- 1Reproductive Medicine Center, Renmin Hospital, Hubei University of Medicine, Shiyan, China
- 2Hubei Clinical Research Center for Reproductive Medicine, Shiyan, China
- 3Biomedical Engineering College, Hubei University of Medicine, Shiyan, China
- 4Biomedical Research Institute, Hubei University of Medicine, Shiyan, China
- 5Hubei Key Laboratory of Embryonic Stem Cell Research, Hubei University of Medicine, Shiyan, China
Objective: To evaluate the dynamics of serum medroxyprogesterone acetate (MPA) concentrations and their influence on serum progesterone (P) levels and pregnancy outcomes in the progestin-primed ovarian stimulation (PPOS) protocol. A total of 116 patients who underwent in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment using the PPOS protocol were included. Serum MPA levels were measured on the third, fifth, and seventh days of MPA use; on the day of human chorionic gonadotropin (hCG) trigger; and two and five days after oocyte pick-up (OPU).
Results: The serum MPA concentration was 2.26 ± 2.11 nmol/L on the hCG trigger day, 0.37 ± 0.40 nmol/L two days after OPU, and zero five days after OPU. There were no statistically significant differences in P levels on the hCG trigger day, total dosage of Gn, duration of Gn, number of oocytes retrieved, number of mature oocytes, fertilization rate, blastocyst progression rate, CPR, ectopic pregnancy rate, early pregnancy loss rate, or live birth rate (LBR) between the two cohorts (P > 0.05).
Conclusion(s): Serum concentrations of MPA had no effect on serum P levels or pregnancy outcomes in patients undergoing the PPOS protocol.
Introduction
In recent years, the progestin-primed ovarian stimulation (PPOS) protocol, which uses oral progestins as a substitute for gonadotropin-releasing hormone (GnRH) analogs to suppress the pituitary gland and inhibit premature luteinizing hormone (LH) surges, has emerged as an alternative to conventional protocols such as the GnRH agonist (GnRH-a) long protocol and antagonist protocol. The PPOS protocol has been applied in cases of oocyte donation (1, 2), fertility preservation (3, 4), and hyper-responders (5). Its advantages include lower gonadotropin consumption (6), the retrieval of more oocytes and good embryos, and a higher cumulative live birth rate (CLBR) in older women (7) and women with diminished ovarian reserve (DOR) (8), as well as comparable pregnancy outcomes in women with normal ovarian reserve (9). Consequently, the PPOS protocol is considered more convenient, effective, and suitable for all in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) patients (6, 10, 11).
Since oral progestins are analogs of progesterone (P), exposure to these drugs throughout the body may impact three stages: the oocyte developmental stage during ovulation induction, the embryo implantation stage, and the early developmental stage of embryos. This exposure could lead to lower oocyte quality, reduced endometrial receptivity (12) and embryonic teratogenicity and toxicity (13). Therefore, a freeze-all and thawed embryo transfer (ET) strategy is routinely implemented in the PPOS protocol.
The grade rating of embryos, the aneuploidy rate, and the clinical pregnancy rate (CPR) can indirectly reflect oocyte quality. Previous studies have shown that the number of blastocysts, euploid blastocyst rates, and CPRs were similar between PPOS patients and those undergoing conventional stimulation cycles (6, 10, 11, 14–19). Additionally, there are concerns regarding the potential risks of progestins on the health and safety of offspring in patients undergoing the PPOS protocol. Some studies have reported no significant differences in neonatal outcomes or congenital malformation rates between the PPOS protocol and traditional protocols (20–24). Moreover, oral progestins are assumed to increase progesterone (P) levels, particularly on the human chorionic gonadotropin (hCG) trigger day, potentially shifting the endometrial implantation window. However, this theory remains controversial (17, 25–38).
The PPOS protocol has been tested with various progestin administration methods, with medroxyprogesterone acetate (MPA) being the most commonly used. Evaluating the potential influence of the MPA dose effect is crucial. Our previous study demonstrated that a degressive administration of MPA based on serum luteinizing hormone (LH) levels could decrease the total MPA dose while preventing preovulation (39). Therefore, it is important to understand the duration of MPA metabolism in the PPOS protocol and assess whether different serum MPA concentrations affect P levels and pregnancy outcomes. We conducted a single-center retrospective cohort study to explore the dynamics of serum MPA levels and to compare P levels on the hCG trigger day and pregnancy outcomes after frozen embryo transfer (FET) between high- and low-MPA groups, which is clinically significant.
Materials and methods
Study design and patients
We conducted a hospital-based retrospective cohort study, adhering to the principles outlined in the Declaration of Helsinki. Data were collected from the Reproductive Medicine Center, Renmin Hospital, Hubei University of Medicine, covering the period from October 2021 to October 2022. All the data were anonymized to ensure patient confidentiality and privacy.
Women who underwent the PPOS protocol were included in the study if they met the following criteria: patients with regular menstrual cycles (25-35 days), aged 20-40 years, body mass index (BMI) 18-28 kg/m², bilateral antral follicle counts (AFCs) 3-20, and normal basal serum levels of follicle-stimulating hormone (FSH) (<10 IU/L) and anti-Müllerian hormone (AMH) (≥1.1 ng/mL) on Day 2 or 3 of the cycle before ovarian stimulation. The exclusion criteria included metabolic disorders, polycystic ovarian syndrome (PCOS), endometriosis, pelvic tuberculosis, congenital uterine malformations, chromosomal abnormalities, single-gene disorders, and immunological diseases.
The ovarian stimulation was started on Day 2 or 3 of the cycle. The detailed treatment of a modified PPOS protocol and endometrial preparation methods for FET used in this study has been reported in our previous research (39).
Moderate/severe OHSS was diagnosed in women who met more than one of the following criteria: clinical ascites, hydrothorax, or dyspnea (exertional or at rest). Biochemical pregnancy was defined as hCG >10 IU/L two weeks after ET. Clinical pregnancy was defined as an intrauterine gestational sac identified by ultrasonography 30 days after ET. Early pregnancy loss was defined as spontaneous pregnancy loss before 12 weeks. Live birth was defined as a living fetus born after 28 weeks of pregnancy.
