- 1Department of Clinical Medicine and Surgery, Federico II University, Napoli, Italy
- 2Service d’Endocrinologie et Diabétologie Pédiatrique, Assistance Publique-Hôpitaux de Paris, Hôpital Robert-Debré, Paris, France
- 3Advanced Biomedical Sciences Department, University Federico II of Naples, Naples, Italy
- 4Department of Neuroscience, Reproductive Sciences and Dentistry, Federico II University, Naples, Italy
- 5Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy
- 6Department of Translational Medical Sciences, Federico II University of Naples, Naples, Italy
- 7Department of Public Health, Federico II University, Naples, Italy
Introduction: Postmenopausal hyperandrogenism (PH) is a rare clinical condition caused by relative or absolute androgen excess after menopause. Tumorous or non-tumorous ovarian diseases can cause PH.
Methods: In this two-section hybrid study, the first section describes the case of a patient with PH caused by an ovarian disease and surgically treated. The second section shows the results of a scoping review with individual patient data (IPD) analysis, which was performed to define the biochemical and clinical features of PH patients with tumorous or non-tumorous ovarian diseases surgically treated. All PH cases caused by anything but ovarian disease and/or without surgical indication and/or without histological diagnosis were excluded.
Results: Due to imaging suspicion, our PH patient underwent robotic hysterectomy with bilateral ovariectomy. A Leydig cell tumor stage 1A was diagnosed. At 6 months after surgery, the PH was resolved. Overall, the IPD analysis included 280 PH patients with ovarian diseases (oPH) surgically treated. Among them, histological examination showed 174 tumorous oPH and 106 non-tumorous oPH. Patients with tumorous oPH showed lower body mass index and lower levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as higher levels of testosterone, dehydroepiandrosterone sulfate (DHEA-S), 17-OH progesterone, and estradiol compared with non-tumorous oPH patients. We defined the levels of testosterone (≥9.8 nmol/L), LH (≤15 mUI/ml), FSH (≤35 mUI/ml), and DHEA-S (≥1.6 μmol/L) able to differentiate between tumorous and non-tumorous oPH patients with suitable sensitivity (≥68.6%) and specificity (≥72.7%). No PH recurrence was described after surgery.
Discussion: The study results provide useful biochemical parameters to support the diagnosis of ovarian tumor in patients with oPH.
1 Introduction
Postmenopausal hyperandrogenism (PH) is a rare clinical condition caused by relative or absolute androgen serum excess after menopause from an adrenal or an ovarian source (1). It is further amplified by a decrease in the sex hormone-binding globulin (SHBG) levels, which increases the free androgen index (2). PH clinically appears with symptoms and signs of virilization. According to the criteria proposed by Markopoulos and colleagues, there are several causes of PH so that it can be generally categorized into non-tumorous or tumorous (3). Non-tumorous PH includes polycystic ovary syndrome, congenital adrenal hyperplasia, ovarian hyperthecosis, states of insulin resistance including obesity, endocrinopathies such as Cushing’s syndrome and acromegaly, and, finally, the use of certain drugs such as testosterone and its analogues, valproic acid, and oxcarbazepine. On the other hand, tumorous PH includes adrenal or ovarian neoplasms (3). As PH can be the sole manifestation of several conditions and due to the lack of standardized diagnostic procedures, a two-section hybrid study was performed. In the first section, we report on the case of a patient with PH caused by an ovarian disease and who was surgically treated. In the second section, a scoping review with individual patient data (IPD) analysis collected and analyzed all PH cases of ovarian source (oPH) requiring surgery and with histological diagnosis to better define the clinical characteristics of the patients with oPH described so far. To our best knowledge, these data are not available in the international literature yet.
2 Materials and methods
2.1 Case report
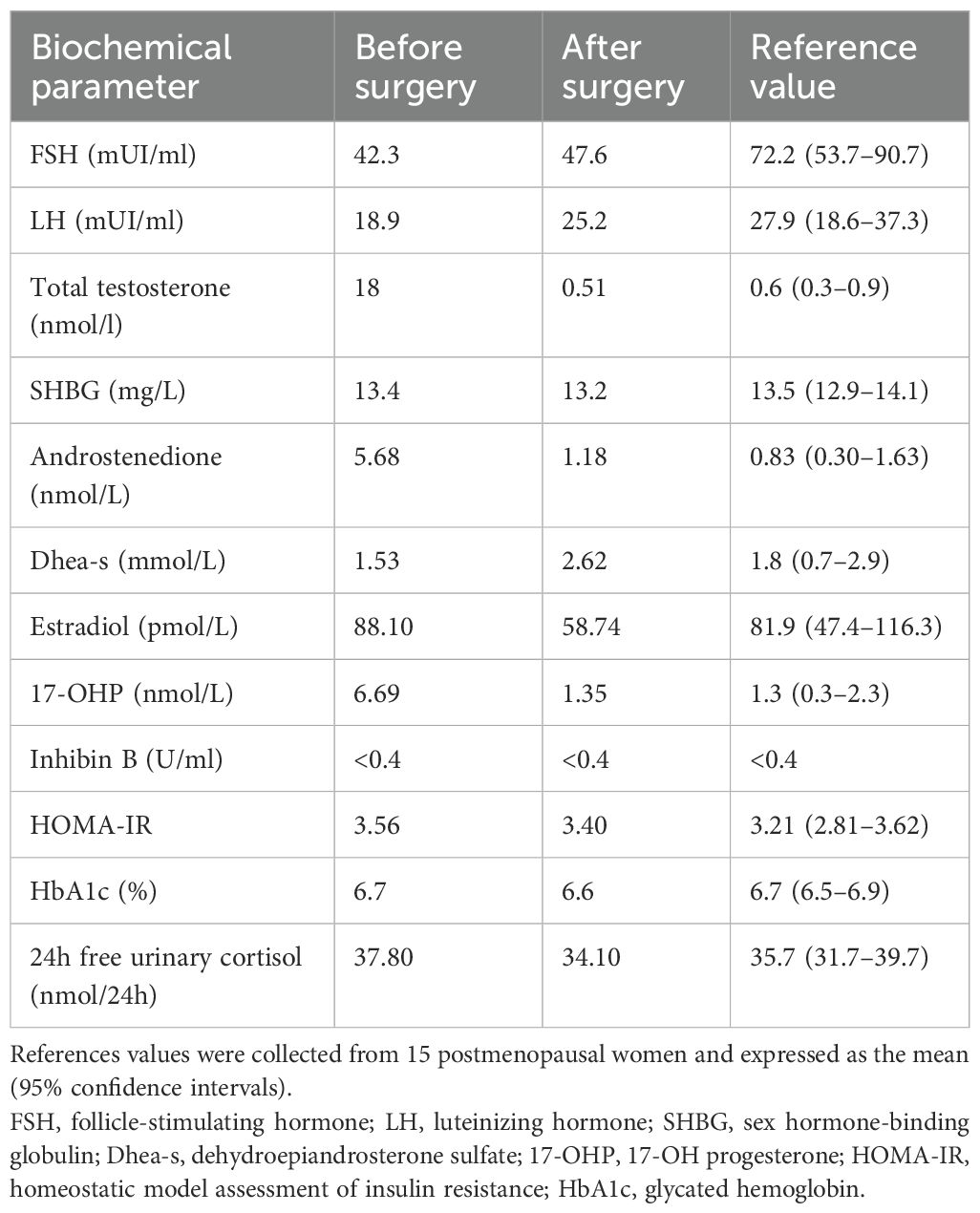
