- 1Endocrine Physiology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Molecular, Cellular and Biomedical Sciences, Sophie Davis School of Biomedical Education, City University of New York School of Medicine, New York, NY, United States
Approximately 28% of individuals with diabetes have osteoporosis. Diabetoporosis, which refers to the diabetes-related decrease in bone quality and quantity, increases the risk of osteoporotic fractures by 600-700% in individuals with type 1 diabetes (T1D) and by 38-70% in those with type 2 diabetes (T2D) compared to non-diabetic individuals. Decreased nitric oxide (NO) bioavailability contributes to diabetoporosis. This review summarizes the potential role of nitrate as a NO donor in preventing and treating diabetic osteoporosis. Evidence suggests that organic and inorganic nitrates have anti-osteoporotic effects in animal models of osteoporosis, as demonstrated by increasing bone mineral density (BMD, 3-42%) and bone weight (6-160%). Observational human studies indicate a lower fracture risk (6-17%) and a higher BMD (3-5%) following organic nitrate administration. Similar protective effects (7-74% reduction in fracture risk and 8-84% increase in BMD) have been observed with nitrate-rich diets. Randomized controlled trials have also shown that nitrate increases circulating bone formation markers; however, no effect on fracture risk has been reported, and increased BMD (8.8%) was reported only in one study. Nitrate converts to nitrite and then to NO (exogenous NO), increasing NO bioavailability in bone. In addition, nitrate increases the expression of endothelial NO synthase (eNOS), thereby increasing the endogenous NO in bone. Nitrate-derived NO promotes bone formation and reduces bone resorption via the NO/cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) signaling pathway. In addition to increasing NO availability, nitrate may enhance plasma insulin levels, reduce hyperglycemia, and improve insulin resistance in diabetes, further contributing to nitrates’ anti-osteoporotic effects in diabetic bone. In conclusion, NO-based interventions such as nitrate may have a potential role in preventing and treating diabetoporosis.
1 Introduction
Approximately 537 million people (10.5% of the global population) had diabetes in 2021, and this number is projected to rise to 783 million (12.2%) by 2045 (1). A meta-analysis of observational studies from 2001 to 2020 (103,334,579 subjects aged 15-105 years in all continents) indicates that the prevalence of osteoporosis is about 23% in women and 12% in men (2). The prevalence of osteoporosis is higher by ~28% (~8-54%) in women and men with diabetes (3). In addition, individuals with type 1 diabetes (T1D) face a 6-7 times higher risk of osteoporotic fractures compared to normal subjects, whereas those with type 2 diabetes (T2D) have an increased risk ranging from 38% to 70% (4–7). Moreover, hip fractures occur 10-15 years earlier in diabetic patients (8), increasing the risk of all-cause mortality by 24% (9) [28% in men and 57% in women (10)] over the following 1-5 years (9–11). Osteoporosis results from a diabetes-related decline in bone quality and quantity (12), often called diabetoporosis (13, 14).
Diabetic patients with osteoporosis are treated with a combination of antidiabetic and anti-osteoporotic medications. The effect of primary antidiabetic drugs, such as metformin and insulin, on fracture risk is inconsistent (15). Meta-analyses of randomized controlled trials (RCTs) and observational studies suggest that these medications have a neutral effect on fracture risk (9); however, some reports indicate an increased risk of fractures (16, 17). On the other hand, there are currently no RCTs evaluating the metabolic effects of anti-osteoporosis medications in diabetic patients. Post hoc analyses of trial data indicate that first-line anti-osteoporotic treatments like bisphosphonates may have a neutral or unfavorable impact on metabolic parameters, including fasting glucose and insulin resistance (18–21). In addition, current anti-osteoporotic treatments are limited by cost, side effects, and efficacy, warranting new strategies for managing diabetoporosis.
Decreased nitric oxide (NO) bioavailability contributes to diabetoporosis (22). In patients with T1D (22) and T2D (23), decreased endothelial NO synthase (eNOS) and increased inducible NOS (iNOS) activity have been observed in bone cells. eNOS-derived NO facilitates bone formation, reduces the risk of osteoporotic fractures, enhances bone healing, and inhibits bone resorption, whereas iNOS-derived NO hinders bone formation and promotes resorption (24–26). The lack of NO bioavailability in diabetic bones is linked to disruptions in specific signaling pathways, suggesting that targeting these pathways might improve bone health. Studies conducted in ovariectomized rats (27) and postmenopausal women (28) show that NO boosting through organic and inorganic nitrates can protect against osteoporosis. Furthermore, inorganic nitrate, particularly in food-derived sources, may indirectly ameliorate hyperglycemia and insulin resistance (29), improving bone quality. The protective effect of NO against osteoporosis in T1D (22) and T2D (23) has been previously reviewed. This paper focuses on the potential role of nitrate (organic and inorganic) in preventing and treating osteoporosis in patients with T1D and T2D.
2 Evidence of anti-osteoporotic effect of nitrate in animal studies
Animal studies have addressed the anti-osteoporotic effects of both organic and inorganic nitrates. Specifically, nitroglycerin (NG), an organic nitrate, improves BMD, bone weight, and bone quality. Both organic and inorganic nitrates may play a beneficial role in mitigating osteoporosis in rat models; however, further research is needed to evaluate the effectiveness of inorganic nitrates.
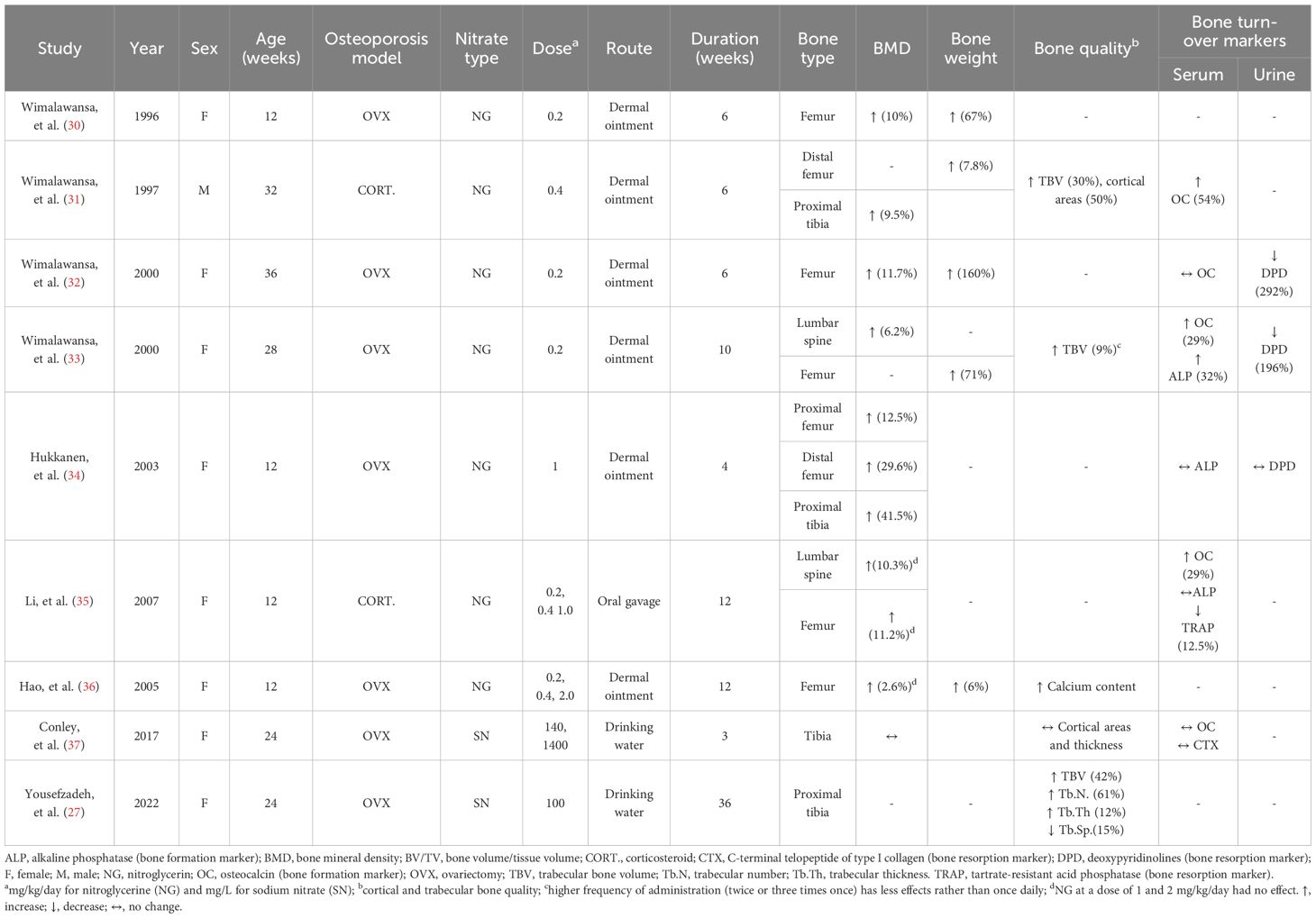
Table 1 summarizes the anti-osteoporotic effects of organic nitrate [NG as single dose (30–34) or multiple doses (35, 36) for 4-12 weeks] and inorganic nitrate (sodium nitrate in drinking water for 3 and 36 weeks) in animal models of osteoporosis, viz., ovariectomy-induced osteoporosis (27, 31–34, 36, 37) and corticosteroid-induced osteoporosis (31, 35). All studies were conducted in rats (aged 12-36 weeks), as recommended by Food and Drug Administration guidelines for induction of animal models of osteoporosis (38) and discussed in our previous report (39). In addition, all studies were conducted in female rats except one study (31) that assessed corticosteroid-induced osteoporosis in male rats. NG has been used as dermal ointment except in one study that used it by oral gavage (35). Regions of interest assessed following NG administration include the femur (30–36), tibia (31, 34), and lumbar spine (33, 35), as these are the main fracture sites in humans and are clinically relevant (39). In the case of sodium nitrate administration, only the tibia has been assessed (27, 37).
