- 1Department of Clinical Laboratory, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou, China
- 2Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Thyroid Breast Surgery, The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, China
Introduction: Serum folate and vitamin B12 levels correlate with the prevalence of fatty liver disease, but it is not clear how they affect mortality. Therefore, this study aimed to investigate the association of serum folate and vitamin B12 concentrations with all-cause mortality in individuals with metabolic dysfunction-associated steatotic liver disease (MASLD).
Methods: MASLD subjects were from the Third National Health and Nutrition Examination Survey (NHANES III) in the United States, and mortality follow-up data were obtained by linkage to death records from the National Death Index. Multivariable Cox proportional regression models and restricted cubic spline (RCS) models were used to evaluate the association of serum folate/vitamin B12 with all-cause mortality in the MASLD population.
Results: 3,636 and 2,125 MASLD individuals were included in the analyses related to serum folate and vitamin B12, respectively. During a follow-up period of more than 20 years, the RCS models demonstrated significant nonlinear associations of both serum folate (P <0.001) and vitamin B12 (P =0.016) with all-cause mortality in MASLD. When their serum concentrations were below the median level, the risk of all-cause mortality decreased with increasing concentration, reaching a lowest risk around the median level, and then leveled off. In the multivariable cox regression model, for vitamin B12, the risk of all-cause mortality was reduced by 42% and 28% in the third and fourth quartile groups, respectively, compared with the lowest quartile group (hazard ratio [HR]=0.58, 95% CI: 0.39-0.86, P =0.008; HR =0.72, 95% CI: 0.54-0.96, P=0.026, respectively). For folate, the risk of all-cause mortality was reduced by 28% in the third quartile compared with the lowest quartile (HR =0.72, 95% CI: 0.57-0.91, P =0.005).
Conclusion: This longitudinal cohort study suggests that low serum folate and vitamin B12 levels in patients with MASLD are significantly associated with an elevated risk of all-cause mortality.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a syndrome of liver disease associated with cardiometabolic dysregulation, previously known as non-alcoholic fatty liver disease (NAFLD) (1). Because of its potential stigmatization and ambiguity in determining etiology, the term NAFLD has been widely criticized (1, 2). Therefore, a new nomenclature framework for fatty liver disease has recently been proposed, in which MASLD is introduced as a new term to replace NAFLD and accompanied by modified diagnostic criteria (1). As the predominant type of steatotic liver disease, the worldwide prevalence of MASLD has been estimated to be more than 30% (3). Alarmingly, MASLD is the leading cause of hepatocellular carcinoma and cirrhosis and is associated with a significant increase in all-cause mortality (4–6). With the rising prevalence of obesity, the incidence of MASLD continues to increase and has resulted in a huge global disease burden (7). The mechanisms underlying the pathogenesis and progression of MASLD are not fully understood; as a multifactorial disease, it is closely related to insulin resistance, oxidative stress, lipid metabolism, and lifestyle environmental factors (8). Although hundreds of drugs are being developed for fatty liver disease, only resmetirom has recently been approved for the treatment of non-alcoholic steatohepatitis, a subtype of NAFLD characterized by hepatitis and liver damage (9). Lifestyle interventions based on exercise and diet modification remain the cornerstone of MASLD management (8, 10). Therefore, it is particularly important to explore new targets for intervention and to identify biomarkers that can be used for risk management in this chronic disease.
Folate, as an important B vitamin, mediates one carbon (1C) metabolic reactions that play a key role in a variety of physiological processes in the body, including nucleic acid and protein synthesis, amino acid homeostasis, redox defense, methylation modification, and immune response (11, 12). Folate deficiency has been found to be strongly associated with a variety of systemic conditions such as cancer, cardiovascular disease, and psychiatric disorders (13–16). In addition, previous studies have demonstrated that folate affects oxidative stress, chronic inflammation, and lipid metabolism in the liver, all of which are pathogenic mechanisms of fatty liver (17–19). The liver is the primary processing and storage site for folate and is critical in the maintenance of folate homeostasis throughout the body (20, 21). Similar to folate, vitamin B12 (also known as cobalamin) is an essential water-soluble vitamin for the maintenance of 1C metabolism, and plays an important role in human health and disease (11, 12). As an integral component of 1C metabolism, vitamin B12 has the ability to support the translocation and storage of folate within the cell (11, 12). Deficiencies of both are often known as the cause of megaloblastic anemia (22). In addition, vitamin B12 is a cofactor for methyl malonyl coenzyme A mutase, which regulates the transfer of long-chain fatty acyl-CoA to the mitochondria and influences lipid metabolic pathways (23). The liver is the major storage organ for vitamin B12, and previous studies have shown that vitamin B12 is associated with hepatocellular carcinoma, cirrhosis, and hepatitis and can independently predict the histologic severity of non-alcoholic steatohepatitis (24–26).
In fact, the relationship between serum folate/vitamin B12 and NAFLD has been explored in several cross-sectional studies. Previous studies have found that serum folate and vitamin B12 are inversely associated with the prevalence of NAFLD and that their levels are lower in patients with NAFLD than in healthy controls (27–30). These studies suggest that folate and vitamin B12 may be involved in the onset and progression of NAFLD. However, no studies have examined the effect of these two essential B vitamins on mortality in patients with NAFLD. Furthermore, for MASLD, this recently proposed concept, its association with serum folate and vitamin B12 has not been reported. To address this research gap, we therefore analyzed the association of serum folate and vitamin B12 levels with long-term all-cause mortality among patients with MASLD in a nationally representative prospective cohort of U.S. adults.
Method
Study population
The third National Health and Nutrition Examination Survey (NHANES III) was a major project conducted by the U.S. National Center for Health Statistics from 1988 to 1994 aimed at providing national estimates of the nutritional and health status of children and adults in the United States (31, 32). To enable the survey population to be nationally representative, NHANES III utilized a stratified multistage complex sampling design (31, 32). NHANES III has been frequently used as an unbiased and high-quality dataset for studies in the field of fatty liver disease (33–38). There are other cycles of NHANES that were not included because hepatic imaging data were not available or the linked follow-up period was insufficient. The study population included in the current study was participants aged 20-74 years who underwent hepatic ultrasound. NHANES III has been approved by the NCHS Institutional Review Board. Documented consent was obtained from all participants (https://www.cdc.gov/nchs/nhanes/irba98.htm). The data that we used were completely de-identified for participants, thus exempting the institutional review board.
Definition of MASLD
The procedure for detecting hepatic steatosis based on hepatic/gallbladder ultrasound is described in detail in the Hepatic Steatosis Ultrasound Images Assessment Procedures Manual of NHANES III (39, 40). Briefly, gallbladder ultrasound was performed on all adults aged 20-74 years who received examinations at the mobile examination center. Hepatic steatosis was assessed based on the following five criteria: (a) liver to kidney contrast; (b) parenchymal brightness; (c) vessel walls definition; (d) deep beam attenuation; and (e) gallbladder wall definition. The original ultrasound video images were reviewed by three ultrasound readers who received standardized training from a board-certified radiologist specializing in liver imaging. Rigorous quality control and quality assurance procedures were used to standardize reading manner among the readers. In the assessment of hepatic steatosis, percentage agreement for intra-rater reliability and inter-rater reliability reached 91.3% and 88.7%, respectively, with kappa coefficients both >0.6 (39). In our study, any degree (mild-severe) of steatosis detected was defined as steatotic liver disease (41–43).
According to the MASLD diagnostic criteria, individuals with steatotic liver disease who had any one of the following five cardiometabolic risk factors were identified as MASLD: (a) body mass index (BMI) ≥25 kg/m2, or waist circumference ≥94 cm (male) or ≥80 cm (female); (b) fasting glucose ≥100 mg/dL, or 2-hour post load glucose levels ≥ 140 mg/dl, or hemoglobin A1c ≥ 5.7%, or type 2 diabetes mellitus, or receiving treatment for type 2 diabetes mellitus; (c) blood pressure ≥ 130/85 mmHg or receiving antihypertensive medication; (d) plasma triglycerides ≥ 150 mg/dL or taking lipid-lowering medications; and (e) plasma HDL-cholesterol ≤40 mg/dl (male) or ≤50 mg/dl (female), or taking lipid-lowering medications (1). Steatosis due to underlying etiologies other than cardiometabolic criteria were excluded, including excessive alcohol consumption (alcohol intake ≥30 g/day for males and ≥20 g/day for females), HBV/HCV infection (serum hepatitis B surface antigen-positive or hepatitis C antibody-positive), and iron overload (transferrin saturation ≥50%). Participants who could not be diagnosed with MASLD because of missing data related to the above cardiometabolic risk factors were excluded.
Measurement of serum folate and victim B12
Both serum folate and vitamin B12 measurements were done by the National Center for Environmental Health using the Bio-Rad Laboratories “Quantaphase II Folate or Folate/B12” radioassay kit (44). The assay was conducted by combining serum samples with 57Co- vitamin B12 and 125I- folate in a solution that contained dithiothreitol and cyanide. All field-collected specimens were frozen and then transported on dry ice and stored at ≤ -20°C after receipt until analysis. Standard procedures for sample collection, storage, processing, analysis, and quality control are described in detail in Laboratory Procedures Used for the NHANES III (44). The coefficients of variation for the long-term accuracy of the NHANES III assays for serum folate and vitamin B12 were 3-6% (at 3-15 ng/mL) and 5-7% (at 300-1500 pg/mL), respectively (44). Folate or vitamin B12 concentrations below 1% or greater than 99% of the overall distribution were considered outliers and these participants were excluded.
