Abstract
Introduction:
Growth Differentiation Factor 15 (GDF15) is a mitokine expressed in response to various stresses whose circulating levels increase with age and are associated with numerous pathological conditions, including muscle wasting and sarcopenia. However, the use of circulating GDF15 (c-GDF15) as a biomarker of sarcopenia is still debated. Moreover, the role of GDF15 intracellular precursor, pro-GDF15, in human skeletal muscle (SM-GDF15) is not totally understood. In order to clarify these points, the association of both forms of GDF15 with parameters of muscle strength, body composition, metabolism and inflammation was investigated.
Methods:
the levels of c-GDF15 and SM-GDF15 were evaluated in plasma and muscle biopsies, respectively, of healthy subjects (HS) and patients with lower limb mobility impairment (LLMI), either young (<40 years-old) or old (>70 years-old). Other parameters included in the analysis were Isometric Quadriceps Strength (IQS), BMI, lean and fat mass percentage, Vastus lateralis thickness, as well as circulating levels of Adiponectin, Leptin, Resistin, IGF-1, Insulin, IL6, IL15 and c-PLIN2. Principal Component Analysis (PCA), Canonical Discriminant Analysis (CDA) and Receiving Operating Characteristics (ROC) analysis were performed.
Results:
c-GDF15 but not SM-GDF15 levels resulted associated with decreased IQS and IGF-1 levels in both HS and LLMI, while only in LLMI associated with increased levels of Resistin. Moreover, in LLMI both c-GDF15 and SM-GDF15 levels were associated with IL-6 levels, but interestingly SM-GDF15 is lower in LLMI with respect to HS. Furthermore, a discrimination of the four groups of subjects based on these parameters was possible with PCA and CDA. In particular HS, LLMI over 70 years or under 40 years of age were discriminated based on SM-GDF15, c-GDF15 and Insulin levels, respectively.
Conclusion:
our data support the idea that c-GDF15 level could be used as a biomarker of decreased muscle mass and strength. Moreover, it is suggested that c-GDF15 has a different diagnostic significance with respect to SM-GDF15, which is likely linked to a healthy and active state.
Introduction
Aging is a complex process characterized by multiple biological alterations, including changes in body composition. In particular, during aging, a decrease of muscle mass and strength and an increase of total fat mass occur (1–3). Over time, these changes may contribute to the onset of different age-related diseases, and in particular sarcopenia (4). The causes of sarcopenia are not yet completely understood, but to date, it is thought to be a multifactorial disease, in which neurological decline, hormone changes, increased inflammatory status, decline in physical activity and poor nutrition, together contribute to its onset (5). Sarcopenia is becoming highly prevalent in the elderly population, with a remarkable impact on the quality of life, since it is associated with increase of falls, poor physical function and difficulties in activities of daily living. Moreover, it is considered as both a precursor and a physical manifestation of frailty, and its diagnostic process is often difficult (6, 7). Given the impact of this pathology, in particular on Western societies that are rapidly aging, and the fact that elderly population is continuously increasing, prevention, early diagnosis and treatment of sarcopenia are becoming fundamental.
A chronic and low-grade state of inflammation, known as “inflammaging” (8), is considered one of the hallmarks of aging and one of the main drivers of many, if not all, age-related diseases, including sarcopenia. In fact, elevated circulating levels of typical inflammaging mediators, such as Interleukin 6 (IL6), Tumor necrosis factor α (TNFα) and C-reactive protein (CRP), have been found in patients with sarcopenia (9–11). However, inflammaging has also a counterpart indicated as anti-inflammaging, whose components are still under investigation, though some of them have been possibly identified, such as Growth Differentiation Factor 15 (GDF15) (12, 13). Recently, a possible association of GDF15 with sarcopenia and muscle mass loss has been reported (14–16). GDF15 is a mitokine, i.e. a stress-related protein mainly secreted in response to mitochondrial stress, and one of the most upregulated proteins during aging and is associated with overall mortality in the elderly (17–19). Human GDF15 gene encodes for a 308 amino acid biologically inactive precursor protein, named pre-pro-GDF15, which is cleaved in the endoplasmic reticulum to pro-GDF15. At the C-terminal a conserved domain of seven consecutive cysteines makes the pro-GDF15 to form an intracellular homodimer (35 kDa) through a single disulfide bond (20). The pro-GDF15 is further processed in the trans-Golgi apparatus and secreted as mature GDF15 homodimer (15 kDa) (21, 22). As mentioned, a tight relationship of GDF15 with inflammation has been found, since it has been shown that its levels increase in response to infections and inflammation, with possible immunoregulatory and immunosuppressive roles, being fundamental in tolerance and adaptation to bacterial and viral infections (23, 24). Other precise biological functions of GDF15 are still poorly defined, but it has been shown that it acts centrally to control appetite and is involved in energy metabolism, with catabolic and cachexia-promoting effects (13, 25). Many studies have associated GDF15 with a plethora of age-related diseases, such as neurodegenerative diseases, cardiovascular diseases, metabolic diseases and cancer (13, 26–29).
As mentioned, an association of the circulating GDF15 (c-GDF15), i.e. the mature GDF15 homodimer, with sarcopenia and muscle atrophy has been suggested. In particular, studies showed that c-GDF15 level could easily predict sarcopenia in patients with chronic obstructive pulmonary disease (30) and in aged mice and humans (31). Moreover, inactivation of GDF15 signaling in a mouse model of cancer-induced cachexia led to improved muscle mass and physical performance (16). However, the usefulness of c-GDF15 as a biomarker of sarcopenia is still debated, as it is not possible to understand which tissue it comes from. In fact, GDF15, as pro-GDF15 form, is expressed by many tissues, including skeletal and cardiac muscles, and consequently it is listed among myokines (32). Moreover, it is not clear what is the role, if any, of intracellular pro-GDF15 protein in human skeletal muscle (SM-GDF15). In particular, it is not clear whether SM-GDF15 expression is just a sign of muscle stress and reflects c-GDF15 levels, or rather it may play a detrimental or beneficial role trying to promote or counteract muscle wasting and weakness. To this aim, in the present study we have analyzed the levels of c-GDF15 and SM-GDF15 in both healthy subjects with an active life-style and patients with lower limb mobility impairment and a sedentary life-style, with different age (from 20 to 96 years). We have found that in patients c-GDF15 level is higher while, surprisingly, SM-GDF15 level is lower compared to healthy subjects. Moreover, c-GDF15 level is inversely correlated with muscle strength in both healthy subjects and patients, supporting its use as a biomarker of sarcopenia and muscle dysfunction.
Methods
Subjects’ description
The subjects were recruited in the framework of the EU Project “MYOAGE”. The study protocol was approved by the Ethical Committee of Istituto Ortopedico Rizzoli, Bologna, Italy (ethical clearance no. 10823 issued on April 26, 2010). All subjects signed an informed consent before entering the study. For this study, ethical aspects for aging research were considered, as illustrated in 33.
Samples from two groups of subjects were used: i) 47 healthy subjects (HS) (mean age 57.1 ± 3.6) divided in 15 subjects <40 years of age (mean age 22.7 ± 0.63) and 32 subjects >70 years of age (mean age 74.3 ± 0.62); ii) 46 patients with lower limb mobility impairment (LLMI) (mean age 61.3 ± 3.6), divided in 21 subjects <40 years of age (mean age 36.3 ± 1.5) and 25 subjects >70 years of age (mean age 82.2 ± 1.6). To ensure the selection of subjects in healthy conditions, the following exclusion criteria were used: presence of comorbidities as detailed in 2, inability to walk a distance of 250m, use of medication, immobilization for 1 week during the last 3 months and orthopedic surgery during the last 2 years. For the patient’s group, exclusion criteria were: the presence of chronic kidney or liver diseases, bleeding disorders, severe diabetes mellitus, rheumatic diseases other than osteoarthritis, neuromuscular disorders, malignancies and systemic infections, chronic steroid use, major psychological problems or history of alcohol or drug abuse, evidence of prior surgery in the involved hip (2).
Height and weight were measured for each subject and BMI was calculated as weight in kilograms divided by the square of the height in meters (kg/m2).
