- Department of Endocrinology, Cardio-Cerebrovascular Disease Hospital, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
Objective: By analyzing the expression levels of circulating microRNAs (miRNAs) in patients with type 2 diabetes mellitus (T2DM) and its correlation with diabetic osteoporosis (DOP), this study aims to identify potential biomarkers for the early prediction and screening of DOP.
Methods: A total of 120 patients with T2DM who received treatment in the endocrinology outpatient/inpatient department between January 2023 and June 2024, along with 90 healthy volunteers, were enrolled in this study. Based on the bone mineral density (BMD), the 120 T2DM patients were divided into three groups: normal group (54 cases), osteopenia group (38 cases), and osteoporosis group (28 cases). The differences in clinical data, laboratory test indicators and miRNA expression differences among the three groups were statistically analyzed, and the high-risk factors for DOP in T2DM patients were analyzed.
Results: Compared to healthy volunteers, patients with T2DM demonstrated significantly decreased levels of P1NP and miR-219a-5p, alongside elevated levels of β-CTX, miR-188-3p, and miR-19a/b. Additionally, miR-335-5p levels were notably reduced in T2DM patients. Among these markers, significant differences were observed in the expression levels of P1NP, β-CTX, and miRNA in T2DM patients. Further analysis revealed distinct expression patterns of miR-188-3p, miR-335-5p, and miR-19a/b across the three T2DM subgroups (osteoporosis, osteopenia, and normal bone density groups). Specifically, miR-188-3p levels were 10.34 ± 1.26 in the osteoporosis group, 8.35 ± 1.33 in the osteopenia group, and 6.55 ± 1.18 in the normal group. Similarly, miR-335-5p levels were 0.44 ± 0.14, 0.67 ± 0.16, and 0.88 ± 0.15, respectively, while miR-19a/b levels were 4.04 ± 1.41, 3.19 ± 1.21, and 2.47 ± 1.24, respectively (P < 0.001 for all comparisons). These miRNAs also exhibited significant correlations with BMD at the hip and lumbar spine (P < 0.001 or P = 0.001), highlighting their potential role in bone metabolism and osteoporosis risk in T2DM patients.
Conclusions: The results suggest that the circulating levels of miR-188-3p, miR-335-5p, and miR-19a/b are significantly associated with the occurrence of DOP in T2DM patients. These miRNAs show potential as biomarkers for the early diagnosis of DOP.
Introduction
With the improvement of living standards and changes in lifestyle, the prevalence of diabetes mellitus (DM) has increased rapidly, and the total number of diabetes patients in China ranks second in the world (1). Among them, type 2 diabetes mellitus (T2DM) accounts for 93.7% of the diabetic population (2). As understanding and research on diabetes and its complications have deepened, the changes in bone metabolism caused by diabetes have gradually received attention. Diabetic osteoporosis (DOP) is a type of disease caused by changes in bone metabolism caused by diabetes, which leads to a decrease in bone mass and destruction of bone structure, and ultimately increases risk of fractures. DOP is considered a type of secondary osteoporosis (3, 4). The number of patients in China continues to increase, and more and more people are suffering from reduced bone mass. If a fracture occurs, it will place a heavy burden on the patient’s family and cause physical and psychological trauma (5, 6).
Currently, the clinical diagnosis and efficacy evaluation of DOP are mostly based on fragility fractures and/or bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DXA) (7). It may be time-consuming to diagnose DOP in the absence of a fragility fracture, and the occurrence of the first fragility fracture also increases the risk of subsequent fractures (8). Early detection and timely clinical intervention in patients with osteoporosis are crucial, as they can substantially mitigate the risks of fractures, thereby preventing physical and psychological trauma to patients. Additionally, such measures can alleviate the economic burden on healthcare systems and society at large. Consequently, the early identification and targeted prevention and management of osteoporosis hold significant clinical and public health importance.
MicroRNAs (miRNAs) are a class of short noncoding single-stranded molecules, typically 18–24 nucleotides in length, that play a key role in gene translation and expression by binding to the 3’untranslated region (3’UTR) of target messenger RNAs (mRNAs) (9). A large number of studies have shown that osteoporosis is associated with abnormal expression of miRNA in bone tissue and blood circulation, and miRNA is involved in the pathogenesis of osteoporosis (10). miRNA may be a potential biomarker for predicting the prognosis of high-risk osteoporotic fractures (11). However, its role in DOP is still unknown. Therefore, this study aims to explore the correlation between circulating miRNA levels and the occurrence of DOP to provide theoretical guidance and basis for early prediction and screening.
