- 1The First Clinical Institute, Zunyi Medical University, Zunyi, China
- 2Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 3Department of Endocrinology, Dongying City District People Hospital, Dongying, China
- 4Department of Endocrinology and Metabolism, Dongying People's Hospital, Dongying, China
- 5Department of Endocrinology, The First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou, China
- 6Department of Endocrinology, Shandong Provincial Hospital, Shandong University, Jinan, China
Objective: The aim of this study was to evaluate the admission indicators and characteristics of individuals diagnosed with type 1 diabetes (T1D) to ascertain potential impact on the choice of glucose control therapy after discharge.
Methods: A total of 398 eligible T1D patients were selected. We conducted multivariable logistic regression analysis to determine the independent influence of predictors on the selection of glucose control therapy after discharge. To explore the influencing factors of different subgroups, we additionally performed subgroup analyses based on gender and age.
Results: Our study revealed that body mass index (BMI) was noteworthy influence factor for prescription of insulin and non-insulin antidiabetic drug (NIAD prescription) in T1D patients of general population [OR = 1.109 (1.033-1.195), p = 0.006], male [OR = 1.166 (1.040−1.318), p = 0.011] and individuals below the age of 30 years [OR = 1.146 (1.020−1.301), p = 0.028]. Diastolic blood pressure (DBP) was a protective factor for NIAD prescription in the general population [OR = 0.971 (0.949-0.992), p = 0.008] and women [OR = 0.955 (0.923−0.988), p = 0.008]. The other risk factor of NIAD prescription in men was dyslipidemia [OR = 4.824 (1.442−22.246), p = 0.020]. Pulse pressure [OR = 1.036 (1.007–1.068), p = 0.016] constituted an additional risk factor of NIAD prescription among individuals below the age of 30 years. The risk factors of NIAD prescription for people aged 30 to 50 years were length of stay [OR = 1.097 (1.014–1.196), p = 0.026] and initial blood glucose [OR = 1.078 (1.007–1.168), p = 0.047]. In the case of individuals aged above 50 years, physicians exhibited a higher tendency to prescribe supplementary non-insulin medications to men [OR = 9.385 (1.501–87.789), p = 0.029].
Conclusions: We identified notable factors that influence discharge prescriptions in patients with T1D. In order to enhance the treatment outcome for the patient, clinicians ought to have a special focus on these indicators or factors.
1 Introduction
Type 1 diabetes (T1D) is an insulin-dependent condition caused by the destruction of pancreatic β-cells, which causes insufficient insulin secretion in the body (1). Approximately 5% to 10% of diabetes cases worldwide are type 1, but its incidence has increased over the past few decades (2, 3). At present, exogenous insulin substitution therapy has become the main treatment for T1D. The systemic adverse effects associated with insulin therapy primarily encompass hypoglycemia, weight gain, edema, refractive error, and anaphylaxis (4).
Hypoglycemia makes it less easy to achieve good results through intensive therapy (5). In addition, intensive therapy can also lead to an increased prevalence of obesity in individuals with T1D (6). Chronic pro-inflammation induced by obesity contributes to the development of insulin resistance (7). A considerable number of patients continue to fall short of attaining optimal glycemic control (8). Several studies have demonstrated the efficacy of metformin in the maintenance or reduction of weight in patients with T1D, as well as its potential to decrease insulin dosages (9–11). GLP-1 receptor agonists (12–14) and sodium-glucose cotransporter 2 inhibitors (15–18) have exhibited effectiveness in facilitating weight loss and diminishing insulin dosage, while also enhancing glycated hemoglobin levels (HbA1c), albeit concurrently elevating the likelihood of ketosis. Non-insulin drugs are not typically recommended in clinical practice, and treatment should be based on insulin therapy and individual patient needs, but what makes a clinician decide to use non-insulin medication? We do not know yet.
People diagnosed with T1D usually require hospital admission due to the occurrence of a sudden onset or certain acute complication (4). Do certain indicators or characteristics observed at admission impact the selection of the discharge treatment plan? The prognoses of T1D have long been different between different genders and ages (19–21). Do these indicators or characteristics have different effects on the choice for different gender and age groups? Hence, a study was undertaken with the primary objective of assessing the admission indicators or characteristics of individuals diagnosed with T1D, aiming to determine their potential influence on the therapy of glucose control upon discharge. As a result, healthcare practitioners are encouraged to prioritize these indicators or characteristics to optimize treatment plans and improve patient outcomes during therapy.
2 Materials and methods
2.1 Study subjects
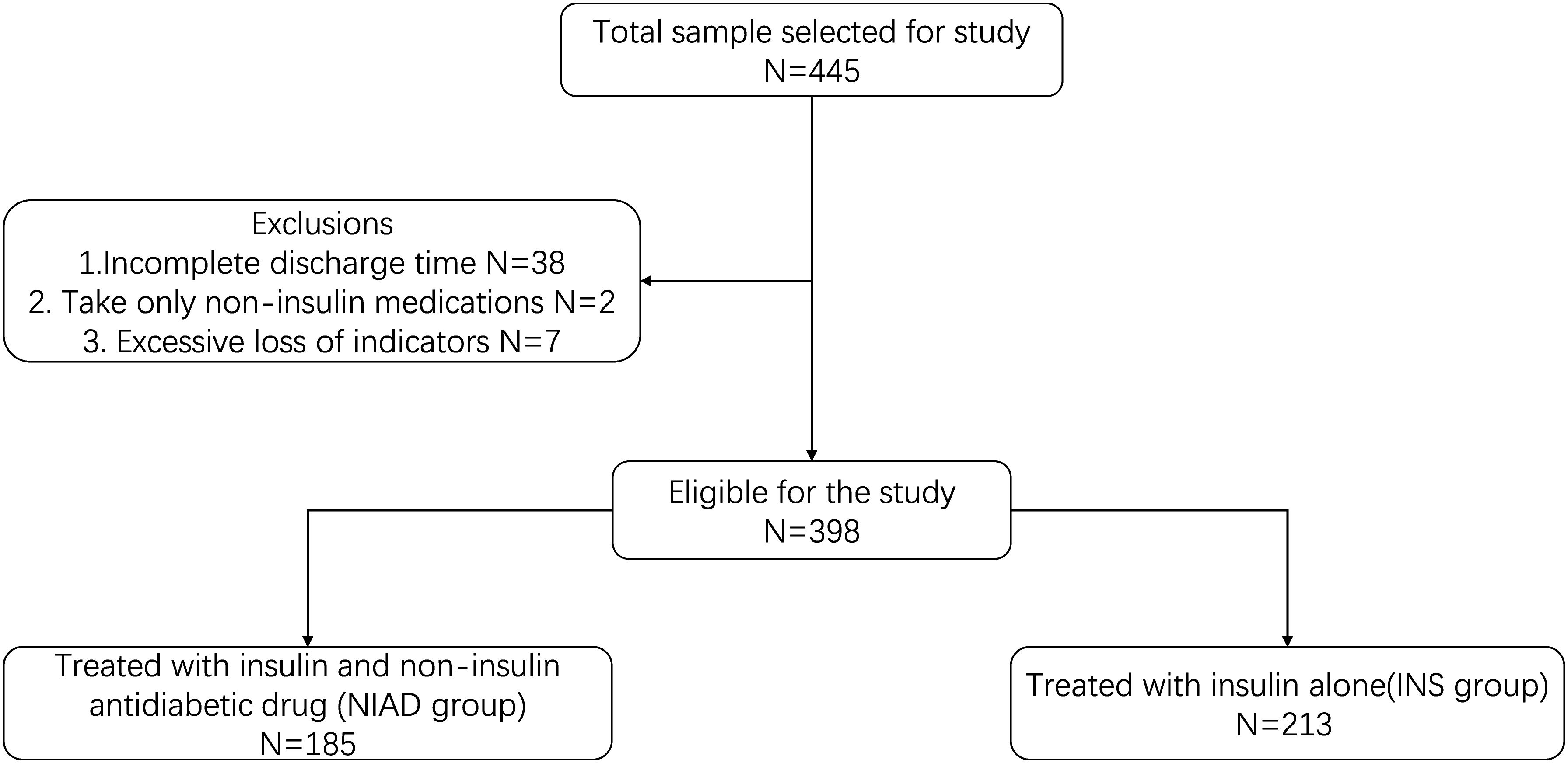
We first selected 445 T1D patients hospitalized in Shandong Provincial Hospital affiliated to Shandong First Medical University (Jinan, China) from January 2008 to December 2018. In the case of multiple hospital records, it is customary to retain only one. Patients with too many missing indicators or features would also be excluded from the study. Finally, this retrospective study comprised a total of 398 patients selected based on exclusion criteria. We conducted a comparative analysis between the group treated with insulin and non-insulin antidiabetic drug (NIAD group) and the group treated with insulin alone (INS group).