Clinical and laboratory data
The following socio-demographic data were included: age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, other), educational level (≤high school, >high school degree), marital status (married, unmarried), family income to poverty ratio (<1, 1-5, >5), smoking status (current smoker, ex-smoker, never smoker), physical activity (active, median, inactive), Healthy Eating Index, and self-reported general health (excellent, very good, good, fair, poor). These data were derived from the baseline questionnaire interviews. For physical activity, leisure-time activities (such as jogging, swimming, riding, calisthenics, and dancing) were categorized into moderate (MET 3-6) and vigorous (MET > 6) types based on intensity ratings (45). The active physical activity level group was defined as engaging in moderate activities at least five times or vigorous activities at least three times per week; the inactive group was defined as no leisure time physical activity; and the moderate group was participants whose physical activity level fell between the active and inactive groups (46). The Healthy Eating Index is an indicator developed by the U.S. Department of Agriculture to measure the overall quality of an individual’s diet, with scores ranging from 0 to 100 (47). In addition to folate and vitamin B12, laboratory tests included as covariates included FIB-4 index, serum triglycerides, and C-reactive protein. FIB-4 was calculated as “(age (years) × AST (U/L))/((PLT [109/L]) × (ALT (U/L))1/2)”. Body measurements body mass index (BMI) and waist circumference were included. Common chronic diseases hypertension, diabetes, and history of heart attack were included. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or taking antihypertensive medication, or having ever been told a diagnosis of hypertension by a physician. Diabetes mellitus was defined as fasting plasma glucose concentration ≥126 mg/dL, or random/casual plasma glucose concentration ≥200 mg/dL, or Oral Glucose Tolerance Test ≥200 mg/dL, or HbA1c ≥6.5%, or taking antidiabetic medication, or ever been informed of a diagnosis of diabetes by a physician.
All-cause mortality
Mortality follow-up data were obtained by linking the unique identifiers of participants in NHANES III with death records from the National Death Index. The follow-up period was from the date that the NHANES interview was performed to the occurrence of a death or December 30, 2019, which is the latest data currently available.
Statistical analysis
Folate and vitamin B12 levels were categorized into four intervals based on quartiles to compare baseline characteristics and mortality status of participants in their respective groups. In comparisons of baseline characteristics, the Rao-Scott chi-squared test was used for dichotomous variables, and the Wilcoxon rank-sum test was used for continuous variables. Cox proportional regression models were applied to examine differences in all-cause mortality among MASLD patients in different serum folate and vitamin B12 quartile groups, with participants in the lowest serum folate and vitamin B12 quartile intervals being used as the reference group, respectively. Since the serum folate and vitamin B12 levels of most MASLD participants fell within the normal ranges suggested by some clinical guidelines for healthy adults (serum folate: 3–20 ng/mL; vitamin B12: 160–950 pg/mL) (48), we did not use the normal ranges for both metrics as a reference. In our analysis, the vast majority of participants in the lowest quartile for serum folate and vitamin B12 were still within the normal range for both markers, with fewer than 10% classified as deficient. Given the differences in sources of folate and vitamin B12, we conducted an analysis based on the combined status of both. Specifically, we classified the levels of each indicator into high-level and low-level groups based on their median values, resulting in the following four combinations: low folate & low vitamin B12 group, low folate & high vitamin B12 group, high folate & low vitamin B12 group, and high folate & high vitamin B12 group. The low folate & low vitamin B12 group was used as the reference group. Age, sex, and race-adjusted models considered age, sex, and race as confounders. Moreover, we developed multivariable Cox models further adjusting for demographic characteristics (educational level, marital status, family income level), lifestyle factors (smoking status, physical activity, Healthy Eating Index, vitamin C intake, and vitamin D intake), body measurements (body mass index, waist circumference), laboratory tests (FIB-4 index, serum triglycerides, C-reactive protein), and health status (self-reported general health, diabetes mellitus, hypertension, history of heart attack). To examine whether the effects of folate and vitamin B12 on mortality in MASLD were age-, sex-, or race-specific, we conducted stratified analyses according to them and analyzed interaction effects. To investigate the dose-response effects of vitamin B12 and folate levels on mortality in patients with MASLD, we used restricted cubic spline (RCS) models adjusted for baseline age, sex, and race/ethnicity, educational level, marital status, family income level, smoking status, physical activity, Healthy Eating Index, FIB-4 index, triglycerides, C-reactive protein, body mass index, waist circumference, self-reported general health, diabetes mellitus, hypertension, and history of heart attack. The respective median values of folate and vitamin B12 were used as reference points.
Sensitivity analyses were performed by excluding participants who died within two years of follow-up to rule out a reverse causal association between folate/vitamin B12 levels and all-cause mortality in patients with MASLD. Primary analyses were repeated (age-, sex-, and race-adjusted Cox regression models, multivariable Cox regression models, and RCS dose-response effect analyses) to examine whether the findings were robust.
We considered the complex survey design of NHANES III and used appropriate sample weights in all statistical analyses to make the results nationally representative. All tests were two-sided and P<0.05 was considered statistically significant. R version 4.3.1 (https://www.r-project.org/) was used to perform all statistical analyses.
Results
A total of 14,797 participants underwent an ultrasound examination, of which 941 were excluded because of missing or un-gradable image data. Of the 13,856 participants with available hepatic/gallbladder ultrasound data, hepatic steatosis was detected in 5016 individuals. After excluding participants with non-cardiometabolic etiologies and missing data on cardiometabolism, folate/vitamin B12 levels, and mortality status, 3636 participants with serum folate data and 2125 participants with serum vitamin B12 data were ultimately included in the formal analysis. The detailed study screening process is displayed in Figure 1.
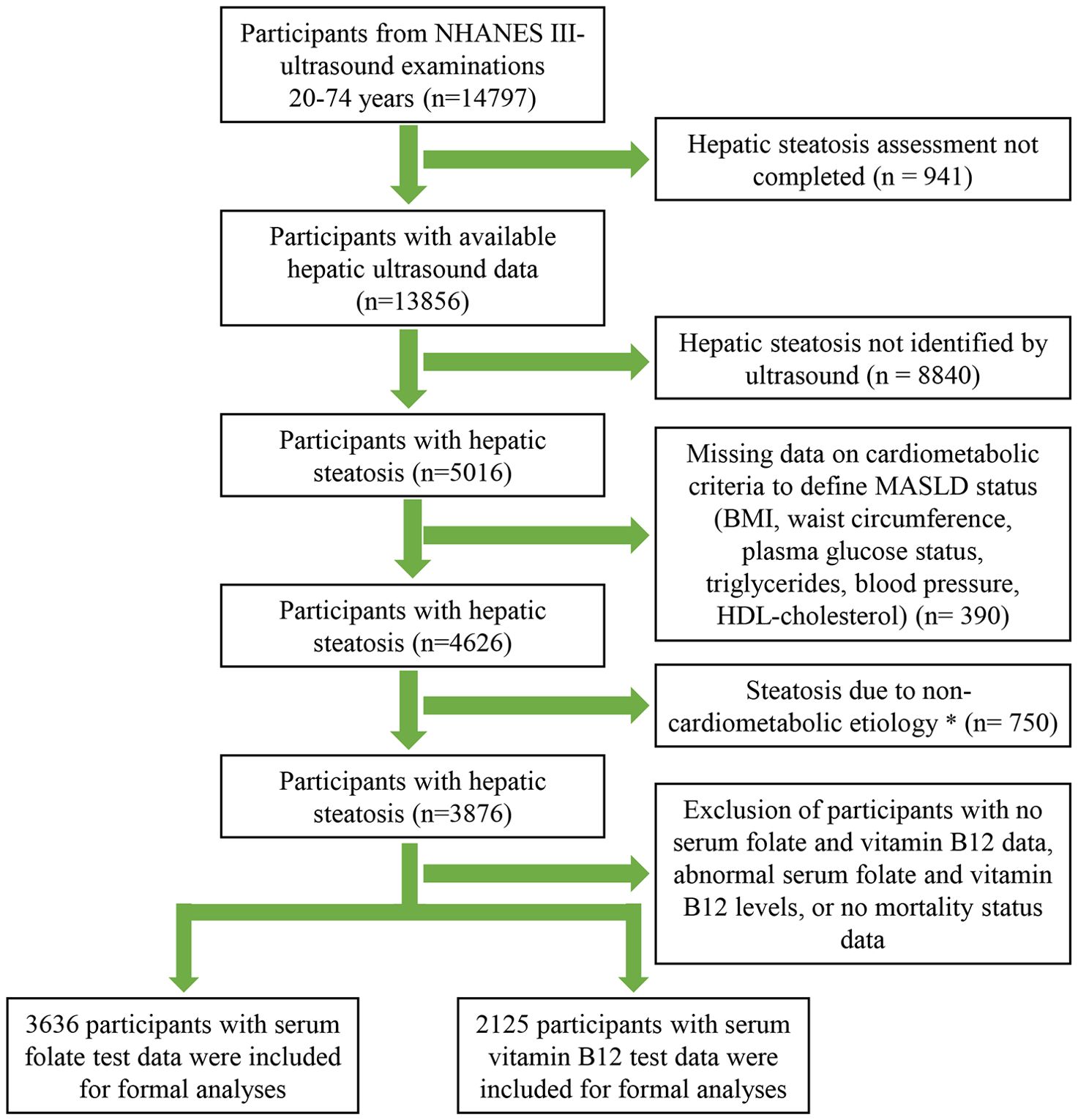
Figure 1. Flow Diagram of Participants Inclusion in the Study. *Alcohol consumption >30 g/day for male and >20 g/day for female, HBV/HCV infection, or serum transferrin saturation >50%.
