- 1Montioring and Evaluation, Wolaita Zone Health Department, Wolaita Sodo, Ethiopia
- 2School of Public Health, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 3School of Public Health, College of Medicine and Health Sciences, Wachemo University, Hossana, Ethiopia
- 4School of Public Health, Walailak University, Nakhon Si Thammarat, Thailand
Background: Globally, non-communicable diseases contribute to about three-quarters of all deaths. Developing nations face particular difficulties with the concurrent presence of diabetes and high blood pressure, problems intensified by poor nutrition habits, lack of physical exercise, and continuing high rates of illnesses like HIV/AIDS and malaria. A significant data gap in primary healthcare (PHC) data has been determined and prioritized by governments seeking to assess and enhance the prevention and management of chronic disease.
Objective: The main aim of the current study is to determine factors related to hypertension among patients with type 2 diabetes in public PHC facilities in Southern Ethiopia.
Methods: A facility-based cross-sectional study was conducted from 6 March 2023 to 5 April 2023 among 409 patients with diabetes. A systematic random sampling technique was employed to select the study participants. The data were cleaned, coded, and entered into Epi-data version 4.6.0.2 and exported to STATA version 14 for analysis. For descriptive statistics, both bivariate and multivariable logistic regression analyses were employed. Variables with a p-value <0.05 in the multivariable logistic regression analysis were declared as significantly associated with outcome variables.
Results: A total of 407 patients with type 2 diabetes mellitus (T2DM) were included in the current study with a response rate of 99.5%, and the mean age of the study participants was 57.1 years (SD ±9.91). The prevalence of hypertension among patients with T2DM was 66.1% with a 95% confidence interval (CI) of 59.9–70.3. The age group of >60 years [adjusted odds ratio (AOR) = 2.09, 95% CI (1.02–4.28)], patients with ≥10 years’ duration of T2DM [AOR = 1.79, 95% CI (1.05–3.03)], body mass index ≥25 kg/m2 [AOR = 4.19, 95% CI (2.10–8.33)], and patients who have a family history of hypertension [AOR = 11.73, 95% CI (5.82–23.66)] were significantly associated with hypertension among patients with type 2 diabetes.
Conclusion: The prevalence of hypertension is high and the majority has poor blood pressure control. Hence, DM care providers and other health sector stakeholders have to work in collaboration to prevent it by designing appropriate strategies especially for those at higher risk of developing hypertension.
Introduction
High blood pressure stands as the third-leading cause of health burden measured in disability-adjusted life years (DALYs). While impacting 972 million adults globally in 2000, projections indicate this may rise to 1.56 billion cases by 2025 (1). Low- and middle-income countries (LMICs) bear 60% of the total impact, reflecting system healthcare and preventive care challenges (1). In sub-Saharan Africa (SSA), the burden reaches up to 38% in some communities, with an estimated 10–20 million hypertensive individuals among 650 million people in SSA (2–4). In Addis Ababa, Ethiopia, hypertension (HTN) burden ranges from 155 to 305, indicating significant regional variability and underdiagnosis (5).
Diabetes mellitus (DM), a chronic condition marked by hyperglycemia, affected 460 million individuals globally in 2019 and categorized as the world’s eighth-leading disability-adjusted fatal disease and ranking as the eighth-leading cause of disability-related mortality (6). By 2021, the International Diabetes Federation (IDF) reported 537 million cases, with health expenditures nearing $966 billion. Undiagnosed cases are rampant, particularly in LMICs (7, 8). In 2022, approximately 54 million adults aged above 18 years in the WHO Africa region had diabetes, with >50% undiagnosed (34 million untreated), exacerbating complications like renal failure and amputation (9). National prevalence was 3.2% in Ethiopia in 2015 by the WHO survey (10).
DM and HTN are worldwide public health issues (11). These have been shown to be two of the main risk factors for cardiocerebrovascular illnesses, which are the top causes of adult mortality and disability (12). DM and HTN are additional problems facing the healthcare system in LMICs (13), attributable to shifts in dietary patterns and physical inactivity (14, 15), and with malaria and HIV on the rise, this region faces a growing dual burden, resulting in significant public health consequences (16). This disease is disproportionately more common in countries with limited resources and underdeveloped healthcare systems (17). In underdeveloped nations, the death rate is increased by 7.2 times when diabetes and HTN coexist. According to epidemiological research, DM increases the risk of HTN (18, 19). An American research, for example, found that up to three-fourths of adults with diabetes also had HTN (20), and in China, approximately 15 million people have both conditions (21).
Type 2 diabetes mellitus (T2DM) and HTN together are especially deadly and greatly increase a person’s cardiovascular risk. High BP and T2DM also raise the risk of acquiring additional diabetes-related conditions, such as renal disease and retinopathy, which can result in blindness (22). Furthermore, the formation of resistant HTN is linked to their cohabitation (23, 24). To reduce the risk of diabetic complications, early identification of HTN and related cardiovascular risk factors is necessary (25). The treatment of HTN’s side effects, such as dialysis, cardiac bypass surgery, and stroke prevention, is an expensive intervention that depletes both private and public funds (26). Early identification and effective management of HTN are associated with significant health and financial benefits (27).
Approximately 4 out of 10 (41%) patients had at least one of the following seven chronic conditions: diabetes, asthma, HTN, and others (28). Effectively managing patients with many chronic diseases in primary care is crucial, as the prevalence of multimorbidity rises with age (29). A significant data gap in primary healthcare (PHC) data has been determined and prioritized by governments seeking to assess and enhance the prevention and care of chronic diseases (5, 30). To effectively prevent and control HTN, PHC settings need to collect comparable and aggregated data on the disease burden from the condition. This study aims to determine factors associated with HTN among patients with T2DM in Southern Ethiopia.
Materials and methods
Research framework, duration, and location
This cross-sectional study enrolled 409 patients with diabetes attending primary hospitals in the Wolaita zone between 6 March and 5 April 2023. The Wolaita zone is located in the southern region of Ethiopia, which is 380 km away from Ethiopia’s capital, Addis Ababa. In the study area, there exist eight primary hospitals providing services to all zonal districts’ population and other population groups near the Wolaita zone.
Population
The study included adult patients with DM aged ≥18 years who were under follow-up at selected public PHC chronic outpatient departments and met the inclusion criteria. Eligible participants had a documented history of at least three consecutive follow-up visits at the study facility. Critically ill patients and pregnant women with DM were excluded during the data collection period.