Outcome variables that have been assessed included bone mineral density (BMD) (30–37), bone weight (30–33, 36), bone quality (27, 31, 33, 36, 37), and circulating bone turnover markers (31–37). Results show that organic nitrate has anti-osteoporotic effects (30–36), as documented by increasing BMD (2.6-41.5%) and bone weight (6-160%), improving bone quality, and affecting circulating and urine bone-related markers in favor of bone formation. In addition, it has been observed that a higher frequency of administration (twice or three times per day) rather than once daily (33), as well as a higher dose of NG compared to lower or moderate doses (35), provide less protection against osteoporosis in rats. Studies addressing the effect of inorganic nitrate on osteoporosis are scant (27, 37), with results indicating that in ovariectomy-induced osteoporosis in female rats, short-term nitrate administration (3 (37) and 4 (27) weeks) does not affect bone quality, but long-term administration [13 and 36 weeks (27)] does.
3 Evidence of the anti-osteoporotic effect of nitrate in human studies
3.1 Human studies: organic nitrates
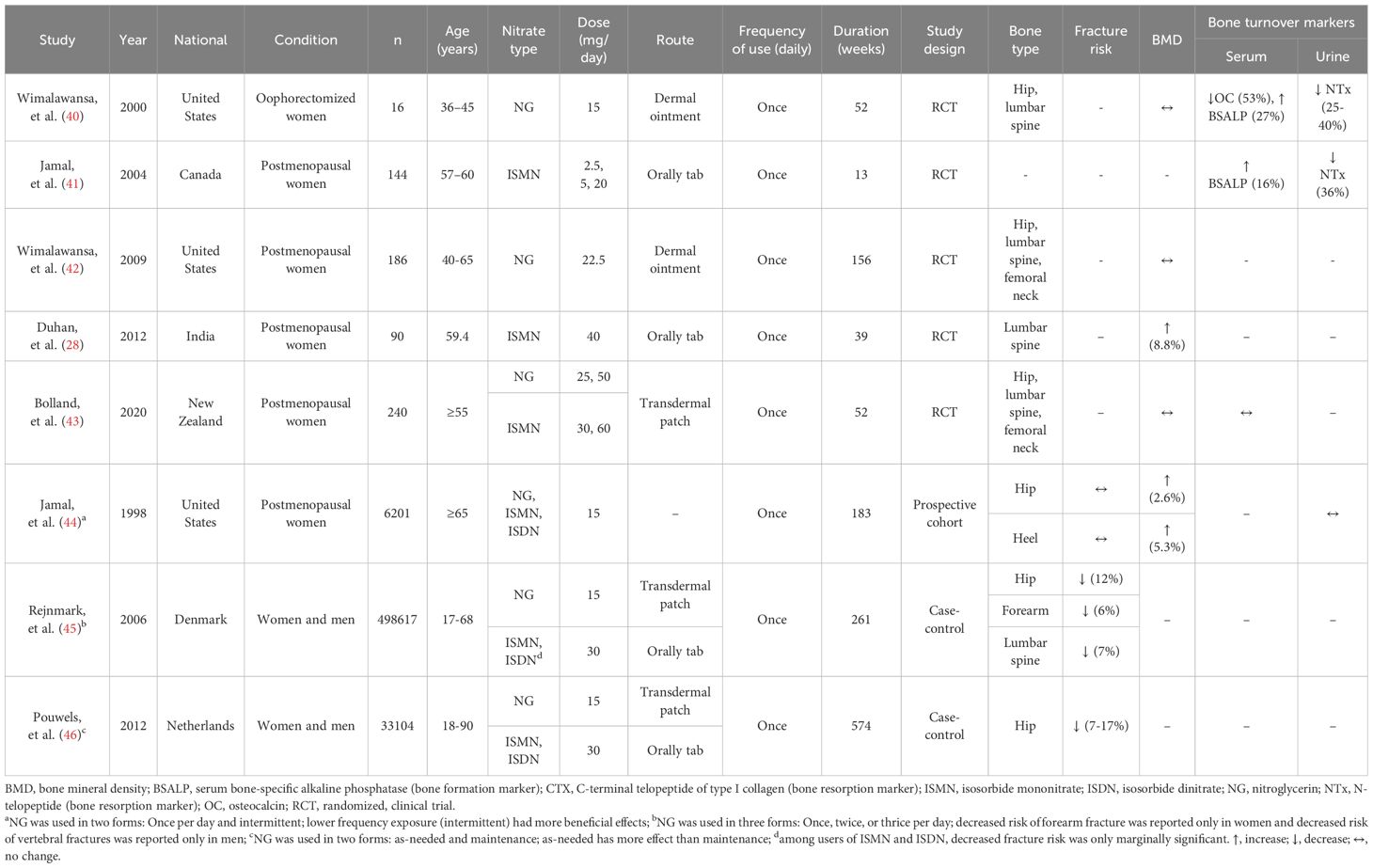
Table 2 summarizes evidence obtained from human studies on the association between organic nitrate and fracture risk, BMD, and bone turnover markers. Two case-control studies conducted in Denmark (45) and the Netherlands (46) compared fracture risk between men and women who consumed NG, isosorbide mononitrate (ISMN), and isosorbide dinitrate (ISDN) compared controls. Results indicate that nitrate consumption is associated with lower fracture risk (6-17%) (45, 46). In addition, a prospective cohort study (with a follow-up mean of 3.5 years) conducted in postmenopausal women in the United States indicated that intermittent nitrate use (NG, ISMN, and ISDN) is associated with higher BMD in the hip (~2.6%) and heel (~5.3%) (44). Five RCTs (28, 40–43) were conducted in postmenopausal women [except one study in young oophorectomized women aged 36-45 years (40)] to find the effect of NG as dermal ointment (40, 42) and transdermal patch (43, 45, 46) as well as ISMN as oral tablets (41) and transdermal patch (43) on BMD and bone turnover markers. Regions of interest were lumbar spine (28, 40, 42, 43), hip (40, 42, 43), and femoral neck (28, 42). None of the five RCTs addressed the effect on fracture risk (28, 40–43). In addition, only one study reported that ISMN (40 mg/kg once daily for 36 weeks) increases BMD in Indian postmenopausal women by 8.8% (28); the others observed no effect on BMD (40–43). Except for two studies (28, 42), all other four RCTs reported changes in bone turnover markers in favor of bone formation, as indicated by an increase in serum bone formation markers [i.e., bone-specific alkaline phosphatase (BSALP), by 15-25%] and decrease in urine bone resorption marker [i.e., N-telopeptide (NTx) by 32-40%]. In this line, a meta-analysis of RCTs that assessed the effect of antiresorptive agents in postmenopausal women indicates that a 40% reduction in bone resorption markers is associated with a 30% decrease in risk fracture (47).
Of note, higher doses of NG (22.5 (42), 25 (43), and 50 (43) mg/day) provide less protection against osteoporosis in humans compared to 15 mg/day (40, 44–46). It has been reported that NG has a narrow therapeutic window for osteoporosis treatment, with optimal dosages of around 15 mg daily (48, 49). Deviations from this dosage, either too low or too high, may result in a lack of efficacy (42, 50, 51). Therefore, the reported ineffectiveness of NG in some instances may be associated with higher doses of NG (22.5 (42), 25 (43), and 50 (43) mg/day). In addition, a case-control study reported that using NG as a fast-acting nitrate in adult women and men had a more significant impact on fracture risk than using slow-release nitrates, such as ISMN and ISDN (45).
3.2 Human studies: inorganic nitrates
According to meta-analyses of observational studies, the Mediterranean diet is associated with a reduced risk of fractures by 20% in the general population (52, 53). These beneficial effects are hypothesized to be attributable to this diet’s high content of calcium, potassium, polyphenols, and fiber (54, 55). Furthermore, the high levels of inorganic nitrate found in fruits and vegetables are involved in the mechanisms underlying the positive effects of these diets (56, 57). Nitrate-rich vegetables account for approximately 85% of dietary nitrate consumption in the human diet (56, 57). These vegetables have potential NO-boosting effects (58) as their nitrate is converted to NO via the nitrate-nitrite-NO pathway (59), which may exert NO-like effects on bone. The protective effects of the Diet to Stop Hypertension (DASH) and Mediterranean diets against cardiovascular disease (60) and T2D (61) are at least partly attributed to the high levels of nitrates (147–1222 mg/day) derived from these nitrate-rich diets. This contrasts with the Western-style diet, which is considered a low-nitrate diet (75 mg/day) (62). For further support, meta-analyses of RCTs indicate that inorganic nitrate and beetroot juice supplementation produce similar blood pressure-lowering effects, while the presence of other bioactive compounds in beetroot juice, such as vitamin C, magnesium, and flavonoids, has minimal additive effects (63).
