- 1Department of Obstetrics and Gynecology, West China Second University Hospital of Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital of Sichuan University, Chengdu, Sichuan, China
- 3National Health Commission (NHC) Key Laboratory of Chronobiology, Sichuan University, Chengdu, Sichuan, China
Background: Endometriosis (EMS) and adenomyosis have adverse effects on women’s fertility. In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are effective treatments for these diseases. Research has shown that different embryo transfer strategies in IVF/ICSI can influence gestational outcomes. This systematic review and meta-analysis aimed to evaluate the impact of freeze-all embryo transfer (FET) versus fresh embryo transfer (ET) strategies in IVF/ICSI cycles for infertile women with EMS and adenomyosis.
Method: A comprehensive search was conducted across PubMed, EMBASE, MEDLINE, Web of Science, Google Scholar, and Chinese databases to identify studies examining different embryo transfer strategies in IVF/ICSI cycles among patients with EMS and adenomyosis. The outcomes analyzed included rates of implantation, clinical pregnancy, miscarriage, and live birth. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using random-effects or fixed-effects models.
Results: In patients with EMS, the results demonstrated that the FET strategy yielded higher clinical pregnancy (OR: 1.25; 95% CI: 1.11, 1.40), live birth rates (OR: 1.31; 95% CI: 1.15, 1.49), and implantation rates (OR: 1.27; 95% CI: 1.05, 1.54) compared to the fresh ET strategy. The miscarriage rate (OR: 0.89; 95% CI: 0.52, 1.52) and the ectopic pregnancy rate (OR: 0.51; 95% CI: 0.24, 1.07) were comparable between groups. For the group of women with adenomyosis, the IVF/ICSI outcomes were comparable between the FET and fresh ET strategies.
Conclusion: In IVF/ICSI, the FET strategy has been associated with more favorable reproductive outcomes compared to the fresh ET strategy in women with EMS. Whereas in women with adenomyosis, pregnancy outcomes were comparable between the FET and fresh ET groups.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024563268, identifier CRD42024563268.
Introduction
Endometriosis (EMS) and adenomyosis are interrelated chronic diseases, both originating from ectopically located intracavitary endometrium. EMS is characterized by the presence of endometrial stroma and glands outside the uterine cavity, while adenomyosis is defined by the infiltration of endometrial tissue within the myometrium (1). Women with EMS and adenomyosis often suffer from subfertility and infertility (2). Up to 35–50% of infertile women are affected by EMS, while the prevalence of adenomyosis in infertile women is reported to be approximately 7.5-24.4% (3). The pathological processes may involve inflammation and fibrosis, immune modulation, altered steroid hormone metabolism, increased oxidative stress, and intrauterine abnormalities. These factors potentially interfere with folliculogenesis, sperm function, embryo transport, and endometrial receptivity (4, 5).
In vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) and embryo transfer is a valid option for infertile women with EMS and adenomyosis (6, 7). The current clinical policy of transferring includes both fresh embryo transfer (ET) and freeze-all embryo transfer (FET). The FET strategy, initially designed to mitigate ovarian hyper-stimulation syndrome, facilitates embryo cryopreservation for subsequent suitable cycles. In IVF/ICSI, the key factors to conception are embryo quality, embryo-endometrium interaction, and endometrial receptivity (8). Recently, heightened attention has been drawn to the elevated sex steroid levels resulting from hyper-stimulation during controlled ovarian hyperstimulation (COS). It may exacerbate endometrial receptivity issues, reducing the likelihood of successful conception. As a results, there has been a proposal that FET approach can separate the COS process from the embryo transfer, thereby serving to circumvent the potential adverse impacts of COS on the endometrium (9). One randomized Controlled Trials (RCTs) involving patients with ovulatory women demonstrated favorable outcomes in either pregnancy outcomes or the incidence of ovarian hyper-stimulation syndrome for the FET group (10). With the advancement of vitrification techniques, FET approach has been applied to patients with EMS and adenomyosis. Bourdon et al. investigated 270 infertile women with EMS undergoing IVF/ICSI. Their results indicated that the FET strategy yielded higher cumulative clinical pregnancy and live birth rates compared to the fresh ET strategy (11). Similarly, a retrospective cohort analysis found that the FET strategy in women with adenomyosis was associated with significantly higher odds of live birth compared to fresh ET (12). However, other studies reported comparable pregnancy outcomes among women with EMS or adenomyosis, regardless of whether FET or fresh ET was used (13–15). Additionally, Roque et al. conducted a systematic review and meta-analysis, demonstrating that FET significantly improved live birth rates compared to fresh ET, particularly in hyper-responders and preimplantation genetic testing for aneuploidy cycles (16). However, the lack of distinction between study populations limited its clinical applicability. To evaluate embryo transfer strategies in infertile patients with endometriosis and adenomyosis, we conducted a systematic review and meta-analysis.
Materials and methods
Protocol and registration
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). The protocol of this meta-analysis was registered in PROSPERO (CRD42024563268).
Study design
We conducted a systematic review and meta-analysis compared the pregnancy outcomes after different embryo transfer strategies in patients with EMS and adenomyosis. In all cases, EMS and adenomyosis were diagnosed by biopsy through surgery or medical imaging evidence like transvaginal ultrasound (TVUS) or magnetic resonance imaging (18). In TVUS, ovarian endometrioma appeared as a persistent unilocular or multilocular cyst with homogeneous low-level echogenicity of the cyst fluid and absent or moderate cyst wall vascularization. Deeply infiltrating endometriosis in the TVUS appeared as thick tissue blocks, nodular formations, or irregularly shaped hypoechoic, commonly affecting the uterosacral ligament, pouch of Douglas, and/or vagina. In addition, adenomyosis showed myometrial cystic areas, hyperechoic islands, linear striations, and buds or irregular/infiltrated endometrial-myometrial junction zones (19–21).
First, we conducted a comparative analysis across the entire study population, including individuals with EMS or adenomyosis. Subsequently, we performed a subgroup analysis based on the included studies. One analysis comprised nine studies focusing on EMS, while the other included two studies on adenomyosis. We separately summarized the gestational outcomes for patients with EMS or adenomyosis.
Search strategy
A systematic search for relevant papers was carried out in PubMed, EMBASE, MEDLINE, Web of Science, Google Scholar, China National Knowledge Infrastructure, Wanfang Data Knowledge Service Platform, and China Biomedical Literature Database. English search keywords included “endometriosis”, “adenomyosis”, “in vitro fertilization/IVF-ET”, “Intracytoplasmic sperm injection/ICSI”, “freeze-all/frozen embryo transfer”, “Fresh Embryo Transfer”, and “pregnancy outcomes”, as showed in support information. No restrictions were placed on the language or publication date of the studies. Additionally, we examined the reference lists of the eligible studies and review articles to identify any additional relevant articles.
Inclusion and exclusion criteria
Studies were included in this meta-analysis if they matched the following inclusion criteria (1): subjects of study were women diagnosed with either EMS or adenomyosis. (2) study focused on the pregnancy outcomes of different embryo transfer types in IVF/ICSI cycles. The exposure group consisted of women undergoing FET, while the control group comprised those undergoing fresh ET. (3) the outcomes include at least one of the following: implantation rate, miscarriage rate, clinical pregnancy rate, ectopic pregnancy rate, or live births rate. (4) randomized controlled trials (RCTs) or cohort studies. exclusion criteria: (1) study involved with donor or recipient oocyte treatments. (2) Review articles, abstracts, letters, conference papers, and case reports were excluded. (3) Necessary data was not available. (4) studies included animal experiments.
Study selection
All titles and abstracts were independently reviewed by two investigators (Yixian Han and Lukanxuan Wu). Studies that potentially met the inclusion criteria were further assessed through full-text review. Any discrepancies were resolved by consulting a third author(Chang Liu). The kappa statistic was used to evaluate inter-examiner agreement in study selection (22). Both researchers manually extracted data from the included studies using specially designed data collection forms. The following data were collected: lead author, publication year, country, study period, study design, intervention, age, body mass index, duration of infertility, diagnosis, EMS phenotype or stage, stimulation protocol, FET protocol, oocyte retrieved, and pregnancy outcomes.