Laboratory measurements on plasma
Blood samples were collected in the morning after an overnight fasting. Plasma samples were obtained after a 15 minutes centrifugation at 2,000 g at 4°C, then rapidly frozen and stored at -80°C until the analysis was performed. Plasma IGF-1, Insulin, Adiponectin, Leptin, Resistin, IL6 and GDF15 concentrations were obtained using commercial ELISA kits (Quantikine R&D Systems), according to manufacturer’s instructions. Circulating Perilipin 2 (c-PLIN2) was measured using the ELISA commercial kit Human ADRP ELISA (E-EL-H0278, Elabscience), according to the manufacturer’s instructions. Each analyte was measured in duplicate for each sample. Plasma IL15 was analyzed using the Simple Plex Human IL-15 Cartridge (ProteinSimple/Bio-Techne) run on an Ella Automated Immunoassay System (ProteinSimple/Bio-Techne), according to manufacturer’s instructions.
Muscle strength
For HS, isometric quadriceps strength (IQS) was measured with a quadriceps chair (Forcelink B.V). Briefly, the subjects were positioned in an upright position, with straps to fix the hips to the chair and the ankle to the force transducer, at the knee angle of 90 degrees. Three trials were conducted to measure maximal voluntary contraction of the quadriceps. Each trial was separated by one minute of rest. The trial with the highest force output was taken for analyses.
For LLMI, the IQS was measured in seated position using a Handifor dynamometer (TRACTEL S.A. Montreuil Cedex – France). After a warming up period, patients were asked to perform three series of 10 contractions, progressively increasing the strength developed. The highest peak torque was withheld for analyses.
Muscle ultrasound measurements
Only for LLMI, ultrasound imaging of the Vastus lateralis (VL) was performed using a portable ultrasound (Mylab25, Esaote) with a 7–10 MHz linear probe. Acquisition was performed by a trained examiner. Muscle thickness was calculated as the vertical distance between muscle superficial and deep aponeuroses, at an equidistant point from right and left borders of the sagittal image.
Dual-energy x-ray absorptiometry
Only for HS, a whole-body scan to detect total fat and lean mass was performed. The scan was performed using Dual-energy X-ray absorptiometry (DXA) (Hologic QDR 4500, version 12.4, Hologic Inc., Bedford), by a trained technician.
Muscle biopsies sampling
Muscle biopsies were taken from VL muscle from 23 HS, after localized anesthesia, and from 16 LLMI, during the operation at the site of surgical incision. All biopsies were immediately frozen in liquid nitrogen and then stored at -80°C.
Protein extraction and western blotting
Proteins were obtained by lysis of about 40mg of frozen tissue using TEAD buffer (Tris-HCl 20 mM pH= 7.5, EDTA 1mM, NaN3 1mM, DTT 1mM) with protease and phosphatase inhibitors (Sigma). Homogenization was performed using a motor driven homogenizer and lysates obtained were then centrifuged at 25,000 g for 1h at 4°C.
10 μg of total proteins were separated on a polyacrylamide gel (4-15% Mini-PROTEAN® TGX™ Precast Protein Gels, Bio-Rad). Proteins were then transferred to a nitrocellulose membrane (Trans-Blot Transfer Medium, Bio-Rad) and then immunoblotted with anti-Mic-1/GDF15 (Cell Signaling) primary antibody. Anti-GAPDH primary antibody (Novus Bio) was used as housekeeping for normalization.
Images acquisition was performed with ChemiDoc Imaging System (Bio-Rad). Band densitometry analysis was performed with Fiji software.
Statistical analysis
Results are shown as mean ± SD or SE. After a Shapiro-Wilk normality test, Student’s t test, and Pearson correlation were used for normally distributed data whereas Mann-Whitney test was used for data that did not follow a normal distribution. SPSS 17.0 for Windows was used for analyses. P values <0.05 were considered statistically significant. The post-hoc power calculation, performed with G*Power software, was 0.81, considering an effect size of 0.30.
To understand the role of c-GDF15 and SM-GDF15 in relation to other parameters (Insulin, Leptin, c-PLIN2, IL6, IL15, Resistin, Adiponectin and BMI) involved in muscle strength and mass loss, a principal component analysis (PCA) was used. The PCA is a multivariate dimension reduction application, mainly aimed at synthesizing data contained in a set of n observed variables (y1,…, yn) by checking a new set of p (p < n) variables (X1,…,Xp), named principal components (PCs). The first PC (PC1) explains the highest variability, while the remaining PCs (PC2, PC3,… PCn; n = number of variables) report for the remaining variability in the data. Each PC is independent and orthogonal to the others. Generally, the first few PCs are sufficient to describe most of the total data variations (34).
Subsequently, a Canonical Discriminant Analysis (CDA) was used to evaluate which of the variables involved in the PCA were able to best discriminate the 4 groups of subjects involved in the test (healthy young subjects, young patients, healthy old subjects and old patients). The CDA is a dimension-reduction technique, related to principal component analysis and canonical correlation, able to perform both univariate and multivariate 1-way analyses. Given a classification character and several interval variables, CDA derives a set of new variables, called canonical functions (CAN), which are linear combinations of the original interval variables.
Moreover, receiver operating characteristics (ROC) curves were constructed to assess the cut-off levels and the discriminatory ability of the above-mentioned parameters in HS and LLMI.
Results
c-GDF15 levels and SM-GDF15 protein follow different trends in patients and healthy subjects
We have previously reported that the level of c-GDF15 in patients with lower limb mobility impairment (LLMI) is higher than that of healthy subjects (HS), and that in both LLMI and HS, older subjects have higher levels of c-GDF15 with respect to younger ones (14). The analysis of c-GDF15 in the present study confirmed previous results, i.e. higher c-GDF15 levels in LLMI vs HS and in >70yrs vs <40yrs subjects (Supplementary Figures 1A–C).
We then wondered whether the same trend was also present in skeletal muscle tissue. To this purpose, we evaluated, in the same subjects, the protein level of the intracellular pro-GDF15 form within skeletal muscle (SM-GDF15), analyzing the whole protein extract from VL biopsies by western blotting. Surprisingly, the level of SM-GDF15 followed a different trend compared to c-GDF15. In particular, LLMI showed a significantly lower level of SM-GDF15 as compared to HS (Figures 1A, B). Moreover, no significant differences were found between younger (<40 yrs) and older (>70yrs) subjects in both HS and LLMI groups (Figures 1C, D). These results indicate that actually GDF15 protein follows different concentration trends in plasma and skeletal muscle. Moreover, no correlation between SM-GDF15 and c-GDF15 was observed (see below).
Figure 1

Western blotting analysis of GDF15 in the skeletal muscle (SM-GDF15). (A) Representative immunoblotting image of GDF15 and GAPDH in the skeletal muscle. (B) Relative protein expression of GDF15 in the SM from 23 healthy subjects (HS) and 16 patients with lower limb mobility impairment (LLMI). (C) Relative protein expression of GDF15 in the SM from 10 HS <40 years (<40) and 13 HS >70 years (>70). (D) Relative protein expression of GDF15 in the SM from 7 LLMI <40 and 9 LLMI >70. The bars represent mean ± SD. The quantification was performed using Fiji software and normalized to GAPDH expression. Student’s t test was applied.
c-GDF15 correlates with markers of inflammation and muscle functionality
We then sought for correlations of c-GDF15 and SM-GDF15 with biochemical, anthropometric and functional parameters [age, BMI, Isometric Quadriceps Strength (IQS), Adiponectin, Leptin, Resistin, Insulin, IL6, IL15, c-PLIN2, IGF-1] related to inflammation, metabolism, body composition and muscle functionality. Mean values of the above-mentioned parameters, analyzed in HS and LLMI, are summarized in Table 1.
Table 1
| HS (47 subjects) | LLMI (46 subjects) | |
|---|---|---|
| Age (years) | 57.1 ± 3.6 | 61.3 ± 3.6 |
| BMI (kg/m2) | 24.9 ± 0.6 | 26.0 ± 0.6 |
| c-GDF15 (pg/ml) | 1168.1 ± 87.6 | 2021.6 ± 249.1 |
| IQS (kg) | 146.5 ± 10.4 | 26.3 ± 1.7 |
| Adiponectin (µg/ml) | 13.7 ± 1.2 | 10.7 ± 1 |
| Leptin (pg/ml) | 11.7 ± 1.9 | 12.2 ± 1.4 |
| Resistin (ng/ml) | 7.1 ± 0.4 | 9.3 ± 1.1 |
| IGF-1 (ng/ml) | 122.5 ± 9.7 | 117.5 ± 8.4 |
| Insulin (µU/ml) | 2.1 ± 0.03 | 12.6 ± 2.1 |
| IL6 (pg/ml) | 3.2 ± 0.2 | 16.7 ± 3.7 |
| IL15 (pg/ml) | 2.0 ± 0.1 | 4.0 ± 1.8 |
| c-PLIN2 (ng/ml) | 32.2 ± 4.9 | 44.7 ± 5.3 |
Summary of biochemical and anthropometric parameters of HS and LLMI patients. Data are expressed ad mean ± SE.
