- 1Department of Doctoral Studies, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
- 2Dr. D Medical Center, Center for Advanced Ultrasound Evaluation, Timisoara, Romania
- 3Center of Molecular Research in Nephrology and Vascular Disease, Faculty of Medicine, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
- 42nd Department of Internal Medicine, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
- 51st Department of Surgery, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
- 6Second Clinic of General Surgery and Surgical Oncology, Emergency Clinical Municipal Hospital, Timisoara, Romania
- 7Endocrinology Unit, Pius Brinzeu Emergency Clinical Hospital, Timisoara, Romania
Introduction: Fine needle aspiration (FNA) is the gold standard method recommended in the diagnosis of thyroid nodules. Bethesda IV cytology results are identified in 7-9% of nodules investigated through FNA, with reported malignancy rate in a wide range of 10-40%. The recommended treatment is either surgical or risk additional molecular testing before surgery. However, a large number of nodules belonging to this category (60-80%) are observed to be benign after surgical excision, which can put the patient at risk of unnecessary surgical morbidity. This study aimed to assess the diagnostic performance of conventional ultrasound, the ACR TI-RADS score and elastography in cases of Bethesda IV cytology on FNA.
Methods: We evaluated ninety-seven consecutive cases with Bethesda category IV results on FNA by using conventional B-mode ultrasound, qualitative strain or shear-wave elastography (Hitachi Preirus Machine, Hitachi Inc., Japan and Aixplorer Mach 30 Supersonic Imagine, Aix-en-Provence, France) and all nodules were classified according to the ACR TI-RADS system. Conventional ultrasound was used to categorize the nodules as potentially malignant based on the following features: hypoechogenicity, inhomogeneity, a taller than wide shape, irregular margins, presence of microcalcifications, an interrupted thyroid capsule and suspicious cervical lymph nodes. Elastography classified nodules with increased stiffness as suspicious for malignancy.
Results: We considered pathology results as the gold standard diagnosis, finding that 32 out of 97 nodules were carcinomas (33%) and 65 out of 97 were benign nodules (67%). The benign group included twenty cases of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Finally, we compared ultrasound data with pathology results, which showed that nineteen out of the 32 malignant nodules presented with increased stiffness on elastography (p=0.0002). On conventional ultrasound, we found that microcalcifications (p=0.007), hypoechogenicity and irregular margins (p=0.006) are features which can distinguish between benign and malignant nodules with statistical significance.
Discussion: Integrating elastography as a parameter of the ACR TI-RADS score in the evaluation of Bethesda category IV nodules showed a sensitivity of 90.62% in detecting thyroid cancer cases (p=0.006). We can conclude that elastographic stiffness as an addition to high risk features observed on conventional ultrasound improves the detection of malignant nodules in cases with Bethesda IV cytology.
1 Introduction
Thyroid nodules are lesions located within the thyroid gland with an ultrasonographically distinct structure compared to the surrounding thyroid parenchyma. Thyroid nodules are a common clinical problem in the general population (1). According to epidemiological studies, the prevalence of thyroid nodules on palpation is low, of approximatively 5% in women and 1% in men living in iodine-sufficient parts of the world. However, the use of high-resolution ultrasound can detect thyroid nodules in up to 50-68% of individuals, with a higher frequency in women and the elderly (2, 3). The most important clinical issue represents differentiating benign from malignant thyroid nodules. Malignant thyroid nodules occur in 5-15% of cases, depending on age, sex, radiation exposure history, family history, and other factors (4, 5). Thyroid cancer is the most common malignant endocrine tumor and accounts for 3.4% of all cancers diagnosed annually. The vast majority of thyroid malignancies (over 90%) are due to differentiated thyroid carcinoma (DTC), which comprises papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) (6, 7).
Initial evaluation of any case where a thyroid nodule is suspected should include a physical examination of the thyroid gland and cervical lymph nodes, assessment for the presence of obstructive symptoms, family history and personal history of risk factors for thyroid cancer. Laboratory evaluation should include a serum thyroid stimulating hormone (TSH) measurement, with further tests such as free thyroxine (FT4), anti-thyroid peroxidase antibodies or TSH receptor antibodies to be used in the presence of a thyroid dysfunction (3, 8).
Ultrasonography is the preferred imaging method for assessing thyroid nodules. Diagnostic neck ultrasound, including the thyroid gland and the central and lateral compartments, should be performed in all patients suspected of having thyroid nodules, or if a nodule was incidentally detected by another imaging modality (3, 8). Ultrasound using a high-frequency, high-resolution transducer can characterize the gland and its nodules very effectively, detecting thyroid nodules of 1.0-2.0 mm, as well as stratify the risk of individual nodules which need to be further evaluated by fine needle aspiration (FNA) (3, 4). Some ultrasound parameters, such as microcalcifications, hypoechogenicity, absence of a halo, increased intranodular vascularity, nodule shape (taller than wide) or irregular margins, have been traditionally associated with an increased risk of malignancy, however, isolated ultrasound features on their own do not provide strong evidence to confirm or rule out a diagnosis of malignancy. The current guidelines recommend the use of a combination of ultrasound features to select thyroid nodules that should be biopsied (9, 10).
Risk stratification systems, established based on ultrasound characteristics, are widely applied to manage thyroid nodules and provide guidance on whether FNA should be performed (11). The American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) uses a point-based method for risk stratification and has been proven to achieve the largest decrease in unnecessary thyroid nodule FNAs. ACR TI-RADS assigns points for each of five ultrasound features (composition, echogenicity, shape, margin, echogenic foci), the sum of which determines a risk category from TR1 (benign) to TR5 (highly suspicious of malignancy) (3, 12). Recommendations for FNA or ultrasound follow-up are based on the nodule’s risk category and its maximum diameter. For risk categories TR1 through TR5 FNA should be recommended according to a size threshold. However, conventional ultrasound examination has limited value for differentiating between benign and malignant thyroid nodules due to its use of a two-dimensional technology (3, 13).
In recent years, ultrasound elastography has been developed as a new technology and applied in clinical practice with good results. Ultrasound elastography is a form of imaging used to analyze the elasticity or stiffness of tissues. The stiffness of a tissue is determined by the structural properties of its matrix. Pathological changes, such as the presence of a tumor or inflammation, alter the tissue composition and structure and increase the lesion stiffness. Thus, ultrasound elastography is a useful evaluation in distinguishing benign from malignant thyroid nodules (14). Based on the fact that on palpation a suspicious thyroid nodule is firm or hard in consistency, stiffness was adopted as indicator of malignancy for elastography (15). In clinical practice, two main thyroid elastography methods are used, depending on which physical quantities are measured: strain elastography (SE) and shear-wave elastography (SWE). SE assesses tissue elasticity through tissue displacement induced by compression. SWE assesses tissue elasticity by measuring the propagation speed of transverse shear waves (16). Considering the increased detection of thyroid nodules in the general population through the widespread use of ultrasound, elastography has emerged as an additional tool for thyroid nodule differentiation, in combination with conventional ultrasound and FNA (14, 15). Diagnostic stiffness information obtained by elastography increases the specificity and positive predictive value of conventional ultrasound (17). However, the WFUMB guideline highlights that not all malignant nodules are stiff, in particular follicular carcinomas tend to be soft or heterogeneous, therefore difficult to distinguish from benign lesions (14). Moreover, current literature provides limited data on the usefulness of ultrasound elastography in the diagnosis of Bethesda category IV nodules (18–20). Conversely, there are rich data suggesting the additional diagnostic value of elastography in thyroid nodules with indeterminate cytology, either grouping Bethesda categories III and IV together or focusing solely on Bethesda category III (21–26).
FNA remains the gold standard method used in the diagnosis of thyroid nodules, due to its high sensitivity and accuracy (27). FNA with a thin-gauge needle (25 or 27) is a safe procedure, without needing to discontinue traditional or novel oral anticoagulant therapy, as well as being cost-effective. It reduces the number of thyroid surgeries on patients with benign lesions, as well as appropriately qualifies patients with thyroid cancer for surgery (3). In 2007 the National Cancer Institute has defined the terminology and morphological criteria for the evaluation of the preparations from the FNA of the thyroid nodules, thereby creating “The Bethesda System for Reporting Thyroid Cytopathology” (TBSRTC). This system is used worldwide to classify cytology findings into six categories (Bethesda I-VI), each having a different malignant potential. Thus, it assists clinicians in understanding a nodule’s risk of malignancy to guide further management (27). The malignant potential of Bethesda category II, V, and VI nodules are well-established, each of these categories having clear recommendations for management (2). On the contrary, Bethesda category III and IV diagnoses are referred to as indeterminate, with a variable malignancy potential and their management depending on the stratification of risk factors (3). The lesions included in Bethesda category IV, defined as follicular neoplasm or suspicious for follicular neoplasm, are characterized by high cellularity, poor or absent colloid and absolute prevalence of microfollicular/trabecular structures, with characteristics suggestive of “follicular neoplasia” (3, 27). Bethesda category IV lesions account for 7-9% of nodules diagnosed through FNA and carry a malignancy rate in a wide range of 10-40% (28). The prevalence of diagnosis for Bethesda category IV has been found to differ in multiple cohorts, from as low as 1.5% to as high as 21.6% (29, 30), while a meta-analysis showed a wide range of 1.2 to 25.3% (mean 10.1%) with a risk of malignancy of 26.1% (31). The third edition of the Bethesda System was published in September 2023, with updates regarding the names of each diagnostic categories as well as their risk of malignancy (32). The cytological examination of all patients in this study was performed prior to the release of this new edition, consequently the results were classified according to the first edition of the Bethesda System (28).
