- 1Department of Botany, Pir Mehr Ali Shah-Arid Agriculture University, Rawalpindi, Pakistan
- 2Department of Botany, Government Graduate College for Women, Jhelum, Pakistan
- 3National Research Center of Intercropping, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
Diabetes mellitus (DM) is a severe metabolic disorder characterized by an increase in blood glucose level due to insufficient insulin production or failure of insulin action on targeted tissues or both. DM impacts male reproductive health across four aspects: ejaculation, erectile dysfunction, structural alterations in reproductive organs, and alterations in semen quality. The population of male individuals with diabetes is steadily rising, paralleled by an increase in fertility issues among men. A WHO report states that diabetes mellitus affects about 171 million (2.8%) persons worldwide. Anti-diabetic medications that are now on the market are expensive and have several negative effects, including cardiac, hepatic, and renal failure in diabetic patients. Keeping in view, this review emphasizes the limitations of currently used synthetic anti-diabetic drugs and provides the progress in the development of phytogenic metallic NPs (NP)in the treatment of diabetes and associated male infertility. To collect data, various databases were examined, including Springer Link, Google Scholar, PubMed, Wiley Online Library, and Science Direct. Several studies and research reports based on nanotechnological approaches in the formulation of anti-diabetic drugs have pointed out the fact that research in the formulation of nanodrugs has improved strategies for combating diabetes and associated male infertility based on the plausible molecular mechanism of action of the drugs. These nanodrugs have been observed to significantly influence regulatory mechanisms through their effects on pancreatic α-amylase, intestinal α-glucosidase, insulin action, and glucose uptake across various in vivo and in vitro systems. Moreover, integrating nanotechnological methodologies with the exploration of herbal compounds further enhances the understanding of their chemical potential. This synergistic approach may pave the way for identifying novel drug candidates with exceptional therapeutic efficacy, offering significant advantages in the management of diabetes and associated male infertility for the betterment of humanity. Furthermore, the personalized design of plant-based metallic NPs has the potential to significantly advance precision medicine techniques for the treatment of male infertility and diabetes.
1 Introduction
Diabetes mellitus (DM) is a serious metabolic disorder characterized by elevated blood glucose levels resulting from insufficient insulin production, impaired insulin action on target tissues, or a combination of both. It is a major risk factor for cardiovascular diseases, which account for approximately 50% of deaths among individuals with diabetes (1, 2). The prevalence of diabetes is increasing worldwide due to an increase in obesity and a sedentary lifestyle. In a report released by (World Health Organization in 2000, it was estimated that over 171 million (2.8%) people are living with diabetes mellitus throughout the world (3, 4). This number is expected to increase to 366 million (4.4%) by 2030. According to the International Diabetes Federation, the incidence of diabetes in Pakistan in 2016 (5, 6), 2018 (1, 7) and 2019 (8, 9) It was 11.77%, 16.98%, and 17.1%, respectively, and in 2022, the prevalence rate of adults was 26.7% in Pakistan (9, 10).
There are two primary forms of diabetes mellitus. Type 1 diabetes mellitus (T1DM), also known as insulin-dependent diabetes, is characterized by the autoimmune destruction of pancreatic beta cells, leading to a complete deficiency of insulin and resulting in chronic hyperglycemia (see Figure 1). Type 2 diabetes mellitus (T2DM), or non-insulin-dependent diabetes, is a metabolic disorder arising from either inadequate insulin production or the body’s inability to effectively utilize insulin, also culminating in elevated blood glucose levels (11). Dietary carbohydrates are broken down during digestion into glucose, which is subsequently absorbed through the walls of the small intestine into the bloodstream. Insulin, a hormone secreted by the pancreas, plays a critical role in facilitating the uptake of glucose into cells throughout the body, where it serves as a primary energy source.
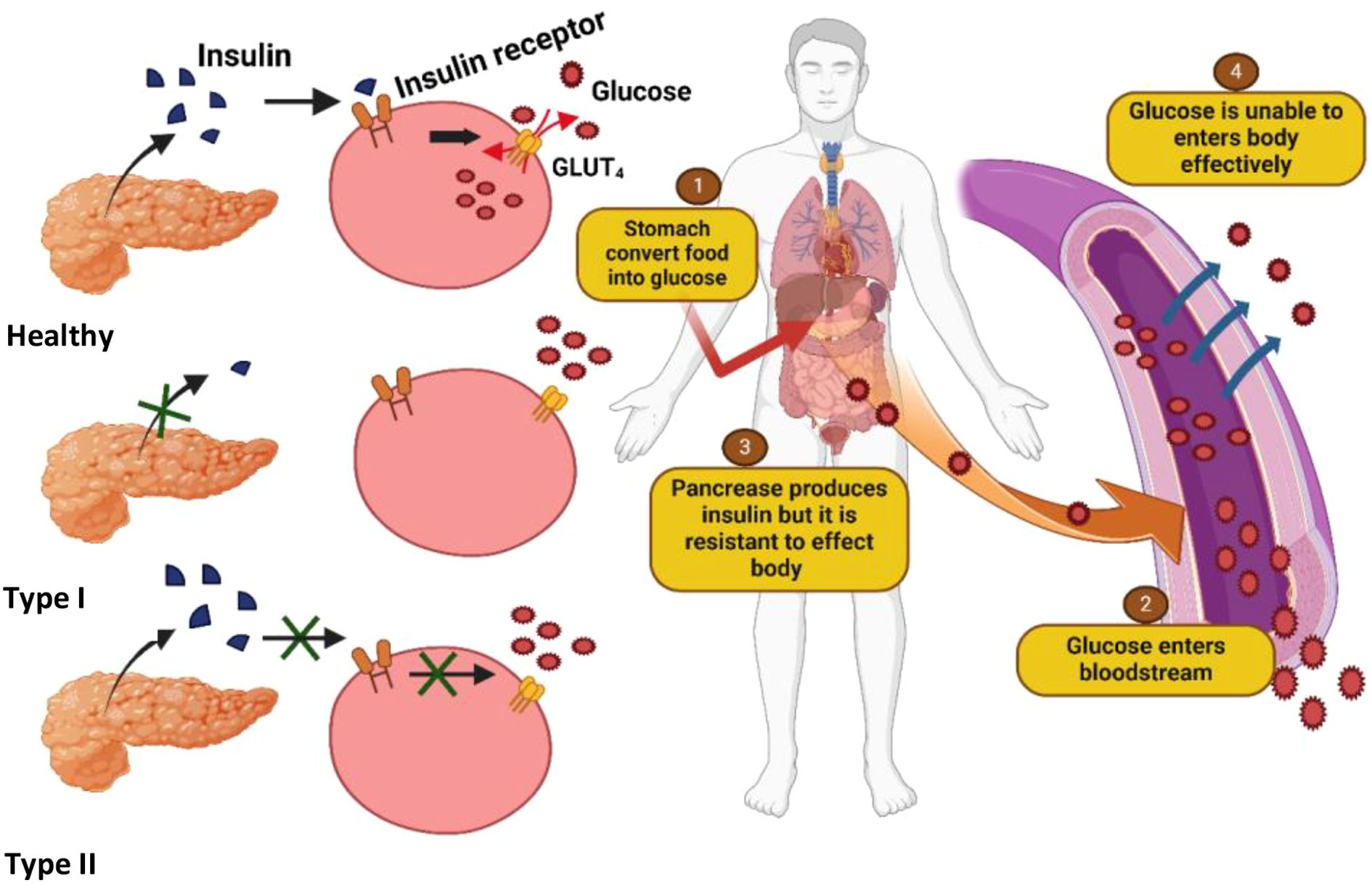
Figure 1. Illustration of differences in insulin production and glucose uptake among normal individuals, Type I diabetics (complete insulin deficiency), and Type II diabetics (insulin resistance and impaired uptake), leading to chronic hyperglycemia.
In individuals with insulin resistance, however, cellular responsiveness to insulin is impaired, hindering glucose uptake and leading to elevated blood sugar levels. Initially, the pancreas compensates for insulin resistance by increasing insulin production; however, over time, this compensatory mechanism fails, leading to sustained hyperglycemia, which is a hallmark of type 2 diabetes (12) (Figure 1). This condition has become a significant public health concern, particularly in developing countries, where rapid urbanization and lifestyle changes, most notably the rising consumption of Western-style diets high in fats, have contributed to the growing prevalence of the disease. It is characterized by hyperglycemia, insulin resistance, and obesity. Obesity results in an imbalance between energy intake and energy expenditure. Besides obesity, it has a strong connection with dyslipidemia and hypertension. The interconnection between these conditions is a major risk factor for cardiovascular diseases (13, 14).
2 Diabetes associated male infertility and its underlying causes
The World Health Organization currently defines infertility as the inability of a sexually active couple (at least three times per month), not using contraception, to achieve pregnancy within one year. About 15% of sexually active couples are infertile (15, 16). And male factor infertility contributes to about 50% of the infertility cases (17). Research suggests that nearly half of male patients with diabetes experience decreased semen quality and impaired reproductive function. Diabetes-induced metabolic disorders can indeed have significant effects on male fertility and reproductive health (18). In recent years, increasing attention has been directed towards its effects on male reproductive health. Diabetes induces metabolic disturbances that contribute to oxidative stress, abnormal zinc metabolism, and insulin resistance (IR). These factors collectively impact male fertility and reproductive health.
Multiple studies conducted using animal models have demonstrated that DM significantly reduces fertility (19–21). This reduction is attributed to decreased sperm concentration and motility, increased seminal plasma abnormalities, and alterations in the normal morphology of sperm cells (22, 23). Additionally, patients with DM may experience other disturbances such as retrograde ejaculation, premature ejaculation, decreased libido, delayed sexual maturation, and impotence (15, 24–26) (Figure 2).
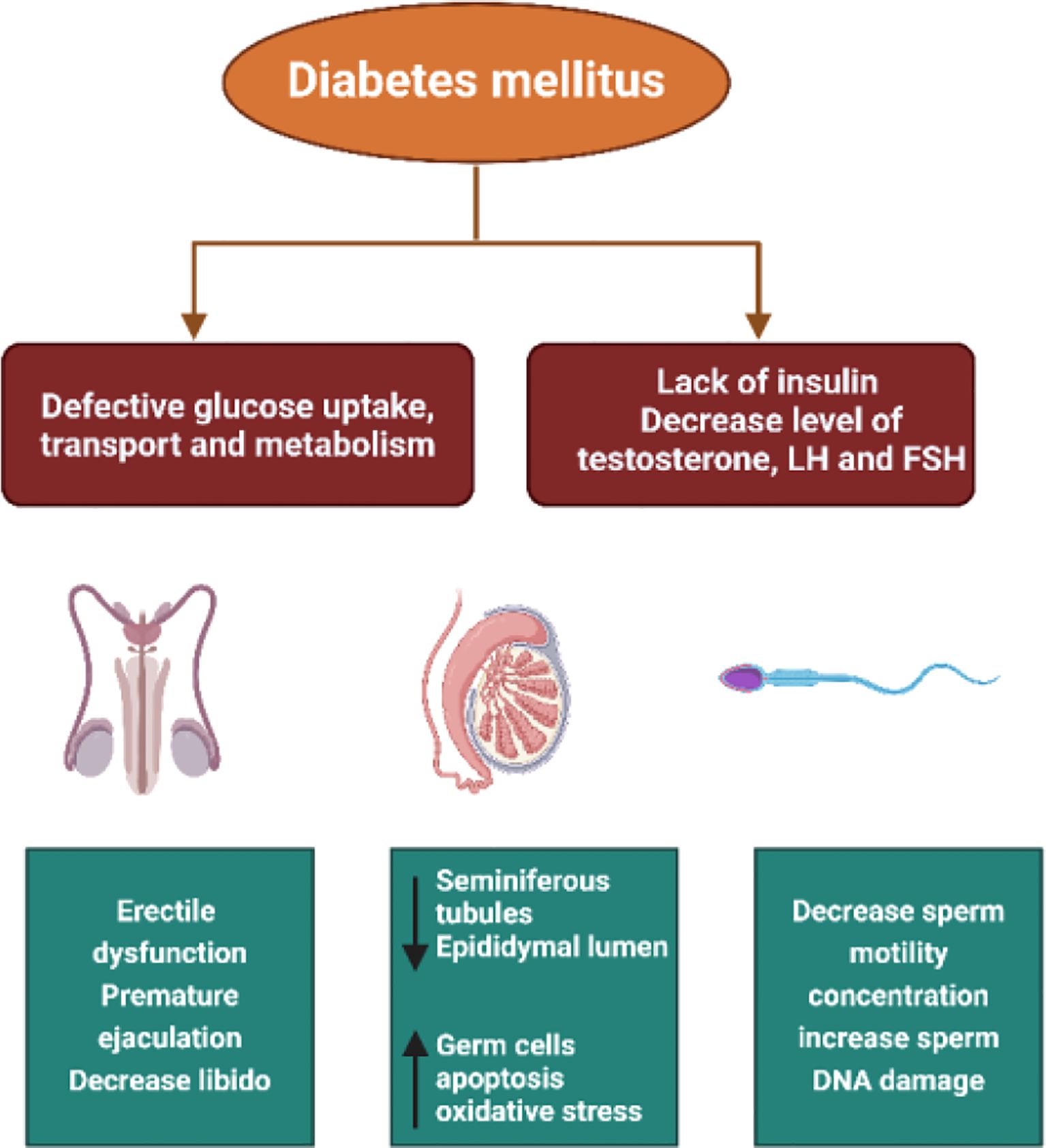
Figure 2. The pathways through which diabetes affects male fertility including oxidative stress, hormonal imbalance, impaired spermatogenesis, erectile dysfunction, and testicular structural damage.
Some reproductive issues associated with diabetes are as follows
2.1 Erectile dysfunction
Among the various reproductive issues associated with diabetes, erectile dysfunction (ED) is a prominent concern. Studies indicate that 59% of diabetic men experience ED. The underlying cause often involves penile nerve thickening or beaded neuropathy. Additionally, decreased serum testosterone levels due to diabetes can negatively affect vascular endothelial function, further contributing to ED (27–32).
2.2 Testicular glucose metabolism and infertility
Hyperglycemia in diabetes alters testicular glucose metabolism. Glycogen, a key regulator of testicular development and spermatogenesis, plays a critical role by mobilizing glucose necessary for germ cell development. Disturbances in testicular carbohydrate metabolism, as observed in diabetes, can significantly contribute to testicular dysfunction and potentially lead to male infertility (33–35).
2.3 Structural and functional changes in reproductive organs
Diabetes induces structural and functional alterations in male reproductive organs. Notably, testicular blood flow velocity decreases, possibly attributed to reduced vascular endothelial growth factor (VEGF) expression (36, 37). Microcirculation disturbances result in testicular morphological and structural changes. In immature rats, diabetes can delay gonadal development, decrease sexual behavior, testosterone synthesis, and promote gonadal atrophy (36, 38). Seminiferous tubules (STs) may show signs of atrophy, thinning of the spermatogenic epithelium, and an increased presence of empty tubules. Some STs may contain multinucleated cells with two or three nuclei, along with evidence of enhanced vascular degeneration and germ cell apoptosis. Sertoli cells, located near the lumen of the STs, may accumulate cytoplasmic debris, and their ultrastructure often reveals irregular basement membranes and a reduced cell population. Structural alterations are also observed in Leydig cells, including irregularly shaped nuclei, abundant heterochromatin, lipid droplets, as well as damaged mitochondria and changes in the endoplasmic reticulum (39).
2.4 Histopathological changes in the testis
Studies investigating male reproductive dysfunction in the context of diabetes primarily focus on changes in testicular morphology. Data from various studies show reductions in the mass of different regions of the testis and a diminished sperm count in testicular tubules. Additionally, hyperglycemia can lead to histological alterations in the epididymal duct, including decreased germ cell populations, reduced stereocilia, clustering of epithelial cells, lipid vacuolization, and inflammation (40). Other histological changes observed in the testis tissue due to diabetes include wrinkled secretory epithelial cells in the prostate, testicular stromal hypertrophy, inflamed cells, prostatic intraepithelial neoplasia, and expanded secretory organelles. Reports in diabetic rodents also demonstrate reductions in the weight of seminal vesicles, as well as decreased weight and mass of testicular tissue. Moreover, the number of Leydig cells decreases, the seminiferous tubules’ diameter and germinal epithelium height decrease, and the volume of interstitial matrix increases in diabetic conditions (41, 42).
Reduced sperm quality is a recognized issue in diabetes, particularly in cases of T1DM. This is linked to various factors, including altered gene expression related to sperm DNA repair, mitochondrial DNA deletions, and decreased sperm motility (43, 44). Insulin levels in the bloodstream have been found to impact the acrosome and plasma membrane of sperm. Diabetic patients often experience reduced sperm motility and abnormal sperm morphology, which can impact fertility.
Hyperglycemia, a common feature of diabetes, affects all stages of spermatogenesis, including spermatogonia proliferation, spermatocyte division, and spermiogenesis. Research has consistently shown that diabetes can lead to decreased sperm count, motility, semen volume, and abnormal sperm morphology. However, some studies suggest that insulin therapy can improve sperm content and motility, while others find that semen volume may or may not be affected by diabetes (45). Diabetes can also cause damage to sperm DNA structure, potentially leading to infertility. Fortunately, controlling blood glucose levels has been shown to restore sperm numbers and motility in diabetic animal models. Normal lipid metabolism is essential for spermatogenesis, and hyperglycemia can disrupt this process, affecting triglyceride hydrolysis, cholesterol esters, and steroid hormones (40).
Hypogonadism, characterized by low testosterone levels, is another issue seen in diabetic men. Testosterone is crucial for male reproductive tissue development, sperm production, and overall sexual health (46, 47). Testosterone deficiency is more common in diabetic men, especially those over 40, and it may contribute to complications associated with diabetes (48). Testicular tissue degradation and small testes size have also been linked to low testosterone levels in diabetic men, impacting fertility and sexual function (49, 50).
2.5 Molecular mechanisms linking glucose metabolism and male reproductive dysfunction in diabetes
Male fertility is affected by Diabetes-induced dysregulation of glucose metabolism through several interconnected molecular pathways:
2.5.1 Pancreatic α-amylase and intestinal α-glucosidase
These enzymes break down complex carbs into glucose, inhibiting their function and lowering hyperglycemia after meals. Prolonged hyperglycemia encourages oxidative stress and glycation end products (AGEs), which harm the seminiferous epithelium, disrupt the function of Leydig and Sertoli cells, and fragment sperm DNA (51).
2.5.2 Insulin action and insulin receptors in testicular tissue
Sertoli and Leydig cells have receptors for insulin and insulin-like growth factor-1 (IGF-1). Insulin resistance impairs testosterone synthesis, breaks down the blood-testis barrier, and decreases spermatogenesis, all of which decrease these cells’ responsiveness. This is partially mediated by the MAPK and PI3K/Akt pathways, which are implicated in cell survival and glucose uptake (52).
2.5.3 Glucose uptake and spermatogenic support
Spermatogenesis depends on Sertoli cells’ uptake of glucose (via the GLUT1 and GLUT3 transporters). The expression of the glucose transporter is dysregulated in diabetic conditions, which causes seminiferous tubules to lose energy. The maturation of germ cells is restricted, and apoptosis is increased (53).
2.5.4 Oxidative stress and inflammation
Prolonged hyperglycemia weakens antioxidant defenses and produces excessive ROS. This oxidative imbalance results in decreased sperm motility, mitochondrial failure, and lipid peroxidation in sperm membranes. Inflammatory cytokines, such as TNF-α and IL-6, also affect the endocrine and exocrine processes of the testicles (54).
2.5.5 Hormonal imbalance
Hypogonadotropic hypogonadism is linked to insulin resistance. It inhibits testosterone synthesis and Sertoli cell support for growing germ cells by decreasing LH and FSH signaling (55).
3 Current diabetic management strategies and their side effects
There are five classes of oral diabetes drugs (OHDs) available that function through four different pathways:
a. Improving insulin secretion in the pancreas (sulfonylurea & non-sulfonylurea)
b. Reducing glucose release from the liver (biguanides)
c. Lowering gastrointestinal absorption of carbohydrates (α-glucosidase inhibitor)
d. enhancing peripheral glucose disposal (biguanides and thiazolidinedione) (56, 57)
All of the medications have side effects (58, 59). Though it is essential to achieve glucose management as soon as possible to reduce the impact of glucose toxic effects, it is also vital to provide treatment to control other associated risks, such as oxidative stress, dyslipidemia, mitochondrial dysfunction, vascular complications, and so on (Table 1) (64–67). Diabetes cannot be cured totally, but its severity and symptoms can be managed with medications and lifestyle changes (68). Thiazolidinediones, Biguanides, Sulfonylureas, meglitinides, α-Glucosidase inhibitors, and Dipeptidyl peptidase-4 (DPP-4) inhibitors. These are some of the most regularly utilized pharmacological medications for the treatment of diabetes. These medicines are administered as the first line of defense to prevent the diabetic state from deteriorating (68, 69).
