- Department of Reproductive Medicine, Yellow River Sanmenxia Affiliated Hospital of Henan University of Science and Technology, Sanmenxia, China
Objective: Ovarian stimulation protocols play a pivotal role in the success of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) treatments. This study compares the clinical outcomes of the long luteal phase GnRH agonist protocol and the flexible GnRH antagonist protocol in patients with normal ovarian reserve.
Methods: This prospective cohort study was conducted at the Reproductive Medicine Center, Sanmenxia Hospital, Yellow River, from March 2021 to September 2023. Patients with normal ovarian reserve were enrolled and randomly assigned by a 1:3 ratio to either the long luteal phase protocol (Group A, n=42) or the flexible antagonist protocol (Group B, n=118). Data on patient characteristics, ovarian response, and embryological outcomes were collected and analyzed. Clinical outcomes, including clinical pregnancy, live birth rates, and ovarian hyperstimulation syndrome (OHSS) incidence, were assessed. Multivariate logistic regression was conducted to identify risk factors associated with clinical pregnancy.
Results: There were no significant differences in baseline characteristics between the two groups (P>0.05). In terms of primary clinical outcomes, there were no significant differences in clinical pregnancy rate (54.8% vs. 56.8%, P=0.092), live birth rate (47.6% vs. 52.5%, P=0.278), or incidence of OHSS (0% vs. 2.5%, P=0.055) between Group A and Group B. Multivariable logistic regression analysis identified significant predictors of clinical pregnancy, including younger age (OR = 0.956, P = 0.042), higher AFC (OR = 1.127, P = 0.018), higher AMH levels (OR = 1.357, P = 0.005), greater endometrial thickness (OR = 1.162, P = 0.021), higher number of oocytes retrieved (OR = 1.234, P = 0.023), and better embryo quality (Grade I-II) (OR = 1.485, P = 0.002). No significant differences were observed between age-related subgroups (P>0.05), but success rates decreased with increasing age, highlighting age as a key factor influencing IVF/ICSI outcomes.
Conclusion: The study found no significant differences in primary clinical outcomes between the two groups. However, younger age, higher AFC, higher AMH levels, greater endometrial thickness, higher number of oocytes retrieved, and better embryo quality were significant predictors of clinical pregnancy.
Introduction
Infertility is a common and distressing condition affecting approximately 10-15% of reproductive-aged couples worldwide. Assisted reproductive technologies (ART), particularly in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), have revolutionized the management of infertility. Central to the success of IVF/ICSI is controlled ovarian hyperstimulation (COH), which aims to recruit multiple follicles to maximize the number of mature oocytes for fertilization (1). Optimal ovarian stimulation protocols are particularly crucial for achieving successful pregnancy outcomes, especially in patients with normal ovarian reserve, who typically have a favorable response to stimulation protocols (1, 2). However, the selection of the most appropriate stimulation protocol for this patient population remains a subject of ongoing debate.
The long luteal phase gonadotropin-releasing hormone agonist (GnRH-a) protocol has long been regarded as the standard approach for ovarian stimulation. This protocol involves the administration of a GnRH-a during the luteal phase of the preceding cycle to downregulate the hypothalamic-pituitary-ovarian axis, suppressing premature luteinizing hormone (LH) surges and allowing for more controlled follicular development. Ovarian stimulation is initiated after sufficient downregulation has been achieved, usually after 14 days of GnRH-a administration (3). Although this protocol has been associated with favorable clinical outcomes, including higher pregnancy and live birth rates, its extended duration and the potential for side effects such as ovarian hyperstimulation syndrome (OHSS) and flare-ups during downregulation pose challenges for some patients (4, 5). In contrast, the flexible GnRH antagonist protocol, developed as an alternative to the traditional GnRH-a protocol, offers a shorter and more patient-friendly approach. This protocol involves the administration of a GnRH antagonist during the follicular phase when the leading follicle reaches a diameter of 12–14 mm, effectively suppressing premature LH surges without the need for extended downregulation. The shorter duration of treatment and reduced risk of OHSS make the antagonist protocol an attractive option for both patients and clinicians (6). Additionally, the antagonist protocol has demonstrated comparable pregnancy outcomes to the agonist protocol in several studies (7, 8). However, concerns remain regarding its effectiveness in specific patient populations, such as those with normal ovarian reserve, where a more aggressive stimulation approach may be beneficial.
Patients with normal ovarian reserve, characterized by adequate antral follicle counts (AFC) and anti-Müllerian hormone (AMH) levels, typically respond well to ovarian stimulation. However, the optimal stimulation protocol for this population remains contentious. While the long luteal phase protocol may offer advantages in terms of controlled follicular development and a more predictable response, it is associated with higher risks of OHSS due to the recruitment of a larger number of follicles. The flexible antagonist protocol, on the other hand, may reduce the risk of OHSS but could lead to suboptimal ovarian response and lower pregnancy rates in some patients (9). As such, there is a need for robust comparative studies to determine which protocol is more effective in achieving successful pregnancy outcomes while minimizing adverse effects in patients with normal ovarian reserve. Recent studies have begun to shed light on this issue (10–13). This study aims to address these gaps by conducting a comprehensive comparison of clinical outcomes between the flexible antagonist protocol and the long luteal phase protocol in patients with normal ovarian reserve. Specifically, we seek to evaluate clinical pregnancy rates, live birth rates, and the incidence of OHSS, as well as secondary outcomes such as embryo quality, fertilization rates, and the number of oocytes retrieved.
Patients and methods
Study design
This prospective cohort study was conducted between March 2021 and September 2023 at the Reproductive Medicine Center, Sanmenxia Hospital, Yellow River. The study was complied with the guidelines of the Declaration of Helsinki and relevant national regulations. The study protocol has been reviewed and approved by the hospital’s institutional ethics committee (No: 2024LLS20240914043), and all participants have provided written informed consent.