Since 2016 the recommended treatment for Bethesda category IV thyroid nodules is either surgical, by total thyroidectomy or lobectomy, or risk assessment with molecular testing before surgery (2). However, a large number of these nodules (60-80%) are observed to be benign after surgical excision, meaning this group of patients is considered to be at risk of unnecessary surgical morbidity (33).
From a clinical point of view, it would be beneficial to determine which Bethesda category IV nodules are at higher risk of malignancy to better guide the management of these nodules, including the decision to undergo surgical treatment versus conservative options. Since there are studies suggestive for the added value of elastography in Bethesda category III lesions (21–25), the aim of this study is to evaluate and compare the diagnostic performance of greyscale ultrasound focusing on high risk features, the ACR TI-RADS score and ultrasound elastography in the detection of malignant nodules which were classified as Bethesda category IV after cytological testing. This will be achieved by analyzing the sensitivity, specificity, positive and negative predictive values of each method. These results might help in guiding the management of thyroid nodules with Bethesda IV cytology results in clinical practice.
The current guidelines recommend a personalized management in cases with Bethesda category IV thyroid nodules, which presents as a challenge for clinicians, who have to take into account multiple variables and decide between a large range of treatment options. The recommendation of surgical treatment is determined by the sum of identified risk factors, either through personal history, clinical examination or molecular testing. Our research raises the question: Is stiffness a viable additional risk factor in the personalized management of Bethesda category IV thyroid nodules?
2 Materials and methods
2.1 Group characteristics
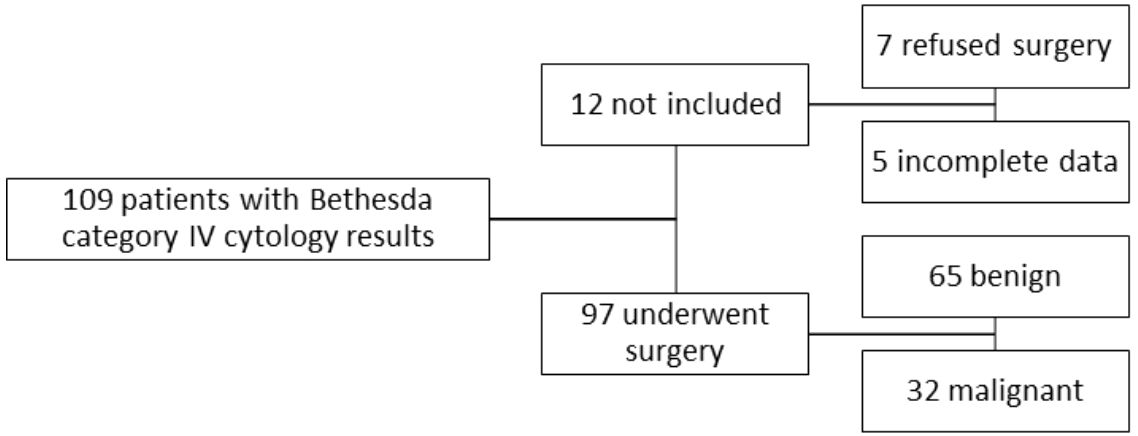
This retrospective study analyzed data from patients evaluated for thyroid nodules in our Endocrinology Clinic (“Dr. D” Medical Center in Timisoara, Romania) from June 2013 until August 2023. Our research included a group of 109 patients between the ages of 25 and 84 years old, all diagnosed by FNA and with their results belonging to the Bethesda category IV. Prior to the FNA procedure, they were examined by conventional ultrasound as well as ultrasound elastography (either strain or shear-wave). Based on their cytology results all of the patients were then referred for surgical intervention, with a recommendation for total thyroidectomy. Ninety-seven of them underwent the operation while the remaining twelve either refused to do so or were lost from our evidence. The study was approved by the Ethics Committee of the Victor Babes University of Medicine and Pharmacy, Timisoara and was performed according to the ethical guidelines of the Helsinki Declaration. A written informed consent was obtained from all patients prior to inclusion.
2.2 Inclusion and exclusion criteria
The present study included only patients who were examined by both conventional ultrasound and elastography in our clinic and had Bethesda category IV cytology results. From the total of 590 FNAs performed in the before mentioned period, 109 cases were Bethesda category IV (18.5%). Ninety-seven of our patients were operated and provided us with the results of the histopathological examination. Out of the ninety-seven patients, sixty-five had benign pathology results and thirty-two were malignant.
We excluded twelve patients who chose not to undergo surgery or who failed to provide us with the histopathology report. Patients who were radiologically examined in a different setting or did not have a complete examination by both conventional ultrasound and elastography were also excluded from this study. We did not include in this study any other Bethesda cytology findings except for category IV. The selection process of the cases is illustrated in Figure 1.
2.3 Conventional ultrasound and elastography examination
Conventional B-mode thyroid ultrasound and strain elastography were performed on the majority of the included patients (77 subjects) with a Hitachi Preirus machine with a 5-15 multi frequency linear probe (Hitachi Medical Corporation, Tokyo, Japan). All subjects were examined by the same practitioner with over 5 years of experience in thyroid ultrasound imaging. The patients were asked to lie supine and keep their neck in a slightly hyper-extended position to fully expose the anterior neck. A pillow was placed behind the neck to help with the hyperextension. Minimal pressure was applied to the neck while acquiring the images and patients were advised not to speak or swallow during the examination. The transverse and longitudinal diameters as well as thyroid volume were measured using grayscale ultrasound (34). Then the presence, location, size (three diameters and volume) and features of each thyroid nodule were assessed. To distinguish between benign and malignant sonographic features, the shape, margin, halo sign, echo structure, homogeneity, echogenicity, calcification and extra capsular invasion of each nodule were examined. The lateral compartments were screened for any abnormal lymph nodes (8). We stratified the risk of each nodule using the ACR TI-RADS system. In the same visit, real-time elastography was performed as a complementary examination to the conventional ultrasound. The patients were asked to hold their breath while the images were acquired. The probe was positioned perpendicular to the skin and light, repetitive compression was applied without lateral movement. The focal zone was placed at or below the level of the nodule. A blue-green-red color map was displayed, with red indicating soft tissue, green indicating intermediate stiffness and blue indicating high stiffness (no strain). A visual scoring system of colors was used to assess the elasticity of the nodule, in this case the 4-point elasticity score (ES) developed by Asteria et al., which showed sensitivities and specificities for thyroid cancer diagnosis of 94.1% and 81% with positive and negative predictive values of 55.2% and 98.2%, respectively (16, 35).
On a smaller proportion of the included patients (20 subjects), conventional B-mode ultrasound (following the above-mentioned technique) and shear-wave elastography were performed using the Aixplorer Mach 30 (Supersonic imagine, Aix-en-Provence, France) machine with and L 18-5 probe (linear, 5-18 MHz). We stratified the risk of each nodule using the ACR TI-RADS system. The “real-time” 2-D SWE technique was used, which allows us to place a larger ROI that can be controlled by the operator, and when activated, a color-coded map of the SWE is displayed in the field of view (FOV). One or more adjustable-measurement ROIs can be placed in the FOV. Soft tissue is displayed in blue and hard tissue is displayed in red. The patient was asked to remain still for a few seconds, a lack of motion being important for acquiring a stable elastogram. The probe was placed perpendicular to the target nodule without pressure, maintaining only slight contact with the skin and four measurements were performed. This method allows for tissue elasticity to be quantitatively estimated and is operator-independent and reproducible, with very good sensitivity and specificity for the diagnosis of thyroid cancer (14, 16, 36–38). For consistency in our study evaluating elastography in thyroid nodules, we employed a qualitative color-coded map to assess stiffness also in the SWE evaluation, similar to the approach used with strain elastography. A visual 4-point scoring system was used to assess the elasticity of each nodule (39).
The suspicious ultrasound features analyzed in this study were hypoechogenicity, inhomogeneity, a taller than wide shape, irregular margins, the presence of microcalcifications, an interrupted thyroid capsule and the presence of suspicious cervical lymph nodes. Regarding the ACR TI-RADS score, we considered categories 2 and 3 to be benign and categories 4 and 5 to be malignant. In regard to the color scoring system, we considered nodules with an ES of 1 or 2 to be benign and those with an ES of 3 or 4 to be malignant. Based on elastography findings, stiff nodules with and ES of 3 or 4 were upgraded to a higher risk category in the TI-RADS + elastography algorithm proposed in this study (18).
2.4 Cytological and histopathological examination
All of the cases underwent ultrasound-guided FNA, which was performed using a 25-gauge needle attached to 10 cc syringes. The patient was asked to lie on their back in a supine position with a pillow underneath their shoulders to obtain the hyperextension of the neck. The skin was thoroughly disinfected and a local anesthetic of 2 g of 5% lidocaine/prilocaine cream was applied locally. The transducer had a sterile cover for the protection of the patient. Firstly, the nodule was located by ultrasound, then the needle was inserted parallel to the probe, using the anterior technique. Confirmation of the needle entering the nodule was observed on ultrasound. The cellular material was gathered using “coring” movements of the needle within the nodule. Each nodule was punctured at least twice, and the obtained material spread on slides (minimum 5 slides per nodule). The slides prepared by aspiration were fixed using 95% ethyl alcohol and stained using May-Grunwald-Giemsa stains for cytological evaluation. All patients signed an informed consent for FNA biopsy. All of the slides containing FNA results were analyzed by the same cytologist with an expertise in thyroid pathology and the Bethesda System for Reporting Thyroid Cytopathology was used to report the results. In this study we included only findings pertaining to the Bethesda category IV.