Sample size determination and sampling procedure
The sample size (n) was calculated using the single population proportion formula, assuming an HTN prevalence of 59.5% among patients with T2DM of another study conducted at DMGH (31) with 95% confidence interval (CI) and 5% margin of error, and 10% of the population size was added to adjust the non-response rate of study participants and to estimate our study sample size. Thus, , and after adding 10% of the non-response rate, the sample used for final study was n = 407.
The study was conducted at seven randomly selected primary hospitals (Tebela, Bele awassa, Bodit, Bedesa, Gesuba, Bitana, and Bomb primary hospital) across the study setting. An SRS method was used to identify the study subjects and 81 adult patients with DM who were followed at Tebela primary hospital, 85 at Bele awassa primary hospital, 92 at Bodit primary hospital, 69 at Gesuba primary hospital, 48 at Bitana primary hospital, 41 at Bedesa primary hospital, and 65 at Bomb primary hospital. The patient’s medical registration number from each primary hospital HTN registration log book was first used to establish a sampling frame. Following that, each hospital received a proportionate share of the computed sample size. Next, a computer-generated specialized random sampling procedure was used to choose research participants from each of the chosen hospitals.
Operational definition
HTN: Participants were classified as hypertensive if they met either of the following criteria: (1) average systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg, or (2) current use of antihypertensive medication (32). HTN staging followed standard classifications: prehypertension (120–139/80–89 mmHg), stage 1 (140–159/90–99 mmHg), stage 2 (160–179/100–109 mmHg), and hypertensive crisis (≥180/110 mmHg) (33). Current smokers were defined as individuals who reported cigarette use at least once during the month preceding the study (34). Poor glycemic control was operationally defined as having a hemoglobin A1c (HbA1c) level exceeding 7% (35).
Patients with diabetes were identified through medical records as individuals with an established diagnosis who were actively engaged in follow-up care at the participating healthcare facilities, as evidenced by possession of a medical record number.
Data collection instrument and procedure
Data were collected using a pretested interviewer-administered questionnaire adapted from relevant literature (31, 36). The study employed a mixed-method approach involving face-to-face interviews, physical examinations, and medical record reviews. Behavioral risk factors were evaluated using the WHO STEPwise surveillance methodology for chronic disease risk factors. Anthropometric measurements included weight, measured to the nearest 0.1 kg using a calibrated scale, and height, measured in meters using a stadiometer with participants standing erect on a level surface. Body mass index (BMI) was subsequently calculated as weight in kilograms divided by height in squared meters.
Blood pressure measurements were obtained using a manual mercury sphygmomanometer, with the left arm positioned at heart level. Participants rested for at least 10 min before measurement (extended to 30 min for those who consumed hot beverages like coffee). Three consecutive readings were taken, and the average of these measurements was used for analysis.
To ensure data quality, the questionnaire was pretested on 5% of the total sample size at a non-selected primary hospital, with reliability assessed using Cronbach’s alpha. Data collection was conducted by four nurses holding bachelor’s degrees, who received 1 day of training on fundamental data collection principles. Supervisors underwent additional training focused on ensuring data completeness, cross-checking entries, and error correction.
Data analysis
The collected data were cleaned using Epi-Data software version 4.6.0.2 before being exported to SPSS version 26 for statistical analysis. Initial analysis involved computation of descriptive statistics, with results presented as frequencies and percentages. Associations between explanatory and outcome variables were examined using bivariate and multivariable logistic regression with a stepwise approach. Statistical significance was determined by an adjusted odds ratio (AOR) with a 95% CI and a p-value of less than 0.05.
Data normality was assessed through visual inspection of Q-Q plots and histograms, with the Q-Q plot approximating the diagonal line and the histogram showing no skewness, confirming normal distribution. Multicollinearity among predictor variables was evaluated using tolerance values greater than 0.1 and variance inflation factors (VIFs) less than 10, indicating no significant collinearity. Model fitness was assessed using the Hosmer–Lemeshow goodness-of-fit test, which yielded a p-value of 0.501 (χ² = 1.44), confirming that the model was well-fitted to the data.
Ethics statement
The Ethical Review Committee (ERC) of the College of Health Science, School of Public Health, Wachemo University performed a review and allowed the study to proceed (ref. no. wcu/000981/15). In addition, formal letters of permission were obtained from the Tebela town administrative health office, the Bele awassa town administrative health office, the Bodit town administrative health office, the Bedesa district health office, the Gesuba town administrative health office, the Bitana district health office, and the Bomb district health office. The letters of permission written from district/town health offices were submitted to the respective hospitals. The study’s objectives and methods were explained to each participant. Additionally, prior to participation, all study participants provided written informed permission. The study’s goal, the thorough review of their medical records, and the potential benefits of the research were also explained to the participants. The data gathered were used for the study’s objectives. All of the information gathered throughout the study was kept private, available only to the research team and utilized only for that reason. The participants’ medical record numbers were utilized instead of personal identifiers for the purpose of gathering data. The study was carried out in compliance with the applicable rules, laws, and Helsinki Declaration principles.
Patient and public involvement
No patients or public groups were involved in the design, analysis, or interpretation of this study, and they were also not involved in the dissemination of the results.
Results
Socio-demographic characteristics of study participants
The study included 407 out of 409 eligible patients with T2DM, yielding a response rate of 99.5%. The mean age of the participants was 57.1 years (± 9.91 SD), with the largest age group (35.6%, n = 145) being 51–60 years old. The cohort consisted of predominantly male (52.1%, n = 212) and urban-dwelling (85.7%, n = 349) individuals. Educationally, 41.0% (n = 167) had attained education beyond a college diploma, while 73.0% (n = 297) were married. Government employees constituted 39.6% (n = 161) of participants, and over half (52.8%, n = 215) reported a monthly income exceeding 5,000 Ethiopian birr (ETB). These characteristics collectively describe an urban-predominant, middle-aged population with relatively high educational attainment and income levels compared to national averages (Table 1).
