- 1Department of Third Internal Medicine, Shirakawa Kosei General Hospital, Shirakawa, Japan
- 2Department of Chest Surgery, Shirakawa Kosei General Hospital, Shirakawa, Japan
Immune checkpoint inhibitors (ICIs) are widely used in cancer treatment; however, they can lead to immune-related adverse events, including immune checkpoint inhibitor-induced type 1 diabetes mellitus (ICI-T1DM). While fulminant T1DM is common in East Asia, ICI-T1DM has predominantly been reported in Western countries. In this report, we present the case of a 66-year-old Japanese man with type 2 diabetes mellitus undergoing dialysis for diabetic nephropathy. The patient was diagnosed with left upper lobe lung cancer, and treatment with nivolumab and ipilimumab was initiated. After 48 days, the patient experienced impaired consciousness and difficulty moving. His blood glucose levels were 815 mg/dL, and metabolic acidosis was detected, leading to a diagnosis of diabetic ketoacidosis. The patient was subsequently treated with continuous intravenous insulin. However, his C-peptide levels rapidly depleted, and new-onset ICI-T1DM was diagnosed. Although most Japanese patients with ICI-T1DM test negative for glutamic acid decarboxylase (GAD) antibodies, this case exhibited a strong positivity. Thus, we reviewed the literature on 15 similar Japanese cases, revealing a mean HbA1c level at onset of 8.7% and a mean time from ICI administration to onset of 9.7 weeks, which was shorter than that in GAD-negative cases. Moreover, human leukocyte antigen typing revealed five cases of DRB1*04:05-DQB1*04:01, including the present case, and one case of DRB1*09:01-DQB1*03:03, both of which were susceptible to T1DM haplotypes. These findings suggest that GAD antibody positivity may be associated with acute onset and disease progression in some cases of Japanese patients with ICI-T1DM. Given that the prediction of new-onset ICI-T1DM is challenging, monitoring GAD antibody levels might be useful. However, further studies with large sample sizes and validation across different racial and ethnic populations are warranted.
1 Introduction
Immune checkpoint inhibitors (ICIs) have been widely used for the treatment of many cancers owing to their potent anti-tumor effects, achieved by inhibiting molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1) (1). However, immune-related adverse events following the use of ICIs have been increasingly reported (2, 3). Fulminant type 1 diabetes mellitus (T1DM) is prevalent in East Asia, including Japan (4). ICI-induced T1DM (ICI-T1DM) has been widely reported in many Western countries, and its onset resembles that of fulminant T1DM (5). ICI-T1DM and acute T1DM are similar yet different conditions classified as “checkpoint inhibitor-associated autoimmune diabetes mellitus (CIADM)” (6), and ICI-T1DM has been recognized as a new category within T1DM.
Glutamic acid decarboxylase (GAD) antibody and human leukocyte antigen (HLA) haplotypes are recognized for their roles in the diagnosis and pathogenesis of T1DM (7, 8). However, their roles in ICI-T1DM have not been yet determined. Baden et al. recently reported that the prevalence of GAD antibody positivity in ICI-T1DM is lower in Japan than in Western countries (9). Moreover, in Western countries, GAD antibody-positive ICI-T1DM has a more acute onset than GAD antibody-negative ICI-T1DM (5, 10–13). However, evidence from Japanese patients is lacking. Although it has been reported that DRB1*04:05-DQB1*04:01 and DRB1*09:01-DQB1*03:03 are common HLA haplotypes in Japanese patients with acute-onset and fulminant T1DM (14, 15), the association between HLA haplotypes and Japanese ICI-T1DM remains unclear.
In this report, we present a case of GAD antibody positivity and T1DM-susceptible haplotype-DRB1*04:05-DQB1*04:01 in ICI-T1DM and review GAD antibody positivity and HLA haplotypes in Japanese ICI-T1DM cases.
2 Case description
A 66-year-old man with a history of diabetes since 43 years of age had been under treatment with antidiabetic agents, including alpha-glucosidase inhibitors, sulfonylureas, and thiazolidinediones. However, owing to inadequate control, he required the administration of a subcutaneous injection of long-acting insulin. At 47 years of age, his serum C-peptide concentration was 0.9 ng/mL. At 59 years of age, he required hemodialysis for diabetic nephropathy. He was treated with 6 units of insulin glargine subcutaneously injected before bedtime and 0.3 mg of voglibose before each meal at another hospital. His fasting blood glucose ranged from 120 to 180 mg/dL, and his HbA1c levels ranged from 6.0 to 7.3%.
