- 1Department of Physical Examination Center, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Neurosurgery, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 3Department of Neurology, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 4Department of Metabolic Regulation, Shinshu University School of Medicine, Matsumoto, Japan
Background: The aging problem is a significant issue and challenge currently faced by the whole world. Hyperhomocysteinemia (HHcy) is a common phenomenon among the older adult. Increasing evidence suggests a link between HHcy and multiple systemic issues in the older adult-related diseases. Therefore, the identification of high-risk factors for HHcy in a healthy screening population can effectively regulate the occurrence, progression of HHcy, thereby reducing the incidence of older adult-related diseases.
Methods: A total of 10,511 individuals who underwent a comprehensive health examination at the Second Hospital of Hebei Medical University, China from 2021 to 2022 were included. Data on gender, age, carotid ultrasound(CCA), blood pressure(BP), body mass index(BMI), serum levels of homocysteine(Hcy), total cholesterol(TC), triglycerides(TG), low-density lipoprotein(LDL-C), high-density lipoprotein(HDL-C), alanine aminotransferase(ALT), aspartate aminotransferase(AST), red blood cells(RBC), creatinine(Cr), urea, uric acid(UA), fasting blood-glucose(FBG), and glycated hemoglobin(GHb) concentration were collected.
Results: Hyperhomocysteinemia was associated with gender, age, BP, CCA, BMI, elevated levels of TC, TG, LDL-C, Cr, urea, UA, as well as decreased levels of HDL-C and RBC. Among these factors male, above 65 years old age, hypertension, carotid artery abnormalities, UA, and Cr were identified as independent risk factors, while HDL-C and RBC were identified as protective factors.
Conclusion: The prevalence of HHcy was very high during routine physical examination especially the senior citizens in Hebei Province, China. Therefore, high-risk populations should be the focus of public health policies, and strengthening early intervention can reduce the occurrence of HHcy, thereby delaying the onset and progression of older adult-related diseases.
1 Introduction
Homocysteine (Hcy), an endogenous amino acid, which can be metabolised enzymatically to either be converted into cysteine or to be remethylated back into methionine (1). Since McCully’s (2) groundbreaking discovery in 1969 highlighted the role of Hcy in altering cases of atherosclerosis, Hcy has emerged as a recognized biomarker and an independent associated with various diseases. Its association with conditions such as coronary artery disease, cardiovascular disease, cerebrovascula disease (3–7), neurocognitive impairment (8, 9), depression, and osteoporotic fractures in the older adult (10, 11) has attracted significant attention from researchers and clinicians. However, to date, the majority of Hcy researches have focused on the correlation between Hcy concentration and diseases, with limited emphasis on epidemiological investigations. Therefore, the prevalence of HHcy in routine health examinations conducted in Hebei, were assessed, with an aim to elucidate the correlation between HHcy and diseases. The associations of Hcy with gender, age, carotid ultrasound, BP, BMI, serum levels of TC, TG, LDL-C, HDL-C, ALT, AST, RBC, Cr, urea, UA, FBG, and GHb concentration were investigated. These findings provide valuable evidence for the prevention and management of HHcy and its associated diseases.
2 Participants
Screen out 10,511 individuals who underwent health check-ups at the Second Hospital of Hebei Medical University from 2021 to 2022 were included in this study. The data collected for these individuals included gender, age,carotid ultrasound, BP,BMI,serum levels of Hcy, TC, TG, LDL-C, HDL-C, ALT, AST, RBC, Cr, urea, UA, FBG, and GHb concentration. Exclusion criteria for individuals affecting Hcy results included recent use of lipid-lowering drugs, folic acid, B-vitamins, and other relevant factors. The study population excluded individuals with severe cardiovascular and cerebrovascular diseases, kidney diseases, thyroid diseases, malignant tumors, as well as those with incomplete information or test results. This study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University. The informed consent to participate was waived by the Ethics Committee of the Second Hospital of Hebei Medical University. All methods were carried out according to relevant guidelines and regulations.
3 Method
3.1 Detection method
Carotid ultrasound results were completed by attending physicians and above, and gender, age, body mass index and blood pressure results were completed by nurses with nursing qualifications. Blood samples were collected in the morning after fasting and were subsequently analyzed for serum red blood cell parameters using a DxH 800 Sysmex blood analyzer. Glycosylated hemoglobin, homocysteine, total cholesterol, triglycerides, low-density lipoprotein, high-density lipoprotein, alanine aminotransferase, aspartate aminotransferase, creatinine, fasting blood glucose, urea, and uric acid were measured using a Cobas 8000 automatic analyzer for biochemical analysis.
