- 1Department of Otolaryngology-Head and Neck Surgery, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
- 2Sydney Medical School, University of Sydney, Sydney, NSW, Australia
- 3Department of Otolaryngology – Head & Neck Surgery, Michael Garron Hospital, Toronto, ON, Canada
- 4Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada
Introduction: Thyroid nodules are extremely common being detected by ultrasonography in up to 67% of the population, with current surgical tenet maintaining that lobectomy is required for large symptomatic benign nodules or autonomously functionally nodules resulting in a risk of hypothyroidism or recurrent laryngeal nerve injury even in high volume centres. The introduction of radiofrequency ablation (RFA) has allowed thermal ablation of both benign and autonomously functioning thyroid nodules with minimal morbidity. The moving shot technique is the most well-established technique in performing RFA of thyroid nodules, and has proven to be safe, efficacious, accurate and successful amongst experienced clinicians. The purpose of this article to propose the use of a novel guide when performing RFA of thyroid nodules in clinical practice utilizing the moving shot technique.
Methods: The technique proposed of RFA involves the use of a 10MHz linear ultrasound probe attached to an 18G guide which provides robust in line visualisation of a 7cm or 10cm radiofrequency probe tip (STARmed, Seoul, Korea) utilizing the trans isthmic moving shot technique. A geometric analysis of the guide has been illustrated diagrammatically.
Results: The use of an 18G radiofrequency probe guide (CIVCO Infiniti Plus™ Needle Guide) maintains in line visualisation of the radiofrequency probe over a cross-sectional area up to 28cm2, facilitating efficient and complete ablation of conceptual subunits during RFA of thyroid nodules.
Discussion: Radiofrequency ablation of thyroid nodules can be performed safely and effectively using the novel radiofrequency probe guide proposed which we believe potentially improves both accuracy and overall efficiency, along with operator confidence in maintaining visualisation of the probe tip, and hence we believe provides a valuable addition to the armamentarium of clinicians wishing to embark on performing RFA of thyroid nodules.
Introduction
Thyroid nodules are common and detectable by ultrasonography in up to 13-67% of individuals (1), with higher frequencies in women and the elderly even when the gland is normal on palpation. The initial work up of thyroid nodules has been well established and includes a thorough clinical history to determine risk factors for malignancy, clinical examination and an assessment of thyroid function followed by ultrasonography of both the thyroid bed and lateral neck. The vast majority do not require treatment, and current treatment paradigm maintains that surgery is required for nodules to confirm either suspicious lesions on fine needle aspiration biopsy (FNA) or on nodules that are confirmed to be malignant. However following FNA confirmation of benign nodules, or in isolated autonomously functioning thyroid nodules (AFTN), the risk of malignancy is low in the order of 2-6%. The majority of solitary toxic nodules represent follicular adenomas with a high prevalence of activating mutations in the TSH receptor and their risk of malignancy is less than 3% (2, 3).
Non-malignant (large benign or autonomously functioning) nodules sometimes, however, do require surgical intervention in the form of partial thyroidectomy (4). This leaves the patient with a small cervical scar and renders up to 22% of patients with hypothyroidism for life (5). Despite advances in surgical training, thyroidectomy still carries with it a 2-5% complication rate, even when performed by high volume surgeons (6), resulting in many patients delaying or avoiding surgery entirely.
With the increasing use of clinician-initiated point of care ultrasonography, ultrasound-guided thermal ablative techniques have been introduced by interventional thyroidologists (surgeons, endocrinologists, and interventional radiologists) to effectively treat and manage benign or AFTN. Radiofrequency ablation (RFA) of the thyroid gland was first introduced in human studies in 2006 (7) and multiple international guidelines (8–13) have since been developed including those most recently released by the American Thyroid Association (ATA) (14). The basic principle of RFA involves using a high-frequency alternating electric current that oscillates resulting in formation of a closed electrical circuit (15). The subsequent activation of radiofrequency power via a probe activates positively charged sodium and potassium ions and negatively charged chloride ions within the adjacent tissue and as they attempt to align with the polarity established by the high frequency electrical current, generate either frictional or resistive heat which is then transmitted and conducted to adjacent tissue (16). This generates heat between 60-100 degrees Celsius around the electrode tip that induces near instantaneous induction of protein coagulation with additional thermal injury via conduction that irreversibly damages cellular enzymes resulting in cell death by coagulative necrosis (15, 16). Perhaps the most established technique in implementing RFA in the thyroid is the moving-shot technique, proposed by Baek et al (17) as a means of maximising the efficiency of frictional heat generated at the electrode tip resulting in a more predictable ablation zone and minimizing risk of damages to adjacent visceral organs and nerves induced by thermal conduction. With this technique, the deepest portion of the nodule is targeted first using a trans-isthmic long-axis technique with a slow withdrawal before the next portion of the nodule is targeted, again from a deep to superficial approach. This technique has a well-established safety profile.
