Abstract
Background:
Type 2 diabetes mellitus (T2DM) is a leading cause of severe complications, projected to affect 693 million adults globally by 2045. Addressing obesity, a key factor in T2DM, through exercise can improve metabolic health and reduce inflammation. This study conducts a Bayesian network meta-analysis to evaluate the long-term effects of various combined interventions on inflammatory markers and metabolic health in overweight or obese individuals with T2DM.
Methods:
We included randomized controlled trials (RCTs) from January 2000 to April 2023 that examined the effects of aerobic training (AT), resistance training (RT), combined aerobic and resistance training (ART), physical-mental training (PMT), whole-body vibration training (WBT), and acupuncture (ACT) on BMI, lipid profiles, fasting blood glucose (FBG), HbA1c, HOMA-IR, IL-6, and TNF-α. A comprehensive literature search was performed in PubMed, Web of Science, CNKI, MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials. Data extraction and quality assessment were independently conducted by two researchers, and Bayesian network meta-analysis was performed using R software.
Results:
A total of 128 RCTs were included. ART showed the most significant improvements in BMI, IL-6, and TNF-α levels. PMT was the most effective in improving lipid profiles (TG, TC, HDL-C, LDL-C) and insulin sensitivity markers (HbA1c, HOMA-IR). The SUCRA rankings indicated ART and PMT as the most beneficial interventions. Meta-regression analysis highlighted that VO2max improvements were closely associated with reductions in BMI and HbA1c.
Conclusion:
ART and PMT demonstrated comprehensive benefits across multiple metabolic and inflammatory outcomes. ART effectively reduced BMI, improved glycemic control, and decreased inflammatory markers through mechanisms involving AMPK and mTOR pathways. PMT improved lipid metabolism and insulin sensitivity by reducing stress hormone levels and modulating endocrine and nervous system functions. A precise exercise prescription combining ART, PMT, AT, RT, and ACT is recommended to optimize metabolic health in T2DM patients. Future research should focus on individualized intervention strategies to enhance clinical outcomes.
Systematic review registration:
PROSPERO, identifier CRD42024539376.
Highlights
-
Exercise can be an effective way to reduce metabolic and inflammatory markers in overweight or obese individuals with T2DM.
-
Different types of exercise, including ART and PMT, have been found to be effective in improving multiple health indicators.
-
ART and PMT were identified as the most beneficial interventions, with ART significantly reducing BMI, IL-6, and TNF-α levels, and PMT improving lipid profiles and insulin sensitivity.
Introduction
Type 2 diabetes (T2DM) is a major contributor to kidney failure, peripheral neuropathy, retinopathy, and cardiovascular disease (CVD) (1). By 2045, it is projected to affect 693 million adults globally (2). T2DM arises from a combination of genetic and environmental factors, with obesity, driven by high-energy diets and sedentary lifestyles, being a significant cause (3, 4). Obesity-induced insulin resistance (IR) leads to increased lipolysis and elevated free fatty acids (FFA), which further worsen IR. Addressing obesity is thus essential for improving T2DM outcomes (5).
Exercise interventions are effective in improving blood sugar levels, weight, blood lipids, and blood pressure in overweight or obese T2DM patients. They also help prevent CVD, reduce mortality, and enhance quality of life (6). Aerobic training (AT) enhances blood sugar control and body composition by improving aerobic capacity and metabolism of fats and glucose, thus boosting insulin sensitivity (7). Resistance training (RT) promotes metabolic health not only by improving body composition—via increased muscle mass, elevated basal metabolic rate (BMR), and enhanced insulin sensitivity—but also by modulating pancreatic β-cell function. Recent studies implicate RT in the regulation of muscle–pancreas crosstalk, suggesting a mechanistic link between skeletal muscle activity and β-cell insulin secretory capacity, ultimately contributing to systemic glucose homeostasis (8). Combined aerobic and resistance training (ART) offers comprehensive benefits, improving both metabolic health and body composition (9). Recently, physical-mental training (PMT) and whole-body vibration training (WBT) have gained attention. Mind-body exercises like yoga and tai chi reduce stress, enhance mental health, and improve insulin sensitivity and blood sugar control by combining physical movement with breath control (10). WBT stimulates muscle contraction through mechanical vibrations, increasing muscle strength and metabolic rate, benefiting weight control and metabolic health (11). Acupuncture therapy (ACT), a traditional Chinese medicine intervention, has shown promise in treating obese T2DM patients. It regulates various metabolic pathways, reduces appetite, slows gastric emptying, improves insulin sensitivity, increases basal metabolic rate (BMR), and accelerates fat consumption through multiple mechanisms¹². Although ACT is not a physical activity-based intervention, it was included in the present study due to its emerging evidence in metabolic regulation and its potential relevance to cardiovascular and autonomic function. Including ACT thus offers a complementary perspective to conventional exercise-based strategies for managing subclinical myocardial dysfunction in individuals with type 2 diabetes (12).
Inflammation, particularly involving interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), plays a crucial role in the pathogenesis of obesity and T2DM (13). Elevated IL-6 and TNF-α levels are closely linked to IR. Exercise interventions reduce IL-6 and TNF-α levels, improving insulin sensitivity by decreasing macrophage infiltration in adipose tissue and modulating pro- and anti-inflammatory factors (14). PMT and WBT also show potential in reducing these inflammatory markers by affecting the neuroendocrine system and mitigating chronic inflammation.
Despite the benefits of these interventions, a systematic comparison is lacking. This study employs a systematic review and meta-analysis to evaluate the impact of combined interventions on T2DM and obesity, aiming to provide a basis for developing more effective intervention strategies.
Methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for network meta-analysis (PRISMA NMA) guidelines (15). The study protocol was registered with PROSPERO (registration number CRD42024539376). Ethical approval and informed consent were deemed unnecessary due to the nature of this research.
Study design and participants
We included randomized controlled trials (RCTs) published from January 2000 to April 2023, focusing on overweight or obese individuals diagnosed with type 2 diabetes mellitus (T2DM). The criteria for overweight and obesity were defined according to ethnicity-specific guidelines: BMI ≥25 kg/m² (overweight) or ≥30 kg/m² (obesity) for Europeans (16), and BMI ≥23 kg/m² (overweight) or ≥27 kg/m² (obesity) for Asians (17). These lower thresholds for Asian populations reflect their higher percentage of body fat and increased cardiometabolic risk at lower BMI levels, as recommended by the World Health Organization Expert Consultation (2004). In studies where BMI data were not reported, body fat percentage (BF%) was used as an alternative index of adiposity. Participants were classified as overweight or obese if BF% was ≥30% for women and ≥25% for men, which are widely accepted cut-off values associated with elevated risk of metabolic syndrome and cardiovascular disease. In studies where BMI was not provided, body fat percentage (BF%) was used (BF% ≥30% for women and ≥25% for men). T2DM diagnosis had to meet the criteria of the International Diabetes Federation (IDF) (18) or the American Diabetes Association (ADA) (19).
Interventions
Interventions included aerobic training (AT), resistance training (RT), combined aerobic and resistance training (ART), physical-mental training (PMT, such as Baduanjin, Tai Chi, yoga, Pilates, etc.), whole-body vibration training (WBT), and acupuncture (ACT), each lasting for a minimum of four weeks. Control groups received either no intervention or standard diabetes medication.
Outcome Measures
Primary outcomes were changes in BMI, lipid profiles (TG, TC, HDL-C, LDL-C), fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR), IL-6, and TNF-α levels pre- and post-intervention. Secondary outcomes included withdrawal risk differences at eight weeks.
Literature search strategy
We systematically searched PubMed, Web of Science, CNKI, MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from January 2000 to April 2024. A combination of subject terms and free terms was used for the search, and only core journals were included in the Chinese literature. References of the included studies were also reviewed to identify additional relevant studies.
Literature screening and data extraction
Two researchers independently screened the literature, extracted data, and cross-checked the results. Disagreements were resolved through discussion with a third party. Authors were contacted to supplement missing data when necessary. Data extraction included: first author, publication year, intervention subjects (sample size, gender, age, BMI), intervention details (type, duration, frequency), and outcome measures. Outcome data were converted into mean and standard deviation (SD) of the pre- and post-intervention differences using the following formula (20, 21):
where M is the mean difference, SD is the standard deviation of the difference, and R is the correlation coefficient (assumed to be 0.5). In cases where original studies did not report the SD of the change between pre- and post-intervention values, we estimated this value using a widely accepted method based on the variance structure of paired data. This approach assumes that each participant provides two correlated measurements (before and after the intervention), and the variability of the change depends not only on the individual SDs at each time point but also on the degree of correlation between them. To account for this correlation, we followed the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions, which advises using an assumed correlation coefficient (R) of 0.5 when the actual value is not reported. This moderate assumption balances the need to reflect within-subject consistency without overestimating or underestimating variability. The use of this method allows for the inclusion of studies lacking direct change score SDs and enables the standardized calculation of intervention effects across trials. It is widely applied in meta-analytical research to maximize data availability and maintain methodological consistency (21).
Risk of bias assessment
The quality of included studies was assessed using the Cochrane Risk of Bias (RoB 2.0) tool, evaluating randomization, allocation concealment, blinding, completeness of outcome data, selective reporting, and other biases. Each domain was rated as “high risk”, “low risk”, or “unclear” (22).
Statistical analysis
Bayesian network meta-analysis was conducted using the Gemtc package in R 4.3.2, with sampling simulated via JAGS 4.3.1 generalized linear models. Missing SDs were calculated from t statistics and p-values of mean differences according to Cochrane guidelines handbooks (23). A random effects model was used to estimate effect sizes and 95% confidence intervals (CI). Surface under the cumulative ranking (SUCRA) values were calculated to rank interventions, and results were presented as ranking tables and forest plots using ggplot2. Publication bias was assessed using comparison-corrected funnel plots, with p< 0.05 considered statistically significant.
Results
Literature search and screening
A total of 8742 articles were retrieved, including 8039 in English and 703 in Chinese. Ultimately, 124 articles (24–48) were included. The literature screening process and results are illustrated in Figure 1.
Figure 1

Flow chart of literature screening.
Basic characteristics of literature and quality assessment results
The 124 included articles are presented in Supplementary Table 1. All patients were treated with conventional medications for diabetes and metabolic syndrome, and the baseline characteristics between the two groups were comparable. The quality and risk assessments are summarized in Figure 2.
Figure 2

Summary of literature quality assessment.
Network relationship and inconsistency analysis
Network relationship diagram
The network evidence diagram with BMI, TG, TC, HDL-C, LDL-C, FBG, HbA1c, HOMA-IR, IL-6, and TNF-α as outcome indicators is presented in Figures 3A–J.
Figure 3

