- 1Department of Endocrinology and Metabolism, Institute of Endocrinology, NHC Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, The First Hospital of China Medical University, Shenyang, China
- 2Department of Endocrinology and Metabolism, Tieling Central Hospital, Tieling, China
Background: HNF4A-MODY constitutes 5%–10% of MODY cases; however, treatment options remain unclearly recommended, and long-term follow-up of patients with HNF4A-MODY is lacking due to limited research. Here, we report a case carrying a new de novo variant of HNF4A. The patient had been using insulin for up to 25 years before genetic diagnosis.
Case description: A 38-year-old man sought consultation due to an increased daily insulin requirement and inadequate glycemic control. At the age of 13, the patent’s parents discovered that he had significantly elevated fasting blood glucose levels accompanied by weight loss. He was subsequently diagnosed with type 1 diabetes and began insulin therapy. At a routine follow-up at age 21, another physician observed that his pancreatic islet function remained preserved, with negative results for diabetes-related antibodies. Consequently, his diagnosis was revised to type 2 diabetes, and the antihyperglycemic therapy was added in metformin and acarbose. Before the current consultation, the patient’s insulin dosage had gradually increased to 80 units per day; however, glycemic control remained unsatisfactory. Whole exome sequencing identified a heterozygous variant, c.272G > A (p.R91H), in exon 3 of the HNF4A gene (NM_175914.5) in the patient. The patient’s treatment regimen was modified to include metformin at a dosage of 1.0 g twice daily, semaglutide at 0.5 mg once weekly, and insulin glargine was gradually discontinued. The patient achieved adequate glycemic control during follow-up.
Conclusion: This case emphasizes that spontaneous HNF4A-MODY is prone to misdiagnosis and the prolonged rate of pancreatic function decline in HNF4A-MODY. Glycemic control and complication progression could be acceptable in HNF4A-MODY cases treated with long-time insulin, but risks of hypoglycemic events, obesity, and atherosclerosis remain. Switching to GLP1RA treatment in HNF4A-MODY still yields a good effect after a prolonged disease course.
1 Introduction
Hepatocyte Nuclear Factor 4 Alpha (HNF4A)-related maturity-onset diabetes of the young (MODY) constitutes approximately 5% to 10% of all MODY cases (1); however, the literature documents only around 20 instances of HNF4A-MODY in China. HNF4A-MODY has demonstrated responsiveness to sulfonylureas (2), and GLP-1 receptor agonists (GLP-1RAs) have proven effective in achieving satisfactory glycemic control in select cases (3, 4). Nevertheless, treatment modalities remain insufficiently characterized due to insufficient research (5). Limited studies have explored the long-term management of patients diagnosed with HNF4A-MODY.
Here, we report a case from China involving a new de novo variant of HNF4A. The patient had been using insulin for up to 25 years before receiving a genetic diagnosis.
2 Case report
At the age of 13, the patent’s parents discovered that he had significantly elevated fasting blood glucose levels (320.4 mg/dL) accompanied by weight loss. He was subsequently diagnosed with type 1 diabetes and began insulin therapy. During this period, he experienced multiple hypoglycemic episodes. At a routine follow-up at age 21, another physician observed that his pancreatic islet function remained preserved, with negative results for diabetes-related antibodies. Consequently, his diagnosis was revised to type 2 diabetes, and the antihyperglycemic therapy was added in metformin and acarbose. At the age of 38, the patient sought consultation due to an increased daily insulin requirement and inadequate glycemic control. Before the current consultation, the patient’s insulin dosage had gradually increased to 80 units per day; however, glycemic control remained unsatisfactory, as indicated by an HbA1c level of 9.6% (Figure 1).
