- Reproductive Medicine Center, Lanzhou University Second Hospital, Lanzhou, China
Objective: To identify potential diagnostic metabolic biomarkers for pregnancy loss (PL) by performing untargeted metabolomics analysis.
Methods: The present study performed untargeted metabolomics analysis on plasma samples from PL patients (n=70) and control subjects (n=122) using liquid chromatography‒mass spectrometry (LC‒MS). Metabolic profiles were evaluated using orthogonal partial least squares discriminant analysis (OPLS-DA), and pathway enrichment analysis was conducted via the KEGG database. LASSO regression was employed to identify significant metabolites, and their diagnostic performance was evaluated through receiver operating characteristic (ROC) curves. Pearson correlation analysis was used to explore the relationships between differentially abundant metabolites and clinical parameters.
Results: In total, 359 metabolites were identified, 57 of which were significantly altered between the control and PL group through OPLS-DA. Differential metabolites were significantly enriched in caffeine metabolism, tryptophan metabolism, and riboflavin metabolism pathways. Key metabolites, such as testosterone glucuronide, 6-hydroxymelatonin, and (S)-leucic acid, exhibited strong diagnostic potential, with AUC values of 0.991, 0.936 and 0.952, respectively, and the combined AUC was 0.993. Furthermore, Pearson correlation analysis revealed a significant negative correlation between the waist‒to‒hip ratio (WHR) and the abundance of testosterone glucuronide (r = -0.291, p = 0.0146), and a significant positive correlation between WHR and (S)-leucic acid (r = 0.248, p = 0.0381) in the PL group.
Conclusion: We identified a panel of plasma metabolites with significant diagnostic potential for PL. These biomarkers may facilitate early, noninvasive diagnosis and offer insights into metabolic dysregulation associated with pregnancy loss.
Introduction
Pregnancy loss (PL) refers to the natural end of a pregnancy before the fetus becomes viable, including all losses from conception until 24 weeks of gestation, affecting approximately 12–15% of recognized pregnancies globally (1, 2). Owing to increased environmental pollution and increasing life stress, the clinical incidence of PL has significantly increased in recent years (3, 4). Despite extensive research, the underlying causes of PL remain poorly understood and are often attributed to a complex interplay of genetic, immunological, and environmental factors (5). The absence of reliable metabolites complicates the early diagnosis and management of PL, leading to profound psychological and physiological impacts on affected women and their families (6).
In the context of PL, metabolomics can reveal critical metabolic alterations associated with this condition (7). Previous research has shown the effectiveness of metabolomics in identifying metabolic signatures for numerous conditions, such as cancer, cardiovascular diseases, autoimmune disease and metabolic disorders (8–11). For example, Lili Zhang et al. investigated serum metabolic profiles in women with recurrent abortion due to antiphospholipid syndrome, and they reported significant disruptions in purine, amino acid, and tyrosine metabolism (12). Another study utilized untargeted GC–MS and targeted liquid chromatography–mass spectrometry (LC–MS) to identify metabolic disturbances associated with recurrent spontaneous abortion. Through this approach, lactic acid and 5-methoxytryptamine were identified as significantly different metabolites between the two groups, and their plasma concentrations were further validated using targeted LC–MS (13). Despite these advances, comprehensive metabolomics analyses specifically targeting PL remain limited.
The integration of metabolomics with well-established analytical techniques, such as LC–MS, enables the detection of subtle metabolic alterations associated with PL. Moreover, the application of machine learning algorithms to metabolomics data has further improved the ability to identify and validate potential metabolites (14). One such machine learning algorithm, least absolute shrinkage and selection operator (LASSO) regression, is particularly effective in managing high-dimensional data characteristic of complex datasets (15, 16). LASSO regression stands out for its ability to perform both variable selection and regularization simultaneously, which helps prevent overfitting while managing large datasets (17). This is especially beneficial in metabolomics, where the number of metabolites often far exceeds the number of samples. By shrinking the coefficients of less relevant variables to zero, LASSO effectively refines the model, focusing on the most significant metabolites (18). This enhances the robustness of the model and increases the reproducibility of identifying the most important metabolites.
The present study aimed to leverage untargeted metabolomics combined with LASSO regression to identify a panel of diagnostic metabolites for PL. By comparing the metabolic profiles of plasma samples from women with a history of PL to those from women who have had normal pregnancies without PL, the present study sought to uncover metabolic signatures that can aid in the early detection and better understanding of PL. In addition, analyses that incorporated clinical parameters, such as age, BMI, and waist–to–hip ratio (WHR), which are potential confounding factors in PL, were performed to explore the relationships between the identified metabolites and these variables. The present findings may provide valuable insights for developing noninvasive diagnostic metabolites for PL.
Methods
Study design and participants
The present study enrolled 192 participants, comprising 122 in the control group and 70 in the PL group. The participants were recruited from Lanzhou University Second Hospital between February 2023 and September 2023. The inclusion criteria for the PL group included women aged 18 to 42 years who had experienced at least one PL in the past six months, in accordance with the ESHRE diagnostic criteria (1). The exclusion criteria for the PL group were as follows: presence of endocrine disorders, infections, or immunological diseases; or a history of the most recent pregnancy ending in an ectopic pregnancy, hydatidiform mole, or congenital defects. The inclusion criteria for the control group were women with no history of PL and at least one successful full-term pregnancy. The exclusion criteria for the control group included the presence of endocrine disorders, infections, or immunological diseases. The present study was approved by the Institutional Review Board of Lanzhou University Second Hospital (ethical approval number: 2023A-553). All patients provided written informed consent.
Sample collection and preparation
Fasting blood samples were collected from participants using EDTA tubes and centrifuged at 1,500×g for 10 minutes at 4°C to obtain plasma, which was then stored at -80°C. For metabolomics analysis, 100 μL of plasma was placed in Eppendorf tubes, mixed with 80% prechilled methanol, and vortexed thoroughly. The samples were incubated on ice for 5 minutes, followed by centrifugation at 15,000×g for 20 minutes at 4°C. A portion of the supernatant was diluted with LC–MS-grade water to reach a 53% methanol concentration. This mixture was transferred to fresh Eppendorf tubes and centrifuged again at 15,000×g for 20 minutes at 4°C. The final supernatant was then injected into the LC–MS/MS system for analysis via an Orbitrap Q Exactive™ HF–X mass spectrometer (19, 20).
UHPLC–MS analysis
UHPLC–MS/MS analysis was performed using a Vanquish UHPLC system coupled with an Orbitrap Q Exactive™ HF-X mass spectrometer (Thermo Fisher, Germany) at Novogene Co., Ltd. Samples were injected onto a Hypersil Gold column (100 × 2.1 mm, 1.9 μm) and separated over a 12-minute linear gradient at 0.2 mL/min. The eluents for positive polarity mode consisted of 0.1% formic acid in water (eluent A) and methanol (eluent B), whereas those for negative polarity mode included 5 mM ammonium acetate (pH 9.0, eluent A) and methanol (eluent B). The solvent gradient was programmed as follows: initial 2% B for 1.5 minutes, increased to 85% B over 3 minutes, increased to 100% B over the next 10 minutes, decreased to 2% B over 0.1 minutes, and maintained at 2% B for 12 minutes. The mass spectrometer was operated in both positive and negative modes with a spray voltage of 3.5 kV, capillary temperature of 320°C, sheath gas flow rate of 35 psi, auxiliary gas flow rate of 10 L/min, S-lens RF level of 60, and auxiliary gas heater temperature of 350°C. High-energy collision dissociation (HCD) was used as the fragmentation method, with an isolation window scanning the m/z range of 100–1,500. Collision energy was applied in three steps, namely, 20 V, 40 V, and 60 V. The quality control (QC) samples were interspersed among the study samples during analysis. The QC samples were used to monitor and assess the data quality by evaluating the correlation between QC injections, ensuring the reliability and consistency of the analytical results. All samples were analyzed in a single batch during the analytical run.
