- 1Department of Endocrinology and Metabolism, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 2Department of Health Services and Management, Dalian Neusoft University of Information, Dalian, Liaoning, China
Objective: To assess the impact of individualized strategy and continuous glucose monitoring (CGM) on glycemic control and mental health(anxiety, depression, pregnancy-related anxiety and diabetes specific quality of life during pregnancy) in patients with diabetes in pregnancy (DIP).
Methods: In this study, 80 pregnant women diagnosed with type 2 diabetes mellitus (T2DM) complicated with pregnancy or gestational diabetes mellitus (GDM) were enrolled. Participants were randomly assigned to either CGM group or self-monitoring of blood glucose (SMBG) group. Blood glucose was regularly monitored for 14 days to guide and adjust hypoglycemic treatment (lifestyle or hypoglycemic agents) of the patients in time. Baseline characteristics were collected after enrollment. Self-rating anxiety scale (SAS), self-rating depression scale (SDS), pregnancy-related anxiety questionnaire (PAQ), diabetes specific quality of life scale (DSQL) were used to evaluate the anxiety, depression, pregnancy-related anxiety and quality of life. Glycemic parameters and scale scores were collected before and after individualized strategy.
Results: FBG and 2hPBG significantly decreased post-intervention in both groups (P<0.001). In the CGM group, the scores of SAS (39.59 ± 7.10 vs 37.15 ± 6.28), PAQ (24.15 ± 6.45 vs 22.59 ± 5.65) and DSQL (47.44 ± 9.01 vs 43.20 ± 9.00) after individualized strategy were significantly lower than those before individualized strategy (P<0.05). The SAS scale scores and PAQ scale scores were positively correlated with blood glucose levels (P<0.05).
Conclusion: The individualized strategy encompasses an insulin titration protocol guided by CGM, coupled with structured lifestyle modifications that address dietary patterns, physical activity and more, combined with short-term glucose monitoring can exert a positive effect on glycemic improvement in the short term and meet the requirements of glycemic control in pregnancy, which has important clinical significance. The combined use of individualized strategy and CGM improves glycemic control and may have protective effects on psychological well-being.
Clinical Trial Registration: https://www.chictr.org.cn, identifier ChiCTR2200060719.
1 Introduction
Diabetes in pregnancy (DIP) is a condition characterized by abnormal glucose metabolism during pregnancy, which includes both pregestational diabetes mellitus (PGDM) and gestational diabetes mellitus (GDM). PGDM denotes that a pregnant woman was diagnosed with diabetes mellitus (DM) prior to pregnancy. GDM is defined as the first occurrence or detection of impaired glucose tolerance during pregnancy. The prevalence of DIP in the U.S. ranges from 6.0% to 9.0%, with GDM constituting 90.0% of cases (1). According to the diagnostic criteria of the International Diabetes and pregnancy Research Group (IADPSG), a study in China in 2013 showed that the incidence of GDM was 17.5% (2). Hyperglycemia in pregnancy can lead to a variety of adverse pregnancy outcomes, such as macrosomia, shoulder dystocia, stillbirth, neonatal respiratory distress syndrome, neonatal hypoglycemia, etc. (1, 3), and is associated with an increased risk of maternal and fetal long-term complications such as type 2 diabetes mellitus (T2DM) (4, 5).
Pregnant women are more likely to be affected psychologically due to changes in physical and social psychological state, with anxiety and depression being more common (6). The global prevalence of prenatal anxiety and depression varied from 6.0% to 57.0% and 8.5% to 44.4%, respectively (7–9). On the other hand, anxiety and depression can cause hypothalamus-pituitary-adrenal dysfunction and then cause abnormal glucose tolerance or insulin resistance (IR) through sympathetic nerve activation (10, 11). GDM is more prone to anxiety and depression (12, 13). Hyperglycemia during pregnancy contributes to anxiety and depression through multiple mechanisms, including lack of awareness of the disease, worry about the health problems of future generations, stress response and so on. Anxiety can affect the mother’s emotional balance and fetal development, and it can also lead to low birth weight, premature birth and other adverse pregnancy outcomes (14–16). Therefore, it is crucial to pay attention to the psychological status of patients with DIP. It is worth mentioning that PGDM and GDM are significantly different in terms of clinical manifestations, treatment measures and clinical prognosis. In addition, the characteristics of glucose metabolism are different at different gestational ages, so it is necessary to analyze the difference of mental health status between them.
Blood glucose monitoring plays an indispensable role in the blood glucose strategy of patients with DM. The most widely used blood glucose monitoring method in a clinic is self-monitoring of blood glucose (SMBG), based on capillary glucose testing. Whereas SMBG can only reflect the instantaneous capillary blood glucose level at that time but cannot recall the overall trend and fluctuation of blood glucose, and some patients cannot stand the pain of fingertip blood glucose monitoring and the economic burden related to blood glucose monitoring. Continuous glucose monitoring (CGM) is a new blood glucose monitoring method that has been used in clinics in recent years, which reflects the whole-day blood glucose level and blood glucose fluctuation by measuring the blood glucose concentration in tissue fluid. The daily glucose trend chart, glucose fluctuation trend and other related data can be obtained to encourage both doctors and patients to evaluate the blood glucose more thoroughly and assist in adjusting of the hypoglycemic treatment to achieve the targets of blood glucose control. CGM and SMBG were used in this study to better understand the blood glucose level of patients with DIP, provide reference for individualized strategy and evaluate the efficacy, anxiety, depression and quality of life of individualized strategy combined with blood glucose monitoring.
2 Materials and methods
2.1 Participants and study design
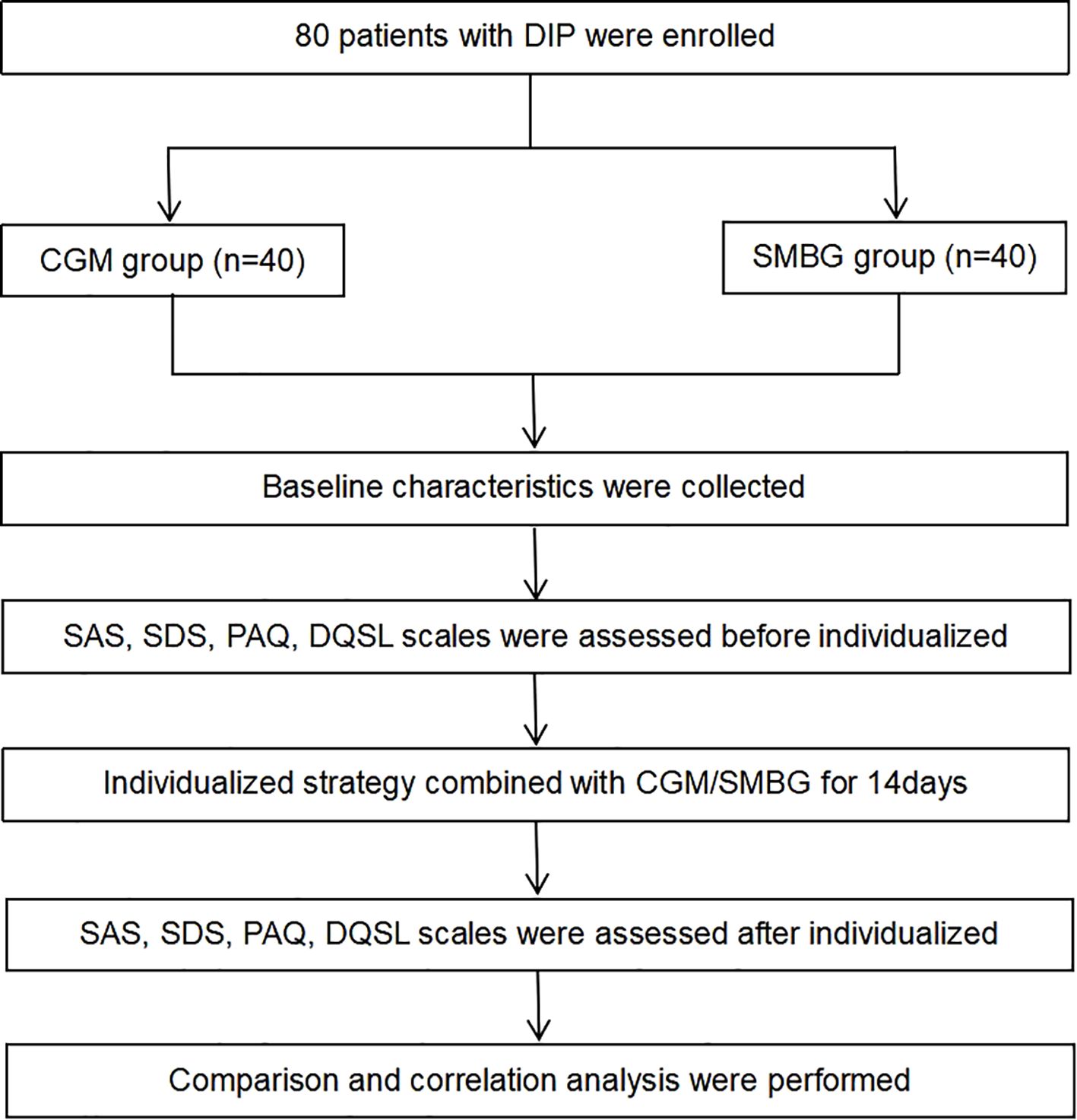
This study enrolled 80 pregnant women who met the diagnosis of DIP (including PGDM and GDM) in the outpatient clinic of the First Affiliated Hospital of Dalian Medical University from June 2022 to July 2022 (Figure 1). This study was approved by the ethics committee of the First Affiliated Hospital of Dalian Medical University. The inclusion criteria included: 1) 18–45 years old; 2) singleton pregnancy; 3) no previous history of mental illness; 4) voluntary use of CGM or SMBG, who have good understanding and communication skills. The exclusion criteria included: 1) anxiety or depression diagnosed before pregnancy; 2) recently experienced severe stress events, complicated with infection, heart failure, kidney insufficiency, or other serious complications; 3) poor compliance. The participants were randomly divided into the CGM group and the SMBG group. The pregnant women in the CGM group used FreeStyle Libre (Abbott Diabetes Care Ltd) to dynamically monitor their blood glucose for 14 consecutive days. By using the scanner, patients can obtain an immediate glucose value, nearly 8 hours of glucose data and a glucose change trend, and the system can automatically save an average of blood glucose every 15 minutes, recording a total of 96 blood glucose values per day, and finally obtaining a 14-day glucose trend chart. The pregnant women in the SMBG group, on the other hand, utilized a home blood glucose meter to track changes in peripheral blood glucose, and recorded fasting and 2-hour postprandial blood glucose for 14 days.
