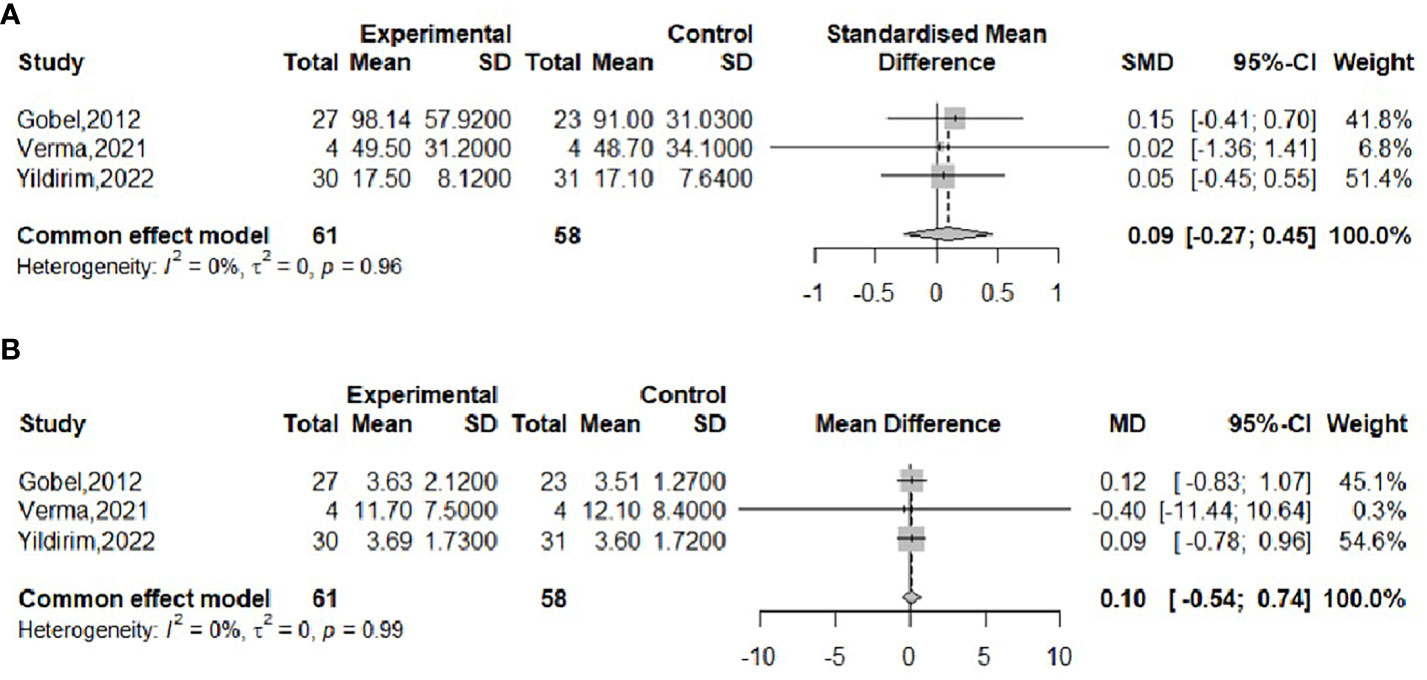
The effect of probiotics on the RI in obese adolescents was analyzed in three studies (16, 17, 23), with 61 in the experimental group and 58 in the control group. No significant heterogeneity was observed when meta-merging was performed (I2 = 0%, P = 0.96), the fixed-effects model was applied for merging, and the effect sizes were merged with SMD due to their inconsistent units, as shown in Figure 8A, with SMD and 95% CI 0.09 (-0.27,0.45), respectively, indicating that there was no effect of probiotic interventions on insulin levels. Begg’s test (P = 0.6015) and Egger’s test (P = 0.5112) indicated no significant publication bias.
The effect of probiotics on HOMA-IR in obese adolescents was analyzed by three studies (16, 17, 23), 61 in the experimental group and 58 in the control group. No significant heterogeneity was observed when meta-merging was performed (I2 = 0%, P=0.99), and a fixed-effects model was applied for merging, as shown in Figure 8B, with a WMD and 95% CI of 0.10 (-0.54, 0.74), respectively, indicating that the probiotic intervention had no effect on HOMA-IR. Begg’s test (P = 0.6015) and Egger’s test (P = 0.3480) indicated no significant publication bias.
