- 1Department of Pediatrics, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 2Department of Child Healthcare, Shunde Women and Children’s Hospital, Guangdong Medical University, Foshan, China
Background: Metabolic disorders are common in individuals with Turner syndrome (TS). Hyperuricemia is associated with metabolic syndrome. This study investigated the serum uric acid (SUA) profile in patients with TS.
Methods: A retrospective observational study was conducted with 145 patients with TS. A total of 72 normal girls were in the control group from 2015 to 2024: 86 TS patients were treated with growth hormone (GH), 80 with stanozolol, and 52 with estrogen.
Results: Hyperuricemia was present in 33.1% (47/145) of patients with untreated TS and in 16.67% (12/72) of the controls (P < 0.001). Multivariable linear regression analysis showed that BMISDS, fasting serum glucose, and eGFR explained 34.4% (model R2 = 0.344) of the total variation in SUA in the untreated TS group. SUA and SUASDS (SUA standard deviation score) levels generally showed a slow rising tendency with age. SUA increased significantly in the first year of stanozolol initiation (P = 0.032), while adding estrogen and stanozolol improved the lipid profile during the whole assessment period.
Conclusion: Girls with TS showed a slow rising tendency in SUA and SUASDS with age and had higher SUA and SUASDS levels and incidence of hyperuricemia compared to their healthy female peers. The independent risk factors for hyperuricemia in pediatric patients with TS were BMISDS, HOMA-IR, glucose, and eGFR. The incidence of hyperuricemia increased in the first year of stanozolol treatment.
1 Introduction
Turner syndrome (TS) affects 25–50 per 100,000 female individuals, which involves multiple organs through all stages of life and is caused by a full or partial deletion of the second X chromosome. The primary problems during childhood and adolescence are short stature and premature ovarian failure, which require growth promotion and sex hormone replacement treatment. Patients with TS are also at an increased risk of obesity, dyslipidemia, metabolic syndrome, and impaired glucose tolerance (1, 2). At the same time, an elevated cardiovascular risk with an atherogenic lipid and carbohydrate profile and impaired endothelial function might occur in their youth (3), which could be mitigated with growth hormone (GH) therapy (4, 5).
Uric acid is the end-product of purine metabolism in humans, and serum uric acid (SUA) differs according to age and sex in childhood and adolescence (6, 7). Elevated serum uric acid level is associated with cardiovascular diseases such as hypertension, atrial fibrillation, chronic kidney disease, heart failure, coronary artery disease, and cardiovascular death (8). Many studies had also demonstrated an association between hyperuricemia and metabolic syndrome (9–11), and it has been well recognized by the 1970s (12) as a component of metabolic syndrome (13). In addition, male, obesity, diastolic blood pressure, and serum triglyceride concentrations were found to be associated with an increased risk of hyperuricemia in a Chinese population (14). As a condition associated with a high risk of renal and cardiovascular diseases and the special treatment of growth promotion and sex hormone replacement in patients with TS, SUA might differ from those of healthy peers. However, to our knowledge, the uric acid profile in individuals with TS was not recognized.
In this retrospective observational study, we analyzed the SUA profile in patients with TS before and after growth promotion and sex hormone replacement treatment.
2 Materials and methods
2.1 Patients
This study enrolled 145 girls (aged 2.75–22.00 years) with a definitively diagnosed TS based on karyotype and 72 prepubertal normal girls (aged 2.00–10.00 years) with a normal karyotype recruited from a tertiary hospital in China from 2015 to 2024. Patients with heart diseases, type 2 diabetes mellitus, resistant hypertension, or using drugs to lower urate were excluded, and those with abnormal thyroid function were all treated and were euthyroid during the study.
Growth promotion therapy with GH (0.33 mg/kg/week) at a mean age of 10.79 ± 3.42 years and an addition of stanozolol (ST, 20–35 μg/kg/day) when diagnosis occurred later than 10 years or older were implemented (15) in this study. Oral estradiol was applied for puberty induction according to guidelines (15) at a mean age of 16.27 ± 2.93 years, with the dose increasing over 2 to 3 years.
This study was approved by the First Affiliated Hospital, Sun Yat-sen University’s Institutional Ethics Committee.
2.2 Study design
In all patients, height and weight were measured by two doctors after training. Serum creatine (Scr), serum uric acid (SUA), serum uric acid standard deviation score (SUASDS) according to SUA reference values in normal Chinese children and adolescents (6, 7), fasting insulin (Ins, mIU/L), fasting glucose (Glu, mmol/L) and lipids (total cholesterol [TC], high-density cholesterol [HDL-c], low-density cholesterol [LDL-c], and triglycerides [TGs]), IGF-1 at the start of GH therapy, and regular evaluations were carried out during GH, stanozolol, and estrogen therapy or a combination of those treatments. The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated as fasting insulin × fasting glucose ÷ 22.5 (16). Scr was used in the calculation of the estimated glomerular filtration rate (eGFR) using the modified Schwartz equation (eGFR in mL/min/1.73 m2 = k × (height in cm/Scr mg/dL), where k = 0.413) (17). Hyperuricemia was defined as SUA higher than 360 µmol/L (6.0 mg/dL) (18–21). The control group was only evaluated once.
2.3 Statistical analysis
SPSS v27.0 and R software were used. Categorical variables were expressed as numbers and frequencies. For continuous variables, normally distributed data were presented as means ± SDs, and non-normally distributed data were shown as medians and interquartile ranges. χ2 test, unpaired Student’s t-test, and Mann–Whitney U-test were applied to determine differences among groups. Correlations were evaluated using Spearman’s or Pearson correlation analysis. By entering SUA as the dependent variable, the independent effects of risk markers on SUA were estimated with stepwise linear regression analysis. The most recent SUA level in untreated TS patients and those collected during different therapies were collected to study the SUA profiles in populations with TS during childhood and adolescence. A p-value <0.05 was considered statistically significant.
