- 1Institute for Fetology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Ultrasound Department, The Fourth Affiliated Hospital of Soochow University, Suzhou, China
- 3Department of Gynecology and Obstetrics, The First Affiliated Hospital of Soochow University, Suzhou, China
- 4Obstetrical Department, Women’s Hospital School of Medicine Zhejiang University, Hangzhou, China
- 5Department of Gynecology and Obstetrics, Taixing People’s Hospital, Taixing, China
- 6Research Institute for Reproductive Health and Genetic Diseases, Maternity and Child Health Care Hospital of Wuxi, Wuxi, China
Gestational diabetes mellitus (GDM) is one of the most common endocrine-related complications during pregnancy, and its prevalence has increased over the past three decades. GDM adversely affects the maternal cardiovascular system, umbilical–placental blood perfusion, and fetal blood flow. We conducted a comprehensive literature search and systematically evaluated and synthesized cardiovascular changes in the mothers, umbilical–placental circulation, and the progeny following exposure to GDM. Multiple pathophysiological mechanisms underlying cardiovascular alteration were investigated, including endothelial dysfunction, insulin resistance, oxidative stress, ion channel abnormalities, inflammation, angiogenic imbalance, and epigenetic modifications. These findings provide valuable insights for developing early intervention strategies and therapeutic approaches to mitigating cardiovascular risks in both mothers and offspring following GDM exposure.
1 Introduction
Gestational diabetes mellitus (GDM) represents the most prevalent metabolic and endocrine disease during pregnancy, affecting approximately 20% of pregnant women in Southeast Asia (1). GDM significantly contributes to increased perinatal morbidity and elevates the risks of adverse outcomes for both mothers and their offspring. The developmental origins of cardiovascular diseases have gained increasing recognition, with numerous studies demonstrating GDM-associated cardiovascular alterations in the mothers and offspring (2, 3). The impacts of GDM on the cardiovascular system and its potential underlying mechanisms have been extensively investigated through both clinical studies and experimental research using animal models. The umbilical–placental circulation, which serves as a crucial link between mother and fetus under GDM conditions, has emerged as a focal point in contemporary research. With the rising global prevalence of GDM, there is a corresponding increase in the risks of GDM-associated cardiovascular complications in both mothers and offspring (4). It is of significant scientific and clinical importance to synthesize existing studies on cardiovascular changes and their underlying mechanisms in the mothers and offspring exposed to GDM, which would enhance our understanding of GDM-induced cardiovascular pathophysiology and potentially identify novel approaches for early prevention and treatments of these disorders. Drawing upon an extensive body of literature, this review firstly exhibited the structural and functional alterations in the cardiovascular system among the mothers, offspring, and umbilical–placental circulation following GDM exposure.
2 Changes in the cardiovascular system after exposure to GDM
2.1 Cardiovascular changes in the mothers
Comprehensive analyses have demonstrated a significant positive association between GDM and cardiovascular diseases (CVDs) (2, 5). Pregnant women with GDM have a higher risk of developing pregnancy-induced hypertension or preeclampsia than those without GDM (6). Accumulating evidence indicates that women with a history of GDM have an elevated risk of developing cardiovascular complications, including coronary artery disease, atherosclerosis, myocardial infarction, ischemic stroke, peripheral artery disease, and heart failure later in life (7–9). These associations will be reviewed in the following sections.
2.1.1 Heart
Women with current or previous GDM have been demonstrated to exhibit subclinical cardiac dysfunction. Clinical studies have revealed that women with current or previous GDM have significant impairments in systolic function and diastolic function of the left ventricle, characterized by decreased global longitudinal strain (GLS, whether endocardial GLS or epicardial GLS) and an increased mitral valve E/E′ ratio (10–12). Cardiac output, ejection fraction, ventricular mass, heart rate, and stroke volume remained unchanged in women with GDM during the second and third trimesters of pregnancy (11, 13, 14). However, in women with a history of GDM, cardiac output and stroke volume were lower, while ejection fraction was higher than that in the control group (12, 15). There were reduced GLS, myocardial deformation, end diastolic/systolic volume, and pulmonary acceleration time in the right ventricle of women with GDM (13, 16), demonstrating the impact of GDM on cardiovascular function. The majority of existing studies have consistently demonstrated that volume, area, contraction function, and ejection fraction of the left atrium were not significantly changed in women with GDM throughout pregnancy (10, 11). Only a few studies have reported either unchanged or decreased left atrial reservoir and conduit strain in women with GDM (17). GDM pregnancies have shown a deterioration of the entire process of ventricular depolarization and repolarization, including increased QT dispersion and a shortened QRS complex (18). Women with a history of GDM demonstrated significantly reduced coronary flow velocity reserve values compared to healthy controls (12). In general, the observed cardiac changes in women with current or previous GDM did not meet established diagnostic criteria for clinical cardiac dysfunction in adults, which were classified as subclinical cardiac abnormalities.
2.1.2 Blood pressure
Women with current or previous GDM are more likely to develop hypertension. Numerous studies have demonstrated that women with current or previous GDM exhibited elevated systolic blood pressure and mean arterial pressure (10, 13, 15). However, this finding remains controversial, as some studies have reported no significant differences in blood pressure during pregnancy and postpartum (11, 19, 20). Compared to the control group, peripheral vascular resistance in women with current or previous GDM remained increased and unchanged (10, 14). These discrepancies might be attributed to variations in study population characteristics, such as gestational weeks (GWs) and sample sizes. Asma et al. reported that among 6,841 pregnancies, the 105 cases who developed GDM had significantly higher systolic blood pressure after adjustment for maternal characteristics during GW 11–13, which might serve as a potential predictor for GDM diagnosis (21).
2.1.3 Uterine artery
The uterine artery plays a crucial role in supplying blood flow to the developing fetus throughout gestation. The pulsatility index has shown inconsistent patterns when comparing GDM with normal pregnancies, including a decrease, an increase, and no changes (19, 22, 23). The resistance index was elevated in GDM pregnancies and leptin-mutation-developed GDM mice (24, 25). Compared to the control group, the ratio of peak systolic velocity to end-systolic blood flow velocity and blood flow were remarkably higher in GDM pregnancies during GW 24–31 (24), while both peak systolic velocity and diastolic velocity were significantly lower. The sensitivity of endothelium-dependent relaxation was significantly impaired in GDM mice (25). Throughout the trimesters, uterine arteries in GDM pregnancies underwent significant changes, and further studies are required to validate these findings. Dysfunction of the uterine artery impairs the utero-placental perfusion and the fetal development. It is still an important question regarding the relationship between uterine artery functions/dysfunction and fetal body weight/growth restriction in GDM.
2.1.4 Carotid artery
In most clinical studies, carotid-femoral pulse wave velocity was increased in women with GDM both during and after pregnancy (26, 27), indicating aggravating arterial stiffness. Women with GDM during pregnancy or a history of GDM had increased carotid intima-media thickness (CIMT), a recognized surrogate marker for future CVD and subclinical atherosclerosis (28, 29). Endothelial function parameters, including the pressure-strain elasticity coefficient, the common carotid stiffness index (β), and the augment index of bilateral common carotid arteries, were significantly elevated in GDM pregnancies, whereas arterial compliance was significantly lower in these patients (27, 30). No significant postpartum differences were observed in the β value and carotid elasticity between the two groups. Overall, the distensibility and elasticity of carotid artery were significantly lower in women with GDM and post-GDM women (31), suggesting an increased risk of subclinical atherosclerosis and stroke (32).
2.1.5 Ocular artery
The vessel density in the central fovea of the superficial and deep retina was remarkably lower in GDM gravidae (33). The central retinal venous diameter was higher, but the artery-to-vein ratio was lower in GDM pregnancies near term (33). The maximum diastolic velocity was significantly higher, while the resistance index was lower in the ophthalmic arteries of women with GDM at GW 28–32 (34). At GW 35–37, women with GDM have been shown to have significantly higher peak systolic velocity ratio in the ophthalmic artery (14). However, some studies reported no significant differences in ophthalmic artery indices in women with GDM, such as the peak systolic velocity ratio delta, pulsatility index, resistance index, peak velocity ratio, peak systolic velocity, and end-diastolic velocity (10, 35). The inconsistent results might be attributed to individual differences (such as GWs and metabolic status) and variations in sample sizes.
2.1.6 Other arteries
The augmentation index, assessed at brachial and radial arteries, has shown inconsistent patterns in women with current or previous GDM. While some studies reported significantly increased augmentation index values, others demonstrated no significant changes compared to healthy controls (21, 36). Pulse wave velocity in the upper limb and aorta was significantly higher in GDM pregnancies at GW 11–13 (21) and in post-GDM women at 5-year follow-up (37). Distensibility of brachial artery was lower in the women with GDM history (31). While some studies reported comparable flow-mediated dilation between GDM and normal pregnancies (38), others have identified lower flow-mediated dilation in post-GDM women compared to the control group (31, 39). Additionally, in GDM mice, maximal endothelium-dependent relaxation was decreased in mesenteric arteries (25). In hypercaloric diet-induced GDM rat, contractile response was impaired, accompanied by altered protein expression of angiotensin type 1 and 2 receptors and cyclooxygenase in the aorta (40). These findings indicate that women with GDM exhibit increased arterial stiffness and impaired vascular function, which may contribute to the increased risk of preeclampsia.
In summary, current evidence strongly supports a significant association between GDM and increased CVD risk in women, including hypertension, coronary artery diseases, atherosclerosis, and subclinical cardiac dysfunction.
2.2 Cardiovascular changes in the progeny
The Barker hypothesis, also known as the developmental origins of health and disease theory, proposes that adverse factors in utero significantly increase risks of CVDs in the offspring (41). Compared to the control group, GDM fetuses are exposed to higher blood glucose in utero, and GDM offspring have been shown an increased risk of congenital heart disease (42) and other CVDs (43). Maternal diabetes during pregnancy increases the rates of early onset of CVDs, particularly hypertension in offspring. GDM exerts long-term effects on offspring blood vessels (cerebral artery, carotid artery, pulmonary artery, aorta, mesenteric artery, and other arteries), both structurally and functionally.
2.2.1 Heart
The majority of studies have demonstrated that fetuses and neonates exposed to GDM had reduced mitral E/A ratio, increased interventricular septal thickness, elevated myocardial performance index, and prolonged isovolumic relaxation time and isovolumic contraction time (44–46). Structural cardiac alterations increased the risk of developing hypertrophic cardiomyopathy and contributed to cardiac systolic and diastolic dysfunction in GDM offspring (47–49). Furthermore, emerging evidence suggests that the right ventricle was more impaired than the left ventricle in GDM offspring (47, 49–51). The right ventricular predominance might be a potential early marker for detecting fetal cardiac dysfunction (48). However, a few studies reported no significant alteration in left ventricular systolic function, myocardial performance index, or E/A ratio in fetuses exposed to GDM (46). The impact of GDM on fetal heart rate remains inconsistent, with reports of both unchanged and increased rates (45, 52). Notably, one study proposed that during the first trimester, fetal heart rate might be highly predictive of GDM (53).
2.2.2 Blood pressure
GDM offspring had higher prevalence of hypertension (43, 54). Children exposed to GDM in utero had elevated systolic blood pressure from 3 years of age (43, 54–57), rather than during the first year of life (58). Streptozotocin diabetes in the pregnant animals resulted in hypertension in adult offspring, with elevated blood pressure from 24 weeks of age and persisting elevated throughout 30 weeks (59). The association between GDM and higher blood pressure remained solid only in male offspring (55), and not in female offspring (60, 61). These findings collectively suggest that GDM-related hypertension in offspring is both age-dependent and sex-dependent.
2.2.3 Cerebral artery
Fetus exposed to GDM demonstrates the hemodynamic alterations, with studies reporting decreased peak systolic velocity in middle cerebral arteries (24, 62, 63) and no significant changes (64). The complicated results were observed in other hemodynamic indices, such as systolic/diastolic ratio, resistance index, and pulsation index (24, 64, 65). Comparative studies reveal that children aged 9–11 years with GDM exposure had increased hypothalamic blood flow (66). Maternal high-sucrose diets consumption during pregnancy induced alterations in cerebral artery function in offspring (67).
2.2.4 Carotid artery
As a primary conduit for cerebral blood supply, the carotid artery exhibits significantly structural and functional changes in GDM progeny. Following intrauterine exposure to GDM, CIMT in neonates was increased or unchanged (68–70). Higher levels of fasting plasma glucose at 26 weeks of gestation were strongly related to increased CIMT in their offspring at the age of 6 years (71).
2.2.5 Pulmonary artery
Maternal hyperglycemia inhibited pulmonary vasculogenesis during fetal development (72). During pregnancy, there was no significant difference in acceleration time and acceleration time/ejection time (73). At 1 year of age, the acceleration time of the pulmonary artery in children born to GDM mothers was significantly lower (74).
2.2.6 Aorta
Previous studies found that in human fetuses exposed to GDM, the propagation velocities of the aortic arch were reduced at GW 34–40 (75). In 3- to 5-day-old human infants born to mothers with diabetes, the intima media thickness of the abdominal aorta was increased, while in 1-year-old offspring of women with GDM, it was not (58, 76). There was an increased aortic augmentation index in the GDM offspring (71). When compared with the aorta of the control offspring, KCl-, endothelin-1-, and noradrenaline-mediated vasoconstriction was potentiated and acetylcholine-mediated vasodilation was reduced in streptozotocin-induced female offspring but not in the male offspring, indicating that GDM programs gender-specific vascular dysfunction in the aorta (77).
2.2.7 Mesenteric artery
The mesenteric vasculature is closely associated with blood pressure, as it constitutes a component of systemic resistance arteries. Offspring of maternal diabetes during pregnancy showed an impaired endothelium-dependent relaxation in mesenteric arteries (25, 59), whereas relaxation to sodium nitroprusside remained unchanged (78). Adult offspring exposed to maternal diabetes during pregnancy had enhanced sensitivity to noradrenaline (78). Maternal high-sucrose diets accelerated vascular stiffness in the aged offspring, characterized by weakened myogenic responses and reduced phenylephrine-stimulated contraction (79).