3.2 Diagnostic criteria
The following definitions are used in this study. HHcy: Serum Hcy concentration ≥15 μmol/L according to the “Guidelines” China cerebrovascular prevention and treatment. red blood cell concentration: male <3.80 1012/L, female <3.80 1012/L; Blood uric acid concentration: male >428 μmol/L, female >357 μmol/L; Creatinine concentration: Male >97 μmol/L, female >73 μmol/L; Urea concentration: Male >8.0 mmol/L, female >7.5 mmol/L; Dyslipidemia: triglyceride concentration ≥1.70 mmol/L, serum TC concentration ≥6.10 mmol/L, serum LDL-C concentration ≥3.37 mmol/L, serum HDL-C male <1.04 mmol/L; High GLU: fasting GLU concentration >6.0 mmol/L; Hemoglobin A1c >6.0%; Alanine transaminase: male >50U/L, female >40U/L; Aspartate transaminase: male >40U/L, female >35U/L. Carotid artery abnormalities: carotid internal membrane thickening and plaque formation.
3.3 Data analysis
“All statistical analyses were performed using SPSS 26.0 software. Continuous variables were expressed as mean and standard deviation, while categorical variables were presented as frequency and percentage. The independent samples t-test was employed to compare the gender, age,CCA, BP,BMI,serum levels of TC,TG, LDL-C, HDL-C, ALT, AST, RBC, Cr, urea, UA, FBG, and GHb concentration between HHcy group and non-HHcy group. The