Total thyroidectomy was recommended to all patients, and was performed by experienced surgeons. All of the surgically resected specimens underwent histopathological examination according to standard procedures. Thyroid tissues were manually processed and fixed in 10% neutralized formaldehyde. Nodules suspected for malignity were embedded in paraffin and sectioned to obtain a thickness of 4 µm. Each tissue section was stained using hematoxylin and eosin (H&E). A team of experienced pathologists from the Pathology Department performed the histological analysis of the slides.
2.5 Data analysis
Microsoft Excel was used for data collection and MedCalc Statistical Software version 20.111 (MedCalc Soft-ware Ltd., Ostend, Belgium) was used for the statistics analysis. The analysis focused on the performance of greyscale ultrasound parameters, the ACR TI-RADS algorithm, elastography and the association of ACR TI-RADS and elastography in distinguishing between benign and malignant thyroid nodules. We determined the true-positive (TP), true-negative (TN), false-negative (FN) and false-positive (FP) diagnoses. Sensitivity (Se) was calculated as TP/(TN+FP), specificity (Sp) as TN/(TN+FP), accuracy as TP+TN/total cases, positive predictive value (PPV) as TP/(TP+FP) and negative predictive value (NPV) was calculated as TN/(TN+FN). Fisher exact 2-tailed test and multiple regression analysis were used for statistical analyses. Area under the receiver operated characteristic curve (AUC-ROC) analysis was performed to evaluate the sensibility and specificity of each parameter in relation to the presence of cancerous nodules. The Fisher’s exact test was used to determine whether any of the analyzed ultrasound and elastography parameters are more likely to be present in malignant versus benign lesions. Prior to the analysis of numerical data, the normality of data distribution was tested using the Shapiro-Wilk test. Consequentially, we performed statistical tests appropriate for the normality of the data in question: medians and the Mann-Whitney test were used to describe the non-normally distributed data. The Pearson correlation coefficient (Phi coefficient) was employed to evaluate the strength and direction of association between binary parameters. The significance threshold was considered a p-value of less than 0.05.
3 Results
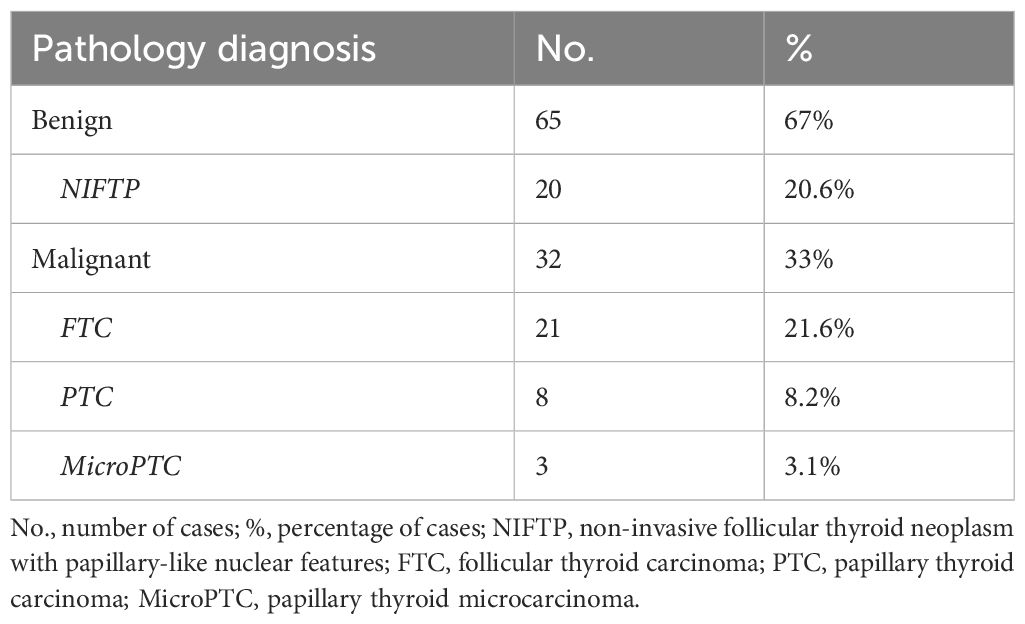
The 97 participants were divided into two groups according to the histological examination of their thyroid nodules, malignant vs benign (see Table 1). Out of the 65 patients (67%) in the benign group, 20 (20.6%) had a histopathological result of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). The cancer group included 32 patients (33%), 21 of them with follicular thyroid carcinoma (FTC) (21.6%), 8 with papillary thyroid carcinoma (PTC) (8.2%) and 3 cases of papillary thyroid microcarcinoma (microPTC) (3.1%); for the further statistical analyses we included PTC and microPTC cases in the same group of 11 patients (11.3%).
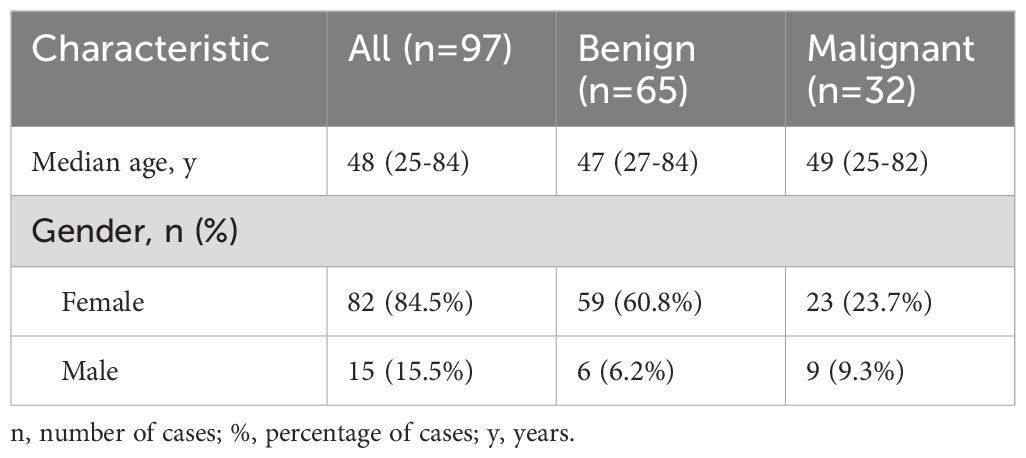
Out of our total 97 patients, 82 were females (84.5%) and 15 were males (15.5%). The cancer group included 23 females (23.7%) and 9 males (9.3%), while in the cancer-free group only 6 of the patients were males (6.2%) and 59 were female (60.8%), as shown in Table 2. The AUC-ROC analysis showed that gender has significant specificity in discriminating between cancerous and benign thyroid nodules, but low sensitivity (AUC=0.59, p=0.03, Se%=28.12, 95% CI=13.7 - 46.7, Sp%=90.77, 95% CI=81.0 - 96.5, PPV%=60, NPV%=72). With regard to age, the median age of the 97 participants was 48 years (limits 25-84 years, see Table 2). No significant differences were detected between the two groups, the median age in the cancer group was 49 years (limits 25-82 years), while in the cancer-free group it was 47 years (limits 27-84 years), p=0.75. The AUC-ROC analysis for age showed that it is not a reliable distinguisher between benign and malignant nodules, p=0.76, with a satisfying sensitivity, but an extremely low specificity (cut-off ≤66 years, AUC=0.52, Se%=78.1, 95% CI=60.0 - 90.7, Sp%=9.23, 95% CI=3.5 - 19.0, PPV%=29.8, NPV%=46.2).
With regard to the nodular maximal diameter and nodular volume as possible discriminators between benign and malignant nodules, the AUC-ROC analysis revealed good sensitivities, but low specificities, and the p-values did not reach significance. Malignant nodules presented a higher median for maximal diameter than benign ones (2.25 cm vs 1.9 cm), but the significance threshold was not met (p=0.78). Moreover, cancerous nodules also presented a higher volume than benign ones, with median values of 3.38 ml vs 2.01 ml, but the difference was still not statistically significant (p=0.46), as illustrated in Table 3.
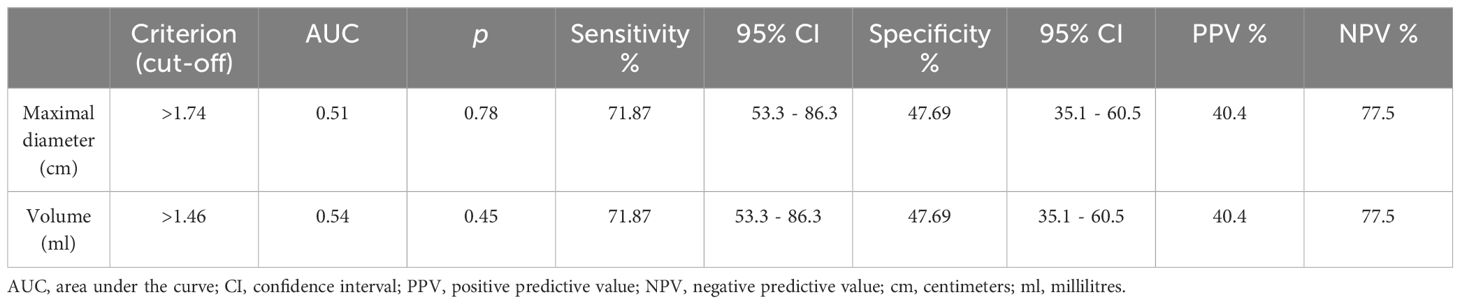
Table 3 The area under the receiver operating characteristic curve analysis and cut-off values for nodular maximal diameter and nodular volume in distinguishing between benign and malignant Bethesda category IV nodules.