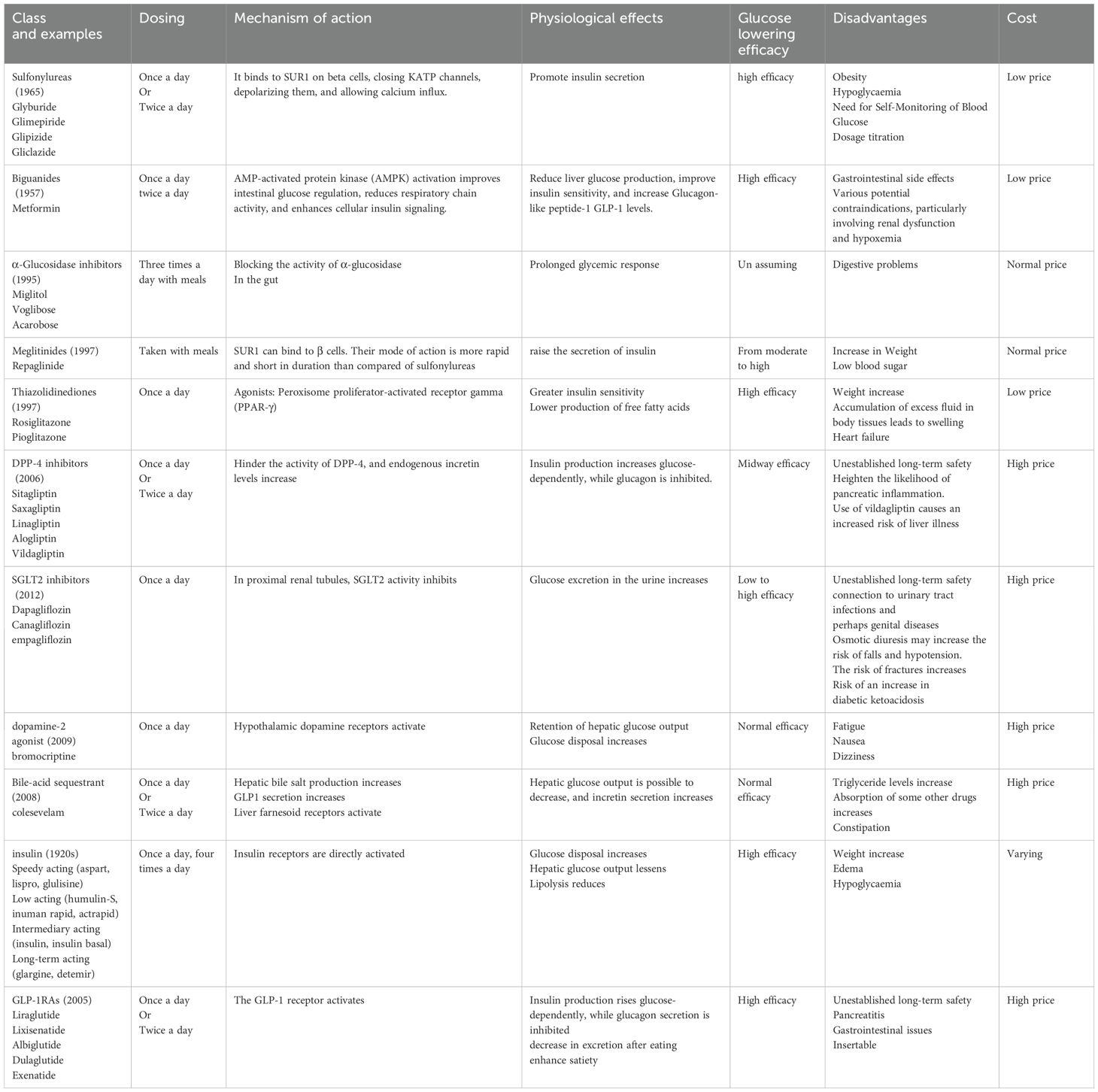
Table 1. A list of the current medications for type 2 diabetes mellitus, along with their disadvantages (44, 48, 60–63).
3.1 Thiazolidinediones
Thiazolidinediones or ‘glitazones’ are a novel class of oral diabetes medications. Thiazolidinediones are insulin sensitizers that function mostly through increasing insulin sensitivity in target organs such as the liver and muscles (70). Pioglitazone, rosiglitazone, and troglitazone are thiazolidinedione-derived drugs. Troglitazone became available in 1997 shortly thereafter removed due to toxicity to the liver (62, 71).
3.1.1 Mode of action
The activation of a transcription factor, peroxisome proliferator-activated receptor (PPAR), is the mechanism by which TZDs exert their anti-diabetic action. This factor affects the transcription of various genes involved in glucose and lipid metabolism and energy balance, such as fatty acyl-CoA synthase, malic enzyme, glucokinase, and glucose transporter 4 (GLUT4), among others. As a result, TZDs decrease insulin resistance (IR) in adipose tissue, muscle, and the liver (70, 71).
3.1.2 Adverse effects of using thiazolidinediones
One of the main adverse effects associated with PPAR receptor activation is the enhanced proliferation of peripheral adipocytes, which leads to increased uptake of free fatty acids. This process can ultimately result in weight gain and an increase in peripheral fat mass (72, 73). Several recent studies and analyses have indicated the potential role of TZD in cardiovascular events in type 2 diabetic patients. In this way, meta-analyses of adverse outcomes from controlled studies have revealed a possible link between thiazolidinedione use and an elevated risk of ischemic myocardial events in diabetes patients (74, 75). Fluid retention is another known side effect associated with the use of thiazolidinediones (TZDs). It is hypothesized that TZD-induced renal edema results from the activation of sodium-coupled bicarbonate reabsorption in the renal proximal tubules. This leads to increased salt and water reabsorption, ultimately causing an expansion in kidney volume (76). The outcomes of these studies caused disagreement as well as confusion about the use of TZDs in diabetic treatment methods (68, 71).
3.2 Biguanide
Biguanides are a pharmacological and pharmaceutical class that relies on the biguanidine molecule (77, 78). These chemicals were first isolated from the plant Galega officinalis (79, 80). Guanidine, the active component of Galega officinalis, was demonstrated to reduce glucose levels in the 1920s and was used for synthesizing many anti-diabetic drugs (78, 81). They are known as another type of insulin sensitizer, and metformin is one of the most commonly used medications for diabetes in this class (68, 72). Biguanides do not act as true hypoglycemic agents (82, 83). Thiazolidinediones (TZDs) lower elevated blood glucose levels in patients with non-insulin dependent diabetes mellitus (NIDDM), but they do not significantly reduce blood glucose levels in non-diabetic individuals unless there has been prolonged fasting (84–89). Biguanides, compared to sulfonylureas, do not increase insulin secretion from p-cells; however, biguanide therapy may result in decreased insulin levels (89, 90).
3.2.1 Adverse effects of biguanide
Symptoms of the gastrointestinal tract (e.g., nausea, vomiting, diarrhea, and abdominal discomfort) are among the side effects linked with the therapeutic use of all biguanides (78, 91). Metformin is seldom associated with acute hepatitis and cholestasis (81, 92–100) Metformin has been linked to malabsorption syndromes, which can result in electrolyte imbalances and vitamin B12 insufficiency (13, 81, 84–86, 90, 101–112) Diarrhea is associated with hypomagnesaemia, hypocalcemia, and hypokalemia (85, 104, 105, 113). Vitamin B12 deficiency can lead to megaloblastic anemia and various neuropathies. The underlying causes of this deficiency are not fully understood but appear to be multifactorial. Contributing factors may include alterations in gut microbiota, reduced gastrointestinal motility, competitive inhibition of B12 absorption, and disruptions in calcium-dependent membrane transport mechanisms in the terminal ileum (106, 113).
Lactic acidosis is an uncommon but possibly hazardous metformin adverse effect. The occurrence of this consequence is quite low: one case per 100,000 individuals receiving treatment (114–119). Lactic acidosis can be produced by very high metformin levels in blood vessels or by any circumstance that causes hypoxia or hepatic insufficiency, restricting the capacity of the body to break down lactate (120). Lactic acidosis usually arises in patients who have persisted in using metformin despite risks (114, 120). Renal insufficiency, indicated by serum creatinine levels of 1.5 mg/dL or higher in men and 1.4 mg/dL or higher in women, is a contraindication for metformin therapy. Additionally, conditions such as severe cardiac or pulmonary insufficiency that lead to reduced peripheral perfusion, lactic acidosis, liver disease, alcohol dependence, or the use of intramuscular contrast agents for radiographic imaging also represent exclusion criteria due to the increased risk of adverse effects, particularly lactic acidosis (80, 121).
3.3 Sulfonylureas
Sulfonylureas are categorized as either first-generation (e.g., tolbutamide and chlorpropamide) or second-generation (e.g., glyburide, gliclazide, glipizide, and glimepiride) (122, 123). Similar to first-generation sulfonylureas (such as tolbutamide, acetohexamide, and chlorpropamide), second-generation sulfonylureas also effectively lower hyperglycemia (124, 125). Second-generation sulfonylureas are preferred over first-generation agents due to their higher potency and more favorable safety profile. First-generation sulfonylureas are associated with a greater risk of adverse effects, including hypoglycemia, weight gain, and fluid retention, making second-generation drugs the recommended choice in clinical practice (126, 127).
3.3.1 Mode of action
Sulfonylureas act by inhibiting the ATP-sensitive K channel (KATP), causing the release of insulin from the cells of the pancreas and therefore lowering blood glucose levels (128–131). Over 90 percent of sulfonylureas in the blood are linked to plasma proteins, causing interactions between drugs with salicylates, sulfonamides, and warfarin (123, 132). While the effectiveness of sulfonylureas varies, they tend to reduce A1C to a comparable extent as metformin, by 1.5 percentage points (117, 133).
3.3.2 Side effects of sulfonylureas
The primary adverse effect associated with sulfonylureas is hypoglycemia. Due to variations in the pharmacotherapeutic properties of different sulfonylurea agents, the risk of hypoglycemic episodes can vary significantly among them (120, 134). The possibility of gaining weight is yet another drawback of sulfonylureas. Many people see an increase of at least 2 kg when taking these drugs (117, 135). It is also to be noted that certain people with sulfonamide allergies show cross reactivity with sulfonylureas; thus, these treatments shouldn’t be used in patients with sulfa allergies. Cross-reactivity with other medicines, such as carbonic anhydrase inhibitors, loop diuretics, and thiazide diuretics, is also possible (120, 136). Sulfonylureas are also linked to an increased risk of cardiovascular disease (68, 137). According to research, while promoting the closure of pancreatic-cell KATP channels to boost insulin secretion, this medicine may also lead to the closure of cardiac KATP channels, resulting in a higher cardiac risk in those people (138, 139).
3.4 Meglitinides
Nateglinide and repaglinide are the two most common meglitinides (glinides) (62, 140–142). The insulinotropic drugs are meglitinide analogues. They were introduced in 1995 and were licensed for clinical use in people with T2DM in 2000. They are secretory substances with a faster anti-hyperglycemic activity and a shorter duration of action than sulfonylurea. As a result, post-prandial hyperglycemia is better managed, and the risk of late hypoglycemia is also decreased (143–146).
3.4.1 Mode of action
Meglitinides attach themselves to Sulfonylurea Receptor 1 (SUR1’s) benzamido site on β cells (123, 147). The first meglitinide analogue approved for clinical use in adults with T2DM was repaglinide. The insulinotropic effect of repaglinide, similar to that of sulfonylureas, operates through ATP-dependent potassium (KATP) channels. Repaglinide stimulates insulin secretion by inhibiting KATP channels in pancreatic β-cells, leading to membrane depolarization and the opening of voltage-gated calcium channels. The resulting influx of calcium increases intracellular calcium levels, triggering the exocytosis of insulin-containing granules (148, 149). Nateglinide, like repaglinide, binds to SURs, blocking KATP channels and promoting insulin secretion, but its pharmacodynamic effects are distinct in several important respects (150, 151).
3.4.2 Adverse effects of meglitinides
Repaglinide and nateglinide studies have revealed different levels of hypoglycemia and, overall, lesser weight gain than sulfonylureas (149, 152–163). Although a topical test-positive delayed-type hypersensitivity reaction to repaglinide has been documented, cutaneous reactions to meglitinides seem to be uncommon. In this case, the fifth day after starting repaglinide, a maculopapular rash developed. Repaglinide was stopped, and systemic corticosteroids and antihistamines were administered instead (158, 164). Six people in a post-marketing monitoring study of nateglinide reported seven cutaneous side effects associated with treatment, including two occurrences of allergic dermatitis and one non-specific rash. There were 892 patients in the study overall (153, 165).
3.5 α-Glucosidase inhibitors
In the early 1990s, acarbose was the first alpha-glucosidase inhibitor to be introduced. Miglitol and voglibose also became available later on. It is common for Asian communities to utilize AGIs, especially those who consume diets that are high in complex carbohydrates (166, 167).
3.5.1 Mechanism of action
In the brush border of enterocytes lining the intestinal villi, α-glucoside inhibitors (AGIs) competitively inhibit α-glucosidase enzymes, preventing the enzymes from cleaving disaccharides and oligosaccharides into monosaccharides (167–170). This process helps to reduce fluctuations in blood glucose levels and decrease the amount of insulin required during meals by slowing down the digestion and absorption of carbohydrates in the lower part of the digestive system (148, 167). Compared to controls, AGI therapy reduces Glucagon-like Peptide (GIP) secretion and increases postprandial Glucose-dependent Insulinotropic Polypeptide (GLP-1) secretion (171–173). Varying α-glucosidase enzymes have distinct affinities for AGIs, resulting in unique activity profiles. For example, acarbose shows a higher affinity for glucoamylase while miglitol is a more effective sucrase inhibitor (167, 174).
3.5.2 Adverse effects of α-glucoside inhibitors
The common gastrointestinal side effects caused by AGIs, such as flatulence, stomach pain, and diarrhea, may lead patients to discontinue their medication (123, 175).
3.6 Dipeptidyl peptidase-4 inhibitors
The DPP-4 inhibitors currently on the market are sitagliptin, vildagliptin, saxagliptin, linagliptin, and alogliptin (176, 177). Japan has granted licenses for two DPP-4 inhibitors, omarigliptin and trelagliptin (178–181).
3.6.1 Mechanism of action
DPP-4 inhibitors increase levels of incretin hormones in circulation, notably GLP-1 and GIP. The “incretin effect” refers to the ability of intestinal variables to increase insulin responses by 50-70% in healthy individuals following a diet (182–184). In T2DM, this effect is significantly reduced. When lipids and carbohydrates are consumed, K cells in the duodenum and jejunum release glucose-dependent insulinotropic polypeptide (GIP) (185–190). Apart from its incretin action, GIP also plays roles in adipogenesis and potentially β-cell proliferation, and reduces stomach acid output (188, 190–198).
3.6.2 Adverse effects
There is an uncertain safety concern over the long run, which may raise the possibility of pancreatitis and an increased risk of liver dysfunction associated with Vildagliptin (123).
4 Plant-based metallic nanoparticles in diabetes management
As previously mentioned, the use of synthetic medicines for diabetes treatment is often hindered by their associated side effects (199, 200). Consequently, the current focus is on exploring the antihyperglycemic potential of medicinal plants in managing diabetes. According to worldwide ethnobotanical studies, approximately 800 plant species are employed for medicinal purposes in preventing diabetes (201, 202). Among these, scientific validation has confirmed that only 450 of these plants possess properties capable of lowering blood glucose levels, with 109 of them having well-documented mechanisms of action (58, 203).
Various treatments are also being employed in a holistic approach that takes into account physical, psychological, and spiritual aspects (204, 205). Notably, between 60% to 80% of the global population utilizes traditional medicines derived from medicinal plants to address various health conditions, including diabetes. There is a multitude of plants known for their anti-diabetic properties (206, 207).
4.1 Preparation and characterization of plant-based metallic nanoparticles
4.1.1 Synthesis methods
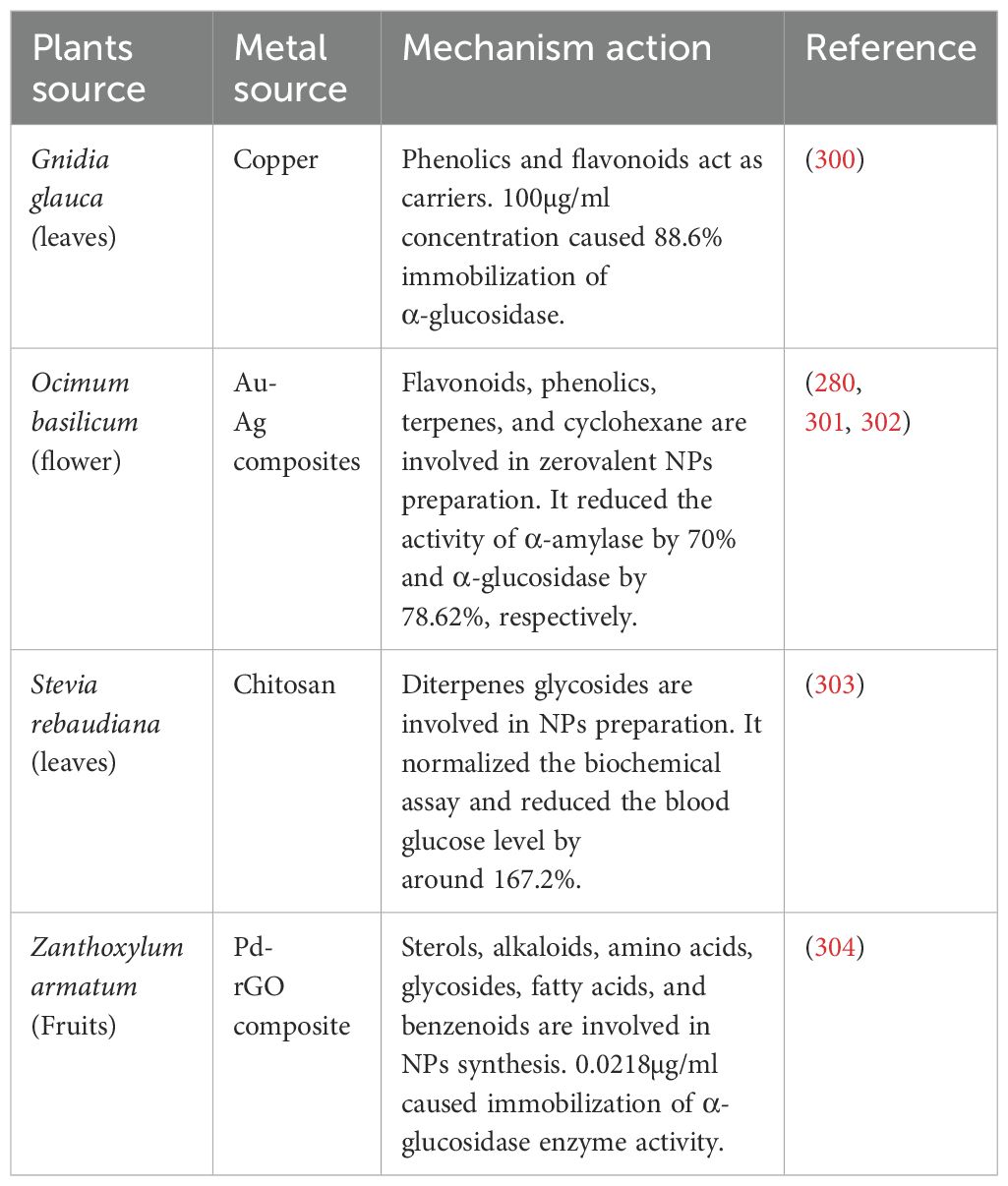
Using green synthesis techniques, plant extracts serve as both capping and reducing agents in the synthesis of plant-based metallic nanoparticles (NPs). The extracts, which are high in alkaloids, terpenoids, phenolics, and flavonoids, are combined with metal precursors such as gold chloride (HAuCl4), zinc sulfate (ZnSO4), or silver nitrate (AgNO3) (208). The pH, temperature, and reaction time are all regulated during the biosynthesis, which is usually shown by a color shift brought on by surface plasmon resonance (for example, AgNPs turning from pale yellow to dark brown) For example, the aqueous leaf extract of Musa paradisiaca is frequently utilized as a stabilizing and reducing agent in the synthesis of zinc oxide nanoparticles (ZnONPs). The plant extract’s bioactive components, including flavonoids, polyphenols, and reducing sugars, convert Zn²+ ions to ZnO nuclei when the zinc nitrate hexahydrate [Zn(NO3)2·6H2O]+ precursor is introduced under carefully monitored conditions (usually 60–80°C, pH ~9). These phytochemicals also cap the developing nanoparticles as the reaction goes on, limiting aggregation and encouraging size control. A color shift (such as a yellowish-white precipitate) usually signals the creation of ZnONPs, and UV-Vis spectroscopy, which shows a distinctive absorption peak at about 360–380 nm, confirms this (209).