Data processing and metabolite identification
The raw data files from UHPLC–MS/MS were processed using Compound Discoverer 3.3 for peak alignment, peak picking, and metabolite quantitation, following the protocols established in previous studies (21, 22). First, retention times and mass-to-charge ratios (m/z) were aligned across samples with a mass tolerance of 5 ppm, a signal intensity tolerance of 30%, and a defined intensity threshold. Metabolite identification was performed by matching accurate m/z values, adduct ions, and MS/MS fragmentation patterns against the mzCloud (https://www.mzcloud.org/), mzVault, and MassList databases. In databases containing fragmentation data, the acquired MS spectra were compared with reference fragment ions and collision energies, ensuring higher confidence annotations. Next, peak intensities were normalized to reduce run-to-run variations and enable consistent comparative analyses. Quality control (QC) samples were injected to monitor instrument stability, and only features with a coefficient of variation (CV) < 30% in the QC samples were retained. Finally, the normalized peak areas of the confidently identified metabolites were used for subsequent statistical analyses.
Data analysis
The identified metabolites were annotated using the KEGG and HMDB databases (https://www.metaboanalyst.ca/). Since our research focus is not on exogenous metabolites, we have excluded them from further analysis. A detailed list of these excluded exogenous metabolites is provided in Supplementary Table 1. To visualize metabolic differences and identify potential metabolic signatures, orthogonal partial least squares discriminant analysis (OPLS-DA) was performed using the ‘ropls’ package in R software. Statistical significance was assessed using the t test, with differentially abundant metabolites selected on the basis of a variable importance in projection (VIP) score > 1 and a p value < 0.05. Heatmaps and volcano plots were generated using the ‘pheatmap’ and ‘ggplot2’ packages in R. Pathway analysis was conducted using the MetaboAnalyst platform (version 6.0), a widely used web-based tool for metabolomics data interpretation. The list of significantly altered metabolites was uploaded using HMDB (Human Metabolome Database) IDs, and these metabolites were assigned on the basis of MS/MS spectral matching and database searches. The KEGG database was used for pathway enrichment analysis. Enrichment analysis was performed using the hypergeometric test to identify overrepresented pathways compared with a reference metabolome; pathways with a p value < 0.05 were considered statistically significant. To assess the biological importance of metabolites within pathways, the relative-betweenness centrality algorithm was applied during pathway topology analysis. The results were visualized via a pathway impact plot, which integrates enrichment and topology analyses to highlight the most significantly impacted pathways.
LASSO regression for metabolites selection and ROC curve analysis
LASSO regression was employed via the ‘glmnet’ package in R to identify significant metabolites associated with PL (23). The data were divided into a training set (60% of the data) and a validation set (40% of the data). Within the training set, 10-fold cross-validation was applied using the ‘caret’ package in R to optimize the model and avoid overfitting (24). The selected features from the LASSO model were then validated using the validation set. ROC curve, sensitivity, specificity, and confusion matrix analyses were conducted using the ‘pROC’ package in R. Additionally, ROC curve analysis on clinical data, including age, BMI, and WHR, was conducted to compare their predictive performance with that of the differentially metabolite abundance-based model.
Correlation analysis
Pearson correlation analysis was performed using the ‘Hmisc’ package in R to investigate the relationships between differentially abundant metabolites and clinical parameters, such as age, BMI, and WHR. Heatmaps were created with the ‘ComplexHeatmap’ package, and significant correlations were visualized using scatter plots generated with the ‘ggplot2’ package, offering insights into the metabolic alterations linked to clinical features in the control and PL group. Additionally, a permutation test with 200 iterations was employed to assess the statistical significance of the correlation coefficient differences between the control group and PL group.
Results
Baseline clinical characteristics
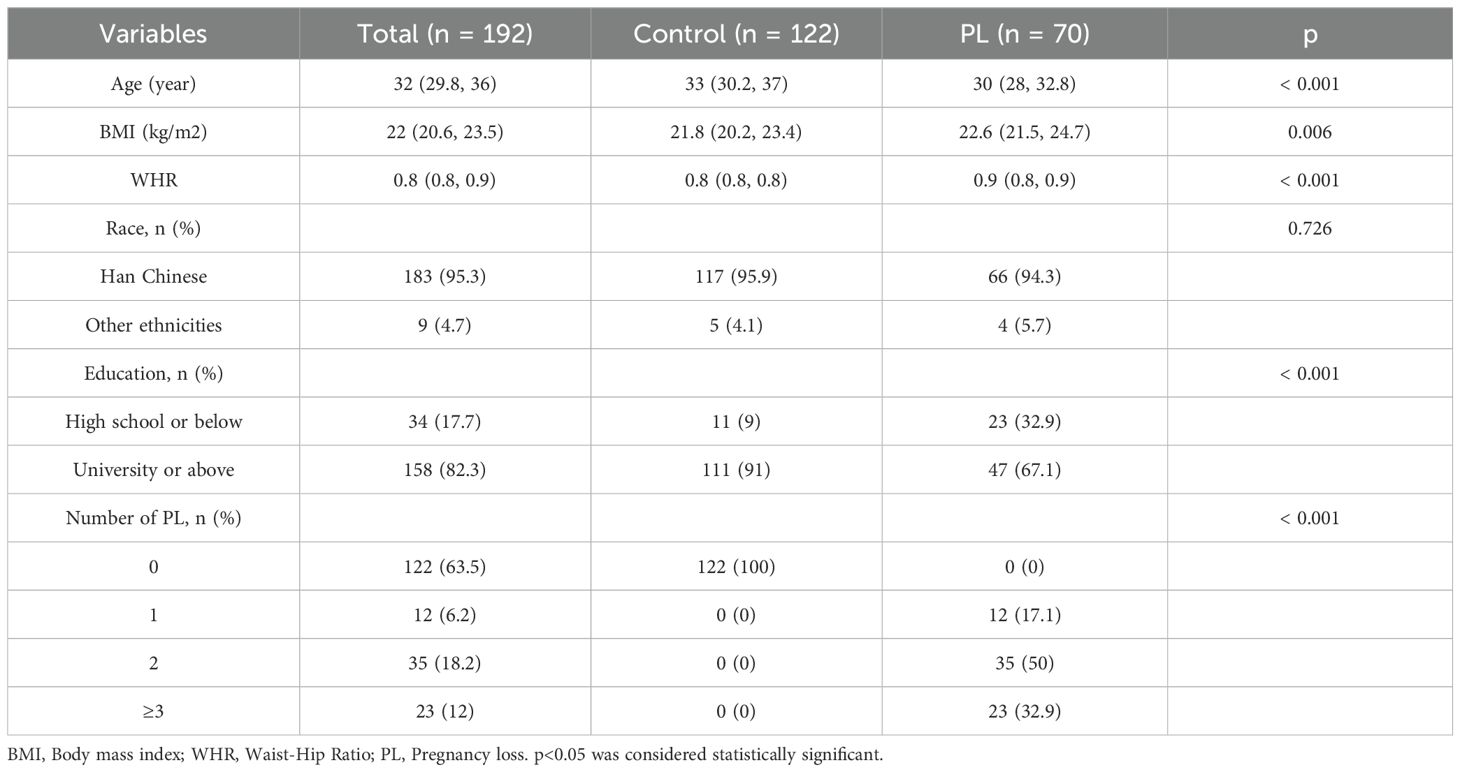
Table 1 presents the baseline clinical characteristics of the study participants, which included 122 women in the control group and 70 women in the PL group. The median age in the PL group was significantly lower than that in the control group (30 years vs. 33 years, p < 0.001). The PL group had a higher median BMI (22.6 kg/m² [Q1-Q3: 21.5-24.7]) than the control group (21.8 kg/m² [Q1-Q3: 20.2-23.4], p = 0.006). Furthermore, the PL group presented a significantly greater WHR (0.9 [Q1-Q3: 0.8-0.9]) than the control group (0.8 [Q1-Q3: 0.8-0.8], p < 0.001). In terms of race, the majority of participants in both groups were Han Chinese, with no significant difference observed between the two groups (p = 0.726). However, education levels significantly differed between the two groups. A significantly greater proportion of women in the control group had a university education or above (91% vs. 67.1%, p < 0.001), whereas the PL group had a greater percentage of participants with a high school education or below (32.9% vs. 9%, p < 0.001). With respect to the number of pregnancy losses, none of the women in the control group experienced a loss, whereas the PL group included participants with varying numbers of losses, including 17.1% with one loss, 50% with two losses, and 32.9% with three or more losses (p < 0.001).