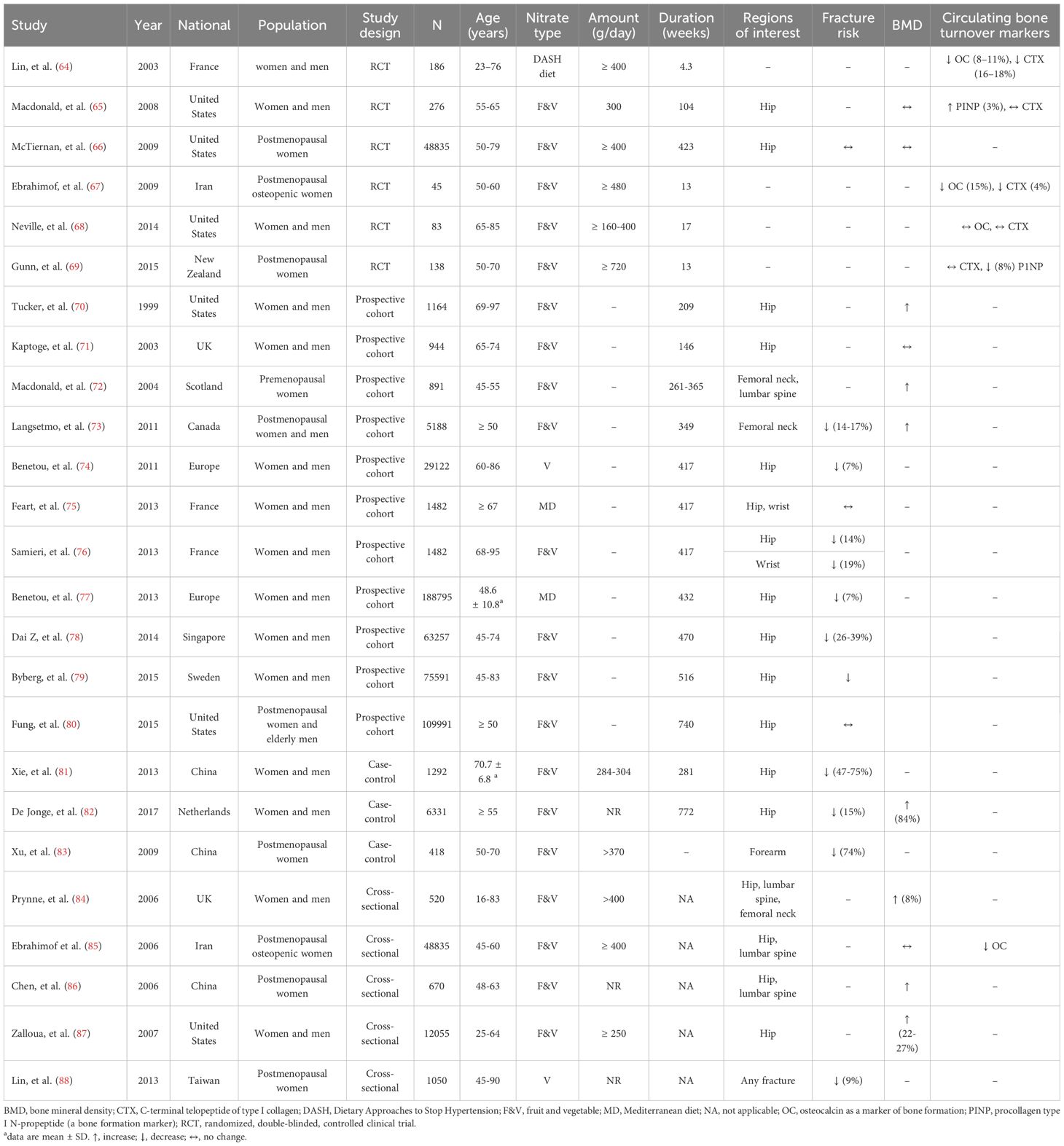
Table 3 summarizes evidence obtained from human studies on the association between inorganic nitrate and fracture risk, BMD, and bone turnover markers. Five cross-sectional studies (84–88) were conducted in the UK (84), Iran (85), China (86), United States (87), and Taiwan (88) in women and men (84, 87), postmenopausal osteopenic women (85), and postmenopausal women (86, 88) who consumed fruit and vegetables. Results indicate that fruit and vegetable consumption as a source of nitrate is associated with lower fracture risk (~9%) (88) and higher BMD (84, 86, 87). Three case-control studies (81–83) conducted in China (81, 83) and the Netherlands (82) indicate that consumption of fruit and vegetables decreases fracture risk (81–83) and increases BMD (82). In addition, 11 prospective cohort studies (70–80) (with a mean follow-up of 146-740 weeks) have been conducted in Women and men (70, 71, 74–79), postmenopausal women (72, 73, 80) and men (73, 80) in the United States (70, 80), Sweden (79), Singapore (78), Europe (74, 77), France (75, 76), UK (71), Scotland (72), and Canada (73) to find association between inorganic nitrate consumption and bone-related parameters. Results indicated that nitrate use [fruit and vegetables (70, 71, 73, 76, 78–80), vegetables (74), and Mediterranean diet (75, 77)] is associated with lower fracture risk (74, 77–79) and higher BMD (70, 72, 73) in the hip (70, 71, 75–80), femoral neck (72, 73), lumbar spine (72), and wrist (75, 76). To summarize, results of observational studies indicate that higher inorganic nitrate consumption is associated with higher BMD (~1.3-8.8%) (70, 72, 73, 82, 84, 86, 87) (in case-control (82), cross-sectional (84, 86, 87) and prospective cohort (70, 72, 73) studies) as well as lower fracture risk (~ 7-74%) (in case-control studies (81–83) and prospective cohort (73, 74, 76–79) studies). In this line, a meta-analysis of cohort studies from Europe and the United States indicates that men and women consuming ≤1 serving per day of fruits and vegetables had a 39% higher risk of hip fractures compared to those consuming >3 and ≤5 serving (89).
Six RCTs [duration range: 30 days (64) to 8.1 years (66)] were conducted in women and men (64, 65, 68), postmenopausal women (66, 69), and postmenopausal osteopenic women (66), to determine the effect of inorganic nitrate in the form of fruit and vegetables (65–69) [except one study with DASH diet (64)] on the fracture risk (66), BMD (65, 66) and circulating bone turnover markers (64, 65, 67–69). The anti-osteoporotic effect of inorganic nitrate has been assessed only in hip (65, 66). Results indicate that inorganic nitrate (300 and ≥ 400 g/day consumption of fruit and vegetables for 104 (65) and 423 (66) weeks) could not decrease the fracture risk (66) and BMD (65, 66). However, the consumption of inorganic nitrate for 4.3 (64), 13 (67), and 104 (65) weeks could decrease serum C-terminal telopeptide of type I collagen (CTX) levels by 4-18% and serum OC levels by 8-15% and increase procollagen type I N-propeptide (PINP) levels by 3-8%. A systematic review and meta-analysis of RCTs and cohort studies (13 studies) in men and women over 50 years of age conducted by Brondani et al. in 2019 reported an association between increasing fruit and vegetable intake by at least one serving per day and lower fracture risk (90).
Table 3 shows that some RCTs indicate no effect of inorganic nitrate consumption on fracture risk, BMD, and serum bone turnover markers. Although participant characteristics may partly explain the lack of a significant effect of inorganic nitrate consumption on bone health outcomes, it seems that dosage inadequacy has a more critical role. Macdonald et al. (65) noted that more than a daily intake of 300 g of fruits and vegetables may be required to impact bone health significantly. Neville et al. (68) suggested that elderly participants (65-85 years) needed a higher dose (400 g/day) and longer treatment duration (over 16 weeks) of fruit and vegetable consumption to see meaningful effects on bone. Research suggests that at least 6.2 g of fresh fruit and vegetables per kg of body weight are needed to inhibit bone resorption. Considering each serving to be 80 g and a body weight of 70 kg, consuming five servings daily (400 g) is necessary to show an effect on bone resorption markers. According to a cohort study, men and women with no fruit or vegetable consumption had an 88% higher hip fracture rate than those consuming five servings daily (79); however, no additional benefits were observed for intakes exceeding five servings (720 g) (69).
3.3 Organic nitrates vs. inorganic nitrates
Organic nitrates are often poorly tolerated, with headache being their primary side effect; in an RCT, 21% of women discontinued the study during the 1-year follow-up because of headaches (43). Furthermore, the anti-osteoporotic effects of organic nitrates diminish with increased frequency (33) and duration (46) of administration. Inorganic nitrates have been suggested as suitable alternatives to organic nitrates (91). Inorganic nitrates have simple ionic structures, are produced endogenously, are present in the diet, and exhibit more prolonged effects without the limitation of tachyphylaxis (92, 93). Inorganic nitrates increase NO bioavailability following reduction to nitrite and then to NO (exogenous NO) and also enhance eNOS-derived NO (endogenous) in bone.
4 Bio-conversation of nitrate to nitrite and then to NO
At least two major pathways contribute to NO production in the human body (1): the L-arginine-NO-oxidative pathway in which NOS enzymes convert L-arginine to NO; (2) the nitrate-nitrite-NO reductive pathway in which the inorganic nitrate and nitrite are reduced to form NO (94).
In addition to the endogenous source (oxidation of NOS-derived NO), nitrate also has an exogenous source (diet, water, and environment) (57). Exogenous nitrate (1700 μmol/day), about 85% of which is derived from vegetables, is almost completely absorbed into circulation in the upper small intestine (duodenum and jejunum), with < 2% reaching the terminal ileum and excreted in feces. ~75% of ingested nitrate is excreted in the urine, while ~25% is transported into the salivary glands and concentrated in saliva (93–96). Oral bacteria utilize nitrate as an alternative electron acceptor during respiration, reducing the anion to nitrite. The saliva’s nitrite subsequently enters the stomach’s acidic environment, where it is reduced to NO and diffuses into the circulation. Considering that 25% of ingested nitrate is taken up from plasma by saliva and that 20% is converted to nitrite in the oral cavity, ~5% of ingested nitrate is converted to nitrite in the oral cavity (93–95). Assuming that all produced nitrite is reduced to NO in the stomach, the contribution of the nitrate-nitrite-NO pathway to overall NO production is estimated to be around 100 μmol/day, compared to the ~1000 μmol/day produced by the NOS-dependent L-arginine-NO pathway (57).
