- 1Department of Andrology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
- 2Department of Burns and Plastic Surgery, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
- 3Department of Radiation Therapy, Dongguan Hospital of Guangzhou University of Chinese Medicine (Dongguan Traditional Chinese Medicine Hospital), Dongguan, China
- 4Surgery and Anesthesia Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
Asthenozoospermia is a severe condition characterized by abnormal sperm motility, contributing to 50% of male infertility cases. Idiopathic asthenozoospermia refers to a form of this condition with no identifiable causes through routine clinical examinations, potentially linked to apoptosis and oxidative stress induced by excessive reactive oxygen species (ROS). At low concentrations, ROS positively influence physiological processes, including sperm mature and motility. However, elevated ROS levels can harm human spermatozoa through oxidative stress, primarily due to the absence of effective DNA damage repair mechanisms and inadequate antioxidant defenses. In this review, we summarize the physiological and pathophysiological roles, endogenous and exogenous sources, and therapeutic strategies related to ROS in idiopathic asthenozoospermia. Ultimately, maintaining a proper balance between ROS concentrations and antioxidants is crucial for ensuring male reproductive health.
1 Introduction
Sperm motility is a crucial capability of human spermatozoa necessary for their journey across the female genital tract post-ejaculation (1), with progressive motility (PR) serving as a key metric (2, 3). In recent years, sperm parameters have witnessed a declining trend, especially with a sharp drop in sperm motility, which, in severe cases, leading to male infertility in severe instances (4). Currently, male infertility is responsible for the 14% of couples experiencing fertility issues (1, 2). Asthenozoospermia (AZS) is a severe condition characterized by abnormal sperm motility, defined by progressive motility of less than 32% (PR<32%) among sperm parameters (2, 3). The majority of patients with male infertility also present with asthenozoospermia. The common causes of AZS include varicocele, endocrine abnormalities, environmental factors, inflammation, drug-induced injury, and certain underlying diseases (5, 6). Nevertheless, in numerous cases, routine clinical examinations fail to identify clear causes, leading to a classification of idiopathic AZS (iAZS) (5).
The exact pathogenesis of iAZS remains unclear, but it is currently believed that excessive reactive oxygen species (ROS) leading to apoptosis and oxidative stress is a key factor in its development. ROS is a group of highly reactive oxygen-containing molecules that include superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radicals (OH-), and singlet oxygen (1O2) (7). Due to their short half-life (8), they cannot be directly detected in human specimens. The OH- is particularly unstable and rapidly reacts with nearby biomolecules. Furthermore, H2O2 is a predominant form of ROS capable of crossing cell membranes to exert effects beyond cellular boundaries (9).
The intracellular levels of ROS are closely regulated by various synthesis and degradation pathways. Maintaining physiological levels of ROS is critical for redox regulation involved in processes such as repair, survival, and differentiation (10). However, when ROS are produced in excess, they can damage sperm cells, leading to impaired motility, DNA fragmentation, and cellular apoptosis, significantly affecting male fertility (11). Additionally, excessive ROS can induce lipid peroxidation in the sperm plasma membrane, which is rich in polyunsaturated fatty acids, disrupting membrane integrity and impairing sperm function and morphology (12). While ROS are often considered detrimental, they also play a vital physiological role in sperm function (13, 14). In spermatozoa, these molecules play essential roles in sperm capacitation, acrosome reaction, and fertilization (15). The challenge lies in the delicate balance between the beneficial and harmful effects of ROS (16). The role of ROS in idiopathic asthenozoospermia remains unclear (17). Furthermore, the mechanisms for maintaining the dynamic balance of ROS in sperm to manage oxidative stress in idiopathic asthenozoospermia require further investigation.
Idiopathic asthenozoospermia (iAZS) may be linked to apoptosis and oxidative stress caused by excessive ROS (18, 19). Nevertheless, the exact pathogenesis of iAZS remains unclear. This review explores the sources of ROS, their physiological and pathological roles in sperm motility, and potential therapeutic strategies targeting ROS in iAZS. By investigating these aspects, we offer new insights for the clinical management of iAZS and provide a comprehensive framework for understanding the complex interplay between ROS and sperm function.
2 Physiological roles of low concentrations of ROS in sperm maturation and motility
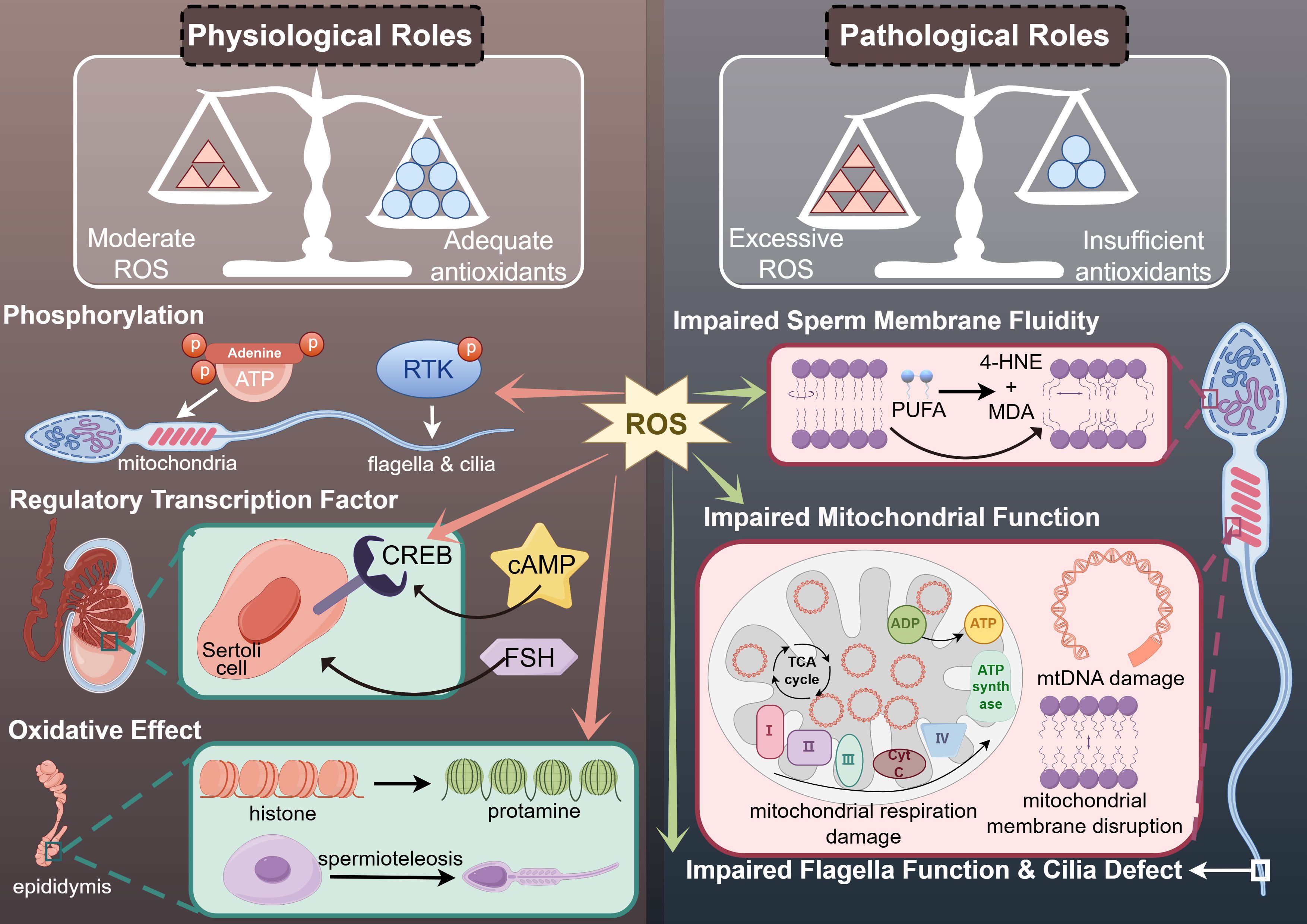
At low concentrations, ROS positively influence physiological processes such as spermatogenesis, sperm motility, and fertilization (20, 21). This process might be associated with phosphorylation, the expression of regulatory transcription factor and oxidative effects (Figure 1) (22).
2.1 Phosphorylation
During spermatogenesis, the DNA replication process of meiosis relies on energy supplied through oxygen consumption and ROS generation in mitochondria, which also provide the necessary ATP for sperm motility (23). The proliferation of spermatogonia and the differentiation of spermatocytes into spermatozoa, including the formation of sperm flagella crucial for motility, depend on various internal environmental factors (24). These factors include receptor-tyrosine kinase (RTK) phosphorylation signaling pathways, which are mediated by ROS in some somatic cells (25). Therefore, physiological concentrations of ROS play beneficial roles by modulating phosphatases and facilitating phosphorylation (Figure 1).
2.2 Regulatory transcription factor
ROS at appropriate concentrations play a crucial role in the transcriptional processes of spermatogenesis by functioning as regulatory transcription factors (26). Several sex hormones, including follicle-stimulating hormone (FSH) and luteinizing hormone (LH), are also involved in the mechanism of transcriptional regulation (27). In the testes, Sertoli cells are the primary targets of FSH (28). The cAMP response element-binding protein (CREB), whose receptors are activated by optimal levels of ROS, serves as a pivotal transcription factor within Sertoli cells under the influence of FSH-mediated signaling pathways (29). However, the role of ROS as regulatory transcription factors varies distinctly from that of various sex hormones (Figure 1) (30).
2.3 Oxidative effect
Sperm maturation in the epididymis is influenced by the oxidative effects of ROS (22). Due to the lack of histone packaging, spermatozoa struggle to maintain the integrity of their genetic material through DNA damage repair processes (21). Consequently, the histone-to-protamine replacement during spermiogenesis provides an alternative mechanism for maintaining genetic stability, which results from the oxidation of small nuclear thiol group proteins in protamine (31). Additionally, ROS, as oxidants derived from redox reactions, are implicated in the formation of chromatin packaging and the mitochondrial capsule—a protective cover surrounding the chromatin and mitochondria (32). Improper formation of these structures can compromise both the integrity and energy generation required for sperm motility, leading to functional impairments in spermatozoa (Figure 1) (33, 34).
3 Pathophysiological roles of ROS in idiopathic asthenozoospermia
Sperm motility is an essential capability of human spermatozoa required for their journey through the female genital tract post-ejaculation (1). Due to the lack of adequate oxygen radical-scavenging enzymes in their cytoplasm, human spermatozoa are highly vulnerable to oxidative stress induced by reactive oxygen species (ROS), leading to idiopathic asthenozoospermia without a clear etiology (35). ROS produced by mitochondrial complex I can cause mitochondrial dysfunction through peroxidation in the mid-piece of spermatozoa, resulting in a rapid depletion of ATP, which adversely affects sperm motility (36, 37). The primary sites of ATP generation in human spermatozoa are the mitochondria, the central part of the flagella, and the sperm head (38). The key metabolic pathways involved are oxidative phosphorylation and glycolysis (18). ATP production via oxidative phosphorylation primarily occurs in the mitochondrial respiratory chain complexes through respiration, while glycolysis takes place in the central part of the flagella (Figure 1) (39, 40).
