- 1Center for Reproductive Medicine, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Henan Key Laboratory of Reproduction and Genetics, First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Reproductive cells play a crucial role in transmitting genetic and epigenetic information from one generation to the next. Oocytes are fundamental to reproduction but human oocytes are difficult to obtain for clinical and research purposes because of ethical restrictions. However, in vitro induction systems have been established to differentiate pluripotent stem cells (PSCs) into human primordial germ cells (hPGCs). These induced hPGCs are referred to as hPGC-like cells. The discovery of ovarian stem cells (OSCs) also opened up a new avenue for studying the development of germline stem cells. In this review, we discuss the latest advances in the development of oocytes in vivo and in vitro, involving PSC-derived PGCs and ovary-isolated OSCs. Specifically, we focus on induction methods and differentiation mechanisms and discuss the associated technical challenges and future directions.
1 Introduction
Oocytes are the precursor cells of eggs in the female reproductive system. Mature oocytes are capable of being fertilized and developing into embryos and offspring (1, 2). The artificial generation of oocytes, which are usually derived from induced pluripotent stem cells (iPSCs) (3) or ovarian stem cells (OSCs) (4), provides significant opportunities for understanding the mechanism of oocyte development and for meeting clinical research needs. Human embryonic stem cells (hESCs) (5, 6), mouse embryonic stem cells (mESCs) (1, 2, 7), human iPSCs (hiPSCs) (6, 8, 9), and mouse iPSCs (miPSCs) (1, 2) can be induced to become primordial germ cell-like cells (PGCLCs), which can be combined with embryonic ovarian somatic cells to form reconstructed ovaries. Regardless of the cell type, the methods used to induce PGCLCs have been extensively developed. These methods commonly trigger the differentiation process towards inner cell mass and germ cells by incorporating factors, such as bone morphogenetic protein 4 (BMP4), leukemia inhibitory factor (LIF), epidermal growth factor (EGF), with or without stem cell factor (SCF) (5, 10, 11). However, the induction of PGCLCs to become oocytes typically needs to be carried out in vivo, for example, by injecting them into the ovaries, oviducts, or subcutaneous tissue of mice (5, 10, 11). Reconstructed ovaries can facilitate the maturation of mPGCLCs into oocytes, leading to the production of fertile offspring through in vitro maturation and fertilization, thereby providing the possibility of in vitro reproduction (1, 2). Therefore, pluripotent stem cells (PSCs) offer new possibilities for in vitro gametogenesis in humans and are also important models for investigating the mechanisms of PGC development.
The total number of follicles in mammals was believed to be established during the perinatal period, and that the production of ovarian oocytes ceased in adult women. However, mounting evidence in both mice (9, 12–14) and humans (4, 15, 16) indicate the presence of OSCs that have the ability to generate new oocytes. OSCs are typically isolated from mice or humans using antibodies against DEAD-box polypeptide 4 (DDX4) followed by magnetic-activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS). Mouse OSCs (mOSCs) can generate fully functional oocytes in vivo, leading to the production of healthy embryos and offspring following fertilization. The transformation of OSCs to oocytes usually takes place within the in vivo environment; for example, by microinjection of mOSCs into the ovaries of recipient mice and allowing development to proceed (4, 14, 17, 18). In contrast, human OSCs (hOSCs) have been injected into human ovarian cortex tissue and then xenotransplanted into mice for development (4, 16, 19). Additionally, OSCs can be cultured ex vivo to successfully produce oocytes (15, 19, 20). These recent discoveries provide a fresh perspective for investigating the development of germline stem cells, and hold great promise for enhancing follicular reserve, preserving fertility, and addressing issues such as infertility and premature ovarian failure.
In this review, we discuss the latest understanding of cells that have the potential to develop into oocytes in vivo and in vitro, including PSC-induced primordial germ cells (PGCs) and ovary-isolated OSCs. Specifically, we focus on induction methods and differentiation mechanisms and discuss the associated technical challenges and future directions.
2 Normal oocyte development
The process of oogenesis begins during fetal life and continues until the end of the female’s reproductive period. During oogenesis, female gametes are produced from PGCs. In human, around 24th day of gestation, PGCs form in the wall of the yolk sac and migrate to the gonadal ridges around the 5th week of gestation. After reaching the primary gonad, cells repeatedly divide mitotically, forming oogonia. Then, meiosis occurs in the oocyte and stops in the bilinear phase of the pre-meiosis phase, which is called primary oocytes. At 20 weeks of gestation, the number of oocytes reaches its peak, with approximately 3.5 million in each ovary. During this period, oocytes enter meiosis, with the majority remaining arrested in the diplotene stage. However, 99.9% of oocytes fail to mature and undergo apoptosis at various stages of development (21, 22). Of these, approximately two-thirds undergo apoptosis between the pachytene stage of meiosis and the formation of the primordial follicle pool. At birth, there are approximately 2 million oogonia in the human female gonad. The majority of these follicles undergo atresia at various stages of follicular development, with only about 400 to 500 follicles capable of maturing and undergoing ovulation. An oocyte arrested at prophase I contains a large nucleus (also called an embryonic or germinal vesicle) with a visible nucleolus.
Folliculogenesis is a distinct sub-process that accompanies and supports oogenesis. Current research indicates that once a primordial follicle is activated, it embarks on a continuous and irreversible developmental trajectory, culminating in one of two outcomes: successful ovulation as a dominant follicle, or arrest at any stage following initiation, followed by atresia. The recruitment, activation, and growth of dormant ovarian follicles are initiated through the c-Kit/kit ligand-PI3K-PTEN-AKT signaling pathway. This pathway suppresses the activity of FOXO3 and mTOR, which are key regulators of follicle dormancy (23, 24). As a result, ovarian follicles progress from the primordial stage through primary and secondary stages, ultimately developing into Graafian follicles.
Prior to ovulation, the oocyte completes the first meiotic and becomes secondary oocytes, arresting at metaphase II (MII) of the second meiosis, waiting for fertilization. Reaching MII is essential for proper chromosome recombination during gamete fusion and zygote formation. The second meiotic division is completed only upon sperm entry into the oocyte.
3 Mechanisms of PGC specification
3.1 Signaling pathways involved in regulating hPGC specification
Our understanding of PGC induction mainly stems from studies in model mammals, including non-human primates, mice, and pigs. Accumulated evidence highlights differences in the cellular and molecular mechanisms governing PGC specification between mice and humans, particularly in terms of gene expression and signaling pathways. For instance, SOX17 is crucial for hPGC specification but not for mPGC induction, while SOX2 is expressed in mPGCs but not in hPGCs. The induction system of hPGCLCs provides a valuable platform to investigate the mechanisms of specialization in hPGCs and hPGCLCs. Studies have shown that the expression of SOX17, TFAP2C, and BLIMP1 are essential for the specialization of hPGCLCs under the influence of BMP signaling.
BMP4: The BMPs belong to the transforming growth factor-beta superfamily and are crucial for embryo development. In the induction of PGCLCs, BMP4 signaling through ALK2/3 is essential. In mice, Bmp4 homozygous knockout embryos exhibit a lack of PGC development, highlighting the pivotal role of BMP4 in PGC induction in vivo. Furthermore, the response of epiblast cells to BMP is dose-dependent during the induction of PGCs (10).
SOX17: SOX17, a member of the SOX (SRY-related HMG box) family of transcription factors, was originally identified as a transcription factor for spermatogenesis. The discovery of SOX17 involvement in hPGC development began with the 4i culture of hPSCs and the in vitro differentiation of hPGCLCs. In vitro induction of hPGCLCs revealed SOX17 to be a key regulator of hPGC fate, while loss of SOX17 impairs hPGC specification. BLIMP1 works downstream of SOX17, which then represses endodermal and somatic genes (5). This pathway works only in hPGCs, and is not necessary for mPGC fate.
The transcription factor EOMES (T-box gene eomesodermin) functions upstream of SOX17 in hPGCLC specification. EOMES is upregulated in iMeLCs and activates SOX17 in response to WNT signaling. Initially expressed in the proximal epiblast before overt primitive streak formation, EOMES later becomes restricted to the primitive streak during gastrulation. While EOMES is expressed in iMeLCs, it is not expressed in PGCLCs or hPGCs. Knockout of EOMES impairs hPGCLC differentiation from hESCs and EOMES most likely acts downstream of WNT and TGFβ signaling pathways (11). This underscores EOMES being critical for human PGCLC specification in a cell-autonomous manner.
BLIMP1: BLIMP1, also known as PRDM1, encodes a zinc finger transcriptional repressor required for anterior endomesodermal cell fate and head induction. It can bind directly to and repress somatic cell proliferation genes. BLIMP1 expression is detected in human fetal gonocytes in the 12th week of gestation and is essential for hPGCLC specification. Mechanistically, BLIMP1 acts downstream of SOX17 to inhibit the developmental processes of neuron differentiation, gastrulation, and embryonic morphogenesis (10). Notably, BLIMP1 action is dose-dependent in hiPSCs. BLIMP1+/- hiPSCs exhibit a phenotype intermediate between that of wild-type and Blimp1-/- hiPSCs. This is consistent with the dose-dependent function of Blimp1 observed in mice. As a complex and highly regulated developmental process, hPGC development involves the coordinated activity of numerous genes within a network. For instance, two other transcription factors, TFAP2C and PRDM14, play indispensable roles in hPGC specification.