Considering that some clinical characteristics and parameters, such as body mass index (BMI), ethnicity (4, 5), and homeostatic model assessment of insulin resistance (HOMA-IR), can influence the serum levels of sexual and pituitary hormones, we enrolled a control group (controls) according to the following criteria. Controls were chosen from among postmenopausal women consecutively referred to the Department of Clinical Medicine and Surgery of Federico II University, from January 1, 2024, to March 31, 2024. The inclusion criteria for the controls were: age ranging from 55 to 65 years, BMI ranging from 28.0 to 32.0 kg/m2, and personal history of type 2 diabetes mellitus (T2DM). All participants provided informed written consent for participation in the study. The serum levels of estradiol (E), follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T), androstenedione (A), and dehydroepiandrosterone sulfate (DHEA-S) were measured with the Advia Centaur XT Immunoassay System Analyzer (Siemens, Munich, Germany), which uses competitive (for E) or direct (for T, A, DHEA-S, FSH, and LH) immunoassay. The chemiluminescent acridinium ester technology was used for reaction quantification. SHBG and 17-OH progesterone (17-OHP) were measured using the IDS iSYS analyzer, which uses direct immunoassay. The chemiluminescent acridinium ester technology was used for reaction quantification. The serum concentration of inhibin B (Inb) was measured using competitive chemiluminescence immunoassay with the YLO IFlash analyzer. The intra- and inter-assay coefficients of variation were 2.4% and 1.4% for FSH, 2.7% and 2.3% for LH, 4.3% and 6.6% for T, 3.0% and 4.6% for SHBG, 3.2% and 4.3% for A, 5.5% and 6.9% for DHEA-S, 4.2% and 1.9% for E, 2.4% and 6.2% for 17-OHP, 5.1% and 4.6% for Inb, respectively.
2.2 Scoping review with IPD analysis
2.2.1 Data sources and search strategy
The scoping review was planned, conducted, and reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (6). A scoping search of the medical literature was performed in Medline, Google Scholar, Google Book, and Cochrane Library (last conducted search February 25, 2024) using the following term: “Postmenopausal Hyperandrogenism.” There were no language restrictions. The reference lists of all identified articles were searched for further relevant publications.
2.2.2 Study selection
Eligible studies were case reports, cases series, and review articles. The review articles were selected for this study to evaluate the state-of-the-art and to find additional articles that could not be found initially by checking the references. Because some individual case reports were included as part of a subsequent case series by the same authors, duplicates were excluded. The predetermined inclusion criteria were: patients with oPH requiring surgery due to severe hyperandrogenism or because of ovarian imaging suspicion. The exclusion criteria were: women with known cause of ovarian hyperandrogenism not subject to surgical treatment, for instance polycystic ovary syndrome, premenopausal hyperandrogenism, hyperandrogenism with any adrenal cause, and/or absence of a histological diagnosis.
2.2.3 Data extraction
The titles and abstracts (when available) of the studies retrieved using the described search strategy were screened independently by two review team members (AF and CDL) to identify studies that potentially meet the inclusion criteria. The full texts of potentially eligible studies were retrieved and independently assessed for eligibility by three review team members (DR, RP, and CDA). Selected studies in languages other than English, French, and Italian (i.e., Chinese, Spanish, Portuguese, German, and Japanese) were translated into English or Italian by a specialist translator. Any disagreement over the eligibility of the studies was resolved through discussion between all review team members. A standardized, pre-piloted form was used to extract relevant clinical data from the included studies. The extracted data included: age at PH diagnosis; BMI; ethnicity (4, 5); age of menarche; pregnancy; age of menopause; occurrence of arterial hypertension (AH) and T2DM; levels of T, DHEA-S, 17-OHP and A, FSH, LH, E, SHBG, HOMA-IR, and glycated haemoglobin (HbA1c); the Ferriman–Galway score; occurrence of androgenic alopecia; occurrence of clitoridomegaly; histological diagnosis; follow-up time; and recurrence. Two review team members (AF and CDL) extracted the data independently, and discrepancies were identified and resolved through discussion with DR and RP. When available, missing data were obtained on request from the study authors by mail. The number of patients with data available for the scoping review is reported in Table 1. Critical appraisal of the case reports and case series included in the scoping review is reported in Supplementary Tables S1 and S2, respectively (7, 8).
