- 1Department of Clinical and Molecular Medicine, University of Rome La Sapienza, Rome, Italy
- 2Diabetes Unit, Sant’Andrea University Hospital, Rome, Italy
- 3Metabolic Fitness Association, Rome, Italy
- 4Department of Human Movement and Sport Sciences, University of Rome ‘Foro Italico’, Rome, Italy
- 5Research Centre for Musculoskeletal Science and Sports Medicine, Department of Life Sciences, Faculty of Science and Engineering, Manchester Metropolitan University, Manchester, United Kingdom
- 6Center for Applied Biological and Exercise Sciences, Faculty of Health and Life Sciences, Coventry University, Coventry, United Kingdom
- 7Centre for Human Performance and Sport, University of Greenwich, London, United Kingdom
- 8Center for Outcomes Research and Clinical Epidemiology (CORESEARCH), Pescara, Italy
- 9Department of Clinical Pharmacology and Epidemiology, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy
Background: Current guidelines for nonalcoholic fatty liver disease (NAFLD) recommend high volumes and/or intensities of physical activity (PA), the achievement of which generally requires participation in supervised exercise training programs that however are difficult to implement in routine clinical practice. Conversely, counselling interventions may be more suitable, but result in only modest increases in moderate-to-vigorous-intensity PA (MVPA). This study assessed whether a counseling intervention for increasing PA and decreasing sedentary time (SED-time) is effective in improving NAFLD markers in people with type 2 diabetes.
Methods: Three-hundred physically inactive and sedentary patients were randomized 1:1 to receive one-month theoretical and practical counseling once-a-year (intervention group) or standard care (control group) for 3 years. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyltranspeptidase (γGT) levels were measured and fatty liver index (FLI), hepatic steatosis index (HSI), and visceral adiposity index (VAI) were calculated. Total PA volume, light-intensity PA (LPA), moderate-to-vigorous-intensity PA (MVPA), and SED-time were objectively measured by an accelerometer.
Results: Throughout the 3-year period, NAFLD markers did not change in the control group, whereas ALT, γGT, FLI, and HSI decreased in the intervention group, with significant between-group differences, despite modest MVPA increases, which however were associated with larger decrements in SED-time and reciprocal increments in LPA. Mean changes in NAFLD markers varied according to quartiles of (and correlated with) changes in MVPA (all markers) and SED-time, LPA, and PA volume (ALT, γGT, and HSI). Mean changes in MVPA or PA volume were independent predictors of changes in NAFLD markers. When included in the models, change in cardiorespiratory fitness and lower body muscle strength were independently associated with some NAFLD markers.
Conclusion: A behavior change involving all domains of PA lifestyle, even if insufficient to achieve the recommended MVPA target, may provide beneficial effects on NAFLD markers in people with type 2 diabetes.
1 Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a broad spectrum of hepatic abnormalities, from simple steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis (1), and is associated with an increased risk of developing hepatocellular carcinoma (2) and extrahepatic disorders such as cardiovascular disease (3). During the last decades, NAFLD has become the most common chronic liver disease worldwide, due to the ongoing epidemics of obesity and type 2 diabetes (4). Both these conditions are strongly associated with (5, 6) and accelerate progression of (7) NAFLD, which is in fact considered as the hepatic expression of the metabolic syndrome (8). For this reason, an international panel of experts has recently proposed to use the term metabolic dysfunction-associated fatty liver disease instead of NAFLD (9).
Current guidelines recommend, in persons with excess adiposity, a weight loss at least 5% and preferably ≥10% through an energy deficit of 500-1000 kcal with adoption of healthy eating patterns and adherence to physical activity (PA) (10–12). Regarding PA, it is advised to participate in a structured exercise program consisting of 3-5 sessions per week (10, 11), with a total duration of 150-300 min of moderate-intensity or, better, 75-150 min of vigorous-intensity aerobic exercise and eventual addition of resistance exercise training (12). Recommendations on PA are based on the evidence from studies (13–16) and meta-analyses (17–20) that engagement in aerobic and/or resistance exercise programs, generally supervised, is associated with beneficial effects on NAFLD/NASH, with significant decreases in intrahepatic fat accumulation as well as in liver enzymes and indices of steatosis and visceral adiposity. Of note, these effects were independent of weight loss (13–20), though losing weight was associated with greater benefits (18, 21, 22) with a dose-response relationship (23). Moreover, these studies were conducted either in the general population or in people with overweight/obesity and/or NAFLD/NASH, which eventually included individuals with type 2 diabetes.
Unfortunately, adherence to PA recommendation is generally poor (24), especially in people with type 2 diabetes (25), pointing to the need for targeted interventions. However, supervised exercise programs, though very effective in favoring the achievement of the high volumes and/or intensities recommended by guidelines (26), are difficult to implement in routine clinical practice. In contrast, counselling interventions may be more suitable for producing a sustained behavior change, but result in only modest increases in moderate-to-vigorous-intensity PA (MVPA) (27–29), which may be insufficient to improve NAFLD. However, sedentary time (SED-time) was found to be associated with NAFLD, independently of MVPA (30, 31), thus suggesting that targeting also this domain of PA behavior by recommending substitution or interruption with time spent in light-intensity PA (LPA) might be effective in ameliorating NAFLD. In the Italian Diabetes and Exercise Study_2 (IDES_2), a counseling intervention targeting both MVPA and SED-time resulted in only modest increases in MVPA (6.4 min·day-1), but larger (0.8 hours·day-1) decreases in SED-time and reciprocal increases in LPA, thus substantially contributing to the 3.3 metabolic equivalents (METs)-hour·week-1 increment in total PA volume (32). These changes were associated with clinically meaningful improvements in physical fitness and glycemic and blood pressure control, with no significant effect on indices of adiposity or lipid profile (32).
This post hoc analysis of the IDES_2 was aimed at evaluating the impact of the counseling intervention and the relative contribution of changes in MVPA and SED-time/LPA on NAFLD markers in people with type 2 diabetes.
2 Materials and methods
Design and methods have been detailed elsewhere (32, 33) and will be briefly reported here.
2.1 Design
The IDES_2 was an open-label, assessor-blinded, parallel, superiority randomized clinical trial that assessed the efficacy of a behavioral intervention in increasing daily PA and reducing SED-time over a 3-year follow-up in individuals with type 2 diabetes.
2.2 Participants
Inclusion criteria were type 2 diabetes of at least one-year duration, age 40-80 years, body mass index (BMI) 27-40 kg/m2, physically inactivity (i.e., insufficient amounts of PA according to current guidelines) and sedentary lifestyle (i.e., more than 8 hours/day spent in any waking behavior characterized by an energy expenditure ≤1.5 metabolic equivalents while in a sitting or reclining posture) for at least 6 months, ability to walk 1.6 Km without assistance, and eligibility after cardiologic evaluation. Exclusion criteria were conditions limiting or contraindicating PA, affect conduct of the trial, reduce lifespan, and/or affect the safety of intervention.
2.3 Randomization and blinding
Three-hundred individuals with type 2 diabetes were recruited in three tertiary referral, outpatients Diabetes Clinics in Rome and randomized 1:1 to either an intervention (INT) group, receiving theoretical and practical exercise counselling, or a control (CON) group, receiving only general physician recommendations. Randomization was stratified by center and, within each center, by age < versus ≥65 years and non-insulin versus insulin treatment, using a permuted-block randomization software.
Participants from both groups received the same treatment regimen, including dietary prescription, to achieve glycemic, lipid, blood pressure (BP), and body weight targets, according to current guidelines (34). Dietary and pharmacological treatment was adjusted at each visit using a pre-specified algorithm.
Physicians, exercise specialists, and participants were not blinded, whereas assessors of accelerometer/diary and biochemical parameters were blinded to group assignment.