3 Results
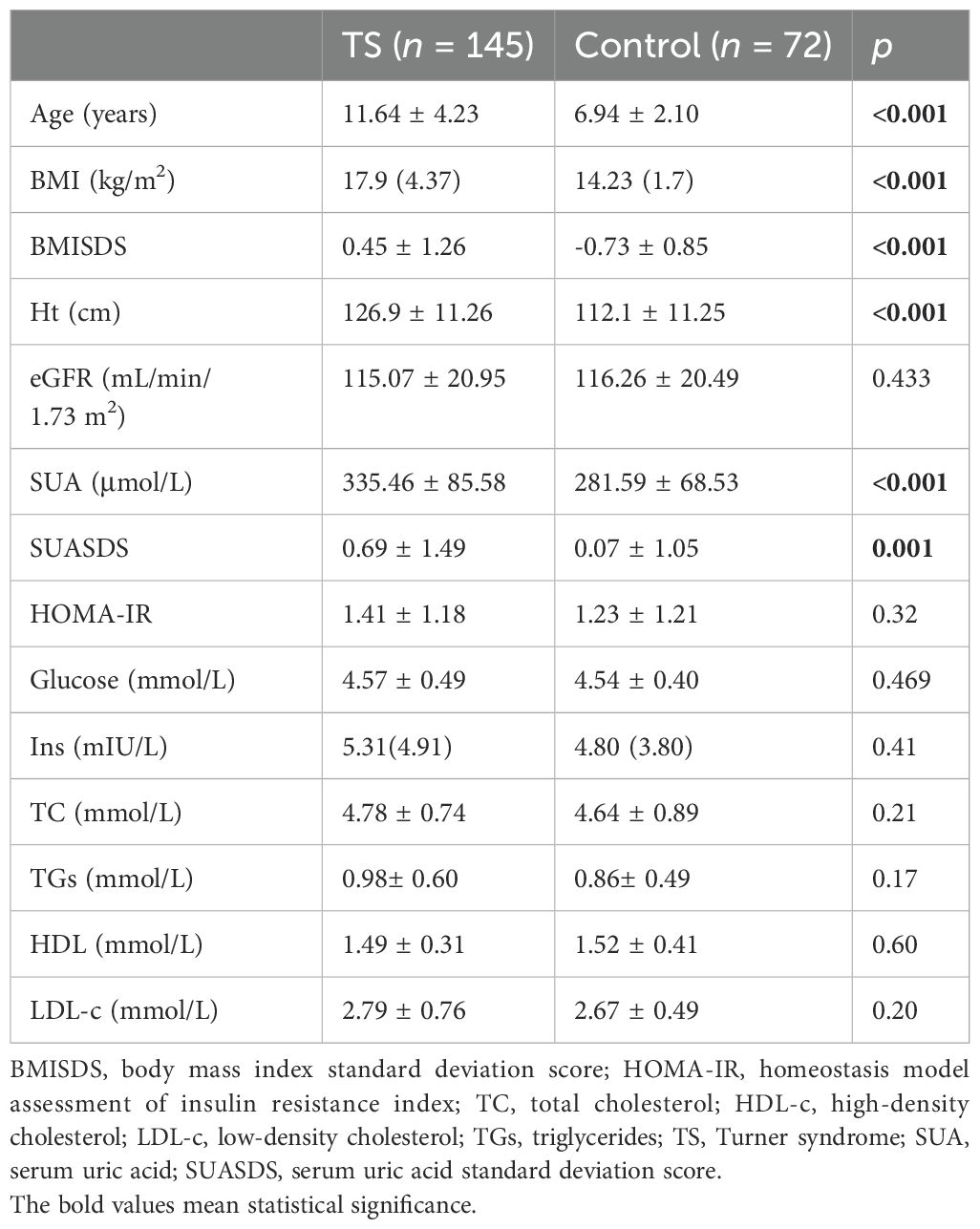
The karyotypes of 145 TS patients were as follows: monosomy (42.76%, 62/145, 45,X), mosaic (4.14%, 6/145, 45,XX/46,XX), variant (20.68%, 30/145, 15 with 46Xq10, five with 46,XiXq, one with 46,X,del(X)(p11), one with 46,X,del(X)(p12), one with 46,X,del(X)(p22.2q27), one with 46,XXp-, one with 45,Xi(X)q1021pstk+, one with 46,X,del(p11.4), one with 45,X,15pstsb+, one with 45Xpsuidic, one with 46,X,del(X)p11.2, 1 with 45,X,1qh), and mosaic with variant (32.41%, 47/145, 16 with 45,X/46Xq10, 10 with 45,X/47,XXX, eight with 46,Xr(X)/45,X, four with 45,X/46,X+mar, three with 46,Xder(X)(p11.2)/45X, one with 45,X/46,X-Xdel(X)(q22), one with 45X/46Xder(X)pter, one with 46,Xidic(X)(p22)/45X, one with 45,X/46,X,iv(X)(qwq.2q26), one with 45,X/46,Xadd(X)(q26), and one with 45,X/46,Xder(X)(q21)). Hyperuricemia was present in 33.1% (48/145) of patients with TS and in 16.67% (12/72) of the control group. The baseline characteristics of untreated TS children (all the included patients with TS had no breast development) and the control group (prepubertal normal girls) are shown in Table 1. A total of 26 patients of TS showed pubertal hair, while their SUA (335.33 ± 88.69 μmol/L vs. 341.24 ± 71.26 μmol/L, P = 0.376) and SUASDS (0.68 ± 1.51μmol/L vs. 0.57 ± 1.23 μmol/L, P = 0.375) was not significantly different with those of patients without pubertal hair growth. Renal abnormality was observed in 22 cases: 10 (41.67%) horseshoe kidney, three (12.5%) kidney duplication, two (8.33%) renal hypodysplasia, two (8.33%) hydronephrosis, one (4.17%) renal cysts, one (4.17%) ectopic kidney, two (8.33%) renal echogenicity enhancement, and one (4.17%) renal resistance index increase. We evaluated the eGFR, SUA, and SUASDS and classified them into group 1 (with renal morphological abnormality, n = 22) and group 2 (without, n = 123). The eGFR in group 1 was lower than that in group 2 (106.98 ± 17.70 mL/min/1.73 m2 vs. 115.70 ± 17.29 mL/min/1.73 m2, P = 0.015); SUA and SUASDS were shown to be not significantly different. No gout was diagnosed during the follow-up.