While all ultrasound features considered as suspicious for malignancy, as well as elastographic stiffness (ES scores 3 and 4) were overall more prevalent in cancerous lesions, the statistical analysis revealed significant results only for microcalcifications (p=0.001) and elastographic stiffness (p=0.0004). Consequently, the presence of microcalcifications and lesions presenting as stiff on elastography could be used as indicators of malignancy in the evaluation of Bethesda category IV thyroid nodules. Table 4 presents descriptive data regarding the presence of each ultrasound feature suspicious for malignancy and elastographic stiffness in the two analyzed groups, as well as the results of the Fisher’s exact test used to compare them.
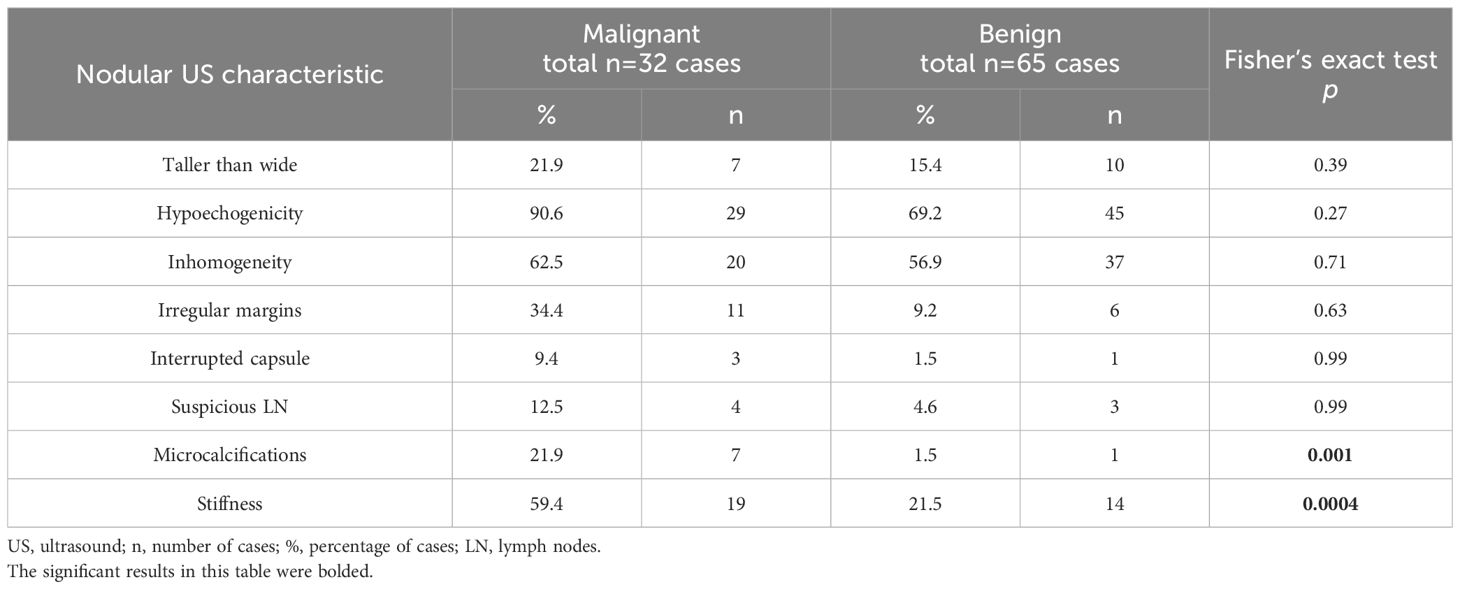
Table 4 The analysis of ultrasound characteristics for each nodule examined; the Fisher’s exact test determines whether the analyzed US characteristics are more likely to be present in one of the two groups.
In terms of the performance of greyscale ultrasound in distinguishing between benign and malignant nodules, we observed that three of the seven high-risk features were statistically significant according to the AUC-ROC analysis: the presence of microcalcifications (p=0.007), hypoechogenicity and irregular margins (p=0.006), as seen in Figure 2 and Table 5. Microcalcifications were highly specific for cancer (Sp%=98.5) and were present in 7 of our malignant nodules. Hypoechogenicity, although with a relatively low specificity, has a significantly high sensitivity and was present in all cases of PTC (Se%=100) and in 18 out of 21 cases of FTC (Se%=85.7). In contrast, irregular or lobulated margins which were present in 11 of the malignant nodules, were observed to have a significant specificity but a quite low sensitivity. In terms of the other four features for which we didn’t find statistically significant data, nodules with a taller than wide shape comprised 7 out of all cancers, this feature presenting a relatively high specificity but a quite low sensitivity. Inhomogeneity was found to offer quite modest results in terms of both sensitivity and specificity; it characterized 20 out of our 32 malignant nodules. The presence of an interrupted capsule and of suspicious lymph nodes were both highly specific features (Sp%=94.46 and Sp%=95.36, respectively), but because of their low prevalence, had a low sensitivity.
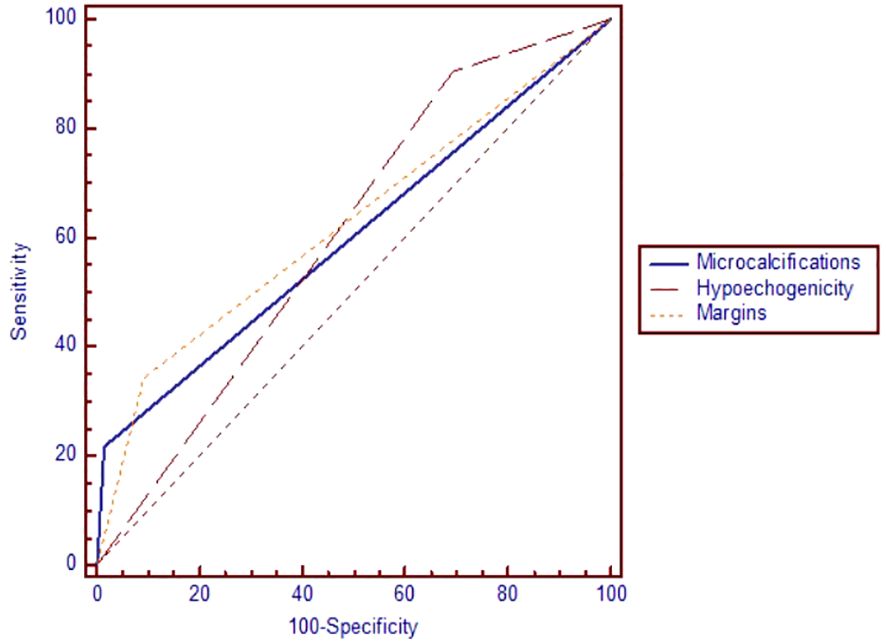
Figure 2 The area under the receiver operating characteristic curve for microcalcifications, hypoechogenicity and irregular margins.
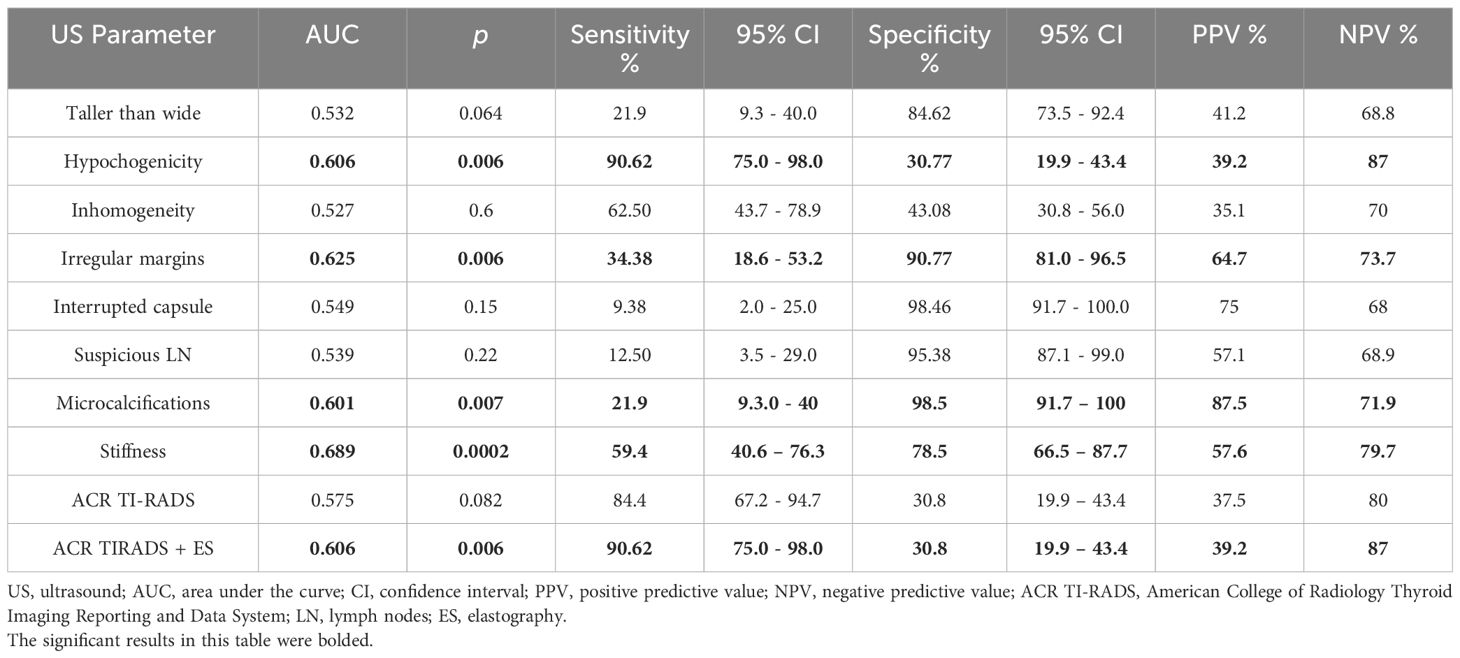
Table 5 The area under the receiver operating characteristic curve analysis for the performance of ultrasound parameters in distinguishing between benign and malignant thyroid nodules.