Materials and methods
Participants
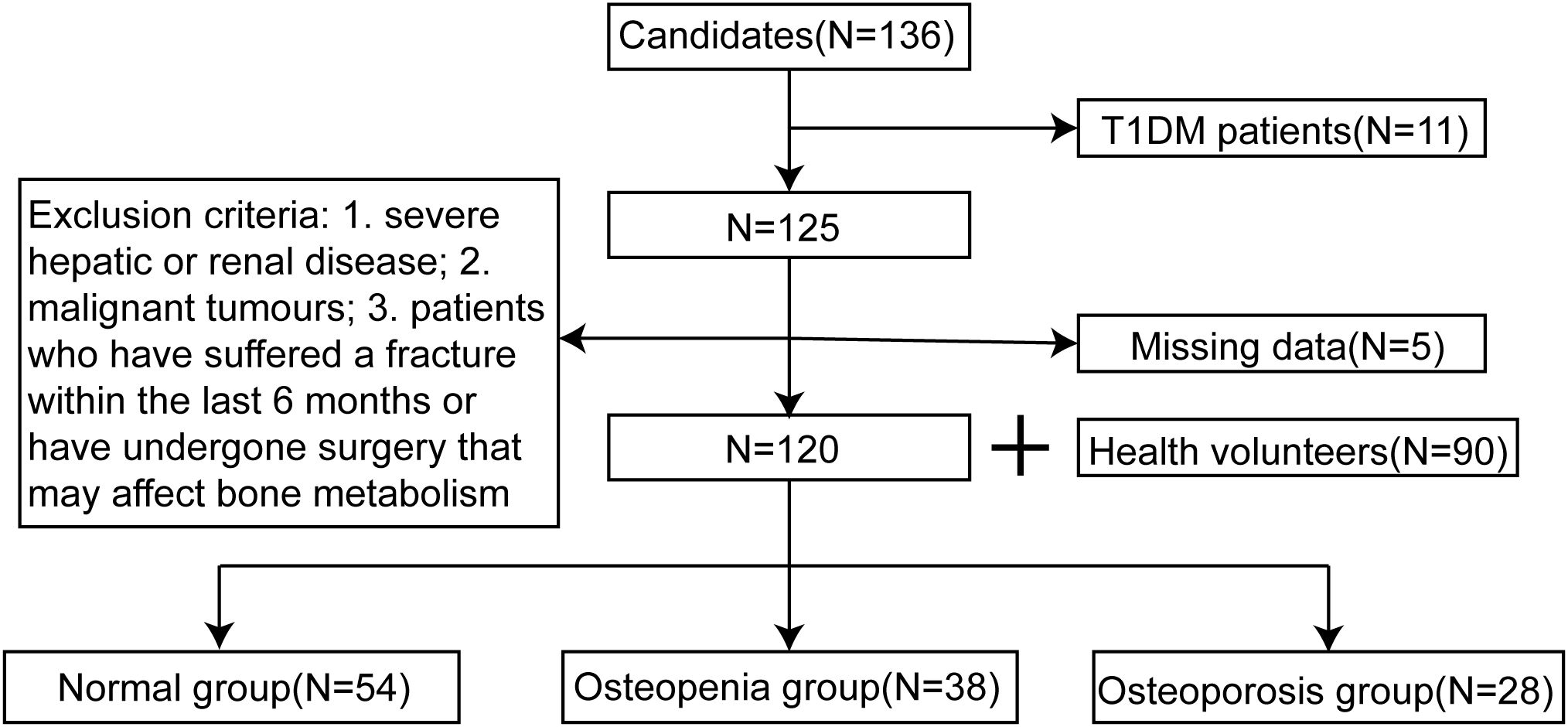
This study retrospectively collected data from 120 patients with T2DM who were treated by the endocrinology department of Cardio-Cerebrovascular Disease Hospital, General Hospital of Ningxia Medical University between January 2023 to June 2024, along with 90 healthy volunteers who were randomly included in the study. The diagnostic criteria for T2DM were as follows: (1) fasting blood glucose levels ≥ 7mmol/L, measured at least twice; (2) typical symptoms such as polydipsia, polyuria, polyphagia, and weight loss, with random blood glucose levels ≥ 11.1 mmol/L; (3) 2h blood glucose levels ≥ 11.1 mmol/L during an oral glucose tolerance test (OGTT) (12). Based on the diagnostic criteria for osteoporosis, the BMD of the patients was measured using dual-energy X-ray absorptiometry (DEXA, equipment Model: Hologic Discovery Wi) to calculate the T-scores. DXA machine calibration follows strict manufacturer’s calibration guidelines and DXA equipment is regularly calibrated and maintained. All operators involved in dual-energy X-ray absorptiometry (DXA) measurements undergo rigorous training and adhere to standardized operating procedures to ensure consistency, accuracy, and reliability in data acquisition and interpretation. The 120 patients with T2DM included in the study were divided into three groups: osteoporosis group (T-score ≤ -2.5 standard deviations), osteopenia group (T-score between -2.5 and -1.0 standard deviations), and normal group (T-score ≥ -1.0 standard deviations) (13). The T-score diagnostic criteria used in this study were based on the 2020 American College of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) Guidelines for the Management of Postmenopausal Osteoporosis. The study was approved by the Ethics Committee of General Hospital of Ningxia Medical University (KYLL-2022-0693).
Collection of clinical data and laboratory test indicators
General clinical data were collected from the study subjects, including age, gender, duration of diabetes, height, weight, body mass index (BMI), and clinical history. Fasting venous blood samples were collected from patients to test for laboratory indicators, including fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), vitamin D3, parathyroid hormone (PTH), bone mineral density (BMD), procollagen type I N-terminal propeptide (P1NP), and serum β-Cross Linked C-telopeptide of type I collagen (β-CTX). All tests were performed by the laboratory of our hospital and the relevant results were issued.
Quantitative detection of miRNA (miR-219a-5p, miR-188-3p, miR-335-5p and miR-19a/b)
Fasting venous blood samples (5 mL) was collected from patients or healthy volunteers within 12 hours of admission, and the serum was separated using a centrifuge (WIGGENS, Germany, model: UNICEN21) and stored at -80°C for later use. The miRNA in serum was extracted using the EasyPure® miRNA Kit (Beijing TransGen Biotech, ER601-01-V2), and the concentration and purity were determined by a spectrophotometer (Dongguan Pubiao Technology Co., Ltd.). The miRNA was reverse transcribed into cDNA by the tailing method using a reverse transcription kit (Takara, 638315). U6 was used as the internal reference gene of miRNA, and the primer sequences are shown in Table 1. The reaction system was prepared according to the instructions of the quantitative kit (Takara), and the PCR amplification conditions were set as follows: 95°C for 30 seconds, 95°C for 15 seconds, 60°C for 30 seconds, 72°C for 30 seconds, for 40 cycles. The relative expression levels of miRNA were analyzed using the 2-ΔΔCt method.