(A) Network relationship diagram of BMI outcome indicators. (B) Network relationship diagram of TG outcome indicators. (C) Network relationship diagram of TC outcome indicators. (D) Network relationship diagram of HDL-C outcome indicators. (E) Network relationship diagram of LDL-C outcome indicators. (F) Network relationship diagram of FBG outcome indicators. (G) Network relationship diagram of HbA1c% outcome indicators. (H) Network relationship diagram of HOMA-IR outcome indicators. (I) Network relationship diagram of IL-6 outcome indicators. (J) Network relationship diagram of TNF-α outcome indicators eta-analysis forest plot.
This study constructed ten separate network meta-analysis models targeting key metabolic and inflammatory outcomes in patients with type 2 diabetes mellitus, including body mass index (BMI), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), glycated hemoglobin (HbA1c%), homeostasis model assessment of insulin resistance (HOMA-IR), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Each network was centered on the control group (CON), which was directly compared with six types of non-pharmacological interventions: aerobic training (AT), resistance training (RT), combined aerobic and resistance training (ART), psychomotor training (PMT), acupuncture (ACT), and whole-body therapy (WBT). The network structures for metabolic outcomes such as BMI, blood lipids, FBG, and HbA1c% were generally dense and well-connected, with frequent direct comparisons between CON and major interventions like AT, PMT, and RT—often supported by more than 15 trials. In addition, several active interventions were directly compared (e.g., AT vs RT, ART vs AT), forming multiple triangular loops that support consistency checks and robust indirect comparisons. The network for HOMA-IR was moderately connected, while those for IL-6 and TNF-α were sparse, with very few direct comparisons among active interventions and limited representation for treatments like ACT and RT. Despite these variations in network density, all interventions were involved in at least one direct or indirect comparison across most outcomes, ensuring broad intervention coverage and acceptable structural comparability. Collectively, these networks provide a strong methodological basis for estimating relative treatment effects, performing SUCRA-based rankings, and conducting subgroup or sensitivity analyses across diverse physiological domains.
Heterogeneity test
This network meta-analysis comprised 124 studies. Heterogeneity tests revealed that studies evaluating the impact of exercise on body composition, glycolipid metabolism, and inflammatory markers among populations with obesity and type 2 diabetes exhibited substantial heterogeneity (I2 > 50%, P< 0.1). Utilizing the data from this meta-analysis, individual studies were sequentially excluded to pinpoint sources of heterogeneity (refer to Table 1). Although some groups displayed persistent heterogeneity, the others were largely homogeneous. To enhance the precision of the findings, a random effects model was employed in the analyses.
Table 1
| Pre-exclusion | Post-exclusion | |||||
|---|---|---|---|---|---|---|
| Outcomes | Comparison | Traditional/Network | I2/% | P | I2/% | P |
| BMI | RT-CON | Pooled (pair-wise) | 84.90% | 0.001 | 43.30% | 0.020 |
| Pooled (network) | 83.80% | 0.001 | 38.50% | 0.030 | ||
| ART-CON | Pooled (pair-wise) | 97.50% | 0.906 | 69.50% | 0.060 | |
| Pooled (network) | 98.50% | 0.906 | 58.50% | 0.084 | ||
| PMT-CON | Pooled (pair-wise) | 91.10% | 0.603 | 91.10% | 0.603 | |
| Pooled (network) | 88.10% | 0.073 | 88.10% | 0.073 | ||
| ACT-CON | Pooled (pair-wise) | 60.50% | 0.05 | 21.20% | 0.000 | |
| Pooled (network) | 60.60% | 0.05 | 17.60% | 0.000 | ||
| AT-CON | Pooled (pair-wise) | 52.20% | 0.05 | 32.20% | 0.027 | |
| Pooled (network) | 39.00% | 0.05 | 39.00% | 0.05 | ||
| WBT-CON | Pooled (pair-wise) | 0.00% | 0.035 | 0.00% | 0.035 | |
| Pooled (network) | 0.00% | 0.075 | 0.00% | 0.075 | ||
| TG | RT-CON | Pooled (pair-wise) | 68.60% | 0.169 | 0.00% | 0.239 |
| Pooled (network) | 99.40% | 0.063 | 47.00% | 0.017 | ||
| ART-CON | Pooled (pair-wise) | 99.40% | 0.075 | 39.40% | 0.013 | |
| Pooled (network) | 99.30% | 0.054 | 33.80% | 0.020 | ||
| PMT-CON | Pooled (pair-wise) | 93.40% | 0.001 | 83.70% | 0.121 | |
| Pooled (network) | 93.00% | 0.005 | 92.40% | 0.031 | ||
| ACT-CON | Pooled (pair-wise) | 25.50% | 0.228 | 25.50% | 0.228 | |
| Pooled (network) | 31.40% | 0.154 | 31.40% | 0.154 | ||
| AT-CON | Pooled (pair-wise) | 99.40% | 0.187 | 92.60% | 0.179 | |
| Pooled (network) | 99.70% | 0.068 | 99.70% | 0.018 | ||
| WBT-CON | Pooled (pair-wise) | 73.80% | 0.075 | 37.20% | 0.041 | |
| Pooled (network) | 73.60% | 0.056 | 39.40% | 0.056 | ||
| TC | RT-CON | Pooled (pair-wise) | 91.70% | 0.061 | 47.20% | 0.061 |
| Pooled (network) | 98.70% | 0.000 | 49.40% | 0.000 | ||
| ART-CON | Pooled (pair-wise) | 96.40% | 0.049 | 96.40% | 0.000 | |
| Pooled (network) | 96.10% | 0.002 | 94.20% | 0.002 | ||
| PMT-CON | Pooled (pair-wise) | 84.80% | 0.211 | 27.40% | 0.120 | |
| Pooled (network) | 87.20% | 0.198 | 37.70% | 0.079 | ||
| ACT-CON | Pooled (pair-wise) | 66.70% | 0.048 | 28.90% | 0.120 | |
| Pooled (network) | 65.30% | 0.051 | 34.60% | 0.014 | ||
| AT-CON | Pooled (pair-wise) | 0.00% | 0.121 | 0.00% | 0.121 | |
| Pooled (network) | 0.00% | 0.154 | 0.00% | 0.154 | ||
| WBT-CON | Pooled (pair-wise) | 89.30% | 0.101 | 34.70% | 0.000 | |
| Pooled (network) | 96.10% | 0.254 | 51.30% | 0.053 | ||
| HDL-C | RT-CON | Pooled (pair-wise) | 91.50% | 0.155 | 41.70% | 0.010 |
| Pooled (network) | 98.50% | 0.103 | 46.40% | 0.032 | ||
| ART-CON | Pooled (pair-wise) | 99.30% | 0.121 | 41.90% | 0.073 | |
| Pooled (network) | 98.30% | 0.231 | 40.90% | 0.183 | ||
| PMT-CON | Pooled (pair-wise) | 91.40% | 0.055 | 34.00% | 0.007 | |
| Pooled (network) | 92.60% | 0.063 | 35.20% | 0.015 | ||
| ACT-CON | Pooled (pair-wise) | 45.90% | 0.061 | 45.90% | 0.061 | |
| Pooled (network) | 50.10% | 0.073 | 43.20% | 0.030 | ||
| AT-CON | Pooled (pair-wise) | 87.20% | 0.059 | 38.90% | 0.024 | |
| Pooled (network) | 86.90% | 0.062 | 34.10% | 0.040 | ||
| WBT-CON | Pooled (pair-wise) | 20.10% | 0.081 | 20.10% | 0.081 | |
| Pooled (network) | 17.60% | 0.095 | 17.60% | 0.095 | ||
| LDL-C | RT-CON | Pooled (pair-wise) | 96.90% | 0.085 | 39.10% | 0.125 |
| Pooled (network) | 98.60% | 0.062 | 40.80% | 0.014 | ||
| ART-CON | Pooled (pair-wise) | 97.80% | 0.077 | 40.00% | 0.121 | |
| Pooled (network) | 97.70% | 0.063 | 39.90% | 0.1200 | ||
| PMT-CON | Pooled (pair-wise) | 93.30% | 0.003 | 35.50% | 0.17 | |
| Pooled (network) | 93.20% | 0.011 | 35.40% | 0.03 | ||
| ACT-CON | Pooled (pair-wise) | 48.60% | 0.095 | 48.60% | 0.025 | |
| Pooled (network) | 48.20% | 0.063 | 48.20% | 0.007 | ||
| AT-CON | Pooled (pair-wise) | 99.20% | 0.002 | 86.40% | 0.069 | |
| Pooled (network) | 98.90% | 0.032 | 81.70% | 0.721 | ||
| WBT-CON | Pooled (pair-wise) | 0.00% | 0.059 | 0.00% | 0.011 | |
| Pooled (network) | 0.00% | 0.063 | 0.00% | 0.072 | ||
| FBG | RT-CON | Pooled (pair-wise) | 100.00% | 0.065 | 43.20% | 0.005 |
| Pooled (network) | 100.00% | 0.051 | 44.70% | 0.019 | ||
| ART-CON | Pooled (pair-wise) | 99.10% | 0.076 | 49.10% | 0.006 | |
| Pooled (network) | 98.90% | 0.084 | 55.00% | 0.014 | ||
| PMT-CON | Pooled (pair-wise) | 99.90% | 0.010 | 41.00% | 0.023 | |
| Pooled (network) | 99.90% | 0.006 | 39.00% | 0.046 | ||
| ACT-CON | Pooled (pair-wise) | 66.00% | 0.056 | 38.80% | 0.014 | |
| Pooled (network) | 65.90% | 0.052 | 42.00% | 0.018 | ||
| AT-CON | Pooled (pair-wise) | 96.40% | 0.008 | 44.20% | 0.062 | |
| Pooled (network) | 95.70% | 0.005 | 32.70% | 0.065 | ||
| WBT-CON | Pooled (pair-wise) | 97.80% | 0.069 | 97.80% | 0.399 | |
| Pooled (network) | 97.40% | 0.076 | 97.40% | 0.395 | ||
| HbA1c% | RT-CON | Pooled (pair-wise) | 99.90% | 0.043 | 99.90% | 0.42 |
| Pooled (network) | 100.00% | 0.052 | 100.00% | 0.421 | ||
| ART-CON | Pooled (pair-wise) | 75.20% | 0.048 | 75.20% | 0.173 | |
| Pooled (network) | 74.40% | 0.039 | 74.40% | 0.165 | ||
| PMT-CON | Pooled (pair-wise) | 98.40% | 0.006 | 98.40% | 0.405 | |
| Pooled (network) | 98.30% | 0.013 | 98.30% | 0.404 | ||
| ACT-CON | Pooled (pair-wise) | 97.20% | 0.133 | 97.20% | 0.393 | |
| Pooled (network) | 97.10% | 0.162 | 97.10% | 0.392 | ||
| AT-CON | Pooled (pair-wise) | 86.40% | 0.002 | 83.70% | 0.120 | |
| Pooled (network) | 93.40% | 0.000 | 79.40% | 0.000 | ||
| WBT-CON | Pooled (pair-wise) | 41.80% | 0.074 | 41.80% | 0.074 | |
| Pooled (network) | 47.00% | 0.069 | 47.00% | 0.069 | ||
| HOMA-IR | RT-CON | Pooled (pair-wise) | 74.00% | 0.052 | 49.20% | 0.037 |
| Pooled (network) | 77.90% | 0.050 | 52.70% | 0.012 | ||
| ART-CON | Pooled (pair-wise) | 95.60% | 0.005 | 38.20% | 0.021 | |
| Pooled (network) | 96.40% | 0.008 | 39.00% | 0.027 | ||
| PMT-CON | Pooled (pair-wise) | 93.50% | 0.008 | 36.10% | 0.010 | |
| Pooled (network) | 93.70% | 0.006 | 36.30% | 0.002 | ||
| ACT-CON | Pooled (pair-wise) | 98.90% | 0.042 | 41.50% | 0.21 | |
| Pooled (network) | 99.00% | 0.038 | 41.60% | 0.01 | ||
| AT-CON | Pooled (pair-wise) | 92.30% | 0.048 | 34.90% | 0.12 | |
| Pooled (network) | 92.00% | 0.045 | 34.60% | 0.37 | ||
| WBT-CON | Pooled (pair-wise) | 57.00% | 0.053 | 57.00% | 0.87 | |
| Pooled (network) | 51.80% | 0.051 | 51.80% | 0.012 | ||
| IL-6 | RT-CON | Pooled (pair-wise) | 13.90% | 0.098 | 13.90% | 0.024 |
| Pooled (network) | 75.90% | 0.152 | 74.30% | 0.004 | ||
| ART-CON | Pooled (pair-wise) | 86.10% | 0.062 | 39.10% | 0.294 | |
| Pooled (network) | 84.50% | 0.048 | 37.50% | 0.09 | ||
| PMT-CON | Pooled (pair-wise) | 89.80% | 0.003 | 52.80% | 0.003 | |
| Pooled (network) | 87.80% | 0.006 | 52.80% | 0.007 | ||
| ACT-CON | Pooled (pair-wise) | 77.80% | 0.095 | 50.80% | 0.005 | |
| Pooled (network) | 89.80% | 0.087 | 46.80% | 0.02 | ||
| AT-CON | Pooled (pair-wise) | 71.50% | 0.031 | 44.50% | 0.013 | |
| Pooled (network) | 88.30% | 0.047 | 41.30% | 0.078 | ||
| TNF-α | RT-CON | Pooled (pair-wise) | NA | NA | NA | NA |
| Pooled (network) | NA | NA | NA | NA | ||
| ART-CON | Pooled (pair-wise) | 69.10% | 0.051 | 34.80% | 0.021 | |
| Pooled (network) | 67.80% | 0.048 | 39.10% | 0.006 | ||
| PMT-CON | Pooled (pair-wise) | NA | NA | NA | NA | |
| Pooled (network) | NA | NA | NA | NA | ||
| ACT-CON | Pooled (pair-wise) | NA | NA | NA | NA | |
| Pooled (network) | NA | NA | NA | NA | ||
| AT-CON | Pooled (pair-wise) | 81.50% | 0.056 | 41.70% | 0.023 | |
| Pooled (network) | 86.10% | 0.048 | 42.80% | 0.003 |
Heterogeneity analysis.
NA, not available.
Inconsistency testing and convergence assessment
Except for the TNF-α outcome indicator, the local inconsistency test results showed P > 0.05, indicating no significant difference between the direct and indirect comparison results. Thus, the consistency model could be used for analysis, while TNF-α was analyzed using an inconsistency model. The PSRFs for all 10 outcome indicators were close to 1, indicating good convergence.
Network meta-analysis results
Body composition
A total of 87 RCTs (70 two-arm, 12 three-arm, and 5 four-arm) (24–28, 31, 32, 35, 36, 38–40, 42–44, 47–58) reported improvements in BMI outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving BMI outcome indicators, RT, ART, ACT, and AT were more effective than the control group as shown in Figure 4A. The SUCRA ranking results showed: ART (0.9332) > ACT (0.7064) > RT (0.6159) > AT (0.4404) > WBT (0.4267) > PMT (0.2907) > CON (0.0867). ART was most likely to be the best intervention measure as shown in Table 2.
Figure 4