Outcome parameters
Serum FSH, LH, E2, and P levels were measured on the first day of stimulation; the third, fifth, and seventh days of MPA use; and the hCG trigger day. Hormone levels were determined using electrochemiluminescence (Beckman Coulter, USA), with all measurements conducted by skilled technicians in accordance with the manufacturer’s instructions. The detection sensitivity limits were as follows: FSH, 0.2 IU/L; LH, 0.2 IU/L; E2, 15 pg/ml; and P, 0.1 ng/ml. The inter- and intra-assay coefficients of variation were less than 10%.
Serum MPA levels were measured on the third, fifth, and seventh days of MPA use; on the hCG trigger day; and two and five days after OPU. MPA was extracted from 1,000 μl of serum and evaporated to dryness, and the reconstituted solution was injected onto a Waters Acquity liquid chromatography (LC) system using an Agilent Zorbax Eclipse-Plus C18 2.1 × 100 mm (3.0 μm) column. MPA and its internal standard were monitored on a QTRAP® 5500 mass analyzer in positive ionization mode. The method was validated according to the FDA Bioanalytical Method Validation guidelines. The calibration curve for MPA had a linear range of 0.10-8.0 μg/l, with a limit of quantification of 40 ng/l. The relative recovery was 76.0%. The inter- and intraday precisions were less than 9.0%.
Statistical methods
All analyses were performed using EmpowerStats (http://www.empowerstats.com) and SPSS 26.0 (IBM, Armonk, NY, USA). Continuous variables are presented as the means with standard deviations or medians with interquartile ranges, and differences among groups were compared using one-way analysis of variance or the Kruskal–Wallis test. A multivariable regression model was constructed to identify factors related to pregnancy outcomes in all participants. Statistical significance was set at a two-sided P value < 0.05. Graphs were created from histograms constructed with GraphPad Prism version 8.4.2 (GraphPad, La Jolla, CA).
Ethics statement
The study protocol was reviewed and approved by the Ethics Committee of Renmin Hospital, Hubei University of Medicine (No: SYRMYY-065). Informed consent was obtained from all patients at the time of enrollment.
Results
In our retrospective cohort study, we achieved a total of 116 OPU cycles and 97 FET cycles. In the remaining 19 patients, no embryos were available for transfer.
Dynamics of serum MPA concentrations
The total dosage of MPA was 21.06 ± 10.38 mg, and the duration of MPA administration was 6.21 ± 1.94 days. The serum MPA concentrations were as follows: 4.27 ± 1.09 nmol/L on the third day, 4.86 ± 1.97 nmol/L on the fifth day, 4.35 ± 2.85 nmol/L on the seventh day, 2.26 ± 2.11 nmol/L on the hCG trigger day, 0.37 ± 0.40 nmol/L two days after OPU, and zero five days after OPU (Supplementary Table 1).
All women were divided into two groups according to a cutoff value of 2.5 nmol/L for serum MPA concentrations on the hCG trigger day. The serum MPA concentrations at any time point were higher in the high-concentration group than that in the low-concentration group, expect on the OPU+5 day (Figure 1).
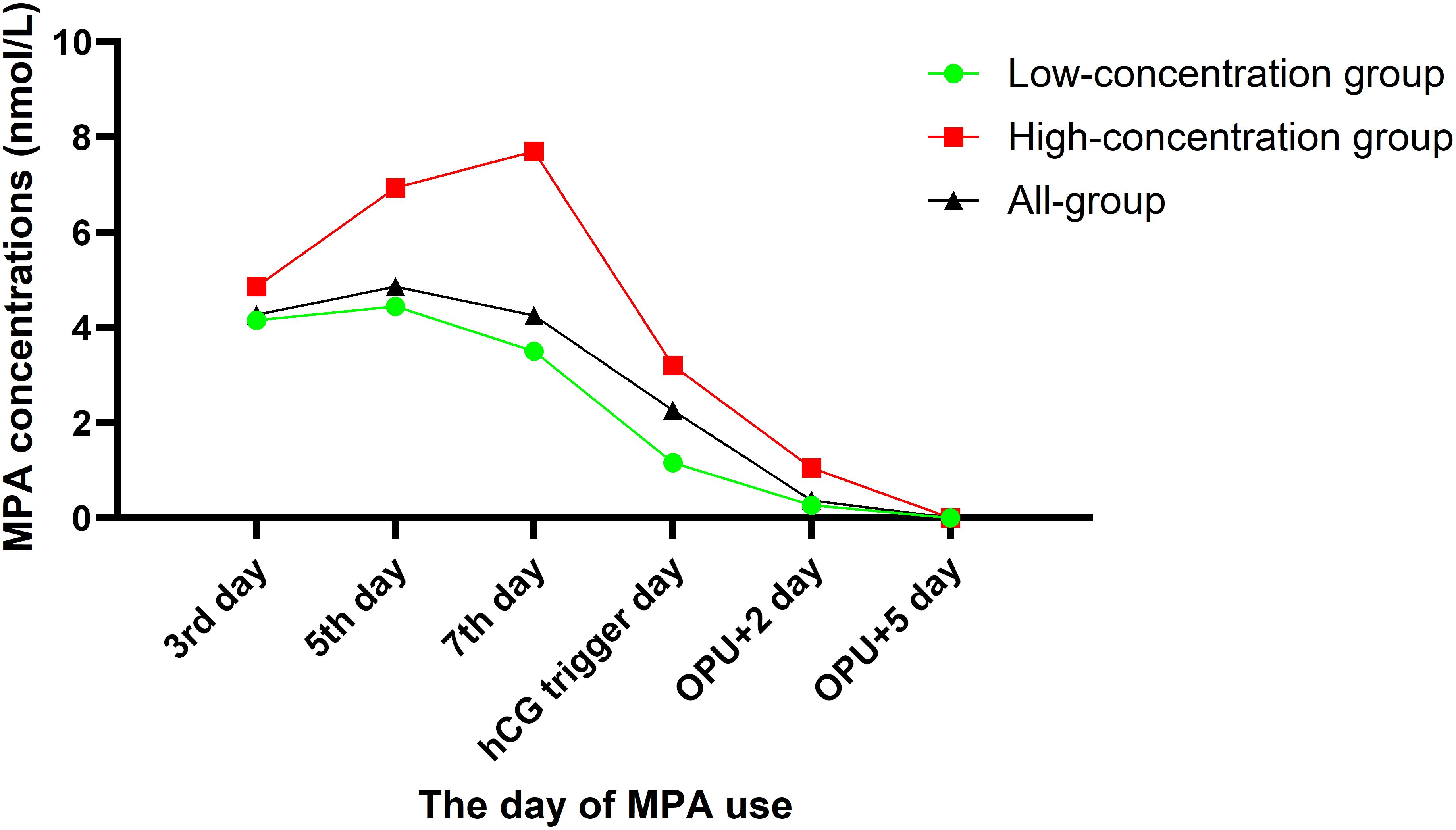
Figure 1. Different groups of the serum MPA concentrations after administration of MPA in the PPOS protocol.