Nitrate reduction to nitrite and then to NO occurs in blood and tissues. Mammalian tissues express nitrate reductase and thus can reduce nitrate to nitrite under normoxic conditions (97). Nitrite reduction to NO can occur via enzymatic and non-enzymatic (i.e., spontaneously in an acidic environment or disproportionation) pathways (57, 96). Enzymes involved in the reduction of nitrite to NO include xanthine oxidoreductase, aldehyde oxidase, deoxygenated hemoglobin and myoglobin, cytochrome P450, cytochrome c, and the mitochondrial respiratory chain) (57, 96). Disproportioination is limited to the stomach and ischemic tissues because of low pKa of nitrite (~3.4) (98). The rate of NO production from nitrite disproportionation in normal tissues with a pH of 7.2 to 7.4 and nitrite concentrations of 10 to 50 μM is 0.05-1 pM/s (99), which is equivalent to about 6 μmol/day in a 70-kg individual. Tissues produce NO from nitrite under normoxia, increasing production in hypoxic conditions (97, 100). Under ischemic conditions, nitrite disproportionation can increase to 4-100 pM/s, but this is still only 5-10% of the maximum NOS-dependent NO production under physiological conditions (99). A decrease in pH enhances NO production from nitrite; in the presence of nitrite (20 μM), a one-unit decrease in pH from 7.0 to 6.0 increases NO generation by ~12 -13 times in liver and heart tissues (100). Thus, non-enzymatic reducing nitrite to NO is crucial in ischemic conditions in which both hypoxia and acidic pH are present. The combined inhibition of xanthine oxidase and aldehyde oxidase decreases NO generation from nitrite by more than 65-70%, indicating the significant contribution of these enzymes to nitrite reduction to NO, with only 15-20% remaining for non-enzymatic reduction of nitrite (100). See published reviews for more details about the nitrate-nitrite-NO reductive pathway (93–95) and the quantitative aspects of NO production (57) (Figure 1).
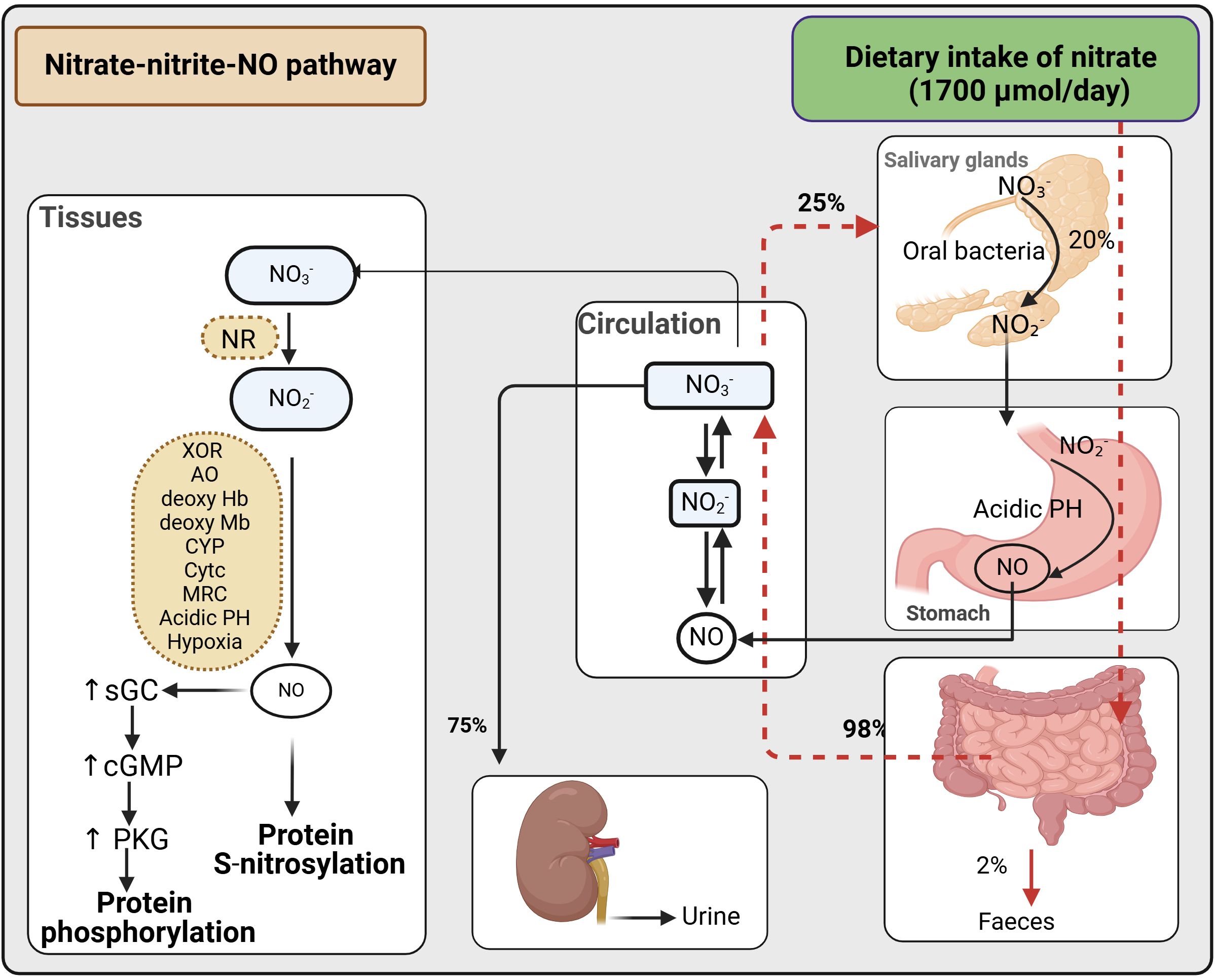
Figure 1. Bio-conversation of nitrate to nitrite and then to NO. cGMP, cyclic guanosine monophosphate; sGC, soluble guanylate cyclase; NO, nitric oxide; PKG, protein kinase G; NR, nitrate reductases; XOR, xanthine oxidoreductase; AO, aldehyde oxidase; deoxy HB, deoxygenated hemoglobin; deoxy MB, deoxygenated myoglobin; CYP, cytochrome P450; Cytc, cytochrome c; MRC, mitochondrial respiratory chain. Created with BioRender.com.
5 Possible mechanisms underlying protective effects of nitrate in diabetoporosis
T1D and T2D exhibit different pathophysiological characteristics; however, they share several features, including hyperglycemia, insulin resistance, and insulin deficiency, which negatively affect bone cells, contributing to the development of diabetoporosis (22, 23). The pathophysiology of diabetoporosis in T1D (22) and T2D (23) have been previously reviewed by us. In brief, both types of diabetes decrease osteoblast-related bone formation and increase osteoclast-related bone resorption. Additionally, reduced levels of transforming growth factor beta (TGF-β) and increased levels of sclerostin in osteocytes have been observed in both forms of diabetes that cause lower osteoblast and higher osteoclast differentiation. Furthermore, decreased bone blood flow, increased inflammation and oxidative stress, advanced glycation end products (AGEs), and bone adiposity contribute to the development of diabetoporosis (22, 23). Regarding gestational diabetes and osteoporosis, it has been reported that a history of gestational diabetes is associated with a higher risk of osteoporosis in postmenopausal women (101). Like T1D and T2D, increased AGEs and inflammation are involved in osteoporosis in subjects with a history of gestational diabetes (101). In addition, due to concerns about the possible adverse effects of nitrate use during pregnancy, including methemoglobinemia, changes in embryonic cells, malignant transformations, and thyroid disorders, the use of dietary nitrate as a common supplement during pregnancy is currently a long way from bench to bedside (102).
As shown in Figure 2, under normal conditions, insulin acts on insulin receptors (IR) and activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in bone cells. Akt phosphorylates and activates eNOS, increasing endogenous NO production in bone cells. NO acts on its receptor (i.e., soluble guanylyl cyclase, sGC) and activates the sGC/cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) signaling pathway, the primary signaling pathway of NO action in the bone. sGC inhibitors block the effect of NO on bone (103), and restoring cGMP synthesis to normal levels in diabetic mice improves bone formation and decreases bone loss (104, 105). eNOS-derived NO increases osteoblast activity in bone as demonstrated by higher levels of ALP and osteocalcin (106, 107) and decreases osteoclast-mediated bone resorption as shown by reduced cathepsin K and collagenase levels in osteoclasts (108, 109). eNOS-derived NO also promotes osteoblast differentiation (110, 111) and inhibits osteoclast differentiation (110, 111). In addition, eNOS-derived NO represses adipogenesis by decreasing adipogenic transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ) and lipoprotein lipase, thus decreasing adipogenesis in bone (112, 113). The ultimate effects of insulin-mediated eNOS activation in bone cells are increased osteoblast-mediated bone formation and decreased osteoclast-mediated bone resorption (114, 115). Supporting the favorable role of insulin on bone, reduced bone formation and increased bone resorption have been reported in rodents with insulin deficiency (115–118). In addition, insulin administration improves bone quality in diabetic rats (119–125).
