- 1Department of Scientific Research Section, The First People’s Hospital of Zhumadian, Affiliated Hospital of Huanghuai University, Zhumadian, China
- 2Institute of Monogenic Disease, School of Medicine, Huanghuai University, Zhumadian, China
- 3Department of Geriatric Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Cardiology, Zhumadian Central Hospital, Affiliated Hospital of Huanghuai University, Zhumadian, China
- 5School of Biological and Food Processing Engineering, Huanghuai University, Zhumadian, China
- 6Department of Scientific Research Section, Zhumadian Central Hospital, Affiliated Hospital of Huanghuai University, Zhumadian, China
- 7School of Medicine, Zhumadian Key Laboratory of Chronic Disease Research and Translational Medicine, Huanghuai University, Zhumadian, China
Objective: To ascertain whether vascular complications and high lipoprotein (a) [Lp(a)] concentrations are related in individuals with early-onset type 2 diabetes mellitus (T2DM).
Methods: This observational cross-sectional study included 591 individuals with early-onset T2DM who were divided into four groups based on Lp(a) values which was measured using immunoturbidimetry and presented as mg/dL: high, >50; intermediate, 30≤Lp(a)<50; low, 10≤Lp(a)<30; and very low, <10. The relationship between the risk of vascular complications and Lp(a) level was examined using a logistic regression model.
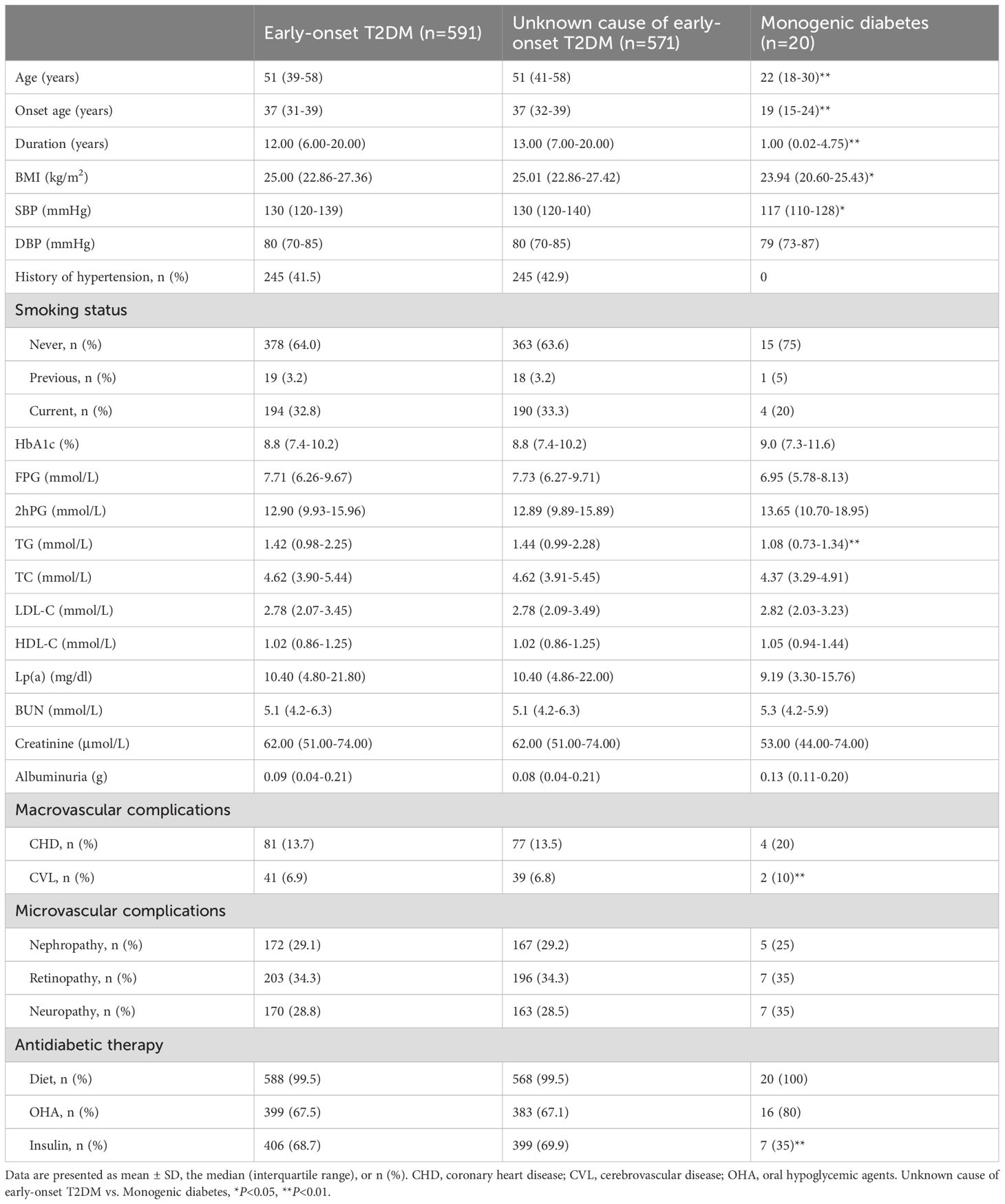
Results: The median age of onset for individuals with early-onset T2DM (n=591) was 37 years, duration of diabetes was 12 years, and glycated hemoglobin (HbA1c) level was 8.8%. The median Lp(a) was 10.40 (4.80-21.80) mg/dL, and Lp(a) concentration did not correlate with age, sex, or glycemic control (P>0.05). Individuals in the low Lp(a) (OR=2.12, 95% CI 1.17-3.84, P<0.05), intermediate Lp(a) (OR=2.76, 95% CI 1.10-6.98, P<0.05) and high Lp(a) (OR=4.79, 95% CI 2.03-11.31, P<0.01) groups had an increased risk of coronary heart disease (CHD) compared with those in the very low Lp(a) group after adjustment. Nevertheless, among individuals with early-onset T2DM, there was no correlation between Lp(a) concentration and the risk of cerebrovascular disease (CVL) and microvascular complications (P>0.05).