When we considered all the subjects analyzed (LLMI+HS), a positive correlation of c-GDF15 with age, IL6 and Resistin and a negative correlation with IGF-1 were observed. As far as SM-GDF15, a positive correlation with Adiponectin and a negative correlation with Insulin were observed (Figures 2A–F).
Figure 2
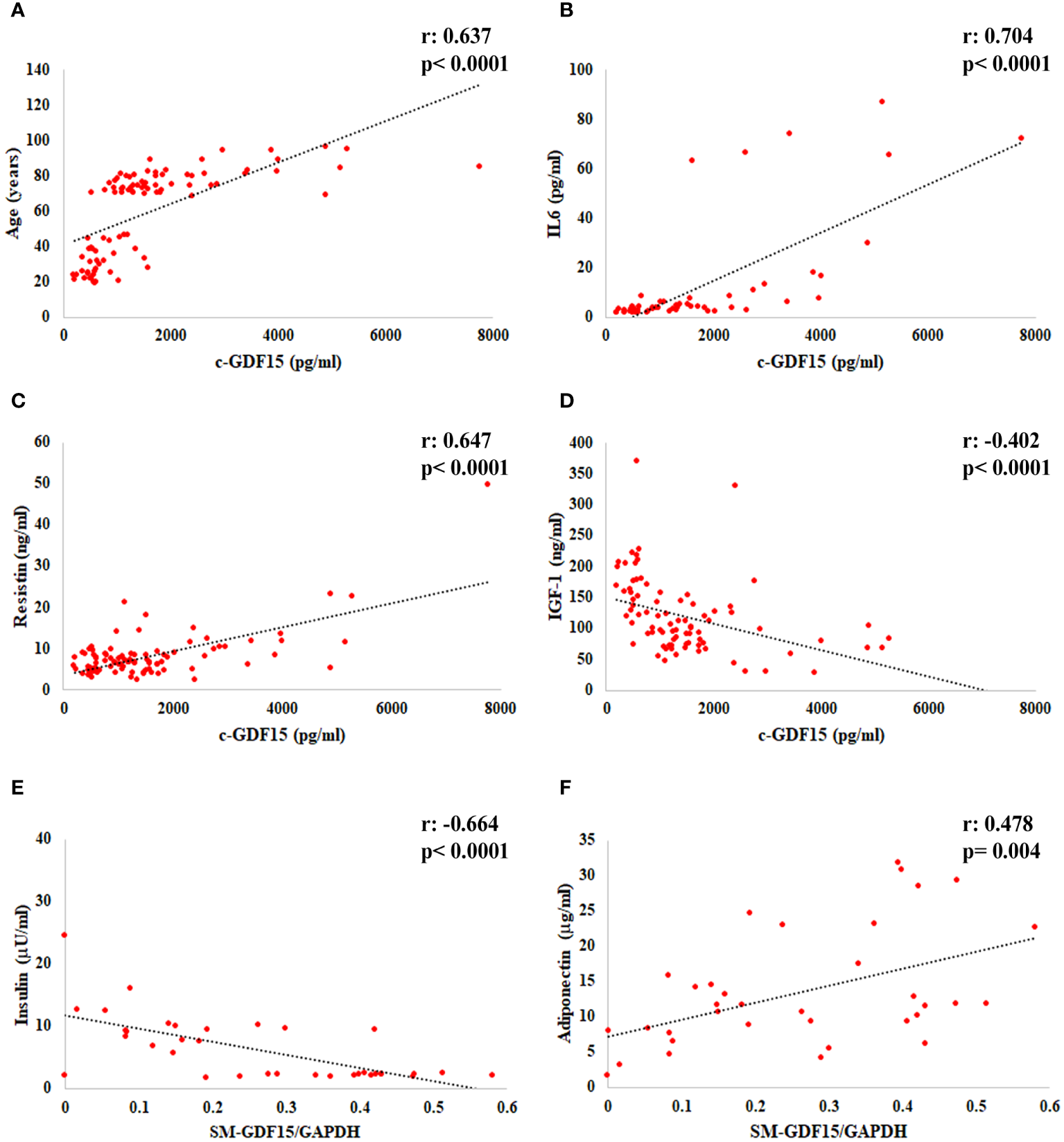
Linear regression analysis of c-GDF15 with (A) age, (B) IL6, (C) Resistin, (D) IGF-1, and of SM-GDF15 with (E) Insulin and (F) Adiponectin, in HS and LLMI. Pearson correlation coefficient (r) and p-value are shown.
When we considered HS group only, a positive correlation of c-GDF15 with age, BMI and IL6 and a negative one with IQS, IQS/BMI and IGF-1 were observed. For this group, data on body composition were available. Interestingly, SM-GDF15 correlated with fat percentage and inversely with lean percentage (27.6% ± 1.3 and 70.4% ± 1.3 of HS body composition on average, respectively) (Table 2).
Table 2
| c-GDF15 | SM-GDF15 | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.756 | < 0.0001 | -0.083 | 0.744 |
| BMI | 0.300 | 0.041 | -0.196 | 0.436 |
| IQS (kg) | -0.325 | 0.026 | -0.426 | 0.078 |
| IQS/BMI | -0.381 | 0.008 | -0.342 | 0.165 |
| Fat % | 0.165 | 0.272 | 0.517 | 0.028 |
| Lean % | -0.118 | 0.435 | -0.527 | 0.025 |
| Adiponectin | 0.122 | 0.413 | 0.322 | 0.192 |
| Leptin | 0.154 | 0.301 | 0.389 | 0.110 |
| Resistin | 0.131 | 0.379 | -0.005 | 0.984 |
| IGF-1 | -0.594 | < 0.0001 | 0.059 | 0.818 |
| Insulin | -0.093 | 0.534 | 0.334 | 0.176 |
| c-PLIN2 | 0.152 | 0.330 | 0.299 | 0.243 |
| IL6 | 0.640 | < 0.0001 | 0.123 | 0.639 |
| IL15 | -0.055 | 0.104 | 0.104 | 0.896 |
| SM-GDF15 | -0.199 | 0.444 | – | – |
Pearson correlations of c-GDF15 and SM-GDF15 with the metabolic and inflammatory parameters in HS (r: Pearson correlation coefficient; p: p-value).
Statistically significant values are indicated in bold.
When we considered LLMI group only, a positive correlation of c-GDF15 with age, Resistin and IL6 and a negative one with IQS, IQS/BMI and IGF-1 were observed. For this group, data on VL thickness were available (1.3mm ± 0.1 on average). An inverse correlation of c-GDF15 with this parameter was found. Furthermore, a positive correlation between SM-GDF15 and IL6 was found (Table 3). Interestingly, higher IL6 plasma levels were observed in LLMI with respect to HS (p= 0.00095, Student’s t test. Supplementary Figure 2).
Table 3
| c-GDF15 | SM-GDF15 | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.714 | < 0.0001 | 0.378 | 0.135 |
| BMI | -0.263 | 0.081 | 0.106 | 0.685 |
| IQS (kg) | -0.405 | 0.019 | -0.188 | 0.628 |
| IQS/BMI | -0.469 | 0.006 | -0.164 | 0.673 |
| Muscle thickness | -0.551 | < 0.0001 | -0.028 | 0.921 |
| Adiponectin | 0.192 | 0.213 | 0.284 | 0.308 |
| Leptin | -0.076 | 0.623 | 0.263 | 0.308 |
| Resistin | 0.689 | < 0.0001 | 0.086 | 0.743 |
| IGF-1 | -0.403 | 0.013 | -0.205 | 0.482 |
| Insulin | -0.144 | 0.350 | -0.421 | 0.092 |
| c-PLIN2 | -0.019 | 0.908 | 0.291 | 0.292 |
| IL6 | 0.687 | < 0.0001 | 0.503 | 0.039 |
| IL15 | -0.105 | 0.493 | -0.336 | 0.188 |
| SM-GDF15 | 0.366 | 0.148 | – | – |
Pearson correlations of c-GDF15 and SM-GDF15 with the metabolic and inflammatory parameters in LLMI (r: Pearson correlation coefficient; p: p-value).
Statistically significant values are indicated in bold.