3.4.4 The effect of probiotics on the lipid profile (TC, TG, LDL, HDL)
The effect of probiotics on TC in obese adolescents was analyzed by five studies (16, 19, 20, 22, 23), of which the Famouri study (19) had a significantly higher TC in the intervention group than in the control group at baseline. This group was excluded, and the remaining four studies were combined (16, 20, 22, 23), with 128 cases in the experimental group and 116 cases in the control group. No significant heterogeneity was observed after performing meta-merging (I2 = 0%, P=0.53), and the fixed-effects model was applied for merging, as shown in Figure 9A, with WMD and 95% CI 0.06 (0.02, 0.09) mmol/L, which indicated that probiotic interventions could elevate TC levels in adolescent obese adolescents. Begg’s test (P =0.1742) and Egger’s test (P =0.5417) indicated no significant publication bias.
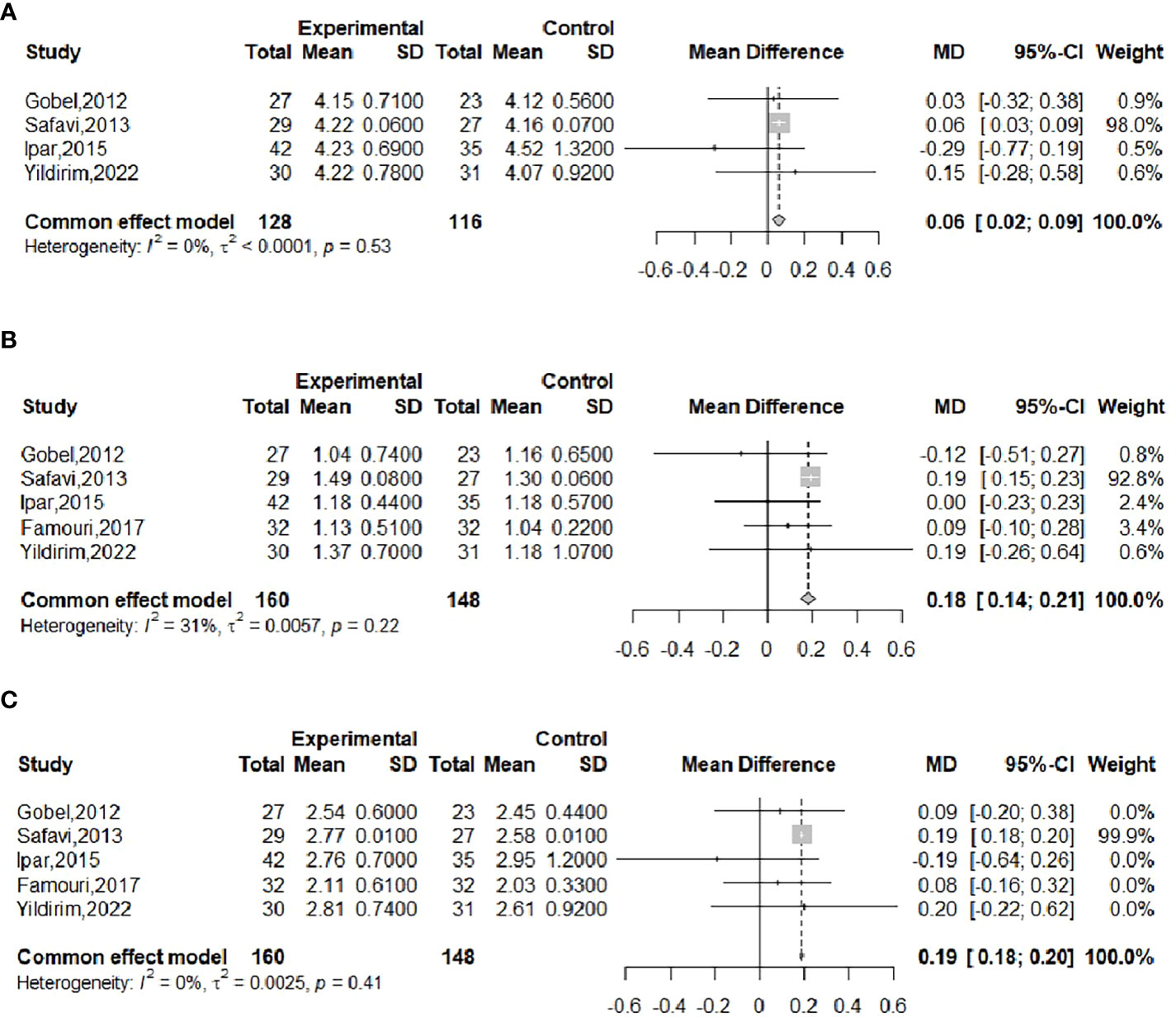
Figure 9 Forest plot of the effect of probiotic supplementation on TC (A). Forest plot of the effect of probiotic supplementation on TG (B). Forest plot of the effect of probiotic supplementation on LDL (C).