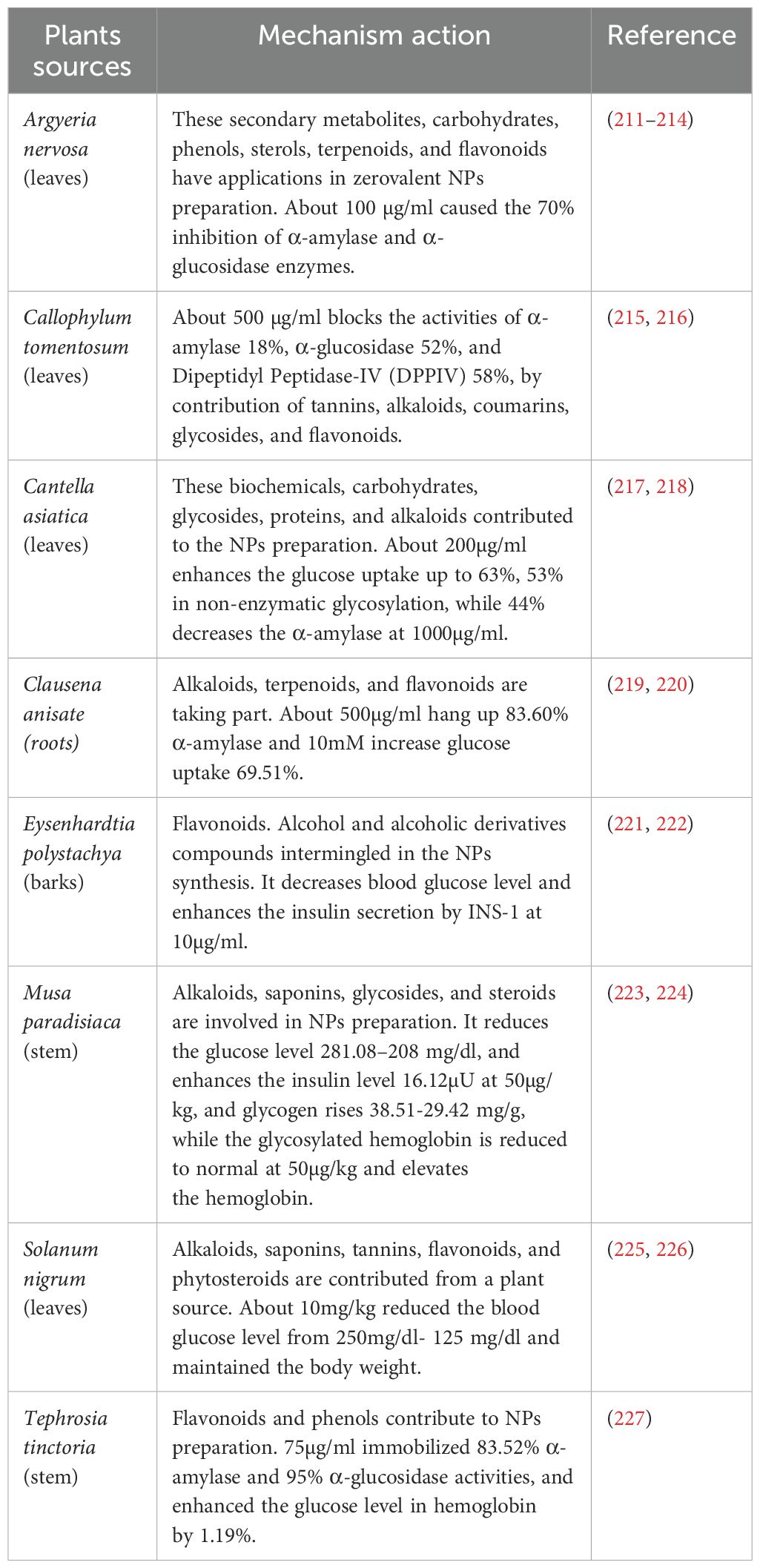
Similarly, Argyria nervosa root extract is used to synthesize silver nanoparticles (AgNPs) by reducing Ag+ ions from silver nitrate (AgNO3). The phytochemicals present, particularly phenolics and alkaloids, donate electrons to reduce Ag+ to metallic Ag⁰, initiating nanoparticle nucleation. Simultaneously, these compounds act as capping agents, forming a protective layer around each nanoparticle that improves colloidal stability and biocompatibility (210) (Tables 2, 3). In these green synthesis systems, plant-derived compounds become physically or chemically associated with the nanoparticle surface. For crude extracts, the nanoparticles serve as nano-carriers, embedding or adsorbing various phytochemicals in their matrix or on their surface. This makes it possible for several bioactives, like enzyme inhibitors, anti-inflammatory drugs, and antioxidants, to be delivered simultaneously in a single nanoformulation (239).
The production of gold nanoparticles (AuNPs) using extract from Typha capensis, which includes bioactive flavonoids and glycosides, is another well-researched example. The gold ion precursor in this method is chloroauric acid (HAuCl4). When the phytochemicals are combined with the plant extract and heated to about 70°C, they convert Au³+ to elemental Au⁰, which starts the nucleation of nanoparticles. The extract’s flavonoids stabilize the suspended nanoparticles by attaching to the gold surface and acting as capping ligands in addition to reducing agents (240). Additionally, certain bioactive substances, like naringenin, can be added to these AuNPs either post-synthesis or from the same extract. Naringenin finds adsorption sites on the surface of the nanoparticles through π-π stacking interactions or hydrogen bonding. Consequently, the plant-derived substance with enhanced cellular absorption and prolonged release is delivered by the nanocarrier system along with the metallic therapeutic activity (e.g., antioxidant, anti-inflammatory from AuNPs) (241).
4.1.2 Characterization techniques
Analyzing the composition and functionality of synthesized NPs using key approaches includes:
4.1.2.1 UV–Visible spectroscopy
Utilized to identify the surface plasmon resonance (SPR) peaks characteristic of metallic nanoparticles to verify the formation of nanoparticles. Peaks for silver nanoparticles typically show up between 400 and 450 nm (242).
4.1.2.2 Fourier transform infrared spectroscopy
Determines the functional groups that are involved in NP stabilization and reduction. This validates how phytochemicals from plant extracts, such as -OH, -COOH, and -NH₂, play a part in NP capping and surface chemistry (243).
4.1.2.3 Dynamic light scattering
Assesses the stability of colloidal particles in solution by measuring hydrodynamic size, zeta potential, and the polydispersity index (PDI) (244).
4.1.2.4 X-ray diffraction
Determines the NPs’ crystalline structure. Specific crystalline planes are represented as peaks in XRD patterns, indicating that the particles are metallic (245).
4.1.2.5 Scanning and transmission electron microscopy
Gives information on particle size distribution and morphology at the nanoscale. The particle shape (spherical, rod-like, etc.) that affects biological interactions is also revealed by TEM (246).
4.1.2.6 Zeta potential analysis
Evaluates the surface charge to determine the stability of dispersion. Zeta potentials of ±30 mV or above indicate strong anti-aggregation stability (247).
4.1.3 Drug loading strategies
From the reference to tables (2–6), plant-based metallic NPs in this review utilize:
4.1.3.1 Crude extracts
The majority of formulations comprise entire plant extracts that contain many phytochemical components, such as alkaloids, flavonoids, and tannins (from, for example, Solanum nigrum, Tephrosia tinctoria, and Eysenhardtia polystachya) (248).
4.1.3.2 Isolated compounds
Numerous studies used particular bioactive substances, including naringenin in AuNPs produced from Typha capensis or silybin from Silybum marianum (240).
4.1.4 Administration routes and formulations
In the preclinical studies reviewed, the following administration routes were commonly reported:
4.1.4.1 Oral administration
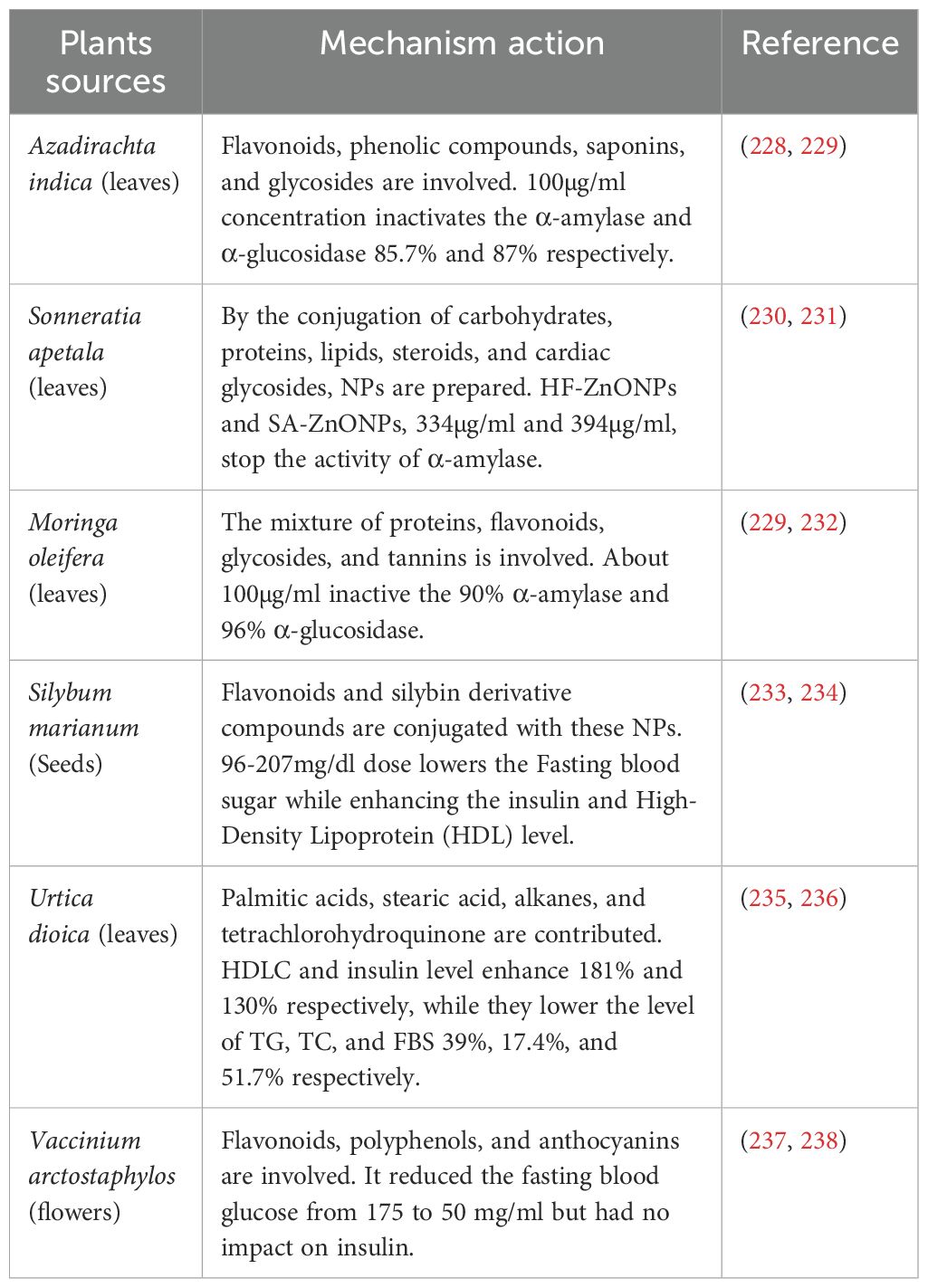
When it comes to administering plant-based metallic nanoparticles in antidiabetic models, this is the most popular method. As an example, ZnONPs produced from Musa paradisiaca were taken orally at a dose of 50 µg/kg, which markedly raised insulin levels and decreased blood glucose (Table 2). When taken orally at a dose of 10 mg/kg, NPs derived from Solanum nigrum extract showed a decrease in blood glucose and a maintenance of body weight in diabetic mice (Table 2). At 100 µg/ml oral dosages, Moringa oleifera ZnONPs demonstrated substantial inhibition of α-amylase and α-glucosidase (Table 3).
4.1.4.2 Intraperitoneal injection
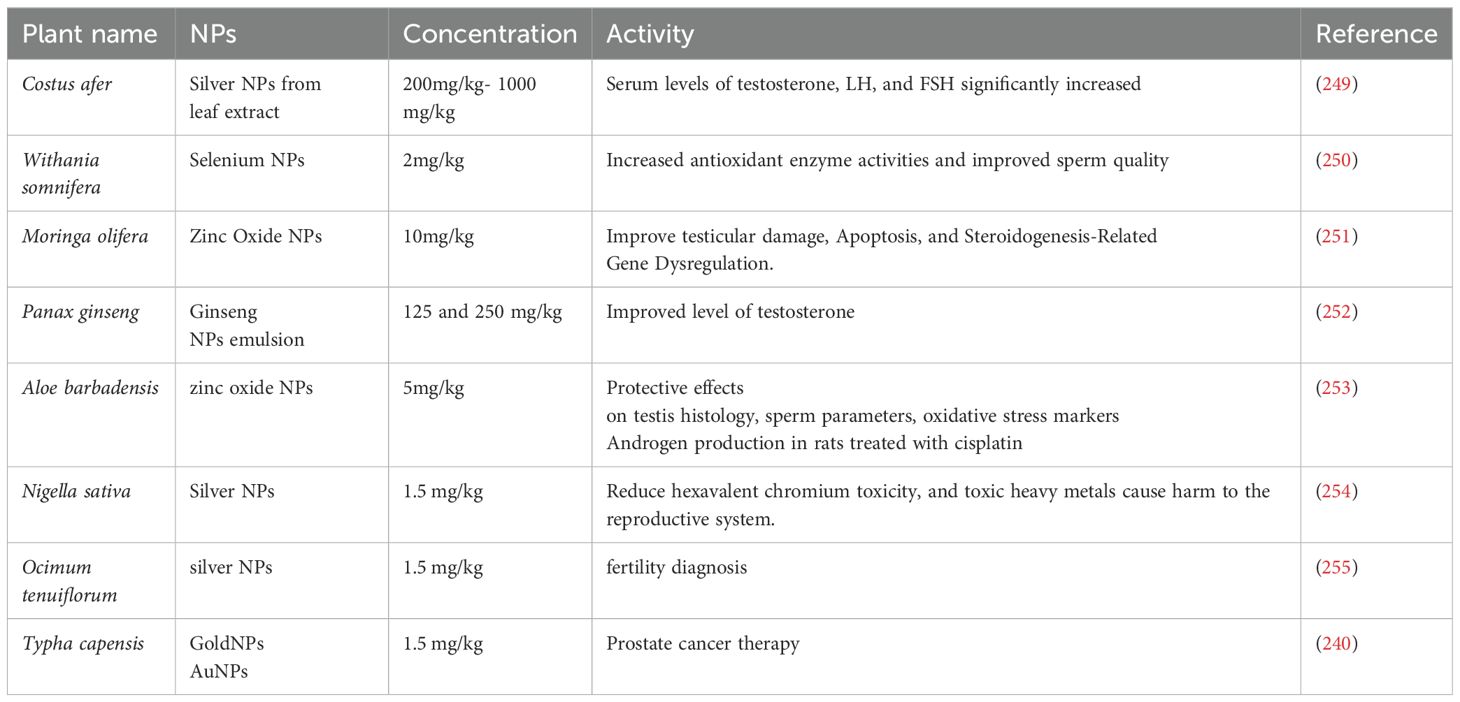
Used, especially in models of male infertility, to evaluate systemic effects on oxidative or hormonal markers. For example, when 200–1000 mg/kg of costus after AgNPs were injected intraperitoneally, the levels of serum testosterone, LH, and FSH rose (Table 4). To enhance sperm quality and antioxidant enzyme activity, diabetic mice were given injections of 2 mg/kg of Withania somnifera-derived SeNPs (Table 4).
4.1.4.3 Other routes
Nigella sativa-mediated AgNPs were orally administered in toxicity reversal studies involving hexavalent chromium exposure (Table 4).
4.1.4.4 Therapeutic advantages of nanoparticle-based delivery
In a study employing ZnONPs made from Moringa oleifera, oral treatment at 100 µg/ml inhibited α-glucosidase by 96% and α-amylase by 90%, suggesting a potent postprandial glucose-lowering action with negligible liver damage (Table 3). Intraperitoneally administered costus after-derived AgNPs at 200 mg/kg significantly decreased testicular lipid peroxidation (MDA) levels and increased endocrine restoration with less oxidative stress, restoring testosterone levels from 2.1 ng/mL in diabetic controls to 4.5 ng/mL (Table 4). Because of their superior stability and targeted administration, quercetin-loaded AuNPs demonstrated a three-fold increase in cellular absorption in β-cells and decreased cytotoxicity to liver cells at equivalent therapeutic doses when compared to free quercetin.
4.1.5 Synergistic mechanisms between plant phytochemicals and metal nanoparticles
Plant-mediated metal nanoparticles have synergistic therapeutic effects that result from the interaction of metal cores (such as Au, Ag, Zn, and Mn) with bioactive phytochemicals (like flavonoids, alkaloids, and phenolics) (208). In addition to improving the solubility and bioavailability of encapsulated plant components, metallic nanoparticles shield them against early degradation. To replicate natural pharmacokinetics, this permits sustained release. In diabetic and cancer animals, for example, AuNPs loaded with naringenin from Typha capensis demonstrated enhanced delivery. Metal nanoparticles (NPs) such as ZnO or AgNPs may reduce oxidative stress by upregulating endogenous antioxidant enzymes (e.g., SOD, CAT, GPx), while plant components frequently scavenge free radicals. By doing so, oxidative damage to cells decreases (240).
For example, in diabetic mice, SeNPs derived from Withania somnifera increased antioxidant defense and decreased ROS, improving sperm quality (250). The inhibition of important enzymes in glucose metabolism (α-amylase, α-glucosidase, and DPP-IV) by both phytochemicals and metal nanoparticles can result in additive or synergistic hypoglycemic effects. The metal ion activity and polyphenolic stabilization of Moringa oleifera-ZnONPs were responsible for the 90% and 96% inhibition of α-amylase and α-glucosidase, respectively. Furthermore, phytochemicals facilitate receptor-mediated endocytosis of NPs by acting as biocompatible capping agents. This improves insulin signaling and tissue regeneration by increasing NP uptake into target cells (such as pancreatic β-cells and Sertoli cells) (256). For instance, male rats’ levels of LH, FSH, and testosterone were raised by Costus after-derived AgNPs. This was probably because of increased bioavailability and hormonal signaling through NP absorption (257).
4.1.6 Structural analogies and comparative efficacy of plant-based actives
The structure and functional characteristics of many active chemicals originating from plants are comparable to those of commercially marketed medications. For instance, thiazolidinediones and flavonoids like quercetin and kaempferol have structural similarities and can bind to PPAR-γ to increase insulin sensitivity (258). The same mechanism that metformin targets, AMPK, is also activated by berberine, and silybin from Silybum marianum has antioxidant and insulin-sensitizing properties similar to those of hepatoprotective drugs (259). In addition to sharing structural similarities with synthetic pharmaceuticals, these substances also modulate insulin signaling, glucose metabolism enzymes (including α-glucosidase and α-amylase), and reproductive hormone pathways.
Due to the synergistic effects of several phytochemicals acting on various targets at once, crude plant extracts frequently exhibit efficacy that is about equal to or even better than isolated substances. For example, formulations using extracts of Azadirachta indica and Moringa oleifera for ZnONPs showed over 85% inhibition of important enzymes involved in the digestion of glucose, which is comparable to that of common medications such as acarbose (260) (Table 3), whereas the extract from Eysenhardtia polystachya increased the release of insulin via INS-1 pancreatic cells (Table 2). Improved bioavailability, wider therapeutic coverage, and natural excipients that promote stability and solubility are all advantages of these multi-component systems (221). However, there are drawbacks to crude extracts as well, including unpredictability, ambiguity in composition, and standardization issues. Curcumin and naringenin, on the other hand, are single-component phytochemicals that provide superior control over nanoparticle formulations, promoting regulatory compliance and reproducibility (261). In general, plant-based bioactives serve as natural substitutes for synthetic medications, and both isolated molecules and crude extracts offer special benefits that can be used strategically depending on the intended therapeutic outcome (262).
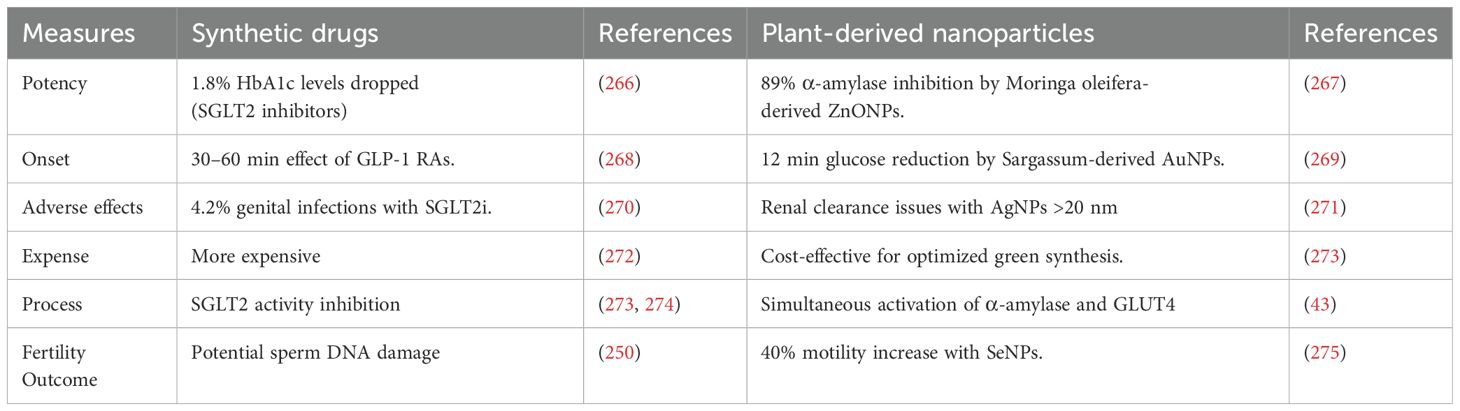
Delivering therapeutic agents to their target sites presents several challenges, including poor bioavailability, in vivo instability, low solubility, limited absorption, target-specific delivery difficulties, and suboptimal therapeutic efficacy. To address these limitations, advanced targeted drug delivery systems have been developed. Among these, nanotechnology has emerged as a promising interdisciplinary approach, offering cost-effective and innovative solutions for precise drug delivery (263). In particular, metal nanoparticles (NPs) have gained significant attention due to their wide-ranging applications in medicine, biology, and physics, making them valuable tools for enhancing the efficiency and specificity of therapeutic interventions (263, 264). The use of plant-based metallic NPs in diabetes treatment offers a promising approach for the precise delivery of therapeutic compounds (Table 2). Derived from natural sources, these NPs are biocompatible and can be engineered to encapsulate insulin, antidiabetic medications, and plant-derived bioactive compounds. This allows for sustained and targeted delivery, mimicking the body’s insulin release patterns, regulating blood glucose levels, and potentially reducing the dosage needed. Additionally, these NPs can help mitigate diabetes-related inflammation and oxidative stress, protect against complications, and enhance the bioavailability of oral medications (265). These phytogenic nanoparticles offer various advantages compared to synthetic antidiabetic drug (Table 5).