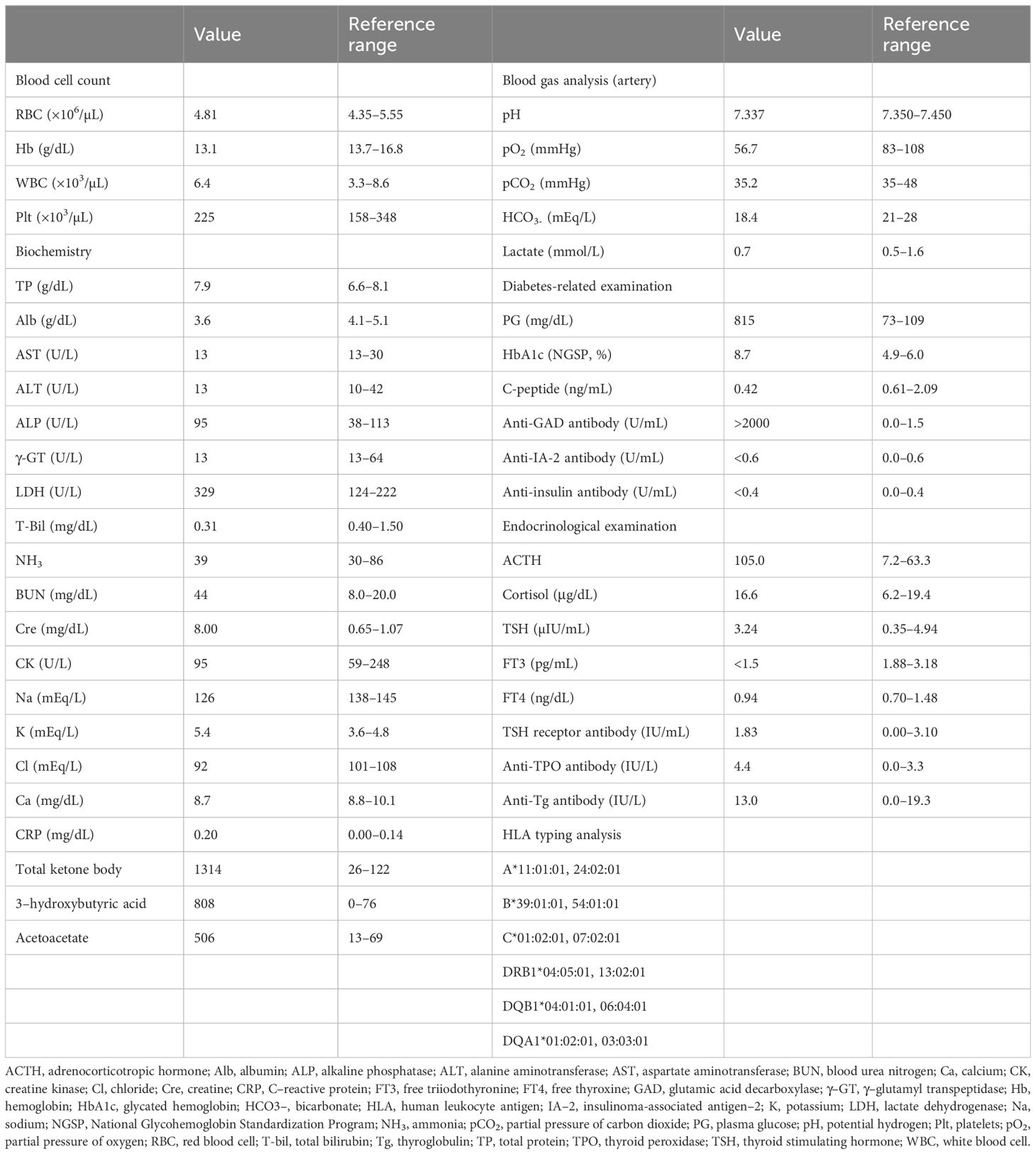
At 66 years of age, at our hospital, immunotherapy with nivolumab and ipilimumab was initiated for left upper lobe lung cancer (c-T2aN1M1a Stage 4A), with the initiation of immunotherapy defined as day 1. After starting immunotherapy, the patient experienced diarrhea and itching, which were considered side effects of immunotherapy. Accordingly, treatment with 10 mg/day of prednisolone was initiated concurrently with the third nivolumab administration on day 43.
On the fifth day of the third nivolumab administration (day 47), the patient began experiencing symptoms of fatigue, followed by difficulty moving the next morning, prompting hospitalization on that day. He had a history of hypertension and dyslipidemia but no relevant family history. His physical parameters were as follows: height, 164 cm; weight, 62.1 kg; body mass index, 23.1 kg/m2; blood pressure, 155/137 mmHg; pulse, 93/min; body temperature, 36.8°C; and oxygen saturation, 95% (ambient air). The patient was unconscious; however, no obvious paralysis or neck rigidity was observed. The blood test results are presented in Table 1. His blood glucose level was 815 mg/dL, with HbA1c elevated to 8.7%. Plasma osmolality was 318 mOsm/L, and ketosis and metabolic acidosis were noted. His serum C-peptide level was 0.42 ng/mL, and the GAD antibody level exceeded 2000 U/mL. Chest radiography and computed tomography (Figure 1) confirmed known lung cancer, with no additional findings. Based on these findings, the patient was clinically diagnosed with diabetic ketoacidosis (DKA).
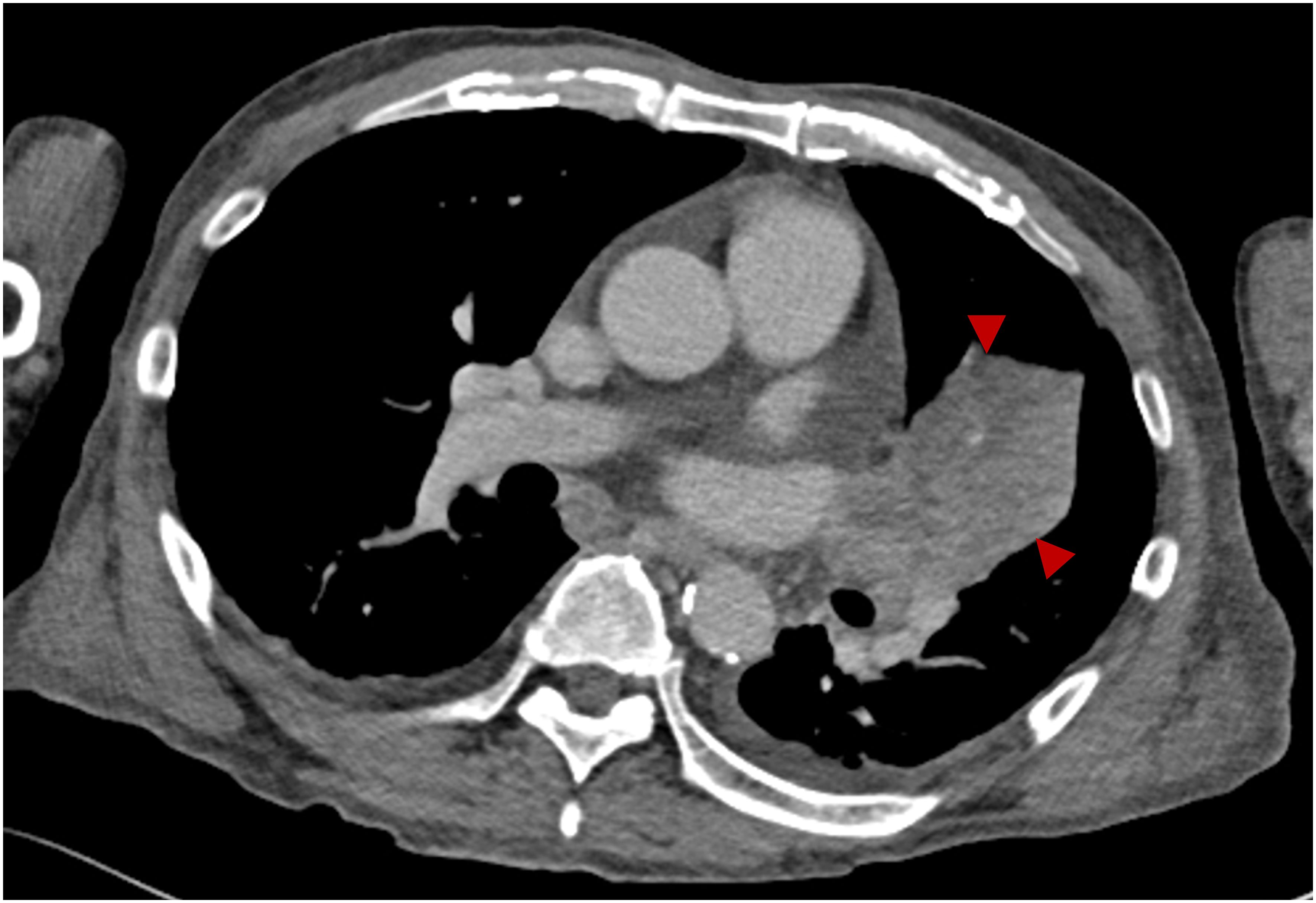
Figure 1 Contrast-enhanced computed tomography on admission. Contrast-enhanced computed tomography showing left upper lobe lung cancer (arrowheads).
