- Department of Pharmacy, Eye & ENT Hospital, Fudan University, Shanghai, China
Objective: To validate the hypothesis proposed by previous studies, which suggests that NSAID use may elevate Klotho levels.
Method: We conducted a cross-sectional study involving 11,626 adults from the National Health and Nutrition Examination Survey (NHANES) 2007-2016. Multivariable linear regression and propensity score analysis were employed to evaluate the association between NSAID use and serum Klotho levels. Additionally, subgroup analyses were performed to assess the consistency of this relationship across various subgroups.
Results: Multivariable linear regression analysis demonstrated that NSAID use was negatively correlated with serum Klotho levels (β = -25.48 [95% CI: -42.00, -8.96], p = 0.003). Additionally, sensitivity analysis results were consistent with the primary analysis. Subgroup analyses did not reveal any statistically significant interactions.
Conclusion: Contrary to previous speculations, the use of NSAIDs is associated with a decrease in serum Klotho levels.
Introduction
The α-Klotho protein, which is encoded by the KL gene, was first identified in 1997 (1, 2). The name α-Klotho is derived from Clotho, the Greek goddess who spins the thread of life, symbolizing its role in the aging process (3). The α-Klotho protein exists in three forms: a full-length transmembrane form, and soluble and secreted isoforms. The membrane-bound α-Klotho is cleaved to produce the soluble form, while the secreted isoform is generated through selective RNA splicing. The soluble and secreted forms share similar structures and together constitute the circulating serum α-Klotho (hereafter referred to as Klotho) (4–6).
Klotho has been implicated in a range of age-related conditions, including Alzheimer’s disease (7, 8), cerebrovascular diseases (9), and Parkinson’s disease (10). Furthermore, research suggests that Klotho plays a protective role in preserving high-frequency hearing (6), mitigating the elevation of blood pressure induced by high salt intake (11), and influencing the development of colorectal cancer (12), cancer metastasis (13), and metabolic disorders (14).
The role of Klotho in longevity and disease prevention has garnered significant interest, prompting researchers to investigate the factors that regulate its expression (15). Studies have shown that inflammation can reduce Klotho expression, leading to speculation that anti-inflammatory drugs may help maintain or even increase Klotho levels (15, 16). However, this hypothesis requires further validation through more precise studies. To test this, we conducted the present study.
Materials and methods
Data sources and study population
The National Health and Nutrition Examination Survey (NHANES) is a nationwide study carried out by the National Center for Health Statistics (NCHS) to provide a representative sampling of the American population’s health and nutrition status (17). The Centers for Disease Control and Prevention (CDC) oversees the survey, employing a complex multistage sampling approach to select a nationally representative sample biennially.
The study was approved by the NCHS Research Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm), with IRB/ERB Protocol Numbers #2005-06 and #2011-17. Participants provided written informed consent in accordance with the Declaration of Helsinki. The data are publicly available through the CDC website: https://www.cdc.gov/nchs/nhanes/ (last accessed on March 1, 2023). We extracted demographic information, survey responses, and laboratory test results, including serum creatinine (Scr) and Serum Klotho concentrations, from the NHANES datasets collected between 2007 and 2016. These years covered all instances in which Klotho concentrations were measured. The sample size was determined based on the available data, with no prior power calculations. This study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting.
Exposure
NSAID usage was obtained from the NHANES Prescription Medications Questions. Participants were asked, “Have you taken or used any prescription medications in the past month?” Those who used NSAIDs were categorized as the exposed group, while those who did not were classified as the control group. In this study, NSAIDs included 17 different drug types, such as acetaminophen, ibuprofen, and aspirin. A comprehensive list of these drugs is provided in Supplementary Table S1.
Serum Klotho protein
Participants followed an overnight fasting protocol in accordance with established guidelines. Blood samples were subsequently collected and immediately stored at -80°C. These samples were first transported on dry ice to the mobile examination center and then transferred to the Northwest Lipid Metabolism and Diabetes Research Laboratories at the University of Washington for analysis. Serum Klotho levels were measured using a commercial ELISA kit from IBL International, Japan. To ensure quality control, each ELISA plate included duplicate samples with either low or high Klotho concentrations, and tests were repeated if any results deviated by more than two standard deviations from the expected values. The assay’s detection limit was 6 pg/mL, and since all samples exceeded this threshold, no imputation was required. Additional details on laboratory methods and quality assurance procedures can be found at: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/SSKL_E.htm.
