- 1Department of Cardiology, Second Xiangya Hospital of Central South University, Changsha, China
- 2Department of General Surgery, Clinical Research Center for Breast Disease, Second Xiangya Hospital of Central South University, Changsha, China
- 3Department of Radiology, The Second Xiangya Hospital of Central South University, Changsha, China
- 4Department of Nutrition, The Second Xiangya Hospital of Central South University, Changsha, China
Background: It remains unknown whether composite-dietary-antioxidant-index (CDAI) is associated with the risk of sarcopenia. This study investigated the association of CDAI with sarcopenia risk among general US adults.
Methods: A total of 10,093 participants were enrolled in the National Health and Nutrition Examination Surveys (NHANES) from 6 survey cycles (2003-2004, 2005-2006, 2011-2012, 2013-2014, 2015-2016 and 2017-2018). Multivariate logistic regression was carried out to examine the relationship between CDAI and the risk of sarcopenia. Restricted cubic spline (RCS) curves were employed to analyze nonlinear relationships.
Results: In a multi-variable logistic regression model adjusting for demographics, lifestyle, economic status and other dietary factors, higher CDAI score was related to a lower risk of sarcopenia among US adults. Compared the highest quartile of CDAI score with the lowest, the OR and 95%CI were 0.49 (0.31-0.75). Furthermore, the RCS demonstrated a linear dose-response relationship between CDAI and sarcopenia (Pnon-linearity=0.92). These results remained consistent across subgroups stratified by age, sex, physical activity, drinking status, body mass index (BMI), smoking habits, energy intake, and Healthy Eating Index (HEI) score. In addition, the favorable associations of CDAI were primarily attributed to Vitamin E intake.
Conclusion: A higher CDAI score was associated with a lower risk of sarcopenia. According to these results, a greater adherence to CDAI may benefit sarcopenia prevention in adults.
1 Introduction
Sarcopenia, characterized by the accelerated loss of skeletal muscle function, strength, and mass, as individuals age, is a significant health concern globally (1). Currently, the prevalence rates of sarcopenia are estimated to vary from 10% to 27%, with severe sarcopenia affecting 2% to 9% of individuals (2), which is significantly associated with elevated risks of falls, functional impairments, frailty, and mortality (3–5).
Oxidative stress is a crucial factor in the development of sarcopenia (6). Impairment of the antioxidant defense mechanisms leads to excess reactive oxygen species (ROS) and oxidative stress within the organism. Excess ROS further destroys muscle cell structure, which may lead to muscle cell loss and decreased muscle strength (7, 8). Adopting a dietary pattern rich in antioxidant nutrients, such as Vitamin C, Vitamin E, carotenoids, selenium, flavonoids and some other plant phytochemicals may prevent the development of sarcopenia via influencing the oxidative damage. Composite dietary antioxidant index (CDAI) has been established as a credible and dependable nutritional instrument for evaluating antioxidants from 6 dietary sources: vitamins A, C, and E, selenium, carotenoids, and zinc (9). Prior studies have demonstrated beneficial associations between CDAI and multiple chronic diseases, such as hypertension (10), chronic kidney disease (CKD) (11), depression (12), cancer (13), coronary heart disease (14) and osteoporosis (15). However, no studies have so far examined the associations of CDAI scores with the risk of sarcopenia.
To explore this issue, we aimed to investigate the relationship between CDAI and sarcopenia risk. We hypothesized that a higher CDAI score was associated with a lower risk of sarcopenia.
2 Methods
2.1 Study population
The National-Health-and-Nutrition-Examination-Survey (NHANES) is a research initiative aimed at assessing the health and nutrition status of both children and adults across America. This survey is conducted annually and represents a nationally diverse sample of around 5,000 individuals from 15 counties. Its distinctiveness lies in its dual approach, combining structured interviews with comprehensive physical examinations. Interviews are conducted within respondents’ homes, while physical measurements are taken at specialized and well-equipped mobile centers, which travel to locations throughout the country (16).
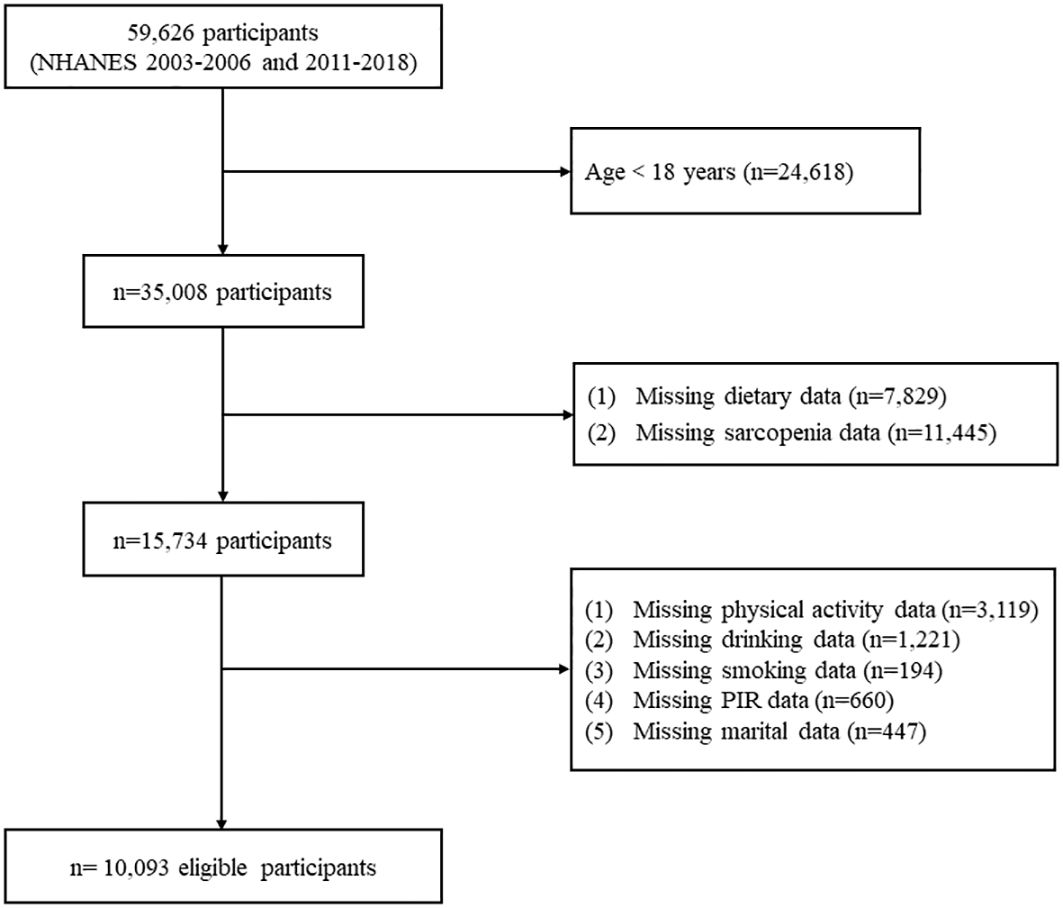
For the current study, we included 59,626 individuals from six NHANES survey cycles spanning from 2003 to 2018 (2003-2004, 2005-2006, 2011-2012, 2013-2014, 2015-2016, and 2017-2018). Exclusions were made for individuals under 18 years old (n=24,618), those lacking dietary data (n=7,829), and without sarcopenia data (n=11,445). Additionally, participants with missing covariate data (n=5,641), such as physical activity, smoking, drinking, poverty income ratio (PIR), and marital status, were further excluded. Finally, 10,093 participants were included in our study, representing approximately 104.7 million noninstitutionalized American citizens (Figure 1). The research protocol received approval from the Ethics Review Board at the National Center for Health Statistics, and all participants signed informed consent (17).