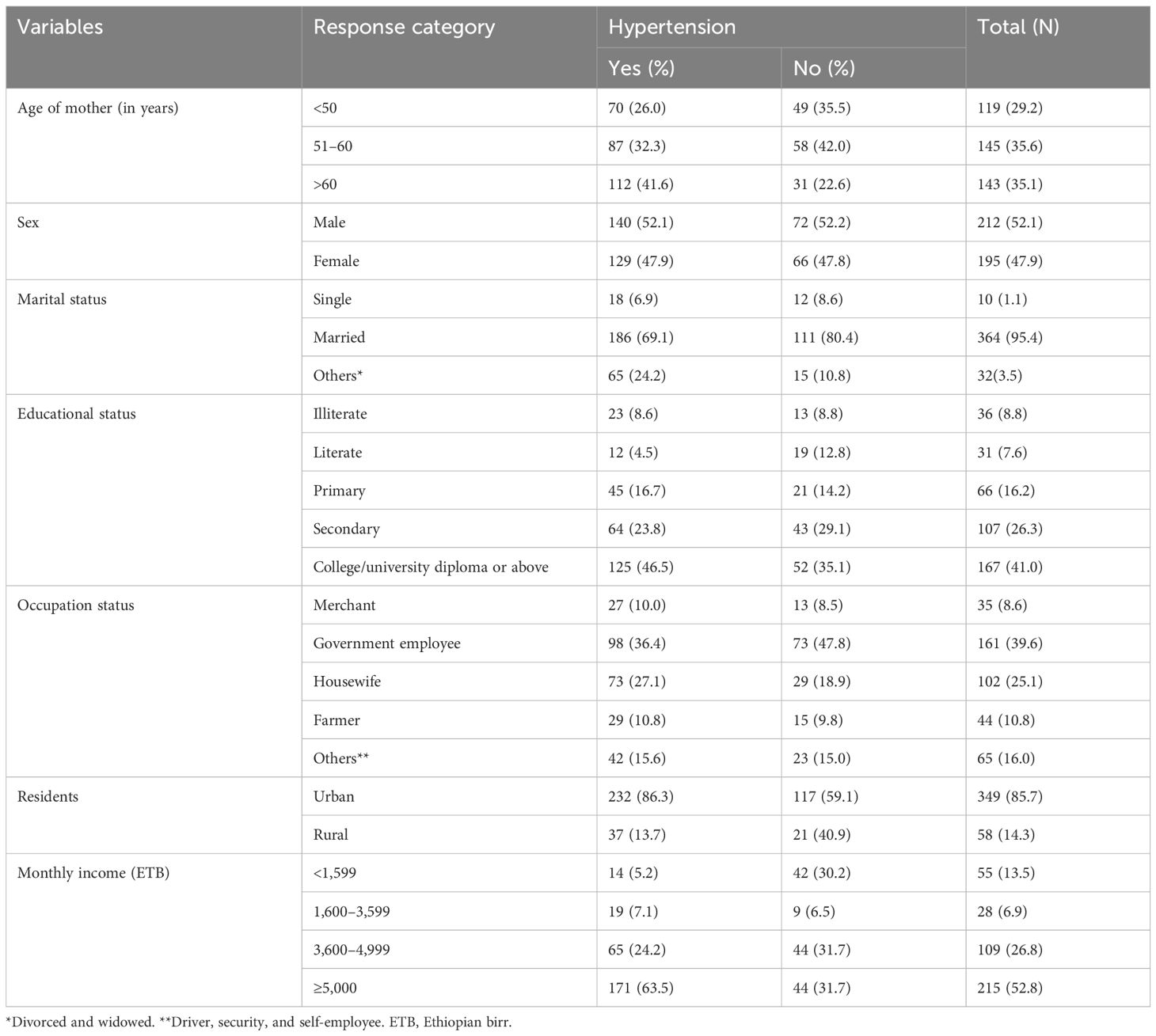
Table 1. Socio-demographic characteristics of patients with type 2 diabetes mellitus: evidence from a resource-limited setting—Southern Ethiopia.
Clinical characteristics of participants
The study population had an average diabetes duration of 10.8 years (± 3.68 SD), ranging from 3 to 25 years. Glycemic control measurements showed a mean HbA1c of 8.2% (± 2.37 SD), with 54.1% (n = 220) of participants exhibiting poor glycemic control (HbA1c >7%). The average fasting blood glucose level was 152.2 mg/dL (± 45.8 SD), while mean blood pressure readings were 131.4/82.5 mmHg (± 16.7/9.0 SD) for systolic and diastolic pressures, respectively. Treatment patterns revealed that 39.8% (n = 162) of patients were on oral hypoglycemic monotherapy, while 37.6% (n = 153) received dual therapy for diabetes management. Anthropometric data indicated that 66.1% (n = 269) of participants fell within the normal BMI range (18.5–24.9 kg/m²). These metabolic parameters collectively demonstrate the clinical characteristics and management profile of the studied diabetic population. Approximately 281 (69.05%) study participants had no history of HTN in the family. Majority of study participants, 403 (99.0%), had no history of chronic kidney disease (CKD), 375 (92.1%) had no history of cardiac heart disease (CHD), 376 (92.4%) had no history of alcohol drinking, and 395 (97.1%) had no history of smoking. Greater than half, 219 (53.8%), of study participants had physical activity of less 3 days per week. Greater than two-thirds, 274 (67.3%), of participants had a history of diabetes for more than 10 years’ duration and 293 (72.0%) participants had a self-glucometer at home. Majority of respondents, 405 (99.5%), had a history of attending health education on DM at a health facility (Table 2).
