- 1Department of Clinical Medicine, St Matthew’s University School of Medicine, Grand Cayman, FL, United States
- 2Division of Endocrinology, Diabetes, and Metabolism, University of Kentucky, Lexington, KY, United States
- 3Pulmonary, Critical Care and Sleep Medicine, Appalachian Regional Healthcare, Hazard, KY, United States
- 4Department of Clinical Medicine, American University of Antigua College of Medicine, Coolidge, AG, United States
- 5Department of Medicine, Division of Endocrinology, Diabetes, and Metabolism, Barnstable Brown Diabetes and Obesity Center, University of Kentucky, Lexington, KY, United States
Background: The literature is rapidly evolving with regards to the endocrine consequences of coronavirus disease 2019 (COVID-19), including diabetes, thyroid dysfunction, adrenal and pituitary disorders. There is evidence suggesting that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to thyroid dysfunction and long-term sequelae. We aimed to review the current evidence and propose a preventive approach based on the published data since the beginning of the COVID-19 pandemic.
Methods: A comprehensive review of literature was conducted using electronic databases PubMed and Google Scholar. Two authors independently used the keywords “Thyroid, Hypothyroidism, Hyperthyroidism, Graves, Thyroid Eye Disease, or Thyroiditis” and “Coronavirus, SARS-CoV-2 or COVID-19” to search these databases. We screened titles and abstracts for initial selection and then reviewed the full text of relevant studies to report the outcomes of published data.
Results: We selected 28 manuscripts. SARS-CoV-2 infection appears similar to other viruses. It affects thyroid function resulting in non-thyroidal illness syndrome, which usually resolves spontaneously. COVID-19 also causes subacute thyroiditis. It may also trigger autoimmunity against the thyroid that leads to autoimmune thyroiditis. Autoimmune thyroiditis or subacute thyroiditis may progress to clinical or subclinical hypothyroidism and clinical or subclinical hyperthyroidism. Patients with pre-existing thyroid dysfunction probably have similar risks of SARS-CoV-2 related adverse outcomes.
Conclusions: Evaluation of thyroid function is important in COVID-19 patients. Improving the efficacy of treatment against acute SARS-CoV-2 infection can reduce the risks of short-term and long-term complications.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier CRD42023447994.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has profoundly impacted society since the beginning of the coronavirus disease 19 (COVID-19) pandemic. Generally, the SARS-CoV-2 infection can present from mild to severe disease and death. It continues to have adverse effects including damage to the endocrine systems. The route of entry of SARS-CoV-2 is mainly via angiotensin-converting enzyme 2 (ACE2). ACE2 is not only a receptor for SARS-CoV-2, but also has protective roles against organ damage by converting angiotensin II into angiotensin 1–7 and down regulating the renin-angiotensin system (1, 2). However, the increased expression of ACE2 and its cofactors, the host proteases transmembrane protease, serine 2 (TMPRSS2) and A Disintegrin and Metalloproteinase 17 (ADAM17), in human tissues can facilitate SARS-CoV-2 entry into human cells, and therefore, increase the risk of organ damage following SARS-CoV-2 infection, including the thyroid gland (1, 3).
The first case of COVID-19 with thyroid dysfunction was reported in May 2020 by Brancatella et al., which was a case of subacute thyroiditis (SAT) following mild, symptomatic SARS-CoV-2 infection (4). There is also an association between COVID-19 and autoimmune diseases, which raises concern regarding a causal relationship between SARS-CoV-2 infection and autoimmune thyroid diseases (5). Basically, the expression of ACE2 and TMPRSS2 on the thyroid follicular cells enables SARS-CoV-2 to invade thyroid cells, and increases the susceptibility of the thyroid gland to SARS-CoV-2 related injuries (6). Furthermore, long COVID is a debilitating complication of acute SARS-CoV-2 infection, possibly due to persistent SARS-CoV-2 reservoir or immune activation (7). Therefore, long COVID can also be associated with further organ damage, such as endocrine organs including the thyroid gland. Although there are studies reporting almost complete recovery of thyroid function following acute SARS-CoV-2 infection, the overlapping symptoms between long COVID and thyroid dysfunction highlight the point that subtle organ damage should be investigated more carefully with pertinent clinical presentations (8).
Preventive and therapeutic strategies may improve the clinical outcomes of acute SARS-CoV-2 infection. However, differences in race and ethnicity, in addition to social and political factors such as economic inequalities, accessibility to healthcare resources, and vaccine hesitancy, were potential drivers of outcome inequalities in different regions of the world (9, 10). There is a concern exists for SARS-CoV-2 mutations, with resistance to antiviral therapy (11). Therefore, proposing a therapeutic approach that is effective against new strains of SARS-CoV-2, and that can be implemented in developing countries is important to prevent acute and chronic complications of COVID-19 infection.