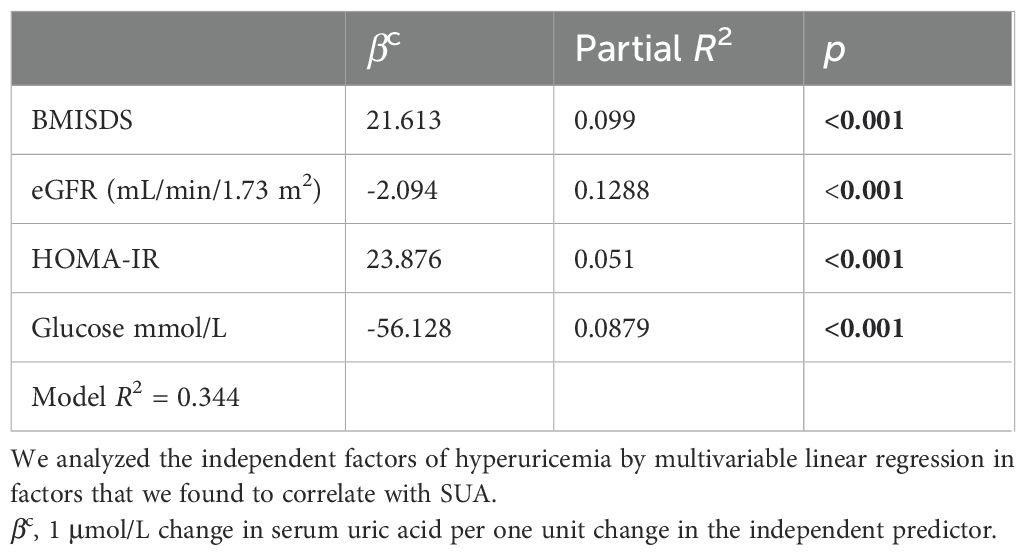
The risk factors for elevated SUA were analyzed. We found that BMI, BMISDS, eGFR, HOMA-IR, glucose, and TGs were associated with SUA (r = 0.34, 0.303, -0.319, -0.185, 0.202, -0.234, and 0.291; P < 0.001, <0.001, <0.001, = 0.024, = 0.029, and = 0.006, respectively) in the TS group, which was not found in the control group. After multicollinearity evaluation, BMI was not entered into the model. The multivariable correlates of SUA were BMISDS (9.94%), glucose (8.70%), HOMA-IR (5.10%), and eGFR (12.88%), which together explained 34.40% (model R2 = 0.344) of the total variation in SUA in the TS group (see Table 2).
SUA in the four groups of different karyotypes (monosomy group, mosaic group, variant group, and mosaic with variant group) showed no significant difference (F = 0.66, P = 0.578).
Age was not linearly associated with SUA in patients with untreated TS. For those untreated patients with TS, the SUA and SUASDS levels showed a slow rising tendency with age, while in the control group, they showed a flat curve within the limit age range (see Figure 1).
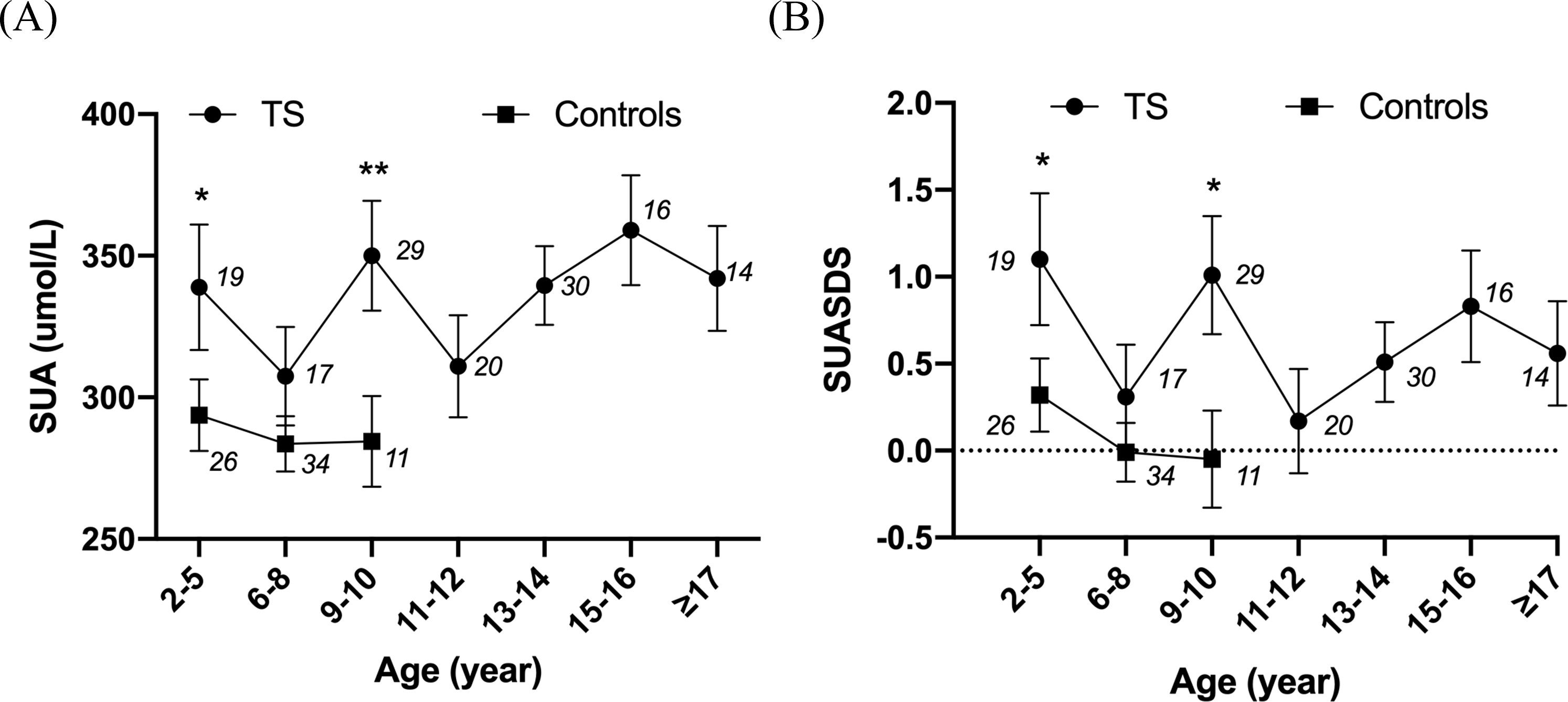
Figure 1. Line chart showing the relationship between SUA and age and SUASDS with age. *P<0.05, **P<0.01. The numbers in the figures are the number of cases in each age group. (A) Relationship of SUA level and age in patients with untreated TS and controls. (B) SUASDS level. 2–5 years: N = 19 (UA: 338.90 ± 96.62 μmol/L, SUASDS 1.10 ± 1.66), 6–8 years: N = 17 (UA: 307.48 ± 71.72 μmol/L, SUASDS 0.31 ± 1.20), 9 to 10 years: N = 29 (UA: 350.74 ± 106.56 μmol/L, SUASDS 1.01 ± 1.83), 11 to 12 years: N = 20 (UA: 311.05 ± 80.80 μmol/L, SUASDS 0.17 ± 0.35), 13 to 14 years: N = 30 (UA: 339.47 ± 79.01 μmol/L, SUASDS 0.51 ± 1.28), 15 to 16 years: N = 16 (UA: 359.00 ± 79.50 μmol/L, SUASDS 0.83 ± 1.26), ≥17 years: N = 14 (UA: 342.29 ± 69.54 μmol/L, SUASDS 0.56 ± 1.13) in patients with TS. 2–5 years: N = 26 (UA: 293.75 ± 64.28 μmol/L, SUASDS 0.32 ± 1.11), 6–8 years: N = 34 (UA: 283.56 ± 57.22 μmol/L, SUASDS -0.01 ± 1.00), 9 to 10 years: N = 11 (UA: 275.83 ± 60.72 μmol/L, SUASDS -0.22 ± 1.06), ≥11 years: N = 1 in controls. SUA and SUASDS levels were higher in patients with TS than those in controls at age of 2–5 years (P = 0.033, 0.033), 6–8 years (P = 0.101, 0.161), and 9 to 10 years (P = 0.003, 0.011).
As age affects SUA profiles, and we studied its changes with treatment of GH, stanozolol, or estrogen treatment after adjusting the patients’ age. No data from the empty control group was available due to the ethical reason of setting empty controls (GH treatment should start early) (22). We found that GH treatment (duration for the estrogen group and GH + estrogen group: 1.61 ± 0.79 vs. 1.16 ± 0.64 years, P = 0.11) did not affect SUA but decreased the insulin sensitivity (PHOMA = 0.02, PIns = 0.014). Adding stanozolol to GH treatment (duration for GH group and GH + stanozolol group: 2.10 ± 1.10 vs. 1.16 ± 0.64 years, P = 0.103) did not affect SUA but decreased the serum glucose level (PGlu = 0.024) and lipid profile (PTC = 0.001, PTGs = 0.032). Adding estrogen (duration for the GH group and GH + estrogen group: 1.66 ± 1.31 vs. 1.16 ± 0.64 years, P = 0.478) also improved TGs (PTGs = 0.005) (see Table 3).
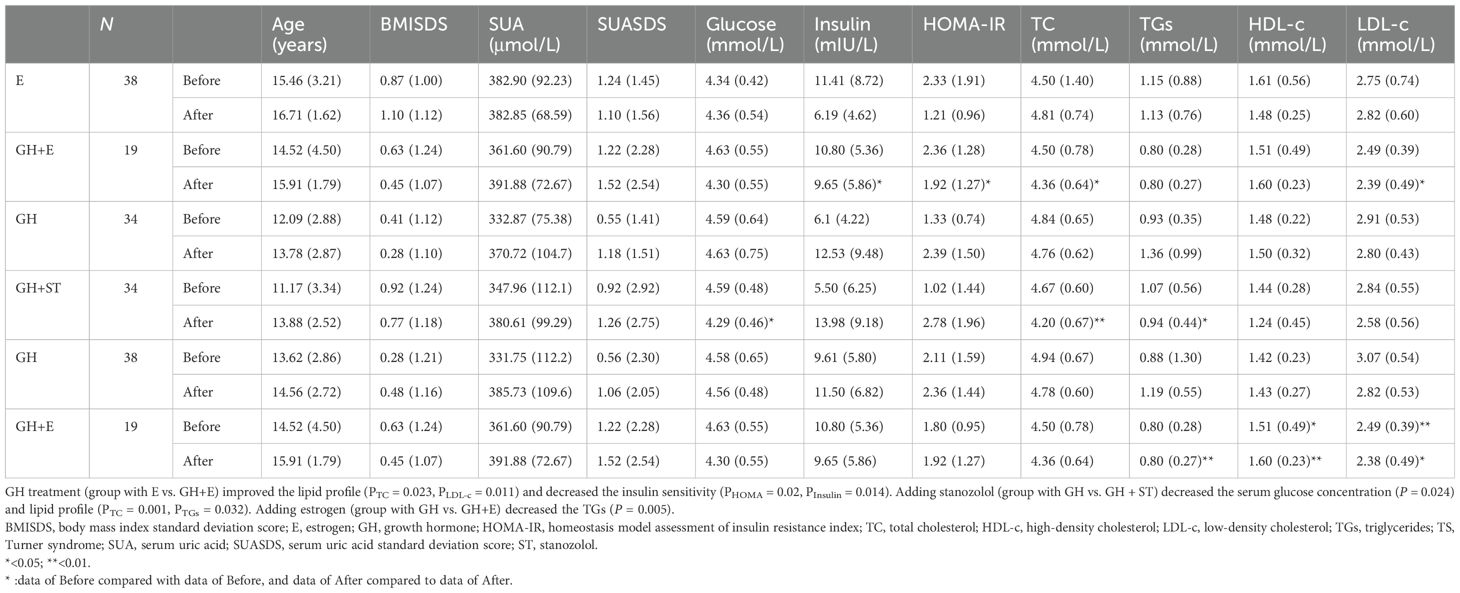
Table 3. Summary of SUA, glucose and lipid metabolism, and the effects of GH therapy, stanozolol, and estrogen in patients with TS.