Covariates
Potential covariates were assessed based on both biological plausibility and existing literature. These factors included demographic variables (such as age, sex, race/ethnicity, education level, poverty-income ratio [PIR], and marital status), health-related behaviors (such as smoking, alcohol consumption, and physical activity), and clinical indicators (including body mass index [BMI], diabetes [DM], hypertension, and estimated glomerular filtration rate [eGFR]).
Age was considered a continuous variable. The participants’ self-reported race/ethnicity was grouped into five categories: Mexican American, other Hispanics, non-Hispanic white, non-Hispanic black, and other racial groups. Educational level was divided into two categories: individuals without a high school diploma and those who had completed high school or obtained a higher level of education. Marital status was classified into two groups: those who were married or living with a partner, and those who were living alone, including individuals who were widowed, divorced, or separated. The Poverty Income Ratio (PIR), which represents the ratio of family income to the federal poverty line, ranged from 0 to 5. Smoking behavior was categorized based on established definitions, with participants classified as never smokers (having smoked fewer than 100 cigarettes), current smokers, or former smokers (having quit after smoking more than 100 cigarettes). Alcohol consumption was assessed with the question, “In the past year, have you consumed at least 12 drinks of any alcoholic beverage?” Those who responded affirmatively were classified as alcohol users. During the physical examination, height and weight were measured, and BMI was subsequently calculated. Physical activity was classified into three intensity levels: sedentary, moderate, and vigorous. Moderate physical activity was defined as at least 10 minutes of movement in the past 30 days that resulted in light sweating or a mild to moderate increase in breathing or heart rate, while vigorous activity required a similar duration of movement but led to heavy sweating or a substantial rise in breathing or heart rate. The diagnosis of DM was based on the criteria established by the American Diabetes Association (18). Participants were categorized as having DM if they fulfilled any of the following conditions: (1) self-reported diabetes diagnosis by a healthcare provider; (2) use of either oral hypoglycemic agents or insulin; or (3) a fasting plasma glucose level ≥ 126 mg/dL, a 75 g oral glucose tolerance test (OGTT) result ≥ 200 mg/dL, or a hemoglobin A1c (HbA1c) level ≥ 6.5%. Hypertension was defined as having a systolic blood pressure ≥ 140 mm Hg and/or a diastolic blood pressure ≥ 90 mm Hg, or as a self-reported diagnosis from a healthcare provider. Chronic kidney disease (CKD) was diagnosed when the eGFR was below 60 mL/min/1.73 m². The eGFR was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: GFR = 142×min [Scr/κ, 1] α × max [Scr/κ, 1]-1.200 × 0.9938Age × 1.012 [if female]. In this formula, κ was set to 0.7 for females and 0.9 for males, α was -0.241 for females and -0.302 for males, while “min” and “max” refer to the minimum and maximum of Scr/κ or 1, respectively (19). Further details on these variables can be accessed through the NHANES website at https://www.cdc.gov/nchs/nhanes/index.htm.