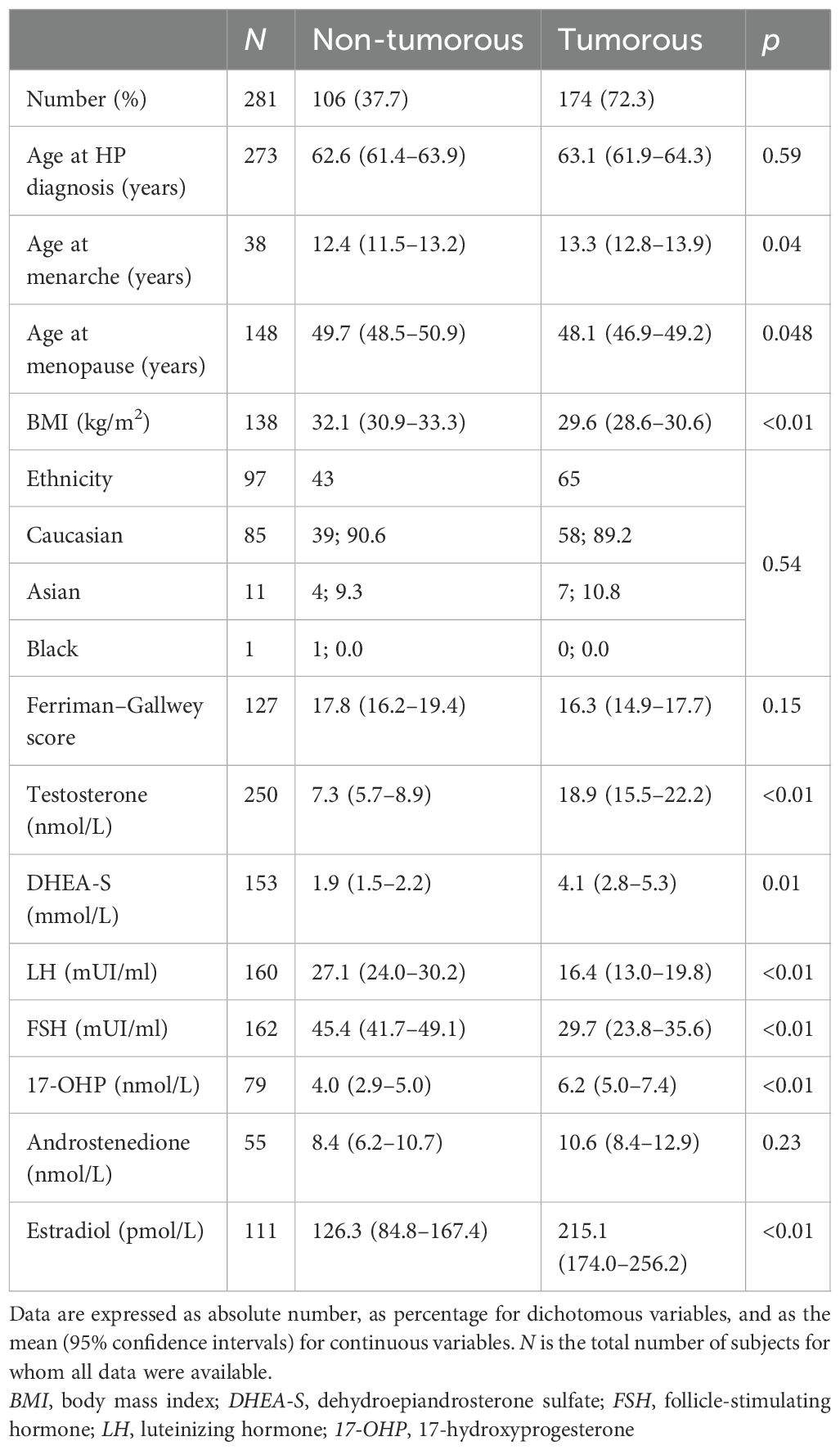
Table 1. Clinical and biochemical parameters of patients with ovarian postmenopausal hyperandrogenism surgically treated and classified into tumorous and non-tumorous according to histological diagnosis.
2.3 Statistical analysis
All statistical analyses were performed by LDE using SPSS (Statistical Package for Social Science), version 29 (International Business Machines Corporation, Armonk, NY, USA). Data from the studies selected for the IPD analysis were extracted and included into a single database. These data were then reanalyzed and combined. Continuous variables were compared using analysis of variance for normally distributed data or the Mann–Whitney test for skewed data. The χ2 test was used to evaluate differences between categorical variables or proportions. Receiver operating characteristic (ROC) analysis was carried out, and the area under the curve (AUC), with its 95% confidence interval (95%CI), was calculated to evaluate the ability of single clinical characteristics to identify patients with tumorous oPH. Subsequently, the optimal cutoff point of the association was identified using ROC analysis. The ROC analysis was performed for each variable with more than 50 values collected. The risk of specific outcomes was estimated using binary logistic regression analysis and expressed as odds ratio (OR) (95% CI). Moreover, the sensitivity and specificity of the tests were calculated with the following formulas: sensitivity = number of true positives/number of true positives + number of false negatives; specificity = number of true negatives/number of true negatives + number of false positives. Data were expressed as absolute numbers, percentages, or the 50th percentile (25th–75th percentile), as appropriate. A p-value <0.05 was considered as statistically significant.