2.2.8 Renal arteries
Doppler ultrasound analysis revealed that the systolic/diastolic ratio, resistance index, and pulsatility index were increased in the renal artery of GDM fetuses (65, 80). Neonates of mothers who maintained strict normoglycemia control during pregnancy and met the other criteria of the GDM management program exhibited no changes in renal volumes, urinary biomarkers of renal functions, or markers of tubular impairment compared to the control group. Conversely, neonates of mothers who did not maintain glycemic control and were non-compliant with the management program exhibited significantly lower renal volumes and higher activities of N-acetyl-β-D-glucosaminidase and cathepsin B (81).
2.2.9 Others
High glucose exposure during pregnancy inhibited the development of the blood vessel plexus and resulted in narrower blood vessel diameter in chick embryo (82).
In conclusion, GDM has been shown to exert both short-term and long-term effects on offspring circulation, which may be age-dependent and gender-specific. The development of CVDs in GDM offspring may be attributed to maternal hyperglycemia. Thus, glycemic control during pregnancy is vital for the cardiovascular health of GDM offspring.
2.3 Umbilical–placental circulation
Umbilical–placental circulation is essential for material exchange between the mother and fetus, typically comprising one umbilical vein, two umbilical arteries, stem placental villi, intermediate villi, and terminal villi. In normal pregnancies, the structure of chorionic villi ensures proper nutrient delivery to the fetus.
GDM is a pathology associated with vascular dysfunction in umbilical–placental circulation. In GDM, placental villi exhibit hypoplasia, with immature villi, abnormal villi branching, and excessive neovascularization (83). It is characterized by an increased distance between the intervillous space and fetal capillaries in the GDM placenta (84). The microvilli of the GDM placenta were disorganized and locally hyperplastic, with some areas showing sparse or even absent microvilli (85). The endoplasmic reticulum and mitochondria of trophoblast cells were significantly swollen, the basement membrane was thickened, and there were varying degrees of hyperplasia in the small placental arteries (83). The barrier integrity of placental vessels was compromised in GDM (86). The structural alterations in placental blood vessels seriously impair blood and oxygen supply between the placenta and fetus, and may be one of the key factors contributing to adverse pregnancy outcomes in GDM (87).
Bahiru et al. reported histopathologic changes in GDM, including umbilical cord crack, disintegration of the endothelium, and crack of umbilical vessels. Endothelial cells in GDM umbilical cords were discontinuous with focal erosions (88). Smooth muscles of GDM umbilical blood vessels appeared disturbed and showed degeneration of their strands (89). The media of GDM umbilical artery showed smooth muscle cells widely separated by connective tissue containing little collagen and few elastic fibers, along with mononuclear cell infiltration. The GDM umbilical vein has a thinner wall and a wider lumen (90).
Alterations in umbilical–placental vessel structures are closely associated with blood flow and vascular tone. Pregnancies complicated by GDM exhibited significantly lower placental volume, vascularization index, and vascularization flow index in the placenta compared to the control group during the first and second trimesters (23, 91). Most studies found that hemodynamic indices of the GDM umbilical artery, such as peak systolic velocity/end-systolic blood flow velocity, resistance index, and pulsation index, were reduced in the third trimester (65, 92). However, Cui et al. reported higher peak systolic velocity/end-systolic blood flow velocity, resistance index, and pulsation index values in the GDM umbilical artery during the third trimester, and also significantly lower peak systolic and minimum diastolic velocities (24). GDM was also reported to have no association with abnormal Doppler indices of placenta circulation (93). One possible explanation for that discrepancy is the existence of individual differences and the varying levels of maternal hyperglycemia.
Miroslav et al. found that in the GDM umbilical artery, cumulative concentrations of 5-HT-mediated vasoconstrictions were significantly attenuated (94), and the concentration–response curve for bradykinin was shifted to the left after endothelial denudation (95). Omar et al. noted that placental vasodilation caused by progesterone via cyclic adenosine monophosphate was significantly reduced (96). Abnormal vessel tone of the umbilical–placental circulation might decrease placental perfusion and the blood flow to the fetus.
Abnormal umbilical–placental circulation in GDM might be one of the most important reasons for cardiovascular changes in progeny and in women.
3 Mechanisms in GDM-related cardiovascular changes
Cardiovascular changes in women with GDM and their offspring were correlated with endothelial dysfunction, insulin resistance, oxidative stress, ion channels, inflammation, angiogenesis, and epigenetic inheritance. These mechanisms could be crucial for the better management of cardiovascular changes in GDM.
3.1 Endothelial dysfunction
Endothelial dysfunction is considered to be a hallmark of vascular disorders. Endothelial dysfunction is widely observed in GDM pregnancies, post-GDM women, the umbilical–placental circulation, and their offspring. Thus, it could be one of the mechanisms underlying GDM-induced CVDs.
GDM pregnancy exhibited impaired endothelium-dependent relaxation to methacholine in mesenteric arteries (25), along with decreased circulating endothelial progenitor cell counts (97), and modified endothelial function markers, such as nitric oxide (NO) and endothelial nitric oxide synthase (eNOS). Reduced bioavailability of NO is a consensus among researchers studying GDM (98). Women with previous GDM displayed lower flow-mediated dilation (39), higher values of markers of endothelial dysfunction, such as E-selectin and intercellular adhesion molecule-1 (ICAM-1) (99), and decreased levels of L-arginine (a critical substrate for NO synthesis) (100). These findings indicated that GDM-related endothelial dysfunction could persist into postpartum.
The umbilical–placental circulation is considered to be part of fetal circulation. Endothelial rupture and erosion were observed in umbilical vessels from GDM pregnancies (88). Fetal endothelial progenitor cells exposed to hyperglycemia in vivo or in vitro formed fewer colonies in culture, and displayed reduced proliferation, migration, and tubule formation (101). Endothelium-dependent relaxation to calcitonin was weaker in GDM umbilical veins than that in the control group (102). When compared to the control group, NO synthase activities were decreased in GDM stem villous vessels (103). The offspring from mothers with diabetes exhibited impaired endothelium-dependent relaxation in mesenteric arteries (59), decreased NO production and lowered eNOS phosphorylation in blood vessels (104), and reduced eNOS functions in regulating vessel tone (105). Thus, it is suggested that GDM-related endothelial dysfunction in the progeny may originate from the prenatal period.
However, there were significantly higher circulating endothelial functional and dysfunctional markers, including von Willebrand factor and eNOS, in GDM umbilical plasma (75, 97). In primary feto-placental endothelial cells from GDM pregnancies, there was a decrease in ICAM-1, a marker of endothelial dysfunction (106). Human umbilical vein endothelial cells (HUVECs) from GDM pregnancy or HUVECs exposed to hyperglycemia showed significantly increased L-arginine transport, enhanced human cationic amino acid transporter-1, and eNOS expression and activities (98, 107, 108). The inconsistent findings may be attributed to different tissues studied and different levels of glycemic control.
The increased circulating endothelial functional markers could originate from the umbilical–placental endothelium. Exosomes isolated from HUVECs of normal pregnancies could inhibit the changes in HUVECs from GDM pregnancies mentioned above. Conversely, exosomes from GDM HUVECs reduced eNOS phosphorylation and increased reactive oxygen species (ROS) generation in cells from normal pregnancy (108). Insulin could reverse GDM-related endothelium abnormalities (109) via activation of insulin receptors (110), A1 adenosine receptors (109), and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (111). The inhibition of endoplasmic reticulum stress and reduction of ROS levels could increase NO production and restore endothelium-dependent vasodilation in offspring of mothers with diabetes (59, 104).
3.2 Insulin resistance
Insulin resistance is a pathophysiological condition in which organs do not respond appropriately to insulin, observed in GDM pregnancies, post-GDM women, and GDM offspring, and the GDM umbilical–placental circulation (77, 112–116). Changes in insulin signaling pathways (Figure 1), such as insulin receptors and substrates, MAPK, JNK, PI3K, AKT, and mTOR, contribute to insulin resistance in GDM (117). In the plasma of women with GDM pregnancy and their offspring, there were alterations in insulin resistance-related factors, such as elevated leptin (118), tumor necrosis factor-α (TNF-α), asprosin (116), and resistin (119). The GDM placenta had increased levels of glucose transporter-4 and glucose transporter-8, and decreased levels of glucose transporters-3, which were one of the mechanisms of insulin resistance (120).
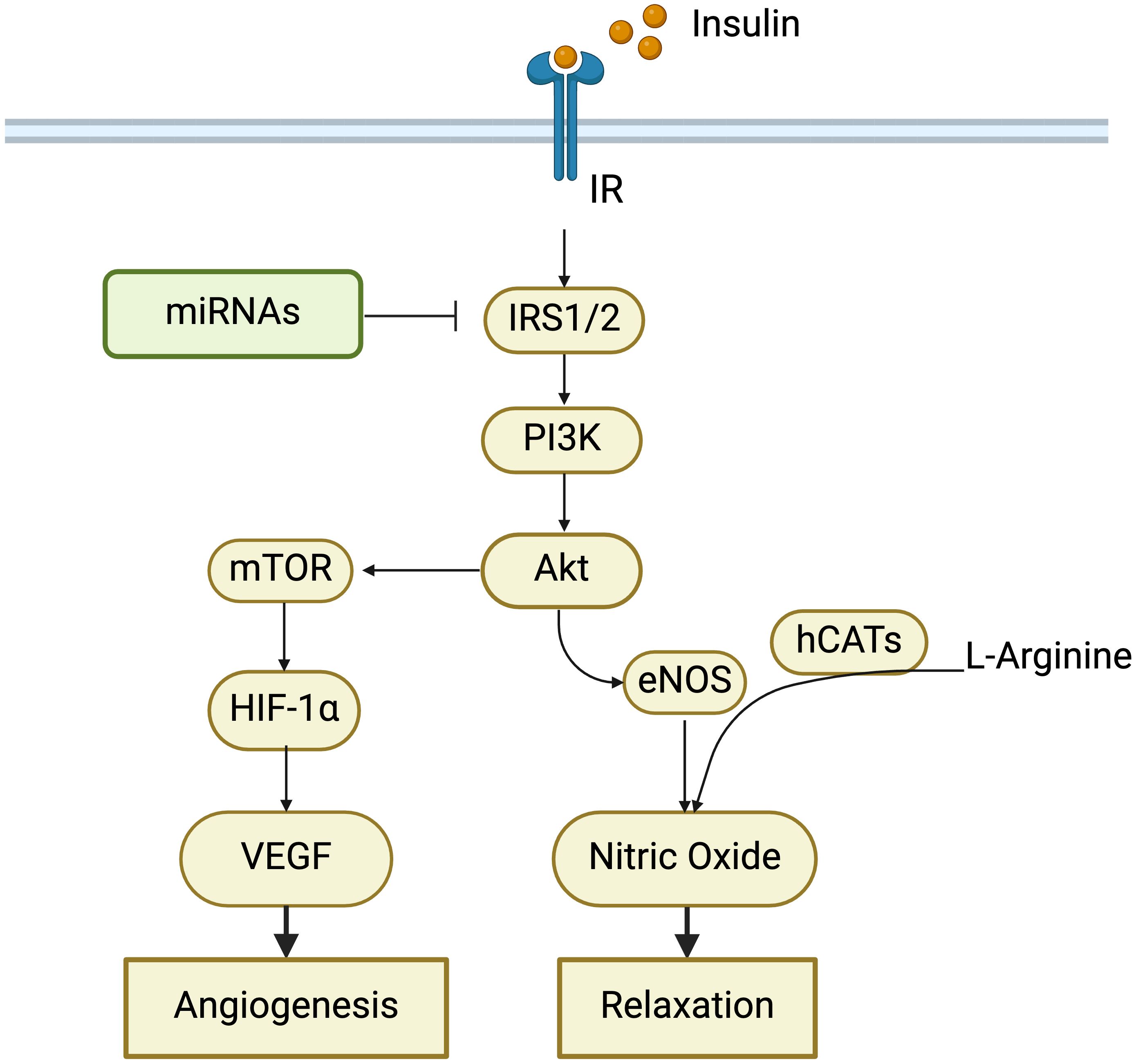
Figure 1. Role of insulin signaling pathway in cardiovascular changes in GDM. Alterations in insulin receptors (IR), insulin receptor substrate proteins 1/2 (IRS1/2), and PI3K-Akt-mTOR pathway contribute to insulin resistance, which has been observed in women with GDM and their offspring. MicroRNAs (miRNAs) can regulate insulin signaling by targeting IRS1/2. Insulin resistance impairs angiogenesis and endothelial dilation, thereby increasing the risk of developing cardiovascular diseases in GDM.
Flow-mediated dilation in brachial arteries in women with previous GDM was correlated inversely with serum markers of insulin resistance (39). Insulin resistance was found to be associated with vascular dysfunction (especially endothelium dysfunction) and arterial stiffness (121–124), thereby increasing the risks of developing CVDs. Increased maternal insulin resistance had a negative impact on placental efficiency in GDM cases (125), which may be due to the expansion of immature villi (126). Astaxanthin and naringenin have the potential to attenuate GDM symptoms by improving insulin sensitivity during pregnancy through adenosine 5′-monophosphate-activated protein kinase (127, 128).
3.3 Oxidative stress
Oxidative stress increases during gestation, and the placenta is considered to be the primary source of ROS generation (129). In the offspring and maternal tissues of GDM pregnancies, there were increased markers of oxidative stress, such as higher levels of circulating free radical production in the mothers and offspring, and reduced catalase activity in the placenta and fetus (59, 130–132).
Maternal hyperglycemia is regarded as an important cause of oxidative stress in GDM. Hyperglycemia contributes to increased ROS synthesis in endothelial cells. HUVECs from GDM showed an increased ROS synthesis, and HUVECs from normal pregnancies exposed to a high extracellular concentration of D-glucose increased NOX-dependent ROS generation (133, 134). Hyperglycemia stimulated ROS production through glucose autoxidation, mitochondrial superoxide production, eNOS uncoupling, and late glycosylation end product-dependent NADPH oxidase activation (135).