OPLS-DA model and permutation test
Figure 1 illustrates the OPLS-DA model and its evaluation through permutation tests. The OPLS-DA plot (Figure 1A) revealed a separation between the control and PL group, indicating distinct metabolic profiles. The x-axis (t1) explained 7.0% of the variance between the two groups, whereas the y-axis (to1) explained 8.0% of the variance within each group. A permutation test with 200 iterations (Figure 1B) was used to evaluate the OPLS-DA model. The histogram shows the frequency distribution of the permuted R2Y and Q2 values, with an R2Y value of 0.932 and an Q2 value of 0.899, which were both significantly higher than those obtained from the permuted models (p = 0.005), confirming the reliability and predictive performance of the model.
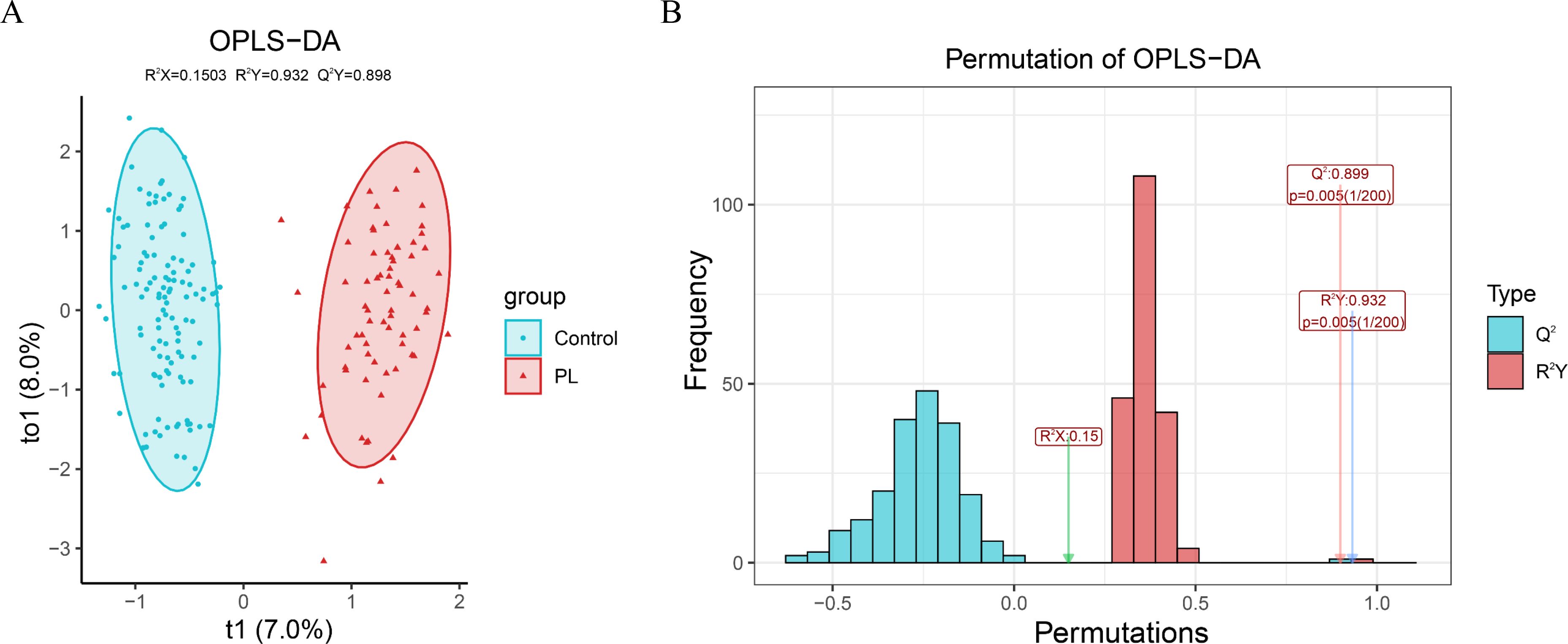
Figure 1. OPLS-DA model and permutation test evaluation. (A) OPLS-DA score plot showing the separation between the PL group and the control group based on their metabolic profiles. (B) Permutation test results with 200 iterations to evaluate the robustness of the OPLS-DA model.
Differential metabolite analysis
Figure 2 shows an in-depth analysis of the differentially abundant metabolites between the control and PL group. In total, 57 differentially abundant metabolites were identified on the basis of VIP> 1 and p values<0.05 (Supplementary Table 2). The heatmap shown in Figure 2A displays the differentially abundant metabolites, revealing distinct clustering patterns between the two groups. In addition, the volcano plot shown in Supplementary Figure S1A illustrates the differentially abundant metabolites using log2-fold changes and -log10 p values. Testosterone glucuronide, 6-hydroxymelatonin, and (S)-leucic acid demonstrated statistical significance and fold changes, with testosterone glucuronide exhibiting the greatest significance and fold change. Among the 57 significantly altered metabolites, 54 were successfully matched to known metabolic pathways in the KEGG database using the MetaboAnalyst platform. The pathway impact plot revealed several significantly impacted pathways, including caffeine metabolism, tryptophan metabolism, and riboflavin metabolism (Figure 2B). The bubble plot shows these pathways, with larger and more vividly colored bubbles indicating pathways with greater impact and significance.