2.2 Assessment of dietary intake and CDAI
Food and nutrient intake data were acquired by professionals through two 24-hour dietary recall questionnaires at each survey cycle. The first questionnaire was administered face-to-face at the respondent’s home, followed by a second one conducted via telephone 3-10 days later (18). Dietary intake of antioxidants and total energy was calculated using the Food and Nutrient Database for Dietary Studies provided by the US Department of Agriculture (19). Antioxidants were sourced exclusively from dietary intake, excluding those from supplements or medications, and the average intake over two days was used for analysis. We included antioxidants data from 6 survey cycles (2003-2004, 2005-2006, 2011-2012, 2013-2014, 2015-2016 and 2017-2018).
To standardize antioxidant intake (including carotenoids, zinc, selenium, vitamins A, C, and E), we subtracted the gender-specific mean and divided by the gender-specific standard deviation. CDAI was calculated by summing the standardized intake of these antioxidants (9), which can be presented in the following formula:
2.3 Ascertainment of sarcopenia
Sarcopenia was defined based on the guidelines established by FNIH and was characterized by appendicular lean mass (ALM) of <0.789 for males and <0.512 for females, after adjustment for body mass index (BMI) (20). ALM was determined as the sum of lean mass from the arm and leg evaluated through Dual-energy X-ray absorptiometry. Sarcopenia data were collected in the same cycle as the antioxidants data.
2.4 Assessment of covariates
Various covariates were collected through interview questionnaires, including age, gender, race, PIR, education, marital status, physical activity, smoking and drinking habits, daily energy intake, and healthy eating index (HEI). Marital status was classified into married, never married, and others. Educational levels were categorized as less than high school, high school, and more than high school. Drinking and smoking status are classified as current, former, or never. Physical activity was measured using total metabolic equivalent of task (MET) for one week. Based on the Global Physical Activity Questionnaire (GPAQ) (21), information was collected on different types of physical activity, such as work activity, transportation modes, and recreational activity. MET scores were assigned for each specific activity. Specifically, moderate and vigorous activities received 4 and 8 points, respectively. In addition, 4 points are assigned for transportation activity, including walking or bicycling (Supplementary Table S1). We calculated the MET (minutes/wk) for each specific activity by multiplying the duration of the specific activity by the corresponding score mentioned above, and then added up the MET (minutes/wk) for each specific activity to obtain the total MET (minutes/wk) (22). Daily energy intake was averaged over two days. The HEI was computed by summing scores for 13 vital dietary components, reflecting compliance to the 2015-2020 Dietary Guidelines for Americans. These comprise nine adequacy components (total fruits, whole fruits, total vegetables, green and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and fatty acids) and four moderation components (refined grains, sodium, added sugars, and saturated fats). A higher HEI score reflects a higher diet quality (23).
Diabetes was diagnosed based on various criteria, including (i) random glucose content ≥11.1 mmol/L; (ii) HbA1c concentration ≥6.5%; (iii) fasting glucose level ≥7.0 mmol/L; (iv) oral glucose tolerance test ≥11.1 mmol/L; or (v) the use of antidiabetic drugs (24). The diagnostic criteria for hypertension included fulfilling one of the following conditions: (i) history of hypertension; (ii) taking antihypertensive medications; (iii) or with average systolic pressure ≥140 mmHg/average diastolic pressure ≥90 mmHg (25). Participants were diagnosed with CKD if the urine albumin/urine was ≥ 3 mg/mmol or if the glomerular filtration rate was < 60 ml/min/1.73 m2 for at least 3 months (26). Cancer was identified by asking “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?”.
2.5 Statistical analysis
The sample weights provided by NHANES were adjusted for different sampling rates, response rates, and different coverage rates among people in the sample. The sample weight for each respondent represents the estimated number of people in the target population, so that accurate national estimates can be obtained from the sample. All analyses incorporated sample weights “wtdr2d”. Baseline variable differences were assessed using Chi-Square and Student t tests. CDAI was divided into quartiles, and logistic regression model was employed to determine OR and 95%CI for the association of CDAI with sarcopenia. To address possible confounding, age (continuous, years) and sex (female and male) were adjusted in model 1. Model 2 further adjusted for race (White, Black, Hispanic, Mexican American and others), marital status (married, never married and others), education (more than high school, high school and less than high school), PIR (continuous), physical activity (continuous, MET-minutes/wk), smoking status (current, former and never), alcohol intake (current, former and never), BMI (continuous, kg/m2), and daily energy intake (continuous, kcal/d). Finally, we additionally adjusted for HEI (continuous) in model 3. Potential nonlinear relationships were explored using restricted cubic splines (RCS) regression with three nodes at the 10th, 50th, and 90th percentiles. In all spline analyses, exposure variables were treated with continuous data, and individuals with extreme first and last percentiles of 2.5 percent were excluded.
Furthermore, we conducted stratified analysis by several key risk factors, including age (<45, ≥45 years), gender (female, male), BMI (<30, ≥30 kg/m2), physical activity (<median, ≥median), alcohol intake (current, former, and never), smoking status (current, former, and never), daily energy intake (<median, ≥median), HEI score (<median, ≥median) and combined chronic diseases (no, yes) by adding an interaction term in model 3. The interaction was assessed in these stratified variables using the likelihood-ratio test.
Several sensitivity analyses were performed to test the robustness of the results. First, we further adjusted for chronic diseases, including diabetes, hypertension, CKD, and cancer. Second, we included populations with missing data on physical activity, smoking, and drinking, PIR, and marital status and utilized multiple imputations. Third, we additionally adjusted for specific dietary intake (including total fruit, total vegetable, whole grain, dairy, red meat, and fiber intake) instead of HEI score in model 3. Finally, we reanalyzed the data by excluding individuals with extreme energy intake (< 1000 kcal/d and > 5000 kcal/d).
All statistical tests were performed with R software (v4.3.1) and P<0.05 was deemed statistically significant.