Oxidative stress in GDM pregnancy could increase cardiovascular risks in the mother and fetus, via endothelial dysfunction, decreased NO bioavailability and inflammation, and altered ion channel activities (Figure 2). ROS increased 4-hydroxynonenal production and damaged the development of coronary artery in pre-gestational diabetes fetus (136). Increased ROS and NADPH activities might cause endothelial dysfunction via the protein kinase C pathway in GDM mothers and their fetuses (137). ROS was found to induce an increase in inflammatory factors, such as interleukin-6 (IL-6) and TNF-α, which were implicated in GDM placental vascular endothelial dysfunction (138, 139). In the offspring exposed to maternal hyperglycemia, NOX4-derived superoxide inhibited large-conductance Ca2+-activated potassium channel (BKCa) activities via the AKT pathway (140). ROS affected transient receptor potential (TRP)-type Ca2+-permeable non-selective cation channels by targeting both membrane lipids and channel proteins in the term syncytiotrophoblast (141). ROS increased the expressions of multiple growth factors and activated multiple stress signals such as JNK and Pim-1, leading to smooth muscle cell proliferation, and regulated angiogenesis through NF-κB/TNF-α signaling pathway and related factors such as IL-6, ICAM-1, and vascular endothelial growth factor (VEGF) (142).
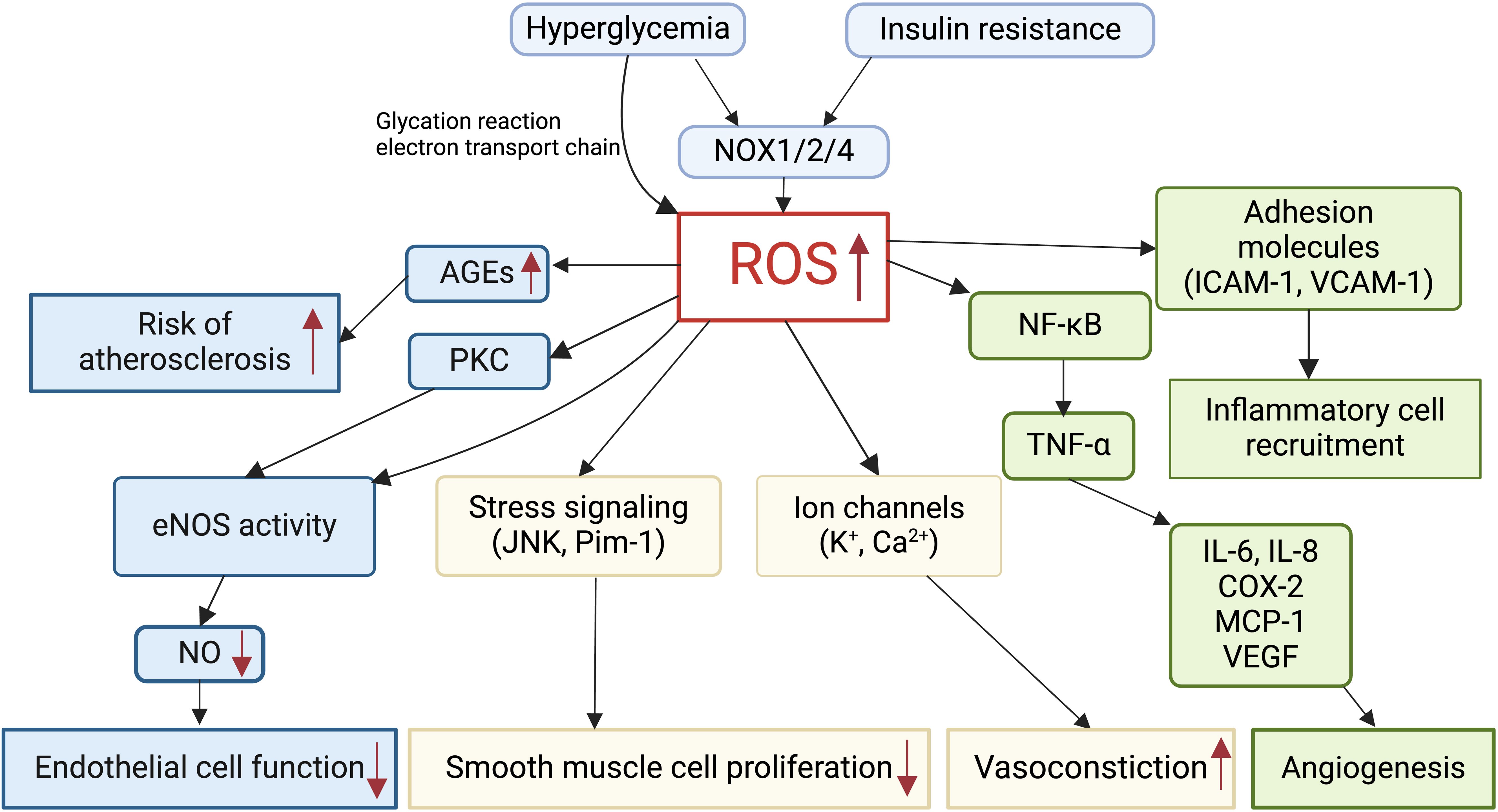
Figure 2. Role of reactive oxygen species (ROS) in cardiovascular alterations in GDM. Hyperglycemia and insulin resistance induce excessive ROS production in GDM via NADPH oxidase (NOX). Elevated ROS levels in GDM impair endothelial cell function, smooth muscle cell proliferation, vasoconstriction, and angiogenesis. ROS mediates the production of advanced glycation end products (AGEs), thereby increasing the risk of atherosclerosis. Furthermore, ROS promotes inflammatory cell recruitment and inflammation in GDM.
3.4 Ion channels
Cation channels, such as K+ and Ca2+ channels, play an important role in the regulation of vessel tone. Li et al. demonstrated that ATP-sensitive potassium channel (KATP) currents and KATP channel-mediated relaxation were impaired in GDM umbilical arteries (143). Djokic et al. found that a K+ channel opener, pinacidil, reduced relaxation in endothelium-denuded HUV compared to those from normal pregnancy, while the expression of KATP channels was decreased in GDM umbilical veins (144). BKCa current density in human umbilical artery smooth muscle cells was significantly reduced, and vasodilation mediated by BKCa agonist NS-1619 was significantly impaired in GDM (145). Changes in inwardly rectifying potassium channel and small-conductance calcium-dependent potassium channel were associated with attenuated bradykinin-mediated contraction in GDM umbilical arteries (95). Some studies reported that polymorphisms of KCNQ1 (rs2237892, rs2237895, and rs2074196) and KCNJ11 (E23K) were associated with GDM (146–149), but others found that gene polymorphisms of KCNJ11 (rs5219) and KCNQ1 (rs2237892, rs151290, rs231841, and rs7929804) were not significant risk factors for the development of GDM (150–152).
Heather found that the reduced rate of Ca2+ bursting in GDM umbilical vein endothelial cells inhibited the functions of NO, thereby leading to vascular dysfunction (153). Moreover, Miroslav et al. found that serotonin-mediated vasoconstriction was significantly attenuated in GDM umbilical arteries, which was associated with the impairment of voltage-gated Ca2+ channels and Na+/K+-ATPase (94). Furthermore, in GDM placenta, mRNA expressions of calcium transporters were downregulated, including TRPV5 and TRPV6, calcium-binding/chaperone proteins, plasma membrane calcium ATPase, inositol triphosphate receptors, and ryanodine receptors (154).
Maternal high-glucose diets during pregnancy altered the frequency and amplitude of BKCa channels, as well as L-type voltage-dependent Ca2+ channel currents in the offspring vasculature (155). Hyperglycemia affected the activities of ion channels in vascular smooth muscle (156). Altered functions, expressions, and polymorphisms of ion channels could contribute to the increased risks of developing CVDs in GDM. The role of ion channels in GDM has primarily been studied in human umbilical and placental vasculature, which is functionally analogous to fetal vasculature and provides insights into offspring cardiovascular programming.
3.5 Inflammation
Inflammatory factors play a key role in the process of GDM and GDM-mediated vascular changes. Many inflammatory factors are closely linked to GDM-mediated vascular injury, including C-reactive protein (CRP), ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and IL-6, among others (157).
Many studies demonstrated that CRP was increased in maternal serum, cord serum, and the placenta of women with GDM (158–161). Higher circulating CRP may predict the risk of GDM development. CRP was one of the significant independent predictors of developing preeclampsia in women with GDM (162). Insulin administration significantly reduced CRP concentration and ameliorated aortic injury in streptozotocin-mediated diabetic rats (163).
ICAM-1 was viewed as a symbol of endothelial dysfunction leading to vascular disorders, and its level was increased in GDM maternal serum and the umbilical–placental circulation (98, 164). Exposure to high glucose could enhance ICAM-1 expression in HUVECs by increasing the release of exosomes (165). Increased ICAM-1 significantly promoted monocyte adhesion to decidual endothelial cells in diabetic pregnancies, which could be inhibited via ICAM-1 silencing (166). In GDM umbilical cords and placental vessels, the immunostaining intensity of ICAM-1 was decreased compared to the control group (164). ICAM-1 protein was lower in primary feto-placental endothelial cells from GDM pregnancy when compared with the control group (106). Decreased ICAM-1 caused by elevated miR-130b-3p from GDM-placenta mesenchymal stem cell-derived exosomes participated in the inhibition of HUVEC proliferation, migration, and angiogenesis (167). Altered ICAM-1 plays an important role in GDM vascular pathology.
VCAM-1 was increased in the maternal serum, umbilical cord, and placenta of patients with GDM (98, 168, 169). After delivery, circulating VCAM-1 remained increased in women with GDM (170). VCAM-1 mRNA and protein levels were unchanged in primary feto-placental endothelial cells from GDM pregnancy when compared with the normal group (106), although other reports showed that VCAM-1 was increased in GDM placenta (171). High glucose stimulated the expression of VCAM-1 in HUVECs (168). Previous studies demonstrated that increased ICAM-1 and VCAM-1 were the first critical step for lymphocyte and endothelial cell interactions (172). Increased VCAM-1 primed diabetic vasculature to have enhanced interaction with circulating monocytes in human endothelial cells cultured with advanced glycation end products (173).
In GDM maternal blood and umbilical cord blood, IL-1β and IL-6 were increased or unchanged (174–178). Moreover, on the third day postpartum, women with GDM were found to have higher circulating IL-1β levels (176). Additionally, GDM placenta showed increased IL-1β and IL-6 expression (159, 179). Increased IL-1β and IL-6 were associated with vascular dysfunction in retinal arteries (180) and in forearm skin vessels (181) from GDM pregnancies. The interaction of IL-6 and TNF-α contributed to endothelial dysfunction in diabetic mice via oxidative stress and reduced eNOS phosphorylation (182). There were decreased IL-37 in the GDM umbilical–placental system (183). IL-37 inhibited the progression of vascular calcification and atherosclerosis in diabetes (184). There were also many changes in inflammatory factors in GDM, such as TNF-α, IL-10, IL-8, and IL-38. Inflammatory factors could affect endothelial functions and vascular calcification, which might finally lead to vascular disease in GDM. More studies are needed to clarify the role of inflammatory factors in GDM vascular dysfunction.
3.6 Angiogenesis
Angiogenesis is a coordinated process of proangiogenic and inhibitory factors. Histopathological analysis indicates excessive angiogenesis in GDM placenta, including increased villous vascularity and elevated number of syncytial knots (185). There were commonly increased proangiogenic factors, including the VEGF-signaling pathway (186, 187), total and active membrane-type matrix metalloproteinase 1 (188), and cognate succinate receptors (189) in GDM placenta, but there was a reduction of anti-angiogenic receptor UNC5b in GDM HUVECs (190). GDM-derived trophoblast showed altered expressions of proangiogenic factors and anti-angiogenic factors (138). Hyperglycemia-induced angiogenesis changes were associated with molecules in trophoblast (191). Exposure to GDM-like conditions enhanced the proangiogenic abilities of human amniotic membrane stem cells (192).
However, some studies reported that when compared with the control group, there was a decrease in angiogenic factors and angiogenesis modulators, such as SIRT1 (193), VEGFA, and VEGFR2 in GDM placenta (194). Maternal hyperglycemia inhibited angiogenesis in fetal pulmonary arteries (72). The HUVECs from GDM pregnancies presented increased apoptosis and decreased proliferation and angiogenesis compared with those from healthy pregnancies (195). Both the GDM conditions and hyperglycemia inhibited HUVEC proliferation, migration, and tube formation via reduced FGF2-induced activation of ERK1/2, and caused apoptosis via increased calcium entry (196, 197).
Alterations in angiogenesis in GDM were closely associated with maternal hyperglycemia, which might lead to abnormal development of both the placenta and the fetus. Abnormal umbilical coiling in GDM was related to the downregulation of the angiogenic factor VEGFA (198). The inconsistent findings may be attributed to variations in tissue types and differences in GDM-like conditions. Therefore, further studies are needed to clarify the mechanisms.
3.7 Epigenetic modification
Epigenetic mechanisms, including DNA methylation, microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and histone modifications, can produce heritable phenotypic changes without altering the DNA sequence.
DNA methylation is widely observed in GDM (199, 200). Exposure to GDM has been shown to alter DNA methylation patterns in human feto-placental arterial and venous endothelial cells, leading to aberrant cellular morphology and impaired barrier function in endothelial cells (201, 202). Notably, the promoter region of estrogen receptor 1 was found to be methylated in decidual vessels of healthy individuals, but not in GDM (203). DNA hypermethylation of HDAC2 was significantly more pronounced in GDM-HUVECs compared to control-HUVECs (204). Sun et al. further demonstrated that the abundance of 5-hydroxymethylcytosine (5hmC) in the umbilical vein of women with GDM was altered, a change linked to DNA methylation-related plasticity through oxidation mediated by ten-eleven translocation enzymes (205). These alterations in DNA methylation and 5hmC levels in GDM reflected the molecular characteristics of “type II diabetes” and “insulin resistance,” contributing to abnormal cardiovascular development and an increased risk of cardio-metabolic diseases later in life.
