- 1Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2Henan Key Laboratory of Reproduction and Genetics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Background: To compare the clinical outcomes between frozen-thawed cleavage embryo transfer and frozen-thawed day 6 blastocyst transfer in patients younger than 35 using the freeze-all strategy without day 5 blastocyst formation.
Methods: This was a retrospective observational analysis performed between January 2018 and December 2022 at the Reproductive and Genetic Specialist Hospital of the First Affiliated Hospital of Zhengzhou University. A total of 576 patients younger than 35 who used the “freeze-all strategy” but produced no day 5 blastocysts were recruited. The patients were divided into 3 groups according to the number and stage of the transferred embryos: double cleavage-stage embryos (Group A), single good-quality day 6 blastocysts (Group B) and single inferior-quality day 6 blastocysts (Group C),and several pregnancy outcomes were measured.
Results: Groups A and B exhibited significantly higher chemical (73.7%, 67.0% versus 51.9%) and clinical pregnancy rates (69.0%, 59.4% versus 44.2%) than Group C. The implantation rate was significantly higher in Group B than in Groups A and C (59.4% versus 45.7%, 43.5%). The live birth rate was significantly higher in Group A than in Group C (59.2% versus 48.1%). The multiple pregnancy rate was significantly higher in Group A than in Groups B and C (34.4% versus 1.6%, 1.5%). The early miscarriage rate was significantly higher in Group C than in Group A and Group B (23.5% versus 8.7%, 12.7%). Premature delivery rates, late miscarriage rates and ectopic pregnancy rates were comparable across groups.
Conclusions: A single good quality day 6 blastocyst transfer was the preferable strategy for the freeze-all strategy patients who younger than 35 and without day 5 blastocyst formation.
1 Introduction
Over the past several decades, with the extension of embryo culture duration in vitro (1, 2) and the broad application of vitrification (3, 4), single blastocyst transfer has been increasingly applied in the clinical setting to avoid ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy (5–8).
Whether to transfer cleavage-stage or blastocyst-stage embryos has become a controversial question in recent years. Some studies suggest that blastocyst-stage embryo transfer is more efficient (9, 10). First, the extended culture of the embryo in vitro promotes embryo self-selection; that is, only embryos with good developmental potential are capable of forming blastocysts (11). Second, in naturally occurring pregnancies, embryo implantation occurs at the hatched-blastocyst stage; therefore, blastocyst-stage embryo transfer may better mimic the physiologic timing of exposure of the embryo to the uterine environment (12). Third, some studies have suggested that blastocyst-stage embryo transfer achieves significantly higher implantation, clinical pregnancy, and live birth rates and comparable miscarriage rates compared with cleavage-stage embryo transfer in high responders (13).
Conversely, some studies indicate that blastocyst-stage embryo transfer is not superior to cleavage-stage embryo transfer (14). First, only 60~80% of cleavage-stage embryos can progress to blastocyst-stage embryos due to self-selection, which may result in a higher incidence of cycle cancellation and lower rates of embryo cryopreservation. Second, some studies have shown that there is no significant difference in live birth, ongoing pregnancy, clinical pregnancy or miscarriage rates between transfers using embryos in these two stages. Third, the extension of the culture period is more time consuming and costly, and two to four days are required for cleavage-stage embryos to develop to blastocyst-stage embryos (15, 16).
Most studies in recent years have indicated that day 5 blastocysts have a higher euploidy rate than day 6 blastocysts (17, 18). Day 5 blastocyst transfer is generally considered to have a higher implantation rate, clinical pregnancy rate and live birth rate than day 6 blastocyst transfer (19), but a limitation of previous studies is that they usually only compared the clinical outcomes within blastocyst-stage and cleavage-stage embryos and did not differentiate between blastocysts at different days of development or between transfers of different numbers of blastocysts. Most embryologists prefer single day 5 blastocyst-stage embryo transfer to achieve higher clinical pregnancy and a lower multiple pregnancy rates, but if patients have no day 5 blastocyst-stage embryos cryopreserved, whether double cleavage-stage embryo transfer or single day 6 blastocyst-stage embryo transfer is more likely to achieve a satisfactory clinical outcome is still a matter of debate.
The aim of this study is to compare the clinical outcomes between frozen-thawed cleavage embryo transfer and frozen-thawed day 6 blastocyst transfer in patients younger than 35 using the freeze-all strategy without day 5 blastocyst formation. We concluded that single good quality day 6 blastocyst transfer was the preferable strategy with a comparable clinical outcomes and significantly lower multiple pregnancies compared with the double cleavage embryo transfer group. This conclusion can give a suggestion for embryologists to provide a more effective selection for the stage of the transferred embryos for who had no day 5 blastocyst cryopreservation.