Statistical analysis
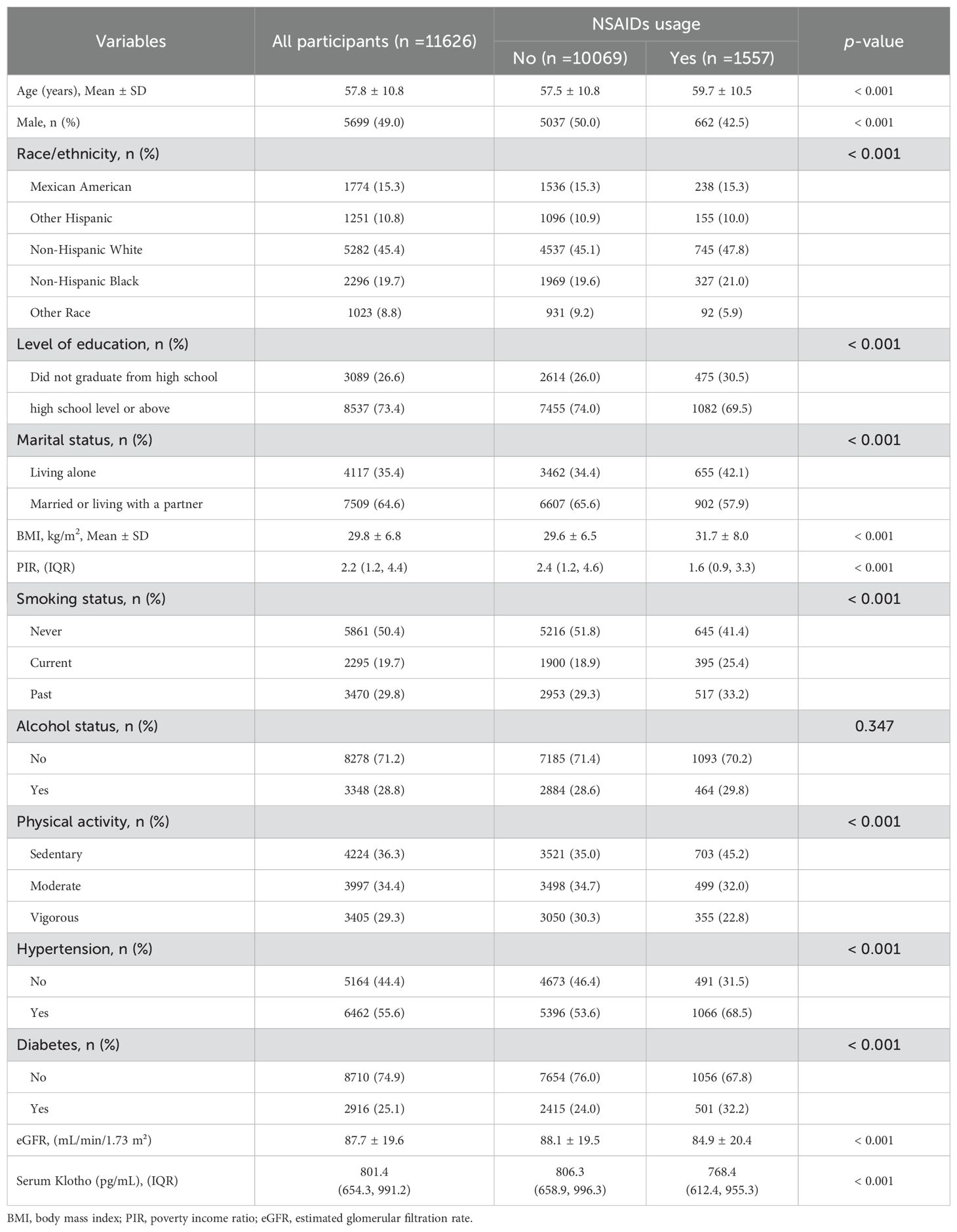
We conducted a descriptive analysis of all participants. Categorical variables are presented as counts and percentages, while continuous variables are summarized as either the mean and standard deviation (SD) for normally distributed data or the median and interquartile range for skewed distributions. To compare categorical variables and continuous variables with different distributions, we applied the chi-square test, one-way ANOVA, and the Kruskal-Wallis test, respectively.
Multivariable linear regression models were applied to investigate the relationship between NSAID use and serum Klotho levels. Additionally, propensity score matching (PSM) and propensity score adjustment were used to assess the impact of NSAID use on serum Klotho levels, while minimizing potential biases related to NSAID allocation and confounding factors (20). A 1:1 nearest neighbor matching algorithm was implemented with a caliper width set at 0.2. The following covariates were included in the propensity score model: age, sex, race, education level, marital status, BMI, PIR, smoking habits, alcohol consumption, physical activity, hypertension, DM, and eGFR. To evaluate the balance achieved by PSM, the standardized mean difference (SMD) was calculated, with an SMD threshold of 0.1 deemed acceptable. Furthermore, we performed a multivariate linear regression analysis to compare serum Klotho levels between populations using NSAIDs, those using other medications, and individuals not using any medication.
Statistical analyses were performed using the R 4.3.2 software package (http://www.R-project.org, The R Foundation) and the Free Software Foundation’s statistics software version 1.7.1. A significance threshold of p < 0.05 was set. The sample size was determined based on the available data, without prior power analysis.