Risk of bias assessment
The quality of the included studies was evaluated using the Newcastle Ottawa scale (NOS) for assessing observational studies based on selection, comparability, and outcome domains. The total score of this scale was 9 points (0–3: poor quality, 4–6: fair quality,7–9: good quality) (23).
Outcome measure
The primary outcomes were clinical pregnancy and live birth rate, and the secondary outcomes were miscarriage, implantation rate, and ectopic pregnancy rate. The implantation rate was defined as the proportion of transferred embryos that were successfully implanted. Clinical pregnancy was confirmed by the presence of at least one gestational sac on ultrasound, including ectopic pregnancy. Ectopic pregnancy was defined as the detection of a gestational sac outside the uterine cavity using TVUS, or clinically diagnosed when no gestational sac is observed within the uterine cavity but serum hCG levels continue to rise. A miscarriage was defined as the loss of gestation before 28 weeks of pregnancy. Live birth was described as a live birth event after 28 weeks.
Statistical analysis
Statistical analysis was performed with Review Manager 5.4 software. All the outcomes in our analysis were binary. Dichotomous data was analyzed using the Mantel–Hansel odds ratio and the CIs between groups. The I² statistic and Cochran’s Q-statistic were used to assess methodologic and clinical heterogeneity across studies. High heterogeneity was defined as I² ≥ 50% or p < 0.10, in which case a random-effects model was applied; otherwise, a fixed-effects model was used. Sensitivity analysis and subgroup analysis were conducted when I² or Cochran’s Q-statistic detected significant heterogeneity. Funnel plot analysis was used to assess the potential publication bias. All statistical tests were two-sided, and p values < 0.05 were considered statistically significant. The GRADE approach was employed to evaluate the quality of each outcome (24). This assessment considered factors such as risk of bias, inconsistency, indirectness, imprecision, and publication bias. Based on the GRADE criteria, the evidence was categorized into four levels: high, moderate, low, and very low quality.
Results
Study selection
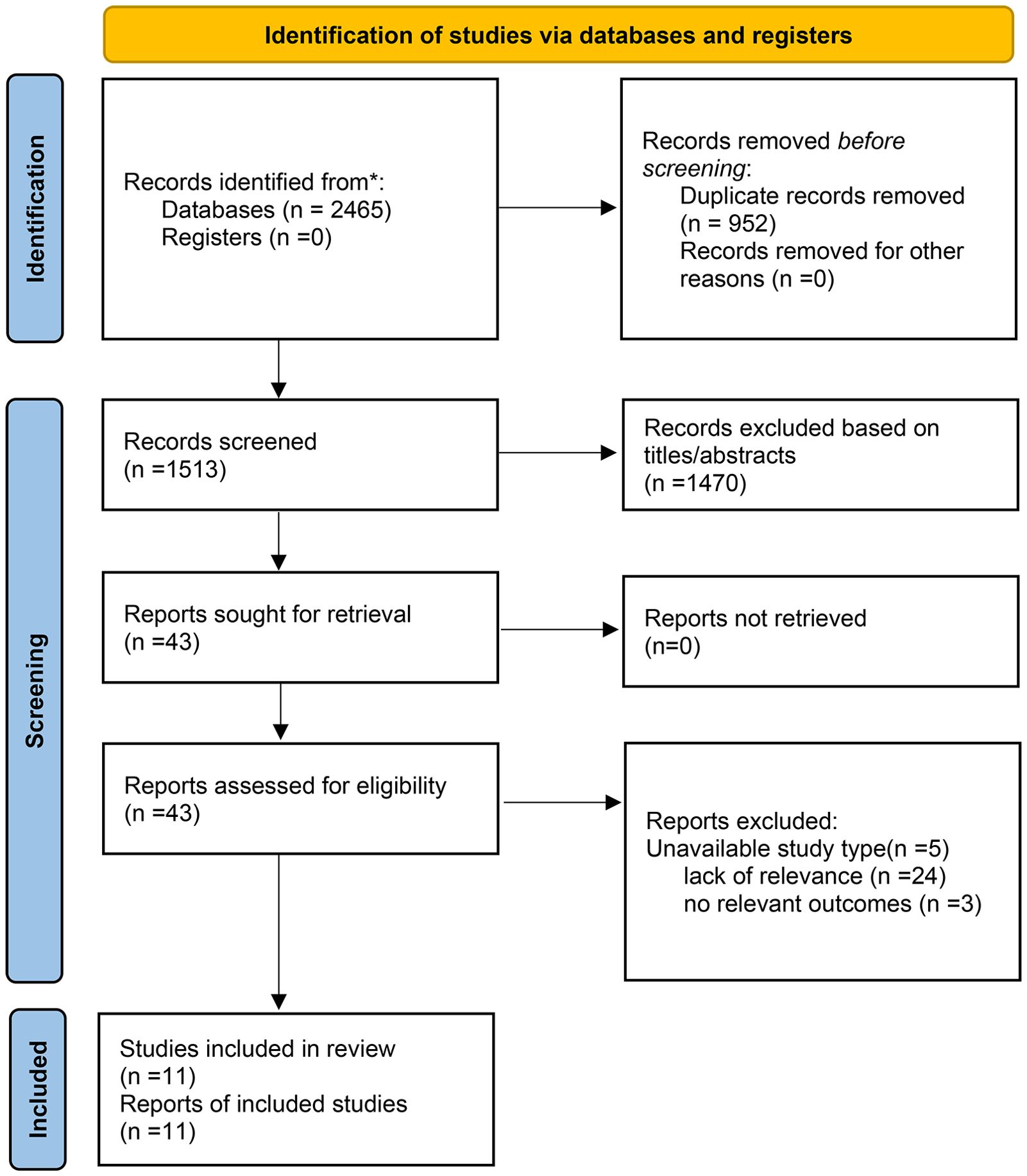
In the initial screening, 2,465 citations were identified across the databases. After removing duplicates, 1,513 articles were evaluated based on their titles and abstracts. Subsequently, 43 articles were retrieved for full-text assessment. After a thorough examination, 5 articles were excluded due to unsuitable study types, 24 for lack of relevance, and 3 for not reporting relevant outcomes. Ultimately, 11 articles that met our eligibility criteria were included in the final analysis. A high level of concordance was observed among the reviewers in terms of data screening and integration (kappa = 0.81). Of the included studies, 4 were published in Chinese and 7 in English. The flow chart of the study selection is demonstrated in Figure 1.
