- 1Department of Gynecology, Fujian Province Key Clinical Specialty for Gynecology, National Key Gynecology Clinical Specialty Building Institution, Fujian Maternity and Child Health Hospital, College of Clinical Medical for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
- 2Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
Background: Cervical cancer, linked to HPV and dysglycemia, lacks clarity on their combined impact. This study explores Ki-67’s role in mediating HPV and dysglycemia effects on cervical cancer risk.
Methods: This study enrolled patients with abnormal cervical cancer screening results, undergoing colposcopy and conization at Fujian Maternity and Child Health Hospital’s Cervical Disease Center from June 2018 to June 2023. Statistical analyses compared baseline characteristics across cervical lesion categories. Multinomial logistic regression examined HPV and dysglycemia associations with LSIL (low-grade squamous intraepithelial lesions), HSIL(high-grade squamous intraepithelial lesions), and cervical cancer, highlighting interaction and mediation analyses involving Ki-67.
Results: A total of 4,115 participants were included: 573 with hyperglycemia, 1,479 with HPV only, and 548 with both HPV and hyperglycemia. Prediabetes and diabetes significantly increased cancer risk (OR: 2.47, 95% CI: 1.75-3.47 and OR: 3.67, 95% CI: 2.41-5.6, respectively). Coexisting hyperglycemia further elevated cervical cancer risk by over three-fold (OR: 3.12, 95% CI: 2.34-4.16) compared to HPV-positive normoglycemics. A significant interaction between hyperglycemia and HPV infection was observed (AP (attributable proportion): 0.69, 95% CI: 0.61-0.77, p<0.001; SI (synergy index): 3.27, 95% CI: 2.5-4.27, p<0.001). Ki-67+ expression accounted for 39.84%, 37.35%, and 55.18% of the total effect of hyperglycemia, HPV, and their combined impact, respectively. Additionally, the combination of dysglycemia and HPV had a significant indirect effect on Ki-67 levels (estimate: 0.08, 95% CI: 0.06- 0.09, p<0.001).
Conclusions: Dysglycemia and HPV infection synergistically elevate cervical cancer risk, possibly influenced by Ki-67. Effective screening and management for both are vital in prevention. Further research is required to validate findings and elucidate molecular mechanisms.
1 Introduction
Cervical cancer stands as a major global health challenge, ranking as the second most prevalent cancer among women worldwide, resulting in over 250,000 deaths annually (1), with an estimated 604,000 new cases and 342,000 deaths reported in 2020 (2). Human papillomaviruses (HPV16 and HPV18) have been identified as potent catalysts in the development of cervical cancer in women (3), underscoring the critical importance of understanding and addressing HPV infection. However, the landscape of cervical cancer risk is not solely defined by viral factors. Diabetes, characterized by metabolic dysregulation and immune alterations, emerges as a significant contributor to the burden of cervical cancer (4, 5).
Contrary to being a mere bystander in cervical carcinogenesis, diabetes is increasingly recognized for its intricate involvement in driving disease progression. The global epidemic of diabetes further compounds the cervical cancer burden, with the condition affecting over 8% of adults worldwide (6). In China alone, recent data from 2018 reveal a notable surge in diabetes prevalence, with rates soaring to 12.4%. Moreover, antecedent diabetes is observed in 38.1% of cases, while diabetes and prediabetes together afflict 50.5% of the population. Strikingly, nearly half of the affected individuals are women, with a weighted percentage of 49.5% (7).
Investigating the intricate relationship between diabetes and cancer is an area of active research, with diverse findings and ongoing debate (8–18). Some studies suggest that diabetes escalates cervical cancer risk (8–10) 8-10, and others also indicate that metformin, a commonly prescribed medication for diabetes, may potentially mitigate this risk (19–21), particularly with cumulative use exceeding 2 years (21). In a cross-sectional study involving 791 women, it was found that diabetes and prediabetes are associated with cervical cancer in patients with high-risk HPV (HR-HPV) combined with high-grade squamous intraepithelial lesions (HGSIL) (22).
Given the substantial global burden of cervical cancer, particularly in developing nations, and the emerging evidence linking diabetes to an increased risk of cervical cancer development and progression, further research is crucial to elucidate the underlying mechanisms and develop effective strategies for prevention, early detection, and management of these intertwined health challenges.
Several studies have highlighted the link between dysglycemia and cervical neoplasia (8–10, 22), yet the potential synergistic effect of dysglycemia on HPV-driven cervical cancer progression remains unexplored. Furthermore, the underlying mechanisms through which dysglycemia may potentiate HPV-mediated cervical carcinogenesis are still elusive. Remarkably, the proliferation marker Ki-67 has been implicated as a crucial player in HPV-driven cervical cancer progression (23). Notably, Ki-67 staining offers improved specificity for triaging HPV-positive women with cervical precancerous lesions (24). Interestingly, Ki-67 has also been found to contribute to the promotion of other malignancies in the context of dysglycemia (25, 26). This intriguing observation raises an important question: Could the potential synergistic effect of dysglycemia on HPV-induced cervical cancer progression be mediated through a mechanism involving Ki-67?
Given the global cervical cancer burden and rising metabolic disorders, exploring dysglycemia’s role alongside HPV in cervical carcinogenesis is crucial. Understanding dysglycemia’s role in HPV-driven cancer and Ki-67’s involvement could inform novel therapies and personalized management. This sheds light on biomarker potential for early detection and risk assessment. Hence, conducting comprehensive research is crucial to investigate the potential synergistic impact of dysglycemia on HPV-driven cervical cancer progression, along with the mechanistic role of Ki-67 in this complex process. Therefore, this study delved into the intricate interplay of Ki-67 in mediating the effects of Human Papillomavirus (HPV) infection and dysglycemia on cervical cancer risk.