Statistical analysis
In this study, data analysis was performed using SPSS 26.0 statistical software. Categorical variables were compared using the chi-square test. For continuous variables, either one-way ANOVA or non-parametric rank-sum tests were applied, depending on whether the data followed a normal distribution. Correlation analysis was conducted using either linear correlation or Pearson correlation, as appropriate. Logistic regression analysis was employed to identify potential risk factors associated with DOP. The predictive performance of relevant factors for DOP diagnosis was assessed using receiver operating characteristic (ROC) curve analysis. P < 0.05 was considered statistically significant.
Results
Feature comparison
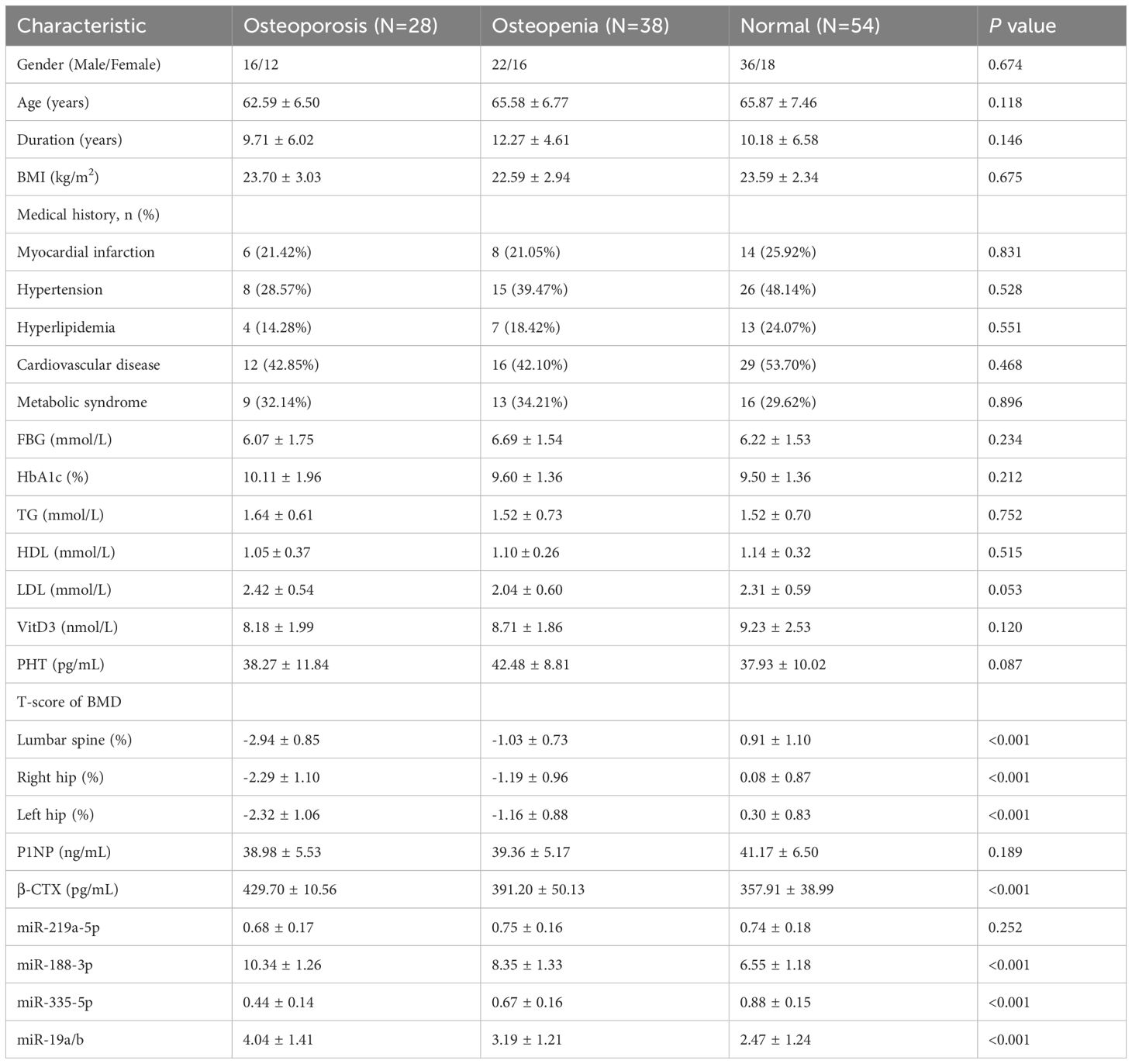
This study included 120 patients with T2DM who were treated by the department of endocrinology at our hospital during xx and 90 healthy subjects. According to the BMD values, the 120 patients were divided into three groups: normal group (54 cases), osteopenia group (38 cases), and osteoporosis group (28 cases), as shown in Figure 1. The differences in the levels of P1NP, β-CTX, and miRNAs in T2DM patients and healthy volunteers were analyzed, as shown in Figure 2. In T2DM patients, P1NP levels decreased, β-CTX levels increased, miR-219a-5p levels decreased, miR-188-3p levels increased, miR-335-5p levels decreased, and miR-19a/b levels increased.