3 Results
3.1 Baseline characteristics of study participants
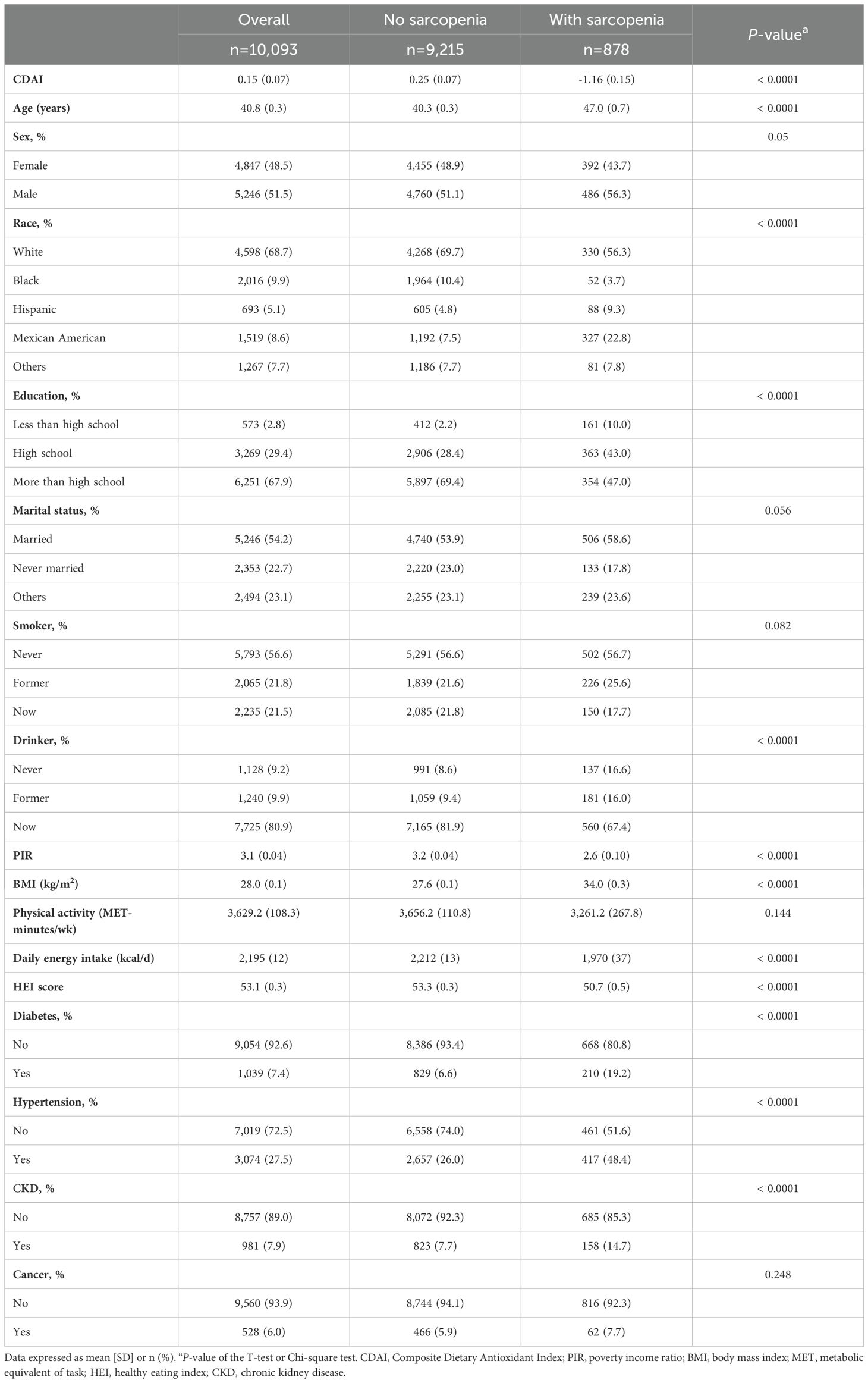
Table 1 displays the baseline characteristics of the participants. The mean age of these participants was 40.8 ± 0.3 years. Among them, 4,847 (48.5%) were females, and around 68.7% of the population identified as White. Compared with the participants without sarcopenia, those with sarcopenia tended to be older, have lower educational levels, income, and CDAI scores, and had higher BMI and a greater risk of chronic disease.
3.2 Relationship between CDAI and sarcopenia
Among the 10,093 participants, 8.7% (878/10,093) were diagnosed with sarcopenia. After adjustment for multiple covariates, such as demographics, lifestyle factors, economic status, energy intake, and HEI (Model 3), comparing with those in the lowest CDAI score, individuals with the highest CDAI score had a decreased risk of sarcopenia. The OR and 95%CI for extreme groups was 0.49 (0.31-0.75) (Table 2). Treating CDAI as a continuous variable, a one-point increase in CDAI score was related to a 5% lower risk of sarcopenia (Table 2). Further, we systematically excluded each of the six components from CDAI individually at a time, and observed that excluding vitamin E substantially attenuated the associations [0.64 (0.40-1.01)] (Table 3).
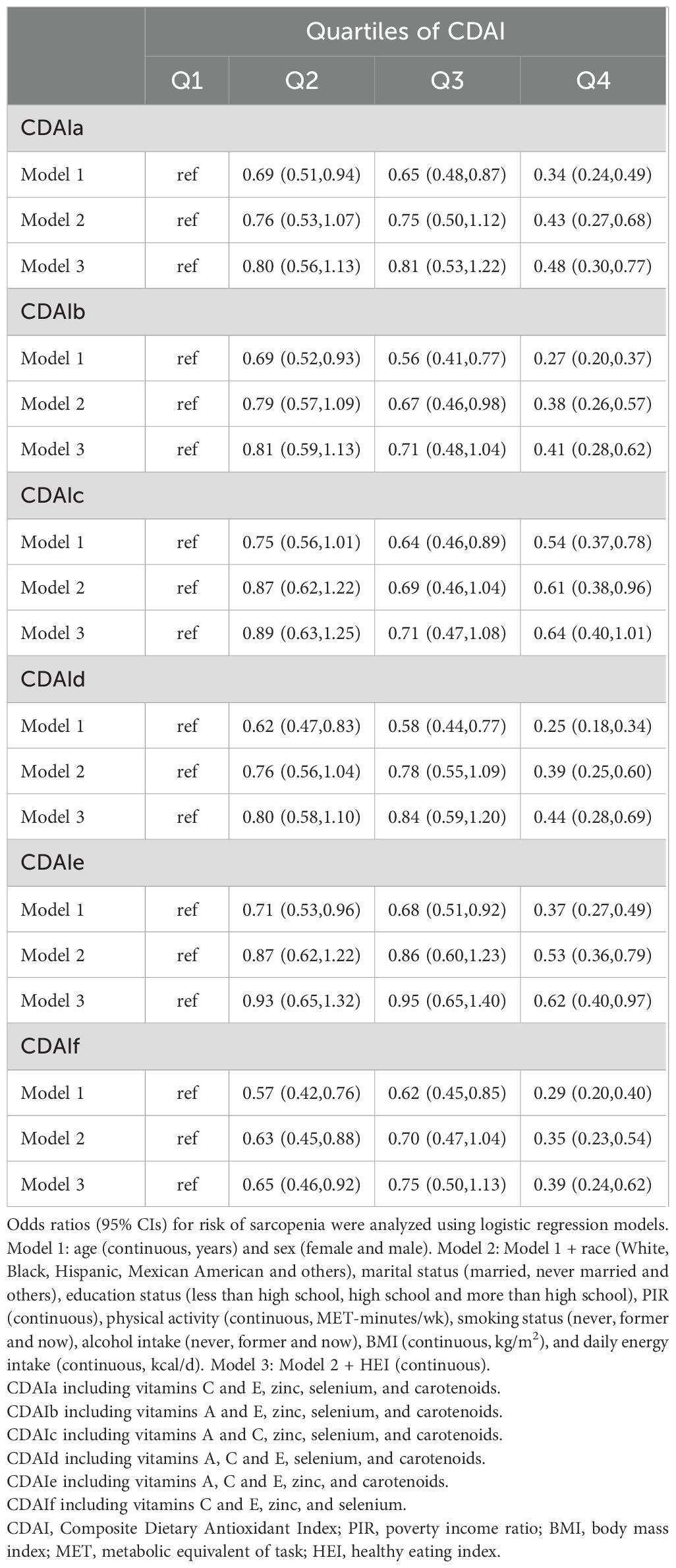
Table 3. Association with sarcopenia after exclusion of each one of 6 components from CDAI by one at a time.
