- 1Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
Purpose: The METS-IR index, a non-insulin-based metabolic score, represents a new marker closely linked to insulin resistance. This study aimed to evaluate the relationship between the METS-IR index and dyslipidemia in individuals diagnosed with Cardiovascular disease (CVD), as well as to delve deeper into how varying glucose metabolic conditions influence this relationship.
Methods: This multicenter retrospective investigation encompassed 214,717 individuals diagnosed with CVD across China, spanning from September 1, 2014, to June 1, 2022, ultimately incorporating 17,632 cases in the conclusive analysis. All cases were grouped according to quartiles of METS-IR. The American College of Cardiology classifies dyslipidemia into four distinct categories: hyper-triglyceridemia (hyper-TG), hyper-cholesterolemia (hyper-TC), hypo-high-density lipoprotein cholesterolemia (hypo-HDL), and hyper-low-density lipoprotein cholesterolemia (hyper-LDL). Dyslipidemia is diagnosed when any one of these conditions is present. Logistic regression analysis was performed to estimate the odds ratio (OR) and 95% confidence interval (CI), assessing the relationship between the METS-IR index and dyslipidemia risk in patients with CVD. To evaluate the precision of the METS-IR index in identifying dyslipidemia, receiver operating characteristic (ROC) curve was produced.
Results: The results of the baseline analysis showed that 11,934 cases had dyslipidemia, with notable variations observed in the clinical and biological attributes among CVD cases (P < 0.05 to < 0.001). Logistic regression analysis showed that the METS-IR index was significantly associated with the risk of dyslipidemia (odds ratio [OR]: 1.14; 95% confidence interval [CI] 1.13-1.15; P < 0.001). The OR for dyslipidemia in Q4 of the METS-IR index was 11.94 (95% CI 10.60-13.45; p < 0.001) compared to Q1. ROC analysis revealing an area under the curve (AUC) of 0.747 (95% CI 0.739-0.754; P < 0.001). The association between the METS-IR index and dyslipidemia proved significant across all glycemic status groups, with the highest OR observed in the Q4 subgroup of cases with NGR (OR: 15.43; 95% CI 12.21-19.49).
Conclusion: The risk of developing dyslipidemia is positively associated with heightened METS-IR levels in individuals afflicted with CVD, and these relationships hold significance across all glycemic metabolic conditions. METS-IR could potentially aid in forecasting the risk of dyslipidemia development in individuals diagnosed with CVD.
Background
Dyslipidemia and Cardiovascular disease (CVD) are chronic conditions that significantly impact public health (1). Rapid socio-economic progress in recent decades has led to a substantial rise in the global burden of dyslipidemia (2). Dyslipidemia, a readily modifiable independent risk factor for CVD (3), can have its associated risks mitigated or postponed through early detection and prompt intervention (4).
Insulin resistance (IR) is considered to have a significant role in the development of dyslipidemia. Within the European Group for Insulin Resistance Research (EGIR), insulin sensitivity, as measured by the hyperinsulinemic-euglycemic clamp method, showed a strong correlation with triglyceride (TG) levels (5, 6). The intricate relationship between IR and alterations in lipid and lipoprotein metabolism contributes to atherosclerotic dyslipidemia, which is believed to elevate the risk of CVD (3).
To date, the established benchmark for evaluating IR is the hyperinsulinemic euglycemic clamp technique (HEC) (7). Conventional approaches to IR evaluation are constrained in terms of their real-world applicability, primarily as a result of their intricacy, invasiveness, and expense. The Metabolic Score for Insulin Resistance (METS-IR) Index is a recently devised indicator that exhibits a stronger correlation with HEC compared to other IR indices not based on insulin (8). However, there is a scarcity of research examining the relationship between METS-IR and dyslipidemia.
Consequently, the objective of this research was to examine the relationship between the METS-IR index and dyslipidemia in a substantial cohort of patients diagnosed with CVD, as well as to assess the impact of varying glucose metabolic conditions on this relationship. Furthermore, this research also determined the precision of the METS-IR index in identifying dyslipidemia among patients diagnosed with CVD.