(A) BMI network meta-analysis forest plot. (B) TG network meta-analysis forest plot. (C) TC network meta-analysis forest plot. (D) HDL-C network meta-analysis forest plot. (E) LDL-C network meta-analysis forest plot. (F) FBG network meta-analysis forest plot. (G) HbA1c% network meta-analysis forest plot. (H) HOMA-IR network meta-analysis forest plot. (I) IL-6 network meta-analysis forest plot. (J) TNF-α network meta-analysis forest plot.
Table 2
| BMI | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | -1.2 (-2.13, -0.28) | -2.23 (-3.16, -1.3) | -0.45 (-1.46, 0.56) | -1.53 (-2.96, -0.09) | -0.82 (-1.55, -0.09) | -0.72 (-3.32, 1.83) |
| 2 | -1.02 (-2.19, 0.15) | 0.76 (-0.58, 2.09) | -0.33 (-2.02, 1.39) | 0.38 (-0.51, 1.29) | 0.48 (-2.28, 3.21) | |
| 3 | 1.78 (0.42, 3.13) | 0.7 (-1.01, 2.41) | 1.41 (0.35, 2.47) | 1.5 (-1.25, 4.23) | ||
| 4 | -1.08 (-2.83, 0.69) | -0.37 (-1.53, 0.8) | -0.27 (-3.06, 2.5) | |||
| 5 | 0.71 (-0.89, 2.31) | 0.8 (-2.16, 3.73) | ||||
| 6 | 0.09 (-2.59, 2.76) | |||||
| 7 | ||||||
| TG | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | -0.29 (-0.93, 0.34) | -0.58 (-1.29, 0.13) | -1 (-1.68, -0.33) | -0.36 (-1.62, 0.91) | -0.29 (-0.8, 0.22) | -1.16 (-3.32, 1) |
| 2 | -0.29 (-1.15, 0.59) | -0.71 (-1.61, 0.2) | -0.07 (-1.47, 1.36) | 0.01 (-0.64, 0.65) | -0.86 (-3.11, 1.38) | |
| 3 | -0.42 (-1.4, 0.54) | 0.22 (-1.23, 1.67) | 0.29 (-0.52, 1.09) | -0.58 (-2.86, 1.68) | ||
| 4 | 0.64 (-0.79, 2.08) | 0.71 (-0.08, 1.5) | -0.15 (-2.42, 2.1) | |||
| 5 | 0.08 (-1.3, 1.42) | -0.79 (-3.32, 1.7) | ||||
| 6 | -0.87 (-3.09, 1.34) | |||||
| 7 | ||||||
| TC | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | -0.43 (-0.79, -0.08) | -0.4 (-0.77, -0.05) | -0.62 (-0.98, -0.25) | -0.55 (-1.48, 0.39) | -0.3 (-0.58, -0.03) | -0.24 (-1.23, 0.76) |
| 2 | 0.02 (-0.43, 0.48) | -0.19 (-0.68, 0.31) | -0.12 (-1.11, 0.89) | 0.13 (-0.22, 0.47) | 0.19 (-0.86, 1.26) | |
| 3 | -0.21 (-0.71, 0.29) | -0.14 (-1.15, 0.87) | 0.1 (-0.31, 0.51) | 0.17 (-0.89, 1.23) | ||
| 4 | 0.07 (-0.93, 1.08) | 0.31 (-0.11, 0.73) | 0.38 (-0.68, 1.44) | |||
| 5 | 0.24 (-0.73, 1.22) | 0.3 (-1.05, 1.68) | ||||
| 6 | 0.07 (-0.96, 1.1) | |||||
| 7 | ||||||
| HDL-C | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | 0.12 (-0.02, 0.25) | 0.2 (0.06, 0.35) | 0.26 (0.1, 0.42) | 0.05 (-0.28, 0.39) | 0.22 (0.11, 0.33) | 0.15 (-0.28, 0.58) |
| 2 | 0.09 (-0.09, 0.27) | 0.14 (-0.06, 0.35) | -0.06 (-0.43, 0.3) | 0.1 (-0.04, 0.24) | 0.03 (-0.42, 0.48) | |
| 3 | 0.06 (-0.15, 0.27) | -0.15 (-0.52, 0.21) | 0.02 (-0.15, 0.18) | -0.06 (-0.51, 0.4) | ||
| 4 | -0.21 (-0.58, 0.16) | -0.04 (-0.22, 0.14) | -0.11 (-0.57, 0.35) | |||
| 5 | 0.17 (-0.19, 0.52) | 0.09 (-0.45, 0.64) | ||||
| 6 | -0.07 (-0.52, 0.38) | |||||
| 7 | ||||||
| LDL-C | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | -0.17 (-0.43, 0.08) | -0.14 (-0.41, 0.13) | -0.72 (-1.03, -0.42) | -0.06 (-0.67, 0.54) | -0.27 (-0.49, -0.06) | -0.29 (-1.08, 0.49) |
| 2 | 0.03 (-0.3, 0.37) | -0.55 (-0.94, -0.16) | 0.11 (-0.55, 0.77) | -0.1 (-0.35, 0.15) | -0.12 (-0.94, 0.72) | |
| 3 | -0.58 (-0.99, -0.19) | 0.08 (-0.6, 0.74) | -0.13 (-0.45, 0.17) | -0.15 (-0.98, 0.67) | ||
| 4 | 0.65 (-0.02, 1.34) | 0.45 (0.1, 0.8) | 0.43 (-0.4, 1.27) | |||
| 5 | -0.21 (-0.86, 0.43) | -0.22 (-1.22, 0.77) | ||||
| 6 | -0.02 (-0.84, 0.8) | |||||
| 7 | ||||||
| FBG | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | -0.65 (-1.18, -0.11) | -0.36 (-0.96, 0.25) | -1.01 (-1.5, -0.52) | -0.8 (-1.65, 0.05) | -0.77 (-1.19, -0.35) | -0.11 (-1.48, 1.25) |
| 2 | 0.29 (-0.43, 1.01) | -0.36 (-1.06, 0.34) | -0.15 (-1.16, 0.85) | -0.12 (-0.68, 0.43) | 0.54 (-0.93, 2) | |
| 3 | -0.65 (-1.42, 0.12) | -0.44 (-1.49, 0.6) | -0.42 (-1.09, 0.26) | 0.25 (-1.26, 1.74) | ||
| 4 | 0.21 (-0.78, 1.19) | 0.24 (-0.36, 0.83) | 0.9 (-0.56, 2.35) | |||
| 5 | 0.03 (-0.92, 0.98) | 0.68 (-0.93, 2.31) | ||||
| 6 | 0.66 (-0.77, 2.09) | |||||
| 7 | ||||||
| HbA1c% | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | -0.35 (-0.56, -0.14) | -0.44 (-0.69, -0.2) | -0.55 (-0.77, -0.32) | -0.32 (-0.92, 0.27) | -0.51 (-0.69, -0.34) | -0.17 (-0.66, 0.32) |
| 2 | -0.09 (-0.37, 0.19) | -0.2 (-0.5, 0.1) | 0.02 (-0.61, 0.65) | -0.17 (-0.38, 0.05) | 0.18 (-0.36, 0.72) | |
| 3 | -0.11 (-0.43, 0.22) | 0.12 (-0.53, 0.76) | -0.07 (-0.34, 0.19) | 0.27 (-0.28, 0.82) | ||
| 4 | 0.22 (-0.41, 0.86) | 0.03 (-0.24, 0.3) | 0.38 (-0.17, 0.91) | |||
| 5 | -0.19 (-0.81, 0.43) | 0.16 (-0.62, 0.93) | ||||
| 6 | 0.35 (-0.18, 0.87) | |||||
| 7 | ||||||
| HOMA-IR | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 1 | -0.75 (-1.42, -0.08) | -1.49 (-2.19, -0.83) | -1.69 (-2.69, -0.7) | -1.25 (-2.25, -0.29) | -0.72 (-1.21, -0.24) | -0.66 (-2.73, 1.37) |
| 2 | -0.74 (-1.56, 0.06) | -0.94 (-2.14, 0.25) | -0.5 (-1.71, 0.66) | 0.03 (-0.63, 0.68) | 0.09 (-2.03, 2.16) | |
| 3 | -0.2 (-1.38, 1.02) | 0.24 (-0.94, 1.44) | 0.78 (0.07, 1.51) | 0.83 (-1.28, 2.95) | ||
| 4 | 0.43 (-0.97, 1.82) | 0.97 (-0.12, 2.06) | 1.03 (-1.24, 3.29) | |||
| 5 | 0.54 (-0.54, 1.64) | 0.6 (-1.67, 2.86) | ||||
| 6 | 0.05 (-1.94, 2.04) | |||||
| 7 | ||||||
Overview of network meta-analysis league tables for each outcome indicator.
Lipid metabolism
A total of 82 RCTs (68 two-arm, 10 three-arm, and 4 four-arm) (24–26, 29, 32, 34, 36–38, 40–42, 47, 50–53, 57–66) reported improvements in TG outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that PMT was more effective than the control group in improving TG outcome indicators as shown in Figure 4B. The SUCRA ranking results showed: PMT (0.8277) > WBT (0.7313) > ART (0.5952) > ACT (0.443) > RT (0.3842) > AT (0.3841) > CON (0.1346). PMT was most likely to be the best intervention measure as shown in Table 2.
A total of 82 RCTs (66 two-arm, 11 three-arm, and 5 four-arm) (24, 25, 28, 32, 34–37, 40–42, 47, 49–53, 55–61, 63–120) reported improvements in TC outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving TC outcome indicators, the effects of RT, ART, PMT, and AT were better than those of the control group as shown in Figure 4C. The SUCRA ranking results showed: PMT (0.8019) > ACT (0.6463) > RT (0.5966) > ART (0.5571) > AT (0.4116) > WBT (0.407) > CON (0.0794). PMT was most likely to be the best intervention measure as shown in Table 3.
Table 3
| IL-6 | |||||
|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT |
| 1 | 1.11 (-0.65, 2.82) | -1.7 (-2.92, -0.58) | -1.73 (-3.48, 0.01) | -0.29 (-3.25, 2.68) | -0.34 (-1.51, 0.8) |
| 2 | -2.82 (-4.62, -1.06) | -2.84 (-5.29, -0.34) | -1.41 (-4.81, 2.09) | -1.45 (-3.24, 0.37) | |
| 3 | -0.03 (-2.07, 2.12) | 1.41 (-1.71, 4.64) | 1.36 (-0.04, 2.83) | ||
| 4 | 1.45 (-2, 4.86) | 1.39 (-0.73, 3.48) | |||
| 5 | -0.05 (-3.25, 3.1) | ||||
| 6 | |||||
| TNF-α | |||||
|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT |
| 1 | 1.35 (-0.62, 3.28) | -0.87 (-1.71, 0.01) | -0.28 (-2.8, 2.32) | -0.02 (-1.83, 1.81) | -0.62 (-1.47, 0.02) |
| 2 | -2.23 (-4.19, -0.18) | -1.63 (-4.81, 1.63) | -1.37 (-4.01, 1.32) | -1.98 (-4.02, -0.04) | |
| 3 | 0.59 (-2.08, 3.3) | 0.85 (-1.18, 2.84) | 0.25 (-0.93, 1.18) | ||
| 4 | 0.26 (-2.88, 3.36) | -0.35 (-3.12, 2.21) | |||
| 5 | -0.59 (-2.68, 1.27) | ||||
| 6 | |||||
List of network meta-analysis league tables for each outcome indicator (sequence).
A total of 85 RCTs (69 two-arm, 11 three-arm, and 5 four-arm) (24–26, 28, 32, 34, 35, 37, 38, 40–42, 47, 49–53, 56–60, 62–65) reported improvements in HDL-C outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving HDL-C outcome indicators, the effects of ART, PMT, and AT were better than those of the control group as shown in Figure 4D. The SUCRA ranking results showed: PMT (0.8063) > AT (0.7139) > ART (0.6559) > WBT (0.5017) > RT (0.3948) > ACT (0.3138) > CON (0.1137). PMT was most likely to be the best intervention measure as shown in Table 3.
A total of 76 RCTs (62 two-arm, 10 three-arm, and 4 four-arm) (24–26, 32, 34, 38, 40–42, 47, 49, 51–53, 56, 58–60, 62–65, 67–75, 77–85, 87–90, 92–103, 121–124) reported improvements in LDL-C outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving LDL-C outcome indicators, the effects of PMT and AT were better than those of the control group as shown in Figure 4E. The SUCRA ranking results showed: PMT (0.9671) > AT (0.6356) > WBT (0.5606) > RT (0.4527) > ART (0.4034) > ACT (0.33) > CON (0.1505). PMT was most likely to be the best intervention measure as shown in Table 3.
A total of 92 RCTs (75 two-arm, 11 three-arm, and 6 four-arm) (24–36, 38–43, 46, 48, 49, 52–54, 56–59, 61, 63, 64, 66–70, 73, 75–83, 121, 125–132) reported improvements in FBG outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving FBG outcome indicators, the effects of RT, PMT, and AT were better than those of the control group as shown in Figure 4F. The SUCRA ranking results showed: PMT (0.8552) > AT (0.6787) > ACT (0.6731) > RT (0.5693) > ART (0.3461) > WBT (0.277) > CON (0.1007). PMT was most likely to be the best intervention measure as shown in Table 4.
Table 4
| BMI | ||||||
|---|---|---|---|---|---|---|
| CON | RT | ART | PMT | ACT | AT | WBT |
| 0.0867 | 0.6159 | 0.9332 | 0.2907 | 0.7064 | 0.4404 | 0.4267 |
| TG | ||||||
|---|---|---|---|---|---|---|
| 0.1346 | 0.3842 | 0.5952 | 0.8277 | 0.443 | 0.3841 | 0.7313 |
| TC | ||||||
|---|---|---|---|---|---|---|
| 0.0794 | 0.5966 | 0.5571 | 0.8019 | 0.6463 | 0.4116 | 0.407 |
| HDL-C | ||||||
|---|---|---|---|---|---|---|
| 0.1137 | 0.3948 | 0.6559 | 0.8063 | 0.3138 | 0.7139 | 0.5017 |
| LDL-C | ||||||
|---|---|---|---|---|---|---|
| 0.1505 | 0.4527 | 0.4034 | 0.9671 | 0.33 | 0.6356 | 0.5606 |
| FBG | ||||||
|---|---|---|---|---|---|---|
| 0.1007 | 0.5693 | 0.3461 | 0.8552 | 0.6731 | 0.6787 | 0.277 |
| HbA1c% | ||||||
|---|---|---|---|---|---|---|
| 0.0652 | 0.4471 | 0.6293 | 0.8171 | 0.478 | 0.7809 | 0.2823 |
| HOMA-IR | ||||||
|---|---|---|---|---|---|---|
| 0.0466 | 0.3906 | 0.795 | 0.8473 | 0.6584 | 0.3662 | 0.396 |
| IL-6 | ||||||
|---|---|---|---|---|---|---|
| 0.3217 | 0.0716 | 0.8574 | 0.8405 | 0.4459 | 0.463 | |
| TNF-α | ||||||
|---|---|---|---|---|---|---|
| 0.3743 | 0.0812 | 0.8329 | 0.5459 | 0.4399 | 0.7258 | |
SUCRA ranking league.
A total of 90 RCTs (74 two-arm, 10 three-arm, and 6 four-arm) (24, 25, 27, 29, 30, 33–35, 37, 38, 41–43, 46, 47, 49–54, 56, 57, 59, 61, 62, 64, 67–70, 73–89, 122, 125, 126, 128–130, 132–135) reported improvements in HbA1c% outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving HbA1c% outcome indicators, the effects of RT, ART, PMT, and AT were better than those of the control group as shown in Figure 4G. The SUCRA ranking results showed: PMT (0.8171) > AT (0.7809) > ART (0.6293) > ACT (0.478) > RT (0.4471) > WBT (0.2823) > CON (0.0652). PMT was most likely to be the best intervention measure as shown in Table 4.
A total of 41 RCTs (34 two-arm, 3 three-arm, and 4 four-arm) (25–27, 40, 41, 43, 45, 48, 53–57, 61, 63, 66, 70, 74, 76, 78–80, 83, 86, 88, 94, 103–105, 112–114, 117, 118, 121, 123, 127, 131, 136–138) reported improvements in HOMA-IR outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving HOMA-IR outcome indicators, the effects of RT, ART, PMT, ACT, and AT were better than those of the control group as shown in Figure 4H. The SUCRA ranking results showed: PMT (0.8473) > ART (0.795) > ACT (0.6584) > WBT (0.396) > RT (0.3906) > AT (0.3662) > CON (0.0466). PMT was most likely to be the best intervention measure as shown in Table 4.
Inflammatory factors
A total of 18 RCTs (15 two-arm, 2 three-arm, and 1 four-arm) (33, 44, 49, 54, 66, 68, 76, 83, 93, 97, 103, 109, 110, 121, 126, 139–141) reported improvements in IL-6 outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that the ART group had better improvements in IL-6 outcome indicators compared to the control group as shown in Figure 4I. The SUCRA ranking results showed: ART (0.8574) > PMT (0.8405) > AT (0.463) > ACT (0.4459) > CON (0.3217) > RT (0.0716). ART was most likely to be the best intervention measure as shown in Table 4.
A total of 14 RCTs (13 two-arm and 1 four-arm) (33, 44, 49, 64, 66, 76, 81, 83, 91, 93, 103, 109, 121, 140) reported improvements in TNF-α outcome indicators for patients with T2DM and overweight or obesity. The network meta-analysis results showed that in terms of improving TNF-α outcome indicators, the crude improvement effect of no intervention was better than that of the control group as shown in Figure 4J. The SUCRA ranking results showed: ART (0.8329) > AT (0.7258) > PMT (0.5459) > ACT (0.4399) > CON (0.3743) > RT (0.0812). ART was most likely to be the best intervention measure as shown in Table 4.
The network meta-analysis SUCRA league table is presented in Table 4.
Meta-regression analysis
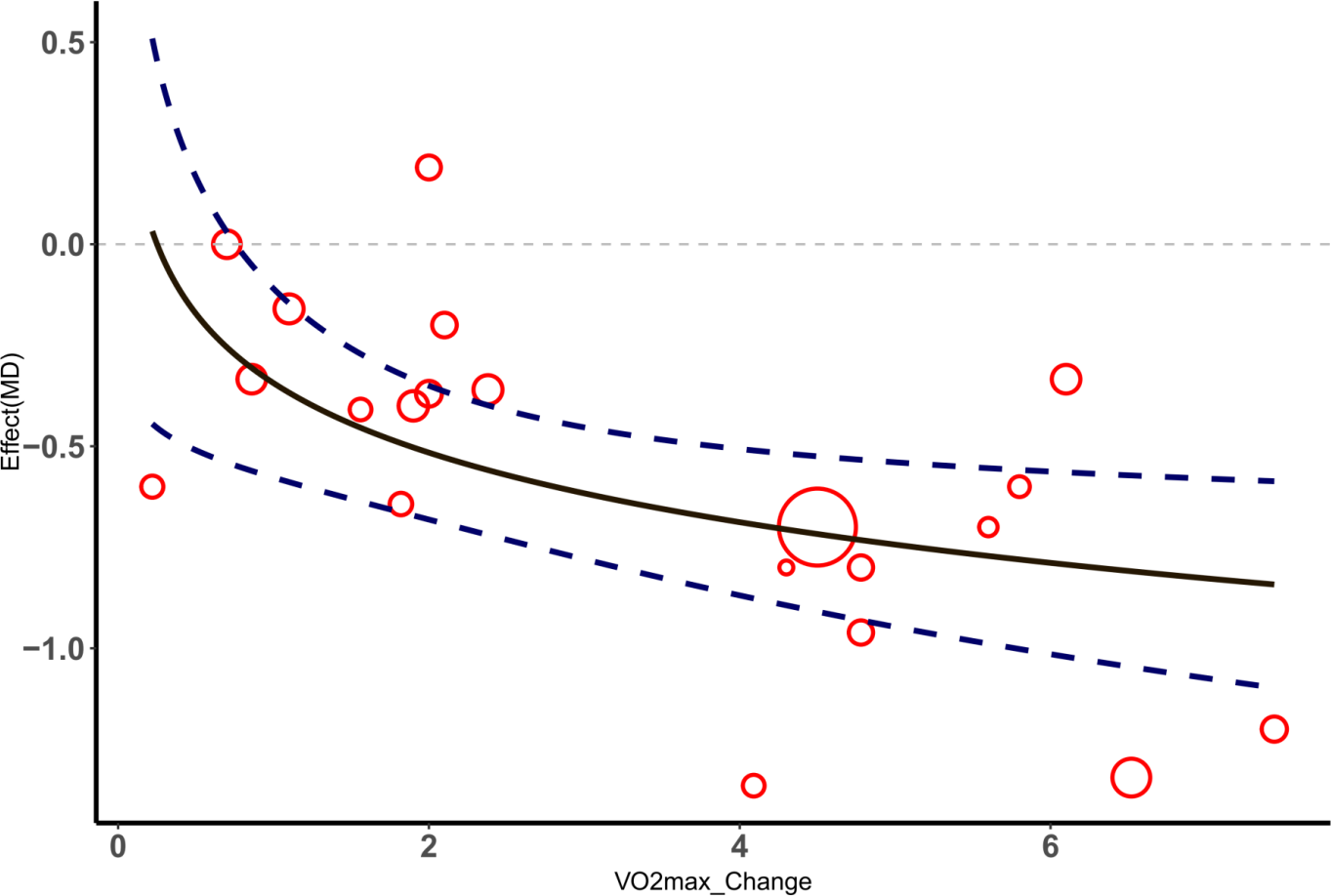
According to the included studies, the change in VO2max significantly impacted the effect size of the selected outcome measures. The nonlinear relationship between the change in VO2max and the effect size of outcome indicators was better captured using a logarithm-transformed regression model. In the meta-regression analysis, the BMI effect size was used as the dependent variable, and the VO2max change value was used as the independent variable. The results showed an R-squared value of 0.4405 and an adjusted R-squared value of 0.4138, indicating that the model explained approximately 44.05% of the BMI change. The F-test results (F=16.53, df=1 and 21, P=0.0005551) indicated that the model was overall significant. For each unit increase in the logarithmic change in VO2max, BMI decreased by 0.5350 units (P< 0.001). Similarly, a meta-regression analysis was performed using the HbA1c% effect size as the dependent variable, and the VO2max change value was used as the independent variable. The results showed an R-squared value of 0.2954 and an adjusted R-squared value of 0.2584, indicating that the model explained approximately 29.54% of the HbA1c% change. The F-test results (F=7.967, df=1 and 19, P=0.01087) indicated that the model was overall significant. For each unit increase in the logarithmic change in VO2max, HbA1c% decreased by 0.24834 units (P< 0.05), as shown in Figures 5, 6.
Figure 5
△ Meta-regression analysis of VO2max and HbA1c%.
Figure 6