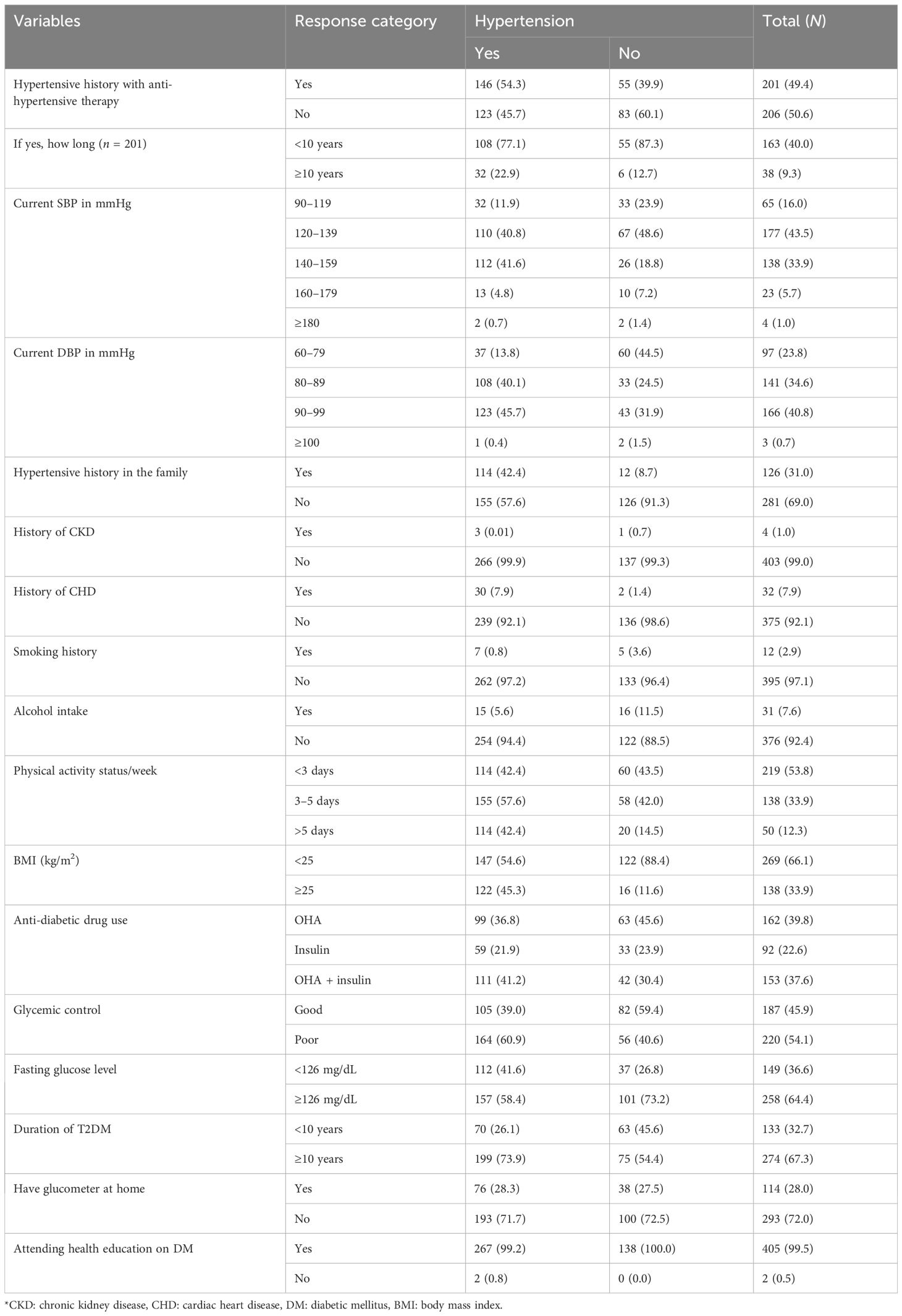
Table 2. Clinical characteristics of patients with type 2 diabetes mellitus: evidence from a resource-limited setting—Southern Ethiopia.
Prevalence and patterns of hypertension among patients with T2DM
HTN was identified in 66.1% (95% CI: 59.9–70.3) of the participants with type 2 diabetes. From these, 68 (25.2%) were newly diagnosed at the time of data collection. The majority, 177 (43.5%), of patients with type 2 diabetes were prehypertensive, followed by stage 1 hypertensive, 138 (33.9%); 23 (5.7%) study participants were stage 2 hypertensive and 4 (1%) were in the hypertensive crisis category.
Statistical analysis of hypertension risk factors
The association between potential predictors and HTN was examined through binary logistic regression analysis. Initial candidate variables considered for the model included age, marital status, family history of HTN, physical activity level, duration of T2DM, BMI, use of anti-diabetic medications, glycemic control status, and fasting blood glucose levels. In the final multivariate analysis, four factors emerged as statistically significant predictors of HTN: age, duration of T2DM, BMI, and family history of HTN.
Advanced age showed a significant association with HTN risk. After adjusting for all other variables in the model, participants aged over 60 years demonstrated 2.09 times greater odds of developing HTN compared to those younger than 50 years (AOR = 2.09; 95% CI: 1.02–4.28; p = 0.044). Elevated BMI also proved to be an important risk factor, with overweight or obese participants (BMI ≥ 25 kg/m²) having 4.19 times higher odds of HTN than those with normal BMI (AOR = 4.19; 95% CI: 2.10–8.33; p = 0.001).
The duration of diabetes showed a significant relationship with HTN risk. Patients with a T2DM duration of 10 years or more had 1.8 times greater odds of developing HTN compared to those with a shorter disease duration (AOR = 1.8; 95% CI: 1.05–3.03; p = 0.03). Most strikingly, family history of HTN emerged as the strongest predictor, with affected participants showing 11.7 times higher odds of developing HTN compared to those without such history (AOR = 11.73; 95% CI: 5.82–23.66; p = 0.001). The notably wide CI for family history may reflect limited precision due to the relatively small subgroup size (n = 126). This finding was further validated using Firth’s penalized regression analysis, which addresses small-sample bias, confirming the strong association (AOR = 10.41; 95% CI: 5.12–21.14) (Table 3).