Characteristics of included studies
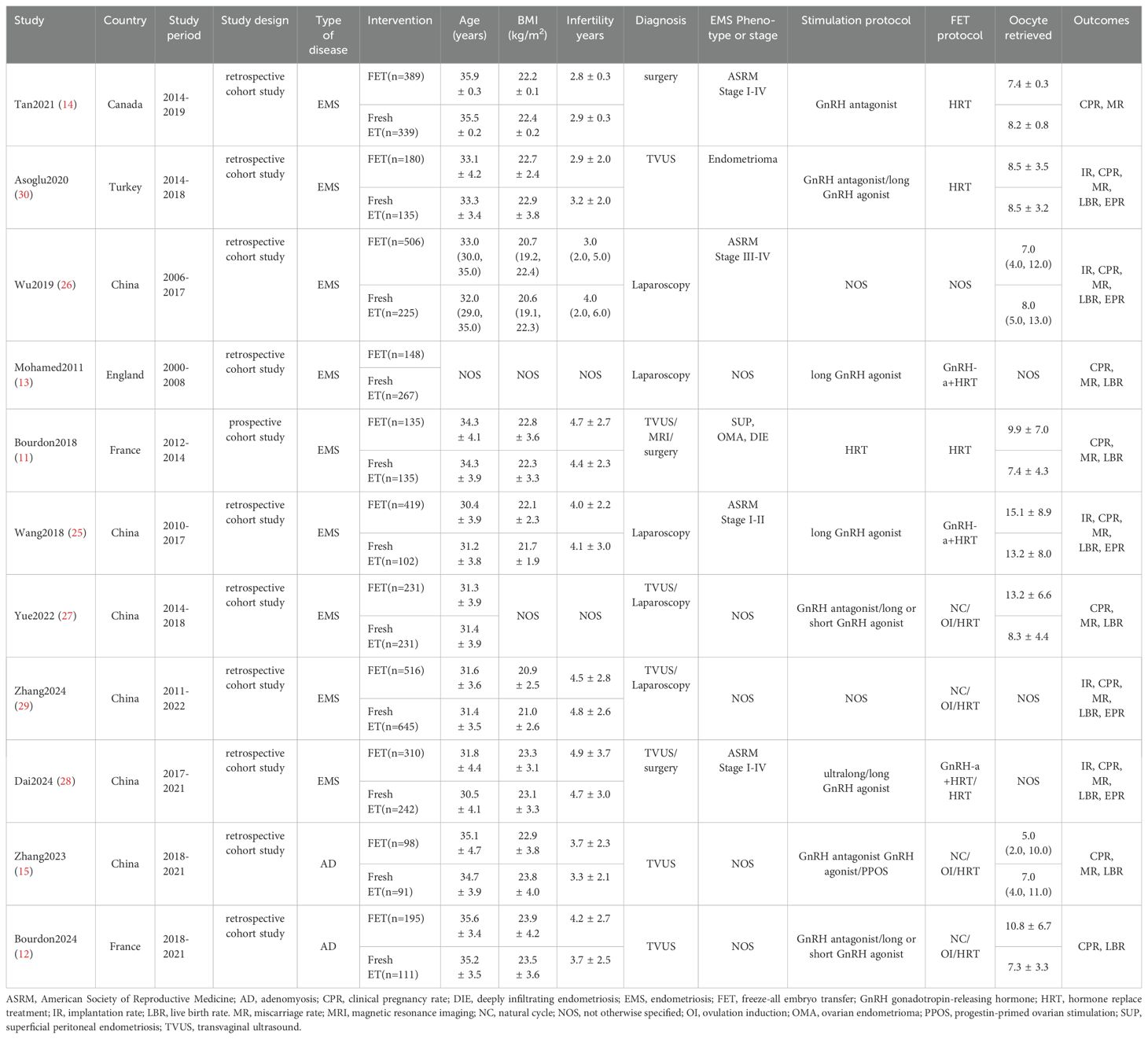
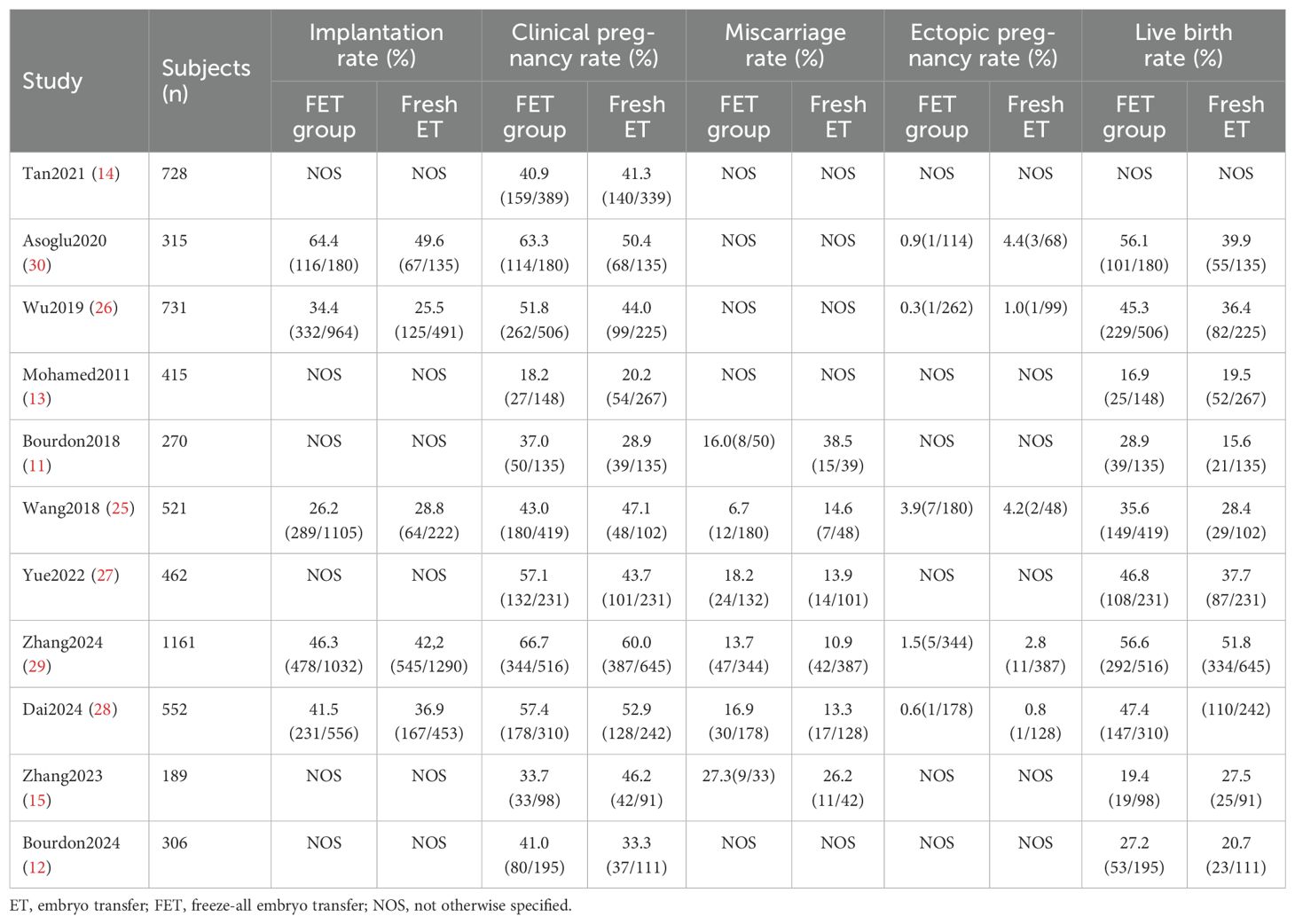
A total of 5650 patients were enrolled, with 3127 undergoing FET and 2523 undergoing fresh ET in the IVF/ICSI cycles. Six studies originated from China (15, 25–29), two from France (11, 12). and the remaining three from Canada, England, and Turkey, respectively (13, 14, 30). Of the eleven studies, two primarily addressed adenomyosis (12, 15), while the remaining nine focused on various types of EMS (8, 11, 13, 14, 25, 27–30). Among the EMS studies, three exclusively included patients with ovarian endometriomas, rASRM stage I-II EMS, or rASRM stage III-IV EMS, respectively (25, 26, 30). In four studies, the diagnosis of EMS was made via laparoscopy or laparotomy, while in five studies, it was determined using either laparoscopy or imaging examinations such as TVUS and magnetic resonance imaging. Adenomyosis was diagnosed by TVUS. Among the included studies, all involved clinical pregnancy rate, five involved embryo implantation rate (25, 26, 28–30), five involved ectopic pregnancy rate (25, 26, 28–30), ten involved live birth rate (11–13, 15, 25–30), and ten involved miscarriage rate (11, 13–15, 25–30). However, the definitions of miscarriage rate varied, with pregnancy loss ranging from 6 to 28 weeks, potentially introducing confounding factors. Therefore, we adopted the most common definition of miscarriage rate, specifically as a pregnancy loss occurring before 28 weeks after clinical pregnancy confirmation, and excluded four studies with differing definitions of miscarriage rate (13, 14, 26, 30). Table 1 presents the characteristics of each study included and Table 2 outlines the main results from each study included in the analysis.