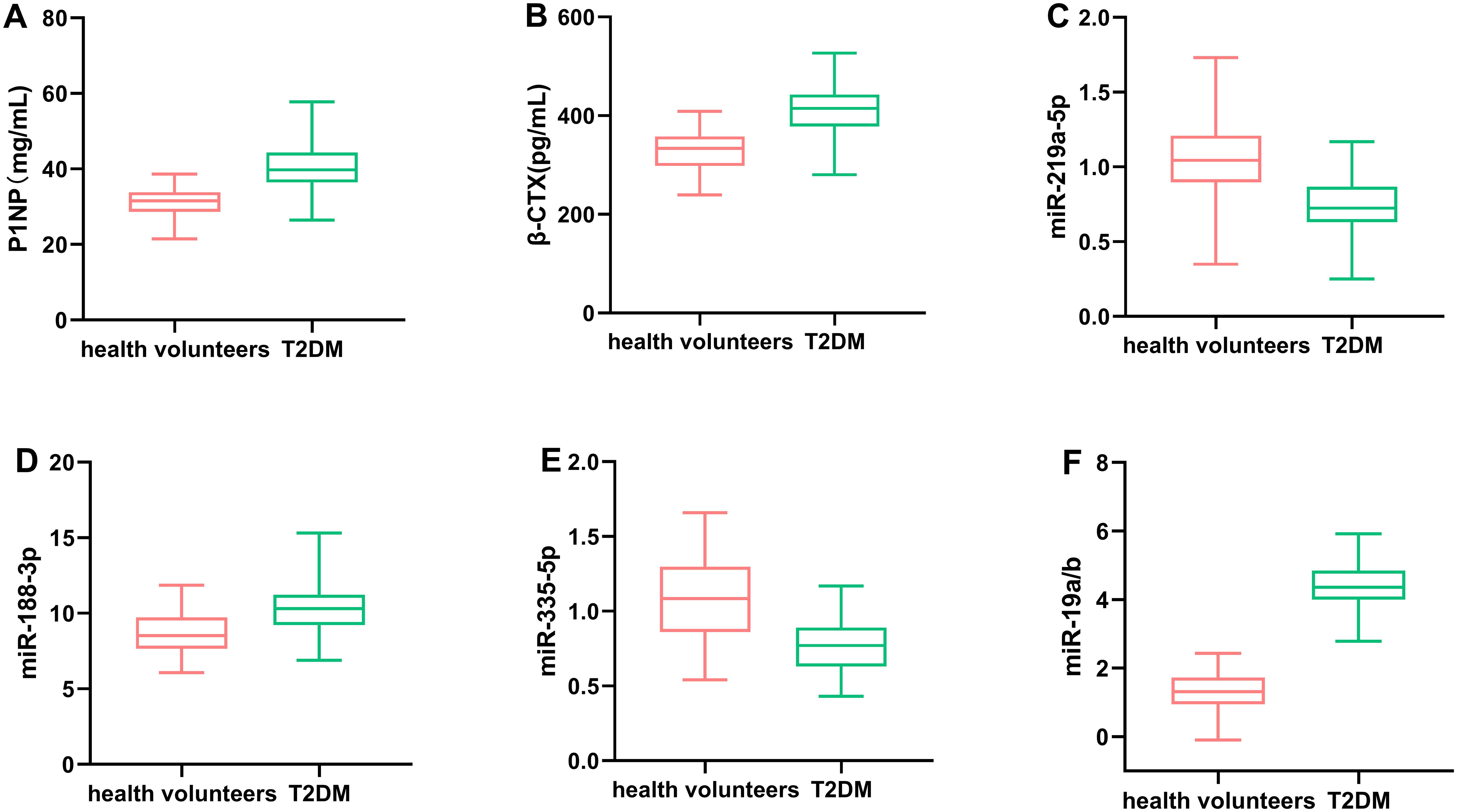
Figure 2. Comparison of the levels of P1NP (A), β-CTX (B), miR-219a-5p (C), miR-188-3p (D), miR-335-5p (E), and miR-19a/b (F) in T2DM patients and healthy volunteers. Circulating levels of miR-188-3p, miR-335-5p, and miR-19a/b were significantly associated with the development of DOP in T2DM patients.
The 120 T2MD patients were divided into three groups based on BMD. The general clinical data and laboratory tests among the three groups are compared in Table 1. There were significant differences in both hip and lumbar BMD among the osteoporosis group, osteopenia group, and normal group (all P values < 0.001). The β-CTX level also decreased with the loss of bone density, and the difference among the three groups was significant (P < 0.001). There was also a significant difference between each of the two groups (p<0.05). In terms of miRNA, there were statistical differences in the levels of miR-188-3p, miR-335-5p, and miR-19a/b among the three groups (all P values < 0.001). There was also a significant difference between each of the two groups (p<0.05). There were no significant differences in other clinical data and laboratory test indicators among the three groups.