The Pearson (Phi) coefficient was calculated between ultrasound parameters and elastography. A moderate, positive association was found between elastography and echogenicity, of approximately 0.408 (p<0.0001). Another positive, weak to moderate correlation was established between elastography and ACR-TIRADS category (phi=0.387, p<0.001), and a positive, weak correlation with homogeneity (phi=0.260, p=0.01).
The statistical significance threshold was not met for the ACR TI-RADS score to be used as standalone risk assessment method (see Table 5). With regard to elastographic stiffness, we found a satisfying specificity, but quite average sensibility (AUC=0.68, p=0.0002, Se=59.4%, Sp=78.5, PPV=57.6%, NPV=79.7%). Considering elastography color scores 1 and 2 as suggestive for benignity and scores 3 and 4 as predictors for malignancy, stiffness evaluation helped us identify 64 nodules belonging to the benign group (score 1 and 2) and 33 nodules to the malignant group (scores 3 and 4). Less than half of the cases (13 out of 32) with a histological diagnosis of malignancy were characterized as soft based on elastography (scores 1 and 2). Overall, the prevalence of cancer in the soft lesions (scores 1 and 2) was 20%. As for stiff lesions, 57% of scores 3 and 4 cases proved to be malignant.
Testing the ROC curves regarding differences between the ACR-TIRADS score, elastographic stiffness and ACR-TIRADS + elastography, rendered the results depicted in Figure 3. Comparing ACR-TIRADS vs ACR-TIRADS + elastography curves did not reveal significant differences, p=0.15. Neither did comparing ACR-TIRADS vs elastographic stiffness (p=0.77), nor ACR-TIRADS + elastography vs elastographic stiffness (p=0.62). However, the AUC-ROC analysis shows that the combined use of the ACR TI-RADS score (which is a sum of high risk features observed on conventional ultrasound) with elastographic stiffness as a complementary method for risk upgrade represents the most sensitive diagnostic algorithm for detecting cancer (AUC=606, p=0.006) (see Table 5). Figure 4 illustrates the simultaneous evaluation of the thyroid nodules included in this study through B-mode ultrasound and elastography (strain and shear-wave).
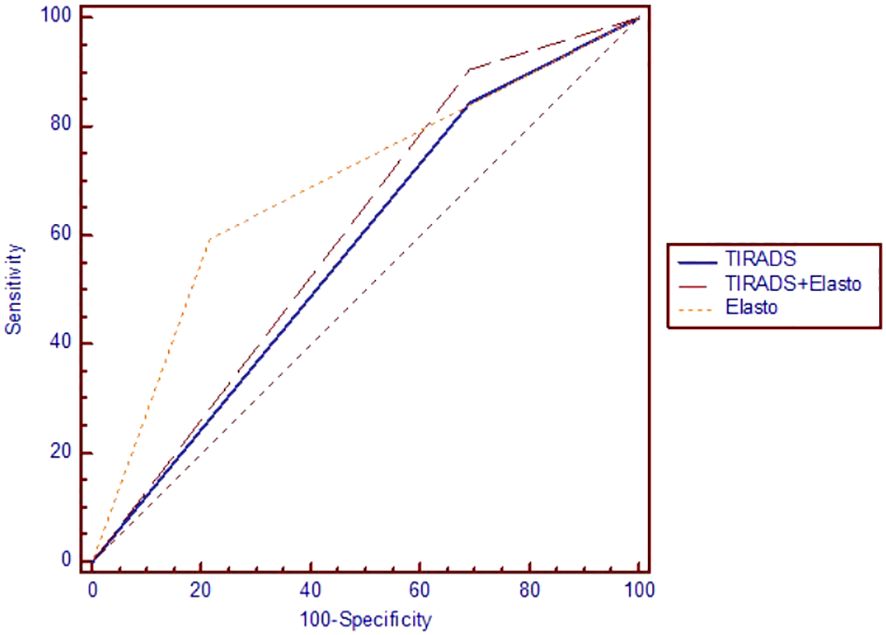
Figure 3 The area under the receiver operating characteristic curve for ACR TI-RADS and elastography.
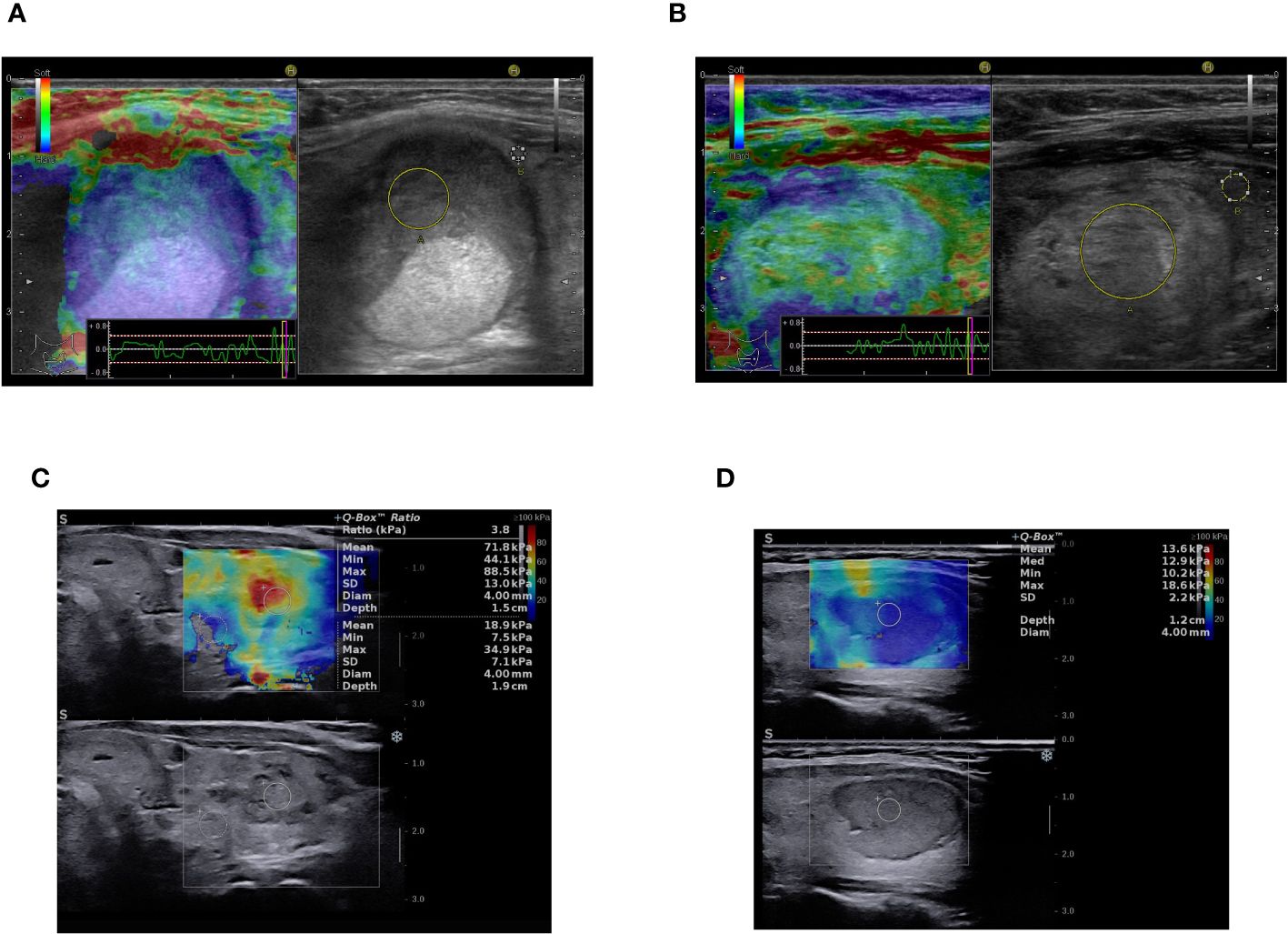
Figure 4 Multiparametric ultrasound-based evaluation: (A) B-mode image on the right - solid, oval, inhomogeneous, hypoechoic thyroid nodule with smooth margins, ACR-TIRADS 4, strain elastography color-map on the left - stiff in elastography (color code blue), asteria 4, ACR-TIRADS + elasography score 5, Histopathology: NIFTP. (B) B-mode image on the right - solid, oval, inhomogeneous, isoechoic thyroid nodule with smooth margins, ACR-TIRADS 3, strain elastography color-map on the left - mostly soft in elastography (code green), asteria 2, ACR-TIRADS + elasography score 3, Histopathology: NIFTP. (C) B-mode image on the bottom - solid, oval, inhomogeneous, hypoechoic thyroid nodule with ill-defined margins, ACR-TIRADS 4, SWE elastography color-map on the upper side of the image - stiff in elastography (code red), asteria 4, ACR-TIRADS + elasography score 5, Histopathology: Follicular thyroid carcinoma. (D) B-mode image on the bottom - solid, oval, homogeneous, hypoechoic thyroid nodule with smooth, lobulated margins, ACR-TIRADS 5, SWE elastography color-map on the upper side of the image - mostly soft in elastography (code blue-yellow), asteria 2, ACR TIRADS + elasography remains score 5, Histopathology: Follicular adenoma.