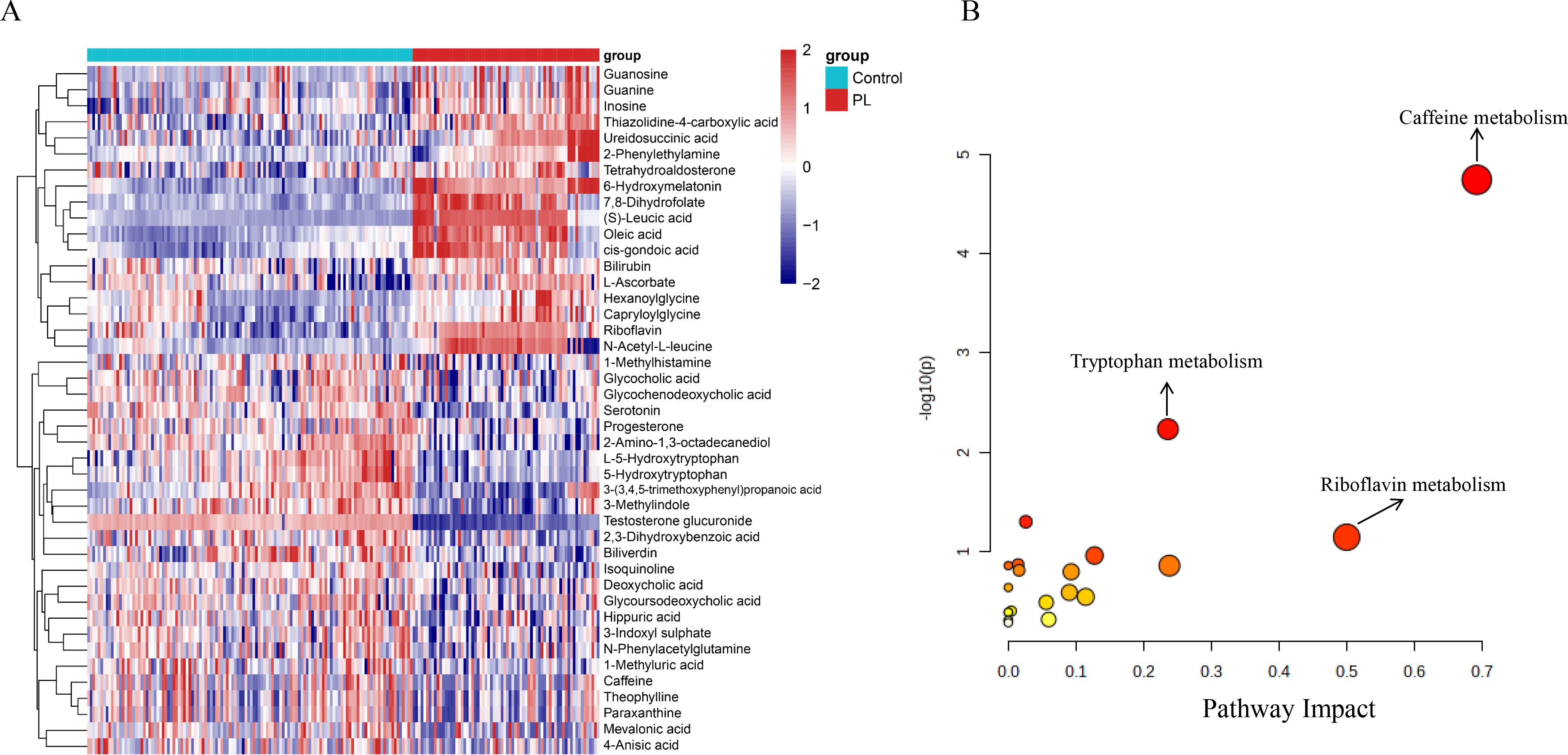
Figure 2. Differential metabolites and pathway enrichment analysis. (A) Heatmap of the top 43 differential metabolites identified between the control and PL group, showing distinct clustering patterns. (B) Pathway enrichment analysis bubble plot. The x-axis represents the pathway impact, and the y-axis represents the -log10 of the p-value.
Screening of differential metabolites
Figure 3 shows the results of LASSO regression and the subsequent analysis of selected metabolites. In Figure 3A, the coefficients of the metabolites are plotted against the log of the regularization parameter (λ), demonstrating the selection process for the most important metabolites in distinguishing between the control and PL group. Figure 3B shows the three most important metabolites selected by the LASSO model, namely, testosterone glucuronide, 6-hydroxymelatonin and (S)-leucic acid.
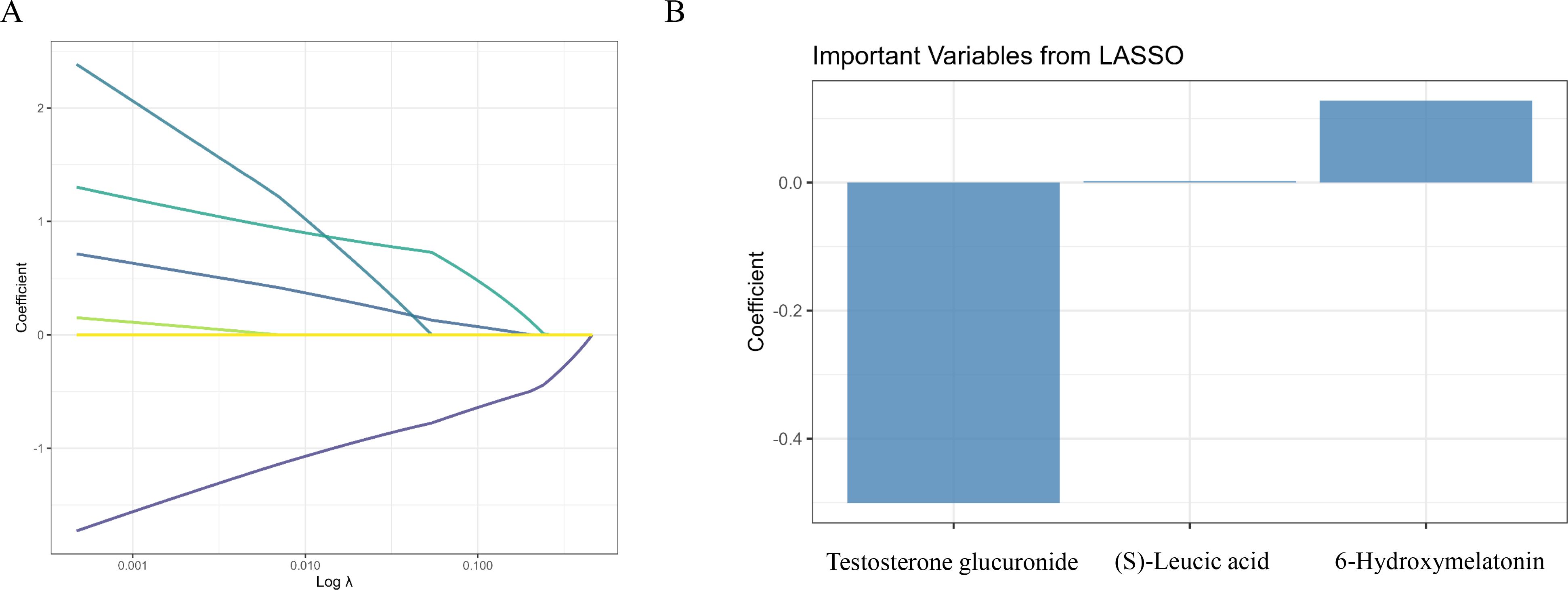
Figure 3. Screening of differential metabolites using LASSO regression. (A) LASSO regression cross-validation plot identifying the optimal λ to minimize deviance. (B) Bar plot of the regression coefficients for the three most important metabolites selected by the LASSO model.
Abundance and diagnostic performance of key metabolites
Figure 4A compares the abundance levels of these metabolites between the control and PL group. Significant differences in abundance were observed for all three metabolites, with p values indicating significant differences (p<0.0001). These findings suggested that these metabolites play crucial roles in the metabolic alterations associated with PL. An ROC curve of the LASSO model revealed that the combined AUC value was 0.993, indicating high predictive accuracy for the selected metabolites in the validation set (Figure 4B). The performance of the optimal metabolite model was evaluated using a confusion matrix (Supplementary Figure S1B) and classification performance indicators (Supplementary Figure S1C). The confusion matrix revealed that the model achieved a sensitivity of 1.0000 and a specificity of 0.9643, indicating high diagnostic accuracy. In addition, the model had an accuracy of 0.9868. The positive predictive value (PPV) was 0.9796, and the negative predictive value (NPV) was 1.0000, reflecting the robust ability of the model to distinguish between the control and PL group.