Results
Characteristics of the participants
After screening the original dataset, a total of 11,626 participants, aged 40 to 79 years, were included in this study (the screening process is detailed in Figure 1). Compared to non-users, NSAID users were older, more likely to be female, had lower levels of education and income, were more likely to live alone, engaged in physical activity less frequently, and had a higher prevalence of smoking, alcohol consumption, hypertension, DM, and a lower eGFR (see Table 1).
Results of the multivariate linear regression analysis of the effect of NSAID use on serum Klotho levels
To minimize confounding effects, we constructed four stepwise-adjusted generalized linear regression models to analyze the independent effects of NSAID use on serum Klotho levels. The effect sizes (β) and 95% confidence intervals (CIs) are presented in Table 2. In the unadjusted model, NSAID use was negatively correlated with serum Klotho levels (β = -35.05 [95% CI -51.56, -18.53]) compared to participants who did not use NSAIDs. After adjusting for confounding factors, this negative association remained consistent.
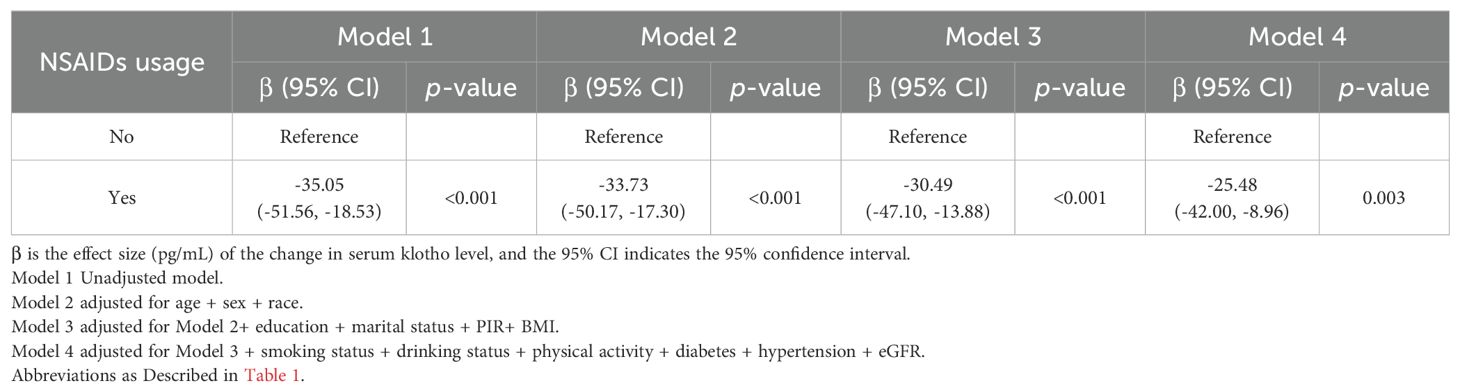
Table 2. Multivariate linear regression analysis of the effect of NSAIDs use on serum Klotho levels.
Subgroup analysis
To further explore these relationships, we conducted stratified analyses based on baseline characteristics to assess the consistency of the association between NSAID use and serum Klotho levels (see Figure 2). No statistically significant interactions were observed in these subgroup analyses examining effect modification.
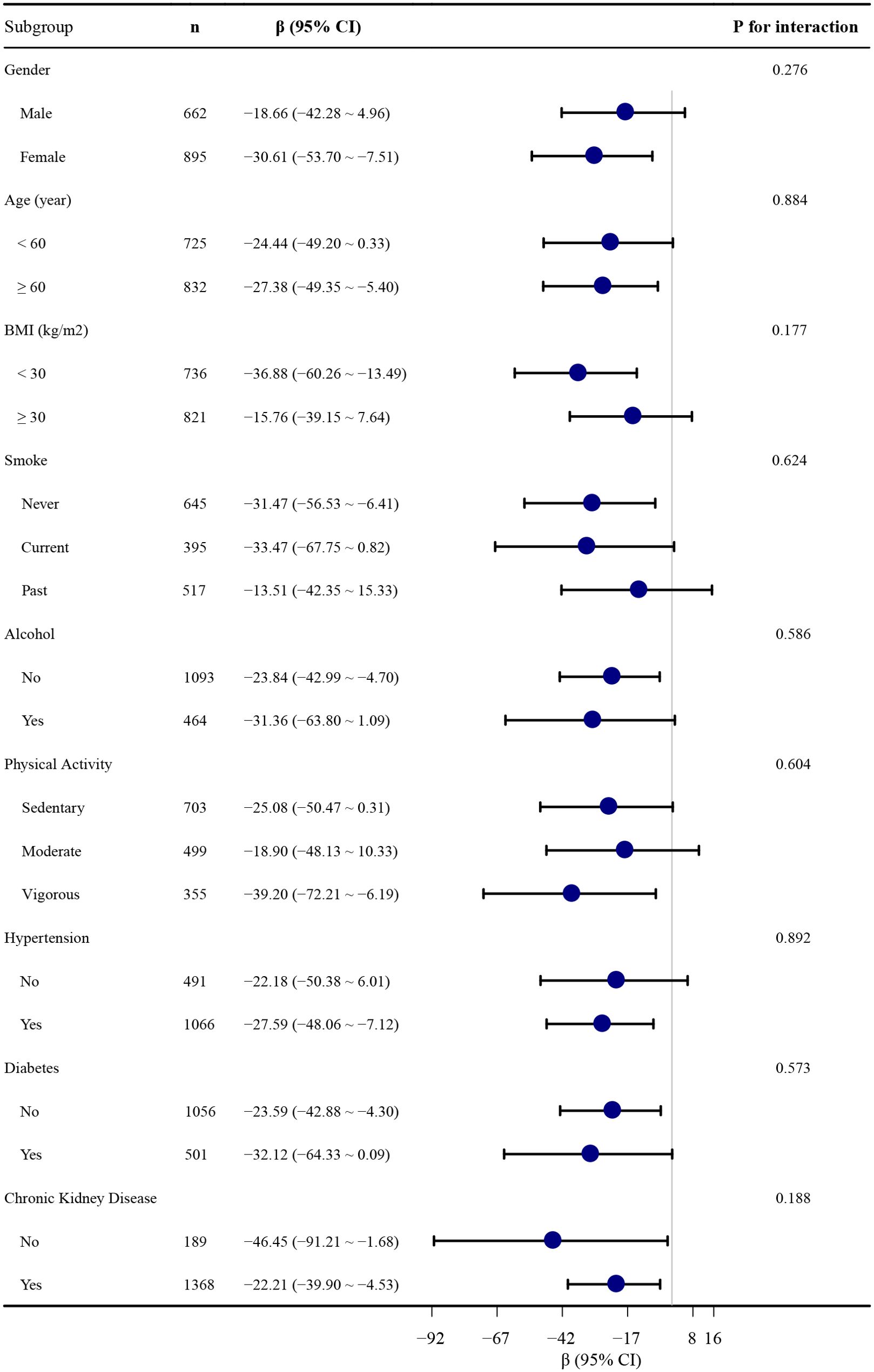
Figure 2. Association between NSAID use and serum S-Klotho levels stratified by baseline characteristics. Each stratification is adjusted for all covariates in Table 2, Model 4, except for the stratification itself.