3.1 Impaired mitochondrial function
The specific activity of mitochondrial enzymes, which depend on the mitochondrial electron transfer chain complexes (ETCs), can influence sperm motility and potentially lead to idiopathic asthenozoospermia (41). Sperm motility has been shown to correlate with oxygen consumption and the efficiency of mitochondrial respiration. Several inhibitors of ETCs have been observed to impair sperm motility. Complex I in the mitochondria is particularly sensitive to excessive ROS (42), and this sensitivity arises because ROS generated through unsaturated fatty acids inhibit complex I (42). Meanwhile, the absence of mitochondrial protein OPA1 leads to disorganization of mitochondrial cristae structure and impaired assembly of ETC Complexes I, III, IV, and V, but does not affect the assembly of Complex II. OPA1 plays a role in the accumulation of mitochondrial ROS and lipid ROS induced by cysteine deprivation. Additionally, ferroptosis is a form of iron-dependent non-apoptotic cell death primarily triggered by the accumulation of intracellular iron and lipid peroxidation. Mitochondria-targeted antioxidants such as SkQ1 and redox mediators like methylene blue can inhibit the production of ROS in Complex I of the mitochondrial electron transport chain, preventing mitochondrial lipid peroxidation and ferroptosis (13).
The primary characteristics of non-motile sperm include the disruption of the mitochondrial dysfunction (43–45). Mitochondrial DNA (mtDNA) damage resulting from interactions between nitric oxide (NO) and superoxide (O2⁻) can also affect sperm motility and function (12, 46). And mtDNA repair is inadequate because of the complete absence of nucleotide-excision repair pathways (47, 48). Mitochondria are crucial for the energy metabolism of sperm, primarily generating ATP through oxidative phosphorylation (OXPHOS) to fuel sperm motility (49). Damage to mitochondrial DNA can result in OXPHOS dysfunction (50), which in turn impairs ATP production and leads to reduced sperm motility (36, 37). Additionally, mitochondrial DNA damage can lead to excessive production of reactive oxygen species (ROS), with elevated ROS levels triggering oxidative stress that further impairs sperm function (51). Such damage may also interfere with the expression and function of mitochondria-related proteins. For instance, mitochondrial transcription factor A (TFAM) is critical for regulating mitochondrial DNA replication and transcription. Abnormal TFAM expression may be linked to mitochondrial DNA damage, consequently affecting sperm vitality (52). Moreover, mitochondrial DNA damage can compromise the structural integrity of mitochondria, resulting in reduced sperm motility (53). Finally, mitochondrial DNA damage can influence sperm survival and function by affecting apoptotic pathways. Research indicates that mitochondrial dysfunction may activate apoptotic signaling pathways, leading to sperm cell death (54). Therefore, mitochondrial DNA damage impacts sperm vitality through various mechanisms, including disruptions in energy metabolism, oxidative stress, alterations in mitochondrial membrane potential, abnormal protein expression, and apoptosis. These interconnected pathways collectively result in reduced sperm vitality, ultimately affecting male fertility.
Furthermore, ROS can compromise the integrity of mitochondrial membranes, potentially activating apoptotic signaling cascades and promoting the release of cytochrome C (55, 56). Apoptosis in spermatozoa is typically initiated by oxidative stress and lipid peroxidation, leading to the production of mitochondrial ROS. This cascade results in a rapid loss of sperm motility, followed by caspase activation and the exposure of phosphatidylserine on the sperm surface (57). The Sperm Chromatin Structure Assay (SCSA) and active Caspase-3 levels correlate with the rate of motility decline post-ejaculation. Elevated levels of these markers suggest a faster decline in motility, indicating that apoptosis significantly impacts sperm vitality (58, 59). The phosphoinositide 3-kinase (PI3K) signaling pathway plays a role in regulating sperm apoptosis. Inhibition of PI3K activity triggers an apoptotic cascade characterized by loss of motility and oxidative DNA damage. Thus, impaired mitochondrial function due to mtDNA damage and mitochondrial apoptosis may be responsible for reduced sperm motility and idiopathic asthenozoospermia (Figure 1) (45).
3.2 Impaired sperm plasma membrane
Sperm plasma membrane may be the major target site of ROS through cascade signaling reaction (60). ROS affects the fluidity and integrity of sperm plasma membrane (12). The membrane fluidity of human spermatozoa depends on the polyunsaturated fatty acids (PUFA) in the sperm plasma membrane (61). Excessive ROS converts PUFA into 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA), byproducts of LPO, to destroy the membrane fluidity of spermatozoa. Meanwhile, the generation of 4-HNE and MDA also impaired mitochondrial electron transfer chain complexes, which resulted in reducing ATP production and corresponding sperm motility, and further increased ROS from mitochondria as a result of oxidative stress (62). Therefore, ROS causes damage to membrane fluidity of human spermatozoa through the generation of MDA and 4-HNE, which in turn lead to idiopathic asthenozoospermia (Figure 1) (63). What’s more, loss of glutathione, a kind of antioxidants in the midpiece of spermatozoa, may also contributes to idiopathic asthenozoospermia (64).
In addition, lipid peroxidation (LPO) induced by excessive ROS could also adversely affect the fluidity of the sperm plasma membrane through oxidative stress (65). This process can result in the complete inactivation of membrane enzymes, subsequently leading to sperm DNA damage (57). Enzymes on the sperm membrane, such as phospholipase C (PLC) and phospholipase D (PLD), play crucial roles in regulating intracellular signal transduction and membrane lipid metabolism (66). Dysfunction of these enzymes can lead to disordered membrane lipid metabolism, increased ROS production, and consequently, oxidative stress and sperm DNA damage (67). For instance, overactivation of PLC can lead to an increase in intracellular calcium ion concentration, which in turn activates a series of downstream signaling pathways and increases ROS production (66). These ROS can attack the unsaturated fatty acids on the sperm membrane, triggering lipid peroxidation reactions that disrupt the membrane’s integrity, ultimately leading to sperm DNA damage (67). Additionally, when the functions of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) are inhibited or their activities are reduced, ROS levels rise, leading to lipid peroxidation of the sperm membrane and consequently affecting the integrity of sperm DNA, resulting in decreased sperm motility (68).
3.3 Sperm DNA fragmentation
In spermatozoa, the integrity of DNA is crucial for protecting genetic material from environmental damage. Uncompacted DNA, due to its open structure, is more susceptible to attack by ROS, prompting mitochondria to produce apoptosis-inducing factor (AIF) and sperm DNA fragmentation (SDF) (69). DNA damage is assessed by the DNA frag-mentation index (DFI) rate using the comet assay, the sperm chromatin dispersion assay, terminal deoxyuridine nick end labeling (TUNEL) assay, and sperm chromatin structure assay (70, 71). Studies have shown that the sperm DFI is significantly negatively correlated with progressive sperm motility (72, 73). Specifically, for every 10% increase in DFI, the probability of male conception may decrease by up to 30% (1, 74, 75). One study found that a sperm DFI greater than 30% is a threshold for a significant decline in conception rates; when DFI exceeds 30%, the success rates of natural conception and intrauterine insemination (IUI) are nearly zero (1, 74, 75). SDF is a type of DNA damage that occurs under conditions involving sperm caspase and endonuclease activity, which subsequently affects the transition from histone to protamine (76). The reduction in sperm motility is also linked to the inhibition of this histone-to-protamine transition (77, 78). In patients with asthenozoospermia, the expression levels of protamine are typically lower, which may affect the motility and fertilization capacity of spermatozoa (79). Thus, SDF is one of the manifestations of idiopathic asthenozoospermia.
The relationship between SDF and reduced sperm motility is complex, involving various molecular mechanisms. Studies suggest that oxidative stress is a primary factor contributing to SDF. It leads to the excessive production of ROS within sperm cells, which attack DNA, causing strand breaks and thereby increasing DNA fragmentation (80). Moreover, oxidative stress can impair mitochondrial function, disrupting energy metabolism and resulting in diminished sperm motility (52). The chromatin packaging state of sperm is another critical factor. Research has shown that sperm with poorly packaged chromatin are more prone to DNA fragmentation and cell death during freeze-thaw processes (81). This inadequate chromatin packaging may be linked to insufficient protamine levels, which are essential for the high degree of chromatin compaction in spermatozoa (82). Additionally, sperm DNA fragmentation is correlated with the age of the sperm. As age advances, the integrity of sperm DNA declines, and DNA fragmentation increases. This is possibly due to age-related oxidative stress and a decline in antioxidant defense mechanisms (83).
3.4 Impaired flagella function
Human spermatozoa can utilize various carbohydrates to generate ATP necessary for sperm motility. However, even in the absence of impaired mitochondrial function, inhibition of glycolysis can also affect sperm motility (84). The flagella, which constitute most of the sperm tail structure, play a crucial role in facilitating sperm motility. For instance, cAMP can promote the phosphorylation of protein kinase A (PKA) in sperm flagella (85), which is followed by the activation of tyrosine kinases and the phosphorylation of tyrosine residues in sperm proteins, including AKAP3, AKAP4, FSIP2, CABYR, and VCP (86, 87). The cyclic AMP (cAMP)-mediated PKA signaling pathway in sperm has been shown to be downregulated due to oxidative stress in idiopathic asthenozoospermic males (84). Levels of cAMP are positively correlated with sperm motility (88). cAMP is activated by intracellular soluble adenylyl cyclase (sAC), which is encoded by the ADCY10 gene under the stimulation of Ca2+, and is essential for sperm motility (89). Mutations in the ADCY10 gene can lead to a decline in sAC, resulting in idiopathic asthenozoospermia[106].
Meanwhile, AKAP3, a structural protein acting as the regulatory subunit of PKA, forms the fibrous sheath, maintaining the structural integrity of the sperm flagella in collaboration with AKAP4 (90). A deficiency in AKAP3 may impair sperm motility due to the abnormal accumulation of DNA and RNA metabolites (91). Mutations in AKAP3 and AKAP4 can lead to structural abnormalities in the sperm tail’s flagella (91). FSIP2 anchors cAMP-mediated PKA into AKAP4 to sustain sperm motility. Mutations in FSIP2, characterized by the absence of CPC, IDA, and ODA, can cause idiopathic asthenozoospermia due to the lack of AKAP4 protein (92). Thus, idiopathic asthenozoospermia can be monitored through AKAP3, AKAP4, and FSIP2 within the cAMP/PKA signaling pathway (93).
Additionally, the glycolysis process in the central part of the flagella provides sufficient ATP for its function to support sperm movement (94). Researchers have found that GPI, MDH1, PGAM1 and PGAM2A, the glycolysis-mediated proteins, were downregulated in the spermatozoa of patients with iAZS (95). Meanwhile, a significant number of glycolytic enzymes, including lactate dehydrogenase, phosphofructokinase, hexokinase, glyceraldehyde-3-phosphate dehydrogenase (GAPD), and phosphoglucose isomerase, have been identified in the sheath of the sperm flagella, maintaining its function (38). Additionally, in seminal plasma, researchers have demonstrated that citric acid, malic acid, succinic acid, which are associated with energy metabolism, and pyruvate were collectively reduced in the iAZS group, while lactate levels were elevated (96). These findings indicate a shift towards anaerobic glycolysis, resulting in decreased production of ATP compared to aerobic catabolism via the tricarboxylic acid cycle (96). This metabolic alteration likely contributes to reduced sperm motility (Figure 1).