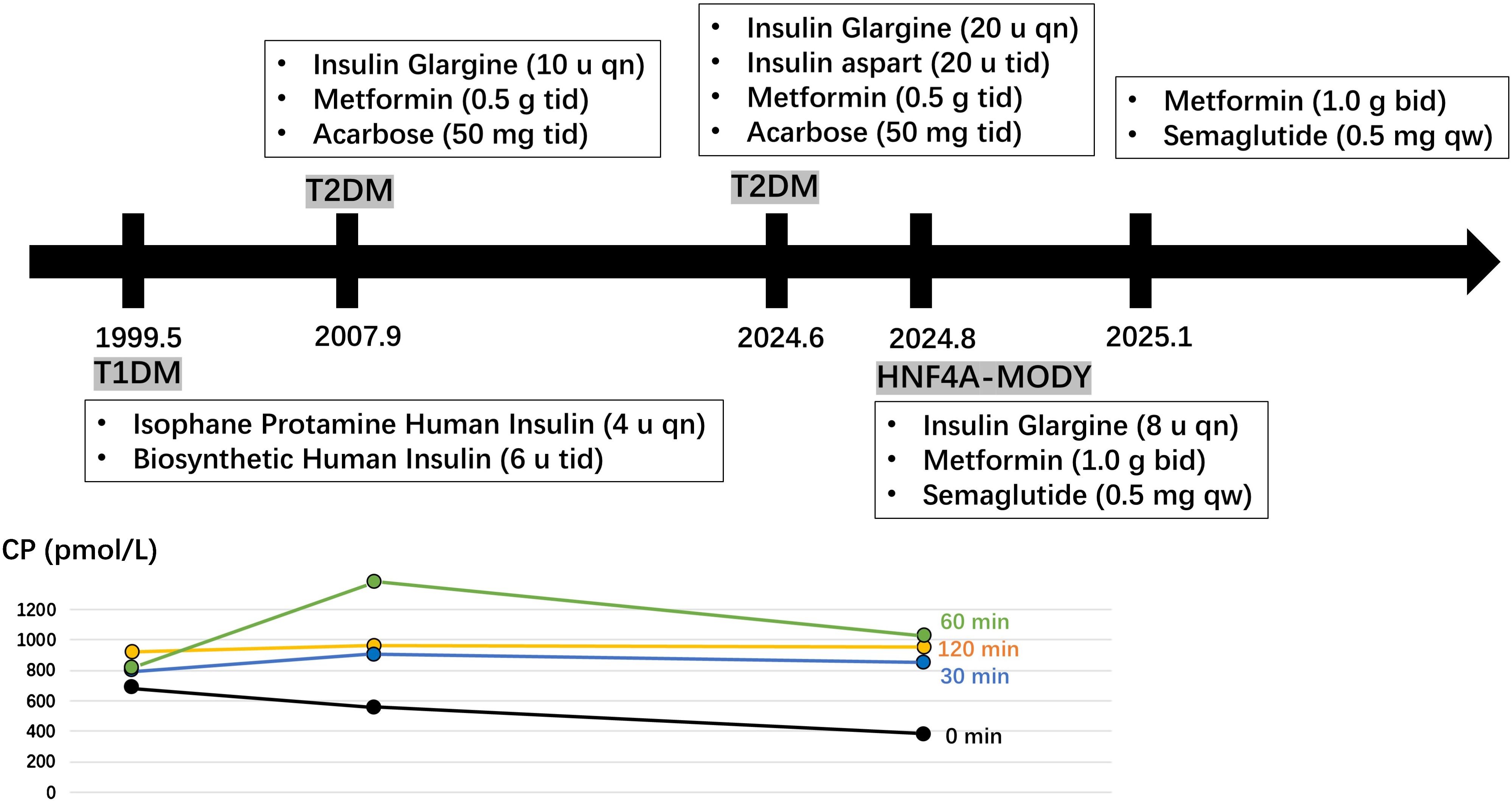
Figure 1. A historical timeline of the patient. Fasting and postprandial C-peptide (CP) levels were measured at the ages of 13, 21, and 38 years.
The patient was born with a birth weight of 4,300 g; however, there was no relevant medical record indicating neonatal hypoglycemia. Additionally, the family reported no history of diabetes. At 21 years of age, the patient had a body mass index (BMI) of 24.5 kg/m²; at 38 years of age, the BMI increased to 28.4 kg/m². His blood pressure was recorded at 145/90 mmHg. Laboratory tests for serum lipid levels, uric acid, diabetic autoimmune antibodies, urinary microalbumin creatinine ratio, and thyroid function, including antibodies, were all within normal ranges. Fasting and postprandial C-peptide levels were measured at ages 13, 21, and 38 years (Figure 1). A carotid ultrasound indicated the presence of atherosclerosis, and a fundoscopic examination revealed scattered macular hemorrhages in both eyes.