Conclusions: In patients with early-onset T2DM, Lp(a) concentration was independently associated with CHD. Lp(a) testing is essential to determine who has a latent high risk of CHD among patients with early-onset T2DM.
Introduction
Lipoprotein (a) [Lp(a)] is a particle that resembles low-density lipoprotein (LDL) containing apolipoprotein B100 (apoB100) covalently attached to highly polymorphic glycoprotein, apolipoprotein(a) [apo(a)] (1). The interethnic variation in Lp(a) levels may be caused by the size of apo(a) and LPA genetic variation (2). Owing to the potent capacity of Lp(a) to transport oxidized phospholipids and its structural similarity to plasminogen, the cholesterol ester-rich Lp(a) is proposed to have stronger proatherogenic properties than LDL-cholesterol (LDL-C) (3, 4). Strong evidences from cross-sectional, prospective cohort, and genetic studies have confirmed that regardless of ethnicity, even at low levels of LDL-C, elevated Lp(a) levels have been identified as a risk factor for cardiovascular disease (CVD) (5–9). Elevated levels of Lp(a) have also been linked to a higher risk of ischemic stroke and poor functional outcomes, based on recent research (10–12). Additionally, at low inflammatory or LDL-C levels, the risk of stroke recurrence associated with high Lp(a) levels is reduced (13).
Limited data regarding Lp(a) in type 1 diabetes mellitus (T1DM) have been reported. Studies have shown that patients with well-controlled T1DM have low Lp(a) levels to T1DM patients with poorer metabolic control, and Lp(a) is regarded as an important risk factor for CVD, albuminuria, and calcified aortic valve disease in individuals with T1DM (14, 15). Lp(a) in type 2 diabetes mellitus (T2DM) presents a significantly more complex scenario. Although plasma Lp(a) levels have been consistently reported to be lower in individuals with T2DM than in nondiabetic controls, compelling evidences suggest that higher circulating Lp (a) levels in individuals with T2DM are markedly related to higher odds of CVD and diabetic nephropathy and retinopathy (16–21). A reduced Lp(a) concentration in patients with T2DM and their increased risk of diabetic complications is called the Lp(a) paradox in T2DM (22). Gene variants associated with lipid and lipoprotein metabolism in individuals with T2DM may help explain their low Lp(a) levels. Consistently, reduced Lp(a), apolipoprotein (AII), and apolipoprotein (CIII) levels have been observed in patients with maturity-onset diabetes of the young (MODY) carrying the HNF4A (Q268X) mutation (23).
Based on recent epidemiological research, the age-standardized incidence of early-onset T2DM has increased remarkably from 117.22 per 100,000 in 1990 to 183.36 per 100,000 in 2019 (24). Monogenic diabetes mainly occurs in patients with early-onset T2DM, with a high mutation detection frequency of 16% (25, 26). Mutations in HNF1A, HNF4A, and HNF1β genes involved in Lp(a) expression account for more than 50% of monogenic diabetes with a known genetic cause, which may lead to the complexity of concentrations of Lp(a) in patients with T2DM (22, 25, 27, 28).
The purpose of this cross-sectional study is to ascertain whether vascular complications of individuals with early-onset T2DM are related to high Lp(a) concentrations, and the association between Lp(a) levels and glycemic control. We tested the hypothesis that high levels of Lp(a) are associated with increased risk of cardiovascular diseases in patients with early-onset T2DM.
Research design and methods
Study population
This observational cross-sectional study recruited patients with early-onset T2DM from the Department of Endocrinology, Zhumadian Central Hospital and the First Affiliated Hospital of Zhengzhou University from June 2021 to June 2023. Early-onset T2DM refers to T2DM that is diagnosed in childhood or early adulthood (29). The participant inclusion criteria were (1) diagnosis of diabetes according to criteria set by the American Diabetes Association (2021) and (2) onset age ≤ 40 years (30). Patients with T1DM and secondary diabetes (such as diabetes induced by exocrine pancreas diseases, drugs, or chemicals) were excluded. Patients without Lp(a), glycated hemoglobin (HbA1c), and fasting plasma glucose (FPG) measurements were also excluded. Finally, 591 patients with early-onset T2DM were included, of which 20 individuals had monogenic diabetes of a known cause. Based on a prior report, individuals with early-onset T2DM were categorized into four groups according to Lp(a) levels: high Lp(a) (≥50 mg/dL), intermediate Lp(a) (≥30 to <50 mg/dL), low Lp(a) (≥10 to <30 mg/dL), and very low Lp(a) (<10 mg/dL) groups (18). This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committees of Zhumadian Central Hospital and the First Affiliated Hospital of Zhengzhou University. Written informed permission was obtained from each patient to take part in the research.
Clinical features and laboratory parameters
Basic information on the study population, such as age, onset age of diabetes, height, weight, blood pressure, smoking history, family history of diabetes, diabetic complications, and hypoglycemic treatment, was obtained from electronic medical records. Various blood samples were collected from patients for laboratory analysis after overnight fasting for 10-12 hours. Using immunoturbidimetry, Lp(a) levels were determined and are presented as mg/dL. For calibration, the Lp(a) protein validation standard was used, and the coefficient of variation of the repeated measurements was less than 10%. An automatic biochemistry analyzer (7600-020; Hitachi, Tokyo, Japan) was used to measure LDL-C, triglyceride, total cholesterol, serum creatinine (SCr), blood urea nitrogen, and high-density lipoprotein cholesterol. Plasma glucose levels were measured using an enzymatic hexokinase method. HbA1c levels were determined using high-performance liquid chromatography (Bio-Rad, Hercules, CA, USA). Urinary protein quantification and urinary microalbumin levels were identified in 24-hour urine samples using immunoturbidimetry.