The effect of probiotics on TG in obese adolescents was analyzed in five studies (16, 19, 20, 22, 23), with 160 in the experimental group and 148 in the control group. There was no significant heterogeneity after performing meta-merging (I2 = 31%, P = 0.22), and the fixed-effects model was applied for merging, as shown in Figure 9B, with WMD and 95% CI: 0.18 (0.14, 0.21) mmol/L, which indicated that probiotic interventions could elevate TG levels in adolescent obese adolescents. Begg’s test (P = 0.6242) and Egger’s test (P = 0.0834) indicated no significant publication bias.
The effect of probiotics on LDL in obese adolescents was analyzed by five studies (16, 19, 20, 22, 23), including 160 cases in the experimental group and 148 cases in the control group, and there was no significant heterogeneity after meta-merging (I2 = 0%, P=0.41). The fixed-effects model was applied to merge them, as shown in Figure 9C, and the WMD and 95% CI were 0.19 (0.18, 0.20) mmol/L, indicating that probiotic intervention can elevate LDL levels in adolescent obese patients. Begg’s test (P = 0.3272) and Egger’s test (P = 0.1077) indicated no significant publication bias.
The effect of probiotics on HDL in obese adolescents was analyzed in five studies (16, 19, 20, 22, 23), with 160 in the experimental group and 148 in the control group. Significant heterogeneity of meta-merge was observed (I2 = 81%, P<0.01), and the random effects model was applied for the merge, as shown in Figure 10A, with WMD and 95% CI -0.01 (-0.11,0.13) mmol/L, suggesting that the probiotic intervention did not have a significant effect on HDL in obese adolescents. To find the source of heterogeneity, sensitivity analysis was performed and found that the heterogeneity was weakened after excluding the Famouri study (19) (Figure 10B). After excluding this literature, the heterogeneity was weakened (I2 = 0%, P=0.84), and the fixed-effects model was applied for the merger, as shown in Figure 10C. The WMD and the 95% CI were -0.05 (-0.09, -0.01) mmol/L, respectively, indicating that the probiotic intervention could reduce HDL levels. This result is very different from the result before excluding the Famouri study, indicating high sensitivity and low robustness of the result. Begg’s test (P = 0.1416) and Egger’s test (P = 0.3392) indicated no significant publication bias.
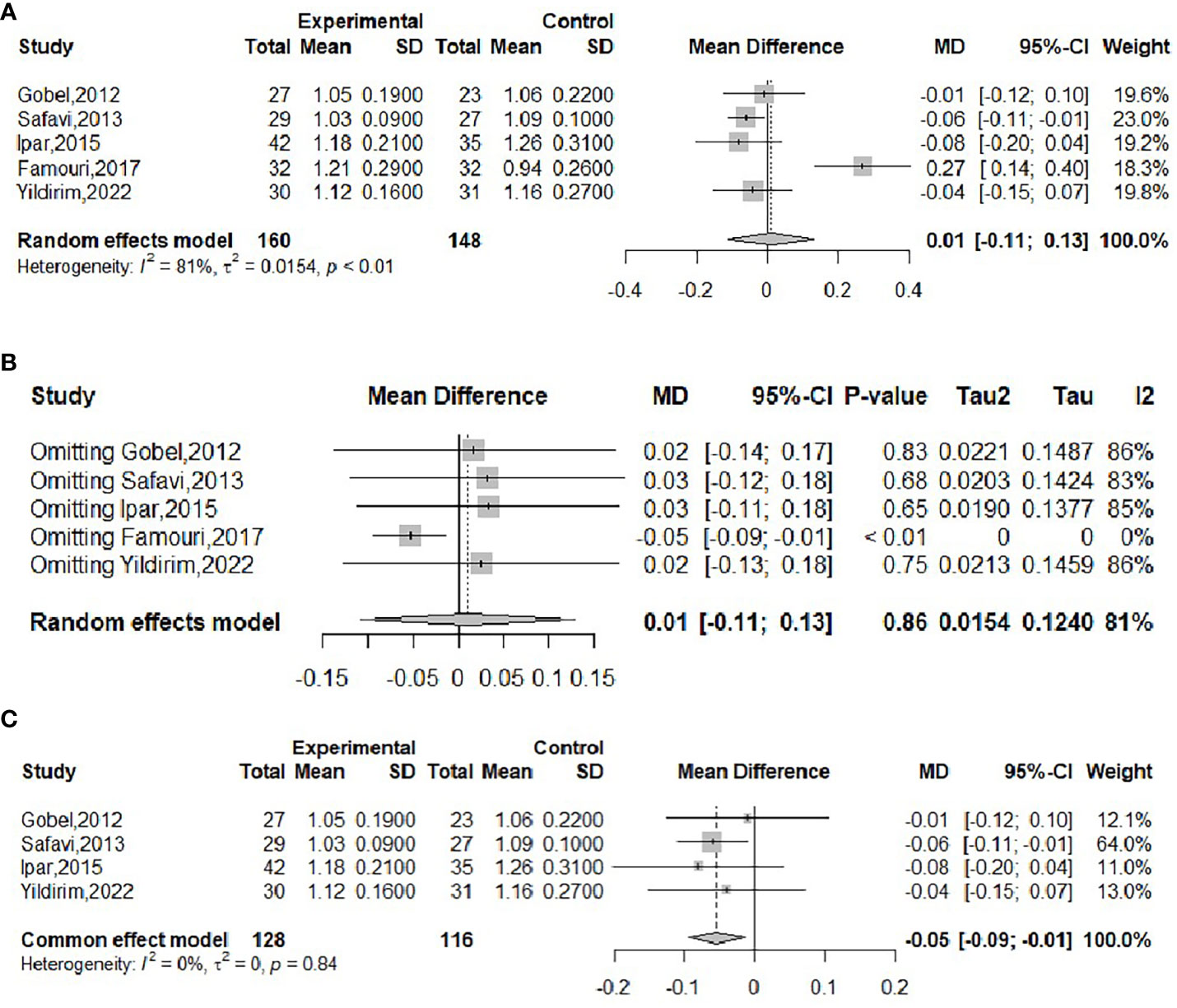
Figure 10 Forest plot of the effect of probiotic supplementation on HDL (A). Sensitivity analysis of the effect of probiotic supplementation on HDL (B). Forest plot of the effect of probiotic supplementation on HDL (after elimination) (C).
4 Discussion
The WHO defines probiotics as nondigestible food ingredients that improve the health of the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already established in the colon, with beneficial effects on the host (24). They are involved in host regulation in different ways: antagonizing the growth of pathogenic microorganisms and competitive adhesion to the intestinal mucosa and epithelium (antimicrobial activity), increasing intestinal mucus production and decreasing intestinal permeability (barrier function), and modulating the immune system of the gastrointestinal tract (immunomodulation) (25–27). All of these mechanisms influence the development of the microbiota to ensure the proper balance between the pathogen and the microorganisms needed for optimal host function. Furthermore, through a review of the literature we found that the most commonly researched and recommended probiotics include Lactobacillus genus and Bifidobacterium genus etc.