2 Materials and methods
2.1 Patients and groups
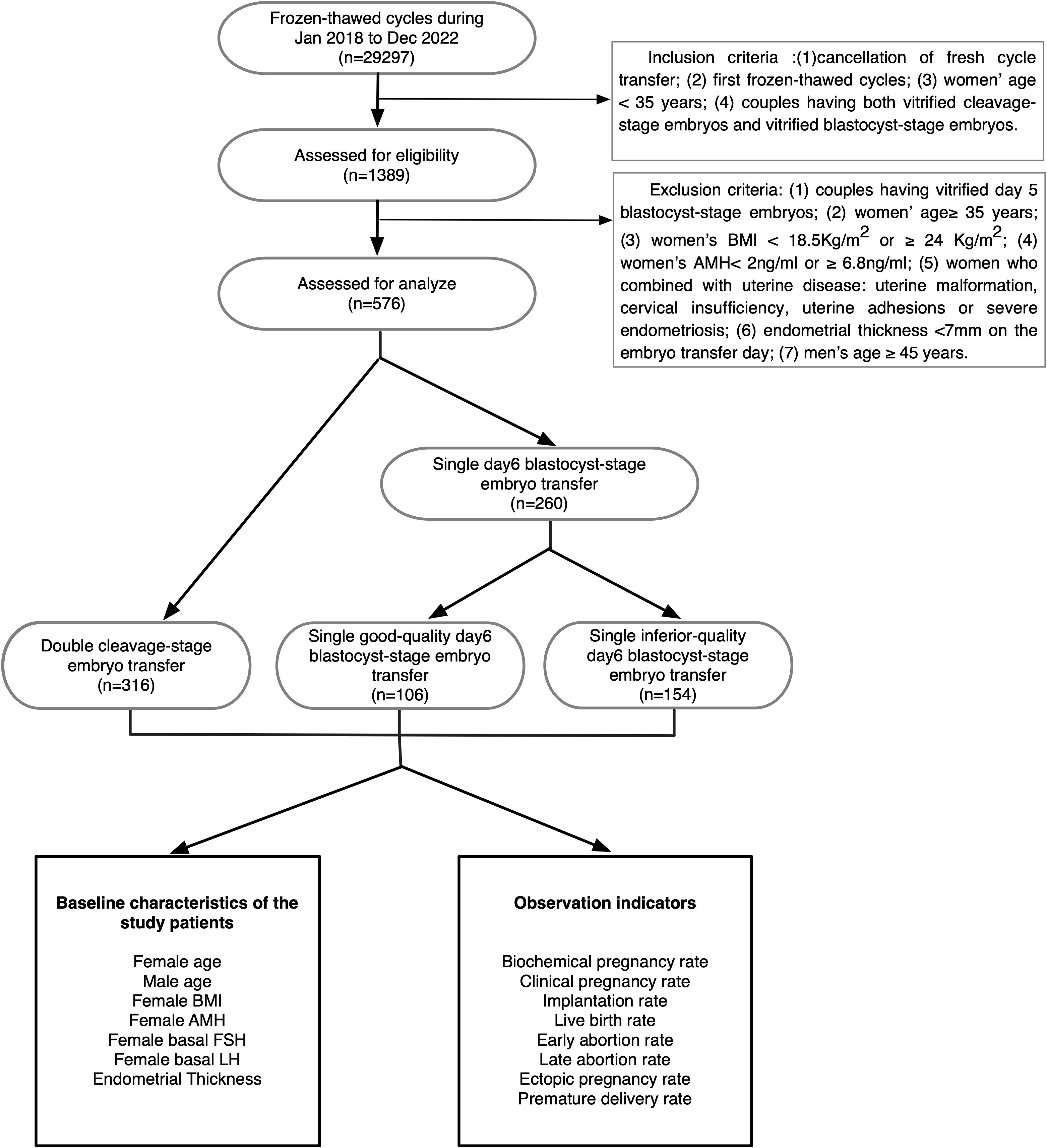
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zheng Zhou University. The data of 29297 couples treated in the Reproductive Medical Center of the First Affiliated Hospital of Zhengzhou University between January 2018 and December 2022 were collected. Eventually, 576 couples were included and analyzed in accordance with the inclusion criteria and exclusion criteria. This study enrolled only patients under 35 years who were undergoing their first frozen-thawed cycle and had at least two cleavage-stage embryos and one blastocyst-stage embryo available at the same time.
The 576 couples were divided into three groups according to the stages and the numbers of embryos transferred: double cleavage-stage embryos (Group A), single good-quality day 6 blastocyst (Group B) and single inferior-quality day 6 blastocyst (Group C). The route of our study is shown in Figure 1.
2.2 Inclusion criteria and exclusion criteria
The inclusion criteria were as follows: (1) cancellation of fresh cycle transfer; (2) first frozen-thawed cycle; (3) female partner aged < 35 years on the day of embryo transfer; and (4) couples having both vitrified cleavage-stage embryos and vitrified blastocyst-stage embryos.
The exclusion criteria were as follows: (1) couples with vitrified day 5 blastocyst-stage embryos; (2) female partner aged ≥ 35 years on the day of embryo transfer; (3) female partner’s body mass index (BMI) < 18.5 kg/m2 or ≥ 24 kg/m2; (4) anti-Mullerian hormone (AMH) < 2 ng/ml or ≥ 6.8 ng/ml; (5) uterine malformation, cervical insufficiency, uterine adhesions or severe endometriosis; (6) endometrial thickness <7 mm on the day of embryo transfer; and (7) male partner aged ≥ 45 years on the day of embryo transfer.
2.3 Vitrification, thawing and embryo culture
Vitrification of both cleavage- and blastocyst-stage embryos was performed following the manufacturer’s instructions (Vitrification Kit, Kitazato, Japan). The thawing process also followed the manufacturer’s instructions (Thawing Kit, Kitazato, Japan). The thawed cleavage or blastocyst embryos were transferred into overnight balanced G-2 Plus (G-2 plus, Vitrolife, Sweden), and every thawed embryo was cultured in overnight balanced G-2 Plus at 37°C and 6% CO2 at least 2 hours before embryo transfer.
2.4 Morphological grading of embryos
Cleavage scoring was performed according to the Peter scoring standard (20); the number of blastomeres is more than 6, the size of blastomeres is slightly uneven, and the cell fragments are < 10% were considered high-quality cleavage-stage embryos. Only high-quality cleavage-stage embryos were cryopreserved. In this study, post-thaw survival for cleavage-stage embryos was defined as more than half of the original cells remaining intact and at least four blastomeres.