△ Meta-regression analysis of VO2max and HbA1c%.
Two-dimensional graph evaluation
This study used two-dimensional plots to evaluate the comprehensive effects of different intervention measures on BMI, HbA1c%, IL-6, and TNF-α. Figure 7 shows the performance of different intervention measures on the outcome indicators of BMI and HbA1c%. Figure 8 shows the performance of different intervention measures on the outcome indicators of IL-6 and TNF-α. In the figures, each intervention is represented by a point of a different color. The position of the point indicates the impact of the intervention on the corresponding indicator, and the error bars indicate its 95% confidence interval. The analysis results in Figures 6 and 7 show that the ART intervention had the best effect on the four selected outcome indicators. Specifically, the ART point is close to the lower left corner in both Figures 7 and 8, indicating that it can significantly reduce the levels of BMI, HbA1c%, IL-6, and TNF-α with a better effect than other interventions. In summary, through the comprehensive evaluation of the two-dimensional graphs, the ART intervention demonstrated the best performance in reducing BMI, HbA1c%, IL-6, and TNF-α, and it is recommended to prioritize this intervention strategy.
Figure 7

Two-dimensional evaluation of the comprehensive intervention effects of different non-drug interventions on BMI and HbA1c%.
Figure 8

Two-dimensional evaluation of the comprehensive intervention effects of different non-drug interventions on IL-6 and TNF-α.
Publication bias assessment
The adjusted funnel plots for publication bias of the 10 outcome indicators are presented in Figures 9-18. The adjusted funnel plots for the studies included in the 10 outcome indicators are relatively symmetrical, with fewer studies falling at the bottom of the funnel. This indicates that there may be a small sample effect or a certain degree of publication bias.
Figure 9