4 The sources of ROS in human ejaculate
ROS is produced through both endogenous and exogenous pathways and play critical roles in sperm function. Human spermatozoa are significant sites of cellular ROS production (97, 98). Meanwhile in the context of iAZS, endogenous ROS are often produced in excess.
4.1 Endogenous sources and their effects on sperm motility
Human ejaculate contains a diverse array of round cell types, including human spermatozoa at various developmental stages, leukocytes, and epithelial cells (97, 98). The ROS contributed by these cells constitute the majority of the endogenous ROS pool, which is predominantly found in seminal plasma. Among them, immature spermatozoa and leukocytes, such as neutrophils and macrophages, are considered major endogenous sources of ROS (99, 100). The mechanisms of endogenous ROS generation in immature spermatozoa and leukocytes lied in two primary pathways: the reduced nicotinamide adenine dinucleotide (NADH)-dependent oxidoreductase system in the mitochondria and the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system located in the spermatozoa plasma membrane (101).
The mitochondrial oxidoreductase system is responsible for the majority of ROS production within human spermatozoa, primarily due to the abundance of mitochondria, which supply continuous energy for sperm motility (101). Mitochondrial ROS generation is fundamentally linked to the process of respiration. NADH oxidoreductase plays a critical role by catalyzing the oxidation of O2 to O2-, a precursor of sperm ROS, while transferring electrons from NADH to coenzyme Q10 (CoQ10) within the mitochondrial respiratory chain (102). If mitochondrial O2 concentrations are elevated, coupled with increased respiratory rates, more superoxide is released (103).
In the plasma membrane of human spermatozoa, NADPH oxidase also contributes to the transformation of O2 to superoxide (104). NOX5, a type of NADPH oxidase located in the acrosome and midpiece region of human spermatozoa, is activated through the binding of Ca2+ to its N-terminal cytoplasmic domain (105). These conformational changes facilitate the generation of superoxide, making the ROS generated by NOX5 as a major component of reactive oxygen species in human spermatozoa (105).
4.1.1 Immature spermatozoa
The synthesis of ROS in semen is influenced by the maturation level of spermatozoa (106). During their development and maturation, damaged or immature spermatozoa may retain residual cytoplasmic droplets, which are remnants of spermatogenesis. These droplets contain glucose-6-phosphate dehydrogenase (G6PD), a cytosolic enzyme that produces an excess of NADPH. This NADPH acts as a substrate for NADPH oxidase, facilitating the conversion of O2 to O2- (107).
A significant concentration of mitochondria is found in the midpiece of spermatozoa, serving as energy reservoirs that support sperm motility (108). The diaphorase enzyme, an oxidoreductase in the mitochondrial respiratory chain, maintains a balance between the oxidized and reduced forms of NADH to sustain sperm motility. However, a reduction in diaphorase enzyme activity can lead to superoxide generation, resulting in mitochondrial dysfunction through ROS-induced oxidative stress, potentially even damaging the mitochondrial integrity of human spermatozoa (109). Damage to the mitochondrial membrane by excessive ROS can further exacerbate ROS generation (110) (Figure 2a).
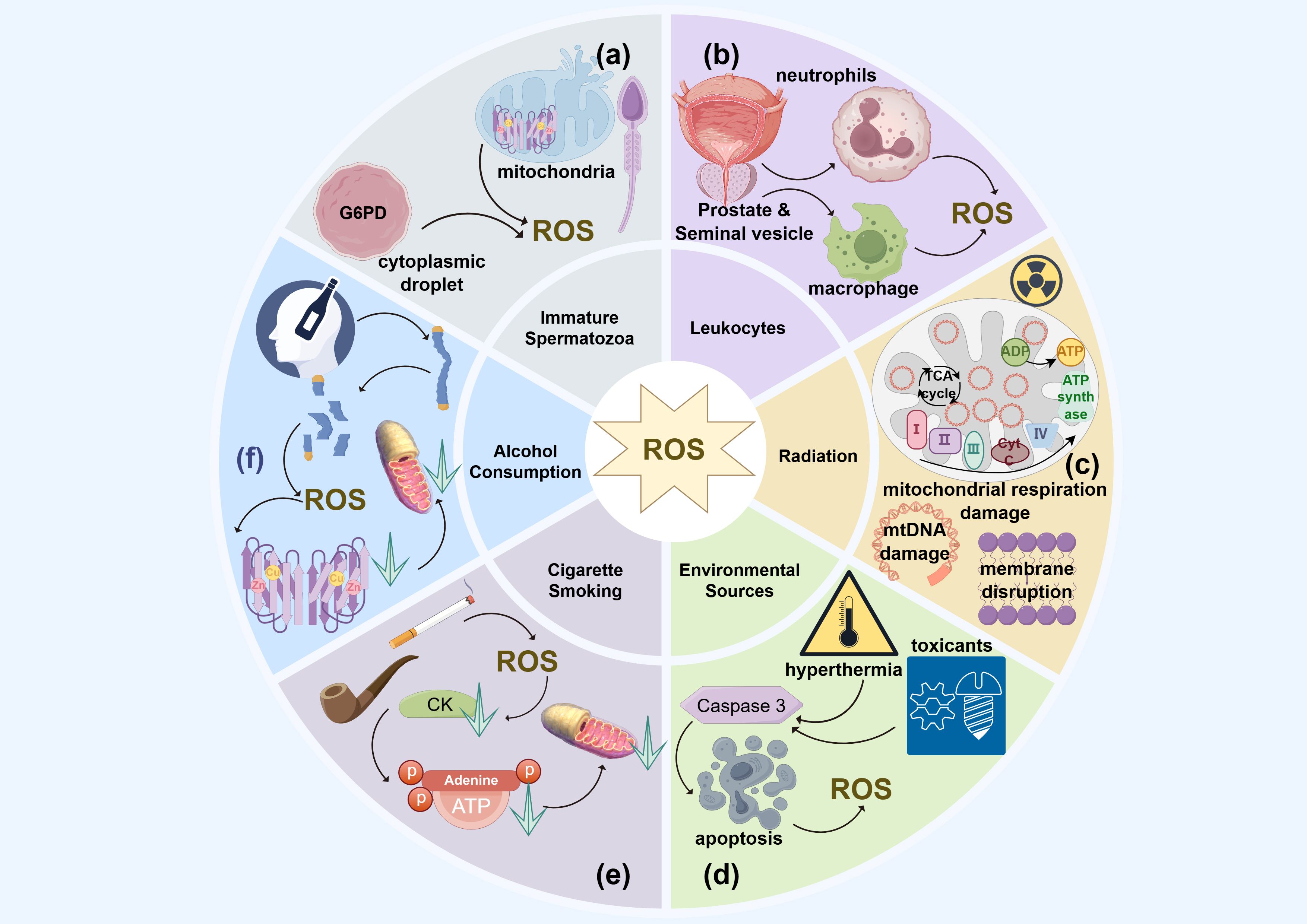
Figure 2. The endogenous and exogenous sources of ROS in idiopathic asthenozoospermia. (a) Immature spermatozoa; (b) Leukemia; (c) Radiation; (d) Environment resources; (e) Smoking; (f) Alcohol consumption.
4.1.2 Leukocyte
In patients with idiopathic asthenozoospermia, less than one million leukocytes per milliliter are typically found in naturally ejaculated semen (1). Most of the leukocytes from the prostate and seminal vesicles are activated leukocytes (106). Activated leukocytes, particularly peroxidase-positive types such as polymorphonuclear neutrophils (PMNs) and macrophages, are significant producers of ROS in human semen (111). These leukocytes enhance the production of NADPH, thereby increasing the activity of NADPH oxidases and resulting in elevated levels of superoxide O2-. Additionally, myeloperoxidase-positive neutrophils contribute to the oxidative conversion of O2 (112).
In addition, leukocyte-mediated signaling can also lead to an imbalance between oxidative and antioxidative processes. Elevated proinflammatory cytokines, such as interleukin (IL)-8, and reduced levels of superoxide dismutase (SOD) promote ROS generation, triggering oxidative stress. This stress, exacerbated by excessive leukocytes, ultimately damages spermatozoa (Figure 2b) (74).
4.2 Exogenous sources of ROS and their effects on sperm motility
The influence of environmental factors on sperm quality and motility represents a complex and multifaceted challenge. Over recent years, an expanding corpus of research has been dedicated to elucidating the mechanisms by which these factors impact male fertility, with particular emphasis on sperm quality and motility. This review specifically examines the roles of environmental factors, encompassing high temperatures, toxicants, and occupational exposures, as well as lifestyle factors, including obesity, smoking, alcohol consumption, and daily electronic radiation, in the generation of ROS and their impact on sperm motility.
4.2.1 Environmental sources
Prolonged exposure to high temperatures and heat radiation can induce scrotal hyperthermia, promoting the generation of ROS. Studies have shown that high summer temperatures are associated with decreased sperm concentration and count, while variations in sunlight duration and humidity can also affect sperm quality (113). Research conducted in Argentina found that changes in sunlight duration and humidity are linked to reductions in sperm concentration, count, motility, and membrane integrity (113). The underlying mechanism involves the upregulation of Caspase 3, which induces apoptosis in Leydig and Sertoli cells of the human testis due to excessive ROS generated by heat stress (114, 115) (Figure 2c).
Chemical toxicants such as phthalates, originating from microplastic pollution, can lead to an overproduction of ROS in human spermatozoa and testicular germlines cells (116, 117). This condition is characterized by a reduction in testicular antioxidants and hormone levels, causing mitochondrial dysfunction and decreased sperm motility as a result of oxidative stress (116, 117). Similarly, heavy metal ions like cadmium, copper, iron, and lead can reduce sperm motility and affect other sperm parameters. These effects are attributed to mitochondrial DNA damage caused by excessive ROS (118–120). A study involving coke oven workers identified a dose-response relationship between exposure to metal mixtures and diminished sperm quality (121). Furthermore, air pollution can also impact sperm motility by compromising the integrity of the spermatic plasma membrane through excessive ROS production. Research conducted in southern China has revealed a significant association between exposure to air pollutants like CO, NO2, O3, PM10, and PM2.5 and reductions in sperm count and motility, particularly during critical periods of sperm development (122) (Figure 2d).
Occupational exposure and pesticides pose a global concern regarding male reproductive health, particularly in industrialized nations (12, 123). Research has indicated that exposure to environmental toxicants such as cadmium, mercury and bisphenol A (BPA) can lead to male infertility, a condition associated with oxidative stress (123). These toxicants instigate oxidative stress, thereby disrupting the normal function of reproductive cells and consequently affecting the quality and motility of sperm (124). For instance, cadmium and mercury can interfere with the intracellular antioxidant defense systems, leading to an overproduction of ROS, which in turn damage sperm DNA and membrane lipids (125). BPA and pesticides may mimic or disrupt endocrine functions, thereby affecting the balance of reproductive hormones and subsequently influencing spermatogenesis (126, 127). Therefore, reducing occupational exposure and the use of pesticides is crucial for the protection of male reproductive health.