2 Materials and methods
2.1 Study population
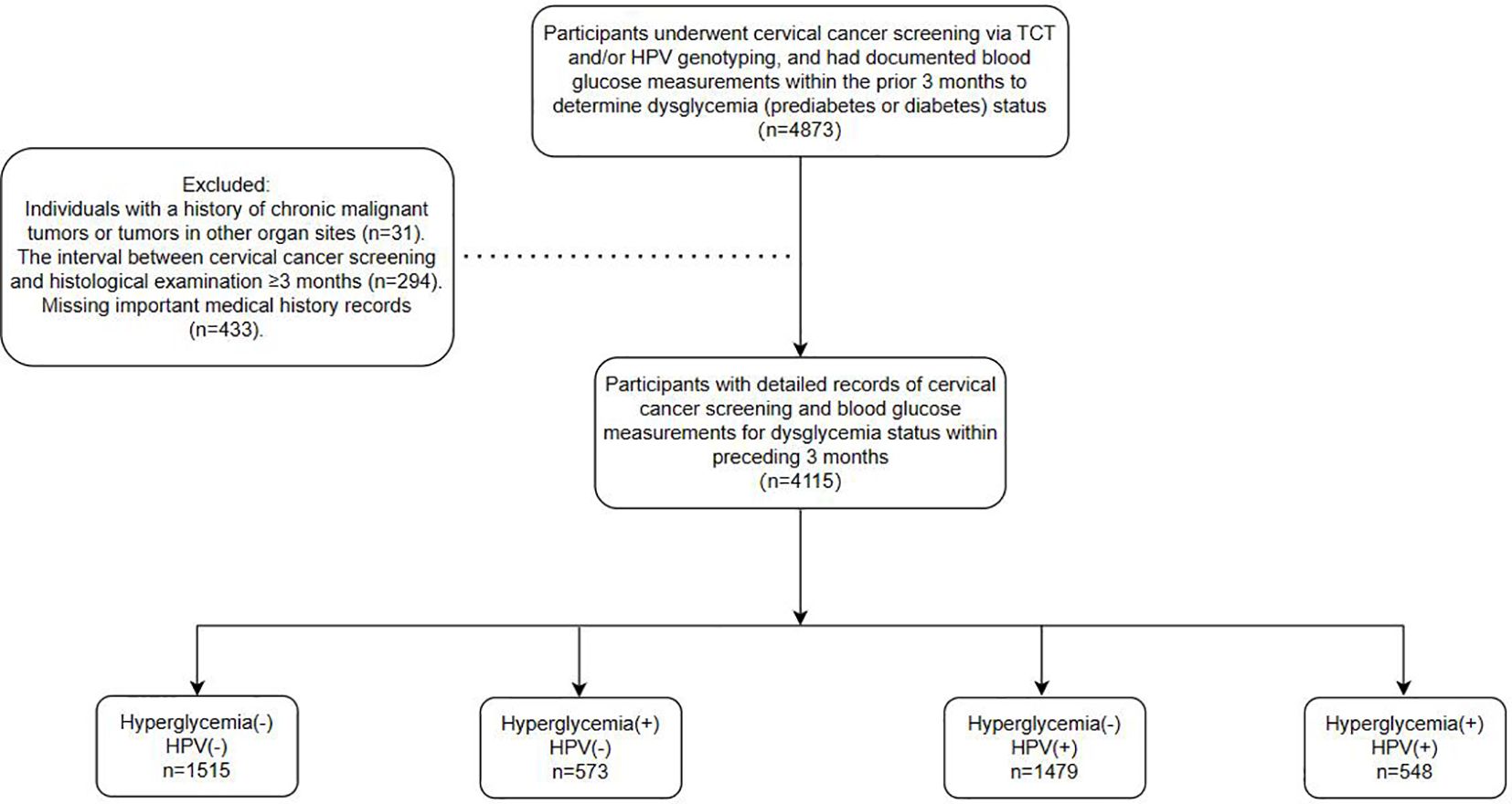
This observational study recruited participants from patients referred to the Cervical Disease Center of Fujian Maternity and Child Health Hospital for colposcopy and conization procedures following abnormal cervical cancer screening results between June 2018 and June 2023. Screening methods employed included the ThinPrep Cytology Test (TCT) and/or HPV genotyping, with a maximum interval of 3 months between screening and histological examination. Furthermore, the study encompassed a total of 4,155 patients. All participants had undergone blood glucose testing within the preceding 3 months, with documented results available. Dysglycemia, including both prediabetes and diabetes, was defined according to the American Diabetes Association (ADA) criteria using SI units: prediabetes as fasting plasma glucose (FPG) between 5.6–6.9 mmol/L and/or HbA1c between 5.7%–6.4%; and diabetes as FPG ≥7.0 mmol/L and/or HbA1c ≥6.5%. Participants with either abnormal FPG or HbA1c were classified as having dysglycemia. Clinical parameters such as age, gravidity, parity, blood glucose profiles (fasting and postprandial levels, and glycosylated hemoglobin), BMI, HPV genotypes, and cervical pathology were obtained from medical records. Ultimately, the study encompassed a cohort of 4155 patients, with the detailed workflow depicted in Figure 1.
Ethical approval was obtained from the Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University (2023KY038), in accordance with the Declaration of Helsinki (2013 revision). Due to the retrospective design, informed consent was waived.
2.2 Serologic detection and HPV genotyping
Fasting and postprandial blood glucose levels were measured to evaluate participants’ glucose metabolism status, providing insights into their glycemic control both in the fasting state and after meals. These measurements help identify individuals with impaired glucose tolerance or diabetes mellitus, important risk factors for cervical cancer. Glycosylated hemoglobin (HbA1c) levels were quantified to assess participants’ long-term glucose control. HbA1c reflects average blood glucose levels over the past 2–3 months and is a valuable indicator of overall glycemic management. Lipid metabolism-related parameters, including cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL-C) levels, were also evaluated. Dyslipidemia, characterized by abnormal lipid profiles, has been associated with an increased risk of various cancers, including cervical cancer. Assessing lipid parameters provides additional insights into participants’ metabolic health and potential cancer risk.
Moreover, HPV detection using polymerase chain reaction-reverse dot blot (PCR-RDB) technology was performed to identify specific HPV genotypes present in cervical samples. HPV genotyping utilized the PCR-RDB HPV genotyping method provided by Yaneng Biotech, distinguishing among 18 types of high-risk HPV (HR-HPV) strains (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and 83). Single identification of a high-risk HPV type indicated positivity, while detection of multiple HPV types in a sample suggested multiple infections.
2.3 Pathological diagnosis and Ki-67 staining in cervical biopsy samples
Colposcopy and cervical biopsy were performed in all patients. Colposcopy and cervical biopsy are performed by qualified attending physicians and above in the Cervical Center of our hospital. Biopsy was performed from the abnormal area using a perforated cervical biopsy tissue forceps. Cervical specimens were processed in a histopathology laboratory, where histopathologists performed a blind diagnosis of HPV status in participants. Two blind senior pathologists independently performed pathological assessments of cervical biopsy, ECC and cone tissue. Histological end points were defined according to WHO Classification of Female Genital Tumors (4th Edition) 2014, and were divided into normal or cervicitis, low-grade cervical intraepithelial neoplasia(LSIL), high-grade cervical intraepithelial neoplasia(HSIL), and cervical cancer.
Ki-67 staining kit was used for immunocytochemical staining of each biopsy tissue specimen. The scoring criteria for the Ki-67 index involve assessing the percentage of positive cells. Typically, an appropriate field of view is selected, and the proportion of Ki-67 positive cells within that field is calculated. This proportion is usually expressed as a percentage. To ensure accuracy, multiple fields are often chosen for scoring, and the results are averaged. Following scoring, the results are verified and recorded. For each sample, the percentage of Ki-67 positive cells is documented as a quantitative measure of the Ki-67 index. For the purpose of this study, high Ki-67 expression was defined as ≥10% positive cells, based on established literature and clinical relevance. The scoring process requires highly trained professionals to ensure accuracy and reliability. Three pathologists typically independently evaluate the Ki-67 index to minimize subjective bias and ensure result consistency.
2.4 Statistical analysis
Participant characteristics were presented as mean ± SD for continuous variables and as frequency and percentage for categorical variables. Baseline characteristics across different categories of cervical lesions were compared using one-way analysis of variance (ANOVA) or the Kruskal-Wallis rank-sum test for continuous variables, and the Chi-squared test or Fisher’s exact test for categorical variables. Multinomial logistic regression was conducted to investigate the associations of HPV and dysglycemia, both separately and in combination, with the occurrence of LSIL, HSIL, and cervical cancer. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated, and trend analysis was performed. Interaction analysis was also employed to assess multiplicative scale, relative excess risk due to interaction (RERI), attribute proportion (AP), and synergy index (SI) for various cervical lesions. Additionally, mediation analysis was conducted to examine whether the combined co-infection of HPV and dysglycemia influences LSIL, HSIL, and cervical cancer through Ki-67 expression. The mediation proportion was estimated using the R package “mediation”, with a 95% confidence interval calculated around the estimate. Mediation diagrams were also generated to illustrate these relationships. Statistical significance was defined as a two-sided test with a significance level of P ≤ 0.05. All statistical analyses were performed using R, version 4.2.2 (http://www.R-project.org).