No correlation was observed with either c-PLIN2 (a marker of adiposity according to 28) or IL15 (a positive modulator of muscle growth, according to 35). Taken together, these data indicate that c-GDF15 is correlated with decreased muscle strength and increased inflammation and could thus represent a biomarker of poor muscle function.
Principal component and canonical discriminant analyses separate HS and LLMI
To better understand the diagnostic role and the possible link of c-GDF15 and SM-GDF15 with the other parameters analyzed, we performed a principal component analysis (PCA) and a canonical discriminant analysis (CDA).
PCA was applied to explore the effect of the parameters analyzed in both HS and LLMI subjects. The first two principal components (PC1 and PC2) described 54% of the total variation (29.6% and 24.8% for PC1 and PC2 respectively). The loading plot of the PCA showed that the parameters analyzed could be grouped into three main groups (Figure 3A). The first group (positive loadings for PC1 and PC2) included c-GDF15, IL6, IL15 and Resistin, which are molecules related to inflammatory responses (23, 36–38). The second group (variables with negative and positive loadings for PC1 and PC2 respectively) included BMI, c-PLIN2 and Leptin, that are related to adipose tissue and its metabolism, as well as energy balance regulation (39, 40). The third group (negative loadings for both PCs) included Adiponectin, an anti-inflammatory protein secreted by adipose tissue, and SM-GDF15. Surprisingly, the score plot of the PCA showed that HS were mainly associated with SM-GDF15 and Adiponectin, while LLMI were associated with all the other parameters. These latter could be further subdivided by age, in particular >70yrs LLMI were mainly associated to c-GDF15, IL6, IL15 and Resistin (Figure 3B).
Figure 3
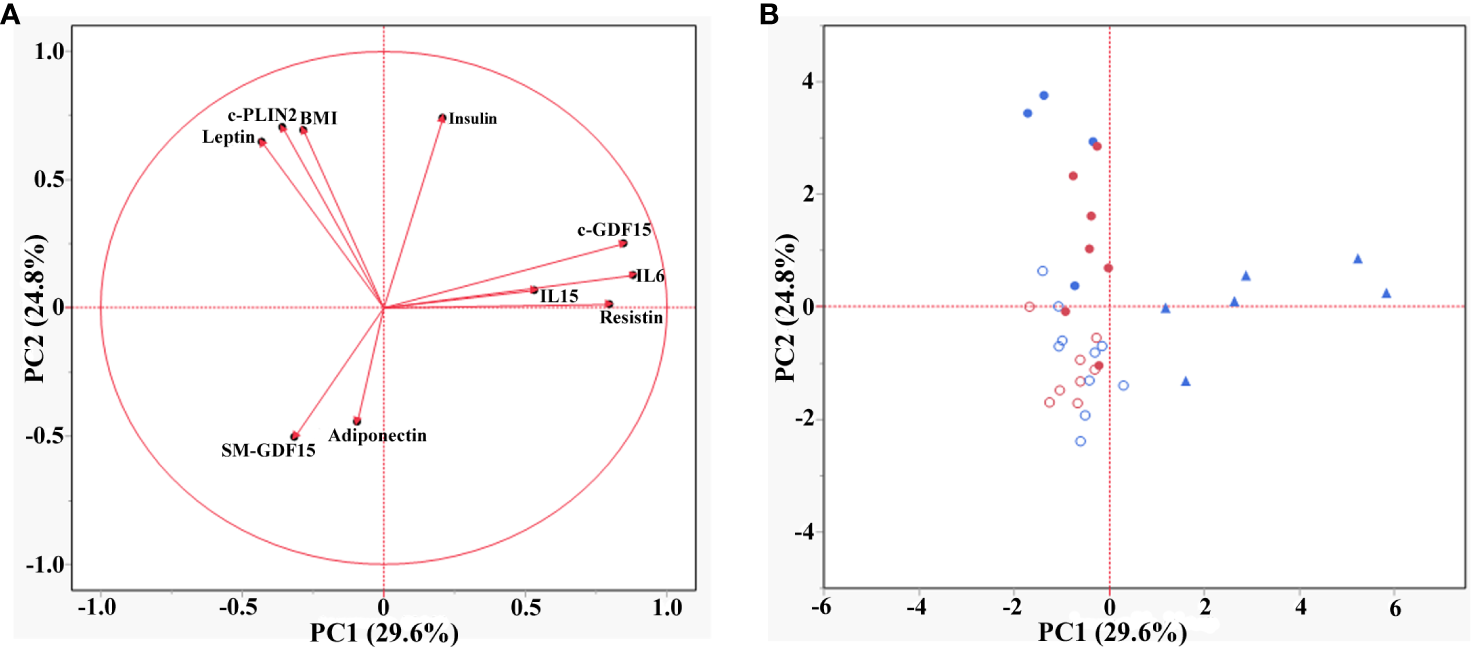
Principal component analysis (PCA) in HS and LLMI. (A) Loading plot of the PCA showing the different groups of parameters. (B) Score plot of the PCA showing the association of the different groups of subjects (<40 years HS, >70 years HS, <40 years LLMI and >70 years LLMI) with the parameters analyzed. Empty red circles are HS <40 years; empty blue circles are HS >70 years; filled red circles are LLMI <40 years; filled blue circles are LLMI >70 years; filled triangles are LLMI >85 years.
CDA showed that the parameters considered could discriminate the subjects into four groups (<40yrs HS, >70yrs HS, <40yrs LLMI and >70yrs LLMI). The canonical 1 accounted for 67.93% of separation and the canonical 2 for 21.17%. In particular, >70yrs LLMI were well separated from all other groups, mainly because of c-GDF15 and Leptin expression levels (Figure 4).
Figure 4
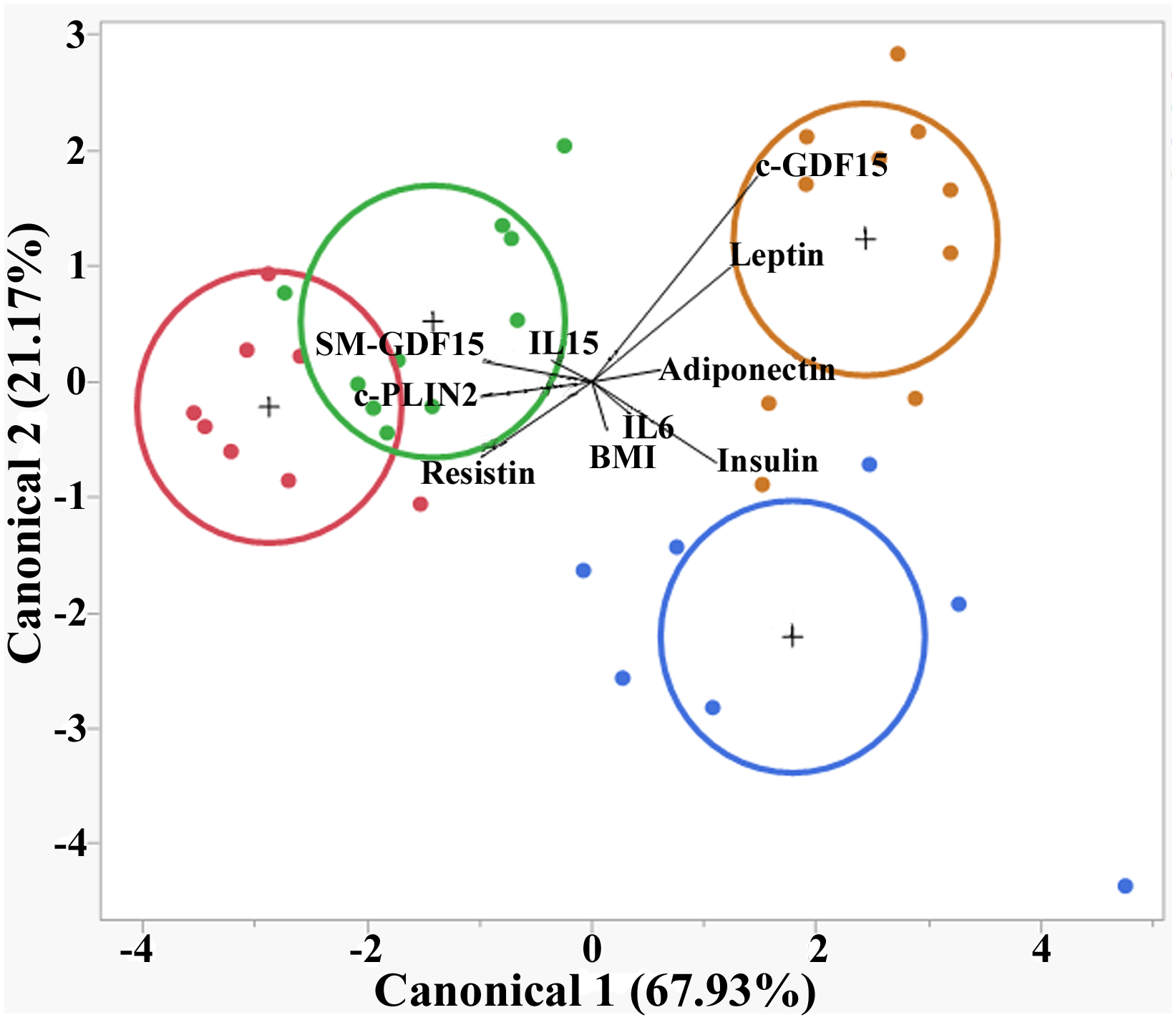
Canonical discriminant analysis (CDA) in HS and LLMI. Discrimination of <40 HS, >70 HS, <40 LLMI and >70 LLMI based on the parameters analyzed. Red circle: <40 HS; green circle: >70 HS; blue circle: <40 LLMI; yellow circle: >70 LLMI.