differences in carotid artery status and blood pressure between the two groups were compared using the chi-square test. The indicators with statistical significance for comparison between groups were selected for stepwise logistic regression analysis to screen for risk factors. Finally, the indicators with statistical significance were the risk factors. A P-value <.05 was considered statistically significant.
3.3.1 Comparison of serum Hcy concentration and prevalence of HHcy in different subjects
The overall prevalence of HHcy among participants was 22% (18.1% in males and 3.9% in females). The detection rate of HHcy in females was lower than that in males with a statistically significant difference (χ2 = 963.174, P <.05). In each age group (35–44 years old, 45–54 years old, 55–64 years old, 65 years old and older), the detection rate of HHcy in males was higher than that in females (P <.05), as shown in Table 1. The plasma Hcy levels all showed a trend of decreasing first and then increasing, Among them, the HHcy detection rate was the highest in the group aged 65 years and above (P <.05), as shown in Figure 1.
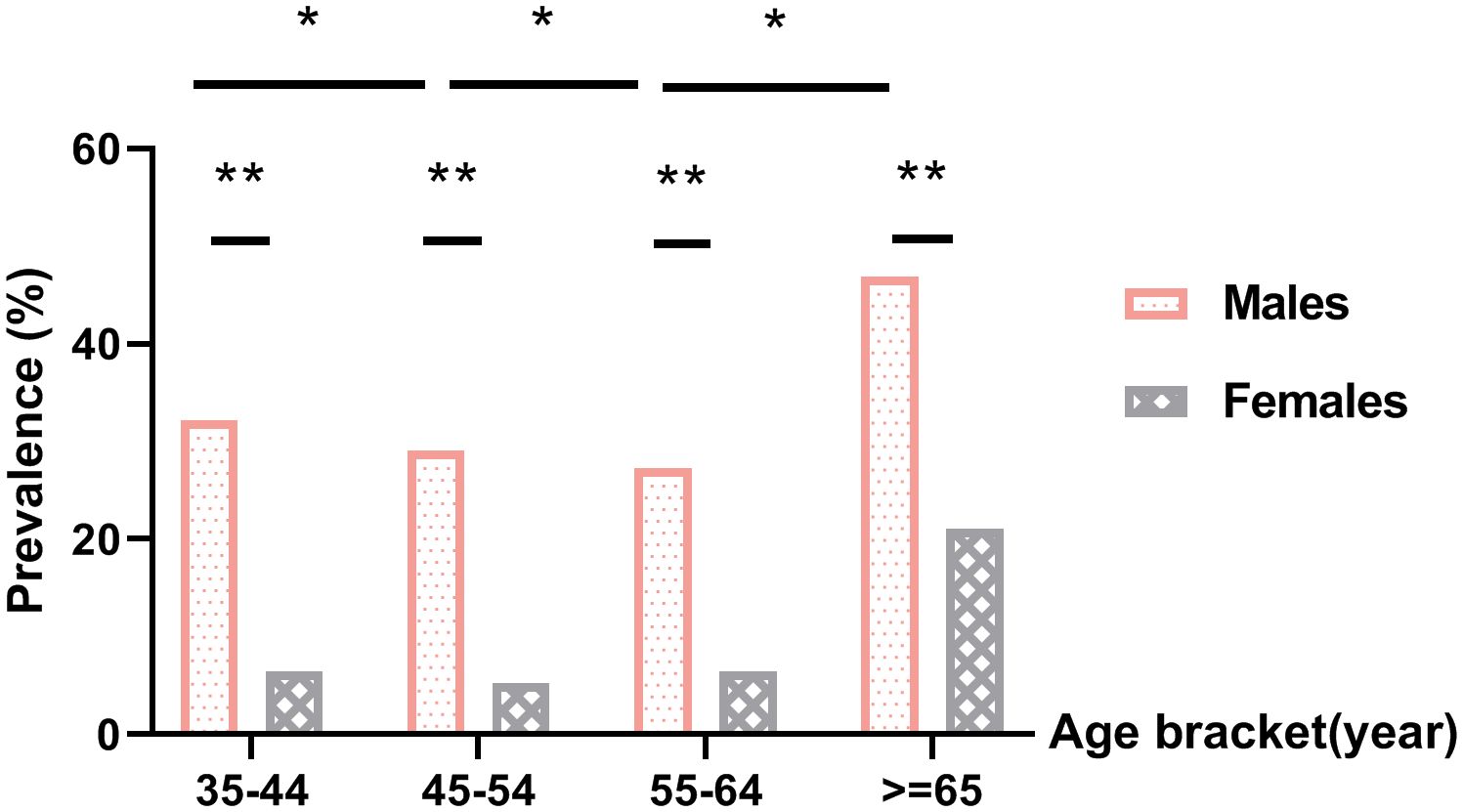
Figure 1. Prevalence of hyperhomocysteinemia. *P<.05 for comparison of HHcy prevalence between different age groups within the same gender, **P<.05 for comparison of HHcy prevalence between males and females in the same age bracket.