Multiple miRNAs are reported to play roles in cardiovascular changes associated with GDM. In a GDM rat model, inhibition of miR-873, which targeted IGFBP2, was shown to regulate insulin resistance and alleviate myocardial injury by activating the PI3K/AKT/mTOR signaling pathway, thereby mitigating the progression of GDM (206). Additionally, decreased levels of placenta-derived exosome miR-140-3p and miR-574-3p in GDM were found to inhibit the proliferation, migration, and tube formation capacity of umbilical vein endothelial cells by targeting VEGFs (207). However, miR-130b-3p exhibited an opposite effect on HUVECs compared to miR-140-3p and miR-574-3p, as its upregulation inhibited HUVEC proliferation and angiogenesis (167). Alterations in cerebrovascular functions in GDM offspring may be attributed to changes in miR-29a-3p and miR-92a-3p levels (208). Although a large number of differentially expressed miRNAs have been identified, further research is needed to elucidate the relationship between miRNAs and cardiovascular changes in GDM (209).
In GDM pregnancies, changes in circulating lncRNAs have been observed, including decreased lncRNA SNHG17 and increased lncRNA SOX2OT, which were strongly associated with adverse outcomes such as intrauterine distress and hypertension (210). Elevated levels of circVEGFC in maternal serum from GDM pregnancies might be linked to hypertension (211). Furthermore, high sucrose intake upregulated angiotensin 1 receptor expression through histone modifications, such as increased H3Ac, H3K4me3, and H3S10ph, as well as decreased H3K9me3, ultimately contributing to hypertension in aged offspring (156).
Epigenetic mechanisms may serve as mediators of persistent metabolic memory in endothelial cells exposed to hyperglycemia (204). These epigenetic modifications affect insulin resistance, angiogenesis, and vascular functions, finally leading to cardiovascular changes in GDM.
4 Prevention and treatment of GDM-related cardiovascular diseases
GDM significantly increases cardiovascular risks in both mothers and offspring. Insulin and metformin are commonly used to treat GDM, improving immediate pregnancy outcomes, and reducing the incidence of pregnancy-related hypertension (212). Metformin treatment in GDM pregnancy is associated with a reduced risk of preeclampsia (213). Additionally, treatment with metformin alone or in combination with insulin has been shown to ameliorate the increased augmentation index in the brachial arteries and aorta during GW 28–36 in GDM pregnancies (214). Insulin-treated GDM pregnancies exhibited a resistance index of umbilical arteries similar to that of the control group (92). This review summarizes the effects of exercise, dietary modification, and probiotics on cardiovascular changes associated with GDM.
4.1 Exercise
Exercise is effective for controlling blood glucose and insulin levels in GDM pregnancies (215, 216). It modestly improved cardiorespiratory fitness in both GDM pregnancies and their fetuses (217), as evidenced by elevated heart rates (218). Moderate-intensity resistance exercise has been found to be beneficial for improving blood pressure in patients with GDM (219). Exercise reduced uterine artery pulsatility indexes in GDM pregnancies (220). During exercise, women with GDM exhibited blunted cerebral oxygenation, which was correlated with macrovascular functions (221). Moderate-intensity exercise improved oxidation capacity in GDM pregnancies (220). Exercise is highly recommended for the management of GDM and has been shown to be beneficial in preventing cardiovascular damage in both mothers and their offspring.
4.2 Dietary modification
Modified dietary interventions favorably influenced maternal glycemia, insulin levels, and fetal birth weight in GDM (222, 223). Compared with the control group, there was an increased augmentation index in the brachial arteries and aorta from GDM pregnancies with diet management, which could be attenuated by treatment with metformin alone or in combination with insulin (214). The resistance index in umbilical arteries was lower in GDM pregnancies managed with diet interventions compared to the control group, whereas no significant difference was observed between the insulin-treated GDM group and the control group (92). In diet alone-controlled GDM placentas, occludin expression was lower than that in placentas from normal pregnancies and metformin-controlled GDM pregnancies (86). These findings indicated that dietary modification alone during pregnancy may not be sufficient to reverse impaired vascular functions and placental barrier integrity. However, a diet rich in monounsaturated fatty acids demonstrated favorable effects on diastolic blood pressure in women with GDM compared to a high-carbohydrate diet (224).
4.3 Probiotics
The consumption of Lactobacillus and Bifidobacterium probiotics decreased fasting plasma glucose, serum insulin levels, insulin resistance, inflammatory factors (such as CRP and IL-6), and oxidative stress markers, while probiotics significantly increased insulin sensitivity, plasma NO levels, and total antioxidant capacity in GDM pregnancies (225–227). However, some studies have reported that probiotic supplementation was not associated with a reduced risk of hypertensive disorders in GDM pregnancies (228, 229). In contrast, excessive probiotic supplementation might increase the risk of preeclampsia in women with GDM (230). The inconsistent findings may be attributed to variations in probiotic strains, dosages, timing of intervention, and individual differences. Probiotics have demonstrated a positive impact on glycemic control, and further research is needed to clarify their role in preventing CVDs in women with GDM and their offspring.
5 Conclusions
GDM exerts both short- and long-term effects on cardiovascular changes in mothers and their offspring. The influence of GDM on offspring may stem from alterations in umbilical–placental circulation and the direct consequences of maternal hyperglycemia. Endothelial dysfunction, insulin resistance, oxidative stress, ion channel abnormalities, inflammation, impaired angiogenesis, and epigenetic modifications collectively contribute to the structural and functional abnormalities of the cardiovascular system in GDM. Early diagnosis and intervention, along with strategies such as exercise, dietary modifications, and probiotics supplementation, may have beneficial effects on GDM-related cardiovascular changes.
Author contributions
ZZ: Conceptualization, Investigation, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. YZ: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing, Software. SH: Writing – original draft, Writing – review & editing, Visualization. ML: Writing – original draft. LL: Writing – original draft. LQ: Writing – original draft. YH: Writing – original draft. ZX: Writing – original draft, Writing – review & editing. JT: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Project administration, Software, Supervision, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported by grants from the Natural Science Foundation of China (82101761) and the Natural Science Foundation of Jiangsu Province (BK20200194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BKCa, large-conductance Ca2+-activated K+ channel; CIMT, carotid intima-media thickness; CRP, C-reactive protein; CVD, cardiovascular disease; eNOS, endothelial nitric oxide synthase; GDM, gestational diabetes mellitus; GLS, global longitudinal strain; GW, gestational week; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; KATP, ATP-sensitive potassium channel; lncRNAs, long noncoding RNAs; miRNAs, microRNAs; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor.
References
1. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
2. Kramer CK, Campbell S, and Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. (2019) 62:905–14. doi: 10.1007/s00125-019-4840-2
3. Moon JH and Jang HC. Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diabetes Metab J. (2022) 46:3–14. doi: 10.4093/dmj.2021.0335
4. Johns EC, Denison FC, Norman JE, and Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. (2018) 29:743–54. doi: 10.1016/j.tem.2018.09.004
5. Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. (2017) 177:1735–42. doi: 10.1001/jamainternmed.2017.2790
6. Chatzakis C, Sotiriadis A, Demertzidou E, Eleftheriades A, Dinas K, Vlahos N, et al. Prevalence of preeclampsia and uterine arteries resistance in the different phenotypes of gestational diabetes mellitus. Diabetes Res Clin Pract. (2023) 195:110222. doi: 10.1016/j.diabres.2022.110222
7. Khoja A, Andraweera PH, Tavella R, Gill TK, Dekker GA, Roberts CT, et al. Pregnancy complications are associated with premature coronary artery disease: linking three cohorts. J Womens Health (Larchmt). (2023) 32:1208–18. doi: 10.1089/jwh.2023.0239
8. Gunderson EP, Sun B, Catov JM, Carnethon M, Lewis CE, Allen NB, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA study. Circulation. (2021) 143:974–87. doi: 10.1161/CIRCULATIONAHA.120.047320
9. Retnakaran R and Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. (2009) 181:371–6. doi: 10.1503/cmaj.090569
10. Anzoategui S, Gibbone E, Wright A, Nicolaides KH, and Charakida M. Midgestation cardiovascular phenotype in women who develop gestational diabetes and hypertensive disorders of pregnancy: comparative study. Ultrasound Obstet Gynecol. (2022) 60:207–14. doi: 10.1002/uog.24929
11. Aguilera J, Semmler J, Coronel C, Georgiopoulos G, Simpson J, Nicolaides KH, et al. Paired maternal and fetal cardiac functional measurements in women with gestational diabetes mellitus at 35-36 weeks’ gestation. Am J Obstet Gynecol. (2020) 223:574.e1– e15. doi: 10.1016/j.ajog.2020.04.019
12. Caliskan M, Turan Y, Caliskan Z, Gullu H, Ciftci FC, Avci E, et al. Previous gestational diabetes history is associated with impaired coronary flow reserve. Ann Med. (2015) 47:615–23. doi: 10.3109/07853890.2015.1099719
13. Company Calabuig AM, Nunez E, Sanchez A, Nicolaides KH, Charakida M, and De Paco Matallana C. Three-dimensional echocardiography and cardiac strain imaging in women with gestational diabetes mellitus. Ultrasound Obstet Gynecol. (2021) 58:278–84. doi: 10.1002/uog.23666
14. Mansukhani T, Arechvo A, Cecchini F, Breim M, Wright A, Nicolaides KH, et al. Vascular phenotype at 35-37 weeks’ gestation in women with gestational diabetes mellitus. Ultrasound Obstet Gynecol. (2023) 61:386–91. doi: 10.1002/uog.26077
15. Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, and Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. (2005) 90:3983–8. doi: 10.1210/jc.2004-2494
16. Buddeberg BS, Sharma R, O’Driscoll JM, Kaelin Agten A, Khalil A, and Thilaganathan B. Impact of gestational diabetes mellitus on maternal cardiac adaptation to pregnancy. Ultrasound Obstet Gynecol. (2020) 56:240–6. doi: 10.1002/uog.21941
17. Li W, Li Z, Liu W, Zhao P, Che G, Wang X, et al. Two-dimensional speckle tracking echocardiography in assessing the subclinical myocardial dysfunction in patients with gestational diabetes mellitus. Cardiovasc Ultrasound. (2022) 20:21. doi: 10.1186/s12947-022-00292-3
18. Medova E, Fialova E, Mlcek M, Slavicek J, Dohnalova A, Charvat J, et al. QT dispersion and electrocardiographic changes in women with gestational diabetes mellitus. Physiol Res. (2012) 61:S49–55. doi: 10.33549/physiolres
19. Sweeting AN, Wong J, Appelblom H, Ross GP, Kouru H, Williams PF, et al. A first trimester prediction model for gestational diabetes utilizing aneuploidy and pre-eclampsia screening markers. J Matern Fetal Neonatal Med. (2018) 31:2122–30. doi: 10.1080/14767058.2017.1336759
20. Rueangjaroen P, Luewan S, Phrommintikul A, Leemasawat K, and Tongsong T. The cardio-ankle vascular index as a predictor of adverse pregnancy outcomes. J Hypertens. (2021) 39:2082–91. doi: 10.1097/HJH.0000000000002907
21. Khalil A, Garcia-Mandujano R, Chiriac R, Akolekar R, and Nicolaides KH. Maternal hemodynamics at 11-13 weeks’ gestation in gestational diabetes mellitus. Fetal Diagn Ther. (2012) 31:216–20. doi: 10.1159/000336692
22. Perez-Martin SM, Quintero-Prado R, Lara-Barea A, Lopez-Tinoco C, Torrejon R, and Bugatto F. Fetal cerebral three-dimensional power Doppler vascularization indices and their relationships with maternal glucose levels in pregnancies complicated with gestational diabetes. Diabetes Vasc Dis Res. (2022) 19:14791641221078109. doi: 10.1177/14791641221078109
23. Wong CH, Chen CP, Sun FJ, and Chen CY. Comparison of placental three-dimensional power Doppler indices and volume in the first and the second trimesters of pregnancy complicated by gestational diabetes mellitus. J Matern Fetal Neonatal Med. (2019) 32:3784–91. doi: 10.1080/14767058.2018.1472226
24. Wei Z, Mu M, Li M, Li J, and Cui Y. Color Doppler ultrasound detection of hemodynamic changes in pregnant women with GDM and analysis of their influence on pregnancy outcomes. Am J Transl Res. (2021) 13:3330–6.