Definitions of diabetic complications
As previously reported, macrovascular complications were defined based on the international Classification of diseases, 10th edition (ICD-10) (15). Macrovascular complications are composites of coronary heart disease (CHD) and cerebrovascular disease (CVL). CHD included angina pectoris (I20), myocardial infarction (I21, I25.2), coronary artery bypass graft history (Z95.1), and coronary angioplasty implant (Z95.5). CVL included ischemic stroke (I63.4, I63.5) and transient ischemic attack (TIA) (G45.9). The microvascular complications of diabetes were defined according to the ADA guidelines (31). Diabetic nephropathy (DN) was defined as the presence of macroalbuminuria [urinary albumin-creatinine ratio (UACR)≥300 mg/g)] or microalbuminuria (30≤UACR<300mg/g) and/or impaired renal function characterized by a decreased estimated glomerular filtration rate (eGFR). Retinopathy confirmed by fundus photography of the retina was defined as diabetic retinopathy (DR). Diabetic peripheral neuropathy (DPN) was diagnosed by a clinician based on the patient’s symptoms and nerve conduction studies (NCS).
Statistical analysis
SPSS 29.0 and GraphPad Prism 8 were used for statistical analyses and graphics production. Values are presented as median (interquartile range) or mean ± SD for continuous variables and as frequency (%) for categorical variables. The distribution pattern was tested using the Kolmogorov-Smirnov test. Student’s t-test was employed to compare the means of the differences between the groups, Mann-Whitney U test for medians, and Pearson χ2 tests for percentages, where appropriate. A logistic regression model was used to assess the correlation between macrovascular and microvascular problems and Lp(a) level. Statistical significance was defined as a P value <0.05.
Results
Clinical and biochemical characteristics of patients with early-onset T2DM
Table 1 displays the characteristics of the 591 patients with early-onset T2DM. Compared with early-onset T2DM patients with an unknown cause, patients with monogenic diabetes had a younger onset age (19 vs. 37 years, P<0.01), shorter duration (1 vs. 13 years, P<0.01), and comparable HbA1c level (9.0 vs. 8.8%, P>0.05). The total prevalence of macrovascular complications in individuals with early-onset T2DM was 18.4%, including 13.7% with CHD and 6.9% with CVL. The total prevalence of microvascular complications was 58.2%, including 29.1% with DN, 34.3% with DR, and 28.8% with DPN. The range of Lp(a) values for those with early-onset T2DM was 10.40 (4.80-21.80) mg/dL, and comparable Lp(a) levels were detected in patients with monogenic diabetes and those with diabetes with an unknown cause [9.19 (3.30-15.76) vs. 10.4 (4.86-22.00) mg/dL, P>0.05] (Table 1).
Lp(a) distribution of patients with early-onset T2DM
Figure 1A illustrates the Lp(a) skewed distribution in patients (n=591) with early-onset T2DM; the median, 80th, and 90th percentiles were 10.4, 25.48, and 43.36 mg/dL, respectively. There were no discernible variations in the concentration of Lp(a) between sexes (Figure 1B). Patients were grouped according to age quartile (Q1: 8-39; Q2: 40-51; Q3: 52-58; and Q4: 59-86 years). Compared with the lowest respective quartile, no significant differences in levels of Lp(a) across age quartile groups were found at the median (Q1: 9.9, Q2: 8.7, Q3: 12.9, Q4: 10.8), 80th percentile (Q1: 24.1, Q2: 22.58, Q3: 29.52, Q4: 24.34), or 90th percentile (Q1: 43.91, Q2: 31.30, Q3: 54.82, Q4: 39.86)] (P>0.05 for all comparisons except for Q3) (Figure 1C).
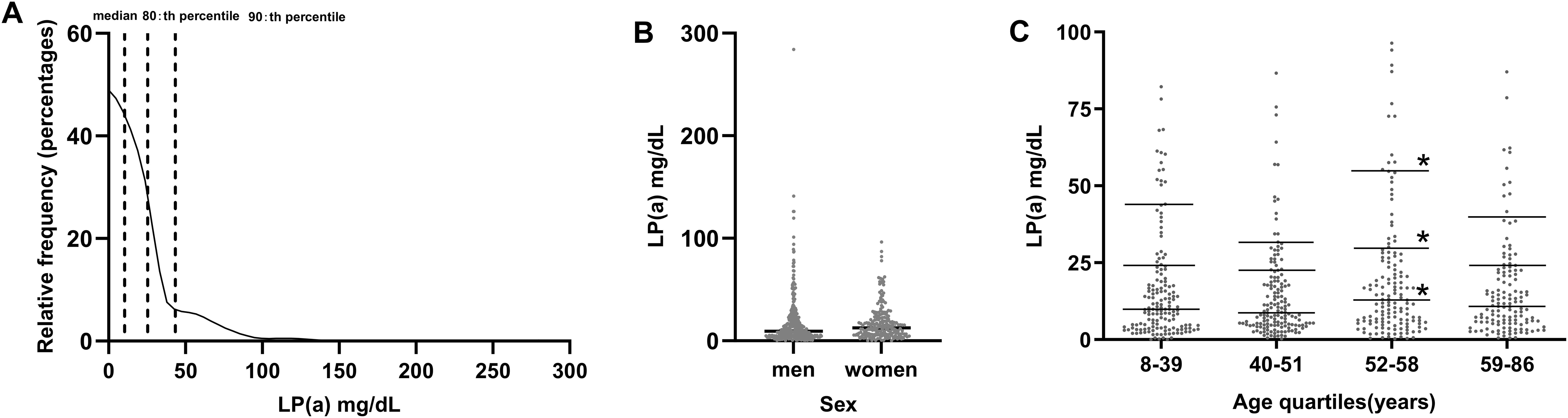
Figure 1. (A) Distribution of lipoprotein (a) [Lp(a)] levels in patients with early-onset T2DM (n=591). (B) Comparison of Lp(a) levels between men and women. (C) Comparison of Lp(a) levels between age quartiles. *P<0.05.