Utilizing Nigella sativa plant extract, Alkhalaf et al. (276) Assessed the green prepared Ag NPs for diabetic neuropathy. A comparison between diabetic neuropathy-induced and stable control groups revealed a significant increase in blood glucose levels, advanced glycation end products (AGEs), and aldose reductase activity, accompanied by a marked reduction in insulin levels. Additionally, inflammatory markers were significantly elevated in the diabetic neuropathy group. Notably, nitrotyrosine levels were substantially lower, suggesting a notable alteration in the oxidative state. Gene expression analysis further demonstrated a significant upregulation of nerve growth factor (NGF) and a downregulation of brain Tyrosine Kinase Receptor A (TrkA) in individuals with diabetic neuropathy compared to healthy controls. Various therapeutic interventions targeting diabetic neuropathy have shown significant improvements across all evaluated biomarkers (276).
Cheng et al. (277) conducted a study involving the manufacturing of Ramulus Mori extract loaded on polyacrylic Gold nanoparticles (AuNPs) as an antidiabetic agent, specifically gestational diabetes mellitus nursing (GDM). Microscopic examination of the livers in diabetic mother rats revealed characteristic alterations in cell layers. However, administering Au NPs at the maternal stage significantly improved. Biochemical analysis indicated that Au–PAA–NPs contributed to the amelioration of alterations in maternal serum glucose concentration. Govindan et al. (278) created eco-friendly Zn-doped Catharanthus Roseus plants, aiming to enhance anti-diabetic properties through inhibiting alpha amylase activity.
A recent study revealed that Allium cepa extract-prepared NPs effectively suppressed the activity of α-amylase and α-glucosidase enzymes. Since α-amylase plays a crucial role in carbohydrate metabolism, inhibiting its activity is considered a promising strategy to lower blood sugar levels. Additionally, these amylase inhibitors act to reduce the absorption of dietary starch in the body. Likewise, α-glucosidase is a key enzyme in carbohydrate metabolism, facilitating the breakdown of disaccharides and oligosaccharides into monosaccharides (279, 280). Furthermore, NPs derived from plants and coated with phytochemicals, and other vital bioactive compounds sourced from plant secondary metabolites may find application in treating foot or limb infections in individuals with diabetes (281, 282).
Silver NPs derived from Argyreia nervosa and Punica granatum (211, 283), and marine algae Colpomenia sinuosa (284, 285) Have been documented for their antidiabetic properties. They exhibited a dose-dependent inhibition of α-amylase and α-glucosidase activities, as compared to the crude plant extracts. Moreover, silver nanoparticles (AgNPs) derived from the stem section of Tephrosia tinctoria were found to inhibit the activities of α-amylase and α-glucosidase, while also enhancing glucose uptake in human red blood cells (286, 287).
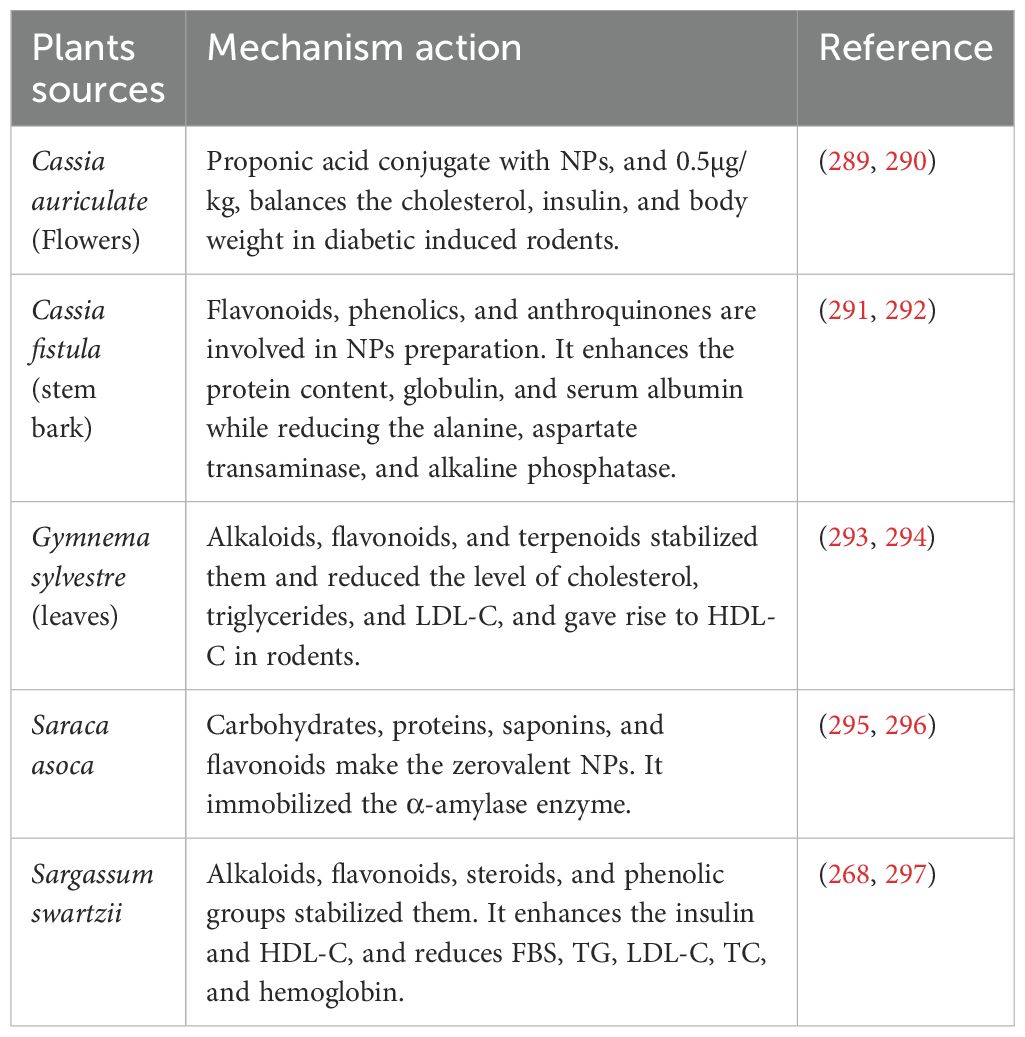
Shanker et al. (288) Reported that Zinc oxide nanoparticles (ZnONPs) derived from different plant sources such as Momordica charantia, Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, and Tamarindus indica have antidiabetic potential by significantly inhibiting the activities of α-amylase and α-glucosidase(Table 3). The anti-diabetic properties of ZnONPs prepared from the leaves of the insulin plant (Costus igneus), by diminished the activities of α-amylase and α-glucosidase, were reported by (282). Gold NPs (AuNPs) obtained from Cassia auriculata (289) And Sargassum swertzii (Dhas et al., 2016) demonstrated antihyperglycemic action in rats after induced diabetes. Gold NPs have been found the application biocompatible, nontoxic, and easily interact with a variety of biomolecules such as amino acids, proteins, enzymes, and DNA, also playing a vital role in their immobilization (Table 6).
Plant-based metallic NPs have been found to heighten antimicrobial resistance in diabetic patients even at lower concentrations, exhibiting lower toxicity to the human body (Table 7). These SNPs induce bacterial cell damage through two mechanisms. Firstly, they adhere to the bacterial cell wall, disrupting permeability and cellular respiration (298). Additionally, they cause damage by interacting with phosphorus- and sulfur-containing compounds like DNA and proteins (299). Based on the Streptomyces achromogenes bacterium, Streptozotocin (STZ) serves as a broad-spectrum antibiotic. Additionally, it functions as a pancreatic beta-cell–specific cytotoxin, making it a commonly used inducer of diabetes in rat models (280).
STZ elevates malondialdehyde (MDA) and nitric oxide (NO) levels while reducing the antioxidant capabilities of catalase (CAT), superoxide dismutase (SOD), GR, and glutathione peroxidase (GPx) in rodents. It utilizes specific receptors such as glucose transporter-2 (GLUT 2) receptors, competing with glucose molecules and leading to AKt phosphorylation, also referred to as protein kinase B. Moreover, STZ triggers apoptosis and cytotoxicity by elevating reactive oxygen species (ROS) and nitric oxide synthase production. It instigates oxidative stress, resulting in lowers testosterone levels, mitochondrial breakage, and DNA destruction through diminishing the antioxidant capacity of CAT, SOD, and other factors. Eventually resulting in the demise of cells. Through the utilization of receptor-mediated endocytosis for internalization, phyto-fabricated metallic NPs reduce the generation of ROS and nitric oxide synthase by boosting the antioxidant capacity of CAT, POD, serum testosterone, and lipid levels in STZ-treated rodents (305).
Furthermore, the synthesis of metallic NPs from plant extracts adds another dimension to the therapeutic potential of these natural remedies. NPs exhibit diverse shapes and sizes, which can influence their pharmacokinetics, biodistribution, and interactions with biological systems. For instance, NPs with smaller sizes may have enhanced cellular uptake and bioavailability compared to larger particles. Additionally, their unique shapes can affect their stability, surface area, and binding affinity to target molecules.
When metallic NPs derived from plant extracts are used as antidiabetic agents, their distinct properties contribute to their efficacy through multiple mechanisms. These mechanisms may include modulating insulin sensitivity, enhancing glucose uptake by cells, inhibiting carbohydrate-digesting enzymes, and protecting pancreatic beta cells from oxidative stress. The specific combination of phytochemicals encapsulated within NPs, along with their size and shape characteristics, determines their overall impact on different regulatory pathways involved in diabetes management (Figure 3).
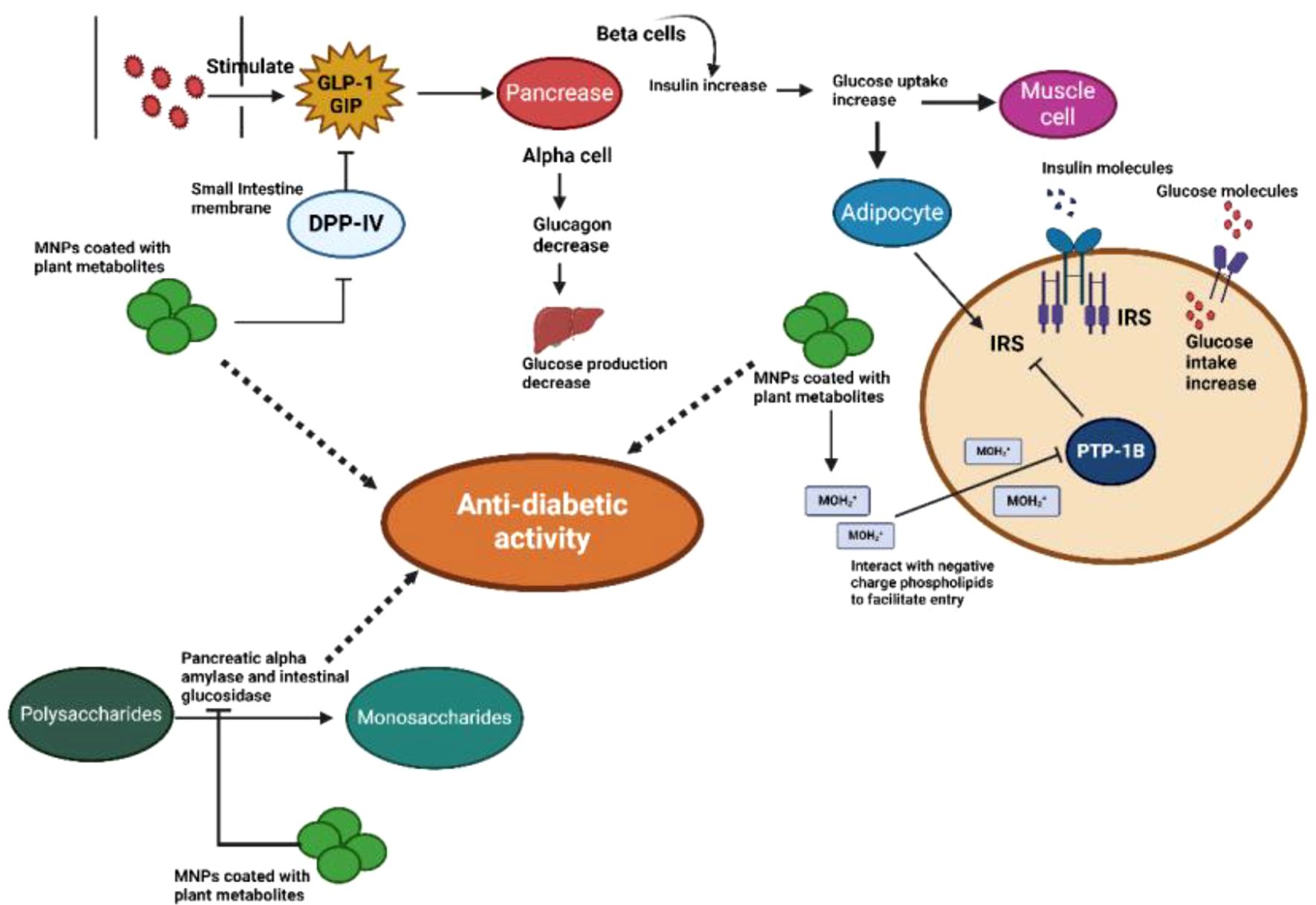
Figure 3. Anti-diabetic activity of plant-based metal NPs: The therapeutic target of monomeric plant peptidase IV (DPPIV), which is implicated in glucose regulation, is MNPs coated with plant metabolites in type 2 diabetes. When glucose or other nutrients are consumed, the intestine secretes two main incretin hormones that trigger the pancreatic β cells to secrete insulin: gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). MNPs cause the serine protease enzyme DPPIV to be inhibited, as well as GLP-1 and GIP, which in turn causes an increase in the amount of insulin secreted by pancreatic β-cells. By inhibiting the PTP-1B enzyme on the ER membrane, MNPs stimulate insulin signaling pathways, which in turn cause adipocytes and muscle cells to absorb glucose. MNPs decrease the release of glucose by inhibiting the α-amylase and α-glucosidase enzymes.
5 Plant-based metallic NPs for the treatment of male infertility
The use of plant-based metallic NPs for the treatment of diabetes-induced male infertility offers numerous potential advantages. They can be customized to deliver bioactive compounds to the male reproductive system, potentially enhancing sperm quality and motility. These NPs may also address inflammation associated with diabetes-induced male infertility. Furthermore, the encapsulation of plant-derived compounds can help regulate blood sugar levels, indirectly improving fertility and reducing side effects (306).
With a prevalence ranging from 2.5% to 12%, male infertility impacts more than 30 million men globally. It stands as the primary cause in 20%-30% of cases and contributes significantly to half of all instances of infertility (307–309). The key determinants of male fertility, including sperm count, quality, motility, and morphology, play crucial roles, and deviations in any of these factors can result in infertility (310). A substantial proportion, up to 90%, of infertile couples contend with issues related to low sperm count and/or poor sperm quality (257). Several factors, such as testicular development, reproductive system diseases, elevated scrotal temperature, immune system and endocrine disorders, as well as lifestyle choices, environmental conditions, and nutritional factors, have been identified as detrimental to sperm parameters, contributing to male infertility (251).
A significant proportion of male infertility cases remain idiopathic, highlighting the ongoing difficulty in determining definitive underlying causes. In such cases, the absence of a clearly defined etiology limits the availability of targeted pharmacological treatments. As a result, clinicians often resort to various empirical strategies aimed at stimulating spermatogenesis, which frequently produce inconsistent and non-standardized outcomes. In some countries, the off-label use of selective estrogen receptor modulators (SERMs), such as tamoxifen and clomiphene citrate, has been explored as a potential therapeutic option for managing male infertility (311). However, it is noteworthy that some drugs commonly used in this context have been linked to side effects that may impact male fertility. Within the realm of traditional medicine, plant-based preparations, including decoctions, concoctions, macerations, or infusions, are frequently employed to address a broad spectrum of ailments. Some of these botanical remedies are specifically utilized in connection with male reproductive health issues, acknowledging the global significance of these concerns as a public health and social challenge (312).
In recent decades, nanotechnology has emerged as a promising avenue for addressing infertility. NPs, characterized by their extremely small size (one billionth part of a meter - 10^-9), offer innovative solutions. While the chemical method of nanoparticle synthesis poses potential harm to human health and the environment, the biological method stands out as an eco-friendly, cost-effective, and reliable alternative. Nanostructures find diverse applications in gene delivery, tissue engineering, drug delivery, biological labeling, protein tracing, pathogen detection, cancer therapy, DNA structure analysis, and serve as contrast agents in magnetic resonance imaging (MRI) and molecular sensing. The green synthesis approach, employing plant extracts, presents notable advantages, including simplicity, cost-effectiveness, environmental friendliness, and reliability (249).
In a recent study by Egbiremhon et al. (313), the focus was on evaluating the impact of Costus afer-AgNPs extracts on male reproductive hormones in rats. The primary aim was to investigate the effects on testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH). The experimental groups, administered varying doses of 200mg, 400mg, 600mg, 800mg, and 1000mg of Costus afer-AgNPs extract per kilogram of body weight, demonstrated a significant elevation in serum levels of testosterone, LH, and FSH compared to the control group (P < 0.05). Remarkably, Costus after-AgNPs not only sustained but also exhibited the potential to augment the concentrations of these crucial reproductive hormones.
In their study, Ali et al. (250) Utilized a secure and non-hazardous approach to synthesize selenium nanoparticles (Se NPs) with the aqueous extract of Withania somnifera roots. The investigation focused on evaluating Se NPs’ potential to enhance antioxidant enzyme function and mitigate DNA damage in sperm, specifically in STZ-induced diabetic mice. The results demonstrated that Se NPs treatment increased antioxidant enzyme activities, improved sperm quality, and stabilized ROS levels in diabetic mice. The green synthesis method using plant extracts emerged as a secure means of producing Se NPs, with Se NPs exhibiting greater benefits compared to both inorganic and organic selenium counterparts.
Mostafa-Hedeab et al. (251) Conducted an assessment of the potential protective role of zinc oxide NPs synthesized via a green method using Moringa oleifera leaf extract (MO-ZNPs) against acrylamide (ACR)-induced reproductive dysfunctions in male rats. The results conclusively demonstrated the protective impact of MO-ZNPs, shielding male rats from ACR-induced reproductive toxicity. This effect was attributed to the suppression of oxidative injury and apoptosis, along with an augmentation in steroidogenesis and sex hormones. In summary, MO-ZNPs emerged as a valuable intervention to mitigate the adverse reproductive effects induced by ACR in male rats.
In their 2019 study, Kamel et al. (252) Addressed the clinically significant testicular toxicity associated with methotrexate (MTX). While previous research indicated ginseng’s potential to stimulate spermatogenesis and prevent chemotherapy-induced testicular injury, the study focused on formulating ginseng into NPs due to its low bioavailability. With limited available data on the protective effects of ginseng or its NPs against MTX-induced testicular toxicity, the findings of this study suggest that both ginseng and ginseng NPs protect against MTX-induced testicular toxicity in rats. This protective effect is attributed to the inhibition of MTX-induced testicular apoptosis, with ginseng NPs exhibiting a superior protective effect compared to ginseng at the given doses.
In their 2023 study, Nauroze et al. (254) aimed to investigate the adverse effects on the reproductive system induced by hexavalent chromium (Cr (VI)) and explore potential ameliorative effects using Nigella sativa and Nigella sativa-mediated silver NPs (AgNP) in male mice (Mus musculus). Clomiphene citrate, a known infertility medication, served as a positive control. The primary objective was to assess the ameliorative potential of orally administered substances, including 50 mg/kg body weight clomiphene citrate (control), chemically synthesized AgNP, Nigella sativa seed extract, and Nigella sativa-mediated AgNP, against the toxic effects of Cr (VI) induced by oral administration of K2Cr2O7 at a dose of 1.5 mg/kg body weight over eight weeks. The administration of Nigella sativa and Nigella sativa-mediated AgNPs demonstrated a reduction in toxicity.
The study by Jha et al. (255) Delves into nanocarrier-mediated targeted delivery and biosensing in reproductive health care. It specifically highlights the encapsulation of silver NPs (AgNPs) from Ocimum tenuiflorum within multiwalled carbon nanotubes (MWCNTs). This approach demonstrates the composite’s efficacy in targeting the intracellular region of sperm cells, suggesting its potential applications in biosensing-based infertility diagnosis. The investigation also confirms the binding and targetability of AgNP to the sperm nucleus, supported by assessments of DNA fragmentation and morphological examinations. The enhanced targeting efficiency and biosensing capabilities position the AgNP-MWCNT composite as a promising candidate for fertility diagnosis applications.
In their 2023 study, Pearce et al. (240) Explore the biomedical applications of green nanotechnology in addressing the challenges associated with the clinical use of naringenin, a flavone recognized for its emerging anti-cancer properties. Naringenin is naturally found in Typha capensis, a South African plant used in traditional medicine. Despite promising in vitro results, the study addresses limitations such as poor oral bioavailability and rapid metabolism. The research focuses on a novel drug delivery approach, reporting the successful synthesis of self-stabilized gold NPs (AuNPs) derived from naringenin. This innovation introduces an effective drug delivery tool with anticipated applications in prostate cancer treatment, aiming to enhance the delivery of anticancer therapeutics, particularly naringenin.
In a study, Majd et al. (253) Address the adverse effects of cisplatin (CP) on male reproductive tissues during cancer treatment. While the potential benefits of zinc oxide NPs (ZnO NPs) in cancer therapy have been extensively explored, limited data exists on the protective effects of green ZnO NPs against CP-induced male reproductive dysfunctions. The research involved comprehensive analyses, including testis histology, sperm parameters, oxidative stress markers, testosterone concentration, and the expression of genes related to steroidogenesis in different experimental groups. The findings indicate that green ZnO NPs exhibit notable protective effects, mitigating testis tissue damage and epididymal sperm disorders induced by CP. Across various factors, green ZnO NPs demonstrated a more potent protective effect compared to other forms of ZnO, suggesting their potential in attenuating CP-induced male reproductive dysfunctions (Table 4).
6 Challenges and considerations
Use of phytogenic metal NPs for treating diabetes and male infertility presents several challenges and considerations, particularly regarding safety and toxicity. Plant-based metal NPs must demonstrate biocompatibility to prevent eliciting immune responses or adverse reactions within the body, which could potentially lead to inflammation or tissue damage.