For serum folate levels, the concentration ranges of the four groups according to the quartile method were: quartile 1 (<3.3 ng/mL), quartile 2 (3.3-4.7 ng/mL), quartile 3 (4.8-7.3 ng/mL), and quartile 4 (>7.3 ng/mL); for serum vitamin B12 levels, the concentration ranges of the four groups were: quartile 1 (<352 pg/mL), quartile 2 (352-457 pg/mL), quartile 3 (458-588 pg/mL), and quartile 4 (>588 pg/mL). In the serum folate analysis, the mean age of participants was 46.94 (SE: 0.43), and in the serum vitamin B12 analysis, the mean age of participants was 46.34 (SE: 0.71). Interestingly, the age tended to be older in the higher quartile serum folate group (P<0.001), whereas there was no significant difference in age among the different vitamin B12 groups (P=0.943). There was a difference in the gender ratio among the different serum vitamin B12 groups, with the high quartile groups (quartile 3 and quartile 4) tending to have a greater proportion of females (P=0.033), whereas the gender ratio was relatively balanced among the different folate groups (P=0.382). When complex sampling was considered, non-Hispanic whites were the most numerous, and there were differences in the ethnic distributions across the different quartile groups for both serum folate and vitamin B12. Detailed study characteristics of the included population are displayed in Table 1.
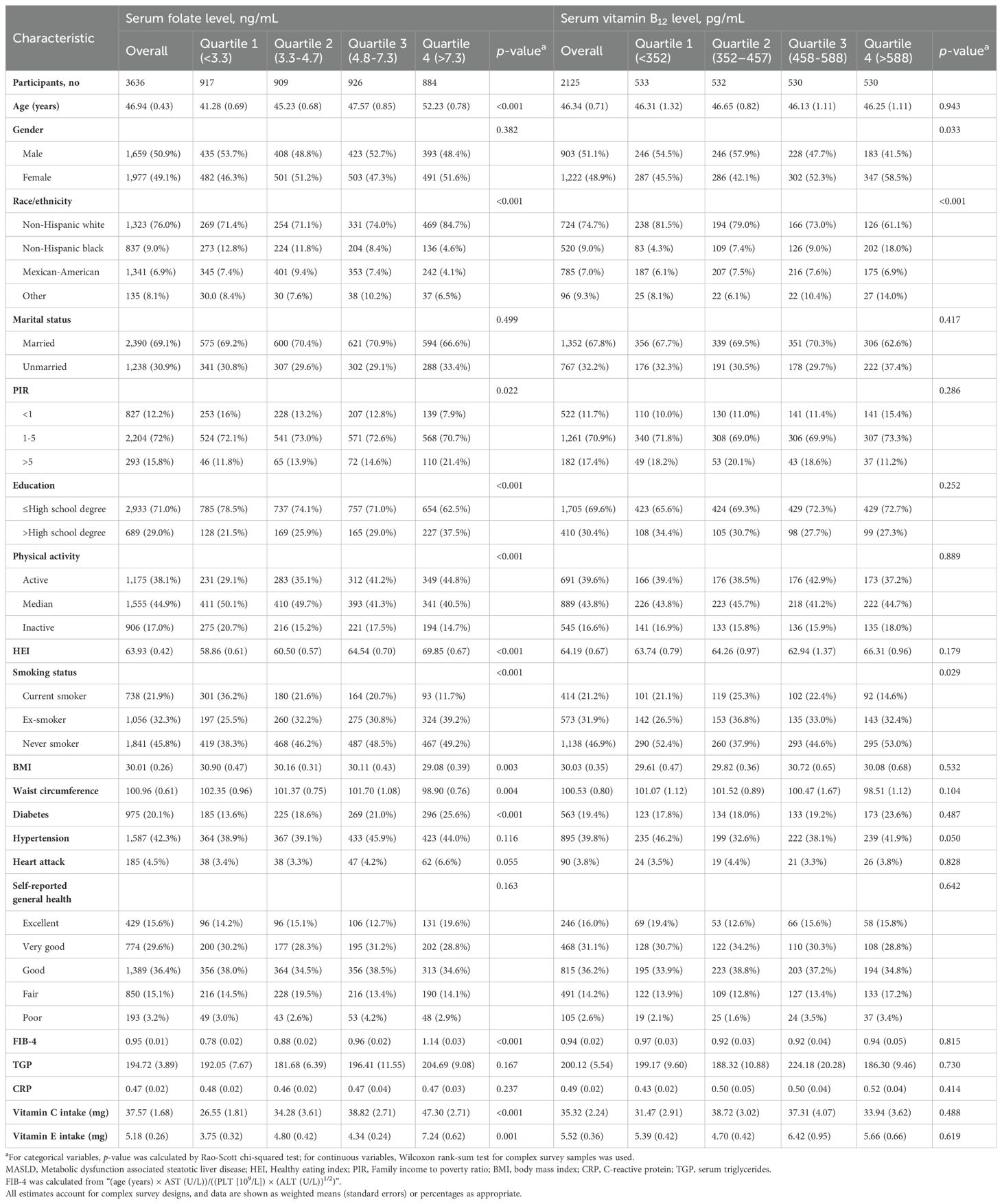
Table 1. Baseline characteristics of participants with MASLD according to the serum folate and vitamin B12 levels.
Serum folate and mortality
During a median 26.08 (IQR:18.08-28) years of follow-up for 3636 participants, 1571 deaths occurred. In age, sex, race-adjusted Cox proportional regression models, the higher folate quartile had a significantly lower risk of mortality compared with the lowest quartile group, with HRs of 0.77 (95% CI: 0.60-0.97, P=0.026) for quartile 2, 0.68 for quartile 3 (95% CI: 0.56-0.82, P<0.001), and 0.74 (95% CI: 0.59-0.94, P=0.011) for quartile 4 (Table 2). In multivariable models further adjusted for other potential confounders, only the quartile 3 group showed a significant reduction in mortality (HR=0.72, 95% CI: 0.57-0.91, P=0.005) (Table 2). The multivariable RCS dose-response analysis showed a significant nonlinear association between serum folate levels and all-cause mortality in patients with MASLD (P for nonlinear <0.001) (Figure 2A). At serum folate levels less than the median value, the risk of all-cause mortality decreased with increasing folate levels, reaching a lowest risk at around 4.7 ng/mL and then slowly increasing.
