- 1Beijing Ophthalmology & Visual Sciences Key Laboratory, Beijing Tongren Eye Center Research Ward, Beijing Tongren Hospital, Beijing Institute of Ophthalmology, Capital Medical University, Beijing, China
- 2Yale University, New Haven, CT, United States
- 3Departments of Medicine (Biomedical Genetics), Boston University School of Medicine, Boston, MA, United States
- 4Departments of Psychiatry and Pharmacology, Alzheimer’s Disease Center, Boston University School of Medicine, Boston, MA, United States
Background: Recent studies suggest that the diabetes might be associated with higher risk for primary open angle glaucoma (POAG) and Alzheimer’s disease (AD). However, studies have not addressed the critical issue of confounding by indication, and associations have not been evaluated in a large cross-sectional study. We started this cross-sectional study included United Kingdom Biobank (UKBB) participants with complete data (2006-2010) for analysis to explore the associations between diabetes mellitus (DM) and POAG and AD by considering depression and diabetic retinopathy (DR) as intermediate factors.
Methods: 28,112 diabetes patients and 471,869 controls without diabetes were included from UKBB. Data on diagnosis of glaucoma, diabetes, depression, Alzheimer’s disease, diabetic retinopathy, apolipoprotein E (APOE) E4 genotypes and data from ophthalmologic examinations were gathered. We further collect the prevalence of DM, DR, depression, POAG and AD, gender, APOE E4 genotypes, C-reactive protein (CRP) levels to analysis.
Results: Depression, AD, and POAG were more prevalent in participants with DM compared with non-DM participants, and if DM patients had DR, the prevalence of those comorbidities was even higher than those without DR (all p<0.05). DM, DR, AD, and POAG were more prevalent in participants with depression compared with non-depression participants. Specifically, if DM patients had depression, the prevalence of DR and AD were even higher than those without depression (all p<0.05). In addition, using age-adjusted multivariable general linear model (GLM), we found DM and depression were associated with a higher prevalence of POAG in females while DM and APOE E4 negative status were associated with a higher prevalence of POAG in males. In both genders, DM, APOE E4, and depression were all associated with higher prevalence of AD in both univariable and multivariable GLM adjusted by age (all p<0.05). DM and depression were all associated with higher CRP, while carrying APOE E4 was associated with lower CRP levels in both univariable and multivariable GLM (all p< 0.001) in all populations.
Conclusions: DR and depression, as comorbidities related to blood-retinal barrier and blood-brain barrier impairment in patients with DM, may play pivotal roles in the development of POAG and AD among DM patients.
Introduction
Glaucoma, affecting over 60 million people globally, is the second leading cause of blindness, with POAG being predominant. Glaucoma is characterized by the progressive loss of retinal ganglion cells (RGCs) through apoptosis (1, 2), which shares molecular similarities with central nervous system (CNS) degenerative disorders like AD, indicating a common pathological mechanism. The retina, an extension of the CNS, and its barrier (inner blood-retinal barrier, iBRB) resemble the blood-brain barrier (BBB) (3). It was reported that circulating immune cell migration through an impaired BBB and glial activation contributes to the progression of AD (4). Similarly, impairment of the iBRB is observed in glaucoma, which has been proven to be pivotal in determining the fate or prognosis of neuroinflammation pathological outcomes for POAG (5). A current theory proposes that when systemic immune and inflammatory components entering the retina or brain through the impaired iBRB or BBB initiate a self-exacerbating cycle of neuroimmune responses, then it would lead to the development of clinical disease phenotypes such as POAG or AD (6). Similar pattern has been stated in previous studies about brain structural changes and neurodegenerative processes in relation to clinical severity and cognitive symptoms in glaucoma (7–10). Although, there is little agreement on the role of chronic systemic diseases in developing of neurodegenerative diseases (11, 12), this novel perspective could enhance our understanding of the etiology, mechanisms, and potential therapies for POAG and AD.
DM, a common systemic disease, is associated with heightened risks of both POAG and AD (13, 14). DR and depression are common comorbidities of DM (15, 16), sharing pathological mechanisms that contribute to the breakdown of iBRB and BBB, potentially playing a causative role in POAG and AD (17). Associations have been demonstrated in previous studies, including several meta-analyses (18, 19), between POAG with AD (20, 21), DM and depression (22). Therefore, we hypothesized that DR and depression, serving as indicators of iBRB and BBB impairment, might act as intermediary processes between DM and POAG and AD, and could possibly accelerate the progression of POAG and AD. Meanwhile, these processes were also interfered with intrinsic factors associated with AD and POAG, such as age, gender and APOE E4 status (23). A coherent pathological picture that explains these complex relationships is likely to describe molecular and mechanistic similarities of these disorders and may pave the way for the development of novel and effective therapies. This study aims to develop a comprehensive model exploring the association between DM and POAG, AD, across different gender groups and APOE E4 genotypes in a large cohort, in which depression and DR serve as intermediate factors, with CRP levels evaluated to gauge inflammatory conditions in different contexts.
Materials and methods
Ethics statement
UKBB received approval from the North West Multi-centre Research Ethics Committee. Recruitment for the UKBB was obtained by written consent. We have full access to de-identified data with permission approved by UKBB as complying with their Access Procedures and Ethics. Our research adheres to the tenets of the Declaration of Helsinki.
Study population
The UKBB is a large-scale prospective cohort study of participants recruited from 2006 through 2010 from across the United Kingdom. POAG, AD, depression, DM, and DR phenotypes were identified through data coding based on the International Classification of Diseases, Tenth Revision (ICD10). Out of the entire UKBB cohort including 502,505 participants, the final sample consisted of 28112 participants with DM and 471869 participants without DM considered as controls after excluding patients lacking DM information. We retrieved visual acuity from both eyes (data fields: 5208 and 5201). The best recorded visual acuity from either eye at the two time points was converted to its logMAR equivalent and used as visual acuity (VA) for subsequent analysis. Circulating CRP levels were measured using high-sensitivity assays [data-field 30710, initial assessment visit (2006–2010)].
Genotyping
DNA microarray genotyping was generated using Axiom arrays (including the UKBB Lung Exome Variant Evaluation (BiLEVE) and UKBB arrays; Thermo Fisher) for the UKBB. APOE alleles (E1, E2, E3, and E4) were determined from 2 relevant Single Nucleotide Polymorphisms (SNPs) within the APOE gene (rs429358 and rs7412; GRCh38 reference genome) (24). Because of the rarity of the E1 allele, rare E1 genotypes (E1E2 and E1E4) were excluded from the analysis. Apolipoprotein SNPs were measured directly from Axiom arrays. Because the relevant APOE SNPs were not included on the Illumina arrays, they were imputed in Minimac3 using Haplotype Reference Consortium r1.1 as a reference panel (rs429358 imputation R2 ¼ 0.93; rs7412 imputation R2 ¼ 0.92). APOE E4 carriers was classified as carrying ≥1 copy (E24 + E34 + E44).
Statistics
Statistical analyses were conducted using R (version 4.2.1) with publicly available packages. Demographic parameters were initially compared through chi-square tests, and independent sample t-tests. The univariable generalized linear model (GLM) was used to analyze factors related to POAG, AD, and CRP levels, adjusting for age (the one recorded at the time of recruitment in this cohort and was utilized in subsequent analysis) in both genders and each gender. Multivariate GLMs were further conducted, incorporating DM/DR, APOE E4 status and depression as variables, adjusting for age and gender. For the allelic regression, an indicator variable for E4 status, defined by the presence or absence of the relevant allele, was included. In this context, the βe4 represents the impact of the E4 allele, indicating a value of 1 or 0 to represent its presence or absence, respectively. Since depression was more likely to be affected by the awareness of the illness and its effect on patients’ daily life, we further explored the association between depression and POAG, DM, DR using GLM, adjusting for age, sex, and VA. Analysis of variance (ANOVA) was used to compare the differences among Models. The threshold for statistical significance (α) was set at p<0.05.
Results
Population
A total of 28,112 patients with DM and 471,869 without DM were included in the study. The DM group was further divided into two subcategories: 2,097 patients with DR and 9,932 with DM but without DR. A final classification round was done for depression status, with 1,874 patients having both depression and DM, and 26,238 with DM but without depression.