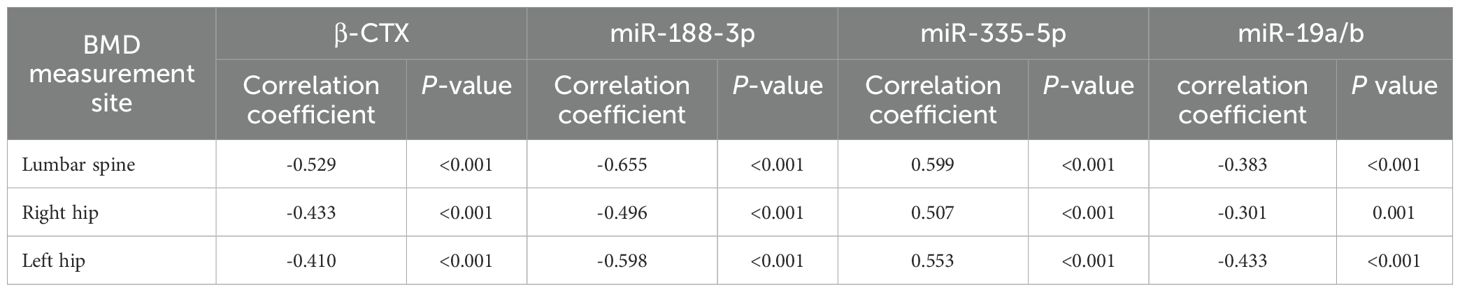
Correlation analysis of hip and lumbar spine mineral density with β-CTX, miR-188-3p, miR-335-5p, and miR-19a/b
In T2DM patients, hip and lumbar spine mineral density were negatively correlated with β-CTX, miR-188-3p, and miR-19a/b (P < 0.001), and hip and lumbar spine mineral density were positively correlated with miR-335-5p (P < 0.001), as shown in Table 2.
Logistic regression analysis of independent predictors for osteoporosis in T2DM and ROC curve evaluation of predictive ability
Logistic regression analysis was performed to evaluate the independent predictors of DOP in T2DM patients using β-CTX and miRNAs. The model was adjusted for age, gender, BMI, duration of diabetes, and HbA1c, and variables were screened using stepwise regression. The results showed that β-CTX, miR-188-3p, miR-335-5p, and miR-19a/b were independent risk factors for DOP (OR: 1.033, 95% CIs: 1.007-1.060; OR: 2.620, 95% CIs: 1.401-4.897; OR: 0.002, 95% CIs: 0.000-0.673; OR: 2.159, 95% CIs: 1.032-4.516, respectively) (Table 3). The ROC curve evaluated the clinical value of these indicators in DOP, among which the combination of the four had the highest diagnostic efficacy (Figure 3). The AUC value of the combined diagnosis was 0.909, sensitivity (82%) and specificity (78%), and the DeLong test demonstrated that the combined index was superior to the single index (P<0.05).
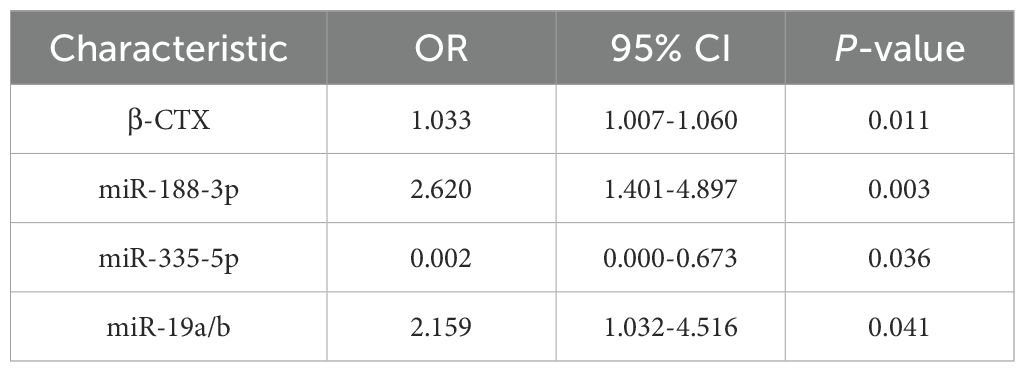
Table 3. Logistic regression analysis reveals independent predictors of osteoporosis in patients with T2DM.
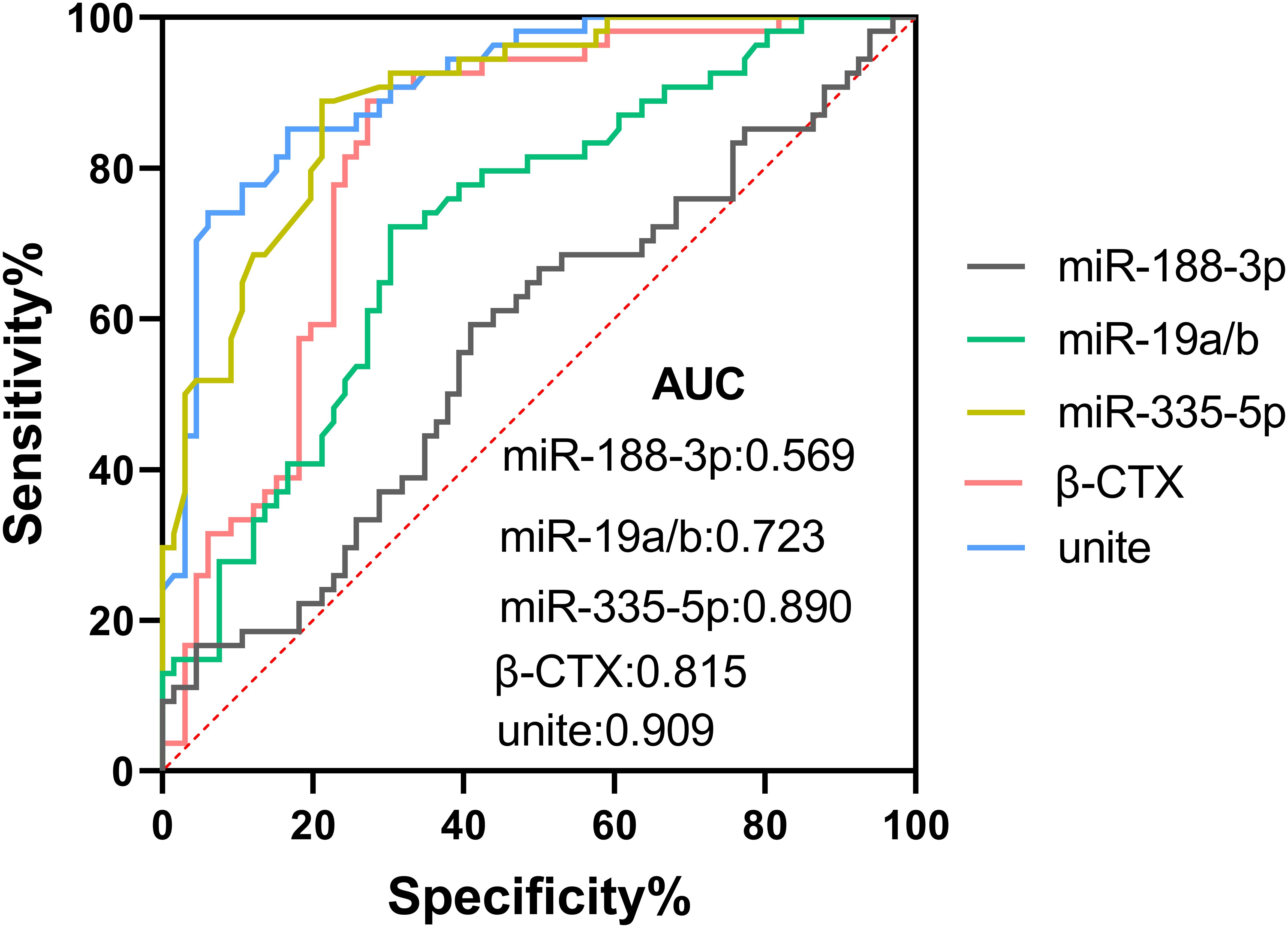
Figure 3. ROC curve analysis of β-CTX, miR-188-3p, miR-335-5p, and miR-19a/b as predictors of osteoporosis in T2DM, joint testing for maximum effectiveness.
Discussion
The incidence of osteoporosis among diabetes patients ranges from 52.1% to 54.68%, with 1/3 to 2/3 of these patients showing reduced bone mineral density (BMD) (14, 15). For diabetic patients, especially elderly patients, bone fractures not only affect their quality of life, but also present treatment challenges, incur high costs, and place significant economic and social burdens on families (16–18). DOP is a preventable and treatable chronic disease with an insidious course. However, once bone loss occurs, it is often irreversible. Therefore, developing scientifically effective screening strategies for high-risk populations is crucial for the prevention, early diagnosis, and treatment of osteoporosis, as well as identifying individuals at increased risk of fractures for early intervention (19). This study identified β-CTX, miR-188-3p, miR-335-5p, and miR-19a/b as potential biomarkers for predicting the occurrence of DOP.