3 Results
3.1 Case report
A postmenopausal 60-year-old woman was referred to the Department of Clinical Medicine and Surgery of the Federico II University in Naples, Italy, for abnormal facial and body hair growth for the past 5 years. She started menstruating at 13 and ended at 50, with regular periods in between. She delivered two male babies through normal vaginal birth. She reported a personal history of T2DM and AH. The physical examination showed androgenic alopecia (second stage according to the Ludwig scale) (9), severe hirsutism (25/36 in the modified Ferriman–Gallwey score) (10), clitoridomegaly (2.5 cm × 3.5 cm) (11), and increased muscle mass. Her BMI was 31.0 kg/m2. In Table 2, we report the biochemical parameters measured in our PH patient according to the criteria proposed by Hirschberg (12). To obtain reliable reference values, the biochemical parameters of our PH patient were compared to the same measured in 15 controls recruited according to the criteria previously mentioned. As shown in Table 2, the circulating levels of androgens, i.e., T, DHEA-S, 17-OHP, and A, measured in our PH patient were higher than the ranges measured in the controls. On the other hand, the FSH and LH levels were lower than the ranges measured in the controls. Serum E, SHBG, HbA1c, and HOMA-IR were withinthe ranges measured in the controls. An adrenocorticotropic hormone (ACTH) 250-µg stimulation test and a dexamethasone 1-mg suppression test were performed according to published criteria, and both tests showed physiological responses (13, 14). A complete blood count (CBC) revealed erythrocytosis, according to the criteria proposed by Babakhanlou et al. (15). Based on the patient’s cardiovascular risk estimated according to the Cardiovascular Risk Chart proposed by the Italian National Institute of Health (16, 17), antiplatelet treatment was prescribed. Pelvic transabdominal ultrasound examination was normal, whereas the transvaginal ultrasonography (TVU) showed the right ovary increasing by size according to age. Pelvic computed tomography (CT) and magnetic resonance (MR) confirmed the increased size of the right ovary. Based on the biochemical and instrumental data, the patient underwent robotic hysterectomy with bilateral ovariectomy (18). Macroscopically, the right ovary was larger than the left (right ovary, 30 mm × 20 mm; left ovary, 20 mm × 10 mm) (Figure 1A). Histological examination showed a Leydig cell tumor (Figures 1B–E) (19). The patient was classified as stage 1A according to the International Federation of Gynecology and Obstetrics (FIGO) criteria (19). As reported in Table 2, 1 week after surgery, all of the biochemical parameters measured in our PH patient returned to normal values, as compared with the controls. The erythrocytosis resolved in 8 weeks, and antiplatelet therapy was dismissed. After 6 months, clinical examination of the patients no longer showed clinical signs of hyperandrogenism.
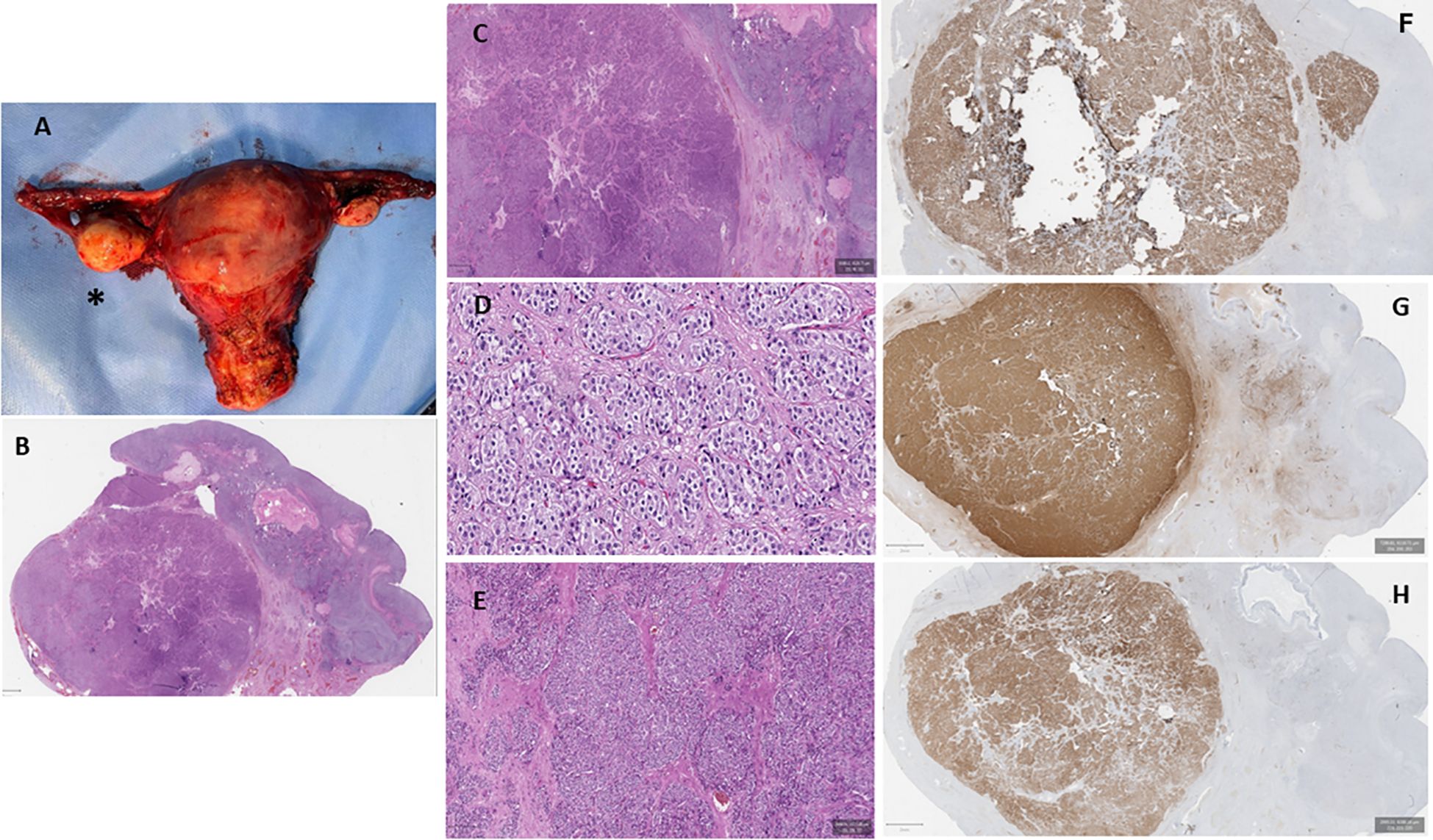
Figure 1. Macroscopic and microscopic appearance of the patient’s internal genitalia. (A) Macroscopic appearance of the uterus, the fallopian tubes, and the ovaries. The asterisk identifies the right ovary, which appears significantly increased compared with the left ovary. (B–E) Ovarian histological examination revealing a well-circumscribed nodular solid tumor composed of groups of large polyhedral, eosinophilic cells with round nuclei and prominent nucleoli [hematoxylin and eosin (H&E) stain: ×2 (B) and ×5 (C)]. Conspicuous fibrous stroma was present [H&E: ×10 (D) and ×20 (E)]. (F–H) Immunohistochemical evaluation showed strong positivity for melanoma antigen recognized by T1 cells (MART1) (F), calretinin (G), and inhibin B (H).