Fasting blood glucose (FBG) and 2 hours postprandial blood glucose (2hPBG) were recorded using CGM or SMBG. To minimize bias, all participants received standardized instructions from the same endocrinologist. Patients sent recorded blood glucose and daily exercise and diet information to the endocrinologist every 1 to 3 days through the WeChat app (application) during the study period. The endocrinologist then gives patients timely lifestyle advice, including diet, exercise instructions and insulin dose adjustments, based on blood glucose control targets during pregnancy. According to American Diabetes Association, blood glucose targets during pregnancy: FPG or pre-prandial blood glucose ≤ 5.3mmol/L, 1h post-prandial ≤ 7.8mmol/L, 2h post-prandial ≤ 6.7mmol/L.
2.2 Hypoglycemic treatment guidance
The dietary principle is the principle of low-glycemic load. Maintain weight gain within a reasonable range through a low-glycemic load diet to avoid hypoglycemia, hyperglycemia and diabetic ketosis. Individual nutrient intake includes: 1) protein: ensure adequate intake of high-quality protein, such as eggs, skim milk, fish and shrimp, beef, mutton, pork, tofu, skinless poultry, etc., which are conducive to the growth and development of the fetus. 2) fat: a high-fat diet is challenging to digest and increases the burden of insulin; limit the intake of high-fat and high-cholesterol foods. 3) carbohydrates: regular and quantitative, preferably buckwheat, oats, whole wheat, brown rice and other hypoglycemic effects; eat less stuffing, noodles, porridge, etc.; avoid desserts, sweets, drinks and excessive intake of fruits rich in monosaccharides. 4) inorganic salts and vitamins: vegetables, nuts, fruits and lean meat are recommended as sources of vitamins, calcium, magnesium and trace elements.
Reasonable diet combined with personalized exercise can effectively reduce blood glucose. Before instructing pregnant women to exercise, first exclude patients with contraindications for exercise during pregnancy, such as heart disease, threatened premature delivery, low progesterone, threatened abortion, fetal intrauterine growth restriction, placental abnormalities, cervical dysfunction, etc. The recommended way of exercise is aerobic exercise such as walking, exercise time in half an hour to one hour after meal, the duration of activity is about 20–30 minutes, to avoid hypoglycemia caused by excessive activity.
After lifestyle interventions, patients with DIP who still failed to achieve blood glucose control targets were treated with insulin based on lifestyle modification. The first choice for basic insulin is hypodermic injection of insulin detemir before bedtime. Insulin glargine or intermediate-acting insulin (Novolin N) should be used instead in the event of an allergic reaction. In addition, the preferred pre-prandial short-acting insulin is insulin aspart subcutaneously injected 5 minutes before meals, and insulin lispro should be used if allergic. During pregnancy, the insulin dose was modified based on blood glucose control targets.
2.3 Baseline characteristics
Baseline characteristics of DIP patients were collected, including age, gestational age, prepregnancy body mass index (BMI), pregnancy type (primipara or multipara), history of abortion, family history of diabetes, glycosylated hemoglobin (HbA1c), hypoglycemic regimen.
2.4 Assessment of glycemic control
FBG and 2hPBG were collected from patients with DIP before and after individualized strategy. CGM-measurements and glycemic variability parameters included time in range (TIR), time above range (TAR), time below range (TBR), average glucose (AG), estimated HbA1c, standard deviation of blood glucose (SDBG), mean amplitude of glucose excursions (MAGE) and coefficient of variation (CV) were also collected.
2.5 Assessment of anxiety, depression and quality of life
Anxiety, depression, pregnancy-related anxiety and diabetes specific quality of life scales were assessed in patients before and after individualized strategy respectively. In this study, patients’ anxiety, depression and quality of life were evaluated by applying self-rating anxiety scale (SAS) (17), self-rating depression scale (SDS) (18), pregnancy-related anxiety questionnaire (PAQ) (19), diabetes specific quality of life scale (DSQL) (20). These scales have been transformed into Chinese versions and are widely used in China with good reliability and validity (21–23). There are 20 items in SAS and SDS, respectively. SAS standard points ≥ 50 were anxiety and SDS standard points ≥ 53 were depression, according to Chinese norms. The PAQ scale, compiled by Chinese scholars, has a total of 13 items, including three aspects of pregnant women worried about fetal health, delivery process and self-care. The total score ≥ 24 was pregnancy-related anxiety, with a higher total score indicating a higher level of pregnancy-related anxiety. The DSQL scale evaluated the quality of life of patients with DIP from physical, psychological, social relations and treatment dimensions, a total of 27 items, with a lower total score indicating a higher level of quality of life.
2.6 Statistical analysis
Statistical analysis was performed using Statistical Package of Social Sciences (SPSS) 26. Data normality was evaluated before using parametric tests. Data with normal distribution were expressed by mean ± standard deviation (± s), and data with non-normal distribution were expressed by medians, and count data were expressed by [n (%)]. T-test was used for continuous variables to compare the difference between the two groups, chi-square test and Fisher’s exact test were used for categorical variables to compare the difference between the two groups. Within-group differences were compared with paired t-test. Pearson correlation analysis was used to analyze the correlation between scale score and other data. All the tests were performed by two-sided test, with a P value<0.05 as the statistical difference evaluation standard.
3 Results
A total of 80 eligible women completed study, including 40 women in the CGM group and 40 women in the SMBG group. Among the 80 participants, 32 had PGDM (all with T2DM), while 48 had GDM.
3.1 Baseline characteristics
3.1.1 Comparison of baseline characteristics between the CGM group and the SMBG group
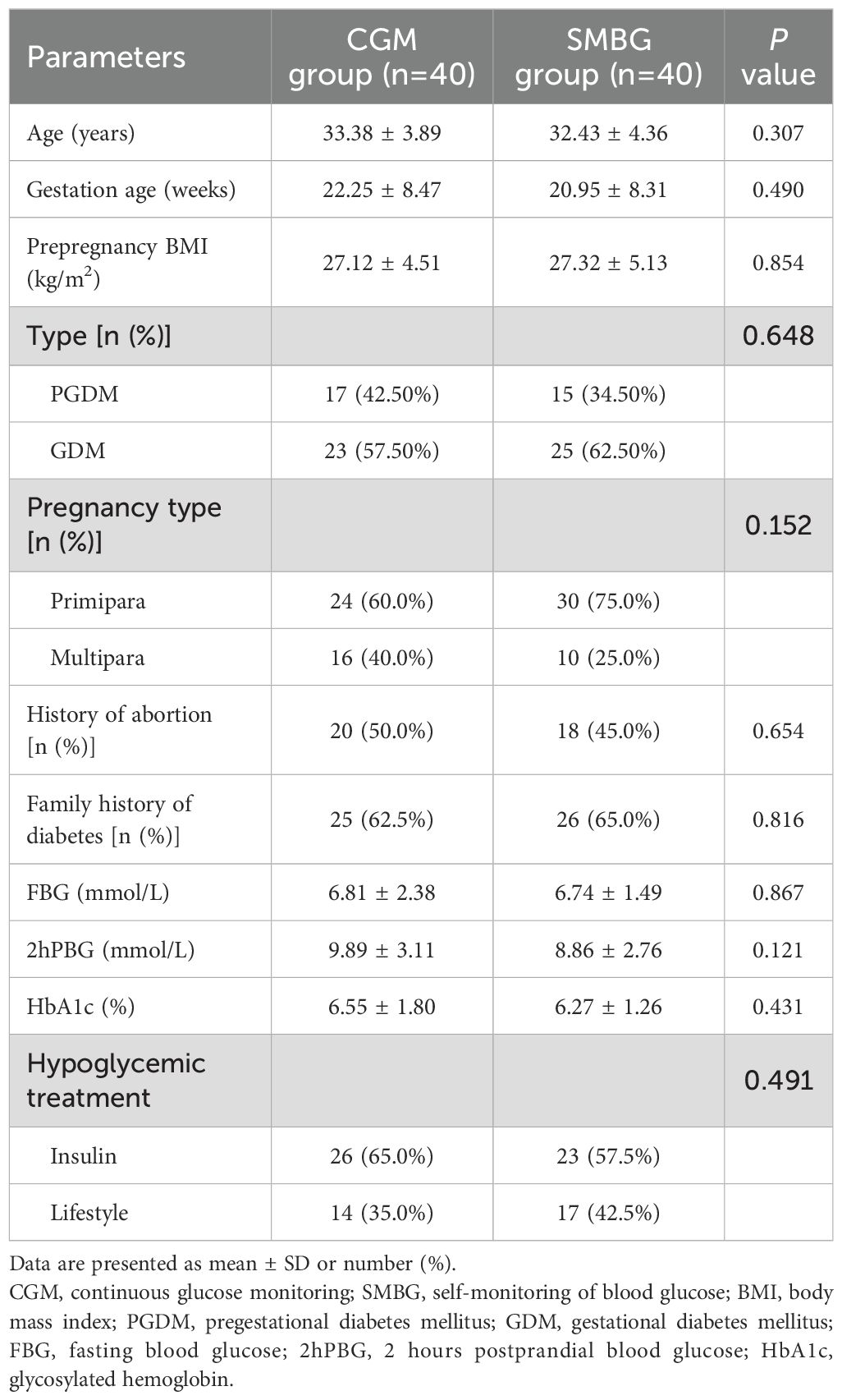
The mean age of DIP patients was 32.90 ± 4.13 years old, and there were 27 (33.75%) patients ≥ 35 years old. The mean pre-pregnancy BMI of the patients was 27.22 ± 4.80kg/m2, and there were 49 patients (61.3%) with pre-pregnancy BMI>25kg/m2. There were 51 patients (63.8%) with a family history of diabetes. There were no statistically significant differences between the CGM group and the SMBG group in age, gestational age, pre-pregnancy BMI, type of DIP, pregnancy type, proportion of abortion history, proportion of family history of diabetes, FBG, 2hPBG, hypoglycemic treatment and HbA1c (P>0.05) (Table 1). There was no difference in baseline between the two groups, indicating comparability of data between the two groups.