Methods
Subjects
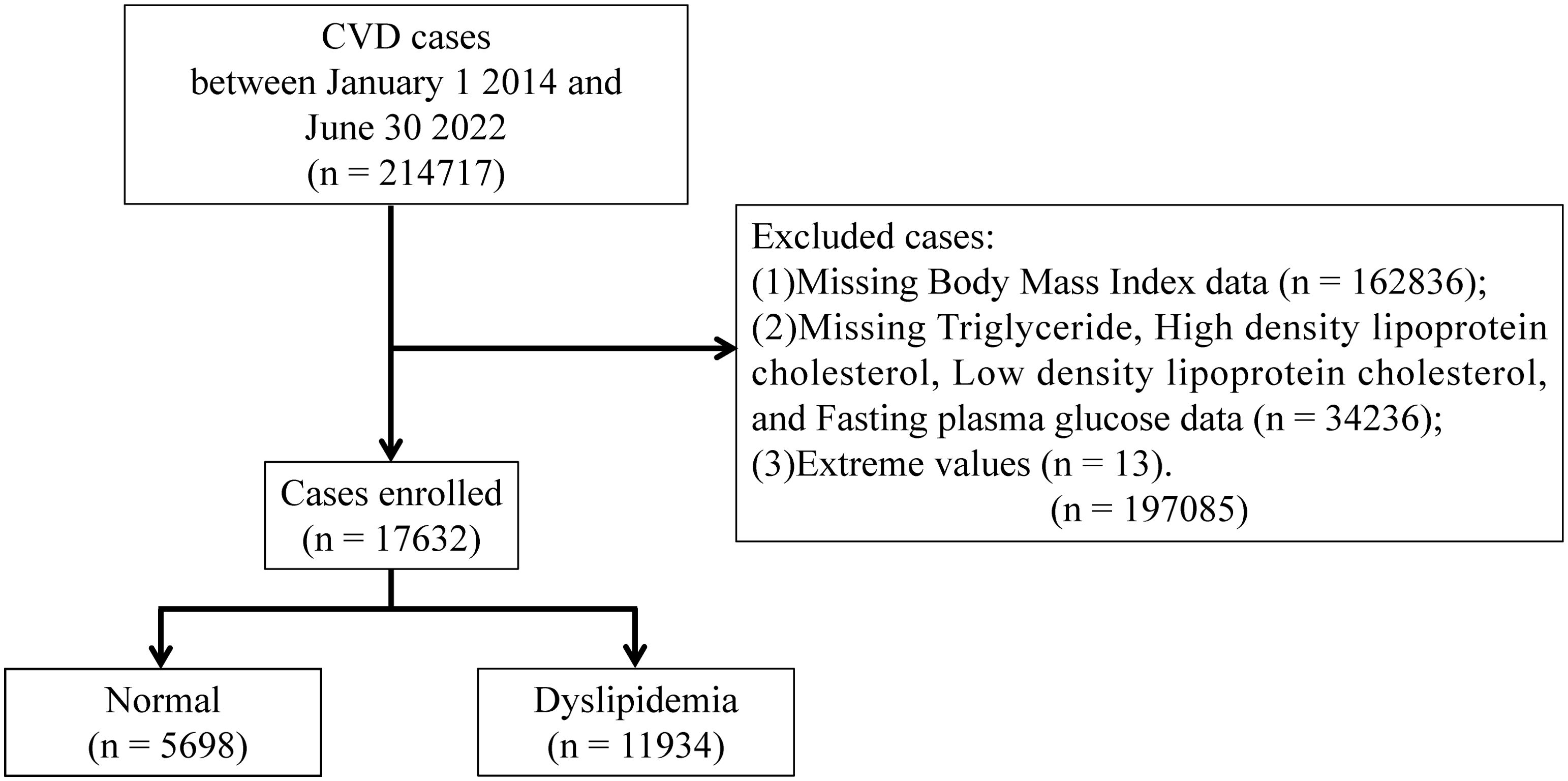
This large-scale multicenter retrospective study population comprised 214,717 CVD cases hospitalized in Tianjin between January 1, 2014, and June 30, 2022. The following cases were excluded: (1) those have extreme values; and (2) those who lacked data on Body Mass Index (BMI), TG, High Density Lipoprotein Cholesterol (HDL-C), Low Density Lipoprotein Cholesterol (LDL-C), and Fasting Plasma Glucose (FPG). Based on previous research, we estimated the overall probability as P≈68%, with an allowable error of δ≈0.15P, leading to a calculated sample size of 41 cases (9). To strengthen the robustness of our findings, we maximized the sample size. Ultimately, a total of 17,632 cases were included in the final analysis. The schematic representation illustrating the cases recruitment process is depicted in Figure 1. Approval for the study was obtained from the Ethics Committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20210007) and registered with the China Clinical Trials Registry (ChiCTR2200058296) and with Clinical Trials. gov (NCT05309343).
Data collection
Age, sex, smoking, drinking, and medication history of cases were collected through standardized questionnaires, which were administered approximately four hours following their admission (10, 11). Systolic blood pressure (SBP), diastolic blood pressure (DBP), FPG, glycated hemoglobin (HbA1c), TC, TG, HDL-C, and LDL-C levels were recorded by practitioners using standardized laboratory methods (12).
Definitions
METS-IR was calculated as (ln [(2 × FPG) + TG)] × BMI)/(ln [HDL-C]) (FPG, TG and HDL-C levels were expressed as mg/dl and BMI as kg/m2 in the equation) (13). BMI was calculated by dividing weight by square height and was expressed in kg/m2. Hyper-triglyceridemia (hyper-TG), hyper-cholestero lemia (hyper-TC), hyper-high-density lipoprotein cholesterolemia (hyper-HDL), hyper-low-density lipoprotein cholesterolemia (hyper-LDL) were defined as TG ≥ 1.7 mmol/L, TC ≥ 5.2 mmol/L, HDL-C < 1.0 mmol/L, and LDL-C ≥ 3.4 mmol/L, respectively, and dyslipidemia if any one of them (14). According to the American Diabetes Association’s Standards for the Medical Treatment of Diabetes (15), diabetes mellitus (DM) is defined as FPG ≥7.0 mmol/L or HbA1c ≥6.5%, prediabetes mellitus (Pre-DM) is defined as 5.6 mmol/L≤FPG ≤ 6.9 mmol/L or 5.7%≤HbA1c ≤ 6.4%, and normal glucose regulation (NGR) is defined as FPG<5.6 mmol/L or HbA1c<5.7%.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables are expressed as percentages. Differences between the groups were calculated using the χ2 test for categorical variables and the t-test Mann-Whitney test or Kruskal-Wallis test for continuous variables. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using logistic regression analysis to test the association between METS-IR index and the risk of dyslipidemia in cases with CVD. To ascertain the precision of the METS-IR index in identifying dyslipidemia among cases diagnosed with CVD, the area under the curve (AUC) was derived from the receiver operating characteristic (ROC) curve analysis. The statistical significance threshold was set at a P-value of less than 0.05. All statistical analyses were performed using the SPSS 24.0 (IBM Corp, New York, NY, USA).