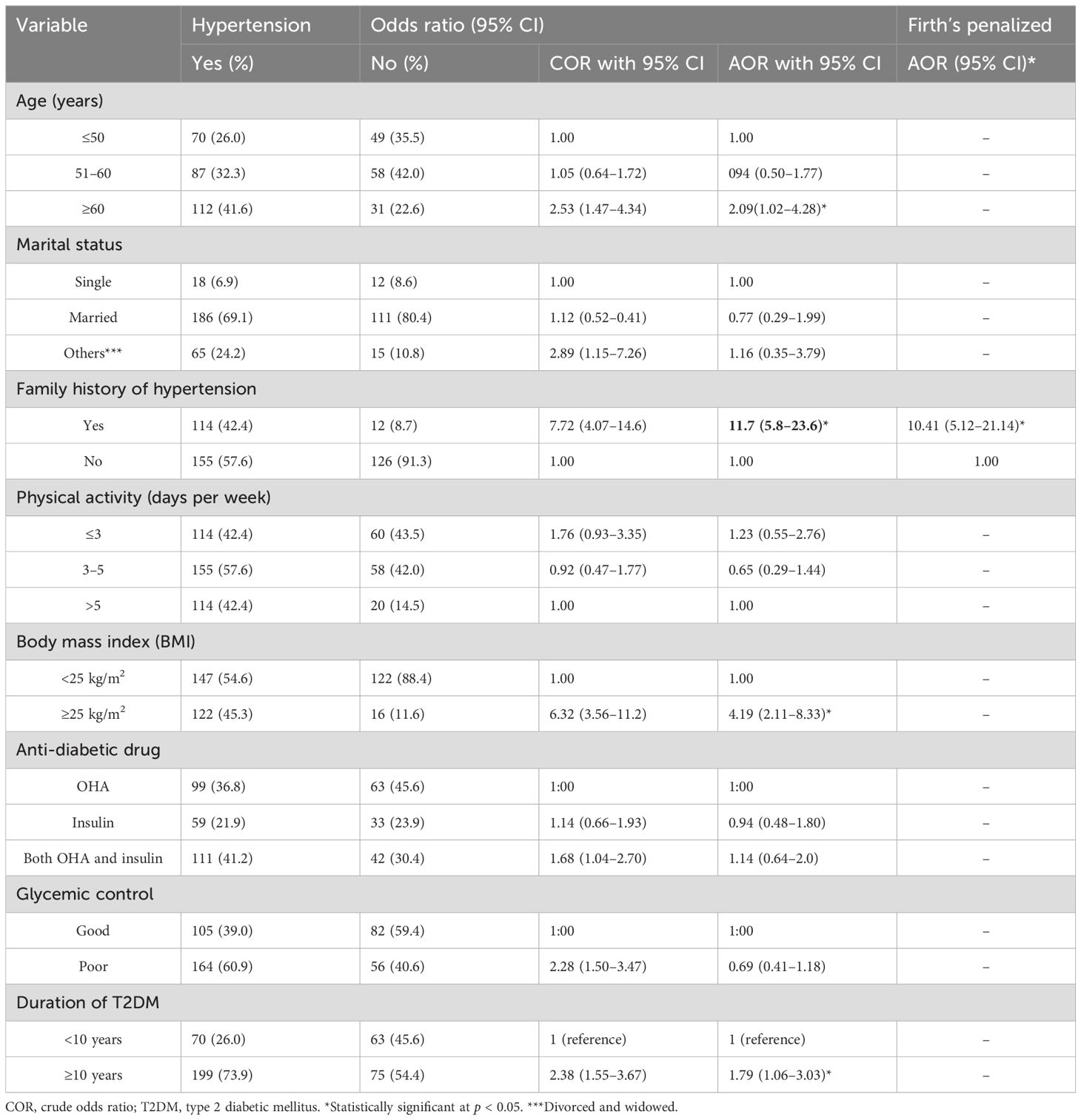
Table 3. Multivariable logistic regression analysis of factors associated with hypertension among patients with type 2 diabetes mellitus: evidence from a resource-limited setting—Southern Ethiopia.
Discussion
This study showed the prevalence of HTN and associated factors among patients with T2DM in public PHC facilities in Southern Ethiopia. The prevalence of HTN in this study was 66.1% with a 95% CI of 59.9–70.3. The current findings are in line with studies in Botswana (63.1%) (32) and in Israel (60.2%) (5). However, the current findings are higher than the prevalence reported in Nigeria (54.2%) (33), Jimma (46.5%) (18), Hosanna (55%) (37), Debor Tabour, Ethiopia (59.5%) (31), Adama, Ethiopia (56.3%) (38), a national diabetes center in Greece (55.6%) (39), and the United States (49.8%) (40). The observed HTN prevalence (66.1%) was lower than that reported in Jordan (72.4%) (41). The substantial differences in HTN prevalence across studies may be attributed to several factors, including variations in diagnostic criteria and distinct population characteristics. Notably, a prospective cohort study conducted in Iraq from August 2008 to April 2011 involving 5,578 patients with T2DM reported an HTN prevalence of 89.6%, with 45.3% of cases being newly identified during the study period (42). The observed discrepancies may stem from variations in diagnostic thresholds (e.g., >130/80 mmHg versus higher cutoffs), differences in study populations’ sociodemographic profiles, and methodological factors including study design, healthcare access patterns, and lifestyle variations among participants. These elements collectively contribute to prevalence rate differences across studies while highlighting the importance of standardized diagnostic criteria and population-specific considerations in HTN research. Such variations underscore the need for careful interpretation of findings relative to each study’s unique context and methodology.
The prevalence of newly diagnosed HTN was 25.2% with a 95% CI of 21.7–29.0. The current findings were in line with studies conducted in Dire Dawa city, eastern Ethiopia (24.43%) (43) and in Gimbi Town, western Ethiopia (24.8%) (44). However, the HTN prevalence identified in our study substantially exceeds the 15.8% rate documented in Ethiopia’s national NCD STEPS survey of the general adult population (45), in Debre-Markos, northwest Ethiopia (12.7%) (31), and Durame town, southern Ethiopia (14%) (46). However, the current findings were lower than those in community-based studies in Addis Ababa, central Ethiopia (newly diagnosed HTN prevalence, 29.2%) (47) and a multicenter facility-based study in Addis Ababa, central Ethiopia (newly diagnosed HTN prevalence, 32.3%) (48). This discrepancy may occur because individuals at risk of HTN are more likely to seek screening at urban tertiary care centers than at PHC facilities. The increased access to specialized services in major urban areas could lead to higher case detection rates compared to peripheral health institutions.
The study demonstrated a significant age-dependent association with HTN, where participants aged >60 years had 2.09 times higher odds of developing HTN (AOR = 2.09; 95% CI: 1.02–4.28) compared to those <50 years. These findings align with previous epidemiological studies conducted in Israel (49), Iraq (42), and Botswana (50), confirming the well-established relationship between advancing age and increased HTN prevalence. The observed association can be attributed to age-related physiological and structural changes in the cardiovascular system. With progressive aging, the vascular system undergoes significant remodeling characterized by endothelial dysfunction, reduced arterial elasticity, and increased vascular stiffness. These changes result from complex pathophysiological alterations affecting all three layers of the arterial wall—the intima, media, and adventitia. Key mechanisms include diminished nitric oxide bioavailability, increased collagen deposition, elastin fragmentation, and medial calcification (51).
This study further demonstrated that individuals with a BMI of ≥25 kg/m² had a significantly elevated risk of developing HTN compared to those with normal BMI. These findings align with previous research conducted in various settings, including Jimma, Gondar, Jijiga, Bayisa State, Nigeria, Kenya, and urban Varanasi (18, 52–57). The pathophysiological basis for this association involves multiple interrelated mechanisms. Excess adiposity contributes to increased cardiovascular risk through endothelial dysfunction, chronic low-grade inflammation, and hemodynamic alterations that collectively promote atherosclerosis (58). Furthermore, overweight and obesity induce insulin resistance and dyslipidemia, characterized by elevated low-density lipoprotein cholesterol deposition in vascular walls. These metabolic disturbances lead to progressive arterial stiffening and luminal narrowing, ultimately resulting in HTN development (32).