Population
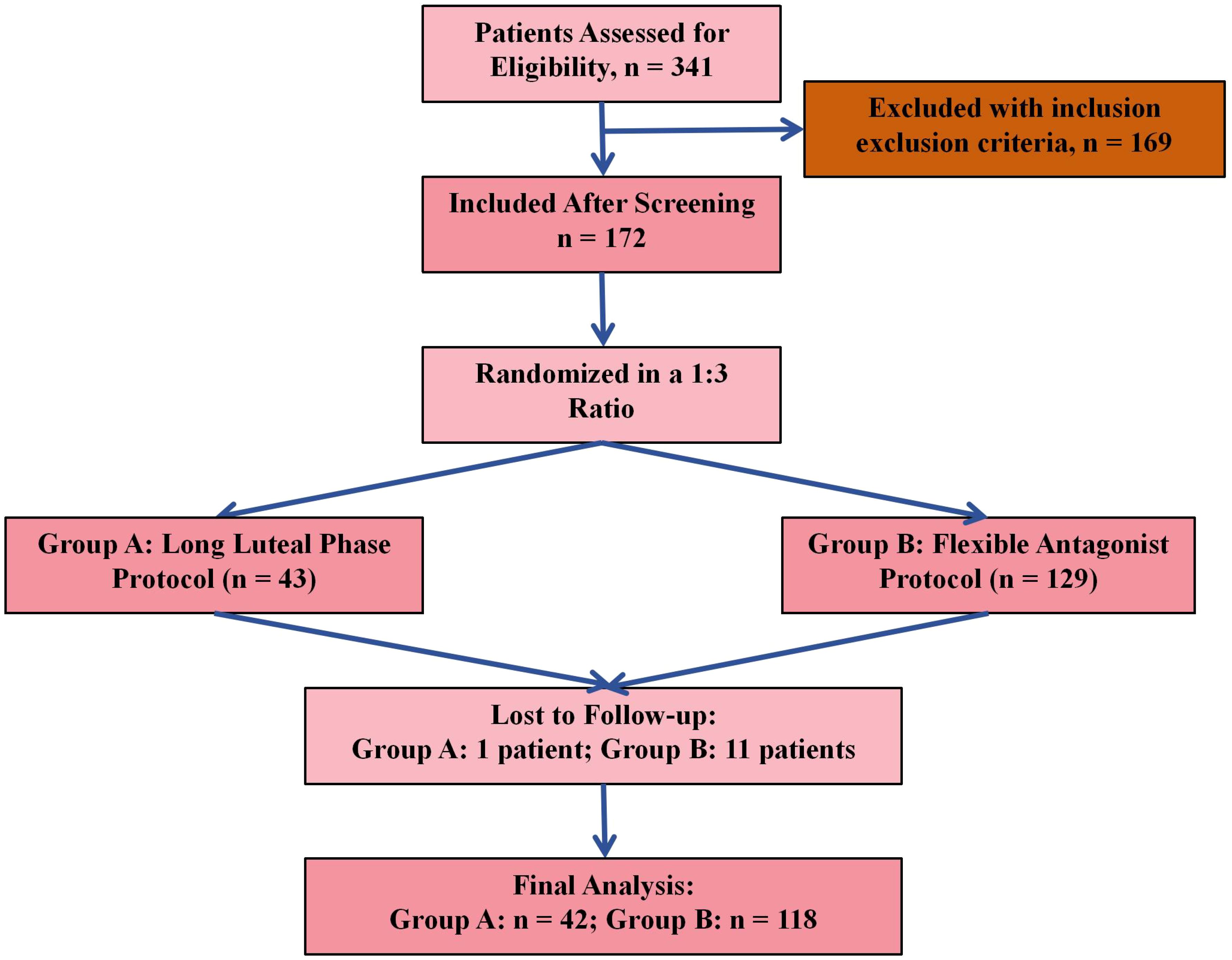
A total of 341 patients were initially assessed for eligibility. Following the application of inclusion and exclusion criteria, 172 patients met the eligibility requirements and were included in the study. Participants were then allocated into two groups using a 1:3 ratio: 43 patients were assigned to Group A (Long Luteal Phase Protocol) and 129 to Group B (Flexible Antagonist Protocol). During follow-up, 1 patient in Group A and 11 patients in Group B were lost to follow-up. As a result, 42 patients in Group A and 118 patients in Group B were included in the final analysis (Figure 1). The 1:3 allocation ratio was based on the proportional use of protocols in clinical practice at our center during the study period. The flexible antagonist protocol (Group B) was more commonly utilized due to its shorter duration and lower risk of OHSS, whereas the long agonist protocol (Group A) was reserved for specific cases.
Inclusion Criteria were as follow: (1)Women included in this study were aged less than 40 years, with normal menstrual cycles (25–35 days) in the preceding three months, and AFC ranging from 8 to 15. (2)Baseline serum (FSH levels were ≤10 U/L, and AMH levels were >1.1 ng/mL. (3)All patients met the established criteria for IVF/ICSI treatment. Exclusion Criteria were as follow: (1) presence of chromosomal abnormalities in either partner, uterine abnormalities (e.g., unicornuate uterus, bicornuate uterus, or septate uterus), (2) any contraindications for IVF-embryo transfer (IVF-ET).
Data collection
Data collection was meticulously carried out to record both biochemical and clinical outcomes following embryo transfer, including key demographic, clinical, and outcome variables. Baseline variables included patient age, body mass index (BMI), duration of infertility, AFC, AMH levels, and baseline serum FSH. During ovarian stimulation, data on gonadotropin dose, stimulation duration, endometrial thickness, estradiol and progesterone levels on trigger day, and the number of follicles ≥18 mm were recorded. Embryology-related variables included the number of oocytes retrieved, mature oocyte count, fertilization rate, and embryo quality on day 3.
Treatment protocols
Long Luteal Phase Protocol: this protocol involved the initiation of a GnRH agonist in the mid-luteal phase of the preceding menstrual cycle to achieve downregulation of the hypothalamic-pituitary-ovarian axis. After 14 days of downregulation, COH was commenced using recombinant human FSH (rFSH) or urinary-derived gonadotropins. The gonadotropin dose was adjusted based on patient age, body weight, and AFC. Ovulation was triggered with human chorionic gonadotropin (hCG) once at least three follicles reached a diameter of ≥18 mm, and oocytes were retrieved 36 hours after the trigger.
Flexible Antagonist Protocol: this protocol began with COH initiated on day 2 or 3 of the menstrual cycle. The starting dose of gonadotropins was individualized according to ovarian response as assessed by transvaginal ultrasound (B-ultrasound) and serum estradiol (E2) levels. When the leading follicle reached 12 mm in diameter, a GnRH antagonist was administered to prevent premature LH surges. Ovulation was triggered using a dual trigger (hCG combined with a GnRH agonist) once two or more follicles reached ≥18 mm, and oocyte retrieval occurred 36 hours post-trigger.
Embryo assessment and transfer
Embryos were assessed on day 3 post-fertilization according to standard morphologic criteria. Grading was based on the number of blastomeres, degree of fragmentation, and uniformity of blastomere size, with grades I and II considered high-quality embryos. Embryos of higher quality were prioritized for transfer. In cases of a high risk for OHSS— characterized by serum E2 levels exceeding 4000 pg/mL or retrieval of more than 15 oocytes— a freeze-all strategy was employed. This involved cryopreservation of all viable embryos and postponement of the embryo transfer to a subsequent frozen embryo transfer (FET) cycle to minimize the risk of OHSS.
Study outcome
Patients with confirmed clinical pregnancies were followed at regular intervals to ensure continued pregnancy viability and to monitor for complications. The first follow-up was conducted at 12 weeks of gestation, focusing on the viability of the pregnancy and the assessment of early fetal development. A second follow-up was carried out at 24 weeks of gestation to further assess fetal growth and maternal health. Finally, all patients were followed up 2 weeks postpartum to document delivery outcomes, including birth weight, gestational age at delivery, and any neonatal complications.
The primary outcomes included: (1) Clinical pregnancy rate: defined as the number of clinical pregnancy cycles per number of transfer cycles × 100%. Clinical pregnancy was confirmed by the presence of at least one gestational sac with a fetal heartbeat detected via transvaginal ultrasound at 6–8 weeks of gestation. This calculation includes both fresh embryo transfer cycles and the first frozen embryo transfer (FET) cycle for patients undergoing a freeze-all strategy, with each patient contributing one transfer cycle. (2) Live birth rate: defined as the number of live births per number of transfer cycles × 100%. A live birth is defined as the delivery of at least one living infant at or beyond 24 weeks of gestation. This calculation includes both fresh embryo transfer cycles and the first FET cycle for patients undergoing a freeze-all strategy, with each patient contributing one transfer cycle, representing a single-cycle liver birth rate. (3) Incidence of moderate to severe OHSS: classified based on standard criteria including symptom severity, ovarian enlargement, and biochemical markers.
The secondary outcomes included: (1) Oocyte metrics: total number of oocytes retrieved, mature oocyte rate (number of mature oocytes per total oocytes retrieved), and fertilization rate. (2) Blastocyst formation rate: The percentage of embryos progressing to the blastocyst stage. (3) Embryo quality: proportion of high-quality embryos (grades I and II). (4) Implantation rate: the number of gestational sacs per number of embryos transferred × 100%. (5) Miscarriage rates: early miscarriage rate (spontaneous loss of pregnancy within 12 weeks) and overall miscarriage rate (spontaneous loss of pregnancy at any gestational age).
Sample size calculation
The sample size was calculated by Pass 11.0 and aimed to detect a 15% difference in clinical pregnancy rate (50% for the long luteal phase protocol vs. 35% for the flexible antagonist protocol) based on prior studies (14, 15). Using a two-proportion z-test with 80% power, a two-sided α = 0.05, and a 1:3 allocation ratio, 144 patients (36 in Group A and 108 in Group B) were required. Accounting for a 10% dropout rate, the target enrollment was increased to 160 patients (40 in Group A and 120 in Group B).