Comparing characteristics between patients with and without DM/depression and between DM patients with and without DR/depression
The comparisons of the demographics and prevalence of depression, AD, and POAG between patients with and without DM and between DM patients with and without DR were presented in Table 1. Older age, more males, and worse VA were presented in patients with DM compared with patients without DM and in DM patients with DR compared with those without DR (all p<0.001). Depression, AD, and POAG were more prevalent in participants with DM compared with non-DM participants, and if DM patients had DR, the prevalence of those comorbidities was even higher than those without DR (all p<0.05). CRP was increased in DM patients (2.54 ± 4.30 vs. 3.49 ± 5.15mg/L; p<0.001), and even higher in those with DR (3.40 ± 4.80 vs. 3.79 ± 5.74mg/L; p<0.001). Though DM patients had a higher percentage of carrying APOE E4 (28.60% vs.26.77%; p<0.001), the distribution of APOE E4 allele status did not differ significantly in DM patients with or without DR (p=0.729) (Table 1).
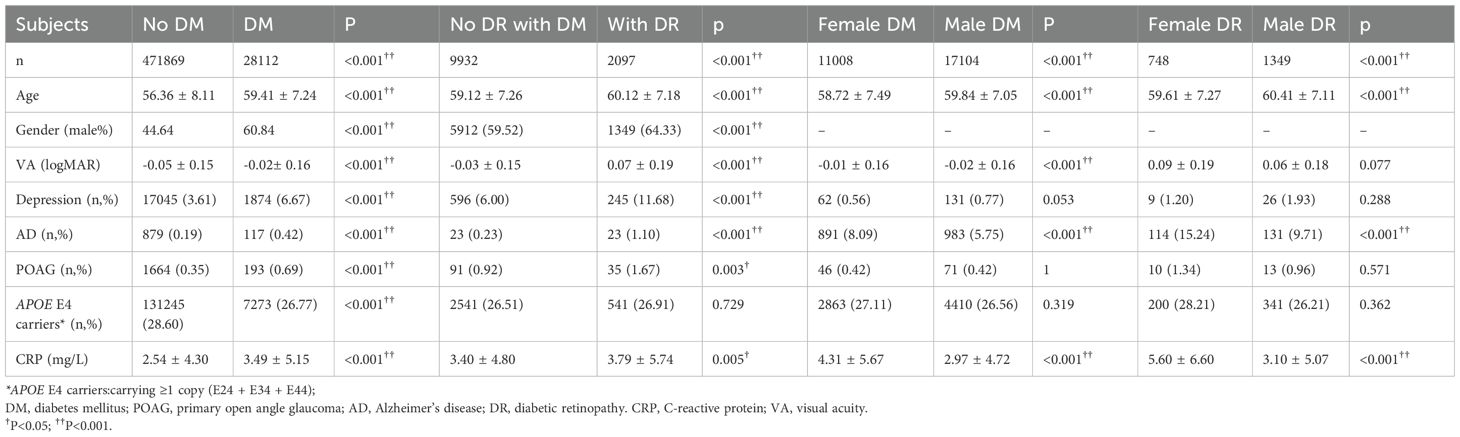
Table 1. Comparison depression, POAG, AD, APOE E4 status and level of CRP in patients with and without DM or DR and in female and male patients with DM or DR.
Table 2 demonstrated the demographics and prevalence of depression, AD, and POAG between patients with and without depression and between DM patients with and without depression. Younger age, more females, and worse VA were presented in patients with depression compared with patients without depression and in DM patients with depression compared with those without depression (All p<0.001). DM, DR, AD, and POAG were more prevalent in participants with depression compared with non-depression participants, and if DM patients had depression, the prevalence of DR and AD were even higher than those without depression (all p<0.05), while the prevalence of POAG was similar (P=0.854). CRP was increased in depression patients (2.55 ± 4.31 vs. 3.65 ± 5.42mg/L; p<0.001), and even higher in DM patients with depression compared with DM patients without depression (3.40 ± 5.08 vs. 4.70 ± 5.95mg/L; p<0.001). The distribution of APOE E4 allele status did not differ significantly in patients with or without depression and in DM patients with or without depression (both p>0.05) (Table 2).
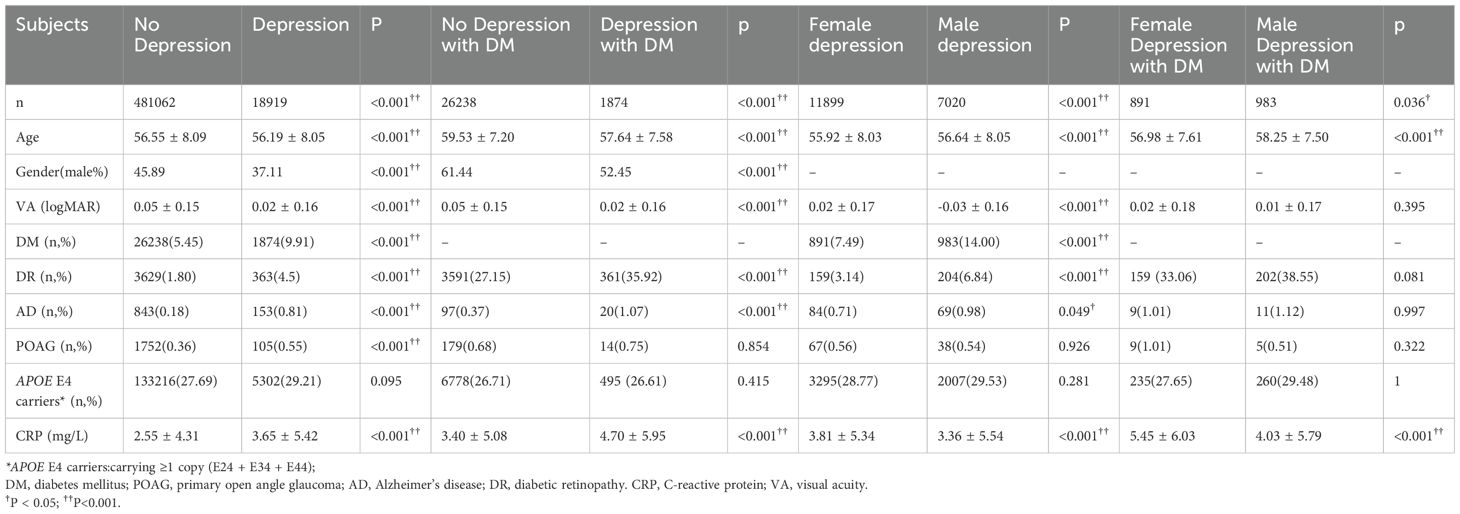
Table 2. Comparison DM, DR, POAG, AD, APOE E4 status and level of CRP in patients with and without depression and/or DM and the gender differences.
Comparing characteristics between females and males in patients with DM or DR or depression
The comparisons of the demographics and prevalence of depression, AD, and POAG between female and male patients with DM or DR were presented in Table 1. In both DM and DR patients, females had younger age, higher CRP levels, and higher prevalence of AD compared with males (all p<0.001). VA in female patients with DM was worse compared with male patients with DM (p<0.001), there was no difference in VA between females and males with DR (p=0.077). No difference in APOE E4 status and the prevalence of depression and POAG between females and males in both DM and DR patients (all p>0.05).
The comparisons of the demographics and prevalence of DM, DR, AD, and POAG between female and male patients with depression were presented in Table 2. In depression patients, females had younger age, worse VA, higher CRP levels, and lower prevalence of DR and AD compared with males (all p<0.001). No difference in APOE E4 status and the prevalence of POAG between females and males in depression patients (both p>0.05). In DM patients with depression, females had younger age and higher CRP levels (both p<0.001), and no differences were detected in VA, APOE E4 status, and the prevalence of DR, AD, and POAG (all p>0.05) between females and males.
Factors associated with the risk of POAG and AD in patients with and without DM/depression and between DM patients with and without DR/depression
Table 3 displayed factors associated with the risk of POAG and AD in patients with/without DM. In the univariate and multivariate GLM (Model 1) adjusted by age, male, DM, and depression were all associated with higher prevalence of POAG and AD (all p<0.05), while carrying APOE E4 is a protective factor for prevalence of POAG with borderline significance (univariable GLM: OR=0.898, p=0.046; multivariate GLM: OR=0.899, p=0.047) but significant risk factor for prevalence of AD (univariate GLM: OR=4.279, p<0.001; multivariate GLM: OR=4.271, p<0.001). In DM patients, being male or having DR were associated with higher prevalence of POAG in the univariate GLM after adjusting for age (both p<0.05). APOE E4 status and depression were not associated with POAG (both p>0.05). In the multivariate GLM (Model 2) after adjusting by age, DR was still associated with higher prevalence of POAG (OR=1.826, p=0.003), while sex, APOE E4 status and depression were not associated with the prevalence of POAG. DR, depression and APOE E4 positive were associated with higher prevalence of AD both in the univariate and multivariate GLM (Model 2) adjusted by age (all p<0.001). However, sex was not associated with AD in DM patients (p=0.324) (Table 3).
