- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Graduate School of Beijing University of Chinese Medicine, Beijing, China
- 3Department of Thyropathy, Sunsimiao Hospital, Beijing University of Chinese Medicine, Tongchuan, Shaanxi, China
Background: Higher thyroid-stimulating hormone (TSH) amidst normal thyroid hormone (TH) levels may contribute to a negative impact on cardiovascular health (CVH). We sought to probe the associations between Life’s Essential 8 (LE8), a newly revised CVH evaluation, and low thyroid function among US adults.
Methods: The datasets from the 2007-2012 National Health and Nutrition Examination Survey (NHANES) were applied to the study. Low-normal thyroid function and subclinical hypothyroidism (SCH) were both regarded to be low thyroid function. Multivariable logistic regressions were utilized to inquire about the relationship between LE8 and low thyroid function.
Results: Among the 6,315 participants (age ≥20 years), 1,375 (21.77%) were ascertained to be low thyroid function. After adjusting possible confounders, a higher LE8 score was linked to a lower probability of experiencing low thyroid function (Odds ratio [OR] for each 10-point increase: 0.923 [95% CI, 0.884-0.964]). A similar correlation was found between the health factors score and low thyroid function (OR for each 10-point increase: 0.905 [95% CI, 0.876-0.935]). Also, scoring better on physical activity (PA), body mass index (BMI), blood lipid, blood glucose (BG), and blood pressure (BP) may be conducive to reducing the rates of low thyroid function. Furthermore, subgroup and sensitivity analyses indicated that the negative correlations were generally robust.
Conclusions: The LE8 score and health factors score were nonlinearly and negatively related to the prevalence concerning low thyroid function. Promoting the regulation of optimum CVH levels could work on mitigating the load of low thyroid function and cardiovascular diseases (CVDs).
1 Introduction
Low thyroid function, a condition of high TSH level in the presence of normal TH level, comprises low-normal thyroid function and SCH (1). TH receptors are situated in myocardial and vascular endothelial organizations, thus slight variations in TH can alter cardiovascular function and affect end-organ regulation (2, 3). It is now commonly recognized that significant hypothyroidism adversely contributes to the morbidity and mortality of CVDs (4). However, a growing body of evidence implies that low thyroid function may also compromise cardiometabolic capacity, dramatically enhancing the danger of hypertension, atherosclerosis, arrhythmias, and other CVDs (5, 6). Meanwhile, it has been anticipated that 19.05 million deaths of CVD worldwide in 2020, which represents an increase of 18.71% from 2010 (7). As a result, proactive screening and monitoring of CVDs in low thyroid function patients may be of assistance in improving CVH.
The American Heart Association (AHA) launched Life’s Simple 7 (LS7) in 2010, which includes 3 health behaviors and 4 health factors to better surveillance of the CVH status of the general population (8). In 2022, in response to the desire to enhance feasibility in practice, the AHA, after more than a decade of accumulated evidence and inspiration, updated the CVH quantitative assessment instrument, known as LE8, to circumvent the limitations of LS7 (9, 10). Compared with the original LS7, LE8 has upgraded the scoring specifications of CVH indicators to refine and continuously track the CVH of individuals (11, 12). Additionally, LE8 further incorporated sleep health to capture the essential role of sleep in human life maintenance and cardiometabolic health (7). Taking into account the close connection between low thyroid function and CVDs, promoting CVH may represent an appropriate means of mitigating thyroid dysfunction and CVD damage. There have been no available studies linking LE8 to low thyroid function. This nationwide representative research evaluated the relationship between the two using NHANES data, aiming to generate novel strategies for the long-term management of low thyroid function and CVDs.
2 Methods
2.1 Study participants
NHANES is a population-based study employing a stratified, sophisticated, and random sampling scheme to deliver a wealth of details about the condition of general health and nutrition in the US. The NHANES study protocol was authorized and affirmed by the National Center for Health Statistics (NCHS) Research Ethics Review Board, and written informed permissions were provided. Public access to more thorough research methodologies and figures is available at https://www.cdc.gov/nchs/nhanes/.
NHANES data from 2007 to 2012 were used in this study. Among the 30,442 participants, 17,713 were ≥20 years. We eliminated 8,936 participants with missing thyroid function data (TSH, FT4), 1,990 participants with missing LE8 data, 207 participants with missing covariates and self-reported histories of CVD data, and 68 pregnant participants. Additionally, 197 participants with FT4 anomalies and TSH <0.34 mIU/L were also removed. In the end, 6,315 participants were incorporated into the research (Figure 1).
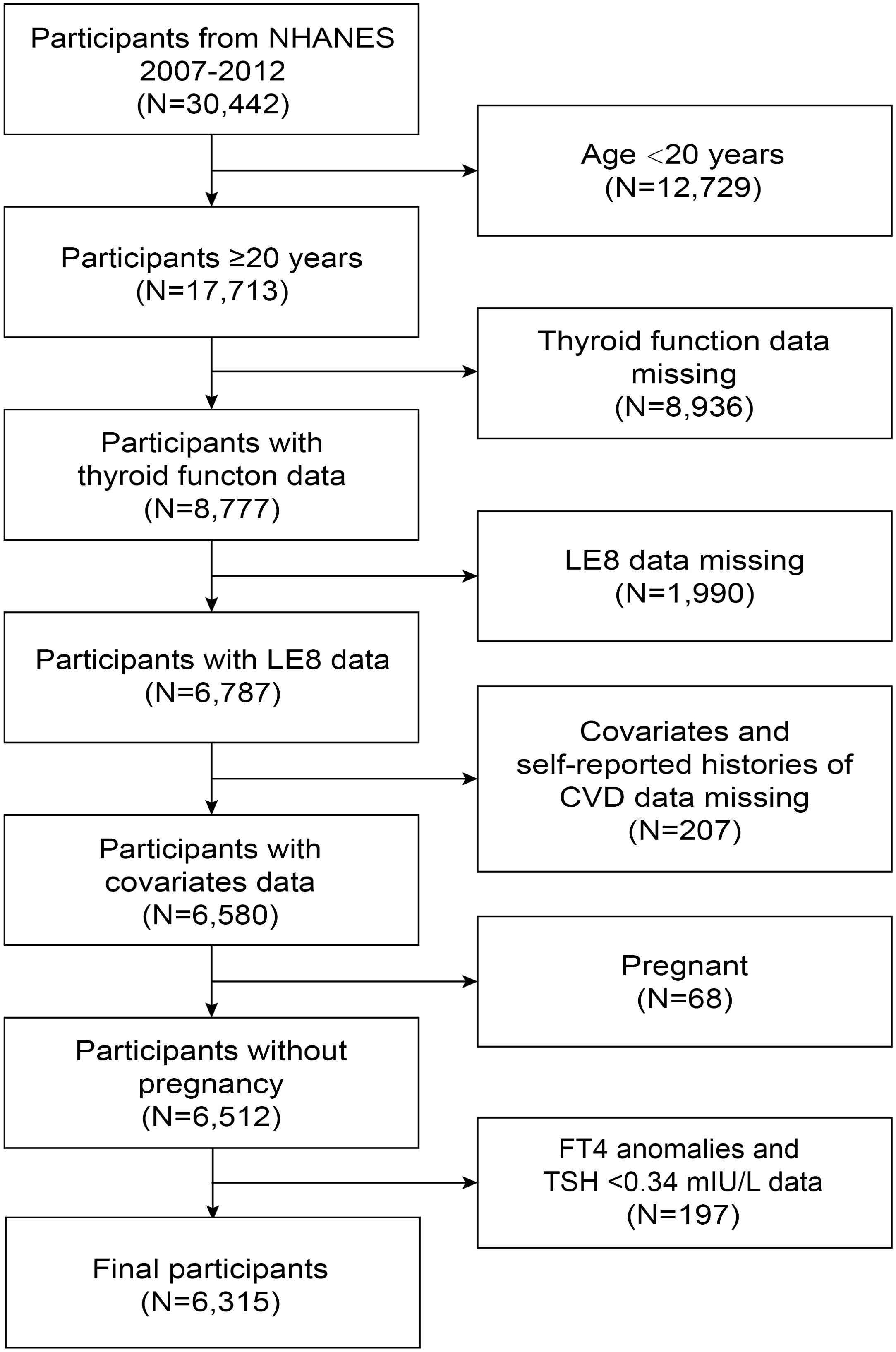
Figure 1. Flowchart of the participant selection from NHANES 2007-2012. NHANES, National Health and Nutrition Examination Survey; FT4, free thyroxine; TSH, thyroid-stimulating hormone; LE8, Life’s Essential 8; CVD, cardiovascular disease.
