- 1Sports Department, Changzhou Vocational Institute of Textile and Garment, Changzhou, Jiangsu, China
- 2Department of Physical Education, Soochow University, Suzhou, Jiangsu, China
Objective: This study aimed to assess the impact of nine exercise interventions (resistance training [BT, ball training [BT], resistance + walking [RT+W alk], resistance + running [RT + Running], resistance + cycling [RT + bicycle], running, and Tai Chi) on insulin sensitivity in patients with diabetes.
Methods: A systematic search of five databases (PubMed, EMBASE, Cochrane, Web of Science, and CNKI) for RCTs investigating the effects of exercise interventions on insulin sensitivity in patients with diabetes was conducted. The quality of the included studies was assessed using the Cochrane Manual version 5.1.0 Risk of Bias Assessment Tool (ROB). Data analysis software was used for the synthesis and analysis.
Results: This Meta-analysis comprised 21 randomized controlled trials involving 1140 participants. Cycling significantly reduced the fasting glucose index in individuals with diabetes (SUCRA score=90.7%). Resistance exercise exhibited superior efficacy in enhancing insulin sensitivity compared with alternative interventions in patients with diabetes (SUCRA score=71.8%). Furthermore, the combination of resistance exercise and running resulted in a noteworthy decrease in HOMA-IR levels (SUCRA score=64.2%).
Conclusion: Cycling, resistance training, and combined aerobic and resistance exercises have been shown to effectively enhance fasting blood glucose levels, insulin secretion, and insulin sensitivity in individuals with diabetes. However, additional studies with longer follow-up periods and more rigorous methodologies are required to further validate these findings.
Systematic review registration: https://ww.crd.york.ac.uk/PROSPERO/, identifier CRD42023450107.
1 Introduction
Diabetes has emerged as a critical global health challenge, with the 2023 Global Burden of Disease Study reporting approximately 529 million affected individuals worldwide and projecting a rise to 1.31 billion by 2050 (1). Type 2 diabetes (T2DM), characterized by insulin resistance and impaired insulin secretion, accounts for over 90% of diabetes cases and imposes substantial economic burdens exceeding $1 trillion USD annually in healthcare expenditures (2, 3).
Physical exercise is a cornerstone of T2DM management, with distinct modalities operating through specific physiological pathways to improve glycemic control. Aerobic exercise enhances insulin sensitivity primarily through GLUT4 translocation in the skeletal muscle, facilitating glucose uptake independent of insulin signaling (4). This process is amplified by mitochondrial biogenesis via the AMPK-PGC1α pathway, which improves oxidative capacity (5), while concurrent reductions in pro-inflammatory cytokines (TNF-α and IL-6) ameliorate adipose tissue dysfunction (6). Resistance training exerts complementary effects through muscle hypertrophy, which expands the glucose storage capacity (7), enhances post-receptor insulin signaling via IRS-1/PI3K/Akt phosphorylation cascades (8), and suppresses hepatic gluconeogenesis (9). Combined aerobic-resistance training synergizes these mechanisms, with recent meta-analyses confirming superior HbA1c reductions compared to single-modality interventions (Δ = -0.17%, p < 0.01) (10). Despite these advances, the comparative efficacy of specific exercise modalities is unclear. This network meta-analysis directly evaluated nine interventions, including resistance training, aerobic modalities (cycling and running), combined regimens, and mind-body exercises, to provide evidence-based guidance for optimizing exercise prescriptions in diabetes care.
2 Materials and methods
This systematic review was registered in the Prospero database (ID: CRD42023450107) under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses (PRISMA-NMA) and the Cochrane Intervention Review.
2.1 Search strategy
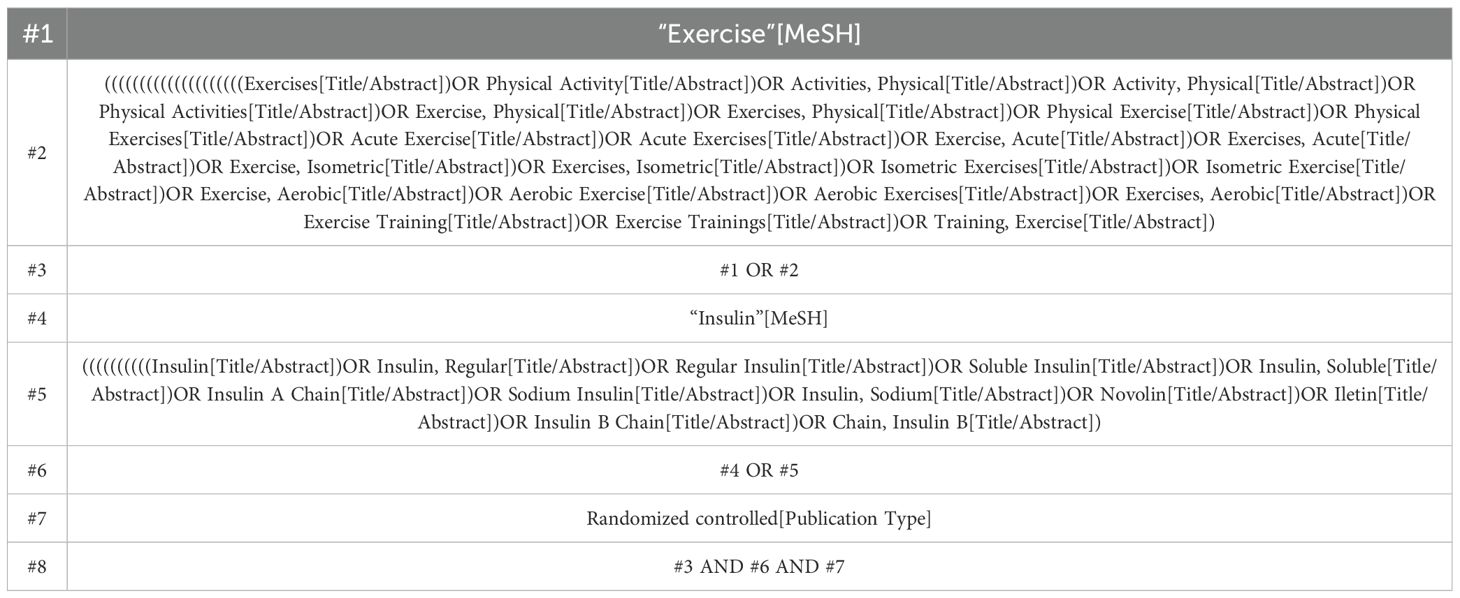
We conducted a comprehensive search across multiple databases, including PubMed, Embase, Cochrane Library, Web of Science, and CNKi, from January 2004 to December 2022, to identify eligible studies. The search keywords were formulated based on the PICOS framework, and the search strategies were developed by PICOS principles: (P) population, diabetic patients; (I) intervention, exercise; (C) comparator, control group receiving only usual care and appropriate rehabilitation measures (placebo or other forms of exercise); and (O) Outcome - Exercise tests in diabetic patients. Finally, we focused on randomized controlled trials as the preferred study design. Taking PubMed as an example, detailed search strategies are provided in Table 1.
2.1.1 Definition of exercise interventions
The nine exercise interventions evaluated in this study are abbreviated as follows:
RT: Resistance training
BT: Ball training
RT+Walk: Combined resistance training and walking
RT+Running: Combined resistance training and running
RT+Bicycle: Combined resistance training and cycling
Bicycle: Cycling training alone
Running: Running training alone
Taichi: Tai Chi practice
CON: Control group (no exercise intervention, routine care only)
All combined training involved sequential sessions of resistance and aerobic exercise within the same day.
2.2 Inclusion criteria
(1) Randomized controlled clinical trials involving patients with diabetes. (2) The experimental group utilizes various exercise methods as interventions for diabetes. (3) The control group receives conventional care only. (4) Active cooperation of participants in the experimental process is required. (5) Outcome measures include at least one of the following: Fasting blood glucose levels (FBG), Homeostasis Model Assessment of insulin Resistance (HOMA-IR), fasting insulin level (FI), and homeostasis model of insulin resistance.
2.3 Exclusion criteria
(1) Papers with incomplete or insufficient data or reporting information are excluded. (2) Non-randomized controlled trials, animal studies, conference reports, literature reviews, abstracts, and protocols are excluded.
2.4 Study selection
The two researchers used NoteExpress, a literature management software, to screen and exclude duplicate articles. Initially, they reviewed the titles and abstracts to exclude non-randomized controlled trials, systematic reviews, conference papers, protocols, and communications while retaining the remaining literature. Subsequently, both researchers independently read through the remaining literature and conducted further screening. Only when there was agreement on inclusion criteria did an article finally get included; otherwise, a third researcher was consulted for discussion and resolution.
2.5 Data extraction
Two researchers independently extracted the data and assessed study quality using the Cochrane Handbook, while a third individual addressed any issues that arose post-data extraction. The extracted data encompassed authorship details (author, year, country of publication), average age, sample size, intervention duration, and outcome indicators such as risk of bias assessment.
2.6 Risk of bias in individual studies
We assessed the literature quality based on the Risk Bias Assessment Tool (ROB) outlined in the Cochrane Manual 5.1.0, considering seven key domains for evaluating randomized controlled trials: (1) Random sequence generation, (2) Allocation concealment, (3) Blinding of participants and personnel, (4) Blinding of outcome assessors, (5) Handling of incomplete outcome data, (6) Selective outcome reporting, and (7) Other potential sources of bias.