3 Results
3.1 The impact of blood glucose levels on HPV infection and cervical lesions
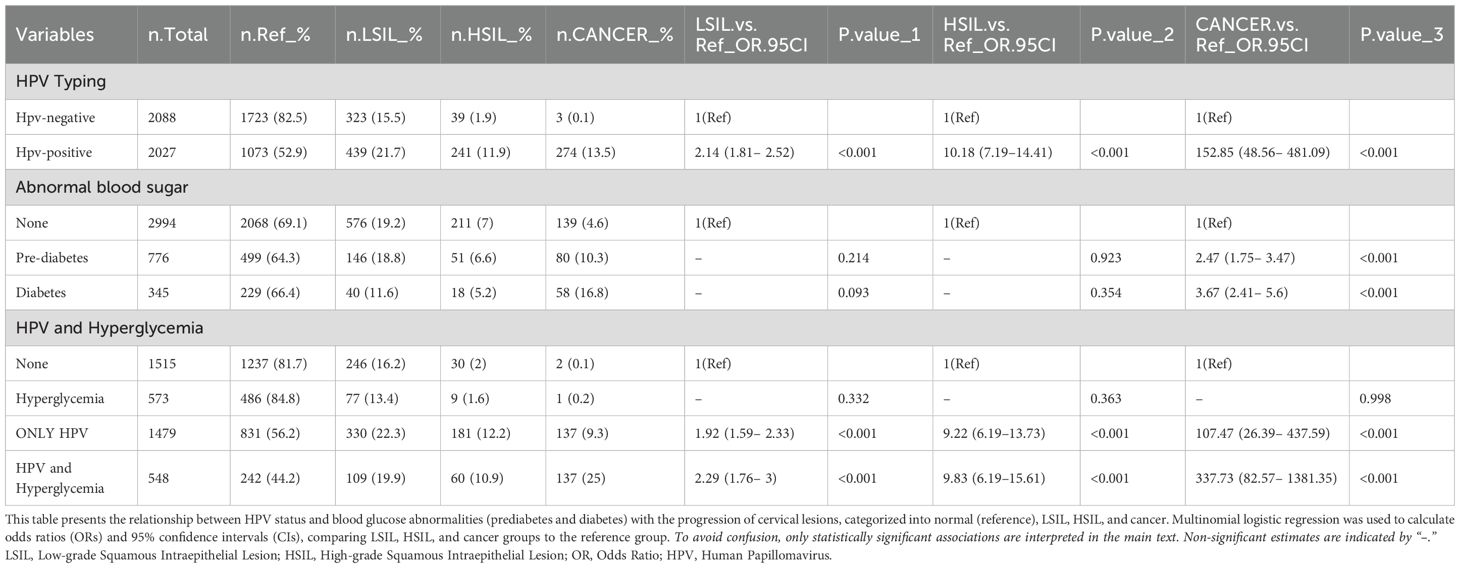
Table 1 provides data on 4,115 individuals, investigating the interplay between HPV infection, cervical lesion severity, and various clinical parameters, notably blood glucose levels. HPV prevalence showed significant associations with cervical lesion severity (p < 0.001). Among the total cohort, 49.3% tested positive for HPV, rising sharply to 98.9% in the cervical cancer group. Similarly, high-risk HPV types 16/18 prevalence increased from 16.7% overall to 65% in the cancer group (p < 0.001). Multiple HPV infections were more prevalent in higher cervical lesion grades (p < 0.001). Dysglycemia emerged as a crucial risk factor. While only 8.4% had diagnosed diabetes, 18.9% exhibited prediabetes based on elevated blood glucose levels. The mean fasting blood glucose was 5.3 mmol/L overall but elevated to 5.9 mmol/L in the cancer group (p < 0.001). Postprandial 2-hour glucose levels were also significantly higher in the cancer group at 8.0 mmol/L compared to 7.0 mmol/L overall (p < 0.001). HbA1c, reflecting longer-term glycemic control, similarly increased with lesion severity from 6.3% to 7.1% in cancer (p < 0.001).
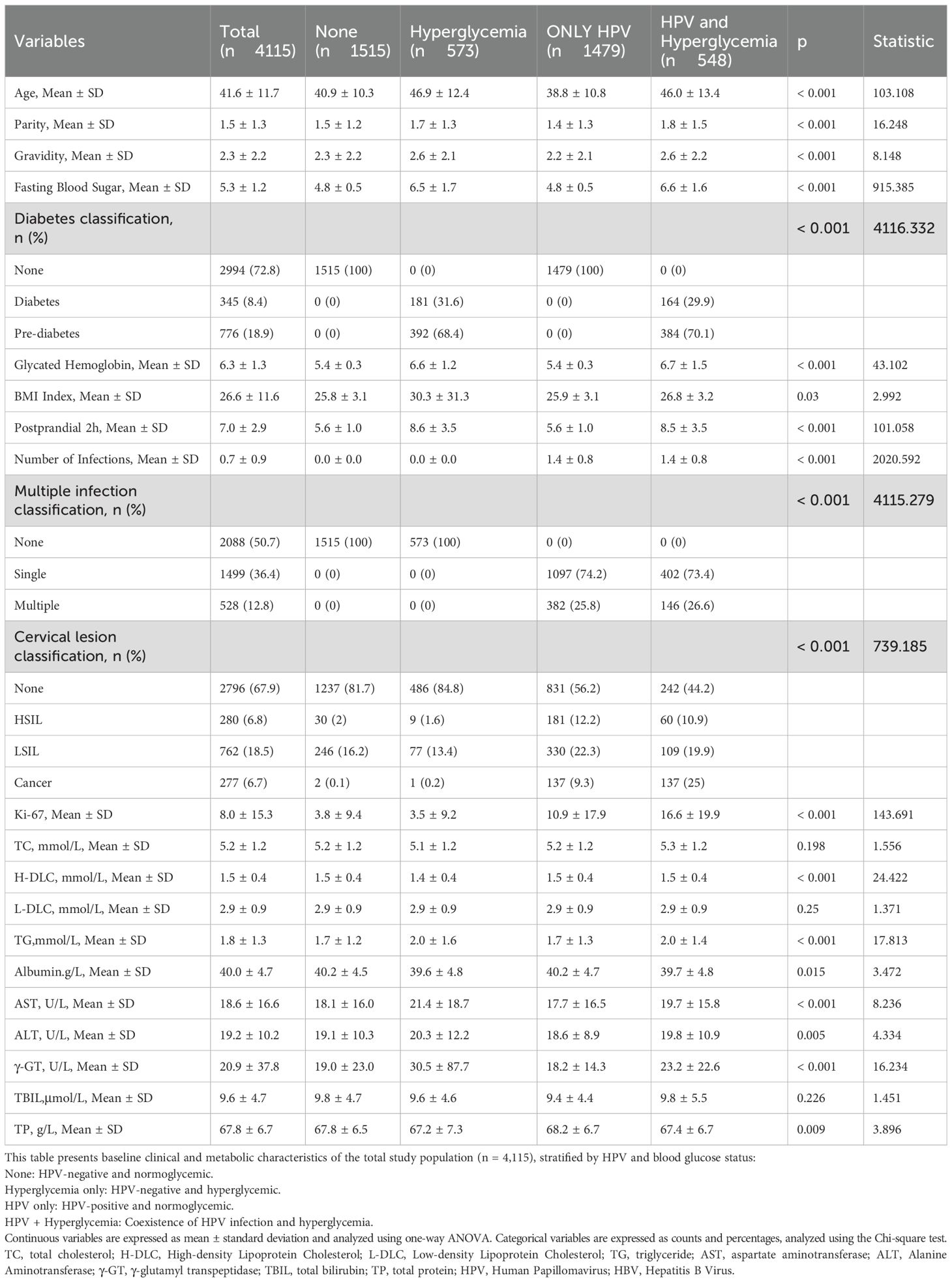
Table 1. Basic characteristics and the impact of blood glucose levels on HPV infection and cervical lesions.