Results
Clinical and biological characteristics
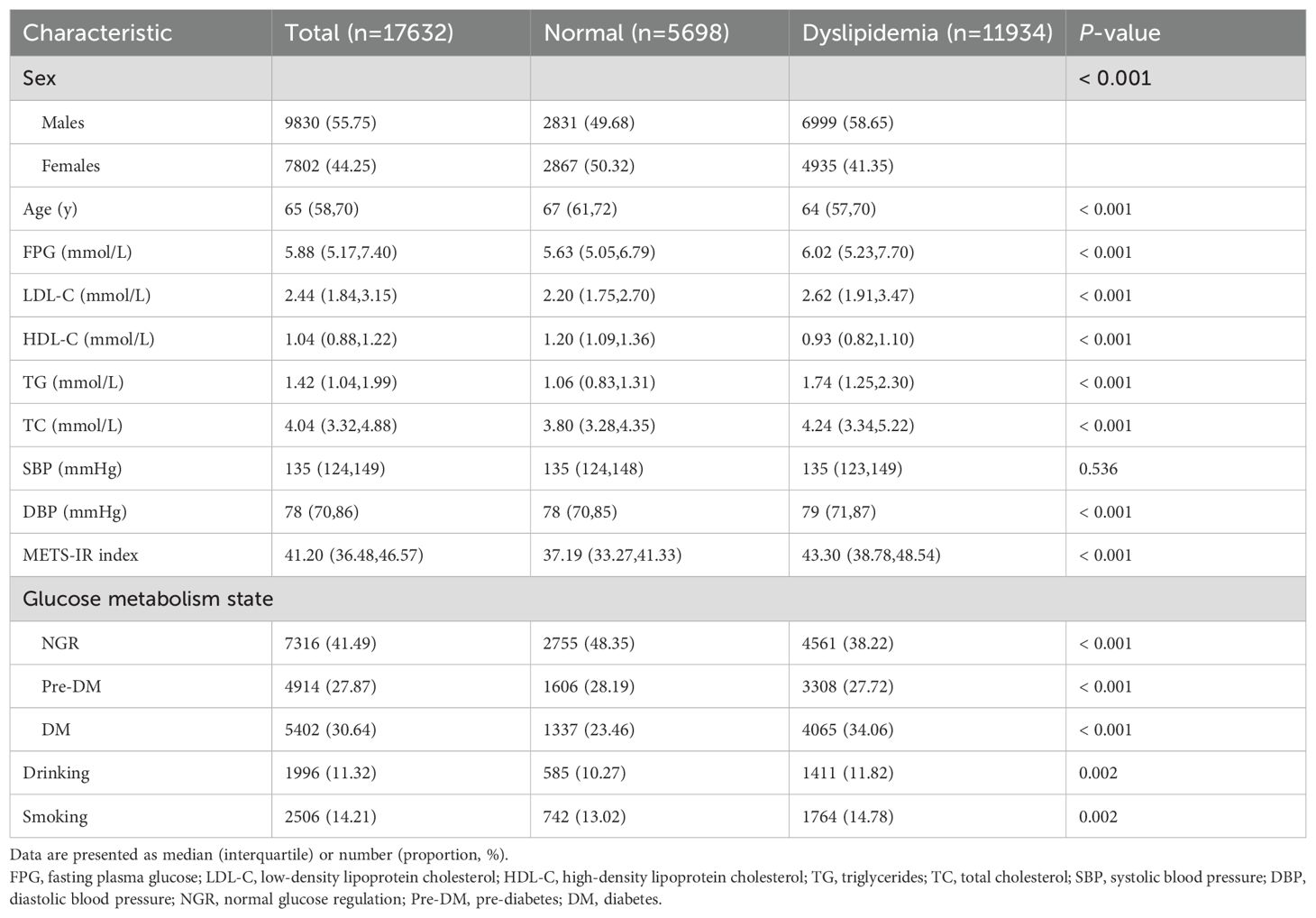
Among the 17,632 cases incorporated into this study, the proportion of males (55.75%) was slightly higher than that of females (44.2%); the median age of cases stood at 65 years, with 11,934 cases exhibiting dyslipidemia. Relative to the normolipidemic cohort, the dyslipidemic group predominantly comprised males, exhibited a lower mean age, registered higher METS-IR scores, and demonstrated a predisposition towards deleterious habits, including smoking and drinking (P < 0.01) (Table 1).