Comparison-correction funnel diagram (BMI).
Figure 10

Comparison-correction funnel diagram (TG).
Figure 11

Comparison-correction funnel diagram (TC).
Figure 12

Comparison-Correction Funnel Diagram (HDL-C).
Figure 13

Comparison-correction funnel diagram (LDL-C).
Figure 14

Comparison-correction funnel diagram (FBG).
Figure 15

Comparison-correction funnel diagram (HbA1c%).
Figure 16

Comparison-correction funnel diagram (HOMA-IR).
Figure 17

Comparison-correction funnel diagram (IL-6).
Figure 18

Comparison-correction funnel diagram (TNF-α).
Discussion
In 1988, Professor Reaven of Stanford University first elucidated the pathogenesis of obesity-related T2DM (142). In overweight or obese individuals, adipose tissue, muscle tissue, and the liver become less sensitive to insulin, causing pancreatic beta cells to secrete more insulin to maintain blood sugar balance (143). If this compensatory hyperinsulinemia persists for a long time, it causes pancreatic β-cell function to gradually decline, reduces insulin secretion, and fails to effectively control blood sugar levels, eventually leading to diabetes (144). With the deepening of research, various theories have been proposed, including the inflammation hypothesis (145), the seborrheic hypothesis, the adipokine hypothesis (146), Gut flora hypothesis (147).
The inflammation hypothesis suggests that obesity is a chronic inflammatory state, and inflammatory factors such as TNF-α, interleukin-1β (IL-1β), and IL-6 can decrease tissue sensitivity to insulin and lead to pathological changes in pancreatic β-cell function (145). The lipid overflow hypothesis posits that when the storage capacity of adipose tissue reaches its limit, FFA overflow and accumulate in non-adipose tissues such as the liver, skeletal muscle, and pancreas, leading to IR and damage to pancreatic β-cell function. The adipokine hypothesis suggests that in obesity, the pattern of adipokines secreted by adipose tissue changes, with an increase in pro-inflammatory factors and a decrease in anti-inflammatory factors, leading to chronic low-grade inflammation and IR (146). The gut microbiota hypothesis suggests that the intestinal microbiota plays an important role in the pathological processes of obesity and T2DM by affecting energy balance, metabolic status, and inflammation levels (147).
The trend of obesity and T2DM among younger individuals is increasingly apparent, which may be closely related to high-calorie diets, sedentary lifestyles, and lack of exercise (148). Based on the above mechanisms, intervention measures for obesity and T2DM have gradually diversified and become increasingly refined. Among them, combined interventions such as AT, RT, ART, PMT, ACT, and WBT have shown significant results in various outcome indicators. This article aims to evaluate the effects of these interventions on body composition, blood lipid metabolism, and inflammatory factors in obese patients with T2DM through a systematic review and network meta-analysis and to provide a scientific basis for optimizing clinical intervention strategies.
Body composition
In patients with T2DM and obesity, changes in body fat and muscle composition are directly related to disease progression and management. Through network meta-analysis, this article found that ART had the most significant effect in improving body composition, but AT, RT, and ACT also showed significant improvements. ART promotes lipolysis and inhibits lipogenesis in adipocytes via multiple pathways, effectively reducing body fat content. Initially, ART enhances the activity of hormone-sensitive lipase (HSL), a crucial enzyme in lipolysis. Elevated HSL activity facilitates the hydrolysis of triacylglycerol, thereby releasing free fatty acids (FFA) and glycerol. Catecholamines, such as epinephrine and norepinephrine, regulate this process by activating adenylyl cyclase (AC) via β-adrenergic receptors, thus increasing intracellular cyclic adenosine monophosphate (cAMP) levels, activating protein kinase A (PKA), and ultimately enhancing HSL phosphorylation and activation (149, 150). Moreover, combined aerobic and resistance exercise significantly enhances HSL by boosting its protein content and enzymatic activity in adipose tissue, further facilitating lipolysis. During exercise, phosphorylation of HSL at sites such as Ser-563 and Ser-660 increases, enhancing its translocation to lipid droplets and augmenting its lipolytic activity. Additionally, exercise facilitates the interaction between HSL and the lipid droplet-associated protein PLIN1, thereby enhancing HSL’s access to triacylglycerol and diacylglycerol substrates within the droplets (151). In addition, ART inhibits the expression and activity of key enzymes involved in lipogenesis in adipose tissue, such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC). Through the AMP-activated protein kinase (AMPK) pathway, ART reduces the expression of sterol regulatory element-binding protein 1c (SREBP-1c) (152), a key regulatory factor in fat synthesis, and decreases the synthesis of nascent fatty acids (153). ART not only improves body composition by reducing the amount of adipose tissue but also optimizes body composition by promoting muscle protein synthesis and enhancing muscle metabolism (154). PGC-1α promotes mitochondrial DNA replication and protein expression by interacting with transcription factors such as nuclear respiratory factor 1 (NRF-1) and mitochondrial transcription factor A (TFAM). ART promotes β-oxidation of fatty acids and increases energy consumption by increasing the activity of fatty acid oxidases such as carnitine palmitoyltransferase 1 (CPT1) in skeletal muscle. The AMPK pathway plays an important role in this process. ART activates AMPK, inhibits the activity of ACC, and reduces the production of malonyl-coenzyme A (malonyl-CoA), thereby releasing the inhibition of CPT1 and promoting fatty acids to enter the mitochondria for oxidative metabolism (155). Additionally, AT and RT also show significant effects in improving body fat composition. AT reduces body fat by increasing whole-body energy consumption and fat oxidation, enhancing the number and function of mitochondria in skeletal muscle cells, and improving fatty acid oxidation capacity in muscles. Additionally, AT positively impacts body fat composition by increasing cardiopulmonary function and improving systemic metabolic status. RT improves the quality of skeletal muscle by increasing the volume and number of muscle fibers and promoting muscle protein synthesis (156). Additionally, RT helps reduce body fat by increasing BMR and resting energy expenditure and improves glucose metabolism by increasing insulin sensitivity (157). ACT, as a traditional Chinese medicine method, has also shown certain effects in improving body composition. ACT helps reduce body fat by regulating the neuroendocrine system and promoting lipolysis and energy metabolism. ACT also regulates body composition by improving insulin sensitivity and regulating the secretory function of adipocytes (158). Additionally, ART further affects body composition by regulating hormone levels. ART significantly improves insulin sensitivity and reduces IR. Its mechanism includes increasing the expression and activity of insulin receptor substrate 1 (IRS-1) and glucose transporter type 4 (GLUT4) in skeletal muscle and adipose tissue, enhancing insulin signaling, thereby promoting glucose uptake and utilization. ART regulates leptin and adiponectin, two hormones secreted by adipose tissue, reducing leptin levels, decreasing leptin resistance, and increasing adiponectin levels. Adiponectin exerts anti-inflammatory and insulin-sensitizing effects through the AMPK pathway (159).
Lipid metabolism
In patients with T2DM and overweight or obesity, dyslipidemia is closely related to IR and is a significant factor in disease progression. This study found through network meta-analysis that different types of training improve blood lipid metabolism, especially PMT, which showed significant improvements in multiple blood lipid metabolism indicators. PMT showed the best effect on multiple indicators such as TG, TC, HDL-C, LDL-C, FBG, HbA1c, and HOMA-IR, which may be related to its unique mechanisms. Recent research indicates that PMT has a positive impact on blood lipid metabolism by comprehensively regulating physical and mental status and improving endocrine and nervous system functions (111). PMT includes yoga, tai chi, and other practices. These exercises reduce the secretion of stress hormones (such as cortisol) and decrease the overactivity of the sympathetic nervous system, thereby improving insulin sensitivity. Research indicates that PMT can significantly increase the expression of IRS-1 and GLUT4, enhance glucose uptake and utilization, and reduce blood sugar and blood lipid levels (160). ART has a significant effect in reducing TG and LDL-C levels and increasing HDL-C levels mainly by increasing AMPK activity, promoting fatty acid oxidation and glucose uptake, and inhibiting FAS and ACC, thereby reducing the synthesis of nascent fatty acids. Additionally, ART contributes to long-term blood lipid management by enhancing muscle strength and metabolic rate and increasing resting energy expenditure (161). AT reduces body fat by increasing whole-body energy consumption and fat oxidation while improving cardiopulmonary function and systemic metabolic status. It also shows significant effects in reducing TG, TC, and LDL-C levels and increasing HDL-C levels. Recent research indicates that AT can improve insulin sensitivity and lipid metabolism by regulating intestinal flora and increasing the production of short-chain fatty acids (SCFA) (162). RT plays an important role in reducing FBG and HbA1c% levels by increasing muscle mass and metabolic rate, enhancing resting energy expenditure, and improving glucose metabolism. The mechanism of RT also includes increasing the number and function of mitochondria in skeletal muscle, promoting fatty acid oxidation and energy consumption, and reducing fat accumulation (68). ACT shows a certain lipid-lowering effect by regulating the neuroendocrine system, improving insulin sensitivity, and promoting adipocyte function. Emerging evidence suggests that acupuncture can enhance lipolysis and improve insulin sensitivity through several mechanisms. These include modulation of the hypothalamic-pituitary-adrenal (HPA) axis, reduction of chronic low-grade inflammation, and regulation of adipokine secretion such as adiponectin and leptin. Furthermore, stimulation of specific acupoints (e.g., ST36, CV12) has been shown to activate AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor gamma (PPAR-γ) signaling pathways, which play key roles in enhancing glucose uptake, fatty acid oxidation, and mitochondrial function. These effects collectively contribute to improved lipid metabolism and insulin action in individuals with obesity or type 2 diabetes (163). In summary, PMT has become the best intervention measure for multiple blood lipid metabolism indicators, which may be attributed to its multi-level and multi-target comprehensive regulatory effects. PMT shows unique advantages in blood lipid management by reducing stress hormone levels, regulating the neuroendocrine system, and improving insulin sensitivity and metabolic function (164). However, the advantages of other interventions such as ART, AT, and RT on specific indicators cannot be ignored. Different types of training methods improve blood lipid metabolism through various metabolic pathways and mechanisms, providing effective non-drug intervention methods for patients with T2DM and obesity. These interventions not only help reduce blood lipid levels but also reduce the risk of diabetes by improving insulin sensitivity and overall metabolic function, providing a comprehensive clinical management strategy.
Inflammatory factors
In the pathogenesis of T2DM and obesity, inflammatory factors, particularly IL-6 and TNF-α, play a crucial role. In obesity and T2DM, the inflammatory response in adipose tissue increases significantly, mainly manifested by the infiltration of pro-inflammatory cytokines and immune cells, thereby accelerating the development of metabolic diseases (165). The results of the network meta-analysis showed that ART had a significant effect in reducing IL-6 levels, but the improvement effect on TNF-α did not reach significance.
Obesity significantly affects the immune system in adipose tissue, exacerbating the inflammatory response. This inflammatory environment attracts more immune cell infiltration, mainly macrophages and T lymphocytes, further aggravating the inflammatory response and inhibiting cellular metabolic function (166). Adipose tissue macrophages (ATMs) are crucial in the inflammatory process caused by obesity, with their infiltration promoting inflammation in adipose tissue. With the progression of obesity, the increase in pro-inflammatory signals prompts M2-type macrophages to transform into an M1-type pro-inflammatory phenotype, inducing adipocytes to secrete pro-inflammatory cytokines such as TNF-α, thereby promoting inflammatory responses and IR (13). This mechanism is particularly evident in patients with T2DM, as the inflammatory response not only aggravates IR but also worsens glycemic control by affecting pancreatic β-cell function. ART significantly reduces IL-6 levels through multiple mechanisms. First, ART enhances the expression of anti-inflammatory cytokines in adipose tissue and inhibits the secretion of IL-6, thereby reducing local and systemic inflammatory responses. Secondly, ART promotes the maintenance of M2 macrophages and the reduction of M1 macrophages in adipose tissue, a shift that helps alleviate the inflammatory response and improve insulin sensitivity (167). Additionally, ART enhances immune regulatory function by regulating the balance of T lymphocytes, reducing the infiltration of CD8+ T cells, and increasing the proportion of CD4+ T cells. At the molecular level, ART works by regulating multiple signaling pathways. ART significantly inhibits the activities of nuclear transcription factor kappa B (NF-κB) and c-Jun N-terminal kinase (JNK) signaling pathways. Inhibition of NF-κB and JNK signaling pathways reduces the expression of IL-6, thereby reducing the inflammatory response (168). Additionally, ART activates the phosphatidylinositol 3-kinase (PI3K)/Akt and AMPK signaling pathways. Activation of these pathways improves insulin sensitivity and enhances glucose and lipid metabolism, further alleviating IR (169). Although ART did not reach a significant difference in reducing TNF-α levels, the forest plot of the network meta-analysis still showed a trend in the improvement effect on TNF-α. This suggests that ART may have a regulatory effect on TNF-α levels, but it does not reach statistical significance. It is worth noting that the improvement of inflammatory factors is crucial to the overall metabolic health of T2DM patients. ART plays an important role in regulating blood sugar and improving metabolic function by reducing IL-6 levels and enhancing insulin sensitivity.
Meta-regression analysis and two-dimensional graph evaluation
Meta-regression analysis results showed that changes in VO2max significantly impacted the effect sizes of BMI and HbA1c%. Increases in VO2max are closely related to improvements in BMI and HbA1c%. As an indicator of aerobic capacity, improvements in VO2max mean enhanced cardiopulmonary function and improved systemic metabolic efficiency. Mechanistically, increasing VO2max can increase the density and function of mitochondria in skeletal muscle, promote the oxidation of fatty acids and the uptake and utilization of glucose, directly leading to the reduction of body fat and stabilization of blood sugar (74). Therefore, the significant reduction effect of increasing VO2maxon BMI and HbA1c% reflects its key role in metabolic regulation.
Two-dimensional graph evaluation further revealed the comprehensive advantages of ART across multiple outcome indicators. In the evaluation of the outcome indicators of BMI, HbA1c%, IL-6, and TNF-α, ART was the best intervention. This indicates that ART has a significant effect in comprehensively improving body composition, glycemic control, and inflammatory status. ART combines the advantages of AT and RT, achieving its therapeutic effects through a variety of molecular mechanisms.