RCS analysis indicated a linear association between CDAI and sarcopenia (P nonlinearity=0.92). As depicted in Figure 2, the risk of sarcopenia decreases with higher CDAI scores. Specifically, the inflection point was identified at CDAI of approximately -0.7, which was associated with an OR of 1. Among the six antioxidant nutrients comprising CDAI, only vitamin A showed a nonlinear relationship with sarcopenia (Supplementary Figure S1).
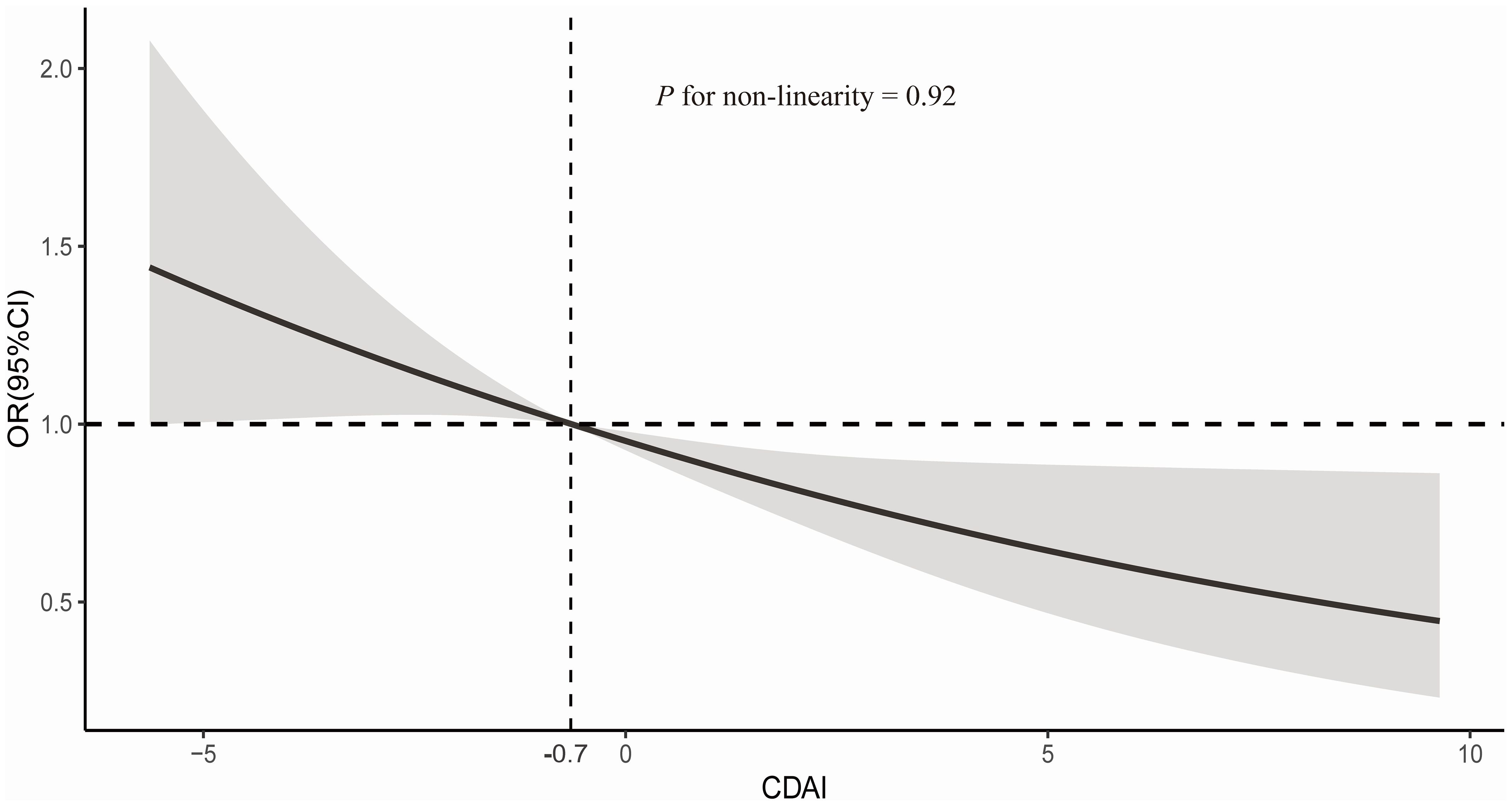
Figure 2. Dose-response relationships between CDAI with sarcopenia. Median CDAI score is reference standard. Odds ratio (OR) and 95%CI are based on logistic regression model adjusted for age (continuous, years) and sex (female and male), race (White, Black, Hispanic, Mexican American and others), marital status (married, never married and others), education status (less than high school, high school and more than high school), PIR (continuous), physical activity (continuous, MET-minutes/wk), smoking status (never, former and now), alcohol intake (never, former and now), BMI (continuous, kg/m2), and daily energy intake (continuous, kcal/d), healthy eating index (continuous). Solid lines indicate OR and shadow indicate 95%CI.
3.3 Subgroup analysis
In subgroup analysis, we observed associations across various strata, including sex (male, female), age (<45, ≥45 years), physical activity (<median, ≥median), alcohol intake (never, former and now), BMI (<30, ≥30 kg/m2), smoking status (never, former and now), HEI (<median, ≥median), energy intake (<median, ≥median) and combined chronic diseases (no, yes). None of the interaction P values between CDAI and these risk factors were statistically significant (all P>0.05) (Figure 3).