Data on ovulation induction process and embryological outcomes
The patient characteristics of the two groups are provided in Table 1. Significant differences were observed in the MPA dose, MPA duration, serum MPA concentration, and type of infertility (P < 0.05). However, there were no statistically significant differences between the two groups in terms of female age, BMI, AMH, AFC, duration of infertility, or insemination method (P > 0.05).
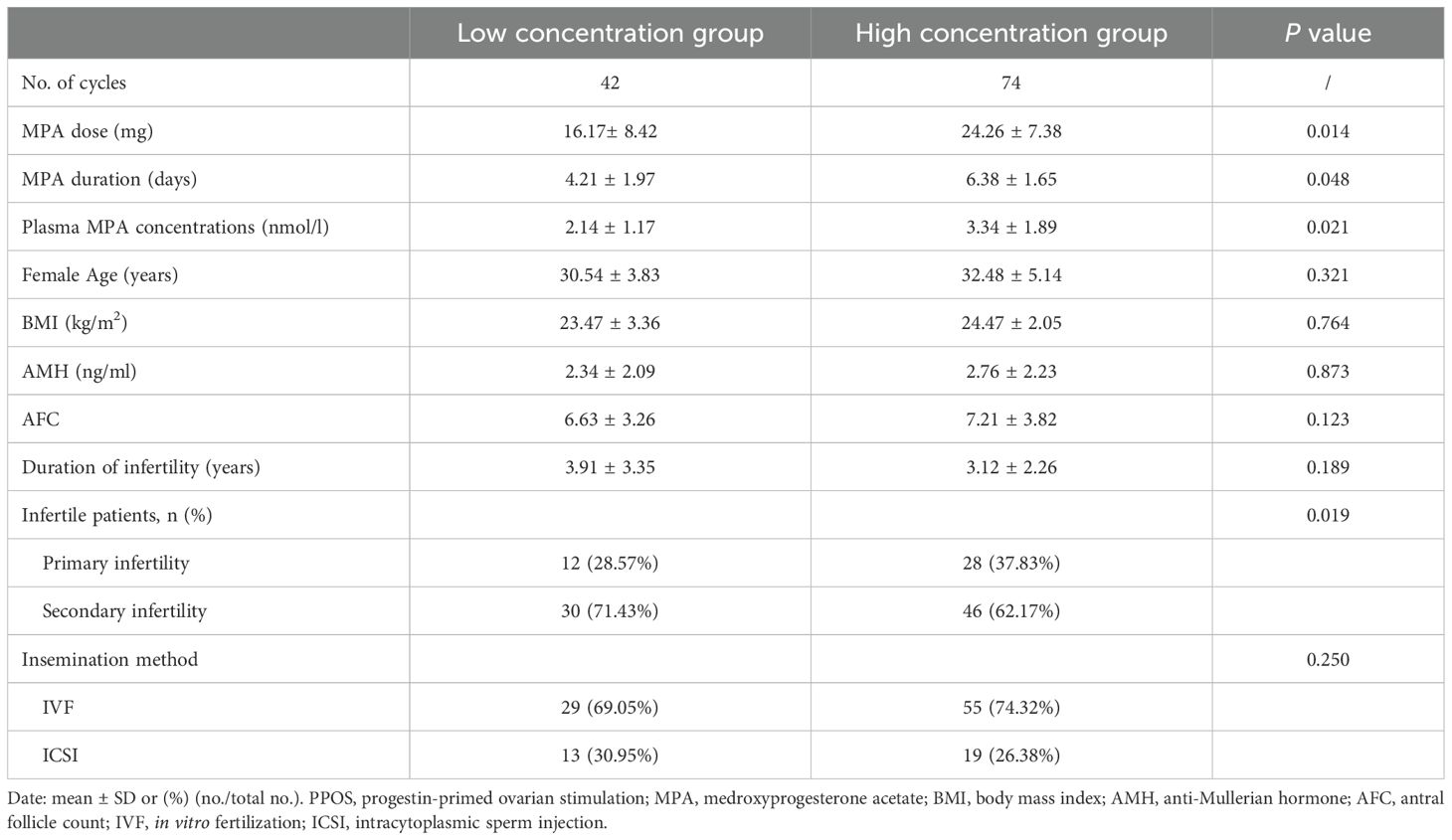
Table 1. Baseline characteristics of women with different serum MPA concentrations on the hCG trigger day in the PPOS protocol.
The ovarian stimulation characteristics of the two groups are summarized in Supplementary Table 2. No statistically significant differences were found between the two groups regarding the total dosage of Gn, duration of Gn, number of oocytes retrieved, number of mature oocytes, fertilization rate, blastocyst progression rate, number of frozen embryos, or moderate/severe OHSS rate (P > 0.05).