3.1.2 Comparison of baseline characteristics between PGDM patients and GDM patients
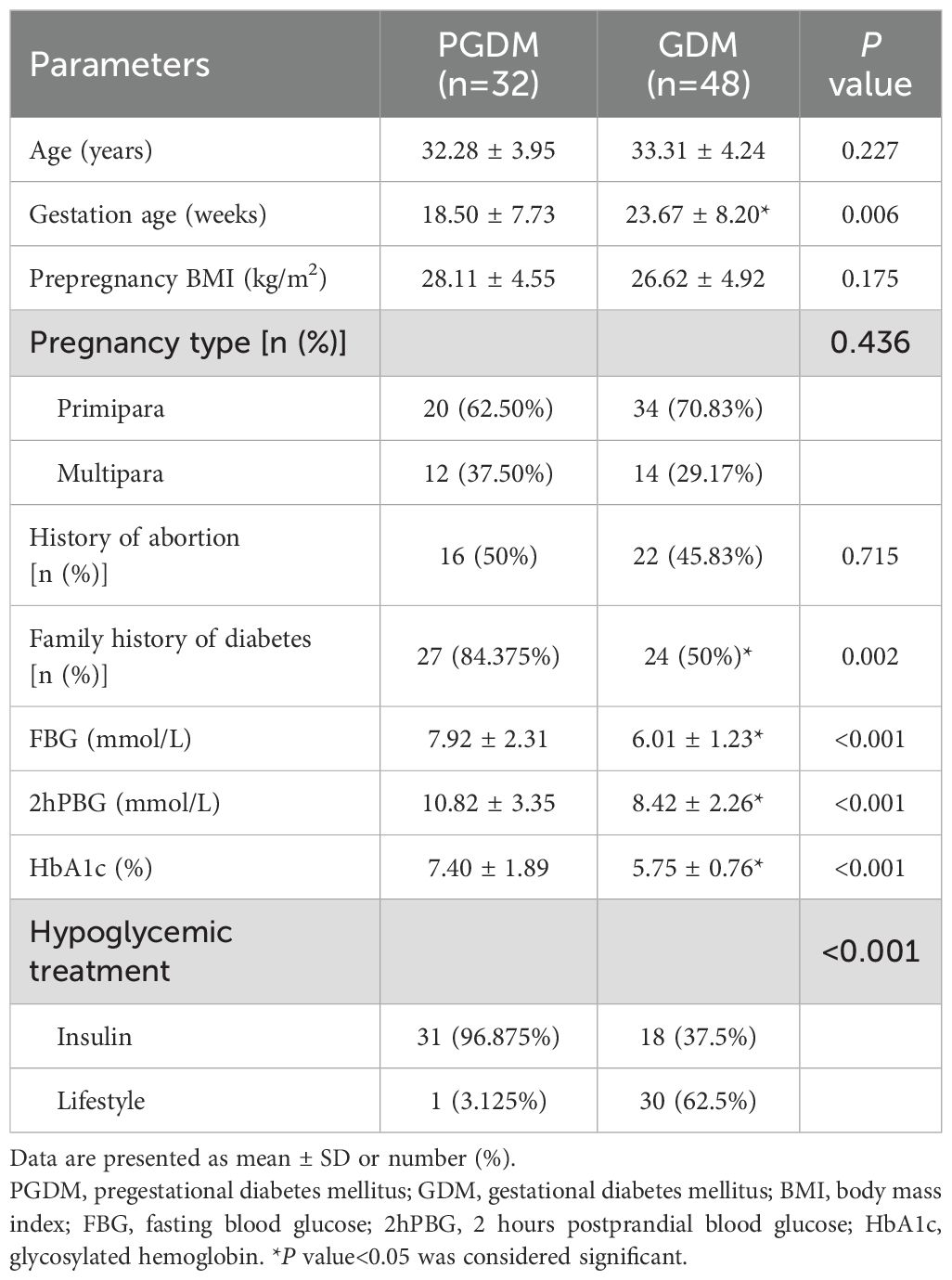
There were 32 patients (40.0%) with PGDM and 48 patients (60.0%) with GDM. There were no significant differences in age, pre-pregnancy BMI, pregnancy type and proportion of abortion history between PGDM patients and GDM patients (P>0.05). The gestational age of PGDM patients was smaller than that of GDM patients, while the proportion of family history of diabetes, FBG, 2hPBG, HbA1c and the proportion of insulin used were significantly higher than those of GDM patients, with statistical significance (P<0.05) (Table 2). This indicated that PGDM presents more significant blood glucose fluctuations and more severe hyperglycemia compared to GDM.
3.2 Glycemic parameters
3.2.1 Comparison of glycemic parameters before and after individualized strategy
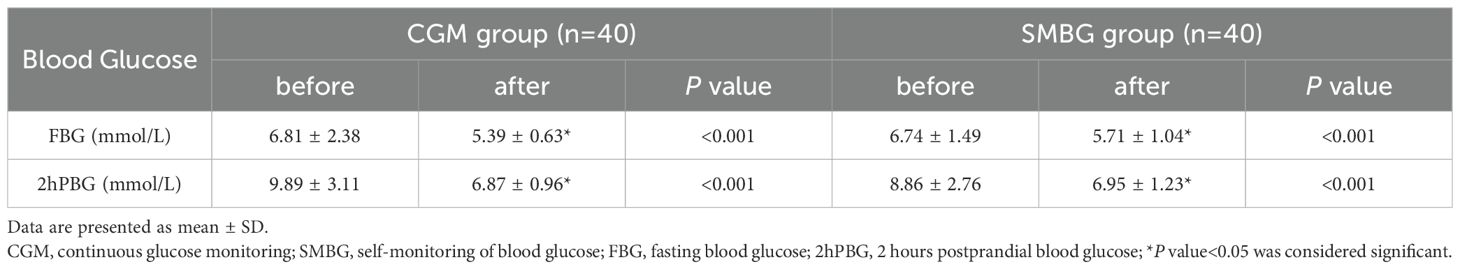
FBG and 2hPBG of the two groups after individualized strategy by different blood glucose monitoring methods (CGM or SMBG) were significantly lower than those before individualized strategy (P<0.001) (Table 3), indicating that individualized strategy exerted a positive effect on glycemic improvement in the short term.
3.2.2 Glycemic variability parameters
Compared with GDM patients, glycemic variability parameters calculated by CGM included TAR, AG, estimated HbA1c, SDBG and MAGE of PGDM patients were significantly higher and TBR was significantly lower (P<0.05); there were no significant difference in TIR and CV between PGDM and GDM (P>0.05) (Table 4). PGDM exhibited higher blood glucose than GDM, with significant blood glucose fluctuation and more severe hyperglycemia.
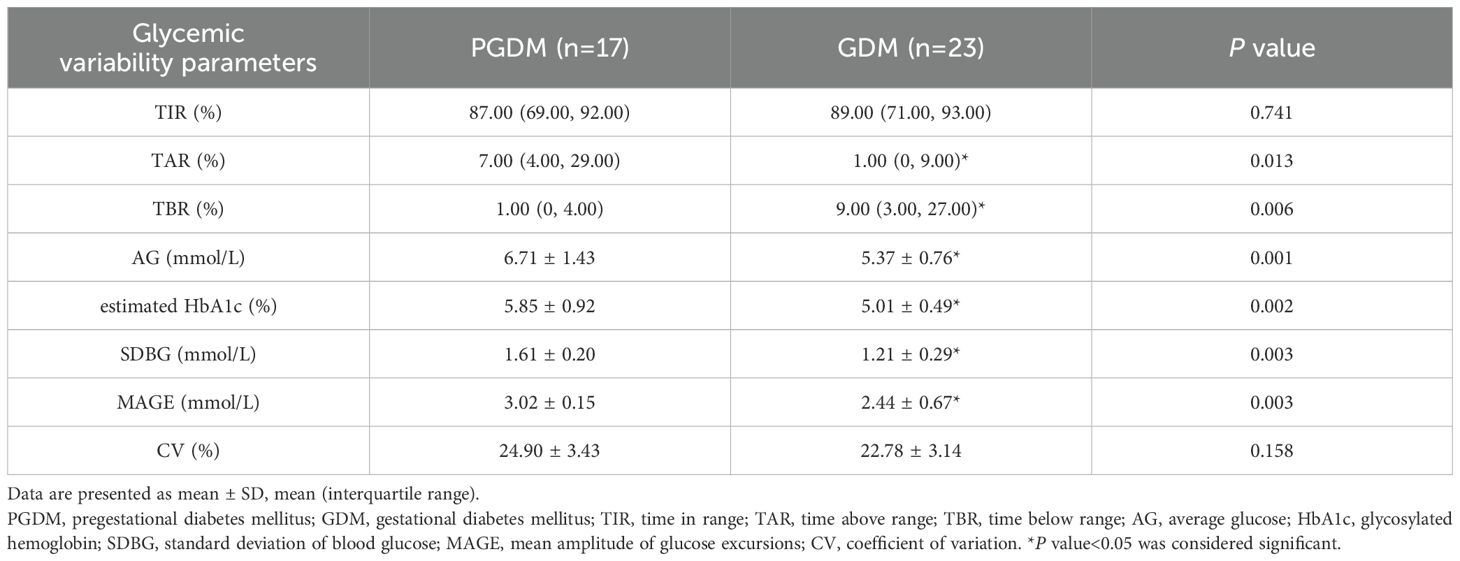
Table 4. Comparison of glycemic variability parameters between PGDM patients and GDM patients in the CGM group.