4.2.2 Lifestyle factors
Lifestyle factors and occupational exposures are considered significant influences on sperm quality. Studies indicate that obesity and irregular sleep patterns are associated with declines in sperm quality (128). In a study involving 1,060 participants, these lifestyle factors were significantly correlated with lower sperm quality (128). Research indicates that obesity leads to an increased accumulation of body fat, thereby triggering oxidative stress. This condition has a negative impact on sperm quality, particularly contributing to the occurrence of AZS (129). The oxidative stress induced by obesity not only affects the quality of sperm but may also exacerbate reproductive dysfunction by influencing the function of the reproductive axis (130). For instance, the disruption of tightly regulated metabolic pathways can lead to adverse reproductive outcomes, such as an inefficient energy supply to germ cells, defects in sperm motility, or arrest of spermatogenesis (129). Moreover, testicular metabolic alterations induced by obesity may also result in mitochondrial dysfunction, which is closely associated with the overproduction of ROS and oxidative stress readily targeting spermatozoa DNA and lipids, thereby contributing to a decrease in sperm quality (129).
Cigarette smoking is a major etiological factor in idiopathic asthenozoospermia. A meta-analysis of 20 studies conducted by Sharma et al. highlighted that cigarette smoking has an overall negative effect on sperm motility and other semen parameters (131). The accumulation of excessive ROS due to hazardous chemicals, carcinogens, and mutagenic substances in tobacco can damage mitochondrial DNA, inducing oxidative stress. Substances such as nicotine in cigarettes ultimately impair sperm motility by causing oxidative damage to the integrity of plasma membranes, altering protein and enzyme conformations and activation, and compromising the mitochondrial DNA sequence integrity. Creatine kinase (CK), a protein serving as a cellular energy reserve for fast ATP buffering and rebuilding in human spermatozoa, exhibits decreased activity in smokers. Elevated levels of reactive oxygen species (ROS) lead to additional oxidative damage to mitochondrial DNA, reducing ATP production and available energy, which, coupled with reduced CK activity, results in a rapid decline in sperm motility. Furthermore, the decline in sperm motility due to smoking is associated with protein phosphorylation, inhibition of histone-to-protamine transition, and disruptions in the expression of microribonucleic acids (miRNAs). Additionally, second-hand smoke also damages sperm motility through mitochondrial DNA damage and methylation, caused by excessive ROS (Figure 2e) (99, 100).
Sperm motility, concentration and morphology are deleteriously affected by excessive alcohol consumption as a result of spermatic chromatin abnormalities through apoptosis, oxidative stress for elevated ROS production and mitochondrial DNA damage (132). Ethanol from alcohol consumption leads to mitochondrial dysfunction and decreased ATP generation in hepatic metabolic processes (110). Cytochrome P450 enzymes (CYP2E), as a kind of catalyst for NADPH oxidase in oxidative stress, promote the concentrations of Cu2+ and Fe3+ under alcohol intake, which, through various pathways, enhance the generation of ROS (133). The generation of nitric oxide (NO) from inducible nitric oxide synthase (iNOS), which is secreted by macrophages, and its metabolite peroxynitrite will induce mitochondrial dysfunction under the stimulus of excessive ROS (Figure 2f) (134).
The biological impact of radiation on sperm motility is influenced by the type of radiation, as well as the dose and duration of exposure (135). In recent years, electronic devices such as mobile phones, computers, and microwave ovens have significantly increased exposure to ionizing radiation (136). It has been demonstrated that mobile phone radiation negatively affects the count, morphology, and motility of spermatogenic cells and spermatozoa (137). This radiation can damage the integrity of the plasma membrane and activate NADPH oxidase, leading to oxidative stress driven by elevated ROS and lipid peroxidation (LPO) (138). Electromagnetic radiation emitted by computers and mobile phones exerts adverse effects on sperm motility, capacitation, and acrosome reaction through oxidative stress induced by radiofrequency. This stress results from damage to mitochondrial DNA and disruptions in the electron transport chain within the mitochondrial respiratory complex (20). Additionally, sperm motility affected by oxidative stress is exacerbated by radiofrequency radiation due to decreased glutathione levels and compromised plasma membrane integrity (136). Furthermore, exposure to microwave radiation for two hours daily over 35 days has been shown to induce oxidative stress in human spermatozoa (Figure 2c) (139).
5 Therapeutic strategy of idiopathic athenozoospermia
Currently, there is no radical treatment for idiopathic asthenozoospermia that can fundamentally preserve sperm motility, primarily due to genetic alterations caused by ROS-mediated oxidative stress (140, 141). However, appropriate antioxidants and healthy lifestyle choices can help protect sperm motility (142). As previously mentioned, unhealthy lifestyle habits and endogenous sources such as immature spermatozoa and leukocytes contribute to excessive ROS in idiopathic asthenozoospermia (143–145).
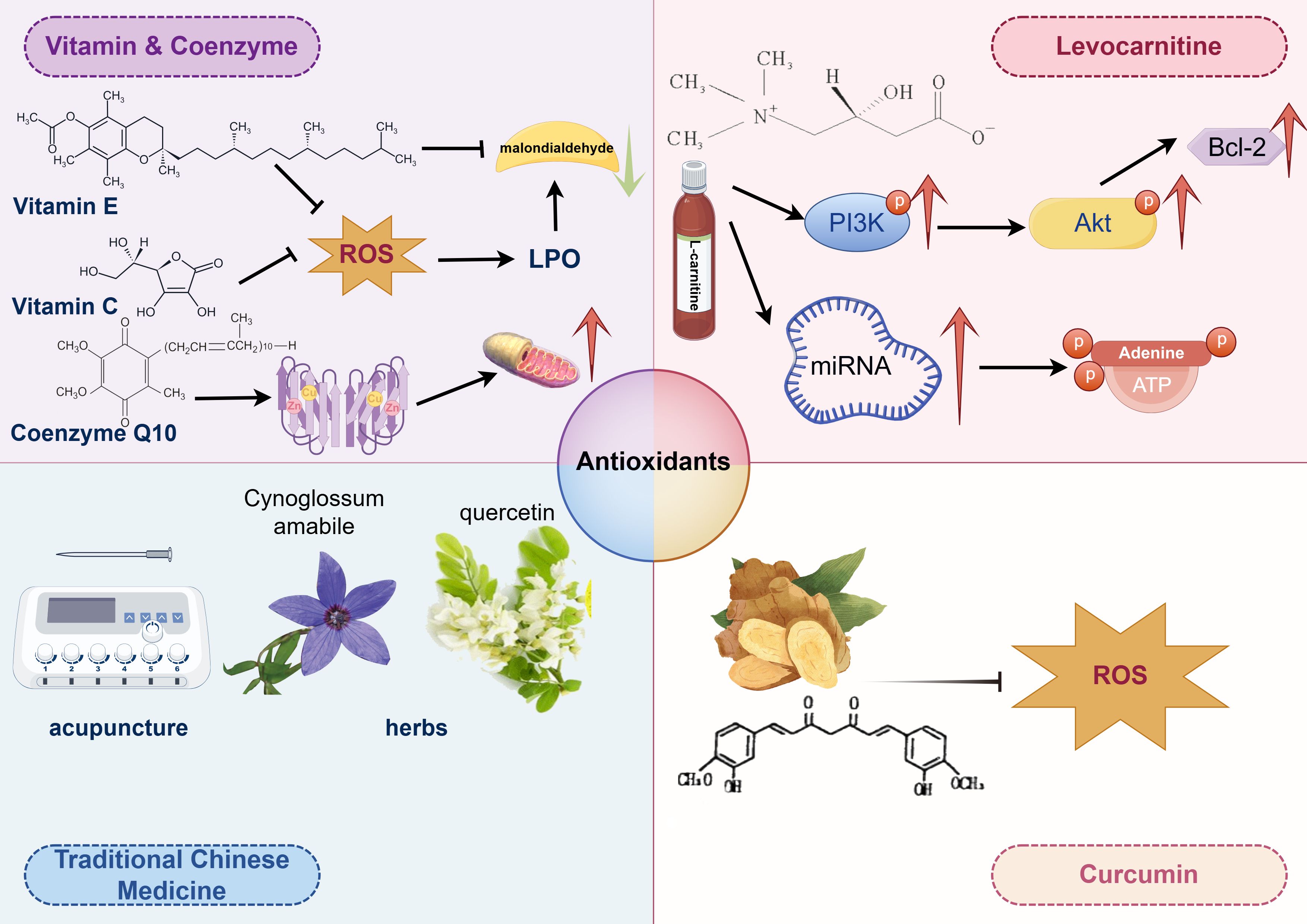
Antioxidants mainly function by suppressing ROS levels, inactivating ROS generated by metabolic processes and enzymatic reactions, thereby preventing lipid peroxidative damage to the plasma membrane of human spermatozoa (36, 37). Most antioxidants primarily have positive effects on reducing ROS levels (146), while a few can also repair oxidative stress damage in human spermatozoa caused by excessive ROS. Agarwal et al. found that approximately 85.6% of urologists and andrologists prescribed oral antioxidants to patients with abnormal semen parameters (147), demonstrating therapeutic effects on idiopathic asthenozoospermia (148, 149). Besides improving sperm motility, antioxidants may also upregulate the expression of fertility-associated sperm proteins in patients with idiopathic asthenozoospermia (150). Antioxidants protecting sperm motility include vitamins E and C, glutathione, hypotaurine, albumin, taurine, as well as superoxide dismutase (SOD) and catalase, while those elevating sperm motility are CoQ10 and N-acetyl cysteine (Figure 3) (12).
5.1 Vitamin C
Vitamin C, as an antioxidant, plays a critical role in alleviating oxidative stress, a recognized factor contributing to male infertility. It safeguards sperm from oxidative damage, thereby enhancing sperm quality and motility. Combination therapies that include Vitamin C have demonstrated promising results in improving sperm motility. For example, one study reported that while individual parameters such as sperm concentration and motility did not show significant changes, a regimen incorporating multiple antioxidants, including Vitamin C, significantly increased the total number of motile sperm (151). Another study emphasized the role of Vitamin C as an adjunct therapy following varicocelectomy, where it significantly improved sperm motility and morphology, highlighting its potential to enhance sperm quality post-surgery (152). Vitamin C effectively mitigates the adverse effects of environmental stressors, such as cigarette smoke and tetrahydrocannabinol exposure. It improved the motility and morphology of sperm exposed to cigarette smoke, underscoring its protective antioxidant properties (153). In vitro studies further demonstrated that Vitamin C could alleviate reductions in sperm motility and kinematics caused by tetrahydrocannabinol, further supporting its role in protecting sperm from various stressors (Figure 3) (153).