This retrospective study used anonymized data with no need for obtaining informed consent from each patient. The Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University approved the project.
2.2 Measurements
The researchers obtained all data from the archival hospital records of patients admitted to Shandong Provincial Hospital affiliated to Shandong First Medical University. Upon admission, healthcare professionals perform a comprehensive assessment, including a thorough medical history review, physical examination, and analysis of blood samples. The calculation of BMI (kg/m2) involves dividing weight by the square of height. Blood pressure was measured following a 5-min period of rest using an electronic sphygmomanometer (HEM-7117; Omron, Kyoto, Japan). The total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), glutamyl transferase (GGT), aspartate aminotransferase (AST), glutamic pyruvic transaminase (ALT), blood glucose, albumin, potassium (K), sodium (Na), chlorine (CI), calcium (Ca), and phosphorus (P) were analyzed using an automatic biochemistry analyzer (Beckman Coulter Analyzer AU58 Series, USA). Glycated hemoglobin (HbA1c) levels were measured using high-performance liquid chromatography (HPLC) with a hemoglobin A1c analyzer manufactured by TOSOH Corporation, Japan. Hemoglobin (Hb) levels were analyzed using a blood cell analyzer (Sysmex Corporation XN-2100, Japan).
2.3 Statistical analysis
Initially, descriptive statistical methods were employed to provide a comprehensive summary of the clinical characteristics and biological indicators observed in the patients. The Kolmogorov–Smirnov test is used to assess the normality of continuous variables prior to conducting parameter tests. Continuous parameters that follow normal distribution are presented as the mean ± standard deviation (SD). Continuous parameters that do not conform to a normal distribution are presented as the median within the interquartile range. Categorical variables are presented in the form of percentages. The entire sample was partitioned into the NIAD group and INS group in order to conduct statistical analysis and construct a model. In order to display the distinction between the NIAD group and the INS group, the Mann–Whitney U-test was employed for continuous variables that do not conform to a normal distribution. The chi-square test or Fisher’s exact test was used to examine the categorical variables.
We conducted multivariable logistic regression analysis to ascertain the independent impact of predictors on the selection of blood glucose control program upon discharge among patients with T1D. The predictors of interest, including age, sex, BMI, smoking history, drinking history, family history of diabetes, dyslipidemia, osteoporosis, SBP, DBP, fatty liver, length of stay and initial blood glucose, are sequentially incorporated into the multivariate logistic regression model. To investigate potential variations in influencing factors across distinct genders and age groups, we additionally developed stepwise logistic regression models for each gender and age group. A two-sided p-value < 0.05 was considered statistically significant. R software (version 4.2.2) was used for all statistical analyses.
3 Results
This study enrolled 398 (NIAD: 185; INS: 213) patients with T1D (Figure 1). The median age of the study population was 30 (21–44). Male patients accounted for approximately 45.72% of the study population. The median length of stay (LOS) was 11 (8–14).
After analysis of the baseline characteristics of the patients, variables with statistical differences between the NIAD and INS groups included sex (p < 0.01), age (p < 0.01), diabetic peripheral vascular disease (p < 0.01), diabetic retinopathy (p = 0.02), fatty liver (p = 0.03), drinking history (p < 0.01), initial blood glucose (p = 0.01), BMI (p < 0.01), Hb (p = 0.03), GGT (p = 0.02) , LOS (p < 0.01) (Table 1).
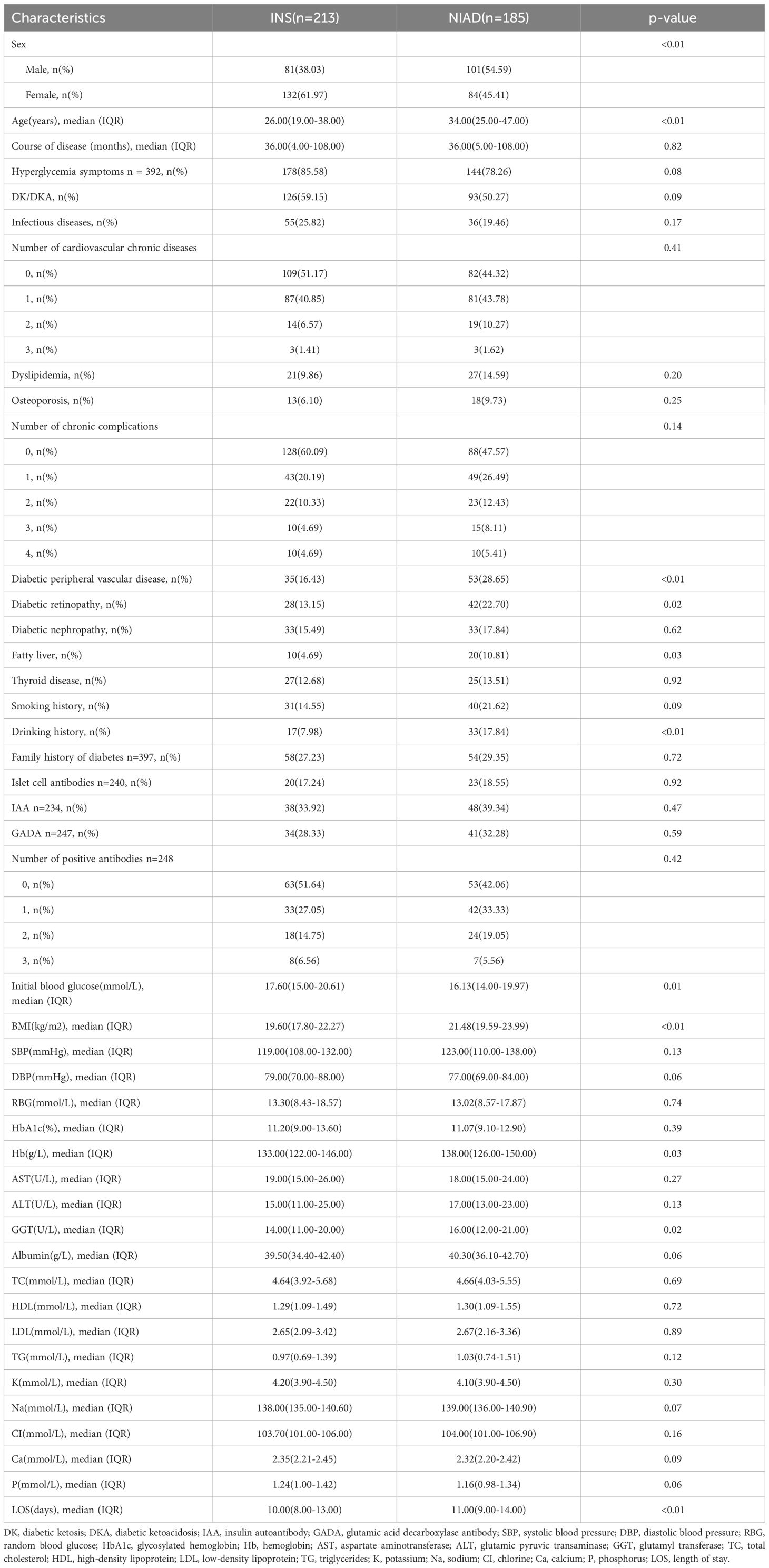
Table 1. Baseline study population characteristics stratified by insulin and non-insulin antidiabetic drugs (NIAD group) and the group treated with insulin alone (INS group) at discharge.