4 Discussion
Ultrasound is the preferred method for the detection of thyroid nodules and in necessary cases can be followed up by ultrasound-guided FNA to gain information for further medical management. FNA helps to reduce unnecessary surgeries and identifies nodules with a high risk of malignancy. The cytology findings of FNA are classified according to the Bethesda system. In case of nondiagnostic or unsatisfactory thyroid FNA results, ultrasound findings can guide the pathologist to manage these particular thyroid nodules (40). However, the risk of malignancy for thyroid nodules classified as Bethesda category IV varies widely. Our study explored whether certain characteristics of thyroid nodules on both ultrasound and elastography can facilitate clinicians to interpret the risk for malignancy of Bethesda category IV nodules and aid in their further management.
Malignancy rates of Bethesda category IV nodules vary from institute to institute, higher rates more commonly found in multicentric studies, from as low as 16.8% (41) to as high as 72.4% (42), however multiple studies reported malignancy rates between 25-50% (43–45). The present study evaluated a cohort of 97 patients with Bethesda category IV cytology findings and a complete histological report. Our results found the malignancy rate for Bethesda category IV nodules (33%) to be only slightly higher than what was outlined by the 2017 Bethesda System for Reporting Thyroid Cytopathology guideline (28) and similar to more recent studies with a large cohort (46), indicating that our nodules were correctly characterized based on cytology, avoiding selection biases. In our series we found 20.6% of NIFTP cases after histological examination, cases which are usually clustered within Bethesda categories III, IV and V, rendering our findings consistent with previous studies (47, 48). However, among the cancer group, follicular cancer was the most common malignant variant, followed by papillary thyroid cancer, which is in contrast to the histopathological distribution reported by other investigators and could be explained by a lower number of cases (43, 49). Malignancy rates carry an important weight in guiding clinicians and surgeons to select cases for surgical treatment or observation and follow-up (50).
In the present study, the median age was 48 years and no significant differences between the cancer and cancer-free group were observed, with a greater proportion of females (82%) compared to males, which is comparable to other published studies (44, 50). According to our results, age cannot be used as a reliable factor in predicting malignancy, findings which differ from several others in specialized literature, where younger age was identified as an independent risk factor for thyroid cancer in indeterminate nodules and an inverse correlation between patient age and malignancy risk for nodules undergoing FNA was reported (51–53). However, we did find that female gender is a significant risk factor for malignancy, which is in line with other studies (53, 54). In terms of thyroid nodule size, our medians of 2.25 cm for malignant nodules and 1.9 cm for benign ones are comparable to other reports (54, 55), but a significant association between nodule size and risk of malignancy cannot be identified, a finding consistent with the results of other investigations (52, 55).
In general, ultrasound features suspicious for malignancy in indeterminate thyroid nodules include hypoechogenicity, inhomogeneity, irregular margins, a taller than wide shape, solid composition, the presence of microcalcifications and extra-capsular extension (2, 56, 57). It was reported that irregular margins, hypoechogenicity and a taller than wide shape were all associated with malignancy in thyroid nodules, the highest positive predictive value being attributed to irregular margins (56, 58). However, specialized literature reports a variable utility of these ultrasound features, with some studies suggesting their potential utility and others stating that specific ultrasound features are poorly predictive of malignancy when used singularly (59, 60). Our results confirm the findings of several studies, which showed hypoechogenicity to be the only feature with a sensitivity of 87.2%, comparable to our finding of 90.62% (61, 62). Multiple studies also report that the presence of microcalcifications and irregular margins are both features that show a high specificity for malignancy, ranging from 90.8% to 96.1%, which was also observed in our study (61, 63), microcalcifications being the feature with the highest specificity (98.5%). It has been demonstrated that a taller than wide shape is the most accurate ultrasound feature for judging the malignancy of a thyroid nodule (57, 60, 62). In our study, we also observed a relationship between the taller than wide nodular shape and the risk of malignancy. Extra-capsular extension is highly reliable sign of malignancy as well as an unfavorable prognostic sign (64). Minimal extra-capsular extension can be suspected on ultrasound examination in the presence of border abutment, contour bulging, or loss of the echogenic border, however clinicians need to be cautious when reporting minimal extension, particularly in case of otherwise benign-appearing nodules (65, 66). Ultrasound examination includes cervical lymph node evaluation, which should be described as normal, indeterminate, or suspicious, and located using the six cervical levels nomenclature. Lymph nodes of high suspicion present cystic areas, microcalcifications, thyroid tissue-like appearance, and anarchic vascularity with the absence of a visible hilum (8, 67). According to multiple studies, the co-presentation of hypoechogenicity, irregular margins, and microcalcifications was associated with a greater risk of cancer than any of these features used in isolation, and had a higher specificity but lower sensitivity for diagnosing malignancy (44, 52, 57, 60).
With regard to ultrasound risk stratification systems, which offer guidance in assessing thyroid nodules and selecting those which need to be further evaluated by FNA (68, 69), we have chosen for our research the point-based ACR TI-RADS scoring system, as it was demonstrated to have the highest diagnostic performance, significantly superior to American Thyroid Association guidelines (70). The question was raised for the possible use of the EU-TIRADS system, taking into account the geographic area of our patients, but further research showed that the ACR TI-RADS system provides a better performance (71). Our research revealed a lower sensitivity, specificity and PPV for the ACR TI-RADS system compared to the results observed in similar studies, due to a high proportion of the nodules in our benign group being described as solid and hypoechogenic and thus classified as ACR TI-RADS category 4, which we considered as suggestive for malignancy. However, our findings are in agreement with the previously mentioned studies, that the ACR TI-RADS system is not able to yield a statistically significant correlation between ultrasound risk category and malignancy (72, 73). Recent data underlines that combining the ACR-TIRADS algorithm with either strain or shear-wave examination maximizes the correct classification of thyroid nodules with similar accuracy between them, while the type of elastography can be chosen according to the preferences of each clinician (74).
The diagnostic performance of evaluating thyroid nodules belonging to intermediate categories, including Bethesda category IV, by ultrasound only has shown poorer outcomes, compared to the nodules classified in the other Bethesda categories, thus highlighting the importance of adding other evaluation methods to these cases (75). Data recorded in multiple studies shows that the majority of Bethesda category IV lesions present as heterogeneous on ultrasound, with usually unsuspicious features, meaning that standalone ultrasound characteristics and the ACR-TIRADS algorithm, while reliable methods to detect papillary thyroid carcinoma, have a lower accuracy for other histological types, including follicular lesions. Due to the reported lack of suspicious ultrasound features in follicular thyroid carcinoma, and acknowledging the limitations of the cytological evaluation in its detection, it is prudent to exercise caution when employing ultrasound for the management of nodules classified as Bethesda category IV (76, 77).
Risk predictions were enhanced by the combined use of conventional ultrasound characteristics with the elastography color score (18–22). Elastography, used as a parameter of the TI-RADS score, increases the diagnostic accuracy of patients with indeterminate thyroid cytology and may lead to a reduction of surgeries in cases with low stiffness (23). In the present study, by including elastography as a parameter of ACR TI-RADS through upgrading nodules described as stiff on elastography (scores 3 and 4), we noticed an increase in specificity (from 84.37% to 90.62%) as well as in the negative predictive value (from 79.2% to 84.2%), which is in line with other findings (23, 24). As mentioned above, since a significant proportion of our ACR-TIRADS score 4 nodules were benign, therefore lowering the specificity of the algorithm itself, only a modest improvement in specificity could be observed when adding stiffness as an additional risk factor. These results reconfirm that including elastography as a parameter of the ACR TI-RADS score can help with the selection of malignant nodules in case of Bethesda IV cytology, thus avoiding unnecessary surgery in these cases, as well as optimizing their personalized management, which can present as a challenge for the clinician (25, 26).
5 Conclusions
Our results demonstrate that while certain ultrasonographic characteristics—like microcalcifications, hypoechogenicity, and irregular margins, along with elastographic stiffness—provide valuable insights into detecting malignancy among Bethesda IV cytology nodules, integrating elastography into the ACR TI-RADS scoring system emerges as the most sensitive diagnostic approach. This method effectively identifies malignant nodules, thereby reducing the incidence of unnecessary surgeries. Considering stiffness as a risk factor further refines ultrasound-based diagnostic accuracy even in this particular cytological category.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Victor Babes University of Medicine and Pharmacy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ML: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. AB: Conceptualization, Methodology, Project administration, Validation, Visualization, Writing – review & editing. MM: Formal analysis, Software, Validation, Visualization, Writing – review & editing, Data curation. ON: Project administration, Resources, Writing – review & editing, Supervision. DS: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Victor Babes University of Medicine and Pharmacy from Timisoara, Romania provided financial support for the publication of this study. The funders had no involvement in the study’s design, implementation, data collection, analysis, interpretation, manuscript preparation, review, approval, or decision to submit for publication.