Hormone profile data
During ovarian stimulation, there were no statistically significant differences in LH, E2, or P levels between the two cohorts at any time point (P > 0.05) (Supplementary Table 3). Linear regression revealed no correlation between serum MPA concentration and P concentration on the hCG trigger day, with correlation coefficient R2 = 0.00078, P = 0.993 (Figure 2).

Figure 2. Scatterplots and correlations (Pearson correlation coefficients) of women with different serum MPA concentrations and P concentrations on the hCG trigger day. (A) All the serum MPA concentration group. (B) Low serum MPA concentration group. (C) High serum MPA concentration group.
Clinical outcome data
Descriptive statistics for the reproductive outcomes of FET are summarized in Table 2. There were no statistically significant differences between the two groups in terms of endometrial preparation method, number of transferred embryos, embryo transfer stage, CPR, ectopic pregnancy rate, early pregnancy loss rate, or live birth rate (LBR) (P > 0.05).
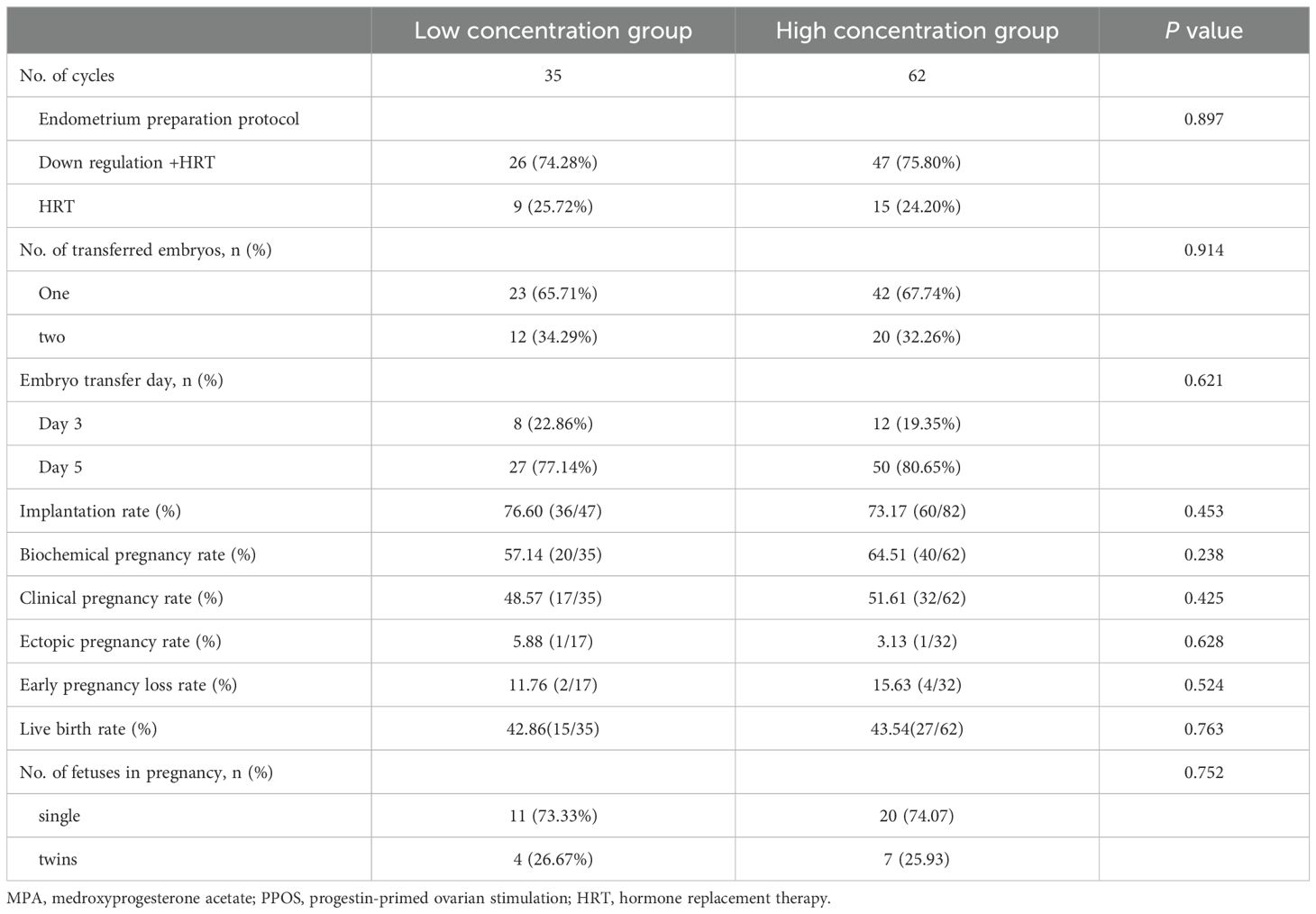
Table 2. Reproductive outcomes of freeze–thaw transplantation cycles in patients with different serum MPA concentrations in the PPOS protocol.
Multiple regression analysis indicated that variations in the total MPA dosage were not significantly related to changes in hormone levels on the hCG trigger day, CPR, or LBR in either the unadjusted or adjusted models.
Discussion
To our knowledge, this is the first report on serum MPA dynamics in patients undergoing the PPOS protocol during IVF/ICSI treatment. We observed a decline in MPA levels during the late follicular stage following a gradual reduction in MPA dosage, with levels becoming undetectable by the fifth day after OPU, coinciding with blastocyst transfer, potentially allowing for fresh ET in the PPOS protocol. Furthermore, both the high- and low-MPA groups presented comparable P levels on the hCG trigger day, suggesting that MPA administration in the PPOS protocol does not affect endometrial receptivity.