Statistical analysis
Continuous variables were described as mean ± standard deviation (SD) for normally distributed data. For data that were not normally distributed, variables were expressed as the median and interquartile range (IQR). Comparisons between the two groups were conducted using independent t-tests for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables were expressed as percentages or proportions. Comparisons between groups were made using chi-square tests or Fisher’s exact test.
Multivariable logistic regression analysis was conducted to identify independent predictors of clinical pregnancy, adjusting for potential confounders. Variables included in the model (age, BMI, AFC, AMH, gonadotropin dose, number of oocytes retrieved, and embryo quality [Grade I-II]) were selected a priori based on their established clinical relevance to IVF/ICSI outcomes, and their potential associations in univariate analyses (P < 0.10). The grouping factor (long luteal phase vs. flexible antagonist protocol) was excluded from the model because the primary study objective was to compare protocol outcomes directly, and the exploratory analyses confirmed that the protocol type was not a significant predictor (P > 0.05). Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were reported.
A subgroup analysis by age (>20–25, 30–35, and 35–<40 years) was performed to assess clinical pregnancy and live birth rates within each age stratum, comparing Group A and Group B using chi-square tests or Fisher’s exact test. All statistical analyses were performed using the latest version of SPSS (IBM, Chicago, USA). A two-tailed P value of less than 0.05 was considered statistically significant.
Results
Comparison of basic characteristics
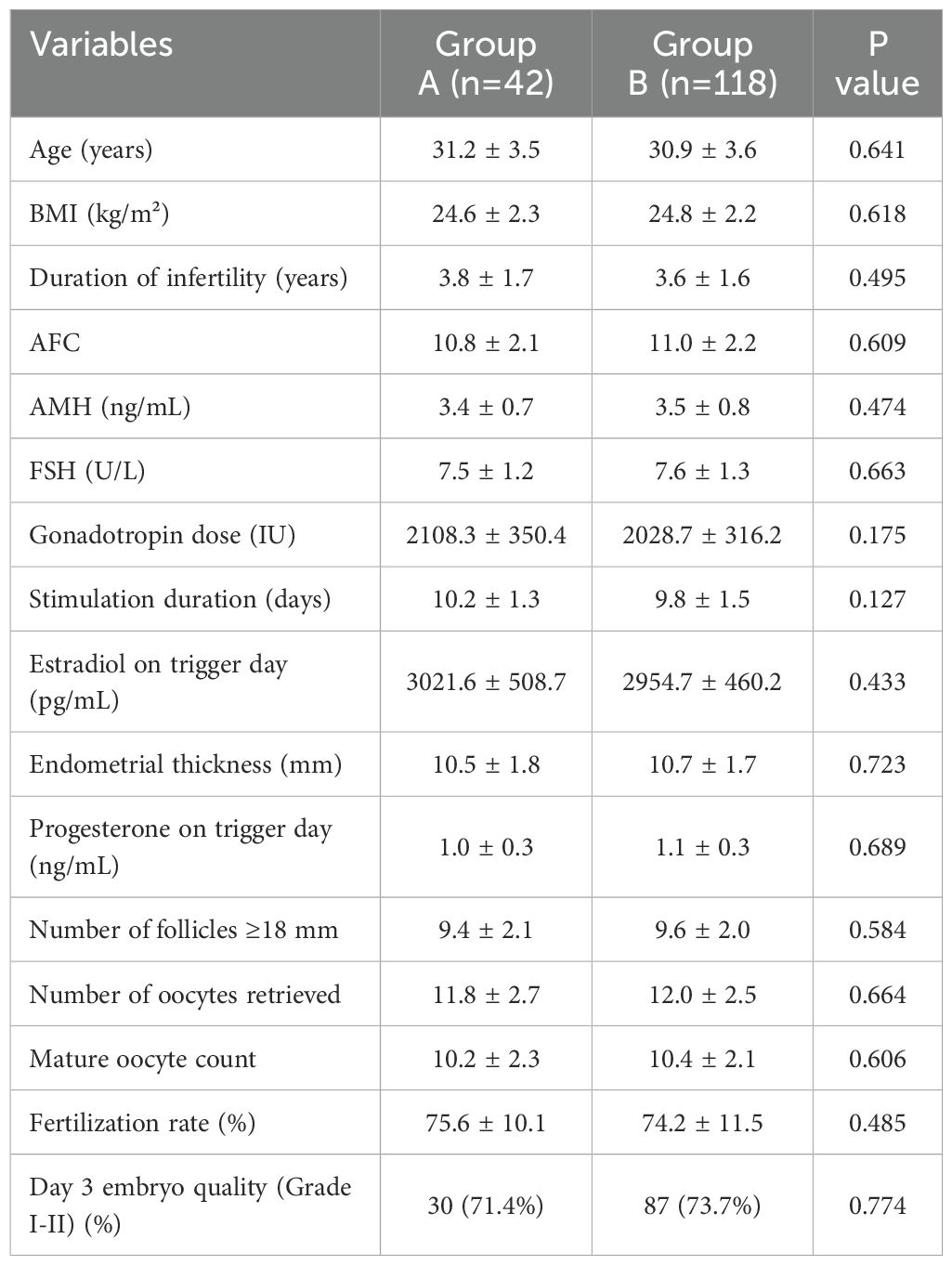
Table 1 showed no significant differences in baseline characteristics between Group A and Group B. Age, BMI, duration of infertility, AFC, AMH, FSH levels, gonadotropin dose, stimulation duration, number of follicles ≥18 mm, oocytes retrieved, mature oocyte count, fertilization rates, and day 3 embryo quality were comparable across both groups (P>0.05).
Comparison of clinical outcomes
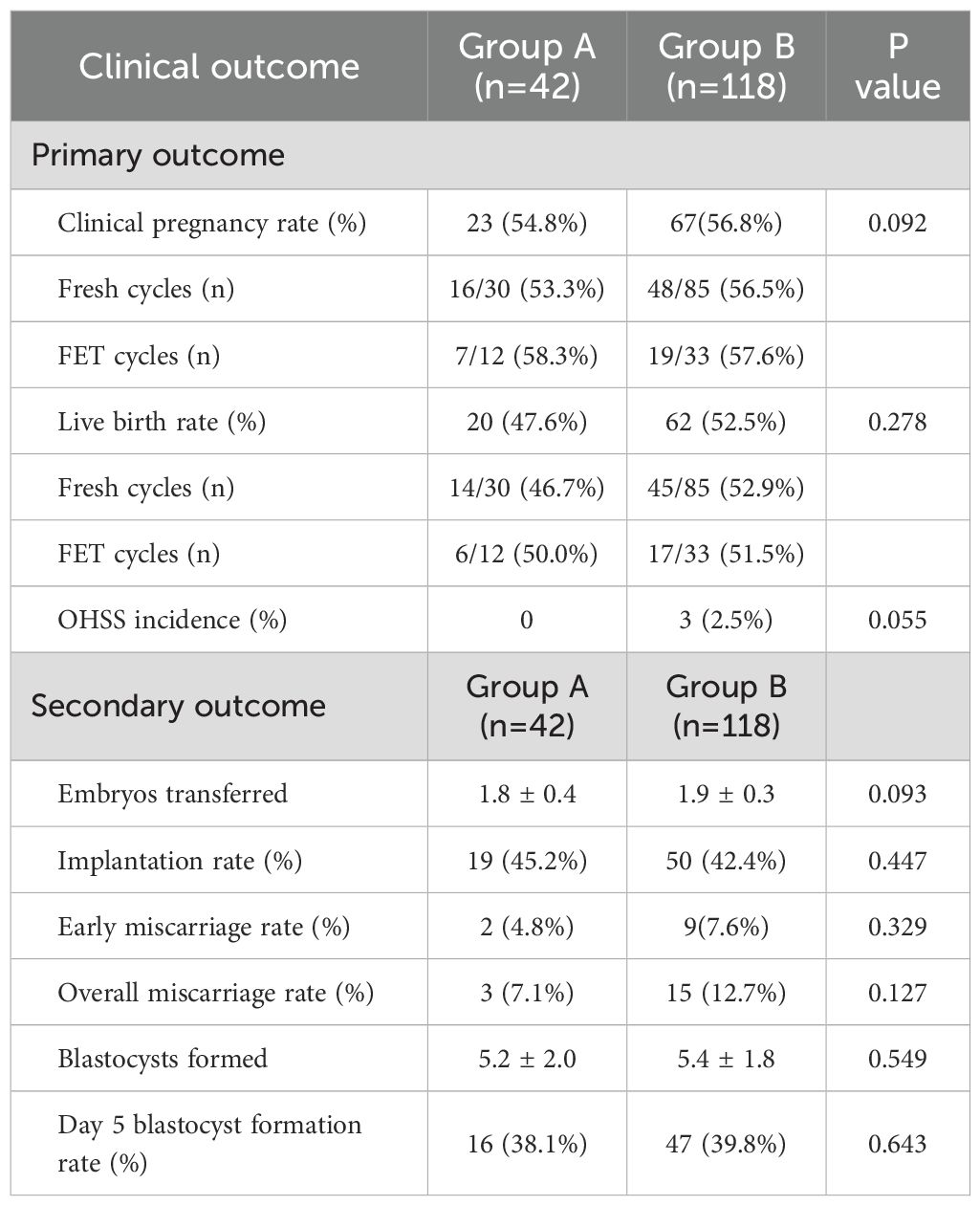
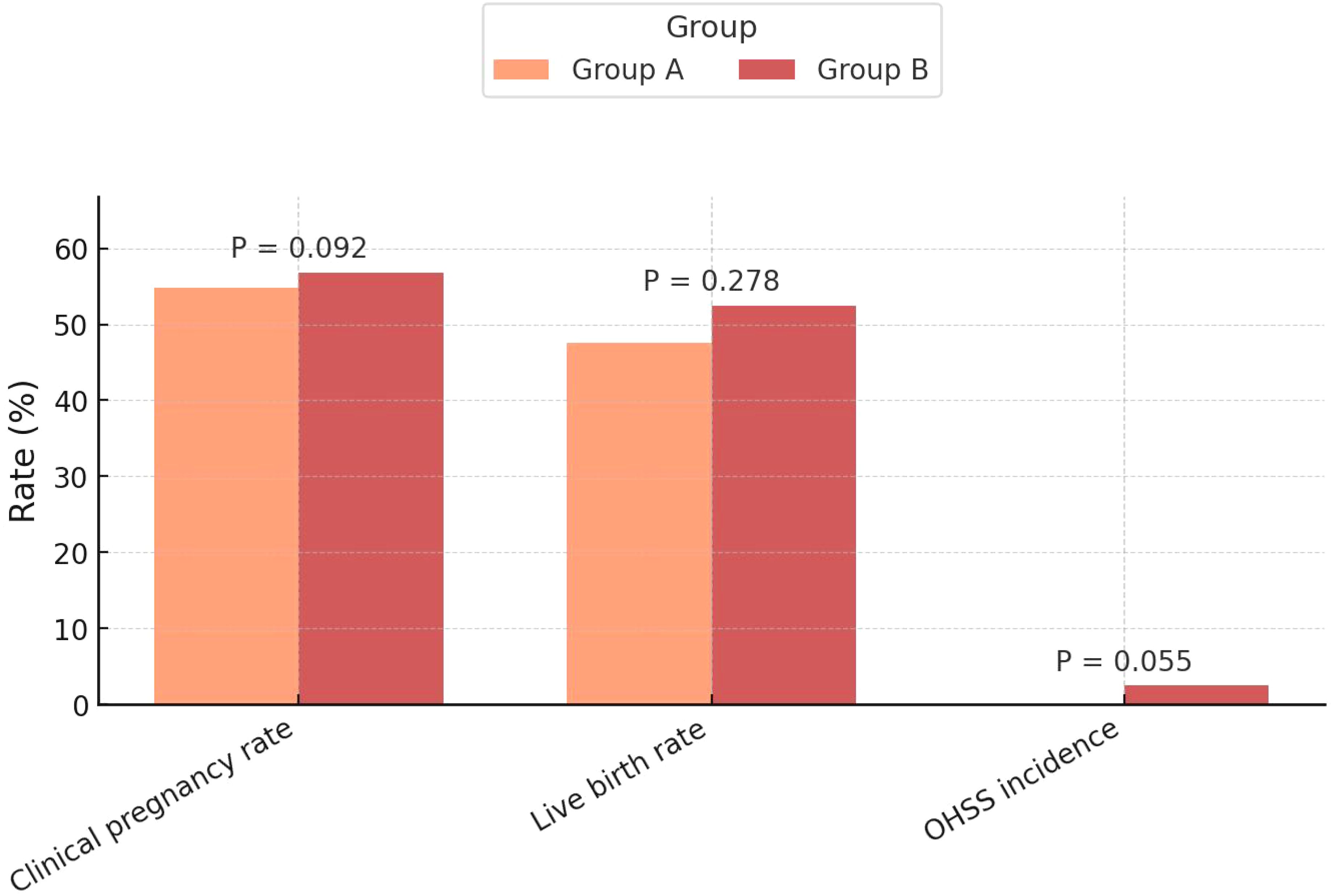
The clinical pregnancy rate was similar between groups (54.8% vs. 56.8%, P=0.092), with fresh cycle rates of 53.3% vs. 56.5% and FET cycle rates of 58.3% vs. 57.6%. Likewise, the live birth rate showed no significant difference (47.6% vs. 52.5%, P=0.278), with fresh cycle rates of 46.7% vs. 52.9% and FET cycle rates of 50.0% vs. 51.5%. These findings indicate comparable efficacy of the two protocols in achieving clinical pregnancy and live births. Additionally, the incidence of OHSS was low, with no cases in Group A and 2.5% in Group B, but this difference did not reach statistical significance (P=0.055) (Table 2, Figure 2).