The purpose of this paper is to provide our approach to safely performing RFA for both benign and AFTN using a moving shot trans-isthmic long-axis technique. More specifically, we provide a pictorial and geometric analysis of the benefits and limitations of a novel ultrasound probe needle guide (CIVCO Infiniti Plus™).
Methods
Indications
RFA at Sunnybrook Health Sciences Centre is performed under ultrasound guidance by a clinician with experience in performing ultrasonography, FNA biopsies of the thyroid gland as well as core biopsies of neck masses. Indications for RFA are in keeping with the most recent ATA consensus statement, namely two thyroid FNA confirmed benign biopsies or AFTN (14).
Procedure preparation
The patient is placed in a supine position with the head elevated at approximately 30 degrees. Grounding pads are then applied to the patient’s upper thigh to establish a complete grounded electrical circuit by also connecting them to the radiofrequency device. The generator is subsequently connected to a straight monopolar RF electrode with an active tip length between 5, 7 or 10mm, depending on the size of the nodule (STARmed Seoul, Korea), and a 7cm RF probe is most commonly employed. A cooling pump is used to circulate saline through the electrode and lower the temperature around the active tip to maximize ablative margin whilst minimizing tissue charring. Local anaesthetic being lidocaine with 1:100,000 adrenaline is injected around the skin at the site of entry of the probe for the trans-isthmic approach followed by a superficial cervical plexus block. Additional anaesthetic and 5% dextrose is then injected under ultrasound guidance using a spinal needle in a pericapsular plane producing a visualised anechoic band that hydrodissects and separates the nodule from the infrahyoid musculature and laterally away from the carotid sheath and a potentially medialized vagus nerve.
Guide technique
A high resolution 10Mhz linear ultrasound probe is then attached to a GE Edwards ultrasound probe guide which on its lateral aspect is directly connected to an 18G fitting guide (CIVCO Infiniti Plus™ Needle Guide) for the RF probe (STARMed Seoul, Korea). (Figures 1, 2) The purpose of the guide is to maintain constant inline visualisation during the procedure, removing the margin of error associated with freely holding the probe. In the results, we will demonstrate the use of this guided technique using a pictorial and geometric analysis of the probe within this guide. This will allow a greater understanding of the pros and cons of using such a guide. Research ethics board approval was not required as patients were not required for this analysis.
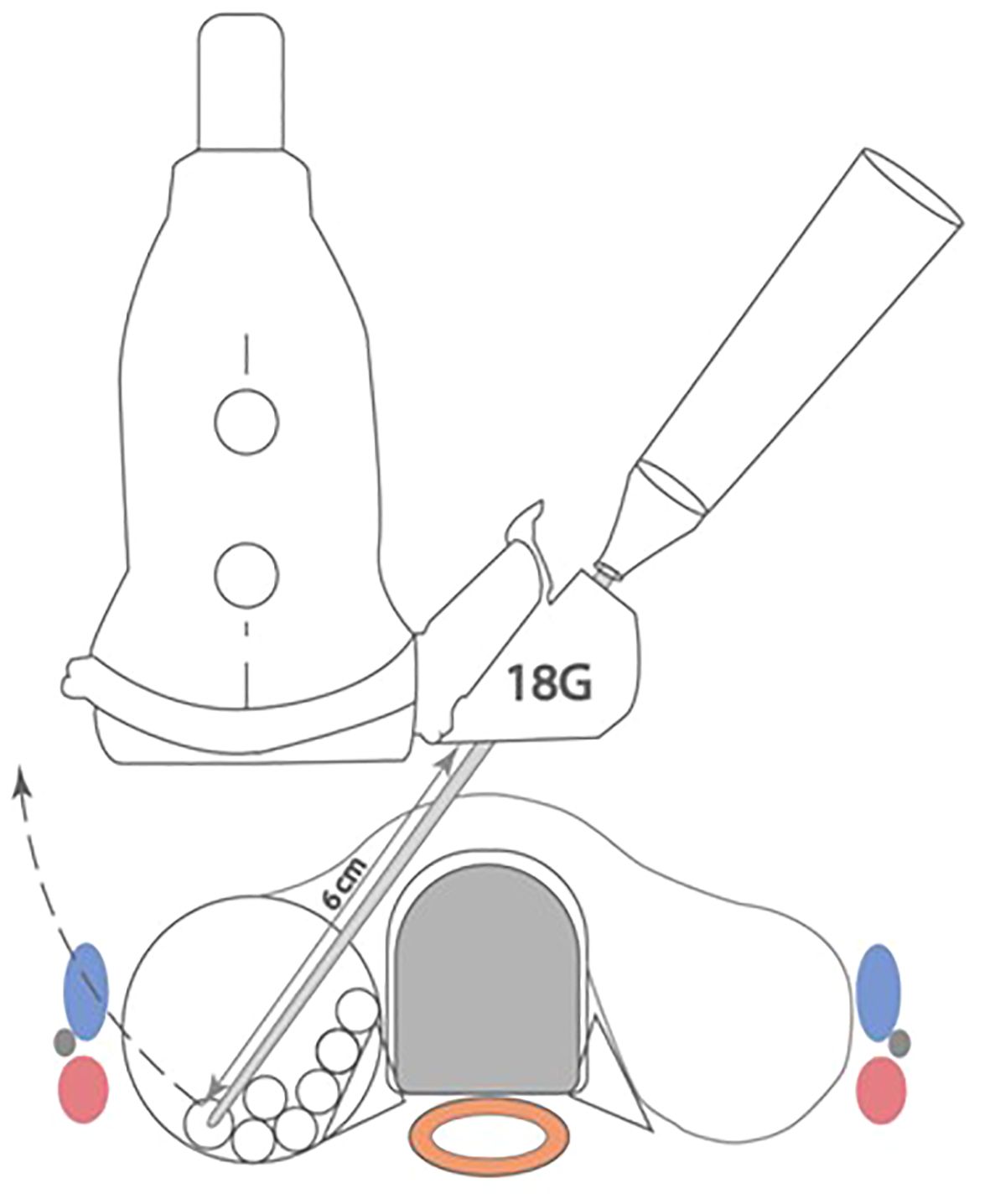
Figure 1 Radiofrequency probe with guide attached demonstrating in line stabilization and visualization during procedure.