25. Stanley JL, Cheung CC, Rueda-Clausen CF, Sankaralingam S, Baker PN, and Davidge ST. Effect of gestational diabetes on maternal artery function. Reprod Sci. (2011) 18:342–52. doi: 10.1177/1933719110393029
26. Vilmi-Kerala T, Lauhio A, Tervahartiala T, Palomaki O, Uotila J, Sorsa T, et al. Subclinical inflammation associated with prolonged TIMP-1 upregulation and arterial stiffness after gestational diabetes mellitus: a hospital-based cohort study. Cardiovasc Diabetol. (2017) 16:49. doi: 10.1186/s12933-017-0530-x
27. Zhou Y, Lan Q, Li Y, Qi L, Dong Y, Zhou H, et al. Clinical value of echo-tracking in gestational diabetes mellitus. Exp Clin Endocrinol Diabetes. (2022) 130:783–8. doi: 10.1055/a-1926-7064
28. Atay AE, Simsek H, Demir B, Sakar MN, Kaya M, Pasa S, et al. Noninvasive assessment of subclinical atherosclerosis in normotensive gravidae with gestational diabetes. Herz. (2014) 39:627–32. doi: 10.1007/s00059-013-3874-3
29. Li JW, He SY, Liu P, Luo L, Zhao L, and Xiao YB. Association of gestational diabetes mellitus (GDM) with subclinical atherosclerosis: a systemic review and meta-analysis. BMC Cardiovasc Disord. (2014) 14:132. doi: 10.1186/1471-2261-14-132
30. Lange A, Brueckmann A, Seeliger C, Jahr R, Hunger-Battefeld W, Schlembach D, et al. PP063. Carotid artery stiffness and elasticity in gestational diabetes. Pregnancy Hypertens. (2013) 3:89–90. doi: 10.1016/j.preghy.2013.04.088
31. Davenport MH, Goswami R, Shoemaker JK, and Mottola MF. Influence of hyperglycemia during and after pregnancy on postpartum vascular function. Am J Physiol Regul Integr Comp Physiol. (2012) 302:R768–75. doi: 10.1152/ajpregu.00115.2011
32. Lee SM, Shivakumar M, Park JW, Jung YM, Choe EK, Kwak SH, et al. Long-term cardiovascular outcomes of gestational diabetes mellitus: a prospective UK Biobank study. Cardiovasc Diabetol. (2022) 21:221. doi: 10.1186/s12933-022-01663-w
33. Evliyaoglu F, Kurt MM, Yilmaz M, and Akpolat C. Evaluation of microvascular density and retinal vessel diameter in gestational and type 2 diabetes using swept-source OCT-A technology. J Fr Ophtalmol. (2022) 45:430–7. doi: 10.1016/j.jfo.2021.06.014
34. Moneta-Wielgos J, Golebiewska J, Brydak-Godowska J, Ciszewska J, Bomba-Opon DA, Wegrzyn P, et al. Doppler flow parameters in orbital arteries in gestational diabetes mellitus patients. J Matern Fetal Neonatal Med. (2014) 27:1075–7. doi: 10.3109/14767058.2013.847916
35. dos Anjos Gde F, Diniz AL, dos Santos MC, and Damian NG. Study of ophthalmic artery hemodynamic pattern in pregnant women with gestational diabetes mellitus. Rev Bras Ginecol Obstet. (2012) 34:473–7. doi: 10.1590/s0100-72032012001000007
36. Savvidou MD, Anderson JM, Kaihura C, and Nicolaides KH. Maternal arterial stiffness in pregnancies complicated by gestational and type 2 diabetes mellitus. Am J Obstet Gynecol. (2010) 203:274.e1–7. doi: 10.1016/j.ajog.2010.06.021
37. Lekva T, Bollerslev J, Norwitz ER, Aukrust P, Henriksen T, and Ueland T. Aortic stiffness and cardiovascular risk in women with previous gestational diabetes mellitus. PLoS One. (2015) 10:e0136892. doi: 10.1371/journal.pone.0136892
38. Garg P, Badhwar S, Jaryal AK, Kachhawa G, Deepak KK, and Kriplani A. The temporal trend of vascular function in women with gestational diabetes. Vasc Med. (2017) 22:96–102. doi: 10.1177/1358863X16678479
39. Anastasiou E, Lekakis JP, Alevizaki M, Papamichael CM, Megas J, Souvatzoglou A, et al. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care. (1998) 21:2111–5. doi: 10.2337/diacare.21.12.2111
40. Tufino C, Villanueva-Lopez C, Ibarra-Barajas M, Bracho-Valdes I, and Bobadilla-Lugo RA. Experimental gestational diabetes mellitus induces blunted vasoconstriction and functional changes in the rat aorta. BioMed Res Int. (2014) 2014:329634. doi: 10.1155/2014/329634
41. Barker DJ, Osmond C, Golding J, Kuh D, and Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. (1989) 298:564–7. doi: 10.1136/bmj.298.6673.564
42. Ji H, Liang H, Yu Y, Wang Z, Yuan W, Qian X, et al. Association of maternal history of spontaneous abortion and stillbirth with risk of congenital heart disease in offspring of women with vs without type 2 diabetes. JAMA Netw Open. (2021) 4:e2133805. doi: 10.1001/jamanetworkopen.2021.33805
43. Yu Y, Arah OA, Liew Z, Cnattingius S, Olsen J, Sorensen HT, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. (2019) 367:l6398. doi: 10.1136/bmj.l6398
44. Ghaderian M, Hemmat M, Behdad S, Saeedi M, and Shahsanaei F. Fetal cardiac functional abnormalities assessed by echocardiography in mothers suffering gestational diabetes mellitus: A systematic review and meta-analysis. Curr Probl Cardiol. (2021) 46:100658. doi: 10.1016/j.cpcardiol.2020.100658
45. Ghandi Y, Habibi D, Nasri K, Alinejad S, Taherahmad H, Arjmand Shabestari A, et al. Effect of well-controlled gestational diabetes on left ventricular diastolic dysfunction in neonates. J Matern Fetal Neonatal Med. (2019) 32:2101–6. doi: 10.1080/14767058.2018.1425832
46. Depla AL, De Wit L, Steenhuis TJ, Slieker MG, Voormolen DN, Scheffer PG, et al. Effect of maternal diabetes on fetal heart function on echocardiography: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2021) 57:539–50. doi: 10.1002/uog.22163
47. Huang P, Deng Y, Feng L, Gao Y, Cheng X, and Liu H. Evaluation of fetal cardiac function in maternal gestational diabetes mellitus by speckle-tracking echocardiography. J Ultrasound Med. (2023) 42:81–9. doi: 10.1002/jum.15994
48. Hou Q, Yan F, Dong X, Liu H, Wu J, Li J, et al. Assessment of fetal cardiac diastolic function of gestational diabetes mellitus using dual-gate Doppler. Med (Baltimore). (2021) 100:e26645. doi: 10.1097/MD.0000000000026645
49. Yovera L, Zaharia M, Jachymski T, Velicu-Scraba O, Coronel C, de Paco Matallana C, et al. Impact of gestational diabetes mellitus on fetal cardiac morphology and function: cohort comparison of second- and third-trimester fetuses. Ultrasound Obstet Gynecol. (2021) 57:607–13. doi: 10.1002/uog.22148
50. Chen Y, Chen Q, Wu Y, Wang H, Fan Q, Lei W, et al. Fetal cardiac geometry and function in pregnancies with well-controlled gestational diabetes mellitus using Fetal HQ. J Matern Fetal Neonatal Med. (2022) 35:8331–7. doi: 10.1080/14767058.2021.1973996
51. Miranda JO, Cerqueira RJ, Ramalho C, Areias JC, and Henriques-Coelho T. Fetal cardiac function in maternal diabetes: A conventional and speckle-tracking echocardiographic study. J Am Soc Echocardiogr. (2018) 31:333–41. doi: 10.1016/j.echo.2017.11.007
52. Buscicchio G, Gentilucci L, Giannubilo SR, and Tranquilli AL. Computerized analysis of fetal heart rate in pregnancies complicated by gestational diabetes mellitus. Gynecol Endocrinol. (2010) 26:270–4. doi: 10.3109/09513590903247840
53. Sirico A, Lanzone A, Mappa I, Sarno L, Slodki M, Pitocco D, et al. The role of first trimester fetal heart rate in the prediction of gestational diabetes: A multicenter study. Eur J Obstet Gynecol Reprod Biol. (2019) 243:158–61. doi: 10.1016/j.ejogrb.2019.10.019
54. Lu J, Zhang S, Li W, Leng J, Wang L, Liu H, et al. Maternal gestational diabetes is associated with offspring’s hypertension. Am J Hypertens. (2019) 32:335–42. doi: 10.1093/ajh/hpz005
55. Li Z, Wu Y, Du B, Yu X, Wang H, Niu Y, et al. Associations of maternal gestational diabetes mellitus with alterations in cardiovascular system in early childhood. Diabetes Metab Res Rev. (2022) 38:e3551. doi: 10.1002/dmrr.v38.6
56. Tsadok MA, Friedlander Y, Paltiel O, Manor O, Meiner V, Hochner H, et al. Obesity and blood pressure in 17-year-old offspring of mothers with gestational diabetes: insights from the Jerusalem Perinatal Study. Exp Diabetes Res. (2011) 2011:906154. doi: 10.1155/2011/906154
57. Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, and Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. (2009) 22:215–20. doi: 10.1038/ajh.2008.326
58. Wacker-Gussmann A, Schopen J, Engelhard J, Sitzberger C, Lienert N, Ewert P, et al. The impact of gestational diabetes in pregnancy on the cardiovascular system of children at one year of age. J Clin Med. (2021) 10:5839. doi: 10.3390/jcm10245839
59. Luo H, Lan C, Fan C, Gong X, Chen C, Yu C, et al. Down-regulation of AMPK/PPARdelta signalling promotes endoplasmic reticulum stress-induced endothelial dysfunction in adult rat offspring exposed to maternal diabetes. Cardiovasc Res. (2022) 118:2304–16. doi: 10.1093/cvr/cvab280
60. de Sa FG, de Queiroz DB, Ramos-Alves FE, Santos-Rocha J, da Silva OA, Moreira HS, et al. Hyperglycaemia in pregnant rats causes sex-related vascular dysfunction in adult offspring: role of cyclooxygenase-2. Exp Physiol. (2017) 102:1019–36. doi: 10.1113/EP086132
61. Yan J, Li X, Su R, Zhang K, and Yang H. Long-term effects of maternal diabetes on blood pressure and renal function in rat male offspring. PLoS One. (2014) 9:e88269. doi: 10.1371/journal.pone.0088269
62. Fatihoglu E, Aydin S, Karavas E, and Kantarci M. Gestational diabetes mellitus and early hemodynamic changes in fetus. J Med Ultrasound. (2021) 29:270–6. doi: 10.4103/JMU.JMU_161_20
63. Dantas AMA, Palmieri ABS, Vieira MR, Souza MLR, and Silva JC. Doppler ultrasonographic assessment of fetal middle cerebral artery peak systolic velocity in gestational diabetes mellitus. Int J Gynaecol Obstet. (2019) 144:174–9. doi: 10.1002/ijgo.2019.144.issue-2
64. Shabani Zanjani M, Nasirzadeh R, Fereshtehnejad SM, Yoonesi Asl L, Alemzadeh SA, and Askari S. Fetal cerebral hemodynamic in gestational diabetic versus normal pregnancies: a Doppler velocimetry of middle cerebral and umbilical arteries. Acta Neurol Belg. (2014) 114:15–23. doi: 10.1007/s13760-013-0221-7
65. Liu F, Liu Y, Lai YP, Gu XN, Liu DM, and Yang M. Fetal hemodynamics and fetal growth indices by ultrasound in late pregnancy and birth weight in gestational diabetes mellitus. Chin Med J (Engl). (2016) 129:2109–14. doi: 10.4103/0366-6999.189057
66. Page KA, Luo S, Wang X, Chow T, Alves J, Buchanan TA, et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care. (2019) 42:1473–80. doi: 10.2337/dc18-2581
67. Wu L, Shi A, Zhu D, Bo L, Zhong Y, Wang J, et al. High sucrose intake during gestation increases angiotensin II type 1 receptor-mediated vascular contractility associated with epigenetic alterations in aged offspring rats. Peptides. (2016) 86:133–44. doi: 10.1016/j.peptides.2016.11.002
68. Atabek ME, Cagan HH, Selver Eklioglu B, and Oran B. Absence of increase in carotid artery intima-media thickness in infants of diabetic mothers. J Clin Res Pediatr Endocrinol. (2011) 3:144–8. doi: 10.4274/jcrpe.v3i3.28
69. Di Bernardo S, Mivelaz Y, Epure AM, Vial Y, Simeoni U, Bovet P, et al. Assessing the consequences of gestational diabetes mellitus on offspring’s cardiovascular health: MySweetHeart Cohort study protocol, Switzerland. BMJ Open. (2017) 7:e016972. doi: 10.1136/bmjopen-2017-016972
70. Epure AM, Di Bernardo S, Mivelaz Y, Estoppey Younes S, Chiolero A, Sekarski N, et al. Gestational diabetes mellitus and offspring’s carotid intima-media thickness at birth: MySweetHeart Cohort study. BMJ Open. (2022) 12:e061649. doi: 10.1136/bmjopen-2022-061649
71. Yuan WL, Lin J, Kramer MS, Godfrey KM, Gluckman PD, Chong YS, et al. Maternal glycemia during pregnancy and child carotid intima media thickness, pulse wave velocity, and augmentation index. J Clin Endocrinol Metab. (2020) 105:dgaa211. doi: 10.1210/clinem/dgaa211
72. Luo Q, Chai X, Xin X, Ouyang W, and Deng F. Maternal hyperglycemia inhibits pulmonary vasculogenesis during mouse fetal lung development by promoting GbetaL Ubiquitination-dependent mammalian target of Rapamycin assembly. Diabetol Metab Syndr. (2023) 15:49. doi: 10.1186/s13098-022-00974-y
73. Han T, Jin XD, Yang JF, and Tang Y. Clinical analysis of fetal lung development index and pregnancy outcome in pregnant women with gestational diabetes mellitus with satisfactory blood glucose control. Contrast Media Mol Imaging. (2022) 2022:5777804. doi: 10.1155/2022/5777804
74. Smith A, Franklin O, McCallion N, Breathnach F, and El-Khuffash A. Assessment of myocardial function in infants of mothers with gestational diabetes mellitus using deformation imaging over the first year of age. J Pediatr. (2023) 263:113645. doi: 10.1016/j.jpeds.2023.113645
75. Chen Y, Huang D, Liu J, Zeng F, Tang G, Lei W, et al. Non-invasive detection of fetal vascular endothelial function in gestational diabetes mellitus. Front Endocrinol (Lausanne). (2021) 12:763683. doi: 10.3389/fendo.2021.763683
76. Triantafyllidou P, Papadopoulou A, Thymara E, Papaevangelou V, Mastorakos G, Papadimitriou A, et al. Aortic intima-media thickness is increased in neonates of mothers with gestational diabetes mellitus: the role of thioredoxin-interacting protein as a marker of oxidative stress. Curr Vasc Pharmacol. (2023) 21:234–45. doi: 10.2174/1570161121666230727150854
77. Segar EM, Norris AW, Yao JR, Hu S, Koppenhafer SL, Roghair RD, et al. Programming of growth, insulin resistance and vascular dysfunction in offspring of late gestation diabetic rats. Clin Sci (Lond). (2009) 117:129–38. doi: 10.1042/CS20080550
78. Holemans K, Gerber RT, Meurrens K, De Clerck F, Poston L, and Van Assche FA. Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia. (1999) 42:81–9. doi: 10.1007/s001250051117
79. Feng X, Li X, Yang C, Ren Q, Zhang W, Li N, et al. Maternal high-sucrose diet accelerates vascular stiffness in aged offspring via suppressing Ca(v) 1.2 and contractile phenotype of vascular smooth muscle cells. Mol Nutr Food Res. (2019) 63:e1900022. doi: 10.1002/mnfr.201900022
80. Sadat Jamal A, Naemi M, Eslamian L, Marsoosi V, Moshfeghi M, Nurzadeh M, et al. The association between fetal renal artery indices in late pregnancy and birth weight in gestational diabetes mellitus: A cohort study. Int J Reprod Biomed. (2022) 20:21–8. doi: 10.18502/ijrm.v20i1.10405
81. Aisa MC, Cappuccini B, Barbati A, Clerici G, Torlone E, Gerli S, et al. Renal consequences of gestational diabetes mellitus in term neonates: A multidisciplinary approach to the DOHaD perspective in the prevention and early recognition of neonates of GDM mothers at risk of hypertension and chronic renal diseases in later life. J Clin Med. (2019) 8:429. doi: 10.3390/jcm8040429
82. Jin YM, Zhao SZ, Zhang ZL, Chen Y, Cheng X, Chuai M, et al. High glucose level induces cardiovascular dysplasia during early embryo development. Exp Clin Endocrinol Diabetes. (2019) 127:590–7. doi: 10.1055/s-0043-109696
83. Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao C, et al. Ultrastructure of placenta of gravidas with gestational diabetes mellitus. Obstet Gynecol Int. (2015) 2015:283124. doi: 10.1155/2015/283124
84. Daskalakis G, Marinopoulos S, Krielesi V, Papapanagiotou A, Papantoniou N, Mesogitis S, et al. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. (2008) 87:403–7. doi: 10.1080/00016340801908783
85. Akarsu S, Bagirzade M, Omeroglu S, and Buke B. Placental vascularization and apoptosis in Type-1 and gestational DM. J Matern Fetal Neonatal Med. (2017) 30:1045–50. doi: 10.1080/14767058.2016.1199676
86. Villota SD, Toledo-Rodriguez M, and Leach L. Compromised barrier integrity of human feto-placental vessels from gestational diabetic pregnancies is related to downregulation of occludin expression. Diabetologia. (2021) 64:195–210. doi: 10.1007/s00125-020-05290-6
87. Carrasco-Wong I, Moller A, Giachini FR, Lima VV, Toledo F, Stojanova J, et al. Placental structure in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165535. doi: 10.1016/j.bbadis.2019.165535
88. Chakraborty SK and Banu LA. Microscopic impacts of gestational diabetes mellitus on the umbilical cord. Mymensingh Med J. (2013) 22:755–60.