3.3.2 Comparison of index mean between HHcy group and non-HHcy group
The CCA, BP, BMI and serum concentrations of UA, urea, Cr, TC, TG, and LDL-C were significantly higher in the HHcy group compared to the non-HHcy group. Conversely, HDL-C and RBC concentrations were significantly lower in the HHcy group than in the non-HHcy group (P<.05). Serum concentrations of FBG, GHb, ALT and AST in HHcy group were not significant compared with those in non-HHcy group, as shown in Table 2.
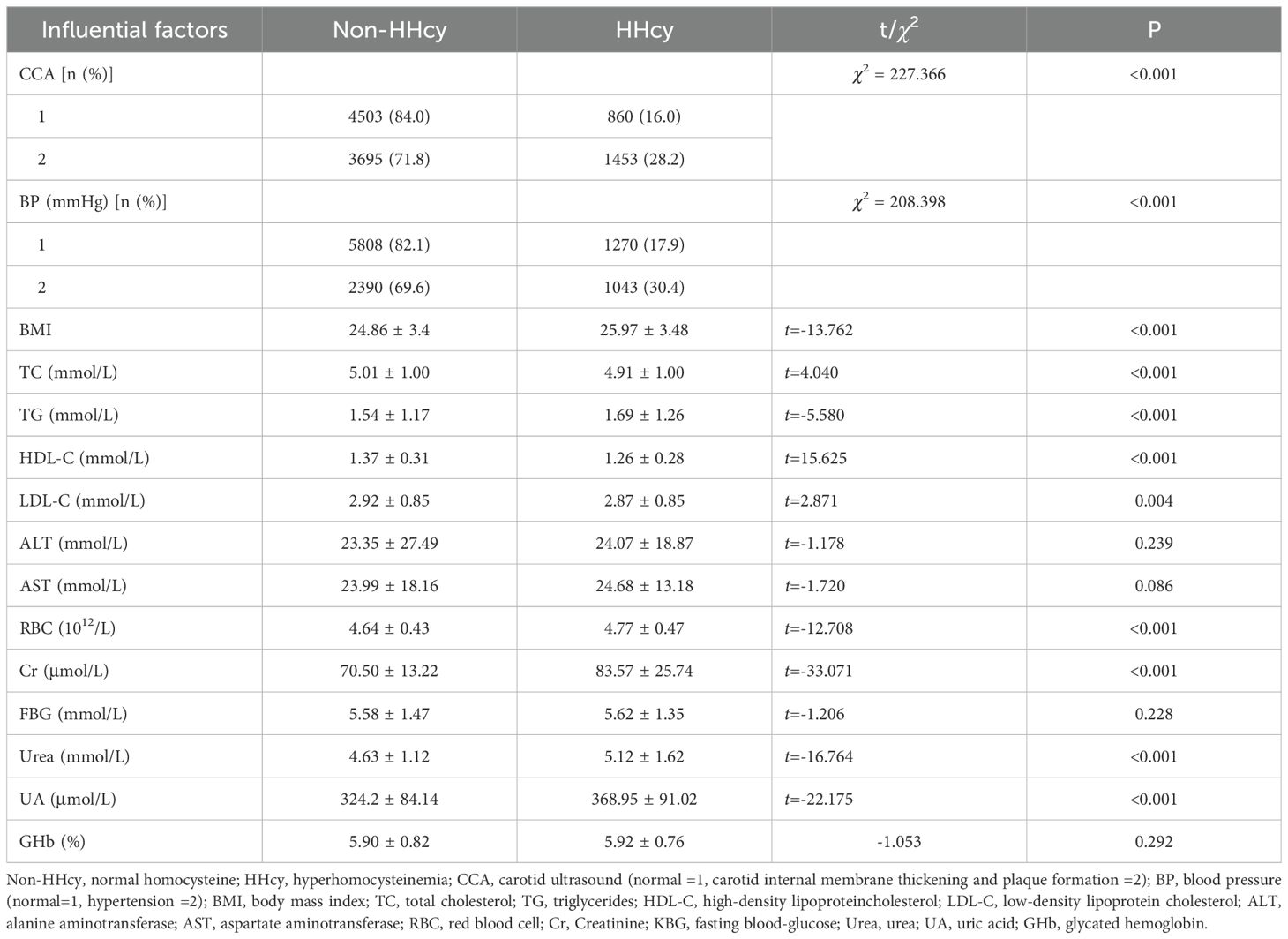
Table 2. Comparisons of CCA, BP, BMI, and serum concentrations of LDL-C, TG, TC, HDL-C, ALT, ASL, RBC, Cr, KFPTT, Ur, UA and GHb between HHcy group with non-HHcy group.
3.3.3 Binary logistic regression analysis of HHcy-related risk factors
The results indicated that male, carotid internal membrane thickening and plaque formation,hypertension and of 65 years old and older age along with elevated UA, Cr levels are risk factors for HHcy; whereas elevated HDL-C levels and RBC count serve as protective factors against HHcy, as shown in Table 3.
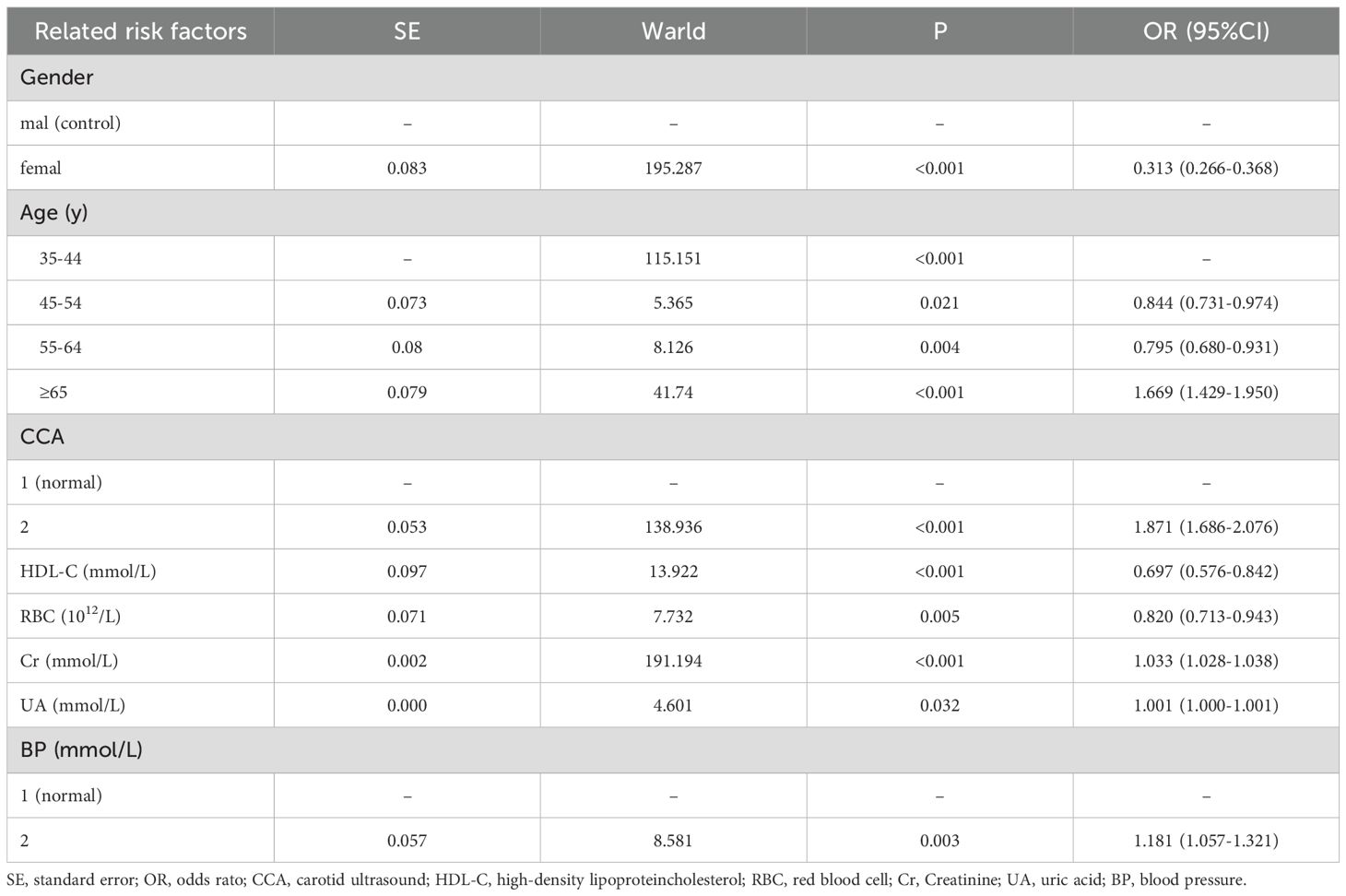
Table 3. Hyperhomocysteinemia related risk factors analyzed by bivariate nonconditional logistic regression model.