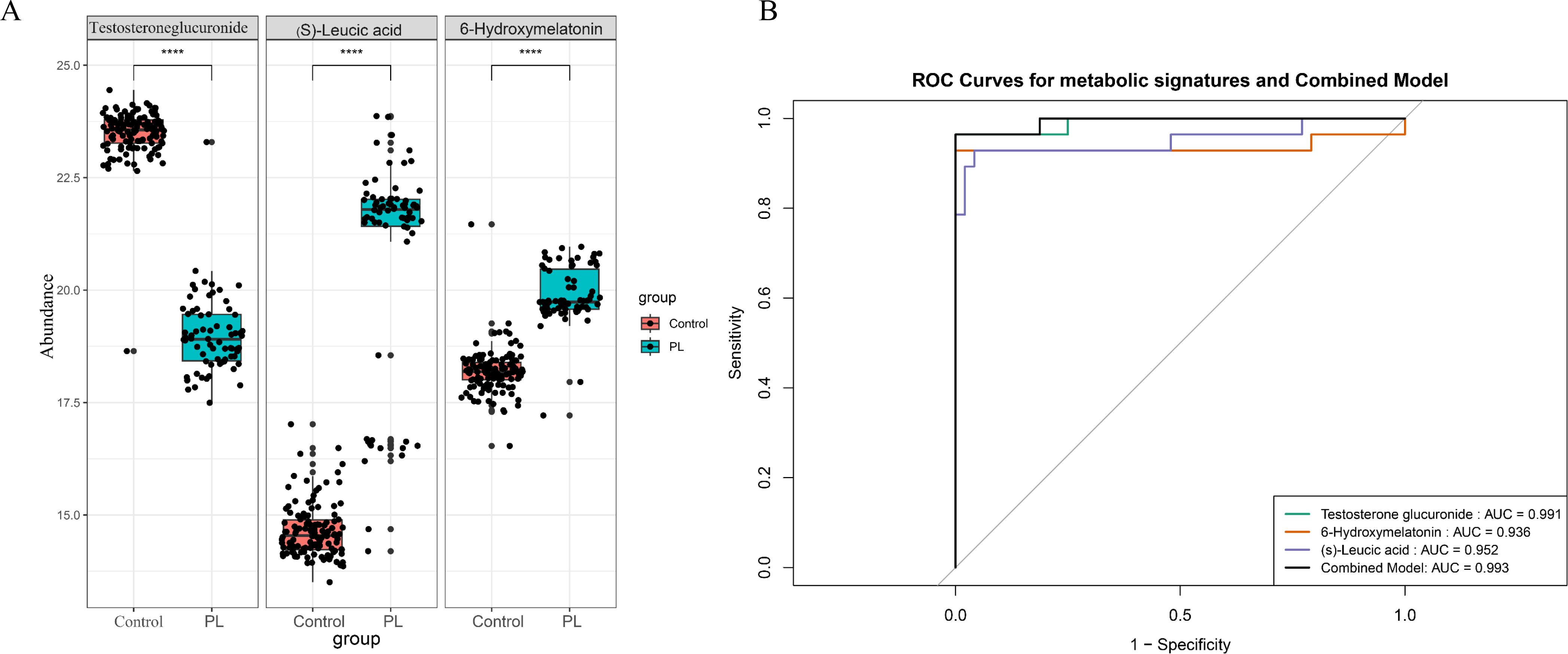
Figure 4. The most important metabolites and their diagnostic performance. (A) Box plots comparing the abundance levels of the three significant metabolites between the control and PL group (****p < 0.0001). (B) ROC curves demonstrating the diagnostic performance of a panel of metabolites in the test dataset.
Comparative diagnostic performance of the key metabolites and clinical parameters
The diagnostic performance of the clinical parameters and metabolites was evaluated via receiver operating characteristic (ROC) curve analysis. Among the clinical parameters, WHR had the highest AUC (0.724), followed by age (AUC = 0.696) and BMI (AUC = 0.435). The combined clinical model achieved an AUC value of 0.775 (Figure 5A). Furthermore, the combined model, which integrated both key metabolites and clinical parameters, achieved an AUC value of 1.000 (Figure 5B).
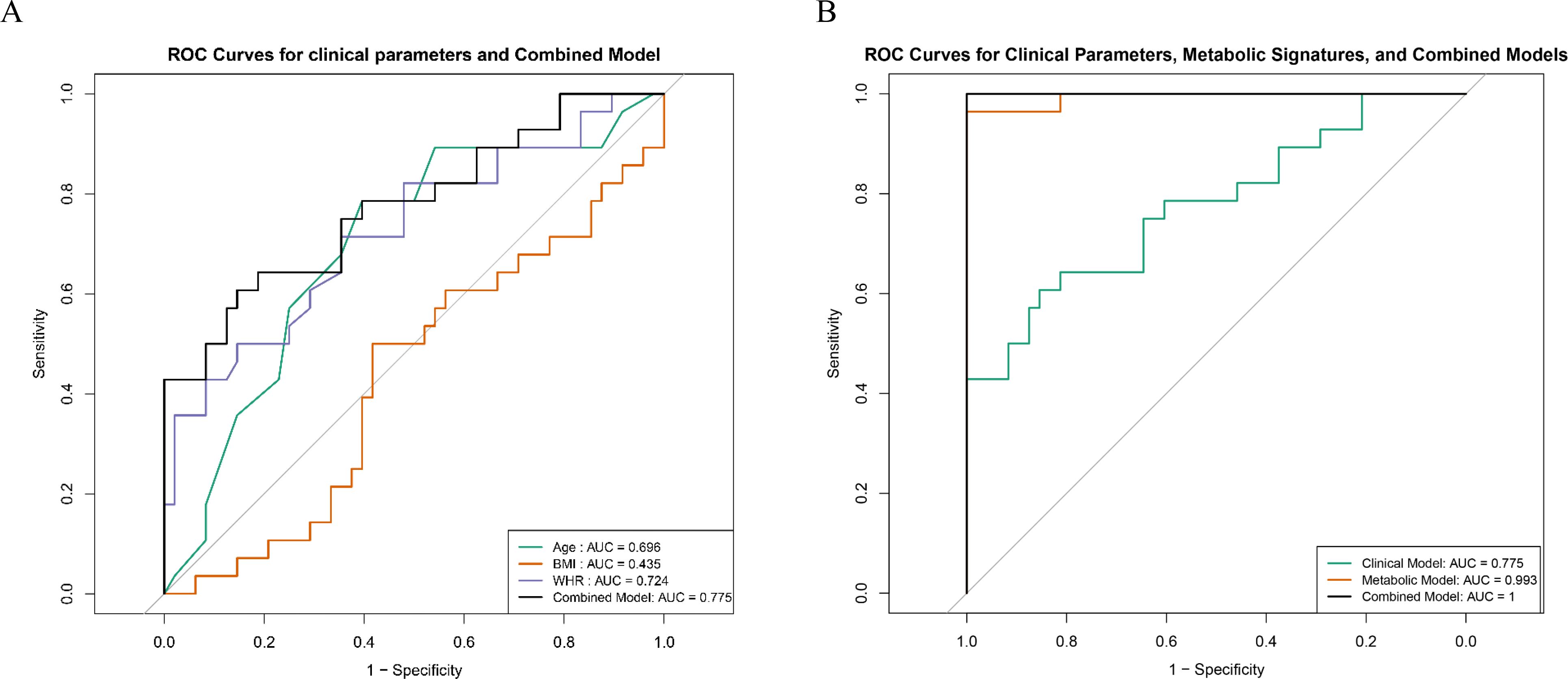
Figure 5. Comparative diagnostic performance of key metabolites and clinical indicators. (A) ROC curves illustrating the diagnostic performance of individual clinical parameters (age, BMI, and WHR). (B) ROC curves comparing the diagnostic performance of combined key metabolites, clinical parameters, and the integrated model.