89. Tenaw Goshu B. Histopathologic impacts of diabetes mellitus on umbilical cord during pregnancy. Pediatr Health Med Ther. (2022) 13:37–41. doi: 10.2147/PHMT.S323812
90. Kadivar M, Khamseh ME, Malek M, Khajavi A, Noohi AH, and Najafi L. Histomorphological changes of the placenta and umbilical cord in pregnancies complicated by gestational diabetes mellitus. Placenta. (2020) 97:71–8. doi: 10.1016/j.placenta.2020.06.018
91. Han Z, Zhang Y, Li X, Chiu WH, Yin Y, and Hou H. Investigation into the predictive potential of three-dimensional ultrasonographic placental volume and vascular indices in gestational diabetes mellitus. Front Endocrinol (Lausanne). (2021) 12:689888. doi: 10.3389/fendo.2021.689888
92. Reitter A, Hajduk B, Geka F, Buxmann H, Schlosser R, and Louwen F. Doppler studies of gestational diabetes in the third trimester. Ultraschall Med. (2011) 32 Suppl 2:E162–8. doi: 10.1055/s-0031-1273415
93. Salvesen DR, Higueras MT, Mansur CA, Freeman J, Brudenell JM, and Nicolaides KH. Placental and fetal Doppler velocimetry in pregnancies complicated by maternal diabetes mellitus. Am J Obstet Gynecol. (1993) 168:645–52. doi: 10.1016/0002-9378(93)90512-H
94. Radenkovic M, Radunovic N, Momcilov P, and Grbovic L. Altered response of human umbilical artery to 5-HT in gestational diabetic pregnancy. Pharmacol Rep. (2009) 61:520–8. doi: 10.1016/S1734-1140(09)70095-7
95. Radenkovic M, Grbovic L, Radunovic N, and Momcilov P. Pharmacological evaluation of bradykinin effect on human umbilical artery in normal, hypertensive and diabetic pregnancy. Pharmacol Rep. (2007) 59:64–73.
96. Omar HA, Ramirez R, Arsich J, Tracy T, Glover D, and Gibson M. Reduction of the human placental vascular relaxation to progesterone by gestational diabetes. J Matern Fetal Investig. (1998) 8:27–30.
97. Mordwinkin NM, Ouzounian JG, Yedigarova L, Montoro MN, Louie SG, and Rodgers KE. Alteration of endothelial function markers in women with gestational diabetes and their fetuses. J Matern Fetal Neonatal Med. (2013) 26:507–12. doi: 10.3109/14767058.2012.736564
98. Di Fulvio P, Pandolfi A, Formoso G, Di Silvestre S, Di Tomo P, Giardinelli A, et al. Features of endothelial dysfunction in umbilical cord vessels of women with gestational diabetes. Nutr Metab Cardiovasc Dis. (2014) 24:1337–45. doi: 10.1016/j.numecd.2014.06.005
99. Bo S, Valpreda S, Menato G, Bardelli C, Botto C, Gambino R, et al. Should we consider gestational diabetes a vascular risk factor? Atherosclerosis. (2007) 194:e72–9. doi: 10.1016/j.atherosclerosis.2006.09.017
100. Mittermayer F, Kautzky-Willer A, Winzer C, Krzyzanowska K, Prikoszovich T, Demehri S, et al. Elevated concentrations of asymmetric dimethylarginine are associated with deterioration of glucose tolerance in women with previous gestational diabetes mellitus. J Intern Med. (2007) 261:392–8. doi: 10.1111/j.1365-2796.2007.01772.x
101. Gui J, Rohrbach A, Borns K, Hillemanns P, Feng L, Hubel CA, et al. Vitamin D rescues dysfunction of fetal endothelial colony forming cells from individuals with gestational diabetes. Placenta. (2015) 36:410–8. doi: 10.1016/j.placenta.2015.01.195
102. Contreras-Duarte S, Carvajal L, Garchitorena MJ, Subiabre M, Fuenzalida B, Cantin C, et al. Gestational diabetes mellitus treatment schemes modify maternal plasma cholesterol levels dependent to women s weight: possible impact on feto-placental vascular function. Nutrients. (2020) 12:506. doi: 10.3390/nu12020506
103. Dollberg S, Brockman DE, and Myatt L. Nitric oxide synthase activity in umbilical and placental vascular tissue of gestational diabetic pregnancies. Gynecol Obstet Invest. (1997) 44:177–81. doi: 10.1159/000291514
104. Yu C, Chen S, Wang X, Wu G, Zhang Y, Fu C, et al. Exposure to maternal diabetes induces endothelial dysfunction and hypertension in adult male rat offspring. Microvasc Res. (2021) 133:104076. doi: 10.1016/j.mvr.2020.104076
105. Katkhuda R, Peterson ES, Roghair RD, Norris AW, Scholz TD, and Segar JL. Sex-specific programming of hypertension in offspring of late-gestation diabetic rats. Pediatr Res. (2012) 72:352–61. doi: 10.1038/pr.2012.93
106. Diaz-Perez FI, Hiden U, Gauster M, Lang I, Konya V, Heinemann A, et al. Post-transcriptional down regulation of ICAM-1 in feto-placental endothelium in GDM. Cell Adh Migr. (2016) 10:18–27. doi: 10.1080/19336918.2015.1127467
107. Contreras-Duarte S, Cantin C, Farias M, and Leiva A. High total cholesterol and triglycerides levels increase arginases metabolism, impairing nitric oxide signaling and worsening fetoplacental endothelial dysfunction in gestational diabetes mellitus pregnancies. Biochim Biophys Acta Mol Basis Dis. (2021) 1867:166216. doi: 10.1016/j.bbadis.2021.166216
108. Saez T, Salsoso R, Leiva A, Toledo F, de Vos P, Faas M, et al. Human umbilical vein endothelium-derived exosomes play a role in foetoplacental endothelial dysfunction in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:499–508. doi: 10.1016/j.bbadis.2017.11.010
109. Guzman-Gutierrez E, Armella A, Toledo F, Pardo F, Leiva A, and Sobrevia L. Insulin requires A1 adenosine receptors expression to reverse gestational diabetes-increased L-arginine transport in human umbilical vein endothelium. Purinergic Signal. (2016) 12:175–90. doi: 10.1007/s11302-015-9491-2
110. Subiabre M, Villalobos-Labra R, Silva L, Fuentes G, Toledo F, and Sobrevia L. Role of insulin, adenosine, and adipokine receptors in the foetoplacental vascular dysfunction in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165370. doi: 10.1016/j.bbadis.2018.12.021
111. Gonzalez M, Rojas S, Avila P, Cabrera L, Villalobos R, Palma C, et al. Insulin reverses D-glucose-increased nitric oxide and reactive oxygen species generation in human umbilical vein endothelial cells. PLoS One. (2015) 10:e0122398. doi: 10.1371/journal.pone.0122398
112. Bianco ME and Josefson JL. Hyperglycemia during pregnancy and long-term offspring outcomes. Curr Diabetes Rep. (2019) 19:143. doi: 10.1007/s11892-019-1267-6
113. Hivert MF, White F, Allard C, James K, Majid S, Aguet F, et al. Placental IGFBP1 levels during early pregnancy and the risk of insulin resistance and gestational diabetes. Nat Med. (2024) 30:1689–95. doi: 10.1038/s41591-024-02936-5
114. Lacroix M, Kina E, and Hivert MF. Maternal/fetal determinants of insulin resistance in women during pregnancy and in offspring over life. Curr Diabetes Rep. (2013) 13:238–44. doi: 10.1007/s11892-012-0360-x
115. Mathew SA and Bhonde R. Mesenchymal stromal cells isolated from gestationally diabetic human placenta exhibit insulin resistance, decreased clonogenicity and angiogenesis. Placenta. (2017) 59:1–8. doi: 10.1016/j.placenta.2017.09.002
116. Zhong L, Long Y, Wang S, Lian R, Deng L, Ye Z, et al. Continuous elevation of plasma asprosin in pregnant women complicated with gestational diabetes mellitus: A nested case-control study. Placenta. (2020) 93:17–22. doi: 10.1016/j.placenta.2020.02.004
117. Villalobos-Labra R, Silva L, Subiabre M, Araos J, Salsoso R, Fuenzalida B, et al. Akt/mTOR role in human foetoplacental vascular insulin resistance in diseases of pregnancy. J Diabetes Res. (2017) 2017:5947859. doi: 10.1155/2017/5947859
118. Yilmaz O, Kucuk M, Ilgin A, and Dagdelen M. Assessment of insulin sensitivity/resistance and their relations with leptin concentrations and anthropometric measures in a pregnant population with and without gestational diabetes mellitus. J Diabetes Complications. (2010) 24:109–14. doi: 10.1016/j.jdiacomp.2009.01.006
119. Jiang S, Teague AM, Tryggestad JB, Lyons TJ, and Chernausek SD. Fetal circulating human resistin increases in diabetes during pregnancy and impairs placental mitochondrial biogenesis. Mol Med. (2020) 26:76. doi: 10.1186/s10020-020-00205-y
120. Ustianowski L, Czerewaty M, Kielbowski K, Bakinowska E, Tarnowski M, Safranow K, et al. Placental expression of glucose and zinc transporters in women with gestational diabetes. J Clin Med. (2024) 13:3500. doi: 10.3390/jcm13123500
121. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. (2021) 119:154766. doi: 10.1016/j.metabol.2021.154766
122. Steinberg HO and Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. (2002) 45:623–34. doi: 10.1007/s00125-002-0800-2
123. Busija DW, Miller AW, Katakam P, and Erdos B. Adverse effects of reactive oxygen species on vascular reactivity in insulin resistance. Antioxid Redox Signal. (2006) 8:1131–40. doi: 10.1089/ars.2006.8.1131
124. Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. (2008) 295:E732–50. doi: 10.1152/ajpendo.90477.2008
125. Tanaka K, Yamada K, Matsushima M, Izawa T, Furukawa S, Kobayashi Y, et al. Increased maternal insulin resistance promotes placental growth and decreases placental efficiency in pregnancies with obesity and gestational diabetes mellitus. J Obstet Gynaecol Res. (2018) 44:74–80. doi: 10.1111/jog.2018.44.issue-1
126. Ehlers E, Talton OO, Schust DJ, and Schulz LC. Placental structural abnormalities in gestational diabetes and when they develop: A scoping review. Placenta. (2021) 116:58–66. doi: 10.1016/j.placenta.2021.04.005
127. Feng W, Wang Y, Guo N, Huang P, and Mi Y. Effects of astaxanthin on inflammation and insulin resistance in a mouse model of gestational diabetes mellitus. Dose Response. (2020) 18:1559325820926765. doi: 10.1177/1559325820926765
128. Li S, Zhang Y, Sun Y, Zhang G, Bai J, Guo J, et al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr Diabetes. (2019) 9:28. doi: 10.1038/s41387-019-0095-8
129. Joo EH, Kim YR, Kim N, Jung JE, Han SH, and Cho HY. Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int J Mol Sci. (2021) 22:10122. doi: 10.3390/ijms221810122
130. Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, and Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. (2006) 27:327–32. doi: 10.1016/j.placenta.2005.01.002
131. Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, and Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. (2011) 15:3061–100. doi: 10.1089/ars.2010.3765
132. Peuchant E, Brun JL, Rigalleau V, Dubourg L, Thomas MJ, Daniel JY, et al. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin Biochem. (2004) 37:293–8. doi: 10.1016/j.clinbiochem.2003.12.005
133. Karbach S, Jansen T, Horke S, Heeren T, Scholz A, Coldewey M, et al. Hyperglycemia and oxidative stress in cultured endothelial cells–a comparison of primary endothelial cells with an immortalized endothelial cell line. J Diabetes Complications. (2012) 26:155–62. doi: 10.1016/j.jdiacomp.2012.03.011
134. Sultan SA, Liu W, Peng Y, Roberts W, Whitelaw D, and Graham AM. The role of maternal gestational diabetes in inducing fetal endothelial dysfunction. J Cell Physiol. (2015) 230:2695–705. doi: 10.1002/jcp.v230.11
135. Giacco F and Brownlee M. Oxidative stress and diabetic complications. Circ Res. (2010) 107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545
136. Moazzen H, Lu X, Liu M, and Feng Q. Pregestational diabetes induces fetal coronary artery malformation via reactive oxygen species signaling. Diabetes. (2015) 64:1431–43. doi: 10.2337/db14-0190
137. Rajaraman B, Ramadas N, Krishnasamy S, Ravi V, Pathak A, Devasena CS, et al. Hyperglycaemia cause vascular inflammation through advanced glycation end products/early growth response-1 axis in gestational diabetes mellitus. Mol Cell Biochem. (2019) 456:179–90. doi: 10.1007/s11010-019-03503-0