In order to evaluate which of the parameters analyzed had the highest discriminative ability in HS and LLMI, we calculated the receiver operating characteristic (ROC) curves, also in order to detect a cut-off for the discriminating parameters considered. The areas under the curves (AUCs) are reported in Tables 4, 5. SM-GDF15 and Adiponectin resulted the most discriminative parameters for HS (Figure 5A), while Insulin and c-GDF15 were those for LLMI (Figure 5B). These data indicate that these parameters can discriminate between LLMI and HS and, interestingly, that c-GDF15 and SM-GDF15 levels seem to characterize LLMI and HS, respectively.
Table 4
| Test result variables | Area (AUCs) | Std. Error | p |
|---|---|---|---|
| Adiponectin | 0.734 | 0.084 | 0.019 |
| Leptin | 0.507 | 0.103 | 0.947 |
| Resistin | 0.480 | 0.106 | 0.843 |
| Insulin | 0.023 | 0.024 | 0.000 |
| c-GDF15 | 0.230 | 0.082 | 0.007 |
| SM-GDF15 | 0.888 | 0.062 | 0.000 |
| IL6 | 0.219 | 0.089 | 0.005 |
| c-PLIN2 | 0.438 | 0.100 | 0.529 |
ROC analysis of metabolic and inflammatory parameters in HS.
Areas under the curves (AUCs) are shown. Null hypothesis: true area = 0.5.
Statistically significant values are indicated in bold.
Figure 5
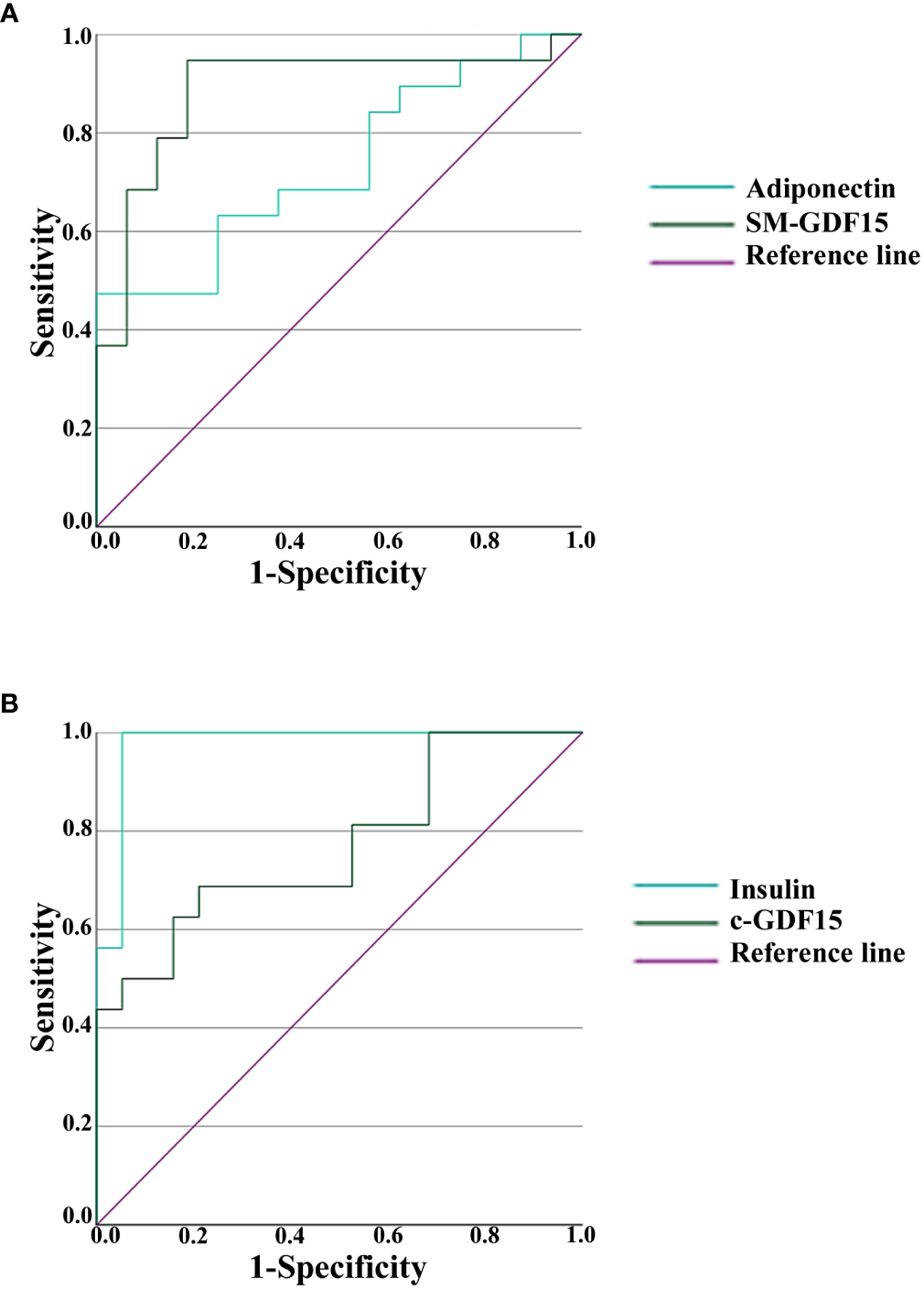
Receiver operating characteristic (ROC) analysis in HS and LLMI. (A) ROC curves of Adiponectin and SM-GDF15 in HS. (B) ROC curves of Insulin and c-GDF15 in LLMI.
Table 5
| Test result variables | Area (AUCs) | Std. Error | p |
|---|---|---|---|
| Adiponectin | 0.266 | 0.084 | 0.019 |
| Leptin | 0.493 | 0.103 | 0.947 |
| Resistin | 0.520 | 0.106 | 0.843 |
| Insulin | 0.977 | 0.024 | 0.000 |
| c-GDF15 | 0.770 | 0.082 | 0.007 |
| SM-GDF15 | 0.112 | 0.062 | 0.000 |
| IL6 | 0.781 | 0.089 | 0.005 |
| c-PLIN2 | 0.562 | 0.100 | 0.529 |
ROC analysis of metabolic and inflammatory parameters in LLMI patients.
Areas under the curves (AUCs) are shown. Null hypothesis: true area = 0.5. The test result variable IL6 has at least one tie between the positive actual state group and the negative actual state group, statistic may be biased.
Statistically significant values are indicated in bold.
Discussion
It has been reported that plasma levels of GDF15 are associated with low muscle function and sarcopenia (14, 41–45). However, it is not yet clear what is the role of SM-GDF15 in muscle function and whether the levels of c-GDF15 are linked to SM-GDF15, given that skeletal muscles constitute the largest body component. Therefore, in this study we addressed the following questions: i. are the levels of SM-GDF15 correlated with those of c-GDF15? ii. are the levels of SM-GDF15 correlated with muscle strength or other parameters related to inflammation or metabolism? iii. is there any change with age in SM-GDF15 and its possible correlations with other parameters? iv. is it possible to discriminate patients suffering of muscle disuse or sarcopenia from healthy controls, as well as young from elderly subjects taking advantage of these parameters?