6.1 Unresolved toxicity profiles
While green-synthesized nanoparticles have demonstrated significant therapeutic potential in laboratory-based research, their unverified toxicological patterns remain a significant risk. Previous studies suggested that such nanoparticles can be involved in cytotoxicity, oxidative stress, and genotoxic alterations within biological systems (314). These nano-formulations have been associated with disruption of cellular architecture, metabolic imbalances, and induction of necrosis (315). Prolonged exposure and systemic accumulation can lead to chronic toxicity, necessitating comprehensive evaluations through longitudinal studies (314). As indicated by research findings that approximately 60% of the administered (AgNPs) accumulated in the liver and spleen of rodents within 28 days (316). Likewise, zinc oxide nanoparticles (ZnONPs) at doses higher than 50 mg/kg were found to induce oxidative stress in the testes of diabetic rats (251).
Assessing the long-term safety of phytogenic NPs is essential, particularly in chronic conditions such as diabetes and male infertility, where prolonged treatment may be necessary. Conducting longitudinal studies becomes essential to thoroughly evaluate any potential cumulative effects and chronic toxicity that may arise from prolonged exposure to these NPs. Understanding the interaction of phytogenic NPs with biological systems is essential for predicting their effects and potential toxicity. This includes studying their pharmacokinetics, biodistribution, metabolism, and excretion pathways. Phytogenic NPs are typically derived from plant-based compounds, which may have their safety profiles and toxicity concerns. Some phytochemicals may exhibit dose-dependent toxicity or interactions with medications. Assessing their potential to induce cytotoxicity, genotoxicity, or immunotoxicity is crucial. While phytogenic NPs hold promises for treating diabetes and male infertility, addressing safety and toxicity considerations is paramount for their successful clinical translation.
6.2 Lack of standardized synthesis protocols
Green synthesized formulation of nanoparticles utilizes diverse techniques notably eco-friendly synthesis routes involving botanical extracts. Developing standardized, reproducible, and scalable synthesis protocols is essential to achieve uniformity and reliability in therapeutic applications (317).
However, the field at the current stage lacks standardized production (317, 318). Factors such as plant species, extraction methods, climate, and reaction conditions can significantly affect the properties and efficacy of the nanoparticles. Aspects like plant taxonomy, extraction techniques, climate, and reaction parameters highly influence the properties and effectiveness of the resulting nanoparticles (318). Natural inconsistencies in raw materials further obstructed the standardization of plant-derived nanoparticles. For instance, shifts in climatic patterns alter the phenolic concentrations in Argyreia nervosa leaf extracts, thereby affecting the efficiency of AgNP biosynthesis (212). To validate reproducibility, protocols must include characterization via dynamic light scattering (DLS) and Fourier-transform infrared spectroscopy (FTIR) to confirm stability and surface functionalization of nanoparticles.
6.3 Insufficient human trial data
Although experimental research shows the potential of plant-derived nanoparticles in addressing diabetes and male infertility, verification from human trials remains limited (319). Most experimental work has relied on in vitro or animal models, restricting it to directly applying these results to human treatment without strong clinical validation (19). Comprehensive human studies are important for exploring how these nanoparticles behave in the body, including their distribution, absorption, metabolism, and possible long-term effects (319).
Robust preclinical and clinical studies are needed to evaluate their efficacy, safety, and long-term effects in patients. Furthermore, the personalized design of plant-based metallic NPs has the potential to significantly advance precision medicine techniques for the treatment of male infertility and diabetes. Utilizing the special advantages of plant-based substances and customizing nanoparticle compositions to match the specific needs of each patient, personalized NPs present a viable way to enhance therapeutic results while lowering risks and enhancing patient safety and satisfaction.
7 Conclusion
In conclusion, diabetes mellitus poses a substantial threat to male reproductive health, affecting critical functions such as ejaculation, erectile performance, reproductive organ integrity, and overall semen quality. While conventional synthetic anti-diabetic medications are widely used, their associated side effects and limitations underscore the urgent need for safer and more effective alternative therapies. Among emerging solutions, plant-derived metallic nanoparticles (NPs) have shown promising antidiabetic potential. These NPs have demonstrated the ability to modulate key regulatory pathways, including the inhibition of pancreatic α-amylase and intestinal α-glucosidase, enhancement of insulin activity, and improved glucose uptake. Such advancements offer considerable promise in addressing the complex interplay between diabetes and male infertility. Although preclinical studies have yielded encouraging results, clinical validation through Phase I/II trials is essential to confirm their safety and efficacy in humans. One promising strategy involves the repurposing of FDA-approved phytochemicals such as curcumin formulated into gold nanoparticles (AuNPs), which may streamline the regulatory approval process. To successfully transition these therapies from the laboratory to widespread clinical use, strong collaboration between nanotechnologists and healthcare professionals is crucial. Furthermore, the development of personalized, plant-based nanoparticle formulations represents a forward-thinking approach in precision medicine, offering tailored treatments for individuals affected by diabetes and infertility. Ultimately, these innovations hold the potential to significantly enhance human health and quality of life. While this review focuses on male infertility associated with diabetes mellitus due to its high prevalence and the abundance of relevant studies, it is important to note that diabetes also significantly affects female reproductive health, including menstrual irregularities, polycystic ovary syndrome (PCOS), and reduced fertility. Future research should explore the potential of plant-based metallic nanoparticles in addressing diabetes-related female reproductive disorders.
Author contributions
AS: Writing – original draft. RQ: Conceptualization, Writing – review & editing. GY: Conceptualization, Writing – review & editing. SR: Writing – review & editing. N-U-AZ: Writing – review & editing. CH: Writing – review & editing. SuR: Writing – review & editing. NN: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Low CY, Gan WL, Lai SJ, Tam RS-M, Tan JF, Dietl S, et al. Critical updates on oral insulin drug delivery systems for type 2 diabetes mellitus. J Nanobiotechnology. (2025) 23:16. doi: 10.1186/s12951-024-03062-7
2. Dey P, Saha MR, Chowdhuri SR, Sen A, Sarkar MP, Haldar B, et al. Assessment of anti-diabetic activity of an ethnopharmacological plant, Nerium oleander, through alloxan-induced diabetes in mice. J ethnopharmacology. (2015) 161:128–37. doi: 10.1016/j.jep.2014.12.012
3. Ali M, Alam MM, Rifat MA, Simi SM, Sarwar S, Amin MR, et al. Prevalence of diabetes and prediabetes in South Asian countries: a systematic review and meta-analysis. Discover Public Health. (2025) 22:39. doi: 10.1186/s12982-025-00426-8
4. Whiting DR, Guariguata L, Weil C, and Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin practice. (2011) 94:311–21. doi: 10.1016/j.diabres.2011.10.029
5. Sooy M, Pyle L, Alonso GT, Broncucia HC, Rewers A, Gottlieb PA, et al. Lower Prevalence of Diabetic Ketoacidosis at Diagnosis in Research Participants Monitored for Hyperglycemia. J Clin Endocrinol Metab. (2024) 110:e80–e6. doi: 10.1210/clinem/dgae158
6. Meo SA, Zia I, Bukhari IA, and Arain SA. Type 2 diabetes mellitus in Pakistan: Current prevalence and future forecast. JPMA J Pakistan Med Assoc. (2016) 66:1637–42.
7. Aamir AH, Ul-Haq Z, Mahar SA, Qureshi FM, Ahmad I, Jawa A, et al. Diabetes Prevalence Survey of Pakistan (DPS-PAK): prevalence of type 2 diabetes mellitus and prediabetes using HbA1c: a population-based survey from Pakistan. BMJ Open. (2019) 9:e025300. doi: 10.1136/bmjopen-2018-025300
8. Sinha R, Angelin P, Anuvi S, and Hifz Ur Rahman M. Prevalence of diabetes distress among type 2 diabetes mellitus patients in India: a systematic review and meta-analysis. Health Psychol Behav Med. (2024) 12:2324091. doi: 10.1080/21642850.2024.2324091
9. Azeem S, Khan U, and Liaquat A. The increasing rate of diabetes in Pakistan: A silent killer. Ann Med Surg. (2022) 79. doi: 10.1016/j.amsu.2022.103901
10. Murtaza G, Riaz S, Zafar M, Ahsan Raza M, Kaleem I, Imran H, et al. Examining the growing challenge: Prevalence of diabetes in young adults (Review). Med Int. (2025) 5:2. doi: 10.3892/mi.2024.201
11. Liang J, He Y, Huang C, Ji F, Zhou X, and Yin Y. The regulation of selenoproteins in diabetes: A new way to treat diabetes. Curr Pharm Design. (2024) 30:1541–7. doi: 10.2174/0113816128302667240422110226
12. Liu Z, Sang X, Liu Y, Yu C, and Wan H. Effect of psychological intervention on glycemic control in middle-aged and elderly patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Primary Care Diabetes. (2024). 18:574–81 doi: 10.1016/j.pcd.2024.09.006
13. Chen Y, Jiang Q, Xing X, Yuan T, and Li P. Clinical research progress on β-cell dysfunction in T2DM development in the Chinese population. Rev Endocrine Metab Disord. (2025) 26:31–53. doi: 10.1007/s11154-024-09914-9
14. Baynes HW. Classification, pathophysiology, diagnosis, and management of diabetes mellitus. J Diabetes Metab. (2015) 6:1–9. doi: 10.2147/DDDT.S316185
15. Wahyuni ES and Setyarini A. Factors Causing Primary Infertility In Couples Of Reproductive Age: Literature Review. Health Access J. (2024) 2):39–48%V 1. doi: 10.31290/haj.v1i2.4520
16. Starc A, Trampuš M, Pavan Jukić D, Grgas-Bile C, Jukić T, and Polona Mivšek A. Infertility and sexual dysfunctions: a systematic literature review. Acta Clinica Croatica. (2019) 58:508–15. doi: 10.20471/acc.2019.58.03.15
17. Agarwal A, Baskaran S, Parekh N, Cho C-L, Henkel R, Vij S, et al. Male infertility. Lancet. (2021) 397:319–33. doi: 10.1016/S0140-6736(20)32667-2
18. Gao Y, Wang C, Wang K, He C, Hu K, and Liang M. The effects and molecular mechanism of heat stress on spermatogenesis and the mitigation measures. Syst Biol Reprod Med. (2022) 68:331–47. doi: 10.1080/19396368.2022.2074325
19. Huang R, Chen J, Guo B, Jiang C, and Sun W. Diabetes-induced male infertility: potential mechanisms and treatment options. Mol Med. (2024) 30:11. doi: 10.1186/s10020-023-00771-x
20. Venditti M, Romano MZ, Boccella S, Haddadi A, Biasi A, Maione S, et al. Type 1 diabetes impairs the activity of rat testicular somatic and germ cells through NRF2/NLRP3 pathway-mediated oxidative stress. Front Endocrinol. (2024) 15:2024. doi: 10.3389/fendo.2024.1399256
21. Facondo P, Di Lodovico E, Delbarba A, Anelli V, Pezzaioli LC, Filippini E, et al. The impact of diabetes mellitus type 1 on male fertility: Systematic review and meta-analysis. Andrology. (2022) 10:426–40. doi: 10.1111/andr.13140
22. Zhu X-B, Niu Z-H, Fan W-M, Sheng C-S, and Chen Q. Type 2 diabetes mellitus and the risk of male infertility: a Mendelian randomization study. Front Endocrinol. (2023) 14:2023. doi: 10.3389/fendo.2023.1279058
23. Shamsi M, Venkatesh S, Kumar R, Gupta N, Malhotra N, Singh N, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J. Biochem. Biophys. (2010) 47:38–43.
24. Anuar NS, Shafie SA, Maznan MAF, Zin NSNM, Azmi NAS, Raoof RA, et al. Lauric acid improves hormonal profiles, antioxidant properties, sperm quality and histomorphometric changes in testis and epididymis of streptozotocin-induced diabetic infertility rats. Toxicol Appl Pharmacol. (2023) 470:116558. doi: 10.1016/j.taap.2023.116558
25. Bisht S and Dada R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front Bioscience-Scholar. (2017) 9:420–47.
26. Dias TIRA. Evaluation of cell death markers and reproductive parameters in models of diabetes mellitus. Portugal: Universidade da Beira Interior (2012).
27. Parmar RS, Verma S, Neelkamal, Pathak VK, and Bhadoria AS. Prevalence of erectile dysfunction in Type 2 diabetes mellitus (T2DM) and its predictors among diabetic men. J Family Med Primary Care. (2022) 11:3875–9. doi: 10.4103/jfmpc.jfmpc_1130_21
28. Bajaj HS, Gerstein HC, Rao-Melacini P, Basile J, Colhoun H, Conget I, et al. Erectile function in men with type 2 diabetes treated with dulaglutide: an exploratory analysis of the REWIND placebo-controlled randomised trial. Lancet Diabetes Endocrinology. (2021) 9:484–90. doi: 10.1016/S2213-8587(21)00115-7
29. Nguyen Ngoc Dang H, Viet Luong T, Kiem Pham A, Trung Le T, Duc Le N, Minh Nguyen H, et al. Exploring the bidirectional link between erectile dysfunction and 10-year cardiovascular risk in men with diabetes and hypertension. Sci Rep. (2024) 14:28816. doi: 10.1038/s41598-024-78182-z
30. Kamdar V and Shah JH. Sexual dysfunction in diabetes. Improving Diabetes Care Clinic. (2014) 312:131–4.
31. Hatzimouratidis K and Hatzichristou D. Erectile dysfunction and diabetes mellitus. Insulin. (2009) 4:114–22. doi: 10.1016/S1557-0843(09)80020-1
32. Kizilay F, Gali HE, and Serefoglu EC. Diabetes and sexuality. Sexual Med Rev. (2017) 5:45–51. doi: 10.1016/j.sxmr.2016.07.002
33. Barkabi-Zanjani S, Ghorbanzadeh V, Aslani M, Ghalibafsabbaghi A, and Chodari L. Diabetes mellitus and the impairment of male reproductive function: Possible signaling pathways. Diabetes Metab Syndrome: Clin Res Rev. (2020) 14:1307–14. doi: 10.1016/j.dsx.2020.07.031
34. Temidayo SO and Du Plessis SS. Diabetes mellitus and male infertility. Asian Pacific J reproduction. (2018) 7:6–14. doi: 10.4103/2305-0500.220978
35. Badejogbin OC, Chijioke-Agu OE, Olubiyi MV, and Agunloye MO. Pathogenesis of testicular dysfunction in diabetes: exploring the mechanism and therapeutic interventions. J Assisted Reprod Genet. (2025) 42:367–79. doi: 10.1007/s10815-024-03314-3
36. Andlib N, Sajad M, and Thakur SC. Association of diabetes mellitus with risk of reproductive impairment in females: A comprehensive review. Acta Histochemica. (2024) 126:152173. doi: 10.1016/j.acthis.2024.152173
37. Long L, Qiu H, Cai B, Chen N, Lu X, Zheng S, et al. Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget. (2018) 9:5321. doi: 10.18632/oncotarget.23915
38. Andlib N, Sajad M, Kumar R, and Thakur SC. Abnormalities in sex hormones and sexual dysfunction in males with diabetes mellitus: A mechanistic insight. Acta Histochemica. (2023) 125:151974. doi: 10.1016/j.acthis.2022.151974
39. He Z, Yin G, Li QQ, Zeng Q, and Duan J. Diabetes mellitus causes male reproductive dysfunction: a review of the evidence and mechanisms. In vivo. (2021) 35:2503–11. doi: 10.21873/invivo.12531
40. Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, and Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Hum Reprod Update. (2018) 24:86–105. doi: 10.1093/humupd/dmx033
41. El Helew EA SHW, M. MA, and Sakkara ZA. Structural changes in testes of Streptozotocin induced diabetic rats and possible protective effect of royal jelly: light and electron microscopic study. Ultrastructural Pathology. (2024) 48:1–15. doi: 10.1080/01913123.2023.2277170
42. Kolahian S, Sadri H, Larijani A, Hamidian G, and Davasaz A. Supplementation of diabetic rats with leucine, zinc, and chromium: effects on function and histological structure of testes. Int J Vitam Nutr Res. (2015) 85:311–21. doi: 10.1024/0300-9831/a000244
43. Lotti F and Maggi M. Effects of diabetes mellitus on sperm quality and fertility outcomes: clinical evidence. Andrology. (2023) 11:399–416. doi: 10.1111/andr.13342
44. Zhou Y, Fu X-M, He D-L, Zou X-M, Wu C-Q, Guo W-Z, et al. Evaluation of urinary metal concentrations and sperm DNA damage in infertile men from an infertility clinic. Environ Toxicol Pharmacol. (2016) 45:68–73. doi: 10.1016/j.etap.2016.05.020
45. Minas A, Camargo M, Alves MG, and Bertolla RP. Effects of diabetes-induced hyperglycemia on epigenetic modifications and DNA packaging and methylation during spermatogenesis; A narrative review. Iranian J Basic Med Sci. (2024) 27:3. doi: 10.22038/IJBMS.2023.69604.15173
46. Lakshmi G and Mahadevan S. Diabetes and Hypogonadism in Males. Apollo Med. (2025) 0:09760016251332088. doi: 10.1177/09760016251332088
47. Kumar P, Kumar N, Thakur DS, and Patidar A. Male hypogonadism: Symptoms and treatment. J advanced Pharm Technol Res. (2010) 1:297–301. doi: 10.4103/0110-5558.72420
48. Xiang Y-X, Xu Z, Xiao R, Yao Y-L, Tang X-J, Fu L-J, et al. Interacting and joint effects of assisted reproductive technology and gestational diabetes mellitus on preterm birth and the mediating role of gestational diabetes mellitus: a cohort study using a propensity score. J Assisted Reprod Genet. (2025) 42:1–10. doi: 10.1007/s10815-024-03342-z
49. Bhasin S, Lincoff AM, Nissen SE, Wannemuehler K, McDonnell ME, Peters AL, et al. Effect of Testosterone on Progression From Prediabetes to Diabetes in Men With Hypogonadism: A Substudy of the TRAVERSE Randomized Clinical Trial. JAMA Internal Med. (2024) 184:353–62. doi: 10.1001/jamainternmed.2023.7862
50. Niwas Jangir R and Chand Jain G. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev. (2014) 10:147–57. doi: 10.2174/1573399810666140606111745
51. Date K. Regulatory functions of α-amylase in the small intestine other than starch digestion: α-glucosidase activity, glucose absorption, cell proliferation, and differentiation. New Insights Into Metab Syndrome: IntechOpen. (2020). doi: 10.5772/intechopen.92660
52. Cannarella R, Condorelli RA, La Vignera S, and Calogero AE. Effects of the insulin-like growth factor system on testicular differentiation and function: A review of the literature. Andrology. (2018) 6:3–9. doi: 10.1111/andr.2018.6.issue-1
53. Mondal S and Bandyopadhyay A. Glucose transporters (GLUTs): underreported yet crucial molecules in unraveling testicular toxicity. Biochimie. (2024) 219:55–62. doi: 10.1016/j.biochi.2023.11.004
54. Dutta S, Sengupta P, Slama P, and Roychoudhury S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int J Mol Sci. (2021) 22:10043. doi: 10.3390/ijms221810043
55. Ni F-D, Hao S-L, and Yang W-X. Molecular insights into hormone regulation via signaling pathways in Sertoli cells: With discussion on infertility and testicular tumor. Gene. (2020) 753:144812. doi: 10.1016/j.gene.2020.144812
56. Lorber DL, ElSayed NA, Bannuru RR, Shah V, Puisis M, Crandall J, et al. Diabetes Management in Detention Facilities: A Statement of the American Diabetes Association. Diabetes Care. (2024) 47:544–55. doi: 10.2337/dci24-0015
57. Cheng AY and Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Cmaj. (2005) 172:213–26. doi: 10.1503/cmaj.1031414
58. Yadav K. Nanotechnology in diabetes Management: Revolutionizing treatment and diagnostics. J Mol Liquids. (2024) 414:126117. doi: 10.1016/j.molliq.2024.126117
59. Prabhakar P, Kumar A, and Doble M. Combination therapy: a new strategy to manage diabetes and its complications. Phytomedicine. (2014) 21:123–30. doi: 10.1016/j.phymed.2013.08.020
60. Dhieb D, Mustafa D, Hassiba M, Alasmar M, Elsayed MH, Musa A, et al. Harnessing Pharmacomultiomics for Precision Medicine in Diabetes: A Comprehensive Review. Biomedicines. (2025) 13:447. doi: 10.3390/biomedicines13020447
61. Xiao J, Li M, Cai R, Huang H, Yu H, Huang L, et al. Smart Pharmaceutical Monitoring System With Personalized Medication Schedules and Self-Management Programs for Patients With Diabetes: Development and Evaluation Study. J Med Internet Res. (2025) 27:e56737. doi: 10.2196/56737
62. Bailey C. The current drug treatment landscape for diabetes and perspectives for the future. Clin Pharmacol Ther. (2015) 98:170–84. doi: 10.1002/cpt.v98.2
63. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. (2015) 38:140–9. doi: 10.2337/dc14-2441
64. Cando LFT, Quebral EPB, Ong EP, Catral CDM, Relador RJL, Velasco AJD, et al. Current status of diabetes mellitus care and management in the Philippines. Diabetes Metab Syndrome: Clin Res Rev. (2024) 18:102951. doi: 10.1016/j.dsx.2024.102951
65. Gardner DSL, Saboo B, Kesavadev J, Mustafa N, Villa M, Mahoney E, et al. Digital Health Technology in Diabetes Management in the Asia–Pacific Region: A Narrative Review of the Current Scenario and Future Outlook. Diabetes Ther. (2025) 16:349–69. doi: 10.1007/s13300-025-01692-0
66. Duckworth WC. Hyperglycemia and cardiovascular disease. Curr Atheroscl Rep. (2001) 3:383–91. doi: 10.1007/s11883-001-0076-x
67. Jain S and Saraf S. Type 2 diabetes mellitus—Its global prevalence and therapeutic strategies. Diabetes Metab Syndrome: Clin Res Rev. (2010) 4:48–56. doi: 10.1016/j.dsx.2008.04.011
68. Kalsi A, Singh S, Taneja N, Kukal S, and Mani S. Current treatments for Type 2 diabetes, their side effects and possible complementary treatments. Int J. (2017) 10:13–18.