2.1 Genetic testing and counseling
Whole exome sequencing was performed to identify a genetic etiology, given that the patient presented with early-onset diabetes, reserved pancreatic function, and negative diabetes-related antibodies. As anticipated, we identified a novel heterozygous variant, c.272G > A (p.R91H), in exon 3 of the HNF4A gene (NM_175914.5) in the patient. Sanger sequencing confirmed the presence of this variant in the patient but not in his parents (Figure 2). Recent versions of genomic databases did not report this missense variant; according to the ACMG/AMP guidelines, it is classified as likely pathogenic (PM1, PM2, PP1, and PP3). Thus, the diagnosis for this patient is classified as HNF4A-MODY.
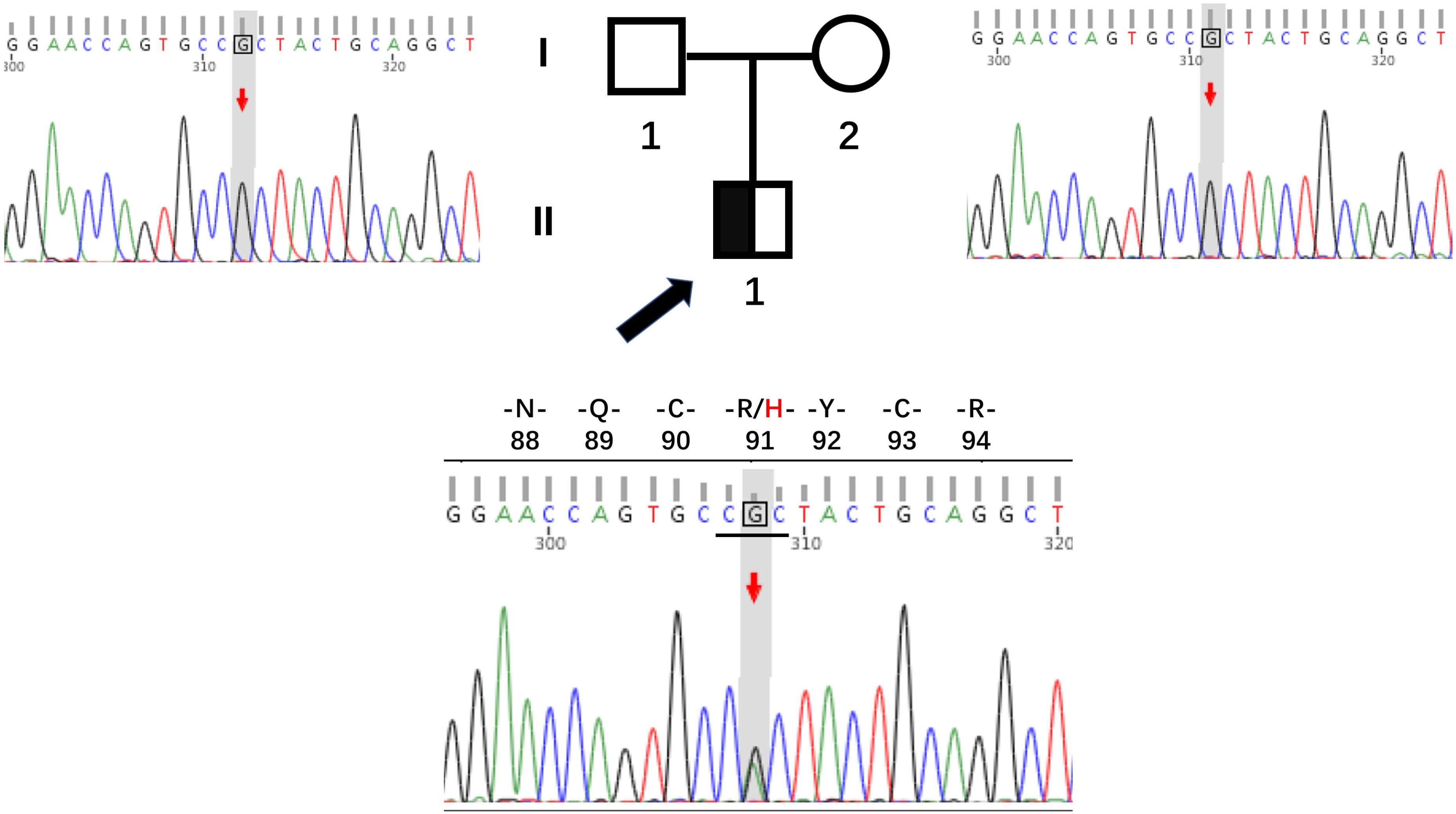
Figure 2. Pedigrees and sequencing chromatograms for the family with the HNF4A mutation R91H. The proband is indicated by a black arrow, with males represented by squares and females by circles.