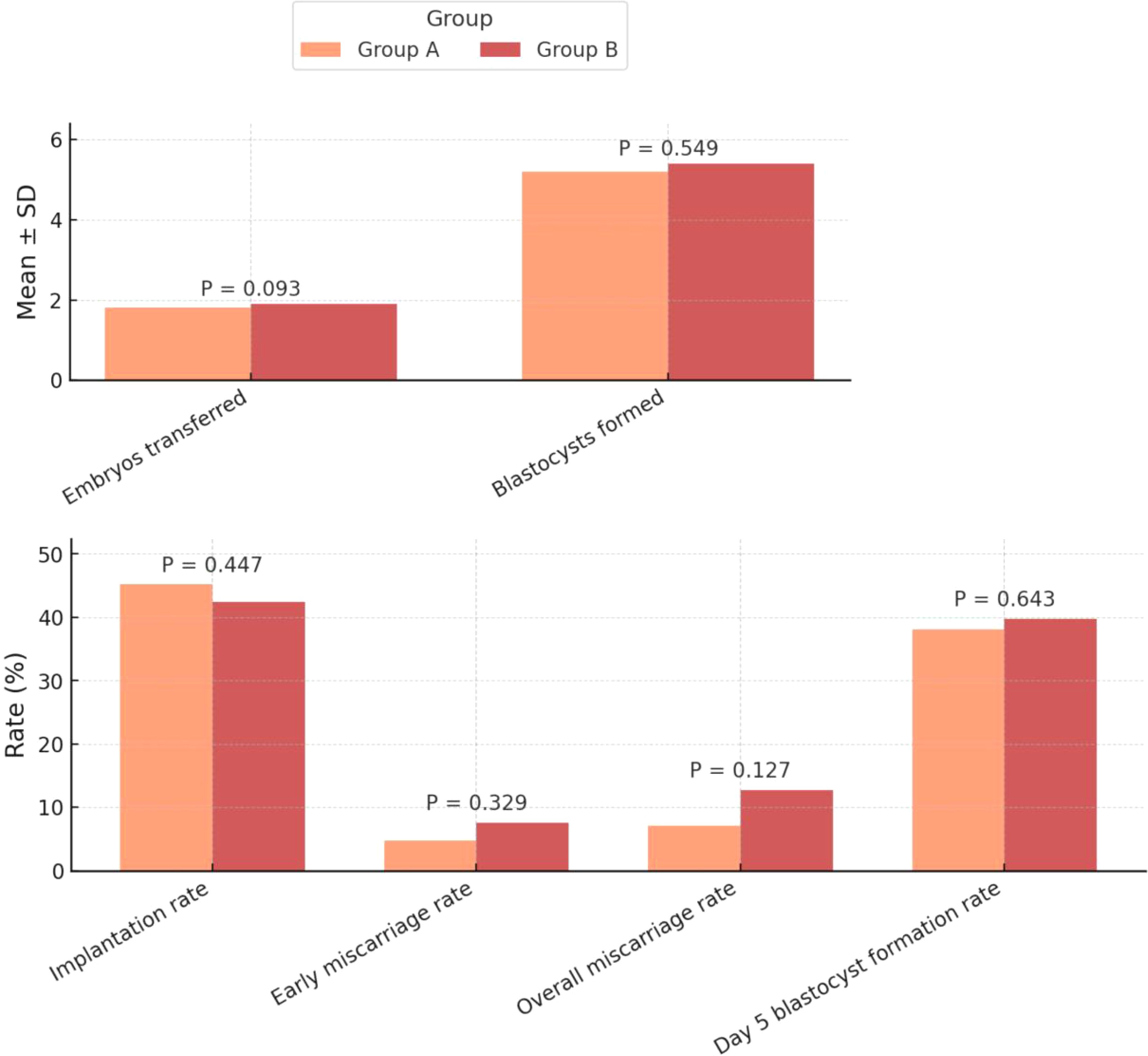
For the secondary outcomes, there were no significant differences in the number of embryos transferred (1.8 ± 0.4 vs. 1.9 ± 0.3, P=0.093) (Figure 3), implantation rate (45.2% vs. 42.4%, P=0.447), or early miscarriage rate (4.8% vs. 7.6%, P=0.329) (Figure 3). Similarly, the overall miscarriage rate (7.1% vs. 12.7%, P=0.127), the number of blastocysts formed (5.2 ± 2.0 vs. 5.4 ± 1.8, P=0.549), and the day 5 blastocyst formation rate (38.1% vs. 39.8%, P=0.643) were all comparable between the two groups (Table 2).
Logistic regression analysis of risk factors for clinical pregnancy
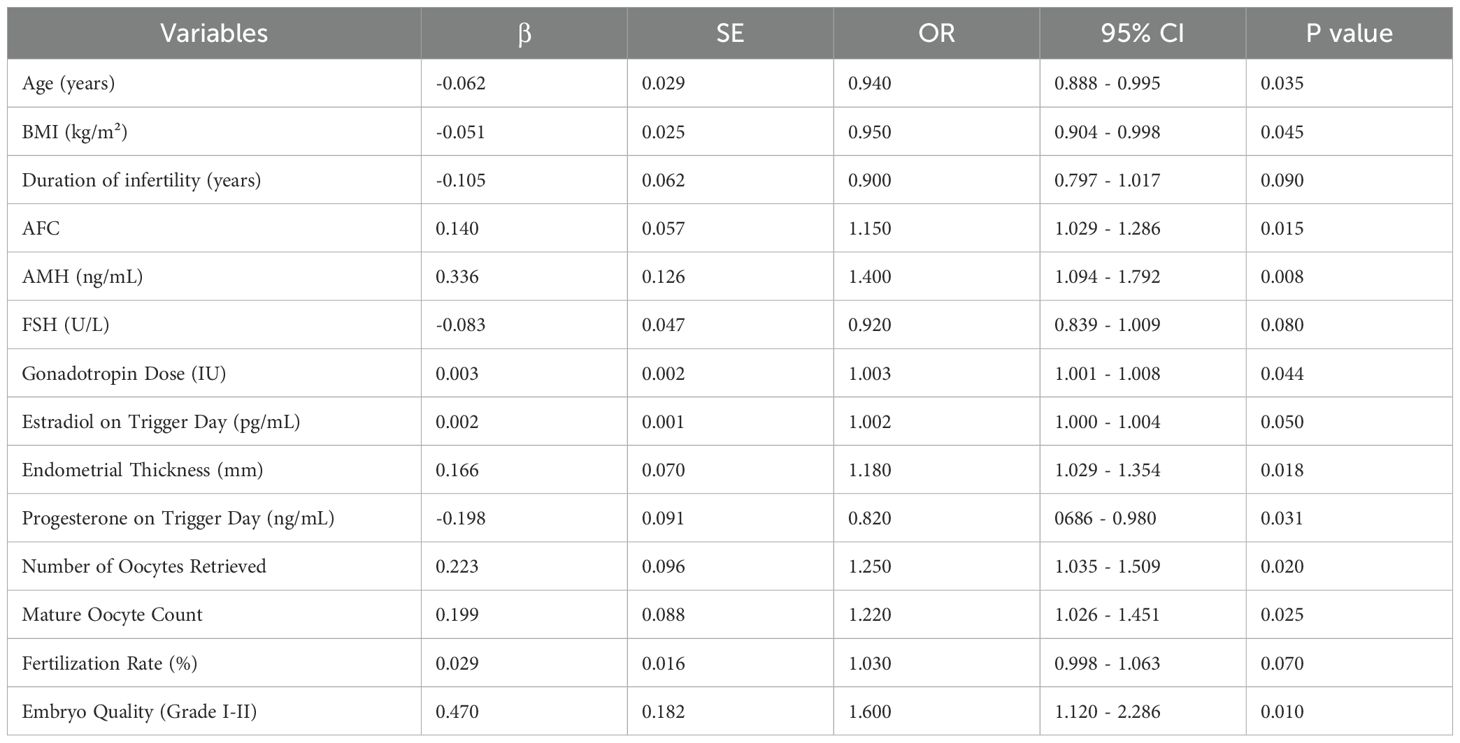
The univariate logistic regression analysis (Table 3) revealed several significant predictors of clinical pregnancy (P < 0.05). Younger age (OR = 0.940, P = 0.035), lower BMI (OR = 0.950, P = 0.045), higher AFC (OR = 1.150, P = 0.015), higher AMH levels (OR = 1.400, P = 0.008), greater endometrial thickness (OR = 1.180, P = 0.018), higher gonadotropin dose (OR = 1.003, P = 0.044), higher estradiol on trigger day (OR = 1.002, P = 0.050), lower progesterone on trigger day (OR = 0.820, P = 0.031), greater number of oocytes retrieved (OR = 1.250, P = 0.020), higher mature oocyte count (OR = 1.220, P = 0.025), and better embryo quality (Grade I-II) (OR = 1.600, P = 0.010) were significantly associated with increased odds of clinical pregnancy. Additionally, duration of infertility (OR = 0.900, P = 0.090), FSH levels (OR = 0.920, P = 0.080), and fertilization rate (OR = 1.030, P = 0.070) showed marginal associations (P < 0.10).
