- 1Department of thyroid surgery, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, China
- 2Department of physical examination center, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, China
Background: Although some evidence suggests a role for vitamin C intake in thyroid diseases, the complex interplay between vitamin C intake and thyroid function remains incompletely understood. The objective of this study was to explore the relationship between vitamin C intake and serum thyroid function in the United States adults.
Methods: A total of 5,878 participants from the National Health and Nutrition Examination Survey (NHANES) between 2007 and 2012 were included in this study. Weighted multivariate linear regression models, subgroup analyses, and interaction terms were used to assess the association between vitamin C intake, evaluated as a continuous and categorical variable, and thyroid function. Additionally, restricted cubic spline (RCS) regression was employed to assess any nonlinear relationship that may exist between vitamin C intake and thyroid function.
Results: After adjusting for covariates, our research found a significant inverse correlation between vitamin C intake and total thyroxine (TT4) (β= -0.182, P= 0.006). Using subgroup analyses, the association was more pronounced among subjects with lower alcohol consumption(β= -0.151, P=0.013, P for interaction = 0.043). In RCS regression, the correlation between vitamin C and TT4 exhibited a distinct reversed L-shaped curve pattern in total participants (P for nonlinear = 0.005) and male adults (P for nonlinear = 0.014). Additionally, we found an inverted U-shaped curve pattern in the relationship between vitamin C intake and FT4 (P for nonlinear = 0.029), while an U-shaped curve relationship was observed between vitamin C consumption and the FT3/FT4 ratio (P for nonlinear = 0.026).
Conclusion: The findings of our study have illustrated a notable correlation between vitamin C intake and thyroid function. A high level of vitamin C intake is associated with a decreased in TT4 levels among American adults, and the association was more pronounced among subjects with lower alcohol consumption. Furthermore, our analysis revealed a nonlinear correlation between the intake of vitamin C and the levels of TT4, FT4, and FT3/FT4 ratio. Our findings support the rationale for making food-based dietary recommendations and maybe provide guidance for diet guidelines with thyroid dysfunction to a certain extent in the future.
1 Introduction
Thyroid, a crucial endocrine gland, regulates various functions in the body, such as metabolism, growth, and development through the secretion of thyroid hormones (1). The incidence of thyroid dysfunction varies by factors including age (2), sex (3), diet (4) geography (5), and genetics (6). For example, women are more likely to have thyroid problems at certain ages, such as puberty, pregnancy, and menopause, due to fluctuating hormone levels. In addition, factors such as environmental pollution (7), poor living habits (8), and trace elements (9, 10) may also increase the risk of thyroid disease. According to epidemiological data, the incidence of thyroid-related diseases has increased and become a health concern of global worldwide. A nationally representative cross-sectional study has revealed that the overall prevalence of thyroid dysfunction among adults stands at 15.17%. Specifically, subclinical hypothyroidism accounts for 12.93% of the cases, overt hypothyroidism comprises about 1.02%, subclinical hyperthyroidism constitutes 0.44%, and overt hyperthyroidism represents 0.78% of the total prevalence (11).
Vitamin C is an essential nutrient that plays a pivotal role in various biochemical processes within the body, including enzymatic activation, antioxidant reactions, and the maintenance of immune functions (12, 13). Unlike other animals, humans lack the ability to synthesize vitamin C internally. Therefore, we must obtain sufficient amounts of vitamin C from food or supplements to maintain good health. In recent years, there has been increasing interest in exploring the potential link between vitamin C and thyroid function and thyroid disorders. Several studies have shown that vitamin C kills thyroid cancer cells by inhibiting signaling pathways via distinct mechanisms (14–16). In addition, lack of vitamin C can make thyroid tissue more susceptible to oxidative stress, which can increase the risk of goiter development. And appropriate supplementation of vitamin C can effectively reduce this oxidative stress and lower the incidence of goiter (17).
The majority of existing studies have concentrated on the effects of vitamin C on specific thyroid disorders or its interaction with thyroid medications, rather than exploring its broader impact on thyroid function and hormone levels. Therefore, the objective of this study was to investigate the correlation between vitamin C intake and serum thyroid function among adults in the United States, employing data obtained from the National Health and Nutrition Examination Survey (NHANES).
2 Methods
2.1 Study population
NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States. Through a combination of interviews and physical examinations, NHANES provides insights into the prevalence of major diseases and conditions, dietary and nutritional patterns, and health-related behaviors in the United States. Conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC), all participants were required to sign the informed consent document prior to the commencement of the investigation.