The blastocysts were evaluated using the Gardner blastocyst scoring system (12), and the post-thaw survival of cryopreserved blastocyst-stage embryos was defined as maintenance of expansion ability. A surviving blastocyst was considered worth transferring if the degree of blastocyst expansion was at least ≥3, the inner cell mass (ICM) was at least grade B and the trophectoderm (TE) was at least grade C. In this study, blastocysts of 3BB and above (grade C without ICM or TE) were defined as good-quality and those with grade C TE were defined as inferior quality.
Both the cleavage scoring and the blastocyst scoring were scored according on the state of embryos before cryopreservation.
2.5 Endometrial preparation
The endometrial preparation plan was selected as appropriate according to the patient’s condition: 1) For patients with a regular menstrual cycle, follicular development and endometrial condition were monitored by transvaginal ultrasound on the 10-12th day of the menstrual cycle. When the diameter of the dominant follicle was between 18–20 mm and a urinary LH (Luteinizing Hormone, LH) peak appeared, or if there was no LH surge, 10000 IU of human chorionic gonadotropin (HCG, Zhuhai,Lizhu) was given to induce ovulation in the morning in order to control the ovulation time. An endometrial thickness ≥7 mm was considered appropriate for embryo transfer. 2) Artificial cycle: Estradiol valerate tablets (Estradiol Valerate, Bayer, Germany) were given 2–4 mg/d on the 2nd-3rd day of the menstrual period or progesterone withdrawal bleeding, the endometrial thickness was monitored by ultrasound, and the dosage of Estradiol valerate tablets was adjusted according to the serum estrogen level and endometrial condition.
2.6 Pregnancy outcome evaluation
Fourteen days after embryo transfer, the serum β-human chorionic gonadotropin (HCG) level was detected to determine whether pregnancy had been established. Chemical pregnancy was defined as β-HCG > 5 U/mL. Clinical pregnancy was diagnosed as the presence of a gestational sac and primitive heart tube pulsation on transvaginal ultrasound examination 35 days after embryo transfer, and multiple pregnancy was judged according to the number of sacs present. If the β-HCG-positive gestational sac was not in the normal position in the uterine cavity, it was considered as ectopic pregnancy; spontaneous abortion was diagnosed if the embryo stopped developing or the fetus had no heartbeat at a later follow-up. Luteal support drugs were given when a pregnancy was confirmed and discontinued in cases of pregnancy loss.
2.7 Outcome indicator calculations
The calculations of the outcome indicators were as follows:
1. Biochemical pregnancy rate = number of biochemical pregnancy cycles/number of transplantation cycles × 100%;
2. Clinical pregnancy rate = number of clinical pregnancy cycles/number of transplantation cycles × 100%;
3. Implantation rate = number of gestational sacs/number of transplanted embryos × 100%;
4. Live birth rate = number of live birth cycles/number of transplantation cycles × 100%;
5. Early abortion rate = number of spontaneous abortions before 12 weeks of pregnancy/number of clinical pregnancy cycles × 100%;
6. Late abortion rate = number of spontaneous abortions from 12 to 28 weeks of pregnancy/number of clinical pregnancy cycles × 100%;
7. Ectopic pregnancy rate = number of ectopic pregnancy cycles/number of clinical pregnancy cycles × 100%;
8. Premature delivery rate = number of live births at 28–36 gestational weeks/number of live birth cycles × 100%.
2.8 Statistical analyses
SPSS 21.0 software (IBM, USA) was used to process the data, and the quantitative data are expressed as the mean standard deviation (X ± S). The independent samples T test was used for comparisons between two groups, and one-way ANOVA was used for comparisons between multiple groups. Univariable logistic regressions were used to analyze the correlation of the three transfer groups with the clinical outcomes. Qualitative data were expressed as percentages (%) and compared using the chi-square test or Fisher’s exact probability method; differences were considered statistically significant at p< 0.05.