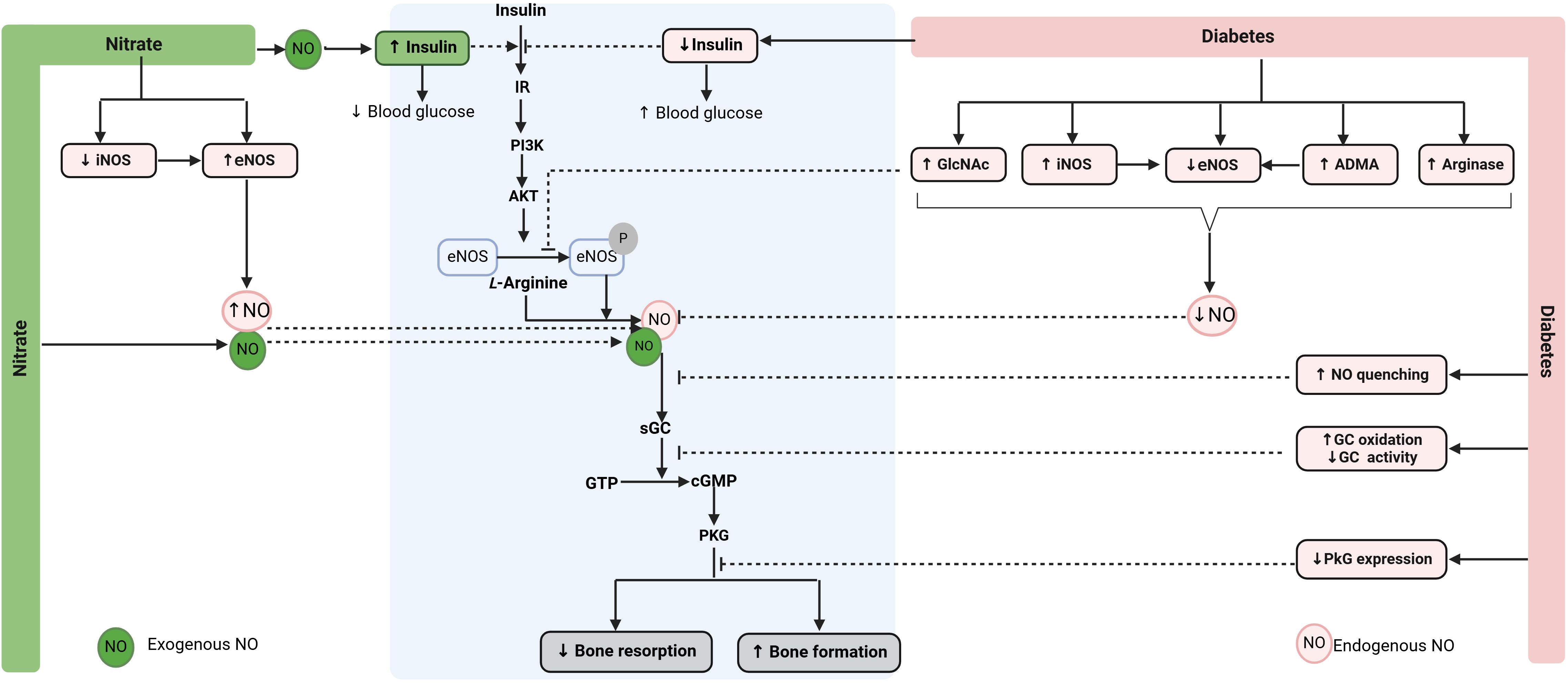
Figure 2. Proposed mechanisms by which nitrate can promote bone health in diabetoporosis. Nitrate is converted to NO (exogenous NO), increasing eNOS activity (endogenous NO), thereby restoring decreased NO bioavailability in diabetic bone. NO also increases circulating insulin and improves insulin resistance, indirectly promoting bone health in diabetes. ADMA, asymmetric dimethylarginine; cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; GTP, guanosine triphosphate; GlcNAc, O-linked N-acetylglucosamine; IR, insulin receptor; iNOS, inducible NOS; sGC, soluble guanylate cyclase; NO, nitric oxide; PKG, protein kinase G. ↑, increase; ↓, decrease. Created with BioRender.com.
Decreased NO bioavailability in diabetic bone is attributed to reduced availability of L-arginine (126), increased activity and expression of arginase [which converts L-arginine to urea and L-ornithine instead of NO] (126), increased levels of the asymmetrical dimethylarginine (ADMA) [which is an endogenous inhibitor of NOS] (127), decreased eNOS expression (105) and activity (128), increased eNOS uncoupling (105), increased expression and activity of iNOS (36), and increased O-linked N-acetylglucosamine (GlcNAc) [which inhibits eNOS phosphorylation] (129, 130). In addition, diabetes enhances AGE-mediated NO quenching (131), increases sGC oxidation that decreases its activity, and decreases PKG expression and activity in diabetic bone (105). These effects blunt PI3K/Akt/eNOS and NO/cGMP/PKG signaling pathways (105, 129), leading to osteoporosis.
Nitrate therapy can potentially restore decreased NO bioavailability in diabetoporosis as it is reduced to nitrite and then to NO (57). Nitrate also decreases iNOS expression and increases eNOS expression, thereby increasing endogenous eNOS-derived NO (132–134). Endogenous and exogenous NO can contribute to increased bone formation and decreased bone resorption via the NO/cGMP/PKG signaling pathway. In addition to increasing NO availability (57), nitrate increases plasma insulin, decreases hyperglycemia, and improves insulin resistance, as demonstrated in animal models of T2D (96, 135, 136). These effects can also contribute to nitrate-mediated health-promoting effects in diabetic bone.
6 Conclusion
Animal and human studies propose anti-osteoporotic effects for organic and inorganic nitrates. Animal studies (Table 1) indicate a 3-42% increase in BMD and a 6-160% increase in bone weight (femur, tibia, and lumbar spine) following the administration of NG and sodium nitrate in rat models of osteoporosis. Observational human studies indicate a 6-17% reduction in fracture risk and a 2.6-5.3% increase in BMD following organic nitrate administration (Table 2). Similar protective effects (a 7-74% reduction in fracture risk and an 8-84% increase in BMD) have been observed with nitrate-rich diets (Table 3). RCTs have shown increased circulating bone formation markers, and one study (Table 2) reported an 8.8% increase in BMD after the administration of ISMN in postmenopausal women; however, no effect on fracture risk has been reported. Inorganic nitrates may exert anti-osteoporotic effects in both T1D and T2D; however, further clinical trials in which BMD and fracture risk are considered the primary outcomes are needed to confirm the efficacy of inorganic nitrate in preventing and treating diabetoporosis.
Author contributions
SJ: Conceptualization, Writing – original draft. KK: Conceptualization, Writing – review & editing. AG: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a grant (Grant Number: 43011549-3) from Shahid Beheshti University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen Y, Zhao W, Hu A, Lin S, Chen P, Yang B, et al. Type 2 diabetic mellitus related osteoporosis: focusing on ferroptosis. J Trans Med. (2024) 22:409. doi: 10.1186/s12967-024-05191-x
2. Salari N, Ghasemi H, Mohammadi L, Behzadi Mh, Rabieenia E, Shohaimi S, et al. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J Biol Chem. (2021) 16:1–20. doi: 10.1074/jbc.M513225200
3. Liu X, Chen F, Liu L, and Zhang Q. Prevalence of osteoporosis in patients with diabetes mellitus: A systematic review and meta-analysis of observational studies. BMC Endocr Disord. (2023) 23:1. doi: 10.1186/s12902-022-01260-8
4. Vestergaard P, Rejnmark L, and Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcified Tissue Int. (2009) 84:45–55. doi: 10.1007/s00223-008-9195-5
5. Shah VN, Shah CS, and Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabetic medicine: J Br Diabetic Assoc. (2015) 32:1134–42. doi: 10.1111/dme.12734
6. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporosis Int. (2007) 18:427–44. doi: 10.1007/s00198-006-0253-4
7. Janghorbani M, Van Dam RM, Willett WC, and Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. (2007) 166:495–505. doi: 10.1093/aje/kwm106
8. Weber DR, Haynes K, Leonard MB, Willi SM, and Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: A population-based cohort study using the health improvement network (Thin). Diabetes Care. (2015) 38:1913–20. doi: 10.2337/dc15-0783
9. Shen Q and Ma Y. Impact of diabetes mellitus on risk of major complications after hip fracture: A systematic review and meta-analysis. Diabetol Metab Syndr. (2022) 14:51. doi: 10.1186/s13098-022-00821-0
10. Tebé C, Martínez-Laguna D, Carbonell-Abella C, Reyes C, Moreno V, Diez-Perez A, et al. The association between type 2 diabetes mellitus, hip fracture, and post-hip fracture mortality: A multi-state cohort analysis. Osteoporosis Int. (2019) 30:2407–15. doi: 10.1007/s00198-019-05122-3
11. Bell DSH and Goncalves E. Why do falls and lower limb fractures occur more frequently in the diabetic patient and how can they be prevented? Diabetes Ther. (2020) 11:1687–94. doi: 10.1007/s13300-020-00877-z
12. Chau DL and Edelman SV. Osteoporosis and diabetes. Clin Diabetes. (2002) 20:153–7. doi: 10.2337/diaclin.20.3.153
13. Wongdee K and Charoenphandhu N. Osteoporosis in diabetes mellitus: possible cellular and molecular mechanisms. World J Diabetes. (2011) 2:41–8. doi: 10.4239/wjd.v2.i3.41
14. Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, Eastell R, et al. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporosis Int. (2018) 29:2585–96. doi: 10.1007/s00198-018-4650-2
15. Wikarek A and Grabarczyk M. Effect of drugs used in pharmacotherapy of type 2 diabetes on bone density and risk of bone fractures. Medicina (Kaunas). (2024) 60:393–406. doi: 10.3390/medicina60030393
16. Loke YK, Singh S, and Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: A meta-analysis. CMAJ. (2009) 180:32–9. doi: 10.1503/cmaj.080486
17. Zhang Z, Cao Y, Tao Y, M E, Tang J, Liu Y, et al. Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res Clin Pract. (2020) 159:107990. doi: 10.1016/j.diabres.2019.107990
18. Sheu A and White CP. Bone metabolism in diabetes: A clinician’s guide to understanding the bone-glucose interplay. Diabetologia. (2024) 68:1493–1506. doi: 10.1007/s00125-024-06172-x
19. Celer O, Akalın A, and Oztunali C. Effect of teriparatide treatment on endothelial function, glucose metabolism and inflammation markers in patients with postmenopausal osteoporosis. Clin Endocrinol. (2016) 85:556–60. doi: 10.1111/cen.13139
20. Paschou SA, Dede AD, Anagnostis PG, Vryonidou A, Morganstein D, and Goulis DG. Type 2 diabetes and osteoporosis: A guide to optimal management. J Clin Endocrinol Metab. (2017) 102:3621–34. doi: 10.1210/jc.2017-00042
21. Anastasilakis A, Goulis D, Koukoulis G, Kita M, Slavakis A, and Avramidis A. Acute and chronic effect of teriparatide on glucose metabolism in women with established osteoporosis. Exp Clin Endocrinol Diabetes. (2007) 115:108–11. doi: 10.1055/s-2007-967090
22. Jeddi S, Yousefzadeh N, Kashfi K, and Ghasemi A. Role of nitric oxide in type 1 diabetes-induced osteoporosis. Biochem Pharmacol. (2022) 197:114888. doi: 10.1016/j.bcp.2021.114888
23. Yousefzadeh N, Jeddi S, Kashfi K, and Ghasemi A. Diabetoporosis: role of nitric oxide. EXCLI J. (2021) 20:764–80. doi: 10.17179/excli2021-3541
24. Aguirre J, Buttery L, O’Shaughnessy M, Afzal F, Fernandez de Marticorena I, Hukkanen M, et al. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol. (2001) 158:247–57. doi: 10.1016/s0002-9440(10)63963-6
25. Hukkanen MV, Platts LA, Fernandez De Marticorena I, O’Shaughnessy M, MacIntyre I, and Polak JM. Developmental regulation of nitric oxide synthase expression in rat skeletal bone. J Bone mineral Res. (1999) 14:868–77. doi: 10.1359/jbmr.1999.14.6.868