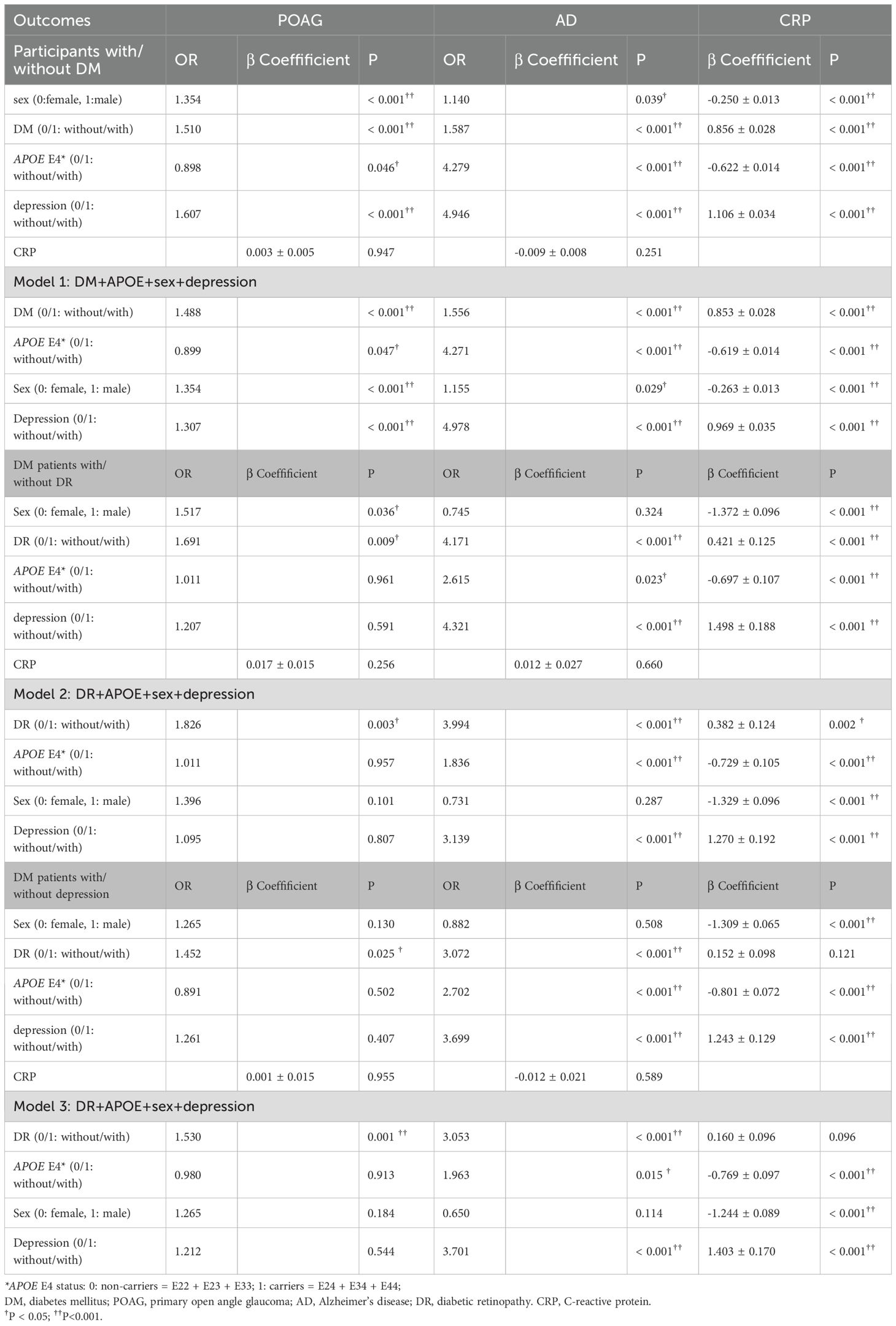
Table 3. Factors associated with POAG, AD and blood CRP level in participants with/without DM and in DM patients with/without DR (general linear model, adjust for age).
Being female, having DM and experiencing depression were all associated with higher CRP, while carrying APOE E4 was associated with lower CRP in both univariate and multivariate GLM (all p< 0.001) in all populations (all participants with/without DM and DM patients with/without DR or depression, Table 3). CRP was not associated with either POAG or AD in all populations (all p>0.05).
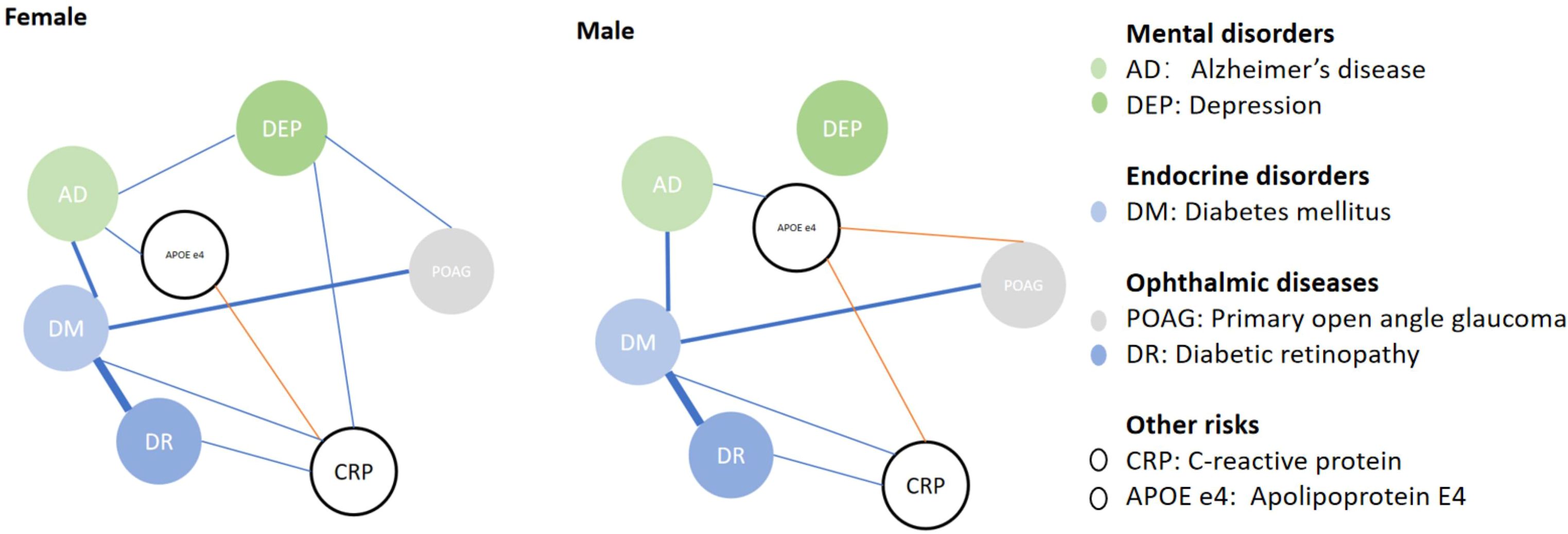
We further analyzed factors associated with POAG, AD and blood CRP levels in different genders (Table 4) and have the association visualized in Figure 1. After adjusting for age, DM was associated with higher prevalence of POAG in both genders (female, ORPOAG=1.504, p<0.001; male, ORPOAG=1.417, p<0.001). APOE E4 was not associated with POAG in females (p=0.433), while it was associated with a lower prevalence of POAG in males (OR=0.863, p=0.045). Only among female patients, depression was associated with a higher prevalence of POAG (ORPOAG=1.980, p<0.001). DM and depression were still associated with a higher prevalence of POAG in females in multivariate GLM adjusted by age (model 4, both p<0.05). While DM and not carrying APOE E4 status were associated with a higher prevalence of POAG in males in multivariate GLM adjusted by age (model 4, both p<0.05). In both genders, DM, carrying APOE E4 and depression were all associated with a higher prevalence of AD in both univariate and multivariate GLM adjusted by age (all p<0.05).