The study demonstrated a significant duration-dependent relationship between T2DM and HTN, with longer diabetes duration correlating with increased HTN prevalence. This finding is consistent with prior research indicating that prolonged T2DM exacerbates metabolic and vascular dysfunction, thereby elevating HTN risk (16, 30, 31). The underlying mechanisms involve progressive metabolic and hemodynamic disturbances associated with chronic hyperglycemia. Extended exposure to insulin resistance, dyslipidemia, and sustained hyperglycemia leads to cumulative microvascular damage, endothelial dysfunction, and sympathetic nervous system overactivation. Additionally, prolonged diabetes duration enhances renin–angiotensin–aldosterone system (RAAS) activity while reducing insulin-mediated vasodilation, further contributing to elevated blood pressure (34).
The study demonstrated a robust association between familial HTN history and HTN development in patients with T2DM. Individuals with a family history of HTN exhibited a 10.4-fold increased likelihood of developing HTN compared to those without such history (AOR = 10.4, 95% CI: 5.12–21.14). These findings align with epidemiological evidence from Sri Lanka (58), Northern India (59), and multiple international studies (60–64). The observed familial aggregation likely reflects both shared genetic susceptibility and common environmental exposures. From a genetic perspective, multiple polygenic variants affecting renal sodium handling, vascular tone regulation, and RAAS system function may contribute to this predisposition (26). Additionally, familial clustering of modifiable risk factors—including dietary patterns (particularly sodium and potassium intake), physical activity levels, and stress coping mechanisms—may synergistically interact with genetic predisposition to elevate HTN risk within families.
Limitation of the study
While this study identified four significant predictors of HTN (age, family history, BMI, and diabetes duration), several potential confounding variables remain unaccounted for in our analysis. These include medication adherence patterns, specific dietary factors (such as sodium intake or DASH diet compliance), and objective measures of physical activity. Such unmeasured variables may represent important modifiers of HTN risk that could influence the observed associations. Future research incorporating these additional parameters would provide a more comprehensive understanding of HTN risk factors in diabetic populations.
Conclusion
This study revealed that over 50% of participants with T2DM also had HTN. Our analysis identified significant associations between HTN and four key factors: advanced age, family history of HTN, elevated BMI, and longer duration of T2DM.
Understanding the global prevalence of HTN in diabetic populations is crucial for formulating effective public health strategies. Such epidemiological data inform both national and international healthcare policies aimed at managing this dual disease burden. HTN and diabetes are well-established, independent risk factors for atherosclerotic cardiovascular diseases, including coronary artery disease and cerebrovascular accidents. When these conditions coexist, they synergistically amplify the risk of serious complications, creating a greater combined effect than either condition alone. Medical practitioners ought to recommend regular blood pressure checks for patients’ relatives and promote preventative measures including dietary improvements and increased physical activity. The Ministry of Health and other concerned policymaker bodies should enhance care through workforce training and support interdisciplinary teams of primary care providers, fund community-based screening approaches, integrate HbA1c testing into routine primary care, develop guidelines prioritizing intensive lifestyle program for patients with overweight and family history of the disease, and continue health advocacies and promote lifestyle modification. Furthermore, future studies should highlight the need for more comprehensive longitudinal studies with detailed risk factor assessment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethical Review Committee (ERC) of College of Health Science, School of Public Health, Wachemo University. Also, formal letters of permission were obtained from Tebela town administrative health office, Beleawassa town administrative health office, Bodit town administrative health office, Bedesa district health office, Gesuba town administrative health office, Bitana district health office and Bomb district health office. The formal letter of permission was written to the respective hospitals. The study’s objectives and methods were explained to each participant. Additionally, prior to participation, all study participants provided written informed permission. The study’s goal, the thorough review of their medical records, and the potential benefits of the research were also explained to the participants. The data gathered was used for the study’s objectives. All of the information gathered throughout the study was kept private, available only to the research team, and utilized only for that reason. Their medical record numbers were utilized instead of personal identifiers for the purpose of gathering data. The study was carried out in compliance with the applicable rules, laws, and Helsinki Declaration principles. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
BY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EI: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. MM: Investigation, Software, Validation, Writing – original draft, Writing – review & editing. AA: Funding acquisition, Methodology, Visualization, Writing – original draft, Writing – review & editing. TY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Hypertension (2019). Available online at: https://www.who.int/news-room/fact-sheets/detail/hypertension (Accessed November 28, 2020).
2. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. (2003) 289:2560–72. doi: 10.1001/jama.289.19.2560
3. Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, and Kengne AP. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension. (2015) 65:291–8. doi: 10.1161/HYPERTENSIONAHA.114.04394
4. Opie LH and Seedat YK. Hypertension in sub-Saharan African populations. Circulation. (2005) 112:3562–8. doi: 10.1161/CIRCULATIONAHA.105.539569
5. Tiruneh SA, Bukayaw YA, Yigizaw ST, Angaw DA, and Widmer RJ. Prevalence of hypertension and its determinants in Ethiopia: A systematic review and meta-analysis. PloS One. (2020) 15:e0244642. doi: 10.1371/journal.pone.0244642
6. WHO Classification of diabetes mellitus . Available online at: https://apps.who.int/iris/handle/10665/325182 (Accessed May 15, 2023).
7. International Diabetes Federation (IDF). (2021). Brussels: Diabetes Atlas International Diabetes Federation.
8. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. (2022) 183:109119.
9. WHO Africa region. Available online at: https://www.afro.who.int/health-topics/diabetes:~:text=In%20the%20WHO%20African%20Region,increase%2C%20further%20straining%20healthcare%20systems. (Accessed October 13, 2025).