Upon admission, a central venous catheter was inserted. As the patient was on dialysis, the infusion was minimized, and treatment focused on a continuous intravenous insulin infusion, ranging from 0.2 to 5.0 U/h. By day 3, the patient’s consciousness had gradually improved. As his blood glucose level decreased and his metabolic acidosis improved, continuous intravenous insulin infusion was terminated, and subcutaneous insulin injection was initiated (14–25 U/day). However, as blood glucose levels fluctuated, insulin injection levels were finely adjusted. On the 17th day of hospitalization (day 64), the patient was finally discharged with multiple daily injections of insulin aspart 6–6-4 and insulin glargine 0–0-0–9. His serum C-peptide levels decreased to 0.26 ng/mL and <0.03 ng/mL on days 63 and 94, respectively. Given his medical history and highly positive anti-GAD antibody test results, we diagnosed the patient with autoimmune T1DM caused by ICIs. HLA typing identified DRB1*04:05-DQB1*04:01, which is reportedly associated with T1DM susceptibility (Table 1).
Following discharge, immunotherapy was resumed on day 85. Unfortunately, tumor growth persisted despite ICI treatment. Therefore, immunotherapy was discontinued six months post-discharge, and chemotherapy with carboplatin and paclitaxel was initiated as the second-line treatment. After completing six courses of treatment, tumor shrinkage was observed, and the patient is still under treatment and follow-up. The clinical course of the patient is summarized in Figure 2.
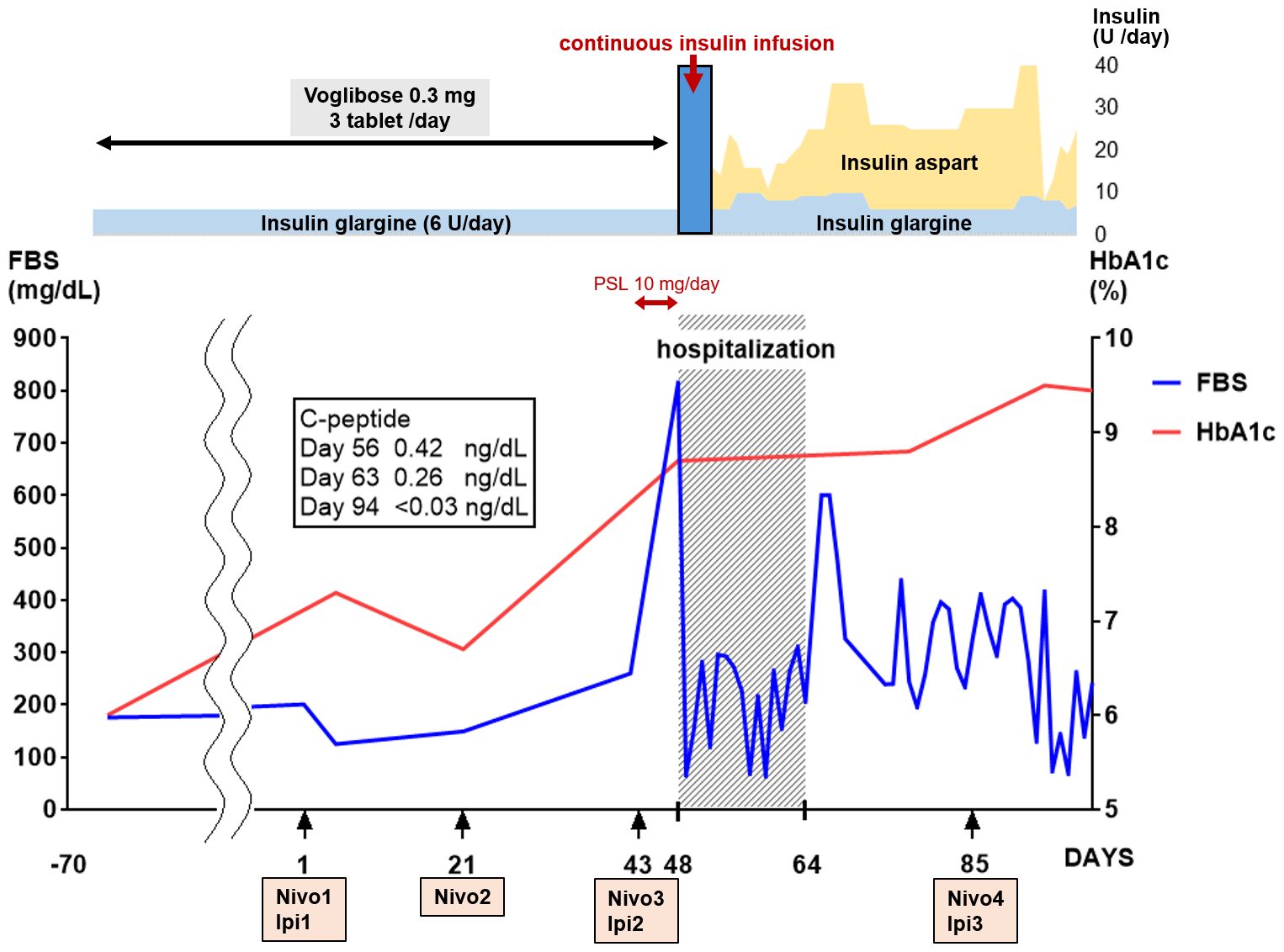
Figure 2 Clinical course. On the 48th day after the start of immunotherapy, the blood glucose level spiked; thereafter, the blood glucose levels fluctuated. The HbA1c and C-peptide levels increased and decreased rapidly, respectively. Continuous intravenous insulin was administered at 0.2 to 5.0 U/h from day 48 to day 50. FBS, fasting blood sugar; HbA1c, glycated hemoglobin; Ipi, ipilimumab; nivolumab, nivolumab.