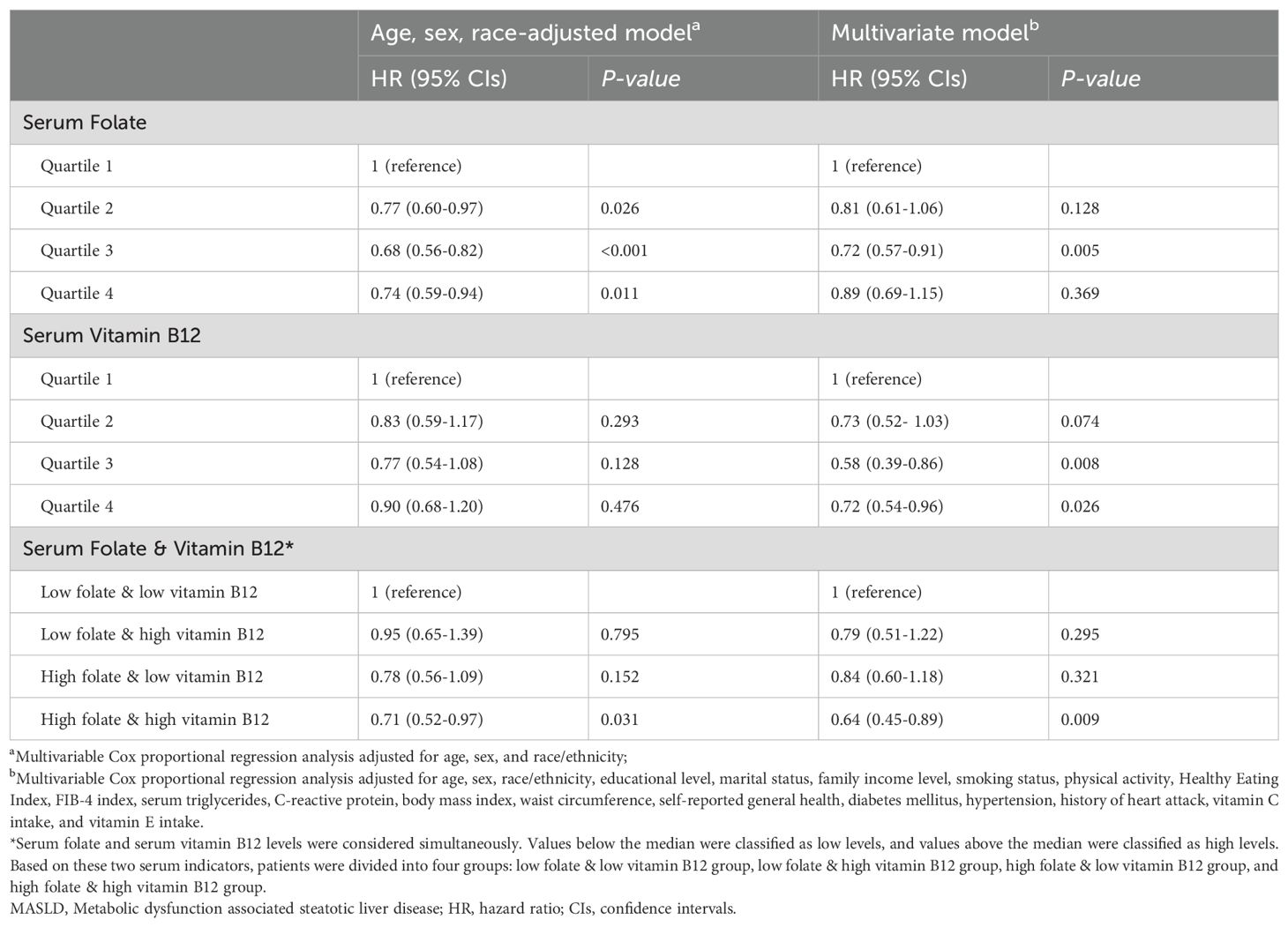
Table 2. Hazard ratios of all-cause mortality by serum folate and vitamin B12 levels among adults with MASLD.
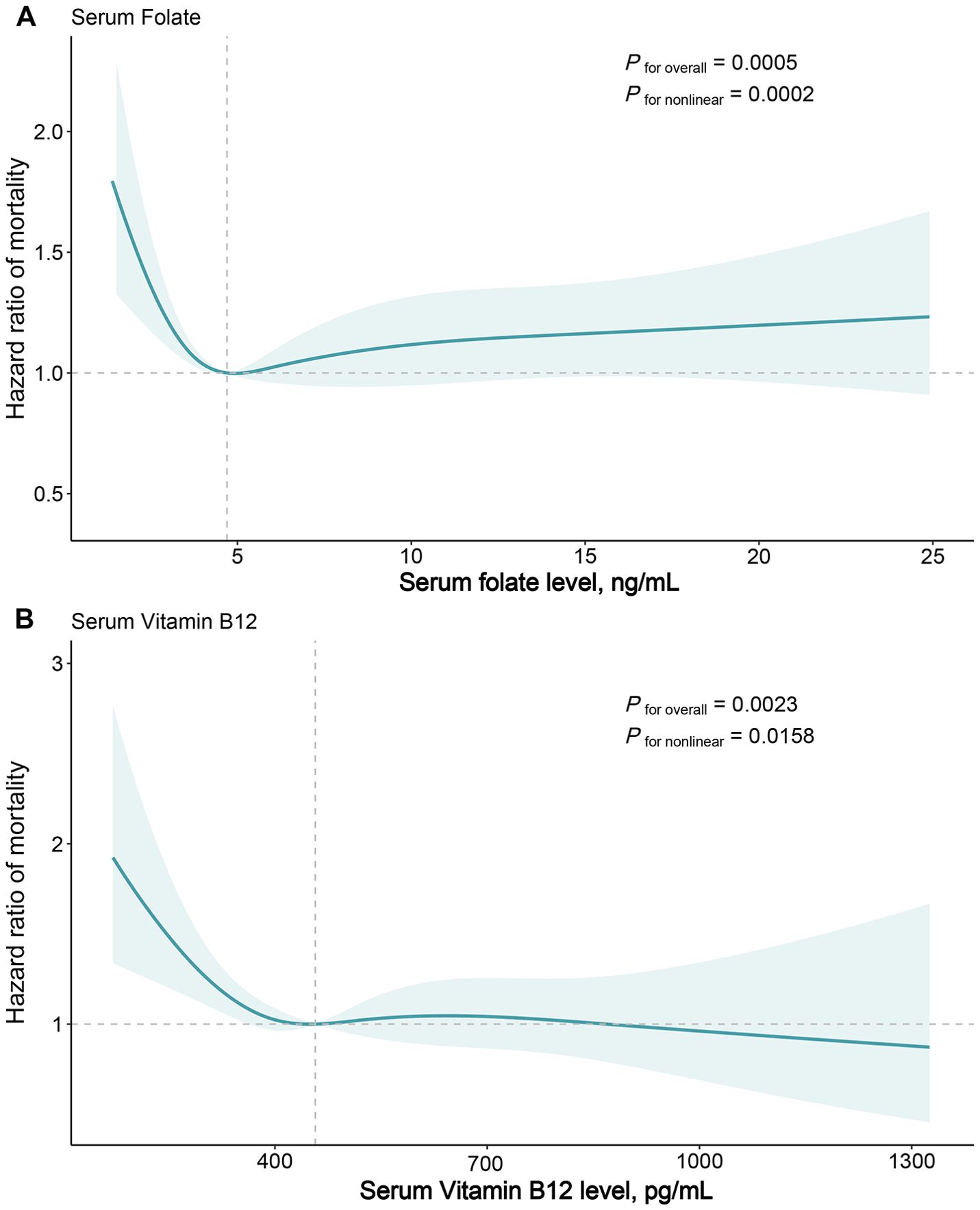
Figure 2. Dose-response Association of Serum Folate (A) and Vitamin B12 (B) Levels with All-cause Mortality in Patients with Metabolic Dysfunction-associated Fatty Liver Disease. Hazard ratios were estimated by multivariable restricted cubic spline models, with knots placed at 5th, 35th, 65th, and 95th percentiles. Solid line represents hazard ratios and shaded areas represents 95% CIs. The reference points are the median values for serum folate (4.7 ng/mL) and serum vitamin B12 (457.0 pg/mL) level. Risk estimates were adjusted for baseline age, sex, and race/ethnicity, educational level, marital status, family income level, smoking status, physical activity, Healthy Eating Index, FIB-4 index, triglycerides, C-reactive protein, body mass index, waist circumference, self-reported general health, diabetes mellitus, hypertension, and history of heart attack.
Serum vitamin B12 and mortality
2125 participants incurred 835 deaths during a median 25.75 (IQR:19.25-26.83) years of follow-up. The age, sex, race-adjusted Cox proportional regression models indicated that higher vitamin B12 quartiles tended to have a lower risk of mortality compared with the lowest quartile group, but did not reach statistical significance (Table 2). After further adjustment for potential confounders, the multivariable Cox model indicated that the quartile 3 (HR=0.58, 95% CI: 0.39-0.86, P=0.008) and quartile 4 (HR=0.72, 95% CI: 0.54-0.96, P=0.026) groups had a significantly lower risk of mortality compared with the lowest quartile group (Table 2). Similar to folate, multivariable RCS models showed a significant nonlinear correlation between serum vitamin B12 concentrations and all-cause mortality (P for nonlinear =0.016), with the risk of mortality decreasing with increasing vitamin B12 levels when serum vitamin B12 concentrations were below the median value, reaching a nadir risk at around 457 pg/mL. In contrast, the change in risk of all-cause mortality was relatively smooth after serum vitamin B12 concentrations were greater than the median value (Figure 2B).
Serum folate and vitamin B12 combination status and mortality
Considering the significant differences in the sources of serum folate and vitamin B12, we next evaluated the combined effects of serum folate and vitamin B12 on MASLD population. In multivariable models adjusted for full potential confounders, compared to the low folate & low vitamin B12 group, both the low folate & high vitamin B12 group and the high folate & low vitamin B12 group tended to have lower all-cause mortality (HR=0.79, 95% CI: 0.51-1.22, and HR=0.84, 95% CI: 0.60-1.18, respectively), although these differences were not statistically significant (Table 2). Interestingly, the high folate & high vitamin B12 group showed a significantly reduced mortality (HR=0.64, 95% CI: 0.45-0.89, P=0.009) (Table 2).
Stratified analysis
In stratified analyses according to age, sex, and race, no significant interaction effects were found for them on the correlation between folate/vitamin b12 and all-cause mortality (Tables 3, 4). However, there were some scenarios of marginal statistical significance. Specifically, the association between folate and mortality in patients with MASLD appeared to be more significant in females (P=0.098). Serum folate was statistically correlated with reduced all-cause mortality in the fourth and third quartiles of the young and middle-aged groups, respectively (Table 3). In the analysis of vitamin b12, the association between elevated serum vitamin b12 concentrations and reduced mortality appeared to be more significant in middle-aged and older adults (P =0.071) (Table 4). In the combined analysis of vitamin B12 and folate, we found that Non-Hispanic Black individuals did not seem to benefit from the simultaneous elevation of both (Table 5).