MPA has historically been used as a contraceptive agent. As a potent synthetic progestin, it exhibits a distinct metabolism compared with that of P due to structural differences. Previous studies have shown that following oral administration of a single dose of MPA, serum levels peak within 1 to 4 hours and then decline rapidly, with a biological half-life of 40-60 hours (25, 40, 41). In our study, MPA was administered from Day 5 of Gn use until the hCG trigger day, resulting in serum levels of 2.26 ± 2.11 nmol/L on the hCG trigger day, 0.37 ± 0.40 nmol/L on OPU+2 day, and undetectable levels on OPU+5 days. Consistent with the literature, our findings indicate rapid clearance of MPA in women undergoing the PPOS protocol. As an analog of P, MPA may exert similar effects. When serum MPA levels decrease to undetectable levels, the P-like activity diminishes, potentially facilitating fresh ET at the blastocyst stage (OPU+5 days) rather than the cleavage stage (OPU+3 days).
Endometrial receptivity can be assessed through morphological observation or biomarker profiling of endometrial function. In a randomized controlled trial (RCT), MPA induced a significant increase in subnuclear vacuolation, a classical effect of P, at oral doses of 2.5 mg, 5 mg, and 10 mg per day during the mid-proliferative stage over 4 days (42). Another study demonstrated that, unlike P, MPA promoted the differentiation of human monocytes toward an M2 phenotype, resembling decidual macrophages that are crucial for successful pregnancy, via extracellular regulated protein kinase (ERK) phosphorylation in both a human monocyte cell line and primary monocytes (43). Furthermore, a transcriptome and biofunctional study using primary human stromal cell cultures revealed the differential expression of 116 genes with P treatment and 251 genes with MPA treatment compared with the vehicle control (44). Both treatments upregulated genes such as SPARCL1, SLC7A8, OMD, FKBP5, THSD7A, LCP1, GPX3, and IL1R1, while downregulating EVT1, NDNF, LYPD1, GBP4, KRT19, SFRP1, and CD34. Notably, both treatments decreased cell viability. Therefore, further investigations are needed to clarify the impact of MPA administration on endometrial receptivity and elucidate the underlying mechanisms, thereby offering insights into the use of MPA during ovulation induction.
The detrimental effects of premature elevation of P on the hCG trigger day during IVF/ICSI treatment have been extensively documented (45). Hence, attention should be focused on serum P levels potentially altered after progestin administration. A study involving women treated by MPA for threatened abortion in the first trimester reported no difference in urine P levels between treated and untreated women (46). The serum P levels remained consistent with the follicular phase levels during and up to 20 days after treatment with intravaginal administration of a single 100 mg dose for 21 days (25). In poor responders, P levels remained low at the LH surge day in natural cycles but were higher in the minimal stimulation MPA group (26). In a comparison of the serum P levels on the hCG trigger day among patients who underwent IVF/ICSI cycles using 4 mg PPOS or short-term protocols, no significant difference was found between the 4 mg PPOS protocol and the short-term protocol, but the P levels were greater in the 10 mg PPOS protocol (27), although contradictory results have been reported (33). Most studies, including self-controlled studies and RCTs, have consistently shown comparable serum P levels on the hCG trigger day between the PPOS group and traditional protocol groups (17, 28–32, 34–37, 39, 47, 48). Similarly, our study revealed no difference in P concentration on the day of ovulation trigger between the high- and low-MPA groups, as defined by serum MPA levels on the hCG trigger day, suggesting that MPA levels do not impact P secretion.
Evidence regarding the safety of MPA use during the first trimester remains limited. A large population study involving 1,016 women revealed no significant difference in congenital abnormalities between women treated orally with MPA at doses of 80–120 mg per day for at least three months (4.1%, 15/366 infants) and those in an untreated group (3.5%, 15/428 infants) (49). Similar results were observed in a female baboon experiment (50). However, clinical reviews have reported male feminization and female masculinization (51), and experiments on cynomolgus monkeys have revealed female pseudohermaphroditism, male hypospadias, and reduced adrenal gland size (52). Our study used a low dosage of 10 mg MPA, which was significantly lower than that used in previous studies. Thus, we hypothesize that at such low doses, MPA may be safe for early embryonic development without embryotoxic or teratogenic effects.
Our study has several limitations. First, the lack of data on blastocyst euploidy rates may impact the precision of our conclusions. Second, we did not measure corresponding P levels at the timepoints of MPA measurement, limiting our understanding of P dynamics following MPA use. Third, molecular-level results from human endometrial tissues were absent. Fourth, our sample size was relatively limited and no formal power analysis was performed, as this was an exploratory retrospective study. Fifth, the patient cohort was heterogeneous and not stratified by PGT-A or oocyte donor cycles, which may reduce the generalizability of our findings. Lastly, as our study focused solely on MPA, extrapolating these results to other progestins should be done with caution.
Conclusion
This retrospective study suggests that serum MPA levels were undetectable on the fifth day after OPU and that serum P levels were unaffected by MPA levels on the hCG trigger day. While these observations may indicate the potential for fresh blastocyst transfer in the PPOS protocol, the findings should be interpreted cautiously given the retrospective nature, modest sample size, and lack of mechanistic confirmation. Further prospective studies are needed to validate the clinical applicability of these findings and their implications for treatment strategy optimization.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed toeWluZ3poYW5naXZmQGdtYWlsLmNvbQ==.
Ethics statement
The studies involving humans were approved by The study protocol was reviewed and approved by the Ethics Committee of Renmin Hospital, Hubei University of Medicine (No: SYRMYY-065). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XC: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XY: Conceptualization, Data curation, Writing – review & editing. HX: Conceptualization, Writing – review & editing. YH: Conceptualization, Writing – review & editing. SJ: Conceptualization, Writing – review & editing. XW: Conceptualization, Writing – review & editing. HP: Conceptualization, Writing – review & editing. BF: Conceptualization, Writing – review & editing. CZ: Conceptualization, Writing – review & editing. HD: Supervision, Writing – review & editing. YZ: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82101726), the Innovation Group of Natural Science Foundation of Hubei Province (Grant No. 2020CFA021) and the Guiding Project of Science and Technology Research Program of Hubei Provincial Department of Education (Grant No. B2017110).