2.7 Subgroup analysis and outcome indicators
We conducted a subgroup analysis to categorize the experiments based on medication status. Specifically, 13 trials received metformin treatment, five received insulin treatment, and the remaining three did not receive any hypoglycemic drugs. The findings of our meta-analysis remained robust across these subgroups, indicating that exercise intervention benefits blood glucose levels independently of drug therapy. Our primary outcome measure was the change in fasting plasma glucose (ΔFPG) levels from baseline (mmol/L). We also compared fasting insulin concentration (ΔFI; μU/ml) and HOMA-IR index (ΔHOMA-IR) between the experimental and control groups.
We quantified between-study heterogeneity using I² statistics. For fasting blood glucose (FBG), I² = 62% (95%CI: 48-75%), indicating moderate heterogeneity. For fasting insulin, I² = 45% (95%CI: 28-59%), suggesting low-moderate heterogeneity. For HOMA-IR, I² = 68% (95%CI: 52-80%), reflecting moderate heterogeneity. These values align with expected variations in exercise interventions across diverse populations.
2.8 Data analysis
Sensitivity analyses excluding studies with high/unclear risk of bias in ≥3 Cochrane domains (n=5 studies) confirmed robustness: FBG reduction with cycling [MD = -50.21 mmol/L, 95%CI -92.15 to -8.27], fasting insulin with RT vs. BT [MD = -25.94 μU/ml, 95%CI -49.83 to -2.05], and HOMA-IR ranking of RT+Running (SUCRA=62.1%). In our included studies involving various exercise interventions, all variables were continuous and expressed as the mean and standard deviation (SD) with a 95% confidence interval (CI) (11). The mean difference (MD) was used to represent the net change in the measured variables between the experimental and control groups, with a negative MD value indicating a greater reduction in the experimental group (12). A random-effects model was employed for the meta-analysis while calculating the SUCRA values to rank the interventions. Funnel plots were used to assess publication bias, and frequency analysis of random-effects models was conducted to evaluate the effectiveness of multiple interventions in addressing potential differences among studies (13).
The effectiveness of multiple interventions in addressing potential differences between studies was evaluated using a frequency analysis of random-effects models (13). Stata software (version 15.1) was employed to model four chains using the Markov chain Monte Carlo (MCMC) method. The fit and consistency of the model were assessed using the Deviation Information Criterion (DIC). Network diagrams illustrating the different motion interventions were generated using Stata software (version 15.1). In case a closed-loop mesh appeared in the network, node splitting analysis was conducted to examine local consistency, with a passing consistency test defined as a P value >0.05. The network diagram consists of nodes and lines connecting them, where the width of each node and connecting line is proportional to the sample size of the respective study (14). Furthermore, the interventions were ranked based on their SUCRA values, and a ranking table was created to compare their relative effectiveness. To assess potential bias between the studies, heterogeneity was examined by constructing a funnel plot (15). The degree of intervention was summarized as an S value representing the area under the cumulative ranking curve; larger values indicated better intervention effects within a scoring range of 0-1. Similarly, the SUCRA values ranged from 0% to 100%, with higher scores indicating superior intervention effects. However, caution should be exercised when interpreting these scores unless genuine clinical differences exist between the interventions (16).
To address potential confounding by exercise duration, we calculated the metabolic equivalents (MET-min) for each intervention using standard compendium values (17). For example:
- Cycling: 8.0 METs
- Running: 10.0 METs
- Resistance training: 6.0 METs
Sensitivity analyses were performed to assess whether duration-adjusted energy expenditure influenced primary outcomes.
3 Results
3.1 Study and identification and selection
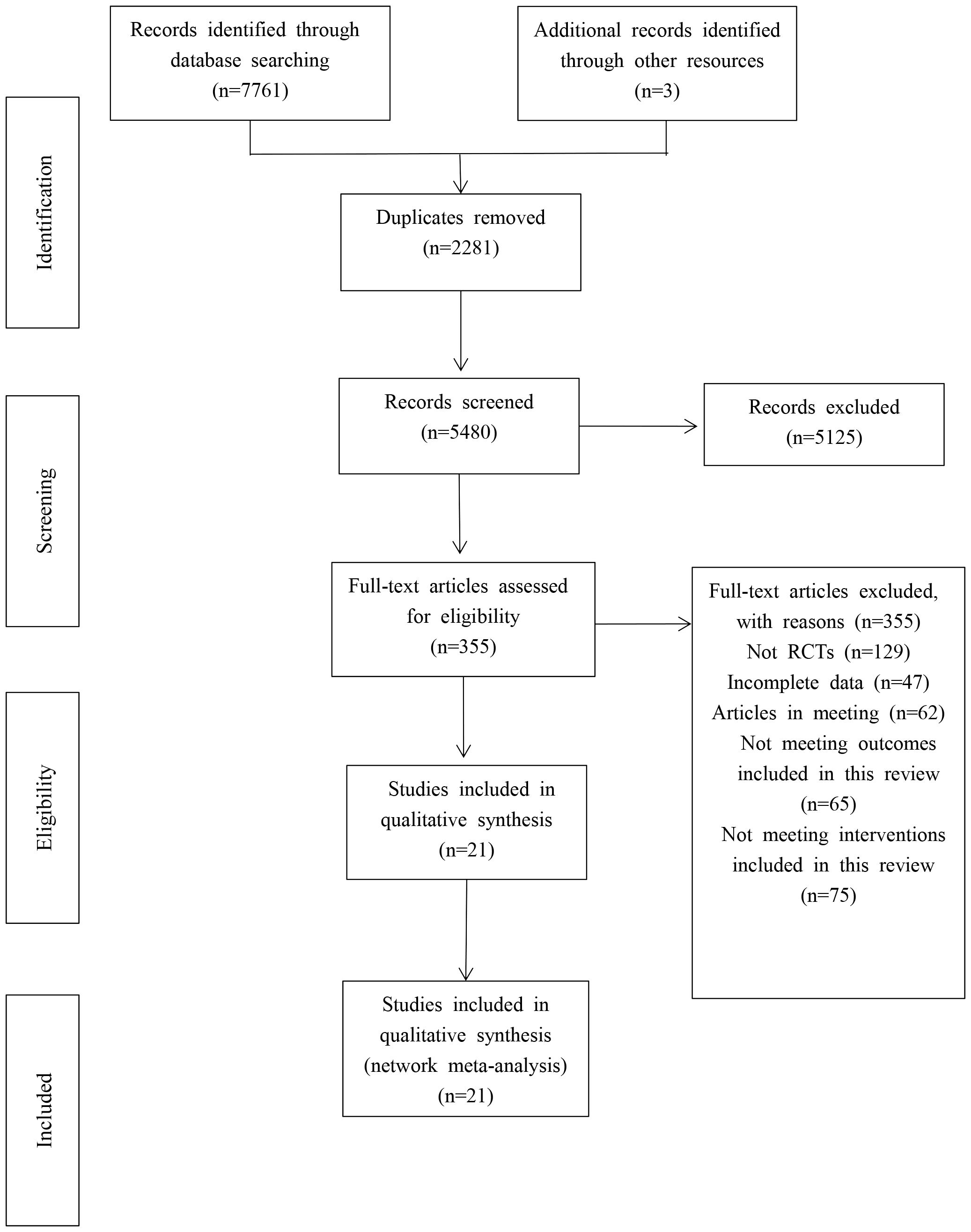
A total of 7761 articles were retrieved from five electronic databases, and three were retrieved. After excluding 2281 duplicate references, 5, 125 articles were eliminated based on the evaluation of their titles and abstracts, resulting in 5480 remaining references. Subsequently, a comprehensive review was performed on the remaining 355 papers by reading them in their entirety. Following this assessment, an additional 334 papers were excluded, ultimately leading to the inclusion of only 21 studies for the meta-analysis. (Show in Figure 1).
3.2 Quality evaluation of the included studies
Given the diverse range of movement modes of these interventions, achieving blinding for both subjects becomes challenging. Consequently, informed consent was obtained from all participants and their families before the experiment.
The risk-of-bias assessment across seven domains is summarized in Figure 2, revealing consistent limitations in participant blinding due to exercise intervention nature.”