Sensitivity analyses
To minimize potential biases related to NSAID allocation and confounding factors, we employed PSM and propensity scores adjustment to assess the impact of NSAID use on serum Klotho levels. PSM resulted in 1,551 well-balanced pairs, with baseline variables comparable across groups (Supplementary Table S2). Additionally, we performed a multivariate linear regression analysis to compare serum Klotho levels among individuals using NSAIDs, those using other medications, and those not using any medications. The results were consistent with the primary analysis, indicating that NSAID use was negatively correlated with serum Klotho levels (see Table 3).

Table 3. Associations between NSAID use and serum Klotho levels: crude, multivariable linear regression, and propensity score analysis.
Discussion
In this study, we investigated the relationship between NSAID use and blood Klotho levels. Our findings revealed a significant negative correlation between NSAID use and blood Klotho levels. We employed multiple analytical methods to validate the results, and both multivariable linear regression and propensity score analyses confirmed the robustness of the findings.
To the best of our knowledge, this is the first study to examine the relationship between NSAID use and blood Klotho levels in participants, thereby challenging previous hypotheses that the use of anti-inflammatory drugs may help maintain or even elevate Klotho expression (15). While existing research has explored the role of inflammation in the reduction of Klotho (16, 21, 22), it was previously hypothesized that anti-inflammatory drugs might help maintain or even increase Klotho expression. However, contrary to this speculation, our study found that NSAID use is associated with a decrease in blood Klotho levels.