5.2 Vitamin E
Vitamin E serves as an oxygen radical scavenger, protecting sperm motility from reactive oxygen species (ROS)-mediated oxidative stress. It prevents the propagation of ROS, thus ensuring the integrity of the membrane and plasma lipoproteins of human spermatozoa (154). The level of malondialdehyde (MDA), a biomarker of lipid peroxidation (LPO) in oxidative stress, can be reduced by Vitamin E, thereby improving sperm motility (155). Additionally, Vitamin E can prevent DNA damage and fragmentation in human spermatozoa and their mitochondria caused by ROS (156, 157). Therefore, Vitamin E is potentially an effective treatment strategy for idiopathic asthenozoospermia, warranting a level B recommendation (Figure 3).
5.3 Coenzyme Q10
Coenzyme Q10 (CoQ10) primarily participates in the electron transport of oxidative phosphorylation during the respiratory process (158). It receives electrons from complex I and complex II, transferring them to complex III, to generate sufficient ATP necessary for maintaining sperm motility. Additionally, CoQ10 plays a role in transferring protons from fatty acids to the matrix (159). CoQ10 may positively influence nutrient uptake through the outer mitochondrial membrane, supporting the mitochondrial function of human spermatozoa (Figure 3) (160, 161).
5.4 Levocarnitine
Levocarnitine is a naturally occurring compound that has been demonstrated to enhance sperm motility, rendering it a promising candidate for the treatment of asthenozoospermia. The enhancement in sperm motility is attributed to several mechanisms, notably its role in energy metabolism and its influence on various molecular pathways. Specifically, levocarnitine upregulates the expression of PI3K, p-Akt, and BCL-2 proteins, thereby decreasing sperm cell apoptosis and improving both sperm count and motility (162). Additionally, levocarnitine modulates the expression of specific miRNAs, such as Hsa-mir-27b-3p and hsa-MIR-206, which are integral to energy metabolism pathways like ATP synthase activity and cAMP signaling. These pathways are crucial for sperm motility, providing a molecular foundation for the effectiveness of levocarnitine in the treatment of asthenozoospermia (163). In a randomized controlled trial, levocarnitine significantly enhanced sperm motility, morphology, and concentration when compared to coenzyme Q10 and vitamin E (164). It also increased testosterone and luteinizing hormone levels, suggesting a more extensive hormonal impact. A meta-analysis corroborated that levocarnitine and its derivatives substantially im-prove sperm motility and morphology relative to placebo, albeit without significant effects on serum hormone levels (165). Although levocarnitine demonstrates significant potential in treating asthenozoospermia, its precise molecular mechanisms remain partially elucidated, necessitating additional research to fully explore its capabilities and long-term effects. Moreover, while levocarnitine is efficacious, its combination with other therapeutic modalities may enhance its benefits, indicating that a multifaceted approach could be more beneficial for patients (Figure 3).
5.5 Curcumin
Curcumin, a natural compound derived from Curcuma longa (turmeric), exhibits numerous biological effects, including anti-inflammatory, antioxidant, anti-proliferative, and anti-metastatic activities. It is also recognized as a scavenger of reactive oxygen species (ROS) in both in vitro and in vivo settings (166, 167). Adequate levels of curcumin can enhance sperm motility by binding to promoters of antioxidant genes, thereby promoting the release of antioxidative enzymes and upregulating the expression of these genes to suppress ROS generation (158). It is also believed to aid in the cryopreservation of spermatozoa. However, excessive curcumin has been reported to mediate oxidative stress in the testes of rats. Notably, it is renowned as a potent non-steroidal contraceptive due to its ability to block sperm motility within the female reproductive tract (168). Therefore, the dosage of curcumin is crucial for regulating sperm motility (Figure 3).
5.6 Traditional Chinese medicine
The clinical application of Traditional Chinese Medicine in enhancing sperm motility involves a multifaceted approach combining herbal medicine, acupuncture, and integrative therapies. These methods have shown promising results in improving sperm motility and overall semen quality, providing a complementary treatment option for male infertility. One study indicated that acupuncture at the Fuxi point combined with tamoxifen citrate tablets significantly improved sperm motility parameters in patients with asthenozoospermia. This combination therapy enhanced sperm motility, average path velocity, and the percentage of motile sperm, outperforming tamoxifen alone (169). Cynoglossum amabile, a traditional Chinese herb, contains bioactive compounds with various pharmacological activities, including anti-inflammatory and cardiovascular effects. Although there is insufficient evidence for its direct application to sperm motility, its traditional use in treating reproductive issues suggests potential benefits (Figure 3) (170).
The multi-target approach of Traditional Chinese Medicine, involving compounds like kaempferol and quercetin, has been shown to regulate hormones, reduce oxidative stress, and improve sperm quality. These components are integral to the effectiveness of Traditional Chinese Medicine in treating male infertility (171). An integrated approach combining data mining, network pharmacology, and experimental validation has identified key components and mechanisms of Traditional Chinese Medicine prescriptions that enhance sperm motility. This approach underscores the multi-component, multi-target strategy of Traditional Chinese Medicine in treating male infertility (171).
While Traditional Chinese Medicine offers promising avenues for improving sperm motility, potential risks must be considered, such as hepatotoxicity associated with certain herbs like Cynoglossum amabile. Further research and clinical trials are needed to validate these therapies and ensure their safety and efficacy in broader applications (170).
6 The limitations and future prospects
Despite significant research into the relationship between ROS and sperm motility, several limitations have also persisted. For instance, accurately measuring ROS levels in semen and effectively assessing the efficacy of antioxidant treatments require further investigation (16). The short half-life of ROS poses challenges for their direct detection in human specimens (8). In addition, despite the availability of various antioxidant therapies for treating idiopathic asthenozoospermia, there is still no more effective clinical strategy to develop sperm motility. Furthermore, individual variability and the complex mechanisms of ROS and oxidative stress add to the challenges of research in this area (16). Consequently, future studies should delve deeper into the specific mechanisms by which oxidative stress affects sperm motility. The development of more effective diagnostic and therapeutic strategies is crucial to enhancing treatment outcomes for male infertility.
In summary, ROS plays a dual role in maintaining sperm motility. A moderate amount of ROS is essential for normal sperm function as they participate in energy acquisition, motility, and the capacitation process. However, excessive ROS can lead to oxidative stress, damaging the lipid bilayer structure of sperm membranes, impairing mitochondrial function, and affecting DNA integrity, which significantly reduces sperm motility and fertilization capacity. Therefore, maintaining an appropriate balance of ROS is crucial for ensuring male reproductive health. Further research should focus on exploring the potential benefits of antioxidant supplementation and its application in improving sperm quality and enhancing the effectiveness of male infertility treatments.
Author contributions
ZW: Conceptualization, Data curation, Formal Analysis, Writing – original draft. DL: Data curation, Formal Analysis, Investigation, Writing – original draft. GZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. ZX: Data curation, Methodology, Project administration, Resources, Writing – original draft. XW: Data curation, Methodology, Writing – original draft. ST: Formal Analysis, Writing – original draft. ZL: Formal Analysis, Investigation, Writing – original draft. XL: Supervision, Validation, Visualization, Writing – review & editing. CS: Funding acquisition, Software, Supervision, Validation, Visualization, Writing – review & editing. SY: Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Hospital Research Fund of SAHSYSU (ZSQYLCKYJJ202317), Research Start-up Fund of Post-doctoral of Shenzhen Municipality and Lateral Research Project of Sun Yat-sen University (ZSQY-XQ-2309-0884)
Acknowledgments
The figures were created using Figdraw 2.0 on Home for Researchers. We thank the investigators whose studies have not yet been cited.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bjorndahl L and Kirkman BJ. The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: ensuring quality and standardization in basic examination of human ejaculates. Fertil Steril. (2022) 117:246–51. doi: 10.1016/j.fertnstert.2021.12.012
2. Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. (2021) 80:603–20. doi: 10.1016/j.eururo.2021.08.014
3. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization (2010). 271 p.
4. Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Jolles M, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update. (2022). doi: 10.1093/humupd/dmac035
5. Jin ZR, Fang D, Liu BH, Cai J, Tang WH, Jiang H, et al. Roles of CatSper channels in the pathogenesis of asthenozoospermia and the therapeutic effects of acupuncture-like treatment on asthenozoospermia. Theranostics. (2021) 11:2822–44. doi: 10.7150/thno.51869
6. Zhang B, Ma H, Khan T, Ma A, Li T, Zhang H, et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J Exp Med. (2020) 217. doi: 10.1084/jem.20182365
7. Sinha K, Das J, Pal PB, and Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. (2013) 87:1157–80. doi: 10.1007/s00204-013-1034-4
8. Dickinson BC and Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. (2011) 7:504–11. doi: 10.1038/nchembio.607
9. Han C, Wang Z, Xu Y, Chen S, Han Y, Li L, et al. Roles of reactive oxygen species in biological behaviors of prostate cancer. BioMed Res Int. (2020) 2020:1269624. doi: 10.1155/2020/1269624
10. Han C, Wang Z, Chen S, Li L, Xu Y, Kang W, et al. Berbamine suppresses the progression of bladder cancer by modulating the ROS/NF-kappaB axis. Oxid Med Cell Longev. (2021) 2021:8851763. doi: 10.1155/2021/8851763
11. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
12. Bisht S, Faiq M, Tolahunase M, and Dada R. Oxidative stress and male infertility. Nat Rev Urol. (2017) 14:470–85. doi: 10.1038/nrurol.2017.69
13. Bui AD, Sharma R, Henkel R, and Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. (2018) 50:e13012. doi: 10.1111/and.13012
14. Bravo A, Quilaqueo N, Jofre I, and Villegas JV. Overtime expression of plasma membrane and mitochondrial function markers associated with cell death in human spermatozoa exposed to nonphysiological levels of reactive oxygen species. Andrologia. (2021) 53:e13907. doi: 10.1111/and.13907
15. Kobashigawa S, Kashino G, Suzuki K, Yamashita S, and Mori H. Ionizing radiation-induced cell death is partly caused by increase of mitochondrial reactive oxygen species in normal human fibroblast cells. Radiat Res. (2015) 183:455–64. doi: 10.1667/RR13772.1
16. Barati E, Nikzad H, and Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol Life Sci. (2020) 77:93–113. doi: 10.1007/s00018-019-03253-8
17. Aitken RJ and Bakos HW. Should we be measuring DNA damage in human spermatozoa? New light on an old question. Hum Reprod. (2021) 36:1175–85. doi: 10.1093/humrep/deab004
18. Chakraborty S and Roychoudhury S. Pathological roles of reactive oxygen species in male reproduction. Adv Exp Med Biol. (2022) 1358:41–62. doi: 10.1007/978-3-030-89340-8_3
19. Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. (2014) 12:33. doi: 10.1186/1477-7827-12-33
20. Aitken RJ, Gibb Z, Baker MA, Drevet J, and Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. (2016) 28:1–10. doi: 10.1071/RD15325
21. Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, et al. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. (1998) 59:1037–46. doi: 10.1095/biolreprod59.5.1037
22. Kovalski NN, de Lamirande E, and Gagnon C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: protective effect of seminal plasma and scavengers. Fertil Steril. (1992) 58:809–16. doi: 10.1016/S0015-0282(16)55332-1
23. Guerriero G, Trocchia S, Abdel-Gawad FK, and Ciarcia G. Roles of reactive oxygen species in the spermatogenesis regulation. Front Endocrinol (Lausanne). (2014) 5:56. doi: 10.3389/fendo.2014.00056
24. Shi Y, Buffenstein R, Pulliam DA, and Van Remmen H. Comparative studies of oxidative stress and mitochondrial function in aging. Integr Comp Biol. (2010) 50:869–79. doi: 10.1093/icb/icq079
25. Fujii J and Imai H. Redox reactions in mammalian spermatogenesis and the potential targets of reactive oxygen species under oxidative stress. Spermatogenesis. (2014) 4:e979108. doi: 10.4161/21565562.2014.979108
26. Lui WY and Cheng CY. Transcription regulation in spermatogenesis. Adv Exp Med Biol. (2008) 636:115–32. doi: 10.1007/978-0-387-09597-4_7
27. Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. (2005) 436:1030–34. doi: 10.1038/nature03894
28. Grimes SR. Testis-specific transcriptional control. Gene. (2004) 343:11–22. doi: 10.1016/j.gene.2004.08.021
29. Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, and Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. (2017) 38:592–607. doi: 10.1016/j.tips.2017.04.005
30. Montano MM, Deng H, Liu M, Sun X, and Singal R. Transcriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implications. Oncogene. (2004) 23:2442–53. doi: 10.1038/sj.onc.1207358
31. Saowaros W and Panyim S. The formation of disulfide bonds in human protamines during sperm maturation. Experientia. (1979) 35:191–92. doi: 10.1007/BF01920608
32. Hutchison JM, Rau DC, and DeRouchey JE. Role of disulfide bonds on DNA packaging forces in bull sperm chromatin. Biophys J. (2017) 113:1925–33. doi: 10.1016/j.bpj.2017.08.050
33. Puglisi R, Tramer F, Carlomagno G, Gandini L, Panfili E, Stefanini M, et al. PHGPx in spermatogenesis: how many functions? Contraception. (2005) 72:291–93. doi: 10.1016/j.contraception.2005.03.002
34. Roveri A, Ursini F, Flohe L, and Maiorino M. PHGPx and spermatogenesis. Biofactors. (2001) 14:213–22. doi: 10.1002/biof.5520140127
35. Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zoolog Sci. (2003) 20:1043–56. doi: 10.2108/zsj.20.1043
36. Ihsan AU, Khan FU, Khongorzul P, Ahmad KA, Naveed M, Yasmeen S, et al. Role of oxidative stress in pathology of chronic prostatitis/chronic pelvic pain syndrome and male infertility and antioxidants function in ameliorating oxidative stress. BioMed Pharmacother. (2018) 106:714–23. doi: 10.1016/j.biopha.2018.06.139
37. Amaral A, Ramalho-Santos J, and St JJ. The expression of polymerase gamma and mitochondrial transcription factor A and the regulation of mitochondrial DNA content in mature human sperm. Hum Reprod. (2007) 22:1585–96. doi: 10.1093/humrep/dem030
38. Goldberg E, Eddy EM, Duan C, and Odet F. LDHC: the ultimate testis-specific gene. J Androl. (2010) 31:86–94. doi: 10.2164/jandrol.109.008367
39. Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. (2017) 84:1039–52. doi: 10.1002/mrd.22871
40. Ferramosca A and Zara V. Bioenergetics of mammalian sperm capacitation. BioMed Res Int. (2014) 2014:902953. doi: 10.1155/2014/902953
41. Barbagallo F, La Vignera S, Cannarella R, Aversa A, Calogero AE, and Condorelli RA. Evaluation of sperm mitochondrial function: A key organelle for sperm motility. J Clin Med. (2020) 9. doi: 10.3390/jcm9020363
42. Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, and Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab. (2008) 93:3199–207. doi: 10.1210/jc.2007-2616
43. Du Plessis SS, Agarwal A, Halabi J, and Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. (2015) 32:509–20. doi: 10.1007/s10815-014-0425-7
44. du Plessis SS, Agarwal A, Mohanty G, and van der Linde M. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J Androl. (2015) 17:230–35. doi: 10.4103/1008-682X.135123
45. Shamsi MB, Kumar R, Bhatt A, Bamezai RN, Kumar R, Gupta NP, et al. Mitochondrial DNA Mutations in etiopathogenesis of male infertility. Indian J Urol. (2008) 24:150–54. doi: 10.4103/0970-1591.40606
46. Wink DA and Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. (1998) 25:434–56. doi: 10.1016/s0891-5849(98)00092-6
47. Allkanjari K and Baldock RA. Beyond base excision repair: an evolving picture of mitochondrial DNA repair. Biosci Rep. (2021) 41. doi: 10.1042/BSR20211320
48. Dianov GL, Souza-Pinto N, Nyaga SG, Thybo T, Stevnsner T, and Bohr VA. Base excision repair in nuclear and mitochondrial DNA. Prog Nucleic Acid Res Mol Biol. (2001) 68:285–97. doi: 10.1016/s0079-6603(01)68107-8
49. Kumar N. Sperm mitochondria, the driving force behind human spermatozoa activities: its functions and dysfunctions - A narrative review. Curr Mol Med. (2023) 23:332–40. doi: 10.2174/1566524022666220408104047
50. Mirshahvaladi S, Topraggaleh TR, Bucak MN, Rahimizadeh P, and Shahverdi A. Quantitative proteomics of sperm tail in asthenozoospermic patients: exploring the molecular pathways affecting sperm motility. Cell Tissue Res. (2023) 392:793–810. doi: 10.1007/s00441-023-03744-y
51. Hussain T, Kandeel M, Metwally E, Murtaza G, Kalhoro DH, Yin Y, et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front Endocrinol (Lausanne). (2023) 14:1070692. doi: 10.3389/fendo.2023.1070692
52. Faja F, Carlini T, Coltrinari G, Finocchi F, Nespoli M, Pallotti F, et al. Human sperm motility: a molecular study of mitochondrial DNA, mitochondrial transcription factor A gene and DNA fragmentation. Mol Biol Rep. (2019) 46:4113–21. doi: 10.1007/s11033-019-04861-0
53. Nagata O, Nakamura M, Sakimoto H, Urata Y, Sasaki N, Shiokawa N, et al. Mouse model of chorea-acanthocytosis exhibits male infertility caused by impaired sperm motility as a result of ultrastructural morphological abnormalities in the mitochondrial sheath in the sperm midpiece. Biochem Biophys Res Commun. (2018) 503:915–20. doi: 10.1016/j.bbrc.2018.06.096
54. Zhang L, Sun Y, Jiang C, Sohail T, Sun X, Wang J, et al. Damage to mitochondria during the cryopreservation, causing ROS leakage, leading to oxidative stress and decreased quality of ram sperm. Reprod Domest Anim. (2024) 59:e14737. doi: 10.1111/rda.14737
55. Vertika S, Singh KK, and Rajender S. Mitochondria, spermatogenesis, and male infertility - An update. Mitochondrion. (2020) 54:26–40. doi: 10.1016/j.mito.2020.06.003
56. Shukla KK, Mahdi AA, and Rajender S. Apoptosis, spermatogenesis and male infertility. Front Biosci (Elite Ed). (2012) 4:746–54. doi: 10.2741/415
57. Aitken RJ and Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol. (2013) 57:265–72. doi: 10.1387/ijdb.130146ja
58. Moradian FZ, Naghdi M, Salehi P, Shahrokhi SZ, Ajami A, Deemeh MR, et al. SCSA results correlated with rate of motility reduction after ejaculation in Asthenozoospermia. Andrologia. (2019) 51:e13146. doi: 10.1111/and.13146
59. Saraswat M, Joenvaara S, Jain T, Tomar AK, Sinha A, Singh S, et al. Human spermatozoa quantitative proteomic signature classifies normo- and asthenozoospermia. Mol Cell Proteomics. (2017) 16:57–72. doi: 10.1074/mcp.M116.061028
60. Moretti E, Collodel G, Fiaschi AI, Micheli L, Iacoponi F, and Cerretani D. Nitric oxide, malondialdheyde and non-enzymatic antioxidants assessed in viable spermatozoa from selected infertile men. Reprod Biol. (2017) 17:370–75. doi: 10.1016/j.repbio.2017.10.003
61. Aitken RJ, Harkiss D, and Buckingham D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil. (1993) 98:257–65. doi: 10.1530/jrf.0.0980257
62. Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, and Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. (2018) 50:e13126. doi: 10.1111/and.13126
63. Potts RJ, Notarianni LJ, and Jefferies TM. Seminal plasma reduces exogenous oxidative damage to human sperm, determined by the measurement of DNA strand breaks and lipid peroxidation. Mutat Res. (2000) 447:249–56. doi: 10.1016/s0027-5107(99)00215-8
64. Adeoye O, Olawumi J, Opeyemi A, and Christiania O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Reprod. (2018) 22:61–6. doi: 10.5935/1518-0557.20180003
65. Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, and Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J. (2007) 93:4225–36. doi: 10.1529/biophysj.107.112565
66. Parrella A, Medrano L, Aizpurua J, and Gomez-Torres MJ. Phospholipase C zeta in human spermatozoa: A systematic review on current development and clinical application. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25021344
67. Aitken RJ. Sperm DNA integrity: a special issue exploring the causes, consequences, and treatment of DNA damage in human spermatozoa. Andrology. (2023) 11:1541–44. doi: 10.1111/andr.13503
68. An BGL, Chapman M, Tilia L, and Venetis C. Is there an optimal window of time for transferring single frozen-thawed euploid blastocysts? A cohort study of 1170 embryo transfers. Hum Reprod. (2022) 37:2797–807. doi: 10.1093/humrep/deac227