Additionally, focusing solely on the HPV-positive subgroups, individuals with concurrent dysglycemia exhibited a significantly higher prevalence of cervical cancer at 25% compared to 9.3% in the only HPV-positive group (p < 0.001). The proliferative marker Ki-67 was also notably elevated at 16.6% in the dysglycemic HPV-positive subgroup. Diabetes classification demonstrated a substantial association with both HPV infection and cervical lesions (p < 0.001), indicating elevated rates of diabetes and prediabetes among individuals with advanced lesions, suggesting an association between dysglycemia and HPV-driven cervical carcinogenesis. Other metabolic parameters such as dyslipidemia and liver enzyme elevations were similarly linked to more severe cervical lesions, potentially reflecting shared metabolic dysfunction. However, BMI did not exhibit significant differences between groups.
In summary, these findings underscore that hyperglycemia and diabetes pose significant risks for HPV persistence and progression to precancerous cervical lesions and cancer. Tight glycemic control may confer protection, as evidenced by the increasing levels of fasting glucose, postprandial glucose, and HbA1c across worsening cervical pathology grades. This emphasizes the importance of metabolic health in HPV immunity and cervical cancer prevention efforts.
3.2 Risk association between diabetes/prediabetes and HPV-driven cervical lesion progression
Table 2 explores the association between blood glucose levels, HPV infection, and cervical lesions. The data examines the relationship between dysglycemia (diabetes and prediabetes), HPV infection status, HPV genotypes, and severity of cervical lesions in 4,115 individuals. Examining HPV genotypes, infection with high-risk HPV markedly increased odds of cervical cancer by over 152-fold compared to HPV negative individuals (OR: 152.85, 95% CI: 48.56–481.09, p<0.001). Among those with dysglycemia, 10.3% had cervical cancer compared to only 4.6% in the normoglycemic group (p<0.001). Prediabetes and diabetes were both significantly associated with increased cancer risk(OR: 2.47, 95% CI: 1.75-3.47 and OR:3.67, 95% CI: 2.41-5.6, respectively). HPV prevalence was significantly higher in the dysglycemic group (p<0.001). When stratified by HPV and dysglycemia status, those with both HPV and dysglycemia had the highest cervical cancer rate at 25% versus 9.3% with HPV alone and 0.1% with neither (p<0.001). The combination of high-risk HPV and dysglycemia appeared to promote cervical lesion progression.
3.3 Subgroup analysis: combined effects of HPV infection and hyperglycemia on cervical lesions
Table 3 stratifies individuals into subgroups based on HPV status and presence of hyperglycemia (diabetes/prediabetes). This allows examination of how hyperglycemia impacts cervical lesion risk in both HPV-positive and HPV-negative settings. Among the HPV-negative subgroups, hyperglycemia alone did not significantly increase risk of low-grade squamous intraepithelial lesions (LSIL) or higher grade cervical lesions compared to normoglycemic controls. However, in the HPV-positive setting, coexisting hyperglycemia drastically elevated cervical cancer risk over 3-fold (OR: 3.12, 95% CI: 2.34-4.16) versus HPV-positive normoglycemics. 25% of the HPV-positive hyperglycemic subgroup had invasive cancer compared to only 9.3% with HPV alone.
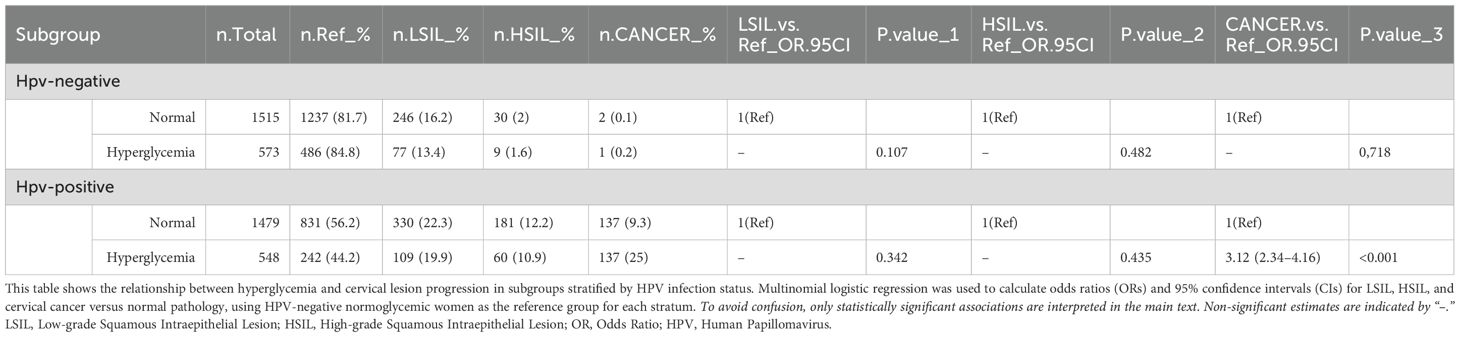
Table 3. Subgroup analysis: combined effects of HPV infection and hyperglycemia on cervical lesions.
3.4 Additive and multiplicative interaction between dysglycemia and HPV infection on cervical cancer risk
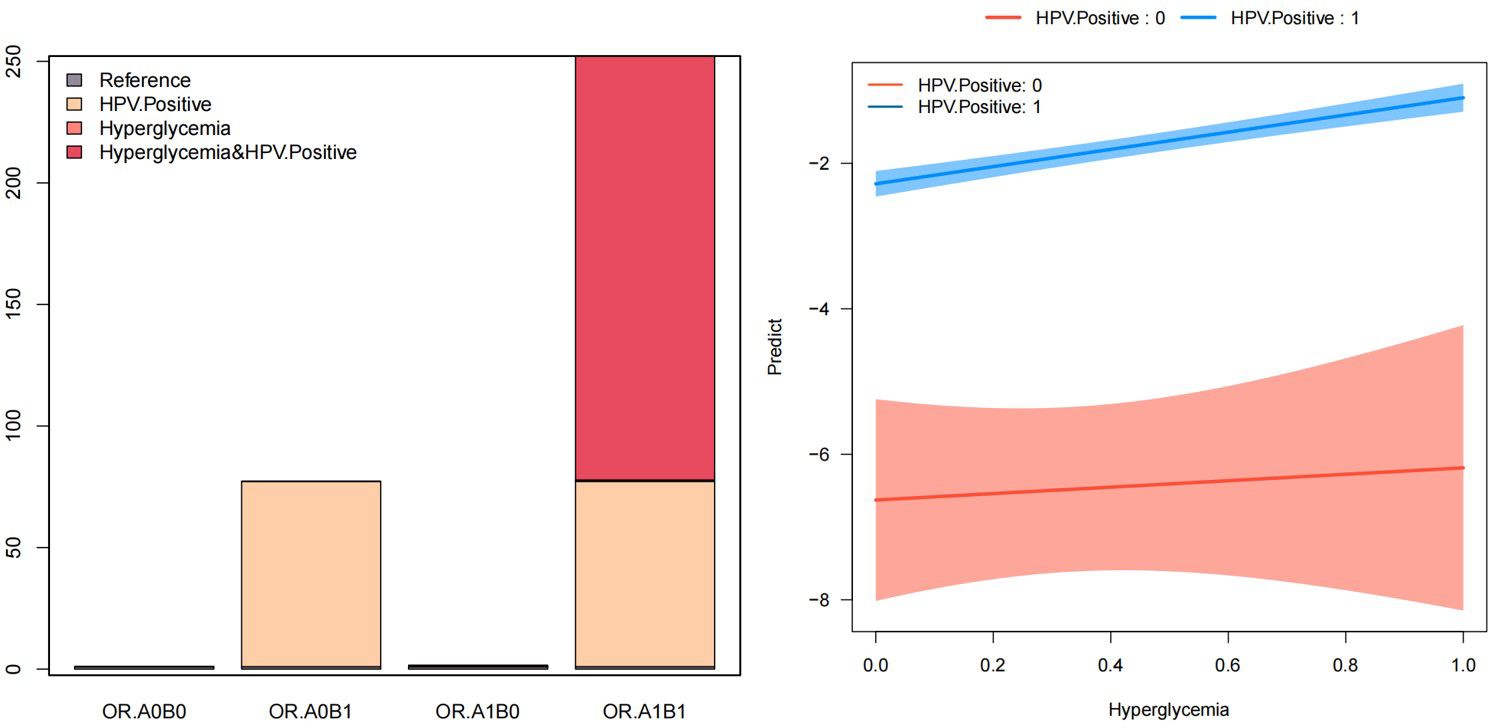
Table 4 and Figure 2 depict the additive and multiplicative interactions between dysglycemia and HPV infection in relation to cervical cancer risk. The OR for women who are HPV positive and have hyperglycemia (OR11) is 252.17 (95% CI: 62.17-1022.81, p < 0.001), indicating a significantly increased risk compared to those without either exposure. Being HPV positive alone (OR01) also substantially increases the odds of cervical cancer by 77.23 times (95% CI: 19.08-312.52, p < 0.001). In contrast, hyperglycemia alone (OR10) does not appear to significantly elevate the risk, and therefore, was excluded from further interpretation.