Acknowledgments
The authors thank these women who taken part in this study and all the staff at the Reproductive Medicine Center, Renmin Hospital, Hubei University of Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1490839/full#supplementary-material
References
1. Giles J, Alama P, Gamiz P, Vidal C, Badia P, Pellicer A, et al. Medroxyprogesterone acetate is a useful alternative to a gonadotropin-releasing hormone antagonist in oocyte donation: a randomized, controlled trial. Fertil Steril. (2021) 116:404–12. doi: 10.1016/j.fertnstert.2021.02.036
2. Beguería R, García D, Vassena R, Rodríguez A. Medroxyprogesterone acetate versus ganirelix in oocyte donation: a randomized controlled trial. Hum Reprod. (2019) 34:872–80. doi: 10.1093/humrep/dez034
3. Huang H, Itaya Y, Samejima K, Ichinose S, Narita T, Matsunaga S, et al. Usefulness of random-start progestin-primed ovarian stimulation for fertility preservation. J Ovarian Res. (2022) 15:2. doi: 10.1186/s13048-021-00935-5
4. Mathieu d’Argent E, Ferrier C, Zacharopoulou C, Ahdad-Yata N, Boudy AS, Cantalloube A, et al. Outcomes of fertility preservation in women with endometriosis: comparison of progestin-primed ovarian stimulation versus antagonist protocols. J Ovarian Res. (2020) 13:18. doi: 10.1186/s13048-020-00620-z
5. Zhu J, Zhang J, Yang J, Li D, Wang C, Elizur SE, et al. A comprehensive evaluation of progestin-primed ovarian stimulation protocol in patients with or without PCOS undergoing in vitro fertilization. Reprod Biol. (2021) 21:100540. doi: 10.1016/j.repbio.2021.100540
6. Ata B, Capuzzo M, Turkgeldi E, Yildiz S, La Marca A. Progestins for pituitary suppression during ovarian stimulation for ART: a comprehensive and systematic review including meta-analyses. Hum Reprod Update. (2021) 27:48–66. doi: 10.1093/humupd/dmaa040
7. Nie Y, Guo W, Shen X, Xie Y, Zeng Y, Gao H, et al. The cumulative live birth rates of 18 593 women with progestin-primed ovarian stimulation-related protocols and frozen-thawed transfer cycles. Hum Reprod Open. (2024) 2024:hoad051. doi: 10.1093/hropen/hoad051
8. Lin G, Zhong X, Li S, Liu X, Xu L. The clinical value of progestin-primed ovarian stimulation protocol for women with diminished ovarian reserve undergoing IVF/ICSI: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1232935. doi: 10.3389/fendo.2023.1232935
9. Ye H, Shi L, Quan X, Hou M, Ma H, Xue S, et al. Cumulative live birth rate of in vitro fertilization cycle via progestin-primed ovarian stimulation versus gonadotropin-releasing hormone antagonist protocol in infertile women with normal ovarian reserve: an open-label, randomized controlled trial. Hum Fertil (Camb). (2024) 27:2316005. doi: 10.1080/14647273.2024.2316005
10. Welp AM, Williams CD, Smith LP, Purcell S, Goodman LR. Oral medroxyprogesterone acetate for the use of ovulation suppression in in vitro fertilization: a cohort trial. Fertil Steril. (2024) 121:806–13. doi: 10.1016/j.fertnstert.2024.01.026
11. Ata B, Kalafat E. Progestin-primed ovarian stimulation: for whom, when and how? Reprod BioMed Online. (2024) 48:103639. doi: 10.1016/j.rbmo.2023.103639
12. Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. (2017) 23:211–20. doi: 10.1093/humupd/dmw047
13. National Toxicology P. NTP Research Reports. In: NTP Research Report on the Scoping Review of Prenatal Exposure to Progestogens and Adverse Health Outcomes: Research Report 17. National Toxicology Program, Research Triangle Park (NC (2020).
14. Vidal MDM, Martínez F, Rodríguez I, Polyzos NP. Ovarian response and embryo ploidy following oral micronized progesterone-primed ovarian stimulation versus GnRH antagonist protocol. A prospective study with repeated ovarian stimulation cycles. Hum Reprod. (2024) 39:1098–104. doi: 10.1093/humrep/deae047
15. Chen ZQ, Zhang Y, Li H, Wang JY, Wang L, Ai A, et al. A randomized controlled trial to compare the live birth rate of the first frozen embryo transfer following the progestin-primed ovarian stimulation protocol vs. the antagonist protocol in women with an anticipated high ovarian response. Fertil Steril. (2024) 121:937–45. doi: 10.1016/j.fertnstert.2024.01.027
16. Li H, Yu M, Zhang W, Chen J, Chen H, Lu X, et al. Comparing blastocyst euploid rates between the progestin-primed and gonadotrophin-releasing hormone antagonist protocols in aneuploidy genetic testing: a randomised trial protocol. BMJ Open. (2024) 14:e079208. doi: 10.1136/bmjopen-2023-079208
17. Giles J, Cruz M, Cobo A, Vidal C, Requena A, Remohi J, et al. Medroxyprogesterone acetate: an alternative to GnRH-antagonist in oocyte vitrification for social fertility preservation and preimplantation genetic testing for aneuploidy. Reprod BioMed Online. (2023) 47:103222. doi: 10.1016/j.rbmo.2023.04.013
18. La Marca A, Capuzzo M, Sacchi S, Imbrogno MG, Spinella F, Varricchio MT, et al. Comparison of euploidy rates of blastocysts in women treated with progestins or GnRH antagonist to prevent the luteinizing hormone surge during ovarian stimulation. Hum Reprod. (2020) 35:1325–31. doi: 10.1093/humrep/deaa068