3.3 The score of SAS, SDS, PAQ and DSQL scales
Among patients with DIP, 7.5% had anxiety, 17.5% had depression, 5.0% had anxiety and depression, and 45.0% had pregnancy-related anxiety.
3.3.1 Comparison of scale scores between the CGM group and the SMBG group before and after individualized strategy
Before and after individualized strategy, there were no significant differences in SAS, SDS, PAQ and DSQL scores between the CGM group and the SMBG group (P>0.05) (Table 5).
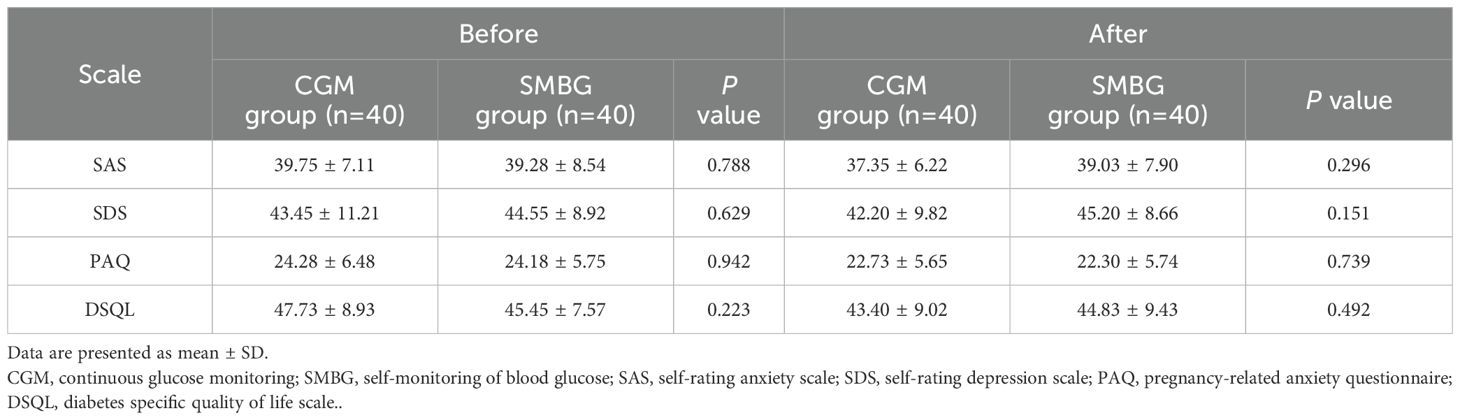
Table 5. Comparison of inter-group scale scores between the CGM group and the SMBG group before and after individualized strategy.
In the CGM group, the scores of SAS, PAQ and DSQL after individualized strategy were significantly lower than those before individualized strategy (P<0.05) (Figure 2); the SDS score were lower than that before individualized strategy, but the difference was not statistically significant (P>0.05) (Table 6). In the SMBG group, the scores of PAQ after individualized strategy were significantly lower than that before individualized strategy (P<0.05); the scores of SAS, SDS and DSQL scales after individualized strategy had no statistical difference compared with those before individualized strategy (P>0.05) (Table 6), indicating that CGM is superior to SMBG in improving anxiety and quality of life.
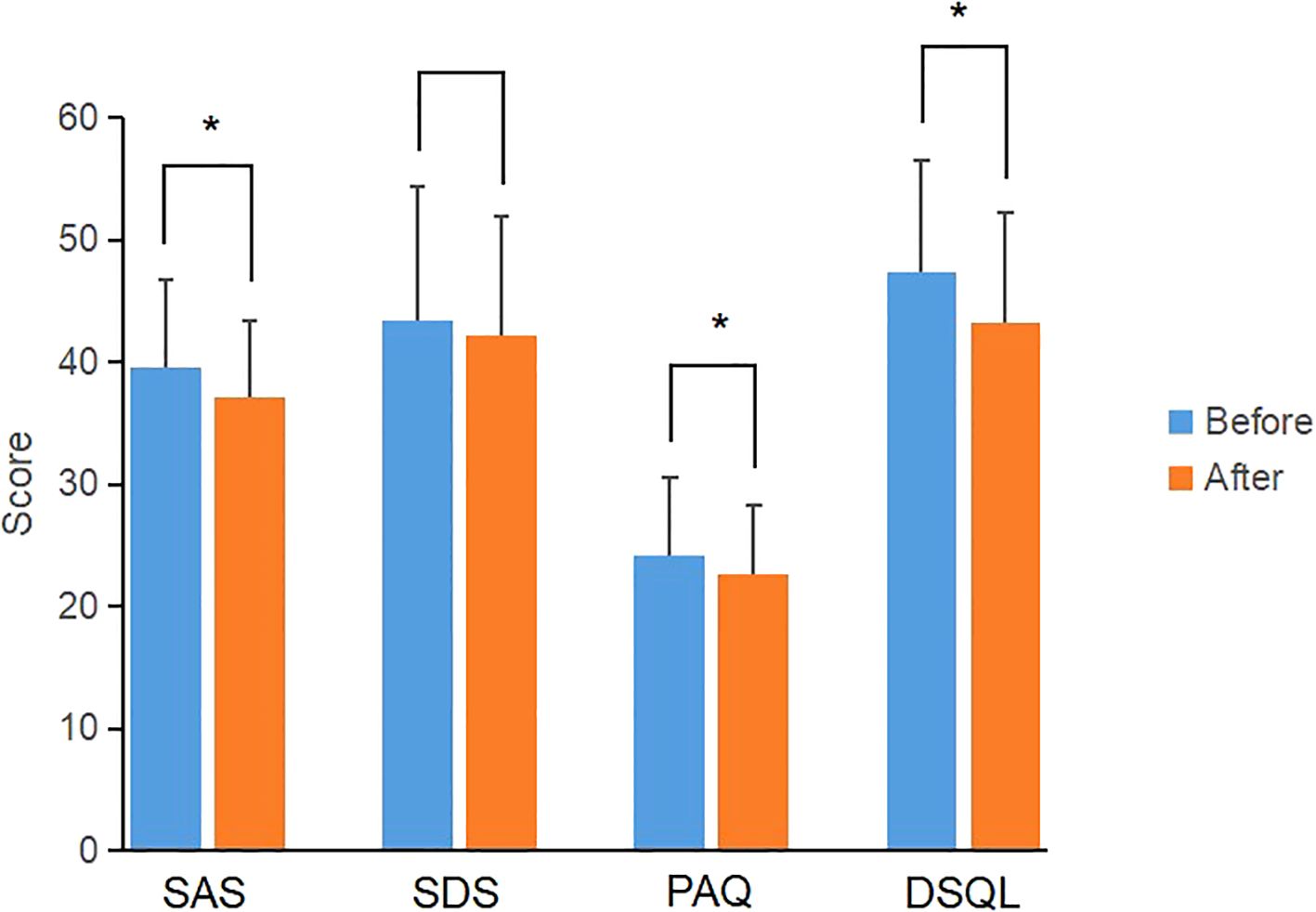
Figure 2. Comparison of scale scores of the CGM group before and after individualized strategy. *P value<0.05 was considered significant.
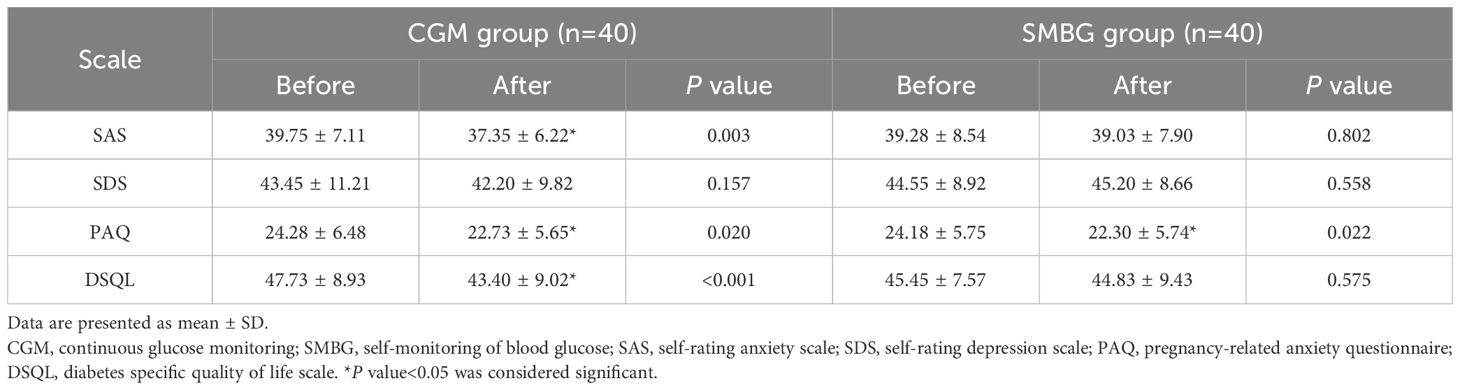
Table 6. Comparison of within-group scale scores between the CGM group and the SMBG group before and after individualized strategy.
3.3.2 Comparison of scale scores between PGDM patients and GDM patients before and after individualized strategy
In the CGM or SMBG group, there were no statistical differences in SAS, SDS, PAQ and DSQL scale scores between PGDM patients and GDM patients in before and after individualized strategy (P>0.05) (Table 7).