3 Discussion
In this report, we describe a case of strong GAD antibody positivity and T1DM-susceptible haplotype DRB1*04:05-DQB1*04: 01 in a Japanese patient with ICI-T1DM. This case presents several notable findings. First, although the prevalence of GAD antibody positivity in ICI-T1DM is lower in Japan than in Western countries, our patient exhibited a high GAD antibody titer, along with a remarkably short duration between the first ICIs and the acute onset of symptoms. Second, we identified the HLA haplotype as DRB1*04:05-DQB1*04:01, susceptible to T1DM haplotypes. Considering these findings, below we have discussed and reviewed the literature on GAD antibody-positive Japanese patients with ICI-T1DM and HLA haplotypes.
3.1 A brief review of ICI-T1DM
ICI-T1DM, also known as CIADM, has recently been recognized as a new category of T1DM (6). ICIs may inhibit PD-1 on the surface of T cells or PD-L1 on the surface of tumor cells, thereby activating T cells and enhancing tumor immunity. As PD-L1 is expressed in Langerhans cells, ICIs may block the PD-1 pathway, potentially activating self-reactive T cells to attack Langerhans cells (11, 16).
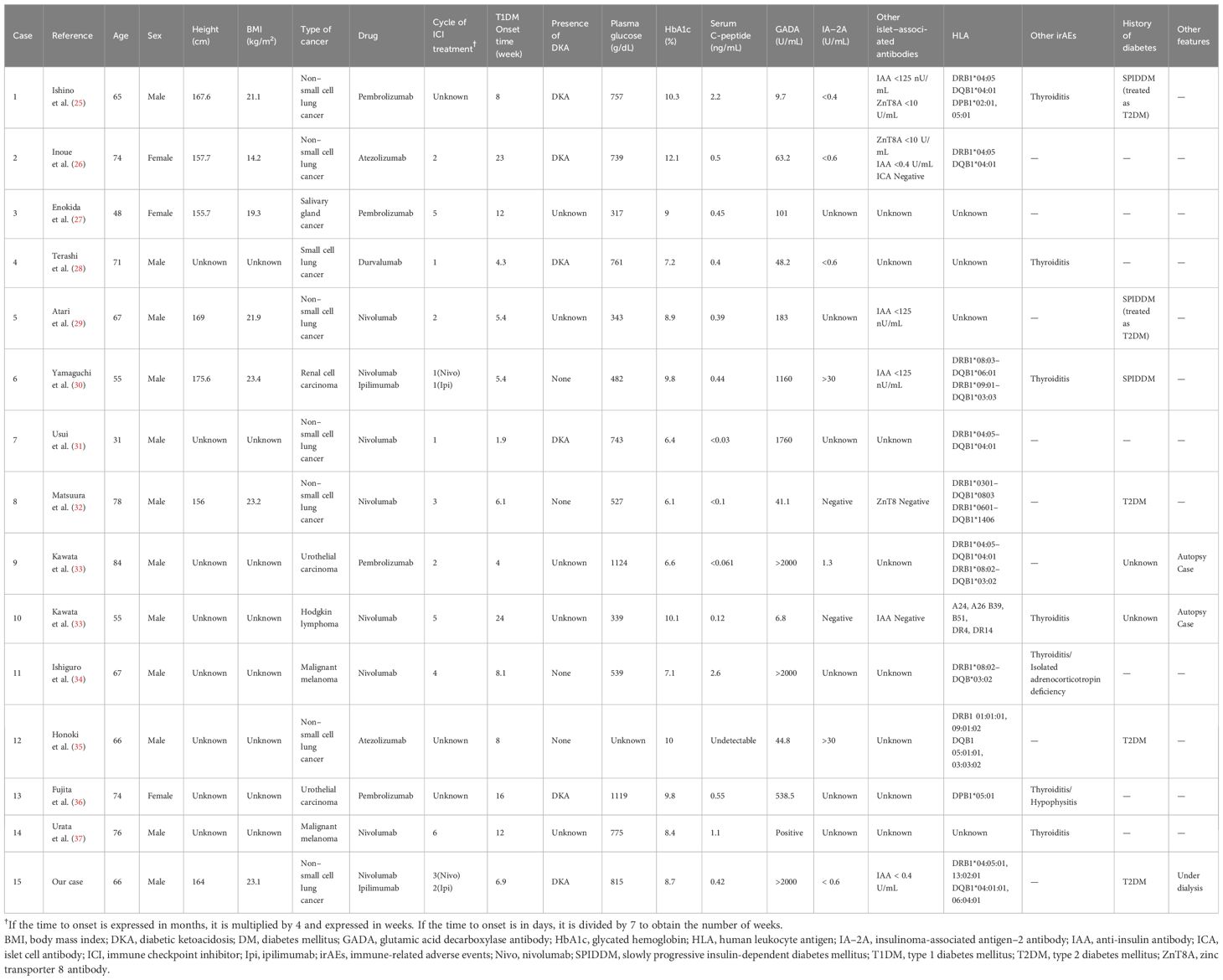
The global prevalence of ICI-T1DM is approximately 1–1.8% (17–20). ICI-T1DM is characterized by an intermediate onset that is between acute-onset and fulminant T1DM, according to the speed of loss of β cells (21, 22). Therefore, ICI-T1DM may have a different pathogenesis from that of acute-onset or fulminant T1DM. Although race (5), islet antibodies (5, 10–13, 23), and HLA haplotypes (5, 10–13, 24) may be important factors in new-onset ICI-T1DM, their roles have not yet been conclusively determined. We have summarized the characteristics of GAD antibody-positive Japanese patients with ICI-T1DM and their associated HLA haplotypes in Table 2.