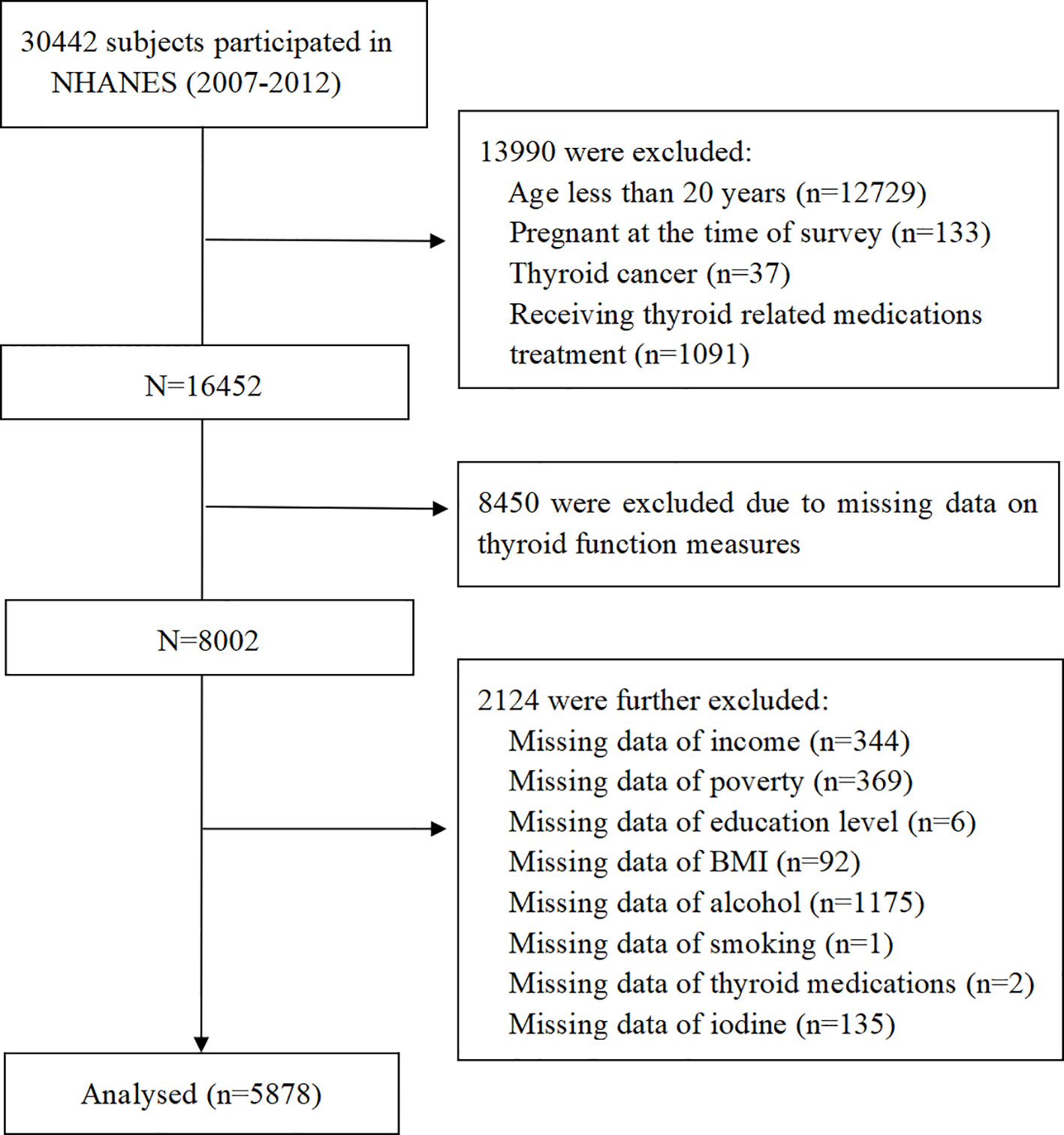
For the current study, a total of 30,442 participants were identified and selected from the NHANES cycles between 2007 and 2012. Exclusion criteria were: (1) age of participants was < 20 years or participants were currently pregnant; (2) participants with thyroid cancer or receiving thyroid-related medication treatment; (3) participants without thyroid measurement data; (4) participants without vitamin C intake data; (5) participants with missing covariate values. Finally, 5,878 participants were incorporated into our analysis (Figure 1).
2.2 Serum thyroid measure
The thyroid panel comprises a series of tests intended for evaluating thyroid gland activity. This panel includes determinations of thyroid stimulating hormone (TSH), total thyroxine (TT4), free thyroxine (FT4), total triiodothyronine (TT3), free triiodothyronine (FT3), thyroglobulin antibodies (TgAb), and thyroid peroxidase antibodies (TPOAb). Thyroid blood specimens were processed, stored and shipped to University of Washington, Seattle, WA. Detailed specimen collection and processing instructions are discussed in the NHANES Laboratory/Medical Technologists Procedures Manual (LPM).
2.3 Dietary vitamin C intake assessment
Dietary vitamin C intake was derived from the Food and Nutrient Database for Dietary Studies (FNDDS), which is utilized to code and analyze dietary intakes for the What We Eat In America (WWEIA), NHANES. Dietary vitamin C intake was calculated based on the average of two 24-hour dietary recalls. The initially collected recall was obtained in-person at the Mobile Examination Center (MEC), while the second interview was conducted over the phone after an interval of 3 to 10 days. In this study, the total intake of vitamin C includes a variety of foods and vitamin C supplements. Furthermore, due to the non-normal distribution characteristics of the vitamin C intake data, we implemented logarithmic transformations in order to obtain normal distributions of the data (named lnVc), which was subsequently confirmed by the conduct of Shapiro-Wilk tests. The lnVc were categorized into quartiles as follows: quartile 1 (0-1.508); quartile 2 (1.509-1.828); quartile 3 (1.829-2.074), and quartile 4 (2.075-3.037).
2.4 Covariates
According to the previous studies, we considered as confounding factors, information on demographics, socioeconomic status, diet, and health conditions, such as age, gender, race/ethnicity, education level, annual family income, alcohol consumption, poverty to income ratio (PIR), body mass index (BMI), urinary iodine concentration (UIC) and smoking (18). Considering the effects of other trace elements on the thyroid function, we also included dietary vitamin A(Va) (19), vitamin D(Vd) (20), vitamin E(Ve) (21), zinc(Zn) (22) and selenium(Se) (23) intake in this study.
Age was grouped into three groups: 20-40, 41-60 and more than 60 years. Education level was classified into three categories: less than high school, high school graduate, more than high school. Race was grouped into non-Hispanic white, non-Hispanic black, Mexican American, and others. Annual family income was divided into three groups (< 25,000, 25,000 to 74,999 and ≥75,000$). Poverty was defined as PIR less than 1.0, and PIR is further classified into four categories(<1.0, 1.0-1.9, 2.0-3.9, ≥4.0). BMI was categories as <25.0, 25.0–29.9, and ≥30 kg/m2. Alcohol consumption was defined as the average daily intake of any type of alcoholic beverage over the past year. A previous study on thyroid function have classified alcohol consumption into three categories of ≤50 g/day, 51 to 100 g/day, and > 100 g/day, and we adopt this classification method as well (24). UIC to determine iodine conditions in participants, which was classified as <100, 100-299 and ≥300 ug/L (25). Smoking habits were categorized as never, former, or now.
2.5 Statistical analysis
All statistical analyses were performed using the R package. Continuous variables were expressed in terms of the mean (standard deviation, SD), while categorical variables were summarized as frequency and percentage. With regard to the baseline characteristics, categorical variables were analyzed using the chi-square test for comparison, and continuous variables were analyzed using the one-way analysis of variance.