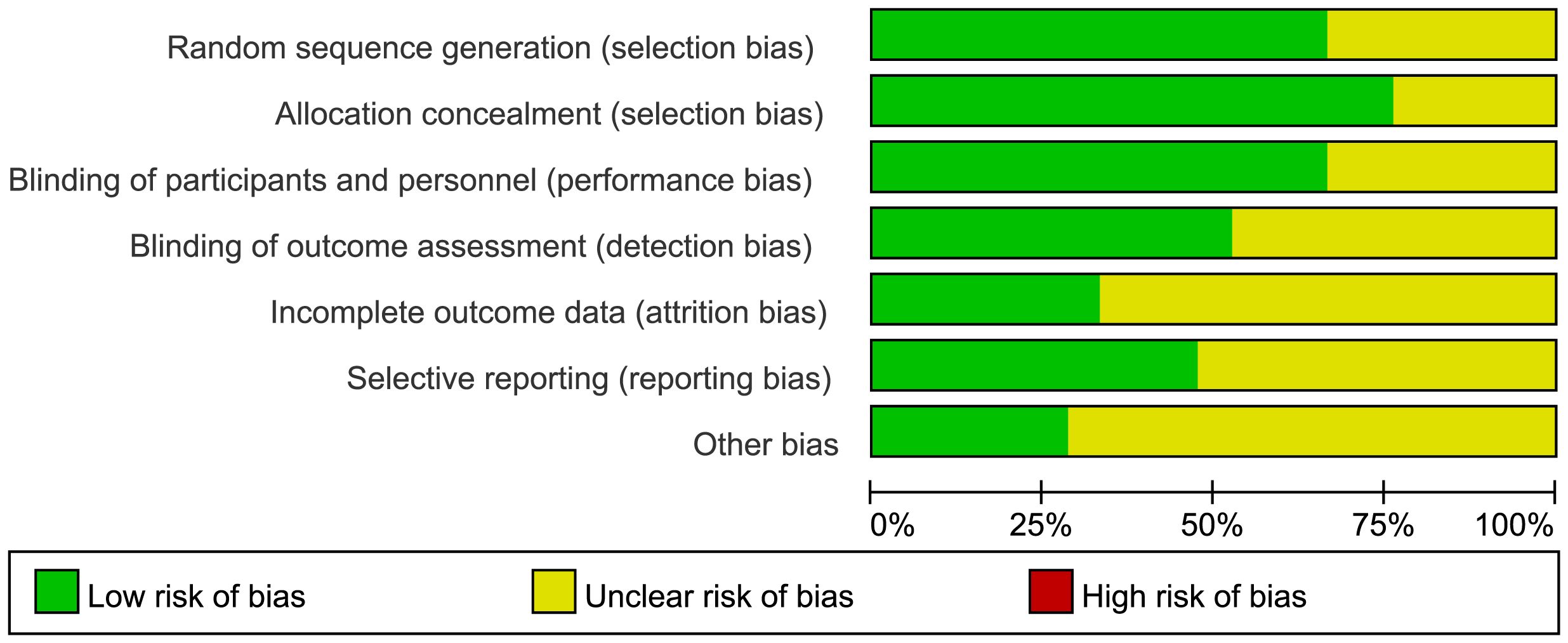
Figure 2. Risk of bias graph (percentage form). The “X-axis" lists bias domains (e.g."Random sequence generation"), and the "Y-axis" represents the "percentage of studies" falling into each risk category(Low/Unclear/High). The color-coded legend (green/yellow/red) explicitly defines each risk level, eliminating the need for additional axis units.
3.3 Features of the included study
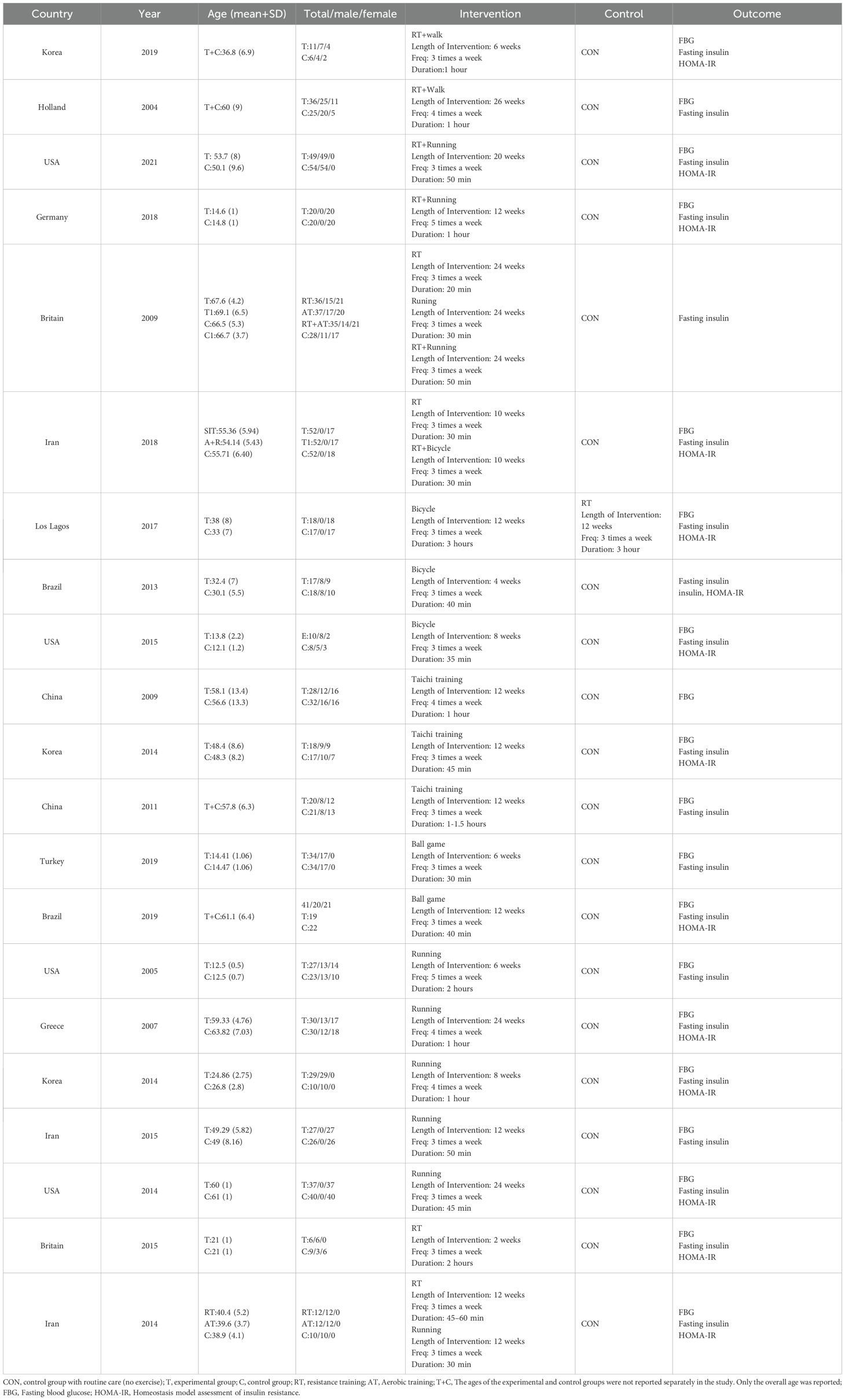
Table 2 presents the baseline characteristics of the included studies. The present study included 21 randomized controlled trials involving 1140 participants. The 21 trials, conducted between 2004 and 2022, encompassed a diverse age range of 10–69 years. The exercise interventions comprised resistance training (RT), aerobic training (such as cycling and running), combination training, Tai Chi, and ball games. The control group received standard treatment and daily care without exercise intervention. Control group interventions consisted of combined resistance and walking exercise training (18, 19), combined resistance and running exercise training (15, 20, 21), combined resistance and cycling exercise training (22), bicycle training (23–25), combined resistance and cycling exercise training along with Tai Chi Qigong practice (24, 26, 27), ball game exercises (28, 29), running exercises (20, 30–34) as well and standalone resistance exercises (22, 30, 31, 35, 36). FBG was employed as an outcome indicator in 19 studies, while fasting insulin was an outcome indicator in all included studies. HOMA-IR was used in 15 studies for evaluation. These studies were conducted in various countries, including China, South Korea, the United States, Brazil, Iran, Turkey, the Netherlands, Greece, the United Kingdom, and Germany. The detailed characteristics of the included studies are provided in Table 2.