2.2 Definition and measurement of low thyroid function
The thyroid function parameters investigated in this study contained FT4 and TSH. FT4 was determined by a two-step enzyme immunoassay standardized to a range of 0.6-1.6 ng/dL, while TSH was assayed utilizing a 3rd generation, two-site immunoenzymatic “sandwich” assay standardized to a value of 0.34-5.6 mIU/L. Strict-normal thyroid function was recognized to be a TSH level of 0.34-2.5 mIU/L and a regular FT4 level. Low thyroid function was viewed to be a TSH level over 2.5 mIU/L and a regular FT4 level, including both low-normal thyroid function and SCH (13).
2.3 Measurement of LE8
The LE8 score is made up of 4 health behaviors (diet, PA, nicotine exposure, and sleep health) and 4 health factors (BMI, blood lipids, BG, and BP). The elaboration on the computation of the LE8 score utilizing NHANES data was described in Supplementary Table 1 (9, 14, 15). The Healthy Eating Index-2015 (HEI-2015) was employed for the assessment of dietary metrics, which was computed utilizing data from two 24-hour dietary recall interviews (16). Supplementary Table 2 outlined the elements and grading criteria for the HEI-2015 (17). Information on the other components of the LE8 came from self-report questionnaires, medical checkups, and gathered blood samples. Each LE8 metric was awarded points from 0 to 100. The overall LE8 score was determined by the unweighted average of the 8 indicators. The AHA proposed quantifying CVH depending on the LE8 score, with 80-100, 50-79, and 0-49 representing high, moderate, and low CVH, respectively (9).
2.4 Study covariates
Potential confounders associated with Life’s Essential 8 and low thyroid function were integrated into the final statistical analyses following previous studies (18, 19). The covariates comprised age, sex, race/ethnicity, education level, marital status, and urine iodine concentration (UIC). For these, age was categorized as <65 years and ≥65 years. Race/ethnicity was stratified into non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic, and Other races. Education level was divided into <high school, high school, and ≥high school. Marital status was separated into three types: married/living with a partner, divorced/separated/widowed, and never married. UIC was dichotomized into <100 ug/L, 100-300 ug/L, and ≥300 ug/L. In addition, this investigation demonstrated the prevalence of diabetes and hypertension among the participants. Diabetes was described as a self-reported diagnosis of diabetes, use of insulin or diabetes medications, glycosylated hemoglobin ≥6.5%, fasting blood glucose ≥126 mg/dL, or two-hour postprandial blood glucose ≥200 mg/dL from an oral glucose tolerance test (20). Also, hypertension was identified as a self-reported diagnosis of hypertension, use of hypertensive medications, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg (21).
2.5 Statistical analysis
The statistical analyses were undertaken using R (version 4.2.3) and EmpowerStats (version 2.0). Participants were organized into two groups according to their TSH status. We used Student’s t-tests for continuous variables that conformed to a normal distribution, nonparametric tests for continuous variables that were not normally distributed, and chi-square tests to characterize baseline characteristics for categorical variables, which were expressed as mean ± standard deviation (SD) or median (interquartile range) for continuous variables, and frequency (percentage) for categorical variables. Multivariable logistic regressions were used to explore the correlation between LE8 and its components with low thyroid function by aligning latent confounders. To ensure that the model does not suffer from the problem of multicollinearity, we included covariates with variance inflation factor (VIF) < 5 in the models. To conduct additional research on the correlation between LE8 and low thyroid function among various cohorts, stratified analyses were performed. Furthermore, we eliminated those who had self-reported histories of CVDs (comprising congestive heart failure, coronary heart disease, angina, and heart attack, N=508) to evaluate the reliability of the outcomes. Then, smoothed curve fitting and threshold effect analyses were carried out. A P-value <0.05 was determined to be statistically significant.
3 Results
3.1 Baseline characteristics of participants
There were 6,315 adults recruited for this research. Table 1 summarizes the baseline characteristics of those individuals categorized as to the thyroid function. The mean ± SD age was 50.23 ± 17.65 years, of which 3,162 (50.07%) participants were females. The mean ± SD LE8 score was 64.80 ± 14.94. The number of participants with low, moderate, and high CVH was 1,031 (16.33%), 4,204 (66.57%), and 1,080 (17.10%), respectively. 1,375 (21.77%) participants were considered to have low thyroid function, much more probably as older, non-Hispanic white, married/living with a partner, with a higher UIC, and in greater prevalence of histories of diabetes, hypertension, and CVDs. Compared to those with low thyroid function, participants with strict-normal thyroid function scored higher on LE8, PA, BMI, blood lipids, BG, and BP.