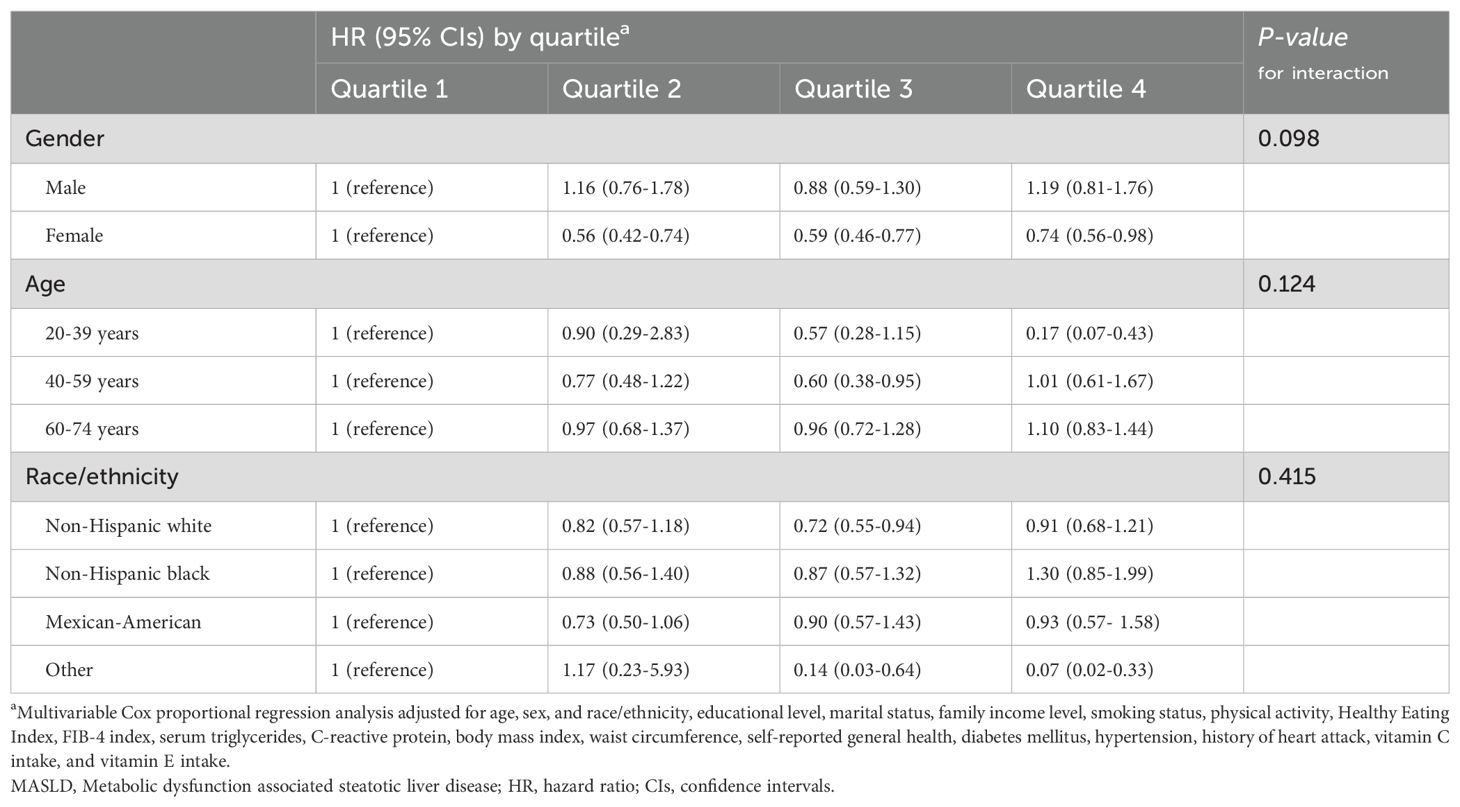
Table 3. Association of serum folate levels with all-cause mortality in different subgroups of patients with MASLD.
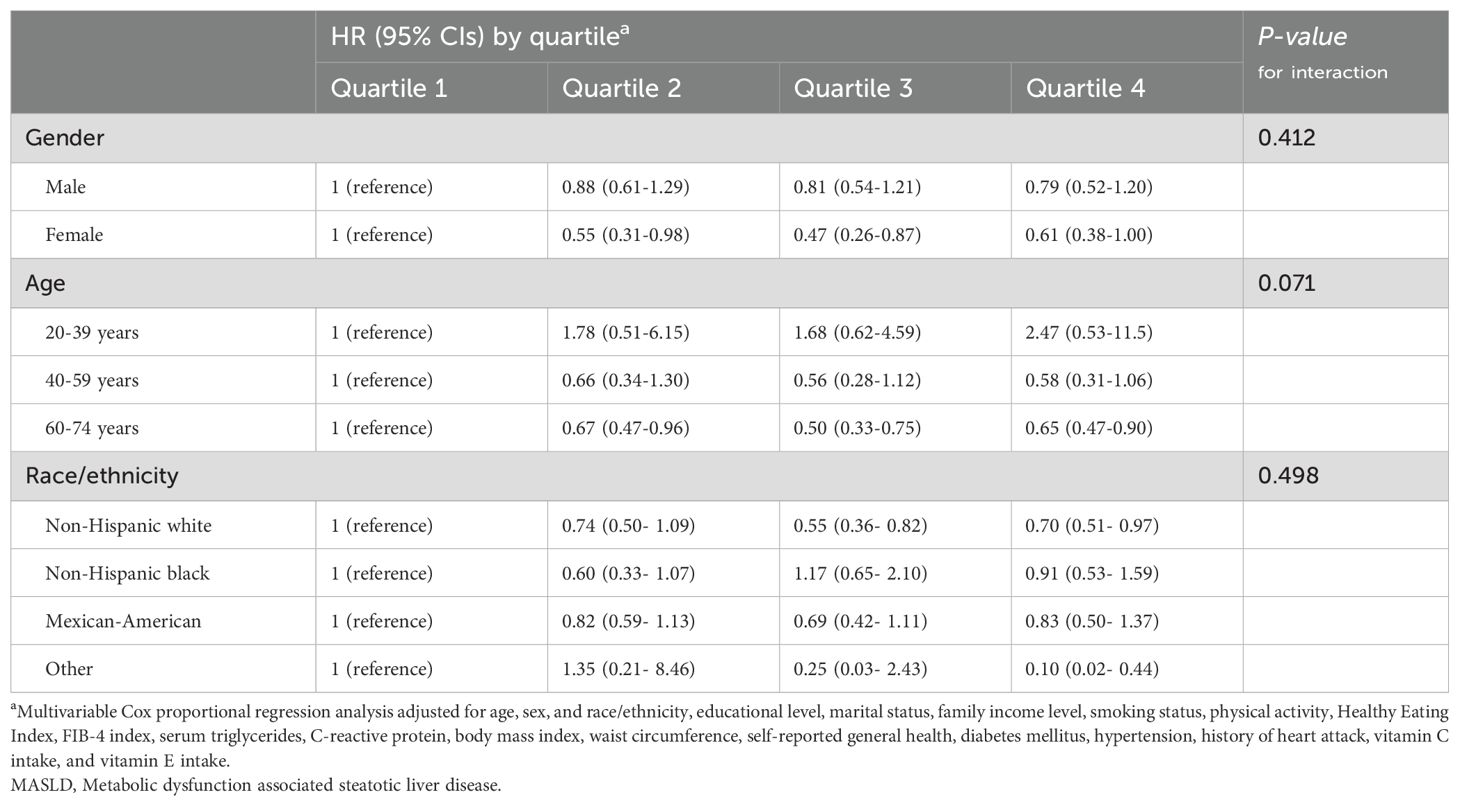
Table 4. Association of serum vitamin B12 levels with all-cause mortality in different subgroups of patients with MASLD.