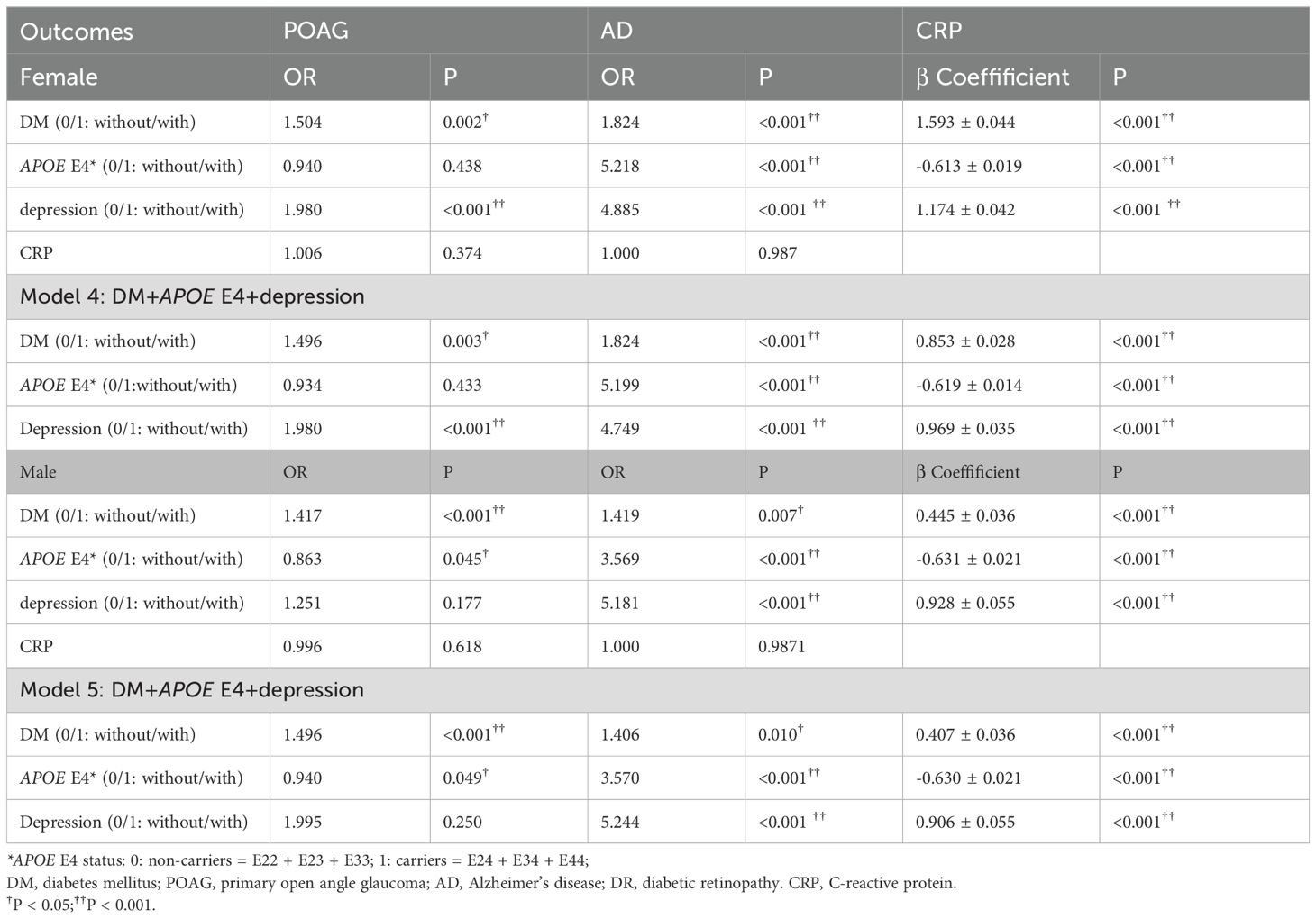
Table 4. Factors associated with POAG, AD and blood CRP level in female and male participants with/without DM (general linear model, adjust for age).
DM and depression were all associated with higher CRP, while carrying APOE E4 was associated with lower CRP level in both univariate and multivariate GLM (all p< 0.001) in both female and male population. CRP level was not associated with either POAG or AD in both female and male populations (all p>0.05).
Factors associated with depression in patients with/without DM
Table 5 displayed factors associated with depression in patients with/without DM. In the univariable GLM adjusted by age, factors like POAG, DM, female, and worse VA were all associated with higher prevalence of depression (ORPOAG=1.580, p<0.001; ORDM=1.952, p<0.001; ORGender=0.697, p<0.001; βVA=1.087 ± 0.086, p<0.001), while carrying APOE E4 was not associated with depression(p=0.100). After adjusting the VA and sex, POAG showed no association with depression (p=0.131). Both DM and DR showed an association with higher prevalence of depression after adjusting the VA and sex (ORDM=2.066, p<0.001; ORDR=2.420, p<0.001).
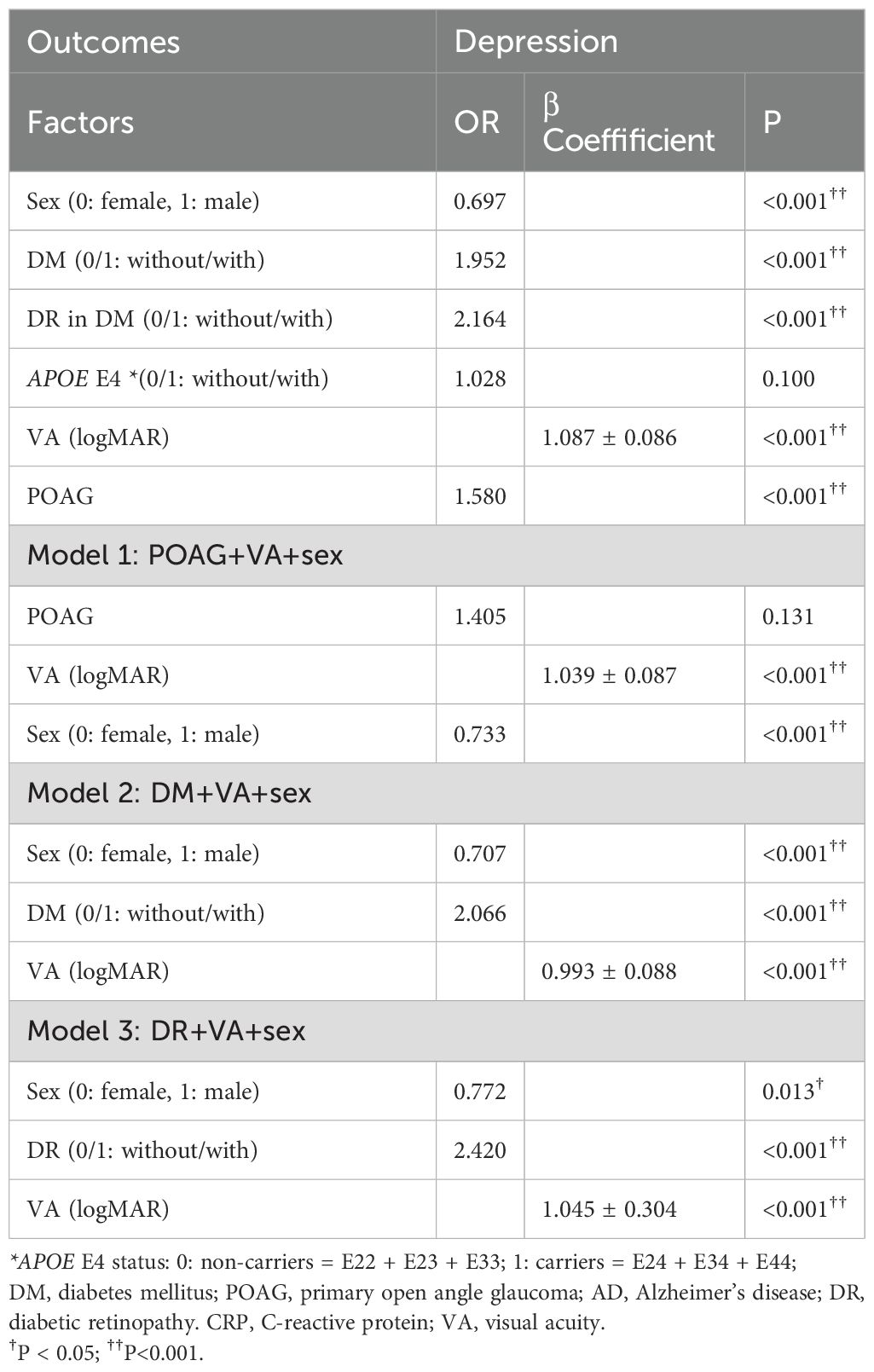
Table 5. Factors associated with depression in patients with/without DM (general linear model, adjust for age).