We retrospectively studied SUA in age-adjusted patients between groups treated with GH and treated with GH combined with stanozolol during the follow-up period. The results showed that the incidence of hyperuricemia was only higher at 1 year after combining stanozolol (see Table 4).
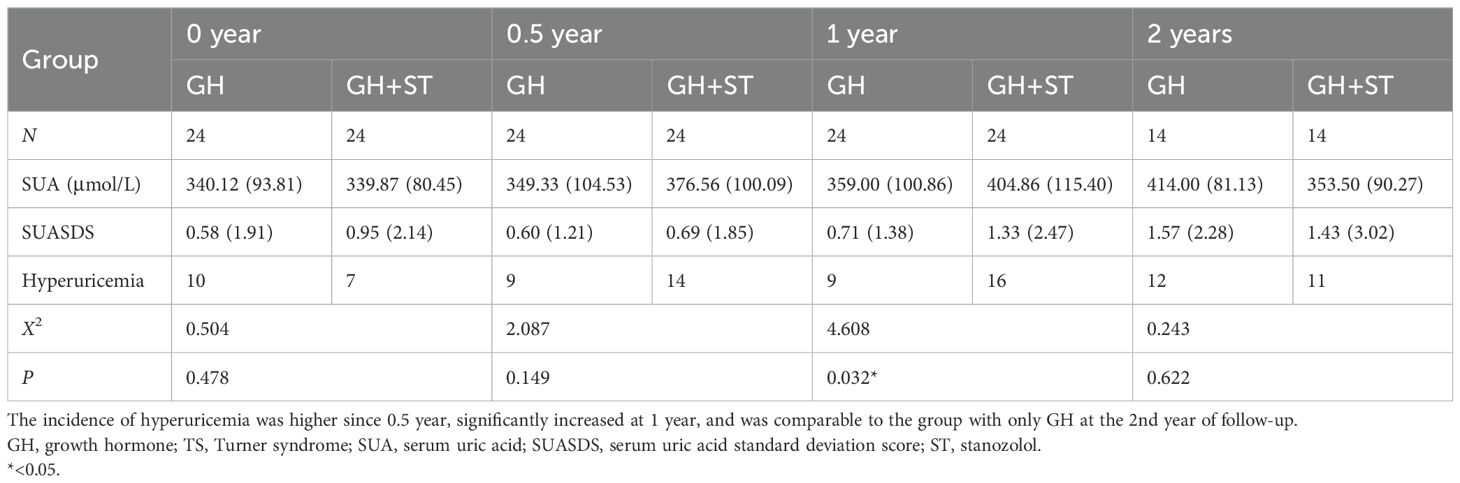
Table 4. SUA profile and incidence of hyperuricemia in age-matched girls with TS before and after stanozolol.
4 Discussion
A range of cardiometabolic risk factors, such as obesity, impaired glucose sensitivity, and dyslipidemia, occur since childhood in individuals with TS and are associated with the risk of atherosclerosis and cardiovascular disease in adulthood (23, 24). Hyperuricemia, as one of those risk factors, is unclear in TS individuals. This study uncovered the uric acid profile in TS individuals, and BMISDS, HOMA-IR, fasting serum glucose, and eGFR were the independent risk factors of hyperuricemia. Growth promotion treatment with GH or ST and estrogen induction therapy did not affect the SUA level except that ST transiently increased the morbidity of hyperuricemia.
SUA was significantly higher in patients with TS than that in normal female peers. Based on the definition of hyperuricemia as an SUA level >6.0 mg/dL, the incidence of hyperuricemia was 33.1% in patients with TS, which was higher than that in normal female peers (16.67%). The incidence of hyperuricemia was 10.0% in a study of Chinese adults (9) and significantly higher in boys (2.7%) than in girls (1.9%) in preadolescent children from a Japanese study (20). The overall prevalence of SUA ≥310 μmol/L (5.16 mg/dL) among children was 10.1% in a study from China in children aged 3 to 6 years (25). Recent research described a mild elevation of SUA with age in normal Chinese girls, and the age-specific mean and upper reference value (97.5th percentile) slightly increased from 275 μmol/L (4.6 mg/dL) and ≤407 μmol/L (6.8 mg/dL) at 2 to 5 years to 308 μmol/L (5.2 mg/dL) and ≤432 μmol/L (7.3 mg/dL) over 14 years, respectively (7). The SUA in TS also showed a slow rising tendency with age. Given the narrow age range in controls, we compared to the age-matched normal girls from the above-mentioned study, and the mean value in TS patients over 10 years was also higher. We did not observe a change in SUA in patients with different karyotypes, suggesting that karyotypes did not affect SUA in girls with TS. To the best of our knowledge, this is the first study showing the SUA profile in patients with TS.
In normal karyotypes of children or adult populations, SUA is strongly associated with obesity, hypertension, high LDL-c, TC, and TGs (20, 26). Our study indicated that BMISDS, HOMA-IR, glucose, and eGFR were independent risk factors for hyperuricemia in patients with TS. Similar to those of previous studies in that BMI is associated with hyperuricemia (27, 28), BMISDS in patients with TS was also higher than that in control, and overweight or obese patients were more likely to have higher uric acid levels. This suggested that overproduction of UA was related to the accumulation of fat in the body of TS. Insulin resistance is an important determinant in the association between hyperuricemia and metabolic syndrome as both fructose-dependent and fructose-independent models that may mediate hyperuricemia with insulin resistance, fatty liver, and dyslipidemia can lead to hyperuricemia by reducing the kidney’s ability to excrete urate (29). Serum uric acid could mediate insulin resistance through the development of mitochondrial oxidative stress in endothelial cells (30). A previous study found that impaired glucose tolerance started in patients with TS since childhood, and the prevalence increased during adolescence, young adulthood, and early adulthood (10%, 16.7%, 21.4%, and 41.2%, respectively) (31). Ibarra-Gasparini D et al. also observed that early insufficient insulin secretion characterizes diabetes mellitus in TS. Interestingly, this study found that glucose level was negatively associated with SUA, of which the mechanism was unclear yet. It is worth noting that the glucose levels in this study were all within normal range, but it could not rule out the possibility that a relatively higher glucose level within the normal range was resulting from a relatively lower insulin level, which is a growth factor in childhood, and it might be associated to malnutrition or emaciation, which are generally signs of a low level of serum uric acid (20, 32–35). Some pediatric patients with TS may have a lower eGFR, which was also found in our study and might affect urate excretion (36). Lastly, although the mechanism of increasing SUA in TS was not fully explored by this study and further research is encouraged to solve the chicken-and-egg dilemma, we identified possible treatment targets of hyperuricemia and insulin resistance in TS.