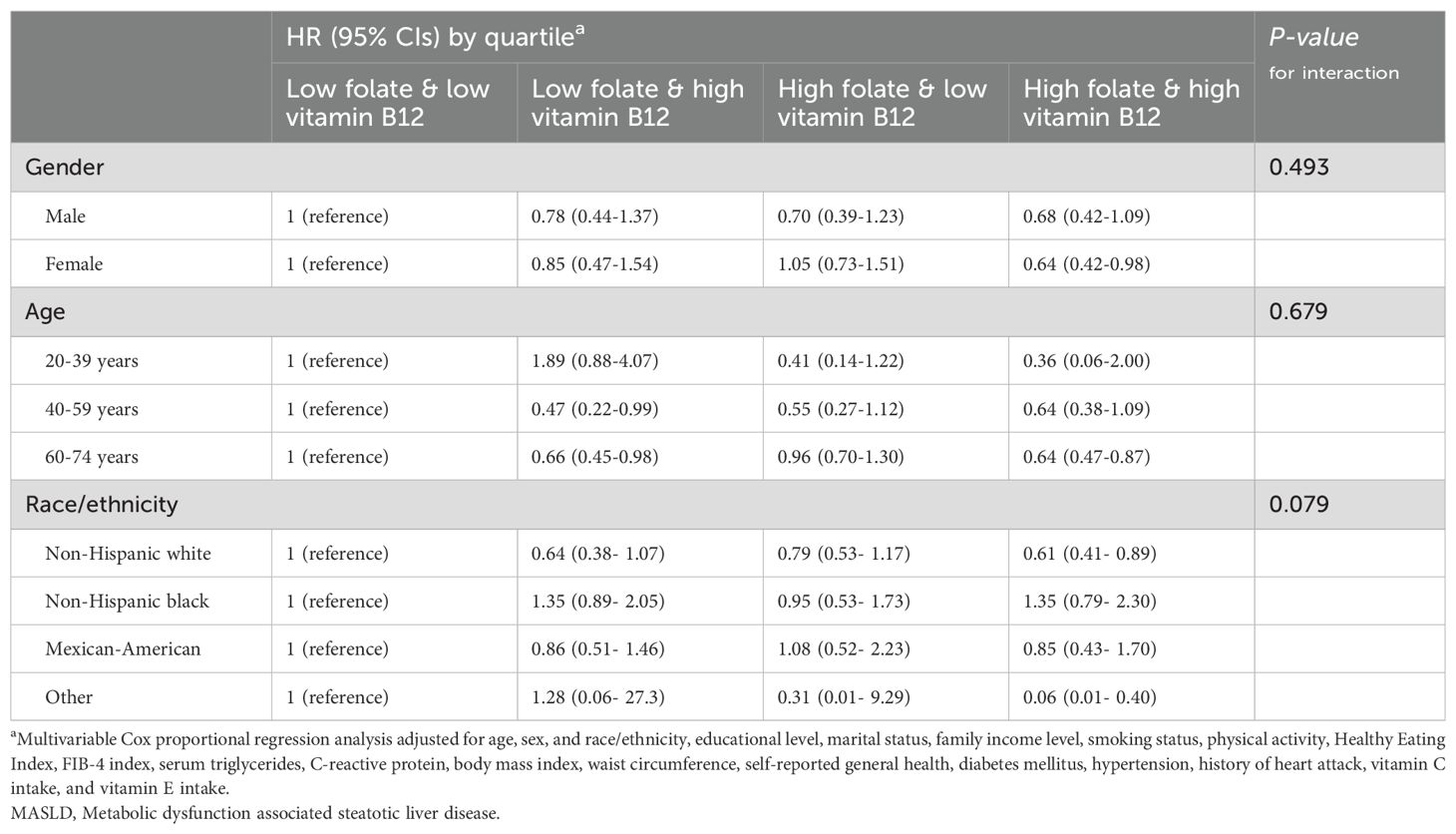
Table 5. Association of serum folate & vitamin B12 levels with all-cause mortality in different subgroups of patients with MASLD.
Sensitivity analysis
We repeated the analyses for the primary findings after excluding participants whose deaths occurred within two years of follow-up. Sensitivity analyses showed that participants who died within a short period of time had little effect on the results of the Cox proportional regression model and the multivariable RCS model, suggesting that the correlation between folate/vitamin b12 and mortality was not confounded by reverse causal effects (Supplementary Table S1, Supplementary Figure S1).
Discussion
To our knowledge, this is the first study to explore the effect of serum folate and vitamin B12 levels on all-cause mortality in patients with MASLD. In this large population-based prospective cohort study with a follow-up of more than 20 years, we found that both serum folate and vitamin B12 concentrations were significantly associated with all-cause mortality in individuals with MASLD. Low serum folate and vitamin B12 concentrations implied worse long-term outcomes for individuals with MASLD. Interestingly, we found that participants with both high folate and high vitamin B12 levels exhibited a more pronounced reduction in mortality compared to those with elevated levels of either folate or vitamin B12 alone. Sensitivity analyses confirmed the robustness of these findings from this study.
Of note, the association between serum folate and mortality shows inconsistent trends between men and women with MASLD. Some previous studies have suggested that estrogen influences folate metabolism and effects, but it is unclear whether this interaction contributes to the observed differences in MASLD patients (49, 50). Additionally, the association between serum vitamin B12 and mortality shows opposite trends in individuals under and over 40 years of age. This may be because MASLD patients over 40 tend to have poorer baseline health and more comorbidities, making the antioxidant, anti-inflammatory, and cardiovascular protective roles of vitamin B12 more critical (51, 52). In contrast, in patients under 40, high folate levels do not appear to offer similar protective effects. In summary, current evidence is insufficient to clarify the specific mechanisms of interaction between age/gender and the effects of these two nutrients in MASLD patients. However, it is important to note that the impact of folate and vitamin B12 on patient outcomes is not always consistent across different demographic groups.
Previous studies have shown that patients with NAFLD have lower serum folate and vitamin B12 concentrations compared with the general population (27–30). Furthermore, higher concentrations of folate/vitamin B12 and severity of liver fibrosis, steatosis, and nonalcoholic steatohepatitis were inversely correlated in patients with fatty liver (26, 53, 54). Thus, our findings may be due to the fact that low folate and vitamin B12 concentrations in MASLD imply a more severe disease status. Folate and vitamin B12 may be useful as biomarkers to independently predict mortality in individuals with MASLD, but more evidence from other geographic areas or ethnicities is needed to support this.
Several published studies have investigated the correlation between folate/vitamin B12 concentrations and mortality in the general population, but the results have been inconsistent. For example, Wolffenbuttel et al. showed that low serum vitamin B12 concentrations were significantly associated with increased all-cause mortality (55), but Flores-Guerrero et al. found that high vitamin B12 concentrations represented increased all-cause mortality in the general population of the city of Groningen, the Netherlands (56). In an elderly population in China, the correlation between serum vitamin B12 and all-cause mortality showed a J-shaped pattern (57). Existing studies exploring the association between folate concentrations in the body and mortality in the general population tend to report beneficial health effects of folate (58). For example, Peng et al. and Song et al. found that higher folate levels were associated with lower all-cause and cause-specific mortality (59, 60). Interestingly, several studies have shown that folate intake is associated with a reduced risk of mortality, whereas vitamin B12 intake did not have this effect (55, 61–63).
Some cross-sectional investigations have found an inverse association between folate intake and the prevalence of NAFLD (28, 29). However, there is no evidence from longitudinal studies that increased folate intake reduces the risk of mortality in individuals with fatty liver disease. Although our study suggests that low folate levels in vivo may be associated with a higher risk of mortality and studies from the general population have shown the benefits of folate intake, more direct clinical evidence is still needed as to whether folate supplementation can be used as a dietary intervention strategy for patients with MASLD. Compared to vitamin B12 deficiency, folate deficiency is more common because the body stores only a small amount of folate, so when the ingested diet is deficient in folate, the body can exhibit folate deficiency within a few months (64). However, insufficient intake is only part of the reason for folate or vitamin B12 deficiency in the body; low serum concentrations of both nutrients may also represent absorption disorders such as celiac disease, pancreatic disease, small bowel resection, endogenous factor deficiencies, or the effects of certain medications such as metformin, methotrexate, and antibiotics (65). Therefore, further studies are still needed to determine whether folate or vitamin B12 supplementation is truly effective as an intervention.
The potential mechanisms by which folate/vitamin B12 affects mortality in MASLD remain to be elucidated. Both play a core role in 1C metabolism, affecting a wide range of physiological activities such as protein and nucleic acid synthesis, methylation of DNA, and post-translational modification of proteins (11, 12). A recent study found that folate/vitamin B12 reduced circulating concentrations of homocysteine and improved autophagy through transmethylation, while serum homocysteine levels were significantly associated with worse liver inflammation and degree of fibrosis (66). Thus, dietary folate/vitamin B12 supplementation in the mouse model slows the progression of non-alcoholic steatohepatitis and reverses inflammation and fibrosis (66). In addition, folate/vitamin B12 is strongly associated with lipid metabolism (17, 67); low folate/vitamin B12 levels are associated with a high prevalence of metabolic syndromes, and their deficiency increases lipid accumulation in adipocytes, leptin production, and inflammatory factors, and thus may influence the progression of fatty liver (17, 67). Furthermore, deficiencies in vitamin b12 and folate increase oxidative stress by raising homocysteine levels (51, 68–70). Since oxidative stress plays an important role in the progression of fatty liver (71), ensuring adequate levels of vitamin b12 and folate may improve the prognosis of patients through this mechanism.