Discussion
DM was found to be associated with heightened risks of both POAG and AD in previous studies, likely due to shared pathological mechanisms such as oxidative stress, inflammation, vascular dysfunction, and impaired insulin signaling pathways (25). Thus, our study supports a current hypothesis that POAG and AD may be thought of as diabetes of the brain (25). Additionally, immune and inflammatory components from the systemic circulation enter the brain and retina through the impaired BBB and BRB, which are also implicated in DM-related DR and depression, and could also initiate a self-exacerbating vicious cycle of neuroimmune responses that lead to the development of POAG and AD (6). In this study, we first explored the associations between DM and POAG, AD, on the conception that DR and depression serve as intermediate factors between these associations. We discovered that patients with DR showed higher prevalence of POAG, together with higher prevalence of AD among DM patients with DR and depression. Moreover, we tried to integrate the effects of APOE E4 allele, gender and CRP into our models to enhance our understanding of the common pathological mechanisms in neurodegeneration and further explain the associations among DM, POAG, and AD (26).
Consistent with our findings, an Indian study identified a positive association between glaucoma and DR in a type 2 diabetes mellitus (T2DM) population (OR=2.62) (27). Additionally, other studies have shown that T2DM patients with POAG have a threefold higher risk of developing DR compared to those without POAG (28). Similarly, research using the Danish Registry of Diabetic Retinopathy found that patients with DM and either glaucoma or ocular hypertension were more likely to develop DR within five years (29). Clinical diagnosis of both POAG and DR are often abrupt, making early detection and monitoring of progression challenging. Whilst these findings do not establish causality, they reinforce the association between POAG and DR. Indeed, we continue to regard the development of DR as a crucial factor in the progression of POAG, particularly concerning the role of the BRB in glaucoma. Normally, the BRB’s integrity limits retinal damage; minor disruptions in the BRB are typically transient and quickly repaired, preventing clinical consequences (5). Thus, age-related BRB breakdown alone may not cause POAG. Histological studies have shown BRB damage in glaucoma models, with abnormalities in retinal pigment epithelium (RPE) cells, increased permeability of retinal vessels, and leukocyte infiltration (2). In animal models of transient high intraocular pressure (IOP) glaucoma, BRB impairment has been linked to T-cell infiltration and progressive ganglion cell death (30, 31). These findings suggested that BRB integrity is crucial in POAG progression and breakdown of BRB was the essential factor in the very early stages of POAG (5, 31). DM, especially DR, can compromise the BRB, leading to neuroinflammation in the retina, which may contribute to glaucoma progression. Early dysfunction of the neurovascular unit (NVU) in diabetes has been observed in animal models and patients, leading to impaired neurovascular coupling, loss of autoregulation, and disruption of the iBRB (32). Factors like hypoxia-ischemia, oxidative stress, and inflammation during DR development contribute to both inner and outer BRB breakdown (18, 33–35). These processes indicate that BRB impairments could even precede the clinical onset of DR. The relationship between POAG severity and a significant decrease in retinal vessel density at ONH and macula level further suggests that microvascular damage in the diabetic retina exacerbates retinal neurodegeneration (36, 37). Persistent microvascular leakage in advanced DR induces chronic neural immune-inflammatory responses, eventually leading to neuronal loss (38). Therefore, the disruption of the iBRB caused by DM might explain the significant association between POAG and DM. The higher prevalence of POAG among DR patients could be due to a longer duration of DM and more severe BRB and microvascular damage. Although the precise timing and relationship between glial activation, BRB impairment, and immune-inflammatory cell infiltration in POAG need further clarification, comprehensive research could shed light on the mechanisms underlying progressive RGC loss in glaucoma associated with age-related systemic diseases like DM and DR,which may also explain the progressive RGC loss in glaucoma patients with well-controlled or normal IOP, where BRB impairment, triggered by high IOP or other systemic factors, is likely a critical factor leading to ongoing neuroinflammation and RGC loss independent of IOP levels.
Meanwhile, our study has corroborated the association between DM and AD. Numerous investigations have shown a higher incidence of cognitive decline in individuals with DM. Longitudinal studies conducted in Japan (39) and a five-year prospective study by Yaffe et al. (40) have robustly established DM as a risk factor for AD and cognitive decline, particularly in the context of metabolic syndrome. Neurovascular changes in the brain, similar to those seen in the BRB in DM patients, are believed to contribute to the higher risk of AD (41). The retina, an extension of the CNS, shares similarities with the BBB, suggesting that DM-related BBB impairment, particularly in patients with DR, could be expected (3, 42, 43). The BBB, composed of endothelial cells, pericytes, and astrocytes, regulates the passage of substances into the brain and protects against harmful signals from the bloodstream (44). Migration of circulating immune cells through an impaired BBB, along with glial activation, contributes to the progression of AD and may explain the increased prevalence observed in DM patients, particularly those with DR. Moreover, depression, which can be induced by DM through the strain of diabetes itself and changes in the hypothalamus–pituitary–adrenal (HPA) axis or BBB structure (45–47), has been identified as a risk factor for AD and is prevalent in DM patients. Chronic social stress has been shown to alter BBB integrity in animal models, promoting behaviors akin to depression (48, 49). This suggests that BBB dysfunction may play a role in the development of depression and could contribute to the association between DM, DR, and depression. Furthermore, BBB damage may exacerbate the impact of depression on AD, similar to the key role of BRB disruption in DR on the progression of POAG. Additionally, the APOE E4 allele, a known risk factor for AD, may contribute to BBB breakdown and neuroinflammation, synergizing with systemic inflammation to promote AD onset (50). Our multivariable GLM analysis, adjusted for age and stratified by sex, demonstrated the complex interplay between DM, depression, APOE E4, and AD, underscoring their roles in the pathogenesis of AD.
Although we did not observe an association between POAG and depression after adjusting for age, sex, and VA, previous studies have indicated a higher prevalence of depression in individuals with POAG (22), and increased risk of glaucoma in patients with major depressive disorder (MDD). In our study, females were more likely to experience depression than males, regardless of DM or DR status. Notably, depression was associated with POAG only in females, possibly due to irregular estrogen levels caused by ovarian hormone fluctuations in patients with depression, since estrogen is essential for maintaining RGCs (51, 52). Additionally, we observed a protective effect of the APOE E4 allele in males against POAG, even with DM, which aligns with previous research indicating a reduced risk of POAG associated with this allele (53). Similar findings were revealed by previous studies which showed a difference of AD prevalence among different gender (54). Interestingly, the APOE E4 allele is a known risk factor for AD, suggesting a mechanistic difference between neurodegenerative diseases affecting the eye and brain. This difference may be due to APOE E4 acting as an example of antagonistic pleiotropy—a gene that provides benefits at one stage of life but later presents disadvantages (55)—since POAG typically manifests at a younger age than AD. The post-menopausal loss of estrogen was key in the increased incidence of AD in women (56). Considering the accelerated aging process post-menopause, particularly in females with DM and depression (57–60), the protective effect of the APOE E4 allele may be diminished in this group. Both clinical researches (61, 62) and transgenic mice model (63, 64) has revealed that combination of APOE4 genotype and female sex often exacerbated outcomes across numerous cognitive loss. Although further studies are needed to clarify these mechanisms, these gender differences highlight the complex interplay of DM, depression, and APOE E4 in the context of POAG. Additionally, the correlation between retinal vascular abnormalities, cognitive impairment, and dementia, as well as the similarity between the BRB and the BBB, suggests that retinal function may serve as a promising biomarker for neurodegenerative diseases (65). Previous clinical studies have already shown that POAG patients are at a higher risk of developing AD compared to controls (66) and vice versa (67). Therefore, considering the earlier and more accessible detection of POAG compared to AD, we propose that POAG could potentially be used as an early screening indicator for AD in patients with DM.