4 Discussion
Studies have demonstrated a significant association between Hcy levels and the occurrence of cardiovascular and cerebrovascular diseases, as well as the severity of cognitive impairment. In the Framingham study, there was a positive correlation observed between Hcy levels and both overall mortality and cardiovascular mortality among older adult individuals. Furthermore, a dose-dependent relationship exists between Hcy levels and the development of CSVD, with elevated homocysteine concentrations being linked with cognitive dysfunction (12, 13). That is, for every 3umol/L increase in plasma Hcy, the incidence of ischemic heart disease increases by 11% (14), and for every 2.5umol/L increase in plasma Hcy concentration, the risk of stroke increases by approximately 20% (15). For every 5umol/L increase in Hcy, a patient’s risk of AD increases by 40%, and individuals with Hcy levels higher than 14umol/L are twice as likely to develop AD 8 years later (16). With the aging problem in China, the older adult are facing great threat of aging-related chronic diseases. Therefore, the investigation of Hcy metabolism in Hebei is bound to play a very important role in the prevention and treatment of cardiovascular and cerebrovascular diseases in middle-aged and older adult people.
In this study, a total of 10,511 individuals who participated in the health examination in the Hebei area were included. The overall prevalence of HHcy in the healthy population was found to be 22.0% (18.1% among men and 3.9% among women). Previous reports have indicated that HHcy incidence in China ranges from 10% to 68% (17, 18,) with higher rates observed among older adult individuals and males, which is consistent with our findings. Gender differences may be attributed to variations in muscle mass, estrogen status, lifestyle factors, and vitamin levels (19). Furthermore, it has been established that homocysteine production is associated with creatinine-creatinine synthesis (20). Males generally exhibit a higher muscle mass, leading to an increasing demand for creatine biosynthesis and subsequently resulting in elevated Hcy production (21). Hormonal disparities between genders may also contribute to sex-related differences. Furthermore, compared to females, Chinese men have a higher prevalence of alcohol consumption and smoking habits, both of which are positively correlated with Hcy concentration (22). Alternatively, discrepancies in folate, vitamin B12, and vitamin B6 status between the sexes could partially account for the observed gender difference (19).
Age is a associated with HHcy; Xu (23) et al.’s study confirmed its effect on the prevalence of HHcy while our study demonstrated that men had significantly higher rates of HHcy than women across different age groups.The plasma levels of Hcy exhibited a pattern of initial decline followed by an increase in both males and females. Bivariate unconditional logistic regression analysis revealed that age was a associated with HHcy. This could potentially be attributed to altered renal function, decreased vitamin levels, and impaired renal metabolism of Hcy (24–27).