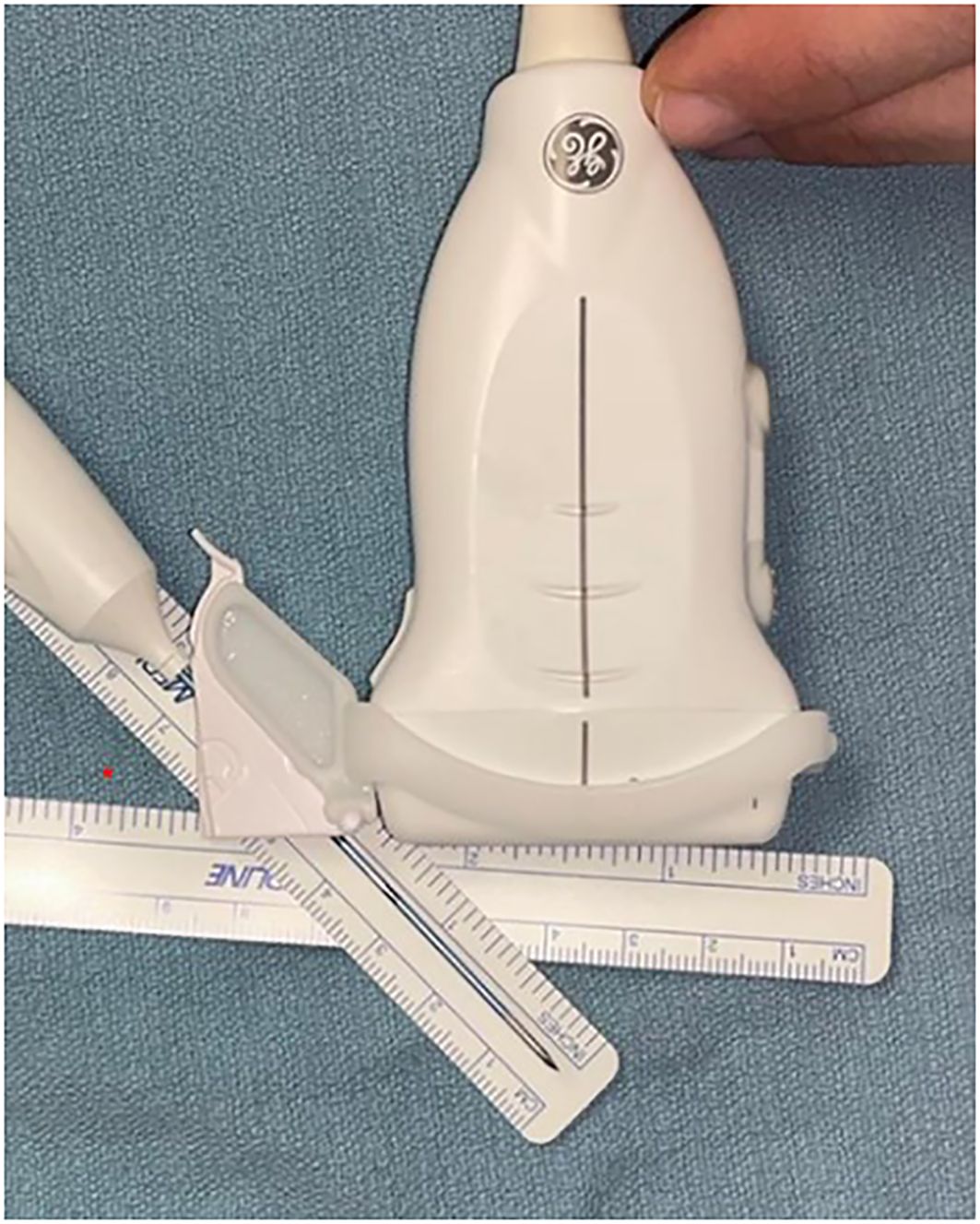
Figure 2 Equipment set-up The linear transducer probe guide is attached directly to an 18G radiofrequency probe guide to produce fixed translatory bodily movements with the electrode tip consistently centred in the long axis, minimizing angulation error during ablation.