3.2 Scoping review
As reported in Figure 2, the search strategy identified 1,060 studies after removal of duplicates. After the exclusion of the studies that did not meet the inclusion criteria, 151 studies were finally included in the qualitative and quantitative syntheses. There were 123 case reports and 28 case series included in the final analysis. The complete list of studies included in the scoping review with IPD analysis is shown in Supplementary Tables S1 and S2 (7, 8).
Overall, 280 patients with oPH were included in the IPD analysis. According to the criteria proposed by Markopoulos et al. (3), 280 patients with oPH were dichotomized according to the histological diagnosis: 174 tumorous oPH and 106 non-tumorous oPH patients (Table 1).
According to the World Health Organization criteria (20), among the patients with tumorous oPH, 127 (72.9%) [mean age = 62.8 (61.4–64.2) years, mean BMI = 29.3 (28.2–30.9) kg/m2] were affected by pure stromal tumors, 14 (8%) [mean age = 62.9 (56.5–69.3) years, mean BMI = 31.1 (24.0–38.1) kg/m2] by epithelial tumors, 11 (6.3%) [mean age = 63.0 (56.4–69.5) years; BMI = 26.0 kg/m2] affected by mixed sex cord stromal tumors, 8 (4.6%) [mean age = 64.2 (50.6–77.7) years; mean BMI = 30.3 (24.0–36.5) kg/m2] by pure sex cord tumors, 3 (1.7%) [mean age = 70 (55–78) years] by germ cell tumors, and 11 (6.3%) [mean age = 67.0 (53.6–80.4) years] affected by not specified virilizing ovarian tumor (VOT). Among the patients with non-tumorous oPH, 94 (88.7%) [mean age = 62.4 (61.1–63.8) years, mean BMI = 32.2 (30.9–33.5) kg/m2] were affected by ovarian hyperthecosis and the remaining 12 (11.3%) [mean age = 63.3 (59.1–67.1) years, mean BMI = 30.4 (25.1–35.7) kg/m2] by ovarian Leydig cell hyperplasia. Follow-up information was available for all oPH patients. The follow-up period after surgery was 3 (1–24) months. Data analyses indicated that no patient showed PH recurrence after surgery, i.e., curative in all cases.
3.2.1 Biochemical characteristics of oPH patients
As shown in Table 1, patients with tumorous oPH showed lower BMI compared with those with non-tumorous oPH. Regarding the biochemical parameters, patients with tumorous oPH showed lower levels of LH and FSH and higher levels of T, DHEA-S, 17-OHP, and E compared with non-tumorous oPH patients (p < 0.05 in all cases) (Table 1). According to previously published criteria, ROC analysis was performed for T, LH, FSH, and DHEA-S. As shown in Figures 3A–D, the AUCs of T, LH, FSH, and DHEA-S were 0.88 (0.76–0.86), 0.78 (0.70–0.84), 0.76 (0.69–0.82), and 0.66 (0.58–0.74), respectively. Consecutively, we suggest the following cutoff points for the identification of tumorous oPH: T ≥ 9.8 nmol/L (sensitivity = 71.3%, specificity = 89.2%), LH ≤ 15 mUI/ml (sensitivity = 68.6%, specificity = 93.2%), FSH ≤ 35 mUI/ml (sensitivity = 73.9%, specificity = 82.4%), and DHEA-S ≥ 1.6 μmol/L (sensitivity = 70.5%, specificity = 72.7%).
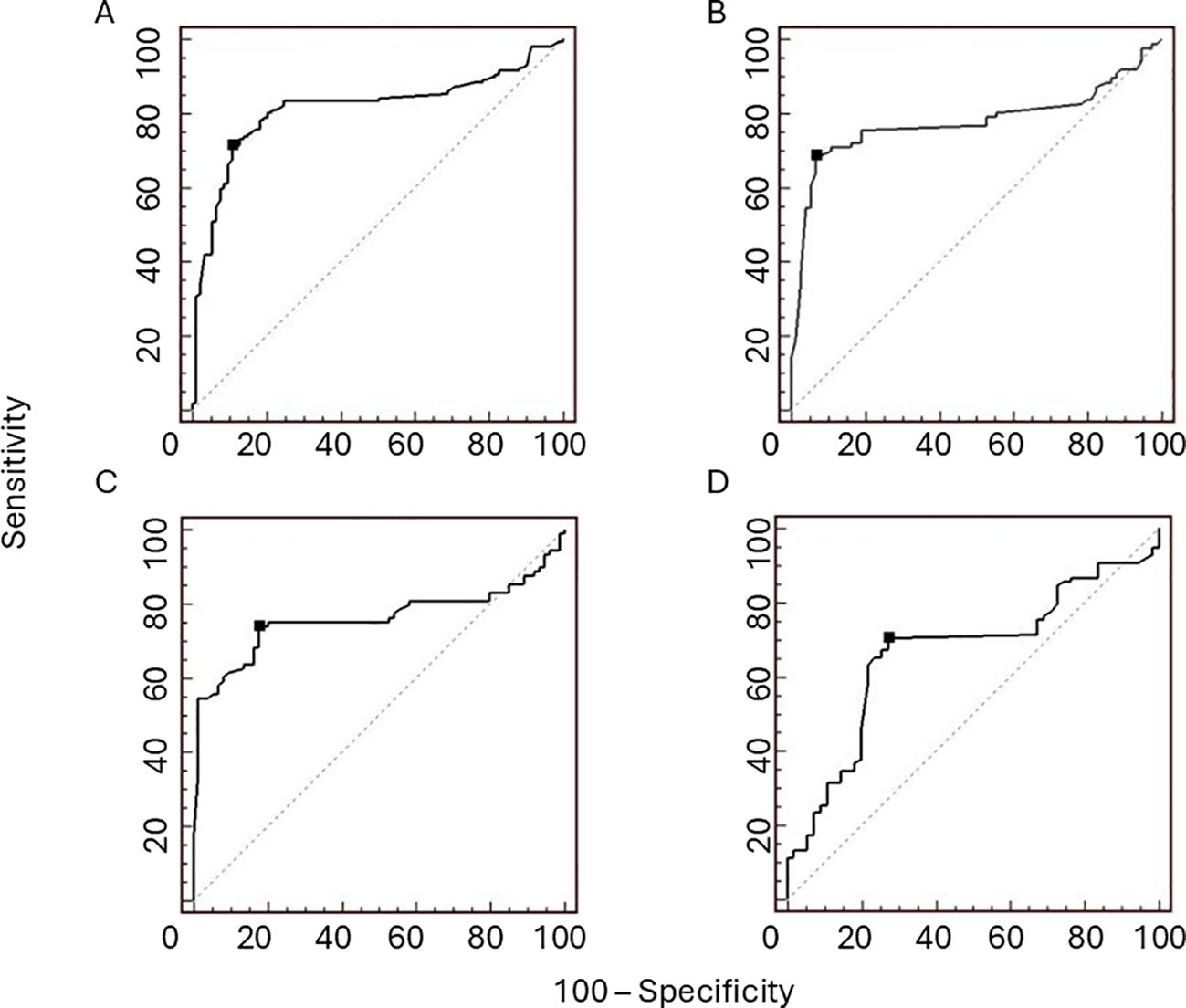
Figure 3. Receiver operating characteristic (ROC) curves for testosterone (T), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and dehydroepiandrosterone sulfate (DHEA-S) in patients with ovarian postmenopausal hyperandrogenism (PH). The areas under curve (AUCs) of T (A), LH (B), FSH (C), and DHEA-S (D) are 0.88 (0.76–0.86), 0.78 (0.70–0.84), 0.76 (0.69–0.82), and 0.66 (0.58–0.74), respectively. The proposed cutoff points (black dots) for differentiating ovarian PH patients with tumours from ovarian PH patients without tumours, with suitable sensitivity and specificity, are T ≥9.8 nmol/L (sensitivity = 71.3%, specificity = 89.2%), LH ≤15 mUI/ml (sensitivity = 68.6%, specificity = 93.2%), FSH ≤35 mUI/ml (sensitivity = 73.9%, specificity = 82.4%), and DHEA-S ≥1.6 μmol/L (sensitivity = 70.5%, specificity = 72.7%).