Quite surprisingly, SM-GDF15 expression evaluated in Vastus lateralis biopsies did not correlate with the levels of c-GDF15 nor with isometric quadriceps strength (IQS) and resulted higher in HS than LLMI samples. This suggests that the contribution of skeletal muscle to the production of c-GDF15 is likely exceeded by the contributions of other organs/tissues. Moreover, given the fact that the expression of SM-GDF15 is apparently higher in HS group, it is possible that in skeletal muscle such an expression is stimulated by fiber contraction, as suggested also by previous data indicating a transient spike of GDF15 expression upon a strenuous physical bout (14). On the contrary, it seems that inactivity induces a much lower expression of SM-GDF15. This conclusion is schematized in Figure 6.
Figure 6
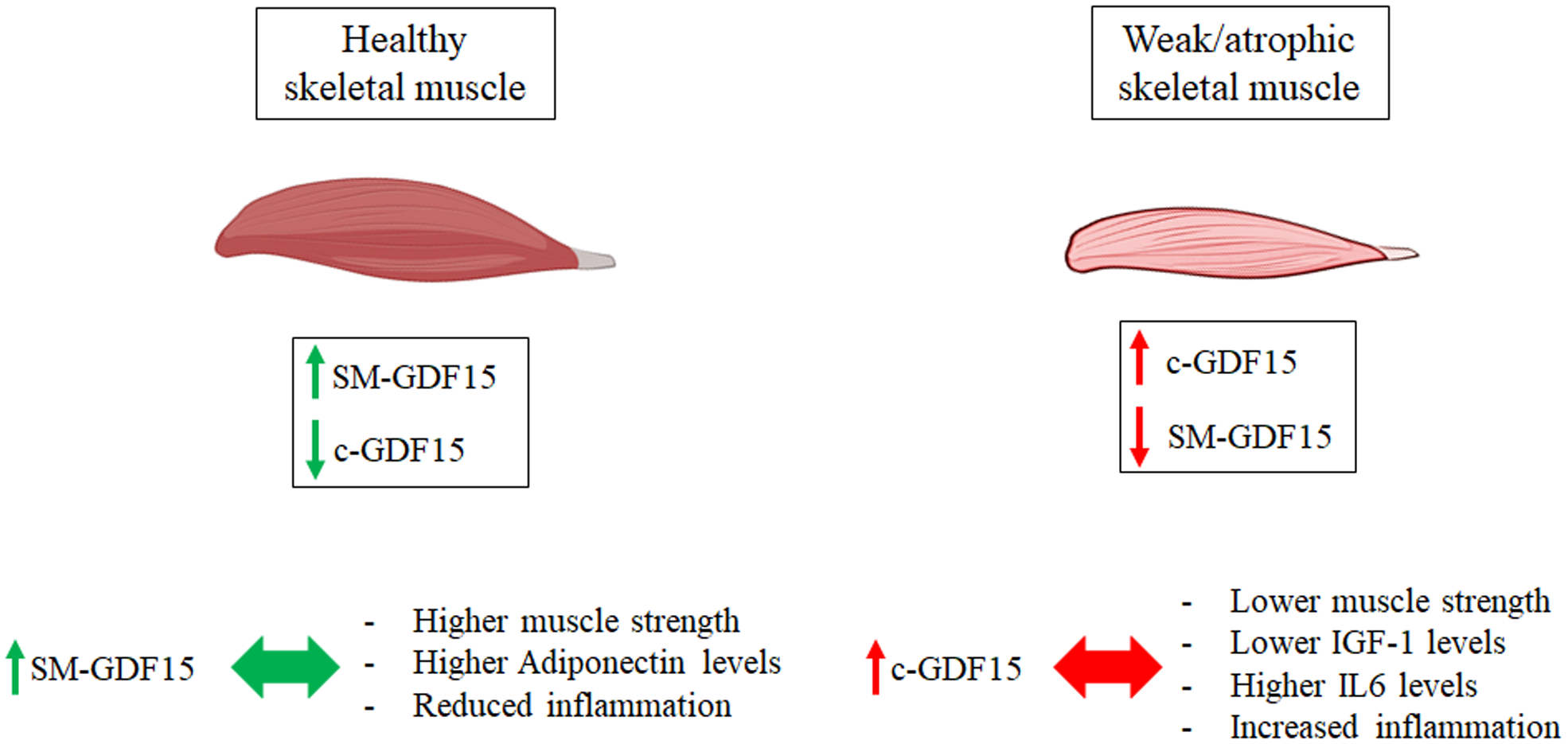
Working hypothesis on the diagnostic roles of c-GDF15 and SM-GDF15. Higher levels of SM-GDF15 and lower levels of c-GDF15 are observed in healthy subjects with functional muscles, and are associated with reduced inflammation, higher muscle strength and higher Adiponectin levels. Lower SM-GDF15 and higher c-GDF15 levels, on the contrary, are observed in patients with LLMI, with atrophic and weak muscles, and are associated with increased inflammation, higher IL6 levels and lower IGF-1 levels and muscle strength.
As far as the correlation with other parameters, when considering LLMI and HS together, c-GDF15 is associated with age, as expected, and with other parameters such as IGF-1 (46), IL6 (47), Resistin (46), a finding that is also not unexpected, as these parameters are associated with age, too. At variance, SM-GDF15 resulted directly associated with Adiponectin and inversely with Insulin. In the case of Adiponectin, it is known that it plays a role in maintaining skeletal muscle health and function, in particular by promoting glucose uptake and fatty acids oxidation in the skeletal muscle (48, 49). Given that a role in promoting lipid oxidation has also been attributed to GDF15 (50), it is possible that the two molecules could have a synergic role in the skeletal muscle. As far as Insulin, it is known that GDF15 improves insulin sensitivity (51, 52), thus it is possible that an inverse correlation exists between Insulin and GDF15. However, it is to note that existing data regard c-GDF15, but not SM-GDF15, therefore this hypothesis has to be further validated. Interestingly, c-GDF15 is inversely associated with IQS in both HS and LLMI, while SM-GDF15 positively correlates with IL6 only in LLMI but not HS group. Together with the observation that SM-GDF15 seems to be higher in HS, these data may suggest that c-GDF15 but not SM-GDF15 is associated with inflammation and decreased muscle function (Figure 6). In particular, the fact that in LLMI, but not in HS group, SM-GDF15 is associated with IL6 may suggest that a normal physical activity induces GDF15 but not IL6 expression, while chronic inactivity induces IL6 but not (or not so much) GDF15. Unfortunately, we do not have data on muscle-specific expression of IL6, however, being IL6 a well-known myokine needed to induce muscle repair (53, 54), it can be inferred that, in absence of overt infections, the large majority of circulating IL6 comes from muscles. Since GDF15 expression is triggered, among others, by mitochondrial stress (55–57), it can be hypothesized that GDF15 expression is induced in contracting myofibers, where an intense mitochondrial activity is present. On the contrary, inactive muscle may display a reduced GDF15 expression but, on the contrary, a higher IL6 expression. Further experiments are needed to formally prove this hypothesis.
It is important to note that in skeletal muscle tissue we were only able to detect the immature and non-cleaved form of GDF15 (pro-GDF15) and not the mature form, which is the one found in the circulation and considered the most active. Thus, we cannot rule out the possibility that SM-GDF15 (being an immature form) is not proportionally connected with the levels of c-GDF15 (therefore accounting for the missing association between the two forms), however, it is known that many other organs and tissues produce this protein, including prostate, kidney, lung, but also senescent cells and many types of cancers (13, 26, 58–60). Therefore, the level of c-GDF15 derives from the sum of all these contributions, reflecting the general health state of a subject rather than muscle health alone.
To further support the idea that the diagnostic meaning of c-GDF15 and SM-GDF15 are different, the PCA, CDA and ROC analysis clearly indicate that these two variables have an opposite direction and can help discriminating between HS and LLMI groups. In particular, c-GDF15 is the main factor for discriminating >70yrs patients, while <40yrs patients seem to be discriminated by other parameters including Insulin and IL6. At variance, SM-GDF15 seems to be associated with muscle activity and can help discriminating healthy, active people.
As a whole, these data suggest that c-GDF15 is associated with decreased muscle strength and mass and can be useful to identify patients with muscle function impairment/sarcopenia, in particular elderly ones (Figure 6).