SUA differs according to age and sex, and children over 12 years old (adolescence) showed an increase in SUA, and girls presented a lower SUA than boys from adolescence to menopause due to the hypouricemic effect of estrogen (6, 37, 38). We found that SUA in patients with TS had an upward trend with increasing age. Our results showed that SUA increased in adolescence in the population without spontaneous puberty initiation and SUASDS increased in people with adolescent hair, although there was no statistically significant difference, indicating the possible reason that the age and sex differences in SUA might be associated with androgenic hormones (6, 39). Nevertheless, our result indicated that the androgenic hormones that affected SUA in female individuals were mainly from adrenal glands rather than ovaries since the puberty of the adrenal gland maximizes in the late teenage years (40). Estrogen could decrease SUA by increasing the renal clearance of uric acid (38). However, the addition of estrogen in our study did not decrease SUA; this might be due to the lack of a uniform dosage that mimics normal puberty, and uric acid clearance needs to be further studied in individuals with TS considering their high risk of renal diseases.
Although there was no significant change in SUA, increased insulin resistance after GH treatment and improved lipid profile with the addition of GH, stanozolol, and estrogen were observed in this study. Increased insulin resistance was also found in a previous study (41). Lipids included TC, and LDL-c decreased with the addition with GH, which was also found in previous studies (42–44). Xiong H et al. observed that stanozolol did not affect lipid metabolism, while TC and TGs were decreased with the addition of stanozolol in this study (45). What is more, Irzyniec TJ et al. showed that hormone replacement therapy (HRT) does not affect lipid metabolism in TS women (44). However, we found that TGs and LDL-c decreased while HDL-c increased with estrogen replacement. The difference among the study results could be caused by the different study populations, which still needs further study for possible mechanisms to be explored. As lipid profile was an important metabolic and cardiovascular risk profile for patients with TS, the study provided supporting evidence that timely treatment with GH and estrogen is beneficial to the metabolic profile in individuals with TS.
Stanozolol is an anabolic–androgenic steroid which increases muscle growth and protein metabolism (46). For growth promotion, stanozolol induced the differentiation of chondrocytes and promoted growth plate development through the activation of JNK/c-Jun/Sox9 signaling (47). SUA was higher in stanozolol-treated individuals than in those not treated with stanozolol, although the difference was not statistically significant, which might be due to the limited sample size. However, when we further studied SUA change at different time points, we found that hyperuricemia increased in the first year of stanozolol treatment, which was consistent with findings in animal experiments (48). However, there was no statistically significant difference in the incidence of hyperuricemia between the stanozolol group and the control group in the second year. It might be related to several factors, of which inevitable statistical bias should be the first to consider due to the limited sample size, leading to underrepresentation of the results. Furthermore, the maximum anabolic effect of stanozolol on girls with TS has not been elucidated, and we speculated that it might take place at approximately 1 year after treatment, which needs further studies in patients with TS. Taken together, attention on SUA is still needed when stanozolol is planned to be used.
4.1 Limitations
This study is a retrospective observational study and not enough to figure out the mechanism of increased SUA profile in patients with TS. The age range of controls was not appropriately matched to those of patients with TS because we only included prepubertal children and aimed to match the ovarian failure station of TS. In addition, this study had a lack of enough sample size of patients during both stanozolol and estrogen treatment and had no data about the periodicity of estrogen and progesterone replacement or in adult individuals with TS. This study also did not consider the interacting effects of parental education, diet, and/or environment. Above all, further studies are needed.
5 Conclusion
Girls with TS showed a slowly rising tendency in SUA and SUASDS with age and have increased SUA and SUASDS levels and a higher incidence of hyperuricemia compared to their healthy female peers. The independent risk factors for hyperuricemia in pediatric patients with TS were BMISDS, HOMA-IR, glucose, and eGFR. The incidence of hyperuricemia increased in the first year of stanozolol treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans (IIT-2022-567) were approved by the First Affiliated Hospital, Sun Yat-sen University’s Institutional Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SG: Resources, Methodology, Writing – original draft, Funding acquisition, Data curation. QC: Resources, Writing – original draft, Supervision, Data curation. JZ: Resources, Methodology, Writing – original draft, Data curation. MW: Writing – original draft, Resources, Investigation, Formal Analysis. RZ: Writing – original draft, Resources, Data curation. BW: Writing – original draft, Resources, Data curation. YL: Writing – original draft, Resources, Data curation. HM: Writing – original draft, Conceptualization. XJ: Writing – original draft, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. All phases of this study was supported by the Medical Science and Technology Foundation of Guangdong Province, China (Grant No. A2021105).
Acknowledgments
We thank our colleague, Li Bin, for the statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMISDS, body mass index standard deviation score; E, estrogen; eGFR, estimated glomerular filtration rate; Glu, glucose; GH, growth hormone; HDL-c, high-density cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance index; Ins, insulin; LDL-c, low-density cholesterol; SUA, serum uric acid; SUASDS, SUA standard deviation score; ST, stanozolol; Scr, serum creatine; TC, total cholesterol; TGs, triglycerides; TS, Turner syndrome.