10. Bantie GM, Wondaye AA, Arike EB, Melaku MT, Ejigu ST, Lule A, et al. Prevalence of undiagnosed diabetes mellitus and associated factors among adult residents of Bahir Dar city, northwest Ethiopia: A community-based cross-sectional study. BMJ Open. (2019) 9:e030158. doi: 10.1136/bmjopen-2019-030158
11. Roglic G. WHO. Global report on diabetes: a summary. Int J Noncommun Dis. (2016) 1:3. doi: 10.4103/2468-8827.184853
12. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2224–60. doi: 10.1016/S0140-6736(12)61766-8
13. Sunkara N and Ahsan CH. Hypertension in diabetes and the risk of cardiovascular disease. Cardiovasc Endocrinol. (2017) 6:33. doi: 10.1097/XCE.0000000000000114
15. Grosso G. Impact of nutritional risk factors on chronic noncommunicable diseases. Eur J Public Health. (2019) 29:ckz185–197. doi: 10.1093/eurpub/ckz185.197
16. Di Cesare M. Global trends of chronic non-communicable diseases risk factors. Eur J Public Health. (2019) 29:ckz185–196. doi: 10.1093/eurpub/ckz185.196
17. Addo J, Smeeth L, and Leon DA. Hypertension in sub-saharan Africa: a systematic review. Hypertension. (2007) 50:1012–8. doi: 10.1161/HYPERTENSIONAHA.107.093336
18. Gudina EK, Michael Y, and Assegid S. Prevalence of hypertension and its risk factors in southwest Ethiopia: a hospital-based cross-sectional survey. Integr Blood Press Control. (2013) 6:111. doi: 10.2147/IBPC.S47298
19. Channanath AM, Farran B, Behbehani K, and Thanaraj TA. State of diabetes, hypertension, and comorbidity in Kuwait: showcasing the trends as seen in native versus expatriate populations. Diabetes Care. (2013) 36:e75–5. doi: 10.2337/dc12-2451
20. Tesfaye B, Alebel A, Gebrie A, Zegeye A, Leshargie CT, Ferede A, et al. Diabetes mellitus and its association with hypertension in ethiopia: a systematic review and meta-analysis. Diabetes Res Clin Pract. (2019) 156:107838. doi: 10.1016/j.diabres.2019.107838
21. Alloubani A, Saleh A, and Abdelhafiz I. Hypertension and diabetes mellitus as a predictive risk factors for stroke. Diabetes Metabol Synd Clin Res Rev. (2018) 12:577–84. doi: 10.1016/j.dsx.2018.03.009
22. Ye N, Jardine MJ, Oshima M, Hockham C, Heerspink HJL, Agarwal R, et al. Blood pressure effects of Canagliflozin and clinical outcomes in type 2 diabetes and chronic kidney disease: insights from the CREDENCE trial. Circulation. (2021) 143:1735–49. doi: 10.1161/CIRCULATIONAHA.120.048740
23. Singal AK, Hasanin M, Kaif M, Wiesner RW, and Kuo Y-F. MELD. Stratified outcomes among recipients with diabetes or hypertension. J Clin Gastroenterol. (2018) 52:67–72. doi: 10.1097/MCG.0000000000000818
24. Jia G and Sowers JR. Hypertension in diabetes: an update of basic mechanisms and clinical disease. Hypertension. (2021) 78:1197–205. doi: 10.1161/HYPERTENSIONAHA.121.17981
25. Ferrannini E and Cushman WC. Diabetes and hypertension: the bad companions. Lancet. (2012) 380:601–10. doi: 10.1016/S0140-6736(12)60987-8
26. Levin G, Kestenbaum B, Ida Chen YD, Jacobs DR Jr, Psaty BM, Rotter JI, et al. Glucose, insulin, and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol. (2010) 172:1144–54. doi: 10.1093/aje/kwq266
27. Long AN and Dagogo-Jack S. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. J Clin Hypertens. (2011) 13:244–51. doi: 10.1111/j.1751-7176.2011.00434.x
28. Wang Z and Hanlin TY. Prevalence of diabetes and hypertension and their interaction effects on cardiocerebrovascular diseases: a cross-sectional study. BMC Public Health. (2021) 21:1224. doi: 10.1186/s12889-021-11122-y
29. Kotiso KS, Degemu N, Gebremedhin S, Taye M, Petros A, Belayneh F, et al. Determinants of hypertension among patients with type 2 diabetes mellitus on followup at Tikur Anbessa Specialized Hospital, Addis Ababa: a case-control study. PloS One. (2021) 16:e0256399. doi: 10.1371/journal.pone.0256399
30. Angaw K, Dadi AF, and Alene KA. Prevalence of hypertension among federal ministry civil servants in Addis Ababa, Ethiopia: a call for a workplace-screening program. BMC Cardiovasc Disord. (2015) 15:76. doi: 10.1186/s12872-015-0062-9
31. Akalu Y and Belsti Y. Hypertension and Its Associated Factors Among Type 2 Diabetes Mellitus Patients at Debre Tabor General Hospital, Northwest Ethiopia. (2020), 1621–31. doi: 10.2147/DMSO.S254537
32. Janakiraman B, Abebe SM, Chala MB, and Demissie SF. Epidemiology of general, central obesity and associated cardio-metabolic risks among University Employees, Ethiopia: a cross-sectional study. Diab. Metabol. Synd. Obes Targets Ther. (2020) 13:343. doi: 10.2147/DMSO.S235981
33. Guideline for the pharmacological treatment of hypertension in adults. Geneva: World Health Organization (2021). Licence: CC BY-NC-SA 3.0 IGO.
34. Al-Nozha MM, Abdullah M, Arafah MR, Khalil MZ, Khan NB, Al-Mazrou YY, et al. Hypertension in Saudi Arabia. Saudi Med J. (2007) 28:77–84.
35. Committee ADAPP. 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes—2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S002
36. Faselis C, Doumas M, and Papademetriou V. Common Secondary Causes of Resistant Hypertension and Rational for Treatment. (2017).
37. Tadesse K, Amare H, Hailemariam T, and Gebremariam T. Prevalence of Hypertension among Patients with Type 2 Diabetes Mellitus and Its Socio Demographic Factors in Nigist Ellen Mohamed Memorial Hospital Hosanna, Southern Ethiopia. J Diabetes Metab. (2018) 9:792. doi: 10.4172/2155-6156.1000792
38. Dedefo A, Galgalo A, Jarso G, and Mohammed A. Prevalence of Hypertension and Its Management Pattern among Type 2 Diabetic Patients Attending, Adama Hospital Medical College, Adama, Ethiopia. J Diabetes Metab. (2018) 9. doi: 10.4172/2155-6156.1000808
39. Skliros EA, Vasibossis A, Loumakis P, Sotiropoulos A, Giannakaki G, and Razis N. Evaluation of hypertension control in Greek primary care units. The VANK study. J Hum Hypertension. (2003) 17:297–8. doi: 10.1038/sj.jhh.1001540
40. Shelley D, Tseng TY, Andrews H, Ravenell J, Wu D, Ferrari P, et al. Predictors of blood pressure control among hypertensives in community health centers. Am J Hypertension. (2011) 24:1318–23. doi: 10.1038/ajh.2011.154
41. Mansour AA. Prevalence and Control of Hypertension in Iraqi Diabetic Patients: A Prospective Cohort Study. J Diabetes Metab Disord. (2019) 4:68–71.