Meta analysis
Clinical pregnancy rate
There were 11 studies investigating the difference in clinical pregnancy rate between FET and fresh ET groups. Compared to fresh ET, FET group improved clinical pregnancy rate (0R: 1.22; 95% CI: 1.09, 1.37; p <0.01; I2 = 48%) (Figure 2A). In the subgroup analysis, the results of women with EMS were consistent with the overall analysis, observing a higher clinical pregnancy rate in FET group (0R: 1.25; 95% CI: 1.11, 1.40; p <0.01; I2 = 38%) (Figure 2B). However, among women with adenomyosis, the clinical pregnancy rate did not significantly differ between the FET and fresh ET groups (0R: 0.92; 95% CI: 0.40, 2.13; p =0.85; I2 = 79%) (Figure 2C).
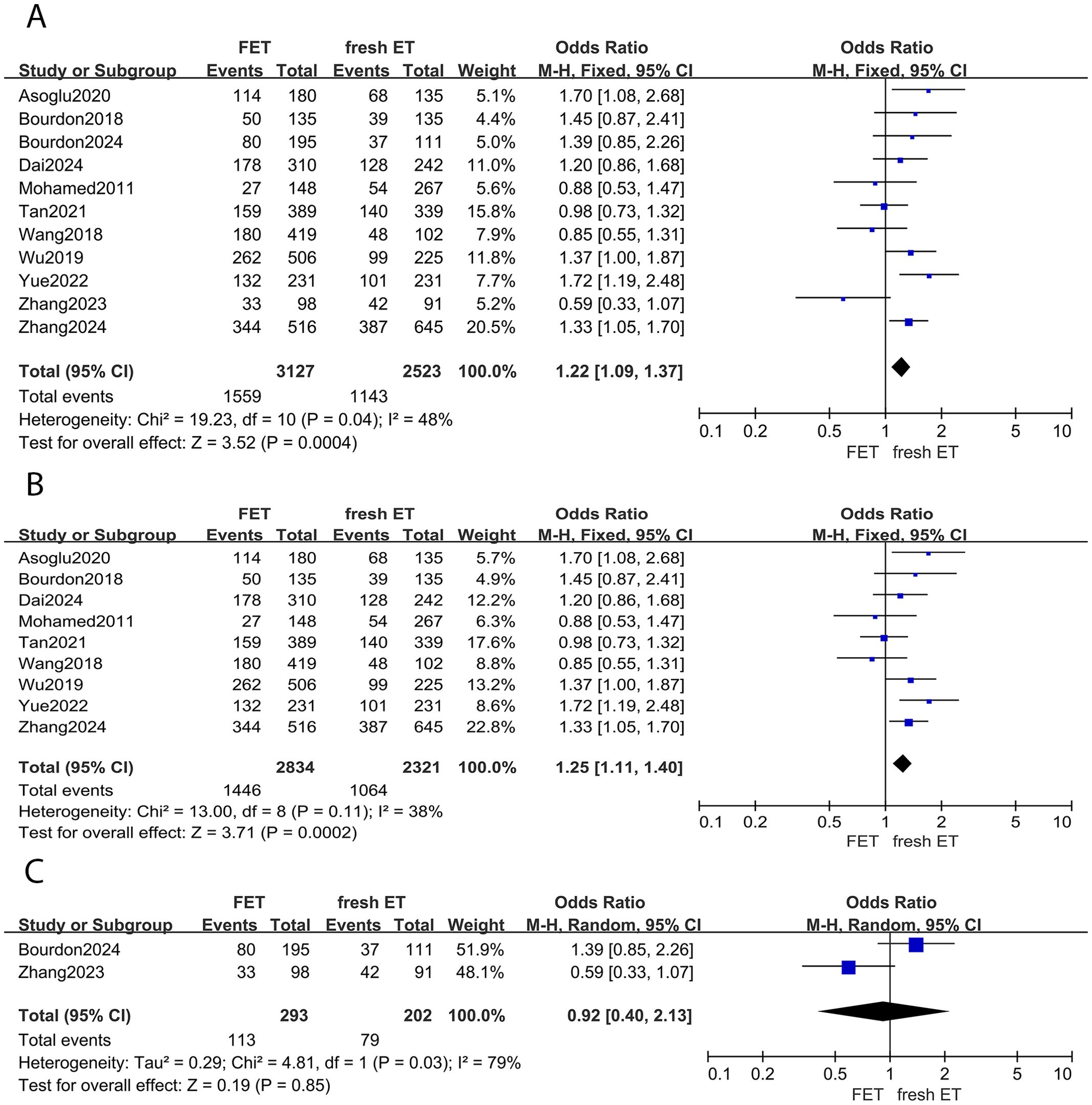
Figure 2. Forest plot for clinical pregnancy rate: (A) FET group versus fresh ET group in patients with endometriosis and adenomyosis; (B) FET group versus fresh ET group in patients with endometriosis; (C) FET group versus fresh ET group in patients with adenomyosis.
Live birth rate
Of the included studies, 10 reports investigated the live birth rate between different embryo transfer groups. A significant increase in live birth rates was observed in women undergoing FET cycles compared to those undergoing fresh ET cycles (OR: 1.29; 95% CI: 1.14, 1.45; p <0.01; I2 = 38%) (Figure 3A). Subgroup analysis consistently showed a higher live birth rate in the FET group among women with EMS (OR: 1.31; 95% CI: 1.15, 1.49; p <0.01; I2 = 32%) (Figure 3B). However, for women with adenomyosis, the live birth rate was similar between groups (OR: 0.98; 95% CI:0.44, 2.16; p =0.95; I2 = 69%) (Figure 3C).
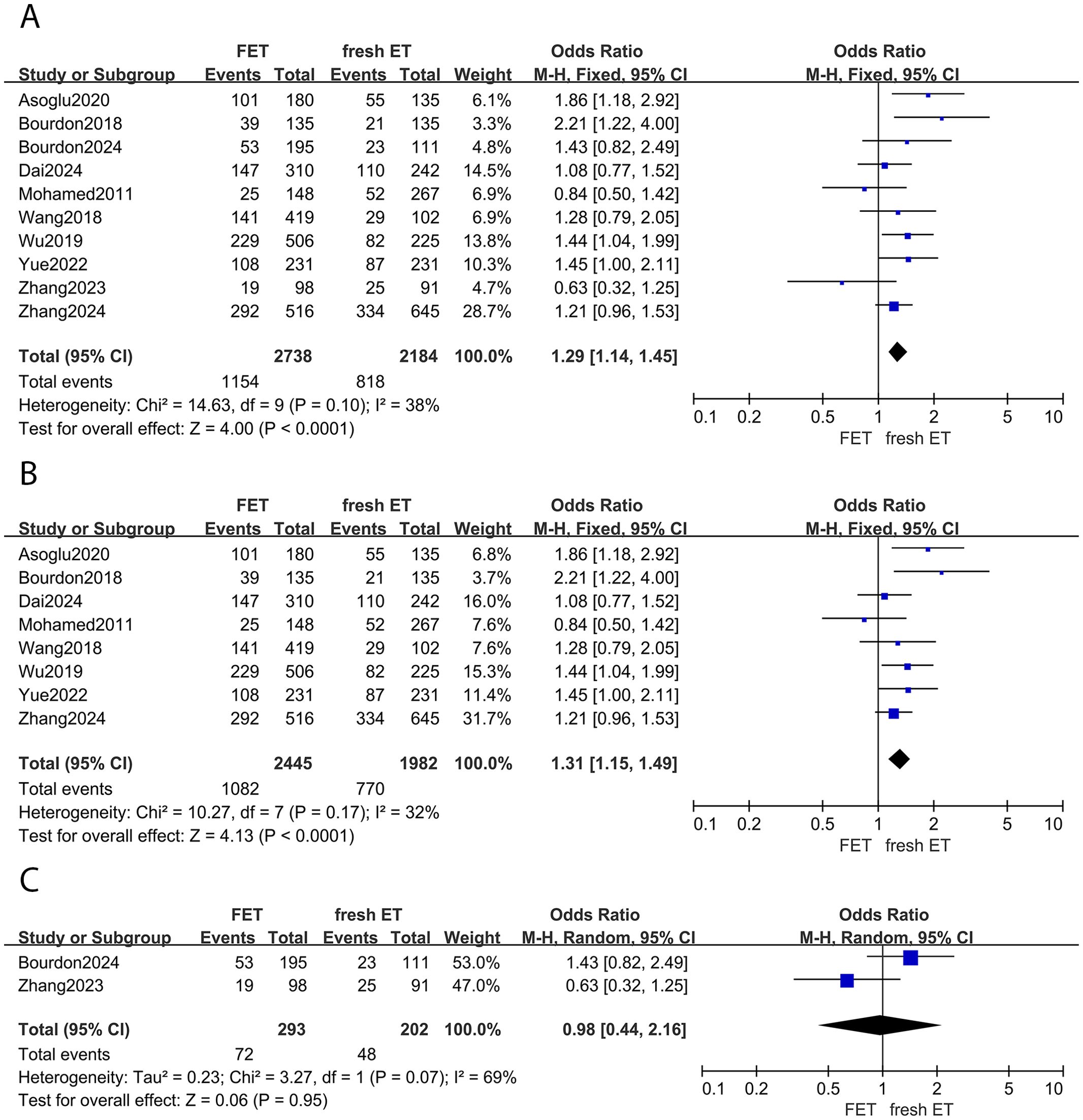
Figure 3. Forest plot for live birth rate: (A) FET group versus fresh ET group in patients with endometriosis and adenomyosis; (B) FET group versus fresh ET group in patients with endometriosis; (C) FET group versus fresh ET group in patients with adenomyosis.