First, ART effectively reduces BMI by activating AMPK and mTOR signaling pathways, increasing muscle synthesis and fat oxidation, increasing lean body mass, and reducing fat storage. Secondly, ART can significantly reduce HbA1c% levels and improve glucose metabolism by enhancing insulin sensitivity, promoting GLUT4 translocation and glucose uptake. Regarding the regulation of inflammatory factors, ART reduces the expression of pro-inflammatory factors and the levels of IL-6 and TNF-α by inhibiting the NF-κB and JNK signaling pathways. Modulation of these signaling pathways plays a key role in alleviating chronic inflammation and IR (153). Specifically, ART reduces the infiltration of M1 macrophages in adipose tissue and promotes the phenotype conversion of M2 macrophages, thereby reducing the inflammatory response. Additionally, ART regulates intestinal flora, increases the production of anti-inflammatory short-chain fatty acids (such as butyric acid), and further alleviates systemic inflammation (167). Moreover, both aerobic and resistance training induce the secretion of a wide range of exercise-induced signaling molecules known as exerkines, including myokines (e.g., irisin, IL-6), adipokines, and hepatokines. These circulating factors serve as mediators of inter-organ communication and play a crucial role in regulating metabolic function. Notably, exerkines influence distant target organs such as the liver, adipose tissue, and particularly the endocrine pancreas, where they enhance β-cell function and insulin secretion, ultimately contributing to improved glycemic control and systemic glucose homeostasis.
Overall, the results of meta-regression analysis and two-dimensional graph evaluation show that increasing VO2max has a significant effect on improving BMI and HbA1c%, and ART shows comprehensive advantages across multiple metabolic and inflammatory indicators. ART employs multiple biomolecular mechanisms to comprehensively improve patients’ health status through metabolic regulation and inflammation relief, providing strong evidence for clinical intervention. Future research should further explore the individualized application of different training methods to optimize intervention strategies and maximize clinical effects.
Precise exercise prescription
Based on the findings of our systematic review and network meta-analysis, a precise and comprehensive exercise prescription was developed to maximize metabolic improvements in patients with T2DM and obesity. The core components—ART, PMT, and ACT—were prioritized due to their high SUCRA rankings and superior effects across multiple outcomes, including BMI, HbA1c%, HOMA-IR, IL-6, and TNF-α. This was supported by multidimensional comparisons (Figures 7, 8) and the consistency of their effects observed in subgroup analyses. To determine the specific frequency, intensity, and duration of each intervention, we examined the protocols of the most effective and methodologically rigorous RCTs included in our analysis. Aerobic training was commonly performed at 40–60% VO2;max for 30–60 minutes per session, 3 times per week, while resistance training was frequently conducted at 50–80% 1RM for similar durations and frequency. The structure of ART, combining moderate-intensity aerobic and resistance training within the same session, was drawn from protocols showing synergistic benefits on insulin sensitivity and body composition. PMT (e.g., Tai Chi, yoga) and ACT (e.g., body and auricular acupuncture targeting acupoints such as Zusanli, Sanyinjiao, Guanyuan, Pishu, and Weishu) were included based on trials demonstrating consistent benefits in inflammatory regulation and glycemic control. In addition, evidence from meta-regression analyses indicated that improvements in VO2;max were significantly associated with reductions in BMI and HbA1c%, further justifying the inclusion of structured aerobic components. Mechanistically, these interventions exerted beneficial effects via AMPK/mTOR and GLUT4 signaling, reduction of macrophage-driven inflammation, and modulation of gut microbiota and exerkines. Together, these findings informed the development of a multi-modal, evidence-based exercise plan tailored to the physiological needs of this population (Table 5).
Table 5
| Type | Frequency | Duration per Session | Intensity | Description |
|---|---|---|---|---|
| Aerobic Exercise (AE) | 3 sessions per week | 30–60 minutes | 40% to 60% VO2max | e.g., running, cycling, swimming |
| Resistance Training (RT) | 3 sessions per week | 30–60 minutes | 50% to 75%-80% 1RM | e.g., using dumbbells, barbells, gym equipment |
| Combined Resistance Training (ART) | 3 sessions per week | 60 minutes | Moderate-intensity AE 30 minutes + RT 30 minutes | Combination of aerobic and resistance training |
| Mind-Body Movement (PMT) | 2–3 sessions per week | 30–60 minutes | Low to Moderate | e.g., yoga, tai chi |
| Acupuncture (ACT) | 2–3 sessions per week | 30–60 minutes | NA | Commonly used body acupuncture and ear acupuncture; main acupoints: Zusanli, Sanyinjiao, Guanyuan, Pishu, Weishu |
Recommendation of precise intervention prescriptions for type 2 diabetes and overweight and obesity.
①It is recommended to exercise 1 hour after a meal, especially in the afternoon. ②Continuously monitor heart rate during exercise to ensure it remains within the target intensity range. ③Warm up and cool down for 10 minutes before and after each exercise session to prevent injuries. ④Regular health assessments should be conducted to adjust exercise intensity and methods, ensuring personalized intervention effects.NA, not available.
Although ACT is not a form of physical exercise in the traditional sense, it was included in the prescription due to its non-pharmacological nature and its complementary effects on metabolic regulation, inflammation, and insulin sensitivity, as demonstrated in our network meta-analysis. Thus, ACT was treated as a structured therapeutic session rather than an exercise modality, but integrated into the weekly schedule due to its evidence-based contribution to systemic metabolic improvements. We acknowledge that the combined frequency of the proposed interventions may appear intensive. However, the prescription is intended to offer flexible combinations tailored to individual schedules. Rather than requiring multiple sessions per day, participants are encouraged to alternate between modalities on different days or combine compatible types (e.g., ART replacing separate AT and RT sessions; PMT on rest days). The proposed format represents an optimal upper boundary based on available evidence, and clinicians are advised to adapt it to patient-specific contexts, considering time availability, occupational demands, and socioeconomic factors.
The recommendation to exercise approximately one hour after a meal, especially in the afternoon, is based on physiological and behavioral considerations. Postprandial exercise has been shown to attenuate glucose spikes, enhance insulin action, and reduce cardiovascular stress. Afternoon timing aligns with improved muscular performance and increased substrate utilization, and is often more feasible in daily routines compared to early mornings. These timing recommendations aim to optimize metabolic responses and improve long-term adherence.
Future Directions
Although the present study did not directly assess cardiac outcomes, subclinical myocardial dysfunction is increasingly recognized in individuals with type 2 diabetes and obesity, often preceding overt cardiac disease. Speckle-tracking echocardiography (STE), particularly the assessment of left ventricular global longitudinal strain (LV-GLS), provides a sensitive tool for detecting early impairments in myocardial contractility, even in patients with preserved ejection fraction (170–172). Future longitudinal studies are warranted to determine whether ART and PMT confer cardioprotective effects by improving LV-GLS or other STE-derived parameters. Exploring this potential link may provide novel insights into integrated interventions for metabolic and cardiac health in diabetes.
Conclusion
This study systematically evaluated the effects of various combined interventions on patients with T2DM and obesity through a network meta-analysis system. The results show that PMT is the most significant in improving blood lipid metabolism, especially in reducing TG, TC, LDL-C, and increasing HDL-C. In addition, ART has shown significant effects in improving multiple health indicators such as BMI, HbA1c%, and IL-6.
From the perspective of biomolecular mechanisms, PMT significantly improves insulin sensitivity and lipid metabolism by reducing stress hormone levels and improving endocrine and nervous system functions. ART activates the AMPK and mTOR signaling pathways, promotes fatty acid oxidation and muscle protein synthesis, reduces fat storage, and increases lean body mass, thereby effectively reducing BMI and HbA1c%. AT improves cardiopulmonary function and systemic metabolic status by increasing whole-body energy consumption and fat oxidation, helping to lower blood lipids and improve glucose metabolism. RT significantly reduces FBG and HbA1c% levels and improves glucose metabolism by increasing muscle mass and metabolic rate.
Based on the results of this study, it is recommended to use PMT and ART as the main interventions, combined with AT and RT to maximize the improvement of metabolic health in patients with T2DM and obesity. Both PMT and ART show comprehensive advantages in multiple outcome indicators and can significantly improve blood lipid metabolism, reduce inflammatory factor levels, and improve insulin sensitivity. Future research should further explore the individualized application of these interventions to optimize intervention strategies, improve clinical effects, and provide more scientific and effective management solutions for patients with T2DM and obesity.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LC: Supervision, Funding acquisition, Writing – review & editing, Writing – original draft. DL: Writing – original draft. ST: Writing – review & editing. LQC: Supervision, Conceptualization, Formal analysis, Project administration, Funding acquisition, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The National Key Research and Development Program of China (2020YFC2006704).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1462104/full#supplementary-material
Abbreviations
AT, Aerobic Training; RT, Resistance Training; ART, Aerobic and Resistance Training; PMT, Physical-Mental Training; ACT, Acupuncture; CON, Control Group; WBT, Whole-Body Training; LDL-C, Low-Density Lipoprotein Cholesterol; HDL-C, High-Density Lipoprotein Cholesterol; FBG, Fasting Blood Glucose; HbA1c, Hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; IL-6, Interleukin 6; TNF-α, Tumor Necrosis Factor Alpha; TC, Total Cholesterol; TG, triglyceride.
References
1
Karan A Bhakkiyalakshmi E Jayasuriya R Sarada D Ramkumar KM . The pivotal role of nuclear factor erythroid 2-related factor 2 in diabetes-induced endothelial dysfunction. Pharmacol Res. (2020) 153:104601. doi: 10.1016/j.phrs.2019.104601
2
Lin X Xu Y Pan X Xu J Ding Y Sun X et al . Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci reports. (2020) 10:1–11. doi: 10.1038/s41598-020-71908-9
3
Ling C Rönn T . Epigenetics in human obesity and type 2 diabetes. Cell Metab. (2019) 29:1028–44. doi: 10.1016/j.cmet.2019.03.009
4
Kopp W . How western diet and lifestyle drive the pandemic of obesity and civilization diseases. In: DeFronzoRAFerranniniEZimmetPAlbertiG, editors Diabetes, metabolic syndrome and obesity: targets and therapy. Auckland, New Zealand: Dove Medical Press (2019). p. 2221–36.
5
Roden M Shulman GI . The integrative biology of type 2 diabetes. Nature. (2019) 576:51–60.
6
Kemps H Kränkel N Dörr M Moholdt T Wilhelm M Paneni F et al . Exercise training for patients with type 2 diabetes and cardiovascular disease: What to pursue and how to do it. A Position Paper of the European Association of Preventive Cardiology (EAPC). Eur J preventive Cardiol. (2019) 26:709–27. doi: 10.1177/2047487318820420
7
Swift DL Houmard JA Slentz CA Kraus WE . Effects of aerobic training with and without weight loss on insulin sensitivity and lipids. PloS One. (2018) 13:e0196637. doi: 10.1371/journal.pone.0196637
8
Keenan S Cooke MB Belski R . The effects of intermittent fasting combined with resistance training on lean body mass: a systematic review of human studies. Nutrients. (2020) 12:2349. doi: 10.3390/nu12082349
9
Hansen D Niebauer J Cornelissen V Barna O Neunhäuserer D Stettler C et al . Exercise prescription in patients with different combinations of cardiovascular disease risk factors: a consensus statement from the EXPERT working group. Sports medicine. (2018) 48:1781–97. doi: 10.1007/s40279-018-0930-4
10
Kong L Ren J Fang S He T Zhou X Fang M . Effects of traditional Chinese mind-body exercise-Baduanjin for type 2 diabetes on psychological well-being: A systematic review and meta-analysis. Front Public Health. (2022) 10:923411. doi: 10.3389/fpubh.2022.923411
11
Gomes-Neto M de Sa-Caputo D Paineiras-Domingos LL Brandao AA Neves MF Marin PJ et al . Effects of whole-body vibration in older adult patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Can J diabetes. (2019) 43:524–9. e2. doi: 10.1016/j.jcjd.2019.03.008
12
Liu J Yao C Wang Y Zhao J Luo H . Non-drug interventions of traditional Chinese medicine in preventing type 2 diabetes: a review. Chin Medicine. (2023) 18:151. doi: 10.1186/s13020-023-00854-1
13
Lempesis IG Georgakopoulou VE . Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J Exp Medicine. (2023) 13:7.
14
Al-Mansoori L Al-Jaber H Prince MS Elrayess MA . Role of inflammatory cytokines, growth factors and adipokines in adipogenesis and insulin resistance. Inflammation. (2022), 1–14. doi: 10.1007/s10753-021-01559-z
15
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) ;372:n71.
16
Hebebrand J Holm J-C Woodward E Baker JL Blaak E Durrer Schutz D et al . A proposal of the European Association for the Study of Obesity to improve the ICD-11 diagnostic criteria for obesity based on the three dimensions etiology, degree of adiposity and health risk. Obesity facts. (2017) 10:284–307. doi: 10.1159/000479208
17
Low S Chin MC Ma S Heng D Deurenberg-Yap M . Rationale for redefining obesity in Asians. Ann Acad Med Singapore. (2009) 38:66.
18
Bergman M Manco M Satman I Chan J Schmidt MI Sesti G et al . International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res Clin practice. (2024) 209:111589.
19
Association AD . Classification and diagnosis of diabetes. Diabetes Care. (2017) 40:S11–24. doi: 10.2337/dc17-S005
20
Borenstein M Hedges LV Higgins JP Rothstein HR . Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons (2021).
21
Yokoyama Y Levin SM Barnard ND . Association between plant-based diets and plasma lipids: a systematic review and meta-analysis. Nutr Rev. (2017) 75:683–98. doi: 10.1093/nutrit/nux030
22
Mickenautsch S Yengopal V . Selection bias risk in randomized controlled trials rated as low bias using risk of bias, version 2 (RoB2) tool. Cureus. (2024) 16. doi: 10.7759/cureus.63581
23
Chandler J Cumpston M Li T Page MJ Welch V . Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley (2019).
24
Bao QW Gong C Shen XZ Wang X . The preventive effect of Tai Chi exercise on osteoporosis in elderly patients with type 2 diabetes. Chin J Gerontol. (2016) 36:3246–8.
25
Chen SH Sun JH . Effects of aerobic exercise combined with resistance training on oxidative stress and glucose-lipid metabolism in patients with type 2 diabetes. Rare Dis J. (2023) 30:95–7.
26
Chen YX . Effect of combined acupuncture and medication on insulin resistance and biochemical metabolism in obese patients with type 2 diabetes. Shanghai J Acupunct Moxibustion. (2013) 32:911–3.
27
Ding X Tu P Wu HP Zhou XQ Si L Wan BH et al . Effect of acupuncture on insulin resistance and adipocytokines in patients with type 2 diabetes. Chin Gen Pract. (2013) 16:1184–6.
28
Duan JH Li ZB Li J . Clinical study of Baduanjin exercise prescription in the treatment of type 2 diabetes. Chin Community Doctors (Med Ed). (2012) 14:218–9.
29
Guan YX Wang SS Ma MN . Effect of Baduanjin exercise intervention on relevant indicators in patients with type 2 diabetes. J Nurs Sci. (2012) 27:23–4.
30
Li QW Ding WG Pei JY Xu DQ Huang LP . Effect of Baduanjin on lower limb muscle strength and body composition in patients with type 2 diabetes. Tianjin J Tradit Chin Med. (2016) 33:661–4.
31
Li YH Cui SM Xu HT Ma J . Clinical observation of acupuncture treatment for obese patients with diabetes. J Clin Acupunct Moxibustion. (2013) 29:30–2.
32
Li TX . Effects of different exercise interventions on blood biochemical indicators in patients with type 2 diabetes. Shandong Sports Sci Technol. (2014) 36:81–4.
33
Luo F . Effects and mechanisms of Baduanjin intervention in elderly patients with type 2 diabetes and hypertension. Chin J Geriatr Health Med. (2021) 19:13–6.