3 Results
3.1 Study population and cycle characteristics
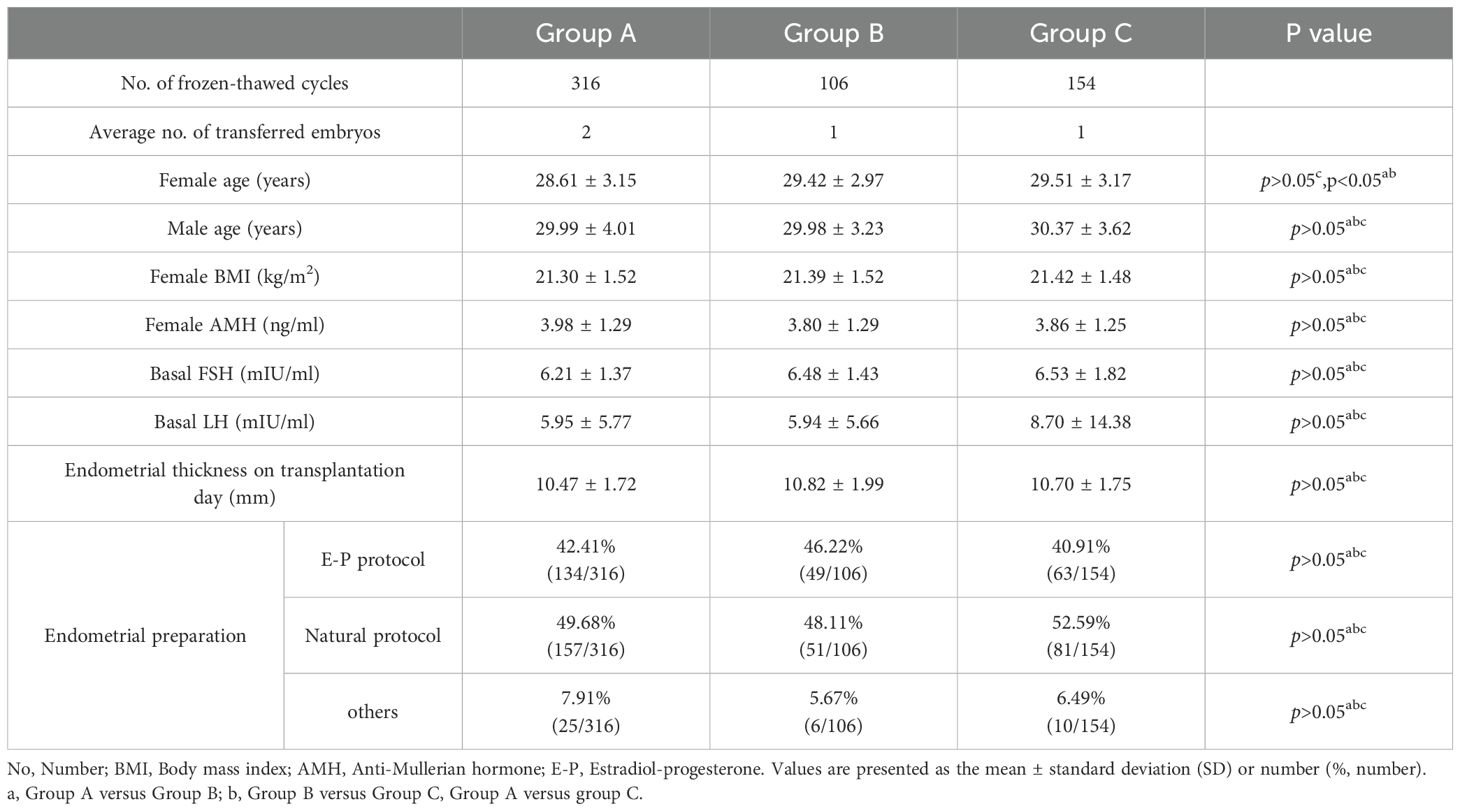
In total, 892 frozen-thawed embryos from 576 patients were retrospectively analyzed (see Table 1 for basic data and cycle characteristics of the patients in the three groups). There were no significant differences among these three groups in terms of male age (years), female BMI (kg/m2), female AMH (ng/ml), female basal FSH (mIU/ml), female basal LH (mIU/ml) or endometrial thickness on transplantation day (mm). The distribution of the endometrial preparation protocols in these three groups was not significantly different, but the female age (years) in Group A was significantly younger compared with the other groups(28.61 ± 3.15 versus 29.42 ± 2.97, 29.51 ± 3.17, p<0.05).
3.2 The comparison of the clinical outcomes between the three transfer groups
Group A and Group B had significantly higher chemical pregnancy and clinical pregnancy rates compared with Group C (73.7%, 67.0% versus 51.9%, p<0.05; 69.0%, 59.4% versus 44.2%, p<0.05). Group B had a significantly higher implantation rate than Group A and Group C (59.4% versus 45.7%, 43.5%, p<0.05). Group A had a significantly higher live birth rate than Group C (59.2% versus 48.1%, p<0.05), but the differences between Group A and Group B and between Group B and Group C were not significant. Group C had a significantly higher early miscarriage rate than Group A and Group B (23.5% versus 8.7%, 12.7%, p<0.05); no significant difference were found in late miscarriage rate and ectopic rate between these three groups. Group A had a significantly higher multiple pregnancy rate than Group B and Group C (34.4% versus 1.6%, 1.5%, p<0.05). Interestingly, the premature delivery rate was apparently higher in Group A than in Group B or C (13.9% versus 5.67%, 12.0%), but the differences were not statistically significant (Figure 2).

Figure 2. Rates of each outcome in the three groups. Group A, double cleavage-stage embryo transfer group; Group B, single day 6 good-quality blastocyst-stage embryo transfer group; Group C, single day 6 inferior-quality blastocyst-stage embryo transfer group. *denotes p<0.05, **denotes p<0.01. The blue bar chart represents Group A; the orange bar chart represents Group B; the grey bar chart represents Group C.
3.3 The correlation of the three transfer groups with the clinical outcomes
In order to compare the efficacy of the different transfer strategies, logistic regression analysis was conducted to detect the association of the clinical outcomes within the three transfer groups (Table 2), adjusted OR were analyzed due to the significantly differences in female ages. From the Table 2, no statistical significant difference was detected with the clinical pregnancy rate, live birth rate, abortion rate between the group A and group B. There were a lower probability of clinical pregnancy (aOR 0.501, 95%CI 0.335-0.749) and live birth (aOR 0.337, 95%CI 0.223-0.509) in the transfer of the Group C compared with the transfer of the Group A, but there was a higher risk of abortion (aOR 2.477, 95%CI 1.198-5.122) in the transfer of the Group C.