Acknowledgments
We would like to express our gratitude to each author for their valuable contributions and acknowledge the support of our respective affiliations, which made this study possible. We would like to extend special thanks to the Victor Babes University of Medicine and Pharmacy from Timisoara, for providing financial support for the publication of this study. We extend our sincere appreciation and wishes of good health to all the patients who agreed to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mu C, Ming X, Tian Y, Liu Y, Yao M, Ni Y, et al. Mapping global epidemiology of thyroid nodules among general population: A systematic review and meta-analysis. Front Oncol. (2022) 12:1029926. doi: 10.3389/fonc.2022.1029926
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
3. Kobaly K, Kim CS, Mandel SJ. Contemporary management of thyroid nodules. Annu Rev Med. (2022) 73:517–28. doi: 10.1146/annurev-med-042220-015032
4. Remonti LR, Kramer CK, Leitão CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. (2015) 25:538–50. doi: 10.1089/thy.2014.0353
5. Grussendorf M, Ruschenburg I, Brabant G. Malignancy rates in thyroid nodules: a long-term cohort study of 17,592 patients. Eur Thyroid J. (2022) 11:e220027. doi: 10.1530/ETJ-22-0027
6. Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. (2019) 48:23–35. doi: 10.1016/j.ecl.2018.10.002
7. Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol (Lausanne). (2020) 11:102. doi: 10.3389/fendo.2020.00102
8. Durante C, Hegedüs L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur Thyroid J. (2023) 12:e230067. doi: 10.1530/ETJ-23-0067
9. Xie C, Cox P, Taylor N, LaPorte S. Ultrasonography of thyroid nodules: a pictorial review. Insights Imaging. (2016) 7:77–86. doi: 10.1007/s13244-015-0446-5
10. Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. (2010) 43:229–vii. doi: 10.1016/j.otc.2010.01.002
11. Floridi C, Cellina M, Buccimazza G, Arrichiello A, Sacrini A, Arrigoni F, et al. Ultrasound imaging classifications of thyroid nodules for Malignancy risk stratification and clinical management: state of the art. Gland Surg. (2019) 8:S233–44. doi: 10.21037/gs.2019.07.01
12. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
13. Nie W, Zhu L, Yan P, Sun J. Thyroid nodule ultrasound accuracy in predicting thyroid Malignancy based on TIRADS system. Adv Clin Exp Med. (2022) 31:597–606. doi: 10.17219/acem/146776
14. Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: part 4. Thyroid Ultrasound Med Biol. (2017) 43:4–26. doi: 10.1016/j.ultrasmedbio.2016.06.022
15. Cantisani V, De Silvestri A, Scotti V, Fresilli D, Tarsitano MG, Polti G, et al. US-elastography with different techniques for thyroid nodule characterization: systematic review and meta-analysis. Front Oncol. (2022) 12:845549. doi: 10.3389/fonc.2022.845549
16. Zhao CK, Xu HX. Ultrasound elastography of the thyroid: principles and current status. Ultrasonography. (2019) 38:106–24. doi: 10.14366/usg.18037
17. Cappelli C, Pirola I, Gandossi E, Agosti B, Cimino E, Casella C, et al. Real-time elastography: a useful tool for predicting Malignancy in thyroid nodules with nondiagnostic cytologic findings. J Ultrasound Med. (2012) 31:1777–82. doi: 10.7863/jum.2012.31.11.1777
18. Huang S, Meng N, Pan M, Yu B, Liu J, Deng K, et al. Diagnostic performances of the KWAK-TIRADS classification, elasticity score, and Bethesda System for Reporting Thyroid Cytopathology of TI-RADS category 4 thyroid nodules. Int J Clin Exp Pathol. (2020) 13:1159–68.
19. Samir AE, Dhyani M, Anvari A, Prescott J, Halpern EF, Faquin WC, et al. Shear-wave elastography for the preoperative risk stratification of follicular-patterned lesions of the thyroid: diagnostic accuracy and optimal measurement plane. Radiology. (2015) 277:565–73. doi: 10.1148/radiol.2015141627
20. Wojtaszek-Nowicka M, Słowińska-Klencka D, Sporny S, Popowicz B, Kuzdak K, Pomoroski L, et al. The efficiency of elastography in the diagnostics of follicular lesions and nodules with an unequivocal FNA result. Endokrynol Pol. (2017) 68:610–22. doi: 10.5603/EP.a2017.0050
21. Borlea A, Stoian D, Cotoi L, Sporea I, Lazar F, Mozos I. Thyroid multimodal ultrasound evaluation—Impact on presurgical diagnosis of intermediate cytology cases. Appl Sci. (2020) 10:3439. doi: 10.3390/app10103439
22. Garino F, Deandrea M, Motta M, Mormile A, Ragazzoni F, Palestini N, et al. Diagnostic performance of elastography in cytologically indeterminate thyroid nodules. Endocrine. (2015) 49:175–83. doi: 10.1007/s12020-014-0438-0
23. Stoian D, Borcan F, Petre I, Mozos I, Varcus F, Ivan V, et al. Strain elastography as a valuable diagnosis tool in intermediate cytology (Bethesda III) thyroid nodules. Diagnostics (Basel). (2019) 9:119. doi: 10.3390/diagnostics9030119
24. Russ G, Royer B, Bigorgne C, Rouxel A, Bienvenu-Perrard M, Leenhardt L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol. (2013) 168:649–55. doi: 10.1530/EJE-12-0936
25. Habib L, Abdrabou A, Geneidi E, Sultan Y. Role of ultrasound elastography in assessment of indeterminate thyroid nodules. Egyptian J Radiol Nucl Med. (2015) 47. doi: 10.1016/j.ejrnm.2015.11.002
26. Moraes PHM, Takahashi MS, Vanderlei FAB, Schelini MV, Chacon DA, Tavares MR, et al. Multiparametric ultrasound evaluation of the thyroid: elastography as a key tool in the risk prediction of undetermined nodules (Bethesda III and IV)-histopathological correlation. Ultrasound Med Biol. (2021) 47:1219–26. doi: 10.1016/j.ultrasmedbio.2021.01.019
27. Wesoła M, Jeleń M. Bethesda System in the evaluation of thyroid nodules: Review. Adv Clin Exp Med. (2017) 26:177–82. doi: 10.17219/acem/27319
28. Cibas ES, Ali SZ. The 2017 bethesda system for reporting thyroid cytopathology. Thyroid. (2017) 27:1341–6. doi: 10.1089/thy.2017.0500
29. Rai K, Park J, Gokhale S, Irshaidat F, Singh G. Diagnostic accuracy of the bethesda system for reporting thyroid cytopathology (TBSRTC): an institution experience. Int J Endocrinol. (2023) 2023:9615294. doi: 10.1155/2023/9615294
30. Inabnet WB 3rd, Palazzo F, Sosa JA, Kriger J, Aspinall S, Barczynski M, et al. Correlating the bethesda system for reporting thyroid cytopathology with histology and extent of surgery: A review of 21,746 patients from four endocrine surgery registries across two continents. World J Surg. (2020) 44:426–35. doi: 10.1007/s00268-019-05258-7
31. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. (2012) 56:333–9. doi: 10.1159/000339959
32. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 bethesda system for reporting thyroid cytopathology. Thyroid. (2023) 33:1039–44. doi: 10.1089/thy.2023.0141
33. Lee SH, Baek JS, Lee JY, Lim JA, Cho SY, Lee TH, et al. Predictive factors of Malignancy in thyroid nodules with a cytological diagnosis of follicular neoplasm. Endocr Pathol. (2013) 24:177–83. doi: 10.1007/s12022-013-9263-x
34. Viduetsky A, Herrejon CL. Sonographic evaluation of thyroid size: A review of important measurement parameters. J Diagn Med Sonography. (2019) 35:206–10. doi: 10.1177/8756479318824290
35. Dietrich CF, Barr RG, Farrokh A, Dighe M, Hocke M, Jenssen C, et al. Strain elastography - how to do it? Ultrasound Int Open. (2017) 3:E137–49. doi: 10.1055/s-0043-119412
36. Ferraioli G, Barr RG, Farrokh A, Radzina M, Cui XW, Dong Y, et al. How to perform shear wave elastography. Part I. Med Ultrason. (2022) 24:95–106. doi: 10.11152/mu-3217
37. Liao LJ, Chen HW, Hsu WL, Chen YS. Comparison of strain elastography, shear wave elastography, and conventional ultrasound in diagnosing thyroid nodules. J Med Ultrasound. (2019) 27:26–32. doi: 10.4103/JMU.JMU_46_18
38. Chang N, Zhang X, Wan W, Zhang C, Zhang X. The preciseness in diagnosing thyroid Malignant nodules using shear-wave elastography. Med Sci Monit. (2018) 24:671–7. doi: 10.12659/msm.904703
39. Zhang YX, Xue JP, Li HZ, Miao JW, Kang CS. Clinical value of shear wave elastography color scores in classifying thyroid nodules. Int J Gen Med. (2021) 14:8007–18. doi: 10.2147/IJGM.S331406
40. Poller DN. Value of cytopathologist review of ultrasound examinations in non-diagnostic/unsatisfactory thyroid FNA. Diagn Cytopathol. (2017) 45:1084–7. doi: 10.1002/dc.23822
41. Godoi Cavalheiro B, Kober Nogueira Leite A, Luongo de Matos L, Palermo Miazaki A, Ientile JM, Kulcsar MAV, et al. Malignancy rates in thyroid nodules classified as bethesda categories III and IV: retrospective data from a tertiary center. Int J Endocrinol Metab. (2017) 16:e12871. doi: 10.5812/ijem.12871
42. Chirayath SR, Pavithran PV, Abraham N, Nair V, Bhavani N, Kumar H, et al. Prospective study of bethesda categories III and IV thyroid nodules: outcomes and predictive value of BRAFV600E mutation. Indian J Endocrinol Metab. (2019) 23:278–81. doi: 10.4103/ijem.IJEM_635_18