2.4 Intervention
Participants in the INT group were engaged in a one-month theoretical and practical counselling, each year for three years. Specifically, the intervention consisted of one individual theoretical counselling session plus eight twice-weekly individual theoretical and practical counselling sessions.
The 30-min theoretical, individual, face-to-face counselling session was held by a diabetologist and consisted of seven steps. Each theoretical and practical counselling session was held by a certified exercise specialist. The theoretical part was aimed at improving knowledge of the effects of exercise on health, conditions contraindicating exercise, difference between habitual and occasional exercise, and essential parameters of wellness such as BP, heart rate, and blood glucose. The practical part served to instruct participants to distinguish the different types of exercise, to evaluate exercise intensity, and to monitor and correct blood glucose imbalances during and after the session.
This approach was designed to promote an increase in any kind of PA, based on individual preference, and a decrease in SED-time through a two-step behavior change, i.e. (1) decreasing SED-time by substituting and/or interrupting it with a wide range of LPAs; and (2) gradually increasing the time spent in purposeful MVPA.
2.5 Measurements
2.5.1 Liver enzymes and indices of steatosis and visceral adiposity
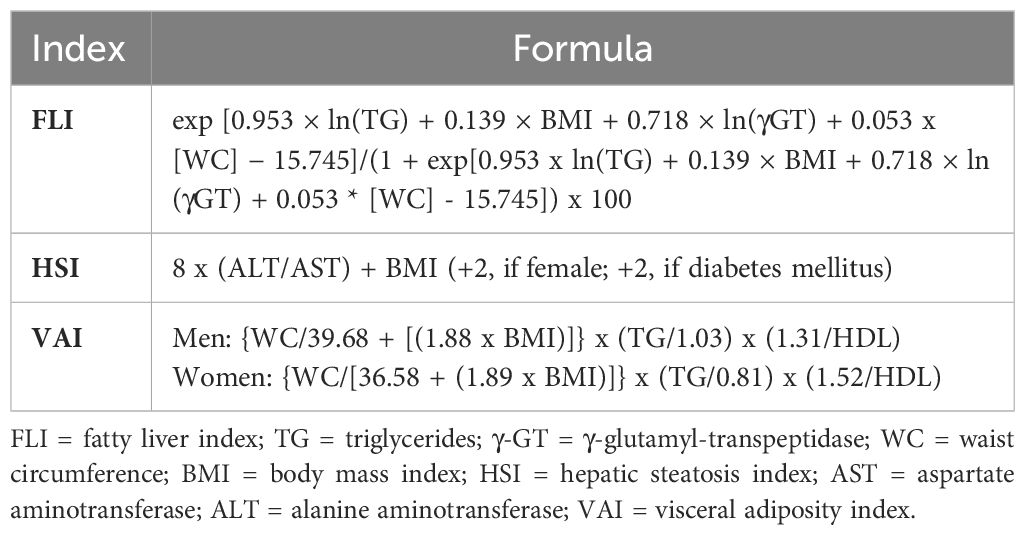
At baseline and every 4 months thereafter, levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyltranspeptidase (γGT) were measured by the use of standard methods (VITROS 5,1 FS Chemistry System, Ortho-Clinical Diagnostics Inc, Raritan, NJ). Values of the following indices were then calculated using the formulas reported in Table 1: fatty liver index (FLI) (35) and hepatic steatosis index (HSI) (36), two validated indices of steatosis, and visceral adiposity index (VAI), a marker of visceral fat distribution and dysfunction (37) that was found to predict liver histology in individuals with NAFLD (38), though not consistently (39). The following cut-off levels for liver enzymes were used (corresponding to the upper limit of laboratory range): 34 IU/L for AST, 55 IU/L for ALT, and 78 IU/L for γGT, whereas FLI, HSI, and VAI values were considered abnormal if ≥60 (intermediate if 30-59), 36, and 1.9, respectively (36, 37, 40).
2.5.2 Physical activity and sedentary behavior
Total PA volume, time spent in LPA and MVPA, and SED-time were measured by the use of an accelerometer (MyWellness Key, Technogym, Cesena, IT) and a daily diary for non-accelerometer recordable activities. Measurements were obtained at baseline and every 4 months thereafter for seven consecutive days, except for the initial 4 months, during which the device was worn for the entire period.
2.5.3 Physical fitness
At baseline and every year thereafter, participants were evaluated for physical fitness by assessing cardiorespiratory fitness (as maximal oxygen uptake, VO2max), upper and lower body muscle strength, and flexibility by maximal treadmill exercise test, isometric test, and bending test, respectively.
2.5.4 Cardiovascular risk factors
At the same time points, the modifiable cardiovascular risk factors hemoglobin A1c, fasting plasma glucose, BMI, waist circumference, triglycerides, total, HDL, and LDL cholesterol, serum creatinine (with calculation of estimated glomerular filtration rate), albumin:creatinine ratio, high-sensitivity C-reactive protein, and systolic and diastolic BP, were measured using standard methods.
2.6 Statistical analysis
Mean changes from baseline throughout the three-year follow-up in liver enzymes and indices, PA/SED-time, physical fitness, and cardiovascular risk factors were calculated for participants who completed the study as the mean values of changes from baseline at each time point (i.e., at 4, 8, 12, 16, 20, 24, 28, 32, and 36 months). Between-group differences in mean changes in AST, ALT, γGT, FLI, HSI, and VAI were assessed by Student’s t test; in addition, differences in liver enzymes and indices throughout the three-year follow-up between INT and CON participants were analyzed by generalized linear mixed models for repeated measures.