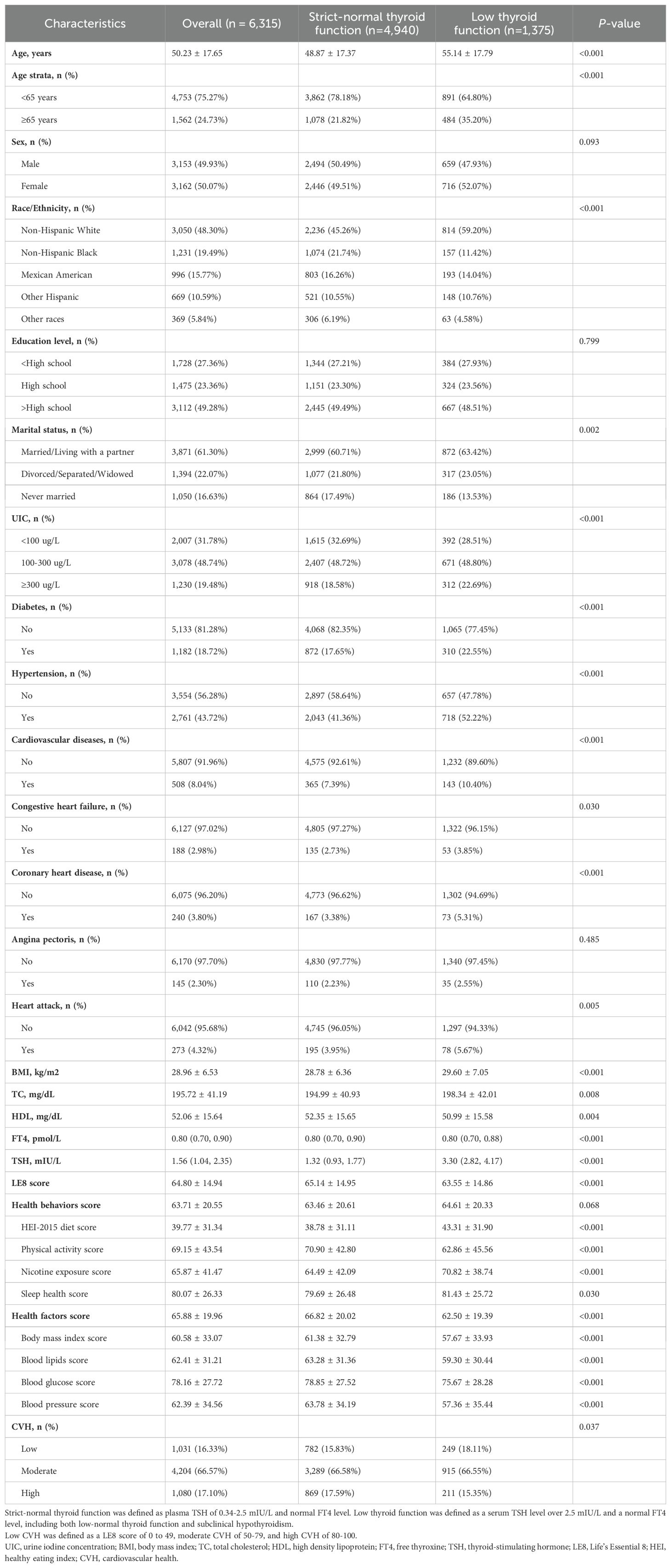
Table 1. Baseline characteristics of the NHANES 2007–2012 study participants (n=6,315) based on TSH status.
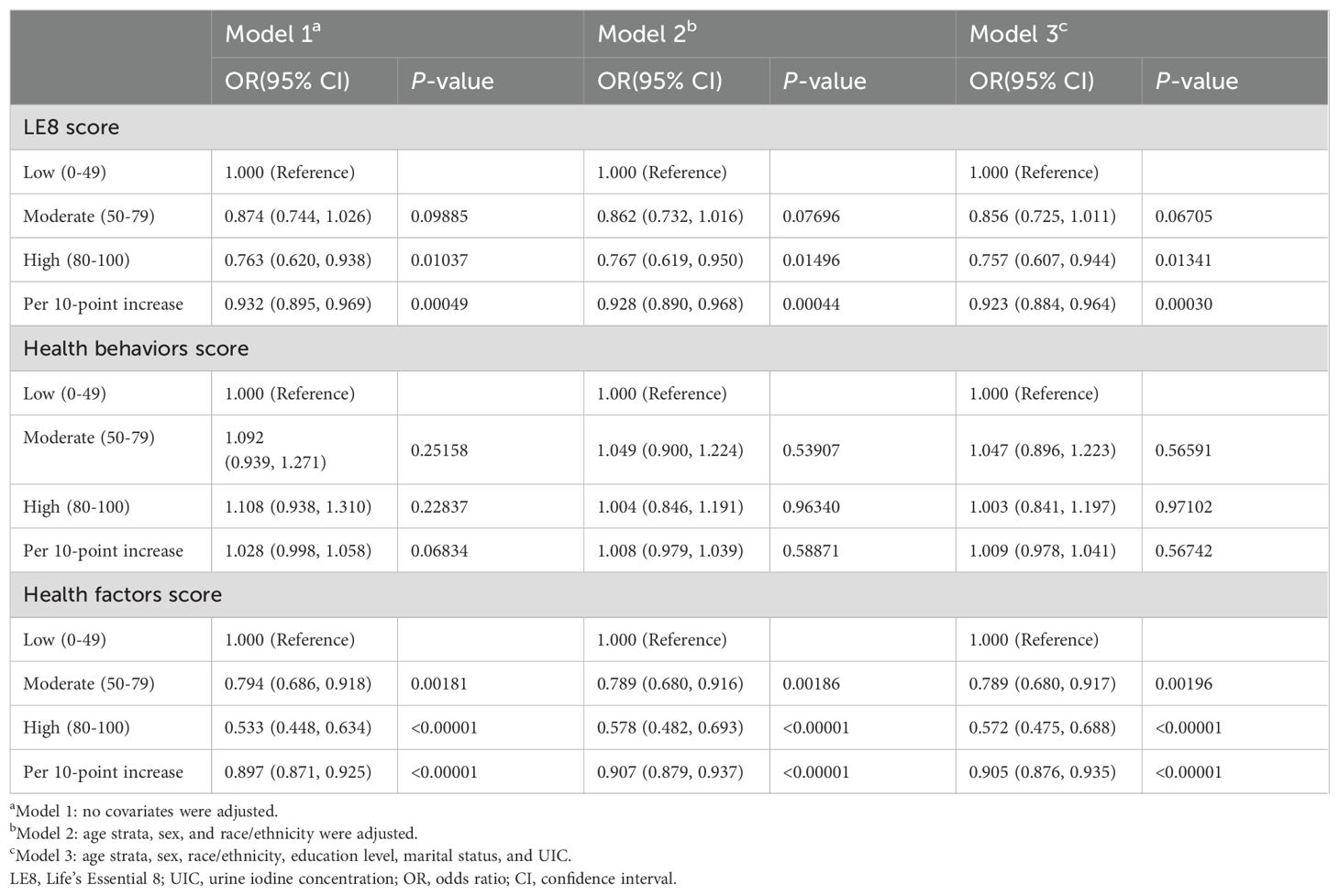
3.2 Association between LE8 and its components with low thyroid function
Table 2 demonstrated the findings of the multivariate regression analyses between the LE8 score and low thyroid function. The completely adjusted model suggested that the high CVH group (OR: 0.757, 95%CI: 0.607-0.944) had a considerably lower probability of low thyroid function in comparison with the low. For every 10-point increase in LE8 score, the risk of developing low thyroid function was reduced by 7.7% (OR: 0.923, 95%CI: 0.884-0.964). However, the health behaviors score did not substantially correlate with low thyroid function in multivariate regression analysis (P>0.05). As for the health factors score, fully adjusted models revealed a significantly lower risk of developing low thyroid function in the moderate (OR: 0.789, 95%CI: 0.680-0.917) and the high (OR: 0.572, 95%CI: 0.475-0.688) health factors groups in comparison to the low. For every 10-point increase in the health factor score, the risk of developing low thyroid function was reduced by 9.5% (OR: 0.905, 95% CI: 0.876-0.935).