In the overall multivariate logistic regression (Figure 2), NIAD prescription was associated with older age, male, and higher BMI (Model 1). After further adjustment for history of smoking and drinking, family history of diabetes, dyslipidemia, osteoporosis, SBP, DBP, not being prescribed NIAD was associated with DBP (Model 3). After final adjustment for initial blood glucose, older age no longer associated with NIAD prescription. Male [OR = 1.853 (1.120-3.079), p = 0.017] and higher BMI [OR = 1.109 (1.033-1.195), p = 0.006] were still associated with NIAD prescription, and DBP was a protective factor for NIAD prescription [OR = 0.971 (0.949-0.992), p = 0.008].

Figure 2. Odds ratio [95% confidence interval] of being discharged with insulin and non-insulin antidiabetic drug among patients with type 1 diabetes. SBP, systolic blood pressure; DBP, diastolic blood pressure; LOS, length of stay. *Statistical significance.
In the multivariate logistic regression analysis conducted on the male group (Figure 3), NIAD prescriptions continued to exhibit a significant association with elevated BMI [OR = 1.166 (1.040−1.318), p = 0.011] and dyslipidemia [OR = 4.824 (1.442−22.246), p = 0.020] after controlling for confounding variables. In the female population (Figure 4), not being prescribed NIAD was associated with DBP [OR = 0.955 (0.923−0.988), p = 0.008]. NIAD prescriptions exhibited a significant association with older age [OR = 1.026 (1.002−1.051), p = 0.038].
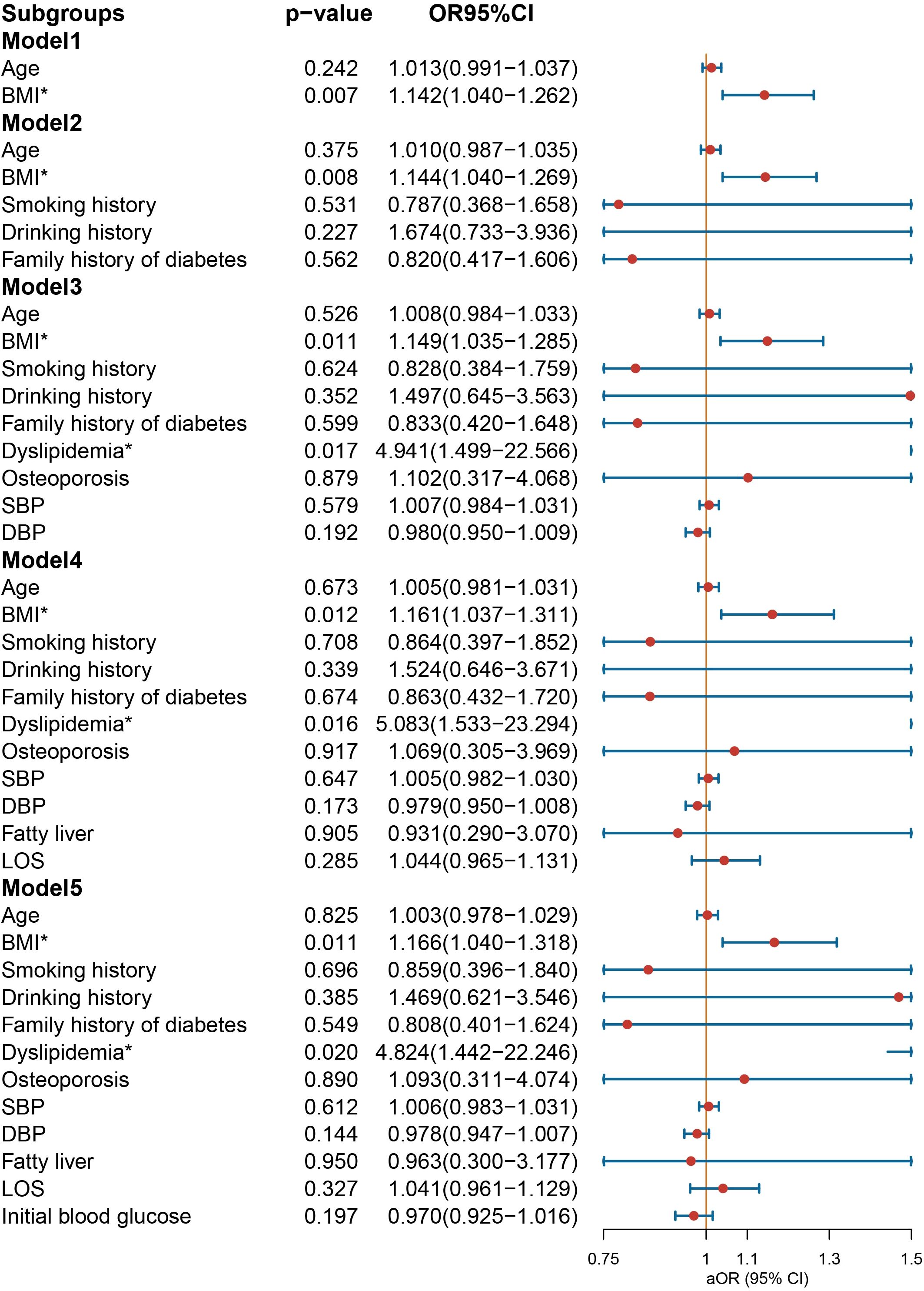
Figure 3. Odds ratio [95% confidence interval] of being discharged with insulin and non-insulin antidiabetic drug among male patients with type 1 diabetes. SBP, systolic blood pressure; DBP, diastolic blood pressure; LOS, length of stay. *Statistical significance.