Correlation between diabetic complications and Lp(a) levels in individuals with early-onset T2DM
In individuals with early-onset T2DM, binary logistic regression was used to examine the relationship between the risk of diabetic complications and plasma Lp(a) levels. In contrast to those in the very low Lp(a) group, individuals in the low (OR=1.91, 95% CI 1.11-3.29, P<0.05), intermediate (OR=3.04, 95% CI 1.30-7.08, P<0.05) and high (OR=3.28, 95% CI 1.53-7.06, P<0.01) Lp(a) groups had a higher CHD risk (Table 2). Lp(a) levels remained independently linked to a higher CHD risk after adjusting for confounding factors [low Lp(a) vs. very low Lp(a) group: OR=2.12, 95% CI 1.17-3.84, P<0.05; intermediate Lp(a) vs. very low Lp(a) group: OR=2.76, 95% CI 1.10-6.98, P<0.05; high Lp(a) vs. very low Lp(a) group: OR=4.79, 95% CI 2.03-11.31, P<0.01]. Nevertheless, independent of adjusting for confounding factors, Lp(a) levels in individuals with early-onset T2DM were not associated with the risk of microvascular complications and CVL (P>0.05).
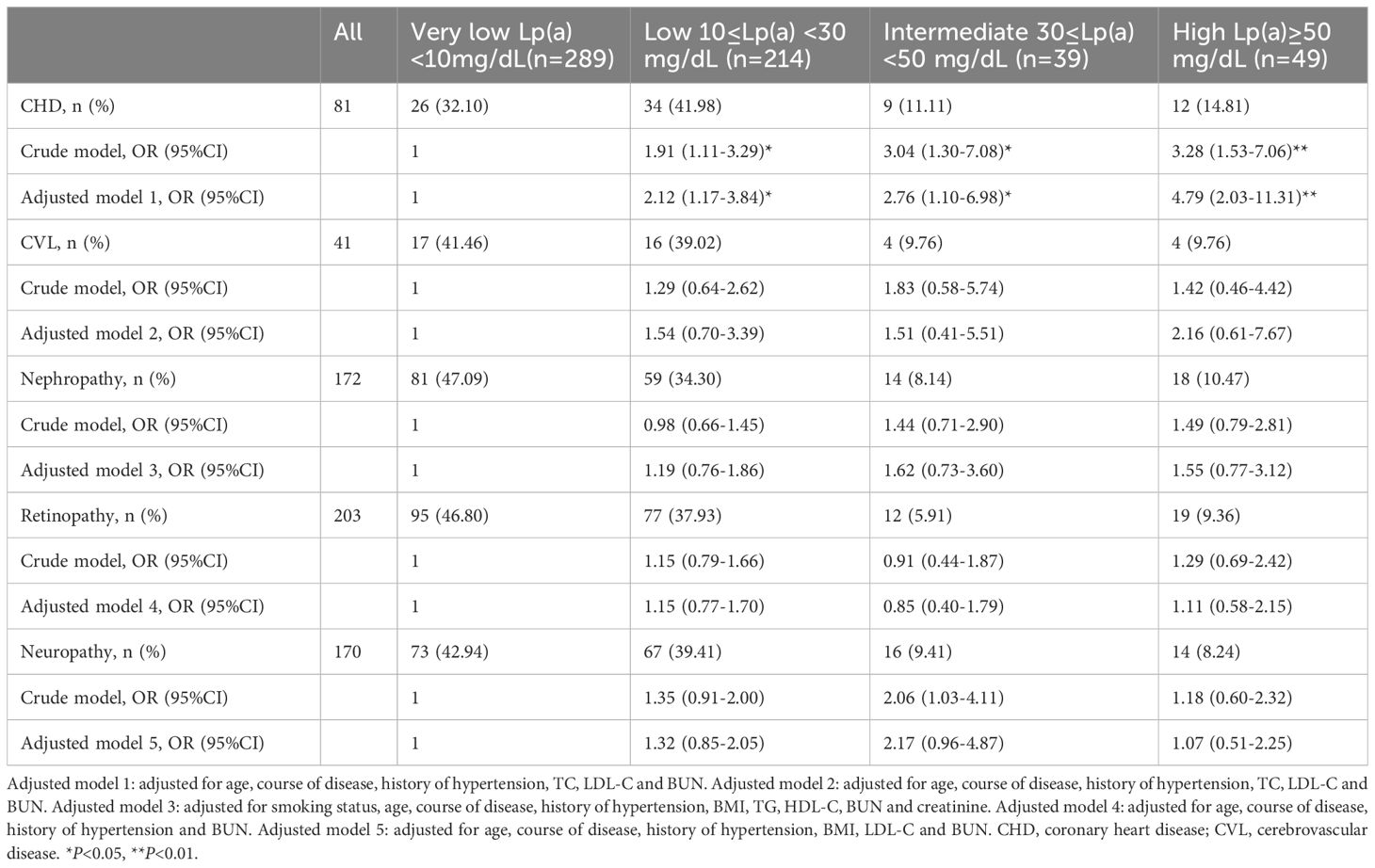
Table 2. Association between Lp(a) levels and diabetic complications in patients with early-onset T2DM(n=591).
Plasma Lp(a) level in relation to glycemic control
As shown in Figures 2A, B, plasma Lp(a) levels in individuals with early-onset T2DM (n=591) were not correlated with HbA1c levels (r=-0.007, P=0.866) and FPG (r=-0.052, P=0.208). Patients were further separated into three groups according to HbA1c level categorized as representing good, <6.9% (n=89); intermediate, 6.9-8.6% (n =208); or poor, >8.6% (n=294) glycemic control, and Lp(a) levels remained uncorrelated with HbA1c levels, with median values at 9.76, 11.10, and 9.60 mg/dL, 80th percentile values at 27.80, 22.70, and 26.20 mg/dL, and 90th percentiles values at 38.50, 37.10, and 48.00 mg/dL, in the three groups, respectively (Figure 2C).