3.2 GAD antibody positivity in acute-onset Japanese ICI-T1DM
In our case, our patient exhibited a high GAD antibody titer with a considerably short symptom onset duration from the first ICIs. The positivity rate of islet-related antibodies in ICI-T1DM is reportedly approximately 26–56%, which is lower than that in T1DM (5, 10–13, 23, 38). Additionally, Qiu et al. reported a positivity rate of 45.7% and 9.5% in islet-related antibodies in Caucasians and Asians with ICI-T1DM, respectively (5). Notably, the positivity rate of islet-related antibodies in ICI-T1DM in Asians is low (5, 9, 24). To further elucidate these characteristics, we searched for Japanese patients with GAD antibody-positive ICI-T1DM.
To the best of our knowledge, so far, 15 cases have been reported, as summarized in Table 2 (25–37). The mean age of the patients was 65.1. The HbA1c level at the time of ICI-T1DM onset was 8.7%, and the duration of ICI-T1DM onset after the first ICIs was 9.7 weeks. DKA was observed in 6 cases, 3 cases had type 2 DM, and 3 cases had slowly progressive insulin-dependent DM. Baden et al. reported that the mean onset duration from the first ICI was 22.1 weeks in 22 Japanese ICI-T1DM cases, with only one patient testing positive for GAD antibodies (9). Similarly, Wu et al. reported a mean onset duration from the first ICI of 21.5 and 15.6 weeks in islet antibody-negative and -positive ICI-T1DM cases, respectively. Therefore, the onset duration in the reviewed patients with ICI-T1DM, including the current case, was short, consistent with previous reports (5, 10–13, 23). In our current case, the onset duration was 47 days (6.7 weeks), which is considerably short.
The prevalence of DKA is reportedly high in cases positive for islet-related antibodies (10, 13). Our review indicated a DKA prevalence of 38.9%, similar to that reported by Baden et al. (9). The reasons behind the differences reported in the clinical courses between GAD-positive and GAD-negative cases in ICI-T1DM remain unclear. In T1DM, anti-islet autoantibodies are generated as a result of β-cell destruction by T cells (39), and GAD antibody titers do not always reflect disease progression in T1DM (39). However, our results suggest that GAD antibodies may serve as surrogate markers of disease progression in some ICI-T1DM cases.
Predicting new-onset ICI-T1DM is difficult, as demonstrated in our case. In a recent retrospective cohort study, Akturk et al. reported that blood glucose monitoring at every ICI infusion visit failed to detect new-onset ICI-T1DM (40). They proposed that continuous glucose monitoring or daily self-monitoring may be useful for some high-risk patients (40). Although we acknowledge their suggestion, in our case, despite daily self-monitoring, early detection of new-onset ICI-T1DM was not achieved. Initially, we attributed the elevated glucose levels to prednisolone, delaying the recognition of acute glucose elevation and subsequent unconsciousness. Therefore, identifying high-risk patients could facilitate early intervention, such as initiating early insulin injections or increasing the dose, to prevent worsening metabolic disorders related to elevated glucose levels.
The onset of ICI-T1DM is unpredictable, suggesting that monitoring GAD antibody titers could serve as a valuable tool for detecting new-onset ICI-T1DM. However, there are currently no clear criteria for the timing of its measurement. One of the reasons for this is that the pattern of change in GAD antibody titer varies, with some cases reported to have been positive before the start of immunotherapy (41, 42), others to have become positive after the start of immunotherapy (43), and still others to have increased antibody titers after the start of immunotherapy (29, 30). Considering this issue, we propose that anti-GAD antibodies should be measured before immunotherapy, and positive cases should be considered high-risk patients and periodically monitored after the start of immunotherapy to confirm that the titer does not increase rapidly. Another issue is that this assessment plan cannot be applied to cases that become positive for GAD antibodies after the start of immunotherapy. As mentioned above, the average period from the start of immunotherapy to the onset of disease in Japanese ICI-T1DM patients with positive GAD antibodies is 9.7 weeks (n=15). In this regard, measuring GAD antibodies once, for example, a few weeks after the start of immunotherapy, may be useful. While it is difficult to suggest a timing for GAD antibody measurement at present, this is a clinically important issue, and further studies are needed in this regard.
In addition, although islet antigen-2 (IA-2) and zinc transporter 8 (ZnT8) antibodies have been reported to be surrogate markers of pancreatic beta cell destruction (39), their titers were not high in our review (Table 2). Wu et al. reported that multiple autoantibodies are less frequently detected in ICI-T1DM than in T1DM (13), possibly indicating low positivity rates for IA-2 and ZnT8 antibodies in patients with ICI-T1DM. Therefore, anti-islet antibodies, such as GAD or IA-2 antibodies, may play different roles in T1DM and ICI-T1DM, and future studies should focus on elucidating their roles.
3.3 T1DM-susceptible haplotype DRB1*04:05-DQB1*04:01 in our patient
The association between HLA haplotypes and ICI-T1DM remains unclear. However, the HLA haplotype of our patient was identified as DRB1*04:05-DQB1*04:01, a T1DM-susceptible haplotype.