3.2.2 Radiological examination of oPH patients
A total of 260 (92.5%) patients underwent pelvis radiological examinations during the oPH diagnostic process. As reported in Table 3, 247 patients with oPH underwent a TVU, 139 a pelvic CT scan, and 107 a pelvic magnetic resonance imaging (MRI). Overall, the pelvic radiological examination identified the ovarian condition that caused PH in 176 patients (59 patients affected by non-tumorous oPH and 117 affected by tumorous oPH). In the case of a radiological finding of macroscopic abnormalities (i.e., increased volume of an ovary and/or abnormal age-corrected ovary size), the OR of tumorous oPH was 2.08 (95%CI = 1.22–3.53, p < 0.01). The sensitivity and specificity of TVU, CT, and MRI for oPH are reported in Table 3.
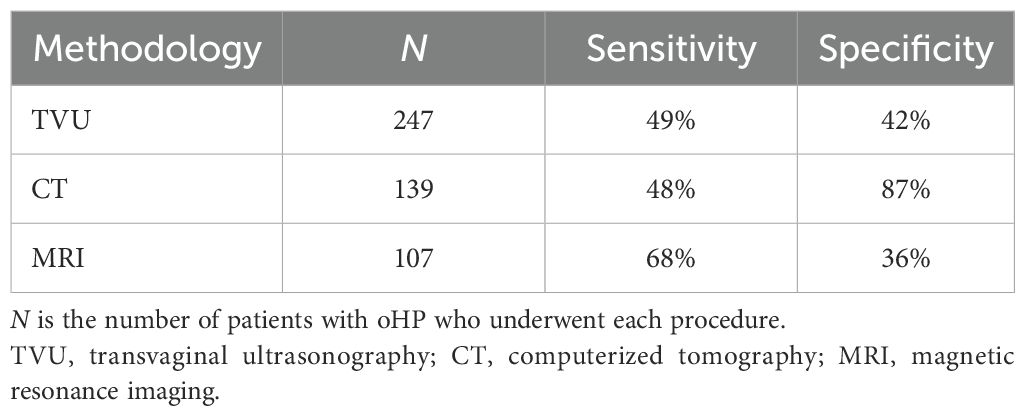
Table 3. Sensitivity and specificity of the radiological methodology in surgically treated ovarian postmenopausal hyperandrogenism (oPH) patients.
4 Discussion
PH is a rare condition that requires a careful and laborious differential diagnosis, considering that it may be dependent on the neoplastic source of androgens. The results of this study provide clinical and biochemical parameters for the identification of tumorous oPH before the surgical results. Indeed, patients with tumorous oPH show lower BMI, LH, and FSH and higher T, E, and DHEA-S compared with non-tumorous oPH patients. Based on the data collected using IPD, we proposed cutoff points for T, DHEA-S, LH, and FSH for the identification of patients with tumorous oPH with good sensitivity and specificity. The female reproduction system is physiologically regulated by intricate neuroendocrine signals involving the hypothalamic–pituitary–ovarian (HPO) axis. Overweight and obesity can disturb the HPO axis, causing metabolic and reproductive disorders, as observed in patients with non-tumorous oPH (21). According to our results, we hypothesize that the interference of adiposity on the HPO axis, mediated by insulin and leptin resistance and by low-grade chronic inflammation (LGCI), is responsible for the occurrence of PH in the case of non-tumorous ovarian conditions (22). On the contrary, patients with tumorous oPH showed simultaneous increases in T and DHEA-S, which can reduce the gonadotropin levels even in the postmenopausal era, as observed in our data collection. The increased DHEA-S levels may be related to the hyper-expression of steroid sulfatase androgen-activating enzymes in tumorous ovarian cells (23, 24). As shown in Table 3, the IPD analysis also indicated that all of the radiologic procedures showed good sensitivity and specificity in identifying ovarian diseases behind oPH. Based on these data and according to Hofland et al. (25), we suggest performing a pelvic exploratory laparotomy after the exclusion of the adrenal PH causes, if any radiological pelvic examination is not conclusive. However, an ovarian cancer causing PH is very likely in cases where ovarian macroscopic abnormalities are reported. No recurrence was reported in oPH patients after surgery.