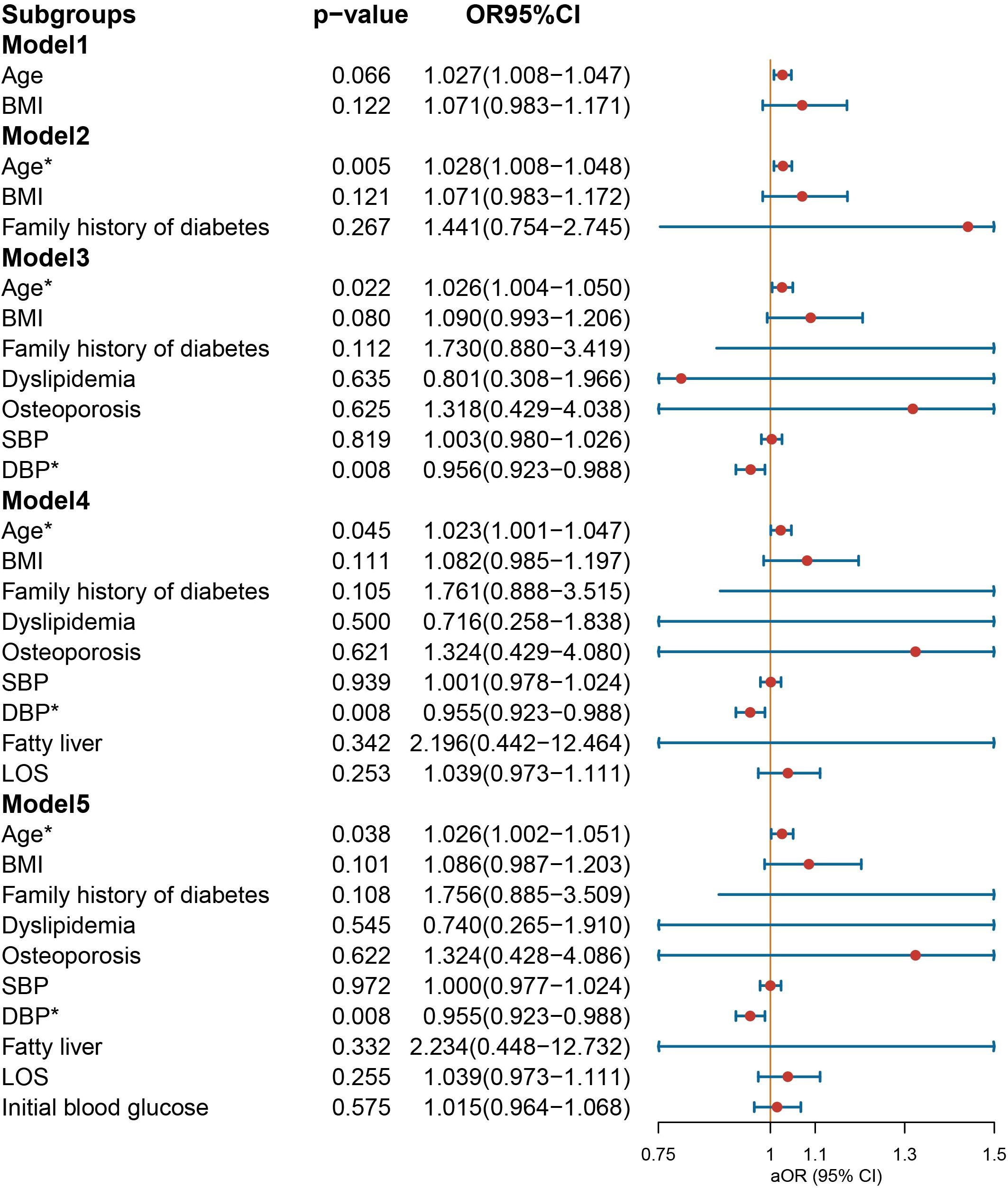
Figure 4. Odds ratio [95% confidence interval] of being discharged with insulin and non-insulin antidiabetic drug among female patients with type 1 diabetes. SBP, systolic blood pressure; DBP, diastolic blood pressure; LOS, length of stay. *Statistical significance.
In the context of age-stratified multivariate regression models, the variables of BMI [OR = 1.146 (1.020–1.301), p = 0.028] and SBP [OR = 1.035 (1.005–1.067), p = 0.022] demonstrated a significant association with the prescription of NIAD in individuals below the age of 30 years (Figure 5). However, DBP was a protective factor for NIAD prescription [OR = 0.943 (0.906–0.980), p = 0.004]. After adjusting for smoking history, drinking history and family history of diabetes in Model 2, the impact of gender was no longer statistically significant. In individuals aged above 30 and below 50 years (Figure 6), the prescription of NIAD was found to be associated with only LOS [OR = 1.097 (1.014–1.196), p = 0.026] and initial blood glucose [OR = 1.078 (1.007–1.168), p = 0.047]. Among individuals aged above 50 years (Figure 7), being male [OR = 9.385 (1.501–87.789), p = 0.029] was identified as a risk factor for the NIAD prescription after adjusting for fatty liver and LOS factors, whereas smoking history [OR = 0.037 (0.001–0.428), p = 0.021] was found to be a protective factor.