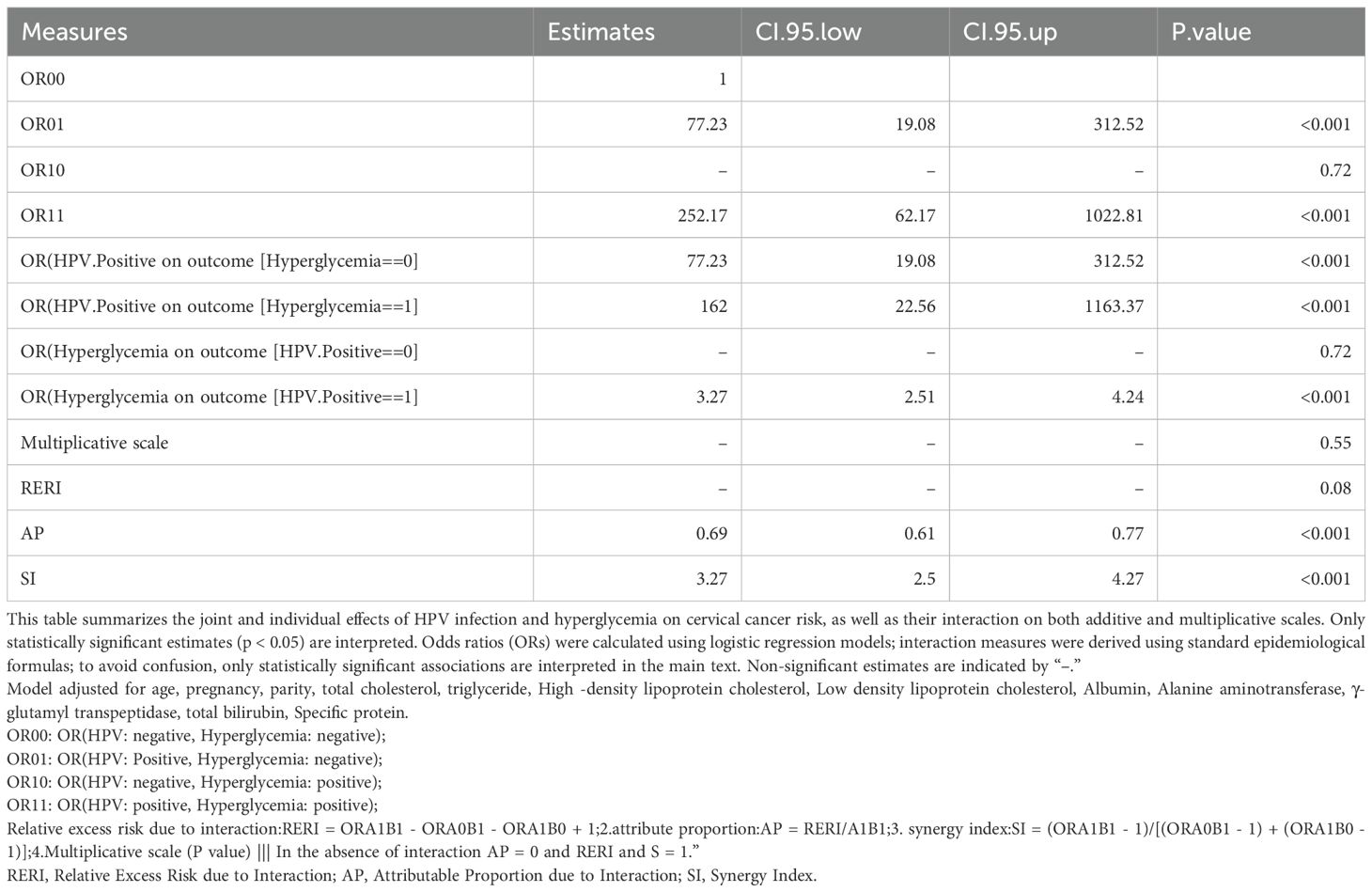
Table 4. Additive and multiplicative interaction between dysglycemia and HPV infection on cervical cancer risk.
Table 4 also provides evidence of a potential interaction between these two factors on cervical cancer risk. The multiplicative scale suggests a multiplicative interaction, though it is not statistically significant. Additionally, the attributable proportion due to interaction (AP) of 0.69 (95% CI: 0.61-0.77, p < 0.001) indicates that 69% of the combined effect is attributable to the interaction between HPV and hyperglycemia. Furthermore, the synergy index (SI) of 3.27 (95% CI: 2.5-4.27, p < 0.001) demonstrates a synergistic effect of these two exposures on cervical cancer risk.
3.5 Role of Ki-67 in mediating the interaction of HPV infection and dysglycemia in cervical cancer
Figure 3 predominantly focuses on the mediation effects of Hyperglycemia, HPV, and their combined impact on cervical cancer through the Ki-67 pathway. Ki-67, a protein associated with cellular proliferation, serves as a critical marker for assessing cancer cell growth and division rates. The mediation analysis conducted in the study yielded significant insights into the role of Ki-67+ in mediating the effects of Hyperglycemia, HPV, and their combined impact on cervical cancer. The results indicated that Ki-67+ accounted for a substantial proportion of the total effect of Hyperglycemia, HPV, and their co-infection on these cervical abnormalities. Specifically, the analysis revealed that Ki-67+ accounted for 39.84%, 37.35%, and 55.18% of the total effect of Hyperglycemia, HPV, and their combined impact on cervical cancer (all P < 0.05, Figure 3). Model adjusted for age, pregnancy, parity, total cholesterol, triglyceride, High -density lipoprotein cholesterol, Low density lipoprotein cholesterol, Albumin, Alanine aminotransferase, γ-glutamyl transpeptidase, total bilirubin, Specific protein. Dysglycemia and HPV similarly demonstrate a significant indirect effect mediated by Ki-67 levels, with an estimate of 0.08 (95% CI: 0.06-0.09, p<0.001). This suggests that abnormal blood glucose elevation and HPV contribute to cervical carcinogenesis, at least partially, by inducing Ki-67 overexpression and aberrant proliferation. Beyond this Ki-67-mediated pathway, dysglycemia exerts a smaller but still significant direct effect of 0.13 (95% CI: 0.011- 0.14, p<0.001) on cervical cancer risk.