69. Wang W, Puri G, Sly B, Samadbeik M, Ng S, Newton J, et al. Optimizing inpatient diabetes management with the diabetes dashboard. JBI Evidence Implementation. (2025) 9900. doi: 10.1097/XEB.0000000000000489
70. Hauner H. The mode of action of thiazolidinediones. Diabetes/metabolism Res Rev. (2002) 18:S10–S5. doi: 10.1002/(ISSN)1520-7560
71. Chiu C-D, Chiu Y-P, Yip H-T, Ji H-R, Cho D-Y, Cheng IH-J, et al. Thiazolidinediones Decrease the Recurrence of Intracerebral Hemorrhage in Type 2 Diabetes Mellitus Patients: A Nested Case-Control Study. Neuroepidemiology. (2024) 59:43–56. doi: 10.1159/000539001
72. Basak S, Murmu A, Matore BW, Roy PP, and Singh J. Thiazolidinedione an auspicious scaffold as PPAR-γ agonist: its possible mechanism to Manoeuvre against insulin resistant diabetes mellitus. Eur J Medicinal Chem Rep. (2024) 11:100160. doi: 10.1016/j.ejmcr.2024.100160
73. Greenfield JR and Chisholm DJ. Thiazolidinediones-mechanisms of action. Aust Prescriber. (2004) 27:67–70.
74. Devajit M and Haradhan Kumar M. Oral Hypoglycaemic Agents: Non-Insulin Medications for Type 2 Diabetes Patients. Innovation Sci Technology. (2024) 3:23–31. doi: 10.56397/IST.2024.01.04
75. Singh S and Loke YK. Thiazolidinediones and cardiovascular disease: balancing benefit and harm. Geriatr Aging. (2008) 11:179–83.
76. Endo Y, Suzuki M, Yamada H, Horita S, Kunimi M, Yamazaki O, et al. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARγ-dependent nongenomic signaling. Cell Metab. (2011) 13:550–61. doi: 10.1016/j.cmet.2011.02.015
77. Naziyah N, Saifulaman M, Faridah F, and Marpaung E. Effectiveness of polyhexamethylene biguanide (PHMB) against odor in diabetic foot ulcer. Malahayati Int J Nurs Health Science. (2024) 7:598–604. doi: 10.33024/minh.v7i5.314
78. Wang GS and Hoyte C. Review of biguanide (metformin) toxicity. J Intensive Care Med. (2019) 34:863–76. doi: 10.1177/0885066618793385
79. Kumar A, Mazumder R, Rani A, Pandey P, and Khurana N. Novel Approaches for the Management of Type 2 Diabetes Mellitus: An Update. Curr Diabetes Rev. (2024) 20:45–61. doi: 10.2174/0115733998261903230921102620
80. Bailey CJ and Turner RC. Metformin. New Engl J Med. (1996) 334:574–9. doi: 10.1056/NEJM199602293340906
81. Bhilare NV, Shedge R, Tambe PM, and More A. Unveiling the potential of prodrug and drug-conjugate strategies in treatment of diabetes mellitus and its complications. Medicinal Chem Res. (2024) 33:337–53. doi: 10.1007/s00044-024-03187-2
82. Bouchi R, Kondo T, Ohta Y, Goto A, Tanaka D, Satoh H, et al. A consensus statement from the Japan Diabetes Society (JDS): a proposed algorithm for pharmacotherapy in people with type 2 diabetes—2nd Edition (English version). Diabetol Int. (2024) 15:327–45. doi: 10.1007/s13340-024-00723-8
83. Vigneri R and Goldfine ID. Role of metformin in treatment of diabetes mellitus. Diabetes Care. (1987) 10:118–22. doi: 10.2337/diacare.10.1.118
84. Di Magno L, Di Pastena F, Bordone R, Coni S, and Canettieri G. The Mechanism of Action of Biguanides: New Answers to a Complex Question. Cancers. (2022) 14:3220. doi: 10.3390/cancers14133220
85. Mehrpour O, Saeedi F, Hoyte C, Hadianfar A, Nakhaee S, and Brent J. Distinguishing characteristics of exposure to biguanide and sulfonylurea anti-diabetic medications in the United States. Am J Emergency Med. (2022) 56:171–7. doi: 10.1016/j.ajem.2022.03.023
86. Jones M, Ionescu CM, Walker D, Wagle SR, Kovacevic B, Chester J, et al. Biguanide Pharmaceutical Formulations and the Applications of Bile Acid-Based Nano Delivery in Chronic Medical Conditions. Int J Mol Sci. (2022) 23:836. doi: 10.3390/ijms23020836
87. Hermann L. Metformin: a review of its pharmacological properties and therapeutic use. Diabete metabolisme. (1979) 5:233–45.
88. Sterne J and Junien J. Metformin: pharmacological mechanisms of the antidiabetic and antilipidic effects and clinical consequences. R Soc Med Int Congr Symp Ser;. (1981). 48:3–16.
89. Karam JH, Matin S, and Forsham PH. Antidiabetic drugs after the university group diabetes program (UGDP). Annu Rev Pharmacol. (1975) 15:351–66. doi: 10.1146/annurev.pa.15.040175.002031
90. Li Y, Zheng H, Liang Y, Xuan M, Liu G, and Xie H. Hyaluronic acid-methacrylic anhydride/polyhexamethylene biguanide hybrid hydrogel with antibacterial and proangiogenic functions for diabetic wound repair. Chin Chem Letters. (2022) 33:5030–4. doi: 10.1016/j.cclet.2022.03.116
91. Zaman MSZ, Hassan PH, Islam SMSI, Ullah MAU, and Rabbani MGR. Comparative Efficacy of Insulin, Biguanides and Sulfonylureas In The Glycemic Control of Newly Diagnosed Diabetes Mellitus Patients in Rajshahi, Bangladesh. J Bio-Science. (2023) 31:39–49. doi: 10.3329/jbs.v31i1.69533
92. Jamiu FFM, Gostevcic N, Hammad YM, and Ahmed SMG. Preoperative management of diabetes mellitus: A comparative narrative review of the recommendations of three professional organizations with Hamad Medical Corporation guidelines. Qatar Med J. (2025) 2025. doi: 10.5339/qmj.2025.23
93. Shareef Z, Murtaza A, Fatima G, Aqib AI, Manzoor Z, Malik MSU, et al. Pharmacological and herbal approach to diabetes mellitus type 2 management: A comparative analysis of conventional therapy and alternative remedy. Annales Pharm Françaises. (2025). doi: 10.1016/j.pharma.2025.03.002
94. Zarrinkamar M, Geran M, and Geran M. Patient Adherence for Oral Combination Therapies in Diabetes Management: A Scoping Review. Health Sci Rep. (2025) 8:e70780. doi: 10.1002/hsr2.70780
95. Bailey CJ. Metformin: Therapeutic profile in the treatment of type 2 diabetes. Diabetes Obes Metab. (2024) 26:3–19. doi: 10.1111/dom.v26.S3
96. Babich MM, Pike I, and Shiffman ML. Metformin-induced acute hepatitis. Am J Med. (1998) 104:490–2. doi: 10.1016/S0002-9343(98)00088-6
97. Nammour FE, Fayad NF, and Peikin SR. Metformin-induced cholestatic hepatitis. Endocrine Practice. (2003) 9:307–9. doi: 10.4158/EP.9.4.307
98. Kutoh E. Possible metformin-induced hepatotoxicity. Am J geriatric pharmacotherapy. (2005) 3:270–3. doi: 10.1016/j.amjopharm.2005.12.002
99. Biyyani RSRS, Battula S, Erhardt CA, and Korkor K. Metformin-induced cholangiohepatitis. Case Rep. (2009) 2009:bcr0920080950. doi: 10.1136/bcr.09.2008.0950
100. Cone CJ, Bachyrycz AM, and Murata GH. Hepatotoxicity associated with metformin therapy in treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Ann Pharmacotherapy. (2010) 44:1655–9. doi: 10.1345/aph.1P099
101. Callaghan T, Hadden D, and Tomkin G. Megaloblastic anaemia due to vitamin B12 malabsorption associated with long-term metformin treatment. Br Med J. (1980) 280:1214. doi: 10.1136/bmj.280.6225.1214
102. Bauman WA, Shaw S, Jayatilleke E, Spungen AM, and Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care. (2000) 23:1227–31. doi: 10.2337/diacare.23.9.1227
103. Ting RZ-W, Szeto CC, Chan MH-M, Ma KK, and Chow KM. Risk factors of vitamin B12 deficiency in patients receiving metformin. Arch Internal Med. (2006) 166:1975–9. doi: 10.1001/archinte.166.18.1975
104. Svare A. A patient presenting with symptomatic hypomagnesemia caused by metformin-induced diarrhoea: a case report. cases J. (2009) 2:1–4. doi: 10.1186/1757-1626-2-156
105. Bouchoucha M, Uzzan B, and Cohen R. Metformin and digestive disorders. Diabetes Metab. (2011) 37:90–6. doi: 10.1016/j.diabet.2010.11.002
106. Mazokopakis EE and Starakis IK. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin practice. (2012) 97:359–67. doi: 10.1016/j.diabres.2012.06.001
107. Pierce SA, Chung AH, and Black KK. Evaluation of vitamin B12 monitoring in a veteran population on long-term, high-dose metformin therapy. Ann Pharmacotherapy. (2012) 46:1470–6. doi: 10.1345/aph.1R223
108. Iftikhar R, Qadir A, Iqbal Z, and Usman H. Prevalence of vitamin B12 deficiency in patients of type 2 diabetes mellitus on metformin: a case control study from Pakistan. Pan Afr Med J. (2014) 16. doi: 10.11604/pamj.2013.16.67.2800
109. Sato Y, Ouchi K, Funase Y, Yamauchi K, and Aizawa T. Relationship between metformin use, vitamin B12 deficiency, hyperhomocysteinemia and vascular complications in patients with type 2 diabetes. Endocrine J. (2013) 60:1275–80. doi: 10.1507/endocrj.EJ13-0332
110. Jounela A, Pirttiaho H, and Palva I. DRUG-INDUCED MALABSORPTION OF VITAMIN B12: VI. Malabsorption of Vitamin B12 during Treatment with Phenformin. Acta Med Scandinavica. (1974) 196:267–9. doi: 10.1111/j.0954-6820.1974.tb01008.x
111. Adams J, Clark J, Ireland J, Kesson C, and Watson W. Malabsorption of vitamin B 12 and intrinsic factor secretion during biguanide therapy. Diabetologia. (1983) 24:16–8. doi: 10.1007/BF00275941
112. Berger W. Incidence of severe sideeffects during therapy with sulfonylureas and biguanides. Hormone Metab Res Supplement series. (1985) 15:111–5.
113. Pan Z-C, Li A-Z, Zeng N-K, Yang X-Q, Xie H-J, Chen J, et al. Structural characteristics and hypoglycemic activity of a polysaccharide from an edible bolete Phlebopus portentosus. Int J Biol Macromolecules. (2025) 142587. doi: 10.1016/j.ijbiomac.2025.142587
114. Mohri T, Okamoto S, Nishioka Y, Myojin T, Kubo S, Higashino T, et al. Risk of Lactic Acidosis in Hospitalized Diabetic Patients Prescribed Biguanides in Japan: A Retrospective Total-Population Cohort Study. Int J Environ Res Public Health. (2023) 20:5300. doi: 10.3390/ijerph20075300
115. Stahl SM, Sy S, and Maguire GA. How and when to treat the most common adverse effects of antipsychotics: Expert review from research to clinical practice. Acta Psychiatrica Scandinavica. (2021) 143:172–80. doi: 10.1111/acps.v143.2
116. Buzea CA, Peter M, Lorena D, and Correll CU. Drug–drug interactions involving combinations of antipsychotic agents with antidiabetic, lipid-lowering, and weight loss drugs. Expert Opin Drug Metab Toxicology. (2022) 18:729–44. doi: 10.1080/17425255.2022.2147425
117. Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. (2006) 29:1963–72. doi: 10.2337/dc06-9912
118. Schwartz S, Fonseca V, Berner B, Cramer M, Chiang Y-K, and Lewin A. Efficacy, tolerability, and safety of a novel once-daily extended-release metformin in patients with type 2 diabetes. Diabetes Care. (2006) 29:759–64. doi: 10.2337/diacare.29.04.06.dc05-1967
119. Salpeter S, Walsh J, Ormiston T, Greyber E, Buckley N, and Salpeter E. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. (2006) 8:538–54. doi: 10.1111/j.1463-1326.2005.00545.x
120. Fowler MJ. Diabetes treatment, part 2: oral agents for glycemic management. Clin diabetes. (2007) 25:131–5. doi: 10.2337/diaclin.25.4.131
121. Nagai Y, Kazumori K, Takeshima T, Iwasaki K, and Tanaka Y. A Claims Database Analysis of Dose-Dependency of Metformin and Incidence of Lactic Acidosis in Japanese Patients with Type 2 Diabetes. Diabetes Ther. (2021) 12:1129–41. doi: 10.1007/s13300-021-01029-7
122. Devajit M and Haradhan Kumar M. Sulfonylureas: A Widely Used Oral Anti-Hyperglycaemic Medication for Type 2 Diabetes Management. J Innov Med Res. (2024) 3:14–9. doi: 10.56397/JIMR/2024.03.02
123. Tahrani AA, Barnett AH, and Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinology. (2016) 12:566–92. doi: 10.1038/nrendo.2016.86
124. Tomlinson B, Gajanan PN, Manson F, Paul C, and Lam CWK. The role of sulfonylureas in the treatment of type 2 diabetes. Expert Opin Pharmacotherapy. (2022) 23:387–403. doi: 10.1080/14656566.2021.1999413
125. Korytkowski MT. Sulfonylurea treatment of type 2 diabetes mellitus: focus on glimepiride. Pharmacotherapy: J Hum Pharmacol Drug Ther. (2004) 24:606–20. doi: 10.1592/phco.24.6.606.34752
126. Baccetti F, Crisafulli C, Andreozzi F, Mannino GC, Nicolucci A, Michelli A, et al. Profiles of sulfonylurea use in Diabetes Mellitus type 2: an analysis of clinical practice over the last 10 years. Diabetes Res Clin Practice. (2024) 214:111781. doi: 10.1016/j.diabres.2024.111781
127. Gerich JE. Oral hypoglycemic agents. New Engl J Med. (1989) 321:1231–45. doi: 10.1056/NEJM198911023211805
128. Patani K, Joharapurkar A, Sundar R, Kshirsagar S, Metiya S, Kumar A, et al. Pharmacological effect of ZYGK1, a novel glucokinase activator, in hyperglycemic and sulfonylurea-resistant type 2 diabetes in mice models. J Diabetes its Complications. (2025) 39:108977. doi: 10.1016/j.jdiacomp.2025.108977
129. Assan Aliyar M, Nadig P, and Bharatham N. In vitro anti-diabetic activity, bioactive constituents, and molecular modeling studies with sulfonylurea receptor1 for insulin secretagogue activity of seed extract of Syzygium cumini (L.). J Herbmed Pharmacol. (2021) 10:304–12. doi: 10.34172/jhp.2021.35
130. Lee S-H, Park S-Y, and Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2022) 46:15–37. doi: 10.4093/dmj.2021.0280
131. Eller-Vainicher C, Cairoli E, Grassi G, Grassi F, Catalano A, Merlotti D, et al. Pathophysiology and management of type 2 diabetes mellitus bone fragility. J Diabetes Res. (2020) 2020. doi: 10.1155/2020/7608964
132. Hong JH, Moon JS, Seong K, and Lim S. Comparison of therapeutic efficacy and safety of sitagliptin, dapagliflozin, or lobeglitazone adjunct therapy in patients with type 2 diabetes mellitus inadequately controlled on sulfonylurea and metformin: Third agent study. Diabetes Res Clin Practice. (2023) 203:110872. doi: 10.1016/j.diabres.2023.110872
133. El-Zahabi MA, Bamanie FH, Ghareeb S, Alshaeri HK, Alasmari MM, Moustafa M, et al. Design, Synthesis, Molecular Modeling and Anti-Hyperglycemic Evaluation of Quinazoline-Sulfonylurea Hybrids as Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) and Sulfonylurea Receptor (SUR) Agonists. Int J Mol Sci. (2022) 23:9605. doi: 10.3390/ijms23179605
134. Haas B, Hass MDS, Voltz A, Vogel M, Walther J, Biswas A, et al. Sulfonylureas exert antidiabetic action on adipocytes by inhibition of PPARγ serine 273 phosphorylation. Mol Metab. (2024) 85:101956. doi: 10.1016/j.molmet.2024.101956
135. Drea A, Jalil M, and Alalwani TAAM. Theoretical investigation for new suggestion derivatives of sulfonylurea drug. AIP Conf Proc. (2024) 3092. doi: 10.1063/5.0199699
136. Saif-Ul Haque M, Khan UZ, and Memon RA. Chapter 4 - Pharmacological management of diabetes. In: Basit A and Ahmedani MY, editors. BIDE’ s Diabetes Desk Book. Elsevier (2024). p. 71–101.
137. Liu Y, Wang Q, Ma J, Li J, Li C, Xie X, et al. Discovery of Novel Sulfonylurea NLRP3 Inflammasome Inhibitor for the Treatment of Multiple Inflammatory Diseases. J Medicinal Chem. (2025) 68:7243–62. doi: 10.1021/acs.jmedchem.4c02813
138. He Z-C, Zhang T, Lu X-F, Li R, Peng W, and Ding F. Assessing the environmental risks of sulfonylurea pollutants: Insights into the risk priority and structure-toxicity relationships. Ecotoxicology Environ Safety. (2025) 292:117973. doi: 10.1016/j.ecoenv.2025.117973
139. Aquilante CL. Sulfonylurea pharmacogenomics in Type 2 diabetes: the influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther. (2010) 8:359–72. doi: 10.1586/erc.09.154
140. Chawla G, Pradhan T, and Gupta O. An Insight into the Combat Strategies for the Treatment of Type 2 Diabetes Mellitus. Mini Rev Medicinal Chem. (2024) 24:403–30. doi: 10.2174/1389557523666230517113936
141. Tahrani AA, Bailey CJ, Del Prato S, and Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet. (2011) 378:182–97. doi: 10.1016/S0140-6736(11)60207-9
142. Campbell I. Nateglinide–current and future role in the treatment of patients with type 2 diabetes mellitus. Int J Clin practice. (2005) 59:1218–28. doi: 10.1111/j.1368-5031.2005.00669.x
143. Okoduwa SIR, Mhya DH, Abdulwaliyu I, Igiri BE, Okoduwa UJ, Arthur DE, et al. Phytomedicine approach for management of diabetes mellitus: an overview of scientifically confirmed medicinal plants with hypoglycaemic properties and their probable mechanism of action. Phytochemistry Reviews (2024).
144. Hodgson S, Williamson A, Bigossi M, Stow D, Jacobs BM, Samuel M, et al. Genetic basis of early onset and progression of type 2 diabetes in South Asians. Nat Med. (2025) 31:323–31. doi: 10.1038/s41591-024-03317-8
145. Dornhorst A. Insulinotropic meglitinide analogues. Lancet. (2001) 358:1709–16. doi: 10.1016/S0140-6736(01)06715-0
146. Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs aging. (2000) 17:411–25. doi: 10.2165/00002512-200017050-00007
147. Faruk O and Khan M. Diabetes and Antidiabetic Drugs. In: Medicinal Chemistry for Pharmacy Students. Bentham Science Publishers (2024).