42. Mansour AA. Prevalence and Control of Hypertension in Iraqi Diabetic Patients : A Prospective Cohort Study. (2019), 68–71.
43. Who J and Consultation FE. Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. (2003) 916:1–49.
44. Biswas T, Islam A, Rawal LB, and Islam SMS. Increasing prevalence of diabetes in Bangladesh: a scoping review. Public Health. (2016) 138:4–11. doi: 10.1016/j.puhe.2016.03.025
45. Alaboud AF, Tourkmani AM, Alharbi TJ, Alobikan AH, Abdelhay O, Al Batal SM, et al. Microvascular and macrovascular complications of type 2 diabetic mellitus in central, Kingdom of Saudi Arabia. Saudi Med J. (2016) 37:1408. doi: 10.15537/smj.2016.12.17062
46. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
47. Afroz A, Alam K, Ali L, Karim A, Alramadan MJ, Habib SH, et al. Type 2 diabetes mellitus in Bangladesh: a prevalence based cost-of-illness study. BMC Health Serv Res. (2019) 19:1–12. doi: 10.1186/s12913-019-4440-3
48. Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London England). (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
49. Nouh F, Omar M, and Younis M. Prevalence of Hypertension among Diabetic Patients in Benghazi: A Study of Associated Factors. Asian J Med Heal. (2017) 6:1–11. doi: 10.9734/AJMAH/2017/35830
50. Janse Van Rensburg Z, Vincent-Lambert C, Razlog R, and Phaladze N. Prevalence of hypertension in a sample of community member in a low-income peri-urban setting in Gaborone, Botswana. J Public Health Afr. (2023) 14:2068. doi: 10.4081/jphia.2023.2068
51. Cheng WL. Interpretation of the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Chin Gen Pract. (2019) 22:2519–23.
52. Abebe SM, Berhane Y, Worku A, and Getachew A. Prevalence and associated factors of hypertension: a cros-sectional community based study in Northwest Ethiopia. PloS One. (2015) 10:e0125210. doi: 10.1371/journal.pone.0125210
53. Asresahegn H, Tadesse F, and Beyene E. Prevalence and associated factors of hypertension among adults in Ethiopia: a community based cross-sectional study. BMC Res Notes. (2017) 10:629. doi: 10.1186/s13104-017-2966-1
54. Egbi OG, Ogoina D, and Oyeyemi A. Prevalence of hypertension and associated factors in a rural community in Bayelsa State. Int J Res Med Sci. (2018) 6:1106. doi: 10.18203/2320-6012.ijrms20181264
55. Ajayi IO, Sowemimo IO, Akpa OM, and Ossai NE. Prevalence of hypertension and associated factors among residents of Ibadan-North Local Government Area of Nigeria. Niger J Cardiol. (2016) 13:67. doi: 10.4103/0189-7969.165168
56. Carmelli D, Robinette D, and Fabsitz R. Concordance, discordance and prevalence of hypertension in World War II male veteran twins. J Hypertens. (1994) 12:323–8. doi: 10.1097/00004872-199403000-00015
57. Singh S, Shankar R, and Singh GP. Prevalence and associated risk factors of hypertension: a cross-sectional study in urban varanasi. Int J Hyp. (2017) 2017:5491838. doi: 10.1155/2017/5491838
58. Katulanda P, Constantine GR, Mahesh JG, Sheriff R, Seneviratne RD, Wijeratne S, et al. Prevalence and projections of diabetes and pre-diabetes in adults in Sri Lanka–Sri Lanka Diabetes, Cardiovascular Study (SLDCS). Diabetes Med. (2008) 25:1062–9. doi: 10.1111/j.1464-5491.2008.02523.x
59. Khanna N, Sharma RS, and Sidhu RS. A study of the basic and derived anthropometric indices among the healthy adults (20–30 years of age) of amritsar city (punjab) having family history of hypertension. Int J Biol Med Res. (2011) 2:743–6.
60. Corvol P, Jeunemaitre X, Charru A, and Soubrier F. Can the genetic factors influence the treatment of systemic hypertension? The case of the renin-angiotensin-aldosterone system. Am J Cardiol. (1992) 70:14D–20D. doi: 10.1016/0002-9149(92)90267-3
61. Stamler R, Stamler J, Riedlinger WF, Algera G, and Roberts RH. Family (parental) history and prevalence of hypertension. Results of a nationwide screening program. JAMA. (1979) 241:43–6. doi: 10.1001/jama.1979.03290270033016
62. Williams RR, Hunt SC, Hopkins PN, Wu LL, Hasstedt SJ, Berry TD, et al. Genetic basis of familial dyslipidemia and hypertension: 15-year results from Utah. Am J Hypertens. (1993) 6:319S–27S. doi: 10.1093/ajh/6.11.319S
63. Masuo K, Mikami H, Ogihara T, and Tuck ML. Familial hypertension, insulin, sympathetic activity, and blood pressure elevation. Hypertension. (1998) 32:96–100. doi: 10.1161/01.HYP.32.1.96
Keywords: hypertension, type 2 diabetes mellitus, primary healthcare, Ethiopia, resource limited setting
Citation: Yakob B, Israel E, Jaldo MM, Abraham A and Yakob T (2025) Factors associated with hypertension among patients with type 2 diabetes: evidence from a resource-limited setting—Southern Ethiopia. Front. Endocrinol. 16:1534852. doi: 10.3389/fendo.2025.1534852
Received: 26 November 2024; Accepted: 08 July 2025;
Published: 01 August 2025.
Edited by:
Alexander Akhmedov, University of Zurich, SwitzerlandReviewed by:
Maria Guadalupe Moreno Treviño, University of Monterrey, MexicoSrividya Velagapudi, University of Zurich, Switzerland
Jamol Uzokov, Ministry of Health of the Republic Uzbekistan, Uzbekistan
Copyright © 2025 Yakob, Israel, Jaldo, Abraham and Yakob. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Begidu Yakob, eWFrb2JlZ2lkdUBnbWFpbC5jb20=
 Begidu Yakob
Begidu Yakob Eskinder Israel
Eskinder Israel Mesfin Menza Jaldo
Mesfin Menza Jaldo Awoke Abraham
Awoke Abraham Tagese Yakob
Tagese Yakob