References
1. Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in turner syndrome. J Clin Epidemiol. (1998) 51:147–58. doi: 10.1016/s0895-4356(97)00237-0
2. Ostberg JE, Thomas EL, Hamilton G, Attar MJ, Bell JD, Conway GS. Excess visceral and hepatic adipose tissue in turner syndrome determined by magnetic resonance imaging: estrogen deficiency associated with hepatic adipose content. J Clin Endocrinol Metab. (2005) 90:2631–5. doi: 10.1210/jc.2004-1939
3. O'Gorman CS, Syme C, Bradley T, Hamilton J, Mahmud FH. Impaired endothelial function in pediatric patients with turner syndrome and healthy controls: a case-control study. Int J Pediatr Endocrinol. (2012) 2012:5. doi: 10.1186/1687-9856-2012-5
4. van Teunenbroek A, de Muinck Keizer-Schrama SM, Aanstoot HJ, Stijnen T, Hoogerbrugge N, Drop SL. Carbohydrate and lipid metabolism during various growth hormone dosing regimens in girls with turner syndrome. Dutch Working Group Growth Hormone. Metab. (1999) 48:7–14. doi: 10.1016/s0026-0495(99)90003-3
5. Wooten N, Bakalov VK, Hill S, Bondy CA. Reduced abdominal adiposity and improved glucose tolerance in growth hormone-treated girls with turner syndrome. J Clin Endocrinol Metab. (2008) 93:2109–14. doi: 10.1210/jc.2007-2266
6. Wilcox WD. Abnormal serum uric acid levels in children. J Pediatr. (1996) 128:731–41. doi: 10.1016/s0022-3476(96)70322-0
7. Ye PY, Zhao XY, Yan YK, Xiao P, Hou DQ, Zhu ZX, et al. [Association between hyperuricemia and incidence risk for cardiometabolic abnormity in children]. Zhonghua Liu Xing Bing Xue Za Zhi. (2021) 42:433–9. doi: 10.3760/cma.j.cn112338-20200825-01094
8. Ye W, Zhou X, Xu Y, Zheng C, Liu P. Serum uric acid levels among chinese children: Reference values and association with Overweight/Obesity. Clin Pediatr (Phila). (2024) 63(12):1684–90. doi: 10.1177/00099228241238510
9. Saito Y, Tanaka A, Node K, Kobayashi Y. Uric acid and cardiovascular disease: A clinical review. J Cardiol. (2021) 78:51–7. doi: 10.1016/j.jjcc.2020.12.013
10. Chen WY, Fu YP, Zhou M. The bidirectional relationship between metabolic syndrome and hyperuricemia in china: A longitudinal study from CHARLS. Endocrine. (2022) 76:62–9. doi: 10.1007/s12020-022-02979-z
11. Mortada I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: an emerging association. Curr Hypertens Rep. (2017) 19:69. doi: 10.1007/s11906-017-0770-x
12. Wei CY, Sun CC, Wei JC, Tai HC, Sun CA, Chung CF, et al. Association between hyperuricemia and metabolic syndrome: An epidemiological study of a labor force population in taiwan. BioMed Res Int. (2015) 2015:369179. doi: 10.1155/2015/369179
13. Herman JB, Medalie JH, Goldbourt U. Diabetes, prediabetes and uricaemia. Diabetologia. (1976) 12:47–52. doi: 10.1007/BF01221964
14. Reaven GM. Role of insulin resistance in human disease (syndrome x): an expanded definition. Annu Rev Med. (1993) 44:121–31. doi: 10.1146/annurev.me.44.020193.001005
15. Li N, Zhang S, Li W, Wang L, Liu H, Li W, et al. Prevalence of hyperuricemia and its related risk factors among preschool children from china. Sci Rep. (2017) 7:9448. doi: 10.1038/s41598-017-10120-8
16. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with turner syndrome: proceedings from the 2016 cincinnati international turner syndrome meeting. Eur J Endocrinol. (2017) 177:G1–1G70. doi: 10.1530/EJE-17-0430
17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
18. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. (2009) 20:629–37. doi: 10.1681/ASN.2008030287
19. Qian Y, Kong YW, Wan NJ, Yan YK. Associations between body mass index in different childhood age periods and hyperuricemia in young adulthood: the china health and nutrition survey cohort study. World J Pediatr. (2022) 18:680–6. doi: 10.1007/s12519-022-00573-x
20. Huang YF, Yang KH, Chen SH, Xie Y, Huang CB, Qing YF, et al. [Practice guideline for patients with hyperuricemia/gout]. Zhonghua Nei Ke Za Zhi. (2020) 59:519–27. doi: 10.3760/cma.j.cn112138-20200505-00449
21. Aoki Y, Sofue T, Kawakami R, Ozaki T, Manabe M, Kanda K, et al. Prevalence and factors related to hypouricemia and hyperuricemia in schoolchildren: results of a large-scale cross-sectional population-based study conducted in japan. Sci Rep. (2022) 12:17848. doi: 10.1038/s41598-022-19724-1
22. Desideri G, Castaldo G, Lombardi A, Mussap M, Testa A, Pontremoli R, et al. Is it time to revise the normal range of serum uric acid levels. Eur Rev Med Pharmacol Sci. (2014) 18:1295–306.