This de novo mutation might originate from early embryonic mutations or parental germline mosaicism. Even though the tests on the parents are negative, there is still an extremely slight risk of recurrence because of the potential mosaicism. We suggest that the parents consider undergoing high-sensitivity testing to rule out low-level mosaicism. However, due to their advanced age, the parents have no plans for another pregnancy and thus have no intention of undergoing further examinations. For the patient, there is a 50% chance that their offspring will develop the disease. If the patient has plans for childbearing, preimplantation genetic testing or prenatal diagnosis options can be considered.
2.2 Treatment and Follow-up
The patient’s treatment regimen was modified to include metformin at a dosage of 1.0 g twice daily, semaglutide at 0.5 mg once weekly, and insulin glargine gradually reduced to 8 units once daily. After six months, the patient achieved adequate glycemic control, as indicated by hemoglobin A1c levels of 7.1%—7.3%. The patient’s achieved a weight loss of 4.2 kilograms, and insulin has been discontinued at present.
The patient expresses a high level of satisfaction with the current treatment regimen and is willing to comply with scheduled follow-up appointments.
3 Discussion
The HNF4A gene encodes HNF4α, a member of the nuclear receptor superfamily of ligand-dependent transcription factors, which play a crucial role in regulating pancreatic insulin secretion (2). HNF4A-MODY accounts for approximately 5% to 10% of all MODY cases; however, it is infrequently observed in the Chinese population (6, 7). We reviewed the literature and identified 23 mutations in the HNF4A gene among Chinese patients (Figure 3). The majority of these mutations are localized within the DNA binding domain (DBD) and ligand binding domain (LBD) regions. Notably, the R91H mutation in this patient is located in the highly conserved DBD region of HNF4α (8) (Figure 3).
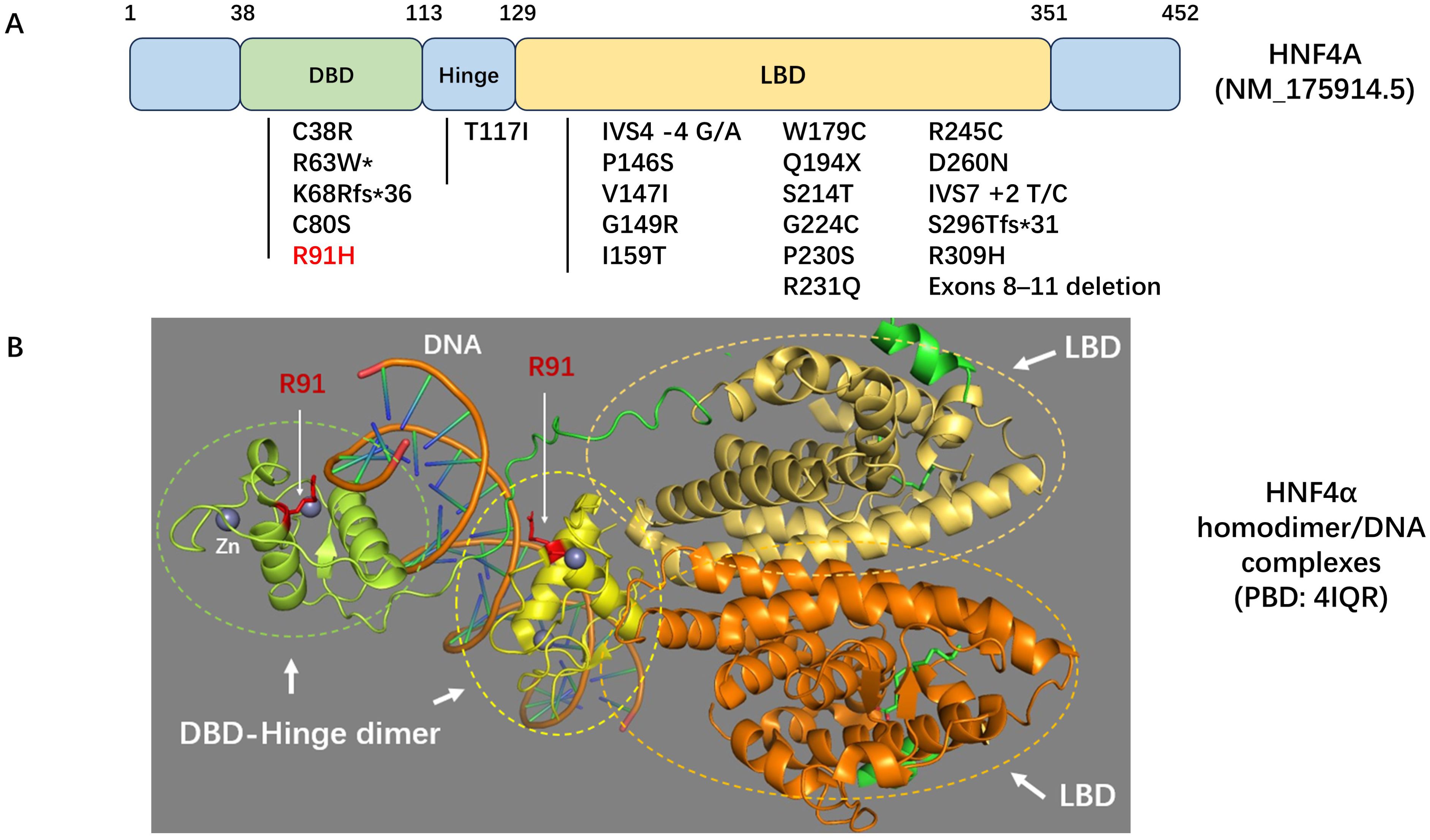
Figure 3. (A) Schematic diagram of the HNF4A gene. The locations of variants identified in Chinese patients with HNF4A-MODY or congenital hyperinsulinemia are indicated. *This condition has also been reported as HNF4A-related Fanconi syndrome. (B) Homodimer/DNA complex model of HNF4α. The ligand binding domains (LBD) are depicted in yellow-orange and orange, respectively. The DNA binding domain (DBD)-hinge dimers are illustrated in green and yellow. Residue R91, a component of the DBD-hinge dimer interface in HNF4α, is highlighted in red. Zinc (Zn) is represented in grey. The Protein Data Bank (PDB) ID code for this model is 4IQR.