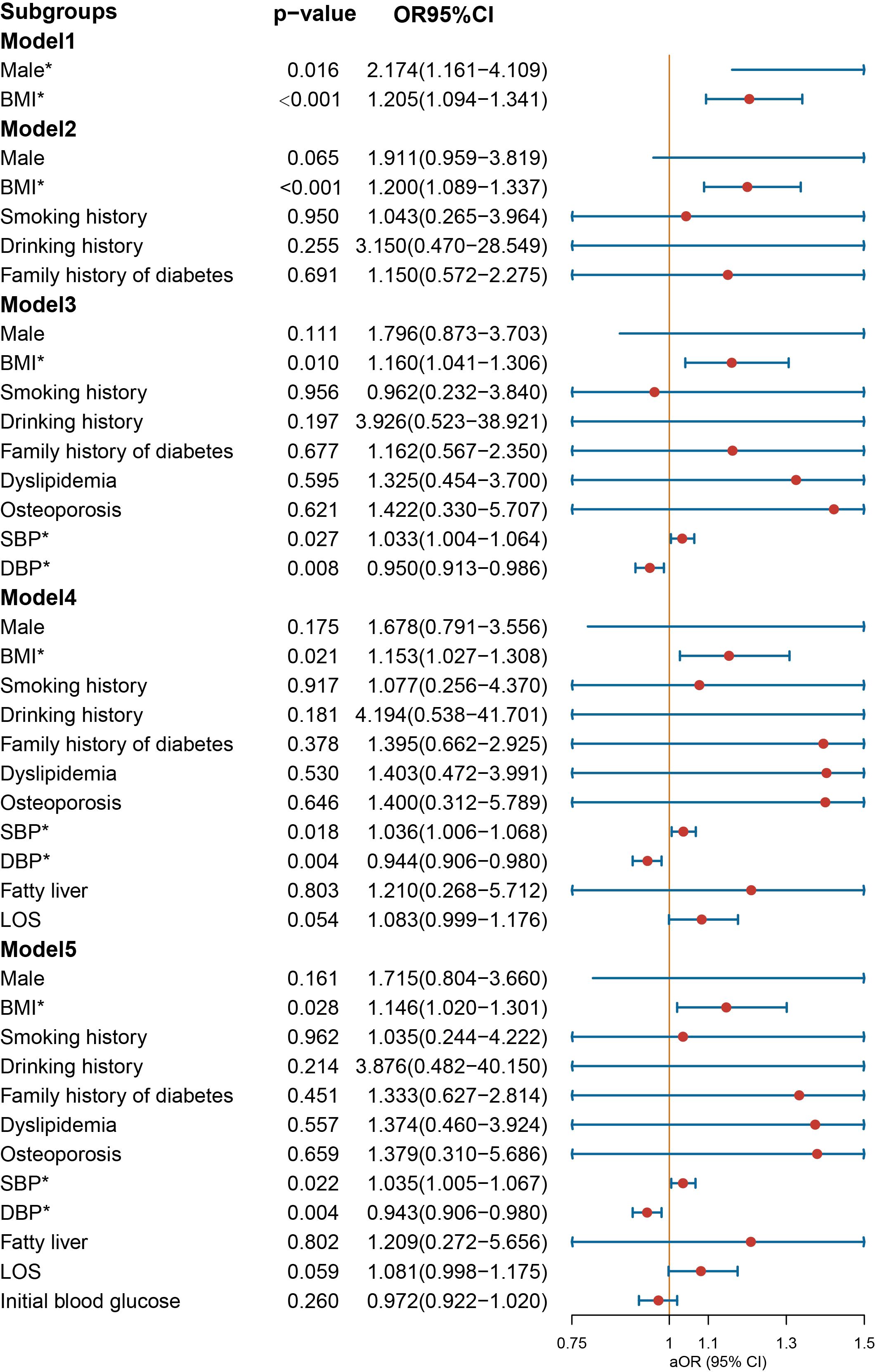
Figure 5. Odds ratio [95% confidence interval] of being discharged with insulin and non-insulin antidiabetic drug among patients below the age of 30 with type 1 diabetes. SBP, systolic blood pressure; DBP, diastolic blood pressure; LOS, length of stay. *Statistical significance.
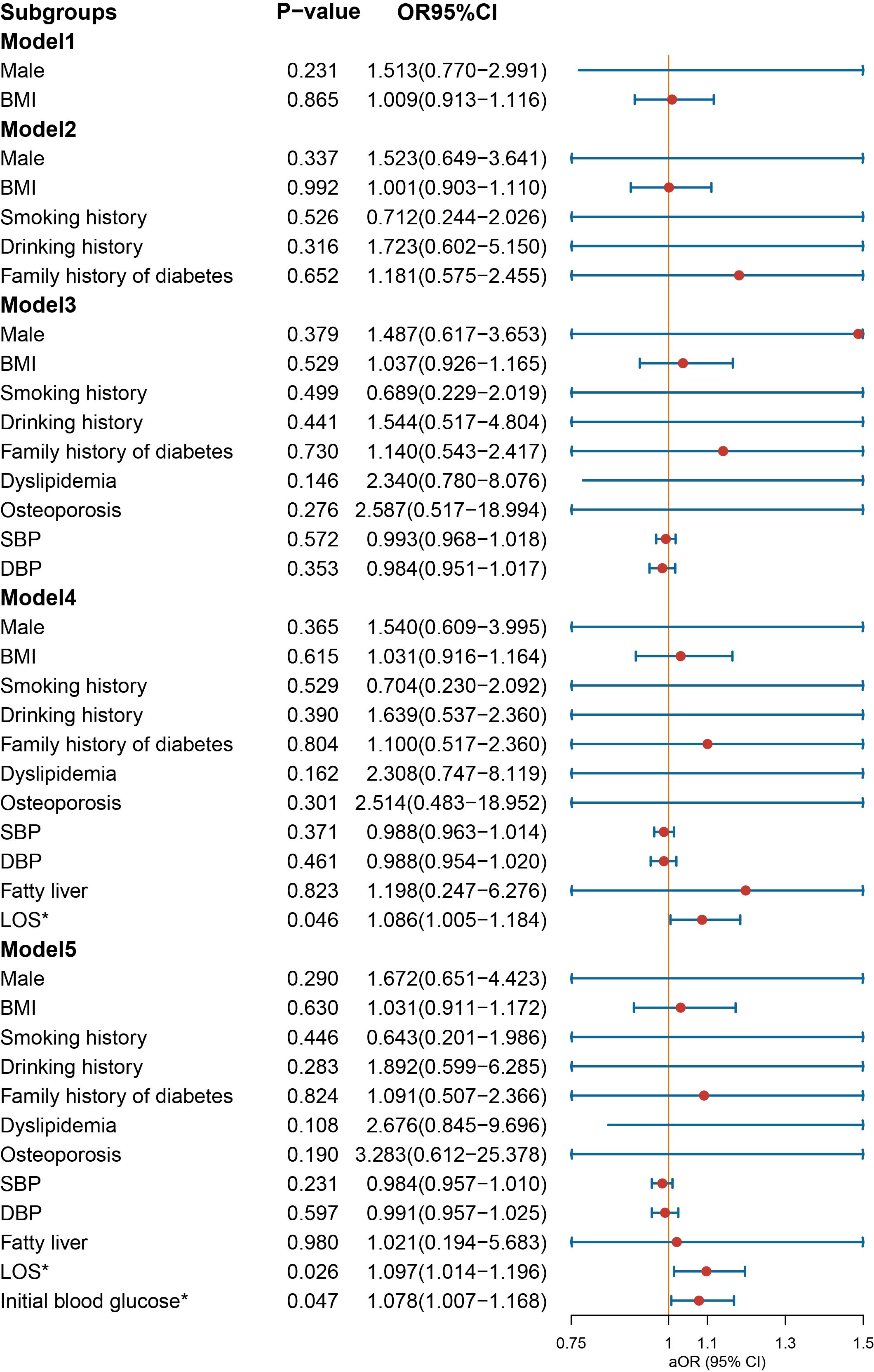
Figure 6. Odds ratio [95% confidence interval] of being discharged with insulin and non-insulin antidiabetic drug among patients aged above 30 and below 50 years with type 1 diabetes. SBP, systolic blood pressure; DBP, diastolic blood pressure; LOS, length of stay. *Statistical significance.