First, the association between HLA haplotypes and T1DM and the frequency of HLA haplotypes vary across racial and ethnic groups (44). For example, DR3 and DR4 are important susceptible HLA haplotypes in Europe and Africa. However, in Japan, DR3 is rare, and instead, DR4 and DR9 are important susceptible HLA haplotypes (45). Additionally, susceptible HLA haplotypes vary by race, even in East Asia. For example, DR4, DR9, and DR3 HLA haplotypes have been reported in Korea (46) but not in Japan. Moreover, acute-onset and fulminant T1DM have different HLA-susceptible haplotypes. Although DRB1*04:05-DQB1*04:01, DRB1*08:02-DQB1*03:02, and DRB1*09:01-DQB1*03:03 are typically detected in Japanese patients with acute-onset T1DM, DRB1*08:02-DQB1*03:02 is not associated with the HLA haplotype of fulminant T1DM (15). Therefore, if ICI-T1DM is categorized as a new T1DM subtype, ICI-T1DM-related HLA haplotypes may differ from those observed in T1DM. Some patients with ICI-T1DM exhibit a T1DM-resistant HLA haplotype (13, 24). In a study conducted in Japan, Inaba et al. reported a high frequency of HLA-DRB1*04:05-DQB1*04:01-DPB1*05:01 or DPA1*02:02-DPB1*05:01 haplotypes in ICI-T1DM (47, 48). In our case, the T1DM-susceptible haplotype-DRB1*04:05-DQB1*04:01 was detected, which may also be associated with the HLA haplotype of Japanese ICI-T1DM.
Second, an association between GAD antibody positivity and HLA haplotypes has been reported. Tsutsumi et al. reported that although both DRB1*04:05-DQB1*04:01 and DRB1*09:01-DQB1*03:03 are frequent in Japanese fulminant T1DM, GAD antibody-positive fulminant T1DM was only significantly associated with DRB1*09:01-DQB1*03:03, even with considerably low GAD antibody positivity rates (14). As shown in Table 2, DRB1*04:05-DQB1*04:01 has been detected in five cases, including ours (25, 26, 31, 33). Yamaguchi et al. reported DRB1*09:01‐DQB1*03:03 in a patient (30). In contrast, Imagawa et al. reported a high frequency of DR9 in Japanese ICI-T1DM cases (49), which is inconsistent with the observations from our review. These findings suggest that susceptible HLA-related haplotypes differ in Japanese patients with ICI-T1DM positive for GAD antibodies, warranting further studies with larger sample sizes.
3.4 Strengths and limitations
In this study, we reviewed Japanese patients with GAD antibody-positive ICI-T1DM and their associated HLA haplotypes. Our study highlights several important findings. First, GAD antibody positivity may be associated with acute onset and disease progression in some cases of Japanese patients with ICI-T1DM. Second, as the prediction of new-onset ICI-T1DM is challenging, monitoring GAD antibody levels might be useful. Third, DRB1*04:05-DQB1*04:01 is a potentially important HLA haplotype of GAD antibody-positive ICI-T1DM in Japanese patients. These findings provide important insights into guiding clinicians in treating patients undergoing ICI therapy.
This study has several strengths. First, as only a few cases of GAD antibody-positive Japanese ICI-DM have been reported, the characteristics of this condition remain unclear. To the best of our knowledge, this is the first review focusing on GAD antibody-positive Japanese patients with ICI-T1DM. Recently, various rare onsets and related cases of ICI-related T1DM have been reported (50–52), and our study adds valuable inputs for understanding the pathogenesis and management of this condition. Second, we have discussed the HLA haplotype of GAD-positive antibodies in a small number of Japanese ICI-T1DM cases, warranting future investigations with larger sample sizes and validation across different racial and ethnic groups.
However, this study has some limitations. First, we could not measure GAD antibody titers before ICI treatment. Second, data on islet–associated autoantibodies other than GAD antibodies are limited. Third, we could not determine the HLA haplotypes of DPA1 and DPB1 owing to phase ambiguity. Fourth, although we carefully searched the literature, some reports did not contain detailed information, and we could not draw detailed clinical manifestations in some cases. Finally, no individual GAD-positive cases were identified in several observational studies.
4 Conclusions
In summary, we present a case of strongly positive GAD antibody and T1DM-susceptible haplotype DRB1*04:05-DQB1*04:01 detected in a Japanese patient with ICI-T1DM. As the high-GAD antibody titer ICI-T1DM may be acute and have a short onset from the first ICIs, clinicians should carefully observe patients. However, our results should be validated in studies with large sample sizes and across different racial and ethnic groups.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Shirakawa Kosei General Hospital Ethics Committee-Decision No HAKURIN23-011, June 19, 2023. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. HH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Supervision. CM: Data curation, Investigation, Writing – review & editing. YK: Supervision, Writing – review & editing. TH: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank Editage for English language editing and HLA LABORATORY (Kyoto, Japan) for conducting the HLA analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ICI, immune checkpoint inhibitor; CTLA-4, cytotoxic T-lymphocyte antigen 4; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; irAE, immune-related adverse event; T1DM, type 1 diabetes mellitus; GAD, glutamic acid decarboxylase; HLA, human leukocyte antigen; ICI-T1DM, immune checkpoint inhibitor-induced type 1 diabetes mellitus; CIADM, checkpoint inhibitor-associated autoimmune diabetes mellitus; IA-2, insulinoma-associated antigen-2; ZnT8, zinc transporter 8.