NSAIDs exert their effects by inhibiting cyclooxygenase (COX) activity (23), an enzyme present in various tissues, including the kidneys, where it plays a crucial role in maintaining essential physiological functions such as renal blood flow (24, 25). Due to COX inhibition, NSAIDs decrease the synthesis of prostaglandins (26–28), which are essential for regulating renal blood flow (RBF) and glomerular filtration rate (GFR). This inhibition may reduce the production of local vasodilators in the kidneys, leading to renal vasoconstriction and a subsequent decline in RBF. (29). This could, in turn, lower Klotho levels. Additionally, NSAIDs may disrupt fluid and electrolyte balance and elevate blood pressure (30, 31), both of which can further affect renal blood flow and serum Klotho levels.
We hypothesize that the reduction in serum Klotho levels associated with NSAID use may be related to the following mechanisms: Klotho protein is primarily expressed in the kidneys, and kidney disease can disrupt renal Klotho expression. Studies have shown that a decrease in kidney mass is associated with reduced Klotho levels (32). Renal injury, a known adverse effect of NSAID use, is linked to both acute and chronic kidney damage (33–35). Therefore, NSAIDs may contribute to a reduction in Klotho levels by exacerbating kidney injury (32).
NSAIDs exert their effects by inhibiting cyclooxygenase (COX) activity, an enzyme expressed in various tissues, including the kidneys, where it is crucial for maintaining fundamental physiological functions such as renal blood flow by inhibiting COX, NSAIDs reduce the synthesis of prostaglandins, which play a key role in regulating renal blood flow (RBF) and glomerular filtration rate (GFR).
In summary, NSAID medications may influence blood Klotho levels through multiple mechanisms, including reductions in renal blood flow and glomerular filtration rate, increased renal injury, and disruptions in fluid and electrolyte balance and blood pressure. These mechanisms may act independently or synergistically, collectively contributing to decreased blood Klotho levels. This observational study was conducted in a human population. It is recommended that future animal experiments be conducted to validate these findings and further explore the underlying mechanisms.
Limitations
This study has several limitations. Firstly, due to its cross-sectional design, we are unable to establish a causal relationship between NSAID use and serum Klotho levels, and there may be unmeasured confounding factors. We suggest that future research consider these potential confounders, such as specific dietary habits and the use of other medications. Additionally, serum Klotho levels were measured at a single time point in the NHANES dataset, and we lacked information on potential fluctuations over time. Various factors could influence Klotho levels, leading to abnormally high or low measurements at the time of assessment. Future research should explore the temporal variability of serum Klotho concentrations. Furthermore, different classes of NSAIDs, with their distinct chemical structures and mechanisms of action, may have differential effects on blood Klotho levels. Subsequent studies will investigate the individual impacts of NSAIDs with varying mechanisms on Klotho levels.
Conclusion
This study provides evidence suggesting an association between NSAID use and lower serum Klotho levels, which contrasts with prior speculations that NSAIDs might elevate Klotho levels. Our findings, based on a large cohort of 11,626 adults from the NHANES dataset, were consistent across various analytical methods, including multivariable linear regression and propensity score analysis. Due to NSAID diversity, individual variability, and underlying conditions, these results should be interpreted cautiously. Further studies are needed to confirm the findings and establish causal links.
Data availability statement
Publicly available datasets are available online for this study. The repository/repositories name and accession numbers are available online at http://www.cdc.gov/nchs/nhanes.htm (accessed on March 1, 2023). Further inquiries can be directed to the corresponding authors.
Ethics statement
The study was approved by the NCHS Research Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm), with IRB/ERB Protocol Numbers #2005-06 and #2011-17. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JY: Conceptualization, Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. HS: Data curation, Writing – review & editing. XX: Data curation, Supervision, Writing – review & editing. TH: Data curation, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the Science and Technology Commission of Shanghai Municipality (No. 23ZR1409100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1531325/full#supplementary-material
References
1. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. (1997) 390:45–51. doi: 10.1038/36285
2. Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, et al. α-klotho expression in human tissues. J Clin Endocrinol Metab. (2015) 100:E1308–1318. doi: 10.1210/jc.2015-1800
3. Kuro-O M. Klotho and calciprotein particles as therapeutic targets against accelerated ageing. Clin Sci (London England: 1979). (2021) 135:1915–27. doi: 10.1042/CS20201453
4. Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. (1998) 242:626–30. doi: 10.1006/bbrc.1997.8019
5. Xu Y, Sun Z. Molecular basis of klotho: From gene to function in aging. Endocr Rev. (2015) 36:174–93. doi: 10.1210/er.2013-1079
6. Yan J, Li L, Ye Q, Huang T. Exploring the link between serum klotho and high-frequency hearing loss in older adults. Laryngosc. (2024) 135(3):1169–76. doi: 10.1002/lary.31851. Preprint.