Ablative technique
The moving shot technique is then implemented by the clinician whereby the nodule is divided into a series of conceptual subunits with smaller subunits at the periphery, paying close attention to the danger triangle as has been previously described (7). The radiofrequency active tip probe (STARMed Seoul, Korea) is percutaneously inserted under direct vision using the guide (Figure 1) via a transisthmic approach beginning at the most posterior and deepest aspect of the nodule. The primary treatment endpoint is a combination of hyperechogenic change including echogenic microbubbles on the ultrasound screen and objective monitoring of tissue impedance level. The clinician then retracts the probe along the guide, consistently visualising the probe tip, and proceeds to ablation of the next conceptual subunit until all units have developed a visualised hyperechogenic focus with high tissue impedance proceeding from deep to superficial, and sparing the subunits contained within the danger triangle.
The patient is then monitored for at least one hour following the procedure to ensure there are no complications encountered and subsequently followed up by phone call within 24 hours of the procedure to monitor for any symptom change.
Results
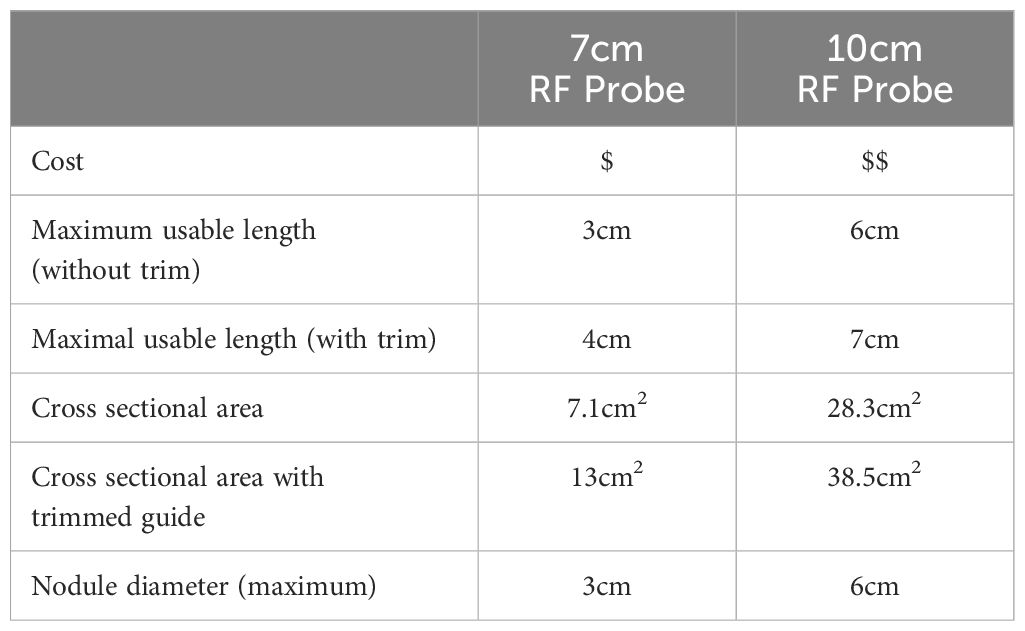
Using the guided technique, a 7cm probe allows a maximum usable length of 3cm and cross-sectional area of 7.1cm2. Trimming of the guide, facilitates a further 1cm of length, resulting in a cross-sectional area of 13cm2. A 10cm probe provides a usable length of 6cm using the guide with a cross-sectional area attainable of 28.3cm2, that can be increased to 38.5cm2 once the guide is trimmed. Accounting for the thickness of the overlying subcutaneous tissue and skin, the guided technique can be safely used to ablate a 3cm nodule with a 7cm probe and up to a 6cm nodule with the 10cm probe once the guide is trimmed. A summary of the geometric analysis of both guides has been provided in Table 1. Figures 3, 4 provide a schematic geometric analysis using the 10cm RF probe (STARMed Seoul, Korea) length of a potential arc of treatment using a 4cm right thyroid nodule as an example with the ability to ablate a total area of 28cm2 using the guide with constant probe tip visualisation. The advantages and disadvantages of such an approach have been summarized in Table 2 below.

Figure 3 The combination of an 18G guide to maintain in line visualisation combined with a 10cm length RF probe (A) provides a working field of approximately 28cm2 whilst applying the moving shot technique. Using a 7cm RF probe, the cross-sectional area achieved is approximately 7cm2 but can be increased to 13cm2 by trimming the guide using sterile scissors as shown in (B).