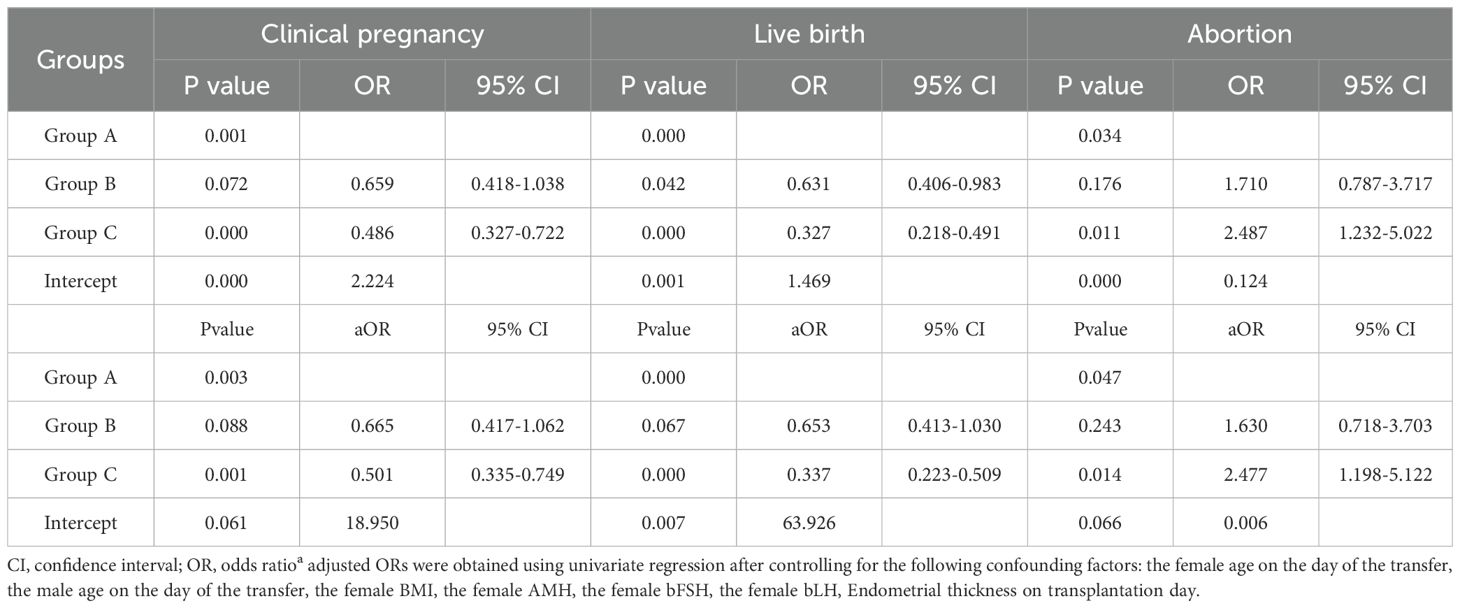
Table 2. Logistic regression analysis of pregnancy, live birth and abortion outcomes between the three groups.
4 Discussion
The cleavage-stage embryo transfer or the day 6 blastocyst-stage embryo transfer is seldom studied in the couples who had no day 5 blastocyst cryopreservation over the past two decades. In this retrospective study, we confirmed that good-quality blastocysts, even day 6 blastocysts, had a significantly higher implantation rate than cleavage-stage embryos and poor-quality day 6 blastocysts, but the implantation rate of poor-quality day 6 blastocysts was comparable to that of cleavage-stage embryos. This conclusion is consistent with those of most studies published in recent decades (21). The main reason for this may be that there is a positive relationship between blastocyst formation and embryo euploidy (20–22), such that cleavage-stage embryos with poor developmental potential often fail to develop into blastocysts (23, 24). In addition, the timing of blastocyst transfer is identical to that in natural pregnancy and synchronized with the endometrial environment (25, 26). Some papers proved that the implantation rate of blastocysts is related not to the developmental stage of the blastocysts but rather to TE quality, which may be because the euploidy rate of blastocysts with a poor TE grade was significantly lower than that of those with a high-quality TE (27–29).
We found that although good-quality day6 blastocyst had a significantly higher implantation rate, the double cleavage-stage embryo transfer can achieve a comparable chemical pregnancy and clinical pregnancy rates compared with to those of a single good-quality day 6 blatstocyst transfer. the main reason for this maybe the increased number of transferred embryos can correspondingly increase the probability of a positive clinical outcome of the embryo transfer, A meta-analysis showed that double cleavage transfer had a apparently higher clinical pregnancy rate and ongoing pregnancy rate compared with the single cleavage transfer (30). In a randomized controlled trial from Aafke also showed in unselected patient, double embryo transfer can resulted in significantly higher pregnancy rates compared with the elective single embryo transfer (31).
Although double cleavage-stage embryo transfer achieved a comparable clinical pregnancy rates and live birth rates compared with single good-quality blastocyst-stage embryo transfer in our study, it was also associated with significantly higher multiple pregnancy rates compared with the other groups. Multiple pregnancy is also associated with a significantly higher risk of perinatal mortality and morbidity, preterm birth, neonatal death and maternal complications such as hypertensive disorders, gestational diabetes and postpartum hemorrhage (32–37). In addition, multiple pregnancy is also associated with social, financial, and psychological implications for new parents, with higher levels of stress and lower quality of life (38). So in recent years, embryologists have focused on selective single embryo transfer (sSET) to reduce multiple pregnancy rates while ensuing stable pregnancy and live birth rates. Some studies showed that in good-prognosis patients or the females under 35 years old who had normal menstrual cycles, single day 5 blastocyst transfer or even a selected single cleavage-stage embryo transfer can achieve a comparable clinical pregnancy rates, live birth rates and cumulative pregnancy rates compared with double embryo transfer (39, 40). Moreover, although the premature delivery rates were not significantly different among these three groups in our data, the highest premature delivery rate was observed for the double cleavage-stage embryo transfer group. Many previous studies have shown that an increased number of transferred embryos is a relevant risk factor for premature delivery (41, 42). The lack of statistical difference in our study may be due to the limited sample size. The multiple pregnancy rates in the two blastocyst-stage transfer groups were not significantly different, besides both of them were significantly lower than the double cleavage-stage transfer group, so it is suggesting that it is the increased number of transferred embryos, not the stage of the transferred embryos is the relevant risk factor for multiple pregnancy.