The nonlinear associations between LE8, health behaviors, and health factors with low thyroid function were presented in Figure 2. The results showed a linear negative relationship between LE8 and health factors and low thyroid function, and there was no significant correlation between health behaviors and low thyroid function.
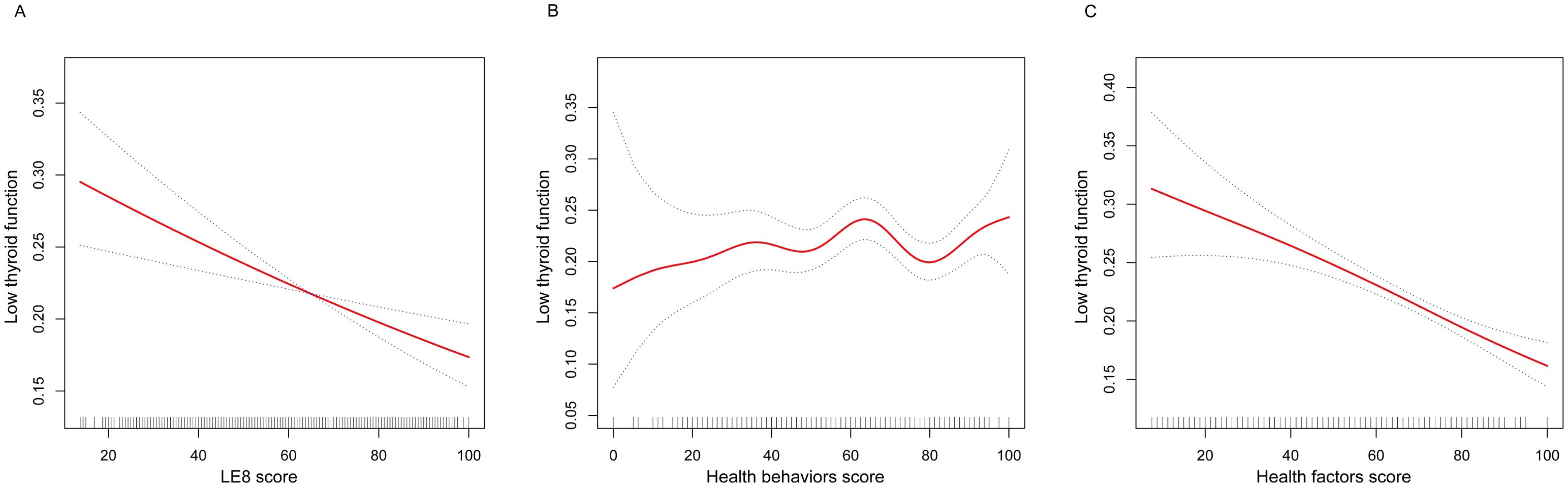
Figure 2. The nonlinear associations between LE8 and its subscales with low thyroid function. (A) The nonlinear associations between LE8 score and low thyroid function. (B) The nonlinear associations between Health behaviors score and low thyroid function. (C) The nonlinear associations between Health factors score and low thyroid function. LE8, Life’s Essential 8.
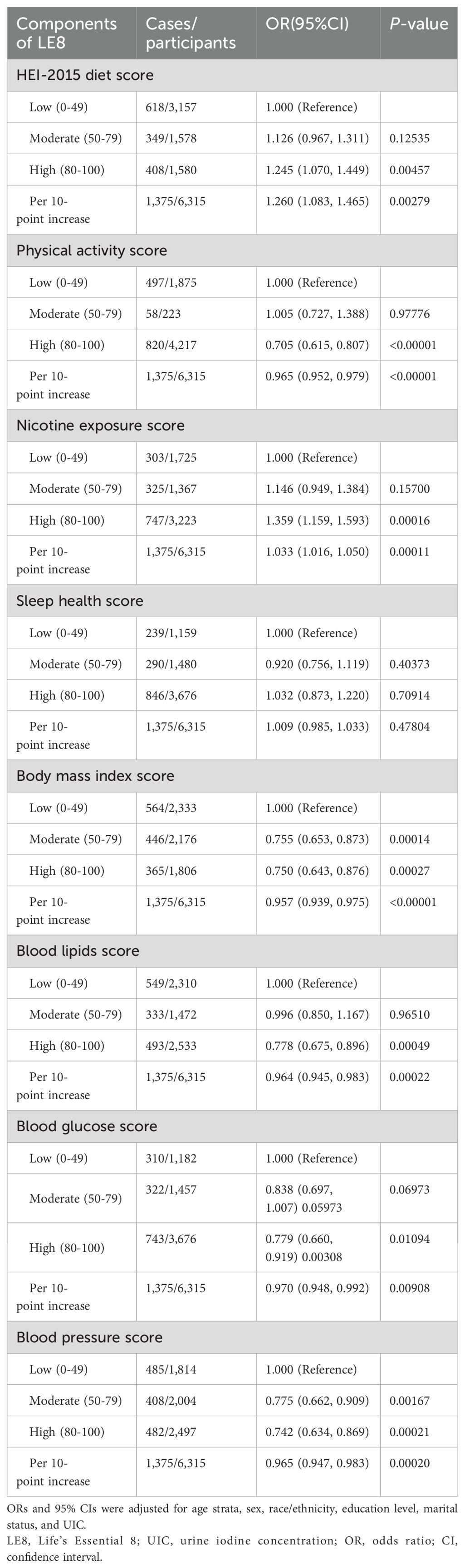
Moreover, the outcomes of the multivariate regression analyses between components of LE8 and low thyroid function were displayed in Table 3. Fully adjusted models suggested that among the LE8 components, elevated scores in PA, BMI, blood lipid, BG, and BP might have favorable effects on diminishing the incidence of low thyroid function.
3.3 Subgroup and sensitivity analysis
The outcomes of the subgroup analyses in Figure 3 illustrated that LE8 was negatively linked to low thyroid function in various subpopulations, which was in agreement with the preliminary findings. Among them, the connection between LE8 and low thyroid function categorized by UIC was displayed in Figure 4. In the UIC<100 ug/L group, an L-shaped curve with an inflection point of 41.25 could be obtained after threshold calculation (Supplementary Table 3). When the LE8 score was below the inflection point, a higher LE8 score manifested a significant correlation with a lower risk of low thyroid function (P<0.05), and were not significant when LE8 scores were above the inflection point. Additionally, in the 100 ≤ UIC < 300 ug/L group, an L-shaped curve with an inflection point of 60 was obtained after threshold calculation (Supplementary Table 3). When the LE8 score was higher than the inflection point, a higher LE8 score performed a significant connection with a lower risk of low thyroid function (P<0.05), and were not significant when LE8 scores were below the inflection point. While in the 300 ug/L≤ UIC group, a curve with an inflection point of 38.13 was obtained after calculating the threshold (Supplementary Table 3). When the LE8 score was higher than the inflection point, a higher LE8 score performed a significant connection with a lower risk of low thyroid function (P<0.05).