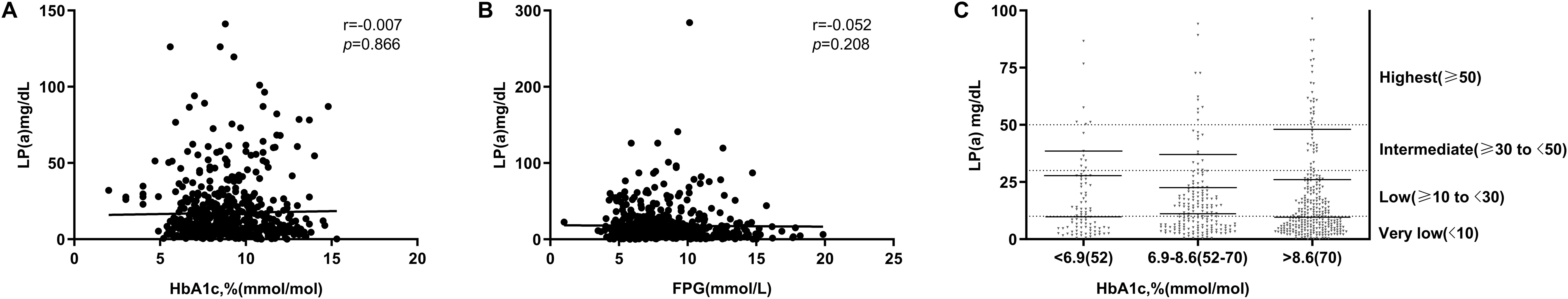
Figure 2. Association between glycemic control and Lp(a) levels. (A, B) Correlation of plasma lipoprotein (a) [Lp(a)] with glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) levels. (C) Absolute values of Lp(a) in each HbA1c group. Patients were divided into three groups according to HbA1c level categorized as representing good, <6.9% (n=89); intermediate, 6.9-8.6% (n =208); or poor, >8.6% (n=294) glycemic control.
Discussion
High Lp(a) is a lipid abnormality primarily caused by variation in the LPA gene, substantial evidence has linked high Lp(a) to an increased risk of CVD (32). Nevertheless, the contribution of Lp(a) to the microvascular and macrovascular consequences of diabetes is still debatable (22). This cross-sectional study demonstrated that the plasma levels of Lp(a) in individuals with early-onset T2DM were not influenced by sex, age, or glycemic control. Moreover, regardless of adjustment for confounders or not, the prevalence of CHD was markedly increased in the high and intermediate Lp(a) groups, while Lp(a) levels were not evidently linked to CVL or diabetic microvascular complications including nephropathy, retinopathy and neuropathy. Therefore, our research provides evidence that in patients with early-onset T2DM, Lp(a) elevation is a risk factor for CHD, regardless of glycemic management.
Although elevated Lp(a) levels are not causally associated with the risk of T2DM, multiple studies have demonstrated that elevated plasma levels of Lp(a) in individuals with T2DM are particularly at an increased risk of CVD (17, 22). In our early-onset T2DM cohort, patients in the very low Lp(a) group had a lower risk of CHD than those in the high and intermediate Lp(a) groups, providing more evidence that high levels of Lp(a) contribute to CVD. Furthermore, a recent study discovered a substantial increase in the likelihood of recurrent cardiovascular events with high levels of Lp(a) in patients with CHD (33). Compared with individuals with little Lp(a) exposure, individuals with T2DM with cumulative Lp(a) exposure are more likely to experience unfavorable cardiovascular outcomes (34). However, the effect of plasma Lp(a) concentration on CVL in patients with diabetes is not as convincing as that on CVD. Despite that raised Lp(a) levels have been reported to be linked to an elevated risk of ischemic stroke, unfavorable functional outcomes, and stroke recurrence, the relation of Lp(a) and CVL in the diabetes cohort was confusing (10–13, 15, 35, 36). In individuals with early-onset T2DM, increased Lp(a) levels did not increase the risk of CVL. Lp(a) concentration was not associated with CVL in patients with early-onset T2DM and T1DM, but was related to the increased risk of ischemic stroke and its unfavorable functional outcome in patients with T2DM, possibly due to the age of onset of diabetes in these individuals (the median of age at diagnosis for early-onset T2DM, T1DM and T2DM, 37 vs. 23 vs.51) (15, 35).
The function of elevated Lp(a) in microvascular problems remains debatable, despite the well-known association between CVD and Lp(a) in individuals with diabetes. Although decreased Lp(a) has previously been reported to be associated with increased microvascular damage, a meta-analysis of 11 observational studies involving 9304 individuals with T2DM indicated that increased Lp(a) in individuals with T2DM was independently related to a greater risk of DN (19, 37). Most epidemiological studies support high circulating Lp(a) concentrations as an independent risk factor for DR, although a correlation between retinopathy and Lp(a) has not been observed in some studies (20, 21). Recently, Shariatzadeh found that anti-inflammatory and anti-angiogenic capacity was affected in patients with T2DM and DR, supporting the unfavorable impact of high Lp(a) levels in DR (38). Unfortunately, we did not observe a relationship between the risk of microvascular disease and the concentration of Lp(a), including DN, DR, and DPN, in Han Chinese individuals with early-onset T2DM. This negative observation may be related to the small sample size and ethnic background in this study, as studies supporting the irrelevance between Lp(a) and microvascular complication also existed the phenomenon of small sample sizes and genetic heterogeneity (19, 21, 39). Additionally, from a genetic perspective, although the LPA single-nucleotide polymorphisms (SNPs) rs3798220 and rs10455872 affect the plasma levels of Lp(a), a prospective genetics-based analysis revealed no evident correlation between the development of microvascular problems in T2DM and LPA SNPs or Lp(a) concentration (40). However, the lack of association in Caucasian populations did not well support our results owing to ethnic heterogeneity. Further large cohort genetic studies conducted in Asian populations will help illuminate the correlation of Lp(a) with diabetic microvascular complications.