138. Loegl J, Nussbaumer E, Cvitic S, Huppertz B, Desoye G, and Hiden U. GDM alters paracrine regulation of feto-placental angiogenesis via the trophoblast. Lab Invest. (2017) 97:409–18. doi: 10.1038/labinvest.2016.149
139. Murthi P, Sarkis R, Lim R, Nguyen-Ngo C, Pratt A, Liong S, et al. Endocan expression is increased in the placenta from obese women with gestational diabetes mellitus. Placenta. (2016) 48:38–48. doi: 10.1016/j.placenta.2016.10.003
140. Feng X, Zhou X, Zhang W, Li X, He A, Liu B, et al. Maternal high-sucrose diets altered vascular large-conductance Ca2+-activated K+ channels via reactive oxygen species in offspring rats. Biol Reprod. (2017) 96:1085–95. doi: 10.1093/biolre/iox031
141. Montalbetti N, Cantero MR, Dalghi MG, and Cantiello HF. Reactive oxygen species inhibit polycystin-2 (TRPP2) cation channel activity in term human syncytiotrophoblast. Placenta. (2008) 29:510–8. doi: 10.1016/j.placenta.2008.02.015
142. Saucedo R, Ortega-Camarillo C, Ferreira-Hermosillo A, Diaz-Velazquez MF, Meixueiro-Calderon C, and Valencia-Ortega J. Role of oxidative stress and inflammation in gestational diabetes mellitus. Antioxidants (Basel). (2023) 12:1812. doi: 10.3390/antiox12101812
143. Li H, Shin SE, Seo MS, An JR, Ha KS, Han ET, et al. Alterations of ATP-sensitive K(+) channels in human umbilical arterial smooth muscle during gestational diabetes mellitus. Pflugers Arch. (2018) 470:1325–33. doi: 10.1007/s00424-018-2154-8
144. Djokic V, Jankovic-Raznatovic S, Novakovic R, Kostic M, Rajkovic J, Labudovic-Borovic M, et al. Effect of gestational diabetes mellitus and pregnancy-induced hypertension on human umbilical vein smooth muscle K(ATP) channels. Exp Mol Pathol. (2019) 111:104323. doi: 10.1016/j.yexmp.2019.104323
145. Li H, An JR, Seo MS, Kang M, Heo R, Park S, et al. Downregulation of large-conductance Ca(2+)-activated K(+) channels in human umbilical arterial smooth muscle cells in gestational diabetes mellitus. Life Sci. (2022) 288:120169. doi: 10.1016/j.lfs.2021.120169
146. Ao D, Wang HJ, Wang LF, Song JY, Yang HX, and Wang Y. The rs2237892 polymorphism in KCNQ1 influences gestational diabetes mellitus and glucose levels: A case-control study and meta-analysis. PloS One. (2015) 10:e0128901. doi: 10.1371/journal.pone.0128901
147. Shin HD, Park BL, Shin HJ, Kim JY, Park S, Kim B, et al. Association of KCNQ1 polymorphisms with the gestational diabetes mellitus in Korean women. J Clin Endocrinol Metab. (2010) 95:445–9. doi: 10.1210/jc.2009-1393
148. Kwak SH, Kim TH, Cho YM, Choi SH, Jang HC, and Park KS. Polymorphisms in KCNQ1 are associated with gestational diabetes in a Korean population. Horm Res Paediatr. (2010) 74:333–8. doi: 10.1159/000313918
149. Shaat N, Ekelund M, Lernmark A, Ivarsson S, Almgren P, Berntorp K, et al. Association of the E23K polymorphism in the KCNJ11 gene with gestational diabetes mellitus. Diabetologia. (2005) 48:2544–51. doi: 10.1007/s00125-005-0035-0
150. Majcher S, Ustianowski P, Malinowski D, Czerewaty M, Tarnowski M, Safranow K, et al. KCNJ11 and KCNQ1 gene polymorphisms and placental expression in women with gestational diabetes mellitus. Genes (Basel). (2022) 13:1315. doi: 10.3390/genes13081315
151. Chon SJ, Kim SY, Cho NR, Min DL, Hwang YJ, and Mamura M. Association of variants in PPARgamma(2), IGF2BP2, and KCNQ1 with a susceptibility to gestational diabetes mellitus in a Korean population. Yonsei Med J. (2013) 54:352–7. doi: 10.3349/ymj.2013.54.2.352
152. Petry CJ, Mooslehner K, Prentice P, Hayes MG, Nodzenski M, Scholtens DM, et al. Associations between a fetal imprinted gene allele score and late pregnancy maternal glucose concentrations. Diabetes Metab. (2017) 43:323–31. doi: 10.1016/j.diabet.2017.03.002
153. Anaya HA, Yi FX, Boeldt DS, Krupp J, Grummer MA, Shah DM, et al. Changes in Ca2+ Signaling and nitric oxide output by human umbilical vein endothelium in diabetic and gestational diabetic pregnancies. Biol Reprod. (2015) 93:60. doi: 10.1095/biolreprod.115.128645
154. Varshney S, Adela R, Kachhawa G, Dada R, Kulshreshtha V, Kumari R, et al. Disrupted placental vitamin D metabolism and calcium signaling in gestational diabetes and pre-eclampsia patients. Endocrine. (2023) 80:191–200. doi: 10.1007/s12020-022-03272-9
155. Wu C, Li J, Bo L, Gao Q, Zhu Z, Li D, et al. High-sucrose diets in pregnancy alter angiotensin II-mediated pressor response and microvessel tone via the PKC/Cav1.2 pathway in rat offspring. Hypertens Res. (2014) 37:818–23. doi: 10.1038/hr.2014.94
156. Nieves-Cintron M, Flores-Tamez VA, Le T, Baudel MM, and Navedo MF. Cellular and molecular effects of hyperglycemia on ion channels in vascular smooth muscle. Cell Mol Life Sci. (2021) 78:31–61. doi: 10.1007/s00018-020-03582-z
157. Kastelan S, Tomic M, Pavan J, and Oreskovic S. Maternal immune system adaptation to pregnancy–a potential influence on the course of diabetic retinopathy. Reprod Biol Endocrinol. (2010) 8:124. doi: 10.1186/1477-7827-8-124
158. Yu N, Cui H, Chen X, and Chang Y. Changes of serum pentraxin-3 and hypersensitive CRP levels during pregnancy and their relationship with gestational diabetes mellitus. PLoS One. (2019) 14:e0224739. doi: 10.1371/journal.pone.0224739
159. Zhang J, Chi H, Xiao H, Tian X, Wang Y, Yun X, et al. Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) single nucleotide polymorphisms (SNPs), inflammation and metabolism in gestational diabetes mellitus in inner Mongolia. Med Sci Monit. (2017) 23:4149–57. doi: 10.12659/MSM.903565
160. Huang X, Li Y, Tong X, Wu Y, Zhang R, Sheng L, et al. Increased circulating IL-32 is associated with placenta macrophage-derived IL-32 and gestational diabetes mellitus. J Clin Endocrinol Metab. (2024) 109:333–43. doi: 10.1210/clinem/dgad531
161. Nelson SM, Sattar N, Freeman DJ, Walker JD, and Lindsay RS. Inflammation and endothelial activation is evident at birth in offspring of mothers with type 1 diabetes. Diabetes. (2007) 56:2697–704. doi: 10.2337/db07-0662
162. Barden A, Singh R, Walters BN, Ritchie J, Roberman B, and Beilin LJ. Factors predisposing to pre-eclampsia in women with gestational diabetes. J Hypertens. (2004) 22:2371–8. doi: 10.1097/00004872-200412000-00020
163. Alzamil NM, Dawood AF, Hewett PW, Bin-Jaliah I, Assiri AS, Abdel Kader DH, et al. Suppression of type 2 diabetes mellitus-induced aortic ultrastructural alterations in rats by insulin: an association of vascular injury biomarkers. Ultrastruct Pathol. (2020) 44:316–23. doi: 10.1080/01913123.2020.1780362
164. Kurt M, Zulfikaroglu E, Ucankus NL, Omeroglu S, and Ozcan U. Expression of intercellular adhesion molecule-1 in umbilical and placental vascular tissue of gestational diabetic and normal pregnancies. Arch Gynecol Obstet. (2010) 281:71–6. doi: 10.1007/s00404-009-1066-4
165. Saez T, de Vos P, Kuipers J, Sobrevia L, and Faas MM. Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta. (2018) 66:26–35. doi: 10.1016/j.placenta.2018.04.010
166. Xie L, Galettis A, Morris J, Jackson C, Twigg SM, and Gallery ED. Intercellular adhesion molecule-1 (ICAM-1) expression is necessary for monocyte adhesion to the placental bed endothelium and is increased in type 1 diabetic human pregnancy. Diabetes Metab Res Rev. (2008) 24:294–300. doi: 10.1002/dmrr.v24:4
167. Gao Z, Wang N, and Liu X. Human placenta mesenchymal stem cell-derived exosome shuttling microRNA-130b-3p from gestational diabetes mellitus patients targets ICAM-1 and perturbs human umbilical vein endothelial cell angiogenesis. Acta Diabetol. (2022) 59:1091–107. doi: 10.1007/s00592-022-01910-2
168. Shan Y, Cui J, Kang X, Tang W, Lu Y, Gao Y, et al. Aquaporin-8 overexpression is involved in vascular structure and function changes in placentas of gestational diabetes mellitus patients. Open Life Sci. (2022) 17:1473–86. doi: 10.1515/biol-2022-0522
169. Siddiqui K, George TP, Nawaz SS, and Joy SS. VCAM-1, ICAM-1 and selectins in gestational diabetes mellitus and the risk for vascular disorders. Future Cardiol. (2019) 15:339–46. doi: 10.2217/fca-2018-0042
170. Kautzky-Willer A, Fasching P, Jilma B, Waldhausl W, and Wagner OF. Persistent elevation and metabolic dependence of circulating E-selectin after delivery in women with gestational diabetes mellitus. J Clin Endocrinol Metab. (1997) 82:4117–21. doi: 10.1210/jcem.82.12.4419
171. Zheng Y, Zhu N, Wang J, Zhao N, and Yuan C. Crocetin suppresses gestational diabetes in streptozotocin-induced diabetes mellitus rats via suppression of inflammatory reaction. J Food Biochem. (2021) 45:e13857. doi: 10.1111/jfbc.13857
172. Tang S, Le-Ruppert KC, and Gabel VP. Expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on proliferating vascular endothelial cells in diabetic epiretinal membranes. Br J Ophthalmol. (1994) 78:370–6. doi: 10.1136/bjo.78.5.370
173. Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. (1995) 96:1395–403. doi: 10.1172/JCI118175
174. Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. (2006) 91:4137–43. doi: 10.1210/jc.2006-0980
175. Teng Y, Xuan S, Jiang M, Tian L, Tian J, and Chang Q. Expression of H(2)S in gestational diabetes mellitus and correlation analysis with inflammatory markers IL-6 and TNF-alpha. J Diabetes Res. (2020) 2020:3085840. doi: 10.1155/2020/3085840
176. Vitoratos N, Valsamakis G, Mastorakos G, Boutsiadis A, Salakos N, Kouskouni E, et al. Pre- and early post-partum adiponectin and interleukin-1beta levels in women with and without gestational diabetes. Hormones (Athens). (2008) 7:230–6. doi: 10.14310/horm.2002.1202
177. Hara Cde C, Franca EL, Fagundes DL, de Queiroz AA, Rudge MV, Honorio-Franca AC, et al. Characterization of natural killer cells and cytokines in maternal placenta and fetus of diabetic mothers. J Immunol Res. (2016) 2016:7154524. doi: 10.1155/2016/7154524
178. Ma Y, Xu S, Meng J, and Li L. Protective effect of nimbolide against streptozotocin induced gestational diabetes mellitus in rats via alteration of inflammatory reaction, oxidative stress, and gut microbiota. Environ Toxicol. (2022) 37:1382–93. doi: 10.1002/tox.23491
179. Huang X, Zha B, Zhang M, Li Y, Wu Y, Zhang R, et al. Decreased monocyte count is associated with gestational diabetes mellitus development, macrosomia, and inflammation. J Clin Endocrinol Metab. (2022) 107:192–204. doi: 10.1210/clinem/dgab657
180. Liu Y, Biarnes Costa M, and Gerhardinger C. IL-1beta is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1beta autostimulation. PLoS One. (2012) 7:e36949. doi: 10.1371/journal.pone.0036949
181. Mrizak I, Arfa A, Fekih M, Debbabi H, Bouslema A, Boumaiza I, et al. Inflammation and impaired endothelium-dependant vasodilatation in non obese women with gestational diabetes mellitus: preliminary results. Lipids Health Dis. (2013) 12:93. doi: 10.1186/1476-511X-12-93
182. Lee J, Lee S, Zhang H, Hill MA, Zhang C, and Park Y. Interaction of IL-6 and TNF-alpha contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One. (2017) 12:e0187189. doi: 10.1371/journal.pone.0187189
183. Yu Z, Liu J, Zhang R, Huang X, Sun T, Wu Y, et al. IL-37 and 38 signalling in gestational diabetes. J Reprod Immunol. (2017) 124:8–14. doi: 10.1016/j.jri.2017.09.011
184. Chai M, Ji Q, Zhang H, Zhou Y, Yang Q, Zhou Y, et al. The protective effect of interleukin-37 on vascular calcification and atherosclerosis in apolipoprotein E-deficient mice with diabetes. J Interferon Cytokine Res. (2015) 35:530–9. doi: 10.1089/jir.2014.0212
185. Jarmuzek P, Wielgos M, and Bomba-Opon D. Placental pathologic changes in gestational diabetes mellitus. Neuro Endocrinol Lett. (2015) 36:101–5.