Miscarriage rate
Five studies provided data on miscarriage rates between the FET and fresh ET groups. The results indicated no statistical difference between groups (OR: 0.92; 95% CI: 0.58, 1.46; p =0.73; I2 = 55%) (Figure 4A). This finding was consistent in the subgroup analysis focusing on patients with EMS (OR: 0.89; 95% CI: 0.52, 1.52; p =0.67; I2 = 64%) (Figure 4B). Moreover, the sensitivity analysis of EMS did not alter the conclusion after eliminating any study. Similarly, among women with adenomyosis, the risk of miscarriage was comparable between the FET and fresh ET groups (OR: 1.06; 95% CI: 0.38, 2.96; p =0.92) (Figure 4C).
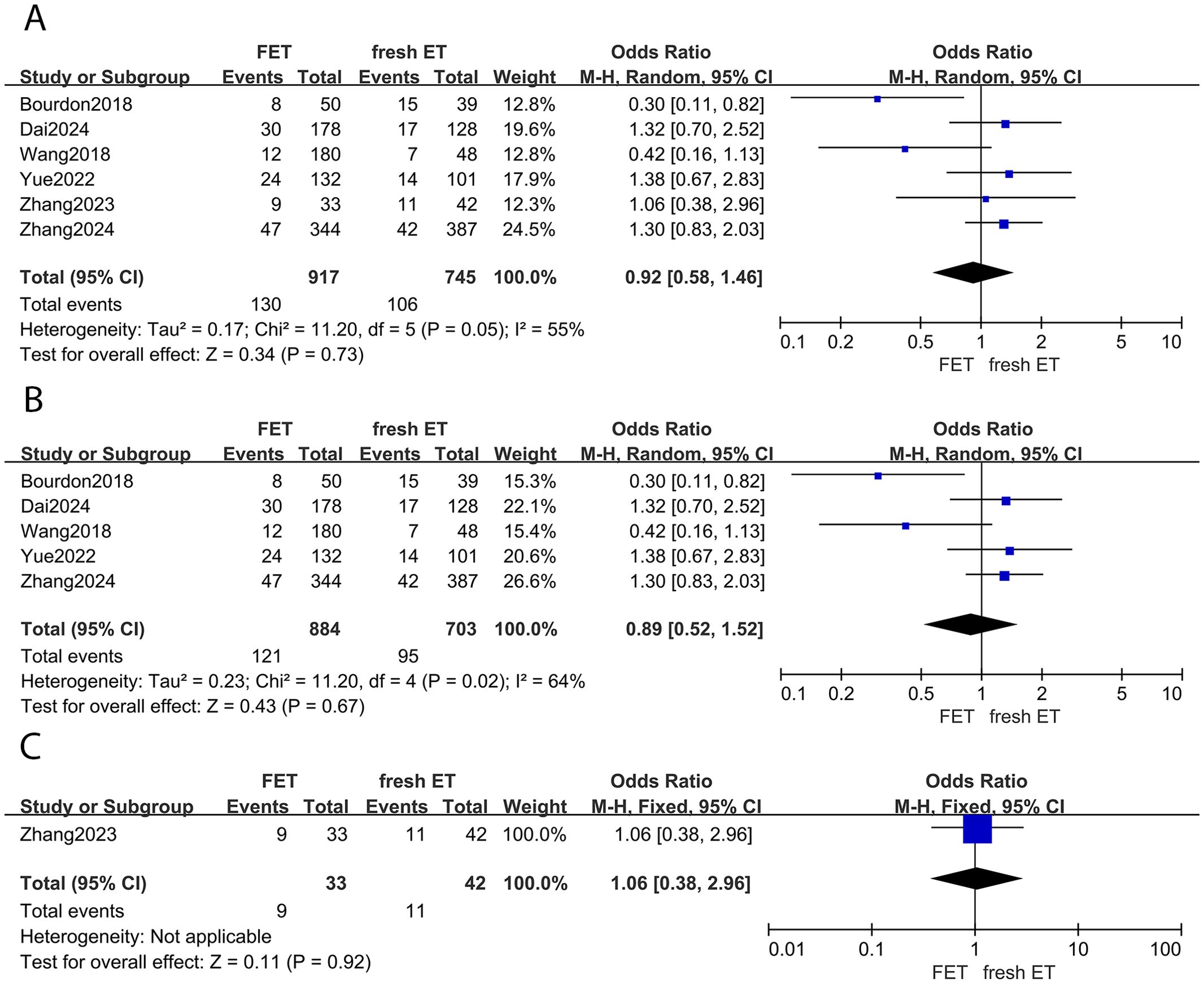
Figure 4. Forest plot for miscarriage rate: (A) FET group versus fresh ET group in patients with endometriosis and adenomyosis; (B) FET group versus fresh ET group in patients with endometriosis; (C) FET group versus fresh ET group in patients with adenomyosis.
Implantation rate
Of the 11 studies included, 5 described the association between different embryo transfer types and implantation rates in patients with EMS. The combined analysis demonstrated that FET group exhibited a higher implantation rate compared to fresh ET group (OR: 1.27; 95% CI: 1.05, 1.54; p =0.01; I2 = 62%) (Supplementary Figure S2). A sensitivity analysis was conducted to address heterogeneity, confirming the findings with consistent results after excluding the study by Wang et al. (25) (OR: 1.32; 95% CI: 1.18, 1.49; p <0.01; I2 = 38%). Data regarding implantation rate specifically in women with adenomyosis were lacking.
Ectopic pregnancy rate
In this meta-analysis, 5 studies were included to investigate the ectopic pregnancy rate between FET and fresh ET groups in women with EMS. The finding indicated a similar ectopic pregnancy rate between groups (OR: 0.51; 95% CI:0.24, 1.07; p =0.08; I2 = 0%) (Supplementary Figure S3). There were no available data to investigate the relationship between ectopic pregnancy rate and different embryo transfer types in women with adenomyosis.
Publication bias assessment of the included studies
Funnel plots for each of the meta-analyses, as shown in Supplementary Figure S1, appeared to be symmetrical, except for the ectopic pregnancy rate, which suggested a potential, though minimal, publication bias likely due to the non-publication of smaller studies.