148. Kumari S, Ravi S, Aditi B, and Mishra A. Exploring plant-based alpha-glucosidase inhibitors: promising contenders for combatting type-2 diabetes. Arch Physiol Biochem. (2024) 130:694–709. doi: 10.1080/13813455.2023.2262167
149. Guardado-Mendoza R, Prioletta A, Jiménez-Ceja LM, Sosale A, and Folli F. State of the art paper The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch Med science. (2013) 9:936–43. doi: 10.5114/aoms.2013.34991
150. Kazi N and Abdelhafiz AH. Oral Hypoglycaemic Therapy. Care Older People Diabetes. (2025), 31–40. doi: 10.1002/9781394205066.ch4
151. Hu S. Interaction of nateglinide with KATP channel in β-cells underlies its unique insulinotropic action. Eur J Pharmacol. (2002) 442:163–71. doi: 10.1016/S0014-2999(02)01499-1
152. Noh J. Pharmacological management of diabetes in older adults. cpp. (2025) 7:13–20. doi: 10.36011/cpp.2025.7.e1
153. Lin T-H, Hung T-Y, Lin L-Y, Lin T-C, Liou Y-J, Hsu Y-J, et al. Dipeptidyl Peptidase-4 Inhibitors and Risk of Heart Failure in Patients With Type 2 Diabetes Mellitus and End-Stage Renal Disease Requiring Dialysis. Pharmacoepidemiology Drug Safety. (2025) 34:e70138. doi: 10.1002/pds.70138
154. Chu PY, Edmondson EK, Flory JH, Huang J, and Hennessy S. Risk of Hypoglycemia Associated With Concomitant Use of Insulin Secretagogues and ACE Inhibitors in Adults With Type 2 Diabetes: A Systematic Review. Clin Pharmacol Ther. (2025) 117:1005–11. doi: 10.1002/cpt.v117.4
155. Gautam T, Shamsad A, Singh R, Raza Baqri SS, and Banerjee M. Pharmacological therapy for Gestational Diabetes Mellitus: A comprehensive overview. Obes Med. (2025) 54:100587. doi: 10.1016/j.obmed.2025.100587
156. Krishnan A, Schneider CV, Mukherjee D, Woreta TA, and Alqahtani SA. Adverse Liver and Renal Outcomes After Initiating SGLT-2i and GLP-1RA Therapy Among Patients With Diabetes and MASLD. J Diabetes. (2025) 17:e70069. doi: 10.1111/1753-0407.70069
157. Timmons J and Boyle J. Sulfonylureas and Meglitinides. Diabetes Drug Notes. (2022), 49–66. doi: 10.1002/9781119785033
158. Razzaghy-Azar M, Nourbakhsh M, Talea A, Mohammad Amoli M, Nourbakhsh M, and Larijani B. Meglitinide (repaglinide) therapy in permanent neonatal diabetes mellitus: two case reports. J Med Case Rep. (2021) 15:535. doi: 10.1186/s13256-021-03052-5
159. Madsbad S, Kilhovd B, Lager I, Mustajoki P, Dejgaard A, and Group SR. Comparison between repaglinide and glipizide in Type 2 diabetes mellitus: a 1-year multicentre study. Diabetic Med. (2001) 18:395–401. doi: 10.1046/j.1464-5491.2001.00490.x
160. Marbury T, Huang W-C, Strange P, and Lebovitz H. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin practice. (1999) 43:155–66. doi: 10.1016/S0168-8227(99)00002-9
161. Meneilly GS. Effect of repaglinide versus glyburide on postprandial glucose and insulin values in elderly patients with type 2 diabetes. Diabetes Technol Ther. (2011) 13:63–5. doi: 10.1089/dia.2010.0105
162. Schwarz S, Gerich J, Marcellari A, Jean-Louis L, Purkayastha D, and Baron M. Nateglinide, alone or in combination with metformin, is effective and well tolerated in treatment-naive elderly patients with type 2 diabetes. Diabetes Obes Metab. (2008) 10:652–60. doi: 10.1111/j.1463-1326.2007.00792.x
163. Bellomo Damato A, Stefanelli G, Laviola L, Giorgino R, and Giorgino F. Nateglinide provides tighter glycaemic control than glyburide in patients with Type 2 diabetes with prevalent postprandial hyperglycaemia. Diabetic Med. (2011) 28:560–6. doi: 10.1111/j.1464-5491.2010.03219.x
164. Rojas P, Sánchez L, Santos A, Góõmez M, Blanco H, and Laguna J. Hypersensitivity to repaglinide. J Investig Allergol Clin Immunol. (2011) 21:245–7.
165. Yang J-K, Wang L, and Group PSC. Nateglinide in combination with metformin in Chinese patients with type 2 diabetes mellitus: a post-marketing surveillance study. Clin Drug Invest. (2013) 33:185–91. doi: 10.1007/s40261-013-0054-4
166. Dirir AM, Daou M, Yousef AF, and Yousef LF. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem Rev. (2022) 21:1049–79. doi: 10.1007/s11101-021-09773-1
167. Krentz AJ and Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. (2005) 65:385–411. doi: 10.2165/00003495-200565030-00005
168. Kashtoh H and Baek K-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants. (2022) 11:2722. doi: 10.3390/plants11202722
169. Bhatnagar A and Mishra A. α-Glucosidase Inhibitors for Diabetes/Blood Sugar Regulation. In: Maheshwari VL and Patil RH, editors. Natural Products as Enzyme Inhibitors: An Industrial Perspective. Springer Nature Singapore, Singapore (2022). p. 269–83.
171. Smith DL, Orlandella RM, Allison DB, and Norian LA. Diabetes medications as potential calorie restriction mimetics—a focus on the alpha-glucosidase inhibitor acarbose. GeroScience. (2021) 43:1123–33. doi: 10.1007/s11357-020-00278-x
172. Ueno H, Tsuchimochi W, Wang H-W, Yamashita E, Tsubouchi C, Nagamine K, et al. Effects of miglitol, acarbose, and sitagliptin on plasma insulin and gut peptides in type 2 diabetes mellitus: a crossover study. Diabetes Ther. (2015) 6:187–96. doi: 10.1007/s13300-015-0113-3
173. Joshi SR, Standl E, Tong N, Shah P, Kalra S, and Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin pharmacotherapy. (2015) 16:1959–81. doi: 10.1517/14656566.2015.1070827
174. Talab F, Ullah S, Alam A, Halim SA, Rehman NU, Zainab, et al. Bio-Oriented Synthesis of Novel Polyhydroquinoline Derivatives as α-Glucosidase Inhibitor for Management of Diabetes. ACS Omega. (2023) 8:6234–43. doi: 10.1021/acsomega.2c05390
175. Ur Rahman S, Alam A, Parveen Z, Zainab, Assad M, Adnan Ali Shah S, et al. Novel acyl hydrazide derivatives of polyhydroquinoline as potent anti-diabetic and anti-glycating agents: Synthesis, in vitro α-amylase, α-glucosidase inhibition and anti-glycating activity with molecular docking insights. Bioorganic Chem. (2024) 150:107501. doi: 10.1016/j.bioorg.2024.107501
176. Kaku K, Shimoda M, Osonoi T, Iwamoto M, and Kaneto H. Efficacy and safety of imeglimin add-on to DPP-4 inhibitor therapy in Japanese patients with type 2 diabetes mellitus: An interim analysis of the randomised, double-blind FAMILIAR trial. Diabetes Obes Metab. (2025) 27:3212–22. doi: 10.1111/dom.16336
177. Palalau AI, Tahrani AA, Piya MK, and Barnett AH. DPP-4 inhibitors in clinical practice. Postgraduate Med. (2009) 121:70–100. doi: 10.3810/pgm.2009.11.2079
178. Abbas G SAM, Mi HQ SAAI, Daijie W, and Hussain H. Dipeptidyl peptidase IV inhibitors as a potential target for diabetes: patent review 2019 – present. Expert Opin Ther Patents. (2025) 35:239–50. doi: 10.1080/13543776.2024.2446226
179. Panou T, Gouveri E, Popovic DS, Papazoglou D, and Papanas N. The Therapeutic Potential of Dipeptidyl Peptidase 4 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists in Diabetic Peripheral Neuropathy. Diabetes Ther. (2025) 16:1077–105. doi: 10.1007/s13300-025-01712-z
180. McKeage K. Trelagliptin: first global approval. Drugs. (2015) 75:1161–4. doi: 10.1007/s40265-015-0431-9
181. Burness CB. Omarigliptin: first global approval. Drugs. (2015) 75:1947–52. doi: 10.1007/s40265-015-0493-8
182. Chen Z, Su X, Cao W, Tan M, Zhu G, Gao J, et al. The Discovery and Characterization of a Potent DPP-IV Inhibitory Peptide from Oysters for the Treatment of Type 2 Diabetes Based on Computational and Experimental Studies. Mar Drugs. (2024) 22:361. doi: 10.3390/md22080361
183. Elrick H, Stimmler L, Hlad C, and Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. (1964) 24:1076–82. doi: 10.1210/jcem-24-10-1076
184. Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. (1986) 63:492–8. doi: 10.1210/jcem-63-2-492
185. Pham TK, Nguyen THT, Yi JM, Kim GS, Yun HR, Kim HK, et al. Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice. Exp Mol Med. (2023) 55:767–78. doi: 10.1038/s12276-023-00958-6
186. Laeeq T, Ahmed M, Sattar H, Zeeshan MH, and Ali MB. Role of SGLT2 Inhibitors, DPP-4 Inhibitors, and Metformin in Pancreatic Cancer Prevention. Cancers. (2024) 16:1325. doi: 10.3390/cancers16071325
187. Razavi M, Wei Y-Y, Rao X-Q, and Zhong J-X. DPP-4 inhibitors and GLP-1RAs: cardiovascular safety and benefits. Military Med Res. (2022) 9:45. doi: 10.1186/s40779-022-00410-2
188. Baggio LL and Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. (2007) 132:2131–57. doi: 10.1053/j.gastro.2007.03.054
189. Fehmann H-C, Göke R, and Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocrine Rev. (1995) 16:390–410. doi: 10.1210/edrv-16-3-390
190. Gautier J-F, Choukem S-P, and Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. (2008) 34:S65–72. doi: 10.1016/S1262-3636(08)73397-4
191. Ye J. Overview of Diabetes mechanism and therapy. Theor Natural Science. (2024) 65:52–5. doi: 10.54254/2753-8818/2024.LA18044
192. Wang Y, Yu C, Zheng X, Wang Y, and Zhang W. Effects of dipeptidyl peptidase 4 inhibitors on the risk of acute respiratory failure in patients with type 2 diabetes mellitus: a meta-analysis of cardiovascular outcomes trials. Endocrine J. (2024) 71:1175–81. doi: 10.1507/endocrj.EJ24-0164
193. Zhang K, Wang K, Zhang C, Teng X, Li D, and Chen M. Exploring the potential mechanism of emetine against coronavirus disease 2019 combined with lung adenocarcinoma: bioinformatics and molecular simulation analyses. BMC cancer. (2022) 22:687. doi: 10.1186/s12885-022-09763-2
194. Saran A, Raisinghani R, Paliwal S, and Sharma S. GLP-1R agonists: recent advances, current gaps, and future challenges. Mol Diversity. (2025), 1–12. doi: 10.1007/s11030-025-11195-6
195. Sun L, Ma Y, Geng C, Gao X, Li X, Ru Q, et al. DPP4, a potential tumor biomarker, and tumor therapeutic target. Mol Biol Rep. (2025) 52:1–13. doi: 10.1007/s11033-025-10235-6
196. Dupre J, Ross S, Watson D, and Brown J. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. (1973) 37:826–8. doi: 10.1210/jcem-37-5-826
197. Tr̈mper A, Tr̈mper K, Trusheim H, Arnold R, Göke B, and Hörsch D. Glucose-dependent insulinotropic polypeptide is a growth factor for β (INS-1) cells by pleiotropic signaling. Mol endocrinology. (2001) 15:1559–70. doi: 10.1210/mend.15.9.0688
199. Bhadouria N, Alam A, and Kaur A. Nanotechnology-based Herbal Drug Formulation in the Treatment of Diabetes Mellitus. Curr Diabetes Rev. (2025) 21:E310124226554. doi: 10.2174/0115733998282162240116202813
200. Banerjee M, Khursheed R, Yadav AK, Singh SK, Gulati M, Pandey DK, et al. A systematic review on synthetic drugs and phytopharmaceuticals used to manage diabetes. Curr Diabetes Rev. (2020) 16:340–56. doi: 10.2174/1573399815666190822165141
201. Stoleru OA, Burlec AF, Mircea C, Felea MG, Macovei I, Hăncianu M, et al. Multiple nanotechnological approaches using natural compounds for diabetes management. J Diabetes Metab Disord. (2024) 23:267–87. doi: 10.1007/s40200-023-01376-1
202. Bilal M, Iqbal MS, Shah SB, Rasheed T, and Iqbal H. Diabetic complications and insight into antidiabetic potentialities of ethno-medicinal plants: a review. Recent patents Inflammation Allergy Drug discovery. (2018) 12:7–23. doi: 10.2174/1872213X12666180221161410
203. Salehi B, Ata A V, Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. (2019) 9:551. doi: 10.3390/biom9100551
204. Paul S, Sarkar I, Sarkar N, Bose A, Chakraborty M, Chakraborty A, et al. Silver nanoparticles in diabetes mellitus: therapeutic potential and mechanistic insights. Bull Natl Res Centre. (2024) 48:33. doi: 10.1186/s42269-024-01182-6
205. Galanter M. Spirituality and the healthy mind: Science, therapy, and the need for personal meaning. Oxford University Press (2005).
206. Veeramani C, El Newehy AS, Aloud AA, Alsaif MA, and Al-Numair KS. Chapter 11 - Nutritional plants, phytochemicals, microorganisms-derived silver nanoparticles and their diabetic managements. In: Kesharwani P, editor. Silver Nanoparticles for Drug Delivery. Academic Press (2024). p. 241–64.
207. Unuofin JO and Lebelo SL. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: an updated review Vol. 2020. Oxidative medicine and cellular longevity (2020).
208. Adeyemi JO, Oriola AO, Onwudiwe DC, and Oyedeji AO. Plant extracts mediated metal-based nanoparticles: synthesis and biological applications. Biomolecules. (2022) 12:627. doi: 10.3390/biom12050627
209. Lyimo G, Ajayi RF, Maboza E, and Adam R. A green synthesis of zinc oxide nanoparticles using Musa paradisiaca and Rooibos extracts. MethodsX. (2022) 9:101892. doi: 10.1016/j.mex.2022.101892
210. Hng HP. Investigation of green synthesized silver nanoaprticles using aqueous leaf extract of Artemisia Argyi for antioxidant and antimicrobial potentials. UTAR (2017).
211. Saratale GD, Saratale RG, Benelli G, Kumar G, Pugazhendhi A, Kim D-S, et al. Anti-diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J Cluster Science. (2017) 28:1709–27. doi: 10.1007/s10876-017-1179-z
212. Krishnamoorthy K, Jayaraman S, Krishnamoorthy R, Manoharadas S, Alshuniaber MA, Vikas B, et al. Green synthesis and evaluation of anti-microbial, antioxidant, anti-inflammatory, and anti-diabetic activities of silver nanoparticles from Argyreia nervosa leaf extract: An invitro study. J King Saud Univ - Science. (2023) 35:102955. doi: 10.1016/j.jksus.2023.102955
213. Kalakonda P, Kathi R, Ligory MG, Dabbeta N, Madipoju N, Mynepally S, et al. Argyreia nervosa-driven biosynthesis of Cu–Ag bimetallic nanoparticles from plant leaves extract unveils enhanced antibacterial properties. Bioprocess Biosyst Engineering. (2024) 47:1307–19. doi: 10.1007/s00449-024-03020-5
214. Mishra AP, Saklani S, Chandra S, and Tiwari P. Total phenolics, flavonoids and antioxidant evaluation in the leaves of Argyreia nervosa Burm. Int J Pharm Sci Rev Res. (2015) 32:34–7.
215. Bauri AK, Butler JH, Cassera MB, and Foro S. Anti-Malarial Activity of Amentoflavone Isolated from Leaf of Calophyllum Tomentosum Wight. Chem Biodiversity. (2025) 22:e202401576. doi: 10.1002/cbdv.202401576
216. Govindappa M, Hemashekhar B, Arthikala M-K, Rai VR, and Ramachandra Y. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Physics. (2018) 9:400–8. doi: 10.1016/j.rinp.2018.02.049
217. Anandan J, Shanmugam R, Alharbi NS, and Thiruvengadam M. Green-synthesized silver nanoparticles from Centella asiatica and Ayapana triplinervis: A novel approach to treating wound infections and reducing antimicrobial resistance. South Afr J Botany. (2025) 177:617–29. doi: 10.1016/j.sajb.2024.12.020
218. Wilson S, Cholan S, Vishnu U, Sannan M, Jananiya R, Vinodhini S, et al. In vitro assessment of the efficacy of free-standing silver nanoparticles isolated from Centella asiatica against oxidative stress and its antidiabetic activity. Der Pharmacia Lettre. (2015) 7:194–205.
219. Pouthika K and Madhumitha G. A review on plant-derived nanomaterials: an effective and innovative insect-resistant strategy for alternate pesticide development. Int J Environ Sci Technology. (2024) 21:2239–62. doi: 10.1007/s13762-023-04998-3
220. Yakoob AT, Tajuddin NB, Hussain MIM, Mathew S, Govindaraju A, and Qadri I. Antioxidant and hypoglycemic activities of clausena anisata (Willd.) Hook F. ex benth. root mediated synthesized silver nanoparticles. Pharmacognosy J. (2016) 8:579–86. doi: 10.5530/pj.2016.6.10
221. Garcia Campoy AH, Perez Gutierrez RM, Manriquez-Alvirde G, and Muñiz Ramirez A. Protection of silver nanoparticles using Eysenhardtia polystachya in peroxide-induced pancreatic β-cell damage and their antidiabetic properties in zebrafish. Int J nanomedicine. (2018) 13:2601–12. doi: 10.2147/IJN.S163714
222. Gutiérrez-Sánchez R, Molina GA, López-Miranda JL, Rodríguez-Torres A, Estévez M, and Esparza R. One-Step DES-Mediated Synthesis of Au Nanoparticles Using Eysenhardtia polystachya Extract and Evaluation of Their Proapoptotic and Anti-Inflammatory Activities. ChemistrySelect. (2024) 9:e202402447. doi: 10.1002/slct.202402447
223. Abidin SZ. Antidiabetic potential and high synergistic antibacterial activity of silver nanoparticles synthesised with Musa Paradisiaca tepal extract. Med J Malaysia. (2021) 76:81.
224. Anbazhagan P, Murugan K, Jaganathan A, Sujitha V, Samidoss CM, Jayashanthani S, et al. Mosquitocidal, antimalarial and antidiabetic potential of Musa paradisiaca-synthesized silver nanoparticles: in vivo and in vitro approaches. J Cluster Science. (2017) 28:91–107. doi: 10.1007/s10876-016-1047-2
225. Pungle R, Nile SH, and Kharat AS. Green synthesis and characterization of Solanum xanthocarpum capped silver nanoparticles and its antimicrobial effect on multidrug-resistant bacterial (MDR) isolates. Chem Biol Drug Design. (2023) 101:469–78. doi: 10.1111/cbdd.v101.3
226. Sengottaiyan A, Aravinthan A, Sudhakar C, Selvam K, Srinivasan P, Govarthanan M, et al. Synthesis and characterization of Solanum nigrum-mediated silver nanoparticles and its protective effect on alloxan-induced diabetic rats. J Nanostructure Chem. (2016) 6:41–8. doi: 10.1007/s40097-015-0178-6
227. Shashidhara V M, Srinivasappa P, Jadhav A H, and Alwarsamy M. Phyto-synthesis of silver nanoparticles from Tephrosia purpurea and its in-vitro biogenic actions. Appl Organometallic Chem. (2023) 37:e7266. doi: 10.1002/aoc.7266
228. Saini M, Yadav S, Rani N, Mushtaq A, Rawat S, Saini K, et al. Biosynthesized zinc oxide nanoparticles using seed and bark extract of Azadirachta indica for antibacterial, photocatalytic and supercapacitor applications. Materials Sci Engineering: B. (2022) 282:115789. doi: 10.1016/j.mseb.2022.115789
229. Rehana D, Mahendiran D, Kumar RS, and Rahiman AK. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst engineering. (2017) 40:943–57. doi: 10.1007/s00449-017-1758-2
230. Khan MS, Dhavan PP, Ratna D, and Shimpi NG. Ultrasonic-assisted biosynthesis of ZnO nanoparticles using Sonneratia alba leaf extract and investigation of its photocatalytic and biological activities. J Cluster Science. (2022) 33:1007–23. doi: 10.1007/s10876-021-02036-1
231. Thatoi P, Kerry RG, Gouda S, Das G, Pramanik K, Thatoi H, et al. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J Photochem Photobiol B: Biol. (2016) 163:311–8. doi: 10.1016/j.jphotobiol.2016.07.029
232. Irfan M, Munir H, and Ismail H. Moringa oleifera gum based silver and zinc oxide nanoparticles: green synthesis, characterization and their antibacterial potential against MRSA. Biomaterials Res. (2021) 25:17. doi: 10.1186/s40824-021-00219-5
233. Jahan N, Rasheed K, Hazafa A, Saleem A, Alamri S, Iqbal MO, et al. Green inspired synthesis of zinc oxide nanoparticles using Silybum marianum (milk thistle) extract and evaluation of their potential pesticidal and phytopathogens activities. PeerJ. (2023) 11:e15743. doi: 10.7717/peerj.15743
234. Arvanag FM, Bayrami A, Habibi-Yangjeh A, and Pouran SR. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L seed extract. Materials Sci Engineering: C. (2019) 97:397–405. doi: 10.1016/j.msec.2018.12.058
235. Najafabadi SS, Doudi M, Tahmourespour A, Amiri G, and Rezayatmand Z. Assessment of Antimicrobial Activity of Chitosan, ZnO, and Urtica dioica–ZnO NPs Against Staphylococcus aureus Isolated from Diabetic Ulcers. Curr Microbiol. (2024) 81:295. doi: 10.1007/s00284-024-03633-9
236. Bayrami A, Haghgooie S, Pouran SR, Arvanag FM, and Habibi-Yangjeh A. Synergistic antidiabetic activity of ZnO nanoparticles encompassed by Urtica dioica extract. Advanced Powder Technology. (2020) 31:2110–8. doi: 10.1016/j.apt.2020.03.004
237. Mohammadi-Aloucheh R, Habibi-Yangjeh A, Bayrami A, Rahim Pouran S, Latifi-Navid S, and Asadi A. Antiproliferative activity of zinc oxide-silver nanocomposite interlinked with Vaccinium arctostaphylos L. fruit extract against cancer cells and bacteria. Chem Papers. (2022) 76:247–57. doi: 10.1007/s11696-021-01852-z
238. Bayrami A, Parvinroo S, Habibi-Yangjeh A, and Rahim Pouran S. Bio-extract-mediated ZnO nanoparticles: microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif cells nanomedicine Biotechnol. (2018) 46:730–9. doi: 10.1080/21691401.2017.1337025
239. Thomas S, Oyedeji AO, Oluwafemi OS, and PJ RJ. Nanotechnology in Herbal Medicine: Applications and Innovations. Elsevier (2023).