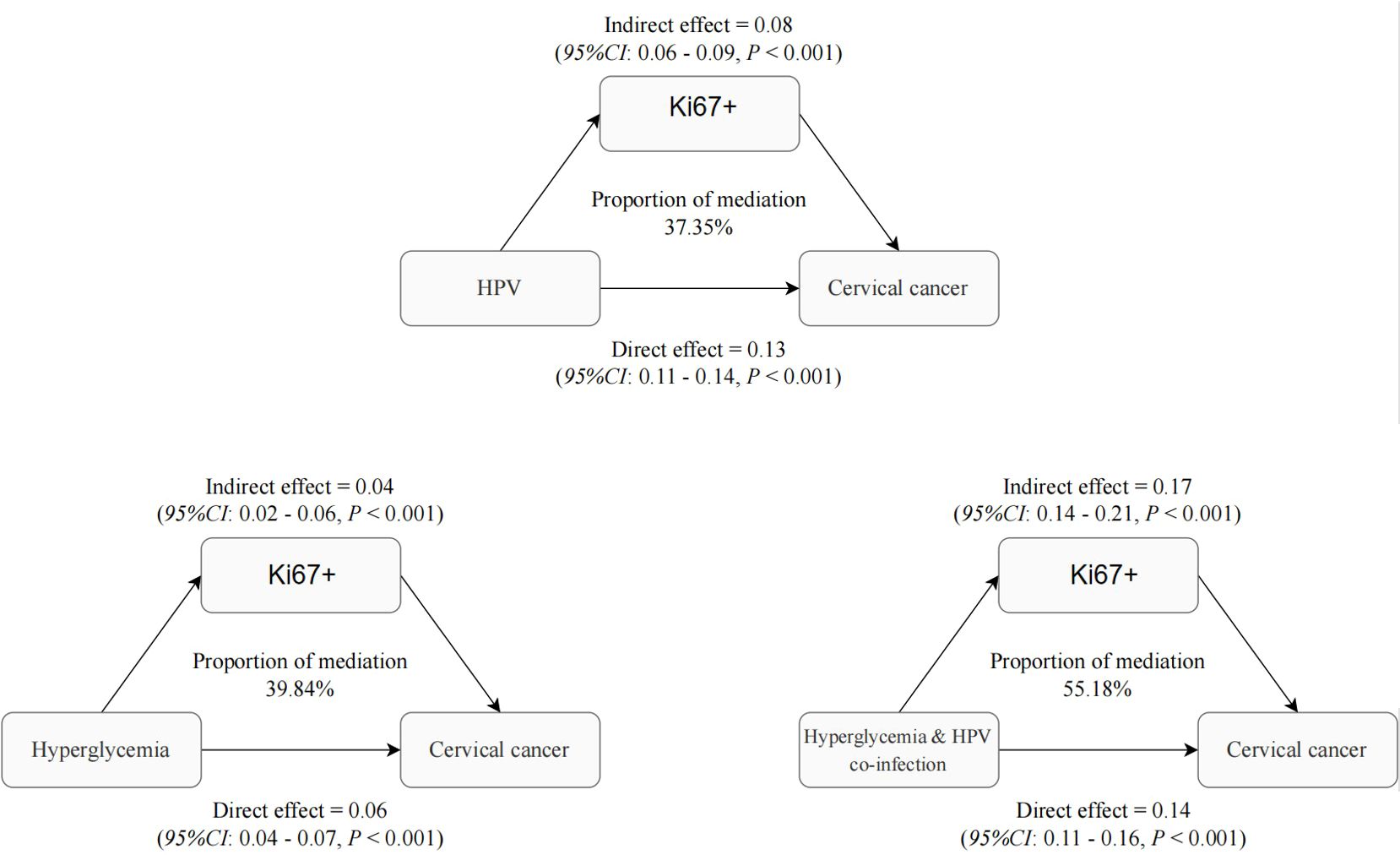
Figure 3. Role of Ki-67 in mediating the interaction of HPV infection and dysglycemia in cervical cancer.
These findings indicate that both HPV and dysglycemia promote cervical cancer development through common mechanisms involving Ki-67 upregulation and increased cellular proliferation, as evidenced by the significant indirect effects. However, each factor also appears to impact carcinogenesis through other direct pathways independent of Ki-67. This analysis provides evidence that Ki-67 overexpression represents a key mechanistic link between HPV, dysglycemia, and cervical cancer development. Nevertheless, additional Ki-67-independent pathways also contribute. Targeting proliferative signaling and improving metabolic health may help disrupt the oncogenic effects of these risk factors and reduce the burden of cervical cancer. Understanding these mediators is crucial for developing effective prevention and therapeutic strategies.
4 Discussion
The present study sheds light on the intricate relationship between HPV infection, dysglycemia, and cervical cancer development, emphasizing the mediating role of Ki-67. HPV infection significantly escalates cervical cancer risk, with an odds ratio surging to 152.85 (95% CI: 48.56–481.09). Consistent with our findings, research by Bruni et al. (2016) and Tan et al. (2018) underscores the significant association between HPV infection and cervical lesion risk. Bruni et al.’s global summary report underscores the relevance of HPV in cervical cancer, while Tan et al.’s study in Malaysian women focuses on HPV prevalence and type distribution (27, 28). Dysglycemia, encompassing both diabetes and prediabetes, emerges as a significant risk factor, with diabetes showing a particularly elevated odds ratio of 3.67 (95% CI: 2.41-5.6) compared to normoglycemic individuals. Similarly, prediabetes significantly increases the risk, with an odds ratio of 2.47 (95% CI: 1.75-3.47). Meanwhile, HPV infection, particularly with high-risk types such as HPV 16/18, substantially increases the risk of cervical cancer. Crucially, the study unveils a synergistic interaction between dysglycemia and HPV infection, amplifying cervical cancer risk beyond their individual effects. Among HPV-positive women, the presence of dysglycemia increases the odds of cervical cancer by over 3-fold compared to HPV-positive individuals with normal glycemic status. This synergy is further supported by measures of additive and multiplicative interaction. The attributable proportion (AP) was 0.71, indicating that approximately 71% of cervical cancer cases among individuals exposed to both dysglycemia and HPV infection may be attributable to their interaction, although again, caution is warranted given the non-significant RERI. Additionally, the synergy index (SI) was 3.45 (95% CI: 2.61–4.56, p < 0.001), providing statistically significant evidence for a multiplicative interaction. Some studies have also found that diabetes increases the risk of cervical cancer. For example, a Danish cohort study of 2,508,321 women found a higher incidence of cervical cancer among those with diabetes (IRR:1.13, 95% CI: 1.00-1.28) (29).Additionally, a meta-analysis of 19 studies reported a relative risk of 1.34 (95% CI: 1.10-1.63) for cervical cancer in women with diabetes (30). Furthermore, a study of 397,783 adults demonstrated a 30% higher prevalence of cervical cancer in diabetic individuals, even after adjusting for confounders (p=0.0011) (31).
Although the risk is relatively low, these studies included the general population, while our focus is on exploring the tumor-promoting effect of hyperglycemia during HPV infection. Another study had similar findings to ours: a retrospective cohort study of 328,994 diabetic individuals noted an increased cervical cancer risk in newly diagnosed type 2 diabetes cases (HR:3.46, 95% CI: 1.10-10.86, p=0.03) (12). However, these studies only found an association between dysglycemia and increased risk of HPV-related cervical cancer, but did not analyze the interaction. Our interaction analysis further confirmed the tumor-promoting effect of dysglycemia on HPV-related cervical cancer. While further research is needed to elucidate the mechanisms, these findings collectively suggest a potential impact of diabetes on cervical cancer risk.
Ki-67 is closely associated with cell proliferation and metastasis, playing a pivotal role in cervical cancer progression (32)and adverse outcomes. Studies consistently link Ki-67 overexpression with advanced cervical cancer stages, lymphatic metastasis, and poor prognosis (33–37). Moreover, both HPV infection and high blood glucose levels are correlated with elevated Ki-67 expression, suggesting a potential mediating role for Ki-67 in the pathways through which HPV and hyperglycemia promote cervical cancer development. Ki-67’s crucial role in cervical cancer progression aligns with our findings. Similar synergistic effects have also been observed in studies investigating the impact of diabetes on the risk of other cancers, where diabetes increases the risk of many cancers (38–42). Additionally, other studies have found that Diabetes Mellitus promotes Ki-67 expression and cell proliferation (26, 43). Our study found a similar effect, and we further analyzed the mediating role of Ki-67 in the increased risk of cervical cancer associated with HPV infection and dysglycemia. In this study, robust mediation analysis revealed Ki-67+ plays a significant role in the collective impact of Hyperglycemia, HPV, and their co-infection on cervical abnormalities. Specifically, Ki-67+ contributed to 39.84%, 37.35%, and 55.18% of the overall effect of Hyperglycemia, HPV, and their combined influence on cervical cancer, respectively.