Weighted multivariate linear regression models were implemented to analyze the potential correlation between lnVc consumption and thyroid function indices. Model 1 was unadjusted for any covariates, whereas Model 2 underwent adjustments for a comprehensive array of factors including age, gender, education level, race/ethnicity, PIR, BMI, UIC, alcohol consumption, annual family income and smoking habits. Based on Model 2, Model 3 further adjusted for additional confounders, including Va, Vd, Ve, Zn and Se. Subgroup analysis, interaction tests and restricted cubic spline (RCS) regression were employed to evaluate the nonlinear association between lnVc and thyroid function. P value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline participant characteristics
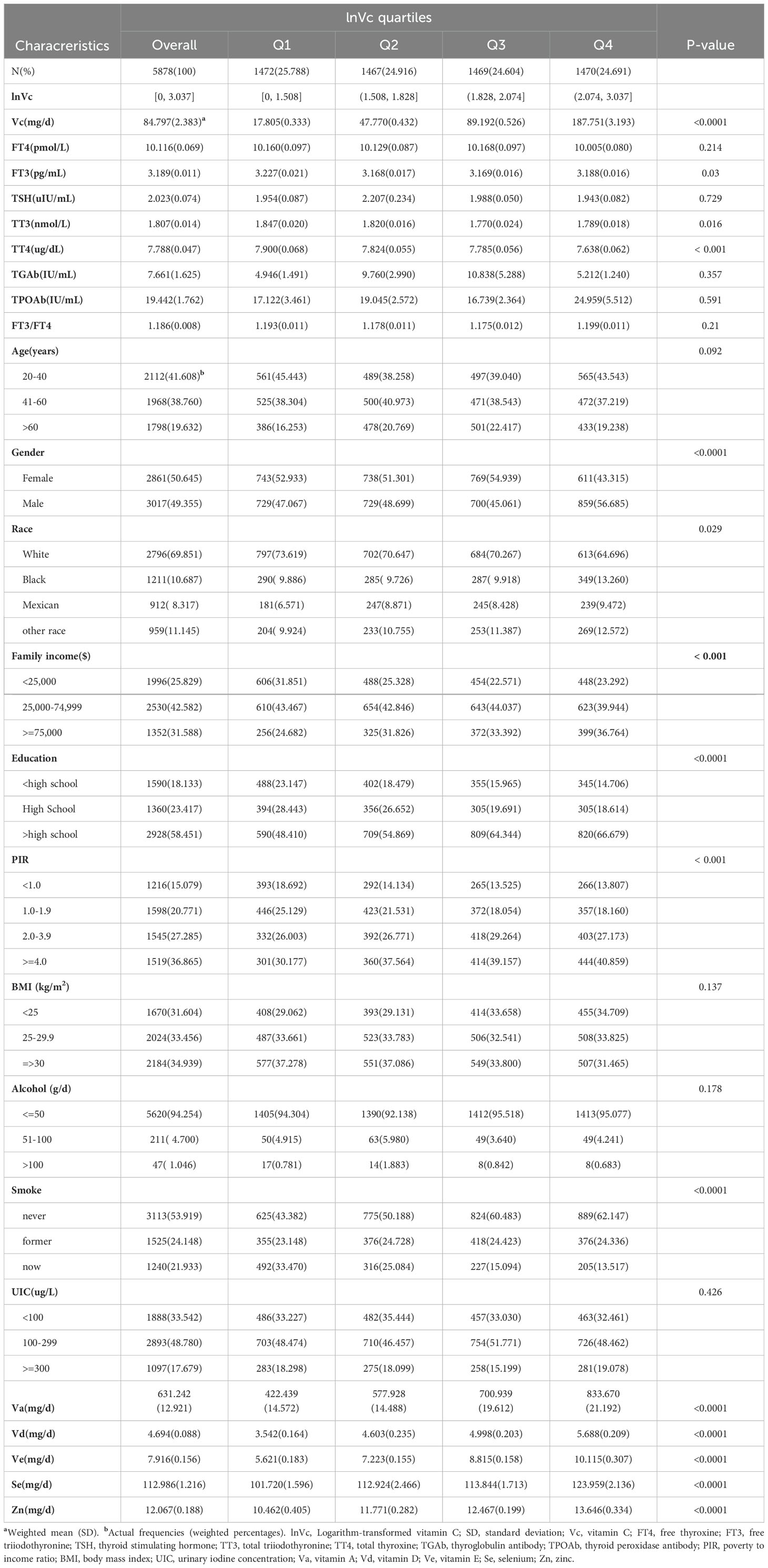
This study included 5,878 US participants aged 20 years or older from NHANES (2007-2012). The baseline characteristics of participants grouped by lnVc quartiles are presented in Table 1. The mean dietary vitamin C intake among these participants was 84.797(2.383) mg/day. For demographic, socioeconomic and dietary condition information, participants in different lnVc quartiles have different Va, Vd, Ve, Zn, Se, gender, race/ethnicity, PIR, annual family income, education level and smoking habits (all P<0.05). For thyroid function indices, differences in FT3, TT3, and TT4 were statistically significant (all P<0.05).
3.2 The correlation between lnVc and thyroid function
The correlation between lnVc and thyroid hormone levels was illustrated in Table 2. In Model 1, a negative relationship was observed between lnVc and TT4 [β= -0.208, 95% CI= (-0.321, -0.094), P<0.001]. After adjusting for a comprehensive array of factors including age, gender, education level, race/ethnicity, PIR, BMI, UIC, alcohol consumption, annual family income and smoking habits in Model 2, this negative association was remained [β= -0.204, 95% CI= (-0.312, -0.096), P<0.001]. Further adjustments for additional confounders in Model 3, including Va, Vd, Ve, Zn and Se, the negative association also remained significant [β= -0.182, 95% CI= (-0.305, -0.059), P= 0.006]. Furthermore, when lnVc was categorized, the negative trend remained statistically significant (P for trend = 0.001). Compared with individuals in quartile 1(Q1), those in quartile 4(Q4) consistently displayed reduced TT4 levels in all models.
In Model 1, lnVc exhibited a negative correlation solely with TT3 [β= -0.057, 95% CI=(-0.100, -0.014), P=0.011]. When lnVc was treated as a categorical variable in Model 1, this inverse relationship remained significant (P for trend = 0.004). However, upon adjusting for potential confounders in Models 2 and 3, this association was no longer observed.
In contrast, when lnVc was used as a categorical variable, the participants in quartile 4(Q4) had a lower FT4 than those in quartile 1(Q1) in Model 2 (P for trend = 0.01) and Model 3 (P for trend = 0.008).