Association between METS-IR index and dyslipidemia
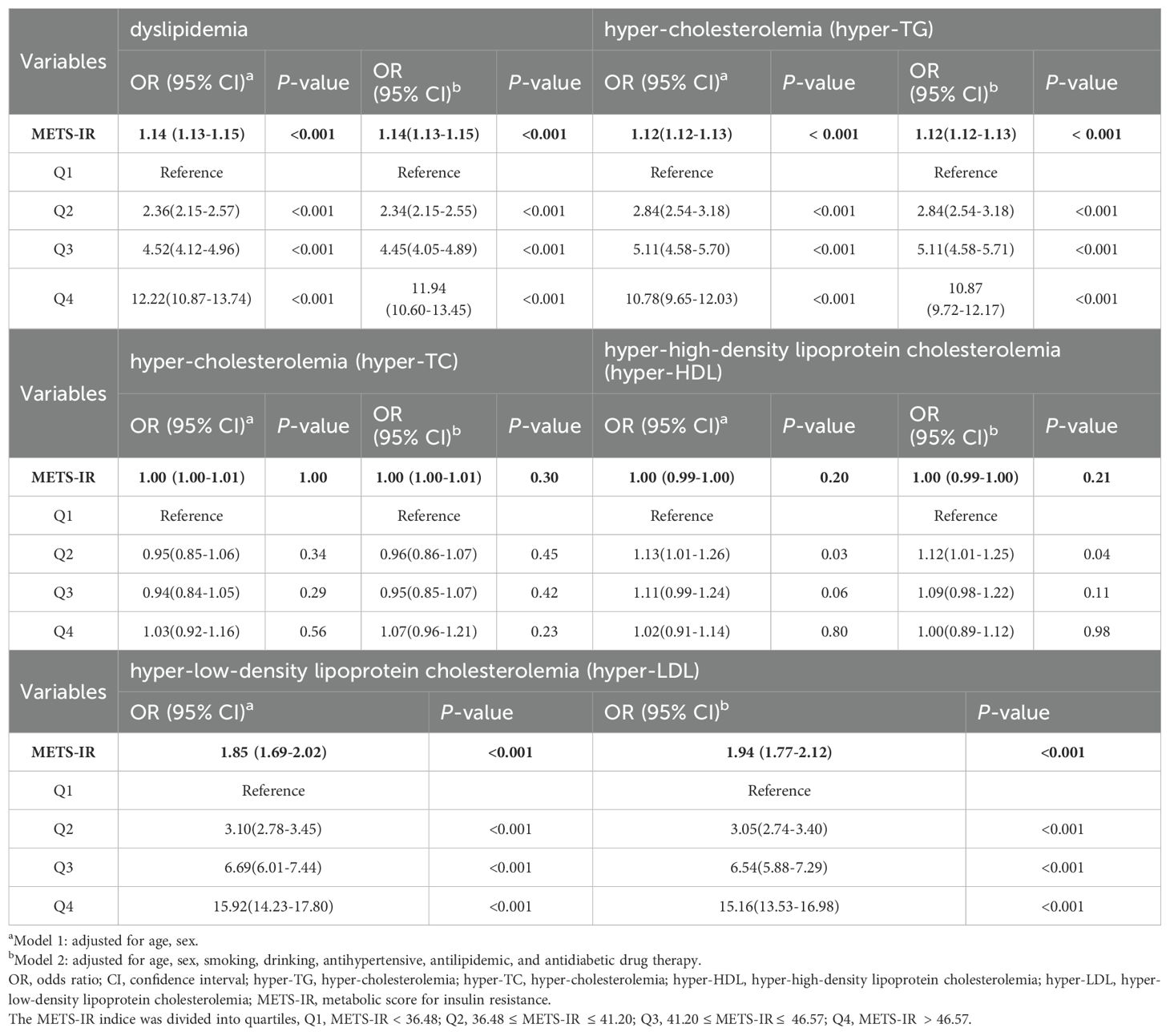
In the context of binary logistic regression analyses, continuous variables revealed a significant and positive association between the METS-IR index and the risk of developing dyslipidemia. Furthermore, the METS-IR index exhibited a positive association with an elevated OR for dyslipidemia in quartiles Q2, Q3, and Q4, with Q1 serving as the reference group. This association persisted as statistically significant even after adjusting for confounding variables. With ascending levels of the METS-IR index, the ORs for dyslipidemia were observed to be 2.34 (95% CI 2.15-2.55), 4.45 (95% CI 4.05-4.89), and 11.94 (95% CI 10.60-13.45), sequentially (all P < 0.001) (Table 2).
Stratified analyses were performed in Table 2, which were conducted with consideration for the disparities among distinct subcategories of dyslipidemia. The METS-IR index exhibited a positive association with the risk of hyper-TG and hyper-LDL, albeit without significant association concerning the concurrent risks of hyper-TG and hyper-TC. The ORs corresponding to the METS-IR index in conjunction with hyper-TG and hyper-TC for quartiles two through four (Q2, Q3, and Q4), respectively, exhibited a persistent upward trend as the METS-IR value increased, with Q1 serving as the baseline comparator. Importantly, these observed associations sustained their statistical significance following the application of adjustments for a range of confounding variables (all P < 0.001).