From our data, it was showed that patients receiving poor-quality day 6 blastocysts had a significantly higher early miscarriage rate than those in the other groups, but the late miscarriage rates and ectopic pregnancy rates were comparable among the three groups. It is believed that higher trophoblast (TE) morphology scores correlate with higher euploidy rates and higher pregnancy rates (43–45), and at present, the evaluation of embryos mainly relies on morphological scores, but some studies demonstrated that even some aneuploidy embryos can also eventually develop into high-quality blastocysts (46). but some papers have showed that in the case of blastocysts transferred after confirmation of euploidy by preimplantation genetic testing (PGT), there is no difference in implantation rate among blastocysts of different development days and different grades; the implantation rate of even poor-quality biopsied blastocysts did not differ significantly from that of high-quality blastocysts (17–27). In the past, it was showed that the prevalence of chromosome abnormalities in women experiencing early miscarriage was as high as 45% (46–48), and the detection of submicroscopic chromosome anomalies in miscarriage samples using molecular techniques has suggested that more than 50% of miscarriages may be due to the application of the array-based gonome-wide techniques such as single nucleotide polymorphism (SNP) (49). However, the proportion of late miscarriages with chromosomal abnormalities of the fetus is lower than that of early miscarriages, as later pregnancy losses more often occur due to s uterine, cervix uteri insufficiency or immune factors (50, 51). So it is suggesting that the significant higher proportion of early abortion in the inferior-quality group may be due to the high proportion of the euploidy state of the transfer embryos.
There are also some limitations in our study. First, although single good-quality blastocyst-stage embryo transfer can achieve a comparable live birth rate and significantly lower multiple pregnancy rates compared with the double cleavage-stage embryo transfer group, it also increases the transfer cancellation rates because no good-quality blastocyst formation occurs in long-term culture in vitro, so the cumulative live birth rates per oocyte pick-up cycle, transfer cancellation rates and duration from oocyte pick-up to clinical pregnancy should be considered, and the maternal pregnancy risk and long-term follow-up studies on infants also need to be considered in the next study. Second, this study is a retrospective analysis, not a randomized controlled study, further prospective studies need to be carried out to verify these findings in multicenter trials with larger numbers of samples. Finally, there are subjective differences in blastocyst morphology scores among different embryologists, which may lead to some bias in the results, a fixed embryologist to evaluate the morphology of the blastocysts in the enrolled couples will eliminate the bias in the results.
In conclusion, based on the current evidence, single good-quality day 6 blastocyst embryo transfer is the optimal strategy for the first warming cycle of the patients who had no day 5 blastocyst cryopreservation, which can achieve a comparable live birth rate and lower the multiple pregnancy risk compared with the double cleavage-stage embryo transfer. our study provided a theoretical foundation for the clinicians and the embryologists to choose the most suitable frozen-thawed transplantation scheme for the patients without day5 blastocyst formation in the fresh cycles.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University [approval no. 2023-KY-0141-001, 26 Feb 2023]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Writing – original draft, Writing – review & editing. TW: Data curation, Writing – original draft. SD: Methodology, Software, Writing – review & editing. HS: Methodology, Software, Writing – review & editing. YS: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the National Natural Science Foundation of China (NO.81601339), Clinical Medical Research Fund of Chinese Medical Association-Reproductive Medicine Research and Developmental Projects for Youth Grant (No.18010400769).
Acknowledgments
The authors sincerely acknowledge the staff of Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University for their diligent work, and all the patients for their selfless participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thomas MR, Sparks AE, Ryan GL, van Voorhis BJ. Clinical predictors of human blastocyst formation and pregnancy after extended embryo culture and transfer. Fertil Steril. (2010) 94:543–8. doi: 10.1016/j.fertnstert.2009.03.051
2. Sills ES, Palermo GD. Human blastocyst culture in IVF: current laboratory applications in reproductive medicine practice. Rom J Morphol Embryol. (2010) 51:441–5.