3.3 RCS analysis
The relationship between lnVc and thyroid hormone levels was further scrutinized through the application of RCS curves. Initially, upon adjusting for potential confounding factors, we observed a reverse U-shaped curve association (P for nonlinear = 0.029) between lnVc and FT4 (Figure 2C). Regarding the strong inverted U-shaped relationship, Figure 2C showed an increase of FT4 levels with increasing lnVc levels, which reached the highest around 1.575 and then decreased thereafter. In addition, the inflection point for lnVc levels in females was 1.811 (P for nonlinear = 0.033) (Figure 2D).
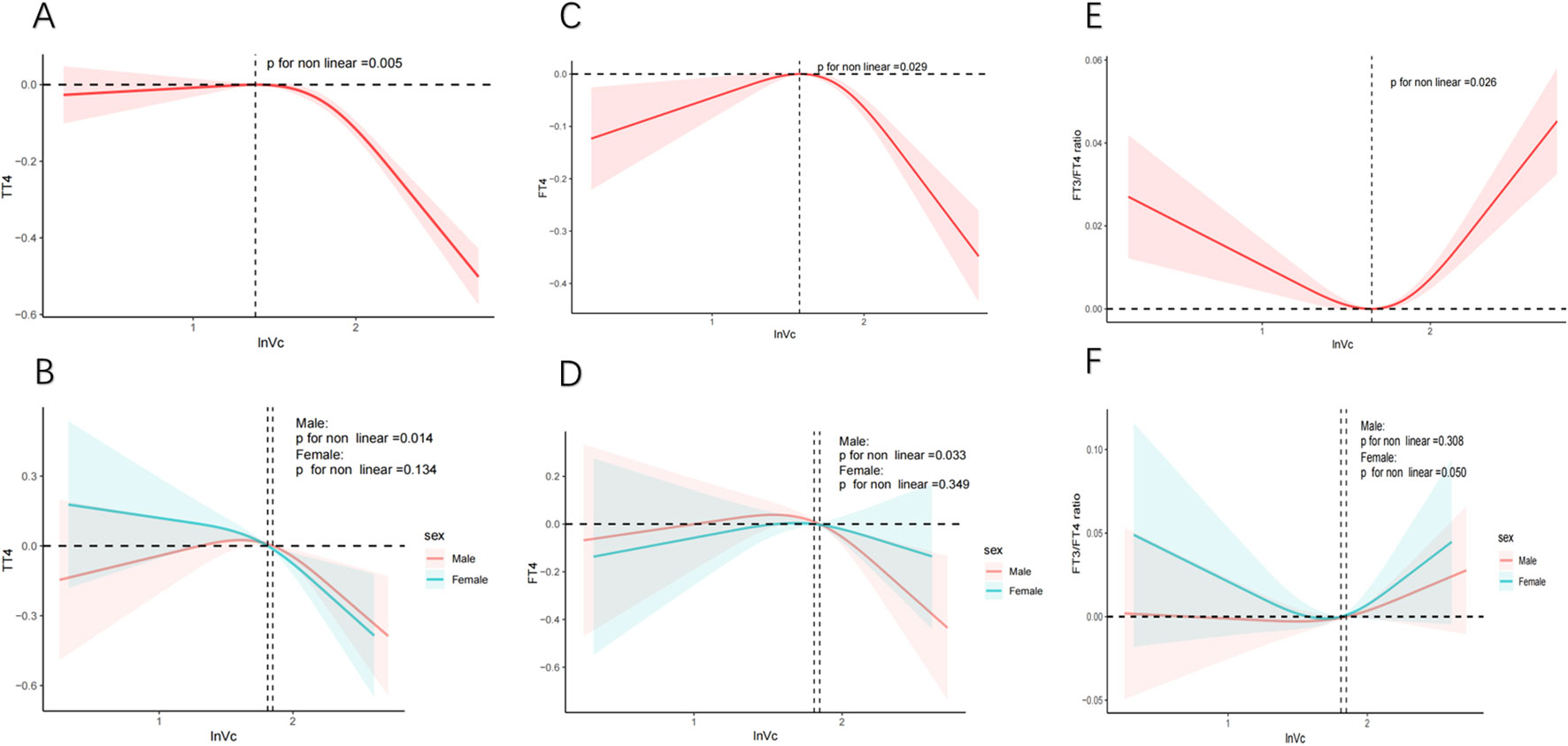
Figure 2. RCS analysis of nonlinear association between lnVc and thyroid function. Notes: The solid line represents the RCS curves between lnVc and thyroid function. The red or blue shaded area symbolize the 95% confidence intervals. Models adjust for age, gender, education level, race/ethnicity, PIR, BMI, UIC, alcohol consumption, annual family income, smoking habits,Va, Vd, Ve, Zn and Se. (A) TT4 of total adults; (B) TT4 of male or female adults; (C) FT4 of total adults; (D) FT4 of male or female adults; (E) FT3/FT4 of total adults; (F) FT3/FT4 of male or female adults.
Second, an U-shaped curve relationship was noticed (P for nonlinear = 0.026) between lnVc and FT3/FT4 ratio (Figure 2E). When the lnVc level was 1.652, the FT3/FT4 ratio was the lowest. And, there was no significant difference between the genders (Figure 2F).
The correlation between lnVc and TT4 exhibited a distinct reversed L-shaped curve pattern in total participants(P for nonlinear = 0.005, Figure 2A) and male adults (P for nonlinear = 0.014, Figure 2B). However, we did not find a nonlinear correlation among TSH, FT3, TT3, TPOAb, TgAb, and lnVc (Supplementary Figures S2).