186. Hosni A, El-Twab SA, Abdul-Hamid M, Prinsen E, AbdElgawad H, Abdel-Moneim A, et al. Cinnamaldehyde mitigates placental vascular dysfunction of gestational diabetes and protects from the associated fetal hypoxia by modulating placental angiogenesis, metabolic activity and oxidative stress. Pharmacol Res. (2021) 165:105426. doi: 10.1016/j.phrs.2021.105426
187. Troncoso F, Acurio J, Herlitz K, Aguayo C, Bertoglia P, Guzman-Gutierrez E, et al. Gestational diabetes mellitus is associated with increased pro-migratory activation of vascular endothelial growth factor receptor 2 and reduced expression of vascular endothelial growth factor receptor 1. PLoS One. (2017) 12:e0182509. doi: 10.1371/journal.pone.0182509
188. Hiden U, Lassance L, Tabrizi NG, Miedl H, Tam-Amersdorfer C, Cetin I, et al. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. J Clin Endocrinol Metab. (2012) 97:3613–21. doi: 10.1210/jc.2012-1212
189. Atallah R, Gindlhuber J, Platzer W, Barnthaler T, Tatzl E, Toller W, et al. SUCNR1 is expressed in human placenta and mediates angiogenesis: significance in gestational diabetes. Int J Mol Sci. (2021) 22:12048. doi: 10.3390/ijms222112048
190. Prieto CP, Casas BS, Falcon P, Villanueva A, Lois P, Lattus J, et al. Downregulation of the netrin-1 receptor UNC5b underlies increased placental angiogenesis in human gestational diabetes mellitus. Int J Mol Sci. (2019) 20:1408. doi: 10.3390/ijms20061408
191. Chang SC and Vivian Yang WC. Hyperglycemia induces altered expressions of angiogenesis associated molecules in the trophoblast. Evid Based Complement Alternat Med. (2013) 2013:457971. doi: 10.1155/2013/457971
192. Klid S, Algaba-Chueca F, Maymo-Masip E, Guarque A, Ballesteros M, Diaz-Perdigones C, et al. The angiogenic properties of human amniotic membrane stem cells are enhanced in gestational diabetes and associate with fetal adiposity. Stem Cell Res Ther. (2021) 12:608. doi: 10.1186/s13287-021-02678-y
193. Alqudah A, Eastwood KA, Jerotic D, Todd N, Hoch D, McNally R, et al. FKBPL and SIRT-1 are downregulated by diabetes in pregnancy impacting on angiogenesis and endothelial function. Front Endocrinol (Lausanne). (2021) 12:650328. doi: 10.3389/fendo.2021.650328
194. Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao L, et al. Expressions of VEGF-A and VEGFR-2 in placentae from GDM pregnancies. Reprod Biol Endocrinol. (2016) 14:61. doi: 10.1186/s12958-016-0191-8
195. Ye HH, Yang SH, and Zhang Y. MEG3 damages fetal endothelial function induced by gestational diabetes mellitus via AKT pathway. Eur Rev Med Pharmacol Sci. (2018) 22:8553–60. doi: 10.26355/eurrev_201812_16617
196. Zhou J, Ni X, Huang X, Yao J, He Q, Wang K, et al. Potential role of hyperglycemia in fetoplacental endothelial dysfunction in gestational diabetes mellitus. Cell Physiol Biochem. (2016) 39:1317–28. doi: 10.1159/000447836
197. Tamareille S, Mignen O, Capiod T, Rucker-Martin C, and Feuvray D. High glucose-induced apoptosis through store-operated calcium entry and calcineurin in human umbilical vein endothelial cells. Cell Calcium. (2006) 39:47–55. doi: 10.1016/j.ceca.2005.09.008
198. Najafi L, Honardoost M, Khajavi A, Cheraghi S, Kadivar M, and Khamseh ME. The association of umbilical coiling and angiogenesis markers: Impact assessment of gestational diabetes. Placenta. (2022) 129:70–6. doi: 10.1016/j.placenta.2022.09.006
199. Kang J, Lee CN, Li HY, Hsu KH, and Lin SY. Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population. Diabetes Res Clin Pract. (2017) 132:127–36. doi: 10.1016/j.diabres.2017.07.034
200. Sevilla-Domingo M, Olivo-Ramirez CG, Huerta-Padilla VM, Gomez-Diaz RA, Gonzalez-Carranza E, Acevedo-Rodriguez GE, et al. Downregulation of SLC16A11 is present in offspring of mothers with gestational diabetes. Arch Med Res. (2022) 53:516–23. doi: 10.1016/j.arcmed.2022.07.002
201. Zhu W, Shen Y, Liu J, Fei X, Zhang Z, Li M, et al. Epigenetic alternations of microRNAs and DNA methylation contribute to gestational diabetes mellitus. J Cell Mol Med. (2020) 24:13899–912. doi: 10.1111/jcmm.v24.23
202. Cvitic S, Novakovic B, Gordon L, Ulz CM, Muhlberger M, Diaz-Perez FI, et al. Human fetoplacental arterial and venous endothelial cells are differentially programmed by gestational diabetes mellitus, resulting in cell-specific barrier function changes. Diabetologia. (2018) 61:2398–411. doi: 10.1007/s00125-018-4699-7
203. Knabl J, Hiden U, Huttenbrenner R, Riedel C, Hutter S, Kirn V, et al. GDM alters expression of placental estrogen receptor alpha in a cell type and gender-specific manner. Reprod Sci. (2015) 22:1488–95. doi: 10.1177/1933719115585147
204. Sultan S and AlMalki S. Analysis of global DNA methylation and epigenetic modifiers (DNMTs and HDACs) in human foetal endothelium exposed to gestational and type 2 diabetes. Epigenetics. (2023) 18:2201714. doi: 10.1080/15592294.2023.2201714
205. Sun M, Song MM, Wei B, Gao Q, Li L, Yao B, et al. 5-Hydroxymethylcytosine-mediated alteration of transposon activity associated with the exposure to adverse in utero environments in human. Hum Mol Genet. (2016) 25:2208–19. doi: 10.1093/hmg/ddw089
206. Han N, Fang HY, Jiang JX, and Xu Q. Downregulation of microRNA-873 attenuates insulin resistance and myocardial injury in rats with gestational diabetes mellitus by upregulating IGFBP2. Am J Physiol Endocrinol Metab. (2020) 318:E723–E35. doi: 10.1152/ajpendo.00555.2018
207. Zhang L, Wu Q, Zhu S, Tang Y, Chen Y, Chen D, et al. Chemerin-Induced Down-Regulation of Placenta-Derived Exosomal miR-140-3p and miR-574-3p Promotes Umbilical Vein Endothelial Cells Proliferation, Migration, and Tube Formation in Gestational Diabetes Mellitus. Cells. (2022) 11:3457. doi: 10.3390/cells11213457
208. Hromadnikova I, Kotlabova K, Dvorakova L, Krofta L, and Sirc J. Substantially altered expression profile of diabetes/cardiovascular/cerebrovascular disease associated microRNAs in children descending from pregnancy complicated by gestational diabetes mellitus-one of several possible reasons for an increased cardiovascular risk. Cells. (2020) 9:1557. doi: 10.3390/cells9061557
209. Hromadnikova I, Kotlabova K, and Krofta L. Cardiovascular disease-associated microRNAs as novel biomarkers of first-trimester screening for gestational diabetes mellitus in the absence of other pregnancy-related complications. Int J Mol Sci. (2022) 23:10635. doi: 10.3390/ijms231810635
210. Ran G, Zhu X, and Qin Y. LncRNA SOX2OT is upregulated in gestational diabetes mellitus (GDM) and correlated with multiple adverse events. Diabetes Metab Syndr Obes. (2021) 14:3989–95. doi: 10.2147/DMSO.S319739
211. She W, Li T, Liu Y, and Liu X. CircRNA circVEGFC is highly expressed in gestational diabetes mellitus (GDM) and it is correlated with multiple adverse events. Diabetes Metab Syndr Obes. (2021) 14:4409–14. doi: 10.2147/DMSO.S334728
212. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, and Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
213. Tarry-Adkins JL, Ozanne SE, and Aiken CE. Impact of metformin treatment during pregnancy on maternal outcomes: a systematic review/meta-analysis. Sci Rep. (2021) 11:9240. doi: 10.1038/s41598-021-88650-5
214. Anness AR, Nath M, Osman MW, Webb D, Robinson T, Khalil A, et al. Does treatment modality affect measures of arterial stiffness in women with gestational diabetes? Ultrasound Obstet Gynecol. (2023) 62:422–9. doi: 10.1002/uog.26234
215. Laredo-Aguilera JA, Gallardo-Bravo M, Rabanales-Sotos JA, Cobo-Cuenca AI, and Carmona-Torres JM. Physical activity programs during pregnancy are effective for the control of gestational diabetes mellitus. Int J Environ Res Public Health. (2020) 17:6151. doi: 10.3390/ijerph17176151
216. Ruchat SM, Davenport MH, Giroux I, Hillier M, Batada A, Sopper MM, et al. Effect of exercise intensity and duration on capillary glucose responses in pregnant women at low and high risk for gestational diabetes. Diabetes Metab Res Rev. (2012) 28:669–78. doi: 10.1002/dmrr.v28.8
217. Avery MD, Leon AS, and Kopher RA. Effects of a partially home-based exercise program for women with gestational diabetes. Obstet Gynecol. (1997) 89:10–5. doi: 10.1016/S0029-7844(97)84256-1
218. Sklempe Kokic I, Ivanisevic M, Kokic T, Simunic B, and Pisot R. Acute responses to structured aerobic and resistance exercise in women with gestational diabetes mellitus. Scand J Med Sci Sports. (2018) 28:1793–800. doi: 10.1111/sms.2018.28.issue-7
219. Huifen Z, Yaping X, Meijing Z, Huibin H, Chunhong L, Fengfeng H, et al. Effects of moderate-intensity resistance exercise on blood glucose and pregnancy outcome in patients with gestational diabetes mellitus: A randomized controlled trial. J Diabetes Complications. (2022) 36:108186. doi: 10.1016/j.jdiacomp.2022.108186
220. Chatzakis C, Sotiriadis A, Fatouros IG, Jamurtas AZ, Deli CK, Papagianni M, et al. The effect of physical exercise on oxidation capacity and utero-placental circulation in pregnancies with gestational diabetes mellitus and uncomplicated pregnancies, a pilot study. Diagnostics (Basel). (2022) 12:1732. doi: 10.3390/diagnostics12071732
221. Kintiraki E, Dipla K, Triantafyllou A, Koletsos N, Grigoriadou I, Poulakos P, et al. Blunted cerebral oxygenation during exercise in women with gestational diabetes mellitus: associations with macrovascular function and cardiovascular risk factors. Metabolism. (2018) 83:25–30. doi: 10.1016/j.metabol.2018.01.009
222. Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, and Fuglsang J. Diet and healthy lifestyle in the management of gestational diabetes mellitus. Nutrients. (2020) 12:3050. doi: 10.3390/nu12103050
223. Yamamoto JM, Kellett JE, Balsells M, Garcia-Patterson A, Hadar E, Sola I, et al. Gestational diabetes mellitus and diet: A systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care. (2018) 41:1346–61. doi: 10.2337/dc18-0102
224. Lauszus FF, Rasmussen OW, Henriksen JE, Klebe JG, Jensen L, Lauszus KS, et al. Effect of a high monounsaturated fatty acid diet on blood pressure and glucose metabolism in women with gestational diabetes mellitus. Eur J Clin Nutr. (2001) 55:436–43. doi: 10.1038/sj.ejcn.1601193
225. Babadi M, Khorshidi A, Aghadavood E, Samimi M, Kavossian E, Bahmani F, et al. The effects of probiotic supplementation on genetic and metabolic profiles in patients with gestational diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Probiotics Antimicrob Proteins. (2019) 11:1227–35. doi: 10.1007/s12602-018-9490-z
226. Kijmanawat A, Panburana P, Reutrakul S, and Tangshewinsirikul C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J Diabetes Investig. (2019) 10:163–70. doi: 10.1111/jdi.2019.10.issue-1
227. Okesene-Gafa KA, Moore AE, Jordan V, McCowan L, and Crowther CA. Probiotic treatment for women with gestational diabetes to improve maternal and infant health and well-being. Cochrane Database Syst Rev. (2020) 6:CD012970. doi: 10.1002/14651858.CD012970.pub2
228. Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care. (2019) 42:364–71. doi: 10.2337/dc18-2248
229. Movaghar R, Farshbaf-Khalili A, Hajizade K, MirzaRezaei ME, and Shahnazi M. The effect of probiotics or synbiotics on the hypertensive disorders of pregnant women with gestational diabetes: A systematic review and meta-analysis. J Caring Sci. (2022) 11:94–104. doi: 10.34172/jcs.2021.027
Keywords: cardiovascular system, gestational diabetes mellitus, progeny, umbilical-placental circulation, mother
Citation: Zhang Z, Zhang Y, Huang S, Li M, Li L, Qi L, He Y, Xu Z and Tang J (2025) Influence of gestational diabetes mellitus on the cardiovascular system and its underlying mechanisms. Front. Endocrinol. 16:1474643. doi: 10.3389/fendo.2025.1474643
Received: 05 August 2024; Accepted: 23 April 2025;
Published: 16 May 2025.
Edited by:
Katsuya Dezaki, Iryo Sosei University, JapanReviewed by:
Faheem Seedat, University of Oxford, United KingdomSheng Xing Ma, Lundquist Institute for Biomedical Innovation, United States
Copyright © 2025 Zhang, Zhang, Huang, Li, Li, Qi, He, Xu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaqi Tang, anF0YW5nQHN1ZGEuZWR1LmNu; dGFuZ2ppYXFpNzVAMTYzLmNvbQ==; Zhice Xu, eHV6aGljZUBzdWRhLmVkdS5jbg==
†These authors have contributed equally to this work
 Ze Zhang
Ze Zhang Yumeng Zhang1†
Yumeng Zhang1† Min Li
Min Li Lingjun Li
Lingjun Li Zhice Xu
Zhice Xu Jiaqi Tang
Jiaqi Tang