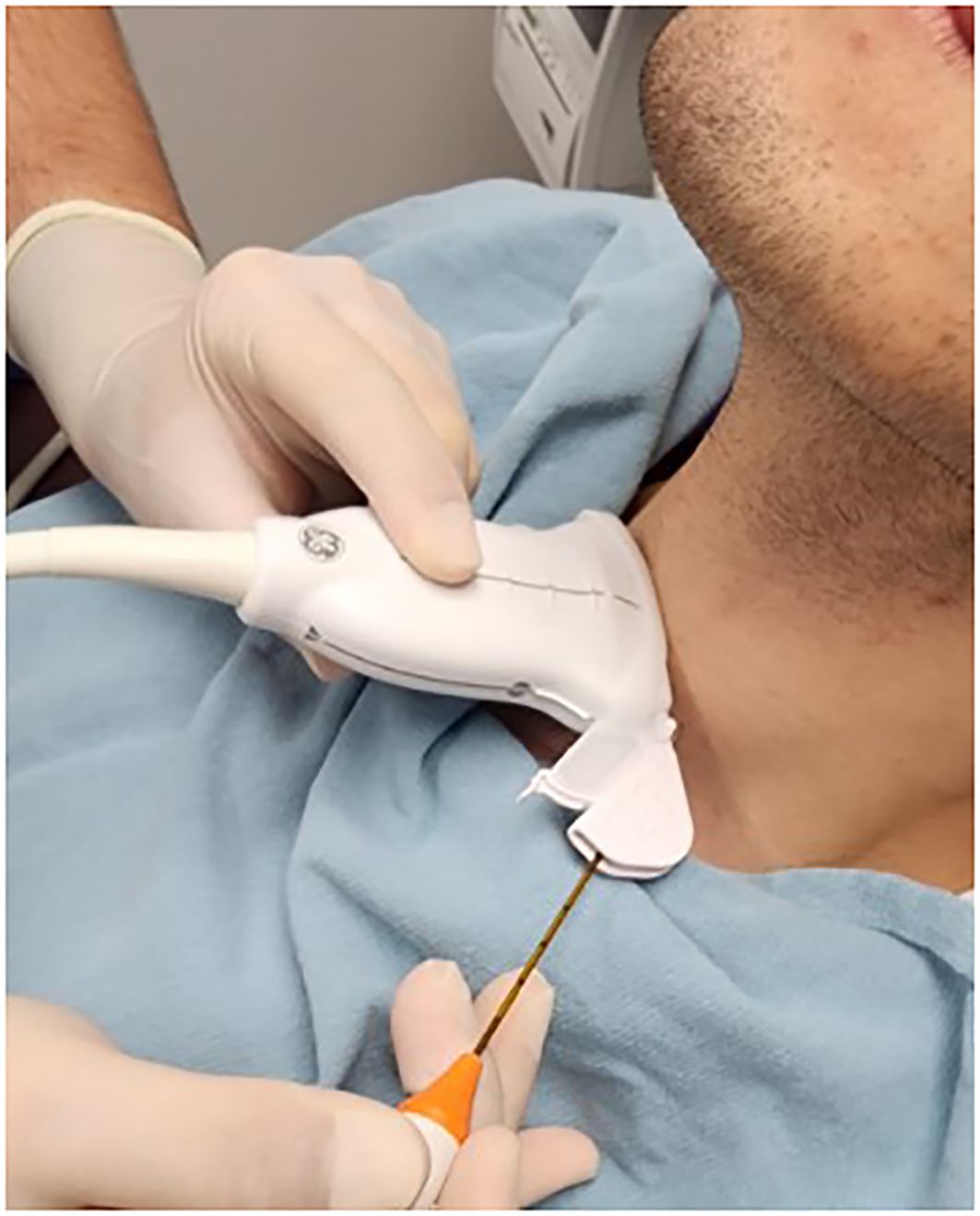
Figure 4 Schematic representation of the use of the RF probe guide in ablation of a 4cm thyroid nodule. A total cross sectional of 28cm2 can be ablated through a single puncture site whilst maintaining in line visualisation of the tip at all times.
Discussion
Radiofrequency ablation of the thyroid gland provides the thyroid interventionalist with a minimally invasive technique to ablate benign thyroid and AFTN in an outpatient setting, without a general anaesthetic, leaving them without a scar and most importantly, avoiding the risk of hypothyroidism. The efficacy of RFA in benign thyroid disease has been previously well established. Most recently, Russell et al. in a multi-institutional prospective collection of 620 patients from a North American population with benign thyroid nodules and a baseline nodule volume of 5.6mL, followed with serial ultrasound and TSH levels for 12 months, demonstrated a median volume reduction rate of 71% at 12 months, with 78% of patients achieving treatment success defined as a volume reduction greater than 50% at 1 year. On multivariate analysis, small nodules (with a treatment volume <10mls) and medium nodules (10-20mls) achieved a success rate of 81% and 87% at 12 months respectively (18). A similar muti-institutional prospective Korean study consisting of 276 patients demonstrated a mean volume reduction of 80% at 12 months with a mean preoperative volume of 14mls and largest diameter of 3.8cm, however, one quarter of these patients also had another ablative procedure (19). When followed by serial ultrasound for 6 years, the mean volume reductions at 24, 36, 48 and 60 months were 84%, 89%, 92% and 95% respectively. Deandrea et al. in their randomized control international collaborative trial across two centres in Italy and Korea demonstrated a mean volume rate reduction of 71% at 6 months (20), and a recent systematic review demonstrated a mean volume reduction of 67-75% at 12 months in patients treated with a single procedure, which increased to 94% in patients with repeated ablations consistent with previous findings (21). Thus, the clinical outcome of volume reduction in benign thyroid nodules has previously well documented.