3.4 Subgroup analysis
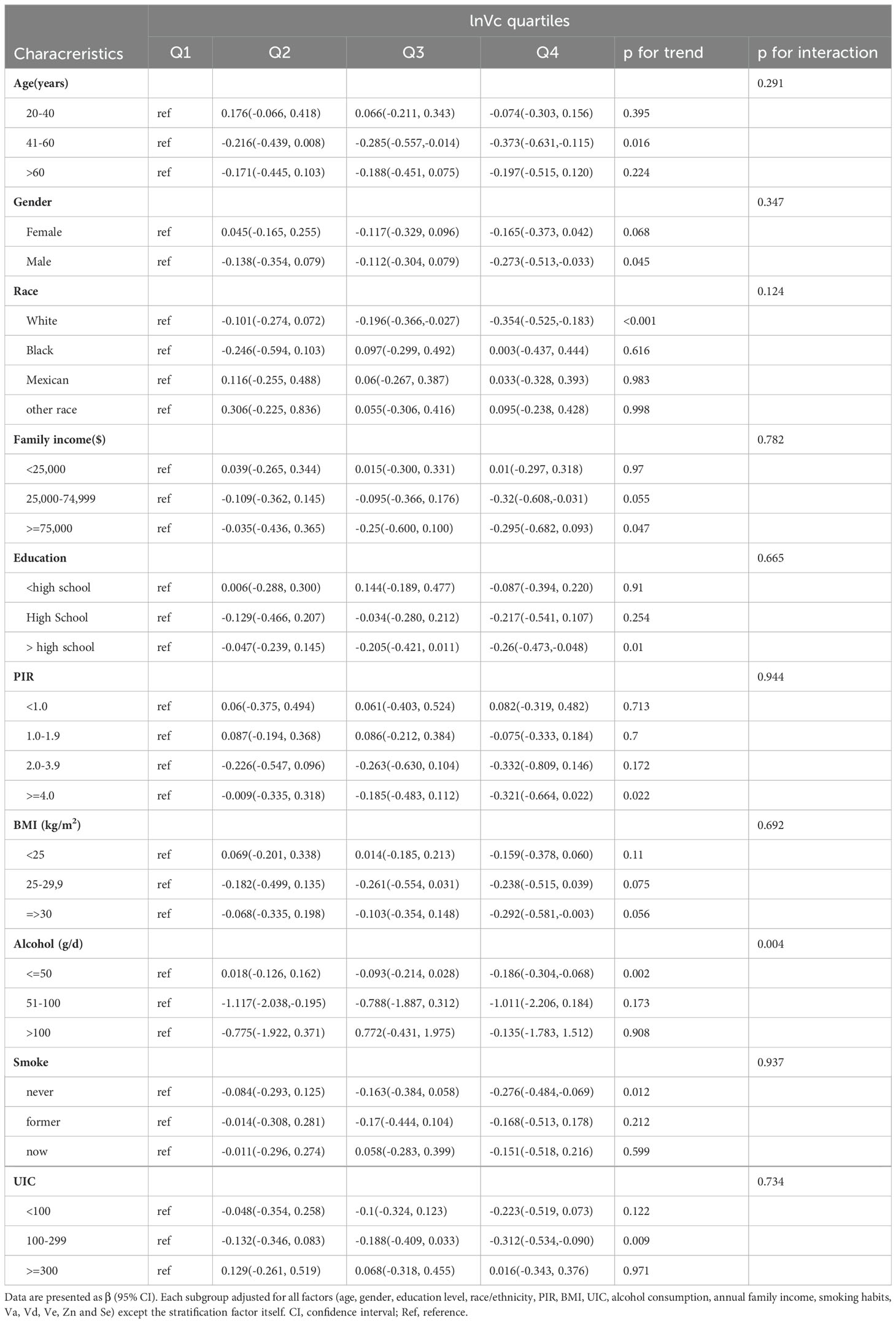
Our research revealed a negative correlation between lnVc and TT4. To further explore this correlation among diverse population groups, we conducted a subgroup analysis utilizing multivariable linear regression. The outcomes of this stratified analysis were presented in Figure 3 and Table 3.
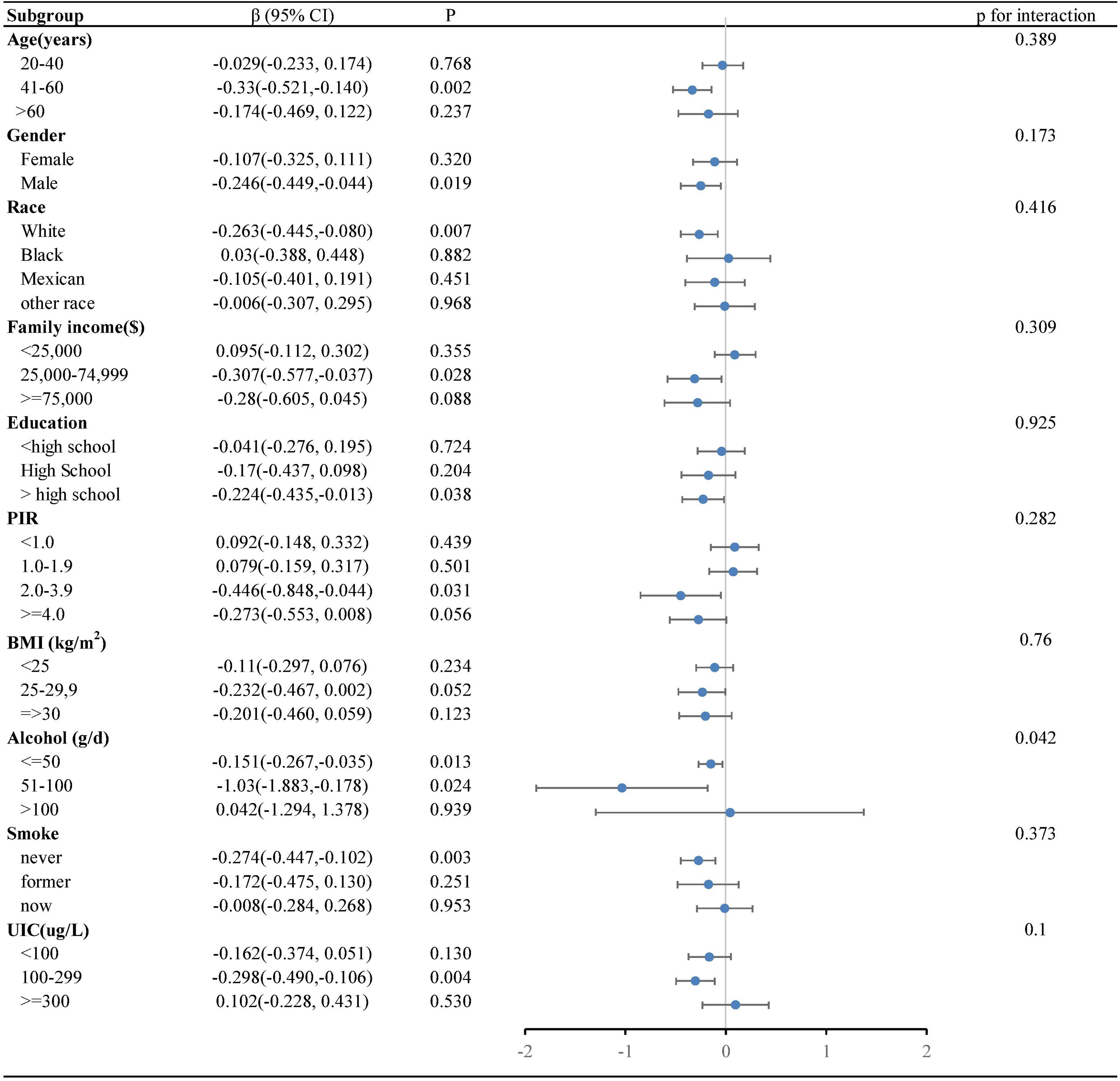
Figure 3. Forest plot of the relationship between lnVc and TT4 in each subgroup. Each subgroup adjusted for all factors (age, gender, education level, race/ethnicity, PIR, BMI, UIC, alcohol consumption, annual family income, smoking habits,Va, Vd, Ve, Zn and Se) except the stratification factor itself.