References
1. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
3. Yin Q, Wu L, Han L, Zheng X, Tong R, Li L, et al. Immune-related adverse events of immune checkpoint inhibitors: a review. Front Immunol. (2023) 14:1167975. doi: 10.3389/fimmu.2023.1167975
4. Imagawa A, Hanafusa T. Fulminant type 1 diabetes-east and west. J Clin Endocrinol Metab. (2023) 108:e1473–e8. doi: 10.1210/clinem/dgad329
5. Qiu J, Luo S, Yin W, Guo K, Xiang Y, Li X, et al. Characterization of immune checkpoint inhibitor-associated fulminant type 1 diabetes associated with autoantibody status and ethnic origin. Front Immunol. (2022) 13:968798. doi: 10.3389/fimmu.2022.968798
6. Wu L, Tsang VHM, Sasson SC, Menzies AM, Carlino MS, Brown DA, et al. Unravelling checkpoint inhibitor associated autoimmune diabetes: from bench to bedside. Front Endocrinol (Lausanne). (2021) 12:764138. doi: 10.3389/fendo.2021.764138
7. Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia. (2017) 60:1370–81. doi: 10.1007/s00125–017-4308–1
8. van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. (2011) 91:79–118. doi: 10.1152/physrev.00003.2010
9. Baden MY, Imagawa A, Abiru N, Awata T, Ikegami H, Uchigata Y, et al. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol Int. (2019) 10:58–66. doi: 10.1007/s13340–018-0362–2
10. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabetes Med. (2019) 36:1075–81. doi: 10.1111/dme.14050
11. Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. (2018) 103:3144–54. doi: 10.1210/jc.2018–00728
12. de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, van der Auwera BJ, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol. (2019) 181:363–74. doi: 10.1530/eje-19–0291
13. Wu L, Tsang V, Menzies AM, Sasson SC, Carlino MS, Brown DA, et al. Risk factors and characteristics of checkpoint inhibitor-associated autoimmune diabetes mellitus (CIADM): A systematic review and delineation from type 1 diabetes. Diabetes Care. (2023) 46:1292–9. doi: 10.2337/dc22–2202
14. Tsutsumi C, Imagawa A, Ikegami H, Makino H, Kobayashi T, Hanafusa T. Class II HLA genotype in fulminant type 1 diabetes: A nationwide survey with reference to glutamic acid decarboxylase antibodies. J Diabetes Investig. (2012) 3:62–9. doi: 10.1111/j.2040-1124.2011.00139.x
15. Kawabata Y, Ikegami H, Awata T, Imagawa A, Maruyama T, Kawasaki E, et al. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. (2009) 52:2513–21. doi: 10.1007/s00125–009-1539–9
16. Fang W, Gao Y, Shi X, Zhang X, Zhou S, Zhu H, et al. Immune checkpoint inhibitors-related pancreatitis with fulminant type 1 diabetes mellitus: case report and literature review. Front Immunol. (2023) 14:1243773. doi: 10.3389/fimmu.2023.1243773
17. Arima H, Iwama S, Inaba H, Ariyasu H, Makita N, Otsuki M, et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J. (2019) 66:581–6. doi: 10.1507/endocrj.EJ19–0163
18. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. (2019) 7:e000591. doi: 10.1136/bmjdrc-2018–000591
19. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. (2018) 67:1471–80. doi: 10.2337/dbi18–0002
20. Zhang Z, Sharma R, Hamad L, Riebandt G, Attwood K. Incidence of diabetes mellitus in patients treated with immune checkpoint inhibitors (ICI) therapy - A comprehensive cancer center experience. Diabetes Res Clin Pract. (2023) 202:110776. doi: 10.1016/j.diabres.2023.110776
21. Imagawa A. Two types of fulminant type 1 diabetes mellitus: Immune checkpoint inhibitor-related and conventional. J Diabetes Investig. (2021) 12:917–9. doi: 10.1111/jdi.13450
22. Tachibana M, Imagawa A. Type 1 diabetes related to immune checkpoint inhibitors. Best Pract Res Clin Endocrinol Metab. (2022) 36:101657. doi: 10.1016/j.beem.2022.101657
23. Lin C, Li X, Qiu Y, Chen Z, Liu J. PD-1 inhibitor-associated type 1 diabetes: A case report and systematic review. Front Public Health. (2022) 10:885001. doi: 10.3389/fpubh.2022.885001
24. Liu YC, Liu H, Zhao SL, Chen K, Jin P. Clinical and HLA genotype analysis of immune checkpoint inhibitor-associated diabetes mellitus: a single-center case series from China. Front Immunol. (2023) 14:1164120. doi: 10.3389/fimmu.2023.1164120
25. Ishino A, Yoneda C, Yoshimoto M, Suda H, Hirano S, Katamine A, et al. Acute exacerbation of Type 1 diabetes and transient thyrotoxicosis around the same time after anti-PD-1 antibody administration in a lung cancer patient. J Jpn Diabetes Soc. (2020) 63:746–53. doi: 10.11213/tonyobyo.63.746
26. Inoue C, Nishihama K, Nakahara H, Okano Y, Tanaka S, Yoshihara A, et al. A case of acute onset Type 1 diabetes diagnosed after the development of diabetic ketoacidosis following the administration of atezolizumab. J Jpn Diabetes Soc. (2020) 63:811–9. doi: 10.11213/tonyobyo.63.811
27. Enokida S, Kumagai K, Mochizuki H, Nakahama T, Ida S, Mine S, et al. A case of mandibular adenocarcinoma with anti-PD-1 immunotherapy-induced Type 1 diabetes successfully treated by carbohydrates counting method. GAKKAISHI JSPEN. (2019) 1:33–7. doi: 10.11244/ejspen.1.1_33
28. Terashi N, Hisakane K, Miyadera K, Kato Y, Terashima Y, Suzuki A, et al. A case of fulminant type 1 diabetes mellitus after the administration of durvalumab for small-cell lung cancer. JJLC. (2022) 62:323–8. doi: 10.2482/haigan.62.323
29. Atari S, Arimura A, Kurano M, Maeda S, Nagatomo R, Arimura H, et al. . doi: 10.1507/endocrine.94.S.Update_64
30. Yamaguchi H, Miyoshi Y, Uehara Y, Fujii K, Nagata S, Obata Y, et al. Case of slowly progressive type 1 diabetes mellitus with drastically reduced insulin secretory capacity after immune checkpoint inhibitor treatment for advanced renal cell carcinoma. Diabetol Int. (2021) 12:234–40. doi: 10.1007/s13340–020-00459–1
31. Usui Y, Udagawa H, Matsumoto S, Imai K, Ohashi K, Ishibashi M, et al. Association of serum anti-GAD antibody and HLA haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J Thorac Oncol. (2017) 12:e41–3. doi: 10.1016/j.jtho.2016.12.015
32. Matsuura N, Koh G, Konishi C, Minamino S, Takahara Y, Harada H, et al. Fulminant onset of insulin-dependent diabetes with positive anti-GAD antibody titers during treatment with nivolumab in a patient with NSCLC. Cancer Immunol Immunother. (2018) 67:1417–24. doi: 10.1007/s00262–018-2203–3
33. Kawata S, Kozawa J, Yoneda S, Fujita Y, Kashiwagi-Takayama R, Kimura T, et al. Inflammatory cell infiltration into islets without PD-L1 expression is associated with the development of immune checkpoint inhibitor-related Type 1 diabetes in genetically susceptible patients. Diabetes. (2023) 72:511–9. doi: 10.2337/db22–0557
34. Ishiguro A, Ogata D, Ohashi K, Hiki K, Yamakawa K, Jinnai S, et al. Type 1 diabetes associated with immune checkpoint inhibitors for Malignant melanoma: A case report and review of 8 cases. Med (Baltim). (2022) 101:e30398. doi: 10.1097/MD.0000000000030398
35. Honoki H, Yagi K, Kambara K, Chujo D, Shikata M, Enkaku A, et al. Anti-programmed death ligand 1 therapy-induced type 1 diabetes presenting with multiple islet-related autoantibodies. J Diabetes Investig. (2020) 11:253–4. doi: 10.1111/jdi.13099
36. Fujita Y, Kamitani F, Yamamoto M, Fukuoka H, Hirota Y, Nishiyama N, et al. Combined hypophysitis and type 1 diabetes mellitus related to immune checkpoint inhibitors. J Endocr Soc. (2023) 7:bvad002. doi: 10.1210/jendso/bvad002
37. Urata S, Ohno R, Ueno H, Tazaki T, Chisaka T, Hirabara Y, et al. New-onset type 1 diabetes mellitus after nivolumab treatment: report of two cases. J Kyushu Pharm. (2020) 74:67–71. Available at: https://search.jamas.or.jp/link/ui/2021126199.