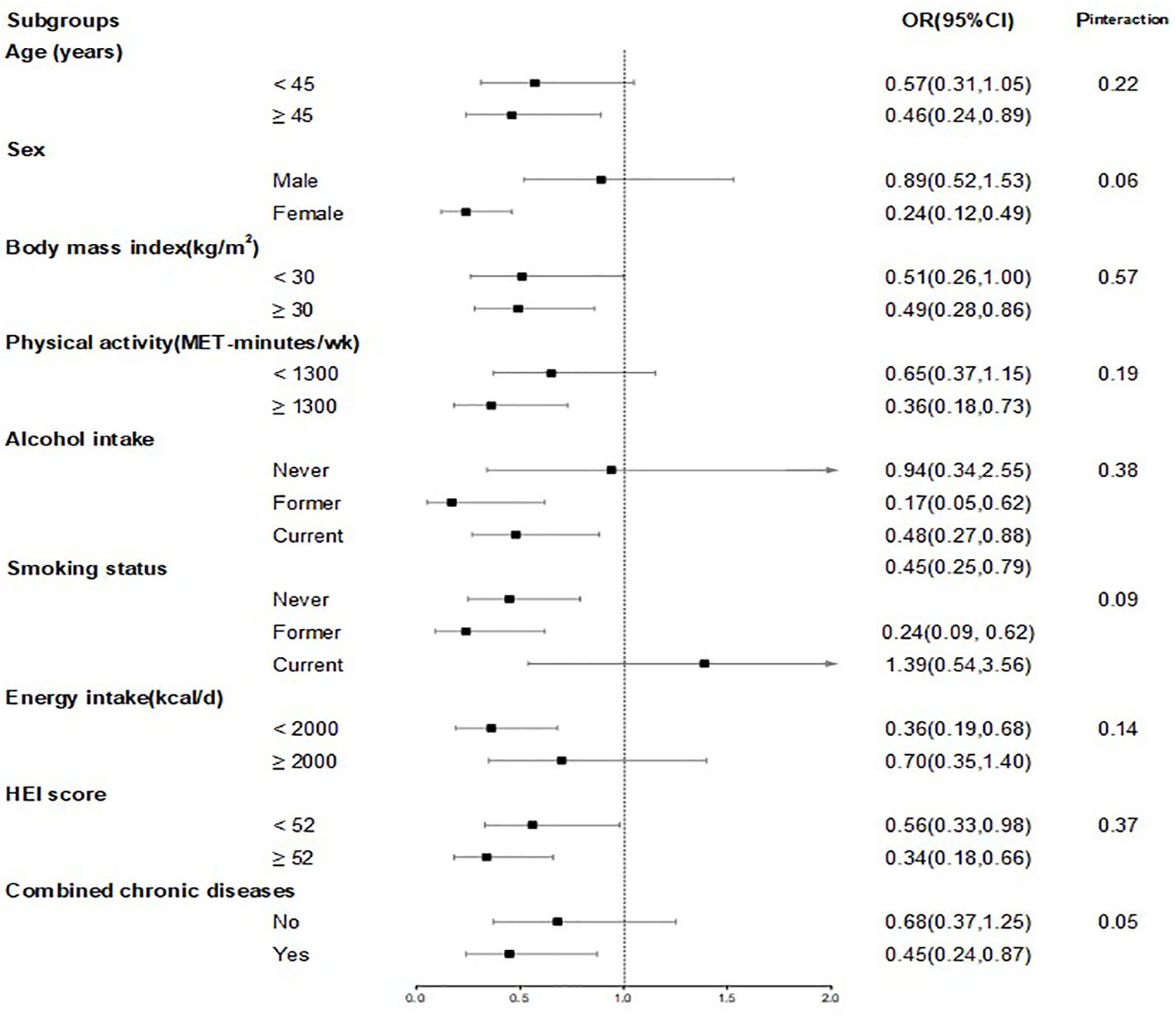
Figure 3. ORs and 95%CIs for CDAI and sarcopenia, stratified by several key risk factors. Model was adjusted for age (continuous, years) and sex (female and male), race (White, Black, Hispanic, Mexican American and others), marital status (married, never married and others), education status (less than high school, high school and more than high school), poverty income ratio (continuous), physical activity (continuous, MET-minutes/wk), smoking status (never, former and now), alcohol intake (never, former and now), BMI (continuous, kg/m2), and daily energy intake (continuous, kcal/d), healthy eating index (continuous). In each stratified analysis, the stratification variable was excluded in the adjustments. Chronic diseases include diabetes, hypertension, CVD and cancer. The OR and 95%CI of each subgroup in the figure from the group with the highest CDAI. Likelihood ratio tests were used for assessment of interaction, and two-sided P values (unadjusted for multiple comparisons) are reported.
3.4 Sensitivity analyses
Firstly, after further adjusting for chronic diseases (including diabetes, hypertension, CKD and cancer), the results remained consistent (Supplementary Table S2). Secondly, when including populations with missing data on physical activity, smoking, drinking, PIR, and marital status and using multiple imputation, similar results were observed (Supplementary Table S2). Thirdly, after further adjusting for specific dietary intake (including fruit, vegetable, whole grain, dairy, red meat, and fiber) instead of HEI, the findings remained unchanged (Supplementary Table S2). Finally, to eliminate the potential impact of outliers, we reconstructed our model by excluding participants with extreme energy intake (<1000 and >5000 kcal/d). The results continued to show a decreasing risk of sarcopenia with increasing CDAI scores (Supplementary Table S3).
4 Discussion
In the present study, a higher CDAI score was associated with a lower risk of sarcopenia. The association was independent of age, sex, race, marital status, education, PIR, BMI, alcohol consumption, smoking behavior, physical activity, energy intake, and HEI. The association was also consistent across stratified groups by age, sex, BMI, physical activity, alcohol consumption patterns, smoking behavior, caloric intake, chronic diseases and HEI. Further, various sensitive analysis demonstrated similar results.
Advancements in the diagnosis and assessment of sarcopenia have been marked by the introduction of diverse methodologies and tools, enhancing our understanding and approach to muscle condition. The diagnosis of sarcopenia has been enriched by a variety of tools and methodologies that assess muscle mass, strength, and functionality (27). The Korean Genome and Epidemiology Study (KoGES) has proposed the muscle-to-fat ratio as a superior metric to BMI for evaluating body composition, particularly in overweight and obese individuals (28). The Framingham Heart Study has further advanced the field by highlighting the efficacy of computed tomography scans and establishing a systematic approach to interpret muscle metrics such as cross-sectional muscle area (CSMA), skeletal muscle index (SMI), skeletal muscle radio attenuation (SMRA), and skeletal muscle gauge (SMG) (29). Although, existing studies utilizing CDAI in the assessment sarcopenia are scarce, studies on related topics exist. A study that included 6,019 participants, also from the NHANES database, found a significant positive association between CDAI and handgrip strength (HGS). Interestingly, further gender-stratified analyses found this association to be significant in male but not female populations (30). Additionally, research involving adults with Metabolic Associated Fatty Liver Disease (MAFLD) using Dual-energy X-ray absorptiometry has revealed that higher CDAI scores are associated with a reduced risk of low muscle mass (31). The above studies support the finding of the current study that CDAI was positively linked to disease with decreased muscle strength, such as sarcopenia.