3.4 Network meta-analysis
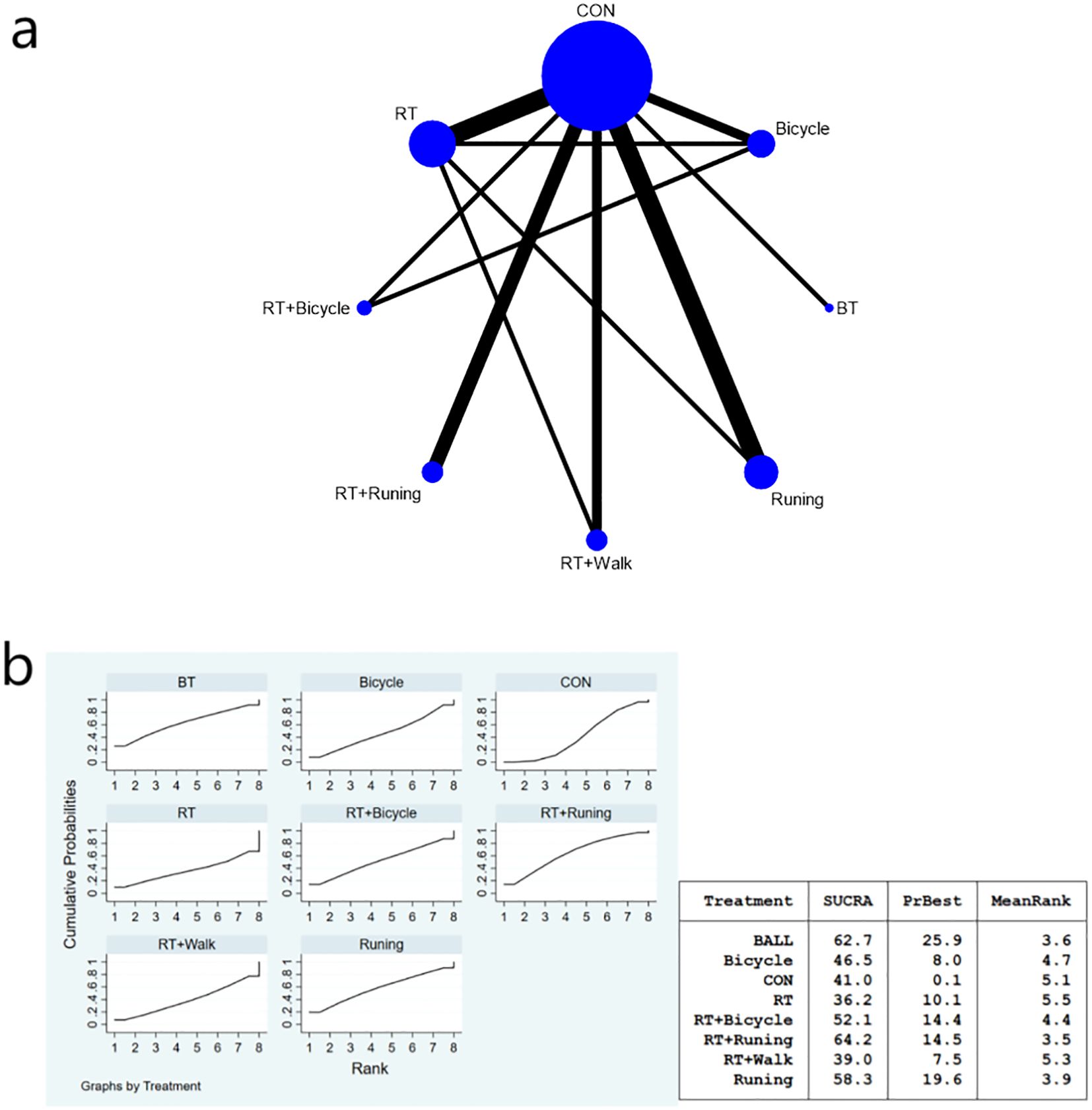
The complete network diagram is shown in Figures 3a, 4a, and 5a.
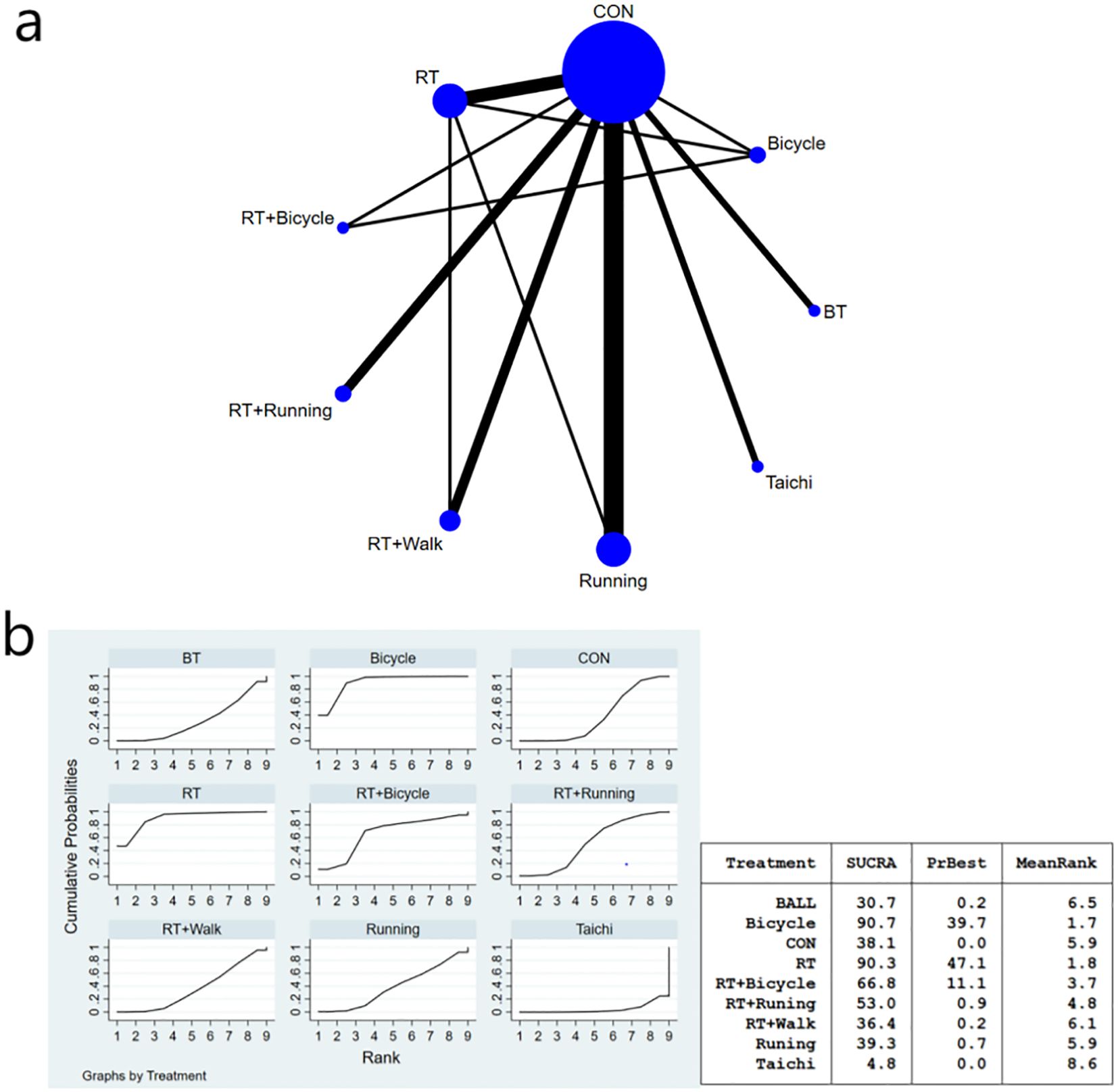
Figure 3. (a) Network meta-analysis figure for FBG; (b) SUCRA plot for FBG. The “X-axis" is labeled "Rank", indicating the relative efficacy ranking of interventions (1=most effective). The “y-axis" is labeled "Cumulative Probability", representing the probability of each intervention being ranked as the best option.
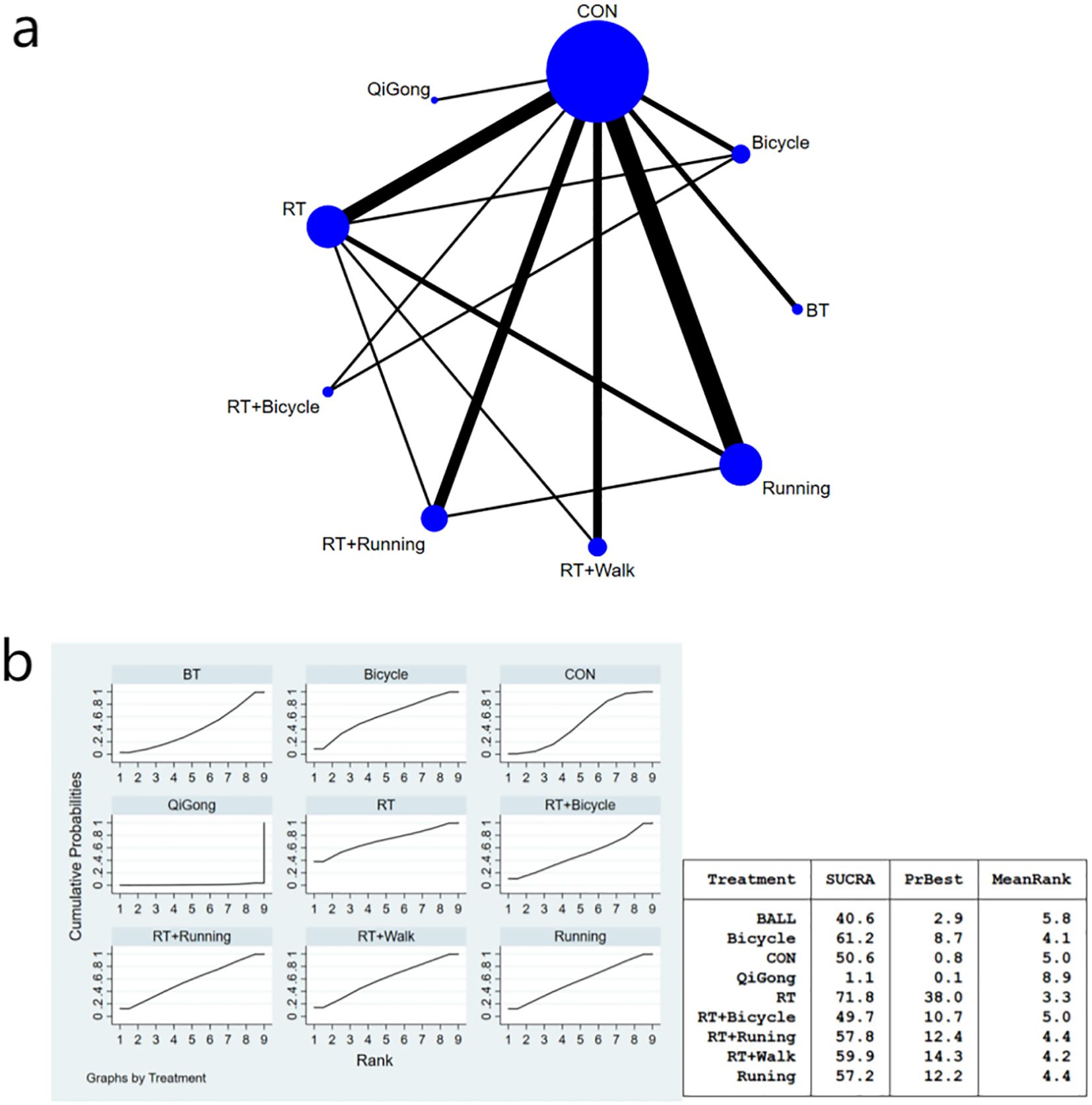
Figure 4. (a) Network meta-analysis figure for fasting insulin; (b) SUCRA plot for fasting insulin. The “X-axis" is labeled "Rank", indicating the relative efficacy ranking of interventions (1=most effective). The “y-axis" is labeled "Cumulative Probability", representing the probability of each intervention being ranked as the best option.