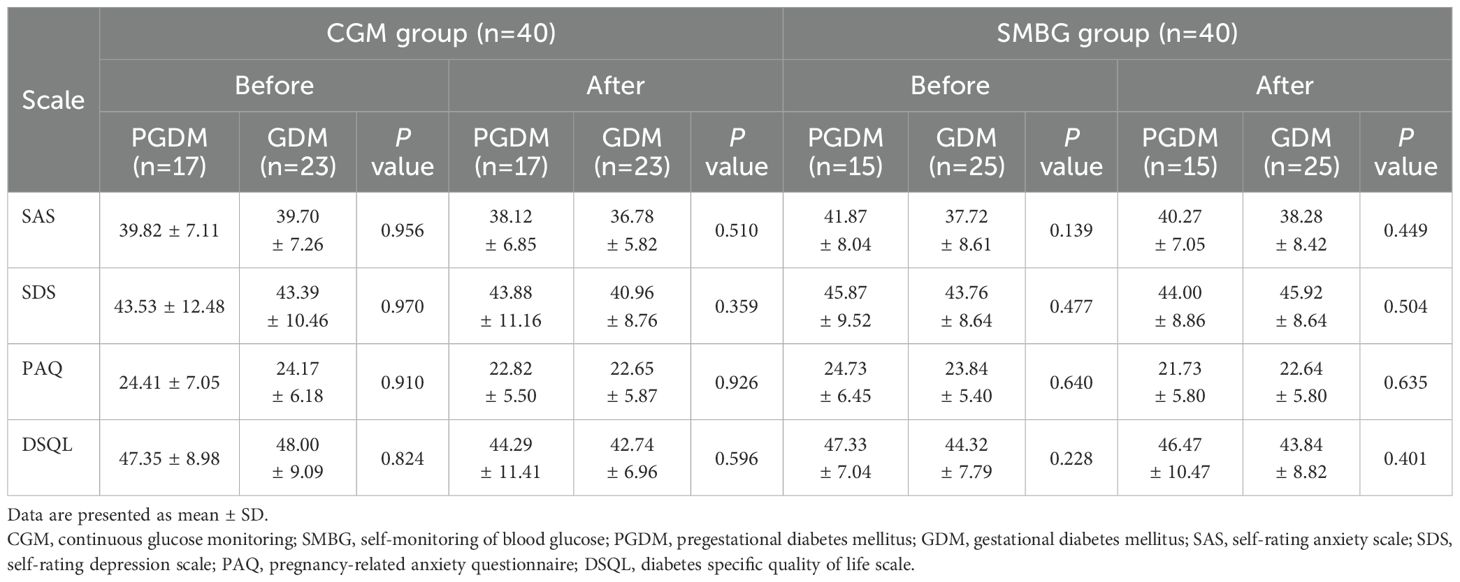
Table 7. Comparison of inter-group scale scores between PGDM patients and GDM patients before and after individualized strategy.
In the CGM group, the scores of SAS, SDS, PAQ and DSQL in PGDM patients after individualized strategy were not significantly different from those before individualized strategy, while the scores of SAS and DSQL in GDM patients were significantly lower than those before individualized strategy (P<0.05) (Figure 3); the scores of SDS and PAQ were lower than those before individualized strategy, but there was no significant difference (P>0.05) (Table 8).
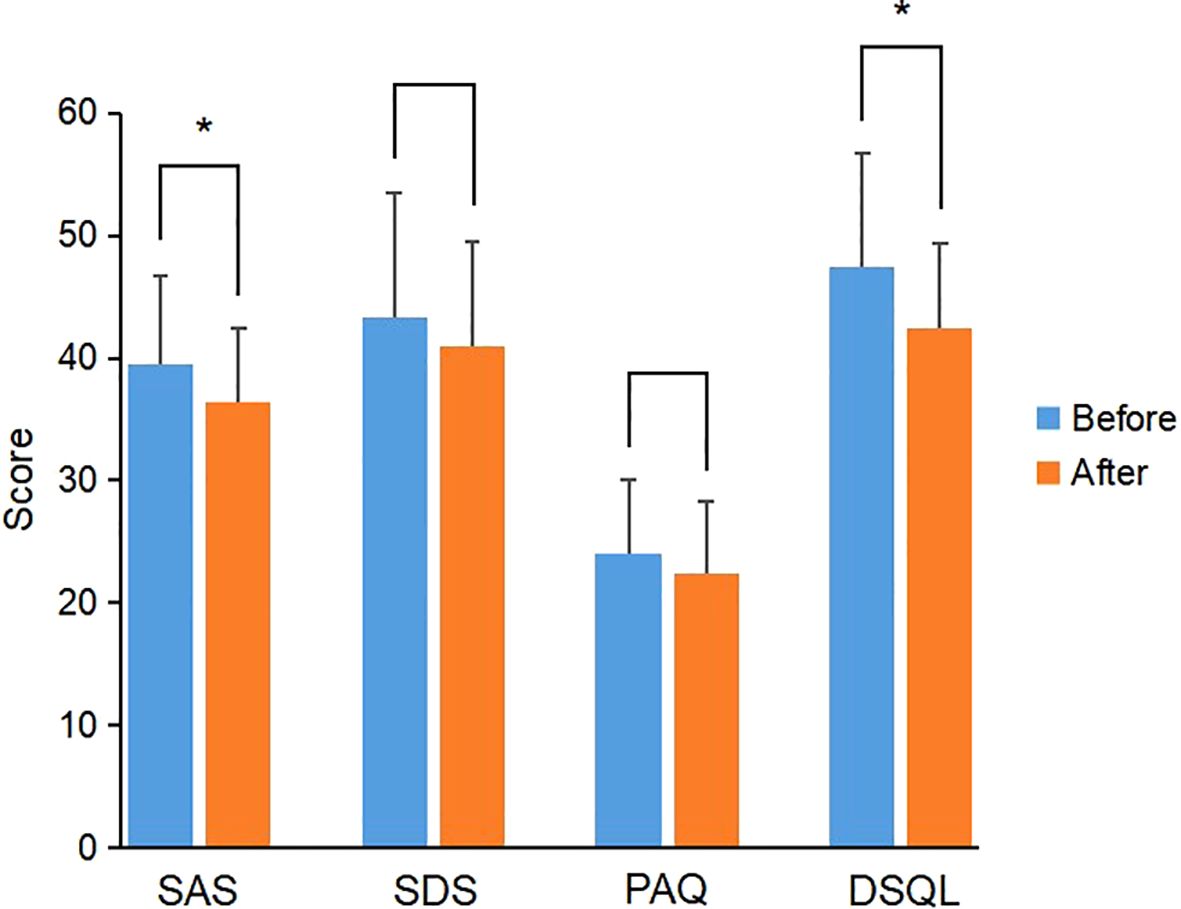
Figure 3. Comparison of scale scores of GDM patients before and after individualized strategy in the CGM group. *P value<0.05 was considered significant..
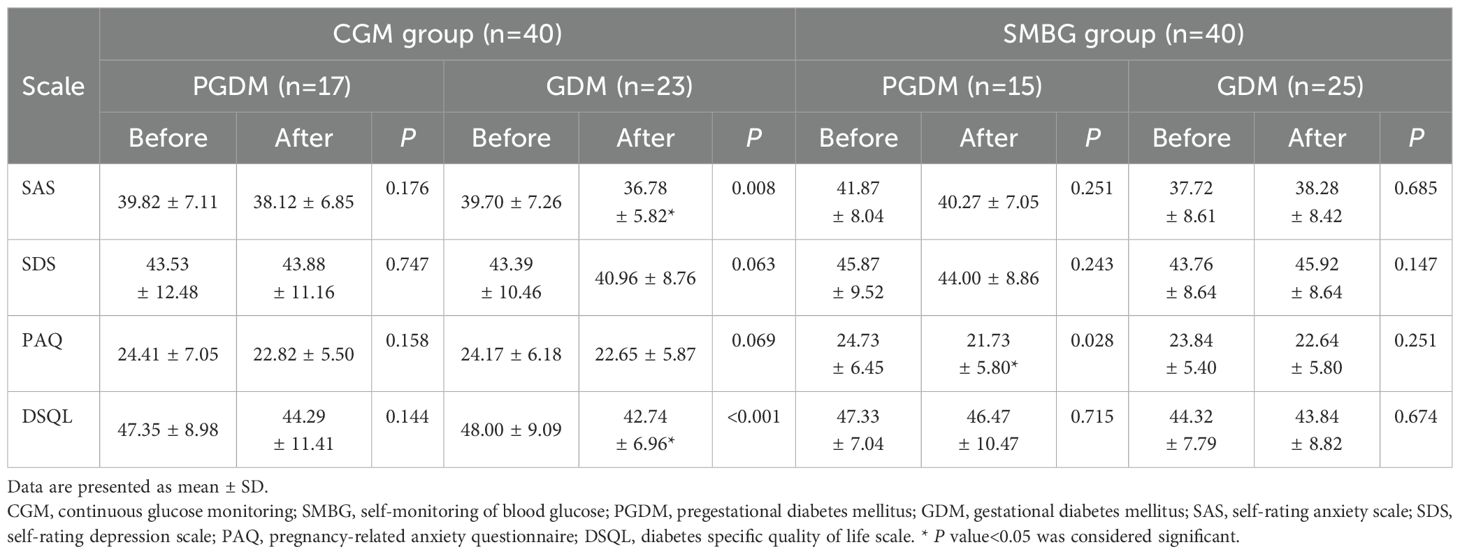
Table 8. Comparison of within-group scale scores between PGDM patients and GDM patients before and after individualized strategy.