26. Brandi ML, Hukkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, et al. Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Natl Acad Sci United States America. (1995) 92:2954–8. doi: 10.1073/pnas.92.7.2954
27. Yousefzadeh N, Jeddi S, Kashfi K, and Ghasemi A. Long-term inorganic nitrate administration protects against ovariectomy-induced osteoporosis in rats. Excli J. (2022) 21:1151–66. doi: 10.17179/excli2022-5082
28. Duhan N, Siwach RC, Yadav K, Dahiya K, Nanda S, and Sirohiwal D. Comparative evaluation of isosorbide mononitrate and alendronate in management of postmenopausal osteoporosis. Arch gynecology obstetrics. (2012) 285:1019–23. doi: 10.1007/s00404-011-2095-3
29. Lundberg JO, Carlström M, and Weitzberg E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. (2018) 28:9–22. doi: 10.1016/j.cmet.2018.06.007
30. Wimalawansa SJ, De Marco G, Gangula P, and Yallampalli C. Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone. (1996) 18:301–4. doi: 10.1016/8756-3282(96)00005-1
31. Wimalawansa SJ, Chapa MT, Yallampalli C, Zhang R, and Simmons DJ. Prevention of corticosteroid-induced bone loss with nitric oxide donor nitroglycerin in male rats. Bone. (1997) 21:275–80. doi: 10.1016/s8756-3282(97)00125-7
32. Wimalawansa SJ. Restoration of ovariectomy-induced osteopenia by nitroglycerin. Calcified Tissue Int. (2000) 66:56–60. doi: 10.1007/s002230050011
33. Wimalawansa S, Chapa T, Fang L, Yallampalli C, Simmons D, and Wimalawansa S. Frequency-dependent effect of nitric oxide donor nitroglycerin on bone. J Bone mineral Res. (2000) 15:1119–25. doi: 10.1359/jbmr.2000.15.6.1119
34. Hukkanen M, Platts LAM, Lawes T, Girgis SI, Konttinen YT, Goodship AE, et al. Effect of nitric oxide donor nitroglycerin on bone mineral density in a rat model of estrogen deficiency-induced osteopenia. Bone. (2003) 32:142–9. doi: 10.1016/S8756-3282(02)00955-9
35. Li Y, Li Y, and Yang W. Preventive effects of nitroglycerine on glucocorticoid-induced osteoporosis in growing rats. J Huazhong Univ Sci Technol Med Sci = Hua zhong ke ji da xue xue bao Yi xue Ying wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. (2007) 27:528–31. doi: 10.1007/s11596-007-0513-3
36. Hao YJ, Tang Y, Chen FB, and Pei FX. Different doses of nitric oxide donor prevent osteoporosis in ovariectomized rats. Clin orthopaedics related Res. (2005) 435):226–31. doi: 10.1097/01.blo.0000153990.74837.73
37. Conley MN, Roberts C, Sharpton TJ, Iwaniec UT, and Hord NG. Increasing dietary nitrate has no effect on cancellous bone loss or fecal microbiome in ovariectomized rats. Mol Nutr Food Res. (2017) 61:1600372. doi: 10.1002/mnfr.201600372
38. Food, Administration D. Guidelines for Preclinical and Clinical Evaluation of Agents Used in the Prevention or Treatment of Postmenopausal Osteoporosis. Rockville, MD: Division of Metabolism and Endocrine Drug Products (1994) p. 125–33. doi: 10.1016/8756-3282(95)00285-l
39. Yousefzadeh N, Kashfi K, Jeddi S, and Ghasemi A. Ovariectomized rat model of osteoporosis: A practical guide. Excli J. (2020) 19:89–107. doi: 10.17179/excli2019-1990
40. Wimalawansa SJ. Nitroglycerin therapy is as efficacious as standard estrogen replacement therapy (Premarin) in prevention of oophorectomy-induced bone loss: A human pilot clinical study. J Bone mineral Res. (2000) 15:2240–4. doi: 10.1359/jbmr.2000.15.11.2240
41. Jamal SA, Cummings SR, and Hawker GA. Isosorbide mononitrate increases bone formation and decreases bone resorption in postmenopausal women: A randomized trial. J Bone mineral Res. (2004) 19:1512–7. doi: 10.1359/jbmr.040716
42. Wimalawansa SJ, Grimes JP, Wilson AC, and Hoover DR. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J Clin Endocrinol Metab. (2009) 94:3356–64. doi: 10.1210/jc.2008-2225
43. Bolland MJ, House ME, Horne AM, Pinel V, Gamble GD, Grey A, et al. Nitrates do not affect bone density or bone turnover in postmenopausal women: A randomized controlled trial. J Bone Mineral Res. (2020) 35:1040–7. doi: 10.1002/jbmr.3982
44. Jamal SA, Browner WS, Bauer DC, and Cummings SR. Intermittent use of nitrates increases bone mineral density: the study of osteoporotic fractures. J Bone mineral Res. (1998) 13:1755–9. doi: 10.1359/jbmr.1998.13.11.1755
45. Rejnmark L, Vestergaard P, and Mosekilde L. Decreased fracture risk in users of organic nitrates: A nationwide case-control study. J Bone mineral Res. (2006) 21:1811–7. doi: 10.1359/jbmr.060804
46. Pouwels S, Lalmohamed A, van Staa T, Cooper C, Souverein P, Leufkens HG, et al. Use of organic nitrates and the risk of hip fracture: A population-based case-control study. J Clin Endocrinol Metab. (2010) 95:1924–31. doi: 10.1210/jc.2009-2342
47. Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, and Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. (2002) 87:1586–92. doi: 10.1210/jcem.87.4.8415
48. Wimalawansa SJ. Nitric oxide: new evidence for novel therapeutic indications. Expert Opin pharmacotherapy. (2008) 9:1935–54. doi: 10.1517/14656566.9.11.1935
49. Wimalawansa SJ. Rationale for using nitric oxide donor therapy for prevention of bone loss and treatment of osteoporosis in humans. Ann New York Acad Sci. (2007) 1117:283–97. doi: 10.1196/annals.1402.066
50. Wimalawansa SJ. Nitric oxide: novel therapy for osteoporosis. Expert Opin pharmacotherapy. (2008) 9:3025–44. doi: 10.1517/14656560802197162
51. Wimalawansa SJ. Skeletal effects of nitric oxide: novel agent for osteoporosis. Principles Bone Biol Elsevier. (2008) . p:1273–310. doi: 10.1016/B978-0-12-373884-4.00007-0
52. Malmir H, Saneei P, Larijani B, and Esmaillzadeh A. Adherence to mediterranean diet in relation to bone mineral density and risk of fracture: A systematic review and meta-analysis of observational studies. Eur J Nutr. (2018) 57:2147–60. doi: 10.1007/s00394-017-1490-3
53. Kunutsor SK, Laukkanen JA, Whitehouse MR, and Blom AW. Adherence to a mediterranean-style diet and incident fractures: pooled analysis of observational evidence. Eur J Nutr. (2018) 57:1687–700. doi: 10.1007/s00394-017-1432-0
54. Fabiani R, Naldini G, and Chiavarini M. Dietary patterns in relation to low bone mineral density and fracture risk: A systematic review and meta-analysis. Adv Nutr (Bethesda Md). (2019) 10:219–36. doi: 10.1093/advances/nmy073
55. Vivekananthan DP, Penn MS, Sapp SK, Hsu A, and Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. (2003) 361:2017–23. doi: 10.1016/S0140-6736(03)13637-9
56. Lundberg JO, Feelisch M, Björne H, Jansson EÅ, and Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. (2006) 15:359–62. doi: 10.1016/j.niox.2006.01.013
57. Ghasemi A. Quantitative aspects of nitric oxide production from nitrate and nitrite. Excli J. (2022) 21:470–86. doi: 10.17179/excli2022-4727
58. Shannon OM, Stephan BCM, Minihane A-M, Mathers JC, and Siervo M. Nitric oxide boosting effects of the Mediterranean diet: A potential mechanism of action. Journals Gerontology: Ser A. (2018) 73:902–4. doi: 10.1093/gerona/gly087
59. Hord NG, Tang Y, and Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. (2009) 90:1–10. doi: 10.3945/ajcn.2008.27131
60. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. New Engl J Med. (2018) 378:e34. doi: 10.1056/NEJMoa1800389
61. Salas-Salvadó J, Bulló M, Babio N, Martínez-González M, Ibarrola-Jurado N, Basora J, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the predimed-reus nutrition intervention randomized trial. Diabetes Care. (2011) 34:14–9. doi: 10.2337/dc10-1288
62. Jackson JK, Patterson AJ, MacDonald-Wicks LK, Oldmeadow C, and McEvoy MA. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: A systematic review and meta-analysis of human evidence. Nutr Rev. (2018) 76:348–71. doi: 10.1093/nutrit/nuy005
63. Siervo M, Lara J, Ogbonmwan I, and Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J Nutr. (2013) 143:818–26. doi: 10.3945/jn.112.170233
64. Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, et al. The dash diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. (2003) 133:3130–6. doi: 10.1093/jn/133.10.3130
65. Macdonald HM, Black AJ, Aucott L, Duthie G, Duthie S, Sandison R, et al. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: A randomized controlled trial. Am J Clin Nutr. (2008) 88:465–74. doi: 10.1093/ajcn/88.2.465
66. McTiernan A, Wactawski-Wende J, Wu L, Rodabough RJ, Watts NB, Tylavsky F, et al. Low-fat, increased fruit, vegetable, and grain dietary pattern, fractures, and bone mineral density: the women’s health initiative dietary modification trial. Am J Clin Nutr. (2009) 89:1864–76. doi: 10.3945/ajcn.2008.26956
67. Ebrahimof S. Effects of increasing fruit and vegetable intake on bone turnover in postmenopausal osteopenic women. Daru. (2009) 17:30–7.