Limitations of the study
This study has some limitations. First of all, the relatively low number of subjects that have been studied and the lack of measurement of some parameters, such as IL6 in skeletal muscle biopsies, due to the tiny amount of biopsy material available. However, the post-hoc power analysis indicated that the sample numerosity was high enough to grant for trustable results. The exact role of GDF15 within the muscle fibers remains poorly elucidated and the present data seem apparently in contrast with the well-known pro-cachectic and catabolic role of GDF15. It is however to note that the conclusions of the majority of studies stem from experiments only considering c-GDF15, not SM-GDF15. Whether the level of this intramuscular form of GDF15 has precise biologic/metabolic effects at muscle level or it is rather a mere marker of muscle activity needs to be better clarified with further studies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethical Committee of Istituto Ortopedico Rizzoli, Bologna, Italy (ethical clearance no. 10823 issued on April 26, 2010). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AC: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. GC: Data curation, Formal analysis, Software, Writing – review & editing. LR: Investigation, Methodology, Writing – review & editing. LT: Investigation, Methodology, Writing – review & editing. SS: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – original draft. MC: Conceptualization, Formal analysis, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work has been co-funded from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society” to SS. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022]. The work was also co-funded from the Italian Ministry of University and Research (MUR) PRIN 2022, Project # 2022KS8T4N “GDF15 as a key player and a potential target to tackle ageing and age-associated diseases: an in silico, in vitro and ex vivo study” to SS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1404047/full#supplementary-material
Supplementary Figure 1ELISA analysis of c-GDF15 levels in healthy subjects (HS) and patients with lower limb mobility impairment (LLMI). (A) c-GDF15 level in LLMI compared to HS. (B) c-GDF15 level in HS of <40 years of age (<40) compared to HS of >70 years of age (>70). (C) c-GDF15 level in LLMI <40 compared to LLMI >70. Data are expressed as mean ± SD. Mann-Whitney test was applied. Each sample was analyzed in duplicate.
Supplementary Figure 2ELISA analysis of plasma IL6 levels in HS and LLMI. Data are expressed as mean ± SD. Student’s t test was applied. Each sample was analyzed in duplicate.
References
1
Kuk JL Saunders TJ Davidson LE Ross R . Age-related changes in total and regional fat distribution. Ageing Res Rev. (2009) 8:339–48. doi: 10.1016/j.arr.2009.06.001
2
Conte M Vasuri F Trisolino G Bellavista E Santoro A Degiovanni A et al . Increased Plin2 expression in human skeletal muscle is associated with sarcopenia and muscle weakness. PloS One. (2013) 8:e73709. doi: 10.1371/journal.pone.0073709
3
Conte M Martucci M Sandri M Franceschi C Salvioli S . The dual role of the pervasive "Fattish" Tissue remodeling with age. Front Endocrinol (Lausanne). (2019) 10:114. doi: 10.3389/fendo.2019.00114
4
Smith L Tully M Jacob L Blackburn N Adlakha D Caserotti P et al . The association between sedentary behavior and sarcopenia among adults aged ≥65 years in lowand middle-income countries. Int J Environ Res Public Health. (2020) 17:1708. doi: 10.3390/ijerph17051708
5
Walston JD . Sarcopenia in older adults. Curr Opin Rheumatol. (2012) 24:623–7. doi: 10.1097/BOR.0b013e328358d59b
6
Wilson D Jackson T Sapey E Lord JM . Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res Rev. (2017) 36:1–10. doi: 10.1016/j.arr.2017.01.006
7
Veronese N Demurtas J Soysal P Smith L Torbahn G Schoene D et al . Special Interest Groups in Systematic Reviews and Meta-analyses for healthy ageing Sarcopenia and Frailty and resilience in older persons of the European Geriatric Medicine Society (EuGMS). Sarcopenia and health-related outcomes: an umbrella review of observational studies. Eur Geriatr Med. (2019) 10:853–62. doi: 10.1007/s41999-019-00233-w
8
Franceschi C Bonafè M Valensin S Olivieri F De Luca M Ottaviani E et al . Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. (2000) 908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x
9
Bian AL Hu HY Rong YD Wang J Wang JX Zhou XZ . A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. (2017) 22:25. doi: 10.1186/s40001-017-0266-9
10
Dalle S Rossmeislova L Koppo K . The role of inflammation in age-related sarcopenia. Front Physiol. (2017) 8:1045. doi: 10.3389/fphys.2017.01045
11
Wang T . Searching for the link between inflammaging and sarcopenia. Ageing Res Rev. (2022) 77:101611. doi: 10.1016/j.arr.2022.101611
12
Conte M Martucci M Chiariello A Franceschi C Salvioli S . Mitochondria, immunosenescence and inflammaging: a role for mitokines? Semin Immunopathol. (2020) 42:607–17. doi: 10.1007/s00281-020-00813-0
13
Conte M Giuliani C Chiariello A Iannuzzi V Franceschi C Salvioli S . GDF15, an emerging key player in human aging. Ageing Res Rev. (2022) 75:101569. doi: 10.1016/j.arr.2022.101569
14
Conte M Martucci M Mosconi G Chiariello A Cappuccilli M Totti V et al . GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front Immunol. (2020) 11:915. doi: 10.3389/fimmu.2020.00915
15
Alcazar J Frandsen U Prokhorova T Kamper RS Haddock B Aagaard P et al . Changes in systemic GDF15 across the adult lifespan and their impact on maximal muscle power: the Copenhagen Sarcopenia Study. J Cachexia Sarcopenia Muscle. (2021) 12:1418–27. doi: 10.1002/jcsm.12823
16
Kim-Muller JY Song L LaCarubba Paulhus B Pashos E Li X Rinaldi A et al . GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. (2023) 42:111947. doi: 10.1016/j.celrep.2022.111947
17
Durieux J Wolff S Dillin A . The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. (2011) 144:79–91. doi: 10.1016/j.cell.2010.12.016
18
Tanaka T Biancotto A Moaddel R Moore AZ Gonzalez-Freire M Aon MA et al . Plasma proteomic signature of age in healthy humans. Aging Cell. (2018) 17:e12799. doi: 10.1111/acel.12799
19
Conte M Ostan R Fabbri C Santoro A Guidarelli G Vitale G et al . Human aging and longevity are characterized by high levels of mitokines. J Gerontol A Biol Sci Med Sci. (2019) 74:600–7. doi: 10.1093/gerona/gly153
20
Mullican SE Rangwala SM . Uniting GDF15 and GFRAL: therapeutic opportunities in obesity and beyond. Trends Endocrinol Metab. (2018) 29:560–70. doi: 10.1016/j.tem.2018.05.002
21
Baek SJ Eling T . Growth differentiation factor 15 (GDF15): A survival protein with therapeutic potential in metabolic diseases. Pharmacol Ther. (2019) 198:46–58. doi: 10.1016/j.pharmthera.2019.02.008
22
Wang D Day EA Townsend LK Djordjevic D Jørgensen SB Steinberg GR . GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. (2021) 17:592–607. doi: 10.1038/s41574-021-00529-7
23
Luan HH Wang A Hilliard BK Carvalho F Rosen CE Ahasic AM et al . GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. (2019) 178:1231–1244.e11. doi: 10.1016/j.cell.2019.07.033
24
Pence BD . Growth differentiation factor-15 in immunity and aging. Front Aging. (2022) 3:837575. doi: 10.3389/fragi.2022.837575
25
Patel S Alvarez-Guaita A Melvin A Rimmington D Dattilo A Miedzybrodzka EL et al . GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. (2019) 29:707–718.e8. doi: 10.1016/j.cmet.2018.12.016
26
Welsh JB Sapinoso LM Kern SG Brown DA Liu T Bauskin AR et al . Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci USA. (2003) 100:3410–5. doi: 10.1073/pnas.0530278100
27
Baggen VJ van den Bosch AE Eindhoven JA Schut AW Cuypers JA Witsenburg M et al . Prognostic value of N-terminal pro-B-type natriuretic peptide, troponin-T, and growth-differentiation factor 15 in adult congenital heart disease. Circulation. (2017) 135:264–79. doi: 10.1161/CIRCULATIONAHA.116.023255
28
Conte M Sabbatinelli J Chiariello A Martucci M Santoro A Monti D et al . Disease-specific plasma levels of mitokines FGF21, GDF15, and Humanin in type II diabetes and Alzheimer's disease in comparison with healthy aging. Geroscience. (2021) 43:985–1001. doi: 10.1007/s11357-020-00287-w
29
Chiariello A Valente S Pasquinelli G Baracca A Sgarbi G Solaini G et al . The expression pattern of GDF15 in human brain changes during aging and in Alzheimer's disease. Front Aging Neurosci. (2023) 14:1058665. doi: 10.3389/fnagi.2022.1058665
30
Deng M Bian Y Zhang Q Zhou X Hou G . Growth differentiation factor-15 as a biomarker for sarcopenia in patients with chronic obstructive pulmonary disease. Front Nutr. (2022) 9:897097. doi: 10.3389/fnut.2022.897097
31
Kim H Kim KM Kang MJ Lim S . Growth differentiation factor-15 as a biomarker for sarcopenia in aging humans and mice. Exp Gerontol. (2020) 142:111115. doi: 10.1016/j.exger.2020.111115
32
Chung HK Ryu D Kim KS Chang JY Kim YK Yi HS et al . Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol. (2017) 216:149–65. doi: 10.1083/jcb.201607110
33
Seppet E Pääsuke M Conte M Capri M Franceschi C . Ethical aspects of aging research. Biogerontology. (2011) 12:491–502. doi: 10.1007/s10522-011-9340-9
34
Joliffe IT . Principal component analysis. 2nd ed. New York: Springer Science & Business Media (2002).