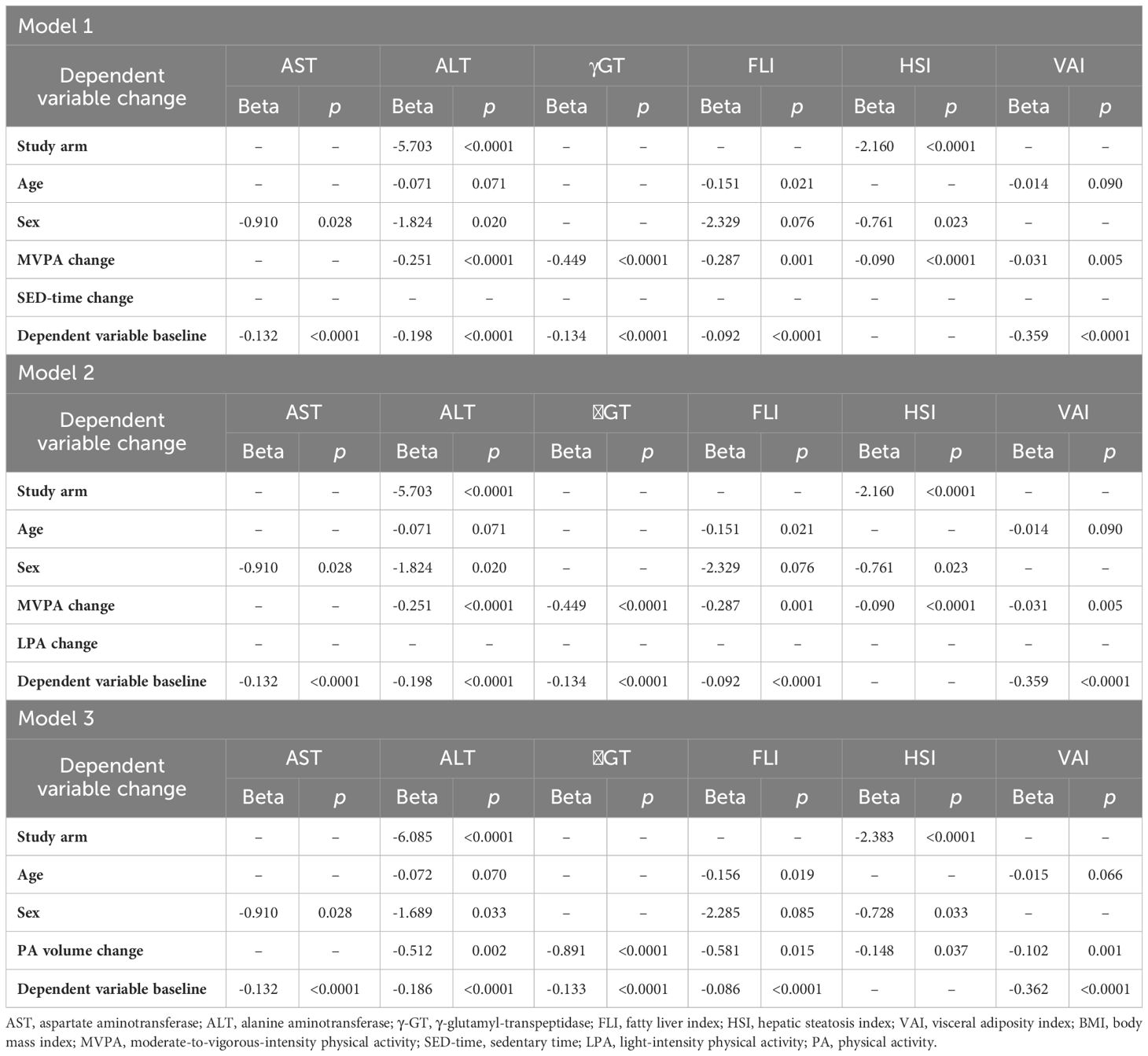
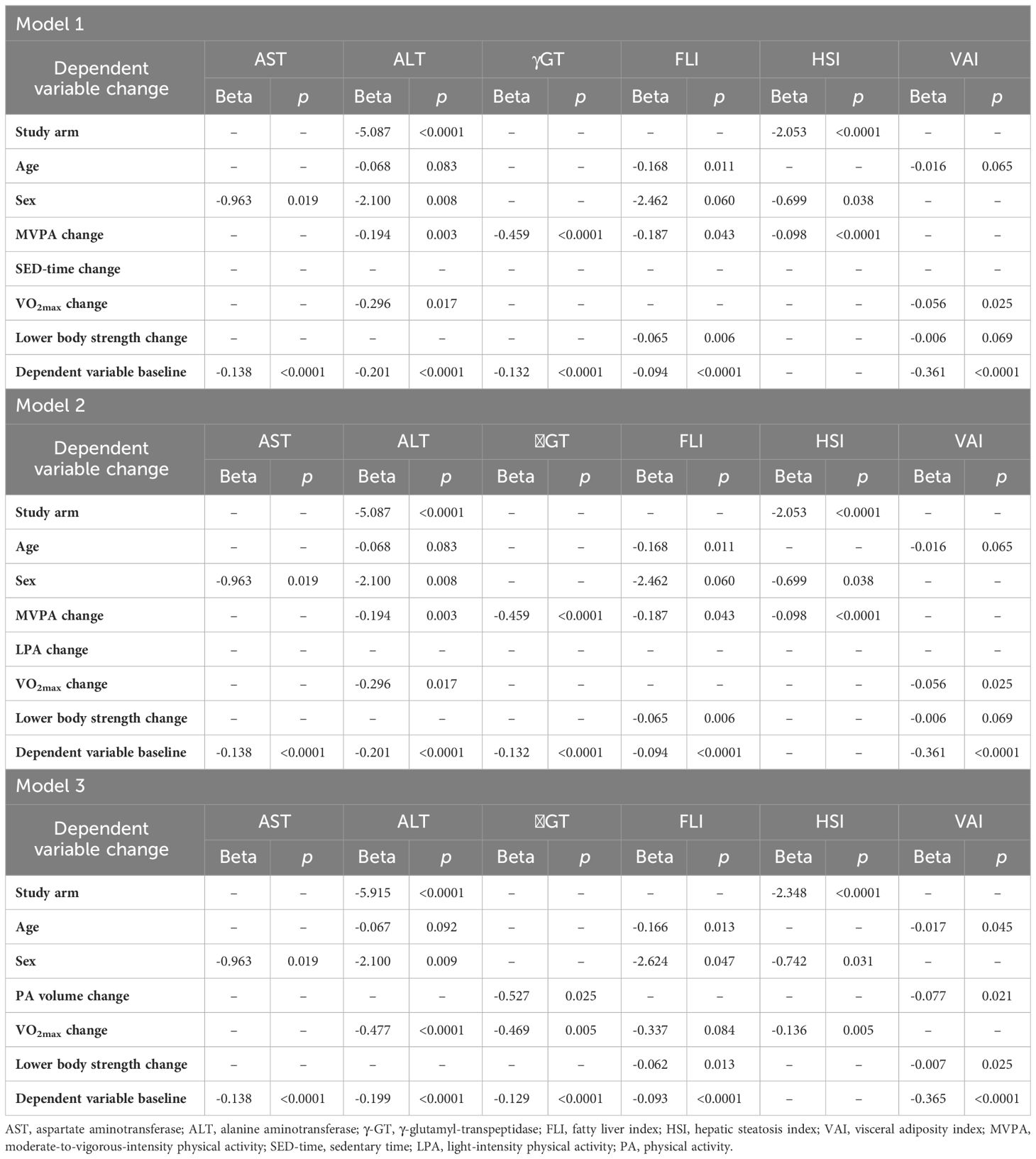
To describe the relationships of changes in liver enzymes and indices with those in PA/SED-time, irrespective of study arm, the mean values of changes in AST, ALT, γGT, FLI, HSI, and VAI were then stratified by quartiles of changes in SED-time, MVPA, LPA, and PA volume in the whole cohort and data were expressed as mean ± SD and analyzed by one-way ANOVA. Moreover, univariate correlations between changes in AST, ALT, γGT, FLI, HSI, and VAI and those in SED-time, MVPA, LPA, and PA volume were assessed by Pearson correlation coefficient. Finally, multivariable linear regression analyses with stepwise backward selection of variables were applied to assess the independent predictors of changes in liver enzymes and indices over the three-year period. Study arm, age, sex, the baseline value of the dependent variable, and changes in SED-time and MVPA were included as covariates in Model 1. Changes in LPA were substituted for changes in SED-time in Model 2, whereas changes in PA volume were substituted for changes in SED-time and MVPA in model 3, respectively. All the analyses were repeated by including in the models either changes in BMI or, alternatively, waist circumference, HbA1c or VO2max and lower body muscle strength. Additional analyses were run to assess the independent effect of treatment with anti-hyperglycemic agents on changes in liver enzymes and indices.
All the p-values <0.05 were considered statistically significant. Statistical analyses were performed with SPSS version 20 (SPSS Inc., Chicago, IL, USA).
3 Results
As previously reported (32), 267 participants completed the study at the final evaluation (CON=134; INT=133), whereas 33 participants (CON=16; INT=17) dropped out for various reasons; of those in the INT group, >90% attended the counselling sessions.