To the best of our knowledge, this current analysis is the initial effort to assess the associations of CDAI with sarcopenia risk. Previous studies have examined several healthful dietary eating indices, such as the Mediterranean diet (MED) index, the Healthy Eating Index 2015 (HEI-2015), the Alternative Healthy Eating Index 2010 (AHEI-2010), Japanese Food Guide Spinning Top (JFG-ST) and the oxidative balance score (OBS). A robust adherence to the Mediterranean Diet Score (MDS) has been linked to improved muscle outcomes, as evidenced by significant differences of 1.7% in fat-free mass percentage (FFM%) and a 9.6% rise in leg explosive power when comparing extreme quartiles of intake (32). According to the 2015-2020 Dietary Guidelines for Americans (DGA), individuals with the highest adherence to the HEI-2015 were 24% less likely to exhibit low grip strength than those in the lowest quartile among US adults (33). Nevertheless, an inverse correlation was observed between adherence to the AHEI-2010 and indigence of sarcopenia according to the 2019 European Working Group on Sarcopenia in Older People (EWGSOP2) criteria among women (34). The context of a 3-year Cohort Study focusing on elderly individuals living in community-dwelling, who were all above the age of 60, revealed that higher JFG-ST adherence scores were more likely to have greater SMI, specifically among the male participants (35). In other words, it is crucial to devise dietary guidelines specifically adopted to each country’s unique circumstances to prevent sarcopenia. More recently, after adjusting for potential confounders via the backward conditional method, no significant linkage was identified between the OBS and the likelihood of developing sarcopenia (36). These results corroborate the coherence of our study with the majority of previous studies, reinforcing the importance of considering dietary patterns in strategies aimed at promoting optimal muscle health throughout the aging process.
The underlying mechanisms of sarcopenia remain elusive despite being recognized as a multifactorial pathogenesis. This complex process involves oxidative stress, inflammation, mitochondrial dysfunction and reduced synthesis within the muscle tissue (37). While a myriad of risk factors, including advancing age, gender, physical activity, and dietary patterns are well-documented, the molecular mechanisms hinge on an aberrant imbalance between muscle protein synthesis and degradation (38). Crucial to muscle mass and function is the integrity of mitochondria. When compromised, they failed to generate reactive oxygen species (ROS) in a homeostatic manner, leading to a decline in cellular function and overall health (39). The consequential mitochondrial dysfunction is linked to impaired energy production and excessive ROS generation, which are key triggers for the phenotypic changes observed in sarcopenia patients. Moreover, dysregulated ROS production further correlates with elevated levels of inflammatory mediators, such as tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), nuclear factor-kappa B (NF-κB), and C-reactive protein (CRP), contributing to a pro-inflammatory state particularly in muscle tissue (40). Given the centrality of mitochondrial health in the pathogenesis of sarcopenia, interventions aimed at enhancing mitochondrial function, such as physical exercise and nutritional strategies, appear particularly promising in alleviating sarcopenia (41). In this context, our study has revealed a significant inverse correlation between higher CDAI scores and the likelihood of developing sarcopenia. This observation suggested that a diet abundant in antioxidants comprising vitamins C and E, as well as carotenoids may counteract the oxidative damage implicated in muscle degradation.
Individuals diagnosed with sarcopenia tend to have lower intake of essential nutrients such as selenium calcium, magnesium, and sodium compared to older adults with normal muscle function (42). Additionally, selenium deficiency has been linked to skeletal muscle disorders. A cross-sectional study found that higher selenium levels were associated with decreased physical limitations (43). Dietary factors are critical in modulating oxidative stress and protecting against ROS and reactive nitrogen species. Systematic reviews and meta-analyses revealed a correlation between consuming antioxidant-rich foods or antioxidant supplementation and a decreased risk of sarcopenia in individuals aged 55 and older (44). Similarly, data from the Korea-National-Health-and-Nutrition-Examination-Survey (2008–2011) demonstrated an opposite relationship between adequate intake of antioxidant nutrients and the incidence of sarcopenia in Korean adults (45). In a randomized, double-anonymized, placebo-controlled pilot study, supplementation with zinc, selenium, vitamin E, and vitamin C for 17 weeks did not significantly impact the two-minute walking test (2-MWT). However, the intervention did improve the maximum voluntary contractile force and sustained endurance limit time of the quadriceps muscles, possibly by improving the antioxidant defense system and decreasing oxidative stress (46). Overall, the literature suggests that individuals with sarcopenia often have nutrient deficiencies, highlighting the potential benefits of antioxidant nutrient interventions for the management of sarcopenia.
Despite limited research on CDAI and sarcopenia, previous research has explored the correlation between CDAI and various muscle-related conditions. Specifically, CDAI is positively correlated with HGS, with noted differences between sex (30). Additionally, higher CDAI scores have been related to a decreased risk of LAM in individuals with metabolic-associated fatty liver disease (31). Consistent with these findings, our study contributes to the growing body of evidence supporting an inverse correlation between CDAI and sarcopenia.
Vitamin C, a crucial water-soluble nonenzymatic antioxidant nutrient, has been found to positively correlate with skeletal muscle measurements among middle-aged and older individuals (47). Nevertheless, conflicting studies exist, with some indicating that vitamin C and E supplementation does not increase lower limb strength or reduce muscle damage in young athletes (48). According to NHANES data, dietary intake of vitamin E, selenium, and zinc is related to HGS in males, while only zinc intake is linked to HGS in females (30). The effectiveness of selenium supplementation in individuals with sarcopenia remains uncertain, as evidenced by observational studies (49). Prospective studies have demonstrated a favorable relationship between higher carotenoid intake and improved grip strength and walking speed among individuals in their middle and older years (50).
Conversely, a systematic review has highlighted the potential benefits of minerals such as magnesium and selenium for preventing and managing sarcopenia in the elderly (51). A positive correlation was observed between increased zinc intake and reduced risk of lower-extremity dysfunction and frailty in older adults, as indicated by a prospective study (52). Consequently, growing evidence suggests that sarcopenia can be prevented and managed through the consumption of vitamin E, vitamin C, and selenium. Subgroup analysis conducted in our study did not reveal any significant interactions among various risk factors, including age, gender, BMI, physical activity, alcohol consumption, smoking habits, chronic diseases and energy intake.
Interestingly, this study revealed that vitamin E significantly impacted the association between CDAI and sarcopenia. Furthermore, an investigation utilizing cross-sectional data originating from the fifth round of the ROAD study found that increased diet consumption of vitamin E and fats in the diet was associated with reduced sarcopenia (53). Another cross-sectional study indicated positive associations between intake of food-derived substances and plasma concentration of vitamin E in skeletal muscle mass, suggesting that dietary intake of vitamin E may play a significant role in preventing sarcopenia (54). Previous research has suggested that vitamin E deficiency may worsen sarcopenia, a condition often linked with aging, marked by decreased muscle strength and mass (55). A cross-sectional investigation employing data derived from the Korean-National-Health-and-Nutrition-Examination-Survey found that community-dwelling adults with lower serum vitamin E levels had weaker grip strength (56).
The notable strengths of our study include a substantial sample size and adjustment for various covariates. In addition, we carried out an array of sensitivity analyses which supported the robustness of our findings. However, our study had inherent limitations that need to be acknowledged. Firstly, our current analysis was conducted based on a cross-sectional study, which could not establish causal relationships. Secondly, the accuracy of 24-hour dietary questionnaires may be compromised by reliance on participants’ memory. Thirdly, our study was conducted among US populations, which may limit the broad applicability of our research findings across other racial/ethnic or socioeconomic groups. Finally, as with all observational studies, we cannot guarantee the absence of any residual confounding elements despite adjusting for dietary, lifestyle and medical history factors in our analysis.