Our study found that probiotic intervention reduced BMI in overweight/obese adolescent patients but had no effect on metabolic indices such as weight, WC, WHR, or body fat%. Among them, no significant heterogeneity was found after meta-merging for WC, WHR, and body fat%, and greater heterogeneity was found for weight and BMI. To find the source of heterogeneity, sensitivity analyses were carried out, and all of them found that the heterogeneity was significantly reduced by exclusion of a particular piece of literature. Among them, the results of the effect on weight were the same before and after the exclusion, but the results of merging the results of BMI appeared to be inconsistent, which indicated that Ipar’s study (20) had a high sensitivity and that the results had a low robustness. Further analysis of Ipar’s study did not find clinical heterogeneity due to the same inclusion or exclusion criteria. When exploring methodological heterogeneity, we found that Ipar’s study had an open label design with a higher risk of bias compared to the rest of the included literature, so this group of data was excluded before combining them, and the combined results showed that probiotic interventions can reduce BMI levels in obese adolescent patients. The above findings are consistent with the results of a previous meta-analysis of children and adolescents (28), which did not analyze BMI together. In a multigroup meta-analysis of adults (29, 30), a significant effect of the application of probiotic ingredients in reducing body weight and BMI was observed. Adolescents are in a special growth and development stage, and during probiotic intervention tracking, adolescents are often accompanied by a simultaneous increase in weight and height, so we did not directly observe a clear decrease in body weight through probiotic intervention, but the decrease in BMI also indirectly reflected the benign effect of probiotics on body weight, and meaningful meta-results were obtained.
In this study, we found that probiotic intervention significantly reduced serum levels of CRP in overweight/obese adolescent patients, while it had no significant effect on IL-6, TNF-α, or FC. CRP, IL-6, TNF-α, and FC are indicators used to reflect the level of inflammation. The low-grade inflammatory response is a key factor in the development of obesity, and many studies have demonstrated that obese patients have an increase in proinflammatory cytokines (31). Previous studies on the effects of probiotic supplementation on inflammatory markers in adult obese patients have also found significant reductions in CRP (32). To our knowledge, this is the first meta-analysis to specifically assess the effect of probiotic supplementation on inflammatory markers in obese adolescents and observe a significant reduction in CRP, and more multicenter, larger sample size clinical trials are needed to demonstrate the efficacy of probiotic supplementation on inflammatory markers in obese adolescents, which in turn can help better guide clinical practice.