23. Gravholt CH, Andersen NH, Christin-Maitre S, Davis SM, Duijnhouwer A, Gawlik A, et al. Clinical practice guidelines for the care of girls and women with turner syndrome. Eur J Endocrinol. (2024) 190:G53–53G151. doi: 10.1093/ejendo/lvae050
24. Pirgon Ö, Atabek ME, Oran B, Güçlü R. Atherogenic lipid profile and systolic blood pressure are associated with carotid artery intima-media thickness in children with turner syndrome. J Clin Res Pediatr Endocrinol. (2008) 1:62–71. doi: 10.4008/jcrpe.v1i2.9
25. Ross JL, Feuillan P, Long LM, Kowal K, Kushner H, Cutler GB Jr.. Lipid abnormalities in turner syndrome. J Pediatr. (1995) 126:242–5. doi: 10.1016/s0022-3476(95)70551-1
26. Civantos Modino S, Guijarro de Armas MG, Monereo Mejías S, Montaño Martínez JM, Iglesias Bolaños P, Merino Viveros M, et al. Hyperuricemia and metabolic syndrome in children with overweight and obesity. Endocrinol Nutr. (2012) 59:533–8. doi: 10.1016/j.endonu.2012.06.010
27. Stelmach MJ, Wasilewska N, Wicklund-Liland LI, Wasilewska A. Blood lipid profile and BMI-z-score in adolescents with hyperuricemia. Ir J Med Sci. (2015) 184:463–8. doi: 10.1007/s11845-014-1146-8
28. Thomazini F, de Carvalho BS, de Araujo PX, Franco M. High uric acid levels in overweight and obese children and their relationship with cardiometabolic risk factors: what is missing in this puzzle. J Pediatr Endocrinol Metab. (2021) 34:1435–41. doi: 10.1515/jpem-2021-0211
29. King C, Lanaspa MA, Jensen T, Tolan DR, Sánchez-Lozada LG, Johnson RJ. Uric acid as a cause of the metabolic syndrome. Contrib Nephrol. (2018) 192:88–102. doi: 10.1159/000484283
30. Asma Sakalli A, Küçükerdem HS, Aygün O. What is the relationship between serum uric acid level and insulin resistance?: a case-control study. Med (Baltimore). (2023) 102:e36732. doi: 10.1097/MD.0000000000036732
31. Lebenthal Y, Levy S, Sofrin-Drucker E, Nagelberg N, Weintrob N, Shalitin S, et al. The natural history of metabolic comorbidities in turner syndrome from childhood to early adulthood: Comparison between 45,X monosomy and other karyotypes. Front Endocrinol (Lausanne). (2018) 9:27. doi: 10.3389/fendo.2018.00027
32. Menon RK, Sperling MA. Insulin as a growth factor. Endocrinol Metab Clin North Am. (1996) 25:633–47. doi: 10.1016/s0889-8529(05)70344-3
33. Zhang LL, Zhang L, Dong J, Zhao Y, Wang XP. Factors contributing to malnutrition in parkinson's disease patients with freezing of gait. Front Neurol. (2022) 13:816315. doi: 10.3389/fneur.2022.816315
34. Ibarra-Gasparini D, Altieri P, Scarano E, Perri A, Morselli-Labate AM, Pagotto U, et al. New insights on diabetes in turner syndrome: results from an observational study in adulthood. Endocrine. (2018) 59:651–60. doi: 10.1007/s12020-017-1336-z
35. Sun L, Wang Y, Zhou T, Zhao X, Wang Y, Wang G, et al. Glucose metabolism in turner syndrome. Front Endocrinol (Lausanne). (2019) 10:49. doi: 10.3389/fendo.2019.00049
36. Izumita Y, Nishigaki S, Satoh M, Takubo N, Numakura C, Takahashi I, et al. Retrospective study of the renal function using estimated glomerular filtration rate and congenital anomalies of the kidney-urinary tract in pediatric turner syndrome. Congenit Anom (Kyoto). (2020) 60:175–9. doi: 10.1111/cga.12384
37. Igarashi T. Normal serum uric acid concentrations for age and sex and incidence of renal hypouricaemia in japanese school children. Pediatr Nephrol. (1993) 7:239–40. doi: 10.1007/BF00864417
38. Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. (1973) 1:449–51. doi: 10.1136/bmj.1.5851.449
39. Mu L, Pan J, Yang L, Chen Q, Chen Y, Teng Y, et al. Association between the prevalence of hyperuricemia and reproductive hormones in polycystic ovary syndrome. Reprod Biol Endocrinol. (2018) 16:104. doi: 10.1186/s12958-018-0419-x
40. Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche–normal variant or forerunner of adult disease. Endocr Rev. (2000) 21:671–96. doi: 10.1210/edrv.21.6.0416
41. Gravholt CH, Naeraa RW, Brixen K, Kastrup KW, Mosekilde L, Jørgensen JO, et al. Short-term growth hormone treatment in girls with turner syndrome decreases fat mass and insulin sensitivity: a randomized, double-blind, placebo-controlled, crossover study. Pediatrics. (2002) 110:889–96. doi: 10.1542/peds.110.5.889
42. Bannink EM, van der Palen RL, Mulder PG, de Muinck Keizer-Schrama SM. Long-term follow-up of GH-treated girls with turner syndrome: metabolic consequences. Horm Res. (2009) 71:343–9. doi: 10.1159/000223419
43. Irzyniec T, Jeż W, Lepska K, Maciejewska-Paszek I, Frelich J. Childhood growth hormone treatment in women with turner syndrome - benefits and adverse effects. Sci Rep. (2019) 9:15951. doi: 10.1038/s41598-019-52332-0
44. Irzyniec TJ, Jeż W. The influence of hormonal replacement and growth hormone treatment on the lipids in turner syndrome. Gynecol Endocrinol. (2014) 30:250–3. doi: 10.3109/09513590.2013.872236
45. Xiong H, Chen HS, Du ML, Li YH, Ma HM, Su Z, et al. Therapeutic effects of growth hormone combined with low-dose stanozolol on growth velocity and final height of girls with turner syndrome. Clin Endocrinol (Oxf). (2015) 83:223–8. doi: 10.1111/cen.12785
46. Bates PC, Chew LF, Millward DJ. Effects of the anabolic steroid stanozolol on growth and protein metabolism in the rat. J Endocrinol. (1987) 114:373–81. doi: 10.1677/joe.0.1140373
47. Zhu S, Long L, Hu Y, Tuo Y, Li Y, Yu Z. GnRHa/Stanozolol combined therapy maintains normal bone growth in central precocious puberty. Front Endocrinol (Lausanne). (2021) 12:678797. doi: 10.3389/fendo.2021.678797
Keywords: Turner syndrome, serum uric acid, lipids, insulin resistance, stanozolol
Citation: Guo S, Chen Q, Zhang J, Wei M, Zheng R, Wang B, Li Y, Ma H and Jiang X (2024) A single-center’s uric acid profile in girls with Turner syndrome. Front. Endocrinol. 15:1442166. doi: 10.3389/fendo.2024.1442166
Received: 01 June 2024; Accepted: 23 October 2024;
Published: 22 November 2024.
Edited by:
Yukihiro Hasegawa, Tokyo Metropolitan Children’s Medical Center, JapanReviewed by:
Kento Ikegawa, Tokyo Metropolitan Children’s Medical Center, JapanMasanobu Kawai, Osaka Women’s and Children’s Hospital, Japan
Copyright © 2024 Guo, Chen, Zhang, Wei, Zheng, Wang, Li, Ma and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyun Jiang, anhpYW95QG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Song Guo
Song Guo Qiuli Chen
Qiuli Chen Jun Zhang
Jun Zhang Meihua Wei
Meihua Wei Rujiang Zheng
Rujiang Zheng Bing Wang1
Bing Wang1 Yanhong Li
Yanhong Li Xiaoyun Jiang
Xiaoyun Jiang