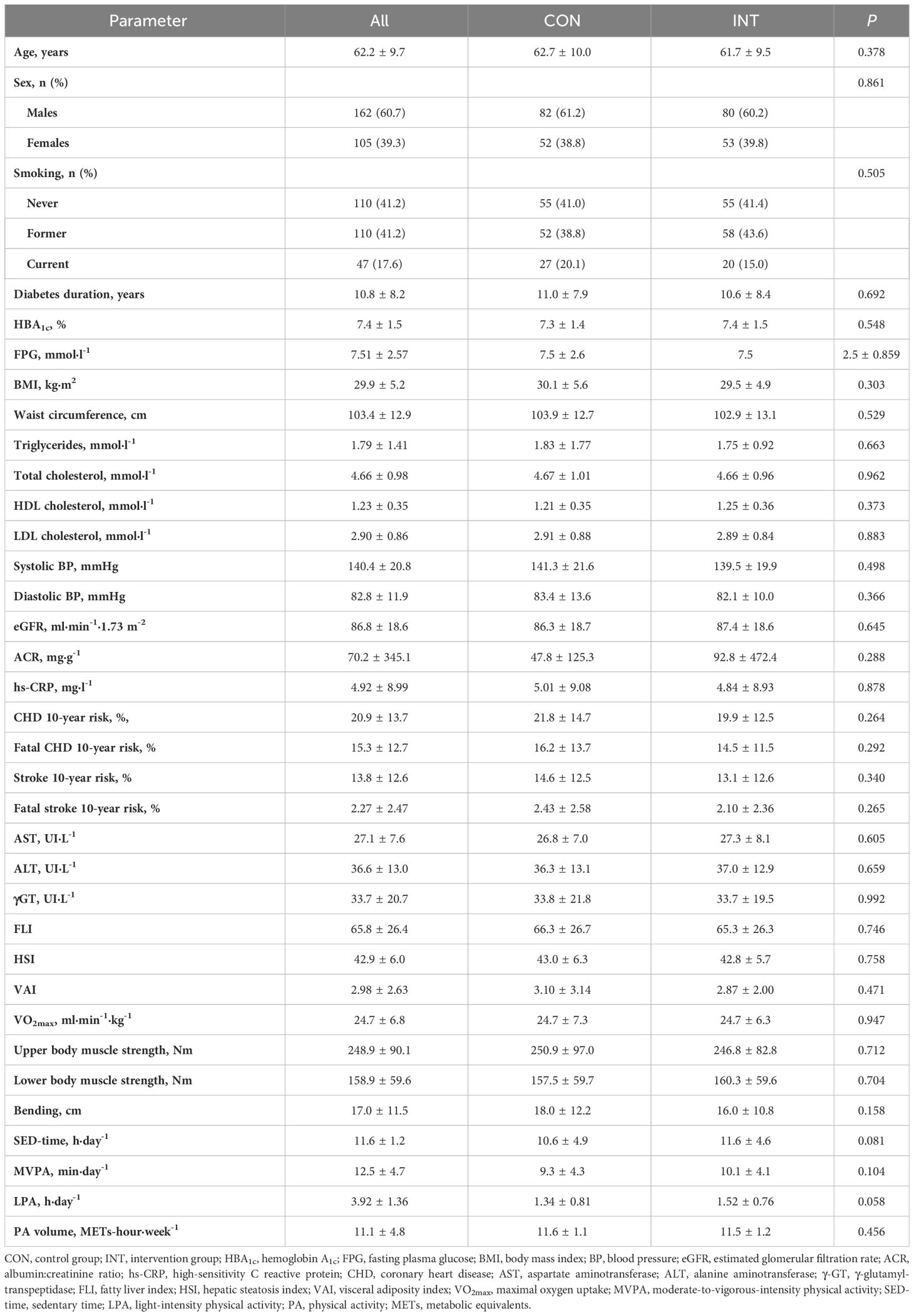
The baseline features of the individuals considered for the present analysis are reported in Table 2.
3.1 Effects of intervention on liver enzymes and indices
No between-group differences were detected at baseline either in liver enzymes and indices or in the parameters used for calculating them (Table 2). The percentages of participants with elevated liver enzymes were low (2.6% for AST, 7.9% for ALT, and 4.9% for γGT), whereas the percentages of those with abnormal indices were high (86.9% for FLI, of whom 26.2% with intermediate values, 89.1% for HSI, and 59.9% for VAI), with no significant differences between the two groups.
Mean changes in liver enzymes and indices were negligible in the CON group, whereas those in the INT group indicated substantial decreases in ALT, γGT, FLI, and HSI, but not AST and VAI, with significant between-group differences (Figure 1). The analysis of liver enzymes and indices throughout the 3-year follow-up period showed no change in the CON group, and significant decreases in ALT, γGT, FLI, and HSI, but not AST and VAI, in the INT group, with significant between-group differences for ALT and HSI only (Figure 2). At end-of-study, the percentages of participants with elevated liver enzyme levels (1.6% for AST, 2,7% for ALT, and 3.5% for γGT) and abnormal indices (84.3% for FLI, of whom 24.7% with intermediate values, and 82.4% for HSI) were lower than at baseline, except for VAI (64.9%). In the CON group, the individuals that became abnormal were more numerous than those that returned normal, whereas the opposite was observed in the INT group (not shown).
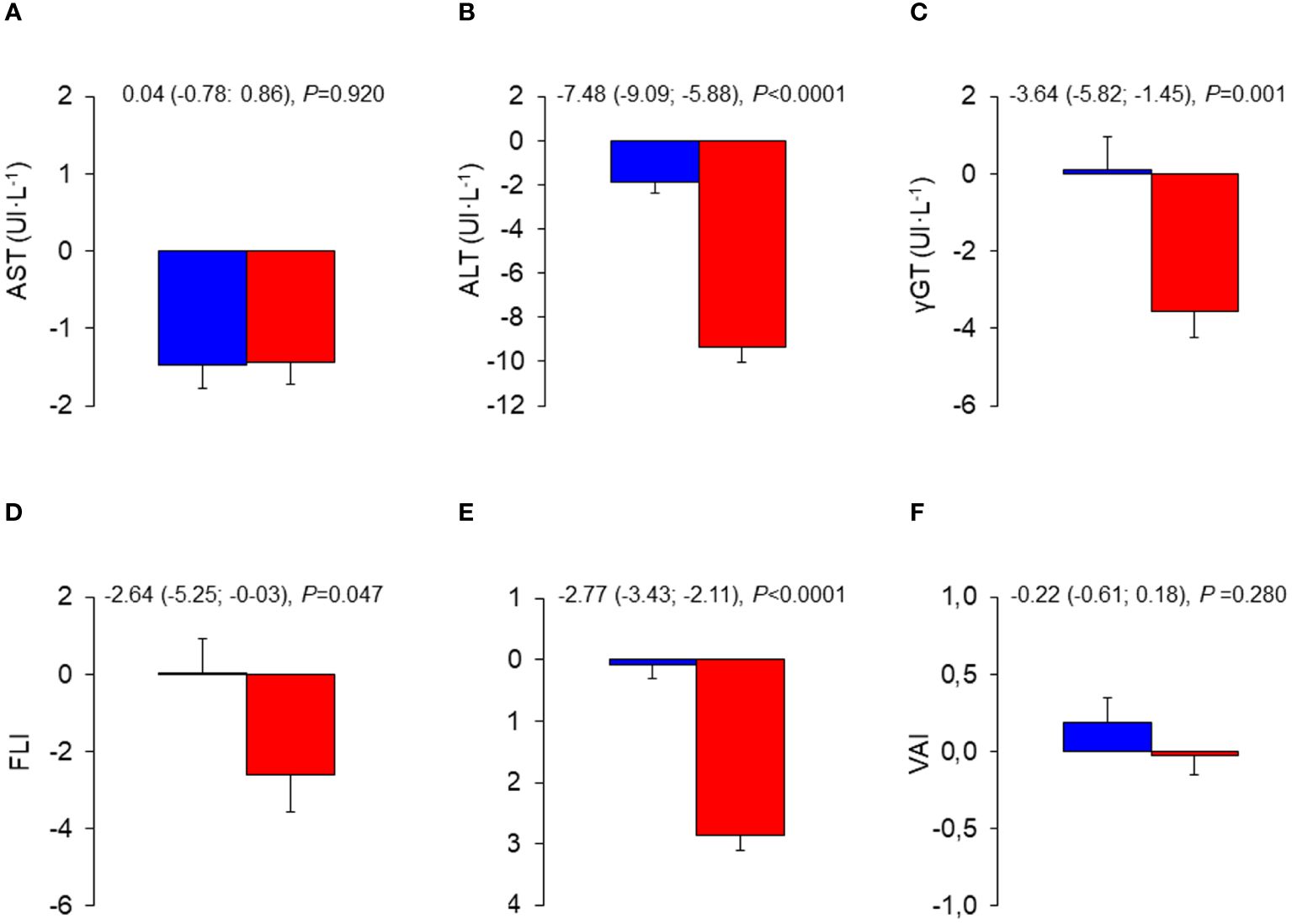
Figure 1 Mean changes from baseline in liver enzymes and indices in the INT and CON group. Baseline to end-of-study changes in AST (A), ALT (B), γ-GT (C), FLI (D), HSI (E), and VAI (F) in CON (blue bars) and INT (red bars) participants. AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, γ-glutamyltranspeptidase; FLI, fatty liver index; HSI, hepatic steatosis index; VAI, visceral adiposity index; CON, control; INT, intervention.