Our study found that probiotic intervention had no effect on improving insulin levels or insulin resistance in overweight/obese adolescent patients. Due to significant heterogeneity in the results of the effect on fasting blood glucose, subgroup analyses were performed to show that the heterogeneity was attenuated by grouping based on the type of intervention and that a reduction in fasting blood glucose was observed in the group supplemented with probiotics alone. In some previous studies, the same benefits of probiotic supplementation on glycemic control in patients were found (33–35). The mechanism may be related to the regulation of glucose metabolism by intestinal flora and its products, for example: 1. Probiotics can increase the secretion of glucagon-like peptide 1 (GLP-1) in the body. GLP-1 is an endogenous gut hormone secreted by L-cells that is key to promoting insulin secretion through entero-proteinotropic effects (36). Several studies have demonstrated the efficacy of GLP-1 agonists (liraglutide) for weight loss in pediatric patients (37). Specifically, probiotics promote GLP-1 secretion through three pathways. First, probiotics are able to produce short-chain fatty acids (SCFAs) by fermenting dietary fiber in the diet, which can promote GLP-1 production (38). Second, probiotics can also indirectly stimulate GLP-1 secretion by fermenting indigestible polysaccharides (39). Third, probiotics convert primary bile acids into secondary bile acids, which activate Takeda G protein receptor 5 and subsequently stimulate GLP-1 secretion (40). 2. Probiotics can improve mitochondrial damage. In animal experiments, lactobacilli were found to improve the morphological structure of mitochondrial damage caused by hyperglycemia. Improved mitochondrial health restored fatty acid β-oxidation, thereby reducing fatty acid accumulation in the liver and improving systemic glucose metabolism (41).
In this study, we found that probiotic intervention adversely affected TC, TG, and LDL in overweight/obese adolescent patients. Because of the significant heterogeneity in the combination of HDL, we further performed sensitivity analyses, and although the results showed that the exclusion of Famouri’s study reduced the heterogeneity, the study did not have a high risk of bias and did not find clinical, methodological or statistical heterogeneity, so we concluded that we could not exclude the data from this group of studies. The final results showed no effect of probiotic intervention on HDL in overweight/obese adolescent patients. Dyslipidemia is an important risk factor for obesity, which may involve elevated levels of TC, LDL, and TG and decreased levels of HDL (42). Past studies have shown that probiotics can improve lipid disorders, such as lowering blood cholesterol levels and improving the antioxidant capacity of LDL (43). Several mechanisms have been proposed for probiotics to lower cholesterol by controlling cholesterol metabolism: 1. Enzymatic action through bile salt hydrolase (BSH) of probiotics. BSH is an enzyme that catalyzes the hydrolysis of glycine and/or taurine-coupled bile salts to amino acid residues and free bile acids (44). Since coupled bile salts are more hydrophilic and are readily absorbed by the gastrointestinal tract, free bile acids are less soluble than coupled bile salts, are less likely to be reabsorbed by the intestinal tract and are more likely to be excreted in the feces (44), which will increase the need to synthesize new bile acids to replace those lost. Since cholesterol is a precursor for the resynthesis of new bile acids, the use of cholesterol to synthesize new bile leads to a decrease in blood cholesterol concentration. 2. The anabolic effect of probiotics on cholesterol in the small intestine can lower serum cholesterol by reducing the absorption of cholesterol in the intestine (45). Some probiotics can produce exopolysaccharides that adhere to cell surfaces and can absorb cholesterol (45). Very little of the cholesterol that is absorbed by the bacterial cells during growth in the small intestine is absorbed by the enterohepatic circulation (43) and thus may lead to lower serum cholesterol in humans. 3. Probiotics can also incorporate cholesterol into cell membranes, lowering blood cholesterol levels (46). 4. Probiotics convert cholesterol to fecal steroids through cholesterol reductase, thus reducing cholesterol absorption and eliminating it from the body with feces (47). 5. Probiotics inhibit cholesterol resynthesis by producing short-chain fatty acids (48). A previously conducted meta-analysis of the effects of a probiotic intervention on lipid profiles found significant reductions in TC, TG and LDL (49). In addition, there are still several studies (50–52) showing that probiotic supplementation in obese or overweight populations significantly reduces TC and LDL, but there is no significant difference in changes in HDL and TG. All these studies have confirmed the beneficial effects of probiotics in regulating the lipid profile, whereas our study found that probiotic intervention had an adverse effect on the regulation of lipids, which is contrary to previous conclusions. To determine the reason for this result, we further analyzed the trials included in the present study and found that the hypolipidemic effect of probiotics was found in all the studies, except for the studies of Gobel’s and Yildirim’s, which did not find any effect of probiotics on lipids. Therefore, we analyzed that the reason for this result may be because for the randomized controlled trials, we considered that the difference between the baseline level of the test group and the control group before the intervention was not statistically significant, so the data of the studies included in the meta-analysis were the data after the intervention of the two groups, whereas the results of the original studies usually compare the difference in the value of the change between the test group and the control group before and after the treatment, and due to the small number of related studies at the moment, for the results of the study, we still need to discuss the credibility of the results of the study further. With differences in experimental design, participant characteristics, and probiotic strains used between studies also contributing to differences in trial results, more well-designed RCTs are still needed to conclusively determine whether the use of probiotic strains is effective in improving lipid levels in obese adolescents.