Quality assessment of the included studies
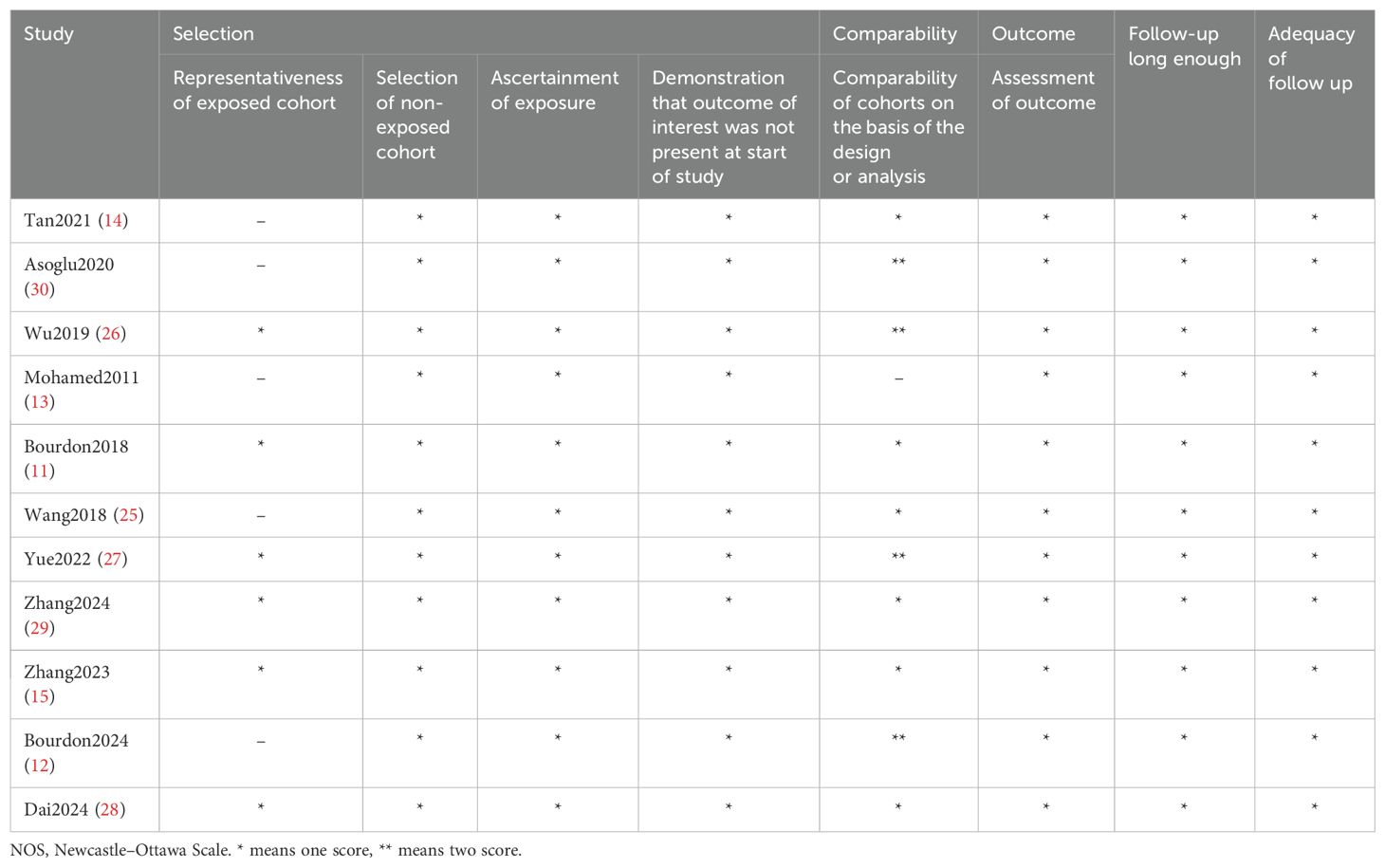
The NOS used for assessing the quality of studies in this meta-analysis is presented in Table 3. The included studies received scores ranging from 7 to 9, indicating they were classified as high-quality studies with a low risk of bias.
GRADE assessment
The quality of evidence for each outcome was evaluated using the GRADE approach as demonstrated in Supplementary Figure S4. The findings revealed that the clinical pregnancy and live birth rates, as well as the ectopic pregnancy rate, following different embryo transfer strategies for patients with EMS and adenomyosis, or EMS alone, were assessed as having moderate certainty of evidence. In contrast, all other outcomes were rated as low or very low certainty of evidence.
Discussion
The objective of this study was to compare pregnancy outcomes between FET and fresh ET cycles in infertile patients with EMS and adenomyosis. The results of the meta-analysis involved 11 studies with a total of 5,650 patients, of which nine focused on endometriosis, while the remaining studies examined adenomyosis. In endometriosis-associated infertility patients undergoing different embryo transfer strategies, FET was associated with increased implantation, clinical pregnancy, and live birth rates, while no significant differences were observed in miscarriage and ectopic pregnancy rates. Compared to previous meta-analysis (31), our findings highlight that FET protocols improve clinical pregnancy rates while maintaining comparable miscarriage rates. These differences may be attributed to the inclusion of a greater number of studies in our analysis. In women with adenomyosis, IVF/ICSI outcomes were similar between FET and fresh ET groups. However, given that only two studies focused on pregnancy outcomes in adenomyosis, these findings should be interpreted with caution.
Due to the elusive nature of their pathogenesis, both EMS and adenomyosis are often referred to as “enigmatic diseases” (32). The question of whether EMS and adenomyosis represent two phenotypes of the same disease or are distinct entities remains a topic of ongoing debate (1, 32, 33). Dysfunction of the myometrial junctional zone and aberrations in the eutopic and heterotopic endometria suggest that adenomyosis and EMS may share a common origin (34). However, a shared origin does not imply they are identical, as they differ significantly in their histological, and clinical manifestations, as well as in their associated risk factors (32). Regardless of whether they are considered the same disease, it is clear that both conditions have a detrimental impact on women’s fertility.
EMS is associated with poor pregnancy outcomes during IVF/ICSI, the mechanisms are highly complex. In addition to alterations in pelvic anatomy and diminished ovarian reserve, changes in the immune microenvironment and reduced endometrial receptivity also play significant roles (35). Implantation is a pivotal aspect of assisted reproductive technology (ART). Supraphysiologic levels of estradiol and progesterone during COS could impair endometrial receptivity and lead to embryo-endometrium asynchrony, thereby reducing implantation rates during IVF/ICSI (36). Meanwhile, EMS, an estrogen-dependent disease, is characterized by inflammation, dysregulated differentiation of endometrial mesenchymal cells, and abnormal epigenetic marks in both the intracavitary endometrium and ectopic endometriotic tissue, all of which are associated with the imbalance between estrogen and progesterone (37). As a result, it is plausible to conjecture that the heightened steroid levels during COS and the intricate pathological characteristic of EMS itself might synergistically impede endometrial receptivity. The FET strategy, an alternative to fresh ET, allows for the separation of COS and embryo transfer. In this way, endometrial development can be controlled more precisely than in cycles of COS with gonadotropins (38). Additionally, embryos are transferred to an environment that has not been exposed to the supraphysiologic hormonal levels associated with COS. Consequently, it has been proposed that FET is advantageous for ART outcomes compared to fresh ET. Yue et al. included 462 patients with EMS to compare pregnancy outcomes between different embryo transfer methods. Their findings indicated that the cumulative clinical pregnancy rate was significantly higher in the FET group compared to the fresh ET group (27). Similarly, a meta-analysis involving six cohort studies revealed that in patients with endometriosis-related infertility, the live birth rate following FET was significantly higher than that of fresh ET. Additionally, the miscarriage rate was statistically lower in the FET group (31). Our study, which incorporated more recent research, confirmed part of these findings, demonstrating consistent improvements in pregnancy outcomes with the FET strategy in patients with endometriosis. However, the miscarriage rate in our analysis was comparable between the FET and fresh ET groups. We considered this discrepancy may arise from variations in the definition of miscarriage rate across studies. To minimize potential confounding, we only included studies that adhered to the most commonly accepted definition of miscarriage, excluding those with differing criteria. In addition, Tan et al. investigated early pregnancy outcomes in fresh versus freeze-all ET for patients with endometriosis, and the results showed comparable outcomes between the two groups (14). We believe that the observed differences may associated with the type of embryos transferred. The study by Tan et al. exclusively included patients who received blastocyst transfers. Previous research has shown that there are significant differences between blastocysts and cleavage-stage embryos in terms of embryo development and synchronization with the endometrium, which could potentially impact pregnancy outcomes (39–41). Additionally, variations in controlled ovarian stimulation protocols and differences in the staging and classification of endometriosis may also influence pregnancy outcomes (8).