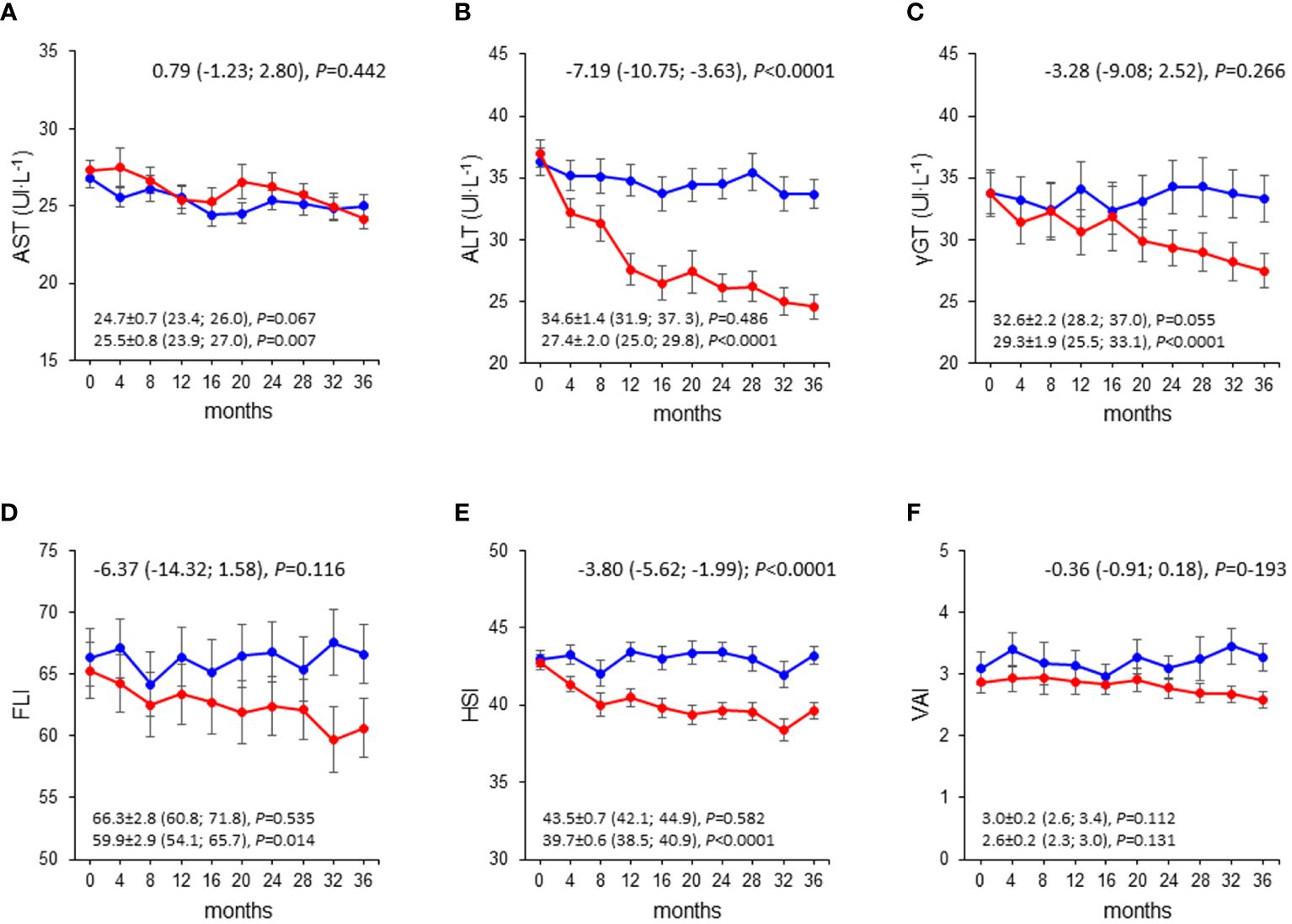
Figure 2 Liver enzymes and indices over the 3-year follow-up in the INT and CON group. Values of AST (A), ALT (B), γ-GT (C), FLI (D), HSI (E), and VAI (F) over the 3-year follow-up in CON (blue circles and lines) and INT (red circles and lines) participants. AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, γ-glutamyltranspeptidase; FLI, fatty liver index; HSI, hepatic steatosis index; VAI, visceral adiposity index; CON, control; INT, intervention.
3.2 Relationships between changes in liver enzymes and indices and changes in PA/SED-time and fitness
Mean changes from baseline in liver enzymes and indices significantly varied according to quartiles of changes in MVPA, with participants falling in quartiles III and IV showing the most marked reductions in these parameters. The same trend was observed according to quartiles of changes in SED-time, LPA, and PA volume, though only for ALT, γGT, and HSI (Table 3).