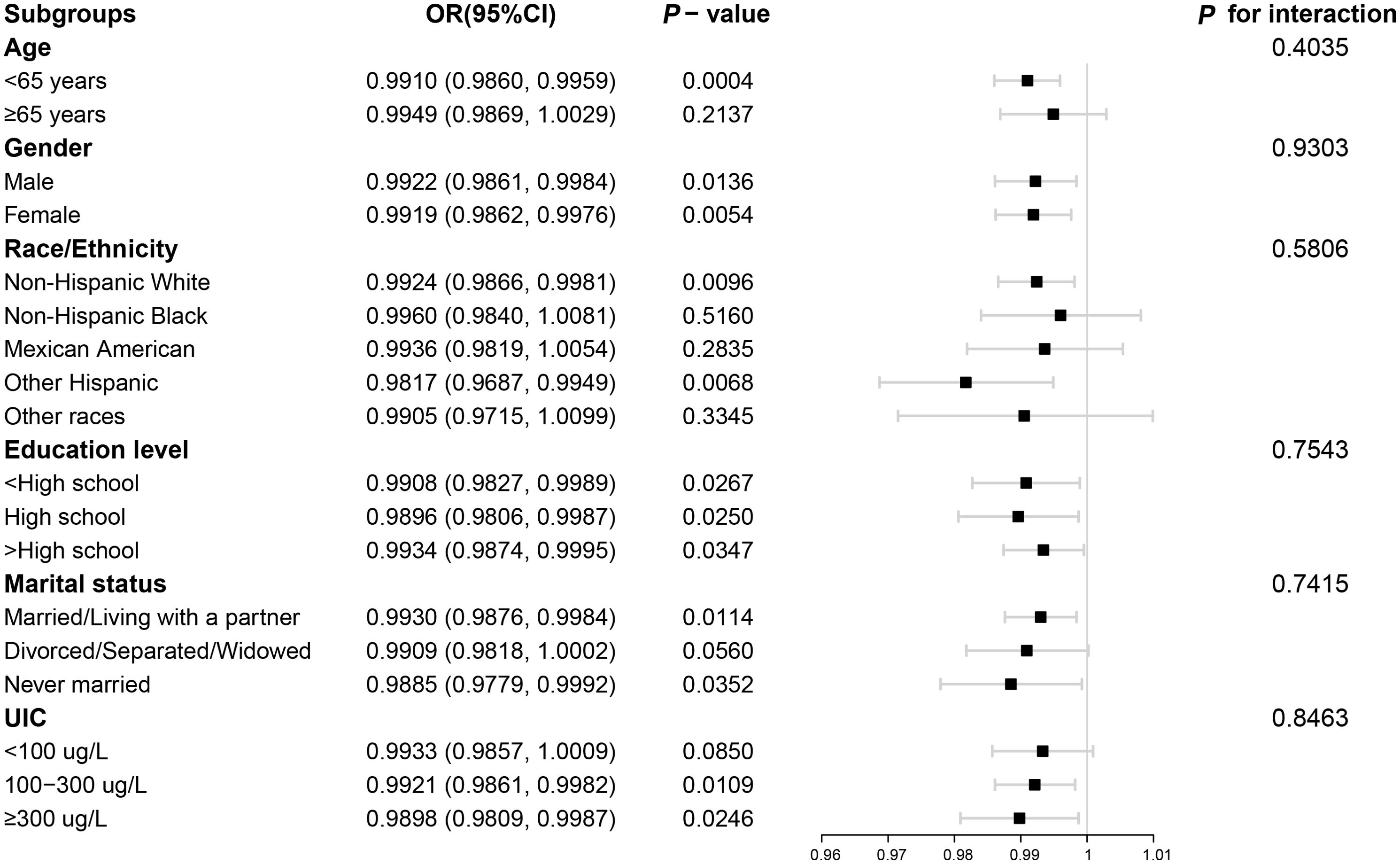
Figure 3. Subgroup analysis for the association between LE8 and low thyroid function. LE8, Life’s Essential 8; UIC, urine iodine concentration.
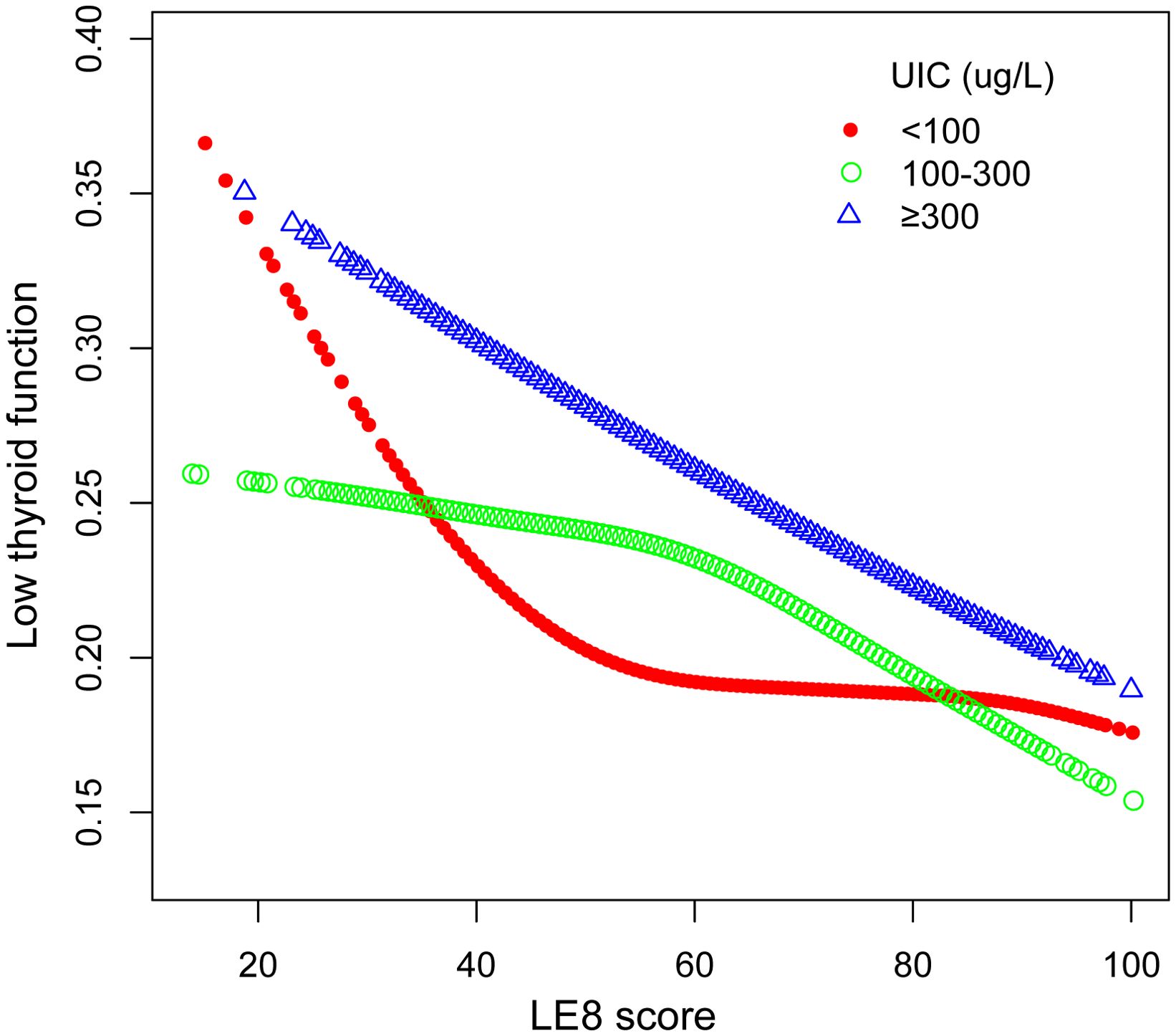
Figure 4. The association between LE8 and low thyroid function stratified by UIC. LE8, Life’s Essential 8; UIC, urine iodine concentration.