In women with adenomyosis, infertility may arise from local endometrial inflammation due to alterations in the eutopic endometrium. Immunologic changes, fibrosis, local hyperestrogenism, and dysperistalsis of the myometrium could be responsible for altered endometrial receptivity and implantation (42, 43). ART is an effective method to improve pregnancy outcomes in infertile adenomyosis-associated patients. In this meta-analysis, we summarized the IVF/ICSI pregnancy outcomes after different embryo transfer strategies. The results indicated that the pregnancy outcomes were comparable between groups. A retrospective cohort analysis involving 306 infertile adenomyosis-associated patients compared the IVF/ICSI outcomes after FET or fresh ET strategies. It also revealed that the rate of clinical pregnancy, miscarriage, and live birth were not significantly different between the two groups (15). However, Bourdon et al. revealed that the FET groups showed higher cumulative ongoing pregnancy rate and cumulative live birth rate versus fresh ET groups in infertile patients affected by adenomyosis (12). They considered that a freeze-all strategy could be beneficial by avoiding the negative effects of ovarian stimulation on an already impaired endometrium. In addition, FET strategy offers an opportunity for gonadotropin-releasing hormone agonist (GnRH-a) pretreatment before embryo transfer, which potentially improve uterine cavity morphology and create a more favorable endometrial environment (44). Due to the limitations of the study design and the small sample size, more rigorous and large-scale multicenter randomized controlled trials are needed to explore this topic.
Overall, it is essential to follow women not only during their journey to achieve pregnancy but throughout their entire reproductive lifespan. Patients with adenomyosis and EMS may experience long-term reproductive health challenges that extend beyond conception, necessitating ongoing monitoring and management to optimize their overall gynecological and reproductive well-being.
Strengths and limitations
This review has several limitations and strengths that may have influenced the findings. The strengths of our study include the following: it is a large-scale meta-analysis based on 11 studies with a novel focus on patients with EMS and adenomyosis. We conducted a systematic literature search adhering to strict inclusion and exclusion criteria, ensuring a rigorous methodology. In addition to reporting the pregnancy outcomes of clinical pregnancy and live birth, we also addressed key reproductive outcomes, such as ectopic pregnancy, which is particularly significant in the context of assisted reproductive treatments. Despite the precautions taken, our study is subject to certain limitations. The quality of each of the included studies varies. Since there are some significant differences in baseline characteristics concerning the age of patients, type of infertility, ovarian reserve function and ovarian protocols, among others. it could introduce confounding factors into the results. Additionally, the included studies differed in their ascertainment of EMS and adenomyosis, without explicitly distinguishing between the two conditions. This lack of distinction may have led to the analysis of gestational outcomes in a mixed group. Furthermore, our study did not examine pregnancy complications or obstetric outcomes, which warrant further investigation.
In clinical practice, factors such as uterine environment, endometrial receptivity, and the patient’s overall health should be carefully considered during IVF/ICSI cycles before embryo transfer. Based on current evidence, for women with EMS undergoing IVF/ICSI, clinicians might prioritize FET protocols over fresh embryo transfer. despite the potential for increased financial costs. Stimulation protocols should be adjusted to optimize oocyte retrieval and subsequent cryopreservation for FET cycles. For women with adenomyosis, pregnancy outcomes appear comparable between FET and fresh ET strategies. Clinicians should evaluate uterine abnormalities using imaging or hysteroscopy to guide decisions regarding endometrial preparation and the appropriate type of embryo transfer.
Heterogeneity exists across the studies due to substantial variations in study design, participant characteristics, and sample size. Although subgroup analyses for EMS and adenomyosis were performed to mitigate some of the heterogeneity, differences in the types of observational studies and patient characteristics, particularly variations in disease severity among EMS or adenomyosis patients, contribute to variability in baseline data, potentially impacting the outcomes. Consequently, randomized controlled trials focusing on different subtypes and severity levels of EMS and adenomyosis are warranted to better evaluate the efficacy and applicability of various embryo transfer strategies.
Conclusion
In IVF/ICSI, the FET strategy has been associated with more favorable reproductive outcomes compared to the fresh ET strategy in women with EMS, whereas in women with adenomyosis, pregnancy outcomes were comparable between the FET and fresh ET groups. However, the current evidence remains limited. More investigations are underscored to confirm our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
YH: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. CL: Funding acquisition, Methodology, Writing – review & editing. DL: Funding acquisition, Software, Visualization, Writing – review & editing. LW: Writing – review & editing. WH: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Key R&D Plan of the Sichuan Provincial Department of Science and Technology (Grant number 2023YFS0072), National Nature Grand. (Grant Number 82071625), and National Key R&D Program of China. (Grant number 2022YFC2704000).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1507252/full#supplementary-material
Supplementary Figure 1 | Funnel plot.
Supplementary Figure 2 | Forest plot for implantation rate in the FET group versus fresh ET group in patients with endometriosis.
Supplementary Figure 3 | Forest plot for ectopic pregnancy rate in the FET group versus fresh ET group in patients with endometriosis.
Supplementary Figure 4 | Summary of findings (GRADE).