69. Paasch U, Sharma RK, Gupta AK, Grunewald S, Mascha EJ, Thomas AJ, et al. Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol Reprod. (2004) 71:1828–37. doi: 10.1095/biolreprod.103.025627
70. Mehdi M, Khantouche L, Ajina M, and Saad A. Detection of DNA fragmentation in human spermatozoa: correlation with semen parameters. Andrologia. (2009) 41:383–86. doi: 10.1111/j.1439-0272.2009.00953.x
71. Middelkamp S, van Tol HTA, Spierings DCJ, Boymans S, Guryev V, Roelen BAJ, et al. Sperm DNA damage causes genomic instability in early embryonic development. Sci Adv. (2020) 6:eaaz7602. doi: 10.1126/sciadv.aaz7602
72. Wang Z, Yu J, Zhu W, Hong X, Xu Z, Mao S, et al. Unveiling the mysteries of extrachromosomal circular DNA: from generation to clinical relevance in human cancers and health. Mol Cancer. (2024) 23:276. doi: 10.1186/s12943-024-02187-5
73. Zhang F, Li J, Liang Z, Wu J, Li L, Chen C, et al. Sperm DNA fragmentation and male fertility: a retrospective study of 5114 men attending a reproductive center. J Assist Reprod Genet. (2021) 38:1133–41. doi: 10.1007/s10815-021-02120-5
74. Baldi E, Gallagher MT, Krasnyak S, and Kirkman-Brown J. Extended semen examinations in the sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: contributing to the understanding of the function of the male reproductive system. Fertil Steril. (2022) 117:252–57. doi: 10.1016/j.fertnstert.2021.11.034
75. Vasconcelos AL, Campbell MJ, Barratt CLR, and Gellatly SA. Do studies published in two leading reproduction journals between 2011 and 2020 demonstrate that they followed WHO5 recommendations for basic semen analysis? Hum Reprod. (2022) 37:2255–63. doi: 10.1093/humrep/deac173
76. Takeshima T, Usui K, Mori K, Asai T, Yasuda K, Kuroda S, et al. Oxidative stress and male infertility. Reprod Med Biol. (2021) 20:41–52. doi: 10.1002/rmb2.12353
77. Wang T, Gao H, Li W, and Liu C. Essential role of histone replacement and modifications in male fertility. Front Genet. (2019) 10:962. doi: 10.3389/fgene.2019.00962
78. Bao J and Bedford MT. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction. (2016) 151:R55–70. doi: 10.1530/REP-15-0562
79. Jodar M, Kalko S, Castillo J, Ballesca JL, and Oliva R. Differential RNAs in the sperm cells of asthenozoospermic patients. Hum Reprod. (2012) 27:1431–38. doi: 10.1093/humrep/des021
80. Derbel R, Sellami H, Sakka R, Ben Slima A, Mkaddem I, Gdoura R, et al. Relationship between nuclear DNA fragmentation, mitochondrial DNA damage and standard sperm parameters in spermatozoa of infertile patients with leukocytospermia. J Gynecol Obstet Hum Reprod. (2021) 50:102101. doi: 10.1016/j.jogoh.2021.102101
81. Hallam J, Burton P, and Sanders K. Poor sperm chromatin condensation is associated with cryopreservation-induced DNA fragmentation and cell death in human spermatozoa. J Clin Med. (2024) 13. doi: 10.3390/jcm13144156
82. Yassine S, Escoffier J, Martinez G, Coutton C, Karaouzene T, Zouari R, et al. Dpy19l2-deficient globozoospermic sperm display altered genome packaging and DNA damage that compromises the initiation of embryo development. Mol Hum Reprod. (2015) 21:169–85. doi: 10.1093/molehr/gau099
83. Ozkosem B, Feinstein SI, Fisher AB, and O’Flaherty C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. (2015) 5:15–23. doi: 10.1016/j.redox.2015.02.004
84. Parte PP, Rao P, Redij S, Lobo V, D’Souza SJ, Gajbhiye R, et al. Sperm phosphoproteome profiling by ultra performance liquid chromatography followed by data independent analysis (LC-MS(E)) reveals altered proteomic signatures in asthenozoospermia. J Proteomics. (2012) 75:5861–71. doi: 10.1016/j.jprot.2012.07.003
85. Martin-Hidalgo D, Serrano R, Zaragoza C, Garcia-Marin LJ, and Bragado MJ. Human sperm phosphoproteome reveals differential phosphoprotein signatures that regulate human sperm motility. J Proteomics. (2020) 215:103654. doi: 10.1016/j.jprot.2020.103654
86. Naz RK and Rajesh PB. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocrinol. (2004) 2:75. doi: 10.1186/1477-7827-2-75
87. Lefievre L, Jha KN, de Lamirande E, Visconti PE, and Gagnon C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J Androl. (2002) 23:709–16. doi: 10.1002/j.1939-4640.2002.tb02314.x
88. Pereira R, Sa R, Barros A, and Sousa M. Major regulatory mechanisms involved in sperm motility. Asian J Androl. (2017) 19:5–14. doi: 10.4103/1008-682X.167716
89. Pozdniakova S and Ladilov Y. Functional significance of the Adcy10-dependent intracellular cAMP compartments. J Cardiovasc Dev Dis. (2018) 5. doi: 10.3390/jcdd5020029
90. Fiedler SE, Dudiki T, Vijayaraghavan S, and Carr DW. Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol Reprod. (2013) 88:41. doi: 10.1095/biolreprod.112.105262
91. Xu K, Yang L, Zhao D, Wu Y, and Qi H. AKAP3 synthesis is mediated by RNA binding proteins and PKA signaling during mouse spermiogenesis. Biol Reprod. (2014) 90:119. doi: 10.1095/biolreprod.113.116111
92. Nsota MJ, Coutton C, Arnoult C, Ray PF, and Toure A. Genetic causes of male infertility: snapshot on morphological abnormalities of the sperm flagellum. Basic Clin Androl. (2019) 29:2. doi: 10.1186/s12610-019-0083-9
93. Maurya S, Kesari KK, Roychoudhury S, Kolleboyina J, Jha NK, Jha SK, et al. Metabolic dysregulation and sperm motility in male infertility. Adv Exp Med Biol. (2022) 1358:257–73. doi: 10.1007/978-3-030-89340-8_12
94. Miller MR, Kenny SJ, Mannowetz N, Mansell SA, Wojcik M, Mendoza S, et al. Asymmetrically positioned flagellar control units regulate human sperm rotation. Cell Rep. (2019) 26:2847. doi: 10.1016/j.celrep.2019.02.075
95. Guo Y, Jiang W, Yu W, Niu X, Liu F, Zhou T, et al. Proteomics analysis of asthenozoospermia and identification of glucose-6-phosphate isomerase as an important enzyme for sperm motility. J Proteomics. (2019) 208:103478. doi: 10.1016/j.jprot.2019.103478
96. Chen L, Wen C, Deng M, Ping-Li, Zhang Z, Zhou Z, et al. Metabolic and transcriptional changes in seminal plasma of asthenozoospermia patients. BioMed Chromatogr. (2020) 34:e4769. doi: 10.1002/bmc.4769
97. Chen S, Allam J, Duan Y, and Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. (2013) 288:191–99. doi: 10.1007/s00404-013-2801-4
98. Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. (2001) 8:851–62. doi: 10.2174/0929867013373039
99. Sengupta P, Roychoudhury S, Nath M, and Dutta S. Oxidative stress and idiopathic male infertility. Adv Exp Med Biol. (2022) 1358:181–204. doi: 10.1007/978-3-030-89340-8_9
100. Agarwal A, Parekh N, Panner SM, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. (2019) 37:296–312. doi: 10.5534/wjmh.190055
101. Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. (2011) 13:43–52. doi: 10.1038/aja.2010.76
102. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. (2003) 552:335–44. doi: 10.1113/jphysiol.2003.049478
103. Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. (2000) 45:314–20.
104. Agarwal A, Virk G, Ong C, and du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. (2014) 32:1–17. doi: 10.5534/wjmh.2014.32.1.1
105. Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, et al. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem. (2012) 287:9376–88. doi: 10.1074/jbc.M111.314955
106. Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. (2001) 16:1922–30. doi: 10.1093/humrep/16.9.1922
107. Rengan AK, Agarwal A, van der Linde M, and du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. (2012) 10:92. doi: 10.1186/1477-7827-10-92
108. Golas A, Malek P, Piasecka M, and Styrna J. Sperm mitochondria diaphorase activity–a gene mapping study of recombinant inbred strains of mice. Int J Dev Biol. (2010) 54:667–73. doi: 10.1387/ijdb.082778ag
109. Aitken RJ and Gibb Z. Sperm oxidative stress in the context of male infertility: current evidence, links with genetic and epigenetic factors and future clinical needs. Minerva Endocrinol (Torino). (2022) 47:38–57. doi: 10.23736/S2724-6507.21.03630-7
110. Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, and Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod BioMed. (2016) 14:231–40. doi: 10.29252/ijrm.14.4.231
111. Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. (2003) 79 Suppl 3:1597–605. doi: 10.1016/s0015-0282(03)00337-6
112. Das A and Roychoudhury S. Reactive oxygen species in the reproductive system: sources and physiological roles. Adv Exp Med Biol. (2022) 1358:9–40. doi: 10.1007/978-3-030-89340-8_2
113. Veron GL, Tissera AD, Bello R, Estofan GM, Hernandez M, Beltramone F, et al. Association between meteorological variables and semen quality: a retrospective study. Int J Biometeorol. (2021) 65:1399–414. doi: 10.1007/s00484-021-02112-1
114. Jalili C, Abbasi A, Rahmani-Kukia N, Andarzi S, Kakebaraie S, and Zamir Nasta T. The relationship between aflatoxin B1 with the induction of extrinsic/intrinsic pathways of apoptosis and the protective role of taraxasterol in TM3 leydig cell line. Ecotoxicol Environ Saf. (2024) 276:116316. doi: 10.1016/j.ecoenv.2024.116316
115. Zhou Z, Kawabe H, Suzuki A, Shinmyozu K, and Saga Y. NEDD4 controls spermatogonial stem cell homeostasis and stress response by regulating messenger ribonucleoprotein complexes. Nat Commun. (2017) 8:15662. doi: 10.1038/ncomms15662
116. Deng C, Zhu J, Fang Z, Yang Y, Zhao Q, Zhang Z, et al. Identification and analysis of microplastics in para-tumor and tumor of human prostate. EBioMedicine. (2024) 108:105360. doi: 10.1016/j.ebiom.2024.105360
117. Zhao Q, Zhu L, Weng J, Jin Z, Cao Y, Jiang H, et al. Detection and characterization of microplastics in the human testis and semen. Sci Total Environ. (2023) 877:162713. doi: 10.1016/j.scitotenv.2023.162713
118. Kumar SB, Chawla B, Bisht S, Yadav RK, and Dada R. Tobacco use increases oxidative DNA damage in sperm - possible etiology of childhood cancer. Asian Pac J Cancer Prev. (2015) 16:6967–72. doi: 10.7314/apjcp.2015.16.16.6967
119. Whitacre DM. Reviews of environmental contamination and toxicology. Preface. Rev Environ Contam Toxicol. (2010) 204:vii–viii. doi: 10.1007/978-1-4419-1440-8
120. Lee E, Ahn MY, Kim HJ, Kim IY, Han SY, Kang TS, et al. Effect of di(n-butyl) phthalate on testicular oxidative damage and antioxidant enzymes in hyperthyroid rats. Environ Toxicol. (2007) 22:245–55. doi: 10.1002/tox.20259
121. Jeng HA, Sikdar S, Huang Y, and Pan C. Mixture analysis of associations between exposure to low levels of multiple metals and semen quality and sperm DNA integrity. J Environ Sci Health Tox Hazard Subst Environ Eng. (2022) 57:318–26. doi: 10.1080/10934529.2022.2061256
122. Huang G, Zhang Q, Wu H, Wang Q, Chen Y, Guo P, et al. Sperm quality and ambient air pollution exposure: A retrospective, cohort study in a Southern province of China. Environ Res. (2020) 188:109756. doi: 10.1016/j.envres.2020.109756