38. Lo Preiato V, Salvagni S, Ricci C, Ardizzoni A, Pagotto U, Pelusi C. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord. (2021) 22:337–49. doi: 10.1007/s11154-020-09618-w
39. Kawasaki E. Anti-islet autoantibodies in type 1 diabetes. Int J Mol Sci. (2023) 24:10012. doi: 10.3390/ijms241210012
40. Akturk HK, Michel K, Couts K, Karakus KE, Robinson W, Michels A. Routine blood glucose monitoring does not predict onset of immune checkpoint inhibitor-induced type 1 diabetes. Diabetes Care. (2024) 47:e29–30. doi: 10.2337/dc23–1964
41. Godwin JL, Jaggi S, Sirisena I, Sharda P, Rao AD, Mehra R, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer. (2017) 5:40. doi: 10.1186/s40425–017-0245–2
42. Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother. (2017) 66:1399–410. doi: 10.1007/s00262–017-2033–8
43. Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. (2016) 4:89. doi: 10.1186/s40425-016-0196-z
44. Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. (2008) 57:1084–92. doi: 10.2337/db07–1331
45. Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev. (2010) 9:A355–65. doi: 10.1016/j.autrev.2009.12.003
46. Kawabata Y, Ikegami H, Kawaguchi Y, Fujisawa T, Shintani M, Ono M, et al. Asian-specific HLA haplotypes reveal heterogeneity of the contribution of HLA-DR and -DQ haplotypes to susceptibility to type 1 diabetes. Diabetes. (2002) 51:545–51. doi: 10.2337/diabetes.51.2.545
47. Inaba H, Kaido Y, Ito S, Hirobata T, Inoue G, Sugita T, et al. Human leukocyte antigens and biomarkers in type 1 diabetes mellitus induced by immune-checkpoint inhibitors. Endocrinol Metab (Seoul). (2022) 37:84–95. doi: 10.3803/EnM.2021.1282
48. Inaba H, Morita S, Kosugi D, Asai Y, Kaido Y, Ito S, et al. Amino acid polymorphisms in human histocompatibility leukocyte antigen class II and proinsulin epitope have impacts on type 1 diabetes mellitus induced by immune-checkpoint inhibitors. Front Immunol. (2023) 14:1165004. doi: 10.3389/fimmu.2023.1165004
49. Imagawa A, Tachibana M. Fulminant type 1 diabetes: recent research progress and future prospects. Diabetol Int. (2020) 11:336–41. doi: 10.1007/s13340–020-00466–2
50. Wen L, Zou X, Chen Y, Bai X, Liang T. Sintilimab-induced autoimmune diabetes in a patient with the anti-tumor effect of partial regression. Front Immunol. (2020) 11:2076. doi: 10.3389/fimmu.2020.02076
51. Qi R, Xu H, Fu X, Yu Y, Lv D, Li Y, et al. Case Report: Keratoacanthoma and type I diabetes secondary to treatment with PM8001, a bifunctional fusion protein targeting TGF-β and PD-L1. Front Oncol. (2023) 13:1046266. doi: 10.3389/fonc.2023.1046266
52. Tanaka T, Nagasu S, Furuta T, Gobaru M, Suzuki H, Shimotsuura Y, et al. Case report: A case of fulminant type 1 diabetes mellitus after COVID-19 vaccination during treatment of advanced gastric cancer: pitfall in managing immune-related adverse events. Front Oncol. (2023) 13:1264281. doi: 10.3389/fonc.2023.1264281
Keywords: immune checkpoint inhibitors, glutamic acid decarboxylase antibody, type 1 diabetes mellitus, human leukocyte antigen haplotype, Japanese
Citation: Yabuki S, Hirai H, Moriya C, Kusano Y and Hasegawa T (2024) Case report: Strong GAD antibody positivity and type 1 diabetes-HLA-susceptible haplotype-DRB1*04:05-DQB1*04:01 in a Japanese patient with immune checkpoint inhibitor-induced type 1 diabetes. Front. Endocrinol. 15:1407192. doi: 10.3389/fendo.2024.1407192
Received: 26 March 2024; Accepted: 07 May 2024;
Published: 22 May 2024.
Edited by:
Benjamin Udoka Nwosu, Hofstra University, United StatesReviewed by:
Marcelo Maia Pinheiro, Centro Universitário de Várzea Grande, BrazilTakao Ando, Nagasaki University Hospital, Japan
Copyright © 2024 Yabuki, Hirai, Moriya, Kusano and Hasegawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroyuki Hirai, aGlyb3l1a2lAZm11LmFjLmpw
†ORCID: Hiroyuki Hirai, orcid.org/0000-0001-5325-3827
 Shunya Yabuki1
Shunya Yabuki1 Hiroyuki Hirai
Hiroyuki Hirai