Correlation analysis between differentially abundant metabolites and clinical parameters
Figure 6 shows the Pearson correlations between differentially abundant metabolites and clinical parameters within the PL group. The heatmap shown in Figure 6A depicts the Pearson correlation coefficients between core differentially abundant metabolites and clinical phenotypes. The scatter plot shown in Figure 6B further illustrates the negative correlation between WHR and testosterone glucuronide, which indicated that higher WHR values were associated with lower levels of testosterone glucuronide (r = -0.291, p = 0.0146), whereas (S)-leucic acid was significantly positively correlated with WHR (r = 0.248, p = 0.0381). Additionally, the correlation of WHR with the levels of testosterone glucuronide or (S)-leucic acid were evaluated in the control group. The scatter plot shown in Supplementary Figure S1D indicates that both correlations were nonsignificant in the control group (WHR vs. testosterone glucuronide: r = –0.0294, p = 0.748; WHR vs. (S)-leucic acid: r = 0.126, p = 0.168), suggesting no evident associations (Supplementary Figures S1D, E). Moreover, a permutation test comparing the correlation coefficients between the control and PL group revealed a statistically significant difference (p = 0.04) in the correlation between WHR and testosterone glucuronide, whereas the correlation between WHR and (S)-leucic acid did not differ significantly (p = 0.28).
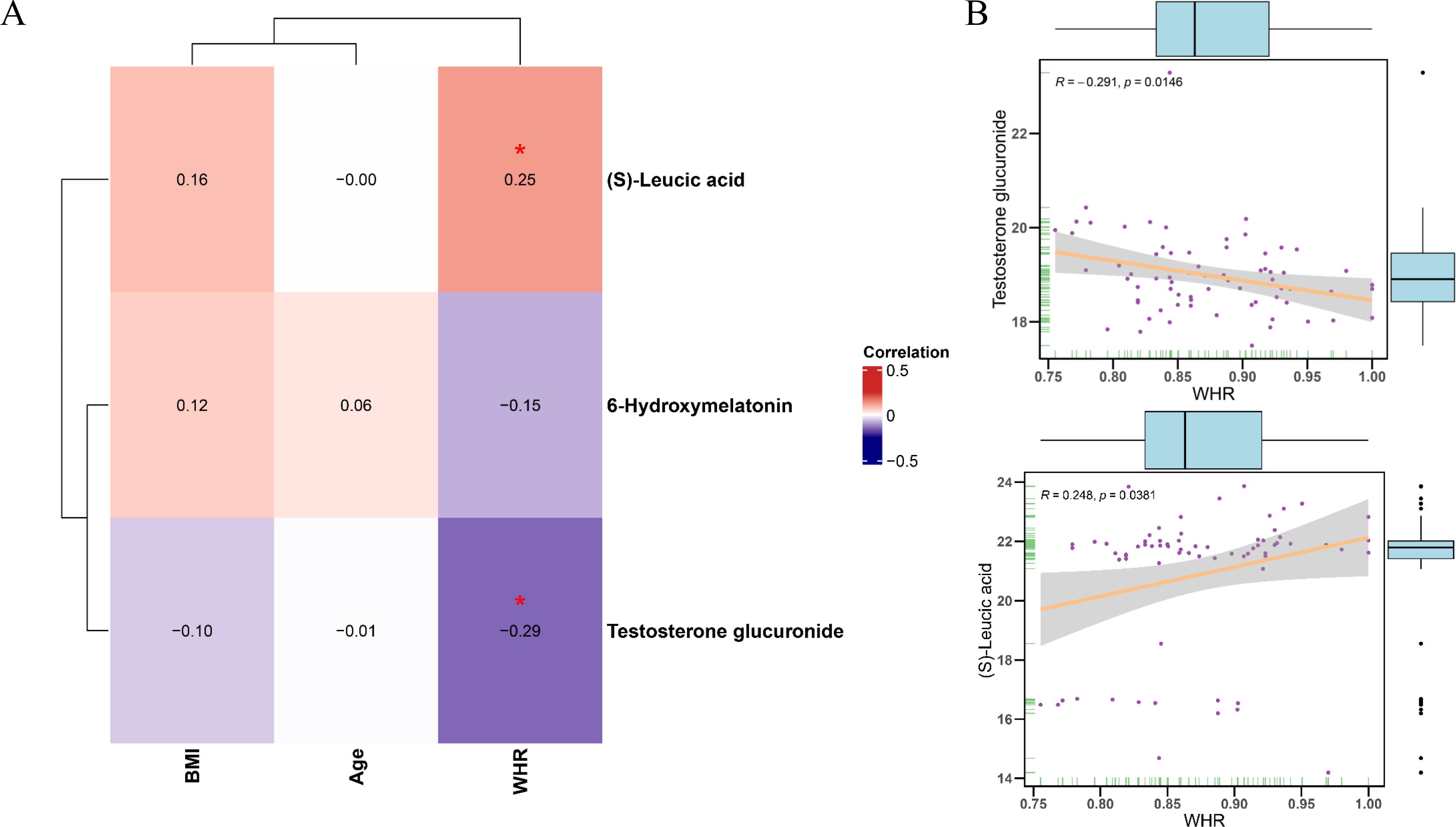
Figure 6. Correlation analysis between differential metabolites and clinical parameters in PL group. (A) Heatmap showing the Pearson correlation coefficients between core metabolites and clinical phenotypes parameters (age, BMI, WHR) in the PL group. (B) Scatter plots illustrating the significant correlations between WHR and testosterone glucuronide levels (negative correlation) and (S)-Leucic acid levels (positive correlation).
Discussion
In the present study, we identified differentially abundant metabolites between the control and PL group, which were primarily enriched in the caffeine metabolism, tryptophan metabolism, and riboflavin metabolism pathways. Caffeine metabolism emerged as a key pathway in the present analysis. Caffeine is widely consumed, and its metabolism involves several enzymes, such as cytochrome P450, which are integral to oxidative stress responses and liver function (25–27). Disruptions in caffeine metabolism may reflect dysregulated detoxification processes or oxidative stress, both of which are critical for maintaining pregnancy. Studies have suggested that high caffeine intake during pregnancy may increase the risk of pregnancy complications, including PL (28). Therefore, the significant alterations in caffeine metabolism observed in PL patients may reflect either direct caffeine exposure or dysregulated detoxification processes that impact pregnancy outcomes.
Amino acids play fundamental roles in various biological processes, including protein synthesis, immune regulation, and neuroendocrine function (29, 30). Our findings revealed significant alterations in amino acid metabolism pathways, with tryptophan metabolism emerging as a key pathway in PL patients. Tryptophan, an essential aromatic amino acid, plays a critical role in protein synthesis, immune regulation, and neuroendocrine function (31). The metabolism of tryptophan primarily proceeds through three major pathways, namely, the kynurenine (KYN) pathway, the serotonin (5-HT) pathway, and the indole pathway (32). The KYN pathway, which dominates tryptophan metabolism, is mediated by several enzymes, including tryptophan-2,3-dioxygenase (TDO) and indoleamine-2,3-dioxygenase (IDO). These enzymes are critical for modulating immune responses (33, 34). Dysregulated IDO activity has been associated with decreased kynurenine levels in PL patients, which may impair immune tolerance and increase the risk of fetal rejection. Additionally, tryptophan serves as a precursor for serotonin synthesis, a key neurotransmitter involved in regulating inflammation and maternal–fetal communication (35). Reduced tryptophan availability in PL patients may compromise serotonin production, exacerbating systemic inflammation and negatively affecting pregnancy outcomes (36). The present findings aligned with these observations, further supporting the critical role of tryptophan metabolism in pregnancy maintenance.
In addition, the present study revealed enrichment in the riboflavin metabolism pathway, highlighting its potential involvement in PL. Riboflavin, also known as vitamin B2, is a precursor for the synthesis of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), which act as essential cofactors for numerous redox enzymes involved in energy metabolism, oxidative stress regulation, and detoxification processes (37). Riboflavin metabolism plays a critical role in redox reactions, energy production, and cellular homeostasis (38, 39). Deficiency in riboflavin has been shown to impair mitochondrial function, resulting in disrupted ATP synthesis and increased production of reactive oxygen species (ROS) (40, 41). This imbalance may contribute to oxidative stress, a condition linked to adverse pregnancy outcomes, such as preeclampsia, intrauterine growth restriction, and spontaneous abortion (42, 43). In the present study, the significant alterations in riboflavin metabolism observed in PL patients may indicate disrupted mitochondrial energy production and heightened oxidative stress, both of which may compromise pregnancy maintenance.