Furthermore, although we attempted to elucidate the connection between DM, POAG, and AD by assessing inflammation levels, represented by blood CRP levels, and hypothesized that proinflammatory cytokine levels might explain breaches in the BRB and BBB, our study did not find any association between blood CRP levels and POAG or AD. Despite observing increased CRP levels in DM, which further increased in patients with DR and depression, particularly among females, no correlation with POAG or AD was identified. We further tried to integrate the effects of APOE E4 allele, gender and CRP into our models to enhance our understanding of the common pathological mechanisms in neurodegeneration and further explain the associations among DM, POAG, and AD (26). CRP is a marker of systemic inflammation and increases with age. Although multiple AD-related genes are associated with the level of CRP, the association between blood CRP levels and risk of AD are not conclusive in the literature, with studies showing both low and high levels of CRP in patients with AD (68). Similarly, peripheral inflammatory markers have been linked to a higher risk of vascular dementia, but not AD (31). A Mendelian randomization study suggested a protective effect of CRP on AD, possibly influenced by the methods used to measure CRP (69). It was reported that in the context influenced by possessing the APOE E4 allele, variations in CRP levels—either transformation or suppression—may lead to reduced blood CRP levels (70, 71). Previous studies have observed an inverse correlation between APOE E4 and blood CRP levels, but only within the lower CRP level range, across both elderly and younger populations (72–74). In a large UK Biobank subsample with elevated CRP levels, it was shown that the effect of genetic variants on CRP diminished as CRP levels increased (75). This suggests that chronic, low-grade inflammation, as indicated by CRP, is strongly influenced by genetic factors such as APOE E4, rather than reflecting CRP’s response to acute infections or other stimuli. This may explain why APOE E4 carriers in our study exhibited lower CRP levels regardless of gender or diabetes status, and why we found no association between CRP levels and AD or POAG. The complex inflammatory profiles involving APOE E4, AD, DM, and POAG may offer insights into the systemic inflammation underlying these distinct but related neurodegenerative processes. Future research investigating other proinflammatory cytokines, such as interleukin-6 (IL-6), and exploring CRP’s role in modulating genetic risk for POAG and AD, may further elucidate these complex interactions (76).
Although the underlying mechanisms still require further investigation, previous clinical studies and our data analysis have come to similar conclusion that POAG might come from the consequence of a primary neurodegenerative disease of the CNS, together with BRB and BBB breakdown (77). This insight opens up potential treatment pathways that focus on neuroprotective molecules rather than IOP-targeted medications for POAG. Currently, Coenzyme Q1 (CoQ10) and citicoline are among the most commonly used neuroprotective agents in POAG treatment. CoQ10, a crucial antioxidant that safeguards proteins and DNA from oxidative stress, has been shown to prevent optic nerve astrocyte activation induced by hydrogen peroxide in vitro while also inhibiting RGC apoptosis and loss in animal models (78). Clinical trials and animal studies further suggest that CoQ10 protects optic nerve head (ONH) astrocytes from oxidative stress primarily by preserving mitochondrial function (79). Similarly, citicoline has demonstrated neuroprotective properties by reducing glutamate-mediated excitotoxicity and oxidative stress through the enhancement of neurotrophin levels and mitochondrial support (78). Preliminary studies indicate that the combination of citicoline and vitamin B12 eye drops can help stabilize neuroretinal degeneration and mitigate microvascular damage in DR patients (80). Given these findings, the combined use of CoQ10 and citicoline presents a promising new strategy for glaucoma treatment. Overall, these emerging therapeutic approaches for POAG further support our findings.
Several limitations were identified in our study, particularly our inability to pinpoint the exact onset times of DM, DR, depression, POAG, and AD. Determining the precise onset of each disease is challenging because symptoms may not manifest in early stages, and recorded onset times often only reflect the age at diagnosis. This limitation has prevented us from fully elucidating the causal relationships and could only presented as possible connection between these conditions. Also, we used ICD-10 codes for ascertaining clinical phenotypes might capture a heterogeneous group of patients. An ideal study sample would include a large, population-based cohort with comprehensive ocular phenotyping and chronic disease phenotypes. However, clinical criteria for diagnosis vary in sensitivity across different diseases, which would still impact the consistency and reliability of our findings. Secondly, in this study, we only included age, sex, CRP, and APOE E4 as variables. However, there are undoubtedly additional variables that could influence the complex interplay among DM, POAG, and AD. We believed our results were still valuable for understanding the shared pathological mechanisms involved in DM, POAG, and AD. Thirdly, although European ancestry represented the predominant ethnic group within this study cohort, ethnic variations in glaucoma prevalence and APOE E4 allele distribution were not addressed, limiting analysis to diverse ethnic groups. Including more diverse populations in future analyses could strengthen the findings and enhance their applicability to global populations.
Conclusions
In summary, our findings suggested that DR and depression, as comorbidities related to BRB and BBB impairment in patients with DM, may play crucial roles in the development of POAG and AD among DM patients. Although the complexities of these interactions require further detailed characterization, they provide valuable insights into the underlying mechanisms and potential shared pathways in the development of POAG, AD, and other neurodegenerative diseases. Enhancing knowledge and awareness of these associations could lead to the development of new avenues in the understanding and management of glaucoma, AD, and DM.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://biobank.ndph.ox.ac.uk/showcase/search.cgi.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/ participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
YS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. XH: Conceptualization, Data curation, Formal Analysis, Project administration, Writing – original draft, Writing – review & editing. WL: Conceptualization, Investigation, Software, Writing – review & editing. JH: Writing – review & editing. WQ: Writing – review & editing. XZ: Writing – review & editing. ZF: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant No.82201171 and Grant No.82171050). The sponsor or funding organization had no role in the design or conduct of this research.
Acknowledgments
This research used data from the UK Biobank Resource: Application 19416.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Qu J, Wang D, and Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. (2010) 91:48–53. doi: 10.1016/j.exer.2010.04.002
2. Okisaka S, Murakami A, Mizukawa A, and Ito J. Apoptosis in retinal ganglion cell decrease in human glaucomatous eyes. Jpn J Ophthalmol. (1997) 41:84–8. doi: 10.1016/s0021-5155(97)00013-0
3. Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, and Terasaki T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem. (2008) 104:147–54. doi: 10.1111/j.1471-4159.2007.05008.x
4. Mietelska-Porowska A and Wojda U. T lymphocytes and inflammatory mediators in the interplay between brain and blood in alzheimer's disease: potential pools of new biomarkers. J Immunol Res. (2017) 2017:4626540. doi: 10.1155/2017/4626540
5. Shi X, Li P, Herb M, Liu H, Wang M, Wang X, et al. Pathological high intraocular pressure induces glial cell reactive proliferation contributing to neuroinflammation of the blood-retinal barrier via the NOX2/ET-1 axis-controlled ERK1/2 pathway. J Neuroinflammation. (2024) 21:105. doi: 10.1186/s12974-024-03075-x
6. Yang X, Yu XW, Zhang DD, and Fan ZG. Blood-retinal barrier as a converging pivot in understanding the initiation and development of retinal diseases. Chin Med J (Engl). (2020) 133:2586–94. doi: 10.1097/CM9.0000000000001015