35
O'Leary MF Wallace GR Bennett AJ Tsintzas K Jones SW . IL-15 promotes human myogenesis and mitigates the detrimental effects of TNFα on myotube development. Sci Rep. (2017) 7:12997. doi: 10.1038/s41598-017-13479-w
36
Park HK Kwak MK Kim HJ Ahima RS . Linking resistin, inflammation, and cardiometabolic diseases. Korean J Intern Med. (2017) 32:239–47. doi: 10.3904/kjim.2016.229
37
Perera PY Lichy JH Waldmann TA Perera LP . The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect. (2012) 14:247–61. doi: 10.1016/j.micinf.2011.10.006
38
Tanaka T Narazaki M Kishimoto T . IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
39
Obradovic M Sudar-Milovanovic E Soskic S Essack M Arya S Stewart AJ et al . Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne). (2021) 12:585887. doi: 10.3389/fendo.2021.585887
40
Conte M Santoro A Collura S Martucci M Battista G Bazzocchi A et al . Circulating perilipin 2 levels are associated with fat mass, inflammatory and metabolic markers and are higher in women than men. Aging (Albany NY). (2021) 13:7931–42. doi: 10.18632/aging.202840
41
Rosenberg BJ Hirano M Quinzii CM Colantuoni E Needham DM Lederer DJ et al . Growth differentiation factor-15 as a biomarker of strength and recovery in survivors of acute respiratory failure. Thorax. (2019) 74:1099–101. doi: 10.1136/thoraxjnl-2019-213621
42
Oba K Ishikawa J Tamura Y Fujita Y Ito M Iizuka A et al . Serum growth differentiation factor 15 level is associated with muscle strength and lower extremity function in older patients with cardiometabolic disease. Geriatr Gerontol Int. (2020) 20:980–7. doi: 10.1111/ggi.14021
43
Herpich C Franz K Ost M Otten L Coleman V Klaus S et al . Associations between serum GDF15 concentrations, muscle mass, and strength show sex-specific differences in older hospital patients. Rejuvenation Res. (2021) 24:14–9. doi: 10.1089/rej.2020.2308
44
Nishikawa R Fukuda T Haruyama A Shibasaki I Yamaguchi S Arikawa T et al . Association between serum GDF-15, myostatin, and sarcopenia in cardiovascular surgery patients. Int J Cardiol Heart Vasc. (2022) 42:101114. doi: 10.1016/j.ijcha.2022.101114
45
Yamamoto H Takeshima F Haraguchi M Akazawa Y Matsushima K Kitayama M et al . High serum concentrations of growth differentiation factor-15 and their association with Crohn's disease and a low skeletal muscle index. Sci Rep. (2022) 12:6591. doi: 10.1038/s41598-022-10587-0
46
Bucci L Yani SL Fabbri C Bijlsma AY Maier AB Meskers CG et al . Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. (2013) 14:261–72. doi: 10.1007/s10522-013-9428-5
47
Ershler WB Keller ET . Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. (2000) 51:245–70. doi: 10.1146/annurev.med.51.1.245
48
Liu Y Sweeney G . Adiponectin action in skeletal muscle. Best Pract Res Clin Endocrinol Metab. (2014) 28:33–41. doi: 10.1016/j.beem.2013.08.003
49
Krause MP Milne KJ Hawke TJ . Adiponectin-consideration for its role in skeletal muscle health. Int J Mol Sci. (2019) 20:1528. doi: 10.3390/ijms20071528
50
Zhang M Sun W Qian J Tang Y . Fasting exacerbates hepatic growth differentiation factor 15 to promote fatty acid β-oxidation and ketogenesis via activating XBP1 signaling in liver. Redox Biol. (2018) 16:87–96. doi: 10.1016/j.redox.2018.01.013
51
Xie B Murali A Vandevender AM Chen J Silva AG Bello FM et al . Hepatocyte-derived GDF15 suppresses feeding and improves insulin sensitivity in obese mice. iScience. (2022) 25:105569. doi: 10.1016/j.isci.2022.105569
52
Sjøberg KA Sigvardsen CM Alvarado-Diaz A Andersen NR Larance M Seeley RJ et al . GDF15 increases insulin action in the liver and adipose tissue via a β-adrenergic receptor-mediated mechanism. Cell Metab. (2023) 35:1327–1340.e5. doi: 10.1016/j.cmet.2023.06.016
53
Pedersen BK . Special feature for the Olympics: effects of exercise on the immune system: exercise and cytokines. Immunol Cell Biol. (2000) 78:532–5. doi: 10.1111/j.1440-1711.2000.t01-11-.x
54
Kistner TM Pedersen BK Lieberman DE . Interleukin 6 as an energy allocator in muscle tissue. Nat Metab. (2022) 4:170–9. doi: 10.1038/s42255-022-00538-4
55
Montero R Yubero D Villarroya J Henares D Jou C Rodríguez MA et al . GDF-15 is elevated in children with mitochondrial diseases and is induced by mitochondrial dysfunction. PloS One. (2016) 11:e0148709. doi: 10.1371/journal.pone.0148709
56
Poulsen NS Madsen KL Hornsyld TM Eisum AV Fornander F Buch AE et al . Growth and differentiation factor 15 as a biomarker for mitochondrial myopathy. Mitochondrion. (2020) 50:35–41. doi: 10.1016/j.mito.2019.10.005
57
Chiariello A Rossetti L Valente S Pasquinelli G Sollazzo M Iommarini L et al . Downregulation of PLIN2 in human dermal fibroblasts impairs mitochondrial function in an age-dependent fashion and induces cell senescence via GDF15. Aging Cell. (2024) 22:e14111. doi: 10.1111/acel.14111. Online ahead of print.
58
Rybicki BA Sadasivan SM Chen Y Kravtsov O Palangmonthip W Arora K et al . Growth and differentiation factor 15 and NF-κB expression in benign prostatic biopsies and risk of subsequent prostate cancer detection. Cancer Med. (2021) 10:3013–25. doi: 10.1002/cam4.3850
59
Radwanska A Cottage CT Piras A Overed-Sayer C Sihlbom C Budida R et al . Increased expression and accumulation of GDF15 in IPF extracellular matrix contribute to fibrosis. JCI Insight. (2022) 7:e153058. doi: 10.1172/jci.insight.153058
60
Valiño-Rivas L Cuarental L Ceballos MI Pintor-Chocano A Perez-Gomez MV Sanz AB et al . Growth differentiation factor-15 preserves Klotho expression in acute kidney injury and kidney fibrosis. Kidney Int. (2022) 101:1200–15. doi: 10.1016/j.kint.2022.02.028
Summary
Keywords
GDF15, biomarkers, muscle health, aging, inflammaging
Citation
Chiariello A, Conte G, Rossetti L, Trofarello L, Salvioli S and Conte M (2024) Different roles of circulating and intramuscular GDF15 as markers of skeletal muscle health. Front. Endocrinol. 15:1404047. doi: 10.3389/fendo.2024.1404047
Received
20 March 2024
Accepted
29 April 2024
Published
14 May 2024
Volume
15 - 2024
Edited by
Angelica Giuliani, Marche Polytechnic University, Italy
Reviewed by
Naresh Chandra Bal, KIIT Universit, India
Elettra Mancuso, University Magna Graecia of Catanzaro, Italy
Updates
Copyright
© 2024 Chiariello, Conte, Rossetti, Trofarello, Salvioli and Conte.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Salvioli, stefano.salvioli@unibo.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.