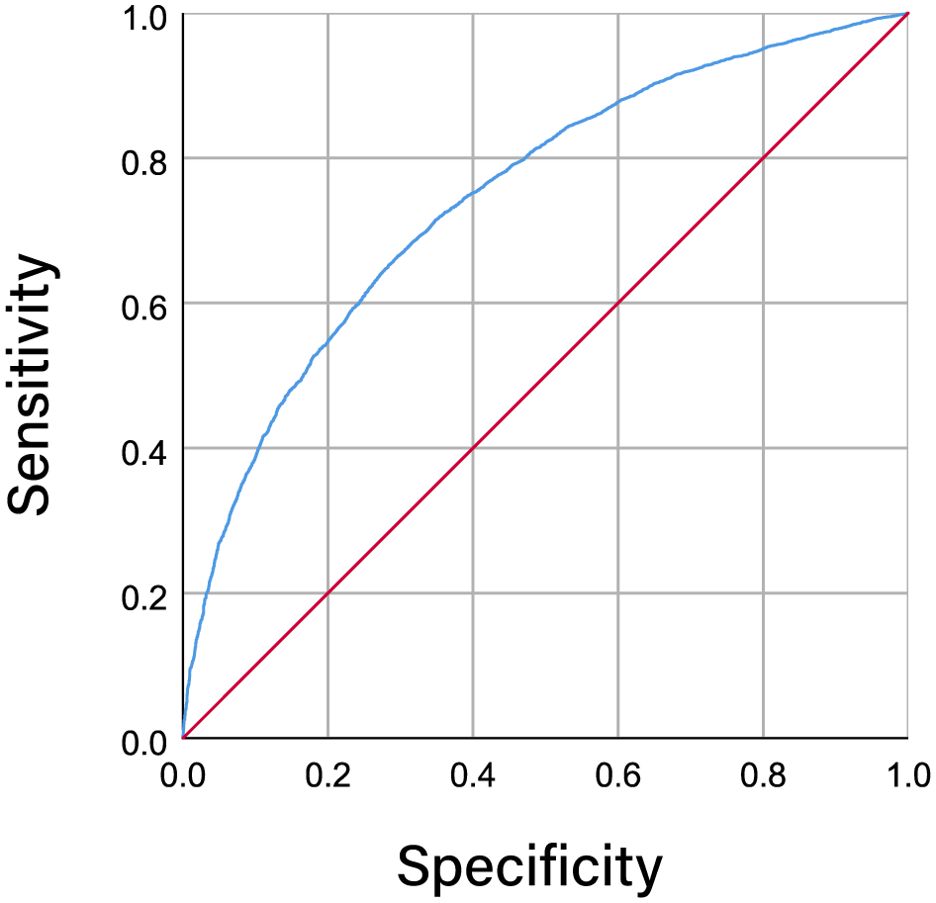
Figure 2 illustrates the ROC curves representing dyslipidemia and the METS-IR index among individuals diagnosed with CVD. The area under the curve (AUC) metric for the METS-IR index was calculated to be 0.747 (95% CI 0.739-0.754; P < 0.001), suggesting a statistically significant capacity of the METS-IR index in identifying and diagnosing dyslipidemic conditions.
Associations between METS-IR index and dyslipidemia in different glucose metabolism states
The employment of binary logistic regression analysis served to evaluate the impact of varying glucose metabolic conditions on the association between the METS-IR index and the presence of dyslipidemia, with findings summarized in Table 3. Concerning the METS-IR index as a continuously measured variable, a statistically significant association was observed with the incidence of dyslipidemia across all distinct categories of glucose metabolic conditions (P < 0.001). Furthermore, in the context where Q1 served as the reference, the ORs associated with the presence of dyslipidemia were observed to escalate in conjunction with ascending levels of the METS-IR index, with Q4 having the highest OR (all P < 0.01). These associations persisted in their significance following the adjustment of the model parameters.
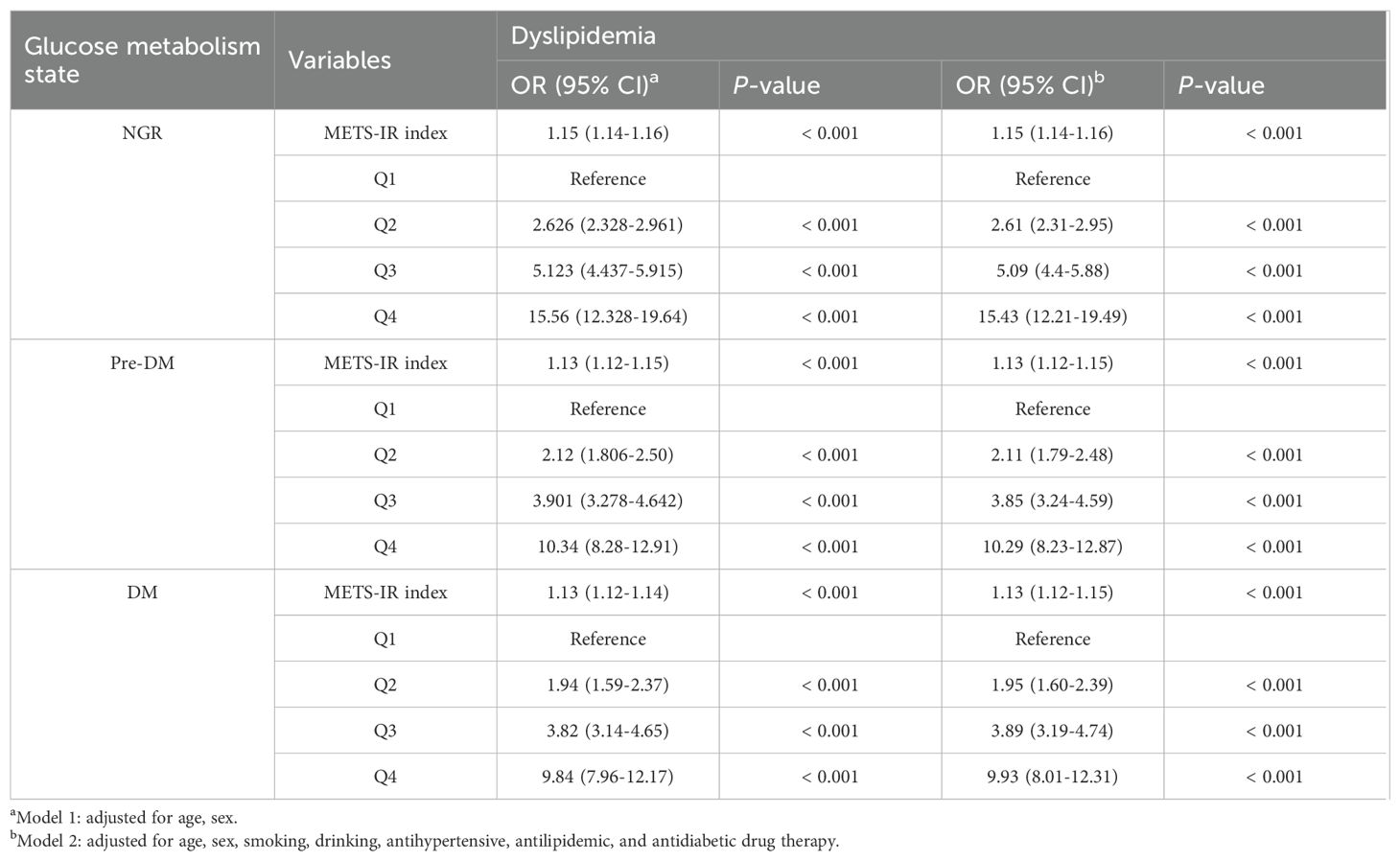
Table 3. Associations between METS-IR index and dyslipidemia in different glucose metabolism states.
Discussion
This is the first study to reveal a significant association between the METS-IR index and dyslipidemia in cases with CVD and to assess this association according to different glucose metabolic states. In the present study, the METS-IR index was significantly associated with dyslipidemia, and this association was particularly significant in different quartiles of the METS-IR index. These associations can still be observed at different levels of glucose metabolism as well. The novelty of this study is to analyze the more convenient and reliable METS-IR index and its association with the risk of dyslipidemia, and directly relates the IR to lipid metabolism changes and advance current knowledge.
Previous observational studies have reported an association between glycolipid metabolism and increased risk of CVD (16, 17). Nuclear magnetic resonance analyses showed that the mean particle size of very low density lipoprotein (VLDL) was larger and the mean particle size of LDL and HDL was smaller in IR individuals compared to insulin-sensitive individuals (18). Besides, postprandial VLDL, Apo B-48, Apo B-100, cholesterol and TG concentrations are more elevated in patients with T2DM compared