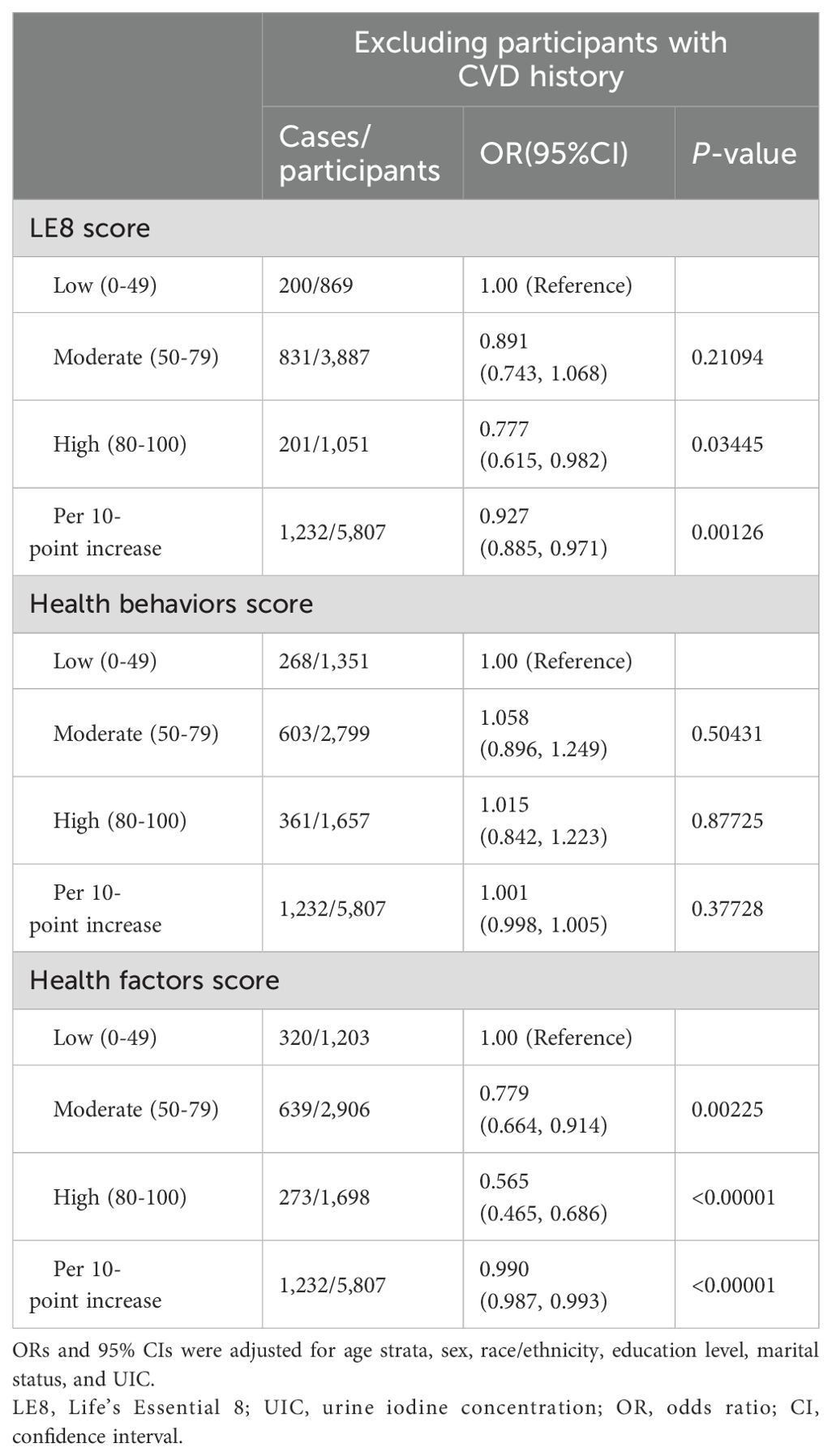
Finally, the results remained robust after removing those who had histories of CVDs in the sensitivity analyses (Table 4).
4 Discussion
This nationwide investigation discovered that the higher LE8 score and the health factors score were related to a reduced hazard of low thyroid function in US adults. Meanwhile, higher scores in PA, BMI, blood lipid, BG, and BP would probably be favorable in terms of a lower incidence of low thyroid function. Subgroup and sensitivity analyses illustrated that the negative relationships were robust overall.
To our knowledge, our research investigated the connection between CVH, which was quantified by the LE8 score, and low thyroid function for the first time. A couple of earlier studies have already discussed the involvement between CVD and low thyroid function. Kosuke et al. discovered that CVDs acted as a mediator connecting SCH and high-normal TSH levels with all-cause mortality among US adults, especially in women and the elderly, suggesting possible threats to the health of patients even with only mildly elevated TSH levels (22). A study anchored in NHANES demonstrated that low thyroid function was related to nonalcoholic fatty liver disease (NAFLD) and was also a separate hazard indicator for heightened all-cause and cardiovascular mortalities in persons suffering from NAFLD (13). Yu-ling et al. also reported a similar negative Impact of low thyroid function on all-cause and cardiovascular deaths among metabolic dysfunction-associated fatty liver disease (MAFLD) populations, emphasizing the significance of reassessing the TSH reference range (23). Anna et al. discovered that among 744 women with normal thyroid function, TSH values at the upper end of the recommended range were linked to worse cardiometabolic profiles, as evidenced by a larger waist circumference, elevated values of BP, total cholesterol (TC), triglycerides (TG), and BG, and decreased values of high-density lipoprotein (HDL-C) (24). A cohort study of diabetic individuals from the US reviewed that high-normal TSH levels were linked to a rise in CVD mortality (25). Another proof gathered through a multi-cohort Mendelian randomization and metabolomics analyses revealed that TSH at the upper limit of the standard range led to poor blood lipid profiles and a higher incidence of CVDs (26).