Furthermore, Hcy has been found to interfere with hepatic lipid metabolism leading to disorders in lipid metabolism. Liao (28) et al.’s study demonstrated that CBS-/-/apoE-/- mice with hyperhomocysteinemia exhibited an increased incidence of atherosclerosis along with reduced HDL levels. In this study, the analysis of the biochemical results revealed that the HHcy group exhibited lower levels of HDL-C compared to the normal group, and a negative correlation was observed between HHcy and HDL-C. Consistent with these findings, each unit increase in HDL-C was associated with a 0.697 times decrease in the risk of HHcy, indicating that HDL-C serves as “good cholesterol” in blood lipids and its reduction is negatively correlated with the risk of atherosclerosis. UA and Cr are crucial indicators for assessing renal function. This study identified UA and Cr as risk factors for HHcy. Malinow et al. (29) proposed that the metabolic product of Hcy was the precursor of UA synthesis. It may be related to the effect of Hcy on renal function and its metabolic pathway and oxidative stress function (30). Xu Shan (31) et al. found that there was a certain correlation between the production and metabolism of plasma Hcy and blood uric acid in the study of the prevalence of hyperuricemia in patients with H-type hypertension. At the same time, because the kidney is also involved in the filtration and metabolism of Hcy, the damage of kidney function will also lead to the increase of Hcy, and there is an interaction between kidney function and Hcy level, which is consistent with the results of this study. Li et al. (32) reported that a high serum UA concentration can acgtivate proinflammatory factors,damage vascular endothelial function,increase oxidative stress,and lead to renal inadequacy.They also reported that Hcy accumulation in the plasma strengthens lipid peroxidation,which further influences kidney function,and Hcy and UA show synergistic effects.
Erythrocytes are the site of homocysteine production. This study found that for each unit increase in red blood cells, the risk of HHcy decreased by 0.820 times, and the mechanism is not clear. Previous research has confirmed a positive correlation between CIMT and plasma Hcy levels (33, 34), with the mechanism involving endothelial injury, oxidative stress, and alterations in lipid metabolism (35). The results of this study are consistent with those findings. Clinical studies have found that the number of individuals with elevated plasma Hcy levels is 2.8 times higher in hypertensive patients compared to the normal population, and high Hcy hyperhomocysteinemia is a associated with hypertension (36). Wilcken’s (37) research also suggests a link between hypertension and gene mutations in MTHFR. In this experiment, a close relationship was found between a history of hypertension and Hcy levels, with both hypertensive cerebrovascular disease patients and the control group exhibiting higher plasma Hcy levels than those without a history of hypertension. Multiple linear regression analysis revealed that hypertension is a associated with HHcy. Simultaneously, a plethora of evidence indicates that hyperhomocysteinemia and hypertension are both crucial and modifiable risk factors for cardiovascular and cerebrovascular events, particularly stroke. The coexistence of these two conditions exponentially increases the risk of cardiovascular and cerebrovascular events. Effectively controlling plasma homocysteine levels in hypertensive individuals may be a pivotal strategy to address the high incidence of stroke.
Li Y, et al.’s study found a close association between HHcy and blood glucose levels as well as transaminase levels (38–41). However, significant differences in blood glucose, glycated hemoglobin, and transaminase between the two population groups have not been detected in this study. This may be due to the uneven distribution in the stratified sampling of subjects or the insignificant impact of blood glucose, glycated hemoglobin, and transaminase on Hcy levels.
5 Conclusion
In summary, the prevalence of HHcy was high in Hebei province, China. Male, above 65 years old age, hypertension, carotid artery abnormalities, elevated Cr, UA, decreased HDL-C and RBC constitute high-risk factors for HHcy. Adopting a rational lifestyle, timely blood pressure reduction and weight management, and implementing effective intervention measures can contribute to lowering HCY levels and thereby reducing the incidence of older adult-related diseases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Second Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The research data were primarily acquired from the Physical Examination Center database of the Second Hospital of Hebei Medical University, with supplementary data collected through telephone follow-ups, all conducted under institutional ethical approval. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HF: Writing – original draft, Writing – review & editing. Xw: Writing – original draft, Writing – review & editing. LY: Data curation, Writing – review & editing. QZ: Writing – original draft. ZW: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Health commission of Hebei province. (No. 20230631).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Refsum H, Ueland PM, Nygard O, and Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. (1998) 49:31–62. doi: 10.1146/annurev.med.49.1.31