68. Neville CE, Young IS, Gilchrist SE, McKinley MC, Gibson A, Edgar JD, et al. Effect of increased fruit and vegetable consumption on bone turnover in older adults: A randomised controlled trial. Osteoporosis Int. (2014) 25:223–33. doi: 10.1007/s00198-013-2402-x
69. Gunn CA, Weber JL, McGill AT, and Kruger MC. Increased intake of selected vegetables, herbs and fruit may reduce bone turnover in post-menopausal women. Nutrients. (2015) 7:2499–517. doi: 10.3390/nu7042499
70. Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, and Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. (1999) 69:727–36. doi: 10.1093/ajcn/69.4.727
71. Kaptoge S, Welch A, McTaggart A, Mulligan A, Dalzell N, Day NE, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. (2003) 14:418–28. doi: 10.1007/s00198-003-1391-6
72. Macdonald HM, New SA, Golden MH, Campbell MK, and Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. (2004) 79:155–65. doi: 10.1093/ajcn/79.1.155
73. Langsetmo L, Hanley DA, Prior JC, Barr SI, Anastassiades T, Towheed T, et al. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged ≥ 50 Y: A population-based cohort study. Am J Clin Nutr. (2011) 93:192–9. doi: 10.3945/ajcn.110.002956
74. Benetou V, Orfanos P, Zylis D, Sieri S, Contiero P, Tumino R, et al. Diet and hip fractures among elderly Europeans in the epic cohort. Eur J Clin Nutr. (2011) 65:132–9. doi: 10.1038/ejcn.2010.226
75. Feart C, Lorrain S, Ginder Coupez V, Samieri C, Letenneur L, Paineau D, et al. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporosis Int. (2013) 24:3031–41. doi: 10.1007/s00198-013-2421-7
76. Samieri C, Ginder Coupez V, Lorrain S, Letenneur L, Allès B, Féart C, et al. Nutrient patterns and risk of fracture in older subjects: results from the three-city study. Osteoporosis Int. (2013) 24:1295–305. doi: 10.1007/s00198-012-2132-5
77. Benetou V, Orfanos P, Pettersson-Kymmer U, Bergström U, Svensson O, Johansson I, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporosis Int. (2013) 24:1587–98. doi: 10.1007/s00198-012-2187-3
78. Dai Z, Wang R, Ang LW, Low YL, Yuan JM, and Koh WP. Protective effects of dietary carotenoids on risk of hip fracture in men: the Singapore Chinese health study. J Bone mineral Res. (2014) 29:408–17. doi: 10.1002/jbmr.2041
79. Byberg L, Bellavia A, Orsini N, Wolk A, and Michaëlsson K. Fruit and vegetable intake and risk of hip fracture: A cohort study of Swedish men and women. J Bone mineral Res. (2015) 30:976–84. doi: 10.1002/jbmr.2384
80. Fung TT and Feskanich D. Dietary patterns and risk of hip fractures in postmenopausal women and men over 50 years. Osteoporosis Int. (2015) 26:1825–30. doi: 10.1007/s00198-015-3081-6
81. Xie HL, Wu BH, Xue WQ, He MG, Fan F, Ouyang WF, et al. Greater intake of fruit and vegetables is associated with a lower risk of osteoporotic hip fractures in elderly Chinese: A 1:1 matched case-control study. Osteoporosis Int. (2013) 24:2827–36. doi: 10.1007/s00198-013-2383-9
82. de Jonge EA and Kiefte-de Jong JC. Dietary patterns explaining differences in bone mineral density and hip structure in the elderly: the Rotterdam study. Am J Clin Nutr. (2017) 105:203–11. doi: 10.3945/ajcn.116.139196
83. Xu L, Dibley M, D’Este C, Phillips M, Porteous J, and Attia J. Food groups and risk of forearm fractures in postmenopausal women in Chengdu, China. Climacteric: J Int Menopause Soc. (2009) 12:222–9. doi: 10.1080/13697130802626958
84. Prynne CJ, Mishra GD, O’Connell MA, Muniz G, Laskey MA, Yan L, et al. Fruit and vegetable intakes and bone mineral status: A cross sectional study in 5 age and sex cohorts. Am J Clin Nutr. (2006) 83:1420–8. doi: 10.1093/ajcn/83.6.1420
85. Ebrahimof S, Houshyarrad A, Hosseinnezhad A, Zandi N, and Kimiagar SM. Fruit and vegetable intake in postmenopausal women with osteopenia. ARYA Atheroscl. (2006) 1, 183–7.
86. Van Dam RM, Chen YM, Ho SC, and Woo JL. Greater fruit and vegetable intake is associated with increased bone mass among postmenopausal Chinese women. Br J Nutr. (2006) 96:745–51.
87. Zalloua PA, Hsu YH, Terwedow H, Zang T, Wu D, Tang G, et al. Impact of seafood and fruit consumption on bone mineral density. Maturitas. (2007) 56:1–11. doi: 10.1016/j.maturitas.2006.05.001
88. Lin C-H, Chen K-H, Chen C-M, Hsu H-C, Chang C-H, Ho C, et al. Insufficient deep-colored vegetable intake is associated with higher fragility fracture rate in postmenopausal Taiwanese women. Int J Gerontology. (2013) 7:75–9. doi: 10.1016/j.ijge.2012.07.007
89. Benetou V, Orfanos P, Feskanich D, Michaëlsson K, Pettersson-Kymmer U, Eriksson S, et al. Fruit and vegetable intake and hip fracture incidence in older men and women: the chances project. J Bone mineral Res. (2016) 31:1743–52. doi: 10.1002/jbmr.2850
90. Brondani JE, Comim FV, Flores LM, Martini LA, and Premaor MO. Fruit and vegetable intake and bones: A systematic review and meta-analysis. PloS One. (2019) 14:e0217223. doi: 10.1371/journal.pone.0217223
91. Münzel T and Daiber A. Inorganic nitrite and nitrate in cardiovascular therapy: A better alternative to organic nitrates as nitric oxide donors? Vasc Pharmacol. (2018) 102:1–10. doi: 10.1016/j.vph.2017.11.003
92. Omar SA, Artime E, and Webb AJ. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide. (2012) 26:229–40. doi: 10.1016/j.niox.2012.03.008
93. McNally B, Griffin JL, and Roberts LD. Dietary inorganic nitrate: from villain to hero in metabolic disease? Mol Nutr Food Res. (2016) 60:67–78. doi: 10.1002/mnfr.201500153
94. Kapil V, Khambata R, Jones D, Rathod K, Primus C, Massimo G, et al. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol Rev. (2020) 72:692–766. doi: 10.1124/pr.120.019240
95. Lundberg JO, Weitzberg E, and Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. (2008) 7:156–67. doi: 10.1038/nrd2466
96. Ghasemi A and Jeddi S. Anti-obesity and anti-diabetic effects of nitrate and nitrite. Nitric Oxide. (2017) 70:9–24. doi: 10.1016/j.niox.2017.08.003
97. Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. (2008) 4:411–7. doi: 10.1038/nchembio.92
98. Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, et al. The emerging biology of the nitrite anion. Nat Chem Biol. (2005) 1:308–14. doi: 10.1038/nchembio1105-308
99. Samouilov A, Kuppusamy P, and Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch Biochem biophysics. (1998) 357:1–7. doi: 10.1006/abbi.1998.0785
100. Li H, Cui H, Kundu TK, Alzawahra W, and Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem. (2008) 283:17855–63. doi: 10.1074/jbc.M801785200
101. Lu B and Zhang L. Association of a history of gestational diabetes mellitus with osteoporosis, bone mineral density, and trabecular bone score in postmenopausal women. Diabetol Metab Syndr. (2023) 15:215. doi: 10.1186/s13098-023-01194-8
102. Bahadoran Z, Mirmiran P, Azizi F, and Ghasemi A. Nitrate-rich dietary supplementation during pregnancy: the pros and cons. Pregnancy hypertension. (2018) 11:44–6. doi: 10.1016/j.preghy.2017.12.010
103. Mancini L, Moradi-Bidhendi N, Becherini L, Martineti V, and MacIntyre I. The biphasic effects of nitric oxide in primary rat osteoblasts are Cgmp dependent. Biochem Biophys Res Commun. (2000) 274:477–81. doi: 10.1006/bbrc.2000.3164
104. Kalyanaraman H, Schall N, and Pilz RB. Nitric oxide and cyclic Gmp functions in bone. Nitric Oxide. (2018) 76:62–70. doi: 10.1016/j.niox.2018.03.007
105. Kalyanaraman H, Schwaerzer G, Ramdani G, Castillo F, Scott BT, Dillmann W, et al. Protein kinase G activation reverses oxidative stress and restores osteoblast function and bone formation in male mice with type 1 diabetes. Diabetes. (2018) 67:607–23. doi: 10.2337/db17-0965