In the SMBG group, the scores of PAQ in patients with PGDM were significantly lower than those before individualized strategy (P<0.05), while the scores of SAS, SDS, PAQ and DSQL in GDM patients after individualized strategy were not significantly different from those before individualized strategy (P>0.05) (Table 8). In addition, the degree of blood glucose elevation and fluctuation in patients with GDM is less than that in PGDM.
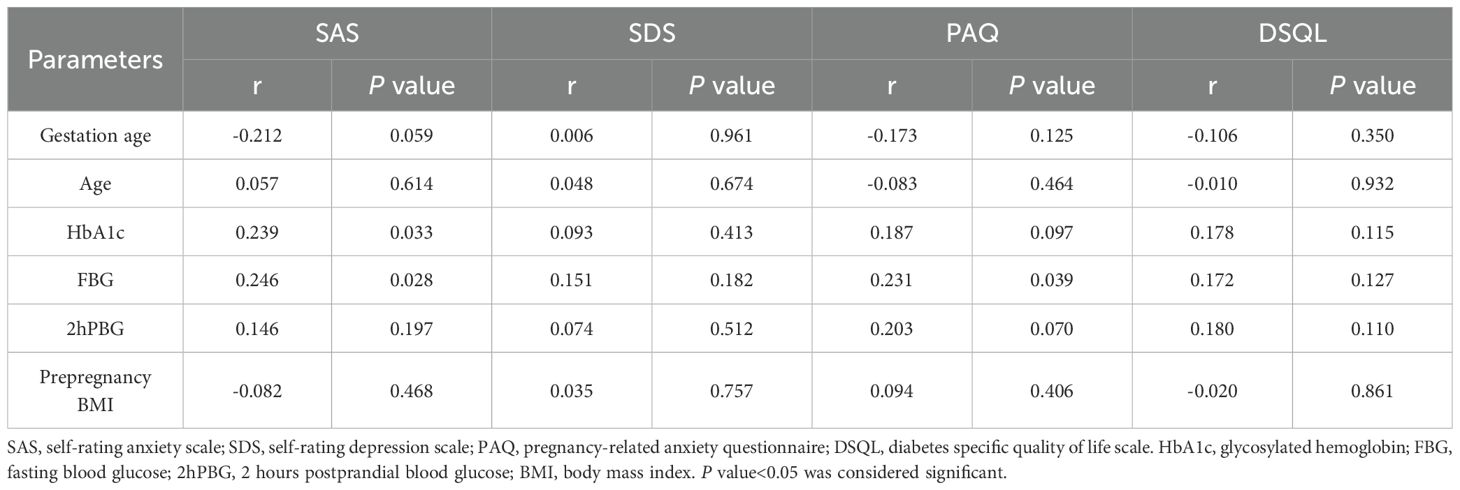
3.3.3 Correlation analysis between SAS, SDS, PAQ, DSQL scale scores and other parameters before individualized strategy
The score of SAS scale was positively correlated with HbA1c, FBG in patients with DIP, and the score of PAQ scale was positively correlated with FBG, indicating that patients with higher blood glucose level tend to have higher anxiety scores. The scores of SDS and DSQL were not significantly correlated with gestational age, age, HbA1c, FBG, 2hPBG and prepregnancy BMI (Table 9).
4 Discussion
Due to physiological factors such as hormone fluctuations, as well as increased sensitivity to family, social and other factors, pregnant women are prone to adverse emotions such as anxiety and depression. In this study, the probabilities of anxiety, depression, anxiety and depression in DIP patients were 7.5%, 17.5% and 5.0%, respectively, which was similar to the probabilities of anxiety, depression, anxiety and depression in early pregnancy found by Tang et al. (7.7%, 10.5%, 4.8%) (24). But Other studies have shown that about 12.0% of pregnant women experience depression and up to 22.0% experience anxiety in late pregnancy (25, 26). Studies reported that the prevalence of maternal depression and anxiety was as high as 27.0% and 24.0%, respectively (27, 28). We emphasize the importance of routine psychological assessment and intervention in the management of DIP.
In addition, Compared with normal GDM pregnant women, GDM pregnant women with anxiety and depression are more prone to adverse outcomes in terms of blood glucose, delivery mode and maternal and infant outcomes during pregnancy (13). A study showed a significant increase in anxiety and depression symptoms among pregnant women during the COVID-19 pandemic, which could have long-term effects on their offspring (59). According to statistics, the probability of depression in patients with DM was 3 times higher than that in healthy people, and the incidence of depression in patients with T1DM was as high as 12.0% (29). Patients with T2DM had a high incidence of anxiety and depression, and patients with adverse emotions had poor compliance, which was detrimental to disease management (60).
Studies have shown that anxiety and depression may be risk factors for GDM (30, 31), but there is no unified conclusion on the correlation between anxiety, depression and GDM at present. Anxiety and depression can lead to hormone imbalance in the body, which seriously affects pregnancy outcomes and blood glucose control of GDM. In addition to physiological factors, psychological factors such as anxiety and depression are also important causes of GDM (32, 33). Anxiety and depression can lead to chronic hypothalamic-pituitary-adrenal (HPA) axis hyperfunction, resulting in increased cortisol release and IR (34), increasing the risk of GDM in pregnant women. At the same time, GDM increases the susceptibility of pregnant women to anxiety and depression, and the likelihood of prenatal or postpartum depression is 2–4 times higher than that without GDM (35–38), which may be related to their awareness of poor blood glucose control and pregnancy complications and adverse pregnancy outcomes (39, 40). However, some studies suggest that anxiety and depression do not increase the probability of GDM in pregnant women (41–44), nor does GDM increase the risk of prenatal or postpartum depression (45, 46). With the implementation of the three-child policy, it is urgent to pay attention to the psychological status of pregnancy and avoid adverse pregnancy outcomes under the guidance of demand. In addition, pregnancy-related anxiety refers to a kind of anxiety and painful emotional experience caused by pregnancy to pregnant women (47). Feng et al. found that pregnancy-related anxiety accounted for 59.1% of GDM patients (48). In this study, the incidence of pregnancy-related anxiety in patients with DIP was 45%, which was higher than that of 31% of normal pregnant women at mid-pregnancy and 29% at late-pregnancy (49).
One study identified three sources of anxiety and depression in patients with GDM: the diagnosis of GDM and perceptions of high-risk pregnancies; glycemic control during dietary intervention; the fear of maternal and infant complications. The study identified the fear of pregnancy complications as the most significant source of stress for GDM. In addition, pregnant women who received insulin treatment were more stressed than those who received dietary intervention only (45). This is consistent with the recent study of Lee et al. (50), which exacerbates patients’ concerns about treatment because of the relationship between insulin and hypoglycemia events. Horsch et al. believed that anxiety was related to FBG (3), which was consistent with the findings of this study that anxiety was positively correlated with HbA1c, FBG, and pregnancy-related anxiety was positively correlated with FBG and 2hPBG. Through the analysis of the three aspects of worrying about fetal health, delivery process and self-care contained in the PAQ scale, it was found that the pregnancy-related anxiety of DIP patients mainly originated from worrying about the physical health of the fetus.
Clinical application of CGM can reduce the risk of hypoglycemia and hyperglycemia as well as blood glucose variability and improve the quality of life of patients (51). CGM contributed to a significant improvement in diabetes specific quality of life in T1DM adults (52). However, one study suggested that the use of well-standardized, structured SMBG could reduce depressive symptoms in a large number of moderately depressed or distressed T2DM patients with poor glycemic control (53). There is no study to observe the effects of individualized strategy through CGM on anxiety, depression and quality of life in DIP patients. This study found that scores on the SAS and PAQ scales were positively correlated with blood glucose parameters, suggesting that effective glycemic control may play a crucial role in mitigating psychological distress in DIP patients. Additionally, this study verified that an individualized strategy combined with CGM can improve anxiety, pregnancy-related anxiety, and diabetes-specific quality of life. The reasons considered are mainly that CGM is easy to monitor blood glucose in patients, which can quickly and painlessly obtain blood glucose value, predict the trend of glucose change, timely detect occult blood glucose abnormalities (hyperglycemia or hypoglycemia), adjust lifestyle and hypoglycemic treatment and optimize treatment effects. Therefore, CGM shows advantages over traditional SMBG in improving anxiety and quality of life. However, no significant improvement in depression was found for the following reasons: the results of this study did not find any correlation between SDS scale scores and blood glucose parameters; there were many factors considering the causes of depression in DIP; the duration of monitoring blood glucose by CGM was short (14 days), and the effect of improving patients’ depression was limited in a short time. In addition, we also found that individualized strategy with CGM played a more significant role in improving anxiety and quality of life in patients with GDM compared with patients with PGDM, probably because most patients with PGDM had taken lifestyle intervention combined with oral drugs or insulin before pregnancy and had a certain degree of understanding of the disease. In addition, the degree of blood glucose elevation and blood glucose fluctuation in GDM patients was less than that in PGDM, so that the results may be better after individualized strategy. Hence, they had a higher acceptance of the disease than patients with GDM and could accept hypoglycemic treatment psychologically, which can improve the anxiety and quality of life of patients to some extent. However, we did not find any improvement in pregnancy-related anxiety in GDM patients in the CGM group, and we hypothesized that this improvement might be supported by larger sample size. Besides, we also indicated improvement in pregnancy-related anxiety in PGDM patients in the SMBG group, as PAQ scores correlated with glycemic parameters. In summary, blood glucose levels are related to the mental health of pregnant women, and good control of blood glucose can improve mental status.