240. Pearce K, Thipe VC, Henkel RR, and Katti KV. Green Nanotechnology as an innovative drug delivery approach for Typha capensis and Naringenin—New class of phytochemical embedded biocompatible gold nanoparticles in prostate cancer therapy. J Drug Delivery Sci Technology. (2023) 80:104100. doi: 10.1016/j.jddst.2022.104100
241. Ajith S, Almomani F, Elhissi A, and Husseini GA. Nanoparticle-based materials in anticancer drug delivery: Current and future prospects. Heliyon. (2023) 9. doi: 10.1016/j.heliyon.2023.e21227
242. Ider M, Abderrafi K, Eddahbi A, Ouaskit S, and Kassiba A. Silver metallic nanoparticles with surface plasmon resonance: synthesis and characterizations. J Cluster Science. (2017) 28:1051–69. doi: 10.1007/s10876-016-1080-1
243. Abraham AN, Sharma TK, Bansal V, and Shukla R. Phytochemicals as dynamic surface ligands to control nanoparticle–protein interactions. ACS omega. (2018) 3:2220–9. doi: 10.1021/acsomega.7b01878
244. Shah R, Eldridge D, Palombo E, and Harding I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J Phys Sci. (2014) 25:59–75.
245. Phung X, Groza J, Stach EA, Williams LN, and Ritchey SB. Surface characterization of metal nanoparticles. Materials Sci Engineering: A. (2003) 359:261–8. doi: 10.1016/S0921-5093(03)00348-4
246. Hadji H and Bouchemal K. Effect of micro-and nanoparticle shape on biological processes. J Controlled Release. (2022) 342:93–110. doi: 10.1016/j.jconrel.2021.12.032
247. Rajeshwari A, Karthiga D, Chandrasekaran N, and Mukherjee A. Anti-aggregation-based spectrometric detection of Hg (II) at physiological pH using gold nanorods. Materials Sci Engineering: C. (2016) 67:711–6. doi: 10.1016/j.msec.2016.05.066
248. Manna I, Das D, Mondal S, and Bandyopadhyay M. Use of Nanoparticles in the Delivery of Plant-Based Therapeutics. In: Advances in Phytonanotechnology for Treatment of Various Diseases. CRC Press (2023). p. 67–126.
249. Oguebie R and Okeke C. Green synthesis of silver nanoparticles using costus afer aqueous leaf extract anditseffectonreproductivefunctions parametersinadultmalewisterrats. GSJ. (2023) 11:718. doi: 10.55248/gengpi.4.1123.113018
250. Mohammed Ali IA, AL-Ahmed HI, and Ben Ahmed A. Evaluation of Green Synthesis (Withania somnifera) of Selenium Nanoparticles to Reduce Sperm DNA Fragmentation Diabetic Mice Induced with Streptozotocin. Appl Sci. (2023) 13:728. doi: 10.3390/app13020728
251. Mostafa-Hedeab G, Behairy A, Abd-Elhakim YM, Mohamed AA-R, Noreldin AE, Dahran N, et al. Green synthesized zinc oxide nanoparticles using moringa olifera ethanolic extract lessens acrylamide-induced testicular damage, apoptosis, and steroidogenesis-related gene dysregulation in adult rats. Antioxidants. (2023) 12:361. doi: 10.3390/antiox12020361
252. Kamel ME, Mohammad HM, Maurice C, and Hagras MM. Ginseng nanoparticles protect against methotrexate-induced testicular toxicity in rats. Egypt J Basic Clin Pharmacol. (2019) 9:101397. doi: 10.32527/2019/101397
253. Erfani Majd N, Hajirahimi A, Tabandeh MR, and Molaei R. Protective effects of green and chemical zinc oxide nanoparticles on testis histology, sperm parameters, oxidative stress markers and androgen production in rats treated with cisplatin. Cell Tissue Res. (2021) 384:561–75. doi: 10.1007/s00441-020-03350-2
254. Nauroze T, Ali S, Kanwal L, Mughal TA, Andleeb S, and Ara C. Pharmacological intervention of biosynthesized Nigella sativa silver nanoparticles against hexavalent chromium induced toxicity in male albino mice. Saudi J Biol Sci. (2023) 30:103570. doi: 10.1016/j.sjbs.2023.103570
255. Jha PK, Jha RK, Rout D, Gnanasekar S, Rana SV, and Hossain M. Potential targetability of multi-walled carbon nanotube loaded with silver nanoparticles photosynthesized from Ocimum tenuiflorum (tulsi extract) in fertility diagnosis. J Drug Targeting. (2017) 25:616–25. doi: 10.1080/1061186X.2017.1306534
256. Hussain SB, Amin H, Naqqash T, Zubair M, and Noor S. Moringa oleifera Mediated Green Synthesis of Zinc Oxide Nanoparticles and their Characterization and Evaluation of Biological Activities. methods. (2021) 17:16–7. doi: 10.9734/AJBGMB/2021/v9i330220
257. Roozbeh N, Rostami S, and Abdi F. A review on herbal medicine with fertility and infertility characteristics in males. Iranian J Obstetrics Gynecology Infertility. (2016) 19:18–32. doi: 10.22038/ijogi.2016.7278
258. Shamsudin NF, Ahmed QU, Mahmood S, Shah SAA, Sarian MN, Khattak MMAK, et al. Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. Int J Mol Sci. (2022) 23:12605. doi: 10.3390/ijms232012605
259. MacDonald-Ramos K, Michán L, Martínez-Ibarra A, and Cerbón M. Silymarin is an ally against insulin resistance: A review. Ann hepatology. (2021) 23:100255. doi: 10.1016/j.aohep.2020.08.072
260. Rehman G, Umar M, Shah N, Hamayun M, Ali A, Khan W, et al. Green synthesis and characterization of silver nanoparticles using Azadirachta indica seeds extract: in vitro and in vivo evaluation of anti-diabetic activity. Pharmaceuticals. (2023) 16:1677. doi: 10.3390/ph16121677
261. Rathi R, Kaur S, and Singh I. A review on co-crystals of herbal bioactives for solubility enhancement: preparation methods and characterization techniques. Crystal Growth Design. (2022) 22:2023–42. doi: 10.1021/acs.cgd.1c01408
262. Najmi A, Javed SA, Al Bratty M, and Alhazmi HA. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules. (2022) 27:349. doi: 10.3390/molecules27020349
263. Siddiqa A, Qureshi R, Raja NI, Khan IA, Ahmad MZ, Rafique S, et al. Liver-boosting potential: chicory compound-mediated silver nanoparticles for hepatoprotection—biochemical and histopathological insights. Front Pharmacol. (2024) 15:1325359. doi: 10.3389/fphar.2024.1325359
264. Jain PK, Huang X, El-Sayed IH, and El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Accounts Chem Res. (2008) 41:1578–86. doi: 10.1021/ar7002804
265. Samrat K, Krishna Murthy T, Divyashri G, Hari Krishna R, and Chandraprabha M. Nanotechnology: Antidiabetics, Antioxidant and Anti-inflammatory. In: Nanomaterials for Sustainable Development: Opportunities and Future Perspectives. Springer (2023). p. 235–63.
266. Akunjee MM, Khosla SG, Nylen ES, and Sen S. SGLT2 inhibitors Use in Kidney Disease: What Did We Learn? Am J Physiology-Endocrinology Metab. (2025) 328:E856–68. doi: 10.1152/ajpendo.00034.2025
267. Kupnicka P, Król M, Żychowska J, Łagowski R, Prajwos E, Surówka A, et al. GLP-1 Receptor Agonists: A Promising Therapy for Modern Lifestyle Diseases with Unforeseen Challenges. Pharmaceuticals. (2024) 17:1470. doi: 10.3390/ph17111470
268. Dhas TS, Kumar VG, Karthick V, Vasanth K, Singaravelu G, and Govindaraju K. Effect of biosynthesized gold nanoparticles by Sargassum swartzii in alloxan induced diabetic rats. Enzyme microbial technology. (2016) 95:100–6. doi: 10.1016/j.enzmictec.2016.09.003
269. Unnikrishnan A, Kalra S, Purandare V, and Vasnawala H. Genital infections with sodium glucose cotransporter-2 inhibitors: occurrence and management in patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. (2018) 22:837–42. doi: 10.4103/ijem.IJEM_159_17
270. Roda E, Barni S, Milzani A, Dalle-Donne I, Colombo G, and Coccini T. Single silver nanoparticle instillation induced early and persisting moderate cortical damage in rat kidneys. Int J Mol Sci. (2017) 18:2115. doi: 10.3390/ijms18102115
271. Dziendzikowska K, Gromadzka-Ostrowska J, Lankoff A, Oczkowski M, Krawczyñska A, Chwastowska J, et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. Journal of Applied Toxicology. (2012) 32:920–8. doi: 10.1002/jat.2758
272. Asam Raza M, Farwa U, Waseem Mumtaz M, Kainat J, Sabir A, and Al-Sehemi AG. Green synthesis of gold and silver nanoparticles as antidiabetic and anticancerous agents. Green Chem Lett Rev. (2023) 16:2275666. doi: 10.1080/17518253.2023.2275666
273. Scheen AJ and Delanaye P. Understanding the protective effects of SGLT2 inhibitors in type 2 diabetes patients with chronic kidney disease. Expert Rev Endocrinol Metab. (2022) 17:35–46. doi: 10.1080/17446651.2022.2014322
274. Dhobale S, Thite T, Laware S, Rode C, Koppikar SJ, Ghanekar R-K, et al. Zinc oxide nanoparticles as novel alpha-amylase inhibitors. J Appl Phys. (2008) 104. doi: 10.1063/1.3009317
275. Horky P, Urbankova L, Bano I, Kopec T, Nevrkla P, Pribilova M, et al. Selenium nanoparticles as potential antioxidants to improve semen quality in boars. Animals. (2023) 13:2460. doi: 10.3390/ani13152460
276. Alkhalaf MI, Hussein RH, and Hamza A. Green synthesis of silver nanoparticles by Nigella sativa extract alleviates diabetic neuropathy through anti-inflammatory and antioxidant effects. Saudi J Biol Sci. (2020) 27:2410–9. doi: 10.1016/j.sjbs.2020.05.005
277. Cheng X, Xu Y, Jia Q, Guo N, Wang Z, and Wang Y. Novel greener approached synthesis of polyacrylic nanoparticles for therapy and care of gestational diabetes. Drug Delivery. (2020) 27:1263–70. doi: 10.1080/10717544.2020.1809555
278. Govindan K, Mina H, and Alavi B. A decision support system for demand management in healthcare supply chains considering the epidemic outbreaks: A case study of coronavirus disease 2019 (COVID-19). Transportation Res Part E: Logistics Transportation Review. (2020) 138:101967. doi: 10.1016/j.tre.2020.101967
279. Santos CMM, Proença C, Freitas M, Araújo AN, Silva AMS, and Fernandes E. 2-Styrylchromones as inhibitors of α-amylase and α-glucosidase enzymes for the management of type 2 diabetes mellitus. Medicinal Chem Res. (2024) 33:600–10. doi: 10.1007/s00044-024-03200-8
280. Malapermal V, Botha I, Krishna SBN, and Mbatha JN. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J Biol Sci. (2017) 24:1294–305. doi: 10.1016/j.sjbs.2015.06.026
281. Patel P, Shah D, Bambharoliya T, Patel V, Patel M, Patel D, et al. A Review on the Development of Novel Heterocycles as α-Glucosidase Inhibitors for the Treatment of Type-2 Diabetes Mellitus. Medicinal Chem. (2024) 20:503–36. doi: 10.2174/0115734064264591231031065639
282. Vinotha V, Iswarya A, Thaya R, Govindarajan M, Alharbi NS, Kadaikunnan S, et al. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J Photochem Photobiol B: Biol. (2019) 197:111541. doi: 10.1016/j.jphotobiol.2019.111541
283. Parvathalu K, Chinmayee S, Preethi B, Swetha A, Maruthi G, Pritam M, et al. Green Synthesis of Silver Nanoparticles Using Argyreia nervosa Leaf Extract and Their Antimicrobial Activity. Plasmonics. (2023) 18:1075–81. doi: 10.1007/s11468-023-01835-8
284. Algotiml R, Gab-Alla A, Seoudi R, Abulreesh HH, El-Readi MZ, and Elbanna K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci Rep. (2022) 12:2421. doi: 10.1038/s41598-022-06412-3
285. Manam S, Tsakok T, Till S, and Flohr C. The association between atopic dermatitis and food allergy in adults. Curr Opin Allergy Clin Immunol. (2014) 14:423–9. doi: 10.1097/ACI.0000000000000095
286. Shashidhara V M, Srinivasappa P, Jadhav A H, and Alwarsamy M. Phyto-synthesis of silver nanoparticles from and its in-vitro biogenic actions. Appl Organometallic Chem. (2023) 37:e7266. doi: 10.3390/molecules29071660
287. Rajaram K, Aiswarya D, and Sureshkumar P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Materials Letters. (2015) 138:251–4. doi: 10.1016/j.matlet.2014.10.017
288. Shanker R, Bhanugopan R, van der Heijden BI, and Farrell M. Organizational climate for innovation and organizational performance: The mediating effect of innovative work behavior. J vocational behavior. (2017) 100:67–77. doi: 10.1016/j.jvb.2017.02.004
289. Venkatachalam M, Govindaraju K, Sadiq AM, Tamilselvan S, Kumar VG, and Singaravelu G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochimica Acta Part A: Mol Biomolecular Spectroscopy. (2013) 116:331–8. doi: 10.1016/j.saa.2013.07.038
290. Sadhu VA, Jha S, Ghosh S, Mehta VN, Park TJ, and Kailasa SK. Senna auriculata extract-assisted biogenic synthesis of yellow emissive gold nanoclusters for quantitative detection of glyphosate in food and environmental samples. Environ Nanotechnology Monit Management. (2024) 22:100964. doi: 10.1016/j.enmm.2024.100964
291. Beg M, Anukul M, Sk N, Nasima AM, Kumar SN, Chandra JG, et al. Biophysical insights into the interaction of human serum albumin with Cassia fistula leaf extracts inspired biogenic potent antibacterial and anticancerous gold nanoparticles. J Biomolecular Structure Dynamics. (2021) 39:4567–81. doi: 10.1080/07391102.2020.1778532
292. Daisy P and Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J nanomedicine. (2012) 7:1189–202. doi: 10.2147/IJN.S26650
293. Malik S, Niazi M, Khan M, Rauff B, Anwar S, Amin F, et al. Cytotoxicity Study of Gold Nanoparticle Synthesis Using Aloe vera, Honey, and Gymnema sylvestre Leaf Extract. ACS Omega. (2023) 8:6325–36. doi: 10.1021/acsomega.2c06491
294. Karthick V, Kumar VG, Dhas TS, Singaravelu G, Sadiq AM, and Govindaraju K. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats—an in vivo approach. Colloids Surfaces B: Biointerfaces. (2014) 122:505–11. doi: 10.1016/j.colsurfb.2014.07.022
295. Majani SS, Sathyan S, Manoj MV, Vinod N, Pradeep S, Shivamallu C, et al. Eco-friendly synthesis of MnO2 nanoparticles using Saraca asoca leaf extract and evaluation of in vitro anticancer activity. Curr Res Green Sustain Chem. (2023) 6:100367. doi: 10.1016/j.crgsc.2023.100367
296. Patra N, Kar D, Pal A, and Behera A. Antibacterial, anticancer, anti-diabetic and catalytic activity of bio-conjugated metal nanoparticles. Adv Natural Sciences: Nanoscience Nanotechnology. (2018) 9:035001. doi: 10.1088/2043-6254/aad12d
297. Mares Briones F, Frutis-Murillo M, López-Miranda JL, López Meza JE, Rosas G, Estevez M, et al. Effect of Sargassum-mediated synthesis on hemolytic activity of Au nanoparticles. MRS Advances. (2025) 10:30–5. doi: 10.1557/s43580-024-01043-4
298. Durán N, Durán M, De Jesus MB, Seabra AB, Fávaro WJ, and Nakazato G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine: nanotechnology Biol Med. (2016) 12:789–99. doi: 10.1016/j.nano.2015.11.016
299. Rajeshkumar S, Bharath L, and Geetha R. Broad spectrum antibacterial silver nanoparticle green synthesis: Characterization, and mechanism of action. In: Green synthesis, characterization and applications of nanoparticles. Elsevier (2019). p. 429–44.
300. Jamdade DA, Rajpali D, Joshi KA, Kitture R, Kulkarni AS, Shinde VS, et al. Gnidia glauca-and Plumbago zeylanica-mediated synthesis of novel copper nanoparticles as promising antidiabetic agents. Adv Pharmacol Pharm Sci. (2019) 2019. doi: 10.1155/2019/9080279
301. Malapermal V, Mbatha JN, Gengan RM, and Anand K. Biosynthesis of bimetallic Au-Ag nanoparticles using Ocimum basilicum (L.) with antidiabetic and antimicrobial properties. Advanced materials Lett. (2015) 6:1050–7. doi: 10.5185/amlett.2015.5997
302. More PR, Zannella C, Folliero V, Foglia F, Troisi R, Vergara A, et al. Antimicrobial Applications of Green Synthesized Bimetallic Nanoparticles from Ocimum basilicum. Pharmaceutics. (2022) 14:2457. doi: 10.3390/pharmaceutics14112457
303. Perumal V, Manickam T, Bang K-S, Velmurugan P, and Oh B-T. Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. Int J Biol macromolecules. (2016) 92:63–9. doi: 10.1016/j.ijbiomac.2016.07.006
304. Hazarika M, Boruah PK, Pal M, Das MR, and Tamuly C. Synthesis of Pd-rGO Nanocomposite for the Evaluation of In Vitro Anticancer and Antidiabetic Activities. ChemistrySelect. (2019) 4:1244–50. doi: 10.1002/slct.201802789
305. Fan D, Li L, Li Z, Zhang Y, Ma X, Wu L, et al. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci Technol advanced materials. (2020) 21:505–14. doi: 10.1080/14686996.2020.1788907
306. Gorain B, Karmakar V, Sarkar B, Dwivedi M, Leong JTL, Toh JH, et al. Biomacromolecule-based nanocarrier strategies to deliver plant-derived bioactive components for cancer treatment: A recent review. Int J Biol macromolecules. (2023) 126623. doi: 10.1016/j.ijbiomac.2023.126623
307. Agarwal A, Roychoudhury S, Bjugstad KB, and Cho C-L. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. (2016) 8:302–18. doi: 10.1177/1756287216652
308. Hosseini H and Abdi F. Experiences of vasectomy: a phenomenological study. North Am J Med Sci. (2012) 4:619. doi: 10.4103/1947-2714.104311
309. Huang B, Wang Z, Kong Y, Jin M, and Ma L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: an analysis of global burden of disease study. BMC Public Health. (2023) 23:2195. doi: 10.1186/s12889-023-16793-3
310. Oyeyemi M, Olukole S, and Esan O. Sperm morphological studies of West African Dwarf Bucks treated with pumpkin plant (Cucurbita pepo). Int J Morphol. (2008) 26:121–6. doi: 10.4067/S0717-95022008000100020
311. Hill S, Arutchelvam V, and Quinton R. Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men. IDrugs. (2009) 12:109–19.
312. Bussmann RW and Glenn A. Medicinal plants used in Northern Peru for reproductive problems and female health. J Ethnobiology Ethnomedicine. (2010) 6:1–12. doi: 10.1186/1746-4269-6-30
313. Egbiremhon B, Sam-Duru Prisca A, Ogbonna E, Joseph R, and Oguebie R. Green Synthesis of Sliver Nanoparticles Using Costus Afer Aqueous Leaf Extract and its Effect on Liver and Kidney Biomarkers in Adult Male Wistar Rat. Int. J. Res. Publ. Rev. (2023) 4:901–7. doi: 10.55248/gengpi.4.1123.113018
314. Gao M, Chang J, Wang Z, Zhang H, and Wang T. Advances in transport and toxicity of nanoparticles in plants. J Nanobiotechnology. (2023) 21:75. doi: 10.1186/s12951-023-01830-5
315. Kang ME, Weng Y, Liu Y, Wang H, Ye L, Gu Y, et al. A review on the toxicity mechanisms and potential risks of engineered nanoparticles to plants. Rev Environ Contamination Toxicology. (2023) 261:5. doi: 10.1007/s44169-023-00029-x
316. Ansari MA, Shukla AK, Oves M, and Khan HM. Electron microscopic ultrastructural study on the toxicological effects of AgNPs on the liver, kidney and spleen tissues of albino mice. Environ Toxicol Pharmacol. (2016) 44:30–43. doi: 10.1016/j.etap.2016.04.007
317. Hano C and Abbasi BH. Plant-based green synthesis of nanoparticles: Production, characterization and applications. MDPI;. (2021) p:31. doi: 10.3390/biom12010031
318. Metha C, Pawar S, and Suvarna V. Green Synthesis of Nanoparticles Using Plant Extracts. Nanomaterials Agroforestry Systems: Springer;. (2025) p:41–66.
Keywords: diabetes mellitus, male infertility, nanotechnology, anti-diabetic drugs, phyto-nanoparticles
Citation: Siddiqa A, Qureshi R, Yasmin G, Rafique S, Zafar N-U-A, Hussain CS, Rehman Su and Naheed N (2025) Unlocking the dual healing powers of plant-based metallic nanoparticles: managing diabetes and tackling male infertility challenges. Front. Endocrinol. 16:1482127. doi: 10.3389/fendo.2025.1482127
Received: 17 August 2024; Accepted: 12 June 2025;
Published: 04 July 2025.
Edited by:
Andrea Orellana-Manzano, Escuela Superior Politécnica del Litoral (ESPOL), EcuadorReviewed by:
Jilai Tian, Nanjing University of Chinese Medicine, ChinaDr. Laxmi Rathor, University of Florida, United States
Copyright © 2025 Siddiqa, Qureshi, Yasmin, Rafique, Zafar, Hussain, Rehman and Naheed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rahmatullah Qureshi, cmFobWF0dWxsYWhxQHVhYXIuZWR1LnBr; cmFobWF0dWxsYWhxQHlhaG9vLmNvbQ==
 Ayesha Siddiqa1
Ayesha Siddiqa1 Shaista Rafique
Shaista Rafique Chudary Sadam Hussain
Chudary Sadam Hussain Sana ur Rehman
Sana ur Rehman