3.4.1 Fasting blood glucose index results of diabetic patients
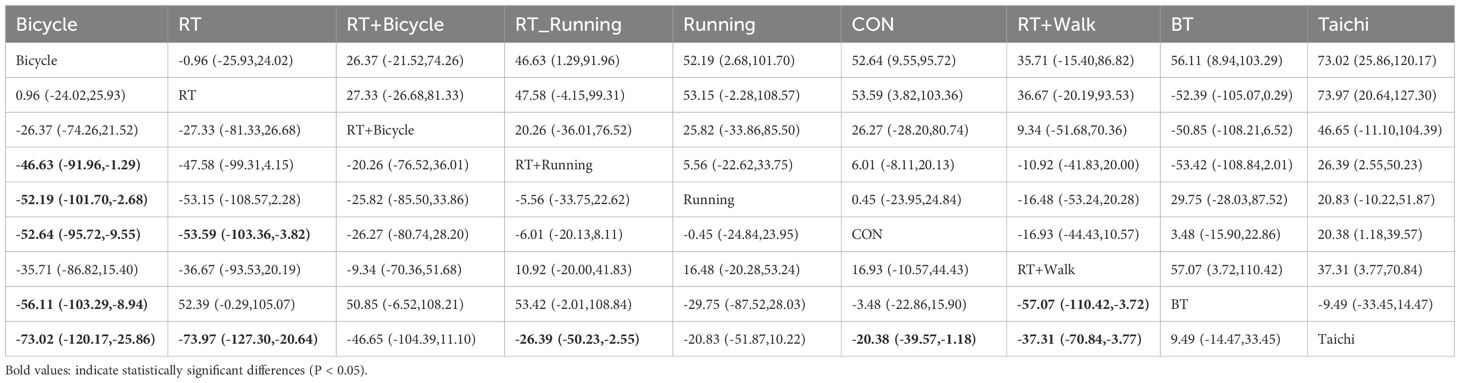
The meta-analysis results demonstrated that the intervention effect in the bicycle group was superior. Specifically, when comparing the cycling group with both anaerobic and running groups [MD=-46.63, 95%CI (-91.96,-1.29)], and when comparing the running group with the cycling group [MD=-52.19, 95%CI (-101.70,-2.68)], significant differences were observed in favor of the bicycle group’s intervention effect (Figure 3b). Furthermore, compared to the control group [MD=-52.64, 95%CI (-95.72,-9.55)], ball games [MD=-56.11, 95%CI (-103.29,-8.94)], and Tai Chi group [MD=-73.02, 95%CI (-120.-17,-25-86)], fasting insulin sensitivity exhibited a more pronounced improvement in insulin sensitivity (Figure 3b). Regarding the SUCRA ranking score (Figure 3b), cycling practice ranked first with a SUCRA score of 90%. Pairwise comparisons between the interventions are presented in Table 3.
Notably, cycling interventions had longer session durations (mean 90 min) compared to running (mean 45 min) and resistance training (mean 50 min). However, after adjusting for MET-minutes, cycling remained superior in reducing FBG [MD = -38.72, 95%CI (-75.15, -2.29)].
3.4.2 Fasting insulin index results of diabetic patients
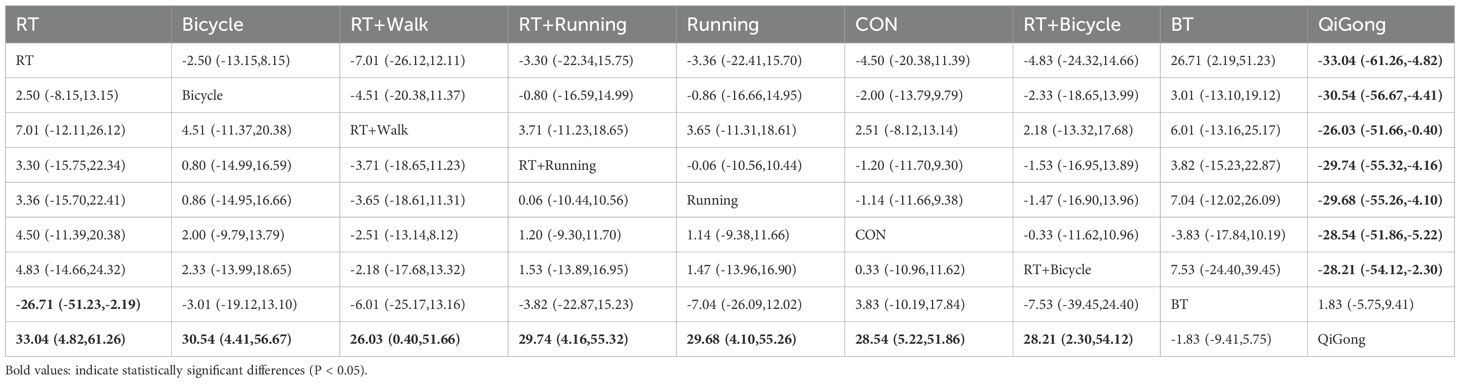
In comparison to ball games, resistance exercise demonstrated a significant impact on enhancing insulin sensitivity [MD=-26.71, 95% CI (-51.23, -2.19)].The Qigong exercise group exhibited significant differences compared to the aerobic exercise group [MD=33.04, 95% CI (4.82, 61.26)], bicycle exercise group [MD=30.54, 95% CI (4.41, 56.67)], aerobic walking combined exercise group [MD=26.03, 95% CI (0.40, 51.66)], aerobic running combined exercise group [MD=29.74, 95% CI (4.16, 55.32)], running exercise group [MD=29.68,95%CI(4.10,55.26)], the general control group[MD=28.54,95%CI(5.22,51.86)], and the aerobic and bicycle combined exercise group [MD=28. 21,95%CI(2.30,54.12)], the results suggest that the Qigong exercise intervention had limited impact on improving insulin sensitivity parameters. The bicycle group (SUCRA: 90.7%) exhibited superior efficacy in enhancing insulin sensitivity parameters, as demonstrated in Figure 4b of the SUCRA analysis. The effect size of the key comparison indicates that, Resistance training vs. Ball training: MD = -26.71 μU/ml, 95%CI (-51.23, -2.19);Qigong vs. Control: MD = -28.54 μU/ml, 95%CI (-51.86, -5.22);Cycling vs. Control: MD = -2.00 μU/ml, 95%CI (-13.79, 9.79). The MD for Resistance Training versus Ball Training and Qigong versus control was statistically significant. A comparison of the various interventions is shown in Table 4.