7. Zhao Y, Zeng CY, Li XH, Yang TT, Kuang X, Du JR. Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimers disease. Aging Cell. (2020) 19:e13239. doi: 10.1111/acel.13239
8. Zhang S, Xia B, Kalionis B, Li H, Zhang X, Zhang X, et al. The role and mechanism of vascular aging in geriatric vascular diseases. Aging Dis. (2024). doi: 10.14336/AD.2024.0717. Preprint.
9. Wei H, Li H, Song X, Du X, Cai Y, Li C, et al. Serum klotho: A potential predictor of cerebrovascular disease in hemodialysis patients. BMC Nephrol. (2019) 20:63. doi: 10.1186/s12882-019-1232-2
10. Sancesario GM, Di Lazzaro G, Grillo P, Biticchi B, Giannella Ev , et al. Biofluids profile of α-klotho in patients with parkinsons disease. Parkinsonism Rel Disord. (2021) 90:62–4. doi: 10.1016/j.parkreldis.2021.08.004
11. Kawarazaki W, Mizuno R, Nishimoto M, Ayuzawa N, Hirohama D, Ueda K, et al. Salt causes aging-associated hypertension via vascular Wnt5a under klotho deficiency. J Clin Invest. (2020) 130:4152–66. doi: 10.1172/JCI134431
12. Arbel Rubinstein T, Shahmoon S, Zigmond E, Etan T, Merenbakh-Lamin K, Pasmanik-Chor M, et al. Klotho suppresses colorectal cancer through modulation of the unfolded protein response. Oncogene. (2019) 38:794–807. doi: 10.1038/s41388-018-0489-4
13. Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. (2011) 286(10):8655-65. doi: 10.1074/jbc.M110.174037
14. Gu H, Jiang W, You N, Huang X, Li Y, Peng X, et al. Soluble klotho improves hepatic glucose and lipid homeostasis in type 2 diabetes, Molecular Therapy. Methods Clin Dev. (2020) 18:811–23. doi: 10.1016/j.omtm.2020.08.002
15. Poursistany H, Azar ST, Azar MT, Raeisi S. The current and emerging klotho-enhancement strategies. Biochem Biophys Res Commun. (2024) 693:149357. doi: 10.1016/j.bbrc.2023.149357
16. Martín-Núñez E, Donate-Correa J, López-Castillo Á, Delgado-Molinos A, Ferri C, Rodríguez-Ramos S, et al. Soluble levels and endogenous vascular gene expression of KLOTHO are related to inflammation in human atherosclerotic disease. Clin Sci (London England: 1979). (2017) 131:2601–9. doi: 10.1042/CS20171242
17. US Centers for Disease Control and Prevention, National Center for Health Statistics. About the national health and nutrition examination survey(2020). Available online at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (Accessed April 7, 2023).