Dual-energy X-ray absorptiometry (DXA) is currently the gold standard for diagnosing osteoporosis. DXA can distinguish between healthy individuals, osteopenia and osteoporosis, but it cannot distinguish between different potential causes of fracture risk. The changes in bone density measured take several weeks or months to be detected (20, 21). The International Osteoporosis Foundation (IOF) recommends the use of type I procollagen N-terminal propeptide (P1NP) and serum type 1 collagen cross-linked C-terminal peptide (β-CTX) as two indicators with good sensitivity. This recommendation has also been included in the “Guidelines for the Diagnosis and Treatment of Primary Osteoporosis”. However, although bone turnover markers can reflect the status of bone metabolism to a certain extent, their specificity and sensitivity are limited by factors such as circadian rhythms and diet (22–24). The fundamental regulatory role of miRNAs in biological functions and their abnormal expression in disease pathogenesis highlight the potential of miRNAs as disease biomarkers, especially in complex syndrome conditions (15, 18, 25). In this study, we analyzed the correlation between miRNAs and BMD and found that both hip and lumbar BMD were correlated with miR-188-3p, miR-335-5p, and miR-19a/b.
Previous studies have suggested that miR-188-3p may be involved in proliferation, apoptosis, and differentiation of chondrocytes in knee osteoarthritis (26). miR-335-5p can downregulate the expression of DKK1, an inhibitor of the Wnt pathway, thereby promoting the differentiation of osteoblasts. It has also been reported that miR-335-5p is closely linked to the growth and development of osteoblasts through signaling pathways such as MAPK, Focal adhesion, and ErbB (27). miR-19a/b can improve the osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporotic rats by regulating Ras homologous gene family members/Rho-associated coiled-coil protein kinase pathway proteins, promote bone mineralization, and facilitate bone formation (28). Despite initial reports of miR-19a/b and miR-335-5p in primary osteoporosis, this is the first time that the diagnostic value of their circulating levels has been validated in patients with secondary osteoporosis in T2DM and their gradient correlation with BMD has been revealed. Similar results were also observed in this study. The levels of miR-188-3p, miR-335-5p, and miR-19a/b were statistically different among the three research groups divided according to bone density loss, and ROC curve analysis showed that their combination with β-CTX can provide higher diagnostic efficacy. miRNA can provide more biological information for diagnosis and personalized treatment, and can serve as potential biomarkers for predicting osteoporosis in T2DM patients. In terms of sensitivity and specificity, although P1NP and β-CTX have been widely used as bone turnover markers in the clinic, they are affected by a variety of factors, such as circadian rhythms and diet, and there are limitations in their specificity and sensitivity. In contrast, the miRNA biomarkers identified in this study, such as miR-188-3p, miR-335-5p, and miR-19a/b, were found to be closely associated with the occurrence of DOP at the expression level in T2DM patients, and the area under the curve (AUC) values for the co-diagnosis of these miRNAs by the analysis of the working characteristics of the subjects (ROC) curve were high, and the sensitivity and specificity also showed a better level of sensitivity and specificity. The combined diagnostic index showed superior performance in predicting DOP compared with single indicators.