3.4.3 Results of the HOMA-IR index for diabetic patients
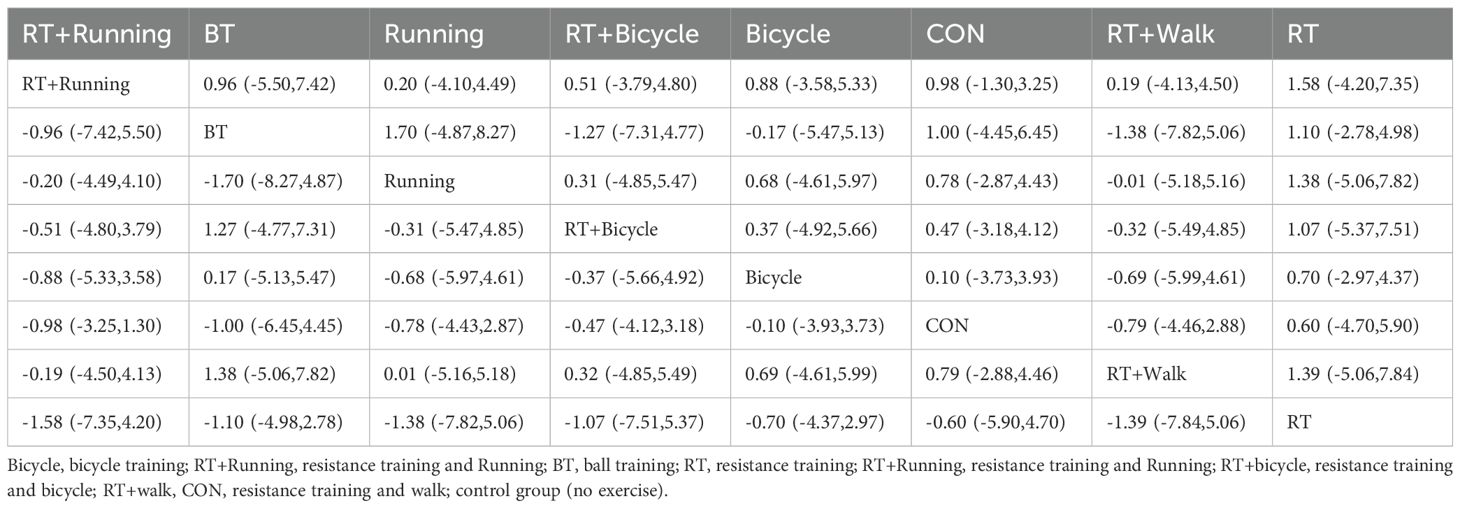
The meta-analysis chart results (Figure 5b) revealed no statistically significant differences in the reduction of HOMA-IR index among the intervention groups. The SUCRA value indicated that the combination of aerobic exercise and running exhibited the highest ranking for reducing HOMA-IR values, thus proving to be the most effective approach compared to other exercises (SUCRA: 64.2%). Ball games ranked next (SUCRA: 62.7%), as shown in Figure 4b. HOMA-IR changes versus control:RT+Running: MD = -1.20, 95%CI (-11.70, 9.30); ball training: MD = -3.83, 95%CI (-17.84, 10.19); Cycling: MD = -0.10, 95%CI (-3.93, 3.73); Although RT+Running had the highest SUCRA ranking, its effect versus control did not reach statistical significance (95%CI crosses zero). The pairwise comparison of the interventions is presented in Table 5.
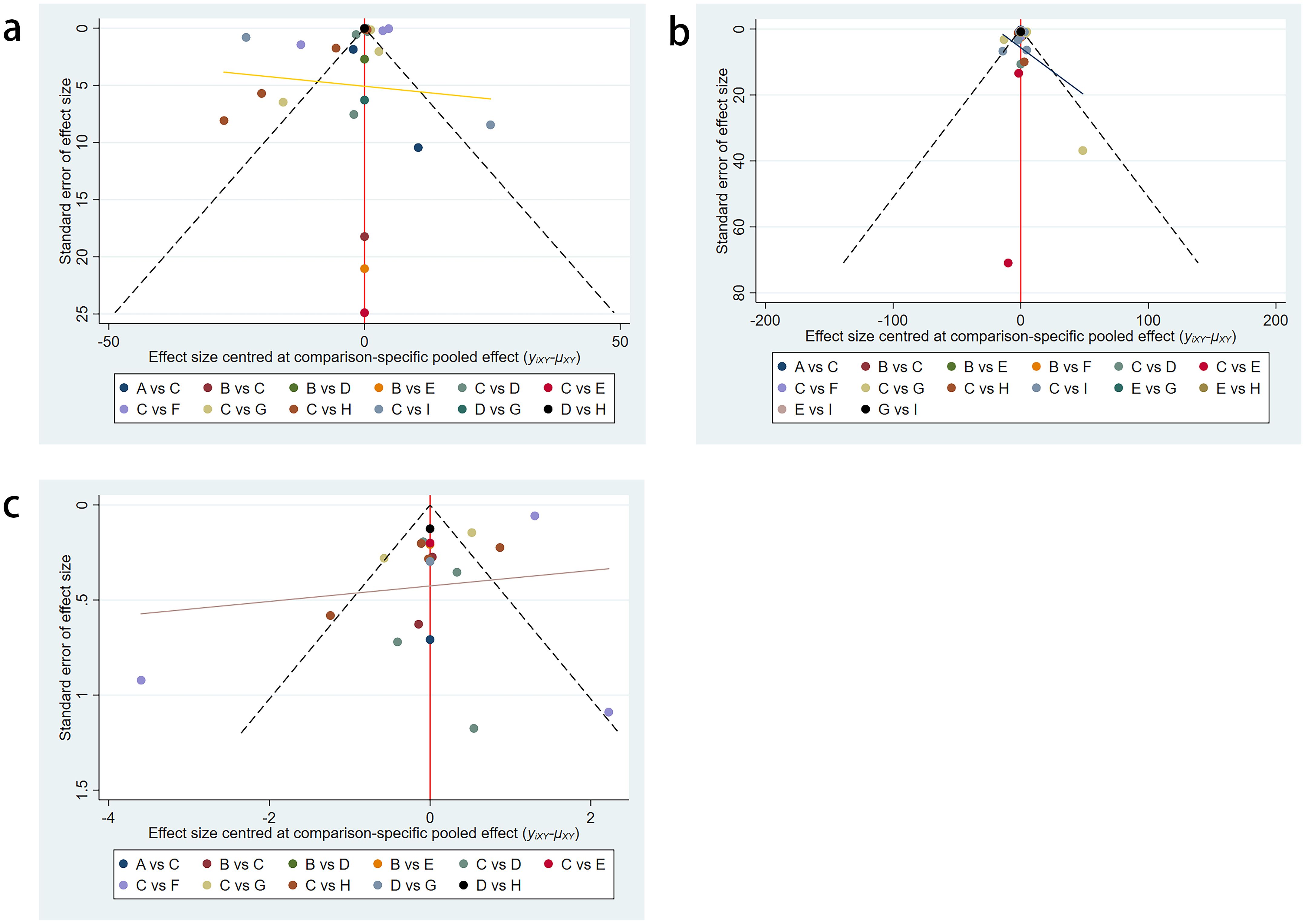
3.5 Publication bias test
The included trials were assessed using the Cochrane risk assessment tool and were determined to have a low-to-moderate risk of bias. Additionally, no significant publication bias was observed in the funnel plots (Figures 6a–c).
4 Discussion
In this meta-review and meta-analysis, we incorporated data from studies conducted across multiple continents, including the United States, Europe, Asia, and Australia, to augment the sample size and enhance the generalizability of our findings. By comparing the effects of nine different exercise interventions, we observed that cycling, resistance exercise, and combined resistance with running exercise exhibited comparatively superior enhancements. Specifically, cycling showed the largest FBG reduction [MD = -52.64 mmol/L vs. control], resistance training significantly improved insulin sensitivity over ball games [MD = -26.71 μU/ml], and RT+Running had the highest probability (SUCRA=64.2%) for HOMA-IR reduction despite non-significant effects versus control [MD = -1.20].
By comparing the effects of nine different exercise interventions on fasting blood glucose, fasting insulin, and HOMA-IR levels among patients with diabetes, we observed that cycling, resistance exercise, and combined resistance with running exercise demonstrated relatively superior improvements in glycemic control indicators, including FPG, FI, and HOMA-IR index. Cycling is most likely to reduce fasting plasma glucose (FPG) levels, which is consistent with previous evidence indicating that cycling recruits a more significant number of type I muscle fibers and improves glucose utilization (37, 38). The extensive engagement of muscles and the absence of weight-bearing characteristics make cycling a safer and more effective exercise option for individuals with type 2 diabetes mellitus (T2DMM) (39). Utilizing bicycles mobilizes large muscle groups and eliminates leg weight-bearing and ground friction, making it remarkably safe and effective for patients with T2DMM. Cycling elicits greater recruitment of type I muscle fibers, which demonstrate higher insulin sensitivity and GLUT4 density (4, 39). Li et al. demonstrated that both high-intensity interval cycling and moderate-intensity cycling significantly reduced fasting glucose in T2DMM patients (40).
During exercise under normoglycemic-hyperinsulinemic conditions, skeletal muscles account for nearly all human glucose uptake. The increase in muscle glucose uptake during exercise is attributed to enhanced contraction activity and increased blood flow within the muscles, which facilitates glucose transport (41). The higher level of glucose utilization observed during cycling compared with running may be due to the greater contraction activity resulting from the larger active muscle mass. Muscle fiber recruitment and glycogen utilization patterns differ among various forms of exercise. It has been discovered that the effect on muscle glycogen supply by type I fibers was superior in the group undergoing cycling interventions compared to those undergoing running interventions. Type I fibers possess higher insulin content, are more sensitive to insulin stimulation, and can recruit more GLUT4 transport proteins, thereby enhancing the skeletal muscle’s ability to take up and transport glucose, an effect associated with increased insulin-stimulated glucose uptake capability (37, 39). These findings suggest that cycling elicits greater recruitment of type I fibers and higher glucose utilization than running does.