TFAP2C: TFAP2C (also known as AP2-GAMMA) is a sequence-specific DNA-binding transcription factor involved in the activation of several developmental genes. Multiple studies have shown that TFAP2C is necessary for the formation of hPGCLCs. TFAP2C-/- hESCs are unable to induce the formation of hPGCLCs. However, when TFAP2C-/- hESC lines are injected into immune-deficient mice, they are capable of teratoma formation. This indicates that TFAP2C is not necessary for exit from primed pluripotency and somatic cell differentiation per se, instead it has a specific effect on the specification of hPGCLCs (25).
One human-specific role for TFAP2C in hPGCLCs involves the opening of naive-specific enhancers and the acquisition of naive-like pluripotency. In mice, the Oct4 locus (also known as Pou5f1) is regulated by alternate enhancers. Specifically, the Oct4 distal enhancer (DE) regulates Oct4 expression in the inner cell mass and mPGCs, while the Oct4 proximal enhancer (PE) regulates Oct4 expression in the post-implantation epiblast of the mouse after implantation. During hPGCLC differentiation, the regulation of OCT4 may involve the activation of DE and NE through TFAP2C binding. However, Chen et al. found that the lack of OCT4 DE does not affect hESC differentiation into hPGCLCs, indicating that the DE is not a major regulator of hPGCLC specification (25). Recently, a naive enhancer (NE) that binds TFAP2C was discovered at the OCT4 locus; however, no TFAP2C-binding NE exists in rodents. Aggregate differentiation without the NE results in a decreased percentage of hPGCLCs and reduced expression of OCT4 RNA and diagnostic germ cell genes, such as NANOS3, DND1, TFAP2C, SOX17, and PRDM1. These data indicate that one of the mechanisms by which TFAP2C regulates human germ cell lineage formation is by opening naive-specific enhancers, with one enhancer corresponding to the NE at the OCT4 locus (25).
TFAP2C may also partially regulate the expression of KLF4 during aggregate differentiation (25). In the absence of TFAP2C, the ground-state naive pluripotent transcription factor KLF4 is not expressed. Through detection of ATAC-seq signals and ChIP-qPCR with anti-TFAP2C antibodies, a new peak of open chromatin located 50 kb upstream of the KLF4 locus was identified. TFAP2C binds to this region, which is referred to as the KLF4 element. Given that the NE at the human OCT4 locus contains three AP2 sites and a KLF site, it is possible that regulation of the NE involves the combinatorial binding of both TFAP2C and possibly a KLF family member (25), although this remains to be determined.
In humans, TFAP2C plays a crucial role in the specification of germline cells, acting upstream of SOX17 through its binding to the SOX17 promoter (26). However, the binding of TFAP2C at the SOX17 locus alone is not sufficient to induce the dynamic upregulation of SOX17 at the time of hPGCLC specification. However, there is coordinately enriched H3K27ac on both sides of the TFAP2C binding site in hPGCLCs, suggesting that this epigenetic regulation of TFAP2C may enable the expression of SOX17 at the point of hPGCLC specification. It is worth noting that BMP signaling can also activate TFAP2C in a SOX17-independent manner, and both SOX17 and TFAP2C function upstream of BLIMP1 in the specification of human germ cells. Collectively, TFAP2C plays a critical role in the specification of PGCs.
PRDM14: PRDM14 is a member of the PRDI-BF1 and RIZ homology domain containing (PRDM) family of transcriptional regulators and is expressed in pre-implantation embryos and PGCs in mice and humans. However, in contrast to what is observed in mPGCs, during the specialization process of hPGCLCs, PRDM14 expression is delayed and significantly reduced in hPGCs (5). This suggests that PRDM14 may not be required for human PGC fate (11), or, alternatively, that low levels of PRDM14 are sufficient for hPGC development. Indeed, the loss of PRDM14 affects differentiation efficiency, leading to downregulation of hPGC marker genes, including UTF1 and NANOG, while the re-expression of PRDM14 can rescue hPGCLC differentiation. In hESCs, knockdown of PRDM14 induces the expression of early differentiation marker genes and suppresses the expression of stem cell markers, whereas overexpression of PRDM14 significantly inhibits the expression of differentiation marker genes. These studies indicate a critical role of PRDM14 in hPGC fate by suppressing the expression of differentiation genes and maintaining hESC pluripotency.
A genome-wide RNA interference screen showed that PRDM14 binds to the proximal enhancer of pluripotency gene OCT4 to regulate its expression in hESCs (27). Notably, PRDM14 regulates hPGC development probably through coordination with both TFAP2C and BLIMP1 because it shares a subset of transcription targets with TFAP2C and BLIMP1 (27), although the exact position of PRDM14 in the regulatory network of hPGC specification remains unknown.
3.2 Epigenetic reprogramming of hPGCs/hPGCLCs
Epigenetic reprogramming is another layer of regulation in hPGC development. Shortly after specification, throughout migration, and towards gonad colonization, epigenetic reprogramming takes place in hPGCs. Global genomic DNA demethylation at week 7 is a major epigenetic event during hPGC development. The inactivated X chromosome is reactivated in female hPGCs at 5.5–9 weeks, which is similar to that in mPGCs. The lowest level of hypomethylation occurs at week 10 for females and week 11 for males. The low levels of methylation are maintained until week 19, but global re-methylation starts in female PGCs at week 11 and in male PGCs at week 19.
hPGCLC-derived oogonia also display hallmarks of epigenetic reprogramming (3), such as genome-wide DNA demethylation, imprint erasure, and removal of aberrant DNA methylation in hPSCs, and acquire an immediate precursory state for meiotic recombination. Furthermore, the inactive X chromosome shows partial progressive demethylation and reactivation.
In the early embryonic development of mammals, there are two waves of DNA methylation reprogramming, one occurring shortly after fertilization, and the other in PGCs. Epigenetic reprogramming is believed to remove the epialleles acquired during previous stages of development, such as those generated during pre-fertilization gametogenesis and those formed by initial differentiation of the epiblast before gastrulation. Ground-state naive pluripotency in both mouse and human cells in vitro is associated with global DNA demethylation, and hPGCs in vivo are confirmed to be fully demethylated. The two stages of global DNA methylation reprogramming for attaining ground-state naive pluripotency may constitute crucial checkpoints in germ cell development. Intriguingly, in the hPGCLC model, the establishment of global DNA methylation reprogramming is not observed up to the fourth day of aggregate differentiation, yet the naive pluripotency marker, KLF4, is expressed in hPGCLCs. This suggests that the switch from a primed pluripotency state toward one that resembles the naive ground state in human germline cells precedes DNA methylation reprogramming. Another possibility is that once germline cells acquire transcriptome and chromatin states resembling ground-state naive pluripotency, they are protected from differentiation cues so as to maintain germline cell identity. This possibility is supported by the observation that human ground-state naive pluripotent stem cells do not readily respond to differentiation cues, leading to the formation of teratomas in immunocompromised mice, and necessitating re-priming to effectively differentiate into embryoid bodies (25).
4 Mechanisms of oocyte maturation
Oocyte maturation, the terminal phase of oogenesis, is essential for achieving fertilization competence and ensuring subsequent embryonic development. This process encompasses coordinated nuclear maturation (meiotic resumption), cytoplasmic maturation, and membrane maturation. The core regulatory mechanism involves the resumption of meiosis I, transitioning the oocyte from prophase I arrest to metaphase II readiness. Oocyte maturation is tightly regulated by a network of signaling pathways, with the LH surge serving as the primary physiological trigger. The LH surge coordinates maturation through three principal mechanisms: The LH surge coordinates maturation through three principal mechanisms: (1) resumption of meiosis I; (2) secretion of growth factors and hormones; and (3) calcium-mediated signaling.
LH surge triggers meiotic resumption in oocytes. The key intracellular signaling molecule that maintains meiotic block in oocytes is cAMP, whose levels are regulated by adenylate cyclases (ACs) and phosphodiesterases (PDEs). ACs are activated through G protein-coupled receptors such as GPR3, promoting the synthesis of cAMP, while PDEs break down cAMP. The inhibitory effect of cAMP is because cAMP can maintain the active state of PKA, thereby inhibiting the activity of MPF or degrading the subunits of MPF. The study found that the LH surge can restart meiosis of oocytes by interfering with the cAMP signaling pathway. However, cAMP in follicles has a dual effect of inhibiting and promoting nuclear maturation. When cAMP continues to rise in oocytes, its meiotic recovery is inhibited, while when cAMP is briefly increased, it promotes meiotic recovery in oocytes. The gap junction between granule cells and oocytes plays an important role in maintaining meiotic block. After the LH surge, the connection between cumulus cells and oocytes decreases, and the oocytes restart meiosis.
LH surge promotes the secretion of growth factors The LH surge increases the secretion of growth factors by acting on the LH receptors on follicle membrane cells and granule cells, thereby promoting the expansion of cumulus and the maturation of oocytes. EGF plays an important role in this process. EGF may cause Cx43 phosphorylation by activating the MAPK pathway, destroying the gap junction between granule cells and oocytes, thereby blocking the entry of meiotic inhibitors into the egg and promoting oocyte maturation.