2. Kuo HK, Sorond FA, Chen JH, Hashmi A, Milberg WP, and Lipsitz LA. The role of homocysteine in multisystem age-related problems: a systematic review. J Gerontol A Biol Sci Med Sci. (2005) 60:1190–201. doi: 10.1093/gerona/60.9.1190
3. Temple ME, Luzier AB, and Kazierad DJ. Homocysteine as a risk factor for atherosclerosis. Ann Pharmacother. (2000) 34:57–65. doi: 10.1345/aph.18457
4. Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, UPson B, Ullmann D, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. (1992) 268:877–81. doi: 10.1001/jama.1992.03490070059042
5. Bostom AG, Rosenberg IH, Silbershatz H, Jacques PF, Selhub J, D'Agostino RB, et al. Nonfasting plasma total homocysteine levels and stroke incidence in older adult persons: the Framingham Study. Ann Intern Med. (1999) 131:352–5. doi: 10.7326/0003-4819-131-5-199909070-00006
6. Nygård O, Vollset SE, Refum H, Stensvold I, Tverdal A, Nordrehaug JE, et al. Total plasma homocysteine and cardiovascular risk profle. The Hordaland Homocysteine Study. JAMA. (1995) 274:1526–33. doi: 10.1001/jama.1995.03530190040032
7. Ganguly P and Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. (2015) 14:6. doi: 10.1186/1475-2891-14-6
8. Hainsworth AH, Yeo NE, Weekman EM, and Wilcock DM. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta. (2016) 1862:1008–17. doi: 10.1016/j.bbadis.2015.11.015
9. Falasca K, Nicola MD, Martino GD, Ucciferri C, Vignale F, Occhionero A, et al. The impact of homocysteine, B12, and D vitamins levels on functional neurocognitive performance in HIV-positive subjects. BMC Infect Dis. (2019) 19:105. doi: 10.1186/s12879-019-3742-8
10. van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, der Klift M, der Jonge R, Lindemans J, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. (2004) 350:2033–41. doi: 10.1056/NEJMoa032546
11. McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, et al. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. (2004) 350:2042–9. doi: 10.1056/NEJMoa032739
12. Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, and Jeong SM. Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology. (2019) 92:e317–25. doi: 10.1212/WNL.0000000000006816
13. Yoo JS, Ryu CH, Kim YS, Kim HJ, Bushnell CH, and Kim HY. Homocysteinemia is associated with the presence of microbleeds in cognitively impaired patients. J Stroke Cerebrovasc Dis. (2020) 29:105302. doi: 10.1016/j.jstrokecerebrovasdis.2020.105302
14. Stehouwer CD and van Guldener C. Does homocysteine cause hypertension? Clin Chem Lab Med. (2003) 41:1408–11. doi: 10.1515/CCLM.2003.216
15. Clarke R, Collins R, Lewington S, Dphil , Donald A, Alfthan G, et al. Homocysteine Studies Collaboration: Homocysteine and risk ischemic heart disease and stroke: a meta-analysis. JAMA. (2002) 288:2015–22. doi: 10.1001/jama.288.16.2015
16. Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer,s disease. N Engl J Med. (2002) 346:476–83. doi: 10.1056/NEJMoa011613
17. Hao L, Ma J, Zhu J, Stampfer MJ, Tian YH, Willett WC, et al. High prevalence of hyperhomocysteinemia in Chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J Nutr. (2007) 137:407–13. doi: 10.1093/jn/137.2.407
18. Liu XD, Gao B, Sun D, Shi M, Ma YY, Liu ZR, et al. Prevalence of hyperhomocysteinaemia and some of its major determinants in Shaanxi Province, China: a cross-sectional study. Br J Nutr. (2015) 113:691–8. doi: 10.1017/S0007114514004218
19. Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin Chem. (2004) 50:3–32. doi: 10.1373/clinchem.2003.021634
20. Brattstrom L, Lindgren A, Israelsson B, Andersson A, and Hultberg B. Homocysteine and cysteine: Determinants of plasma levels in middle-aged and older adult subjects. J Intern Med. (1994) 236:633–41.
21. Rauh M, Verwied S, Knerr I, Dorr HG, Sonnichsen A, and Koletzko B. Homocysteine concentrations in a German cohort of 500 individuals: Reference ranges and determinants of plasma levels in healthy children and their parents. Amino Acids. (2001) 20:409–18. doi: 10.1007/s007260170037
22. Hao L, Ma J, Stampfer MJ, Ren A, Tian Y, Willett WC, et al. Geographical, seasonal and gender differences in folate status among Chinese adults. J Nutr. (2003) 133:3630–5. doi: 10.1093/jn/133.11.3630