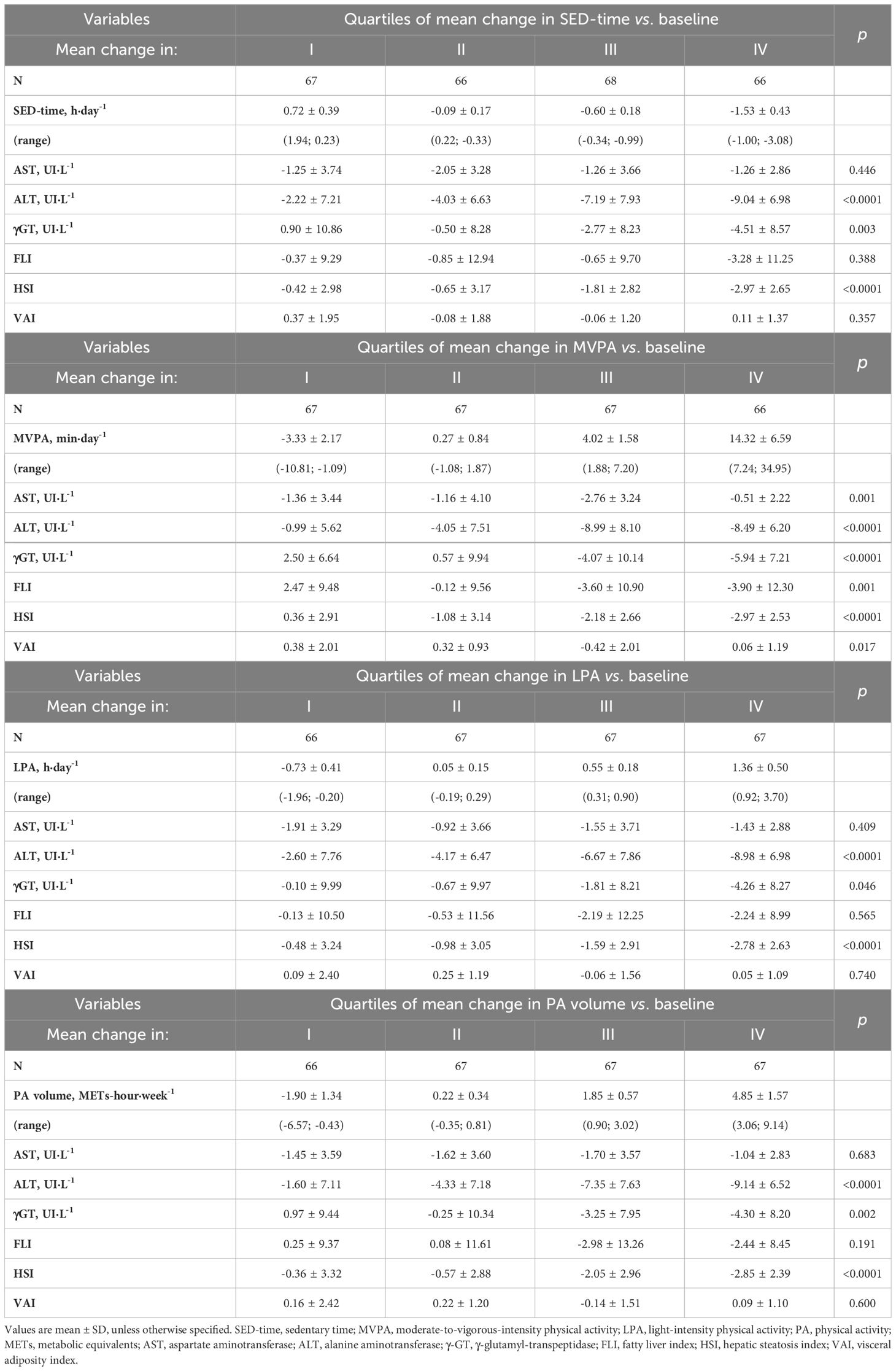
Table 3 Mean changes from baseline in liver enzymes and indices according to quartiles of mean changes from baseline in SED-time, MVPA, LPA, or PA volume.
Likewise, at univariate analysis (Table 4), mean changes from baseline in ALT, γGT, and HSI significantly correlated with mean changes in SED-time and, inversely, with those in MVPA, LPA, and total PA volume, whereas changes in FLI and VAI correlated significantly only with changes in MVPA.
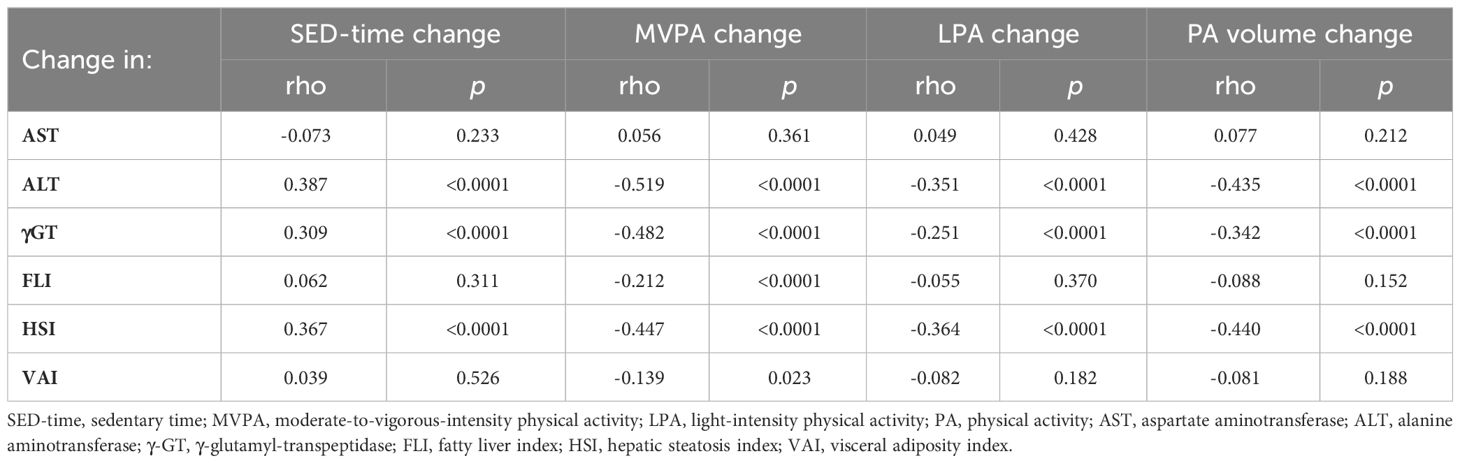
Table 4 Univariate correlation between mean changes from baseline in liver enzymes and indices and those in SED-time, MVPA, LPA, or PA volume.
At multivariable linear regression analysis (Table 5), change in MVPA, but not change in SED-time (in Model 1) or LPA (in Model 2), was an independent predictor of changes in ALT, γGT, FLI, HSI, and VAI, but not AST. Removal of MVPA change from the models resulted in a significant association of change in SED-time with changes in ALT, γGT, HSI, and VAI or of change in LPA with changes in ALT and VAI (not shown). In Model 3, change in PA volume was an independent predictor of changes in the same parameters as MVPA in Model 1 and 2. In addition, study arm, age, and sex were variably associated with changes in liver enzymes and indices, whereas the baseline value of the dependent variable was significantly associated with its change over the study follow-up, except for HSI. When included in the models, change in BMI was significantly associated with changes in AST, ALT (in Model 2 only), γGT, FLI, and HSI, change in waist circumference was significantly associated with change in FLI, HSI, and VAI, and change in HbA1c was significantly associated with changes in γGT, FLI, HSI, and VAI, without substantially modifying the relationships between changes in MVPA or PA volume with changes in liver enzymes and indices (not shown). Moreover, among the antihyperglycemic agents, only glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and thiazolidinediones (TDZs), which were used in 14 CON versus 20 INT (P=0.261) and 33 CON versus 17 INT (P=0.013) participants, respectively, at any time during the 3-year period, were independently associated with changes in NAFLD markers, in particular HSI for GLP-1 RAs and VAI for both, without any significant impact on the association between changes in these indices and those in MVPA or PA volume. Finally, when included in the models, change in VO2max was associated with changes in ALT and VAI in Model 1 and 2 and changes in ALT, γGT, and HSI in Model 3, whereas change in lower body muscle strength was an independent predictor of changes in FLI and, in Model 3 only, VAI, without substantially modifying the relationships between changes in MVPA or PA volume with changes in liver enzymes and indices (Table 6).
4 Discussion
This post hoc analysis of the IDES_2 showed that sustained decreases in SED-time and increases in PA promoted by a behavioral counseling were associated with improvements in NAFLD markers, with significant reductions of ALT, γGT, FLI, and HSI, whereas VAI was unchanged, consistent with the lack of effect of the intervention on indices of adiposity such as BMI and waist circumference (32). Though relatively modest, the improvements in NAFLD markers were similar to those reported in previous studies (41, 42) and meta-analyses (17, 19) for supervised exercise programs of shorter duration. In particular, in the previous IDES (42), enzyme levels did not change, whereas FLI and VAI significantly decreased in the supervised exercise intervention group, but not in the control group, in a PA volume-dependent manner.