34
Ma YF Wu SX Wen YL Zhang L Yang XH . Clinical observation of Baduanjin intervention for peripheral neuropathy in type 2 diabetes. Mod J Integr Tradit Chin West Med. (2021) 30:3266–71.
35
Pan HS Feng YC . Clinical study on Baduanjin exercise prescription in rehabilitation treatment of patients with type 2 diabetes. J Guangzhou Univ Tradit Chin Med. (2008) 03):196–9.
36
Tang XY Fan GJ . Observation on the efficacy of traditional Chinese medicine combined with acupuncture in the treatment of obese type 2 diabetes. Chin Med Herald. (2009) 15:65–6.
37
Wang YG Liu LJ Kou ZJ Wang T . Observation of the therapeutic effect of Baduanjin fitness Qigong in adjuvant treatment of type 2 diabetes. Chin J Sports Med. (2007) 02):208–10.
38
Wei DL . Study on the effect of Tai Chi Rouli Ball exercise on physical fitness of patients with type 2 diabetes. J Nanjing Sport Inst (Nat Sci). (2012) 11:8–11.
39
Xu J Zhao L . Observation on the efficacy of acupuncture treatment for obesity-type type 2 diabetes. J Shandong Univ Tradit Chin Med. (2011) 35:325–6.
40
Yang Y Liu YX . Clinical observation of Bo’s abdominal acupuncture for obese type 2 diabetes. Chin Acupunct. (2015) 35:330–4.
41
Yi WM Zhang XH Xie PF Zhou H Shang XZ Liu TH . Clinical study on Baduanjin exercise prescription in improving pancreatic β-cell function in type 2 diabetes patients with qi-yin deficiency syndrome. Chin J Tradit Chin Med. (2019) 34:1828–31.
42
Zhou LB Zhang JQ Zhao XL Huang ZZ Ao TF Shen M . Study on the effect of Baduanjin exercise intervention on home-based elderly patients with type 2 diabetes. Liaoning J Tradit Chin Med. (2011) 38:1564–5.
43
Zhou PN Peng PM Wang RD . Observation of the efficacy of acupuncture in the treatment of newly diagnosed obese type 2 diabetes. J Clin Acupunct Moxibustion. (2013) 29:21–3.
44
Abd El-Kader SM Al-Jiffri OH Al-Shreef FM . Aerobic exercises alleviate symptoms of fatigue related to inflammatory cytokines in obese patients with type 2 diabetes. Afr Health Sci. (2015) 15:1142–8. doi: 10.4314/ahs.v15i4.13
45
Ae-K A Ss G . Efficacy of whole body vibration exercises versus aerobic training on glycemic control in overweight women with type II diabetes. Med J Cairo University. (2019) 87:455–63.
46
Ahn S Song R . Effects of tai chi exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J Altern Complementary Medicine. (2012) 18:1172–8.
47
Al Ozairi E Alsaeed D Al Roudhan D Jalali M Mashankar A Taliping D et al . The effect of home-based resistance exercise training in people with type 2 diabetes: A randomized controlled trial. Diabetes/Metabolism Res Rev. (2023) 39:e3677. doi: 10.1002/dmrr.v39.7
48
AminiLari Z Fararouei M Amanat S Sinaei E Dianatinasab S AminiLari M et al . The effect of 12 weeks aerobic, resistance, and combined exercises on omentin-1 levels and insulin resistance among type 2 diabetic middle-aged women. Diabetes Metab J. (2017) 41:205. doi: 10.4093/dmj.2017.41.3.205
49
Annibalini G Lucertini F Agostini D Vallorani L Gioacchini A Barbieri E et al . Concurrent aerobic and resistance training has anti-inflammatory effects and increases both plasma and leukocyte levels of IGF-1 in late middle-aged type 2 diabetic patients. Oxid Med Cell longevity. (2017) 2017:3937842. doi: 10.1155/2017/3937842
50
Arora E Shenoy S Sandhu J . Effects of resistance training on metabolic profi le of adults with type 2 diabetes. Indian J Med Res. (2009) 129:515–9.
51
Bacchi E Negri C Targher G Faccioli N Lanza M Zoppini G et al . Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. (2013) 58:1287–95. doi: 10.1002/hep.26393
52
Bacchi E Negri C Zanolin ME Milanese C Faccioli N Trombetta M et al . Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care. (2012) 35:676–82. doi: 10.2337/dc11-1655
53
Balducci S Zanuso S Nicolucci A De Feo P Cavallo S Cardelli P et al . Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Internal medicine. (2010) 170:1794–803.
54
Banitalebi E Kazemi A Faramarzi M Nasiri S Haghighi MM . Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. (2019) 217:101–9. doi: 10.1016/j.lfs.2018.11.062
55
Bassi D Mendes RG Arakelian VM Caruso FCR Cabiddu R Júnior JCB et al . Potential effects on cardiorespiratory and metabolic status after a concurrent strength and endurance training program in diabetes patients—a randomized controlled trial. Sports Medicine-Open. (2016) 2:1–13. doi: 10.1186/s40798-016-0052-1
56
Cauza E Hanusch-Enserer U Strasser B Ludvik B Metz-Schimmerl S Pacini G et al . The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med rehabilitation. (2005) 86:1527–33.
57
Chen S-C Ueng K-C Lee S-H Sun K-T Lee M-C . Effect of t’ai chi exercise on biochemical profiles and oxidative stress indicators in obese patients with type 2 diabetes. J Altern complementary medicine. (2010) 16:1153–9.
58
da Silva CA Ribeiro JP Canto JCA da Silva RE Junior GBS Botura E et al . High-intensity aerobic training improves endothelium-dependent vasodilation in patients with metabolic syndrome and type 2 diabetes mellitus. Diabetes Res Clin Practice. (2012) 95:237–45.
59
Botton CE Umpierre D Rech A Pfeifer LO MaChado CL Teodoro JL et al . Effects of resistance training on neuromuscular parameters in elderly with type 2 diabetes mellitus: A randomized clinical trial. Exp gerontology. (2018) 113:141–9. doi: 10.1016/j.exger.2018.10.001
60
Byrkjeland R Stensæth K-H Anderssen S Njerve IU Arnesen H Seljeflot I et al . Effects of exercise training on carotid intima-media thickness in patients with type 2 diabetes and coronary artery disease. Influence carotid plaques. Cardiovasc diabetology. (2016) 15:1–8. doi: 10.1186/s12933-016-0336-2
61
Cassidy S Thoma C Hallsworth K Parikh J Hollingsworth KG Taylor R et al . High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. (2016) 59:56–66. doi: 10.1007/s00125-015-3741-2
62
Chien Y-H Tsai C-J Wang D-C Chuang P-H Lin H-T . Effects of 12-week progressive sandbag exercise training on glycemic control and muscle strength in patients with type 2 diabetes mellitus combined with possible sarcopenia. Int J Environ Res Public Health. (2022) 19:15009. doi: 10.3390/ijerph192215009
63
Danasegaran M Pal GK Sahoo J Pal P Nanda N Renugasundari M . Effects of 12 weeks practice of yoga on heart rate variability in males with type 2 diabetes receiving oral antidiabetic drugs: A randomized control trial. J Altern Complementary Medicine. (2021) 27:1105–15. doi: 10.1089/acm.2020.0489
64
de Lemos Muller CH Rech A Botton CE Schroeder HT Bock PM Farinha JB et al . Heat-induced extracellular HSP72 release is blunted in elderly diabetic people compared with healthy middle-aged and older adults, but it is partially restored by resistance training. Exp Gerontology. (2018) 111:180–7. doi: 10.1016/j.exger.2018.07.014
65
del Pozo-Cruz B Alfonso-Rosa RM del Pozo-Cruz J Sañudo B Rogers ME . Effects of a 12-wk whole-body vibration based intervention to improve type 2 diabetes. Maturitas. (2014) 77:52–8.
66
Dieli-Conwright CM Courneya KS Demark-Wahnefried W Sami N Lee K Buchanan TA et al . Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J Clin Oncology. (2018) 36:875–83. doi: 10.1200/JCO.2017.75.7526
67
Domínguez-Muñoz FJ Villafaina S García-Gordillo MA Hernández-Mocholi MÁ Collado-Mateo D Adsuar JC et al . Effects of 8-week whole-body vibration training on the HbA1c, quality of life, physical fitness, body composition and foot health status in people with T2DM: A double-blinded randomized controlled trial. Int J Environ Res Public Health. (2020) 17:1317. doi: 10.3390/ijerph17041317
68
Donges C Duffield R Drinkwater E . Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci sports exercise. (2010) 42:304–13.
69
Gouveia SSV de Morais Gouveia GP Souza LM da Costa BC Iles B Pinho VA et al . The effect of pilates on metabolic control and oxidative stress of diabetics type 2–A randomized controlled clinical trial. J Bodywork Movement Therapies. (2021) 27:60–6.
70
Gowri MM Rajendran J Srinivasan AR Bhavanani AB Meena R . Impact of an integrated yoga therapy protocol on insulin resistance and glycemic control in patients with type 2 diabetes mellitus. Rambam Maimonides Med J. (2022) 13.
71
Gram B Christensen R Christiansen C Gram J . Effects of nordic walking and exercise in type 2 diabetes mellitus: a randomized controlled trial. Clin J Sport Medicine. (2010) 20:355–61.
72
Hoseini R Rahim HA Ahmed JK . Decreased inflammatory gene expression accompanies the improvement of liver enzyme and lipid profile following aerobic training and vitamin D supplementation in T2DM patients. BMC Endocrine Disord. (2022) 22:245. doi: 10.1186/s12902-022-01152-x
73
Hsieh P-L Tseng C-H Tseng YJ Yang W-S . Resistance training improves muscle function and cardiometabolic risks but not quality of life in older people with type 2 diabetes mellitus: a randomized controlled trial. J Geriatric Phys Ther. (2018) 41:65–76. doi: 10.1519/JPT.0000000000000107
74
Hsu CH Yang CB Chen MH Tsao TH . Accumulated short bouts of walking in older adults with type 2 diabetes: Effects on glycosylated hemoglobin (HbA1c) and homeostasis model assessment of insulin resistance (HOMA-IR). Res Gerontological Nursing. (2023) 16:250–8. doi: 10.3928/19404921-20230503-04
75
Johansen MY MacDonald CS Hansen KB Karstoft K Christensen R Pedersen M et al . Effect of an intensive lifestyle intervention on glycemic control in patients with type 2 diabetes: a randomized clinical trial. Jama. (2017) 318:637–46. doi: 10.1001/jama.2017.10169
76
Jorge MLMP de Oliveira VN Resende NM Paraiso LF Calixto A Diniz ALD et al . The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. (2011) 60:1244–52. doi: 10.1016/j.metabol.2011.01.006
77
Kadoglou N Fotiadis G Kapelouzou A Kostakis A Liapis C Vrabas I . The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabetic Medicine. (2013) 30:e41–50. doi: 10.1111/dme.2013.30.issue-2
78
Kadoglou N Vrabas I Sailer N Kapelouzou A Fotiadis G Noussios G et al . Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. (2010) 36:144–51. doi: 10.1016/j.diabet.2009.11.004
79
Kadoglou NP Fotiadis G Athanasiadou Z Vitta I Lampropoulos S Vrabas IS . The effects of resistance training on ApoB/ApoA-I ratio, Lp (a) and inflammatory markers in patients with type 2 diabetes. Endocrine. (2012) 42:561–9. doi: 10.1007/s12020-012-9650-y
80
Kadoglou NP Vrabas IS Kapelouzou A Lampropoulos S Sailer N Kostakis A et al . The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci monitor: Int Med J Exp Clin Res. (2012) 18:CR290. doi: 10.12659/MSM.882734
81
Karstoft K Clark MA Jakobsen I Müller IA Pedersen BK Solomon TP et al . The effects of 2 weeks of interval vs continuous walking training on glycaemic control and whole-body oxidative stress in individuals with type 2 diabetes: a controlled, randomised, crossover trial. Diabetologia. (2017) 60:508–17. doi: 10.1007/s00125-016-4170-6
82
Kaur N Majumdar V Nagarathna R Malik N Anand A Nagendra HR . Diabetic yoga protocol improves glycemic, anthropometric and lipid levels in high risk individuals for diabetes: a randomized controlled trial from Northern India. Diabetol Metab Syndrome. (2021) 13:1–10. doi: 10.1186/s13098-021-00761-1
83
Krause M Rodrigues-Krause J O’Hagan C Medlow P Davison G Susta D et al . The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur J Appl Physiol. (2014) 114:251–60. doi: 10.1007/s00421-013-2769-6
84
Kumpatla S Michael C Viswanathan V . Effect of Yogasanas on glycaemic, haemodynamic and lipid profile in newly diagnosed subjects with type 2 diabetes. Int J Diabetes Developing Countries. (2015) 35:181–8.
85
Kwon HR Min KW Ahn HJ Seok HG Lee JH Park GS et al . Effects of aerobic exercise vs. resistance training on endothelial function in women with type 2 diabetes mellitus. Diabetes Metab J. (2011) 35:364. doi: 10.4093/dmj.2011.35.4.364
86
Lam P Dennis SM Diamond TH Zwar N . Improving glycaemic and BP control in type 2 diabetes: the effectiveness of tai chi. Aust J Gen Practice. (2008) 37:884.
87
Leehey DJ Collins E Kramer HJ Cooper C Butler J McBurney C et al . Structured exercise in obese diabetic patients with chronic kidney disease: a randomized controlled trial. Am J nephrology. (2016) 44:54–62. doi: 10.1159/000447703
88
Li M Zheng Q Miller JD Zuo P Yuan X Feng J et al . Aerobic training reduces pancreatic fat content and improves β-cell function: A randomized controlled trial using IDEAL-IQ magnetic resonance imaging. Diabetes/Metabolism Res Rev. (2022) 38:e3516. doi: 10.1002/dmrr.v38.4
89
Li S Yuan S Zhang J Xu F Zhu F . The effect of periodic resistance training on obese patients with type 2 diabetic nephropathy. Sci Reports. (2024) 14:2761.
90
Li Y Xie K Zeng X Ding L Wang Y Lu L et al . Effect of Zuo’s warming Yang acupuncture therapy combined with lifestyle interventions on prediabetes: A randomized controlled trial. Complementary Therapies Medicine. (2023) 78:102985.
91
Lucotti P Monti LD Setola E Galluccio E Gatti R Bosi E et al . Aerobic and resistance training effects compared to aerobic training alone in obese type 2 diabetic patients on diet treatment. Diabetes Res Clin practice. (2011) 94:395–403.
92
Ma X Li M Liu L Lei F Wang L Xiao W et al . A randomized controlled trial of Baduanjin exercise to reduce the risk of atherosclerotic cardiovascular disease in patients with prediabetes. Sci Reports. (2022) 12:19338.
93
Magalhães JP Santos DA Correia IR Hetherington-Rauth M Ribeiro R Raposo JF et al . Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: a secondary analysis from a 1-year randomized controlled trial. Cardiovasc diabetology. (2020) 19:1–11. doi: 10.1186/s12933-020-01136-y
94
Martins RA Neves AP Coelho-Silva MJ Veríssimo MT Teixeira AM . The effect of aerobic versus strength-based training on high-sensitivity C-reactive protein in older adults. Eur J Appl Physiol. (2010) 110:161–9. doi: 10.1007/s00421-010-1488-5
95
MdL M Spivakoski CS Réus BdS DMdS A Mattje PN Hohl A et al . Effect of whole body vibration on clinical and metabolic outcomes in adults with type 2 diabetes: an observational pilot trial. Pract Diabetes. (2021) 38:20–5.
96
Mitranun W Deerochanawong C Tanaka H Suksom D . Continuous vs interval training on glycemic control and macro-and microvascular reactivity in type 2 diabetic patients. Scandinavian J Med Sci sports. (2014) 24:e69–76. doi: 10.1111/sms.2014.24.issue-2
97
Moayedi F Taghian F Jalali Dehkordi K Hosseini SA . Cumulative effects of exercise training and consumption of propolis on managing diabetic dyslipidemia in adult women: a single-blind, randomized, controlled trial with pre–post-intervention assessments. J Physiol Sci. (2023) 73:17. doi: 10.1186/s12576-023-00872-6
98
Mohammadi A Bijeh N Moazzami M khodaei K Rahimi N . Effect of exercise training on spexin level, appetite, lipid accumulation product, visceral adiposity index, and body composition in adults with type 2 diabetes. Biol Res nursing. (2022) 24:152–62. doi: 10.1177/10998004211050596
99
Ng CL Goh S-Y Malhotra R Østbye T Tai ES . Minimal difference between aerobic and progressive resistance exercise on metabolic profile and fitness in older adults with diabetes mellitus: a randomised trial. J physiotherapy. (2010) 56:163–70. doi: 10.1016/S1836-9553(10)70021-7
100
Okada S Hiuge A Makino H Nagumo A Takaki H Konishi H et al . Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscl thrombosis. (2010) 17:828–33.
101
Olioso D Dauriz M Bacchi E Negri C Santi L Bonora E et al . Effects of aerobic and resistance training on circulating micro-RNA expression profile in subjects with type 2 diabetes. J Clin Endocrinol Metab. (2019) 104:1119–30. doi: 10.1210/jc.2018-01820
102
VNd O Bessa A Jorge MLMP RJdS O de Mello MT De Agostini GG et al . The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiology Nutrition Metab. (2012) 37:334–44. doi: 10.1139/h2012-004
103
Ploydang T Khovidhunkit W Tanaka H Suksom D . Nordic walking in water on cerebrovascular reactivity and cognitive function in elderly patients with type 2 diabetes. Med Sci Sports Exercise. (2023). doi: 10.1249/MSS.0000000000003216
104
Ranasinghe C Devage S Constantine GR Katulanda P Hills AP King NA . Glycemic and cardiometabolic effects of exercise in South Asian Sri Lankans with type 2 diabetes mellitus: A randomized controlled trial Sri Lanka diabetes aerobic and resistance training study (SL-DARTS). Diabetes Metab Syndrome: Clin Res Rev. (2021) 15:77–85. doi: 10.1016/j.dsx.2020.12.011
105
Rezaeeshirazi R . Aerobic versus resistance training: Leptin and metabolic parameters improvement in type 2 diabetes obese men. Res Q Exercise Sport. (2022) 93:537–47.
106
Rodríguez-Reyes G García-Ulloa AC Hernández-Jiménez S Alessi-Montero A Carrera LN Rojas-Torres F et al . Effect of whole-body vibration training on transcutaneous oxygen levels of the foot in patients with type 2 diabetes: A randomized controlled trial. J Biomechanics. (2022) 139:110871. doi: 10.1016/j.jbiomech.2021.110871