This case emphasizes that spontaneous HNF4A-MODY is highly susceptible to misdiagnosis as either type 1 or type 2 diabetes mellitus. Currently, there are recommended tools for screening the clinical risk for MODY, such as the AACM strategy, which includes the age of onset, autoantibody to islet antigen, C-peptide and metabolic syndrome (9), and the MODY probability calculator (10). However, these tools have demonstrated inadequate efficacy in distinguishing MODY cases (11). Moreover, epidemiological research has indicated that de novo mutations in the MODY genes may occur more frequently than previously estimated (12). In individuals suspected of having MODY, characterized by clinical indicators such as macrosomia, early onset, absence of obesity, non-insulin dependence, and negative diabetic autoimmune antibodies—regardless of family history—a genetic assessment should be considered.
There is a paucity of research investigating the long-term follow-up and management strategies for patients diagnosed with HNF4A-MODY. Prolonged follow-up of this patient indicated a gradual decline in pancreatic function associated with HNF4A-MODY. Sulfonylureas are recognized as the standard first-line therapy for HNF4A-MODY (2). Due to the patient’s obesity, we kept metformin in the treatment regimen. Additionally, we gradually reduced the insulin dosage and avoided sulfonylurea medications that stimulate insulin secretion, in order to avoid the risk of atherosclerotic cardiovascular disease (ASCVD) associated with weight gain. It is reasonable to hypothesize that patients with HNF4A-MODY may respond positively to meglitinides and GLP-1RAs; however, there is currently no empirical evidence to support this hypothesis. While glycemic control and the progression of complications can be effectively managed in HNF4A-MODY patients receiving long-term insulin therapy, the potential risks of hypoglycemic episodes, obesity, and atherosclerosis remain significant concerns. Furthermore, the shift to GLP-1RA treatment in individuals with HNF4A-MODY continues to exhibit beneficial outcomes, even after an extended period of disease progression.