LASSO algorithms were further employed to refine the selection of significant metabolites. The present analysis identified testosterone glucuronide, 6-hydroxymelatonin and (S)-leucic acid as key metabolites with strong diagnostic potential. These metabolites demonstrated high area under the curve (AUC) values in the ROC curve analysis, indicating their utility as noninvasive metabolites for PL and surpassing the diagnostic accuracy of clinical indicators alone. Testosterone glucuronide is a conjugated form of testosterone involved in androgen metabolism (44). In the context of PL, altered levels of testosterone glucuronide may reflect disruptions in hormonal regulation. 6-Hydroxymelatonin is a metabolite of melatonin, a hormone known for regulating sleep–wake cycles and reproductive functions (45). Changes in melatonin metabolism have been linked to various reproductive disorders, suggesting that alterations in melatonin metabolism in PL patients may indicate disruptions in circadian rhythms and reproductive health. (S)-Leucic acid is a metabolite of leucine, a branched-chain amino acid, and it has been studied for its anabolic effects (46), particularly in promoting muscle protein synthesis. However, the role of (S)-leucic acid in reproductive health and pregnancy remains largely unexplored. The present findings suggested that (S)-leucic acid may be involved in metabolic pathways that are critical for maintaining a healthy pregnancy.
Correlation analysis revealed a significant negative association between waist–to–hip ratio (WHR) and testosterone glucuronide in the PL group, implying that an elevated WHR may coincide with lower levels of testosterone glucuronide. Such disruptions could impair maternal–fetal communication and contribute to adverse pregnancy outcomes. Of note, this relationship was not observed in the control group, highlighting the specificity of metabolic alterations in PL patients. Although (S)-leucic acid was also positively correlated with WHR in the PL group, the difference in correlation coefficients compared with those in the control group was not statistically significant, possibly reflecting the limited sample size. Nevertheless, the apparent involvement of (S)-leucic acid in metabolic pathways relevant to pregnancy warrants further study to clarify its mechanistic role in PL and to assess its potential diagnostic value.
In conclusion, the present findings suggest that caffeine metabolism, tryptophan metabolism and riboflavin metabolism pathway may play important roles in the pathophysiology of PL, Moreover, testosterone glucuronide, 6-hydroxymelatonin, and (S)-leucic acid showed potential as noninvasive diagnostic metabolites. Further validation in larger cohorts is necessary to confirm these findings and to better understand the role of metabolic alterations in the pathophysiology of PL.
Limitation
Despite the promising findings, this study has several limitations. Firstly, the sample size is relatively small, which may limit the generalizability of the results and prevent detailed stratification analyses, such as differentiating metabolic variations among PL cases with different recurrence histories. Secondly, the study design is cross-sectional, which prevents us from establishing causal relationships between the identified metabolic alterations and PL. Although we applied OPLS-DA and LASSO regression to prioritize key metabolites and minimize irrelevant variation and multicollinearity, these methods cannot fully account for confounding factors such as age, BMI, and WHR. We employed a permutation test to further address this issue, but future studies incorporating matched or statistically adjusted designs are warranted. Additionally, we did not collect detailed dietary information or account for variations in education level, which may influence metabolic profiles. Finally, the study focuses solely on plasma metabolites and includes limited clinical data. Future studies should aim to integrate multi-omics approaches and more comprehensive clinical datasets to provide a deeper understanding of the molecular mechanisms underlying PL.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Lanzhou University Second Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ND: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Writing – original draft. XY: Formal analysis, Investigation, Project administration, Software, Supervision, Validation, Writing – original draft. RW: Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. FW: Data curation, Funding acquisition, Resources, Visualization, Writing – review & editing, Conceptualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Science Foundation of Lanzhou University (Grant No. 071100132 and 071100186), the Medical Innovation and Development Project of Lanzhou University (Grant No. lzuyxcx-2022-137), and the Science Foundation of Lanzhou University Second Hospital (Grant No. YJS-BD-19).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1518043/full#supplementary-material
References
1. Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. (2018) 2018:hoy004. doi: 10.1093/hropen/hoy004
2. Zhao QY, Li QH, Fu YY, Ren CE, Jiang AF, Meng YH. Decidual macrophages in recurrent spontaneous abortion. Front Immunol. (2022) 13:994888. doi: 10.3389/fimmu.2022.994888
3. Grippo A, Zhang J, Chu L, Guo Y, Qiao L, Zhang J, et al. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev Environ Health. (2018) 33:247–64. doi: 10.1515/reveh-2017-0033
4. Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. (2021) 397:1658–67. doi: 10.1016/S0140-6736(21)00682-6
5. Pinar MH, Gibbins K, He M, Kostadinov S, Silver R. Early pregnancy losses: review of nomenclature, histopathology, and possible etiologies. Fetal Pediatr Pathol. (2018) 37:191–209. doi: 10.1080/15513815.2018.1455775
6. Farren J, Mitchell-Jones N, Verbakel JY, Timmerman D, Jalmbrant M, Bourne T. The psychological impact of early pregnancy loss. Hum Reprod Update. (2018) 24:731–49. doi: 10.1093/humupd/dmy025
7. Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA. Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom. (2016) 27:1897–905. doi: 10.1007/s13361-016-1469-y
8. Perera TRW, Bromfield EG, Gibb Z, Nixon B, Sheridan AR, Rupasinghe T, et al. Plasma lipidomics reveals lipid signatures of early pregnancy in mares. Int J Mol Sci. (2024) 25(20):11073. doi: 10.3390/ijms252011073
9. Palisi A, Grimaldi M, Sabatini P, Montoro P, Scrima M, Rodriquez M, et al. A serum nuclear magnetic resonance-based metabolomic signature of antiphospholipid syndrome. J Pharm BioMed Anal. (2017) 133:90–5. doi: 10.1016/j.jpba.2016.11.002
10. Sun Y, Zhang X, Hang D, Lau HC, Du J, Liu C, et al. Integrative plasma and fecal metabolomics identify functional metabolites in adenoma-colorectal cancer progression and as early diagnostic biomarkers. Cancer Cell. (2024) 42:1386–1400.e1388. doi: 10.1016/j.ccell.2024.07.005
11. Culler KL, Sinha A, Filipp M, Giro P, Allen NB, Taylor KD, et al. Metabolomic profiling identifies novel metabolites associated with cardiac dysfunction. Sci Rep. (2024) 14:20694. doi: 10.1038/s41598-024-71329-y
12. Zhang L, Li Y, Lin X, Jia C, Yu X. Liquid Chromatography/Mass Spectrometry based serum metabolomics study on recurrent abortion women with antiphospholipid syndrome. PloS One. (2019) 14:e0225463. doi: 10.1371/journal.pone.0225463