LH surge promotes hormone secretion LH surges can also promote the secretion of a variety of hormones in granule cells, which are crucial to the maturation of oocytes. The LH surge promotes the synthesis of progesterone, steroid hormone and androgens, etc., can promote the maturation of oocytes. Follicular fluid meiosis activating sterol (FF-MAS) is a key factor secreted by granule cells to promote oocyte maturation. Studies have found that LH surge promotes the increase of FF-MAS. Recent research confirms that FF-MAS not only drives meiotic progression from MI to MII, but also stabilizes oocytes at the MII stage to facilitate fertilization.
LH surge regulates calcium-mediated signaling Calcium ions (Ca²+) play a key role in the maturation of oocyte nucleus. Nuclear maturation is dependent on calmodulin and Ca2+, which enhances the regulatory effect of cAMP by regulating the phosphodiesterase activity of oocytes. The release of Ca²+ intracellularly is one of the earliest signs of meiotic initiation. Under the action of the LH surge, phosphatidylinositol on the surface of the granule cell membrane hydrolyzes to generate Ca²+-released ligands, including diacylglycerol and inositol triphosphate (IP3). After IP3 binds to the receptor, Ca²+ of granules can be released and injected into the oocyte. After IP3 binds to the receptor, Ca²+of granules can be released from the cell and injected into the oocyte. IP3 can also enter the egg cell through the coupling pathway of gap junctions, stimulating the release of Ca²+in the oocyte; Ca²+may also enter the oocyte directly through gap junctions, and the eggs react to Ca²+induced Ca²+release.
5 In vitro oocyte induction from PSCs
PGCs are the foundational cells of the germline that are established during the early stages of embryonic development. PGCs play a crucial role in ensuring the generation of new organisms and are the source of germline totipotency (27). Animal models can provide valuable insights into human PGCs; however, the cellular and molecular mechanisms of PGC specialization observed in model animals often do not fully reflect the biology of human PGCs. Ethical considerations limit the study of human PGC development in vivo at early stages. However, in vitro induction systems have been developed to differentiate hESCs (5, 6) and iPSCs (3, 6, 8, 10) into human PGCs. These induced PGCs are known as human PGCLCs, and represent true in vitro counterparts of PGCs. Single-cell transcriptomics and cell lineage tracing have been used to elucidate the lineage trajectory and mechanisms of PGC specialization in the hPGCLC induction system. These studies indicate great promise for the future generation of functional human gametes from hPGCLCs in vitro. The progress made with hPGCLCs not only deepens our understanding of human reproduction but also provides a novel approach for treating infertility (Table 1).
5.1 In vitro induction and expansion of PGCLCs
5.1.1 Methodologies for inducing mPGCLCs
Mouse oocytes can be induced from mouse PSCs (1, 2, 7). First, mESCs are stimulated with activin and fibroblast growth factor (FGF) to induce them to become epiblast-like cells (EpiLCs). Then, under the influence of BMP4, LIF, SCF, and EGF, mEpiLCs differentiate into mPGCLCs during the migration stage. The characteristics of these mPGCLCs are similar to those of E9.5 mPGCs. Essentially the same protocol can be used to induce oocytes from miPSCs (Figure 1).
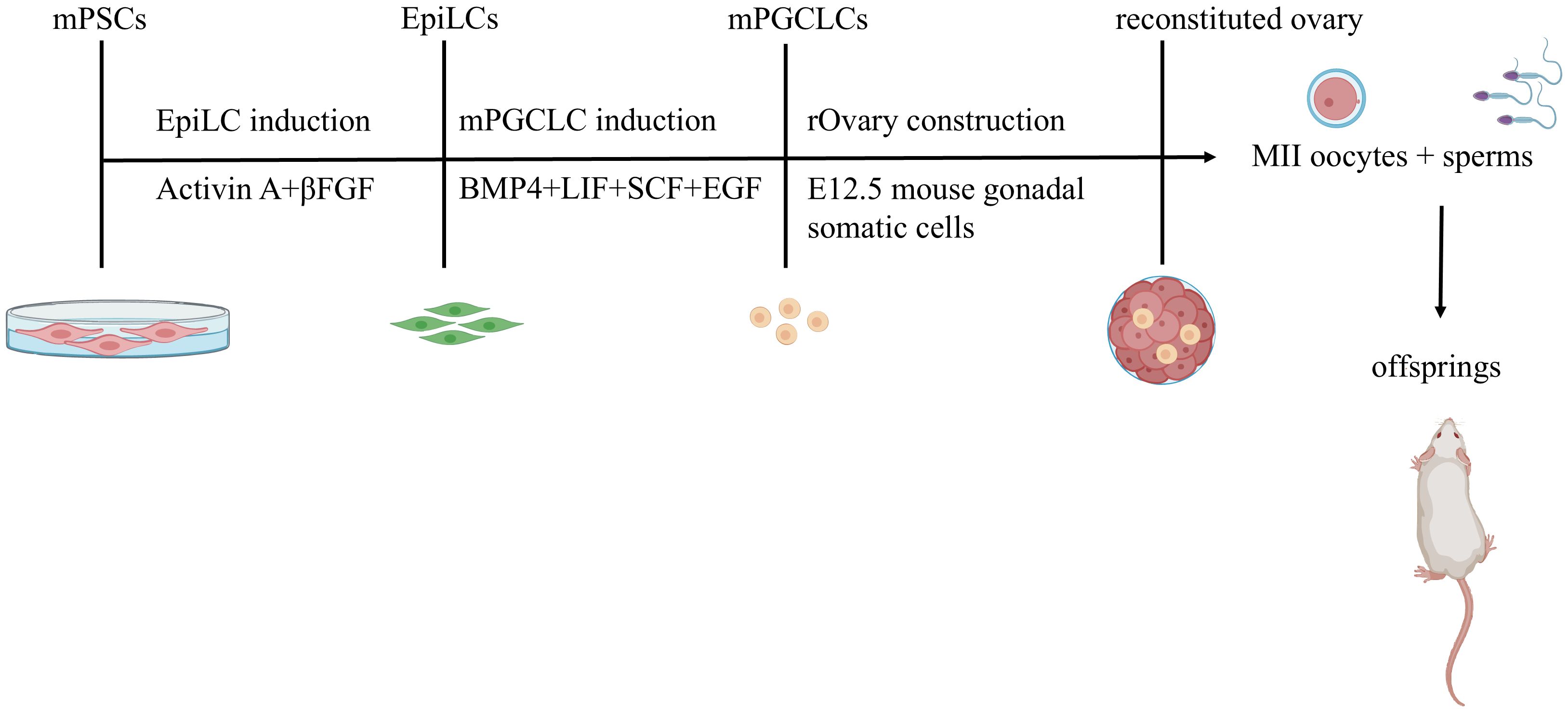
Figure 1. In Vitro Reconstitution of Functional Oocytes from Mouse Pluripotent Stem Cells with Live Offspring Generation. Mouse embryonic stem cells (mESCs) and induced pluripotent stem cells (iPSCs) are differentiated into epiblast-like cells (EpiLCs) using activin A and β FGF. EpiLCs subsequently undergo primordial germ cell-like cell (mPGCLC) specification via BMP4, LIF, SCF, and EGF stimulation during migration. mPGCLCs co-cultured with mouse embryonic ovarian somatic cells self-organize into reconstituted ovaries(rOvaries). These structures support in vitro maturation or in vivo transplantation (ovarian/subcutaneous sites) to generate fertile oocytes.
5.1.2 Methodologies for inducing hPGCLCs
In a similar way, hPGCLCs can be derived from hESCs and hiPSCs. There are two main strategies for inducing hPGCLCs: the incipient mesoderm-like cell (iMeLC) strategy (3, 10, 25, 26, 28, 29) and the 4i strategy (5, 11).
The iMeLC strategy (Figure 2). This strategy is a two-phase induction process from hPSCs to iMeLCs and then to hPGCLCs (27). First, hPSCs are cultured under conventional conditions. During the first phase, hPSCs are induced with activin A (ACTA) and CHIR (a WNT signaling agonist) to form incipient mesoderm-like cells (iMeLCs),which exhibit a similar state to EpiLCs induced from mouse ESCs/iPSCs. In the second stage from iMeLCs to hPGCLCs, iMeLCs are cultured in medium supplemented with BMP4, SCF, LIF, and EGF to induce their differentiation into hPGCLCs.