In the subgroup analysis (Figure 3), alcohol consumption plays a pivotal role in modulating the association between lnVc and TT4. Remarkably, this significant association was more apparent in subjects with lower alcohol consumption[β= -0.151, 95% CI= (-0.267, -0.035), P=0.013, P for interaction = 0.042]. Furthermore, when lnVc was used as a categorical variable (Table 3), the groups with lower alcohol consumption (< 50 g/d) and higher lnVc consumption showed a more substantial reduction in TT4 levels compared to other groups[β= -0.186, 95% CI= (-0.304, -0.068), P = 0.002, P for interaction = 0.004].
4 Discussion
This cross-sectional study was conducted to investigate the potential association between vitamin C intake and thyroid function among 5,878 participants from the NHANES between 2007 and 2012. The findings of this study demonstrated a significant inverse correlation between vitamin C intake and TT4 levels. Furthermore, the subgroup analysis and interaction terms suggested that the association was more pronounced in subjects with lower alcohol consumption. In addition, our investigation also uncovered a nonlinear relationship between vitamin C and thyroid function markers, including TT4, FT4,and the FT3/FT4 ratio.
In previous studies, most scholars focused on studying the relationship between vitamin C intake and thyroid cancers (16, 26), goitre (17), benign thyroid diseases (27), hyperthyroidism and hypothyroidism (28), with few exploring the effects of vitamin C intake on thyroid function in normal individuals. Karimi et al. (29) showed that the concentration of anti-thyroid peroxidase antibodies significantly decreased in autoimmune thyroiditis patients treated with vitamin C compared to those receiving a placebo. Jubiz et al. (30) evaluated the effect of vitamin C administration in hypothyroid patients with elevated TSH levels, showing that vitamin C can increase serum T4, T3, and decrease TSH. The findings of these studies support the potential role of vitamin C in the treatment of thyroid-related diseases.
Our research revealed a significant inverse correlation between vitamin C intake and TT4, but we did not observe significant differences in TPOAb and TSH levels between the four groups. This could be attributed to several factors. First, it is possible that the participants in our study did not have sufficient vitamin C intake. Low levels of vitamin C in the body may limit its beneficial effects on thyroid function, even with supplementation. Furthermore, the dose and duration of vitamin C intake in our study may have been insufficient to observe significant changes in TPOAb and TSH levels. In the studies mentioned, participants were administered 500mg of vitamin C daily for months. In contrast, our study only used the average vitamin C intake over a period of two days. Longer-term and higher-dose vitamin C supplementation may be necessary to observe significant improvements in thyroid function and auto-antibody levels. Moreover, our study population consisted of individuals with normal thyroid function. It is possible that the effects of vitamin C on thyroid function may be more pronounced in individuals with pre-existing thyroid conditions.
In our study, we found an inverted U-shaped curve relationship between vitamin C and FT4, while an U-shaped curve relationship was noticed between vitamin C and FT3/FT4 ratio. An increased of FT4 levels with increasing lnVc levels, which reached the highest around 1.575 and then decreased thereafter. When the lnVc levels were less than 1.652, the FT3/FT4 ratio decreased with the increase of lnVc levels. The Food and Drug Administration (FDA) increased the vitamin C recommendations to 90 mg/day for men and 75 mg/day for women. And the study suggests that raising the existing daily recommended amount of vitamin C to 200 milligrams per day would have a positive impact on the function of the immune system (31). Excessive intake of vitamin C may also have adverse side effects, such as kidney stones, nausea, and diarrhea (32, 33).
The subgroup analysis and interaction terms indicated that the association between vitamin C intake and TT4 levels was more apparent in subjects with lower alcohol consumption. This finding suggests that alcohol consumption plays a pivotal role in modulating the association between vitamin C and thyroid function. Previous studies have shown that vitamin C levels are decreased in alcoholics, excessive alcohol consumption can interfere with the absorption and utilization of vitamin C, leading to its depletion in the body (34–37).
Moreover, gender-specific subgroup analysis showed that women are more likely to be affected from vitamin C intake in maintaining healthy thyroid function. However, no significant interaction effects were observed. Previous studies have demonstrated that the prevalence of thyroid disorders, including hypothyroidism, hyperthyroidism (38), thyroid nodules (39) and thyroid cancer (40), are vary significantly between men and women. These finding could be attributed to gender-related differences in thyroid physiology and metabolism, as well as dietary habits and hormonal status (41–43). Metsios et al. (44) found that a brief exposure to moderate passive smoke significantly increased the TT3 and FT4 levels compared to the control group. Gruppen et. al (45) studies also demonstrated that smokers with ≤20 cigarettes per day were associated with higher serum FT4, FT3 and lower TSH compared to nonsmokers. In our study, subgroup analysis revealed that the impact of vitamin C on thyroid function was particularly evident among non-smokers. This suggests that smokers may benefit more from increasing their intake of vitamin C to maintain healthy thyroid function.