19. Wang N, Lin J, Zhu Q, Fan Y, Wang Y, Fu Y, et al. Comparison of neonatal outcomes and live-birth defects after progestin-primed ovarian stimulation versus conventional ovarian stimulation for in vitro fertilization: A large retrospective cohort study. Med (Baltimore). (2018) 97:e11906. doi: 10.1097/MD.0000000000011906
20. Zolfaroli I, Ferriol GA, Mora JH, Cano A. Impact of progestin ovarian stimulation on newborn outcomes: a meta-analysis. J Assist Reprod Genet. (2020) 37:1203–12. doi: 10.1007/s10815-020-01755-0
21. Liang Z, Wang Y, Kuang Y. Live-birth outcomes and congenital malformations after progestin-primed ovarian stimulation in maternal endometriosis. Drug Des Devel Ther. (2020) 14:5459–67. doi: 10.2147/DDDT.S263138
22. Zhang J, Mao X, Wang Y, Chen Q, Lu X, Hong Q, et al. Neonatal outcomes and congenital malformations in children born after human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Arch Gynecol Obstet. (2017) 296:1207–17. doi: 10.1007/s00404-017-4537-z
23. Du M, Zhang J, Ren B, Guan Y. Comparison of the neonatal outcomes of progestin-primed ovarian stimulation and flexible GnRH antagonist protocols: a propensity score-matched cohort study. Front Endocrinol (Lausanne). (2023) 14:1156620. doi: 10.3389/fendo.2023.1156620
24. Chai W, Liao M, Feng G, Wei M, Shi W, Wang Y, et al. Comparable pregnancy loss and neonatal birthweights in frozen embryo transfer cycles using vitrified embryos from progestin-primed ovarian stimulation and gnRH analogue protocols: A retrospective cohort study. J Clin Med. (2022) 11:6151. doi: 10.3390/jcm11206151
25. Victor A, Johansson ED. Pharmacokinetic observations on medroxyprogesterone acetate administered orally and intravaginally. Contraception. (1976) 14:319–29. doi: 10.1016/0010-7824(76)90099-8
26. Chen Q, Wang Y, Sun L, Zhang S, Chai W, Hong Q, et al. Controlled ovulation of the dominant follicle using progestin in minimal stimulation in poor responders. Reprod Biol Endocrinol. (2017) 15:71. doi: 10.1186/s12958-017-0291-0
27. Wen X, Kuang Y, Zhou L, Yu B, Chen Q, Fu Y, et al. Lipidomic components alterations of human follicular fluid reveal the relevance of improving clinical outcomes in women using progestin-primed ovarian stimulation compared to short-term protocol. Med Sci Monit. (2018) 24:3357–65. doi: 10.12659/MSM.906602
28. Xi Q, Tao Y, Qiu M, Wang Y, Kuang Y. Comparison between PPOS and gnRHa-long protocol in clinical outcome with the first IVF/ICSI cycle: A randomized clinical trial. Clin Epidemiol. (2020) 12:261–72. doi: 10.2147/CLEP.S226414
29. Chen Q, Chai W, Wang Y, Cai R, Zhang S, Lu X, et al. Progestin vs. Gonadotropin-Releasing Hormone Antagonist for the Prevention of Premature Luteinizing Hormone Surges in Poor Responders Undergoing in vitro Fertilization Treatment: A Randomized Controlled Trial. Front Endocrinol (Lausanne). (2019) 10:796. doi: 10.3389/fendo.2019.00796
30. Chen YM, Qi QR, Xie QZ, Yang YF, Xia Y, Zhou XD. Effect of progestin-primed ovarian stimulation protocol on outcomes of aged infertile women who failed to get pregnant in the first IVF/ICSI cycle: A self-controlled study. Curr Med Sci. (2018) 38:513–8. doi: 10.1007/s11596-018-1908-z
31. Dong M, Sun L, Huang L, Wang F, Zhang X, Liu F. Fixed gonadotropin-releasing hormone antagonist protocol versus flexible progestin-primed ovarian stimulation protocol in patients with asynchronous follicular development during controlled ovulation stimulation: A retrospective study. Front Endocrinol (Lausanne). (2021) 12:690575. doi: 10.3389/fendo.2021.690575
32. Cui L, Lin Y, Wang F, Chen C. Effectiveness of progesterone-primed ovarian stimulation in assisted reproductive technology: a systematic review and meta-analysis. Arch Gynecol Obstet. (2021) 303:615–30. doi: 10.1007/s00404-020-05939-y
33. Dong J, Wang Y, Chai WR, Hong QQ, Wang NL, Sun LH, et al. The pregnancy outcome of progestin-primed ovarian stimulation using 4 versus 10 mg of medroxyprogesterone acetate per day in infertile women undergoing in vitro fertilisation: a randomised controlled trial. BJOG. (2017) 124:1048–55. doi: 10.1111/bjo.2017.124.issue-7
34. Wang Y, Chen Q, Wang N, Chen H, Lyu Q, Kuang Y. Controlled ovarian stimulation using medroxyprogesterone acetate and hMG in patients with polycystic ovary syndrome treated for IVF: A double-blind randomized crossover clinical trial. Med (Baltimore). (2016) 95:e2939. doi: 10.1097/MD.0000000000002939
35. Zhang S, Yin Y, Li Q, Zhang C. Comparison of cumulative live birth rates between gnRH-A and PPOS in low-prognosis patients according to POSEIDON criteria: A cohort study. Front Endocrinol (Lausanne). (2021) 12:644456. doi: 10.3389/fendo.2021.644456
36. Guo H, Gao H, Li J, Cong Y, Chen Q, Wang Y, et al. Impacts of medroxyprogesterone acetate on oocytes and embryos: matched case-control study in women with stage III-IV ovarian endometriosis undergoing controlled ovarian hyperstimulation for in vitro fertilization. Ann Transl Med. (2020) 8:377. doi: 10.21037/atm.2020.02.15