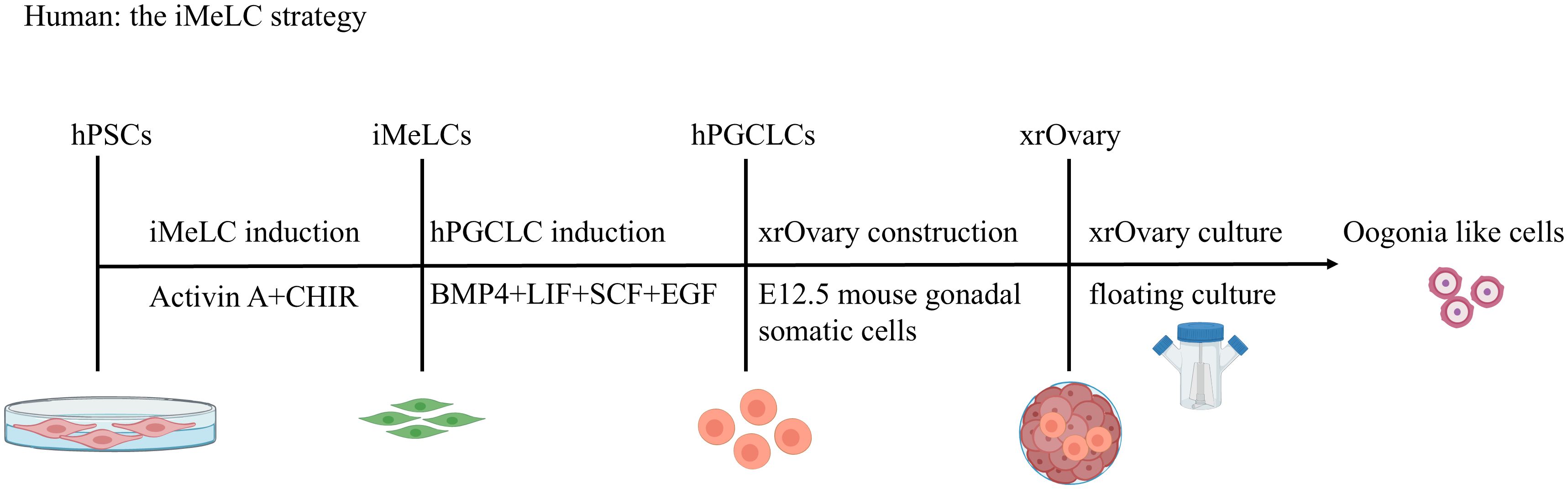
Figure 2. Stepwise Induction of hPGCLCs via iMeLC and Specification of Oogonia-like Cells. The iMeLC strategy is a two-phase induction protocol. Phase 1: activin A and CHIR-driven differentiation of human pluripotent stem cells (hPSCs) to incipient mesoderm-like cells (iMeLCs). Phase 2: BMP4/SCF/LIF/EGF-mediated induction of human primordial germ cell-like cells (hPGCLCs) from iMeLCs. hPGCLCs subsequently differentiate into oogonia-like cells in xenogeneic ovarian reconstitution cultures with mouse embryonic ovarian somatic cells.
The duration of ACTA and CHIR stimulation is critical for hPSCs to acquire a capacitated iMeLC state. Longer stimulation results in excessive upregulation of mesodermal/endodermal properties and depletes the capacity for BTAG(+) cell induction (10). Both ACTA and CHIR/WNT3A are essential; however, both BMP4 and bFGF are detrimental to iMeLC induction. Notably, inhibition of FGF receptor (FGFR) signaling by a specific inhibitor (FGFRi, PD173074) during iMeLC induction leads to more robust proliferation/survival of cells in the aggregates (10).
The 4i strategy (Figure 3). The 4i (four inhibitor) strategy refers to the use of four inhibitors. These are CHIR99021, a GSK-3 inhibitor; PD0325901, a MEK inhibitor; SB203580, a p38 MAPK inhibitor; and SP600125, a JNK inhibitor. hESCs are cultured in a medium containing these four inhibitors and pre-induced with TGFβ and bFGF. These pre-induced cells are then further guided towards hPGCLCs by culture in Glasgow’s minimum essential medium (GMEM) supplemented with BMP2/BMP4, LIF, SCF, and EGF. 4i culture is a key step that renders cells competent to acquire the hPGC fate (10). In the pre-induction step, activin A can be used as a substitute for TGFβ and bFGF (5).For hPGCLC induction, BMP4 signaling through activin receptor-like kinase 2/3 (ALK2/3) is essential. BMP2, but not BMP7 or BMP8A, can replace the role of BMP4 at an essentially identical concentration (10).
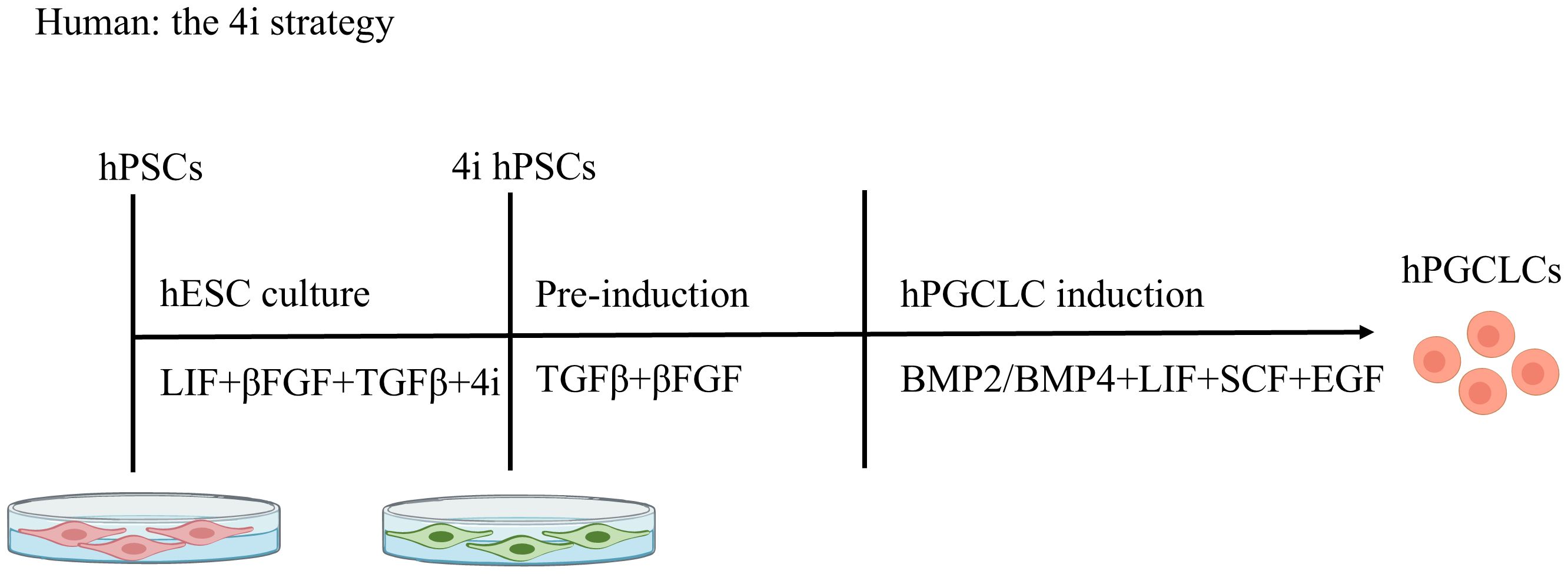
Figure 3. Stepwise Induction of hPGCLCs: The 4i strategy. Human pluripotent stem cells (hPSCs) are cultured in medium containing four inhibitors ("4i") and primed with TGF-β and bFGF. These pre-induced cells are then differentiated into primordial germ cell-like cells (hPGCLCs) using BMP2/BMP4, LIF, SCF, and EGF. The 4i comprises: CHIR99021 (GSK-3 inhibitor); PD0325901 (MEK inhibitor); SB203580 (p38 MAPK inhibitor); SP600125 (JNK inhibitor).
Notably, Sasaki et al. pointed out that hPSCs produced by the 4i strategy are not in the postulated naive state, but are in a type of peri-gastrulating epiblast-like state, similar to iMeLCs (10); mesodermal markers rather than genes for naive pluripotency are upregulated in these cells. Therefore, primed hESCs or 4i-cultured hESCs consistently generate hPGCLCs from iMeLCs or mesendoderm precursors (pre-MEs) (25). Meanwhile, the starting culture conditions (4i on mouse embryonic fibroblasts versus primed media on mouse embryonic fibroblasts) ultimately yields PGCLCs with similar transcriptional identities (11). RNA-seq analysis showed that PGCLCs generated from either primed media- or 4i-cultured hESCs clustered together, and were distinct from the undifferentiated hESCs. This means that hESC-derived PGCLCs generated through the iMeLC or 4i strategy exhibit similar molecular profiles.
Accordingly, recent studies have demonstrated the in vitro specification and reconstitution of the mouse germline by PSCs; mESCs/iPSCs with ground state pluripotency were induced into pre-gastrulation epiblast-like cells, which were in turn induced into PGCLCs with robust capacity for oogenesis and the generation of offspring. These findings indicate a conceptual framework and feasibility for the reconstitution of human germ cell development in vitro (10).
5.1.3 Varied PGCLC induction efficiency among PSC types
Different PSCs exhibit distinct potentials for PGCLC generation. Previous studies have systematically characterized the PGCLC-inducing capacities across various PSC types, including mESCs, mouse epiblast stem cells (EpiSCs), naïve hPSCs, and primed hPSCs. mEpiSCs retain characteristics of the original epiblast cells and serve as a potential source for generating germ cell-like cells in vitro. Under self-renewing conditions, cells positive for STELLA (also known as PGC7/DPPA3), a marker of established PGCs, can be derived from mEpiSCs. However, the emergence of these positive cells occurs at a low frequency (1.5%) even in the presence of BMP4, and the functional relevance of these cells in vivo remains uncharacterized (30). In contrast, EpiLCs derived from naive mESCs demonstrate significant germline potential. When cultured with BMP4, the induction efficiency of PGCLCs can reach up to 40% (30).