3. Zhu L, Xi Q, Zhang H, Li Y, Ai J, Jin L. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod BioMed Online. (2013) 27:154–60. doi: 10.1016/j.rbmo.2013.04.006
4. Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril. (2020) 113:241–7. doi: 10.1016/j.fertnstert.2019.12.009
5. Li Y, Liu S, Lv Q. Single blastocyst stage versus single cleavage stage embryo transfer following fresh transfer: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2021) 267:11–7. doi: 10.1016/j.ejogrb.2021.10.004
6. Glujovsky D, Farquhar C. Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril. (2016) 106:244–50. doi: 10.1016/j.fertnstert.2016.06.029
7. Cornelisse S, Fleischer K, Repping S, Mastenbroek S. An informed decision between cleavage-stage and blastocyst-stage transfer in IVF requires data on the transfers of frozen-thawed embryos. Hum Reprod. (2018) 33:1370. doi: 10.1093/humrep/dey112
8. Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol. (2017) 49:583–91. doi: 10.1002/uog.17327
9. Clua E, Rodriguez I, Arroyo G, Racca A, Martinez F, Polyzos NP. Blastocyst versus cleavage embryo transfer improves cumulative live birth rates, time and cost in oocyte recipients: a randomized controlled trial. Reprod BioMed Online. (2022) 44:995–1004. doi: 10.1016/j.rbmo.2022.01.001
10. Rao J, Qiu F, Tian S, Yu Y, Zhang Y, Gu Z, et al. Clinical outcomes for Day 3 double cleavage-stage embryo transfers versus Day 5 or 6 single blastocyst transfer in frozen-thawed cycles: a retrospective comparative analysis. J Int Med Res. (2022) 49:3000605211062461. doi: 10.1177/03000605211062461
11. Shi W, Zhang W, Li N, Xue X, Liu C, Qu P, et al. Comparison of perinatal outcomes following blastocyst and cleavage-stage embryo transfer: analysis of 10 years’ data from a single centre. Reprod BioMed Online. (2019) 38:967–78. doi: 10.1016/j.rbmo.2018.12.031
12. Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. (1998) 69:84–8. doi: 10.1016/s0015-0282(97)00438-x
13. Eftekhar M, Mohammadi B, Tabibnejad N, Lahijani MM. Frozen-thawed cleavage stage versus blastocyst stage embryo transfer in high responder patients. Zygote. (2020) 28:511–5. doi: 10.1017/S0967199420000428
14. Dirican EK, Olgan S, Sakinci M, Caglar M. Blastocyst versus cleavage transfers: who benefits? Arch Gynecol Obstet. (2022) 305:749–56. doi: 10.1007/s00404-021-06224-2
15. Neuhausser WM, Vaughan DA, Sakkas D, Hacker MR, Toth T, Penzias A. Non-inferiority of cleavage-stage versus blastocyst-stage embryo transfer in poor prognosis IVF patients (PRECiSE trial): study protocol for a randomized controlled trial. Reprod Health. (2020) 17:16. doi: 10.1186/s12978-020-0870-y
16. Levi-Setti PE, Cirillo F, Smeraldi A, Morenghi E, Mulazzani GEG, Albani E. No advantage of fresh blastocyst versus cleavage stage embryo transfer in women under the age of 39: a randomized controlled study. J Assist Reprod Genet. (2018) 35:457–65. doi: 10.1007/s10815-017-1092-2
17. Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. (2014) 29:1173–81. doi: 10.1093/humrep/deu033
18. Nazem TG, Sekhon L, Lee JA, Overbey J, Pan S, Duke M, et al. The correlation between morphology and implantation of euploid human blastocysts. Reprod BioMed Online. (2019) 38:169–76. doi: 10.1016/j.rbmo.2018.10.007
19. He Y, Tang Y, Liu H, Liu J, Mao Y. No advantage of single day 6 good-quality blastocyst transfer versus single day 5 poor-quality blastocyst transfer in frozen-thawed cycles stratified by age: a retrospective study. BMC Pregn Childbirth. (2023) 23:79. doi: 10.1186/s12884-023-05387-x
20. Petersen BM, Boel M, Montag M, Gardner DK. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on Day 3. Hum Reprod. (2016) 31:2231–44. doi: 10.1093/humrep/dew188
21. Blake D, Farquhar C, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. (2016) 30:CD002118. doi: 10.1002/14651858.CD002118.pub5
22. Huang J, Zhao N, Wang X, Qiao J, Liu P. Chromosomal characteristics at cleavage and blastocyst stages from the same embryos. J Assist Reprod Genet. (2015) 32:781–7. doi: 10.1007/s10815-015-0450-1
23. Jiang X, Cai J, Liu L, Liu Z, Wang W, Chen J, et al. Does conventional morphological evaluation still play a role in predicting blastocyst formation? Reprod Biol Endocrinol. (2022) 20:68. doi: 10.1186/s12958-022-00945-y
24. Liu J, Zhou Y, Tong L, Wang X, Li Y, Wang H. Developmental potential of different embryos on day 3: a retrospective study. J Obstet Gynaecol. (2022) 42:3322–7. doi: 10.1080/01443615.2022.2125291
25. Racowsky C, Combelles CM, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod BioMed Online. (2003) 6:323–31. doi: 10.1016/s1472-6483(10)61852-4
26. Zhu D, Zhang J, Cao S, Zhang J, Heng BC, Huang M, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles–time for a new embryo transfer strategy? Fertil Steril. (2011) 95:1691–5. doi: 10.1016/j.fertnstert.2011.01.022
27. Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. (2016) 31:2245–54. doi: 10.1093/humrep/dew183
28. Kim MK, Park JK, Jeon Y, Choe SA, Lee HJ, Kim J, et al. Correlation between morphologic grading and euploidy rates of blastocysts, and clinical outcomes in in vitro fertilization preimplantation genetic screening. J Korean Med Sci. (2019) 34:e27. doi: 10.3346/jkms.2019.34.e27
29. Liu Y, Zhang X, Xu Y, Li R, Cai B, Ding C, et al. Similar implantation competence in euploid blastocysts developed on day 5 or day 6 in young women: a retrospective cohort study. Hum Fertil (Camb). (2023) 26:918–26. doi: 10.1080/14647273.2022.2022454