This study suggests that resistance training is beneficial for improving insulin utilization in patients with type 2 diabetes. Compared to conventional exercise, resistance training can more effectively promote skeletal muscle glucose utilization and uptake due to its ability to increase muscle mass and cross-sectional area (42, 43), thereby facilitating insulin signaling and peripheral tissue glucose uptake (44, 45). Resistance training can augment glucose phosphorylation in skeletal muscle cells, facilitating the conversion of blood sugar into simple sugars, thereby promoting optimal insulin secretion and maintaining blood sugar homeostasis (44, 45). Long-term (>12 weeks) high-intensity resistance training has been shown to significantly enhance insulin sensitivity and sustain physical function for a duration that surpasses that of aerobic exercise (46). The findings of various studies have demonstrated that engagement in resistance exercise can significantly enhance metabolic health during weight recovery, including the reduction of fasting blood glucose levels and enhancement of insulin sensitivity (47). In a 24-week study, a comparison between resistance training and aerobic exercise revealed that the former enhanced insulin sensitivity and glucose uptake in muscles mediated by insulin (46). In general, resistance training enhances insulin sensitivity and improves fasting glucose levels in individuals diagnosed with type 2 diabetes (46, 48).
The combination of running and anaerobic exercise demonstrated superior efficacy in alleviating insulin resistance, as supported by a significant reduction in the HOMA-IR index, indicating an enhanced improvement in insulin sensitivity. The underlying mechanisms potentially involve augmented lipid oxidation and glycogen utilization (38), improved mitochondrial function (49), and enhanced muscle mass and cardiorespiratory fitness (40). Type 2 diabetes is characterized by insulin resistance (IR) and relative insulin insufficiency, leading to glucose intolerance and subsequent elevation of blood glucose levels (40). However, preserving islet β-cell function may be pivotal in preventing T2DM onset (49, 50). Notably, the combined impact of running and resistance training on β-cell function surpasses that achieved through either aerobic or resistance training alone (51), likely attributable to the prolonged duration and heightened intensity associated with combined training regimens. Low-load high-repetition resistance training has emerged as an alternative form of aerobic-based resistance training capable of promoting muscle hypertrophy and strength gains similar to those observed with high-load low-repetition protocols (51, 52). In the context of combined training approaches, increased fat loss during resistance exercise aids in augmenting glucose uptake while concurrently enhancing skeletal muscle mitochondrial oxidative capacity. This synergistic effect maximizes reductionions in body fat content while expediting glycogen consumption during aerobic exercise sessions (53).
5 Advantages and limitations
The methodology employed in this study was highly rigorous and systematic. We conducted a comprehensive search across five electronic databases, strictly adhering to predefined criteria, and identified 21 articles encompassing a substantial sample size of 1140 patients with diabetes. To ensure accuracy, the selected articles underwent double-checking procedures, and we incorporated various specific joint exercise measures targeting both aerobic and anaerobic activities, thereby providing updated and more comprehensive evidence-based recommendations on how exercise can effectively reduce blood glucose levels and enhance insulin sensitivity.
Nevertheless, certain limitations of this meta-analysis should be acknowledged.
1. Cycling duration confounder: The observed superiority of cycling (e.g., FBG MD=-52.64 vs control) must be interpreted in the context of its typically longer session durations (35 min-3 h vs 30 min-2 h for running). While our MET-adjusted analysis suggested that duration alone did not fully explain efficacy (54), energy expenditure differentials remained a potential confounder.
2. Surrogate markers: Reliance on FBG/FI/HOMA-IR rather than gold-standard measures (for example, hyperinsulinemic-eug clamps);
3. Language bias: The inclusion of the CNKI database may limit generalizability, although funnel plots showed symmetry.
4. Blinding impossibility: Participant blinding was unattainable due to the nature of the exercise intervention.
Future directions: (a) Match interventions by MET-minutes to isolate modality effects; (b) validate findings with direct insulin sensitivity measures; (c) extend follow-up beyond 6 months.
6 Conclusion
Our study conducted a systematic review and network meta-analysis to compare the effects of different exercise interventions on glycemic control in patients with diabetes. The results demonstrated that cycling, resistance training, and combined resistance and aerobic training effectively improved fasting blood glucose levels, insulin levels, and insulin resistance. These findings have significant implications for the management of diabetes. We recommend prioritizing cycling to reduce blood glucose levels, incorporating resistance training to enhance insulin sensitivity, and implementing combined training to address insulin resistance. Exercise has been proven effective in regulating glycemia and should be widely recommended as an essential non-pharmacological treatment for individuals with diabetes.
Future studies should further validate the benefits of diverse exercise regimens on blood glucose regulation in a broader population. The genetic background and type of diabetes may influence individual variations in response to exercise interventions; thus, we advocate for future trials with expanded sample sizes encompassing various ethnicities to corroborate our current findings. Additionally, exploring the interaction between exercise and antidiabetic drugs is imperative. Longitudinal trials with larger sample sizes are also necessary to investigate the long-term effects of exercise interventions on maintaining blood glucose control among patients with diabetes while providing more personalized exercise recommendations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
YP: Writing – original draft, Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Writing – review & editing. PW: Writing – review & editing, Investigation, Software, Visualization. CY: Writing – review & editing, Resources, Software. CL: Writing – review & editing, Formal Analysis, Resources, Software.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pan Y, Liu C, Wang P, and Yue C. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the global burden of disease study 2021. Lancet. (2023) 402:203–34. doi: 10.1016/s0140-6736(23)01301-6
2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. Idf diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
3. Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. (2018) 41:963–70. doi: 10.2337/dc17-1962
4. Kirwan JP, Heintz EC, Rebello CJ, and Axelrod CL. Exercise in the prevention and treatment of type 2 diabetes. Compr Physiol. (2023) 13:4559–85. doi: 10.1002/cphy.c220009
5. Memme JM, Erlich AT, Phukan G, and Hood DA. Exercise and mitochondrial health. J Physiol. (2021) 599:803–17. doi: 10.1113/jp278853
6. Kapoor-Narula U and Lenka N. Cancer stem cells and tumor heterogeneity: deciphering the role in tumor progression and metastasis. Cytokine. (2022) 157:155968. doi: 10.1016/j.cyto.2022.155968
7. McLeod JC, Stokes T, and Phillips SM. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front Physiol. (2019) 10:645. doi: 10.3389/fphys.2019.00645
8. Jiao Y, Wang S, Wang X, Yin L, Zhang YH, Li YZ, et al. The M(6)a reader Ythdc2 promotes Sirt3 expression by reducing the stabilization of Kdm5b to improve mitochondrial metabolic reprogramming in diabetic peripheral neuropathy. Acta Diabetol. (2023) 60:387–99. doi: 10.1007/s00592-022-01990-0
9. Petersen MC, Vatner DF, and Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. (2017) 13:572–87. doi: 10.1038/nrendo.2017.80
10. Zhao XP, Chang SY, Pang Y, Liao MC, Peng J, Ingelfinger JR, et al. Hedgehog interacting protein activates sodium-glucose cotransporter 2 expression and promotes renal tubular epithelial cell senescence in a mouse model of type 1 diabetes. Diabetologia. (2023) 66:223–40. doi: 10.1007/s00125-022-05810-6
11. Hurst C, Weston KL, McLaren SJ, and Weston M. The effects of same-session combined exercise training on cardiorespiratory and functional fitness in older adults: A systematic review and meta-analysis. Aging Clin Exp Res. (2019) 31:1701–17. doi: 10.1007/s40520-019-01124-7
12. Schober P, Boer C, and Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. (2018) 126:1763–8. doi: 10.1213/ane.0000000000002864
13. Association AD. 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2020. Diabetes Care. (2020) 43:S32–S6. doi: 10.2337/dc20-s003
14. Association AD. 5. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. (2019) 42:S46–s60. doi: 10.2337/dc19-S005
15. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American college of sports medicine and the American diabetes association: joint position statement executive summary. Diabetes Care. (2010) 33:2692–6. doi: 10.2337/dc10-1548
16. Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, and Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. (2003) 26:2977–82. doi: 10.2337/diacare.26.11.2977