5 Conclusion
Our data indicated a beneficial association between CDAI and the incidence of sarcopenia among US adults. Whether the beneficial association exists in other populations warrants further investigation.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by the Ethics Review Board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KW: Formal analysis, Methodology, Software, Writing – original draft. QZ: Conceptualization, Supervision, Writing – original draft. ZJ: Conceptualization, Supervision, Writing – review & editing. SL: Supervision, Writing – review & editing. HT: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Hunan Province of China (Grant Nos.2022JJ30838).
Acknowledgments
We would like to thank the participants and staff of the National-Health-and-Nutrition-Examination-Survey for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1442586/full#supplementary-material
References
1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. (1997) 127:990S–1S. doi: 10.1093/jn/127.5.990S
2. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
3. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
4. Yang A, Lv Q, Chen F, Wang Y, Liu Y, Shi W, et al. The effect of vitamin D on sarcopenia depends on the level of physical activity in older adults. J Cachexia Sarcopenia Muscle. (2020) 11:678–89. doi: 10.1002/jcsm.12545
5. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
6. Duranti G. Oxidative stress and skeletal muscle function. Int J Mol Sci. (2023) 24:10227. doi: 10.3390/ijms241210227
7. Damiano S, Muscariello E, La Rosa G, Di Maro M, Mondola P, Santillo M. Dual role of reactive oxygen species in muscle function: can antioxidant dietary supplements counteract age-related sarcopenia? Int J Mol Sci. (2019) 20:3815. doi: 10.3390/ijms20153815
8. Danieli MG, Antonelli E, Piga MA, Cozzi MF, Allegra A, Gangemi S. Oxidative stress, mitochondrial dysfunction, and respiratory chain enzyme defects in inflammatory myopathies. Autoimmun Rev. (2023) 22:103308. doi: 10.1016/j.autrev.2023.103308
9. Maugeri A, Hruskova J, Jakubik J, Kunzova S, Sochor O, Barchitta M, et al. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: A cross-sectional assessment in the Kardiovize study. Free Radical Biol Med. (2019) 131:274–81. doi: 10.1016/j.freeradbiomed.2018.12.018
10. Wu M, Si J, Liu Y, Kang L, Xu B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertension. (2023) 45:2233712. doi: 10.1080/10641963.2023.2233712
11. Wang M, Huang Z-H, Zhu Y-H, He P, Fan Q-L. Association between the composite dietary antioxidant index and chronic kidney disease: evidence from NHANES 2011-2018. Food Funct. (2023) 14:9279–86. doi: 10.1039/D3FO01157G
12. Zhao L, Zhang X, Guo S, Han K, Sun Y, Li X, et al. Relationship between composite dietary antioxidant index and depression among overweight and obese adults. J Affect Disord. (2023) 341:358–65. doi: 10.1016/j.jad.2023.08.140
13. Yu Y-C, Paragomi P, Wang R, Jin A, Schoen RE, Sheng L-T, et al. Composite dietary antioxidant index and the risk of colorectal cancer: Findings from the Singapore Chinese Health Study. Int J Cancer. (2022) 150:1599–608. doi: 10.1002/ijc.33925
14. Ma R, Zhou X, Zhang G, Wu H, Lu Y, Liu F, et al. Association between composite dietary antioxidant index and coronary heart disease among US adults: a cross-sectional analysis. BMC Public Health. (2023) 23:2426. doi: 10.1186/s12889-023-17373-1
15. Chen Y, Tang W, Li H, Lv J, Chang L, Chen S. Original Composite dietary antioxidant index negatively correlates with osteoporosis among middle-aged and older US populations. Am J Trans Res. (2023) 15:1300–8.
16. Centers for Disease Control and Prevention [CDC]. National health and nutrition examination survey 2024. Available online at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm#intro. (Accessed May 20, 2024).
17. Centers for Disease Control and Prevention [CDC]. NCHS research ethics review board approval 2024. Available online at: https://www.cdc.gov/nchs/nhanes/irba98.htm. (Accessed May 20, 2024).
18. Centers for Disease Control and Prevention [CDC]. Measuring guides for the dietary recall interview 2024. Available online at: https://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm#print. (Accessed May 20, 2024).
19. Ahuja JKC, Moshfegh AJ, Holden JM, Harris E. and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr. (2013) 143:241S–9S. doi: 10.3945/jn.112.170043
20. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. Journals Gerontology: Ser A. (2014) 69:547–58. doi: 10.1093/gerona/glu010
21. Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Activity Health. (2009) 6:790–804. doi: 10.1123/jpah.6.6.790
22. Feng X, Wu W, Zhao F, Xu F, Han D, Guo X, et al. Association between physical activity and kidney stones based on dose–response analyses using restricted cubic splines. Eur J Public Health. (2020) 30:1206–11. doi: 10.1093/eurpub/ckaa162
23. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Dietetics. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
24. Zhou Y, Gu K, Zhou F. Dietary flavonoid intake and cancer mortality: A population-based cohort study. Nutrients. (2023) 15:976. doi: 10.3390/nu15040976
25. Zhou N, Xie Z-P, Liu Q, Xu Y, Dai S-C, Lu J, et al. The dietary inflammatory index and its association with the prevalence of hypertension: A cross-sectional study. Front Immunol. (2023) 13. doi: 10.3389/fimmu.2022.1097228
26. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
27. Coletta G, Phillips SM. An elusive consensus definition of sarcopenia impedes research and clinical treatment: A narrative review. Ageing Res Rev. (2023) 86:101883. doi: 10.1016/j.arr.2023.101883
28. Jhee JH, Joo YS, Han SH, Yoo TH, Kang SW, Park JT. High muscle-to-fat ratio is associated with lower risk of chronic kidney disease development. J Cachexia Sarcopenia Muscle. (2020) 11:726–34. doi: 10.1002/jcsm.12549
29. Tonnesen PE, Mercaldo ND, Tahir I, Dietrich AW, Amayri W, Graur A, et al. Muscle reference values from thoracic and abdominal CT for sarcopenia assessment: the framingham heart study. Invest Radiol. (2024) 59:259–70. doi: 10.1097/RLI.0000000000001012
30. Wu D, Wang H, Wang W, Qing C, Zhang W, Gao X, et al. Association between composite dietary antioxidant index and handgrip strength in American adults: Data from National Health and Nutrition Examination Survey (NHANES, 2011-2014). Front Nutr. (2023) 10:1147869. doi: 10.3389/fnut.2023.1147869