References
1. Bulun SE, Yildiz S, Adli M, Chakravarti D, Parker JB, Milad M, et al. Endometriosis and adenomyosis: shared pathophysiology. Fertil Steril. (2023) 119:746–50. doi: 10.1016/j.fertnstert.2023.03.006
2. Vercellini P, Viganò P, Bandini V, Buggio L, Berlanda N, Somigliana E. Association of endometriosis and adenomyosis with pregnancy and infertility. Fertil Steril. (2023) 119:727–40. doi: 10.1016/j.fertnstert.2023.03.018
3. Moawad G, Kheil MH, Ayoubi JM, Klebanoff JS, Rahman S, Sharara FI. Adenomyosis and infertility. J Assist Reprod Genet. (2022) 39:1027–31. doi: 10.1007/s10815-022-02476-2
4. Kokcu A. Possible effects of endometriosis-related immune events on reproductive function. Arch Gynecol Obstet. (2013) 287:1225–33. doi: 10.1007/s00404-013-2767-2
5. Vigano P, Corti L, Berlanda N. Beyond infertility: obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil Steril. (2015) 104:802–12. doi: 10.1016/j.fertnstert.2015.08.030
6. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: endometriosis. Hum Reprod Open. (2022) 2022:hoac009. doi: 10.1093/hropen/hoac009
7. Squillace ALA, Simonian DS, Allegro MC, Borges EJ, Bianchi PHM, Bibancos M. Adenomyosis and in vitro fertilization impacts - A literature review. JBRA Assist Reprod. (2021) 25:303–9. doi: 10.5935/1518-0557.20200104
8. de Ziegler D, Pirtea P, Carbonnel M, Poulain M, Cicinelli E, Bulletti C, et al. Assisted reproduction in endometriosis. Best Pract Res Clin Endocrinol Metab. (2019) 33:47–59. doi: 10.1016/j.beem.2018.10.001
9. Richter KS, Shipley SK, McVearry I, Tucker MJ, Widra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. (2006) 86:862–6. doi: 10.1016/j.fertnstert.2006.02.114
10. Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. (2018) 378:126–36. doi: 10.1056/NEJMoa1705334
11. Bourdon M, Santulli P, Maignien C, Gayet V, Pocate-Cheriet K, Marcellin L, et al. The deferred embryo transfer strategy improves cumulative pregnancy rates in endometriosis-related infertility: A retrospective matched cohort study. PLoS One. (2018) 13:e0194800. doi: 10.1371/journal.pone.0194800
12. Bourdon M, Santulli P, Maignien C, Bordonne C, Millischer AE, Chargui A, et al. The “freeze-all” strategy seems to improve the chances of birth in adenomyosis-affected women. Fertil Steril. (2024) 121:460–9. doi: 10.1016/j.fertnstert.2023.11.039
13. Mohamed AM, Chouliaras S, Jones CJ, Nardo LG. Live birth rate in fresh and frozen embryo transfer cycles in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. (2011) 156:177–80. doi: 10.1016/j.ejogrb.2011.01.020
14. Tan J, Cerrillo M, Cruz M, Cecchino GN, Garcia-Velasco JA. Early pregnancy outcomes in fresh versus deferred embryo transfer cycles for endometriosis-associated infertility: A retrospective cohort study. J Clin Med. (2021) 10:344. doi: 10.3390/jcm10020344
15. Zhang Q, Chen Q, Li T, Jia Z, Bu X, Liu Y, et al. Pregnancy outcomes of freeze-all versus fresh embryo transfer in women with adenomyosis: A retrospective study. J Clin Med. (2023) 12:1740. doi: 10.3390/jcm12051740
16. Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. (2019) 25:2–14. doi: 10.1093/humupd/dmy033
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Martire FG, Russo C, Selntigia A, Nocita E, Soreca G, Lazzeri L, et al. Early noninvasive diagnosis of endometriosis: dysmenorrhea and specific ultrasound findings are important indicators in young women. Fertil Steril. (2023) 119:455–64. doi: 10.1016/j.fertnstert.2022.12.004
19. Piketty M, Chopin N, Dousset B, Millischer-Bellaische AE, Roseau G, Leconte M, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod. (2009) 24:602–7. doi: 10.1093/humrep/den405
20. Guerriero S, Condous G, van den Bosch T, Valentin L, Leone FP, Van Schoubroeck D, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. (2016) 48:318–32. doi: 10.1002/uog.15955
21. Exacoustos C, Zupi E, Piccione E. Ultrasound imaging for ovarian and deep infiltrating endometriosis. Semin Reprod Med. (2017) 35:5–24. doi: 10.1055/s-0036-1597127
22. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). (2012) 22:276–82. doi: 10.11613/issn.1846-7482
23. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
24. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
25. Wang F, Ke X, Li M. Clinical outcome of first frozen-thawed embryo transfer cycle after whole embryo cryopreservation in patients with mild endometriosis. J Reprod Med. (2018) 27:248–53. doi: 10.3969/j.issn.1004-3845.2018.03.01
26. Wu J, Yang X, Huang J, Kuang Y, Wang Y. Fertility and neonatal outcomes of freeze-all vs. Fresh embryo transfer in women with advanced endometriosis. Front Endocrinol (Lausanne). (2019) 10:770. doi: 10.3389/fendo.2019.00770
27. Yue C, Yu Q, Zhang Y, Xu X, Yan L. Comparison of pregnancy outcomes between frozen-all and fresh blastocyst transfer in patients with endometriosis infertility. Prog Obstetrics Gynecol. (2022) 31:171–5. doi: 10.13283/j.cnki.xdfckjz.2022.03.003
28. Dai F, Guo Y, Tian X, Liu Q, Zheng B, Ma Y, et al. Comparison of clinical outcomes between fresh and freeze-thaw embryo transplantation in endometriosis. J Reprod Med. (2024) 33:148–54. doi: 10.3969/j.issn.1004-3845.2024.02.002
29. Zhang L, Huang H, Lin J, Gao H, He X, Shi Y, et al. Study on embryo transfer strategies in patients with endometriosis-related infertility. Maternal Child Health Care China. (2024) 39:1816–9. doi: 1001-4411.2024.10.019
30. Asoglu MR, Celik C, Bahceci M. Frozen blastocyst transfer improves the chance of live birth in women with endometrioma. Gynecol Endocrinol. (2020) 36:902–6. doi: 10.1080/09513590.2020.1781082
31. Chang Y, Shen M, Wang S, Li X, Duan H. Association of embryo transfer type with infertility in endometriosis: a systematic review and meta-analysis. J Assist Reprod Genet. (2022) 39:1033–43. doi: 10.1007/s10815-022-02460-w
32. Habiba M, Benagiano G, Guo SW. An appraisal of the tissue injury and repair (TIAR) theory on the pathogenesis of endometriosis and adenomyosis. Biomolecules. (2023) 13:975. doi: 10.3390/biom13060975
33. Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis–prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod. (2005) 20:2309–16. doi: 10.1093/humrep/dei021
34. Benagiano G, Brosens I. Adenomyosis and endometriosis have a common origin. J Obstetrics Gynecol India. (2011) 61:146–52. doi: 10.1007/s13224-011-0030-y
35. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. (2012) 39:535–49. doi: 10.1016/j.ogc.2012.10.002
36. Montoya-Botero P, Polyzos NP. The endometrium during and after ovarian hyperstimulation and the role of segmentation of infertility treatment. Best Pract Res Clin Endocrinol Metab. (2019) 33:61–75. doi: 10.1016/j.beem.2018.09.003
37. Zubrzycka A, Zubrzycki M, Perdas E, Zubrzycka M. Genetic, epigenetic, and steroidogenic modulation mechanisms in endometriosis. J Clin Med. (2020) 9:1309. doi: 10.3390/jcm9051309
38. Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. (2013) 99:156–62. doi: 10.1016/j.fertnstert.2012.09.003
39. Yin H, Jiang H, He R, Wang C, Zhu J, Luan K. The effects of fertilization mode, embryo morphology at day 3, and female age on blastocyst formation and the clinical outcomes. Syst Biol Reprod Med. (2015) 61:50–6. doi: 10.3109/19396368.2014.967368
40. Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol. (2017) 49:583–91. doi: 10.1002/uog.17327
41. Li Y, Liu S, Lv Q. Single blastocyst stage versus single cleavage stage embryo transfer following fresh transfer: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2021) 267:11–7. doi: 10.1016/j.ejogrb.2021.10.004
42. Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. (2011) 95:2228–2235, 2235.e2221. doi: 10.1016/j.fertnstert.2011.02.051
43. Guo SW. Cracking the enigma of adenomyosis: an update on its pathogenesis and pathophysiology. Reproduction. (2022) 164:R101–r121. doi: 10.1530/rep-22-0224
Keywords: endometriosis, adenomyosis, assist reproductive technology, freeze-all, embryo transfer, pregnancy outcomes
Citation: Han Y, Liu C, Liu D, Wu L and Huang W (2025) Pregnancy outcomes in freeze-all versus fresh embryo transfer cycles of women with adenomyosis and endometriosis: a systemic review and meta-analysis. Front. Endocrinol. 16:1507252. doi: 10.3389/fendo.2025.1507252
Received: 07 October 2024; Accepted: 22 April 2025;
Published: 14 May 2025.
Edited by:
María Laura Ribeiro, CONICET Centro de Estudios Farmacológicos y Botánicos (CEFYBO), ArgentinaReviewed by:
Tao-Hsin Tung, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, ChinaMarzieh Ghasemi, Zahedan university of Medical Sciences, Iran
Matteo Giorgi, University of Siena, Italy
Copyright © 2025 Han, Liu, Liu, Wu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, d2VpaHVhbmc2NEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yixian Han
Yixian Han Chang Liu
Chang Liu Dong Liu1,2,3
Dong Liu1,2,3 Lukanxuan Wu
Lukanxuan Wu Wei Huang
Wei Huang