The efficiency of generating hPGCLCs in vitro varies significantly depending on the stem cell type and induction strategy. Conventional hESCs spontaneously differentiate into hPGCLCs at a low efficiency of approximately 5% without directed induction (5). In contrast, direct induction of naive hiPSCs yields 15% BTAG+cells by day 4 (D4) (10). When hESCs are treated with a 4i culture system, the proportion of cells co-expressing TNAP and NANOS3 increases to 45.5% (5). The most efficient approach, the iMeLC strategy, achieves up to 60% BTAG+ cells by D4 (10). Furthermore, Zhu (31) utilized a novel type of formative PSCs (fPSCs) derived from human extended pluripotent stem cells (hEPSCs) to induce PGCLCs. These fPSCs, termed AF9-hPSCs, exhibit intermediate pluripotency features and demonstrate transcriptomic similarity to human E8-E9 epiblast cells. Under BMP4 treatment, the PGCLC induction efficiency reached 26.6% by day 4 (31).
The previously mentioned hPGCLCs derived from early progenitor cells were induced as three-dimensional aggregates in the presence of growth factors, including BMP4,usually using high BMP4 concentrations (200–500 ng/mL). In contrast, Vijayakumar (6) reports a simplified two-dimensional monolayer culture system that generates consistent and reproducible NANOS3-mCherry+ hPGCLCs with 20–30% purity within 3.5 days of in vitro differentiation, even at a 25-fold lower BMP4 concentration.
As mentioned earlier, 4i-cultured hPSCs reside in a peri-gastrulation epiblast-like state, similar to iMeLCs (10). hESCs/iPSCs have differentiation potential and other properties distinct from mESCs/iPSCs and bear a primed pluripotency with similarity to mouse EpiSCs, which resemble post-gastrulation epiblasts (10). The robust induction of hPGCLCs from hiPSCs in a primed pluripotent state, particularly through iMeLCs, is therefore surprising because EpiSCs with primed pluripotency exhibit little, if any, competence to achieve a germ cell fate (10). Further investigation indicated that hiPSCs bear properties intermediate between those of EpiLCs and EpiSCs (10). Chen et al. recently showed that iMeLCs in the lineage trajectory represent a transitional pluripotent state, exhibiting characteristics of both naive and primed hESCs. This state is known as the “germinal pluripotent state” (26).
5.1.4 In vitro expansion of PGCLCs
The difficulty in in vitro expansion of PGCs has been a major obstacle in advancing PGC biology research. Studies have shown that chemical agents (7, 28) including selective inhibitors for PDE4 (e.g., Rolipram), agonists for retinoic acid (RA) signaling, and Forskolin, combined with cytokines (10, 28) such as SCF, LIF, and EGF, exert additive effects on the proliferation of PGCLCs. Ohta et al. successfully established a method for the in vitro expansion of mPGCLCs by stimulating the intracellular production of cyclic AMPs (cAMPs) with forskolin and rolipram (7). Their study demonstrated that mPGCLCs could proliferate for over a week in vitro, expanding up to ~50-fold. Subsequently, Yusuke Murase explored the in vitro expansion conditions for hPGCLCs (28). Their results showed that hPGCLCs can be propagated by a magnitude of at least ~1×106 fold (~20 doublings) during a period of ~4 months with the maintenance of early hPGC properties in the presence of SCF, bFGF, and forskolin. The significant differences in expansion levels and culture durations between mPGCLCs and hPGCLCs may reflect fundamental differences in the intrinsic properties of human and mouse embryonic germ cells: human embryonic germ cells reach 7,000,000 in females (14–19 weeks) and 2,000,000 in males (19 weeks), whereas mouse embryos develop only 25,000 germ cells in both sexes by E13.5 (28). This discrepancy may be due to the unique long-term proliferation mechanisms of human cells or the intrinsic regulatory differences that limit the cell mitotic cycle in mouse cells.
5.1.5 Markers of hPGCs/hPGCLCs
Multiple proteins located on the cell membrane of human PGCs have been used to enrich PGCs from single-cell suspensions of human embryonic and fetal tissues, as well as from in vitro PGCLCs. These include cKIT, TNAP, PDPN, CD38, ITGA6 (also known as Integrinα6), and EPCAM. Combinations of multiple markers can improve isolation accuracy. Combinations, such as NANOS3/NANOG/SOX17, NANOS3/TNAP, and OCT4/TFAP2C have been used as markers for hPGCLCs, and NANOG/SOX17/TFAP2C (N/S/T) as markers for hPGCs. Additionally, EpCAM and Integrinα6 are markers for hPGCLC purification. hPGCLCs can be isolated in vitro (10) and from embryonic ovaries (11) using EPCAM and Integrinα6.
hPGCs express fibroblast growth factor receptor 3 (FGFR3) (29), but this expression is downregulated as PGCs progress into prophase I of meiosis I. FACS using FGFR3 antibodies can be used to enrich for PGCs from prenatal ovaries. FGFR3 can therefore serve as a diagnostic surface marker to improve PGCLC differentiation protocols.
5.2 mPGCLC-derived oocytes and generation of offspring
As mentioned earlier, the method to induce mouse PSCs into mPGCLCs has been extensively developed. mPGCLCs can generate fully functional oocytes in vitro. When co-cultured with mouse embryonic ovarian somatic cells, aggregates of mPGCLCs and ovarian somatic cells are often referred to as reconstructed ovaries. Reconstructed ovaries can induce oocyte production either through complete in vitro culture or re-implantation into mouse ovaries or subcutaneous tissue (1, 2). The entire process is divided into three stages: in vitro differentiation, in vitro growth, and in vitro maturation. During in vitro differentiation, mPGCLCs differentiate into primary oocytes at the secondary follicle stage, and during the in vitro growth stage, they further differentiate into mature oocytes. MII oocytes derived from both mESCs and miPSCs can be fertilized to produce offspring, demonstrating successful in vitro oogenesis.
However, during development, approximately half of PGCLC-derived oocytes/zygotes fail to extrude the second polar body. After in vitro fertilization, this results in the digynic triploid (MMP) phenotype or the digynic diploid (MM) phenotype with failed fertilization. This defect contributes to a low birth rate from the 2-cell embryos derived from PGCLCs. Further investigations of the underlying mechanisms of this failure are needed (1). These advances in mice serve as a basis for in vitro reconstitution of human germ cell development.
5.3 hPGCLC-derived early oocytes
hPGCLCs progressively differentiate into oogonia-like cells during long-term in vitro culture in xenogeneic reconstituted ovaries (xrOvary) with mouse embryonic ovarian somatic cells (3, 28). In this system, hPGCLCs are first aggregated with mouse embryonic ovarian somatic cells at E12.5 and then cultured under floating conditions in GK15 (GMEM+15% KSR) containing Y-27632 in a U-bottom 96-well plate for 2 days to form xrOvaries. Finally, xrOvaries are transferred to Transwell-COL membrane inserts and cultured in minimum essential medium-alpha (MEM-α) containing fetal bovine serum (FBS), 2-mercaptoethanol, L-ascorbic acid, and penicillin/streptomycin, under liquid–gas interface conditions.
qPCR or RNA-seq analysis of c30ag7,c30ag35, and c30ag77 cells (The term 'c30ag7' means hPGCLCs cultured in vitro for 30 days [c30], followed by 7 days of aggregation culture with mouse embryonic ovarian somatic cells [ag7].The same naming rule applies to subsequent terms), indicates that marker genes for oogonia/gonocytes, including DPPA3, PRAME, PIWIL2, DAZL, and DDX4, were more rapidly up-regulated compared with in d6 hPGCLC-derived cells (28). Whole-genome bisulfite sequence analysis revealed the genome-wide DNA methylation 5-methylcytosine (5mC) profiles of hPGCLC-derived cells in xrOvaries. The genome-wide 5mC levels in hPGCLC-derived c30ag77 cells reach approximately 15% (28), a value equivalent to that of human oogonia/gonocytes after 7–10 weeks of development and of ag120 cells (approximately 13%) (3, 28). Unsupervised hierarchical clustering, principle component analysis, and the expression profiles of a gene set that characterizes developmental progression from hPGCLCs to oogonia/gonocytes provided a consistent outcome: c30ag35 and c30ag77 cells represent early- and late-stage oogonia/gonocyte-like cells, respectively (28). The above results from both studies collectively indicate that ag120 and c30ag77 cells from long-term cultures are likely oocytes. These two studies offer fresh insights into human in vitro oogenesis.
6 In vitro oocyte induction from OSCs
Traditional views hold that most female mammals lose the ability to produce oocytes at birth and only have a limited reserve of oocytes. However, in 2004, Johnson et al. (28) reported the presence of reproductive cells with mitotic activity in the ovaries of both juvenile and adult mice, indicating their ability to continuously update the follicle pool. These mitotically active germ cells are referred to as OSCs [also known as female germline stem cells (FGSCs) or oogonial stem cells]. Subsequent studies provided further evidence of OSCs in mice and humans (4, 9, 12–16), and these cells were fertilizable and capable of generating embryos in murine models. Furthermore, hOSCs injected into human ovarian cortex tissue and then transplanted into mice, resulted in the cultivation of primordial follicles. Therefore, FGSCs have significant value in basic research and may provide new strategies for treating ovarian dysfunction and infertility, as well as delaying female aging (Figure 4; Table 2).