31. Guo J, Shi L, Sun Y. Association of composite dietary antioxidant index and muscle mass in individuals with metabolic associated fatty liver disease. Clin Res Hepatol Gastroenterol. (2024) 48:102284. doi: 10.1016/j.clinre.2024.102284
32. Andreo-Lopez MC, Contreras-Bolivar V, Garcia-Fontana B, Garcia-Fontana C, Munoz-Torres M. The influence of the mediterranean dietary pattern on osteoporosis and sarcopenia. Nutrients. (2023) 15:3224. doi: 10.3390/nu15143224
33. Bigman G, Ryan AS. Healthy eating index-2015 is associated with grip strength among the US adult population. Nutrients. (2021) 13:3358. doi: 10.3390/nu13103358
34. Ghoreishy SM, Koujan SE, Hashemi R, Heshmat R, Motlagh AD, Esmaillzadeh A. Relationship between healthy eating index and sarcopenia in elderly people. BMC Geriatrics. (2023) 23:25. doi: 10.1186/s12877-023-03734-3
35. Huang CH, Okada K, Matsushita E, Uno C, Satake S, Martins BA, et al. Dietary patterns and muscle mass, muscle strength, and physical performance in the elderly: A 3-year cohort study. J Nutr Health Aging. (2021) 25:108–15. doi: 10.1007/s12603-020-1437-x
36. Mahmoodi M, Shateri Z, Nazari SA, Nouri M, Nasimi N, Sohrabi Z, et al. Association between oxidative balance score and sarcopenia in older adults. Sci Rep. (2024) 14:5362. doi: 10.1038/s41598-024-56103-4
37. Zhang H, Qi G, Wang K, Yang J, Shen Y, Yang X, et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem Pharmacol. (2023) 214:115664. doi: 10.1016/j.bcp.2023.115664
38. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Directors Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
39. Rimessi A, Previati M, Nigro F, Wieckowskic MR, Pinton P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int J Biochem Cell Biol. (2016) 81:281–93. doi: 10.1016/j.biocel.2016.06.015
40. Elizabeth Jimenez-Gutierrez G, Edith Martinez-Gomez L, Martinez-Armenta C, Pineda C, Angelica Martinez-Nava G, Lopez-Reyes A. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells. (2022) 11:2359. doi: 10.3390/cells11152359
41. Holloway GP. Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Sports Med. (2017) 47:S13–21. doi: 10.1007/s40279-017-0693-3
42. Santiago ECS, Roriz AKC, Ramos LB, Ferreira AJF, Oliveira CC, Gomes-Neto M. Comparison of calorie and nutrient intake among elderly with and without sarcopenia: A systematic review and meta-analysis. Nutr Rev. (2021) 79:1338–52. doi: 10.1093/nutrit/nuaa145
43. Garcia-Esquinas E, Carrasco-Rios M, Ortola R, Sotos Prieto M, Perez-Gomez B, Gutierrez-Gonzalez E, et al. Selenium and impaired physical function in US and Spanish older adults. Redox Biol. (2021) 38:101819. doi: 10.1016/j.redox.2020.101819
44. Besora-Moreno M, Llaurado E, Valls RM, Tarro L, Pedret A, Sola R. Antioxidant-rich foods, antioxidant supplements, and sarcopenia in old-young adults ≥ 55 years old: A systematic review and meta- analysis of observational studies and randomized controlled trials. Clin Nutr. (2022) 41:2308–24. doi: 10.1016/j.clnu.2022.07.035
45. Yoo S, Kim D-Y, Lim H. Sarcopenia in relation to nutrition and lifestyle factors among middle-aged and older Korean adults with obesity. Eur J Nutr. (2020) 59:3451–60. doi: 10.1007/s00394-020-02179-3
46. Passerieux E, Hayot M, Jaussent A, Carnac G, Gouzi F, Pillard F, et al. Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: A double-blind randomized controlled clinical trial. Free Radical Biol Med. (2015) 81:158–69. doi: 10.1016/j.freeradbiomed.2014.09.014
47. Lewis LN, Hayhoe RPG, Mulligan AA, Luben RN, Khaw KT, Welch AA. Lower dietary and circulating vitamin C in middle- and older-aged men and women are associated with lower estimated skeletal muscle mass. J Nutr. (2020) 150:2789–98. doi: 10.1093/jn/nxaa221
48. de Oliveira DCX, Rosa FT, Simões-Ambrósio L, Jordao AA, Deminice R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition. (2019) 63-64:29–35. doi: 10.1016/j.nut.2019.01.007
49. Liu S, Zhang L, Li S. Advances in nutritional supplementation for sarcopenia management. Front Nutr. (2023) 10. doi: 10.3389/fnut.2023.1189522
50. Sahni S, Dufour AB, Fielding RA, Newman AB, Kiel DP, Hannan MT, et al. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: The Framingham Offspring Study. Am J Clin Nutr. (2021) 113:437–45. doi: 10.1093/ajcn/nqaa288
51. van Dronkelaar C, Fultinga M, Hummel M, Kruizenga H, Weijs PJM, Tieland M. Minerals and sarcopenia in older adults: an updated systematic review. J Am Med Directors Assoc. (2023) 24:1163–72. doi: 10.1016/j.jamda.2023.05.017
52. Vega-Cabello V, Caballero FF, Lana A, Arias-Fernandez L, Banegas JR, Rodriguez-Artalejo F, et al. Association of zinc intake with risk of impaired physical function and frailty among older adults. Journals Gerontology Ser a-Biological Sci Med Sci. (2022) 77:2015–22. doi: 10.1093/gerona/glac014
53. Otsuka Y, Iidaka T, Horii C, Muraki S, Oka H, Nakamura K, et al. Dietary intake of vitamin E and fats associated with sarcopenia in community-dwelling older Japanese people: A cross-sectional study from the fifth survey of the ROAD study. Nutrients. (2021) 13:1730. doi: 10.3390/nu13051730
54. Mulligan AA, Hayhoe RPG, Luben RN, Welch AA. Positive associations of dietary intake and plasma concentrations of vitamin E with skeletal muscle mass, heel bone ultrasound attenuation and fracture risk in the EPIC-norfolk cohort. Antioxidants. (2021) 10:159. doi: 10.3390/antiox10020159
55. Chung E, Mo H, Wang S, Zu Y, Elfakhani M, Rios SR, et al. Potential roles of vitamin E in age-related changes in skeletal muscle health. Nutr Res. (2018) 49:23–36. doi: 10.1016/j.nutres.2017.09.005
Keywords: observational study, composite dietary antioxidant index, sarcopenia, NHANES, public health
Citation: Wang K, Zhou Q, Jiang Z, Liu S and Tang H (2024) The inverse associations between composite-dietary-antioxidant-index and sarcopenia risk in US adults. Front. Endocrinol. 15:1442586. doi: 10.3389/fendo.2024.1442586
Received: 02 June 2024; Accepted: 30 August 2024;
Published: 17 September 2024.
Edited by:
Angelica Giuliani, Marche Polytechnic University, ItalyReviewed by:
Jacopo Sabbatinelli, Università Politecnica delle Marche, ItalyDeborah Ramini, Italian National Research Center on Aging (INRCA-IRCCS), Italy
Matilde Sbriscia, Università Politecnica delle Marche, Italy, in collaboration with reviewer DR
Copyright © 2024 Wang, Zhou, Jiang, Liu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanfen Tang, dGFuZ2hhbmZlbjA4MjZAY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Kang Wang
Kang Wang Qin Zhou
Qin Zhou Zhongbiao Jiang3
Zhongbiao Jiang3 Hanfen Tang
Hanfen Tang