RFA has also recently expanded its use in achieving not only volume reduction of thyroid nodules, but biochemical euthyroidism of patients with AFTN, where the risk of malignancy is exceedingly low. In a systematic review and meta-analysis conducted by Cesareo et al, the pooled rate of patients with TSH normalization was 57% with a pooled volume reduction rate of 79% at one year (22). The rate of success of RFA in AFTN has been significantly correlated not only with volume rate reduction but also with size of nodule, with nodules more likely to respond to treatment when baseline volume is <12mls (23). This has been postulated to relate to the ability to achieve complete ablation of conceptual subunits in smaller volume nodules, minimizing the risk of untreated autonomously hyperfunctioning residual tissue. Therefore, RFA has now been recommended as a treatment option in the majority of internationally established guidelines.8-14 Our practice is currently limited to patients with biopsy confirmed benign nodules or toxic adenomas that are less than 20mls in volume and located away from the danger triangle given the potential for undertreating hyperfunctioning ablation subunits to avoid risk of thermal injury.
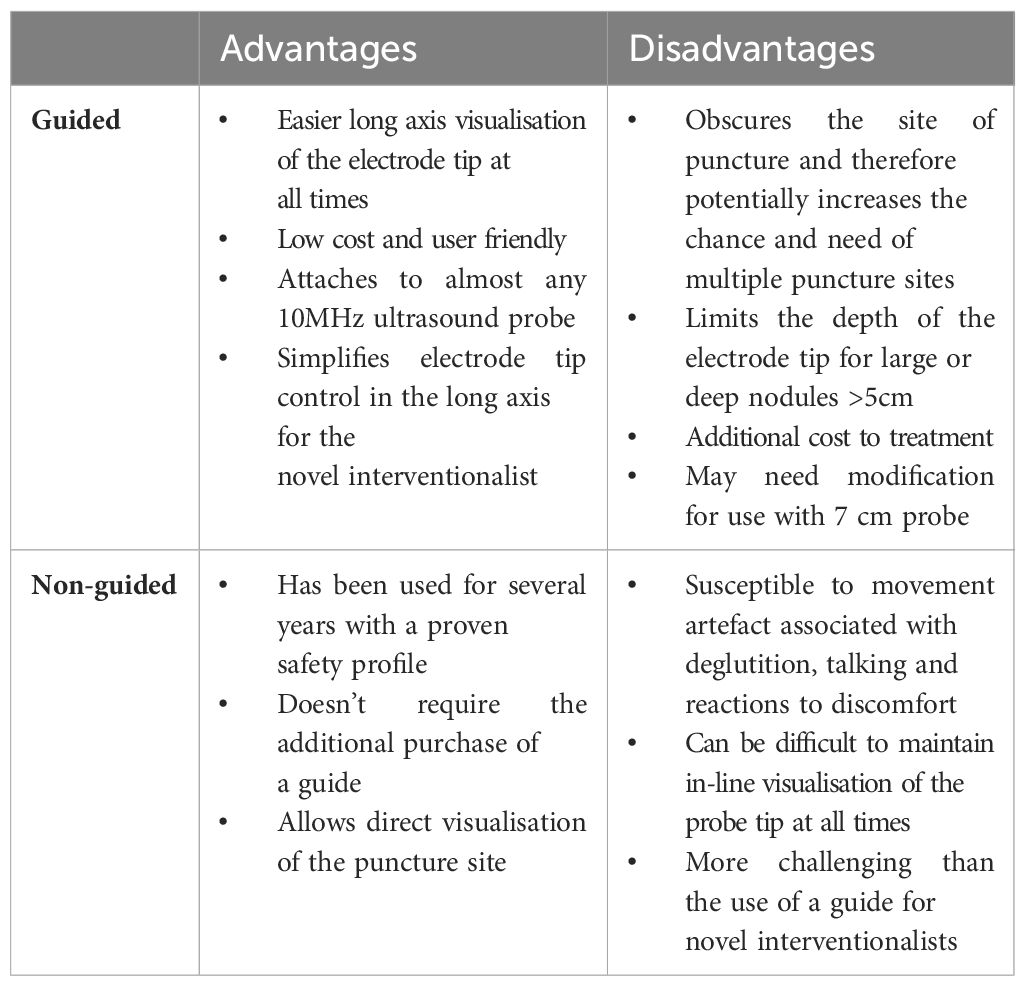
Current consensus guidelines have established there is a significant learning curve for thyroid ablation required prior to providing optimal treatment for patients (8). Complete ablation of all conceptual subunits optimizes patient outcomes by increasing the success of the procedure and decreasing the need for additional treatment. The most recent ATA consensus statement indicates that clinical efficacy requires approximately 30 cases based on the results of three previously published series (24–26). We agree with the aforementioned studies and all our procedures are currently performed by two Otolaryngology Head and Neck Surgeons with significant previous expertise in both US and FNA. In addition to this, Improvements in volume rate reduction in benign thyroid nodules has also been attributed to expertise in electrode manoeuvrability, the amount of energy applied to each conceptual subunit and the use of colour doppler during the procedure to identify viable thyroid tissue (22). We feel that our novel guided technique proposed has several advantages. Firstly, the use of a radiofrequency probe guide attached to a high frequency linear ultrasound transducer ensures visualisation of the needle under direct vision for the entirety of the procedure. The probe can then be angled to target separate subunits of varying depth through the same puncture site. We have found the stability gained by the guide improves operator confidence in ablation of smaller conceptual subunits in close proximity to the recurrent laryngeal nerve and the carotid artery, areas often left unattended due to risk of injury. Secondly, the placement of a fixed guide negates the human error in movement often unavoidable during both talking and swallowing which we have found to be a significant advantage. It also facilitates ablation of multiple subunits through a single-entry site by rotating the probe in a counterclockwise fashion, commencing inferiorly and moving superiorly, yet maintaining direct vision. Further to this, the restraint of a guide facilitates the need for a systematic approach to ablation requiring the clinician to subdivide the outlined nodule into horizontal tracts spaced approximately 1cm apart, ensuring a more complete ablation. One of the challenges we recognise in the implementation of a novel interventional technique, pertains to not only familiarity with instrumentation but also the ability to consistently replicate the same result under varied conditions in patients with variable anatomy and we feel the use of a guide aids in