Moreover, the early diagnosis of DOP facilitated by the detection of these miRNAs holds significant promise in preventing fractures. By identifying individuals at high risk of DOP before the onset of clinical symptoms, clinicians can implement timely interventions, such as lifestyle modifications, pharmacological therapies, and regular bone density monitoring, to slow down or halt the progression of DOP. This proactive approach not only improves the quality of life for patients by reducing the likelihood of fractures but also alleviates the economic and social burdens associated with fracture-related complications. Therefore, the integration of miRNA biomarkers into routine clinical practice for the early diagnosis of DOP represents a crucial step forward in the management of this debilitating condition.
In contrast to previous studies, we found that the suppression of miR-335-5p expression in DOP may be affected by both the hyperglycemic microenvironment and insulin resistance, which provides a new perspective to understand the ‘dual risk factor’ of diabetic bone metabolism. It has been shown that miRNAs such as miR-188-3p, miR-335-5p and miR-19a/b are closely related to the processes of bone formation and bone resorption, and they may affect the functions of osteoblasts and osteoclasts by regulating specific signaling pathways (29). However, the specific mechanisms of the role of these miRNAs in the development of DOP in T2DM patients are not fully understood.
This study has limitations due to its retrospective design, small sample size, and lack of systematic quality control. The results may be influenced by other factors, leading to variability in conclusions. In addition, the small sample size of the osteoporosis subgroup in this study may have affected the validity of analyses of rare covariates (e.g., severe vitamin D deficiency). Unfortunately, due to the limitations of the study design and scope of data collection, the effect of menopausal status on miRNA expression and bone metabolic markers was not systematically analyzed in this paper. Postmenopausal women are known to be at high risk for osteoporosis and fractures. Hormonal changes associated with menopause can have a significant impact on bone metabolism, which may alter the expression patterns of miRNAs and other DOP-related biomarkers. Therefore, the results of this study, while valuable, may not fully reflect the complexity of DOP in postmenopausal women with T2DM. Future studies with a specific focus on postmenopausal women will allow us to better understand the specific role of miRNAs in DOP in this high-risk subgroup and develop more targeted screening and intervention strategies. Future research will aim to validate these findings in multi-center, large-sample, prospective studies. Although this study controlled for confounders as much as possible through baseline characteristic comparisons and covariate adjustment, unrecorded variables (e.g., exercise intensity, dietary calcium intake) may have influenced the results. Further validation in conjunction with a prospective design is needed in the future. Additionally, U6 may not be suitable as a universal internal reference across all pathological conditions. Future studies will incorporate the combined use of multiple internal reference genes, such as miR-16 and RNU48, to enhance methodological rigor and standardization.
Conclusion
The findings of this study demonstrate that circulating levels of miR-188-3p, miR-335-5p, and miR-19a/b are significantly associated with the development of DOP in patients with T2DM. These miRNAs exhibit promising potential as biomarkers for early diagnosis of DOP.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of General Hospital of Ningxia Medical University (KYLL-2022-0693). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YL: Conceptualization, Data curation, Funding acquisition, Project administration, Validation, Writing – original draft, Writing – review & editing. LG: Formal Analysis, Investigation, Methodology, Writing – review & editing. DZ: Resources, Software, Visualization, Writing – review & editing. HL: Data curation, Formal Analysis, Methodology, Validation, Writing – review & editing. LS: Data curation, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was supported by Natural Science Foundation of Ningxia Province (No. 2024AAC03585).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. (2021) 326:2498–506. doi: 10.1001/jama.2021.22208
2. Ruze R, Liu T, Zou X, Song J, Chen Y, Xu R, et al. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne). (2023) 14:1161521. doi: 10.3389/fendo.2023.1161521
3. Prasad TN, Arjunan D, Pal R, and Bhadada SK. Diabetes and osteoporosis. Indian J Orthop. (2023) 57:209–17. doi: 10.1007/s43465-023-01049-4
4. Kalra S, Joshi A, and Kapoor N. Osteoporosis and diabetes: The dual pandemics. J Pak Med Assoc. (2022) 72:1663–4. doi: 10.47391/JPMA.22-86
5. Schacter GI and Leslie WD. Diabetes and osteoporosis: part II, clinical management. Endocrinol Metab Clin North Am. (2021) 50:287–97. doi: 10.1016/j.ecl.2021.03.006
6. Schacter GI and Leslie WD. Diabetes and osteoporosis: part I, epidemiology and pathophysiology. Endocrinol Metab Clin North Am. (2021) 50:275–85. doi: 10.1016/j.ecl.2021.03.005