However, there are also limitations to using the NHANES database for this research. Firstly, the vitamin C intake relies on self-reported dietary intake data, which may be subject to inaccuracies and biases. This can affect the reliability and validity of the findings. Secondly, even though we have tried our best to eliminate potential confounders, there are still some factors that cannot be fully controlled. These factors may have an impact on the relationship between vitamin C and thyroid function. Finally, we are unable to firmly establish the precise causal relationship between vitamin C intake and thyroid function due to the retrospective cross-sectional nature of this study.
5 Conclusion
In conclusion, this cross-sectional study suggested the association between vitamin C and thyroid function. A high level of vitamin C intake is associated with a decrease in TT4 levels among adults in the United States, and the association was more pronounced in subjects with lower alcohol consumption. In addition, our analysis revealed a nonlinear correlation between the intake of vitamin C and the levels of TT4, FT4, and FT3/FT4 ratio. In the future, more studies are needed to further explore the causal relationship.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JW: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. CJ: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. QW: Formal analysis, Investigation, Methodology, Writing – review & editing. XL: Data curation, Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We appreciate the participants and staff of NHANES.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1462251/full#supplementary-material
References
1. Prezioso G, Giannini C, Chiarelli F. Effect of thyroid hormones on neurons and neurodevelopment. Horm Res Paediatr. (2018) 90:73–81. doi: 10.1159/000492129
2. Fu J, Zhang G, Xu P, Guo R, Li J, Guan H, et al. Seasonal changes of thyroid function parameters in women of reproductive age between 2012 and 2018: A retrospective, observational, single-center study. Front Endocrinol (Lausanne). (2021) 12:719225. doi: 10.3389/fendo.2021.719225
3. Chaker L, Korevaar TI, Medici M, Uitterlinden AG, Hofman A, Dehghan A, et al. Thyroid function characteristics and determinants: the rotterdam study. Thyroid. (2016) 26:1195–204. doi: 10.1089/thy.2016.0133
4. Bellastella G, Scappaticcio L, Caiazzo F, Tomasuolo M, Carotenuto R, Caputo M, et al. Mediterranean diet and thyroid: an interesting alliance. Nutrients. (2022) 14:4130. doi: 10.3390/nu14194130
5. Deng Y, Li H, Wang M, Li N, Tian T, Wu Y, et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw Open. (2020) 3:e208759. doi: 10.1001/jamanetworkopen.2020.8759
6. Medici M, Visser TJ, Peeters RP. Genetics of thyroid function. Best Pract Res Clin Endocrinol Metab. (2017) 31:129–42. doi: 10.1016/j.beem.2017.04.002
7. Benvenga S, Antonelli A, Vita R. Thyroid nodules and thyroid autoimmunity in the context of environmental pollution. Rev Endocr Metab Disord. (2015) 16:319–40. doi: 10.1007/s11154-016-9327-6
8. Yeo Y, Han K, Shin DW, Kim D, Jeong SM, Chun S, et al. Changes in smoking, alcohol consumption, and the risk of thyroid cancer: A population-based korean cohort study. Cancers (Basel). (2021) 13:2434. doi: 10.3390/cancers13102343
9. Zhou Q, Xue S, Zhang L, Chen G. Trace elements and the thyroid. Front Endocrinol (Lausanne). (2022) 13:904889. doi: 10.3389/fendo.2022.904889
10. Wróblewski M, Wróblewska J, Nuszkiewicz J, Pawłowska M, Wesołowski R, Woźniak A. The role of selected trace elements in oxidoreductive homeostasis in patients with thyroid diseases. Int J Mol Sci. (2023) 24:4840. doi: 10.3390/ijms24054840
11. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of mainland China. Thyroid. (2020) 30:568–79. doi: 10.1089/thy.2019.0067
12. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. (2017) 9:1211. doi: 10.3390/nu9111211
13. Kaźmierczak-Barańska J, Boguszewska K, Adamus-Grabicka A, Karwowski BT. Two faces of vitamin C-antioxidative and pro-oxidative agent. Nutrients. (2020) 12:1501. doi: 10.3390/nu12051501
14. Su X, Li P, Han B, Jia H, Liang Q, Wang H, et al. Vitamin C sensitizes BRAF(V600E) thyroid cancer to PLX4032 via inhibiting the feedback activation of MAPK/ERK signal by PLX4032. J Exp Clin Cancer Res. (2021) 40:34. doi: 10.1186/s13046-021-01831-y
15. Su X, Shen Z, Yang Q, Sui F, Pu J, Ma J, et al. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics. (2019) 9:4461–73. doi: 10.7150/thno.35219
16. Tronci L, Serreli G, Piras C, Frau DV, Dettori T, Deiana M, et al. Vitamin C cytotoxicity and its effects in redox homeostasis and energetic metabolism in papillary thyroid carcinoma cell lines. Antioxidants (Basel). (2021) 10:809. doi: 10.3390/antiox10050809
17. Alicigüzel Y, Ozdem SN, Ozdem SS, Karayalçin U, Siedlak SL, Perry G, et al. Erythrocyte, plasma, and serum antioxidant activities in untreated toxic multinodular goiter patients. Free Radic Biol Med. (2001) 30:665–70. doi: 10.1016/s0891-5849(00)00509-8
18. Soldin OP, Goughenour BE, Gilbert SZ, Landy HJ, Soldin SJ. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. (2009) 19:817–23. doi: 10.1089/thy.2009.0023
19. Capriello S, Stramazzo I, Bagaglini MF, Brusca N, Virili C, Centanni M. The relationship between thyroid disorders and vitamin A.: A narrative minireview. Front Endocrinol (Lausanne). (2022) 13:968215. doi: 10.3389/fendo.2022.968215
20. Li Y, Sun J, Jiao Y, Li N, Zhao W. Impaired sensitivity to thyroid hormones is associated with decreased vitamin D levels in the euthyroid population. J Clin Endocrinol Metab. (2024) 109:691–700. doi: 10.1210/clinem/dgad607
21. Liu S, Lu C, He L, Shan Z, Teng W, Li Y, et al. Vitamin E intake and prevalence rates of thyroid dysfunction and autoimmune thyroiditis: A cross-sectional analysis of NHANES data. Thyroid. (2024) 34:753–63. doi: 10.1089/thy.2023.0561
22. Beserra JB, Morais JBS, Severo JS, Cruz KJC, de Oliveira ARS, Henriques GS, et al. Relation between zinc and thyroid hormones in humans: a systematic review. Biol Trace Elem Res. (2021) 199:4092–100. doi: 10.1007/s12011-020-02562-5
23. Liu F, Wang K, Nie J, Feng Q, Li X, Yang Y, et al. Relationship between dietary selenium intake and serum thyroid function measures in U.S. adults: Data from NHANES 2007-2012. Front Nutr. (2022) 9:1002489. doi: 10.3389/fnut.2022.1002489
24. Wu J, Jia C, Zhang Z, Hou Z, Cui Y. The relationship between dietary total flavonoids and thyroid function in U.S.adults, NHANES 2007-2010. PloS One. (2024) 19:e0303169. doi: 10.1371/journal.pone.0303169