The plasma concentration of Lp(a) is genetically determined and remains almost constant throughout the life of an individual; thus, significant inter-individual as well as intra- and inter-ethnic differences are observed in the concentrations of Lp(a), but they are not influenced by age or sex (41). No discernible difference in plasma Lp(a) levels of early-onset T2DM patients was observed between the men and women, or among different age levels in our study. Additionally, Lp(a) concentrations showed no differences among patients with good, intermediate, or poor glycemic control, as indicated by HbA1c, suggesting that the improvement in glycemic control was not accompanied by a reduction in Lp(a). Previous research showing no correlation between Lp(a) levels and glycemic management in individuals with T2DM lends credence to our findings (22). However, Littmann found that metabolic control in patients with T1DM was associated with plasma levels of Lp(a) (15). This could be due to the effect of insulin on the production of Lp(a) in the liver and poor renal function in patients with T1DM. The levels of Lp(a) are mostly elevated in patients with T1DM owing to metabolic effects; nevertheless, publications have consistently reported that plasma Lp(a) levels are lower in patients with T2DM than in controls (22). Lp(a) levels in our early-onset T2DM cohort [10.40 (4.80-21.80) mg/dL] were also lower than that of controls with normal blood glucose regulation [15.2 (6.8–35.5) mg/dL] and patients with diabetes [14.7 (6.6–33.8) mg/dL] reported by Jin (18). The early-onset T2DM cohort included patients with monogenic diabetes resulting from mutations in the HNF1β, HNF4A, and HNF1A genes associated with Lp(a) expression, which may help explain the lower levels of Lp(a) (22, 25, 27, 28). Consistently, low Lp(a) levels [9.19 (3.30-15.76) mg/dL] were detected in patients with monogenic diabetes with these gene mutations (n=9). Furthermore, patients with MODY with the HNF4A (Q268X) mutation have decreased plasma Lp(a) concentrations (23).
The role of high Lp(a) levels in CHD and adverse cardiovascular outcomes in individuals with T2DM with cumulative Lp(a) exposure has prompted an ongoing search for strategies to reduce Lp(a) levels. Two randomized, double-blind trials covering 13 research centers found that in individuals with high levels of Lp(a), antisense oligonucleotides targeting apolipoprotein (a) were effective in lowering Lp(a) concentrations and Lp(a)-related cardiovascular risk (42). Subsequent investigations revealed that in people with CVD and increased Lp(a) levels, the hepatocyte-directed antisense oligonucleotide lowered the levels of Lp(a) in a dose-dependent manner (43). These results support the favorable impact of declining Lp(a) levels on cardiovascular risk in patients with high Lp(a) levels. In addition, Yu et al. recently found that evolocumab therapy brings beneficial changes to plaque components in patients with T2DM and attenuates pericoronary adipose tissue (PCAT) density, which may be caused by a decrease in Lp(a) (44). A worse prognosis and higher probability of cardiac mortality are associated with elevated PCAT density (45). Owing to their prolonged illness duration and early onset age, patients with early-onset T2DM are more prone to cardiovascular events (46). Moreover, individuals with early-onset diabetes have a significantly increased risk of heart failure, and excessive cardiorenal risk factors may be the main drivers of the progression of heart failure (47). Therefore, Lp(a) treatment for early-onset T2DM in patients with high Lp(a) concentrations may reduce long-term cardiovascular risk to some extent.
However, the current study had several limitations. Firstly, This is a cross-sectional study, and we cannot provide evidence for a causative role of Lp(a) for CHD in patients with early-onset T2DM. Secondly, it is also limited in sample size, especially the size of the intermediate and high Lp(a) groups, which may be the main reason that the irrelevance between Lp(a) and microvascular complication. Thirdly, we only incorporated 20 patients with monogenic diabetes, which is difficult to highlight the effect of mutations in HNF1β, HNF4A, and HNF1A genes associated with Lp(a) expression on Lp(a) concentration in patients with early-onset T2DM. In addition, the use of both immunoassay-based and mass spectrometry (MS)-based test for Lp(a) may be more convincing, since MS-based test expressed in molar units is the gold standard method for Lp(a) measurement.
In conclusion, in our cross-sectional study, increased Lp(a) levels were associated with an elevated risk of cardiovascular disease in patients with early-onset T2DM, independent of glycemic control. Future prospective studies with a larger sample size including more cases with monogenic diabetes can further elucidate the precise role of Lp(a) in the vascular complication of early-onset T2DM patients.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committees of Zhumadian Central Hospital and the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JuZ: Conceptualization, Funding acquisition, Investigation, Project administration, Writing – original draft, Writing – review & editing. JS: Data curation, Formal Analysis, Software, Writing – original draft. JY: Data curation, Writing – original draft. YZ: Data curation, Writing – review & editing. JiZ: Validation, Writing – review & editing. XL: Data curation, Validation, Writing – review & editing. HQ: Software, Validation, Writing – review & editing. KZ: Formal Analysis, Investigation, Writing – review & editing. HS: Project administration, Supervision, Writing – review & editing. YY: Conceptualization, Validation, Writing – review & editing. HC: Supervision, Investigation, Writing – review & editing. LY: Conceptualization, Investigation, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Key Science and Technology Program of Henan Province, China (No.232102310004, No. 232102320321 and No. 212102310764), the Natural Science Foundation of Henan province, China (No. 242300421528), the Higher Education Teaching Reform Project of Henan province, China (2024SJGLX0477) and the Young Teacher Foundation of Huanghuai University.