The potential mechanisms underlying the effect of CGM on mental health could be expanded through hypothetical pathways. The proposed dual-pathway model integrates “physiological feedback”, “psychosocial mediators” and bidirectional feedback loops. 1) Physiological Feedback a. Glycemic Stability and Stress Response: The hypothalamic-pituitary-adrenal axis is thought to play a vital role in glucose homeostasis and diabetes. Stress reduces glucose and total cholesterol (TC) levels in female rats under the same behavioral tests (54). While human studies exhibit lower TIR with higher serum cortisol (P< 0.001) in T2DM patients (55). b. Neurotransmitter Modulation: CGM-driven hypoglycemia prevention preserves tryptophan availability for serotonin synthesis. Compared with the TIR-H (TIR > 70%) group, the TIR-L (TIR< 50%) group exhibits lower serum levels of 5-hydroxy-L-tryptophan and more (56). c. Psychosocial Mediators: CGM can enhance precise monitoring of diabetes symptoms associated with dysglycemia, diabetes-related complications, and mental conditions within the realm of precision medicine (57). 2) Pychosocial Mediators a. Self-Efficacy and Cognitive Liberation: CGM empowers patients to predict glycemic trends, reducing “decision fatigue” from frequent self-monitoring and enhancing confidence in “daily activities”. b. Anxiety Mitigation: Animals that have previously experienced recurrent hypoglycemia exhibit an increase in norepinephrine levels in the amygdala during hypoglycemia, accompanied by increased anxiety (58). 3) Bidirectional Feedback Loops: Emerging models suggest a virtuous cycle, CGM-enhanced glycemic control → improved mood → better adherence → sustained glycemic benefits (Figure 4).
The innovation of this study is that it is the first to explore the positive significance of individualized strategy combined with CGM on anxiety and diabetes specific quality of life in patients with DIP. The limitations of this study include: 1) This trial was a single-center study; 2) The sample size of this study was small, and the analysis of risk factors for anxiety and depression is limited; 3) The duration of the study was only 14 days to meet the clinical requirements of smoothly lowering blood glucose in the short term, and the improvement of blood glucose and partial psychological status was observed. However, if the individualized strategy combined with CGM was longer, its effect on the improvement of psychological status and quality of life might be more obvious, and its clinical significance on the physical and mental regulation of patients would be more significant.
Although this study has confirmed the short-term psychological benefits of CGM, several unresolved issues persist. Future research should focus on the following areas.
1) Extend the follow-up period to assess the persistence of psychological benefits. Future research should include long-term longitudinal studies (such as/e.g. 1–3 years postpartum) to determine whether the psychological protective effects of CGM are enduring and to explore whether they reduce the risk of postpartum depression; 2) Explore the Impact of CGM on Different Subgroups of DIG. It may impose a different psychological burden compared to GDM and PGDM (such as/e.g,T1DM or T2DM). Future studies should stratify the analysis of the differential impact of CGM on these subgroups and assess whether psychological support strategy need to be tailored accordingly; 3) Combine digital psychological intervention and optimize the clinical utility of CGM. Real-time data can be integrated with mobile health technology, such as developing an AI-based emotional warning system that provides immediate psychological counseling when abnormal blood glucose fluctuations are detected, or recommends relaxation training, thus forming a “blood glucose-psychological” dual management model; 4) Focus on the clinical significance of CGM beyond blood glucose control. Currently, the assessment of CGM primarily concentrates on metabolic indicators, including HbA1c, TIR, etc. Moving forward, a broader range of psychosocial indicators should be incorporated to comprehensively evaluate the clinical value of CGM.
In conclusion, the combination of individualized strategy and regular blood glucose monitoring (CGM or SMBG) enables DIP patients to achieve better blood glucose control in the short term and avoid the effects of hyperglycemia on the fetus and pregnant woman. As for the management of gestational diabetes, it is crucial to pay attention to the patient’s mental health along with the patient’s blood glucose level. CGM appears to be an effective tool for glycemic control and may contribute to improved mental health in DIP patients. A multidisciplinary approach, integrating endocrinology, obstetrics, and mental health support, is essential for optimizing DIP management. We call on researchers, clinicians, and policymakers to jointly advance the following actions. Incorporate mental health indicators into the clinical assessment system of CGM; Conduct multicenter, long-term follow-up studies to clarify the impact of CGM on postpartum mental states; Develop intelligent management tools that integrate CGM with psychological support to optimize the overall care model for DIP.
5 Conclusion
The individualized strategy combined with short-term glucose monitoring can positively impact glycemic improvement in the short term and meet the requirements of glycemic control in pregnancy, which has important clinical significance. The combined use of individualized strategy and CGM improves glycemic control and may have protective effects on psychological well-being.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was given by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University with the following reference number: PJ-KS-KY-2022-113(X). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ML: Investigation, Methodology, Writing – review & editing. TC: Investigation, Methodology, Writing – original draft. SW: Supervision, Writing – review & editing. DL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by Scientific Research Fund of Liaoning Provincial Education Department (JYTMS20230576).
Acknowledgments
We would like to thank all participants for their willingness to cooperate throughout the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1470473/full#supplementary-material
References
1. Practice bulletin no. 180: gestational diabetes mellitus. Obstet Gynecol. (2017) 130:e17–37. doi: 10.1097/AOG.0000000000002159
2. Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care. (2013) 36:586–90. doi: 10.2337/dc12-1157
3. Rosenstein MG, Cheng YW, Snowden JM, Nicholson JM, Doss AE, and Caughey AB. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol. (2012) 206:309 e1–7. doi: 10.1016/j.ajog.2012.01.014
4. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. (2000) 49:2208–11. doi: 10.2337/diabetes.49.12.2208
5. England LJ, Dietz PM, Njoroge T, Callaghan WM, Bruce C, Buus RM, et al. Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Am J Obstet Gynecol. (2009) 200:365 e1–8. doi: 10.1016/j.ajog.2008.06.031
6. Consonni EB, Calderon IM, Consonni M, De Conti MH, Prevedel TTs, and Rudge MV. A multidisciplinary program of preparation for childbirth and motherhood: maternal anxiety and perinatal outcomes. Reprod Health. (2010) 29:728. doi: 10.1186/1742-4755-7-28
7. Biaggi A, Conroy S, Pawlby S, and Pariante CM. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J Affect Disord. (2016) 191:62–77. doi: 10.1016/j.jad.2015.11.014
8. Coll CVN, da Silveira MF, Bassani DG, Netsi E, Wehrmeister FC, Barros FC, et al. Antenatal depressive symptoms among pregnant women: Evidence from a Southern Brazilian population-based cohort study. J Affect Disord. (2017) 209:140–6. doi: 10.1016/j.jad.2016.11.031
9. Ghaffar R, Iqbal Q, Khalid A, Saleem F, Hassali MA, Baloch NS, et al. Frequency and predictors of anxiety and depression among pregnant women attending tertiary healthcare institutes of Quetta City, Pakistan. BMC Womens Health. (2017) 17:51. doi: 10.1186/s12905-017-0411-1
10. Ravid E, Salzer L, Arnon L, Eisner M, Wiznitzer A, Weller A, et al. Is there an association between maternal anxiety propensity and pregnancy outcomes? BMC Pregnancy Childbirth. (2018) 18:287. doi: 10.1186/s12884-018-1925-8
11. Berge LI and Riise T. Comorbidity between type 2 diabetes and depression in the adult population: directions of the association and its possible pathophysiological mechanisms. Int J Endocrinol. (2015) 2015:164760. doi: 10.1155/2015/164760
12. Varner M. Improving the effectiveness of lifestyle interventions for gestational diabetes mellitus prevention (what would Janus do)? BJOG. (2019) 126:321. doi: 10.1111/1471-0528.15489
13. OuYang H, Chen B, Abdulrahman AM, Li L, and Wu N. Associations between gestational diabetes and anxiety or depression: A systematic review. J Diabetes Res. (2021) 2021:9959779. doi: 10.1155/2021/9959779
14. Berle JØ, Mykletun A, Daltveit AK, Rasmussen S, Holsten F, and Dahl AA. Neonatal outcomes in offspring of women with anxiety and depression during pregnancy. A linkage study from The Nord-Trondelag Health Study (HUNT) and Medical Birth Registry of Norway. Arch Womens Ment Health. (2005) 8:181–9. doi: 10.1007/s00737-005-0090-z
15. Graignic-Philippe R, Dayan J, Chokron S, Jacquet AY, and Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev. (2014) 43:137–62. doi: 10.1016/j.neubiorev.2014.03.022
16. Hanley GE and Oberlander TF. Neurodevelopmental outcomes following prenatal exposure to serotonin reuptake inhibitor antidepressants: a “social teratogen” or moderator of developmental risk? Birth Defects Res A Clin Mol Teratol. (2012) 94:651–9. doi: 10.1002/bdra.23032
17. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
18. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