As the first large-scale investigation into the association of serum folate and vitamin B12 levels with all-cause mortality in patients with MASLD, the main strengths of the current study are that it has a follow-up time of more than 20 years, adjusts for a variety of potential confounders, and takes into account the complex sampling design to enable the included samples to be more representative and to facilitate the generalization of the current findings. However, some limitations need to be considered. First, data on serum folate and vitamin B12 concentrations were based on a single measurement, so we were unable to assess the impact of dynamic changes in both concentrations on mortality. Second, because of the lack of histologic examination and natural history information, we were unable to analyze whether the effect of folate/vitamin B12 on mortality was confounded by the severity and duration of MASLD. Third, we did not exclude drug-induced hepatic steatosis because we could not establish a causal association between the two in the current cohort. Fourth, some of the non-statistically significant findings, especially in stratified analyses, may be related to the limited sample size. More studies are needed in the future to minimize type 2 error. Fifth, due to the limited availability of medication information, we cannot rule out the potential impact of drugs on vitamin B12 and folate levels. Additionally, the vitamin B12 profile of vegetarians may differ from that of the general population. Since we were unable to identify vegetarians within our cohort, it is unclear whether the current findings can be generalized to this group. Finally, despite our attempts to eliminate reverse causality by excluding participants who died within two years of follow-up, the current results are still not representative of a causal association between serum folate/vitamin B12 and mortality due to the inherent limitations of observational studies.
Conclusions
Our results suggest a nonlinear association of serum folate and vitamin B12 levels with all-cause mortality in MASLD. Avoiding low serum folate and vitamin B12 concentrations may be potentially beneficial for reducing the risk of mortality in patients with MASLD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics Institutional Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JZ: Writing – review & editing, Data curation, Methodology, Formal analysis, Validation, Investigation, Software. XL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LD: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing, Software. PL: Writing – review & editing, Methodology, Supervision, Project administration, Validation, Investigation. JD: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Investigation, Visualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the participants and staff of NHANES for their valuable contributions to the scientific study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1426103/full#supplementary-material
References
1. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 79:1542–56. doi: 10.1016/j.jhep.2023.06.003
2. Younossi ZM, Alqahtani SA, Alswat K, Yilmaz Y, Keklikkiran C, Funuyet-Salas J, et al. Global survey of stigma among physicians and patients with nonalcoholic fatty liver disease. J Hepatol. (2024) 80:419–30. doi: 10.1016/j.jhep.2023.11.004
3. Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/S2468-1253(22)00165-0
4. Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwälder M, El-Serag HB, et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. (2023) 20:487–503. doi: 10.1038/s41575-023-00754-7
5. Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
6. Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. (2020) 111S:154170. doi: 10.1016/j.metabol.2020.154170
7. Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. (2018) 38:47–51. doi: 10.1111/liv.13643
8. Nassir F. NAFLD: mechanisms, treatments, and biomarkers. Biomolecules. (2022) 12:824. doi: 10.3390/biom12060824
9. Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. (2024) 390:497–509. doi: 10.1056/NEJMoa2309000
10. McPherson S, Armstrong MJ, Cobbold JF, Corless L, Anstee QM, Aspinall RJ, et al. Quality standards for the management of non-alcoholic fatty liver disease (NAFLD): consensus recommendations from the British Association for the Study of the Liver and British Society of Gastroenterology NAFLD Special Interest Group. Lancet Gastroenterol Hepatol. (2022) 7:755–69. doi: 10.1016/S2468-1253(22)00061-9
11. Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. (2017) 25:27–42. doi: 10.1016/j.cmet.2016.08.009
12. Lyon P, Strippoli V, Fang B, Cimmino L. B vitamins and one-carbon metabolism: Implications in human health and disease. Nutrients. (2020) 12:1–24. doi: 10.3390/nu12092867
13. Alam C, Kondo M, O’Connor DL, Bendayan R. Clinical implications of folate transport in the central nervous system. Trends Pharmacol Sci. (2020) 41:349–61. doi: 10.1016/j.tips.2020.02.004
14. Bender A, Hagan KE, Kingston N. The association of folate and depression: A meta-analysis. J Psychiatr Res. (2017) 95:9–18. doi: 10.1016/j.jpsychires.2017.07.019
15. Pieroth R, Paver S, Day S, Lammersfeld C. Folate and its impact on cancer risk. Curr Nutr Rep. (2018) 7:70–84. doi: 10.1007/s13668-018-0237-y
16. Liu Y, Geng T, Wan Z, Lu Q, Zhang X, Qiu Z, et al. Associations of serum folate and vitamin B12Levels with cardiovascular disease mortality among patients with type 2 diabetes. JAMA Netw Open. (2022) 5:e2146124. doi: 10.1001/jamanetworkopen.2021.46124
17. da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. Novel insights on interactions between folate and lipid metabolism. BioFactors. (2014) 40:277–83. doi: 10.1002/biof.1154
18. Chen L, Zhang Z, Hoshino A, Zheng HD, Morley M, Arany Z, et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat Metab. (2019) 1:404–15. doi: 10.1038/s42255-019-0043-x
19. Zaccherini G, Aguilar F, Caraceni P, Clària J, Lozano JJ, Fenaille F, et al. Assessing the role of amino acids in systemic inflammation and organ failure in patients with ACLF. J Hepatol. (2021) 74:1117–31. doi: 10.1016/j.jhep.2020.11.035
20. Steinberg SE, Campbell CL, Hillman RS. Kinetics of the normal folate enterohepatic cycle. J Clin Invest. (1979) 64:83–8. doi: 10.1172/JCI109467
21. Zaitsev AV, Martinov MV, Vitvitsky VM, Ataullakhanov FI. Rat liver folate metabolism can provide an independent functioning of associated metabolic pathways. Sci Rep. (2019) 9:7657. doi: 10.1038/s41598-019-44009-5
22. Socha DS, DeSouza SI, Flagg A, Sekeres M, Rogers HJ. Severe megaloblastic anemia: Vitamin deficiency and other causes. Cleve Clin J Med. (2020) 87:153–64. doi: 10.3949/ccjm.87a.19072
23. Kozyraki R, Cases O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie. (2013) 95:1002–7. doi: 10.1016/j.biochi.2012.11.004
24. Cui LH, Quan ZY, Piao JM, Zhang TT, Jiang MH, Shin MH, et al. Plasma folate and vitamin B12 levels in patients with hepatocellular carcinoma. Int J Mol Sci. (2016) 17:1032. doi: 10.3390/ijms17071032
25. Raza S, Tewari A, Rajak S, Sinha RA. Vitamins and non-alcoholic fatty liver disease: A molecular insight. Liver Res. (2021) 5:62–71. doi: 10.1016/j.livres.2021.03.004
26. Mahamid M, Mahroum N, Bragazzi NL, Shalaata K, Yavne Y, Adawi M, et al. Folate and B12 levels correlate with histological severity in NASH patients. Nutrients. (2018) 10:440. doi: 10.3390/nu10040440
27. Yao B, Lu X, Xu L, Jiang Y. Association of serum folate with prevalence of non-alcoholic fatty liver disease among adults (NHANES 2011–2018). Front Nutr. (2023) 10:1141156. doi: 10.3389/fnut.2023.1141156
28. Liu Z, Zeng Y, Shen S, Wen Y, Xu C. Association between folate and non-alcoholic fatty liver disease among US adults: a nationwide cross-sectional analysis. Chin Med J (Engl). (2023) 136:233–5. doi: 10.1097/CM9.0000000000002516
29. Chen Y, Xiang L, Luo L, Qin H, Tong S. Correlation of nonalcoholic fatty liver disease with dietary folate and serum folate in U.S. Adults: cross-sectional analyses from national health and nutrition examination survey 2009–2018. Metab Syndr Relat Disord. (2023) 21:389–96. doi: 10.1089/met.2023.0024
30. Koplay M, Gulcan E, Ozkan F. Association between serum Vitamin B12 levels and the degree of steatosis in patients with nonalcoholic fatty liver disease. J Investig Med. (2011) 59:1137–40. doi: 10.2310/JIM.0b013e31822a29f5
31. CDC/NCHS. Plan and operation of the NHANES III, 1988-94, national center for health statistics. Vital Health Stat. (1994), 1–407.
32. CDC/NCHS. Analytic And Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988-94). Hyattsville, MD, USA: National Center for Health Statistics Centers for Disease Control and Prevention (1996).
33. Younossi ZM, Paik JM, Stepanova M, Ong J, Alqahtani S, Henry L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol. (2024) 80:694–701. doi: 10.1016/j.jhep.2024.01.014
34. Sripongpun P, Kim WR, Mannalithara A, Charu V, Vidovszky A, Asch S, et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. Hepatology. (2023) 77:256–67. doi: 10.1002/hep.32545
35. Van Kleef LA, De Knegt RJ, Brouwer WP. Metabolic dysfunction-associated fatty liver disease and excessive alcohol consumption are both independent risk factors for mortality. Hepatology. (2023) 77:942–8. doi: 10.1002/hep.32642
36. Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. (2021) 19:2172–2181.e6. doi: 10.1016/j.cgh.2021.05.029
37. Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. (2020) 40:2082–9. doi: 10.1111/liv.14548
38. Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: Prospective cohort study. BMJ. (2011) 343:1245. doi: 10.1136/bmj.d6891
39. National Center for Health Statistics. Third National Health and Nutrition Examination Survey: Hepatic Steatosis Ultrasound Images Assessment Procedures Manual. Available online at: http://www.cdc.gov/nchs/data/nhanes/nhanes3/Hepatic_Steatosis_Ultrasound_Procedures_Manual.pdf (Accessed January 22, 2024).