Acknowledgments
The authors thank all of the participants in the study for their dedication and contribution to the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. (2016) 57:1339–59. doi: 10.1194/jlr.R067314
2. Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res. (2016) 57:1111–25. doi: 10.1194/jlr.R051904
3. Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. (2019) 16:305–18. doi: 10.1038/s41569-018-0153-2
4. Björnson E, Adiels M, Taskinen MR, Burgess S, Chapman MJ, Packard CJ, et al. Lipoprotein(a) is markedly more atherogenic than LDL: an apolipoprotein B-based genetic analysis. J Am Coll Cardiol. (2024) 83:385–95. doi: 10.1016/j.jacc.2023.10.039
5. Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. (2009) 361:2518–28. doi: 10.1056/NEJMoa0902604
6. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. (2009) 301:2331–9. doi: 10.1001/jama.2009.801
7. Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. (2016) 57:1953–75. doi: 10.1194/jlr.R071233
8. Saleheen D, Haycock PC, Zhao W, Rasheed A, Taleb A, Imran A, et al. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol. (2017) 5:524–33. doi: 10.1016/S2213-8587(17)30088-8
9. Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. (2022) 43:3925–46. doi: 10.1093/eurheartj/ehac361
10. Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. (2019) 74:54–66. doi: 10.1016/j.jacc.2019.03.524
11. Arora P, Kalra R, Callas PW, Alexander KS, Zakai NA, Wadley V, et al. Lipoprotein(a) and risk of ischemic stroke in the REGARDS study. Arterioscler Thromb Vasc Biol. (2019) 39:810–8. doi: 10.1161/ATVBAHA.118.311857
12. Jiang X, Xu J, Hao X, Xue J, Li K, Jin A, et al. Elevated lipoprotein(a) and lipoprotein-associated phospholipase A2 are associated with unfavorable functional outcomes in patients with ischemic stroke. J Neuroinflammation. (2021) 18:307. doi: 10.1186/s12974-021-02359-w
13. Xu J, Hao X, Zhan R, Jiang X, Jin A, Xue J, et al. Effect of lipoprotein(a) on stroke recurrence attenuates at low LDL-C (Low-density lipoprotein) and inflammation levels. Stroke. (2022) 53:2504–11. doi: 10.1161/STROKEAHA.121.034924
14. Kollerits B, Auinger M, Reisig V, Kästenbauer T, Lingenhel A, Irsigler K, et al. Lipoprotein(a) as a predictor of cardiovascular disease in a prospectively followed cohort of patients with type 1 diabetes. Diabetes Care. (2006) 29:1661–3. doi: 10.2337/dc06-0546
15. Littmann K, Wodaje T, Alvarsson M, Bottai M, Eriksson M, Parini P, et al. The association of lipoprotein(a) plasma levels with prevalence of cardiovascular disease and metabolic control status in patients with type 1 diabetes. Diabetes Care. (2020) 43:1851–8. doi: 10.2337/dc19-1398
16. Schwartz GG, Szarek M, Bittner VA, Bhatt DL, Diaz R, Goodman SG, et al. Relation of lipoprotein(a) levels to incident type 2 diabetes and modification by alirocumab treatment. Diabetes Care. (2021) 44:1219–27. doi: 10.2337/dc20-2842
17. Ye Z, Haycock PC, Gurdasani D, Pomilla C, Boekholdt SM, Tsimikas S, et al. The association between circulating lipoprotein(a) and type 2 diabetes: is it causal? Diabetes. (2014) 63:332–42. doi: 10.2337/db13-1144
18. Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. (2019) 42:1312–8. doi: 10.2337/dc19-0274
19. Ren X, Zhang Z, Yan Z. Association between lipoprotein (A) and diabetic nephropathy in patients with type 2 diabetes mellitus: A meta-analysis. Front Endocrinol (Lausanne). (2021) 12:633529. doi: 10.3389/fendo.2021.633529
20. Tu WJ, Liu H, Liu Q, Cao JL, Guo M. Association between serum lipoprotein(a) and diabetic retinopathy in han chinese patients with type 2 diabetes. J Clin Endocrinol Metab. (2017) 102:2525–32. doi: 10.1210/jc.2016-4015
21. Gholami Chahkand MS, Esmaeilpour Moallem F, Qezelgachi A, Seifouri K, Pesaran Afsharian A, Sheikhzadeh F, et al. Lipoprotein (a) as a predictor of diabetic retinopathy in patients with type 2 diabetes: A systematic review. Diabetes Vasc Dis Res. (2023) 20:14791641231197114. doi: 10.1177/14791641231197114
22. Kostner KM, Kostner GM. Lp(a) and the risk for cardiovascular disease: focus on the lp(a) paradox in diabetes mellitus. Int J Mol Sci. (2022) 23:3584. doi: 10.3390/ijms23073584
23. Shih DQ, Dansky HM, Fleisher M, Assmann G, Fajans SS, Stoffel M. Genotype/phenotype relationships in HNF-4alpha/MODY1: haploinsufficiency is associated with reduced apolipoprotein (AII), apolipoprotein (CIII), lipoprotein(a), and triglyceride levels. Diabetes. (2000) 49:832–7. doi: 10.2337/diabetes.49.5.832
24. Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, et al. Global burden of type 2 diabetes in adolescents and young adults, 1990-2019: systematic analysis of the Global Burden of Disease Study 2019. BMJ. (2022) 379:e072385. doi: 10.1136/bmj-2022-072385
25. Donath X, Saint-Martin C, Dubois-Laforgue D, Rajasingham R, Mifsud F, Ciangura C, et al. Next-generation sequencing identifies monogenic diabetes in 16% of patients with late adolescence/adult-onset diabetes selected on a clinical basis: a cross-sectional analysis. BMC Med. (2019) 17:132. doi: 10.1186/s12916-019-1363-0
26. Bansal V, Gassenhuber J, Phillips T, Oliveira G, Harbaugh R, Villarasa N, et al. Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals. BMC Med. (2017) 15:213. doi: 10.1186/s12916-017-0977-3