30. Gerris J, de Neubourg D, Mangelschots K, van Royen E, van de Meerssche M, Valkenburg M. prevention of twin preganncy after IVF or ICSI sperm injection based on strict embryo criteria a prospective randomized clinical trial. Hum Reprod. (1999) 14:2581–7. doi: 10.1093/humrep/14.10.2581
31. van Montfoort AP, Fiddelers AA, Janssen JM, Derhaag JG, Dirksen CD, Dunselman GA, et al. In unselected patients, elective single embryo transfer prevents all multiples, but results in significantly lower pregnancy rates compared with double embryo transfer: a randomized controlled trial. Hum Reprod. (2006) 21:338–43. doi: 10.1093/humrep/dei359
32. Ma S, Peng Y, Hu L, Wang X, Xiong Y, Tang Y, et al. Comparisons of benefits and risks of single embryo transfer versus double embryo transfer: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2022) 20:20. doi: 10.1186/s12958-022-00899-1
33. Kawwass JF, Badell ML. Maternal and fetal risk associated with assisted reproductive technology. Obstet Gynecol. (2018) 132:763–72. doi: 10.1097/AOG.0000000000002786
34. Campbell D. A review of maternal complications of multiple pregnancy. Twin Res. (2001) 4:146–9. doi: 10.1375/1369052012371
35. Norwitz ER, Edusa V, Park JS. Maternal physiology and complications of multiple pregnancy. Semin Perinatol. (2005) 29:338–48. doi: 10.1053/j.semperi.2005.08.002
36. Devine PC, Malone FD. Maternal complications associated with multiple pregnancy. Clin Obstet Gynecol. (2004) 47:227–36. doi: 10.1097/00003081-200403000-00023
37. Wei J, Wu QJ, Zhang TN, Shen ZQ, Liu H, Zheng DM, et al. Complications in multiple gestation pregnancy: a cross-sectional study of ten maternal-fetal medicine centers in China. Oncotarget. (2016) 7:30797–803. doi: 10.18632/oncotarget.9000
38. Sitler C, Lustik M, Levy G, Pier B. Single embryo transfer versus double embryo transfer: a cost-effectiveness analysis in a non-IVF insurance mandated system. Mil Med. (2020) 185:e1700–5. doi: 10.1093/milmed/usaa119
39. Huang X, Liu R, Shen W, Cai Y, Ding M, Sun H, et al. An elective single cleavage embryo transfer strategy to minimize twin live birth rate based on a prediction model from double cleavage embryos transfer patients. J Matern Fetal Neonatal Med. (2022) 35:1775–82. doi: 10.1080/14767058.2020.1770215
40. Kawamura T, Mori M, Arichi A, Tajima Y, Karasawa Y, Suga K, et al. Elective single embryo transfer: comparison of blastocyst and cleavage-stage embryo transfer. Reprod Med Biol. (2005) 4:197–201. doi: 10.1111/j.1447-0578.2005.00105.x
41. Chen P, Hu KL, Jin J, Chen R, Xu Q, Zhao W, et al. Risk factors for twin pregnancy in women undergoing double cleavage embryo transfer. BMC Pregn Childbirth. (2022) 22:264. doi: 10.1186/s12884-022-04606-1
42. Kamath MS, Mascarenhas M, Kirubakaran R, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. (2020) 8:CD003416. doi: 10.1002/14651858.CD003416.pub5
43. Yoshida IH, Santos M, Berton CZ, Chiarella CL, Tanada MS, BCordts E, et al. Can trophectoderm morphology act as a predictor for euploidy? JBRA Assist Reprod. (2018) 22:113–5. doi: 10.5935/1518-0557.20180036
44. Thompson SM, Onwubalili N, Brown K, Jindal SK, McGovern PG. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): a national study. J Assist Reprod Genet. (2013) 30:1577–81. doi: 10.1007/s10815-013-0100-4
45. Voullaire L, Collins V, Callaghan T, McBain J, Williamson R, Wilton L. High incidence of complex chromosome abnormality in cleavage embryos from patients with repeated implantation failure. Fertil Steril. (2007) 87:1053–8. doi: 10.1016/j.fertnstert.2006.11.043
46. Viotti M. Preimplantation genetic testing for chromosomal abnormalities: aneuploidy, mosaicism, and structural rearrangements. Genes (Basel). (2020) 11:602. doi: 10.3390/genes11060602
47. Rai R, Regan L. Recurrent miscarriage. Lancet. (2006) 368:601–11. doi: 10.1016/S0140-6736(06)69204-0
48. Jackson T, Watkins E. Early pregnancy loss. JAAPA. (2022) 34:22–7. doi: 10.1097/01.JAA.0000733216.66078.ac
49. van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta. (2012) 1822:1951–9. doi: 10.1016/j.bbadis.2012.07.001
50. Hirayama E, Ebina Y, Kato K, Akabane-Nakagawa K, Okuyama K. Cervical polyps in early pregnancy are a risk factor for late abortion and spontaneous preterm birth: a retrospective cohort study. Int J Gynaecol Obstet. (2022) 156:64–70. doi: 10.1002/ijgo.13608
Keywords: cleavage-stage embryo transfer, blastocyst-stage embryo transfer, single embryo transfer, transfer strategy, frozen-thawed transplantation
Citation: Liu Y, Wang T, Dai S, Shi H and Sun Y (2025) Frozen-thawed double cleavage-stage or frozen-thawed single day 6 blastocyst stage embryo transfer: which is preferable for patients younger than 35 without day 5 blastocyst formation? Front. Endocrinol. 16:1473854. doi: 10.3389/fendo.2025.1473854
Received: 31 July 2024; Accepted: 18 April 2025;
Published: 14 May 2025.
Edited by:
Eytan R. Barnea, BioIncept, LLC, United StatesCopyright © 2025 Liu, Wang, Dai, Shi and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingpu Sun, c3lwMjAwOEB2aXAuc2luYS5jb20=
†These authors share first authorship
‡ORCID: Taojun Wang, orcid.org/0009-0002-7233-183X
Shanjun Dai,
orcid.org/0000-0002-3000-3603
 Yan Liu
Yan Liu Taojun Wang1,2†‡
Taojun Wang1,2†‡ Hao Shi
Hao Shi Yingpu Sun
Yingpu Sun