40. National Center for Health Statistics. Third National Health and Nutrition Examination Survey: Gallbladder Ultrasonography Procedure Manual (1988). Available online at: http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/gallblad.pdf (Accessed January 22, 2024).
41. Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. (2021) 75:1284–91. doi: 10.1016/j.jhep.2021.07.035
42. Alvarez CS, Graubard BI, Thistle JE, Petrick JL, McGlynn KA. Attributable fractions of nonalcoholic fatty liver disease for mortality in the United States: results from the third national health and nutrition examination survey with 27 years of follow-up. Hepatology. (2020) 72:430–40. doi: 10.1002/hep.31040
43. Konyn P, Alshuwaykh O, Dennis BB, Cholankeril G, Ahmed A, Kim D. Gallstone disease and its association with nonalcoholic fatty liver disease, all-cause and cause-specific mortality. Clin Gastroenterol Hepatol. (2023) 21:940–948.e2. doi: 10.1016/j.cgh.2022.04.043
44. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS).Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey(NHANES III), 1988-1994 (1996). Hyattsville, MD: U.S: Department of Health and Human Services, Centers for Disease Control and Prevention. Available online at: https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/labman.pdf (Accessed January 22, 2024).
45. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. (1993) 25:71–4. doi: 10.1249/00005768-199301000-00011
46. Yi Y, Wang C, Ding Y, He JH, Lv YQ, Chang Y. Diet was less significant than physical activity in the prognosis of people with sarcopenia and metabolic dysfunction-associated fatty liver diseases: Analysis of the National Health and Nutrition Examination Survey III. Front Endocrinol (Lausanne). (2023) 14:1101892. doi: 10.3389/fendo.2023.1101892
47. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS).NHANES III Healthy Eating Index Data File Series 11, No. 6A (2000). Hyattsville, MD: U.S: Department of Health and Human Services, Centers for Disease Control and Prevention. Available online at: https://wwwn.cdc.gov/nchs/data/nhanes3/6a/hei-acc.pdf (Accessed January 22, 2024).
48. Marcogliese AN, Yee DL. Resources for the hematologist: interpretive comments and selected reference values for neonatal, pediatric, and adult populations. In: Hoffman R, Benz EJ, Silberstein LE, et al, editors. Hematology: Basic Principles and Practice, 7th ed. Rochester, United States: Elsevier (2018). chap 162. doi: 10.1016/B978-0-323-35762-3.00162-1
49. Pourié G, Martin N, Bossenmeyer-Pourié C, Akchiche N, Guéant-Rodriguez RM, Geoffroy A, et al. Folate- and vitamin B12-deficient diet during gestation and lactation alters cerebellar synapsin expression via impaired influence of estrogen nuclear receptor α. FASEB J. (2015) 29:3713–25. doi: 10.1096/fj.14-264267
50. Kelley KM, Rowan BG, Ratnam M. Modulation of the folate receptor alpha gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Res. (2003) 63:2820–8.
51. van de Lagemaat EE, de Groot LCPGM, van den Heuvel EGHM. Vitamin B12 in eelation to oxidative stress: A systematic review. Nutrients. (2019) 11:482. doi: 10.3390/nu11020482
52. Liu S, An P. Untangling the uncertainty in B vitamins for stroke prevention: folic acid fortification, dosage, and their interaction? Am J Clin Nutr. (2024) 119:593–4. doi: 10.1016/j.ajcnut.2024.01.005
53. Yang S, Ye Z, Liu M, Zhang Y, Wu Q, Zhou C, et al. Associations of different serum folate forms with indices of nonalcoholic fatty liver disease and advanced fibrosis. Obes Res Clin Pract. (2023) 17:58–65. doi: 10.1016/j.orcp.2023.01.004
54. Chen X, Lu J, Xu Q, Chen B, Shen L. The association between serum folate and ultrasound - defined hepatic steatosis. Ann Med. (2023) 55:456–62. doi: 10.1080/07853890.2023.2168042
55. Wolffenbuttel BHR, Heiner-Fokkema MR, Green R, Gans ROB. Relationship between serum B12 concentrations and mortality: Experience in NHANES. BMC Med. (2020) 18:307. doi: 10.1186/s12916-020-01771-y
56. Flores-Guerrero JL, Minović I, Groothof D, Gruppen EG, Riphagen IJ, Kootstra-Ros J, et al. Association of plasma concentration of vitamin B12 with all-cause mortality in the general population in the Netherlands. JAMA Netw Open. (2020) 3:e1919274. doi: 10.1001/jamanetworkopen.2019.19274
57. Xu K, Liu X, Liu J, Zhang Y, Ding X, Li L, et al. Association between serum vitamin B12 and risk of all-cause mortality in elderly adults: a prospective cohort study. BMC Geriatr. (2021) 21:497. doi: 10.1186/s12877-021-02443-z
58. Bo Y, Zhu Y, Tao Y, Li X, Zhai D, Bu Y, et al. Association between folate and health outcomes: an umbrella review of meta-analyses. Front Public Heal. (2020) 8:550753. doi: 10.3389/fpubh.2020.550753
59. Peng Y, Dong B, Wang Z. Serum folate concentrations and all-cause, cardiovascular disease and cancer mortality: A cohort study based on 1999-2010 National Health and Nutrition Examination Survey (NHANES). Int J Cardiol. (2016) 219:136–42. doi: 10.1016/j.ijcard.2016.06.024
60. Song S, Song BM, Park HY. Associations of serum folate and homocysteine concentrations with all-cause, cardiovascular disease, and cancer mortality in men and women in Korea: the cardiovascular disease association study. J Nutr. (2023) 153:760–70. doi: 10.1016/j.tjnut.2023.01.023
61. Bo Y, Xu H, Zhang H, Zhang J, Wan Z, Zhao X, et al. Intakes of folate, vitamin B6, and vitamin B12 in relation to all-cause and cause-specific mortality: A national population-based cohort. Nutrients. (2022) 14:2253. doi: 10.3390/nu14112253
62. Zhang B, Dong H, Xu Y, Xu D, Sun H, Han L. Associations of dietary folate, vitamin B6 and B12 intake with cardiovascular outcomes in 115664 participants: a large UK population-based cohort. Eur J Clin Nutr. (2023) 77:299–307. doi: 10.1038/s41430-022-01206-2
63. Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A. Dietary folate and vitamin B6 and B12 intake in relation to mortality from cardiovascular diseases: Japan collaborative cohort study. Stroke. (2010) 41:1285–9. doi: 10.1161/STROKEAHA.110.578906
64. Shulpekova Y, Nechaev V, Kardasheva S, Sedova A, Kurbatova A, Bueverova E, et al. The concept of folic acid in health and disease. Molecules. (2021) 26:3731. doi: 10.3390/molecules26123731
65. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders: British Committee for Standards in Haematology. Br J Haematol. (2014) 166:496–513. doi: 10.1111/bjh.12959
66. Tripathi M, Singh BK, Zhou J, Tikno K, Widjaja A, Sandireddy R, et al. Vitamin B12 and folate decrease inflammation and fibrosis in NASH by preventing syntaxin 17 homocysteinylation. J Hepatol. (2022) 77:1246–55. doi: 10.1016/j.jhep.2022.06.033
67. Boachie J, Adaikalakoteswari A, Samavat J, Saravanan P. Low vitamin b12 and lipid metabolism: Evidence from pre-clinical and clinical studies. Nutrients. (2020) 12:1–20. doi: 10.3390/nu12071925
68. Bito T, Misaki T, Yabuta Y, Ishikawa T, Kawano T, Watanabe F. Vitamin B12 deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans. Redox Biol. (2017) 11:21–9. doi: 10.1016/j.redox.2016.10.013
69. Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. (2017) 3:17040. doi: 10.1038/nrdp.2017.40
70. Rogers EJ, Chen S, Chan A. Folate deficiency and plasma homocysteine during increased oxidative stress. N Engl J Med. (2007) 357:421–2. doi: 10.1056/NEJMc066569
Keywords: metabolic dysfunction-associated steatotic liver disease, folate, vitamin B12, all-cause mortality, cohort study
Citation: Zhu J, Liao X, Du L, Lv P and Deng J (2024) Associations of serum folate and vitamin B12 levels with all-cause mortality among patients with metabolic dysfunction associated steatotic liver disease: a prospective cohort study. Front. Endocrinol. 15:1426103. doi: 10.3389/fendo.2024.1426103
Received: 30 April 2024; Accepted: 11 November 2024;
Published: 05 December 2024.
Edited by:
Stanisław Surma, Medical University of Silesia, PolandReviewed by:
Celeste Annemarie De Jager Loots, Imperial College London, United KingdomOmar A. Obeid, American University of Beirut, Lebanon
Naheed Aryaeian, Iran University of Medical Sciences, Iran
Copyright © 2024 Zhu, Liao, Du, Lv and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengju Lv, cGpsdkB6enUuZWR1LmNu; Jian Deng, ZHJkZW5namlhbkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Jiaxin Zhu1†
Jiaxin Zhu1† Xinyi Liao
Xinyi Liao Lei Du
Lei Du Jian Deng
Jian Deng