to their non-diabetic peers (19). Furthermore, A 13-year follow-up study reported that metabolic syndrome due to IR is a key risk factor for CVD, leading to approximately twice the mortality rate from CVD (20). A study by the EGIR found that the HOMA-IR index was strongly correlated with TG concentrations (5). These show a strong link between insulin and lipid levels in CVD patients. Our study supports this by more thoroughly demonstrating that the HOMA-IR index is associated with dyslipidemia in CVD patients.
There are several pathways that may explain the observed association between HOMA-IR index and dyslipidemia. Insulin resistance increases hepatic glucose output and conversely enhances triglyceride synthesis and VLDL release. Intracellular accumulation of lipids triggers activation of novel protein kinase C and subsequently leads to impaired insulin signaling. In the liver, insulin normally stimulates glucose uptake, glucose oxidation and glycogen synthesis, inhibits gluconeogenesis, and stimulates triglyceride synthesis and secretion via VLDL (21). IR is a generic term which implies that adipose tissue, skeletal muscle, liver and pancreas are less responsive to the action of insulin.IR increases hepatic TG synthesis, and increased TG synthesis is differentially correlated with increased hepatic Apo B-100 production (22, 23). This results in hypertriglyceridemia, a variable increase in the number of particles reflected by VLDL apolipoprotein B-100, and a decrease in HDL-C concentration (24). Furthermore, IR is also associated with elevated hepatic triglyceride lipase (HTGL), which may lead to accelerated HDL clearance and abnormal HDL-C (25).
A variety of lipids such as TG and TC, as well as HDL and LDL, are involved in the regulation of microvascular function, and dyslipidemia reduces coronary flow reserve and capillary density, induces apoptosis of capillary endothelial cells, and ultimately leads to impaired left ventricular (LV) function (26), exacerbating the condition of CVD patients. In addition, dyslipidemia leads to myocardial ultrastructural alterations through a variety of mechanisms. High fat and high cholesterol (HFHC) diets increase serum TC and free fatty acid (FFA) levels, leading to systemic oxidative stress and a pro-inflammatory state, which in turn leads to apoptosis and cardiac injury (27, 28). Long-term prospective epidemiological studies have consistently shown that the incidence of coronary heart disease is significantly lower in individuals with a favorable lipid profile (29). Prevention and rational management of dyslipidemia can significantly alter cardiovascular morbidity and mortality.