13. Li X, Yin M, Gu J, Hou Y, Tian F, Sun F. Metabolomic profiling of plasma samples from women with recurrent spontaneous abortion. Med Sci Monit. (2018) 24:4038–45. doi: 10.12659/MSM.907653
14. Reel PS, Reel S, Pearson E, Trucco E, Jefferson E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol Adv. (2021) 49:107739. doi: 10.1016/j.biotechadv.2021.107739
15. Zhou Y, Shi W, Zhao D, Xiao S, Wang K, Wang J. Identification of immune-associated genes in diagnosing aortic valve calcification with metabolic syndrome by integrated bioinformatics analysis and machine learning. Front Immunol. (2022) 13:937886. doi: 10.3389/fimmu.2022.937886
16. Xiong T, Han S, Pu L, Zhang TC, Zhan X, Fu T, et al. Bioinformatics and machine learning methods to identify FN1 as a novel biomarker of aortic valve calcification. Front Cardiovasc Med. (2022) 9:832591. 10.3389/fcvm.2022.832591
17. Li Z, Sillanpää MJ. Overview of LASSO-related penalized regression methods for quantitative trait mapping and genomic selection. Theor Appl Genet. (2012) 125:419–35. doi: 10.1007/s00122-012-1892-9
18. Tan J, Shu M, Liao J, Liang R, Liu S, Kuang M, et al. Identification and validation of a plasma metabolomics-based model for risk stratification of intrahepatic cholangiocarcinoma. J Cancer Res Clin Oncol. (2023) 149:12365–77. doi: 10.1007/s00432-023-05119-w
19. Want EJ, O’Maille G, Smith CA, Brandon TR, Uritboonthai W, Qin C, et al. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal Chem. (2006) 78:743–52. doi: 10.1021/ac051312t
20. Barri T, Dragsted LO. UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: effect of experimental artefacts and anticoagulant. Anal Chim Acta. (2013) 768:118–28. doi: 10.1016/j.aca.2013.01.015
21. Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. (2011) 6:1060–83. doi: 10.1038/nprot.2011.335
22. Want EJ, Wilson ID, Gika H, Theodoridis G, Plumb RS, Shockcor J, et al. Global metabolic profiling procedures for urine using UPLC-MS. Nat Protoc. (2010) 5:1005–18. doi: 10.1038/nprot.2010.50
23. Wei K, Cao Y, Kong X, Liu C, Gu X. Exploration and validation of immune and therapeutic-related hub genes in aortic valve calcification and carotid atherosclerosis. J Inflammation Res. (2024) 17:6485–500. doi: 10.2147/JIR.S462546
24. Desaire H. How (Not) to generate a highly predictive biomarker panel using machine learning. J Proteome Res. (2022) 21:2071–4. doi: 10.1021/acs.jproteome.2c00117
25. Coppola P, Kerwash E, Cole S. Use of physiologically based pharmacokinetic modeling for hepatically cleared drugs in pregnancy: regulatory perspective. J Clin Pharmacol. (2023) 63 Suppl 1:S62–s80. doi: 10.1002/jcph.v63.S1
26. Barcelos RP, Lima FD, Carvalho NR, Bresciani G, Royes LF. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr Res. (2020) 80:1–17. doi: 10.1016/j.nutres.2020.05.005
27. Li YF, Ouyang SH, Tu LF, Wang X, Yuan WL, Wang GE, et al. Fang ZZ et al: Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics. (2018) 8:5713–30. doi: 10.7150/thno.28778
28. Jafari A, Naghshi S, Shahinfar H, Salehi SO, Kiany F, Askari M, et al. Relationship between maternal caffeine and coffee intake and pregnancy loss: A grading of recommendations assessment, development, and evaluation-assessed, dose-response meta-analysis of observational studies. Front Nutr. (2022) 9:886224. doi: 10.3389/fnut.2022.886224
29. Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. (2018) 19(4):954. doi: 10.3390/ijms19040954
30. Kelly B, Pearce EL. Amino assets: how amino acids support immunity. Cell Metab. (2020) 32:154–75. doi: 10.1016/j.cmet.2020.06.010
31. Xue C, Li G, Zheng Q, Gu X, Shi Q, Su Y, et al. Tryptophan metabolism in health and disease. Cell Metab. (2023) 35:1304–26. doi: 10.1016/j.cmet.2023.06.004
32. Seo SK, Kwon B. Immune regulation through tryptophan metabolism. Exp Mol Med. (2023) 55:1371–9. doi: 10.1038/s12276-023-01028-7
33. Salminen A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev. (2022) 75:101573. doi: 10.1016/j.arr.2022.101573
34. Mellor AL, Lemos H, Huang L. Indoleamine 2,3-dioxygenase and tolerance: where are we now? Front Immunol. (2017) 8:1360. doi: 10.3389/fimmu.2017.01360
35. Li Y, Yu X, Shi J, Zhao J, Li L. The role of aryl hydrocarbon receptors in nutrient metabolism and immune regulation at the maternal-fetal interface. Placenta. (2024) 154:9–17. doi: 10.1016/j.placenta.2024.05.134
36. Latifimehr M, Rastegari AA, Zamani Z, Esfahani PF, Nazari L. The investigation of metabonomic pathways of serum of Iranian women with recurrent miscarriage using (1)H NMR. BioMed Res Int. (2021) 2021:3422138. doi: 10.1155/2021/3422138
37. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. (2003) 77:1352–60. doi: 10.1093/ajcn/77.6.1352
38. Pinto JT, Cooper AJ. From cholesterogenesis to steroidogenesis: role of riboflavin and flavoenzymes in the biosynthesis of vitamin D. Adv Nutr. (2014) 5:144–63. doi: 10.3945/an.113.005181
39. Lee TY, Farah N, Chin VK, Lim CW, Chong PP, Basir R, et al. Medicinal benefits, biological, and nanoencapsulation functions of riboflavin with its toxicity profile: A narrative review. Nutr Res. (2023) 119:1–20. doi: 10.1016/j.nutres.2023.08.010
40. Smedts HP, Rakhshandehroo M, Verkleij-Hagoort AC, de Vries JH, Ottenkamp J, Steegers EA, et al. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects. Eur J Nutr. (2008) 47:357–65. doi: 10.1007/s00394-008-0735-6
41. Chan J, Deng L, Mikael LG, Yan J, Pickell L, Wu Q, et al. Low dietary choline and low dietary riboflavin during pregnancy influence reproductive outcomes and heart development in mice. Am J Clin Nutr. (2010) 91:1035–43. doi: 10.3945/ajcn.2009.28754
42. Joo EH, Kim YR, Kim N, Jung JE, Han SH, Cho HY. Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int J Mol Sci. (2021) 22(18):10122. doi: 10.3390/ijms221810122
43. Wu F, Tian FJ, Lin Y, Xu WM. Oxidative stress: placenta function and dysfunction. Am J Reprod Immunol. (2016) 76:258–71. doi: 10.1111/aji.2016.76.issue-4
44. Wang R, Hartmann MF, Wudy SA. Targeted LC-MS/MS analysis of steroid glucuronides in human urine. J Steroid Biochem Mol Biol. (2021) 205:105774. doi: 10.1016/j.jsbmb.2020.105774
45. Magliocco G, Le Bloc’h F, Thomas A, Desmeules J, Daali Y. Simultaneous determination of melatonin and 6-hydroxymelatonin in human overnight urine by LC-MS/MS. J Chromatogr B Analyt Technol BioMed Life Sci. (2021) 1181:122938. doi: 10.1016/j.jchromb.2021.122938
Keywords: pregnancy loss, untargeted metabolomics, LASSO regression, metabolic signature, diagnosis
Citation: Ding N, Yang X, Wang R and Wang F (2025) Metabolomics profiling identifies diagnostic metabolic signatures for pregnancy loss: a cross-sectional study from northwestern China. Front. Endocrinol. 16:1518043. doi: 10.3389/fendo.2025.1518043
Received: 27 October 2024; Accepted: 24 March 2025;
Published: 10 April 2025.
Edited by:
María Laura Ribeiro, CONICET Centro de Estudios Farmacológicos y Botánicos (CEFYBO), ArgentinaReviewed by:
Elena Sabini, University of Pennsylvania, United StatesJie Cao, Shanxi Medical University, China
Copyright © 2025 Ding, Yang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wang, ZXJ5X2Z3YW5nQGx6dS5lZHUuY24=
 Nan Ding
Nan Ding Xin Yang
Xin Yang Ruifang Wang
Ruifang Wang Fang Wang
Fang Wang