The current research presented further evidence supporting the relationship between CVH and low thyroid function utilizing an updated CVH evaluation index. We observed that the LE8 score and the health factors score had a negative correlation with low thyroid function, confirming the previous reports. Also, intensive management of PA, BMI, blood lipids, BG, and BP is of vital importance in controlling the incidence of low thyroid function. An investigation noted that women with SCH had a remarkable reduction in PA duration, steps taken, grip and quadriceps strength, and functional motor ability in comparison to the healthy control subjects (27). The result of a Mendelian randomization revealed that obesity was one of the risk factors for hypothyroidism, and individuals with higher BMI had a heightened threat of developing hypothyroidism (28). Another meta-analysis concluded that SCH appeared to be connected to an elevated hazard of metabolic syndrome components such as obesity, hypertension, high TG values, and low HDL-C values (29). Besides, a prospective study of 72,003 participants found that after controlling for risk factors, individuals with high-normal TSH values experienced a 15% greater chance of developing prediabetes (30). In addition, the consequences of our subgroup analyses revealed that the negative correlation between the LE8 score and low thyroid function was more pronounced in women under 65 years of age and unmarried participants, suggesting that enhanced monitoring of CVD and thyroid function in such populations might have long-term benefits. Moreover, for the population with UIC in the normal range (100 ≤ UIC < 300 ug/L), the OR correlated between the LE8 score and low thyroid function declined smoothly in the lower part of the score and sharply in the higher part, which implied that more stringent CVH criteria were likely to be desirable for the generalized population. It was worth pointing out that in this study, we defined the TSH normal range more strictly, and the findings also showed that appropriately decreasing the maximum limit of the TSH benchmark range would be advantageous for identifying individuals at an increased potential risk of CVDs.
It is hypothesized that there are a couple of potential explanations for the relationship between CVD and low thyroid function. Higher levels of TSH are related to many CVD risk elements such as dyslipidemia, hypertension, diminished myocardial systolic and diastolic function, endothelial dysfunction, insulin resistance, etc. (2, 6). TSH can regulate lipid metabolism by combining with specific TSH receptors on the surface of hepatocytes and adipocytes, which primarily leads to raised levels of proprotein convertase subtilisin/kexin type 9 (PCSK9), liver 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-COA) reductase (HMGCR), and hormone-sensitive lipase (HSL), as well as reduced levels of cholesterol 7α-hydroxylase (CYP7A1), inducing cholesterol accumulation and inhibiting clearance (31). Meanwhile, low thyroid function can result in decreased cardiac systolic and diastolic function by modulating the expression of contractile proteins and calcium uptake in cardiomyocytes (3). Furthermore, higher levels of TSH are also likely to promote endothelial dysfunction by augmenting endothelin (ET-1) levels and decreasing nitric oxide (NO) levels, which can contribute to atherosclerosis, increased peripheral vascular resistance, and raised blood pressure (32, 33). What’s more, it has been described that low thyroid function can bring about obvious insulin resistance, and the latter interacts with hyperhomocysteinemia (HHcy), which may provoke vascular endothelial damage and interfere with the process of atherosclerosis and CVD by increasing oxidative stress, stimulating endoplasmic reticulum stress, affecting epigenetic modifications, and altering protein function (34, 35). The mentioned mechanisms provide partially convincing evidence for our study, while more definite principles are expected to be further explored in depth.
Our current investigation has certain limitations. Firstly, even after controlling for a few possible confounders, the cross-sectional character of the research prevented us from concluding a causal and longitudinal association between LE8 and low thyroid function, and we could not completely discard the risk of bias caused by other confounders. Therefore, prospective research with larger sample sizes and longitudinal measurements is warranted to validate our findings. Secondly, the evaluations of certain indicators in LE8 were questionnaire-based, which were susceptible to recall bias. What’s more, as the complex effects of childhood and pregnancy on thyroid function have not yet been validly determined, we excluded underage and pregnant participants, and further explorations for these populations are called for in the future. In addition, the active form of thyroid, triiodothyronine, was not included in our study, and its levels may have important implications for CVH. We plan to explore more comprehensive indicators of thyroid function, including triiodothyronine, in future studies. Nonetheless, our study has several strengths. We employed a sufficient amount of a nationally representative sample to make our results more generalizable. Subgroup and sensitivity analyses were also carried out to augment the reliability of our outcomes.
5 Conclusion
In conclusion, the LE8 score and health factors score were nonlinearly and negatively correlated with the prevalence of low thyroid function. Also, high scores in PA, BMI, blood lipids, BG, and BP assessments should emphasized in the LE8 components. This study suggested that elevated TSH levels might have an implication on CVH even with normal thyroid function. Therefore, the significance of reassessing the TSH reference range should be emphasized. LE8, which is a clinically feasible and comprehensive indicator for improving CVH, may help patients identify the risk of low thyroid function at an early stage and play a potentially meaningful role in the promotion of thyroid health.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XF: Conceptualization, Data curation, Methodology, Software, Writing – original draft. RH: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – original draft. SF: Methodology, Supervision, Writing – original draft. ZD: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. JZ: Resources, Supervision, Validation, Writing – review & editing. JS: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the "Dual Chain Integration" project of Shaanxi Provincial Administration of Traditional Chinese Medicine (Grant No. 2022-SLRH-LG-005), the Key Research and Development Program of Shaanxi Provincial Science and Technology Department (Grant No. 2023-ZDLSF-56), and the project of Shaanxi Provincial Administration of Traditional Chinese Medicine (Grant No. SZY-KJCYC-2023-074), and Shaanxi Province Natural Science Basic Research Program (Grant No. 2024JC-YBMS-676).
Acknowledgments
We would like to thank all members who contributed to this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1437386/full#supplementary-material
References
1. Kim D, Yoo ER, Li AA, Fernandes CT, Tighe SP, Cholankeril G, et al. Low-normal thyroid function is associated with advanced fibrosis among adults in the United States. Clin Gastroenterol Hepatol. (2019) 17:2379–81. doi: 10.1016/j.cgh.2018.11.024
2. Paschou SA, Bletsa E, Stampouloglou PK, Tsigkou V, Valatsou A, Stefanaki K, et al. Thyroid disorders and cardiovascular manifestations: an update. Endocrine. (2022) 75:672–83. doi: 10.1007/s12020-022-02982-4
3. Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. (2018) 71:1781–96. doi: 10.1016/j.jacc.2018.02.045