However, participants in supervised exercise studies were engaged in moderate-to vigorous intensity training or high intensity interval training at least three times a week, thus accumulating large amounts of MVPA, which were shown to be required for obtaining significant decreases in intrahepatic fat accumulation (43). Conversely, in the IDES_2, increments in MVPA were much lower, though sustained over three years, and not sufficient to achieve the recommended target of at least 150-300 min per week. Thus, the novel information provided by our study is that even small increments in MVPA obtained by adopting and maintaining a more active lifestyle can be effective in improving NAFLD markers in individuals with type 2 diabetes, suggesting that “some (MVPA) is better than nothing”. Yet, the findings that participants falling in quartiles III and IV of MVPA change showed the most marked reductions in liver enzymes and indices and that the increase in MVPA was a strong independent predictor of improvements in NAFLD markers confirm the concept that “the more (MVPA) the better”.
Moreover, the modest changes in MVPA were accompanied by larger decrements in SED-time and reciprocal increments in LPA resulting in substantial increases in total PA volume. These changes were also associated with changes in liver enzymes and indices, consistent with previous evidence that sedentary behavior or, inversely, overall PA level are associated with presence of NAFLD or increased liver enzymes (30, 31, 44, 45). These findings suggest that NAFLD may also benefit from reallocation of SED-time to LPA. In fact, though changes in SED-time or LPA were not independent predictors of variations in NAFLD markers, a role for decreases in SED-time through the reciprocal increases in LPA is supported by the independent association of liver enzymes and indices with total PA volume, of which increments in LPA were a main contributor.
Another important finding of this study is that changes in VO2max and lower body muscle strength predicted changes in NAFLD markers beyond changes in PA/SED-time, thus suggesting that the improvements in physical fitness resulting from the behavioral modification provided additional benefits. This is consistent with the observations that cardiorespiratory fitness is associated with presence of NAFLD, as assessed by FLI, independent of MVPA (46), and that baseline VO2max is an independent predictor of reduction in liver fat from a lifestyle intervention in individuals with NAFLD (47). Moreover, changes in BMI and, to a lesser extent, waist circumference were also associated, independent of changes in PA/SED-time, with changes in NAFLD markers, including the indices calculated using these parameters.
Several mechanisms have been hypothesized for explaining the beneficial effects on NAFLD of increasing PA and physical fitness and decreasing SED-time in the absence of significant reductions in total and central fat mass. These mechanisms include amelioration of insulin resistance at both the liver and adipose tissue level and increased mitochondrial biogenesis and capillarization favoring fatty acid uptake, β-oxidation, and triglyceride storage at the muscle level (48). The importance of muscle in the pathogenesis of NAFLD is supported by the inverse association of muscle mass and grip strength with risk of severe NAFLD (49) and the correlation between muscle fat accumulation and NASH severity (50). These mechanisms might be operating also for modest increases in MVPA, provided that they are sustained in the long-term.
The main strength of this study is the evaluation of changes in liver enzymes and indices of steatosis associated with long-term, sustained changes in PA/SED-time, as measured objectively by the use of an accelerometer. Other strengths concern the trial design, including the application of an intervention targeting both PA and SED-time, based on solid theoretical grounds, and using several behavioral change techniques, the specific training of investigators, the long study duration, and the large sample size (32, 33). However, this study has some limitations. First, the use of surrogate measures, such as liver enzymes and indices of steatosis, instead of direct methods, such as the gold standard histology or imaging techniques, does not allow to draw definite conclusions regarding the effect of the intervention on NAFLD/NASH. Second, the lack of data on platelet count did not allow calculation of indices of liver fibrosis. Third, generalization requires further investigation and validation in different cohorts or settings. Fourth, results might have been affected by unmeasured confounders, for instance diet, which was not considered in data analysis, though participants received dietary prescriptions and adherence to diet was verified at intermediate visits.
5 Conclusion
This post hoc analysis of the IDES_2 showed that, in individuals with type 2 diabetes, a counseling intervention for increasing PA and decreasing SED-time was effective in ameliorating NAFLD, as suggested by the improvements in liver enzymes and indices of steatosis over 3 years. Changes in MVPA, though modest, were the main predictors of changes in these surrogate measures, but also the greater decreases in SED-time contributed to this effect, possibly through the reciprocal increases in LPA that resulted in a relatively large increment in total PA volume. These findings indicate that a behavior change involving all domains of PA lifestyle, even if insufficient to achieve the recommended MVPA target, may also provide beneficial effects on NAFLD/NASH in people with type 2 diabetes, eventually combined with treatment with GLP-1 RAs and/or TDZs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Sant’Andrea University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. MV: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. LM: Data curation, Formal analysis, Investigation, Writing – review & editing. CG: Data curation, Formal analysis, Investigation, Writing – review & editing. MS: Investigation, Methodology, Resources, Validation, Writing – review & editing. GO: Investigation, Methodology, Resources, Validation, Writing – review & editing. CI: Investigation, Validation, Writing – review & editing. SM: Investigation, Visualization, Writing – review & editing. SZ: Investigation, Methodology, Resources, Validation, Writing – review & editing. AN: Data curation, Formal analysis, Software, Writing – review & editing. SB: Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing, Resources. GP: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Writing – original draft.
Group members of Italian Diabetes Exercise Study 2 (IDES_2) Investigators
See Supplementary Data Sheet 1 for a complete list of the IDES_2 Investigators.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Metabolic Fitness Association, Monterotondo, Rome, Italy. The sponsor had no role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, and approval of the manuscript.
Conflict of interest
SZ is an employee of Technogym. AN reported grant from Artsana, Astra-Zeneca, Eli Lilly, Novo Nordisk, and Sanofi Aventis and personal fees from Eli Lilly and Novo Nordisk. SB reported personal fees from Astra-Zeneca, Eli Lilly, Novo Nordisk, and Takeda. GP reported personal fees from Astra-Zeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dome, Mylan, Sigma-Tau, and Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1393859/full#supplementary-material
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CON, control; FLI, fatty liver index; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; γGT, γ-glutamyltranspeptidase; HSI, hepatic steatosis index; IDES_2, Italian Diabetes and Exercise Study_2; INT, intervention; LPA, light-intensity physical activity; METs, metabolic equivalents; MVPA, moderate-to-vigorous-intensity physical activity; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PA, physical activity; SED-time, sedentary time; TDZs, thiazolidinediones; VAI, visceral adiposity index; VO2max, maximal oxygen uptake.