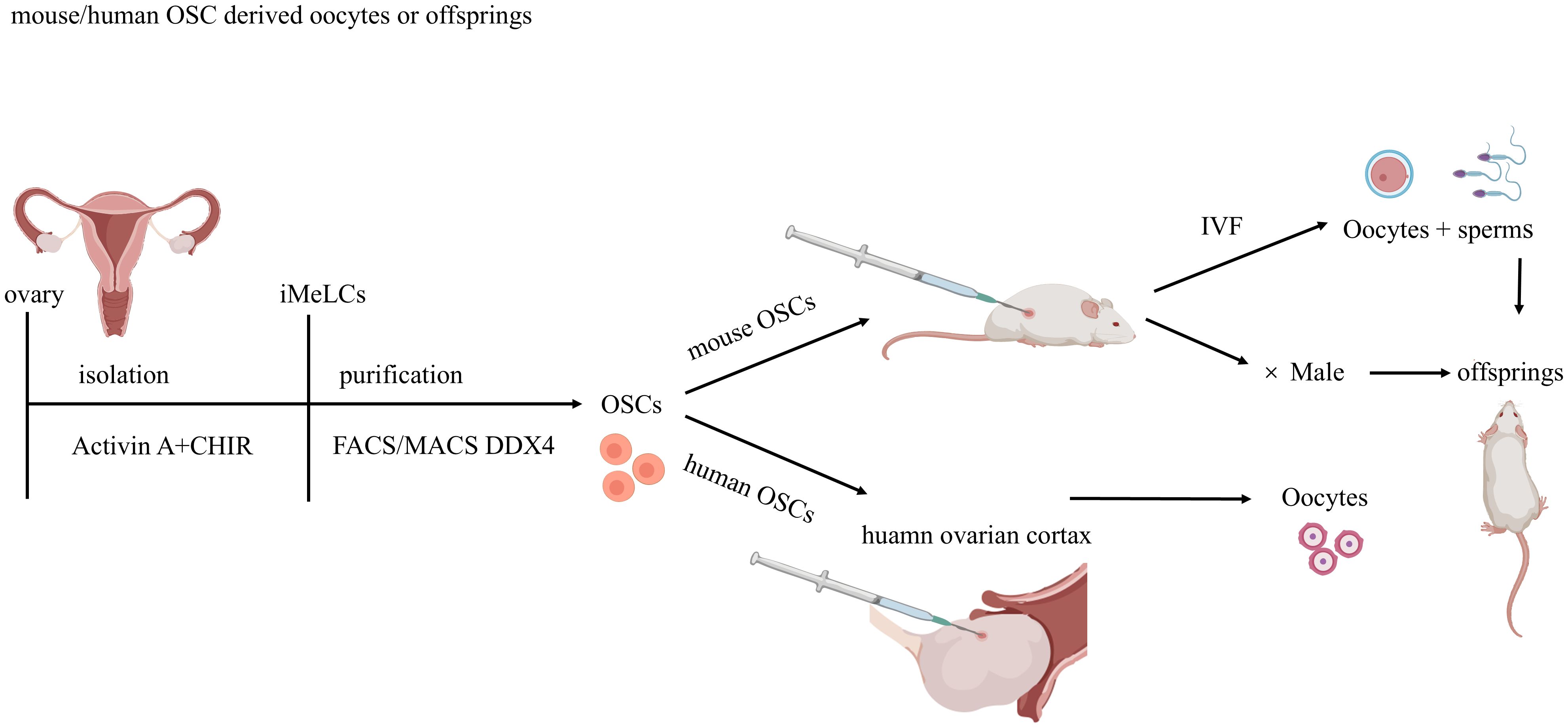
Figure 4. Methodologies for Ovarian Stem Cell Isolation, Purification, and Transplantation. Ovarian stem cells (OSCs) are isolated from murine /human ovaries using DDX4 antibody-based sorting via magnetic-activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS).Mouse OSCs injected into recipient mouse ovaries undergo complete oogenesis, yielding functional oocytes that produce viable embryos and offspring post-fertilization. Human OSCs are injected into human ovarian cortex fragments and xenotransplanted into immunodeficient mice for further development.
Whether or not OSCs exist in the adult mammalian ovary has been the subject of much debate. About 10 primary research papers questioned the existence of OSCs and/or postnatal oogenesis in mammals (32–37). A study rooted in single-cell RNA sequence analysis (scRNA-seq) of adult human ovarian cortical tissue claimed that OSCs do not exist and that other groups mistakenly identified perivascular cells (PVCs) as germ cells after isolating them via magnetic-assisted or fluorescence-activated cell sorting (38). However, follow-up studies have shown that human PVCs and germ cells separate into distinct clusters in scRNA-seq due to their non-overlapping gene expression profiles, preventing the misidentification of PVCs as OSCs in functional characterization studies (39). Other research groups mistakenly used PVCs, rather than germ cells, to isolate the original germ lineage cells using DDX4 antibody-based sorting. There may be several reasons for this: Firstly, OSCs are extremely rare in adult ovaries and can be difficult to identify. Secondly, due to improper workflow, the high degree of cell damage and death leads to non-specific antibody binding in FACS. Finally, the intrinsic autofluorescence of PVCs generates false-positive signals, resulting in their enrichment (39).
6.1 Isolation and culture of OSCs
6.1.1 OSC isolation and purification
To isolate OSCs, a two-step enzymatic digestion method employing collagenase and trypsin is often used for the efficient digestion of ovary tissue (13). Subsequently, MACS or FACS techniques are used to purify and separate cells.
Both FACS and MACS rely on antibody-based detection of externally exposed protein epitopes on OSCs. In contrast to MACS, FACS yields significantly higher cell viability and purity. Notably, MACS remains widely adopted for isolating ovarian germ stem cells from heterogeneous populations. This technique combines magnetic beads with antibodies targeting specific membrane antigens. Multiple studies have successfully isolated OSCs using this approach (13, 15, 17, 19, 40), though potential contamination with non-target cells should be considered. Marker analysis of the DDX4-positive viable cell fraction obtained by MACS revealed several oocyte-specific mRNAs (4). This most likely reflects either a non-specific physical carry-over of small oocytes with OSCs during column washing and flushing or reactivity of cytoplasmic DDX4 in plasma membrane-compromised (damaged) oocytes with the antibody recognizing C-terminal DDX4. In addition, MACS also does not distinguish between viable and damaged or dead cells, and does not allow simultaneous assessment of other cellular features, such as yield, size or co-expression of additional markers. To overcome these limitations, White et al. explored using FACS for OSC isolation, building upon the MACS methodology (4). Dead-cell exclusion and cell size-based inclusion can be performed simultaneously using FACS, further enhancing the viability and purity of the cell population obtained. White and colleagues used an antibody directed against the COOH-terminus of Ddx4/DDX4 to purify rare mitotically active cells with gene expression profiles consistent with PGCs from adult mouse ovaries and human ovarian cortical tissues via FACS. In addition, unlike the oocyte contamination observed when OSCs were isolated by MACS using the antibody recognizing C-terminal DDX4, use of this antibody with FACS provides a superior strategy to obtain OSCs free of oocytes.
In addition to the validated and independently verified OSC purification method based on DDX4 antibodies, an antibody targeting the extracellular domain of interferon-induced transmembrane protein 3 (IFITM3/Fragilis), a marker for PGCs, can also be used with greater efficiency to isolate mOSCs (40). In recent years, OSCs have been isolated from mouse or human ovaries by MACS or FACS based on anti-DDX4 antibodies. However, Zhang and Wu explored a novel isolation method using endogenous fluorescent proteins rather than exogenous antibodies to isolate DDX4-positive cells from postnatal ovaries of lineage-tracing mice (Ddx4-Cre;mT/mG mice) (14). Subsequent experiments demonstrated that these isolated cells could establish cell lines capable of generating offspring.
6.1.2 OSC culture
Mouse and human OSCs are often established on mitotically inactivated STO feeder cells [derived from Sandos inbred mice (SIM) 6-thioguanine-resistant, ouabain-resistant embryonic fibroblasts]. Although an underlying monolayer of somatic feeder cells is not absolutely required for the successful establishment of mouse or human OSCs in vitro, the initial rate of proliferation is greatly enhanced using a co-culture system (16). The culture medium for OSCs usually consists of MEM-α, FBS, sodium pyruvate, non-essential amino acids (NEAA), L-glutamine, β-mercaptoethanol (β-ME), LIF, EGF, glial cell line-derived neurotrophic factor (GDNF), basic FGF and penicillin (4, 13, 18, 20). Some media also incorporate transferrin and insulin (4, 13).