4. Jabbar A, Pingitore A, Pearce SHS, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. (2017) 14:39–55. doi: 10.1038/nrcardio.2016.174
5. van Tienhoven-Wind LJN, Dullaart RPF. Low-normal thyroid function and the pathogenesis of common cardio-metabolic disorders. Eur J Clin Invest. (2015) 45:494–503. doi: 10.1111/eci.12423
6. Manolis AA, Manolis TA, Melita H, Manolis AS. Subclinical thyroid dysfunction and cardiovascular consequences: an alarming wake-up call? Trends Cardiovasc Med. (2020) 30:57–69. doi: 10.1016/j.tcm.2019.02.011
7. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: A report from the American heart association. Circulation. (2023) 147:e93–e621. doi: 10.1161/CIR.0000000000001123
8. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association’s strategic impact goal through 2020 and beyond. Circulation. (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
9. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the American heart association’s construct of cardiovascular health: A presidential advisory from the American heart association. Circulation. (2022) 146:e18–43. doi: 10.1161/CIR.0000000000001078
10. Zhang Y, Ning N, Fan X, Huang R, Ye Y, He Y, et al. Age-dependent interaction between life’s essential 8 and chronic kidney disease: A national cross-sectional analysis. Prev Med. (2023) 177:107763. doi: 10.1016/j.ypmed.2023.107763
11. Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, et al. Association of the American heart association’s new “Life’s essential 8” with all-cause and cardiovascular disease-specific mortality: prospective cohort study. BMC Med. (2023) 21:116. doi: 10.1186/s12916-023-02824-8
12. Cai Z, Liu Z, Zhang Y, Ma H, Li R, Guo S, et al. Associations between life’s essential 8 and abdominal aortic calcification among middle-aged and elderly populations. J Am Heart Assoc. (2023) 12:e031146. doi: 10.1161/JAHA.123.031146
13. Kim D, Vazquez-Montesino LM, Escober JA, Fernandes CT, Cholankeril G, Loomba R, et al. Low thyroid function in nonalcoholic fatty liver disease is an independent predictor of all-cause and cardiovascular mortality. Am J Gastroenterol. (2020) 115:1496–504. doi: 10.14309/ajg.0000000000000654
14. Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, et al. Status of cardiovascular health in us adults and children using the American heart association’s new “Life’s essential 8” Metrics: prevalence estimates from the national health and nutrition examination survey (Nhanes), 2013 through 2018. Circulation. (2022) 146:822–35. doi: 10.1161/CIRCULATIONAHA.122.060911
15. Ren Y, Cai Z, Guo C, Zhang Y, Xu H, Liu L, et al. Associations between life’s essential 8 and chronic kidney disease. J Am Heart Assoc. (2023) 12:e030564. doi: 10.1161/JAHA.123.030564
16. Zhan JJ, Hodge RA, Dunlop AL, Lee MM, Bui L, Liang D, et al. Dietaryindex: A user-friendly and versatile R package for standardizing dietary pattern analysis in epidemiological and clinical studies. bioRxiv. (2023). doi: 10.1101/2023.08.07.548466
17. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: hei-2015. J Acad Nutr Diet. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
18. Zhao G, Wang Z, Ji J, Cui R. Effect of coffee consumption on thyroid function: nhanes 2007-2012 and mendelian randomization. Front Endocrinol (Lausanne). (2023) 14:1188547. doi: 10.3389/fendo.2023.1188547
19. Wang M, Lu X, Zheng X, Xu C, Liu J. The relationship between sleep duration and thyroid function in the adult us population: nhanes 2007-2012. PloS One. (2023) 18:e0291799. doi: 10.1371/journal.pone.0291799
20. Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from nhanes. Diabetes Care. (2022) 45:1453–61. doi: 10.2337/dc21-2371
21. Cao Y, Li P, Zhang Y, Qiu M, Li J, Ma S, et al. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: results from nhanes. Front Immunol. (2023) 14:1087345. doi: 10.3389/fimmu.2023.1087345
22. Inoue K, Ritz B, Brent GA, Ebrahimi R, Rhee CM, Leung AM. Association of subclinical hypothyroidism and cardiovascular disease with mortality. JAMA Netw Open. (2020) 3:e1920745. doi: 10.1001/jamanetworkopen.2019.20745
23. Chen Y-L, Tian S, Wu J, Li H, Li S, Xu Z, et al. Impact of thyroid function on the prevalence and mortality of metabolic dysfunction-associated fatty liver disease. J Clin Endocrinol Metab. (2023) 108:e434–e43. doi: 10.1210/clinem/dgad016
24. Boggio A, Muzio F, Fiscella M, Sommariva D, Branchi A. Is thyroid-stimulating hormone within the normal reference range a risk factor for atherosclerosis in women? Intern Emerg Med. (2014) 9:51–7. doi: 10.1007/s11739-011-0743-z
25. Zhu P, Lao G, Chen C, Luo L, Gu J, Ran J. Tsh Levels within the Normal Range and Risk of Cardiovascular and All-Cause Mortality among Individuals with Diabetes. Cardiovasc Diabetol. (2022) 21:254. doi: 10.1186/s12933-022-01698-z
26. van Vliet NA, Bos MM, Thesing CS, Chaker L, Pietzner M, Houtman E, et al. Higher thyrotropin leads to unfavorable lipid profile and somewhat higher cardiovascular disease risk: evidence from multi-cohort Mendelian randomization and metabolomic profiling. BMC Med. (2021) 19:266. doi: 10.1186/s12916-021-02130-1
27. Tanriverdi A, Ozcan Kahraman B, Ozsoy I, Bayraktar F, Ozgen Saydam B, Acar S, et al. Physical activity in women with subclinical hypothyroidism. J Endocrinol Invest. (2019) 42:779–85. doi: 10.1007/s40618-018-0981-2
28. Qiu Y, Liu Q, Luo Y, Chen J, Zheng Q, Xie Y, et al. Causal association between obesity and hypothyroidism: A two-sample bidirectional Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1287463. doi: 10.3389/fendo.2023.1287463
29. Ding X, Zhao Y, Zhu C-Y, Wu L-P, Wang Y, Peng Z-Y, et al. The association between subclinical hypothyroidism and metabolic syndrome: an update meta-analysis of observational studies. Endocr J. (2021) 68:1043–56. doi: 10.1507/endocrj.EJ20-0796
30. Chang CH, Yeh YC, Shih SR, Lin JW, Chuang LM, Caffrey JL, et al. Association between thyroid dysfunction and dysglycaemia: A prospective cohort study. Diabetes Med. (2017) 34:1584–90. doi: 10.1111/dme.13420
31. Liu H, Peng D. Update on dyslipidemia in hypothyroidism: the mechanism of dyslipidemia in hypothyroidism. Endocr Connect. (2022) 11:e210002. doi: 10.1530/EC-21-0002
32. Gong N, Gao C, Chen X, Wang Y, Tian L. Adipokine expression and endothelial function in subclinical hypothyroidism rats. Endocr Connect. (2018) 7:295–304. doi: 10.1530/EC-18-0007
33. Chrysant SG. The current debate over treatment of subclinical hypothyroidism to prevent cardiovascular complications. Int J Clin Pract. (2020) 74:e13499. doi: 10.1111/ijcp.13499
34. Ebrahimpour A, Vaghari-Tabari M, Qujeq D, Moein S, Moazezi Z. Direct correlation between serum homocysteine level and insulin resistance index in patients with subclinical hypothyroidism: does subclinical hypothyroidism increase the risk of diabetes and cardio vascular disease together? Diabetes Metab Syndr. (2018) 12:863–7. doi: 10.1016/j.dsx.2018.05.002
Keywords: low thyroid function, cardiovascular health, life’s essential 8, NHANES, cross-sectional study
Citation: Fang X, Hu R, Fei S, Ding Z, Zhao J and Shang J (2024) Associations between cardiovascular health and low thyroid function among US adults: a population-based study. Front. Endocrinol. 15:1437386. doi: 10.3389/fendo.2024.1437386
Received: 28 May 2024; Accepted: 11 September 2024;
Published: 27 September 2024.
Edited by:
Joseph V. Martin, Rutgers University Camden, United StatesReviewed by:
Cristina Martín-Arriscado Arroba, Fundación Investigación Biomédica Hospital 12 de Octubre, SpainAnthony Martin Gerdes, New York Institute of Technology, United States
Copyright © 2024 Fang, Hu, Fei, Ding, Zhao and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianwei Shang, c2hhbmdqdzIwMjRAMTYzLmNvbQ==; Jiuli Zhao, MTM1MjA2Njk5MzJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaoxiao Fang
Xiaoxiao Fang Rui Hu3†
Rui Hu3†