107
Sabag A Way KL Sultana RN Keating SE Gerofi JA Chuter VH et al . The effect of a novel low-volume aerobic exercise intervention on liver fat in type 2 diabetes: a randomized controlled trial. Diabetes Care. (2020) 43:2371–8. doi: 10.2337/dc19-2523
108
Saberipour B Gheibizadeh M Maraghi E Moradi L . Comparing the effect of walking and yoga on clinical and laboratory parameters in men with type II diabetes: A randomized controlled clinical trial. Jundishapur J Chronic Dis Care. (2020) 9. doi: 10.5812/jjcdc.99977
109
Saeidi A Soltani M Daraei A Nohbaradar H Haghighi MM Khosravi N et al . The effects of aerobic-resistance training and broccoli supplementation on plasma dectin-1 and insulin resistance in males with type 2 diabetes. Nutrients. (2021) 13:3144. doi: 10.3390/nu13093144
110
Sharma S Bhardwaj S Gupta A Katoch VM Sharma KK Gupta R . Influence of 24-week yoga intervention on cardiovascular risk factors and inflammatory markers in type 2 diabetes. Int J Yoga. (2023) 16:27–33.
111
Sharma S Bhardwaj S Jangir S Gupta B . Influence of yoga on status of lipid indices in type 2 diabetes mellitus subjects. Int J Diabetes Developing Countries. (2020) 40:410–5.
112
Silveira-Rodrigues JG Pires W Gomes PF Ogando PHM Melo BP Aleixo IMS et al . Combined exercise training improves specific domains of cognitive functions and metabolic markers in middle-aged and older adults with type 2 diabetes mellitus. Diabetes Res Clin Practice. (2021) 173:108700. doi: 10.1016/j.diabres.2021.108700
113
Sukala WR Page R Rowlands DS Krebs J Lys I Leikis M et al . South Pacific Islanders resist type 2 diabetes: comparison of aerobic and resistance training. Eur J Appl Physiol. (2012) 112:317–25. doi: 10.1007/s00421-011-1978-0
114
Sun X Yan T Li Z Zhou S Peng W Cui W et al . Effects of endurance exercise and vitamin D supplementation on insulin resistance and plasma lipidome in middle-aged adults with type 2 diabetes. Nutrients. (2023) 15:3027. doi: 10.3390/nu15133027
115
Vinetti G Mozzini C Desenzani P Boni E Bulla L Lorenzetti I et al . Supervised exercise training reduces oxidative stress and cardiometabolic risk in adults with type 2 diabetes: a randomized controlled trial. Sci reports. (2015) 5:9238. doi: 10.1038/srep09238
116
Viswanathan V Sivakumar S Prathiba AS Devarajan A George L Kumpatla S . Effect of yoga intervention on biochemical, oxidative stress markers, inflammatory markers and sleep quality among subjects with type 2 diabetes in South India: Results from the SATYAM project. Diabetes Res Clin practice. (2021) 172:108644. doi: 10.1016/j.diabres.2020.108644
117
Wang Y Wang L Yan J Yuan X Lou QQ . Aerobic training increases hippocampal volume and protects cognitive function for type 2 diabetes patients with normal cognition. Exp Clin Endocrinol Diabetes. (2023) 131:605–14.
118
Winding KM Munch GW Iepsen UW Van Hall G Pedersen BK Mortensen S . The effect of low-volume high-intensity interval training versus endurance training on glycaemic control in individuals with type 2 diabetes. Diabetes Obesity Metab. (2018) 20:1–27. doi: 10.1111/dom.2018.20.issue-5
119
Yuan X Dai X Liu L Hsue C Miller JD Fang Z et al . Comparing the effects of 6 months aerobic exercise and resistance training on metabolic control and β-cell function in Chinese patients with prediabetes: a multicenter randomized controlled trial. J Diabetes. (2020) 12:25–37. doi: 10.1111/1753-0407.12955
120
Zhang Y Fu FH . Effects of 14-week Tai Ji Quan exercise on metabolic control in women with type 2 diabetes. Am J Chin medicine. (2008) 36:647–54.
121
Firouzjaei A Li G Wang N Liu W Zhu B . Comparative evaluation of the therapeutic effect of metformin monotherapy with metformin and acupuncture combined therapy on weight loss and insulin sensitivity in diabetic patients. Nutr diabetes. (2016) 6:e209–e. doi: 10.1038/nutd.2016.16
122
Larose J Sigal R Khandwala F Kenny G . Comparison of strength development with resistance training and combined exercise training in type 2 diabetes. Scandinavian J Med Sci sports. (2012) 22:e45–54. doi: 10.1111/j.1600-0838.2011.01412.x
123
Li M Li J Xu Y Gao J Cao Q Ding Y et al . Effect of 5: 2 regimens: energy-restricted diet or low-volume high-intensity interval training combined with resistance exercise on glycemic control and cardiometabolic health in adults with overweight/obesity and type 2 diabetes: A three-arm randomized controlled trial. Diabetes Care. (2024) 47:1074–83. doi: 10.2337/dc24-0241
124
Plotnikoff R Eves N Jung M Sigal R Padwal R Karunamuni N . Multicomponent, home-based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. Int J obesity. (2010) 34:1733–41. doi: 10.1038/ijo.2010.109
125
Baldi J Snowling N . Resistance training improves glycaemic control in obese type 2 diabetic men. Int J sports medicine. (2003) 24:419–23.
126
Bhati P Hussain ME Deepak K Masood S Anand P . Progressive resistance training ameliorates deteriorating cardiac autonomic dysfunction, subclinical inflammation and endothelial dysfunction in type 2 diabetes mellitus: A randomized control trial. Diabetes Metab Syndrome: Clin Res Rev. (2023) 17:102778. doi: 10.1016/j.dsx.2023.102778
127
Brooks N Layne JE Gordon PL Roubenoff R Nelson ME Castaneda-Sceppa C . Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. (2007) 4:19. doi: 10.7150/ijms.4.19
128
Chen Y Qin J Tao L Liu Z Huang J Liu W et al . Effects of tai chi chuan on cognitive function in adults 60 years or older with type 2 diabetes and mild cognitive impairment in China: A randomized clinical trial. JAMA network Open. (2023) 6:e237004–e. doi: 10.1001/jamanetworkopen.2023.7004
129
Gholami F Nikookheslat S Salekzamani Y Boule N Jafari A . Effect of aerobic training on nerve conduction in men with type 2 diabetes and peripheral neuropathy: A randomized controlled trial. Neurophysiologie Clinique. (2018) 48:195–202. doi: 10.1016/j.neucli.2018.03.001
130
Hegde SV Adhikari P Kotian SM Shastry R . Effects of yoga versus sham yoga on oxidative stress, glycemic status, and anthropometry in type 2 diabetes mellitus: A single-blinded randomized pilot study. Int J yoga Ther. (2020) 30:33–9. doi: 10.17761/D-18-2020-00018
131
Keerthi GS Pal P Pal GK Sahoo JP Sridhar MG Balachander J . Effect of 12 Weeks of yoga therapy on quality of life and Indian diabetes risk score in normotensive Indian young adult prediabetics and diabetics: randomized control trial. J Clin Diagn research: JCDR. (2017) 11:CC10. doi: 10.7860/JCDR/2017/29307.10633
132
Ku Y Han K Ahn H Kwon H Koo B Kim H et al . Resistance exercise did not alter intramuscular adipose tissue but reduced retinol-binding protein-4 concentration in individuals with type 2 diabetes mellitus. J Int Med Res. (2010) 38:782–91. doi: 10.1177/147323001003800305
133
Hirosaki M Ohira T Wu Y Eguchi E Shirai K Imano H et al . Laughter yoga as an enjoyable therapeutic approach for glycemic control in individuals with type 2 diabetes: A randomized controlled trial. Front Endocrinology. (2023) 14:1148468. doi: 10.3389/fendo.2023.1148468
134
Kamat K Kage V Sequeira S . Calisthenics versus Pilates training on glycemic control and body fat in overweight individuals with type 2 diabetes mellitus. Physiotherapy Pract Res. (2023) 44:99–108. doi: 10.3233/PPR-220688
135
Lee K Lee S Song C . Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp medicine. (2013) 231:305–14.
136
Motahari Rad M Bijeh N Attarzadeh Hosseini SR Raouf Saeb A . The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch Physiol Biochem. (2023) 129:424–33. doi: 10.1080/13813455.2020.1829649
137
Patil SG Aithala MR Naregal GV Shanmukhe AG Chopade SS . Effect of yoga on cardiac autonomic dysfunction and insulin resistance in non-diabetic offspring of type-2-diabetes parents: A randomized controlled study. Complementary therapies Clin practice. (2019) 34:288–93.
138
Riahy S . The effects of 12 weeks of high-intensity interval training and moderate-intensity continuous training on FGF21, irisin, and myostatin in men with type 2 diabetes mellitus. Growth Factors. (2024) 42:24–35. doi: 10.1080/08977194.2023.2279163
139
Martins FM de Paula Souza A Nunes PRP Michelin MA Murta EFC Resende EAMR et al . High-intensity body weight training is comparable to combined training in changes in muscle mass, physical performance, inflammatory markers and metabolic health in postmenopausal women at high risk for type 2 diabetes mellitus: a randomized controlled clinical trial. Exp gerontology. (2018) 107:108–15. doi: 10.1016/j.exger.2018.02.016
140
Peña A Olson ML Ayers SL Sears DD Vega-López S Colburn AT et al . Inflammatory mediators and type 2 diabetes risk factors before and in response to Lifestyle intervention among latino adolescents with obesity. Nutrients. (2023) 15:2442. doi: 10.3390/nu15112442
141
Sawangwong P Tungsukruthai S Nootim P Sriyakul K Phetkate P Pawa KK et al . The effects of 12-week traditional thai exercise (Ruesi dadton) on glycemic control and inflammatory markers in prediabetes: A randomized controlled trial. Life. (2023) 13:2166. doi: 10.3390/life13112166
142
Reaven GM . Role of insulin resistance in human disease. Diabetes. (1988) 37:1595–607.
143
Chandrasekaran P Weiskirchen R . The role of obesity in type 2 diabetes mellitus—An overview. Int J Mol Sci. (2024) 25:1882. doi: 10.3390/ijms25031882
144
Ruze R Liu T Zou X Song J Chen Y Xu R et al . Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front endocrinology. (2023) 14:1161521.
145
Rohm TV Meier DT Olefsky JM Donath MY . Inflammation in obesity, diabetes, and related disorders. Immunity. (2022) 55:31–55.
146
Chadt A Scherneck S Joost H-G Al-Hasani H . Molecular links between obesity and diabetes:”diabesity”. In: PatelVBPreedyVR, editors. Type 2 Diabetes: Methods and Protocols. New York, NY: Humana Press (2015).
147
Longo S Rizza S Federici M . Microbiota-gut-brain axis: Relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta diabetologica. (2023) 60:1007–17. doi: 10.1007/s00592-023-02088-x
148
Beltrán-Carrillo VJ Megías Á González-Cutre D Jiménez-Loaisa A . Elements behind sedentary lifestyles and unhealthy eating habits in individuals with severe obesity. Int J Qual Stud Health Well-being. (2022) 17:2056967.
149
Pistor KE . The effects of endurance exercise training on adipose tissue metabolism. Toronto, ON (Canada): York University (2012).
150
Talanian JL Tunstall RJ Watt MJ Duong M Perry CG Steinberg GR et al . Adrenergic regulation of HSL serine phosphorylation and activity in human skeletal muscle during the onset of exercise. Am J Physiology-Regulatory Integr Comp Physiol. (2006) 291:R1094–R9. doi: 10.1152/ajpregu.00130.2006
151
Tsiloulis T Watt MJ . Exercise and the regulation of adipose tissue metabolism. Prog Mol Biol Trans science. (2015) 135:175–201.
152
Yang K Liu C Shao J Guo L Wang Q Meng Z et al . Would Combination Be Better: Swimming Exercise and Intermittent Fasting Improve High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Obese Rats via the miR-122-5p/SREBP-1c/CPT1A Pathway. Diabetes Metab Syndrome Obesity. (2024), 1675–86. doi: 10.2147/DMSO.S448165
153
Nikroo H Hosseini SRA Fathi M Sardar MA Khazaei M . The effect of aerobic, resistance, and combined training on PPAR-α, SIRT1 gene expression, and insulin resistance in high-fat diet-induced NAFLD male rats. Physiol behavior. (2020) 227:113149. doi: 10.1016/j.physbeh.2020.113149
154
D’Alleva M Vaccari F Graniero F Giovanelli N Floreani M Fiori F et al . Effects of 12-week combined training versus high intensity interval training on cardiorespiratory fitness, body composition and fat metabolism in obese male adults. J Exercise Sci Fitness. (2023) 21:193–201. doi: 10.1016/j.jesf.2023.01.004
155
MacInnis MJ Egan B Gibala MJ . The effect of training on skeletal muscle and exercise metabolism. Exercise Metab. (2022), 215–42.
156
Bellicha A van Baak MA Battista F Beaulieu K Blundell JE Busetto L et al . Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: An overview of 12 systematic reviews and 149 studies. Obesity Rev. (2021) 22:e13256. doi: 10.1111/obr.v22.S4
157
Izquierdo M Merchant R Morley JE Anker S Aprahamian I Arai H et al . International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J nutrition Health aging. (2021) 25:824–53.
158
Song S Li R Cao B Zhang J Kim Y Kim B et al . Mechanism of electroacupuncture regulating IRS-1 phosphorylation in skeletal muscle to improve insulin sensitivity. Evidence-Based Complementary Altern Medicine. (2021) 2021:8631475.
159
Wondmkun YT . Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndrome Obesity. (2020), 3611–6. doi: 10.2147/DMSO.S275898
160
Anderson JG Taylor AG . The metabolic syndrome and mind-body therapies: a systematic review. J Nutr Metab. (2011) 2011:276419. doi: 10.1155/2011/276419
161
Khalafi M Sakhaei MH Kazeminasab F Rosenkranz SK Symonds ME . Exercise training, dietary intervention, or combined interventions and their effects on lipid profiles in adults with overweight and obesity: a systematic review and meta-analysis of randomized clinical trials. Nutrition Metab Cardiovasc Diseases. (2023) 33:1662–83. doi: 10.1016/j.numecd.2023.05.024
162
Yao T Wang H Lin K Wang R Guo S Chen P et al . Exercise-induced microbial changes in preventing type 2 diabetes. Sci China Life Sci. (2024) 67:892–9. doi: 10.1007/s11427-022-2272-3
163
Lian X Wang M Yuan M Li X Chen D Zhong J et al . Clinical effect of acupuncture combined with medication on diabetic peripheral neuropathy and its influence on serum lipid metabolism. Zhongguo Zhen jiu= Chin Acupuncture Moxibustion. (2024) 44:503–12.
164
Wu S Wang L He Y Shi F Zhuang H Mei L et al . Effects of different mind-body exercises on glucose and lipid metabolism in patients with type 2 diabetes: A network meta-analysis. Complementary Therapies Clin Pract. (2023) 101802. doi: 10.37766/inplasy2023.6.0023
165
Liu N Yan X Lv B Wu Y Hu X Zheng C et al . A study on the association between gut microbiota, inflammation, and type 2 diabetes. Appl Microbiol Biotechnol. (2024) 108:213. doi: 10.1007/s00253-024-13041-5
166
Domazet SL Olesen TB Stidsen JV Svensson CK Nielsen JS Thomsen RW et al . Low-grade inflammation in persons with recently diagnosed type 2 diabetes: The role of abdominal adiposity and putative mediators. Diabetes Obesity Metab. (2024) 26:2092–101. doi: 10.1111/dom.15514
167
Al-Mhanna SB Batrakoulis A Ghazali WSW Mohamed M Aldayel A Alhussain MH et al . Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: a systematic review and meta-analysis. PeerJ. (2024) 12:e17525. doi: 10.7717/peerj.17525
168
Krüger K Mooren FC Eder K Ringseis R . Immune and inflammatory signaling pathways in exercise and obesity. Am J lifestyle medicine. (2016) 10:268–79.
169
Duft RG Bonfante ILP Palma-Duran SA Chacon-Mikahil MPT Griffin JL Cavaglieri CR . Moderate-intensity combined training induces lipidomic changes in individuals with obesity and type 2 diabetes. J Clin Endocrinol Metab. (2024), dgae177. doi: 10.1210/clinem/dgae177
170
Silverii GA Toncelli L Casatori L Bossini R Nannelli F Pala L et al . Assessment of left ventricular global longitudinal strain in patients with type 2 diabetes: Relationship with microvascular damage and glycemic control. Nutr Metab Cardiovasc Dis. (2022) 32:994–1000. doi: 10.1016/j.numecd.2022.01.014
171
Gupta A Jeyaprakash P Ghoreyshi-Hefzabad SM Pathan F Ozawa K Negishi K . Left ventricular longitudinal systolic dysfunction in children with type 1 diabetes mellitus: A systematic review and meta-analysis. J Diabetes Complications. (2023) 37:108528. doi: 10.1016/j.jdiacomp.2023.108528
172
Sonaglioni A Bordoni T Naselli A Nicolosi GL Grasso E Bianchi S et al . Influence of gestational diabetes mellitus on subclinical myocardial dysfunction during pregnancy: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2024) 292:17–24. doi: 10.1016/j.ejogrb.2023.11.007
Summary
Keywords
type 2 diabetes mellitus, obesity, insulin resistance, aerobic training, resistance training, combined aerobic and resistance training
Citation
Cui L, Lu D, Tan S and Cao L (2025) Comparative effectiveness of various combined interventions for type 2 diabetes and obesity: a systematic review and network meta-analysis. Front. Endocrinol. 16:1462104. doi: 10.3389/fendo.2025.1462104
Received
09 July 2024
Accepted
14 April 2025
Published
06 August 2025
Volume
16 - 2025
Edited by
Elise Catherine Brown, Oakland University, United States
Reviewed by
Gabriela Alves Bronczek, State University of Campinas, Brazil
Andrea Sonaglioni, IRCCS MultiMedica, Italy
Updates
Copyright
© 2025 Cui, Lu, Tan and Cao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sijie Tan, tansijie2003@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.