18. American Diabetes Association Professional Practice Committee. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S002
19. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
20. Yang Q, Zheng J, Wen D, Chen X, Chen W, Chen W, et al. Association between metformin use on admission and outcomes in intensive care unit patients with acute kidney injury and type 2 diabetes: A retrospective cohort study. J Crit Care. (2021) 62:206–11. doi: 10.1016/j.jcrc.2020.12.007
21. Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol: JASN. (2011) 22:1315–25. doi: 10.1681/ASN.2010101073
22. Oh HJ, Nam BY, Lee MJ, Kim CH, Koo HM, Doh FM, et al. Decreased circulating klotho levels in patients undergoing dialysis and relationship to oxidative stress and inflammation. Peritoneal Dialysis International: J Int Soc Peritoneal Dialysis. (2015) 35:43–51. doi: 10.3747/pdi.2013.00150
23. Arfeen M, Srivastava A, Srivastava N, Khan RA, Almahmoud SA, Mohammed HA. Design, classification, and adverse effects of NSAIDs: A review on recent advancements. Bioorg Med Chem. (2024) 112:117899. doi: 10.1016/j.bmc.2024.117899
24. Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens. (2006) 15:34–40. doi: 10.1097/01.mnh.0000186852.15889.1a
25. Elewa M, Shehda M, Hanna PA, Said MM, Ramadan S, Barakat A, et al. Development of a selective COX-2 inhibitor: from synthesis to enhanced efficacy via nano-formulation. RSC Adv. (2024) 14:32721–32. doi: 10.1039/d4ra06295g
26. Tajdari M, Peyrovinasab A, Bayanati M, Ismail Mahboubi Rabbani M, Abdolghaffari AH, Zarghi A, et al. Dual COX-2/TNF-α Inhibitors as promising anti-inflammatory and cancer chemopreventive agents: A review. Iranian J Pharm Res: IJPR. (2024) 23:e151312. doi: 10.5812/ijpr-151312
27. Dong L, Malkowski MG. Coupling subunit-specific states to allosteric regulation in homodimeric cyclooxygenase-2. Biochemistry. (2025) 64(6):1380–92. doi: 10.1021/acs.biochem.4c00821. Preprint.
28. Yui K, Imataka G, Ichihashi M. Prostaglandins: biological action, therapeutic aspects, and pathophysiology of autism spectrum disorders. Curr Issues Mol Biol. (2025) 47:71. doi: 10.3390/cimb47020071
29. Drożdżal S, Lechowicz K, Szostak B, Rosik J, Kotfis K, Machoy-Mokrzyńska A, et al. Kidney damage from nonsteroidal anti-inflammatory drugs-Myth or truth? Review of selected literature. Pharmacol Res Perspect. (2021) 9:e00817. doi: 10.1002/prp2.817
30. Frishman WH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am J Cardiol. (2002) 89:18D–25D. doi: 10.1016/s0002-9149(02)02233-6
31. Morgan T, Anderson A. The effect of nonsteroidal anti-inflammatory drugs on blood pressure in patients treated with different antihypertensive drugs. J Clin Hypertens (Greenwich Conn. (2003) ) 5:53–7. doi: 10.1111/j.1524-6175.2003.00514.x
32. Castillo RF. Pathophysiologic implications and therapeutic approach of klotho in chronic kidney disease: A systematic review. Lab Invest J Tech Methods Pathol. (2023) 103:100178. doi: 10.1016/j.labinv.2023.100178
33. Lefebvre C, Hindié J, Zappitelli M, Platt RW, Filion KB. Non-steroidal anti-inflammatory drugs in chronic kidney disease: a systematic review of prescription practices and use in primary care. Clin Kidney J. (2020) 13:63–71. doi: 10.1093/ckj/sfz054
34. LaForge JM, Urso K, Day JM, Bourgeois CW, Ross MM, Ahmadzadeh S, et al. Non-steroidal anti-inflammatory drugs: clinical implications, renal impairment risks, and AKI. Adv Ther. (2023) 40:2082–96. doi: 10.1007/s12325-023-02481-6
Keywords: NSAIDs, cyclooxygenase, inflammation, aging, α-Klotho (Klotho)
Citation: Yan J, Sun H, Xin X and Huang T (2025) NSAID use may decrease serum Klotho levels. Front. Endocrinol. 16:1531325. doi: 10.3389/fendo.2025.1531325
Received: 21 November 2024; Accepted: 27 March 2025;
Published: 16 April 2025.
Edited by:
James Harper, Sam Houston State University, United StatesReviewed by:
Juan Wang, Shanghai University, ChinaZhiran Ju, Zhejiang University of Technology, China
Adi Nurmesa, Padjadjaran University, Indonesia
Copyright © 2025 Yan, Sun, Xin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingchao Yan, amluZ2NoYW8ueWFuQGZkZWVudC5vcmc=; Taomin Huang, dGFvbWluaHVhbmdAMTYzLmNvbQ==
†ORCID: Jingchao Yan, orcid.org/0000-0002-5689-9989
Hong Sun, orcid.org/0000-0001-9015-0480
Xiu Xin, orcid.org/0009-0003-7176-9159
Taomin Huang, orcid.org/0000-0003-4185-7732
 Jingchao Yan
Jingchao Yan Hong Sun
Hong Sun Xiu Xin†
Xiu Xin†