This review summarizes the incidence of thyroid dysfunction following acute SARS-CoV-2 infection, including outcomes in different countries. Gender and vaccine status are other variables that are considered in this review, and may play a role in susceptibility to thyroid disorders. Finally, this article will discuss the pros and cons of current therapeutic approaches, and introduce a new strategy to mitigate SARS-CoV-2-related complications.
Materials and methods
Searches strategies
We conducted a comprehensive review of literature according to PRISMA guidelines, identifying studies via PubMed and Google Scholar (12). The review was registered on the International Prospective Register of Systematic Reviews (PROSPERO; CRD42023447994) (13). The search was limited to English literature. The timeline for searching the database was between January 2020 and March 2024. A comprehensive search was conducted by using standardized terms, such as “Thyroid, Hypothyroidism, Thyrotoxicosis, Hyperthyroidism, Graves’ Disease, Thyroid Eye Disease, Graves’ Ophthalmopathy, Thyroiditis” and “Coronavirus, SARS-CoV-2 or COVID-19”.
Study selection and data extraction
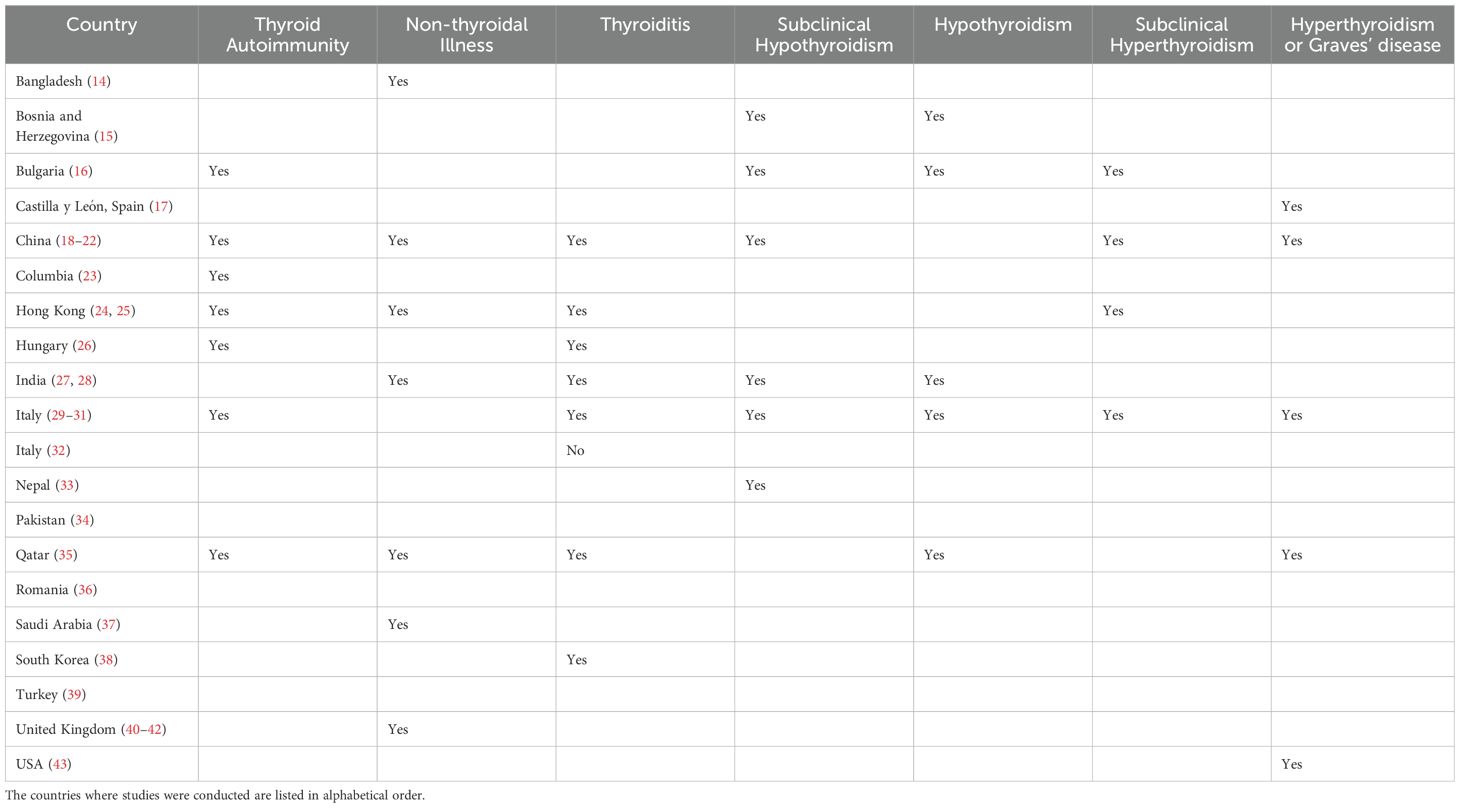
Two investigators independently searched PubMed and Google by reviewing titles and abstracts. We looked at the studies’ participants, type, outcomes and interventions to select appropriate manuscripts. The full text of selected articles was assessed afterward. The third author made a cross-check to confirm the consistency of search results and exclusion of duplicates or irrelevant studies. We reviewed the published systemic reviews from early 2022 (Deng L 2024, Ganie MA 2024, Lui DTW 2024, Ashrafi S 2024, Lampropoulou E 2024, Singhal V 2023, Wei J 2023, Vamshidhar IS 2023, Lee ZC 2023, Chen K 2023, Takedani K 2023, Meftah E 2023, Bellamkonda A 2023, Li Z 2023, Darvishi M 2022, Triantafyllidis KK 2022, Patrizio A 2022, Ando Y 2022, Tutal E 2022) and references of selected studies to include all potentially relevant manuscripts during the selection