References
1. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA. 2 S. Nonalcoholic steatohepatitis: A review. JAMA. (2020) 323:1175–83. doi: 10.1001/jama.2020.2298
2. Shah PA, Patil R, Harrison SA. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology. (2023) 77:323–38. doi: 10.1002/hep.32542
3. Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. Nonalcoholic fatty liver disease and cardiovascular risk: A scientific statement from the american heart association. Arterioscler Thromb Vasc Biol. (2022) 42:e168–85. doi: 10.1161/ATV.0000000000000153
4. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
5. Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2023) 8:20–30. doi: 10.1016/S2468-1253(22)00317-X
6. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021
7. Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol. (2018) 68:238–50. doi: 10.1016/j.jhep.2017.11.012
8. Cariou B, Byrne CD, Loomba R, Sanyal AJ. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes Metab. (2021) 23:1069–83. doi: 10.1111/dom.14322
9. Eslam M, Sanyal AJ, George J, International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
10. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
11. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. (2022) 28:528–62. doi: 10.1016/j.eprac.2022.03.010
12. Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. (2021) 160:912–8. doi: 10.1053/j.gastro.2020.11.051
13. Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. (2015) 63:174–82. doi: 10.1016/j.jhep.2015.02.022
14. Sung KC, Ryu S, Lee JY, Kim JY, Wild SH, Byrne CD. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol. (2016) 65:791–7. doi: 10.1016/j.jhep.2016.05.026
15. Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. (2009) 50:1105–12. doi: 10.1002/hep.23129
16. Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. (2011) 60:1278–83. doi: 10.1136/gut.2011.242073
17. Babu AF, Csader S, Lok J, Gómez-Gallego C, Hanhineva K, El-Nezami H, et al. Positive effects of exercise intervention without weight loss and dietary changes in nafld-related clinical parameters: A Systematic Review and Meta-Analysis. Nutrients. (2021) 13:3135. doi: 10.3390/nu13093135
18. Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. (2012) 57:157–66. doi: 10.1016/j.jhep.2012.02.023
19. Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism. (2017) 68:119–32. doi: 10.1016/j.metabol.2016.12.006
20. Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. (2017) 66:142–52. doi: 10.1016/j.jhep.2016.08.023
21. Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. (2013) 59:536–42. doi: 10.1016/j.jhep.2013.04.013
22. Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern Med. (2016) 176:1074–82. doi: 10.1001/jamainternmed.2016.3202
23. Koutoukidis DA, Koshiaris C, Henry JA, Noreik M, Morris E, Manoharan I, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. (2021) 115:154455. doi: 10.1016/j.metabol.2020.154455
24. Du Y, Liu B, Sun Y, Snetselaar LG, Wallace RB, Bao W. Trends in adherence to the physical activity guidelines for americans for aerobic activity and time spent on sedentary behavior among US adults, 2007 to 2016. JAMA Netw Open. (2019) 2:e197597. doi: 10.1001/jamanetworkopen.2019.7597
25. Mu L, Cohen AJ, Mukamal KJ. Resistance and aerobic exercise among adults with diabetes in the U. S Diabetes Care. (2014) 37:e175–e176. doi: 10.2337/dc14-0619
26. Umpierre D, Ribeiro PA, Kramer CK, Leitão CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. (2011) 305:1790–9. doi: 10.1001/jama.2011.576
27. Harris T, Kerry SM, Limb ES, Furness C, Wahlich C, Victor CR, et al. Physical activity levels in adults and older adults 3-4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care. PloS Med. (2018) 15:e1002526. doi: 10.1371/journal.pmed.1002526
28. Khunti K, Griffin S, Brennan A, Dallosso H, Davies MJ, Eborall HC, et al. Promoting physical activity in a multi-ethnic population at high risk of diabetes: the 48-month PROPELS randomised controlled trial. BMC Med. (2021) 19:130. doi: 10.1186/s12916-021-01997-4
29. Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. (2011) 378:129–39. doi: 10.1016/S0140-6736(11)60442-X
30. Ryu S, Chang Y, Jung HS, Yun KE, Kwon MJ, Choi Y, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. (2015) 63:1229–37. doi: 10.1016/j.jhep.2015.07.010
31. Li J, Hua S, Chen GC, Strizich G, Kuniholm MH, Shan Z, et al. Objectively measured sedentary time, physical activity and liver enzyme elevations in US Hispanics/Latinos. Liver Int. (2020) 40:1883–94. doi: 10.1111/liv.14514
32. Balducci S, D'Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, et al. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: the IDES_2 randomized clinical trial. JAMA. (2019) 321:880–90. doi: 10.1001/jama.2019.0922
33. Balducci S, Sacchetti M, Haxhi J, Orlando G, Zanuso S, Cardelli P, et al. The Italian Diabetes and Exercise Study 2 (IDES-2): a long-term behavioral intervention for adoption and maintenance of a physically active lifestyle. Trials. (2015) 16:569. doi: 10.1186/s13063-015-1088-0
34. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. (2012) 35 Suppl 1:S11–63. doi: 10.2337/dc12-s011
35. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
36. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. (2010) 42:503–8. doi: 10.1016/j.dld.2009.08.002
37. Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. (2010) 33:920–2. doi: 10.2337/dc09-1825
38. Petta S, Amato MC, Di Marco V, Cammà C, Pizzolanti G, Barcellona MR, et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. (2012) 35:238–47. doi: 10.1111/j.1365-2036.2011.04929.x
39. Vongsuvanh R, George J, McLeod D, van der Poorten D. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. J Hepatol. (2012) 57:392–8. doi: 10.1016/j.jhep.2012.03.013
40. Amato MC, Giordano C, Pitrone M, Galluzzo A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. (2011) 10:183. doi: 10.1186/1476-511X-10-183
41. Nath P, Panigrahi MK, Sahu MK, Narayan J, Sahoo RK, Patra AA, et al. Effect of exercise on NAFLD and its risk factors: comparison of moderate versus low intensity exercise. J Clin Transl Hepatol. (2020) 8:120–6. doi: 10.14218/JCTH.2019.00012
42. Balducci S, Cardelli P, Pugliese L, D'Errico V, Haxhi J, Alessi E, et al. Volume-dependent effect of supervised exercise training on fatty liver and visceral adiposity index in subjects with type 2 diabetes The Italian Diabetes Exercise Study (IDES). Diabetes Res Clin Pract. (2015) 109:355–63. doi: 10.1016/j.diabres.2015.05.033
43. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. (2015) 149:367–378.e5. doi: 10.1053/j.gastro.2015.04.005
44. Kim D, Vazquez-Montesino LM, Li AA, Cholankeril G, Ahmed A. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology. (2020) 72:1556–68. doi: 10.1002/hep.31158
45. Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. (2007) 30:683–8. doi: 10.2337/dc06-2032
46. Kerr CJ, Waterworth SP, Brodie D, Sandercock GRH, Ingle L. The associations between physical activity intensity, cardiorespiratory fitness, and non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2021) 36:3508–14. doi: 10.1111/jgh.15672
47. Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. (2009) 58:1281–8. doi: 10.1136/gut.2008.151977
48. Johnson NA, George J. Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology. (2010) 52:370–81. doi: 10.1002/hep.23711
49. Petermann-Rocha F, Gray SR, Forrest E, Welsh P, Sattar N, Celis-Morales C, et al. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J Hepatol. (2022) 76:1021–9. doi: 10.1016/j.jhep.2022.01.010
Keywords: type 2 diabetes, nonalcoholic fatty liver disease, liver enzymes, physical activity, sedentary behavior
Citation: Haxhi J, Vitale M, Mattia L, Giuliani C, Sacchetti M, Orlando G, Iacobini C, Menini S, Zanuso S, Nicolucci A, Balducci S and Pugliese G (2024) Effect of sustained decreases in sedentary time and increases in physical activity on liver enzymes and indices in type 2 diabetes. Front. Endocrinol. 15:1393859. doi: 10.3389/fendo.2024.1393859
Received: 29 February 2024; Accepted: 07 May 2024;
Published: 24 May 2024.
Edited by:
Fernando Martin-Rivera, University of Valencia, SpainReviewed by:
Cesare Berra, MultiMedica (IRCCS), ItalyValeria Grancini, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Italy
Copyright © 2024 Haxhi, Vitale, Mattia, Giuliani, Sacchetti, Orlando, Iacobini, Menini, Zanuso, Nicolucci, Balducci and Pugliese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Pugliese, Z2l1c2VwcGUucHVnbGllc2VAdW5pcm9tYTEuaXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Jonida Haxhi
Jonida Haxhi Martina Vitale
Martina Vitale Lorenza Mattia1,2
Lorenza Mattia1,2 Massimo Sacchetti
Massimo Sacchetti Carla Iacobini
Carla Iacobini Stefano Menini
Stefano Menini Silvano Zanuso
Silvano Zanuso Antonio Nicolucci
Antonio Nicolucci Stefano Balducci
Stefano Balducci Giuseppe Pugliese
Giuseppe Pugliese