The pathogenesis of dyslipidemia includes IR, hyperinsulinemia and abnormal levels of adipokines, with IR being a prominent feature of the metabolic syndrome and T2DM-type diabetes mellitus, which also leads to the development of CVD (30). Several mechanisms by which IR exacerbates atherosclerosis have been elucidated, including systemic inflammation, endothelial dysfunction and oxidative stress (31). The HEC technique is the gold standard for the assessment of IR, while HOMA-IR is the most widely used method due to its technically costly and time-consuming nature. All this explains why the comprehensive monitoring index of METS-IR is useful in developing strategies for primary prevention and management of dyslipidemia in patients with CVD.
Limitations
The current study has several limitations. First, although we accounted for numerous potential confounding factors, dyslipidemia may still be influenced by other variables, such as systemic lupus erythematosus, hereditary dyslipidemia, or cardiac catheterization. Second, this study was conducted in a Chinese population, where racial differences may exist. Third, potential Neyman bias should be considered. Therefore, well-designed randomized controlled trials are needed to validate these results.
Conclusion
The risk of developing dyslipidemia is associated with elevated levels of METS-IR in individuals with coronary artery disease, and this correlation is significant across glucose metabolism states. METS-IR may help predict the risk of developing dyslipidemia in individuals with coronary artery disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Approval for the study was obtained from the Ethics Committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20210007) and registered with the China Clinical Trials Registry (ChiCTR2200058296) and with Clinical Trials. gov (NCT05309343). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study had no direct contact with the subjects and only patient hospitalization information was collected. Patients have been informed that the test results are only used for analysis and exploration, not as any auxiliary basis for diagnosis, and not for any commercial use. The results of the study will remove any character with the subject identification to ensure that personal privacy is not compromised, so there will be no objective risk to the subject.
Author contributions
LY: Writing – original draft. YL: Data curation, Writing – original draft. RG: Data curation, Writing – original draft. TY: Data curation, Writing – original draft. GP: Data curation, Writing – original draft. YH: Methodology, Writing – original draft. SG: Methodology, Supervision, Writing – review & editing. RY: Methodology, Supervision, Writing – review & editing. ZL: Conceptualization, Supervision, Validation, Writing – review & editing. LL: Conceptualization, Methodology, Supervision, Writing – review & editing. CY: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant number: 82074140, 82104565, and 82204142) and Tianjin Hongrentang Pharmaceutical Co., Ltd., Tianjin, China (grant number: HX2020-16). The authors declare that this study received funding from Tianjin Hongrentang Pharmaceutical Co., Ltd., Tianjin, China. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank all the participants in the study and the members of the survey teams, as well as the financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC, Area under the curve; CVD, Cardiovascular disease; BMI, Body Mass Index; CI, Confidence intervals; DBP, Diastolic blood pressure; DM, Diabetes mellitus; EGIR, European Group for Insulin Resistance Research; FFA, free fatty acid; HbA1c, glycated hemoglobin; FPG, Fasting Plasma Glucose; HDL-C. High density lipoprotein cholesterol; HEC, Hyperinsulinemic euglycemic clamp technique; HFHC, High fat and high cholesterol; HTGL, Hepatic triglyceride lipase; hyper-TG, Hyper-triglyceridemia; hyper-TC, hyper-cholestero lemia; hyper-HDL, hyper-high-density lipoprotein cholesterolemia; hyper-LDL, hyper-low-density lipoprotein cholesterolemia; IR, Insulin resistance; LDL-C, Low density lipoprotein cholesterol; LV, Left ventricular; METS-IR, Metabolic Score for Insulin Resistance; NGR, Normal glucose regulation; OR, Odds ratios; Pre-DM, Prediabetes mellitus; ROC, Receiver operating characteristic; SBP. Systolic blood pressure; TG, Triglyceride; VLDL, very low density lipoprotein.
References
1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: A report from the american heart association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
2. World Health Organization. Noncommunicable diseases: risk factors. The Global Health Observatory (2021). Available at: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-risk-factors (Accessed June 12, 2024).
3. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidemias. Nat Rev Cardiol. (2021) 18:689–700. doi: 10.1038/s41569-021-00541-4
4. Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. (2019) 42:1312–8. doi: 10.2337/dc19-0274
5. Baldeweg SE, Golay A, Natali A, Balkau B, Del Prato S, Coppack SW. Insulin resistance, lipid and fatty acid concentrations in 867 healthy Europeans. European Group for the Study of Insulin Resistance (EGIR). Eur J Clin Invest. (2000) 30:45–52. doi: 10.1046/j.1365-2362.2000.00597.x
6. Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. (2011) 34:1869–74. doi: 10.2337/dc10-2234
7. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. (2008) 294:E15–26. doi: 10.1152/ajpendo.00645.2007
8. Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. (2018) 178:533–44. doi: 10.1530/EJE-17-0883
9. Yu L, Li Z, Yang R, Pan G, Cheng Q, He Y, et al. Impaired sensitivity to thyroid hormones is associated with elevated blood glucose in coronary heart disease. Front Endocrinol (Lausanne). (2022) 13:895843. doi: 10.3389/fendo.2022.895843
10. Fan AZ, Ruan WJ, Chou SP. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev Med. (2019) 118:336–43. doi: 10.1016/j.ypmed.2018.11.022
11. Li Z, He Y, Wang S, Li L, Yang R, Liu Y, et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. (2022) 21:38. doi: 10.1186/s12933-022-01470-3
12. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. (2020) 30:160–4. doi: 10.1016/j.tcm.2019.05.003
13. Xu C, Song G, Hu D, Li G, Liu Q, Tang X. Association of METS-IR with incident hypertension in non-overweight adults based on a cohort study in Northeastern China. Eur J Public Health. (2022) 32:884–90. doi: 10.1093/eurpub/ckac140
14. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. Circulation. (2019) 139:e1082–143. doi: 10.1161/CIR.0000000000000625
15. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2014) 37 Suppl 1:S81–90. doi: 10.2337/dc14-S081
16. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17:122. doi: 10.1186/s12933-018-0762-4
17. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. (2018) 98:2133–223. doi: 10.1152/physrev.00063.2017
18. Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. (2005) 111:3465–72. doi: 10.1161/CIRCULATIONAHA.104.512079
19. Rivellese AA, De Natale C, Di Marino L, Patti L, Iovine C, Coppola S, et al. Exogenous and endogenous postprandial lipid abnormalities in type 2 diabetic patients with optimal blood glucose control and optimal fasting triglyceride levels. J Clin Endocrinol Metab. (2004) 89:2153–9. doi: 10.1210/jc.2003-031764
20. Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. (2004) 110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E
21. Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. (2010) 375:2267–77. doi: 10.1016/S0140-6736(10)60408-4
22. Grundy SM, Mok HY, Zech L, Steinberg D, Berman M. Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J Clin Invest. (1979) 63:1274–83. doi: 10.1172/JCI109422
23. Kissebah AH, Alfarsi S, Adams PW. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in man: normolipemic subjects, familial hypertriglyceridemia and familial combined hyperlipidemia. Metabolism. (1981) 30:856–68. doi: 10.1016/0026-0495(81)90064-0
24. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. (2012) 32:2104–12. doi: 10.1161/ATVBAHA.111.241463
25. Baynes C, Henderson AD, Anyaoku V, Richmond W, Hughes CL, Johnston DG, et al. The role of insulin insensitivity and hepatic lipase in the dyslipidemia of type 2 diabetes. Diabetes Med. (1991) 8:560–6. doi: 10.1111/j.1464-5491.1991.tb01652.x
26. Fauchier L, de Labriolle A. Cholesterol levels and cholesterol lowering in idiopathic dilated cardiomyopathy. Eur Heart J. (2005) 26:1931–2. doi: 10.1093/eurheartj/ehi422
27. Han Q, Yeung SC, Ip MSM, Mak JCW. Dysregulation of cardiac lipid parameters in high-fat high-cholesterol diet-induced rat model. Lipids Health Dis. (2018) 17:255. doi: 10.1186/s12944-018-0905-3
28. Moretti S, Renga G, Oikonomou V, Galosi C, Pariano M, Iannitti RG, et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. (2017) 8:14017. doi: 10.1038/ncomms14017
29. Tripathy JP, Thakur JS, Jeet G, Chawla S, Jain S, Pal A, et al. Burden and risk factors of dyslipidemia-results from a STEPS survey in Punjab India. Diabetes Metab Syndr. (2017) 11 Suppl 1:S21–7. doi: 10.1016/j.dsx.2016.08.015
30. Filippatos TD, Florentin M, Georgoula M, Elisaf MS. Pharmacological management of diabetic dyslipidemia. Expert Rev Clin Pharmacol. (2017) 10:187–200. doi: 10.1080/17512433.2017.1263565
Keywords: METS-IR, insulin resistance, dyslipidemia, glucose metabolic states, cardiovascular disease
Citation: Yu L, Liu Y, Guo R, Yang T, Pan G, He Y, Gao S, Yang R, Li Z, Li L and Yu C (2025) The metabolic syndrome-insulin resistance index: a tool for identifying dyslipidemia across varied glucose metabolic score in patients with cardiovascular disease. Front. Endocrinol. 16:1473308. doi: 10.3389/fendo.2025.1473308
Received: 30 July 2024; Accepted: 15 April 2025;
Published: 12 May 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Fatemeh Ayoobi, Rafsanjan University of Medical Sciences, IranZina Ali, Al-Ameed University, Iraq
Copyright © 2025 Yu, Liu, Guo, Yang, Pan, He, Gao, Yang, Li, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu Li, bHpoeXpfMTk5NUAxNjMuY29t; Lin Li, bGxiaWFuamlAMTYzLmNvbQ==; Chunquan Yu, eWNxdGp1dGNtQGZveG1haWwuY29t
†These authors share first authorship
 Lu Yu1†
Lu Yu1† Rongrong Yang
Rongrong Yang Zhu Li
Zhu Li Chunquan Yu
Chunquan Yu