6.2 Characterization of OSCs
As germline stem cells, OSCs not only maintain oogenesis, but also have the characteristics of adult stem cells. The proportion of OSCs is very small, accounting for about 0.014% ± 0.002% of cells in mouse ovaries (4). The main characteristics of mouse and human OSCs are summarized as follows. 1) OSCs are similar to spermatogonial stem cells (SSCs) in morphology, with a larger nucleus and less cytoplasm. 2) Most OSCs are positive for PRDM1/BLIMP1, DPPA3/STELLA, IFITM3/Fragilis, DAZL, OCT4, REX1/ZFP42 and TERT. However, OSCs do not express oocyte specific markers, such as newborn ovary homeobox (NOBOX), zona pellucida glycoprotein 1–3 (ZP1–3), growth differentiation factor 9 (GDF9), NANOG, SSEA1 or SOX2, c-KIT, FIGLA, and synaptonemal complex protein 1–3 (SCP1–3). Oocyte contamination can be excluded by examining oocyte-specific markers. 3) FGSCs show high telomerase activity and most of them have a normal karyotype. 4) FGSCs are also positive for alkaline phosphatase staining. 5) OSCs show a female imprinting pattern. Differentially methylated regions in two maternally imprinted regions (Igf2r and Peg10 regions) and two paternally imprinted regions (H19 and Rasgrf1 regions) were observed, indicating that the maternally imprinted regions were partially methylated and the paternally imprinted regions were demethylated in OSCs.
6.3 Oocytes derived from OSCs
As mentioned earlier, the generation of oocytes from OSCs typically necessitates an in vivo environment. mOSCs can be microinjected into the ovaries of recipient mice. In contrast, hOSCs need to be injected into human ovarian cortex tissue first, and then xenotransplanted into immunodeficient mice to achieve development.
6.3.1 Intraovarian injection of mouse OSCs and generation of offspring
Multiple independent studies have confirmed that mouse OSCs can differentiate into functional oocytes in vivo, yielding viable embryos and offspring. To identify oocytes derived from OSCs, OSCs are often transformed to express fluorescent reporter genes, such as GFP. After stable integration of GFP, GFP-expressing mOSCs can be directly microinjected into the ovaries of infertile female mice pre-treated with cyclophosphamide and busulfan for further development. Determination of oocyte generation includes the following three approaches. First, histological evaluation of ovaries injected with OSCs to observe GFP-positive oocytes. Second, induction of ovulation using gonadotropins, followed by in vitro fertilization of these oocytes with wild-type sperm and monitoring embryo development. Third, mating trials were conducted by pairing recipient female mice receiving intraovarian GFP-OSC injections with wild-type male mice. Conventional PCR-based genotyping is then performed to determine whether the oocyte fertilized in vivo to produce a given offspring was derived from the recipient (wild-type) or from the transplanted OSCs (GFP-positive).
In addition to transplanting OSCs back into ovaries, in vitro methods for generating oocytes from mOSCs have also been explored (4). These include the in vitro culture of OSCs with ovarian somatic cells to form organoids (20). Within these organoids, OSCs gradually develop into follicles and after 3D culture, these follicles mature into complexes containing immature oocytes, known as ovarian follicle-oocyte complexes. Mature oocytes can then be successfully obtained using in vitro maturation techniques, in vitro fertilized, and the embryos transplanted. The success rate of the in vitro fertilization was not significantly different from that of the control group (49.9% and 46.2%, respectively). Offspring born from the transplants exhibited normal weight, methylation patterns, and fertility.
6.3.2 Injection of human OSCs into human ovarian cortical tissue and xenografting
mOSCs have been extensively researched, and in vitro and in vivo protocols have been developed for the induction of mOSCs to form oocytes or embryos that can produce offspring. However, few studies have examined oocyte formation from hOSCs. The conventional method is to inject hOSCs into human ovarian cortical tissue and then transplant them into mouse ovaries (4) or subcutaneous sites (16, 19) for further development. This produces GFP-positive follicles in xenografted OSC tissues and shows that hOSCs, like mOSCs, have the potential to develop into oocytes and to supplement the follicular pool. hOSCs have recently been used to further explore the in vitro development of human oocytes.
It is worth mentioning that Ding (19) established FGSC lines from scarce ovarian cortical tissues that exist in follicular aspirates (faFGSCs), which are produced and discarded by in vitro fertilization centers around the world. A three-step differentiation protocol for differentiating oocytes from faFGSCs was developed. First, faFGSCs were cultured on a granulosa cell monolayer for 3 days in medium containing bFGF and retinoic acid (RA) and thereafter, for 6 days in the presence of bFGF, EGF, insulin, transferrin, PMSG (pregnant mare’s serum gonadotropin), and hCG (human chorionic gonadotropin). Subsequently, E2 (17-β-estradiol), progestogen, and human follicular fluid were added to the medium and culture continued for 3–6 days. Oocyte-like cells appeared continuously during the differentiation process, and some oocyte-like cells (4.9%) were observed to have germinal vesicle-like structures. These findings indicate that the rare ovarian tissue in follicular aspirates can be used as an alternative source of FGSCs, which is a promising solution for the shortage of adult human ovarian cortex tissue. Furthermore, human germinal vesicle oocytes could be developed in vitro from faFGSCs under defined culture conditions.
6.3.3 Analysis of in vitro-derived oocytes
The expression of oocyte-specific markers in oocytes derived from human and mOSCs is routinely analyzed using PCR or immunofluorescence cytochemistry to confirm their cellular identity. Oocytes derived from mouse and human OSCs typically express key markers such as DDX4, KIT, YBX2 (also known as MSY2 and as CONTRIN in humans), NOBOX, LHX8, GDF9, ZP1, ZP2, and ZP3.
7 Conclusion and perspectives
In the last decade, rising infertility rates in developed countries of 12%–24% have increased efforts to establish in vitro conditions for self-renewal and differentiation of PGCs. Significant progress has been made in producing oocytes in vitro using PSCs. The successful generation of oogonia-like cells from hPSCs offers a new possibility for human in vitro gametogenesis. The discovery of OSCs has opened up new areas of research in human reproductive biology, which will address infertility issues and women’s reproductive health. This will provide women with more choices. For example, OSCs can be cryopreserved for future treatment of infertility. Moreover, purified human OCS can act on the ovaries themselves, increasing the reserve capacity of ovarian function.
Although mOSC-derived oocyte development has been extensively studied, further exploration is needed to apply findings in mice to humans. First, unlike the “naïve” state characteristic of mouse ESCs and iPSCs, hESCs and hiPSCs exhibit a “primed” state and exist as heterogeneous populations with varied differentiation potential. Second, the in vivo gonadal environment is essential for meiosis and, to generate functional oocytes from PSCs, the PSCs need to be transplanted into an ovary, which is restricted by ethical and safety considerations.
In addition, the internal gonadal environment is necessary for meiosis. PGCLCs obviously need to interact with fetal gonadal somatic cells to differentiate into mature oocytes (1, 2). Studies in mice have shown that PGCLCs introduced in adult ovarian tissue generate only immature oocytes that arrest and degenerate at very early stages of follicle development. Therefore, obtaining human fetal gonadal tissue and making it available for any type of clinical platform involving germ cell development from PSCs remains to be addressed. In contrast, OSCs are capable of generating mature oocytes when introduced into adult ovarian tissue, removing the need for fetal ovarian somatic cells. However, OSCs alone are not capable of differentiation into fully functional eggs. OSCs, like all other stem cells, require parallel incorporation of appropriate somatic cell partners or transplantation in vivo to be successful. Without question, the most important of these partners are primitive or undifferentiated granulosa cells (sometimes referred to as pregranulosa cells), which are capable of interacting with newly generated oocytes to form primordial follicle-like structures. A new method to differentiate hiPSCs into granulosa-like cells may offer more possibilities for oocyte development (41).
Finally, the specification of PGCLCs from ESCs or iPSCs may fail to account for the importance of the germline mitochondrial DNA (mtDNA) bottleneck in ensuring maternal passage of ‘clean’ mtDNA generation after generation (42). Even in mice, it remains to be determined, if nuclear reprogramming of differentiated somatic cells into iPSCs for subsequent generation of PGCLCs generates germline cells that effectively carry out the process of maternal mitochondrial inheritance. In other words, studies must be performed to demonstrate that offspring produced from iPSC-derived eggs are not burdened from the outset with compromised mitochondrial genomes (43). In comparison, OSCs show some advantages in this respect. Unlike ESCs and iPSCs, OSCs are unipotent and programed from the start as a germ lineage; therefore, these cells require no directed differentiation or genetic manipulation to achieve a germline identity that is capable of oogenesis (43).
In addition, the source of OSCs needs to be resolved. Research on hOSCs is limited by the difficulty of obtaining ovarian tissue and related ethical issues. The previously mentioned faFGSCs (19) can serve as an alternative source of OSCs. Furthermore, studies in mice suggest that bone marrow and peripheral blood may be a potential source of germ cells (44, 45). Bone marrow or peripheral blood transplantation were performed on female mice sterilized by chemotherapy. After transplantation, donor-derived oocytes were produced in the recipient’s ovaries. Their morphology, enclosure within follicles, and expression of germ cell- and oocyte-specific markers collectively support these cells as bona fide oocytes. However, mating experiments revealed that all offspring were derived from the recipient germline. Donor-derived oocytes were only observed in immature follicles up to the preantral stage of development but never observed in mature antral or Graafian follicles from which ovulated eggs are derived (45). These findings indicate that bone marrow transplantation and peripheral blood transplantation can improve follicular development and fertility, but that bone marrow stem cell-derived oocytes do not contribute to the pool of ovulated eggs used for reproduction (43).