27. Wade DP, Lindahl GE, Lawn RM. Apolipoprotein(a) gene transcription is regulated by liver-enriched trans-acting factor hepatocyte nuclear factor 1 alpha. J Biol Chem. (1994) 269:19757–65. doi: 10.1016/S0021-9258(17)32086-0
28. Iwasaki N, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Yano N, et al. Liver and kidney function in Japanese patients with maturity-onset diabetes of the young. Diabetes Care. (1998) 21:2144–8. doi: 10.2337/diacare.21.12.2144
29. Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, et al. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. (2023) 11:768–82. doi: 10.1016/S2213-8587(23)00225-5
30. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-S002
31. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S151–67. doi: 10.2337/dc21-S011
32. Szarek M, Reijnders E, Jukema JW, Bhatt DL, Bittner VA, Diaz R, et al. Relating lipoprotein(a) concentrations to cardiovascular event risk after acute coronary syndrome: A comparison of 3 tests. Circulation. (2024) 149:192–203. doi: 10.1161/CIRCULATIONAHA.123.066398
33. He J, Yang M, Song C, Zhang R, Yuan S, Li J, et al. Lipoprotein(a) is associated with recurrent cardiovascular events in patients with coronary artery disease and prediabetes or diabetes. J Endocrinol Invest. (2024) 47:883–94. doi: 10.1007/s40618-023-02203-3
34. Wang P, Yuan D, Zhang C, Jia S, Song Y, Tang X, et al. Association between cumulative lipoprotein(a) exposure and adverse cardiovascular outcomes in patients with prediabetes or diabetes. iScience. (2023) 26:106117. doi: 10.1016/j.isci.2023.106117
35. Wang H, Zhao J, Gui Y, Yan H, Yan Z, Zhang P, et al. Elevated lipoprotein (a) and risk of poor functional outcome in chinese patients with ischemic stroke and type 2 diabetes. Neurotox Res. (2018) 33:868–75. doi: 10.1007/s12640-017-9850-6
36. Dordonne S, Mergeayfabre M, Hafsi N, Ntoutoum A, Salazar-Cardozo C, Casse O, et al. Impact of lipoprotein(a) on macrovascular complications of diabetes in a multiethnic population in the french amazon. J Diabetes Res. (2023) 2023:8111521. doi: 10.1155/2023/8111521
37. Hermans MP, Ahn SA, Rousseau MF. The mixed benefit of low lipoprotein(a) in type 2 diabetes. Lipids Health Dis. (2017) 16:171. doi: 10.1186/s12944-017-0564-9
38. Shariatzadeh M, Nagtzaam NMA, van Vark-van der Zee L, van Holten-Neelen C, Verhoeven AJM, Dehairs J, et al. Altered functionality of lipoprotein(a) impacts on angiogenesis in diabetic retinopathy. Invest Ophthalmol Vis Sci. (2023) 64:8. doi: 10.1167/iovs.64.5.8
39. Chandni R, Ramamoorthy KP. Lipoprotein(a) in type 2 diabetic subjects and its relationship to diabetic microvascular complications. World J Diabetes. (2012) 3:105–9. doi: 10.4239/wjd.v3.i5.105
40. Singh SS, Rashid M, Lieverse AG, Kronenberg F, Lamina C, Mulder MT, et al. Lipoprotein(a) plasma levels are not associated with incident microvascular complications in type 2 diabetes mellitus. Diabetologia. (2020) 63:1248–57. doi: 10.1007/s00125-020-05120-9
41. Hussain Z, Iqbal J, Liu H, Zhou HD. Exploring the role of lipoprotein(a) in cardiovascular diseases and diabetes in Chinese population. Int J Biol Macromol. (2023) 233:123586. doi: 10.1016/j.ijbiomac.2023.123586
42. Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. (2016) 388:2239–53. doi: 10.1016/S0140-6736(16)31009-1
43. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. (2020) 382:244–55. doi: 10.1056/NEJMoa1905239
44. Yu MM, Zhao X, Chen YY, Tao XW, Ge JB, Jin H, et al. Evolocumab attenuate pericoronary adipose tissue density via reduction of lipoprotein(a) in type 2 diabetes mellitus: a serial follow-up CCTA study. Cardiovasc Diabetol. (2023) 22:121. doi: 10.1186/s12933-023-01857-w
45. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. (2018) 392:929–39. doi: 10.1016/S0140-6736(18)31114-0
46. Dibato JE, Montvida O, Zaccardi F, Sargeant JA, Davies MJ, Khunti K, et al. Association of cardiometabolic multimorbidity and depression with cardiovascular events in early-onset adult type 2 diabetes: A multiethnic study in the U.S. Diabetes Care. (2021) 44:231–9. doi: 10.2337/dc20-2045
Keywords: lipoprotein(a), coronary heart disease, cerebrovascular disease, microvascular complications, early-onset type 2 diabetes mellitus
Citation: Zhang J, Sang J, Jiang Y, Zheng Y, Zhang J, Liu X, Qiu H, Zhao K, Sun H, Yang Y, Chen H and Yang L (2025) Elevated plasma concentrations of lipoprotein (a) are associated with cardiovascular diseases in patients with early-onset type 2 diabetes mellitus. Front. Endocrinol. 16:1434745. doi: 10.3389/fendo.2025.1434745
Received: 18 May 2024; Accepted: 03 April 2025;
Published: 30 April 2025.
Edited by:
Bo Zhu, Boston Children’s Hospital and Harvard Medical School, United StatesReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaHerald Midzi, Family Health International 360, Zimbabwe
Copyright © 2025 Zhang, Sang, Jiang, Zheng, Zhang, Liu, Qiu, Zhao, Sun, Yang, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Chen, Y2xvY2tyZW5AMTI2LmNvbQ==; Lei Yang, eWFuZ2xlaUBodWFuZ2h1YWkuZWR1LmNu
†These authors have contributed equally to this work
 Juan Zhang
Juan Zhang Jingjing Sang1,2†
Jingjing Sang1,2†