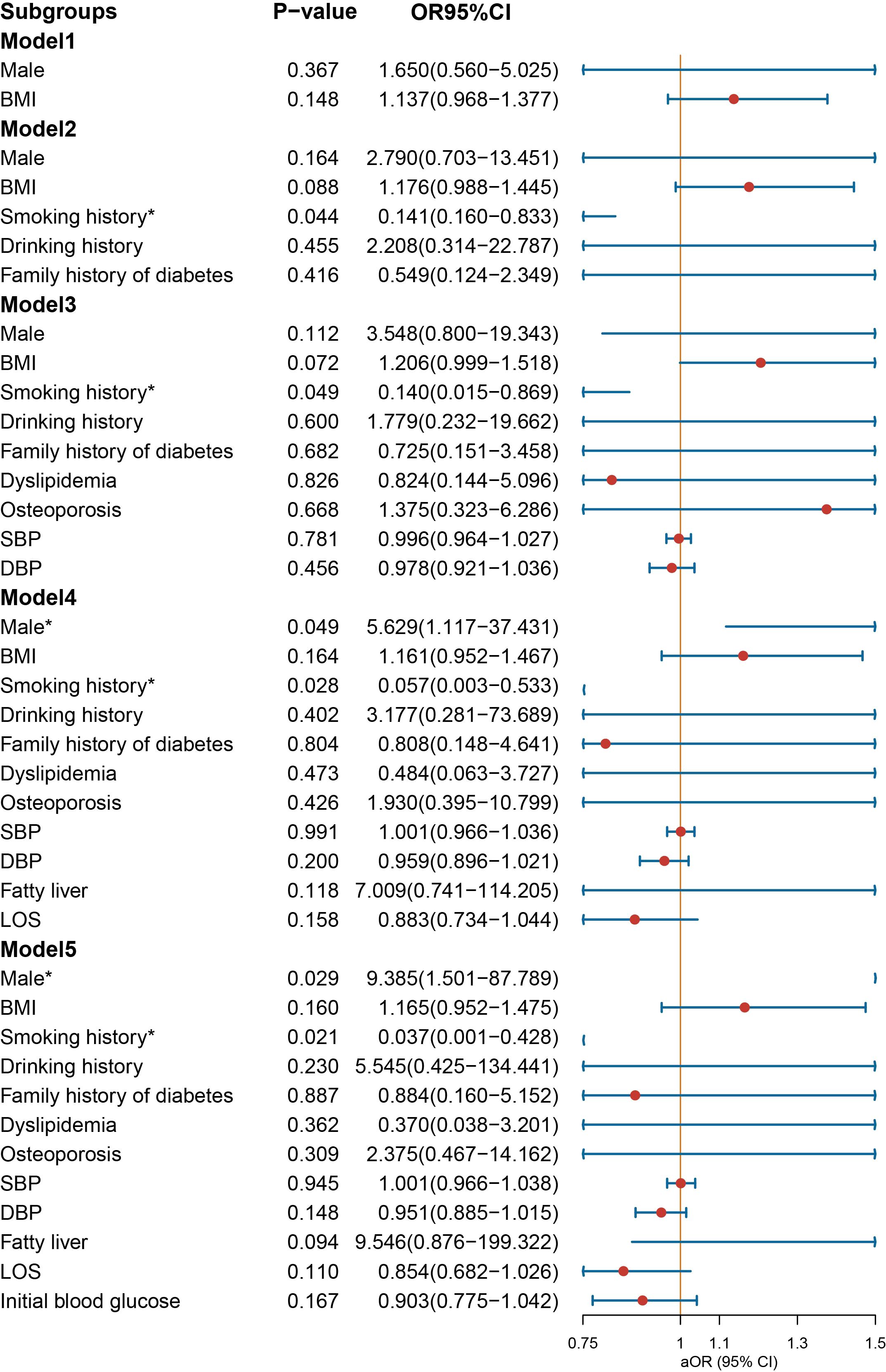
Figure 7. Odds ratio [95% confidence interval] of being discharged with insulin and non-insulin antidiabetic drug among patients aged above 50 with type 1 diabetes. SBP, systolic blood pressure; DBP, diastolic blood pressure; LOS, length of stay. *Statistical significance.
4 Discussion
In this study, we investigated the indicators and characteristics impacting clinicians’ choice of non-insulin hypoglycemic medications for individuals diagnosed with T1D. Differences exist in the primary influencing indicators and characteristics among various populations upon admission, which doctors should prioritize when selecting treatment options. To our knowledge, there is a lack of research on the influencing factors of discharge prescription for T1D.
In our study, we found that the higher the BMI, the more likely it was to receive additional non-insulin treatment at discharge. Similar results were observed in both male and individuals younger than 30 years. Chronic pro-inflammation induced by obesity contributes to the development of insulin resistance (7), which has a negative impact on our subsequent treatment. Intensive insulin therapy has been found to correlate with increased body weight (22), hindering effective weight management in patients. Insulin dose is largely determined by body weight. If patients are overweight and exhibit inadequate compliance, the administered insulin dosage may be insufficient, thereby increasing the likelihood of hyperglycemia. In order to minimize the occurrence of these events, clinicians are more likely to add non-insulin drugs.
We found an inverse relationship between DBP and the proportion of individuals choosing non-insulin drugs combined with insulin regimens. Significant results were also observed in women and individuals younger than 30 years. However, the average amplitude of glycemic excursions exhibited a significant independent correlation with the alteration in aortic diastolic blood pressure (23). Elevated DBP is a risk factor for retinopathy (24). Patients with diabetic complications were also more commonly observed to have higher DBP (25). Patients with diabetes complications appear to have greater blood glucose variability (26, 27), thus necessitating increased focus on the management of both blood glucose and blood pressure. However, the results of this study suggest that clinicians seem to have insufficient cognition, and larger studies are needed to support our hypothesis. Several studies have indicated that certain non-insulin drugs such as metformin (28), GLP-1 agonists (29, 30), and DPP-4 inhibitors (31) do not improve blood glucose variability and diastolic blood pressure level. Hence, physicians may show limited interest in non-insulin medications for individuals with elevated DBP, as these medications may not provide optimal supplementary control over both blood glucose and blood pressure.
We observed an intriguing trend among individuals younger than 30 years: DBP emerged as a protective factor for receiving a prescription for non-insulin medications, while SBP was identified as a risk factor. This finding is often difficult to explain. Thus, we reconstructed a model to investigate the impact of pulse pressure (PP) and other factors on the likelihood of receiving prescriptions for non-insulin drugs (Supplementary Figure 1). After adjusting for various factors, PP consistently remained stable across the several models and emerged as a significant risk factor influencing the outcome. PP serves as an estimate of arterial stiffness (32, 33) and is considered a risk factor for cardiovascular complications among patients with T1D (33, 34). Nevertheless, certain non-insulin medications, such as metformin, exhibit the ability to impede the progression of arterial thickening and exert a certain impact on the development of atherosclerosis (35, 36). Therefore, for patients with high pulse pressure, doctors are more likely to prescribe non-insulin drugs.
Insufficient glycemic control is associated with dyslipidemia (37); thus, additional non-insulin hypoglycemic drugs are necessary to improve blood glucose management. The administration of insulin therapy continues to present the drawback of unstable regulation of blood glucose levels (22, 31, 38). Simultaneously, obesity is linked to dyslipidemia (39), while the administration of insulin therapy may induce weight gain, thereby negatively impacting the regulation of blood lipids in patients. Nevertheless, many non-insulin hypoglycemic agents, including metformin (40), GLP1 agonists (41), and SGLT-2 inhibitors (42), have been shown to have favorable effects on lipid profiles. They may be the reason why T1D patients with dyslipidemia are more likely to obtain non-insulin drug prescriptions.
The length of hospital stay is usually related to the severity of the disease. Higher blood glucose levels at the initial diagnosis often correlate with weaker blood glucose control and necessitate larger insulin doses. Hence, it may be necessary for patients to use non-insulin medications in order to decrease their insulin dosage and ensure the efficacy of blood glucose regulation.