23. Xu R, Huang F, Wang Y, Liu Q, Lv Y, and Zhang Q. Gender- and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China. Sci Rep. (2020) 10:17401. doi: 10.1038/s41598-020-74596-7
24. Challa F, Getahun T, Sileshi M, Nigassie B, Geto Z, Ashibire G, et al. Prevalence of hyperhomocysteinaemia and associated factors among Ethiopian adult population in a 2015 national survey. BioMed Res Int. (2020) 2020:9210261. doi: 10.1155/2020/9210261
25. Jung S, Je Y, Giovannucci E, Rosner B, Ogino Sh, and Cho E. Derivation and validation of homocysteine score in U.S. Men and women. J Nutr. (2015) 145:96–104. doi: 10.3945/jn.114.192716
26. Nurk E, Tell GS, Vollset SE, Nygård O, Refsum H, Nilsen RM, et al. Changes in lifestyle and plasma total homocysteine: The Hordaland Homocysteine Study. Am J Clin Nutr. (2004) 79:812–9. doi: 10.1093/ajcn/79.5.812
27. Wang Y, Li X, Qin X, Cai YF, He ML, Sun LM, et al. Prevalence of hyperhomocysteinaemia and its major determinants in rural Chinese hypertensive patients aged 45–75 years. Br J Nutr. (2013) 109:1284–93. doi: 10.1017/S0007114512003157
28. Liao D, Tan HM, Hui RT, Li ZH, Jiang XH, Gaubatz J, et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I protein synthesis and enhancing HDL cholesterol clearance. Circ Res. (2006) 99:598–606. doi: 10.1161/01.RES.0000242559.42077.22
29. Malinow MR, Levenson J, Giral P, Nieto FJ, Razavian M, Segond P, et al. Role of blood pressure, uric acid,and hemorheological parameters on plasma homocyst(e)ine concentration. Atherosclerosis. (1995) 114:175–83. doi: 10.1016/0021-9150(94)05481-W
30. Zhang Y and Hong XQ. Analysis of the status and influencing factors of hyperhomocysteinemia in community population aged ≥30 years in Hunan Province. China Public Health. (2019) 25:391–7. doi: 10.11847/zgggws1119196
31. Xu S, Wang CY, Zhang CH, Liu SY, Peng XL, Chen ZW, et al. Comparison of the prevalence of hyperuricemia in patients with essential hypertension, hypertension with hyperhomocysteinemia and healthy people. South China Prevent Med. (2014) 40:563–5. doi: 10.13217/j.scjpm.2014.0563
32. Li Y, Shen J, Shen YL, and Zhang AQ. Significance of hyperhomocysteinemia in patients with coronary heart disesse. zhongguo YIke Daxue xuebao. (2014) 10:885–8. doi: 10.3969/j.issn.0258-4646.2014.10.005
33. Kjeldsen SE, Julius S, Hedner T, and Hansson L. Stroke is more common than myocardial infarction in hypertension:analysis based on 11 major randomized intervention trials. Blood Press. (2001) 10:190–2. doi: 10.1080/08037050152669684
34. Hu DY and Xu XP. Effective control of “H” type hypertension-a new idea for stroke prevention. China Internal Med. (2008) 47:976–7. doi: 10.3321/j.issn:0578-1426.2008.12.005
35. Homocysteine Lowering Trialsts’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine:a meta-analysis of the randomized trials. Am J Clin Nutr. (2005) 82:806–12. doi: 10.1093/ajcn/82.4.806
36. Medis S, Athauda SB, Naser M, and Takahashi K. Association between hyperhomocysteinemia and hypertension in Sri Lankans. J Int Med Res. (1999) 27:38–44. doi: 10.1177/030006059902700105
37. Wilcken DE, Wang XL, Sim AS, and McCredie RM. Distribution in healthy and coronary population of the methylenetrahydrofolate reductase (MTHFR) C677T mutation. Arterioscler Thromb Vasc Biol. (1996) 16:878–82. doi: 10.1161/01.ATV.16.7.878
38. Kim HC, Kang DR, Nam CM, Hur NW, Shim JS, Jee SH, et al. Elevated serum aminotransferase level as a predictor of intracerebral hemorrhage: Korea medical insurance corporation study. Stroke. (2005) 36:1642–7. doi: 10.1161/01.STR.0000173404.37692.9b
39. Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer C, Heine RJ, et al. Alanine aminotransferase predicts coronary heart disease events:a 10-year follow-up of the Hoorn Study. Atherosclerosis. (2007) 191:391–6. doi: 10.1016/j.atherosclerosis.2006.04.006
40. Han QW, Fang B, and Chen YF. Effects of Metformin on plasma homocysteine level in patients with diabetes mellitus. Zhong guo Dong mai Ying hua Za zhi. (2005) 13:636–8. doi: 10.3969/j.issn.1007-3949.2005.05.029
Keywords: red blood cells, hyperhomocysteinemia, high-density lipoprotein, healthy population, risk factor
Citation: Feng H, Wang X, Yu L, Zheng Q and Wang Z (2025) Study on the relationship between homocysteine and general metabolic indexes in healthy population in Hebei Province, China. Front. Endocrinol. 16:1523157. doi: 10.3389/fendo.2025.1523157
Received: 18 November 2024; Accepted: 23 July 2025;
Published: 13 August 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Pranay Goel, Indian Institute of Science Education and Research, IndiaKhadijeh Irandoust, Imam Khomeini International University, Iran
Carlo De Matteis, University of Bari Aldo Moro, Italy
Copyright © 2025 Feng, Wang, Yu, Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongru Feng, ZmVuZ2hvbmdydTIwMjRAMTYzLmNvbQ==
 Hongru Feng
Hongru Feng Xiaoliang Wang2
Xiaoliang Wang2 Lili Yu
Lili Yu