While our study provides valuable insights into the complex interplay between HPV infection, dysglycemia, Ki-67 expression, and cervical cancer risk, several limitations should be acknowledged. Firstly, the cross-sectional design of our study limits our ability to establish causal relationships between the variables examined. Longitudinal studies with a prospective design would offer a more robust approach to elucidate the temporal sequence of events and infer causality. Secondly, while our analysis controlled for several confounding factors, residual confounding remains a possibility. Factors such as dietary habits, medication use, and comorbidities not accounted for in our analysis may influence the observed associations. Additionally, our study focused primarily on Ki-67 expression as a marker of proliferation, overlooking other potential molecular mechanisms involved in cervical carcinogenesis. Future research incorporating a broader array of biomarkers and molecular pathways would provide a more comprehensive understanding of the underlying mechanisms. Furthermore, our study population consisted primarily of individuals from a single geographic region, which may limit the generalizability of our findings to other populations with different demographic and socioeconomic characteristics. Moreover, the study’s sample size may have limited statistical power, particularly for subgroup analyses and interaction analyses. Larger sample sizes would allow for more robust assessments of effect modification and subgroup differences. Lastly, the lack of information on HPV genotypes beyond HPV 16/18 and detailed glycemic control measures, precluded a more nuanced analysis of these variables’ impact on cervical cancer risk. Despite these limitations, our study contributes valuable insights into the complex etiology of cervical cancer and underscores the need for further research to address these limitations and confirm our findings in diverse populations using longitudinal designs and comprehensive molecular analyses.
5 Conclusion
Dysglycemia and HPV infection synergistically elevate cervical cancer risk, providing compelling evidence of their independent and synergistic effects, likely mediated by Ki-67. These findings highlight the necessity for comprehensive screening and management of both HPV and dysglycemia in cervical cancer prevention. The synergistic interaction between these two factors significantly amplifies the risk of developing cervical cancer, surpassing their individual impacts. The Ki-67 marker appears to play a crucial role in mediating this synergistic effect, accounting for a substantial proportion of the combined impact on cervical cancer development. Further research is needed to elucidate the underlying molecular mechanisms and validate these findings across diverse populations and ethnic groups. Ultimately, targeting Ki-67-mediated pathways and exploring novel therapeutic interventions that modulate these pathways may offer promising avenues to mitigate the elevated cervical cancer risk observed in individuals with concurrent HPV infection and dysglycemic conditions such as prediabetes or diabetes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University (2023KY038). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YuZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Supervision, Validation, Writing – original draft. HL: Conceptualization, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft. YiZ: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft. QY: Data curation, Formal Analysis, Investigation, Writing – review & editing. YS: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. XZ: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. LS: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding for this research was provided by the National Key Clinical Specialty Construction Program of China (Gynecology) and the Joint Funds for Science and Technology Innovation of Fujian Province, with grant number 2023Y9394.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao M, Wu Q, Hao Y, Hu J, Gao Y, Zhou S, et al. Global, regional, and national burden of cervical cancer for 195 countries and territories, 2007-2017: findings from the Global Burden of Disease Study 2017. BMC Womens Health. (2021) 21:419. doi: 10.1186/s12905-021-01571-3
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Gillani SW, Zaghloul HA, Ansari IA, Abdul MIM, Sulaiman SAS, Baig MR, et al. Multivariate analysis on the effects of diabetes and related clinical parameters on cervical cancer survival probability. Sci Rep. (2019) 9:1084. doi: 10.1038/s41598-018-37694-1
4. Turhan Cakir A, Sel G, Balci S, Harma M, and Harma MI. Evaluation of HPV, smear and colposcopy results in patients with diabetes. Diabetes Metab Syndr. (2022) 16:102335. doi: 10.1016/j.dsx.2021.102335
5. Chen YH, Wang PH, Chen PN, Yang SF, and Hsiao YH. Molecular and cellular mechanisms of metformin in cervical cancer. Cancers (Basel). (2021) 13:1–3. doi: 10.3390/cancers13112545
6. Reinholdt K, Thomsen LT, Munk C, Dehlendorff C, Carstensen B, Jørgensen ME, et al. Incidence of HPV-related anogenital intraepithelial neoplasia and cancer in men with diabetes compared with the general population. Epidemiology. (2021) 32:705–11. doi: 10.1097/EDE.0000000000001375
7. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. Jama. (2021) 326:2498–506. doi: 10.1001/jama.2021.22208
8. Vrachnis N, Iavazzo C, Iliodromiti Z, Sifakis S, Alexandrou A, Siristatidis C, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Arch Gynecol Obstet. (2016) 293:239–46. doi: 10.1007/s00404-015-3858-z
9. Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, et al. Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample mendelian randomization study. Diabetes. (2020) 69:1588–96. doi: 10.2337/db20-0084
10. Anastasi E, Filardi T, Tartaglione S, Lenzi A, Angeloni A, and Morano S. Linking type 2 diabetes and gynecological cancer: an introductory overview. Clin Chem Lab Med. (2018) 56:1413–25. doi: 10.1515/cclm-2017-0982
11. Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. Jama. (2008) 300:2754–64. doi: 10.1001/jama.2008.824
12. Johnson JA, Bowker SL, Richardson K, and Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. (2011) 54:2263–71. doi: 10.1007/s00125-011-2242-1
13. Ranc K, Jørgensen ME, Friis S, and Carstensen B. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia. (2014) 57:927–34. doi: 10.1007/s00125-014-3186-z
14. Shu X, Ji J, Li X, Sundquist J, Sundquist K, and Hemminki K. Cancer risk among patients hospitalized for Type 1 diabetes mellitus: a population-based cohort study in Sweden. Diabetes Med. (2010) 27:791–7. doi: 10.1111/j.1464-5491.2010.03011.x
15. Hsu PC, Lin WH, Kuo TH, Lee HM, Kuo C, and Li CY. A population-based cohort study of all-cause and site-specific cancer incidence among patients with type 1 diabetes mellitus in Taiwan. J Epidemiol. (2015) 25:567–73. doi: 10.2188/jea.JE20140197
16. Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson AM, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. (2016) 59:980–8. doi: 10.1007/s00125-016-3884-9
17. Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. (2005) 92:2070–5. doi: 10.1038/sj.bjc.6602611
18. Chen S, Tao M, Zhao L, and Zhang X. The association between diabetes/hyperglycemia and the prognosis of cervical cancer patients: A systematic review and meta-analysis. Med (Baltimore). (2017) 96:e7981. doi: 10.1097/MD.0000000000007981
19. Mkuu RS, Hall JM, Galochkina Z, Cho HD, Staras SAS, Lee JH, et al. Does the intersectionality of race/ethnicity and type 2 diabetes increase the odds of a cervical cancer diagnosis? A nested case-control study of a florida statewide multisite EHR database. Healthcare (Basel). (2023) 11:4–8. doi: 10.3390/healthcare11131863
20. Kim HM, Kang MJ, and Song SO. Metformin and cervical cancer risk in patients with newly diagnosed type 2 diabetes: A population-based study in Korea. Endocrinol Metab (Seoul). (2022) 37:929–37. doi: 10.3803/EnM.2022.1613
21. Tseng CH. Metformin use and cervical cancer risk in female patients with type 2 diabetes. Oncotarget. (2016) 7:59548–55. doi: 10.18632/oncotarget.10934
22. Yue C, Zhang C, Ying C, and Jiang H. Diabetes associated with cervical carcinoma among high-risk HPV-infected patients with cytologically diagnosed high grade squamous intraepithelial lesion. Front Endocrinol (Lausanne). (2022) 13:993785. doi: 10.3389/fendo.2022.993785
23. Ferrandina G, Ranelletti FO, Larocca LM, Maggiano N, Fruscella E, Legge F, et al. Tamoxifen modulates the expression of Ki67, apoptosis, and microvessel density in cervical cancer. Clin Cancer Res. (2001) 7:2656–61.