Several studies have shown that female patients exhibit higher insulin sensitivity compared to male patients (43–45). Although insulin sensitivity decreases with age in both genders, women tend to maintain comparatively higher insulin sensitivity than men (43). To some extent, this can explain why doctors are more likely to prescribe insulin for female patients. Smoking has been found to elevate the likelihood of cardiovascular disease among individuals with T1D due to its impact on blood glucose, blood lipids, and the facilitation of endothelial dysfunction (46). Non-insulin medications exhibit certain cardiovascular advantages (4, 28, 47), thereby proving beneficial for individuals who smoke.
An ideal adjuvant treatment for T1D would improve HbA1c levels without hypoglycemia, facilitate weight loss in obese patients, and decrease the risk of diabetes-related complications (48). Treatment with insulin alone usually does not achieve this effect (4). Thus, it is not uncommon that non-insulin drugs for T2D are also used as adjunctive treatments for T1D in clinical practice (49–51). For this reason, clinicians should pay more attention to the significant indicators or factors highlighted in this article for their future work with T1D patients.
There are some limitations in our research. Firstly, it is imperative to acknowledge that the research data exclusively originate from a single-center data and potentially susceptible to inherent bias. Secondly, other potential variables that could influence the patient’s discharge treatment prescription, including the patient’s potential reluctance to utilize non-insulin medications, need to be considered. Thirdly, because of the nature of retrospective studies, we are unable to determine the specific reasons why clinical doctors prescribe or do not prescribe non-insulin drugs. Fourthly, we did not conduct a detailed analysis of individual non-insulin medications, and there are potential differences between the factors of different non-insulin drugs.
In conclusion, we identified the indicators and characteristics present at admission that exerted influence on the acquisition of non-insulin prescriptions upon discharge, alongside conducting subgroup analyses based on gender and age. During the course of treatment, the clinician should prioritize these indicators or factors in order to enhance the treatment outcome for the patient.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University. No informed consent was required because the study was conducted retrospectively and anonymized.
Author contributions
YC: Writing – original draft. HL: Writing – original draft. XL: Writing – original draft. XJ: Writing – original draft. JH: Writing – review & editing. JD: Writing – review & editing. CX: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Key R&D Program of China (2023YFC2506000, 2023YFC2506006), Shandong Provincial Natural Science Foundation (ZR2023QH396), The Health Science and Technology Plan project of the Inner Mongolia Autonomous Region (202201411), and Inner Mongolia Higher Education Association Higher education research project (NMGJXH-2022XB020).
Acknowledgments
We kindly acknowledge the participants and researchers who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor XW declared a past co-authorship with the authors JH & CX.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1381248/full#supplementary-material
Supplementary Figure 1 | Odds ratio [95% confidence interval] of being discharged with insulin and non-insulin antidiabetic drug among patients below the age of 30 with type 1 diabetes after rebuilding the model. LOS, length of stay. *Statistical significance.
References
1. Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clinics North America. (2005) 52:1553–78. doi: 10.1016/j.pcl.2005.07.006
2. Daneman D. Type 1 diabetes. Lancet (London England). (2006) 367:847–58. doi: 10.1016/S0140-6736(06)68341-4
3. Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. (2019) 62:408–17. doi: 10.1007/s00125-018-4763-3
4. Society CD, Association CE, Endocrinology CSo, Society CP, Zhiguang Z. Guidelines for the diagnosis and treatment of type 1 diabetes mellitus in China (2021 edition). Chin J Diabetes Mellitus. (2022) 14:1143–250. doi: 10.3760/cma.j.cn115791-20220916-00474
5. Awoniyi O, Rehman R, Dagogo-Jack S. Hypoglycemia in patients with type 1 diabetes: epidemiology, pathogenesis, and prevention. Curr Diabetes Rep. (2013) 13:669–78. doi: 10.1007/s11892-013-0411-y
6. Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabetic medicine: J Br Diabetic Assoc. (2010) 27:398–404. doi: 10.1111/j.1464-5491.2010.02956.x
7. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2021) 137:111315. doi: 10.1016/j.biopha.2021.111315
8. Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. (2015) 38:971–8. doi: 10.2337/dc15-0078
9. Qiu L, Ling P, Yang D, Luo S, Zheng X, Liang H, et al. Current status of metformin in addition to insulin therapy in adult patients with type 1 diabetes mellitus: An analysis from the Guangdong Type 1 Diabetes Mellitus Translational Medicine Study. J Diabetes. (2020) 12:754–60. doi: 10.1111/1753-0407.13025
10. Lund SS, Tarnow L, Astrup AS, Hovind P, Jacobsen PK, Alibegovic AC, et al. Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized study. PloS One. (2008) 3:e3363. doi: 10.1371/journal.pone.0003363
11. Jacobsen IB, Henriksen JE, Beck-Nielsen H. The effect of metformin in overweight patients with type 1 diabetes and poor metabolic control. Basic Clin Pharmacol Toxicol. (2009) 105:145–9. doi: 10.1111/j.1742-7843.2009.00380.x
12. Ahrén B, Hirsch IB, Pieber TR, Mathieu C, Gómez-Peralta F, Hansen TK, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. (2016) 39:1693–701. doi: 10.2337/dc16-0690
13. Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care. (2016) 39:1702–10. doi: 10.2337/dc16-0691
14. Nauck MA, Meier JJ. GLP-1 receptor agonists in type 1 diabetes: a MAG1C bullet? Lancet Diabetes Endocrinol. (2020) 8:262–4. doi: 10.1016/S2213-8587(20)30043-7
15. Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. (2017) 377:2337–48. doi: 10.1056/NEJMoa1708337
16. Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. (2018) 41:2560–9. doi: 10.2337/dc18-1749
17. Taylor SI, Blau JE, Rother KI, Beitelshees AL. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol. (2019) 7:949–58. doi: 10.1016/S2213-8587(19)30154-8
18. Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the north american inTandem1 study. Diabetes Care. (2018) 41:1970–80. doi: 10.2337/dc18-0343
19. Miller RG, Costacou T. Glucose management and the sex difference in excess cardiovascular disease risk in long-duration type 1 diabetes. Curr Diabetes Rep. (2019) 19:139. doi: 10.1007/s11892-019-1240-4