This study has strengths and limitations. The IPD procedure guarantees the best opportunity to summarize the results of multiple case studies and case series, offering a large amount of data in the case of rare conditions (26, 27). Furthermore, IPD analysis allows the evaluation of the predictive value of multiple individual variables, such as, in patients with PH, the T, DHEA-S, LH, and FSH levels, and the standardization of the statistical analysis (28, 29). On the other hand, the heterogeneity of the data, the lack of centralized measurements of the instrumental and biochemical parameters, and the missing information with regard to the assay methods are considered limitations because these do not ensure uneven data. Lastly, one other limit is represented by the selected inclusion and exclusion criteria, which are restricted to a subset of HP patients with ovarian diseases requiring a surgical approach. Indeed, the surgical indication suggests a more severe hyperandrogenism and/or an imaging suspicion of ovarian abnormality, so that just a subset of women with PH were included (operated oPH women with a conclusive histological diagnosis), impairing the generalizability of the results.
6 Conclusion
PH is a rare clinical condition that requires long and delicate diagnostic procedures. For the first time, the current study provided an interpretation of some commonly used biochemical parameters to properly address the diagnostic assessment of oPH. In particular, lower BMI, LH, and FSH and higher T, E, and DHEA-S can be used to distinguish tumorous oPH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving humans because the study is a systematic review with a case report. No ethical approval was needed for the systematic review. We collected the informed consent from the patient involved in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AFo: Funding acquisition, Writing – original draft, Writing – review & editing. LD’E: Formal Analysis, Methodology, Software, Writing – review & editing. CL: Funding acquisition, Writing – original draft. AFi: Data curation, Funding acquisition, Methodology, Writing – original draft. AB: Resources, Writing – original draft. VA: Validation, Writing – original draft. AV: Funding acquisition, Resources, Writing – original draft. NV: Funding acquisition, Visualization, Writing – original draft. GF: Investigation, Visualization, Writing – review & editing. PV: Data curation, Resources, Writing – original draft. MA: Resources, Writing – original draft. RC: Data curation, Resources, Writing – original draft. FG: Project administration, Resources, Writing – original draft. PG: Project administration, Visualization, Writing – original draft. PF: Formal Analysis, Writing – review & editing. AS: Resources, Writing – original draft. RP: Conceptualization, Data curation, Project administration, Writing – review & editing. DR: Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to Miss Rosanna Scala for syntactic and grammatical revision of the manuscript, native English speaker and Editor Assistant Nutrition, Metabolism and Cardiovascular Diseases and for Elsevier Editor (https://www.nmcd-journal.com/content/edboard).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1495930/full#supplementary-material
References
1. Fogle RH, Stanczyk FZ, Zhang X, and Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. (2007) 92:3040–3. doi: 10.1210/jc.2007-0581
2. Gershagen S, Doeberl A, Jeppsson S, and Rannevik G. Decreasing serum levels of sex hormone-binding globulin around the menopause and temporary relation to changing levels of ovarian steroids, as demonstrated in a longitudinal study. Fertil Steril. (1989) 51:616–21. doi: 10.1016/s0015-0282(16)60609-x
3. Markopoulos MC, Kassi E, Alexandraki KI, Mastorakos G, and Kaltsas G. Hyperandrogenism after menopause. Eur J Endocrinol. (2015) 172:R79–91. doi: 10.1530/EJE-14-0468
4. Dubey P, Reddy S, Sharma K, Johnson S, Hardy G, and Dwivedi AK. Polycystic ovary syndrome, insulin resistance, and cardiovascular disease. Curr Cardiol Rep. (2024) 26:483–95. doi: 10.1007/s11886-024-02050-5
5. Wolf WM, Wattick RA, Kinkade ON, and Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/Ethnicity. Int J Environ Res Public Health. (2018) 15:2589. doi: 10.3390/ijerph15112589
6. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
7. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E and Munn Z, editors. JBI Manual for Evidence Synthesis. JBI (2020). doi: 10.46658/JBIMES-20-08
8. Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. (2020) 18:2127–33. doi: 10.11124/JBISRIR-D-19-00099
9. Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. (2011) 6:S1–57. doi: 10.1111/j.1610-0379.2011.07802.x
10. Spritzer PM, Marchesan LB, Santos BR, and Fighera TM. Hirsutism, normal androgens and diagnosis of PCOS. Diagnostics (Basel). (2022) 12:1922. doi: 10.3390/diagnostics12081922
11. Horejsí J. Acquired clitoral enlargement, Diagnosis and treatment. Ann N Y Acad Sci. (1997) 816:369–72. doi: 10.1111/j.1749-6632.1997.tb52163.x
12. Hirschberg AL. Approach to investigation of hyperandrogenism in a postmenopausal woman. J Clin Endocrinol Metab. (2023) 108:1243–53. doi: 10.1210/clinem/dgac673
13. Degitz K, Placzek M, Arnold B, Schmidt H, and Plewig G. Congenital adrenal hyperplasia and acne in male patients. Br J Dermatol. (2003) 148:1263–6. doi: 10.1046/j.1365-2133.2003.05369.x
14. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. (2008) 93:1526–40. doi: 10.1210/jc.2008-0125
15. Babakhanlou R, Verstovsek S, Pemmaraju N, and Rojas-Hernandez CM. Secondary erythrocytosis. Expert Rev Hematol. (2023) 16:245–51. doi: 10.1080/17474086.2023.2192475
16. Il Progetto cuore, carte del rischio cardiovascolare. Available online at: https://www.cuore.iss.it/valutazione/carte (Accessed 02 September 2024).
17. Hirschberg AL. Hyperandrogenism and cardiometabolic risk in pre- and postmenopausal women-what is the evidence? J Clin Endocrinol Metab. (2024) 109:1202–13. doi: 10.1210/clinem/dgad590
18. Bankar GR and Keoliya A. Robot-assisted surgery in gynecology. Cureus. (2022) 14:e29190. doi: 10.7759/cureus.29190
19. Tokunaga H, Shimada M, Ishikawa M, and Yaegashi N. TNM classification of gynaecological Malignant tumours, eighth edition: changes between the seventh and eighth editions. Jpn J Clin Oncol. (2019) 49:311–20. doi: 10.1093/jjco/hyy206
20. Adhikari L and Hassell LA. WHO classification. WHO website Pathology Outlines (2021). Available online at: https://www.pathologyoutlines.com/topic/ovarytumorwhoclassif.html.