17. Brisson NM, Krahl LAN, Krämer M, Reichenbach JR, and Duda GN. Eighteen-month changes in physical activity, body weight, quadriceps strength, and gait biomechanics during the Covid-19 pandemic. Med Sci Sports Exerc. (2023) 55:1366–74. doi: 10.1249/mss.0000000000003160
18. García-Hermoso A, Saavedra JM, Escalante Y, Sánchez-López M, and Martínez-Vizcaíno V. Endocrinology and adolescence: aerobic exercise reduces insulin resistance markers in obese youth: A meta-analysis of randomized controlled trials. Eur J Endocrinol. (2014) 171:R163–71. doi: 10.1530/eje-14-0291
19. Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. (2002) 25:1729–36. doi: 10.2337/diacare.25.10.1729
20. Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: A randomized controlled trial. Arch Intern Med. (2009) 169:122–31. doi: 10.1001/archinternmed.2008.558
21. Zanuso S, Sacchetti M, Sundberg CJ, Orlando G, Benvenuti P, and Balducci S. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. A review of the evidence. Br J Sports Med. (2017) 51:1533–8. doi: 10.1136/bjsports-2016-096724
22. Banitalebi E, Kazemi A, Faramarzi M, Nasiri S, and Haghighi MM. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. (2019) 217:101–9. doi: 10.1016/j.lfs.2018.11.062
23. Trussardi Fayh AP, Lopes AL, Fernandes PR, Reischak-Oliveira A, and Friedman R. Impact of weight loss with or without exercise on abdominal fat and insulin resistance in obese individuals: A randomised clinical trial. Br J Nutr. (2013) 110:486–92. doi: 10.1017/s0007114512005442
24. Kim SH, Lee SH, Ahn KY, Lee DH, Suh YJ, Cho SG, et al. Effect of lifestyle modification on serum chemerin concentration and its association with insulin sensitivity in overweight and obese adults with type 2 diabetes. Clin Endocrinol (Oxf). (2014) 80:825–33. doi: 10.1111/cen.12249
25. Álvarez C, Ramírez-Campillo R, Ramírez-Vélez R, and Izquierdo M. Effects and prevalence of nonresponders after 12 weeks of high-intensity interval or resistance training in women with insulin resistance: A randomized trial. J Appl Physiol (1985). (2017) 122:985–96. doi: 10.1152/japplphysiol.01037.2016
26. Hung JW, Liou CW, Wang PW, Yeh SH, Lin LW, Lo SK, et al. Effect of 12-week tai chi chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med. (2009) 41:924–9. doi: 10.2340/16501977-0445
27. Liu X, Miller YD, Burton NW, Chang JH, and Brown WJ. Qi-gong mind-body therapy and diabetes control. A randomized controlled trial. Am J Prev Med. (2011) 41:152–8. doi: 10.1016/j.amepre.2011.04.007
28. de Sousa MV, Fukui R, Dagogo-Jack S, Krustrup P, Zouhal H, and da Silva MER. Biomarkers of Insulin Action During Single Soccer Sessions before and after a 12-Week Training Period in Type 2 Diabetes Patients on a Caloric-Restricted Diet. Physiol Behav. (2019) 209:112618. doi: 10.1016/j.physbeh.2019.112618
29. Dundar A, Kocahan S, and Sahin L. Associations of apelin, leptin, irisin, ghrelin, insulin, glucose levels, and lipid parameters with physical activity during eight weeks of regular exercise training. Arch Physiol Biochem. (2021) 127:291–5. doi: 10.1080/13813455.2019.1635622
30. Carrel AL, Clark RR, Peterson SE, Nemeth BA, Sullivan J, and Allen DB. Improvement of fitness, body composition, and insulin sensitivity in overweight children in a school-based exercise program: A randomized, controlled study. Arch Pediatr Adolesc Med. (2005) 159:963–8. doi: 10.1001/archpedi.159.10.963
31. Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. (2007) 14:837–43. doi: 10.1097/HJR.0b013e3282efaf50
32. Motahari-Tabari N, Ahmad Shirvani M, Shirzad EAM, Yousefi-Abdolmaleki E, and Teimourzadeh M. The effect of 8 weeks aerobic exercise on insulin resistance in type 2 diabetes: A randomized clinical trial. Glob J Health Sci. (2014) 7:115–21. doi: 10.5539/gjhs.v7n1p115
33. Ryan AS, Ge S, Blumenthal JB, Serra MC, Prior SJ, and Goldberg AP. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. J Am Geriatr Soc. (2014) 62:607–14. doi: 10.1111/jgs.12749
34. Kim YS, Nam JS, Yeo DW, Kim KR, Suh SH, and Ahn CW. The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin Endocrinol (Oxf). (2015) 82:686–94. doi: 10.1111/cen.12601
35. Nikseresht M, Agha-Alinejad H, Azarbayjani MA, and Ebrahim K. Effects of nonlinear resistance and aerobic interval training on cytokines and insulin resistance in sedentary men who are obese. J Strength Cond Res. (2014) 28:2560–8. doi: 10.1519/jsc.0000000000000441
36. Gonzalez JT, Barwood MJ, Goodall S, Thomas K, and Howatson G. Alterations in whole-body insulin sensitivity resulting from repeated eccentric exercise of a single muscle group: A pilot investigation. Int J Sport Nutr Exerc Metab. (2015) 25:405–10. doi: 10.1123/ijsnem.2014-0211
37. Tsintzas K, Simpson EJ, Seevaratnam N, and Jones S. Effect of exercise mode on blood glucose disposal during physiological hyperinsulinaemia in humans. Eur J Appl Physiol. (2003) 89:217–20. doi: 10.1007/s00421-002-0781-3
38. AbouAssi H, Slentz CA, Mikus CR, Tanner CJ, Bateman LA, Willis LH, et al. The effects of aerobic, resistance, and combination training on insulin sensitivity and secretion in overweight adults from strride at/Rt: A randomized trial. J Appl Physiol (1985). (2015) 118:1474–82. doi: 10.1152/japplphysiol.00509.2014
39. Li J, Cheng W, and Ma H. A comparative study of health efficacy indicators in subjects with T2dm applying power cycling to 12 weeks of low-volume high-intensity interval training and moderate-intensity continuous training. J Diabetes Res. (2022) 2022:9273830. doi: 10.1155/2022/9273830
40. Mann S, Beedie C, Balducci S, Zanuso S, Allgrove J, Bertiato F, et al. Changes in insulin sensitivity in response to different modalities of exercise: A review of the evidence. Diabetes Metab Res Rev. (2014) 30:257–68. doi: 10.1002/dmrr.2488
41. Liang M, Pan Y, Zhong T, Zeng Y, and Cheng ASK. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: A systematic review and network meta-analysis. Rev Cardiovasc Med. (2021) 22:1523–33. doi: 10.31083/j.rcm2204156
42. Fonseca RM, Roschel H, Tricoli V, de Souza EO, Wilson JM, Laurentino GC, et al. Changes in exercises are more effective than in loading schemes to improve muscle strength. J Strength Cond Res. (2014) 28:3085–92. doi: 10.1519/jsc.0000000000000539
43. Kristiansen MS, Uhrbrand A, Hansen M, Shiguetomi-Medina JM, Vissing K, Stødkilde-Jørgensen H, et al. Concomitant changes in cross-sectional area and water content in skeletal muscle after resistance exercise. Scand J Med Sci Sports. (2014) 24:e260–8. doi: 10.1111/sms.12160
44. Wang C, Guelfi KJ, and Yang HX. Exercise and its role in gestational diabetes mellitus. Chronic Dis Transl Med. (2016) 2:208–14. doi: 10.1016/j.cdtm.2016.11.006
45. Huifen Z, Yaping X, Meijing Z, Huibin H, Chunhong L, Fengfeng H, et al. Effects of moderate-intensity resistance exercise on blood glucose and pregnancy outcome in patients with gestational diabetes mellitus: A randomized controlled trial. J Diabetes Complications. (2022) 36:108186. doi: 10.1016/j.jdiacomp.2022.108186
46. Jiahao L, Jiajin L, and Yifan L. Effects of resistance training on insulin sensitivity in the elderly: A meta-analysis of randomized controlled trials. J Exerc Sci Fit. (2021) 19:241–51. doi: 10.1016/j.jesf.2021.08.002
47. Warner SO, Linden MA, Liu Y, Harvey BR, Thyfault JP, Whaley-Connell AT, et al. The effects of resistance training on metabolic health with weight regain. J Clin Hypertens (Greenwich). (2010) 12:64–72. doi: 10.1111/j.1751-7176.2009.00209.x
48. Chobanyan-Jürgens K, Scheibe RJ, Potthast AB, Hein M, Smith A, Freund R, et al. Influences of hypoxia exercise on whole-body insulin sensitivity and oxidative metabolism in older individuals. J Clin Endocrinol Metab. (2019) 104:5238–48. doi: 10.1210/jc.2019-00411
49. Malin SK, Rynders CA, Weltman JY, Barrett EJ, and Weltman A. Exercise intensity modulates glucose-stimulated insulin secretion when adjusted for adipose, liver and skeletal muscle insulin resistance. PloS One. (2016) 11:e0154063. doi: 10.1371/journal.pone.0154063
50. Malin SK, Hinnerichs KR, Echtenkamp BG, Evetovich TK, and Engebretsen BJ. Effect of adiposity on insulin action after acute and chronic resistance exercise in non-diabetic women. Eur J Appl Physiol. (2013) 113:2933–41. doi: 10.1007/s00421-013-2725-5
51. Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PloS One. (2010) 5:e12033. doi: 10.1371/journal.pone.0012033
52. Schoenfeld BJ, Peterson MD, Ogborn D, Contreras B, and Sonmez GT. Effects of low- vs. High-load resistance training on muscle strength and hypertrophy in well-trained men. J Strength Cond Res. (2015) 29:2954–63. doi: 10.1519/jsc.0000000000000958
53. Schellenberg ES, Dryden DM, Vandermeer B, Ha C, and Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med. (2013) 159:543–51. doi: 10.7326/0003-4819-159-8-201310150-00007
Keywords: exercise, insulin resistance, diabetes patients, network meta-analysis, systematic review
Citation: Pan Y, Wang P, Yue C and Liu C (2025) Effect of nine different exercise interventions on insulin sensitivity in diabetic patients: a systematic review and mesh meta-analysis. Front. Endocrinol. 16:1409474. doi: 10.3389/fendo.2025.1409474
Received: 30 March 2024; Accepted: 06 August 2025;
Published: 28 August 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Gianpaolo De Filippo, Hôpital Robert Debré, FranceKayvan Khoramipour, Miguel de Cervantes European University, Spain
Copyright © 2025 Pan, Wang, Yue and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunlin Yue, cHlreXlkczY2NkAxNjMuY29t; Chen Liu, Mjc2MjA4OTE2QHFxLmNvbQ==
 Yikang Pan1
Yikang Pan1 Chen Liu
Chen Liu