25. World Health O. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: World Health Organization (2007).
26. Guo D, Liao Y, Na J, Wu L, Yin Y, Mi Z, et al. The involvement of ascorbic acid in cancer treatment. Molecules. (2024) 29:2295. doi: 10.3390/molecules29102295
27. Moncayo R, Kroiss A, Oberwinkler M, Karakolcu F, Starzinger M, Kapelari K, et al. and zinc in benign thyroid diseases and of selenium in Malignant thyroid diseases: Low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma. BMC Endocr Disord. (2008) 8:2. doi: 10.1186/1472-6823-8-2
28. Erdamar H, Demirci H, Yaman H, Erbil MK, Yakar T, Sancak B, et al. The effect of hypothyroidism, hyperthyroidism, and their treatment on parameters of oxidative stress and antioxidant status. Clin Chem Lab Med. (2008) 46:1004–10. doi: 10.1515/cclm.2008.183
29. Karimi F, Omrani GR. Effects of selenium and vitamin C on the serum level of antithyroid peroxidase antibody in patients with autoimmune thyroiditis. J Endocrinol Invest. (2019) 42:481–7. doi: 10.1007/s40618-018-0944-7
30. Jubiz W, Ramirez M. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metab. (2014) 99:E1031–4. doi: 10.1210/jc.2013-4360
31. Eggersdorfer M. What is the optimal intake of vitamin C? Proc Nutr Soc. (2020) 79:E622. doi: 10.1017/S0029665120005716
32. Khoshnam-Rad N, Khalili H. Safety of vitamin C in sepsis: a neglected topic. Curr Opin Crit Care. (2019) 25:329–33. doi: 10.1097/mcc.0000000000000622
33. Sestili MA. Possible adverse health effects of vitamin C and ascorbic acid. Semin Oncol. (1983) 10:299–304.
34. Navasumrit P, Ward TH, Dodd NJ, O’Connor PJ. Ethanol-induced free radicals and hepatic DNA strand breaks are prevented in vivo by antioxidants: effects of acute and chronic ethanol exposure. Carcinogenesis. (2000) 21:93–9. doi: 10.1093/carcin/21.1.93
35. Suresh MV, Sreeranjit Kumar CV, Lal JJ, Indira M. Impact of massive ascorbic acid supplementation on alcohol induced oxidative stress in Guinea pigs. Toxicol Lett. (1999) 104:221–9. doi: 10.1016/s0378-4274(98)00377-4
36. Sandoval C, Farías J, Zamorano M, Herrera C. Vitamin supplements as a nutritional strategy against chronic alcohol consumption? An updated review. Antioxidants (Basel). (2022) 11:564. doi: 10.3390/antiox11030564
37. Susick RL Jr., Zannoni VG. Effect of ascorbic acid on the consequences of acute alcohol consumption in humans. Clin Pharmacol Ther. (1987) 41:502–9. doi: 10.1038/clpt.1987.65
38. Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. (2018) 14:301–16. doi: 10.1038/nrendo.2018.18
39. Li Y, Jin C, Li J, Tong M, Wang M, Huang J, et al. Prevalence of thyroid nodules in China: A health examination cohort-based study. Front Endocrinol (Lausanne). (2021) 12:676144. doi: 10.3389/fendo.2021.676144
40. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. (2022) 10:264–72. doi: 10.1016/s2213-8587(22)00035-3
41. Ferrari SM, Fallahi P, Antonelli A, Benvenga S. Environmental issues in thyroid diseases. Front Endocrinol (Lausanne). (2017) 8:50. doi: 10.3389/fendo.2017.00050
42. Liu N, Ma F, Feng Y, Ma X. The association between the dietary inflammatory index and thyroid function in U.S. Adult Males. Nutrients. (2021) 13:3330. doi: 10.3390/nu13103330
43. Sgrò P, Sansone M, Parisi A, Sartorio A, Sansone A, Romanelli F, et al. Supra-physiological rhGH administration induces gender-related differences in the hypothalamus-pituitary-thyroid (HPT) axis in healthy individuals. J Endocrinol Invest. (2016) 39:1383–90. doi: 10.1007/s40618-016-0489-6
44. Metsios GS, Flouris AD, Jamurtas AZ, Carrillo AE, Kouretas D, Germenis AE, et al. A brief exposure to moderate passive smoke increases metabolism and thyroid hormone secretion. J Clin Endocrinol Metab. (2007) 92:208–11. doi: 10.1210/jc.2006-0762
Keywords: vitamin C, thyroid function, alcohol, NHANES, cross-sectional analysis
Citation: Wu J, Jia C, Wang Q and Li X (2024) Association between vitamin C intake and thyroid function among U.S. adults: a population-based study. Front. Endocrinol. 15:1462251. doi: 10.3389/fendo.2024.1462251
Received: 09 July 2024; Accepted: 21 October 2024;
Published: 07 November 2024.
Edited by:
Joao Dts Anselmo, Hospital do Divino Espírito Santo, PortugalReviewed by:
Inês Mendes, Hospital do Divino Espírito Santo, PortugalI Machado, Divino Espirito Santo Hospital, Portugal
Copyright © 2024 Wu, Jia, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, bGl4aW5zeWp6eEAxNjMuY29t
†These authors share first authorship
 Jie Wu
Jie Wu Chuyu Jia
Chuyu Jia Qiang Wang
Qiang Wang Xin Li
Xin Li