Author contributions
MZ: Writing – review & editing. MT: Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82101742) for Meixiang Zhang and the Henan Province Medical Science and Technology Co-construction Project (No. LHGJ20190117) for Meixiang Zhang.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, and Saitou M. Offspring from oocytes derived from in vitro primordial germ cell–like cells in mice. Science. (2012) 338:971–5. doi: 10.1126/science.1226889
2. Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. (2016) 539:299–303. doi: 10.1038/nature20104
3. Yamashiro C, Sasaki K, Yabuta Y, Kojima Y, Nakamura T, Okamoto I, et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science. (2018) 362:356–60. doi: 10.1126/science.aat1674
4. White YAR, Woods DC, Takai Y, Ishihara O, Seki H, and Tilly JL. Oocyte formation by mitotically-active germ cells purified from ovaries of reproductive age women. Nat Med. (2012) 18:413–21. doi: 10.1038/nm.2669
5. Irie N, Weinberger L, Tang WWC, Kobayashi T, Viukov S, Manor YS, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. (2015) 160:253–68. doi: 10.1016/j.cell.2014.12.013
6. Vijayakumar S, Sala R, Kang G, Chen A, Pablo MA, Adebayo AI, et al. Monolayer platform to generate and purify primordial germ-like cells in vitro provides insights into human germline specification. Nat Commun. (2023) 14:5690. doi: 10.1038/s41467-023-41302-w
7. Ohta H, Kurimoto K, Okamoto I, Nakamura T, Yabuta Y, Miyauchi H, et al. In vitro expansion of mouse primordial germ cell-like cells recapitulates an epigenetic blank slate. EMBO J. (2017) 36:1888–907. doi: 10.15252/embj.201695862
8. Kobayashi M, Kobayashi M, Odajima J, Shioda K, Hwang YS, Sasaki K, et al. Expanding homogeneous culture of human primordial germ cell-like cells maintaining germline features without serum or feeder layers. Stem Cell Rep. (2022) 17:507–21. doi: 10.1016/j.stemcr.2022.01.012
9. Guo K, Li CH, Wang X, and He DJP. Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod. (2016) 22:316–28. doi: 10.1093/molehr/gaw015
10. Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell. (2015) 17:178–94. doi: 10.1016/j.stem.2015.06.014
11. Chen D, Liu W, Lukianchikov A, Hancock GV, Zimmerman J, Lowe MG, et al. Germline competency of human embryonic stem cells depends on eomesodermin. Biol Reprod. (2017) 97:850–61. doi: 10.1093/biolre/iox138
12. Johnson J, Canning J, Kaneko T, Pru JK, and Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. (2004) 428:145–50. doi: 10.1038/nature02316
13. Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. (2009) 11:631–6. doi: 10.1038/ncb1869
14. Zhang C and Wu J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Hum Reprod. (2016) 22:457–64. doi: 10.1093/molehr/gaw030
15. Silvestris E, Cafforio P, D’Oronzo S, Felici C, Silvestris F, and Loverro G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: single-cell isolation and molecular characterization. Hum Reproduction. (2018) 33:464–73. doi: 10.1093/humrep/dex377
16. Woods DC and Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. (2013) 8:966–88. doi: 10.1038/nprot.2013.047
17. Wu C, Xu B, Li X, Ma W, Zhang P, Chen X, et al. Tracing and characterizing the development of transplanted female germline stem cells in vivo. Mol Ther. (2017) 25(6):1408–19. doi: 10.1016/j.ymthe.2017.04.019
18. Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. (2011) 3:132–41. doi: 10.1093/jmcb/mjq043
19. Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, et al. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. (2016) 6(1):28218. doi: 10.1038/srep28218
20. Li X, Zheng M, Xu B, Li D, Shen Y, Nie Y, et al. Generation of offspring-producing 3D ovarian organoids derived from female germline stem cells and their application in toxicological detection. Biomaterials. (2021) 279:121213. doi: 10.1016/j.biomaterials.2021.121213
21. Morita Y and Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. (1999) 213:1–17. doi: 10.1006/dbio.1999.9344
22. Pei Z, Deng K, Xu C, and Zhang S. The molecular regulatory mechanisms of meiotic arrest and resumption in Oocyte development and maturation. Reprod Biol Endocrinol. (2023) 21:90. doi: 10.1186/s12958-023-01143-0
23. Ford EA, Beckett EL, Roman SD, McLaughlin EA, and Sutherland JM. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction. (2020) 159:R15–29. doi: 10.1530/REP-19-0201
24. Adhikari D and Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. (2010) 9:1673–4. doi: 10.4161/cc.9.9.11626
25. Chen D, Liu W, Zimmerman J, Pastor WA, Kim R, Hosohama L, et al. The TFAP2C-regulated OCT4 naive enhancer is involved in human germline formation. Cell Rep. (2018) 25:3591–3602.e5. doi: 10.1016/j.celrep.2018.12.011
26. Chen D, Sun N, Hou L, Kim R, Faith J, Aslanyan M, et al. Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep. (2019) 29:4568–4582.e5. doi: 10.1016/j.celrep.2019.11.083
27. Cheng H. Germline stem cells in human. Signal Transduction Targeted Ther. (2022) 7(1):345. doi: 10.1038/s41392-022-01197-3
28. Murase Y, Yabuta Y, Ohta H, Yamashiro C, Nakamura T, Yamamoto T, et al. Long-term expansion with germline potential of human primordial germ cell-like cells in vitro. EMBO J. (2020) 39:e104929. doi: 10.15252/embj.2020104929
29. Chitiashvili T, man HF, Dror I, Plath K, and Clark A. FGFR3 is expressed by human primordial germ cells and is repressed after meiotic initiation to form primordial oocytes. Stem Cell Rep. (2022) 17:1268–78. doi: 10.1016/j.stemcr.2022.04.015
30. Hayashi K, Ohta H, Kurimoto K, Aramaki S, and Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. (2011) 146:519–32. doi: 10.1016/j.cell.2011.06.052
31. Zhu P, Zhang B, Sun R, Wang J, Liu Z, Liu X, et al. Derivation of new pluripotent stem cells from human extended pluripotent stem cells with formative features and trophectoderm potential. Cell Proliferation. (2023) 56:e13480. doi: 10.1111/cpr.13480
32. Hernandez SF, Vahidi NA, Park S, Weitzel RP, Tisdale J, Rueda BR, et al. Characterization of extracellular DDX4- or Ddx4-positive ovarian cells. Nat Med. (2015) 21:1114–6. doi: 10.1038/nm.3966
33. Zhang H, Panula S, Petropoulos S, Edsgärd D, Busayavalasa K, Liu L, et al. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med. (2015) 21:1116–8. doi: 10.1038/nm.3775
34. Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, and Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci USA. (2012) 109:12580–5. doi: 10.1073/pnas.1206600109
35. Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. (2006) 298:149–54. doi: 10.1016/j.ydbio.2006.06.023
36. Zhang H, Liu L, Li X, Busayavalasa K, Shen Y, Hovatta O, et al. Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice. Proc Natl Acad Sci USA. (2014) 111:17983–8. doi: 10.1073/pnas.1421047111
37. Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, et al. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. (2007) 306:112–20. doi: 10.1016/j.ydbio.2007.03.006
38. Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Obstetrical Gynecological Survey. (2020) 75:354–5. doi: 10.1097/OGX.0000000000000805
39. Alberico H, Fleischmann Z, Bobbitt T, Takai Y, Ishihara O, Seki H, et al. Workflow optimization for identification of female germline or oogonial stem cells in human ovarian cortex using single-cell RNA sequence analysis. Stem Cells. (2022) 40:523–36. doi: 10.1093/stmcls/sxac015
40. Kang Z. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev. (2011) 20:2197–204. doi: 10.1089/scd.2011.0091
41. Pierson Smela MD, Kramme CC, Fortuna PR, Adams JL, Su R, Dong E, et al. Directed differentiation of human iPSCs to functional ovarian granulosa-like cells via transcription factor overexpression. eLife. (2023) 12:e83291. doi: 10.7554/eLife.83291
42. Martin JJ, Woods DC, and Tilly JL. Implications and current limitations of oogenesis from female germline or oogonial stem cells in adult mammalian ovaries. (2019) 8(2):93. doi: 10.3390/cells8020093
43. Akahori T, Woods DC, and Tilly JL. Female fertility preservation through stem cell-based ovarian tissue reconstitution in vitro and ovarian regeneration in vivo. Reprod Health. (2019) 13:1179558119848007. doi: 10.1177/1179558119848007
44. Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. (2005) 122:303–15. doi: 10.1016/j.cell.2005.06.031
45. Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. JCO. (2007) 25:3198–204. doi: 10.1200/JCO.2006.10.3028
Keywords: primordial germ cell-like cells, ovarian stem cells, pluripotent stem cells, oogenesis, oocyte
Citation: Tian M and Zhang M (2025) Advances in in vitro oocyte generation from pluripotent stem cells and ovarian stem cells. Front. Endocrinol. 16:1515253. doi: 10.3389/fendo.2025.1515253
Received: 22 October 2024; Accepted: 20 May 2025;
Published: 11 August 2025.
Edited by:
Fred Sinowatz, Ludwig Maximilian University of Munich, GermanyReviewed by:
Dejin Zheng, Michigan State University, United StatesChun Hang Chen, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2025 Tian and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meixiang Zhang, bXh6MzI5QDE2My5jb20=
 Mingxin Tian
Mingxin Tian Meixiang Zhang
Meixiang Zhang