7. Shevroja E, Cafarelli F P, Guglielmi G, and Hans D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine. (2021) 74:20–8. doi: 10.1007/s12020-021-02806-x
8. Rasmussen NH and Vestergaard P. Diabetes and osteoporosis - Treating two entities: A challenge or cause for concern? Best Pract Res Clin Rheumatol. (2022) 36:101779. doi: 10.1016/j.berh.2022.101779
9. Ho PTB, Clark IM, and Le LTT. MicroRNA-based diagnosis and therapy. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23137167
10. Li Q C, Li C, Zhang W, Pi W, and Han N. Potential effects of exosomes and their microRNA carrier on osteoporosis. Curr Pharm Des. (2022) 28:899–909. doi: 10.2174/1381612828666220128104206
11. Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi G, Zhichen W, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res. (2020) 53:40. doi: 10.1186/s40659-020-00309-z
12. Harreiter J and Roden M. Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023). Wien Klin Wochenschr. (2023) 135:7–17. doi: 10.1007/s00508-022-02122-y
13. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. (2020) 26:1–46. doi: 10.4158/GL-2020-0524SUPPL
14. Paschou SA and Vryonidou A. Diabetes mellitus and osteoporosis. Minerva Endocrinol. (2019) 44:333–5. doi: 10.23736/S0391-1977.19.03040-2
15. Wu EL, Cheng M, Zhang XJ, Wu TG, and Zhang L. The role of non-coding RNAs in diabetes-induced osteoporosis. Differentiation. (2023) 133:98–108. doi: 10.1016/j.diff.2023.08.002
16. Mohsin S, Baniyas MM, AlDarmaki RS, Tekes K, Kalasz H, Adeghate EA, et al. An update on therapies for the treatment of diabetes-induced osteoporosis. Expert Opin Biol Ther. (2019) 19:937–48. doi: 10.1080/14712598.2019.1618266
17. Jackuliak P, Kovarova M, Kuzma M, and Payer J. Osteoporosis in diabetes mellitus patients. Vnitr Lek. (2021) 67:291–5. doi: 10.36290/vnl.2021.076
18. Martiniakova M, Biro R, Penzes N, Sarocka A, Kovacova V, Mondockova V, et al. Links among obesity, type 2 diabetes mellitus, and osteoporosis: bone as a target. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25094827
19. Ala M, Jafari RM, and Dehpour AR. Diabetes mellitus and osteoporosis correlation: challenges and hopes. Curr Diabetes Rev. (2020) 16:984–1001. doi: 10.2174/1573399816666200324152517
20. Brandt IAG, Starup-Linde J, Andersen SS, and Viggers R. Diagnosing osteoporosis in diabetes-A systematic review on BMD and fractures. Curr Osteoporos Rep. (2024) 22:223–44. doi: 10.1007/s11914-024-00867-1
21. Khosla S, Samakkarnthai P, Monroe DG, and Farr JN. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat Rev Endocrinol. (2021) 17:685–97. doi: 10.1038/s41574-021-00555-5
22. Guo H, Wang C, Jiang B, Ge S, Cai J, Zhou Y, et al. Association of insulin resistance and beta-cell function with bone turnover biomarkers in dysglycemia patients. Front Endocrinol (Lausanne). (2021) 12:554604. doi: 10.3389/fendo.2021.554604
23. Wen Y, Li H, Zhang X, Liu P, Ma J, Zhang L, et al. Correlation of osteoporosis in patients with newly diagnosed type 2 diabetes: A retrospective study in chinese population. Front Endocrinol (Lausanne). (2021) 12:531904. doi: 10.3389/fendo.2021.531904
24. Pinto D, Alshahrani M, Chapurlat R, Chevalley T, Dennison E, Camargos BM, et al. The global approach to rehabilitation following an osteoporotic fragility fracture: A review of the rehabilitation working group of the International Osteoporosis Foundation (IOF) committee of scientific advisors. Osteoporos Int. (2022) 33:527–40. doi: 10.1007/s00198-021-06240-7
25. Daamouch S, Emini L, Rauner M, and Hofbauer LC. MicroRNA and diabetic bone disease. Curr Osteoporos Rep. (2022) 20:194–201. doi: 10.1007/s11914-022-00731-0
26. Ji F, Zhu L, Pan J, Shen Z, Yang Z, Wang J, et al. hsa_circ_0026827 promotes osteoblast differentiation of human dental pulp stem cells through the beclin1 and RUNX1 signaling pathways by sponging miR-188-3p. Front Cell Dev Biol. (2020) 8:470. doi: 10.3389/fcell.2020.00470
27. Zhang K, Qiu W, Li H, Li J, Wang P, Chen Z, et al. MACF1 overexpression in BMSCs alleviates senile osteoporosis in mice through TCF4/miR-335-5p signaling pathway. J Orthop Translat. (2023) 39:177–90. doi: 10.1016/j.jot.2023.02.003
28. Taipaleenmaki H, Saito H, Schroder S, Maeda M, Mettler R, Ring M, et al. Antagonizing microRNA-19a/b augments PTH anabolic action and restores bone mass in osteoporosis in mice. EMBO Mol Med. (2022) 14:e13617. doi: 10.15252/emmm.202013617
Keywords: type 2 diabetes mellitus, osteoporosis, miRNA, early diagnosis, diabetic osteoporosis (DOP)
Citation: Li Y, Gan L, Zhao D, Lei H and Sha L (2025) Circulating microRNAs as a potential biomarker for osteoporosis in patients with type 2 diabetes mellitus: a retrospective clinical study. Front. Endocrinol. 16:1534725. doi: 10.3389/fendo.2025.1534725
Received: 26 November 2024; Accepted: 06 June 2025;
Published: 04 August 2025.
Edited by:
Ali Ghasem-Zadeh, University of Melbourne, AustraliaReviewed by:
Rahul Kumar Maurya, Washington University in St. Louis, United StatesAhmet Fatih Durmusoglu, Istanbul Medipol University, Türkiye
Copyright © 2025 Li, Gan, Zhao, Lei and Sha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqi Li, eXVraWxlZTQxN0AxNjMuY29t
 Yuqi Li
Yuqi Li Lu Gan
Lu Gan Liping Sha
Liping Sha