24. Ouh YT, Kim HY, Yi KW, Lee NW, Kim HJ, Min KJ, et al. Enhancing cervical cancer screening: review of p16/ki-67 dual staining as a promising triage strategy. Diagnostics (Basel). (2024) 14:2–3. doi: 10.3390/diagnostics14040451
25. Yan X, Gao Z, Zhou Y, Gao F, and Li Q. Expressions of IGF-1R and Ki-67 in breast cancer patients with diabetes mellitus and an analysis of biological characteristics. Pak J Med Sci. (2022) 38:281–6. doi: 10.12669/pjms.38.1.4718
26. Sancakli Usta C, Turan G, Hocaoglu M, Bulbul CB, Kılıc K, Usta A, et al. Differential expressions of ki-67, bcl-2, and apoptosis index in endometrial cells of women with and without type II diabetes mellitus and their correlation with clinicopathological variables. Reprod Sci. (2021) 28:1447–56. doi: 10.1007/s43032-020-00423-z
27. Crosbie EJ, Einstein MH, Franceschi S, and Kitchener HC. Human papillomavirus and cervical cancer. Lancet. (2013) 382:889–99. doi: 10.1016/S0140-6736(13)60022-7
28. Tan SC, Ismail MP, Duski DR, Othman NH, and Ankathil R. Prevalence and type distribution of human papillomavirus (HPV) in Malaysian women with and without cervical cancer: an updated estimate. Biosci Rep. (2018) 38:3–8. doi: 10.1042/BSR20171268
29. Reinholdt K, Thomsen LT, Munk C, Dehlendorff C, Aalborg GL, Carstensen B, et al. Incidence of human papillomavirus-related anogenital precancer and cancer in women with diabetes: A nationwide registry-based cohort study. Int J Cancer. (2021) 148:2090–101. doi: 10.1002/ijc.33365
30. Starup-Linde J, Karlstad O, Eriksen SA, Vestergaard P, Bronsveld HK, de Vries F, et al. CARING (CAncer Risk and INsulin analoGues): the association of diabetes mellitus and cancer risk with focus on possible determinants - a systematic review and a meta-analysis. Curr Drug Saf. (2013) 8:296–332. doi: 10.2174/15748863113086660071
31. Li C, Balluz LS, Ford ES, Okoro CA, Tsai J, Zhao G, et al. Association between diagnosed diabetes and self-reported cancer among U.S. adults: findings from the 2009 Behavioral Risk Factor Surveillance System. Diabetes Care. (2011) 34:1365–8. doi: 10.2337/dc11-0020
32. Mehdi HK, Raju K, and Sheela SR. Association of P16, Ki-67, and CD44 expression in high-grade squamous intraepithelial neoplasia and squamous cell carcinoma of the cervix. J Cancer Res Ther. (2023) 19:S260–s267. doi: 10.4103/jcrt.jcrt_43_21
33. Yu JQ, Zhou Q, Zheng YF, and Bao Y. Expression of vimentin and ki-67 proteins in cervical squamous cell carcinoma and their relationships with clinicopathological features. Asian Pac J Cancer Prev. (2015) 16:4271–5. doi: 10.7314/APJCP.2015.16.10.4271
34. Wu Y, Lv M, Qian T, and Shen Y. Correlation analysis of Ki67 and CK7 expression with clinical characteristics and prognosis of postoperative cervical adenocarcinoma patients. Ann Palliat Med. (2021) 10:9544–52. doi: 10.21037/apm-21-1974
35. Li H, Shen H, Xu Q, Deng D, Wang S, Lu Y, et al. Expression of Pin1 and Ki67 in cervical cancer and their significance. J Huazhong Univ Sci Technolog Med Sci. (2006) 26:120–2. doi: 10.1007/BF02828056
36. Liang SN, Huang YJ, Liu LL, and Liu X. Study on the correlation between the expression of Ki67 and FasL and prognosis of cervical carcinoma. Genet Mol Res. (2015) 14:8634–9. doi: 10.4238/2015.July.31.11
37. Wu J, Wang R, Chen W, Wu Y, and Xiao L. Immunohistochemical markers Ki67 and P16 help predict prognosis in locally advanced cervical cancer. Eur J Obstet Gynecol Reprod Biol. (2024) 294:210–6. doi: 10.1016/j.ejogrb.2024.01.030
38. Beuschlein F, Weigel J, Saeger W, Kroiss M, Wild V, Daffara F, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. (2015) 100:841–9. doi: 10.1210/jc.2014-3182
39. Simbolo M, Bilotta M, Mafficini A, Luchini C, Furlan D, Inzani F, et al. Gene expression profiling of pancreas neuroendocrine tumors with different ki67-based grades. Cancers (Basel). (2021) 13:2054. doi: 10.3390/cancers13092054
40. Huan C, Cui G, Lu C, Qu X, and Han T. Role of Ki-67 in acromegalic patients with hyperprolactinemia: retrospective analysis in 61 Chinese Patients. Pak J Pharm Sci. (2015) 28:719–23.
41. Yamauchi Y, Kodama Y, Shiokawa M, Kakiuchi N, Marui S, Kuwada T, et al. Rb and p53 execute distinct roles in the development of pancreatic neuroendocrine tumors. Cancer Res. (2020) 80:3620–30. doi: 10.1158/0008-5472.CAN-19-2232
42. Clay MR, Pinto EM, Fishbein L, Else T, and Kiseljak-Vassiliades K. Pathological and genetic stratification for management of adrenocortical carcinoma. J Clin Endocrinol Metab. (2022) 107:1159–69. doi: 10.1210/clinem/dgab866
Keywords: Dysglycemia, HPV, Ki-67, cervical cancer, Interaction OR00: OR(HPV: negative, Hyperglycemia: negative), OR01: OR(HPV: Positive Hyperglycemia: negative), OR10: OR(HPV: negative, Hyperglycemia: positive), OR11: OR(HPV: positive, Hyperglycemia: positive)
Citation: Zhang Y, Zhang J, Li H, Zhuang Y, You Q, Su Y, Zheng X and Li S (2025) Synergistic impact of dysglycemia and HPV on cervical cancer risk: a potential mediating role of Ki-67. Front. Endocrinol. 16:1422881. doi: 10.3389/fendo.2025.1422881
Received: 04 July 2024; Accepted: 01 September 2025;
Published: 06 October 2025.
Edited by:
Stefano Palomba, Sapienza University of Rome, ItalyReviewed by:
Marta Caretto, University of Pisa, ItalyMaria Magdalena Montt-Guevara, University of Pisa, Italy
Copyright © 2025 Zhang, Zhang, Li, Zhuang, You, Su, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangqin Zheng, Wmhlbmd4cTEyMTVAMTYzLmNvbQ==; Yanzhao Su, c3V5YW56aGFvNjA5QDE2My5jb20=; Suyu Li, TGlzdXl1MjAwOEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yulong Zhang
Yulong Zhang Junxin Zhang1†
Junxin Zhang1† Haibo Li
Haibo Li YiLing Zhuang
YiLing Zhuang Qianru You
Qianru You Xiangqin Zheng
Xiangqin Zheng Suyu Li
Suyu Li