37. Ye H, Tian H, He W, Lyu Q, Kuang Y, Chen Q, et al. Progestin-primed milder stimulation with clomiphene citrate yields fewer oocytes and suboptimal pregnancy outcomes compared with the standard progestin-primed ovarian stimulation in infertile women with polycystic ovarian syndrome. Reprod Biol Endocrinol. (2018) 16:53. doi: 10.1186/s12958-018-0373-7
38. Yu S, Long H, Chang HY, Liu Y, Gao H, Zhu J, et al. New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum Reprod. (2018) 33:229–37. doi: 10.1093/humrep/dex367
39. Zhang Y, Li H, Zhu S, Jiang S, Zhao W, Wang X, et al. The comparison between fixed versus degressive doses of medroxyprogesterone acetate combined with letrozole in patients of progestin-primed ovarian stimulation protocol: a propensity score-matched study. Front Endocrinol (Lausanne). (2023) 14:1295787. doi: 10.3389/fendo.2023.1295787
40. Hiroi M, Stanczyk FZ, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR Jr. Radioimmunoassay of serum medroxyprogesterone acetate (Provera) in women following oral and intravaginal administration. Steroids. (1975) 26:373–86. doi: 10.1016/0039-128X(75)90082-3
41. Johansson ED, Johansen PB, Rasmussen SN. Medroxyprogesterone acetate pharmacokinetics following oral high-dose administration in humans: a bioavailability evaluation of a new MPA tablet formulation. Acta Pharmacol Toxicol (Copenh). (1986) 58:311–7. doi: 10.1111/j.1600-0773.1986.tb00115.x
42. Zalányi S Jr., Aedo AR, Johannisson E, Landgren BM, Diczfalusy E. Pituitary, ovarian and endometrial effects of graded doses of medroxyprogesterone acetate administered on cycle days 7 to 10. Contraception. (1986) 33:567–78. doi: 10.1016/0010-7824(86)90045-4
43. Tsai YC, Tseng JT, Wang CY, Su MT, Huang JY, Kuo PL. Medroxyprogesterone acetate drives M2 macrophage differentiation toward a phenotype of decidual macrophage. Mol Cell Endocrinol. (2017) 452:74–83. doi: 10.1016/j.mce.2017.05.015
44. Houshdaran S, Chen JC, Vallvé-Juanico J, Balayan S, Vo KC, Smith-McCune K, et al. Progestins related to progesterone and testosterone elicit divergent human endometrial transcriptomes and biofunctions. Int J Mol Sci. (2020) 21:2625. doi: 10.3390/ijms21072625
45. Xu J, Zhang C, Wang S, Zhang S. Impact of progesterone concentration on human chorionic gonadotropin trigger day on clinical outcomes with one top-quality cleavage-stage embryo or blastocyst transfer in fresh in vitro fertilization cycles. Front Endocrinol (Lausanne). (2023) 14:1085287. doi: 10.3389/fendo.2023.1085287
46. Yovich JL, Willcox DL, Wilkinson SP, Poletti VM, Hähnel R. Medroxyprogesterone acetate does not perturb the profile of steroid metabolites in urine during pregnancy. J Endocrinol. (1985) 104:453–9. doi: 10.1677/joe.0.1040453
47. Wang L, Yin M, Liu Y, Chen Q, Wang Y, Ai A, et al. Effect of Frozen Embryo Transfer and Progestin-primed Ovary Stimulation on IVF outcomes in women with high body mass index. Sci Rep. (2017) 7:7447. doi: 10.1038/s41598-017-07773-w
48. Huang TC, Huang MZ, Seow KM, Yang IJ, Pan SP, Chen MJ, et al. Progestin primed ovarian stimulation using corifollitropin alfa in PCOS women effectively prevents LH surge and reduces injection burden compared to GnRH antagonist protocol. Sci Rep. (2021) 11:22732. doi: 10.1038/s41598-021-02227-w
49. Yovich JL, Turner SR, Draper R. Medroxyprogesterone acetate therapy in early pregnancy has no apparent fetal effects. Teratology. (1988) 38:135–44. doi: 10.1002/tera.1420380206
50. Tarara R. The effect of medroxyprogesterone acetate (Depo-Provera) on prenatal development in the baboon (Papio anubis): a preliminary study. Teratology. (1984) 30:181–5. doi: 10.1002/tera.1420300205
51. Schardein JL. Congenital abnormalities and hormones during pregnancy: a clinical review. Teratology. (1980) 22:251–70. doi: 10.1002/tera.1420220302
Keywords: progestin-primed ovarian stimulation, medroxyprogesterone acetate, progesterone level, MPA concentrations, pregnancy outcome
Citation: Chen X, Yan X, Xu H, Hu Y, Jiang S, Wang X, Peng H, Feng B, Zhang C, Diao H and Zhang Y (2025) Serum concentrations of medroxyprogesterone acetate were undetectable on OPU+5 days and had no effect on the serum progesterone level in patients undergoing the progestin-primed ovarian stimulation protocol. Front. Endocrinol. 16:1490839. doi: 10.3389/fendo.2025.1490839
Received: 03 September 2024; Accepted: 24 April 2025;
Published: 14 May 2025.
Edited by:
Gedis Grudzinskas, Independent Researcher, London, United KingdomReviewed by:
Robert Najdecki, Assisting Nature IVF Clinic, GreeceDongmei Tian, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2025 Chen, Yan, Xu, Hu, Jiang, Wang, Peng, Feng, Zhang, Diao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhang, eWluZ3poYW5naXZmQGdtYWlsLmNvbQ==; Honglu Diao, aGxkaWFvMTk3NkBob3RtYWlsLmNvbQ==; Changjun Zhang, Y2hhbmdqdW56aGFuZ0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Xin Chen
Xin Chen Xu Yan1,2,3,4,5†
Xu Yan1,2,3,4,5† Xiaoning Wang
Xiaoning Wang Changjun Zhang
Changjun Zhang Ying Zhang
Ying Zhang