7. Minosse S, Garaci F, Martucci A, Lanzafame S, Di Giuliano F, Picchi E, et al. Primary open angle glaucoma is associated with functional brain network reorganization. Front Neurol. (2019) 10:1134. doi: 10.3389/fneur.2019.01134
8. Mancino R, Cesareo M, Martucci A, Di Carlo E, Ciuffoletti E, Giannini C, et al. Neurodegenerative process linking the eye and the brain. Curr Med Chem. (2019) 26:3754–63. doi: 10.2174/0929867325666180307114332
9. Di Cio F, Garaci F, Minosse S, Passamonti L, Martucci A, Lanzafame S, et al. Reorganization of the structural connectome in primary open angle Glaucoma. NeuroImage Clin. (2020) 28:102419. doi: 10.1016/j.nicl.2020.102419
10. Martucci A, Cesareo M, Toschi N, Garaci F, Bagetta G, and Nucci C. Brain networks reorganization and functional disability in glaucoma. Prog Brain Res. (2020) 257:65–76. doi: 10.1016/bs.pbr.2020.07.007
11. Yin J, Li H, and Guo N. Prevalence of depression and anxiety disorders in patients with glaucoma: A systematic review and meta-analysis based on cross-sectional surveys. Actas Esp Psiquiatr. (2024) 52:325–33. doi: 10.62641/aep.v52i3.1561
12. Kim HK, Lee W, Ryu IH, Kim JK, Kim H, and Yoo TK. Association between metformin use and the risk of developing open-angle glaucoma among patients with diabetes: a retrospective cohort study and meta-analysis. Int Ophthalmol. (2024) 44:6. doi: 10.1007/s10792-024-02945-w
13. Law SK, Hosseini H, Saidi E, Nassiri N, Neelakanta G, Giaconi JA, et al. Long-term outcomes of primary trabeculectomy in diabetic patients with primary open angle glaucoma. Br J Ophthalmol. (2013) 97:561–6. doi: 10.1136/bjophthalmol-2012-302227
14. Diniz Pereira J, Gomes Fraga V, Morais Santos AL, Carvalho MDG, Caramelli P, and Braga Gomes K. Alzheimer's disease and type 2 diabetes mellitus: A systematic review of proteomic studies. J Neurochem. (2021) 156:753–76. doi: 10.1111/jnc.15166
15. Song P, Yu J, Chan KY, Theodoratou E, and Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. (2018) 8:10803. doi: 10.7189/jogh.08.010803
16. Cooper DH, Ramachandra R, Ceban F, Di Vincenzo JD, Rhee TG, Mansur RB, et al. Glucagon-like peptide 1 (GLP-1) receptor agonists as a protective factor for incident depression in patients with diabetes mellitus: A systematic review. J Psychiatr Res. (2023) 164:80–9. doi: 10.1016/j.jpsychires.2023.05.041
17. Harerimana NV, Liu Y, Gerasimov ES, Duong D, Beach TG, Reiman EM, et al. Genetic evidence supporting a causal role of depression in alzheimer’s disease. Biol Psychiatry. (2022) 92:25–33. doi: 10.1016/j.biopsych.2021.11.025
18. Zhao YX and Chen XW. Diabetes and risk of glaucoma: systematic review and a Meta-analysis of prospective cohort studies. Int J Ophthalmol. (2017) 10:1430–5. doi: 10.18240/ijo.2017.09.16
19. Zhou M, Wang W, Huang W, and Zhang X. Diabetes mellitus as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. PloS One. (2014) 9:e102972. doi: 10.1371/journal.pone.0102972
20. Della Santina L, Inman DM, Lupien CB, Horner PJ, and Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci. (2013) 33:17444–57. doi: 10.1523/JNEUROSCI.5461-12.2013
21. Poon WW, Blurton-Jones M, Tu CH, Feinberg LM, Chabrier MA, Harris JW, et al. beta-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol Aging. (2011) 32:821–33. doi: 10.1016/j.neurobiolaging.2009.05.012
22. Liu CH, Kang EY, Lin YH, Wu WC, Liu ZH, Kuo CF, et al. Association of ocular diseases with schizophrenia, bipolar disorder, and major depressive disorder: a retrospective case-control, population-based study. BMC Psychiatry. (2020) 20:486. doi: 10.1186/s12888-020-02881-w
23. Ravipati K, Chen Y, and Manns JR. Reassessing diabetes and APOE genotype as potential interacting risk factors for alzheimer's disease. Am J Alzheimers Dis Other Demen. (2022) 37:15333175211070912. doi: 10.1177/15333175211070912
24. Tisato V, Zuliani G, Vigliano M, Longo G, Franchini E, Secchiero P, et al. Gene-gene interactions among coding genes of iron-homeostasis proteins and APOE-alleles in cognitive impairment diseases. PloS One. (2018) 13:e0193867. doi: 10.1371/journal.pone.0193867
25. Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, and Giau VV. Type 3 diabetes and its role implications in alzheimer's disease. Int J Mol Sci. (2020) 21:3165. doi: 10.3390/ijms21093165
26. Gemmati D, Longo G, Gallo I, Silva JA, Secchiero P, Zauli G, et al. Host genetics impact on SARS-CoV-2 vaccine-induced immunoglobulin levels and dynamics: The role of TP53, ABO, APOE, ACE2, HLA-A, and CRP genes. Front Genet. (2022) 13:1028081. doi: 10.3389/fgene.2022.1028081
27. Behera UC, Bhattacharjee H, Das T, Gilbert C, Murthy GVS, Rajalakshmi R, et al. group Ss. Spectrum of Eye Disease in Diabetes (SPEED) in India: A prospective facility-based study. Report 4. Glaucoma in people with type 2 diabetes mellitus. Indian J Ophthalmol. (2020) 68:S32–6. doi: 10.4103/ijo.IJO_1948_19
28. Abikoye TM, Oluleye TS, Aribaba OT, Musa KO, Idowu OO, and Onakoya AO. Is primary open-angle glaucoma a risk factor for diabetic retinopathy? Int Ophthalmol. (2020) 40:3233–40. doi: 10.1007/s10792-020-01507-0
29. Sperling S, Stokholm L, Thykjaer AS, Pedersen FN, Moller S, Laugesen CS, et al. Bidirectional 5-year risks of diabetic retinopathy, glaucoma and/or ocular hypertension: Results from a national screening programme. Acta Ophthalmol. (2023) 101:384–91. doi: 10.1111/aos.15300
30. Xu H, Manivannan A, Liversidge J, Sharp PF, Forrester JV, and Crane IJ. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J Neuroimmunol. (2003) 142:47–57. doi: 10.1016/s0165-5728(03)00258-3
31. Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. (2007) 28:1810–20. doi: 10.1016/j.neurobiolaging.2006.08.012
32. Nguyen TT, Kawasaki R, Kreis AJ, Wang JJ, Shaw J, Vilser W, et al. Correlation of light-flicker-induced retinal vasodilation and retinal vascular caliber measurements in diabetes. Invest Ophthalmol Vis Sci. (2009) 50:5609–13. doi: 10.1167/iovs.09-3442
33. Huber JD, VanGilder RL, and Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. (2006) 291:H2660–8. doi: 10.1152/ajpheart.00489.2006
34. Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, and Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. (2003) 74:70–6. doi: 10.1136/jnnp.74.1.70
35. Acharya NK, Levin EC, Clifford PM, Han M, Tourtellotte R, Chamberlain D, et al. Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: beneficial effects of the LpPLA2 inhibitor darapladib. J Alzheimers Dis. (2013) 35:179–98. doi: 10.3233/JAD-122254
36. Ramm L, Jentsch S, Peters S, Sauer L, Augsten R, and Hammer M. Dependence of diameters and oxygen saturation of retinal vessels on visual field damage and age in primary open-angle glaucoma. Acta Ophthalmol. (2016) 94:276–81. doi: 10.1111/aos.12727
37. Martucci A, Giannini C, Di Marino M, Sorge RP, Aiello F, Scuteri D, et al. Evaluation of putative differences in vessel density and flow area in normal tension and high-pressure glaucoma using OCT-angiography. Prog Brain Res. (2020) 257:85–98. doi: 10.1016/bs.pbr.2020.07.006
38. Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. (2004) 18:1450–2. doi: 10.1096/fj.03-1476fje
39. Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. (2011) 77:1126–34. doi: 10.1212/WNL.0b013e31822f0435
40. Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. (2004) 292:2237–42. doi: 10.1001/jama.292.18.2237
41. Kopf D and Frolich L. Risk of incident Alzheimer's disease in diabetic patients: a systematic review of prospective trials. J Alzheimers Dis. (2009) 16:677–85. doi: 10.3233/JAD-2009-1011
42. Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. (2003) 161:653–60. doi: 10.1083/jcb.200302070
43. Argaw AT, Gurfein BT, Zhang Y, Zameer A, and John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. (2009) 106:1977–82. doi: 10.1073/pnas.0808698106
44. Segarra M, Aburto MR, and Acker-Palmer A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. (2021) 44:393–405. doi: 10.1016/j.tins.2020.12.002
45. Hasan SS, Clavarino AM, Dingle K, Mamun AA, and Kairuz T. Diabetes mellitus and the risk of depressive and anxiety disorders in Australian women: A longitudinal study. J Womens Health (Larchmt). (2015) 24:889–98. doi: 10.1089/jwh.2015.5210
46. Arshad AR and Alvi KY. Frequency of depression in type 2 diabetes mellitus and an analysis of predictive factors. J Pak Med Assoc. (2016) 66:425–9.
47. Dunlavey CJ. Introduction to the hypothalamic-pituitary-adrenal axis: healthy and dysregulated stress responses, developmental stress and neurodegeneration. J Undergrad Neurosci Educ. (2018) 16:R59–60.
48. Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. (2017) 20:1752–60. doi: 10.1038/s41593-017-0010-3
49. Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci U S A. (2020) 117:3326–36. doi: 10.1073/pnas.1914655117
50. Zhou X, Shi Q, Zhang X, Gu L, Li J, Quan S, et al. ApoE4-mediated blood-brain barrier damage in Alzheimer's disease: Progress and prospects. Brain Res Bull. (2023) 199:110670. doi: 10.1016/j.brainresbull.2023.110670
51. Fotesko K, Thomsen BSV, Kolko M, and Vohra R. Girl power in glaucoma: the role of estrogen in primary open angle glaucoma. Cell Mol Neurobiol. (2022) 42:41–57. doi: 10.1007/s10571-020-00965-5
52. Albert KM and Newhouse PA. Estrogen, stress, and depression: cognitive and biological interactions. Annu Rev Clin Psychol. (2019) 15:399–423. doi: 10.1146/annurev-clinpsy-050718-095557
53. Margeta MA, Letcher SM, Igo RP Jr., Cooke Bailey JN, Pasquale LR, Haines JL, et al. Association of APOE with primary open-angle glaucoma suggests a protective effect for APOE epsilon4. Invest Ophthalmol Vis Sci. (2020) 61:3. doi: 10.1167/iovs.61.8.3
54. Belloy ME, Andrews SJ, Le Guen Y, Cuccaro M, Farrer LA, Napolioni V, et al. APOE genotype and alzheimer disease risk across age, sex, and population ancestry. JAMA Neurol. (2023) 80:1284–94. doi: 10.1001/jamaneurol.2023.3599
55. Riedel BC, Thompson PM, and Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. (2016) 160:134–47. doi: 10.1016/j.jsbmb.2016.03.012
56. Paganini-Hill A and Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. (1996) 156:2213–7. doi: 10.1001/archinte.1996.00440180075009
57. Poehlman ET and Tchernof A. Traversing the menopause: changes in energy expenditure and body composition. Coron Artery Dis. (1998) 9:799–803. doi: 10.1097/00019501-199809120-00004
58. Kara A, Unal D, Simsek N, Yucel A, Yucel N, and Selli J. Ultra-structural changes and apoptotic activity in cerebellum of post-menopausal-diabetic rats: a histochemical and ultra-structural study. Gynecol Endocrinol. (2014) 30:226–31. doi: 10.3109/09513590.2013.864270
59. Herson M and Kulkarni J. Hormonal agents for the treatment of depression associated with the menopause. Drugs Aging. (2022) 39:607–18. doi: 10.1007/s40266-022-00962-x
60. An SY, Kim Y, Kwon R, Lim GY, Choi HR, Namgoung S, et al. Depressive symptoms and suicidality by menopausal stages among middle-aged Korean women. Epidemiol Psychiatr Sci. (2022) 31:e60. doi: 10.1017/S2045796022000439
61. Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E genotype and sex risk factors for alzheimer disease: A meta-analysis. JAMA Neurol. (2017) 74:1178–89. doi: 10.1001/jamaneurol.2017.2188
62. Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, et al. Alzheimer's disease genetics C, the alzheimer's disease neuroimaging I. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. (2018) 75:989–98. doi: 10.1001/jamaneurol.2018.0821
63. Nam KN, Wolfe CM, Fitz NF, Letronne F, Castranio EL, Mounier A, et al. Integrated approach reveals diet, APOE genotype and sex affect immune response in APP mice. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:152–61. doi: 10.1016/j.bbadis.2017.10.018
64. Maldonado Weng J, Parikh I, Naqib A, York J, Green SJ, Estus S, et al. Synergistic effects of APOE and sex on the gut microbiome of young EFAD transgenic mice. Mol Neurodegener. (2019) 14:47. doi: 10.1186/s13024-019-0352-2
65. Cheung CY, Ikram MK, Chen C, and Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res. (2017) 57:89–107. doi: 10.1016/j.preteyeres.2017.01.001
66. Lin IC, Wang YH, Wang TJ, Wang IJ, Shen YD, Chi NF, et al. Glaucoma, Alzheimer's disease, and Parkinson's disease: an 8-year population-based follow-up study. PloS One. (2014) 9:e108938. doi: 10.1371/journal.pone.0108938
67. Cesareo M, Martucci A, Ciuffoletti E, Mancino R, Cerulli A, Sorge RP, et al. Association between alzheimer's disease and glaucoma: A study based on heidelberg retinal tomography and frequency doubling technology perimetry. Front Neurosci. (2015) 9:479. doi: 10.3389/fnins.2015.00479
68. Tao Q, Ang TFA, DeCarli C, Auerbach SH, Devine S, Stein TD, et al. Association of chronic low-grade inflammation with risk of alzheimer disease in apoE4 carriers. JAMA Netw Open. (2018) 1:e183597. doi: 10.1001/jamanetworkopen.2018.3597
69. Ligthart S, Vaez A, Vosa U, Stathopoulou MG, de Vries PS, Prins BP, et al. Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am J Hum Genet. (2018) 103:691–706. doi: 10.1016/j.ajhg.2018.09.009
70. Tao Q, Alvin Ang TF, Akhter-Khan SC, Itchapurapu IS, Killiany R, Zhang X, et al. Impact of C-reactive protein on cognition and alzheimer disease biomarkers in homozygous APOE varepsilon4 carriers. Neurology. (2021) 97:e1243–52. doi: 10.1212/WNL.0000000000012512
71. Lima TA, Adler AL, Minett T, Matthews FE, Brayne C, and Marioni RE. C-reactive protein, APOE genotype and longitudinal cognitive change in an older population. Age Ageing. (2014) 43:289–92. doi: 10.1093/ageing/aft193
72. Wang Y, Grydeland H, Roe JM, Pan M, Magnussen F, Amlien IK, et al. Associations of circulating C-reactive proteins, APOE epsilon4, and brain markers for Alzheimer's disease in healthy samples across the lifespan. Brain Behav Immun. (2022) 100:243–53. doi: 10.1016/j.bbi.2021.12.008
73. Martiskainen H, Takalo M, Solomon A, Stancakova A, Marttinen M, Natunen T, et al. Decreased plasma C-reactive protein levels in APOE epsilon4 allele carriers. Ann Clin Transl Neurol. (2018) 5:1229–40. doi: 10.1002/acn3.639
74. Haan MN, Aiello AE, West NA, and Jagust WJ. C-reactive protein and rate of dementia in carriers and non carriers of Apolipoprotein APOE4 genotype. Neurobiol Aging. (2008) 29:1774–82. doi: 10.1016/j.neurobiolaging.2007.04.020
75. Dumitrescu L, Mahoney ER, Mukherjee S, Lee ML, Bush WS, Engelman CD, et al. Genetic variants and functional pathways associated with resilience to Alzheimer's disease. Brain. (2020) 143:2561–75. doi: 10.1093/brain/awaa209
76. Huang J, Tao Q, Ang TFA, Farrell J, Zhu C, Wang Y, et al. The impact of increasing levels of blood C-reactive protein on the inflammatory loci SPI1 and CD33 in Alzheimer's disease. Transl Psychiatry. (2022) 12:523. doi: 10.1038/s41398-022-02281-6
77. Nucci C, Martucci A, Cesareo M, Mancino R, Russo R, Bagetta G, et al. Brain involvement in glaucoma: advanced neuroimaging for understanding and monitoring a new target for therapy. Curr Opin Pharmacol. (2013) 13:128–33. doi: 10.1016/j.coph.2012.08.004
78. Martucci A, Mancino R, Cesareo M, Pinazo-Duran MD, and Nucci C. Combined use of coenzyme Q10 and citicoline: A new possibility for patients with glaucoma. Front Med (Lausanne). (2022) 9:1020993. doi: 10.3389/fmed.2022.1020993
79. Zhang X, Tohari AM, Marcheggiani F, Zhou X, Reilly J, Tiano L, et al. Therapeutic potential of co-enzyme Q10 in retinal diseases. Curr Med Chem. (2017) 24:4329–39. doi: 10.2174/0929867324666170801100516
Keywords: diabetes mellitus, primary open angle glaucoma, Alzheimer’s disease, APOE E4 allele, UK biobank
Citation: Shi Y, He X, Liu W, Hu J, Qiu WQ, Zhang X and Fan Z (2025) Associations of diabetes mellitus with primary open angle glaucoma and Alzheimer’s disease: a large cohort study in UK biobank. Front. Endocrinol. 16:1506560. doi: 10.3389/fendo.2025.1506560
Received: 14 October 2024; Accepted: 07 July 2025;
Published: 24 July 2025.
Edited by:
Bert B. Little, University of Louisville, United StatesReviewed by:
Alessio Martucci, University of Rome Tor Vergata, ItalyAjay Vikram Singh, Federal Institute for Risk Assessment (BfR), Germany
Jan Lešták, Eye Clinic JL, Czechia
Copyright © 2025 Shi, He, Liu, Hu, Qiu, Zhang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Fan, ZmFuemhpZ2FuZ0BtYWlsLmNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yan Shi
Yan Shi Xinyue He
Xinyue He William Liu
William Liu Junming Hu3
Junming Hu3 Wei Qiao Qiu
Wei Qiao Qiu Xiaoling Zhang
Xiaoling Zhang Zhigang Fan
Zhigang Fan