106. Inoue A, Hiruma Y, Hirose S, Yamaguchi A, and Hagiwara H. Reciprocal regulation by cyclic nucleotides of the differentiation of rat osteoblast-like cells and mineralization of nodules. Biochem Biophys Res Commun. (1995) 215:1104–10. doi: 10.1006/bbrc.1995.2577
107. Pun KK, Lau P, and Ho PWM. The characterization, regulation, and function of insulin receptors on osteoblast-like clonal osteosarcoma cell line. J Bone Mineral Res. (1989) 4:853–62. doi: 10.1002/jbmr.5650040610
108. Gyurko R, Shoji H, Battaglino RA, Boustany G, Gibson FC 3rd, Genco CA, et al. Inducible nitric oxide synthase mediates bone development and P. Gingivalis-induced alveolar bone loss. Bone. (2005) 36:472–9. doi: 10.1016/j.bone.2004.12.002
109. Percival MD, Ouellet M, Campagnolo C, Claveau D, and Li C. Inhibition of cathepsin K by nitric oxide donors: evidence for the formation of mixed disulfides and a sulfenic acid. Biochemistry. (1999) 38:13574–83. doi: 10.1021/bi991028u
110. Hikiji H, Shin WS, Oida S, Takato T, Koizumi T, and Toyo-oka T. Direct action of nitric oxide on osteoblastic differentiation. FEBS Lett. (1997) 410:238–42. doi: 10.1016/S0014-5793(97)00597-8
111. Yang S, Guo L, Su Y, Wen J, Du J, Li X, et al. Nitric oxide balances osteoblast and adipocyte lineage differentiation via the Jnk/Mapk signaling pathway in periodontal ligament stem cells. Stem Cell Res Ther. (2018) 9:118. doi: 10.1186/s13287-018-0869-2
112. Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, and McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. (2005) 146:3622–31. doi: 10.1210/en.2004-1677
113. Fowlkes JL, Bunn RC, Liu L, Wahl EC, Coleman HN, Cockrell GE, et al. Runt-related transcription factor 2 (Runx2) and Runx2-related osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology. (2008) 149:1697–704. doi: 10.1210/en.2007-1408
114. Li H, Liu D, Zhao CQ, Jiang LS, and Dai LY. Insulin potentiates the proliferation and bone morphogenetic protein-2-induced osteogenic differentiation of rat spinal ligament cells via extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Spine. (2008) 33:2394–402. doi: 10.1097/BRS.0b013e3181838fe5
115. Thrailkill KM, Lumpkin CK Jr., Bunn RC, Kemp SF, and Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. (2005) 289:E735–E45. doi: 10.1152/ajpendo.00159.2005
116. Botolin S and McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. (2007) 148:198–205. doi: 10.1210/en.2006-1006
117. Thrailkill KM, Liu L, Wahl EC, Bunn RC, Perrien DS, Cockrell GE, et al. Bone formation is impaired in a model of type 1 diabetes. Diabetes. (2005) 54:2875–81. doi: 10.2337/diabetes.54.10.2875
118. Carvalho FR, Calado SM, Silva GA, Diogo GS, Moreira da Silva J, Reis RL, et al. Altered bone microarchitecture in a type 1 diabetes mouse model Ins2akita. J Cell Physiol. (2019) 234:9338–50. doi: 10.1002/jcp.27617
119. Nyman JS, Kalaitzoglou E, Clay Bunn R, Uppuganti S, Thrailkill KM, and Fowlkes JL. Preserving and restoring bone with continuous insulin infusion therapy in a mouse model of type 1 diabetes. Bone Rep. (2017) 7:1–8. doi: 10.1016/j.bonr.2017.07.001
120. Hie M, Iitsuka N, Otsuka T, and Tsukamoto I. Insulin-dependent diabetes mellitus decreases osteoblastogenesis associated with the inhibition of Wnt signaling through increased expression of Sost and Dkk1 and inhibition of Akt activation. Int J Mol Med. (2011) 28:455–62. doi: 10.3892/ijmm.2011.697
121. Rybaczek T, Tangl S, Dobsak T, Gruber R, and Kuchler U. The effect of parathyroid hormone on osseointegration in insulin-treated diabetic rats. Implant dentistry. (2015) 24:392–6. doi: 10.1097/ID.0000000000000288
122. Erdal N, Gürgül S, Demirel C, and Yildiz A. The effect of insulin therapy on biomechanical deterioration of bone in streptozotocin (Stz)-induced type 1 diabetes mellitus in rats. Diabetes Res Clin Pract. (2012) 97:461–7. doi: 10.1016/j.diabres.2012.03.005
123. Dixit PK and Ekstrom RA. Decreased breaking strength of diabetic rat bone and its improvement by insulin treatment. Calcified Tissue Int. (1980) 32:195–9. doi: 10.1007/bf02408541
124. Rao Sirasanagandla S, Ranganath Pai Karkala S, Potu BK, and Bhat KM. Beneficial effect of Cissus quadrangularis linn. On osteopenia associated with streptozotocin-induced type 1 diabetes mellitus in male wistar rats. Adv Pharmacol Sci. (2014) 2014:483051–66. doi: 10.1155/2014/483051
125. Bortolin RH, Freire Neto FP, Arcaro CA, Bezerra JF, da Silva FS, Ururahy MAG, et al. Anabolic effect of insulin therapy on the bone: osteoprotegerin and osteocalcin up-regulation in streptozotocin-induced diabetic rats. Basic Clin Pharmacol Toxicol. (2017) 120:227–34. doi: 10.1111/bcpt.12672
126. Bhatta A, Sangani R, Kolhe R, Toque HA, Cain M, Wong A, et al. Deregulation of arginase induces bone complications in high-fat/high-sucrose diet diabetic mouse model. Mol Cell Endocrinol. (2016) 422:211–20. doi: 10.1016/j.mce.2015.12.005
127. Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, and Wilcox CS. Expression of ng,Ng-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin ii receptor blockers. Diabetes. (2008) 57:172–80. doi: 10.2337/db06-1772
128. Mordwinkin NM, Meeks CJ, Jadhav SS, Espinoza T, Roda N, diZerega GS, et al. Angiotensin-(1-7) administration reduces oxidative stress in diabetic bone marrow. Endocrinology. (2012) 153:2189–97. doi: 10.1210/en.2011-2031
129. Ma P, Gu B, Xiong W, Tan B, Geng W, Li J, et al. Glimepiride promotes osteogenic differentiation in rat osteoblasts via the Pi3k/Akt/Enos pathway in a high glucose microenvironment. PLoS One. (2014) 9:e112243–e. doi: 10.1371/journal.pone.0112243
130. Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, and Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. (2001) 108:1341–8. doi: 10.1172/JCI11235
131. Bucala R, Tracey KJ, and Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. (1991) 87:432–8. doi: 10.1172/JCI115014
132. Ren X, Ding Y, and Lu N. Nitrite attenuated peroxynitrite and hypochlorite generation in activated neutrophils. Eur J Pharmacol. (2016) 775:50–6. doi: 10.1016/j.ejphar.2016.02.020
133. Yang T, Peleli M, Zollbrecht C, Giulietti A, Terrando N, Lundberg JO, et al. Inorganic nitrite attenuates nadph oxidase-derived superoxide generation in activated macrophages via a nitric oxide-dependent mechanism. Free Radical Biol Med. (2015) 83:159–66. doi: 10.1016/j.freeradbiomed.2015.02.016
134. Shokri M, Jeddi S, Faridnouri H, Khorasani V, Kashfi K, and Ghasemi A. Effect of nitrate on gene and protein expression of nitric oxide synthase enzymes in insulin-sensitive tissues of type 2 diabetic male rats. Endocrine Metab Immune Disord Drug Targets. (2021) 21:2220–30. doi: 10.2174/1871530321666210622155649
135. Ghasemi A, Afzali H, and Jeddi S. Effect of oral nitrite administration on gene expression of snare proteins involved in insulin secretion from pancreatic islets of male type 2 diabetic rats. Biomed J. (2022) 45:387–95. doi: 10.1016/j.bj.2021.04.004
Keywords: diabetoporosis, fracture risk, nitric oxide, nitrate, osteoporosis
Citation: Jeddi S, Kashfi K and Ghasemi A (2025) The potential role of nitrate, a nitric oxide donor, in the prevention and treatment of diabetic osteoporosis. Front. Endocrinol. 16:1480838. doi: 10.3389/fendo.2025.1480838
Received: 14 August 2024; Accepted: 21 February 2025;
Published: 12 June 2025.
Edited by:
Bingzi Dong, The Affiliated Hospital of Qingdao University, ChinaReviewed by:
Greta Varchi, Consiglio Nazionale delle Ricerche (Bologna), ItalyAmedea Barozzi Seabra, Federal University of ABC, Brazil
Maria Christou, University Hospital of Ioannina, Greece
Copyright © 2025 Jeddi, Kashfi and Ghasemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asghar Ghasemi, R2hhc2VtaUBzYm11LmFjLmly; Khosrow Kashfi, S2FzaGZpQG1lZC5jdW55LmVkdQ==
 Sajad Jeddi
Sajad Jeddi Khosrow Kashfi
Khosrow Kashfi Asghar Ghasemi
Asghar Ghasemi