Thus far, we searched for a meta-analysis (28) published in 2019 on the effectiveness of probiotic interventions on overweight/obesity in adolescents, which applied the means and standard deviations of the values of changes in anthropometric and metabolic indices at baseline and at the end of the intervention and did not find any good effect of the application of probiotic-based supplements in terms of the improvement of waist circumference, body weight, body fat percentage, fasting glucose, and lipid profile (LDL, HDL, TC, TG). After reviewing the relevant literature, we concluded that the included studies were randomized controlled trials, that there were no significant differences in the effect indicators at baseline, so postintervention means and standard deviations were applied, and that the meta-analysis described above was published in 2019, whereas we reviewed relevant RCTs up to 2023. Therefore, there were some differences in the included RCTs, and unlike the results of the above studies, in addition to the above effect indicators, we analyzed the effects of probiotics on parameters including BMI, WHR, insulin, and HOMA-IR, CRP, IL-6, TNF-α, and FC and found a beneficial effect of probiotic interventions in decreasing the effect indicators, such as BMI, FBG, and CRP, in adolescent obese patients. From this, we concluded that probiotic interventions may have a positive role in improving metabolic and inflammatory markers in adolescent obese patients. The main limitations of our meta-analysis included the diversity of probiotic interventions used in different studies, the subgroups of studies and the duration of participant follow-up, and due to the small number of studies, we were unable to group studies by probiotic strain and duration of intervention follow-up. In addition, the effect of confounding variables, including dietary patterns and lifestyle, intraindividual strain differences, and individual genotypes, on the effectiveness of probiotic interventions is unknown, and future studies may analyze this in more detail based on patient and intervention characteristics.
5 Conclusion
According to our results, probiotic supplementation was beneficial in managing metabolic indicators such as fasting blood glucose, body mass index and inflammation-related C-reactive protein in overweight or obese adolescents. Further large scale studies are warranted to confirm present findings and to identify the effects and mechanisms to provide more precise evidence for clinical intervention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
YD: Writing – original draft. LN: Writing – original draft. XZ: Writing – review & editing. LW: Writing – review & editing. YM: Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Shandong Province of China (Grant No. ZR2017LH023); Higher Educational Science and Technology Program of Shandong Province, China (Grant No. J17KA246); Shandong Provincial Key Laboratory of Endocrinology and Lipid Metabolism (Grant No. SDkeylab-Endo&LiMe2019-01); Medical as well as Academic Promotion Program of Shandong First Medical University (Grant No. 2019QL017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics (2018) 141(3):e20181916. doi: 10.1542/peds.2017-3459
2. Garnett SP, Baur LA, Jones AM, Hardy LL. Trends in the prevalence of morbid and severe obesity in Australian children aged 7-15 years, 1985-2012. PloS One (2016) 11(5):e0154879. doi: 10.1371/journal.pone.0154879
3. Lee EY, Yoon KH. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med (2018) 12(6):658–66. doi: 10.1007/s11684-018-0640-1
4. Horesh A, Tsur AM, Bardugo A, Twig G. Adolescent and childhood obesity and excess morbidity and mortality in young adulthood-a systematic review. Curr Obes Rep (2021) 10(3):301–10. doi: 10.1007/s13679-021-00439-9
5. Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, et al. Gut microbiota and obesity: A role for probiotics. Nutrients (2019) 11(11):2690. doi: 10.3390/nu11112690
6. Preveden T, Scarpellini E, Milic N, Luzza F, Abenavoli L. Gut microbiota changes and chronic hepatitis C virus infection. Expert Rev Gastroenterol Hepatol (2017) 11(9):813–9. doi: 10.1080/17474124.2017.1343663
7. Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature (2013) 500(7464):585–8. doi: 10.1038/nature12480
8. Thiennimitr P, Yasom S, Tunapong W, Chunchai T, Wanchai K, Pongchaidecha A, et al. Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition (2018) 54:40–7. doi: 10.1016/j.nut.2018.03.005
9. Green M, Arora K, Prakash S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci (2020) 21(8):2890. doi: 10.3390/ijms21082890
10. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr (2006) 83(6):1256–64. doi: 10.1093/ajcn/83.6.1256
12. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. 1996. Clin Orthop Relat Res (2007) 455:3–5.