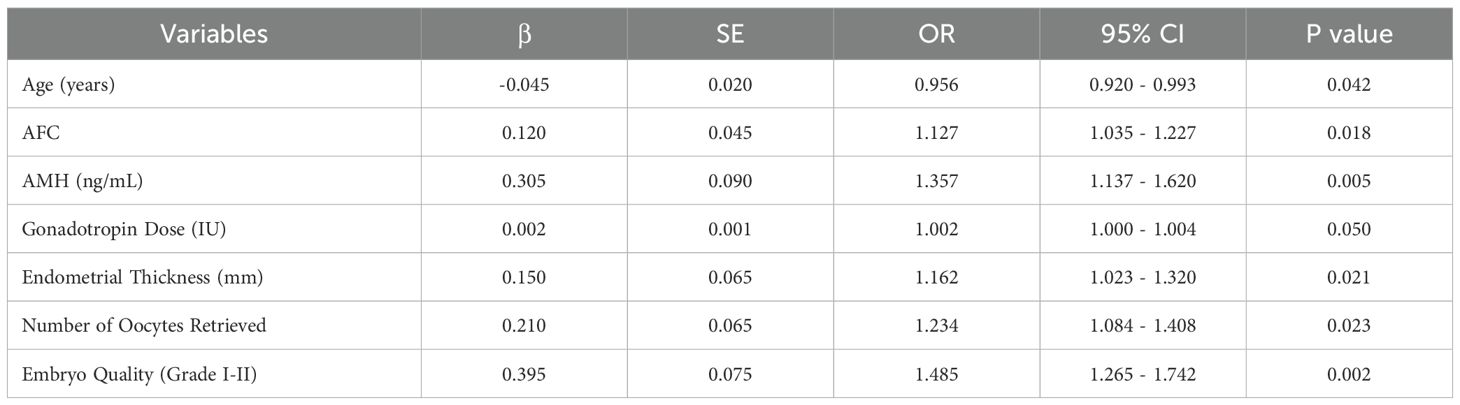
Variables with P < 0.10 in the univariate logistic regression analysis were included in the multivariable logistic regression analysis, and the results (Table 4) demonstrated that younger age (OR = 0.956, P = 0.042), higher AFC (OR = 1.127, P = 0.018), higher AMH levels (OR = 1.357, P = 0.005), greater endometrial thickness (OR = 1.162, P = 0.021), greater number of oocytes retrieved (OR = 1.234, P = 0.023), and better embryo quality (Grade I-II) (OR = 1.485, P = 0.002) were significant independent predictors of clinical pregnancy (P < 0.05). Gonadotropin dose (OR = 1.002, P = 0.050) showed trends toward significance but did not reach statistical significance (P > 0.05).
Subgroup analyses
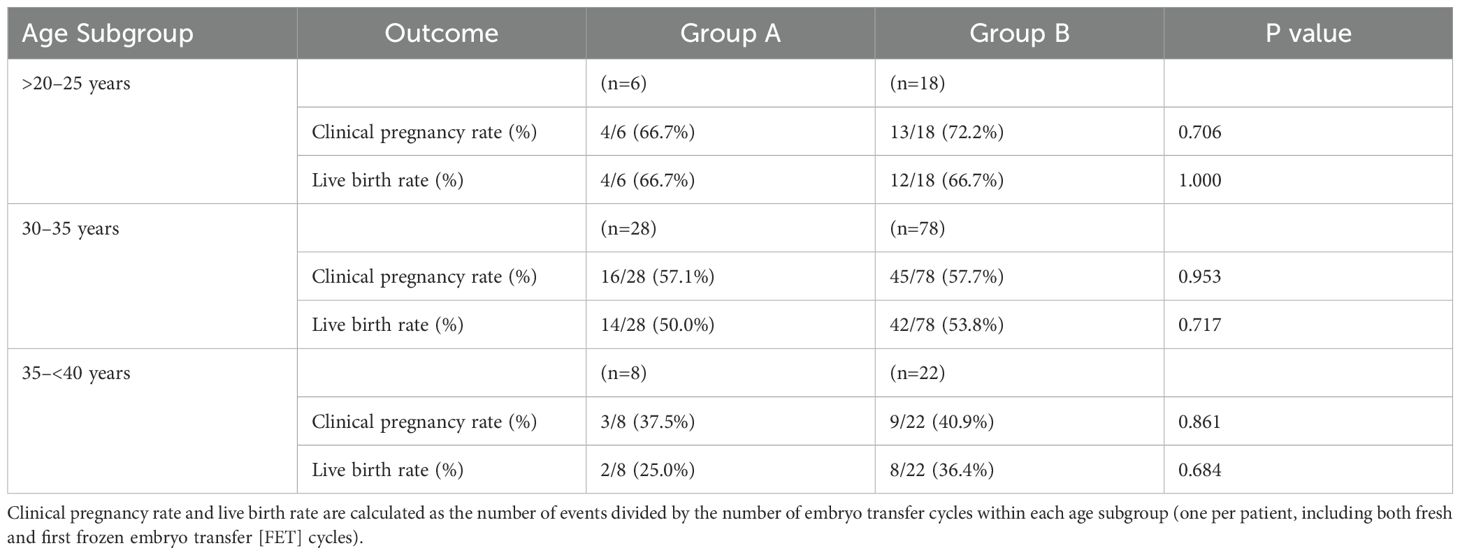
Table 5 presents the age-related subgroup analysis of clinical pregnancy and live birth rates for Group A and Group B, stratified into >20–25, 30–35, and 35–<40 years subgroups. In the >20–25 years subgroup, clinical pregnancy rates were 66.7% (Group A, n=6) vs. 72.2% (Group B, n=18, P=0.706), and live birth rates were both 66.7% (P=1.000). For the 30–35 years subgroup, clinical pregnancy rates were 57.1% (Group A, n=28) vs. 57.7% (Group B, n=78, P=0.953), and live birth rates were 50.0% vs. 53.8% (P=0.717). In the 35–<40 years subgroup, clinical pregnancy rates were 37.5% (Group A, n=8) vs. 40.9% (Group B, n=22, P=0.861), and live birth rates were 25.0% vs. 36.4% (P=0.684). No significant differences were observed between groups, but success rates decreased with increasing age, highlighting age as a key factor influencing IVF/ICSI outcomes.
Discussion
This study compares two commonly used ovarian stimulation protocols— the long luteal phase GnRH agonist protocol and the flexible GnRH antagonist protocol— in patients with normal ovarian reserve undergoing IVF/ICSI. Our findings demonstrate no significant differences between the two protocols in terms of clinical pregnancy rates, live birth rates, or incidence of OHSS. Significant predictors of clinical pregnancy were identified, including AFC, AMH levels, endometrial thickness, number of oocytes retrieved, and embryo quality, while increasing age negatively impacted pregnancy outcomes.
Our study is consistent with the growing body of literature demonstrating that both the GnRH agonist and antagonist protocols are effective for ovarian stimulation in patients with normal ovarian reserve (14–16). However, the flexibility and patient-centered advantages of the antagonist protocol, such as shorter treatment duration and lower risk of OHSS, suggest that it may be preferable for many patients (17–19). Our findings align with several studies that have found no significant differences in pregnancy or live birth rates between the GnRH agonist and antagonist protocols in patients with normal ovarian reserve (20–23). Lambalk et al. (14) and Venetis et al (15) both conducted systematic reviews and meta-analyses, reporting that although antagonist protocols lead to shorter treatment cycles and lower OHSS rates, pregnancy outcomes are comparable between the protocols. Similarly, Kadoura et al. (24) found no differences in live birth rates between the two protocols, though the antagonist protocol reduced patient discomfort and treatment burden. The absence of significant differences in OHSS incidence in our study is supported by earlier findings from studies such as those by Olivennes et al. (25), which suggested that the antagonist protocol is particularly beneficial in preventing severe OHSS in high responders. A systematic review by Engmann et al. (26) reinforced this view, demonstrating the antagonist protocol’s role in enhancing safety without compromising efficacy.
Our findings of equivalent clinical outcomes between the GnRH agonist and antagonist protocols in patients with normal ovarian reserve resolve apparent contradictions in the literature. While some studies suggest advantages for the agonist protocol in populations with diminished ovarian reserve or recurrent implantation failure (27, 28), others report comparable efficacy across protocols in broader populations (14, 15). The equivalence observed in our study is attributable to the focus on patients with normal ovarian reserve, who respond well to both protocols, as evidenced by similar clinical pregnancy (54.8% vs. 56.8%, P=0.092) and live birth rates (47.6% vs. 52.5%, P=0.278). Systematic reviews by Lambalk et al. (14) and Venetis et al. (15) support this equivalence, demonstrating no significant differences in live birth rates, while Kadoura et al. (24) further confirm comparable outcomes in polycystic ovary syndrome patients, a group with robust ovarian response. The antagonist protocol’s shorter treatment duration and lower OHSS incidence, as noted by Nie et al. (20), enhance its appeal without compromising efficacy, providing clinicians with flexibility to tailor treatments based on patient preferences and risk profiles.