43. Zahid A, Shafiq W, Nasir KS, Loya A, Raza SA, Sohail S, et al. Malignancy rates in thyroid nodules classified as Bethesda categories III and IV; a subcontinent perspective. J Clin Transl Endocrinol. (2021) 23:100250. doi: 10.1016/j.jcte.2021.100250
44. Yaprak Bayrak B, Eruyar AT. Malignancy rates for Bethesda III and IV thyroid nodules: a retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC Endocr Disord. (2020) 20:48. doi: 10.1186/s12902-020-0530-9
45. Özdemir A, Uyan M, Kalcan S, Çolakoglu M, Pergel A. Malignancy rates and risk factors in Bethesda category IV thyroid nodules: Is lobectomy enough in an endemic region? J Exp Clin Med. (2022) 39:749–54. doi: 10.52142/omujecm.39.3.30
46. Delman AM, Turner KM, Ammann AM, Sisak S, Farooqui Z, Holm TM. The national rate of Malignancy among Bethesda III, IV, and V thyroid nodules is higher than expected: A NSQIP analysis. Surgery. (2023) 173:645–52. doi: 10.1016/j.surg.2022.06.049
47. Faquin WC, Wong LQ, Afrogheh AH, Ali SZ, Bishop JA, Bongiovanni M, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of Malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol. (2016) 124:181–7. doi: 10.1002/cncy.21631
48. Maletta F, Massa F, Torregrossa L, Duregon E, Casadei GP, Basolo F, et al. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol. (2016) 54:134–42. doi: 10.1016/j.humpath.2016.03.014
49. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
50. Pereira C. Malignancy rates in thyroid nodules classified as bethesda III and IV; correlating fine needle aspiration cytology with histopathology. Prague Med Rep. (2022) 123:243–9. doi: 10.14712/23362936.2022.22
51. Talmor G, Badash I, Zhou S, Kim YJ, Kokot NC, Hsueh W, et al. Association of patient characteristics, ultrasound features, and molecular testing with Malignancy risk in Bethesda III-V thyroid nodules. Laryngoscope Investig Otolaryngol. (2022) 7:1243–50. doi: 10.1002/lio2.847
52. Rago T, Scutari M, Latrofa F, Loiacono V, Piaggi P, Marchetti I, et al. The large majority of 1520 patients with indeterminate thyroid nodule at cytology have a favorable outcome, and a clinical risk score has a high negative predictive value for a more cumbersome cancer disease. J Clin Endocrinol Metab. (2014) 99:3700–7. doi: 10.1210/jc.2013-4401
53. Bessey LJ, Lai NB, Coorough NE, Chen H, Sippel RS. The incidence of thyroid cancer by fine needle aspiration varies by age and gender. J Surg Res. (2013) 184:761–5. doi: 10.1016/j.jss.2013.03.086
54. Nagarkatti SS, Faquin WC, Lubitz CC, Morales Garcia D, Barbesino G, Ross DS, et al. Management of thyroid nodules with atypical cytology on fine-needle aspiration biopsy. Ann Surg Oncol. (2013) 20:60–5. doi: 10.1245/s10434-012-2601-2
55. Kiernan CM, Solórzano CC. Bethesda Category IV III. and V thyroid nodules: can nodule size help predict Malignancy? J Am Coll Surg. (2017) 225:77–82. doi: 10.1016/j.jamcollsurg.2017.02.002
56. Chng CL, Kurzawinski TR, Beale T. Value of sonographic features in predicting Malignancy in thyroid nodules diagnosed as follicular neoplasm on cytology. Clin Endocrinol (Oxf). (2015) 83:711–6. doi: 10.1111/cen.12692
57. Kuru B, Kefeli M. Risk factors associated with Malignancy and with triage to surgery in thyroid nodules classified as Bethesda category IV (FN/SFN). Diagn Cytopathol. (2018) 46:489–94. doi: 10.1002/dc.23923
58. Maia FF, Matos PS, Pavin EJ, Vassallo J, Zantut-Wittmann DE. Value of ultrasound and cytological classification system to predict the Malignancy of thyroid nodules with indeterminate cytology. Endocr Pathol. (2011) 22:66–73. doi: 10.1007/s12022-011-9159-6
59. Rocha TG, Rosario PW, Silva AL, Nunes MB, Silva TH, de Oliveria PHL, et al. Ultrasonography classification of the american thyroid association for predicting Malignancy in thyroid nodules >1cm with indeterminate cytology: A prospective study. Horm Metab Res. (2018) 50:597–601. doi: 10.1055/a-0655-3016
60. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. (2014) 99:1253–63. doi: 10.1210/jc.2013-2928
61. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and Malignant thyroid nodules: US differentiation–multicenter retrospective study. Radiology. (2008) 247:762–70. doi: 10.1148/radiol.2473070944
62. Li F, Pan D, Wu Y, Peng J, Li Q, Gui X, et al. Ultrasound characteristics of thyroid nodules facilitate interpretation of the Malignant risk of Bethesda system III/IV thyroid nodules and inform therapeutic schedule. Diagn Cytopathol. (2019) 47:881–9. doi: 10.1002/dc.24248
63. Popowicz B, Klencki M, Lewiński A, Słowińska-Klencka D. The usefulness of sonographic features in selection of thyroid nodules for biopsy in relation to the nodule’s size. Eur J Endocrinol. (2009) 161:103–11. doi: 10.1530/EJE-09-0022
64. Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol. (2010) 17:386–91. doi: 10.1245/s10434-009-0832-7
65. Youngwirth LM, Adam MA, Scheri RP, Roman SA, Sosa JA. Extrathyroidal extension is associated with compromised survival in patients with thyroid cancer. Thyroid. (2017) 27:626–31. doi: 10.1089/thy.2016.0132
66. Kamaya A, Tahvildari AM, Patel BN, Willmann JK, Jeffrey RB, Desser TS. Sonographic detection of extracapsular extension in papillary thyroid cancer. J Ultrasound Med. (2015) 34:2225–30. doi: 10.7863/ultra.15.02006
67. Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. (2013) 2:147–59. doi: 10.1159/000354537
68. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. (2016) 375:614–7. doi: 10.1056/NEJMp1604412
69. Ahn HS, Kim HJ, Kim KH, Lee YS, Han SJ, Kim Y, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. (2016) 26:1535–40. doi: 10.1089/thy.2016.0075
70. Lauria Pantano A, Maddaloni E, Briganti SI, Differences between ATA. AACE/ACE/AME and ACR TI-RADS ultrasound classifications performance in identifying cytological high-risk thyroid nodules. Eur J Endocrinol. (2018) 178:595–603. doi: 10.1530/EJE-18-0083
71. Marina M, Zatelli MC, Goldoni M, Del Rio P, Corcione L, Martorana D, et al. Combination of ultrasound and molecular testing in Malignancy risk estimate of Bethesda category IV thyroid nodules: results from a single-institution prospective study. J Endocrinol Invest. (2021) 44:2635–43. doi: 10.1007/s40618-021-01571-y
72. Yang W, Fananapazir G, LaRoy J, Wilson M, Campbell MJ. Can the american thyroid association, K-tirads, and acr-tirads ultrasound classification systems be used to predict Malignancy in bethesda category IV nodules? Endocr Pract. (2020) 26:945–52. doi: 10.4158/EP-2020-0024
73. Huang EYF, Kao NH, Lin SY, Jang IJH, Kiong KL, See A, et al. Concordance of the ACR TI-RADS classification with bethesda scoring and histopathology risk stratification of thyroid nodules. JAMA Netw Open. (2023) 6:e2331612. doi: 10.1001/jamanetworkopen.2023.31612
74. Mena G, Montalvo A, Ubidia M, Olmedo J, Guerrero A, Leon-Rojas JE. Elastography of the thyroid nodule, cut-off points between benign and Malignant lesions for strain, 2D shear wave real time and point shear wave: a correlation with pathology, ACR TIRADS and Alpha Score. Front Endocrinol (Lausanne). (2023) 14:1182557. doi: 10.3389/fendo.2023.1182557
75. Park SY, Hahn SY, Shin JH, Ko EY, Oh YL. The diagnostic performance of thyroid US in each category of the bethesda system for reporting thyroid cytopathology. PloS One. (2016) 11:e0155898. doi: 10.1371/journal.pone.0155898
76. Leoncini A, Camponovo C, Gamarra E, Piticchio T, Ruinelli L, Rotondi M, et al. NIFTP-adjusted risk estimation of Bethesda thyroid cytology categories should consider the indication for FNA according to TIRADS. Endocrine. (2024). doi: 10.1007/s12020-024-03800-9
Keywords: thyroid nodule, Bethesda IV cytology, fine needle aspiration, ACR TI-RADS, thyroid ultrasound, thyroid elastography, follicular neoplasm
Citation: Latia M, Borlea A, Mihuta MS, Neagoe OC and Stoian D (2024) Impact of ultrasound elastography in evaluating Bethesda category IV thyroid nodules with histopathological correlation. Front. Endocrinol. 15:1393982. doi: 10.3389/fendo.2024.1393982
Received: 29 February 2024; Accepted: 10 May 2024;
Published: 28 May 2024.
Edited by:
Cristina Alina Silaghi, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Ana Valea, Iuliu Hatieganu University of Medicine and Pharmacy Cluj-Napoca, RomaniaBoris Brkljacic, University of Zagreb, Croatia
Copyright © 2024 Latia, Borlea, Mihuta, Neagoe and Stoian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica Simina Mihuta, c2ltaW5hLm1paHV0YUB1bWZ0LnJv
 Monica Latia
Monica Latia Andreea Borlea
Andreea Borlea Monica Simina Mihuta
Monica Simina Mihuta Octavian Constantin Neagoe
Octavian Constantin Neagoe Dana Stoian
Dana Stoian