13. Guyatt GH, Sackett DL, Sinclair JC, Hayward R, Cook DJ, Cook RJ. Users' guides to the medical literature. IX. A method for grading health care recommendations. Evidence-Based Med Working Group JAMA. (1995) 274(22):1800–4. doi: 10.1001/jama.1995.03530220066035
14. Walker E, Hernandez AV, Kattan MW. Meta-analysis: Its strengths and limitations. Cleve Clin J Med (2008) 75(6):431–9. doi: 10.3949/ccjm.75.6.431
15. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ (1997) 315(7121):1533–7. doi: 10.1136/bmj.315.7121.1533
16. Yildirim G, Dinleyici M, Vandenplas Y, Dinleyici EC. Effects of multispecies synbiotic supplementation on anthropometric measurements, glucose and lipid parameters in children with exogenous obesity: A randomized, double blind, placebo-controlled clinical trial (Probesity-2 trial). Front Nutr (2022) 9:898037. doi: 10.3389/fnut.2022.898037
17. Verma A, Nelson MT, DePaolo WR, Hampe C, Roth CL. A randomized double-blind placebo controlled pilot study of probiotics in adolescents with severe obesity. J Diabetes Metab Disord (2021) 20(2):1289–300. doi: 10.1007/s40200-021-00855-7
18. Jones RB, Alderete TL, Martin AA, Geary BA, Hwang DH, Palmer SL, et al. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: a 16-week, randomized, placebo-controlled trial. Pediatr Obes (2018) 13(11):705–14. doi: 10.1111/ijpo.12273
19. Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr (2017) 64(3):413–7. doi: 10.1097/MPG.0000000000001422
20. Ipar N, Aydogdu SD, Yildirim GK, Inal M, Gies I, Vandenplas Y, et al. Effects of synbiotic on anthropometry, lipid profile and oxidative stress in obese children. Benef Microbes (2015) 6(6):775–82. doi: 10.3920/BM2015.0011
21. Kelishadi R, Farajian S, Safavi M, Mirlohi M, Hashemipour M. A randomized triple-masked controlled trial on the effects of synbiotics on inflammation markers in overweight children. Jornal Pediatria. (2014) 90(2):161–8. doi: 10.1016/j.jped.2013.07.003
22. Safavi M, Farajian S, Kelishadi R, Mirlohi M, Hashemipour M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: A randomized triple-masked controlled trial. Int J Food Sci Nutr (2013) 64(6):687–93. doi: 10.3109/09637486.2013.775224
23. Gobel RJ, Larsen N, Jakobsen M, Molgaard C, Michaelsen KF. Probiotics to adolescents with obesity: effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr (2012) 55(6):673–8. doi: 10.1097/MPG.0b013e318263066c
24. Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, et al. FAO Technical meeting on prebiotics. J Clin Gastroenterol (2008) 42 Suppl 3 Pt 2:S156–9. doi: 10.1097/MCG.0b013e31817f184e
25. Cerdo T, Garcia-Santos JA G, Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients (2019) 11(3):635. doi: 10.3390/nu11030635
26. Markowiak P, Slizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients (2017) 9(9):1021. doi: 10.3390/nu9091021
27. Saez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int J Mol Sci (2016) 17(6):928. doi: 10.3390/ijms17060928
28. Mohammadi H, Ghavami A, Hadi A, Askari G, Symonds M, Miraghajani M. Effects of pro-/synbiotic supplementation on anthropometric and metabolic indices in overweight or obese children and adolescents: A systematic review and meta-analysis. Complement Ther Med (2019) 44:269–76. doi: 10.1016/j.ctim.2019.05.008
29. Borgeraas H, Johnson LK, Skattebu J, Hertel JK, Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev (2018) 19(2):219–32. doi: 10.1111/obr.12626
30. Wang ZB, Xin SS, Ding LN, Ding WY, Hou YL, Liu CQ, et al. The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: A systematic review and meta-analysis. Evid Based Complement Alternat Med (2019) 2019:3862971. doi: 10.1155/2019/3862971
31. Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr (2019) 10(suppl_1):S17–30. doi: 10.1093/advances/nmy078
32. Mazidi M, Rezaie P, Ferns GA, Vatanparast H. Impact of probiotic administration on serum C-reactive protein concentrations: systematic review and meta-analysis of randomized control trials. Nutrients (2017) 9(1):20. doi: 10.3390/nu9010020
33. Ruan Y, Sun J, He J, Chen F, Chen R, Chen H. Effect of probiotics on glycemic control: A systematic review and meta-analysis of randomized, controlled trials. PloS One (2015) 10(7):e0132121. doi: 10.1371/journal.pone.0132121