20. Nattero-Chávez L, Insenser M, Quintero Tobar A, Fernández-Durán E, Dorado Avendaño B, Fiers T, et al. Sex differences and sex steroids influence on the presentation and severity of cardiovascular autonomic neuropathy of patients with type 1 diabetes. Cardiovasc Diabetol. (2023) 22:32. doi: 10.1186/s12933-023-01766-y
21. Schütt M, Fach EM, Seufert J, Kerner W, Lang W, Zeyfang A, et al. Multiple complications and frequent severe hypoglycaemia in ‘elderly’ and ‘old’ patients with Type 1 diabetes. Diabetic medicine: J Br Diabetic Assoc. (2012) 29:e176–9. doi: 10.1111/j.1464-5491.2012.03681.x
22. The Diabetes Control and Complications Trial Research Group. Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care. (1995) 18:1415–27. doi: 10.2337/diacare.18.11.1415
23. Gordin D, Rönnback M, Forsblom C, Mäkinen V, Saraheimo M, Groop PH. Glucose variability, blood pressure and arterial stiffness in type 1 diabetes. Diabetes Res Clin Practice. (2008) 80:e4–7. doi: 10.1016/j.diabres.2008.01.010
24. Hainsworth DP, Bebu I, Aiello LP, Sivitz W, Gubitosi-Klug R, Malone J, et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care. (2019) 42:875–82. doi: 10.2337/dc18-2308
25. Gomes MB, Calliari LE, Conte D, Correa CL, Drummond KRG, Mallmann F, et al. Diabetes-related chronic complications in Brazilian adolescents with type 1 diabetes. A multicenter cross-sectional study. Diabetes Res Clin Practice. (2021) 177:108895. doi: 10.1016/j.diabres.2021.108895
26. Mi SH, Su G, Li Z, Yang HX, Zheng H, Tao H, et al. Comparison of glycemic variability and glycated hemoglobin as risk factors of coronary artery disease in patients with undiagnosed diabetes. Chin Med J. (2012) 125:38–43. doi: 10.3760/cma.j.issn.0366-6999.2012.01.008
27. Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. (2010) 12:288–98. doi: 10.1111/j.1463-1326.2009.01160.x
28. Yang D, Yan J, Deng H, Yang X, Luo S, Zheng X, et al. Effects of metformin added to insulin in adolescents with type 1 diabetes: an exploratory crossover randomized trial. J Diabetes Res. (2020) 2020:7419345. doi: 10.1155/2020/7419345
29. Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. (2016) 4:221–32. doi: 10.1016/S2213-8587(15)00436-2
30. Harrison LB, Mora PF, Clark GO, Lingvay I. Type 1 diabetes treatment beyond insulin: role of GLP-1 analogs. J Invest medicine: Off Publ Am Fed Clin Res. (2013) 61:40–4. doi: 10.2310/JIM.0b013e318279b7d6
31. Frandsen CS, Dejgaard TF, Madsbad S. Non-insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus. Lancet Diabetes Endocrinol. (2016) 4:766–80. doi: 10.1016/S2213-8587(16)00039-5
32. Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol. (2002) 40:773–9. doi: 10.1016/S0735-1097(02)02009-0
33. Gordin D, Wadén J, Forsblom C, Thorn L, Rosengård-Bärlund M, Tolonen N, et al. Pulse pressure predicts incident cardiovascular disease but not diabetic nephropathy in patients with type 1 diabetes (The FinnDiane Study). Diabetes Care. (2011) 34:886–91. doi: 10.2337/dc10-2013
34. Schram MT, Chaturvedi N, Fuller JH, Stehouwer CD. Pulse pressure is associated with age and cardiovascular disease in type 1 diabetes: the Eurodiab Prospective Complications Study. J Hypertension. (2003) 21:2035–44. doi: 10.1097/00004872-200311000-00012
35. Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. (2017) 5:597–609. doi: 10.1016/S2213-8587(17)30194-8
36. Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. (2003) 348:2294–303. doi: 10.1056/NEJMoa022314
37. Tolonen N, Forsblom C, Thorn L, Wadén J, Rosengård-Bärlund M, Saraheimo M, et al. Relationship between lipid profiles and kidney function in patients with type 1 diabetes. Diabetologia. (2008) 51:12–20. doi: 10.1007/s00125-007-0858-y
38. Heller SR, Amiel SA, Mansell P. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. U.K. Lispro Study Group. Diabetes Care. (1999) 22:1607–11. doi: 10.2337/diacare.22.10.1607
39. Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism: Clin Experimental. (2019) 92:71–81. doi: 10.1016/j.metabol.2018.11.005
40. Nagi DK, Yudkin JS. Effects of metformin on insulin resistance, risk factors for cardiovascular disease, and plasminogen activator inhibitor in NIDDM subjects. A study of two ethnic groups. Diabetes Care. (1993) 16:621–9. doi: 10.2337/diacare.16.4.621
41. Rizzo M, Chandalia M, Patti AM, Di Bartolo V, Rizvi AA, Montalto G, et al. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc Diabetol. (2014) 13:49. doi: 10.1186/1475-2840-13-49
42. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
43. Kautzky-Willer A, Brazzale AR, Moro E, Vrbíková J, Bendlova B, Sbrignadello S, et al. Influence of increasing BMI on insulin sensitivity and secretion in normotolerant men and women of a wide age span. Obes (Silver Spring Md). (2012) 20:1966–73. doi: 10.1038/oby.2011.384
44. Clausen JO, Borch-Johnsen K, Ibsen H, Bergman RN, Hougaard P, Winther K, et al. Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest. (1996) 98:1195–209. doi: 10.1172/JCI118903
45. Kautzky-Willer A, Handisurya A. Metabolic diseases and associated complications: sex and gender matter! Eur J Clin Invest. (2009) 39:631–48. doi: 10.1111/j.1365-2362.2009.02161.x
46. Clair C, Rigotti NA, Meigs JB. Smoking cessation, weight change, and risk of cardiovascular disease–reply. Jama. (2013) 310:323. doi: 10.1001/jama.2013.7945
47. Avogaro A, de Kreutzenberg SV, Morieri ML, Fadini GP, Del Prato S. Glucose-lowering drugs with cardiovascular benefits as modifiers of critical elements of the human life history. Lancet Diabetes Endocrinol. (2022) 10:882–9. doi: 10.1016/S2213-8587(22)00247-9
48. Sjöholm Å. Using adjuvant pharmacotherapy in the treatment of type 1 diabetes. Expert Opin Pharmacother. (2021) 22:2143–8. doi: 10.1080/14656566.2021.1939679
49. Harris K, Boland C, Meade L, Battise D. Adjunctive therapy for glucose control in patients with type 1 diabetes. Diabetes Metab syndrome obesity: Targets Ther. (2018) 11:159–73. doi: 10.2147/DMSO
50. Tosur M, Redondo MJ, Lyons SK. Adjuvant pharmacotherapies to insulin for the treatment of type 1 diabetes. Curr Diabetes Rep. (2018) 18:79. doi: 10.1007/s11892-018-1041-1
Keywords: type 1 diabetes, factor, insulin, prescriptions, therapy
Citation: Cheng Y, Li H, Liu X, Jin X, Han J, Du J and Xu C (2024) Exploring the influencing factors of non-insulin drug prescriptions in discharged patients with type 1 diabetes. Front. Endocrinol. 15:1381248. doi: 10.3389/fendo.2024.1381248
Received: 03 February 2024; Accepted: 09 August 2024;
Published: 25 September 2024.
Edited by:
Xiaoqin Wu, University of Texas Health Science Center at Houston, United StatesReviewed by:
Sueziani Binte Zainudin, Sengkang General Hospital, SingaporeMohammad Jeeyavudeen, University of Edinburgh, United Kingdom
Copyright © 2024 Cheng, Li, Liu, Jin, Han, Du and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Xu, ZG9jdG9yeHVjaGFvQDE2My5jb20=; Jing Du, MjgzODY2MTBAcXEuY29t; Junming Han, anVubWluZzY5MDlAMTI2LmNvbQ==
 Yikang Cheng
Yikang Cheng Haizhen Li
Haizhen Li Xin Liu4
Xin Liu4 Junming Han
Junming Han Chao Xu
Chao Xu