123. Wong EWP and Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. (2011) 32:290–99. doi: 10.1016/j.tips.2011.01.001
124. Uwamahoro C, Jo J, Jang S, Jung E, Lee W, Bae J, et al. Assessing the risks of pesticide exposure: implications for endocrine disruption and male fertility. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25136945
125. Knapke ET, Magalhaes DDP, Dalvie MA, Mandrioli D, and Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology. (2022) 465:153017. doi: 10.1016/j.tox.2021.153017
126. Martenies SE and Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: a systematic review. Toxicology. (2013) 307:66–73. doi: 10.1016/j.tox.2013.02.005
127. Fang Q, Wang C, and Xiong Y. Polystyrene microplastics induce male reproductive toxicity in mice by activating spermatogonium mitochondrial oxidative stress and apoptosis. Chem Biol Interact. (2024) 396:111043. doi: 10.1016/j.cbi.2024.111043
128. Yang W, Duan Z, Li G, Geng H, Gao Y, Shen Q, et al. Association of lifestyle and occupational exposure factors with human semen quality: a cross-sectional study of 1060 participants. Syst Biol Reprod Med. (2024) 70:150–63. doi: 10.1080/19396368.2024.2357348
129. Rato L, Alves MG, Cavaco JE, and Oliveira PF. High-energy diets: a threat for male fertility? Obes Rev. (2014) 15:996–1007. doi: 10.1111/obr.12226
130. Salmon AB. Beyond diabetes: does obesity-induced oxidative stress drive the aging process? Antioxidants (Basel). (2016) 5. doi: 10.3390/antiox5030024
131. Sharma R, Harlev A, Agarwal A, and Esteves SC. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 world health organization laboratory methods for the examination of human semen. Eur Urol. (2016) 70:635–45. doi: 10.1016/j.eururo.2016.04.010
132. Guthauser B, Boitrelle F, Plat A, Thiercelin N, and Vialard F. Chronic excessive alcohol consumption and male fertility: a case report on reversible azoospermia and a literature review. Alcohol. (2014) 49:42–4. doi: 10.1093/alcalc/agt133
133. Manzo-Avalos S and Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. (2010) 7:4281–304. doi: 10.3390/ijerph7124281
134. Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, et al. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol. (2006) 291:G857–67. doi: 10.1152/ajpgi.00044.2006
135. Angelopoulou R, Lavranos G, and Manolakou P. ROS in the aging male: model diseases with ROS-related pathophysiology. Reprod Toxicol. (2009) 28:167–71. doi: 10.1016/j.reprotox.2009.04.003
136. Kesari KK, Agarwal A, and Henkel R. Radiations and male fertility. Reprod Biol Endocrinol. (2018) 16:118. doi: 10.1186/s12958-018-0431-1
137. Gautam R, Singh KV, Nirala J, Murmu NN, Meena R, and Rajamani P. Oxidative stress-mediated alterations on sperm parameters in male Wistar rats exposed to 3G mobile phone radiation. Andrologia. (2019) 51:e13201. doi: 10.1111/and.13201
138. Desai NR, Kesari KK, and Agarwal A. Pathophysiology of cell phone radiation: oxidative stress and carcinogenesis with focus on male reproductive system. Reprod Biol Endocrinol. (2009) 7:114. doi: 10.1186/1477-7827-7-114
139. Chauhan P, Verma HN, Sisodia R, and Kesari KK. Microwave radiation (2.45 GHz)-induced oxidative stress: Whole-body exposure effect on histopathology of Wistar rats. Electromagn Biol Med. (2017) 36:20–30. doi: 10.3109/15368378.2016.1144063
140. Gunes S and Esteves SC. Role of genetics and epigenetics in male infertility. Andrologia. (2021) 53:e13586. doi: 10.1111/and.13586
141. Panner SM, Ambar RF, Agarwal A, and Henkel R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia. (2021) 53:e13706. doi: 10.1111/and.13706
142. Bisconti M, Simon JF, Grassi S, Leroy B, Martinet B, Arcolia V, et al. Influence of risk factors for male infertility on sperm protein composition. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms222313164
143. Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, et al. Male infertility. Lancet. (2021) 397:319–33. doi: 10.1016/S0140-6736(20)32667-2
144. Carson SA and Kallen AN. Diagnosis and management of infertility: A review. JAMA. (2021) 326:65–76. doi: 10.1001/jama.2021.4788
145. Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. (2018) 16:10–20. doi: 10.1016/j.aju.2017.12.004
146. Zini A, San GM, and Baazeem A. Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet. (2009) 26:427–32. doi: 10.1007/s10815-009-9343-5
147. Agarwal A, Finelli R, Selvam M, Leisegang K, Majzoub A, Tadros N, et al. A global survey of reproductive specialists to determine the clinical utility of oxidative stress testing and antioxidant use in male infertility. World J Mens Health. (2021) 39:470–88. doi: 10.5534/wjmh.210025
148. Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Panner SM, et al. Utility of antioxidants in the treatment of male infertility: clinical guidelines based on a systematic review and analysis of evidence. World J Mens Health. (2021) 39:233–90. doi: 10.5534/wjmh.200196
149. Arafa M, Agarwal A, Majzoub A, Panner SM, Baskaran S, Henkel R, et al. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants (Basel). (2020) 9. doi: 10.3390/antiox9030219
150. Agarwal A, Panner SM, Samanta L, Vij SC, Parekh N, Sabanegh E, et al. Effect of antioxidant supplementation on the sperm proteome of idiopathic infertile men. Antioxidants (Basel). (2019) 8. doi: 10.3390/antiox8100488
151. Terai K, Horie S, Fukuhara S, Miyagawa Y, Kobayashi K, and Tsujimura A. Combination therapy with antioxidants improves total motile sperm counts: A Preliminary Study. Reprod Med Biol. (2020) 19:89–94. doi: 10.1002/rmb2.12308
152. Cyrus A, Kabir A, Goodarzi D, and Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J Urol. (2015) 41:230–38. doi: 10.1590/S1677-5538.IBJU.2015.02.07
153. Alagbonsi AI and Olayaki LA. Vitamin C ameliorates tetrahydrocannabinol-induced spermatotoxicity. in-vitro BMC Nutr. (2020) 6:59. doi: 10.1186/s40795-020-00387-y
154. Suleiman SA, Ali ME, Zaki ZM, El-Malik EM, and Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. (1996) 17:530–37. doi: 10.1002/j.1939-4640.1996.tb01830.x
155. Mirzaei KK, Reza TA, Abbasi SA, and Mirjalili A. Protective effect of vitamin E on oxidative stress and sperm apoptosis in diabetic Mice. Int J Reprod BioMed. (2019) 17. doi: 10.18502/ijrm.v17i2.3990
156. Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. (2016) 15:71. doi: 10.1186/s12937-016-0186-5
157. Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, and Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. (2005) 26:349–53. doi: 10.2164/jandrol.04146
158. Koppers AJ, Mitchell LA, Wang P, Lin M, and Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. (2011) 436:687–98. doi: 10.1042/BJ20110114
159. Alahmar AT and Singh R. Comparison of the effects of coenzyme Q10 and Centrum multivitamins on semen parameters, oxidative stress markers, and sperm DNA fragmentation in infertile men with idiopathic oligoasthenospermia. Clin Exp Reprod Med. (2022) 49:49–56. doi: 10.5653/cerm.2021.04910
160. Gou C, Zhou Z, Chen Z, Wang K, Chen C, Chen B, et al. Studies on improving semen quality and increasing pregnancy chances through the in vitro addition of L-carnitine and coenzyme Q10 to semen in patients with asthenozoospermia. Basic Clin Androl. (2022) 32:17. doi: 10.1186/s12610-022-00167-7
161. Bruno C, Basile U, Vergani E, Napodano C, Oliva A, Gulli F, et al. Inflammation and oxidative stress in seminal plasma: search for biomarkers in diagnostic approach to male infertility. J Pers Med. (2022) 12. doi: 10.3390/jpm12060857
162. Wang J, Bao B, Meng F, Deng S, Dai H, Feng J, et al. The mechanism analysis using PI3K/AKT pathway for the effects of levocarnitine in the treatment of spermatogenic dysfunction. Andrologia. (2022) 54:e14290. doi: 10.1111/and.14290
163. Liu L, Li T, Li F, Zhao X, Zhang R, Liu J, et al. The influence of l-carnitine on the expression of miRNAs in asthenospermia spermatozoa and the network regulation of the associated molecules. Andrologia. (2020) 52:e13478. doi: 10.1111/and.13478
164. Ma L and Sun Y. Comparison of L-Carnitine vs. Coq10 and Vitamin E for idiopathic male infertility: a randomized controlled trial. Eur Rev Med Pharmacol Sci. (2022) 26:4698–704. doi: 10.26355/eurrev_202207_29194
165. Wei G, Zhou Z, Cui Y, Huang Y, Wan Z, Che X, et al. A meta-analysis of the efficacy of L-carnitine/L-acetyl-carnitine or N-acetyl-cysteine in men with idiopathic asthenozoospermia. Am J Mens Health. (2021) 15:1013921067. doi: 10.1177/15579883211011371
166. Sharma A, Arambula JF, Koo S, Kumar R, Singh H, Sessler JL, et al. Hypoxia-targeted drug delivery. Chem Soc Rev. (2019) 48:771–813. doi: 10.1039/c8cs00304a
167. Amalraj A, Pius A, Gopi S, and Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J Tradit Complement Med. (2017) 7:205–33. doi: 10.1016/j.jtcme.2016.05.005
168. Zhou Q, Wu X, Liu Y, Wang X, Ling X, Ge H, et al. Curcumin improves asthenozoospermia by inhibiting reactive oxygen species reproduction through nuclear factor erythroid 2-related factor 2 activation. Andrologia. (2020) 52:e13491. doi: 10.1111/and.13491
169. Luo J, Luo Y, Dong C, Qi G, Zhong L, Liu F, et al. Enhancing sperm motility parameters in patients with asthenospermia: A combined approach of acupuncture at Fusiguan point and tamoxifen citrate tablets. Arch Esp Urol. (2024) 77:142–47. doi: 10.56434/j.arch.esp.urol.20247702.19
170. Fan Y, Wang M, Zhang Q, Ouyang S, Mao W, Xu C, et al. Traditional uses, phytochemistry, pharmacology, toxicity and clinical application of traditional Chinese medicine Cynoglossum amabile: a review. Front Pharmacol. (2024) 15:1325283. doi: 10.3389/fphar.2024.1325283
Keywords: reactive oxygen species, idiopathic asthenozoospermia, oxidative stress, sperm motility, antioxidants, male infertility
Citation: Wang Z, Li D, Zhou G, Xu Z, Wang X, Tan S, Li Z, Li X, Song C and Yuan S (2025) Deciphering the role of reactive oxygen species in idiopathic asthenozoospermia. Front. Endocrinol. 16:1505213. doi: 10.3389/fendo.2025.1505213
Received: 02 October 2024; Accepted: 25 April 2025;
Published: 21 May 2025.
Edited by:
Shun Bai, University of Science and Technology of China, ChinaReviewed by:
Gennaro Lettieri, University of Naples Federico II, ItalyBilal ÇİĞ, Ahi Evran University Medicine Faculty Department of Physiology, Türkiye
Copyright © 2025 Wang, Li, Zhou, Xu, Wang, Tan, Li, Li, Song and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Yuan, eXVhbnNvbmdAc3lzdXNoLmNvbQ==; Changze Song, c29uZ2NoekBtYWlsLnN5c3UuZWR1LmNu; Xiaoli Li, bGl4aWFvbGlAc3lzdXNoLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zilong Wang
Zilong Wang Dandan Li
Dandan Li Guoyi Zhou1†
Guoyi Zhou1† Xinkun Wang
Xinkun Wang Changze Song
Changze Song Song Yuan
Song Yuan