The absence of protocol-specific differences in our study contrasts with studies reporting advantages for one protocol over the other, a discrepancy attributable to differences in patient populations and study methodologies (14, 15, 27, 28). Studies observed improved outcomes with the agonist protocol in patients with diminished ovarian reserve, where prolonged downregulation may enhance follicular synchronization (27, 28). In contrast, our study’s focus on patients with normal ovarian reserve, who exhibit robust responses to both protocols, likely minimizes differential effects. Additionally, our prospective design with randomization and standardized clinical protocols, such as endometrial thickness ≥8 mm and a freeze-all strategy for high-risk cases, reduced variability that may amplify protocol-specific differences in retrospective or heterogeneous studies. Systematic reviews (14, 15) corroborate that protocol equivalence is more pronounced in normal responders, supporting our findings and highlighting the importance of patient selection in ART outcomes.
The results suggest that both protocols are equally effective for patients with normal ovarian reserve, providing flexibility in treatment options. The flexible antagonist protocol offers distinct advantages, including reduced treatment burden and a lower incidence of severe OHSS. These benefits are particularly relevant in modern clinical practice, where patient comfort and safety are paramount. The role of AFC, AMH, and embryo quality as predictors of success in IVF/ICSI cycles has been well documented in previous studies. AFC and AMH have been shown to be reliable markers of ovarian reserve and predictors of ovarian response to stimulation (29). Our findings further emphasize the importance of these markers in guiding treatment decisions, particularly in identifying patients who may benefit from individualized gonadotropin dosing. Age remains a critical factor influencing clinical pregnancy outcomes, as demonstrated by the negative association between increasing age and pregnancy success in our study. This is consistent with the extensive literature highlighting the impact of maternal age on oocyte quality and reproductive outcomes (30, 31). As age increases, the cumulative impact of genetic and cellular changes within oocytes leads to reduced implantation potential and increased miscarriage rates (32). Although ovarian reserve markers like AFC and AMH provide valuable information, they cannot entirely mitigate the age-related decline in fertility.
Additionally, endometrial factors, such as endometrial thickness, and hormonal levels, such as progesterone on trigger day, are critical for pregnancy success, particularly in fresh embryo transfer cycles (33). In our study, endometrial thickness was standardized (≥8 mm) for fresh transfers, and progesterone levels were monitored to defer fresh transfers in cases of elevation (>1.5 ng/mL), employing a freeze-all strategy to optimize outcomes. These factors were not included in the logistic regression model (Table 3) due to their standardization across groups and lack of significant differences in preliminary analyses. However, we acknowledge that variability in endometrial receptivity or subtle progesterone elevations may still influence outcomes, and their exclusion from the regression analysis is a limitation. Future studies should incorporate these factors as variables to further elucidate their impact on pregnancy outcomes in patients with normal ovarian reserve.
Our findings have several important implications for clinical practice. The comparable efficacy of the two protocols means that clinicians have the flexibility to select a protocol based on patient preferences, clinical workflow, and the risk of OHSS. The antagonist protocol, with its shorter duration and lower complication risk, may be more suitable for patients seeking a less invasive treatment approach (33). The importance of embryo quality in predicting clinical pregnancy further emphasizes the need for careful embryo selection and grading during IVF/ICSI cycles. As demonstrated in several studies, morphologic criteria remain a critical component of embryo assessment, contributing significantly to treatment success. Our study also highlights the value of tailoring ovarian stimulation protocols to individual patient characteristics, including age and ovarian reserve markers. By adjusting stimulation protocols based on these factors, clinicians can optimize outcomes while minimizing the risk of complications such as OHSS.
Strengths and limitations
This study’s prospective design and large sample size are key strengths, allowing for rigorous data collection and robust comparisons between the two protocols. The random assignment of patients to each protocol minimized selection bias and enhanced the generalizability of the findings to other patient populations with normal ovarian reserve (34). The randomization methhod ensured balanced baseline characteristics and minimized the selection bias. Additionally, the use of multivariable logistic regression further adjusted for potential confounders, reducing the risk of bias in identifying predictors of clinical pregnancy.
However, there are several limitations to consider. First, the study was conducted at a single center, which may limit the external validity of the findings. Multi-center studies are needed to confirm these results in more diverse populations and clinical settings. Second, while the sample size was sufficient for the primary outcome, it may be considered moderate for detecting smaller differences in secondary outcomes, such as implantation or blastocyst formation rates, potentially limiting the study’s power for these endpoints. Nevertheless, the observed non-significant differences in primary outcomes (P > 0.05) suggest that the sample size was appropriate for the study’s main objectives. Additionally, while the study focused on short-term outcomes such as clinical pregnancy and live birth rates, it did not assess long-term neonatal outcomes or maternal health beyond the postpartum period. These outcomes are crucial for evaluating the overall safety and effectiveness of ovarian stimulation protocols. Another limitation is the potential for unmeasured confounders, such as genetic factors or variations in laboratory techniques, that may have influenced the outcomes. Future studies should aim to control for these variables and include more detailed assessments of patient and embryological characteristics.
Future research should focus on further refining ovarian stimulation protocols to improve patient outcomes and safety (35, 36). Randomized controlled trials with larger, more diverse populations are needed to confirm the equivalence of the GnRH agonist and antagonist protocols in different patient groups, including those with diminished ovarian reserve and those undergoing multiple IVF cycles.
Conclusion
In conclusion, this prospective cohort study found no significant differences in clinical pregnancy or live birth rates between the long luteal phase GnRH agonist protocol and the flexible GnRH antagonist protocol in patients with normal ovarian reserve undergoing IVF/ICSI. While both protocols are effective, the flexible antagonist protocol offers a more patient-friendly approach, with shorter treatment duration and lower risk of OHSS. Clinicians should consider individual patient characteristics, including age, ovarian reserve markers, endometrial factors, and personal preferences, when selecting the most appropriate protocol. Further research is needed to confirm these findings in larger, multi-center studies and to explore the long-term outcomes associated with different ovarian stimulation protocols.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Yellow River Sanmenxia Affiliated Hospital of Henan University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YT: Conceptualization, Data curation, Funding acquisition, Writing – original draft. JH: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. XT: Formal analysis, Methodology, Validation, Visualization, Writing – review & editing. SF: Investigation, Methodology, Writing – original draft. ML: Investigation, Methodology, Writing – original draft. YC: Data curation, Investigation, Methodology, Writing – original draft. ZC: Conceptualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Our study was funded by 2022 Sanmenxia City Major Science and Technology Project (2022001006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, and Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PloS Med. (2012) 9:e1001356. doi: 10.1371/journal.pmed.1001356
2. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. (2017) 32:1786–801. doi: 10.1093/humrep/dex234
3. de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2012†. Hum Reprod. (2020) 35:1900–13. doi: 10.1093/humrep/deaa090
4. Huang MC, Tzeng SL, Lee CI, Chen HH, Huang CC, Lee TH, et al. GnRH agonist long protocol versus GnRH antagonist protocol for various aged patients with diminished ovarian reserve: A retrospective study. PloS One. (2018) 13:e0207081. doi: 10.1371/journal.pone.0207081
5. Gorodeckaja J, Neumann S, McCollin A, Ottolini CS, Wang J, Ahuja K, et al. High implantation and clinical pregnancy rates with single vitrified-warmed blastocyst transfer and optional aneuploidy testing for all patients. Hum Fertil (Camb). (2020) 23:256–67. doi: 10.1080/14647273.2018.1551628
6. Tarlatzis BC and Kolibianakis EM. GnRH agonists vs antagonists. Best Pract Res Clin Obstet Gynaecol. (2007) 21:57–65. doi: 10.1016/j.bpobgyn.2006.08.002
7. Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. (2016) 22:687–708. doi: 10.1093/humupd/dmw025
8. Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, and Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. (2006) 12:333–40. doi: 10.1093/humupd/dml001
9. Esinler I, Bozdag G, Esinler D, Lale KS, and Yarali H. Luteal-long GnRH agonist versus flexible-multidose GnRH antagonist protocols for overweight and obese patients who underwent ICSI. J Obstet Gynaecol. (2015) 35:297–301. doi: 10.3109/01443615.2014.958439
10. Moraloglu O, Kilic S, Karayalçin R, Yuksel B, Tasdemir N, Işik A, et al. Comparison of GnRH agonists and antagonists in normoresponder IVF/ICSI in Turkish female patients. Adv Ther. (2008) 25:266–73. doi: 10.1007/s12325-008-0028-8
11. Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. GnRH antagonists are safer than agonists: an update of a Cochrane review. Hum Reprod Update. (2011) 17:435. doi: 10.1093/humupd/dmr004
12. Broekmans FJ, Soules MR, and Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. (2009) 30:465–93. doi: 10.1210/er.2009-0006
13. Roque M, Valle M, Guimarães F, Sampaio M, and Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. (2015) 103:1190–3. doi: 10.1016/j.fertnstert.2015.01.04
14. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. (2017) 23:560–79. doi: 10.1093/humupd/dmx017
15. Venetis CA, Storr A, Chua SJ, Mol BW, Longobardi S, Yin X, et al. What is the optimal GnRH antagonist protocol for ovarian stimulation during ART treatment? A systematic review and network meta-analysis. Hum Reprod Update. (2023) 29:307–26. doi: 10.1093/humupd/dmac040
16. Santos-Ribeiro S, Mackens S, Popovic-Todorovic B, Racca A, Polyzos NP, Van Landuyt L, et al. The freeze-all strategy versus agonist triggering with low-dose hCG for luteal phase support in IVF/ICSI for high responders: a randomized controlled trial. Hum Reprod. (2020) 35:2808–18. doi: 10.1093/humrep/deaa226
17. Şahin S, Selçuk S, Devranoğlu B, Kutlu T, Kuyucu M, and Eroğlu M. Comparison of long GnRH agonist versus GnRH antagonist protocol in poor responders. Turk J Obstet Gynecol. (2014) 11:203–6. doi: 10.4274/tjod.80090
18. Qiao J, Zhang Y, Liang X, Ho T, Huang HY, Kim SH, et al. A randomised controlled trial to clinically validate follitropin delta in its individualised dosing regimen for ovarian stimulation in Asian IVF/ICSI patients. Hum Reprod. (2021) 36:2452–62. doi: 10.1093/humrep/deab155
19. Nyboe Andersen A, Nelson SM, Fauser BC, García-Velasco JA, Klein BM, and Arce JC. ESTHER-1 study group. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. (2017) 107:387–96. doi: 10.1016/j.fertnstert.2016.10.033
20. Nie Y, Guo W, Shen X, Xie Y, Zeng Y, Gao H, et al. The cumulative live birth rates of 18 593 women with progestin-primed ovarian stimulation-related protocols and frozen-thawed transfer cycles. Hum Reprod Open. (2023) 2024:hoad051. doi: 10.1093/hropen/hoad051
21. Pundir J, Sunkara SK, El-Toukhy T, and Khalaf Y. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod BioMed Online. (2012) 24:6–22. doi: 10.1016/j.rbmo.2011.09.017
22. Trenkić M, Popović J, Kopitović V, Bjelica A, Živadinović R, and Pop-Trajković S. Flexible GnRH antagonist protocol vs. long GnRH agonist protocol in patients with polycystic ovary syndrome treated for IVF: comparison of clinical outcome and embryo quality. Ginekol Pol. (2016) 87:265–70. doi: 10.17772/gp/62205
23. Kim CH, Moon JW, Kang HJ, Ahn JW, Kim SH, Chae HD, et al. Effectiveness of GnRH antagonist multiple dose protocol applied during early and late follicular phase compared with GnRH agonist long protocol in non-obese and obese patients with polycystic ovary syndrome undergoing IVF/ICSI. Clin Exp Reprod Med. (2012) 39:22–7. doi: 10.5653/cerm.2012.39.1.22
24. Kadoura S, Alhalabi M, and Nattouf AH. Conventional GnRH antagonist protocols versus long GnRH agonist protocol in IVF/ICSI cycles of polycystic ovary syndrome women: a systematic review and meta-analysis. Sci Rep. (2022) 12:4456. doi: 10.1038/s41598-022-08400-z
25. Janssens RM, Brus L, Cahill DJ, Huirne JA, Schoemaker J, and Lambalk CB. Direct ovarian effects and safety aspects of GnRH agonists and antagonists. Hum Reprod Update. (2000) 6:505–18. doi: 10.1093/humupd/6.5.505
26. Gardner DK and Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R and Mortimer D, editors. Towards Reproductive Certainty: Fertility and Genetics Beyond. Parthenon Press, Carnforth (2020). p. 378–88.
27. Pacchiarotti A, Selman H, Valeri C, Napoletano S, Sbracia M, Antonini G, et al. Ovarian stimulation protocol in IVF: an up-to-date review of the literature. Curr Pharm Biotechnol. (2016) 17:303–15. doi: 10.2174/1389201017666160118103147
28. Diedrich K, Ludwig M, and Felberbaum RE. The role of gonadotropin-releasing hormone antagonists in in vitro fertilization. Semin Reprod Med. (2001) 19:213–20. doi: 10.1055/s-2001-18040
29. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. (2011) 26:1270–83. doi: 10.1093/humrep/der037
30. Broer SL, Broekmans FJ, Laven JS, and Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. (2014) 20:688–701. doi: 10.1093/humupd/dmu020
31. Roque M, Bianco B, Christofolini DM, Barchi Cordts E, Vilarino F, Carvalho W, et al. Pharmacogenetic algorithm for individualized controlled ovarian stimulation in assisted reproductive technology cycles. Panminerva Med. (2019) 61:76–81. doi: 10.23736/S0031-0808.18.03496-1
32. Xu B, Geerts D, Hu S, Yue J, Li Z, Zhu G, et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod. (2020) 35:1306–18. doi: 10.1093/humrep/deaa086
33. Grisendi V and La Marca A. Individualization of controlled ovarian stimulation in vitro fertilization using ovarian reserve markers. Minerva Ginecol. (2017) 69:250–8. doi: 10.23736/S0026-4784.17.04044-8
34. La Marca A and Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. (2014) 20:124–40. doi: 10.1093/humupd/dmt037
35. Havrljenko J, Kopitovic V, Trninic Pjevic A, Milatovic S, Kalember S, Katanic F, et al. The effectiveness of the gnRH agonist/antagonist protocols for different poseidon subgroups of poor ovarian responders. J Clin Med. (2025) 14:2026. doi: 10.3390/jcm14062026
36. Ngwenya O, Lensen SF, Vail A, Mol BWJ, Broekmans FJ, and Wilkinson J. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane Database Syst Rev. (2024) 1:CD012693. doi: 10.1002/14651858.CD012693.pub3
Keywords: ovarian stimulation protocols, clinical pregnancy, GnRH agonist, GnRH antagonist, IVF/ICSI outcomes, normal ovarian reserve
Citation: Tian Y, Hao J, Tu X, Feng S, Li M, Chen Y and Cao Z (2025) Comparison of clinical outcomes between flexible antagonist protocol and long luteal phase protocol in patients with normal ovarian reserve function: a prospective cohort study. Front. Endocrinol. 16:1526895. doi: 10.3389/fendo.2025.1526895
Received: 12 November 2024; Accepted: 02 June 2025;
Published: 08 August 2025.
Edited by:
Bo Huang, Huazhong University of Science and Technology, ChinaReviewed by:
Sarmed Al-Samerria, University of Arizona, United StatesWei Wang, Second Hospital of Hebei Medical University, China
Copyright © 2025 Tian, Hao, Tu, Feng, Li, Chen and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Tian, dGlhbnlpbmcxMTM4QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Ying Tian
Ying Tian Jing Hao†
Jing Hao†