19. Zhu J, Li Y, Wang R, Zhang J, Liu C, Li H, et al. The mediating role of family functions between pregnancy-related anxiety and sleep quality: A cross-sectional study. Nat Sci Sleep. (2024) 16:279–89. doi: 10.2147/NSS.S443612
20. Yu Y, Feng L, Shao Y, Tu P, Wu HP, Ding X, et al. Quality of life and emotional change for middle-aged and elderly patients with diabetic retinopathy. Int J Ophthalmol. (2013) 6:71–4. doi: 10.3980/j.issn.2222-3959.2013.01.15
21. Zhang P, Lou P, Chang G, Chen P, Zhang L, Li T, et al. Combined effects of sleep quality and depression on quality of life in patients with type 2 diabetes. BMC Fam Pract. (2016) 17:40. doi: 10.1186/s12875-016-0435-x
22. Tao M and Gao J. Reliability and validity of revised self rating Anxiety Scale. Chin J Nerv Ment Dis. (1994) 20(5):3. doi: CNKI:SUN:ZSJJ.0.1994-05-022
23. Lee HC, Chiu HF, Wing YK, Leung CM, Kwong PK, and Chung DW. The Zung Self-rating Depression Scale: screening for depression among the Hong Kong Chinese elderly. J Geriatr Psychiatry Neurol. (1994) 7:216–20. doi: 10.1177/089198879400700404
24. Tang Y, Lan X, Zhang Y, Zhou F, Cai C, Zhang J, et al. Anxiety and depression on gestational diabetes mellitus in early pregnancy. Wei Sheng Yan Jiu. (2020) 49:179–84. doi: 10.19813/j.cnki.weishengyanjiu.2020.02.002
25. Palladino CL, Singh V, Campbell J, Flynn H, and Gold KJ. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. (2011) 118:1056–63. doi: 10.1097/AOG.0b013e31823294da
26. Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, and Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. (2017) 219:86–92. doi: 10.1016/j.jad.2017.05.003
27. Ecklund-Flores L, Myers MM, Monk C, Perez A, Odendaal HJ, and Fifer WP. Maternal depression during pregnancy is associated with increased birth weight in term infants. Dev Psychobiol. (2017) 59:314–23. doi: 10.1002/dev.21496
28. Alqahtani AH, Al Khedair K, Al-Jeheiman R, Al-Turki HA, and Al Qahtani NH. Anxiety and depression during pregnancy in women attending clinics in a University Hospital in Eastern province of Saudi Arabia: prevalence and associated factors. Int J Womens Health. (2018) 10:101–8. doi: 10.2147/IJWH.S153273
29. Roy T and Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. (2012) 142 Suppl:S8–21. doi: 10.1016/S0165-0327(12)70004-6
30. Byrn M and Penckofer S. The relationship between gestational diabetes and antenatal depression. J Obstet Gynecol Neonatal Nurs. (2015) 44:246–55. doi: 10.1111/1552-6909.12554
31. Horsch A, Kang JS, Vial Y, Ehlert U, Borghini A, Marques-Vidal P, et al. Stress exposure and psychological stress responses are related to glucose concentrations during pregnancy. Br J Health Psychol. (2016) 21:712–29. doi: 10.1111/bjhp.12197
32. Bowers K, Laughon SK, Kim S, Mumford SL, Brite J, Kiely MZ, et al. The association between a medical history of depression and gestational diabetes in a large multi-ethnic cohort in the United States. Paediatr Perinat Epidemiol. (2013) 27:323–8. doi: 10.1111/ppe.12057
33. Gilbert L, Gross J, Lanzi S, Quansah DY, Puder J, and Horsch A. How diet, physical activity and psychosocial well-being interact in women with gestational diabetes mellitus: an integrative review. BMC Pregnancy Childbirth. (2019) 19:60. doi: 10.1186/s12884-019-2185-y
34. Robinson DJ, Coons M, Haensel H, Vallis M, and Yale JF. Diabetes and mental health. Can J Diabetes. (2018) 42 Suppl:1S130–S141. doi: 10.1016/j.jcjd.2017.10.031
35. Hjelm K, Berntorp K, Frid A, Åberg A, and Apelqvist J. Beliefs about health and illness in women managed for gestational diabetes in two organisations. Midwifery. (2008) 24:168–82. doi: 10.1016/j.midw.2006.12.008
36. Hirst JE, Tran TS, Do MA, Rowena F, Morris JM, and Jeffery HE. Women with gestational diabetes in Vietnam: a qualitative study to determine attitudes and health behaviours. BMC Pregnancy Childbirth. (2012) 12:81. doi: 10.1186/1471-2393-12-81
37. Hinkle SN, Buck Louis GM, Rawal S, Zhu Y, Albert PS, and Zhang C. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia. (2016) 59:2594–602. doi: 10.1007/s00125-016-4086-1
38. Azami M, Badfar G, Soleymani A, and Rahmati S. The association between gestational diabetes and postpartum depression: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2019) 149:147–55. doi: 10.1016/j.diabres.2019.01.034
39. Kim C, Brawarsky P, Jackson RA, Fuentes-Afflick E, and Haas JS. Changes in health status experienced by women with gestational diabetes and pregnancy-induced hypertensive disorders. J Womens Health (Larchmt). (2005) 14:729–36. doi: 10.1089/jwh.2005.14.729
40. Dalfra MG, Nicolucci A, Bisson T, Bonsembiante B, and Lapolla A. Quality of life in pregnancy and post-partum: a study in diabetic patients. Qual Life Res. (2012) 21:291–8. doi: 10.1007/s11136-011-9940-5
41. Martini J, Knappe S, Beesdo-Baum K, Lieb R, and Wittchen HU. Anxiety disorders before birth and self-perceived distress during pregnancy: associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Hum Dev. (2010) 86:305–10. doi: 10.1016/j.earlhumdev.2010.04.004
42. Bayrampour H, Salmon C, Vinturache A, and Tough S. Effect of depressive and anxiety symptoms during pregnancy on risk of obstetric interventions. J Obstet Gynaecol Res. (2015) 41:1040–8. doi: 10.1111/jog.12683
43. Ghimire U, Papabathini SS, Kawuki J, Obore N, and Musa TH. Depression during pregnancy and the risk of low birth weight, preterm birth and intrauterine growth restriction- an updated meta-analysis. Early Hum Dev. (2021) 152:105243. doi: 10.1016/j.earlhumdev.2020.105243
44. Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. (2006) 68:938–46. doi: 10.1097/01.psy.0000244025.20549.bd
45. Arafa A and Dong JY. Depression and risk of gestational diabetes: A meta-analysis of cohort studies. Diabetes Res Clin Pract. (2019) 156:107826. doi: 10.1016/j.diabres.2019.107826
46. Hui AL, Sevenhuysen G, Harvey D, and Salamon E. Stress and anxiety in women with gestational diabetes during dietary management. Diabetes Educ. (2014) 40:668–77. doi: 10.1177/0145721714535991
47. Blackmore ER, Gustafsson H, Gilchrist M, Wyman C, and G O'Connor T. Pregnancy-related anxiety: Evidence of distinct clinical significance from a prospective longitudinal study. J Affect Disord. (2016) 197:251–8. doi: 10.1016/j.jad.2016.03.008
48. Fu F, Yan P, You S, Mao X, Qiao T, Fu L, et al. The pregnancy-related anxiety characteristics in women with gestational diabetes mellitus: why should we care? BMC Pregnancy Childbirth. (2021) 21:424. doi: 10.1186/s12884-021-03887-2
49. Zhou C, Weng J, Tan F, Wu S, Ma J, Zhang B, et al. Pregnancy-related anxiety among Chinese pregnant women in mid-late pregnancy under the two-child policy and its significant correlates. J Affect Disord. (2020) 276:272–8. doi: 10.1016/j.jad.2020.07.099
50. Lee KW, Ching SM, Hoo FK, Ramachandran V, Chong SC, Tusimin M, et al. Prevalence and factors associated with depressive, anxiety and stress symptoms among women with gestational diabetes mellitus in tertiary care centres in Malaysia: a cross-sectional study. BMC Pregnancy Childbirth. (2019) 19:367. doi: 10.1186/s12884-019-2519-9
51. Rodbard D. Continuous glucose monitoring: A review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. (2017) 19:S25–37. doi: 10.1089/dia.2017.0035
52. Polonsky WH, Hessler D, Ruedy KJ, and Beck RW. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. (2017) 40:736–41. doi: 10.2337/dc17-0133
53. Fisher L, Polonsky W, Parkin CG, Jelsovsky Z, Amstutz L, and Wagner RS. The impact of blood glucose monitoring on depression and distress in insulin-naive patients with type 2 diabetes. Curr Med Res Opin. (2011) 27 Suppl:339–46. doi: 10.1185/03007995.2011.619176
54. da Silva CC, Lazzaretti C, Fontanive T, Dartora DR, Bauereis B, and Gamaro GD. Estrogen-dependent effects on behavior, lipid-profile, and glycemic index of ovariectomized rats subjected to chronic restraint stress. Behav Processes. (2014) 103:327–33. doi: 10.1016/j.beproc.2014.01.022
55. Liang Y, Liang J, Jiang W, Wang W, Yang X, Liu Y, et al. Stronger association between morning serum cortisol level and diurnal time in range in type 2 diabetes? Diabetol Metab Syndr. (2024) 16:290. doi: 10.1186/s13098-024-01515-5
56. Ma L, Liu J, Deng M, Zhou L, Zhang Q, and Xiao X. Metabolomics analysis of serum and urine in type 1 diabetes patients with different time in range derived from continuous glucose monitoring. Diabetol Metab Syndr. (2024) 16:21. doi: 10.1186/s13098-024-01257-4
57. Hermanns N, Ehrmann D, Kulzer B, Klinker L, Haak T, and Schmitt A. Somatic and mental symptoms associated with dysglycaemia, diabetes-related complications and mental conditions in people with diabetes: Assessments in daily life using continuous glucose monitoring and ecological momentary assessment. Diabetes Obes Metab. (2025) 27:61–70. doi: 10.1111/dom.15983
58. McNay E. Recurrent hypoglycemia increases anxiety and amygdala norepinephrine release during subsequent hypoglycemia. Front Endocrinol (Lausanne). (2015) 6:175. doi: 10.3389/fendo.2015.00175
59. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, and Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. (2020) 277:5–13. doi: 10.1016/j.jad.2020.07.126
Keywords: diabetes in pregnancy, gestational diabetes mellitus, continuous glucose monitoring, anxiety, depression, quality of life
Citation: Liu M, Chen T, Wang S, Li N and Liu D (2025) To assess the impact of individualized strategy and continuous glucose monitoring on glycemic control and mental health in pregnant women with diabetes. Front. Endocrinol. 16:1470473. doi: 10.3389/fendo.2025.1470473
Received: 25 July 2024; Accepted: 26 May 2025;
Published: 18 June 2025.
Edited by:
Ma Jianhua, Nanjing Medical University, ChinaReviewed by:
Bianca Leca, University Hospitals Coventry and Warwickshire NHS Trust, United KingdomPaul Nsiah, University of Cape Coast, Ghana
Copyright © 2025 Liu, Chen, Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Liu, bGl1ZGFuZGFuMzAzMTE0QDE2My5jb20=; Na Li, TGluYTcyMTEyNEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Mengxue Liu
Mengxue Liu Tong Chen1†
Tong Chen1† Dan Liu
Dan Liu