achieving this. Further to this, we feel that in the infancy of clinicians adopting radiofrequency into their practice, such an approach facilitates consistency, adaptability and accounts for potential movement artefact otherwise experienced during deglutition, speech or as a reaction to discomfort experienced by the patient during the procedure. This is further enhanced by the use of a 10cm radiofrequency probe allowing an arc of rotation of 6cm, not otherwise achieved with shorter probes, highlighting its use in large benign nodules. However, the 7cm radiofrequency probe can also be used with modifications made to the needle guide. Of course, an individualized approach is required based on the patient’s body habitus, the size and depth of the nodule and the clinician needs to remain cognizant of potential complications including nodule rupture, hematoma, nerve and tracheal injury which remain of paramount importance.
There is a currently a large amount of literature supporting radiofrequency ablation of thyroid nodules, however this is only amongst high volume thyroid interventionalists. As the use of RFA becomes more widespread amongst surgeons, a guided approach with the electrode tip fixed within the centre of the long axis field consistently, produces translatory bodily movement of both transducer and RF probe endorsing confidence in achieving accurate subunit ablation. This will allow the thyroid interventionalists to consistently ablate more conceptual subunits and potentially improve the efficiency of RFA in the management of both benign and AFTN. Further prospective studies will aid in elucidating this as the technology increases in adoption rates across North America.
Conclusion
We present a novel radiofrequency probe guide which is simple and easy to attach to an ultrasound probe that provides a valuable addition to the head and neck surgeons armamentarium in RFA of thyroid nodule.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
LS: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. KH: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. DE: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. AE: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor PP-A declared a shared parent affiliation with all authors at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. (2008) 13:105–12. doi: 10.1634/theoncologist.2007-0212
2. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of Malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. (2006) 91:4295–301. doi: 10.1210/jc.2006-0527
3. Erdoğan MF, Anil C, Ozer D, Kamel N, Erdoğan G. Is it useful to routinely biopsy hot nodules in iodine deficient areas? J Endocrinol Invest. (2003) 26:128–31.
4. Sarkis LM, Norlen O, Sywak M, Delbridge L. Minimally invasive thyroid nodulectomy reduces post-operative hypothyroidism when compared with thyroid lobectomy. ANZ J Surg. (2017) 87:360–3. doi: 10.1111/ans.12904
5. Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. (2012) 97:2243–55. doi: 10.1210/jc.2012-1063
6. Bergenfelz A, Jansson S, Kristoffersson A, Mårtensson H, Reihnér E, Wallin G, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. (2008) 393:667–73. doi: 10.1007/s00423-008-0366-7
7. Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. (2006) 16:361–7. doi: 10.1089/thy.2006.16.361
8. Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. Guideline committee for the Korean society of thyroid radiology (KSThR) and Korean society of radiology. 2017 thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. (2018) 19:632–55. doi: 10.3348/kjr.2018.19.4.632
9. Papini E, Crescenzi A, D'Amore A, Deandrea M, De Benedictis A, Frasoldati A, et al. Italian guidelines for the management of non-functioning benign and locally symptomatic thyroid nodules. Endocr Metab Immune Disord Drug Targets. (2023) 23:876–85.
10. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. AACE/ACE/AME task force on thyroid nodules. American association of clinical endocrinologists, american college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. (2016) 22:622–39. doi: 10.4158/EP161208.GL
11. Dobnig H, Zechmann W, Hermann M, Lehner M, Heute D, Mirzaei S, et al. Radiofrequency ablation of thyroid nodules: "Good Clinical Practice Recommendations" for Austria : An interdisciplinary statement from the following professional associations: Austrian Thyroid Association (ÖSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (ÖGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med Wochenschr. (2020) 170:6–14. doi: 10.1007/s10354-019-0682-2
12. Orloff LA, Noel JE, Stack BC Jr, Russell MD, Angelos P, Baek JH, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and Malignant thyroid disease: an international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck. (2021) 44:633–60.
13. Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. (2020) 9:172–85. doi: 10.1159/000508484
14. Sinclair CF, Baek JH, Hands KE, Hodak SP, Huber TC, Hussain I, et al. General principles for the safe performance, training, and adoption of ablation techniques for benign thyroid nodules: an American thyroid association statement. Thyroid. (2023) 33:1150–70. doi: 10.1089/thy.2023.0281
15. Shin JH, Baek JH, Ha EJ, Lee JH. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. (2012) 2012:919650. doi: 10.1155/2012/919650
16. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. (2001) 13:129–47. doi: 10.1016/S0929-8266(01)00126-4
17. Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. (2009) 33:1971–7. doi: 10.1007/s00268-009-0130-3
18. Russell JO, Desai DD, Noel JE, Hussein M, Toraih E, Seo S, et al. Radiofrequency ablation of benign thyroid nodules: A prospective, multi-institutional North American experience. Surgery. (2024) 175:139–45. doi: 10.1016/j.surg.2023.07.046
19. Jung SL, Baek JH, Lee JH, Shong YK, Sung JY, Kim KS, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: A prospective multicenter study. Korean J Radiol. (2018) 19:167–74. doi: 10.3348/kjr.2018.19.1.167
20. Deandrea M, Sung JY, Limone P, Mormile A, Garino F, Ragazzoni F, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: A randomized controlled international collaborative trial. Thyroid. (2015) 25:890–6. doi: 10.1089/thy.2015.0133
21. Monpeyssen H, Alamri A, Ben Hamou A. Long-term results of ultrasound-guided radiofrequency ablation of benign thyroid nodules: state of the art and future perspectives-A systematic review. Front Endocrinol (Lausanne). (2021) 12:622996. doi: 10.3389/fendo.2021.622996
22. Cesareo R, Palermo A, Benvenuto D, Cella E, Pasqualini V, Bernardi S, et al. Efficacy of radiofrequency ablation in autonomous functioning thyroid nodules. A systematic review and meta-analysis. Rev Endocr Metab Disord. (2019) 20:37–44. doi: 10.1007/s11154-019-09487-y
23. Cesareo R, Naciu AM, Iozzino M, Pasqualini V, Simeoni C, Casini A, et al. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. Int J Hyperthermia. (2018) 34:617–23. doi: 10.1080/02656736.2018.1430868
24. Kuo CY, Liu CL, Tsai CH, Cheng SP. Learning curve analysis of radiofrequency ablation for benign thyroid nodules. Int J Hyperthermia. (2021) 38:1536–40. doi: 10.1080/02656736.2021.1993358
25. Bom WJ, Joosten FBM, van Borren MMGJ, Bom EP, van Eekeren RRJP, de Boer H. Radiofrequency ablation for symptomatic, non-functioning, thyroid nodules: A single center learning curve. Endocr Connect. (2022) 11:e210304. doi: 10.1530/EC-21-0304
Keywords: thyroid, nodule, radiofrequency ablation, guide, benign, autonomous
Citation: Sarkis LM, Higgins K, Enepekides D and Eskander A (2024) A novel guided approach to radiofrequency ablation of thyroid nodules: the Toronto Sunnybrook experience. Front. Endocrinol. 15:1402605. doi: 10.3389/fendo.2024.1402605
Received: 17 March 2024; Accepted: 10 July 2024;
Published: 24 July 2024.
Edited by:
Pia Pace-Asciak, Department of Otolaryngology – Head and Neck Surgery, University of Toronto, CanadaReviewed by:
Marco Stefano Demarchi, Hôpitaux Universitaires de Genève (HUG), SwitzerlandEmrah Karatay, Marmara University Istanbul Pendik Education and Research Hospital, Türkiye
Copyright © 2024 Sarkis, Higgins, Enepekides and Eskander. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antoine Eskander, YW50b2luZS5lc2thbmRlckBzdW5ueWJyb29rLmNh
†These authors have contributed equally to this work
 Leba Michael Sarkis
Leba Michael Sarkis Kevin Higgins1†
Kevin Higgins1†