21. Lonardo MS, Cacciapuoti N, Guida B, Di Lorenzo M, Chiurazzi M, Damiano S, et al. Hypothalamic-ovarian axis and adiposity relationship in polycystic ovary syndrome: physiopathology and therapeutic options for the management of metabolic and inflammatory aspects. Curr Obes Rep. (2024) 13:51–70. doi: 10.1007/s13679-023-00531-2
22. Chen X, Xiao Z, Cai Y, Huang L, and Chen C. Hypothalamic mechanisms of obesity-associated disturbance of hypothalamic-pituitary-ovarian axis. Trends Endocrinol Metab. (2022) 33:206–17. doi: 10.1016/j.tem.2021.12.004
23. Valenzuela Scheker E, Kathuria A, Esnakula A, Sasano H, Yamazaki Y, Tevosian S, et al. Expression of key androgen-activating enzymes in ovarian steroid cell tumor, not otherwise specified. J Investig Med High Impact Case Rep. (2020) 8:2324709620933416. doi: 10.1177/2324709620933416
24. Calvillo-Robledo A, Pedernera E, Morales-Vásquez F, Pérez-Montiel D, Gómora MJ, Almaraz MÁ, et al. Simultaneous expression of steroid sulfatase and androgen receptor reduced overall survival of patients with epithelial ovarian tumors. J Ovarian Res. (2021) 14:98. doi: 10.1186/s13048-021-00840-x
25. Hofland M, Cosyns S, De Sutter P, Bourgain C, and Velkeniers B. Leydig cell hyperplasia and Leydig cell tumour in postmenopausal women: report of two cases. Gynecol Endocrinol. (2013) 29:213–5. doi: 10.3109/09513590.2012.705375
26. Rendina D, Abate V, Cacace G, D’Elia L, De Filippo G, Del Vecchio S, et al. Tumor-induced osteomalacia: A systematic review and individual patient’s data analysis. J Clin Endocrinol Metab. (2022) 107:e3428–36. doi: 10.1210/clinem/dgac25
27. Abate V, Vergatti A, De Filippo G, Damiano V, Menale C, D’Elia L, et al. Clinical characteristics of Malignant phosphaturic mesenchymal tumor causing tumor-induced osteomalacia. J Clin Endocrinol Metab. (2024) 109:e1006–11. doi: 10.1210/clinem/dgad690
28. Stewart LA and Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof. (2002) 25:76–97. doi: 10.1177/0163278702025001006
Keywords: androgens, testosterone, dehydroepiandrosterone sulphate, 17-OH progesterone, follicle-stimulating hormone, luteinizing hormone
Citation: Forte A, D’Elia L, De Luca C, Fiore A, Barbato A, Abate V, Vergatti A, Verde N, De Filippo G, Venetucci P, De Angelis MC, Di Crescenzo RM, Grasso F, Giuseppe P, Formisano P, Di Spiezio Sardo A, Pivonello R and Rendina D (2025) Postmenopausal ovarian hyperandrogenism of surgically treated patients: a case report and scoping review with individual patient’s data analysis. Front. Endocrinol. 16:1495930. doi: 10.3389/fendo.2025.1495930
Received: 13 September 2024; Accepted: 11 July 2025;
Published: 01 August 2025.
Edited by:
Ahmet Fatih Durmusoglu, Istanbul Medipol University, TürkiyeReviewed by:
Monica Livia Gheorghiu, Carol Davila University of Medicine and Pharmacy, RomaniaErkut Attar, Yeditepe University, Türkiye
Copyright © 2025 Forte, D’Elia, De Luca, Fiore, Barbato, Abate, Vergatti, Verde, De Filippo, Venetucci, De Angelis, Di Crescenzo, Grasso, Giuseppe, Formisano, Di Spiezio Sardo, Pivonello and Rendina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosario Pivonello, cm9zYXJpby5waXZvbmVsbG9AdW5pbmEuaXQ=; Domenico Rendina, ZG9tZW5pY28ucmVuZGluYUB1bmluYS5pdA==
†These authors have contributed equally to this work
‡ORCID: Angelo Forte, orcid.org/0000-0003-0838-4478
Lanfranco D’Elia, orcid.org/0000-0002-1782-0211
Carmine De Luca, orcid.org/0009-0004-6875-9133
Antonella Fiore, orcid.org/0009-0007-4845-2902
Antonio Barbato, orcid.org/0000-0002-4660-821X
Veronica Abate, orcid.org/0000-0003-0085-0067
Anita Vergatti, orcid.org/0009-0009-6574-9120
Nunzia Verde, orcid.org/0000-0002-8509-0409
Gianpaolo De Filippo, orcid.org/0000-0003-1313-5599
Pietro Venetucci, orcid.org/0000-0002-0372-4069
Maria Chiara De Angelis, orcid.org/0000-0002-5817-2468
Perruolo Giuseppe, orcid.org/0000-0003-0479-8729
Pietro Formisano, orcid.org/0000-0001-7020-6870
Attilio Di Spiezio Sardo, orcid.org/0000-0001-6485-5735
Rosario Pivonello, orcid.org/0000-0002-9632-1348
Domenico Rendina, orcid.org/0000-0002-0331-0392
 Angelo Forte1‡
Angelo Forte1‡ Lanfranco D’Elia
Lanfranco D’Elia Antonio Barbato
Antonio Barbato Veronica Abate
Veronica Abate Anita Vergatti
Anita Vergatti Nunzia Verde
Nunzia Verde Gianpaolo De Filippo
Gianpaolo De Filippo Maria Chiara De Angelis
Maria Chiara De Angelis Perruolo Giuseppe
Perruolo Giuseppe Rosario Pivonello
Rosario Pivonello Domenico Rendina
Domenico Rendina