In summary, we have identified a novel de novo heterozygous missense mutation in the HNF4A gene in a Chinese patient diagnosed with MODY. This finding contributes to the existing mutation spectrum associated with HNF4A. This case highlights the susceptibility of spontaneous HNF4A-MODY to misdiagnosis, as well as the prolonged decline in pancreatic function characteristic of this condition. Notably, transitioning to GLP-1RA treatment in individuals with HNF4A-MODY continues to demonstrate positive outcomes, even after an extended disease duration.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found here: https://doi.org/10.6084/m9.figshare.29236490.
Ethics statement
The studies involving humans were approved by The hospital ethics committee of China Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Writing – original draft. AL: Writing – original draft. XW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the patient and his family members who agreed to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Naylor R, Knight Johnson A, del Gaudio D, Adam M, Feldman JM, Mirzaa G, et al. Maturity-Onset Diabetes of the Young Overview. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al, editors. GeneReviews® [Internet] Seattle (WA) (1993).
2. Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, Lumb PJ, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. (2005) 48:878–85. doi: 10.1007/s00125-005-1738-y
3. Broome DT, Tekin Z, Pantalone KM, and Mehta AE. Novel use of GLP-1 receptor agonist therapy in HNF4A-MODY. Diabetes Care. (2020) 43:e65. doi: 10.2337/dc20-0012
4. Ostoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care. (2014) 37:1797–805. doi: 10.2337/dc13-3007
5. Naylor RN, Patel KA, Kettunen JLT, Mannisto JME, Stoy J, Beltrand J, et al. Precision treatment of beta-cell monogenic diabetes: a systematic review. Commun Med (Lond). (2024) 4:145. doi: 10.1038/s43856-024-00556-1
6. Wang X, Wang T, Yu M, Zhang H, Ping F, Zhang Q, et al. Screening of HNF1A and HNF4A mutation and clinical phenotype analysis in a large cohort of Chinese patients with maturity-onset diabetes of the young. Acta Diabetol. (2019) 56:281–8. doi: 10.1007/s00592-018-1232-x
7. Liang H, Zhang Y, Li M, Yan J, Yang D, Luo S, et al. Recognition of maturity-onset diabetes of the young in China. J Diabetes Investig. (2021) 12:501–9. doi: 10.1111/jdi.13378
8. Chandra V, Huang P, Potluri N, Wu D, Kim Y, and Rastinejad F. Multidomain integration in the structure of the HNF-4alpha nuclear receptor complex. Nature. (2013) 495:394–8. doi: 10.1038/nature11966
9. Liu Y, Xie Z, Sun X, Wang Y, Xiao Y, Luo S, et al. A new screening strategy and whole-exome sequencing for the early diagnosis of maturity-onset diabetes of the young. Diabetes Metab Res Rev. (2021) 37:e3381. doi: 10.1002/dmrr.v37.5
10. Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, and Hattersley AT. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia. (2012) 55:1265–72. doi: 10.1007/s00125-011-2418-8
11. Chen Y, Zhao J, Li X, Xie Z, Huang G, Yan X, et al. Prevalence of maturity-onset diabetes of the young in phenotypic type 2 diabetes in young adults: a nationwide, multi-center, cross-sectional survey in China. Chin Med J (Engl). (2023) 136:56–64. doi: 10.1097/CM9.0000000000002321
Keywords: HNF4A, maturity-onset diabetes of the young, mutation, case report, GLP1RA
Citation: Luo J, Li A and Wang X (2025) Case Report: long-term misdiagnosis and follow-up of a patient with HNF4A-MODY carrying a new de novo mutation. Front. Endocrinol. 16:1509135. doi: 10.3389/fendo.2025.1509135
Received: 10 October 2024; Accepted: 20 May 2025;
Published: 16 June 2025.
Edited by:
Frances M. Sladek, University of California, Riverside, United StatesReviewed by:
Ali Chakera, Brighton and Sussex University Hospitals NHS Trust, United KingdomLacramioara Butnariu, Grigore T. Popa University of Medicine and Pharmacy, Romania
Copyright © 2025 Luo, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Wang, d2xpdHRsZXBlYXJAMTYzLmNvbQ==
 Jing Luo1,2
Jing Luo1,2 Xiaoli Wang
Xiaoli Wang