34. Samah S, Ramasamy K, Lim SM, Neoh CF. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract (2016) 118:172–82. doi: 10.1016/j.diabres.2016.06.014
35. Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: A meta-analysis of 12 randomized controlled trials. Med Sci Monit (2017) 23:3044–53. doi: 10.12659/MSM.902600
36. Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi XH. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. BioMed Pharmacother. (2020) 125:109914. doi: 10.1016/j.biopha.2020.109914
37. Singhal S, Kumar S. Current perspectives on management of type 2 diabetes in youth. Children (Basel). (2021) 8(1):37. doi: 10.3390/children8010037
38. Burcelin R, Serino M, Chabo C, Blasco-Baque V, Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol (2011) 48(4):257–73. doi: 10.1007/s00592-011-0333-6
39. Okeke F, Roland BC, Mullin GE. The role of the gut microbiome in the pathogenesis and treatment of obesity. Glob Adv Health Med (2014) 3(3):44–57. doi: 10.7453/gahmj.2014.018
40. Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med (2018) 215(2):383–96. doi: 10.1084/jem.20171965
41. Rodrigues RR, Gurung M, Li Z, Garcia-Jaramillo M, Greer R, Gaulke C, et al. Transkingdom interactions between Lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes. Nat Commun (2021) 12(1):101. doi: 10.1038/s41467-020-20313-x
42. Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med (2017) 167(11):ITC81–96. doi: 10.7326/AITC201712050
43. Lye HS, Kuan CY, Ewe JA, Fung WY, Liong MT. The improvement of hypertension by probiotics: effects on cholesterol, diabetes, renin, and phytoestrogens. Int J Mol Sci (2009) 10(9):3755–75. doi: 10.3390/ijms10093755
44. Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes (2016) 7(1):22–39. doi: 10.1080/19490976.2015.1127483
45. Pigeon RM, Cuesta EP, Gililliand SE. Binding of free bile acids by cells of yogurt starter culture bacteria. J Dairy Sci (2002) 85(11):2705–10. doi: 10.3168/jds.S0022-0302(02)74357-9
46. Taghizadeh M, Asemi Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: a randomized controlled clinical trial. Hormones (Athens). (2014) 13(3):398–406. doi: 10.14310/horm.2002.1489
47. Lye HS, Rusul G, Liong MT. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J Dairy Sci (2010) 93(4):1383–92. doi: 10.3168/jds.2009-2574
48. Naito E, Yoshida Y, Kunihiro S, Makino K, Kasahara K, Kounoshi Y, et al. Effect of Lactobacillus casei strain Shirota-fermented milk on metabolic abnormalities in obese prediabetic Japanese men: a randomised, double-blind, placebo-controlled trial. Biosci Microbiota Food Health (2018) 37(1):9–18. doi: 10.12938/bmfh.17-012
49. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis. Clin Nutr (2018) 37(2):532–41. doi: 10.1016/j.clnu.2017.02.015
50. Yan S, Tian Z, Li M, Li B, Cui W. Effects of probiotic supplementation on the regulation of blood lipid levels in overweight or obese subjects: a meta-analysis. Food Funct (2019) 10(3):1747–59. doi: 10.1039/C8FO02163E
51. Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med (2015) 47(6):430–40. doi: 10.3109/07853890.2015.1071872
52. Pourrajab B, Fatahi S, Dehnad A, Kord VH, Shidfar F. The impact of probiotic yogurt consumption on lipid profiles in subjects with mild to moderate hypercholesterolemia: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis (2020) 30(1):11–22. doi: 10.1016/j.numecd.2019.10.001
Keywords: probiotic, obesity, overweight, adolescent, meta-analysis
Citation: Duan Y, Wang L, Ma Y, Ning L and Zhang X (2024) A meta-analysis of the therapeutic effect of probiotic intervention in obese or overweight adolescents. Front. Endocrinol. 15:1335810. doi: 10.3389/fendo.2024.1335810
Received: 09 November 2023; Accepted: 08 January 2024;
Published: 30 January 2024.
Edited by:
Ningning Hou, Affiliated Hospital of Weifang Medical University, ChinaReviewed by:
Shuiqiao Liu, University of Texas Southwestern Medical Center, United StatesLi Zhang, Indiana Biosciences Research Institute, United States
Copyright © 2024 Duan, Wang, Ma, Ning and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinhuan Zhang, a2F0aHkwNDE4QDE2My5jb20=; Lei Ning, RnlubDc5QDEyNi5jb20=
†These authors contributed equally to this work
 Yuanqing Duan1†
Yuanqing Duan1† Xinhuan Zhang
Xinhuan Zhang