process. We looked at [a] clinical research articles, such as clinical trials, cohort, cross-sectional, case-control studies and case series, [b] review articles including mini-reviews, systematic reviews and meta-analyses, [c] opinion and commentary articles, like editorials, commentaries, perspectives, and letters to editor that discussed the incidence and outcomes of thyroid disorders following COVID-19 infection or vaccination. Studies were excluded if they did not provide enough information about the prevalence and outcomes of thyroid disorders following acute SARS-CoV-2 infection. We finally reported a summary of the results of the clinical research articles (Tables 1, 2), such as retrospective, cross-sectional, and prospective studies, which include the first author, journal, year of publication, country of origin, study design, number of participants, type of intervention, age, gender, outcomes, type of thyroid disorders and other relevant results. We considered sex and vaccine status to assess high risk-populations for SARS-CoV-2 infection induced thyroid disorders. We excluded case reports and case series with less than 10 cases. Figure 1 shows the flowchart for our review.
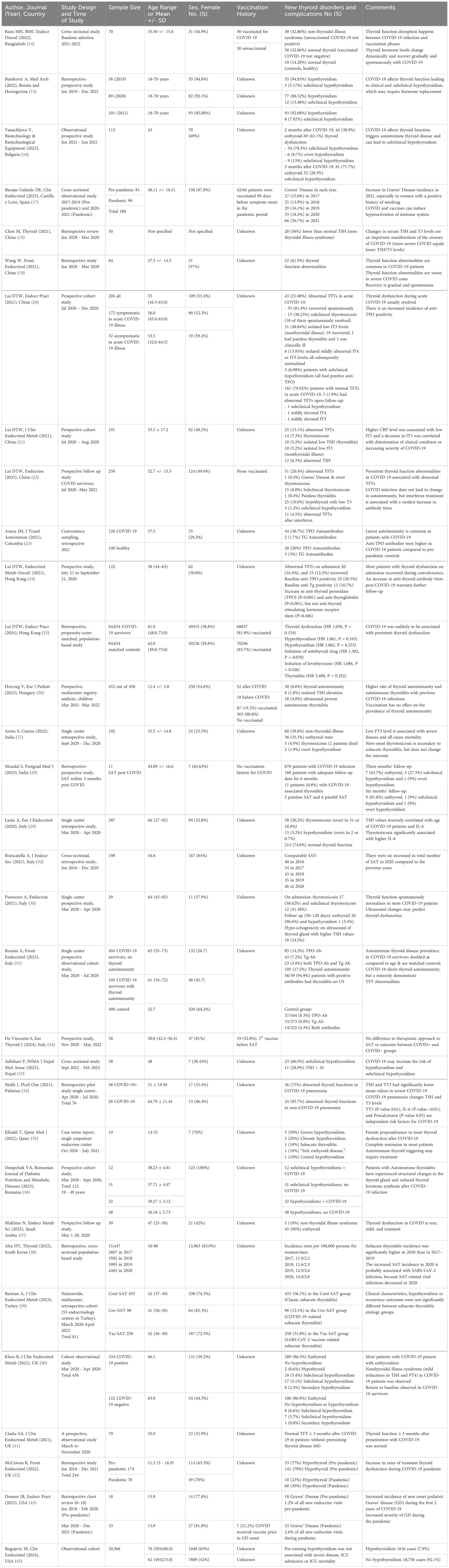
Table 2. Summary of the outcomes of research about thyroid dysfunction following acute SARS-CoV-2 infection, listing the countries in alphabetical order.
Quality assessment was performed using ROBINS (Risk of Bias in Non-randomized Studies of Exposure) or the involvement of a third author. All studies are nonrandomized studies, which may introduce a considerable risk of bias into the review. We generated the review questions, produced review-specific guidance, constructed a flow diagram for the study selection, and then judged bias and applicability. We looked at the following domains: sample size, age, sex, vaccination history, follow-up duration, study design, methods of case selection, outcome measurements, and interventions. We found significant heterogeneity among the studies after considering a subgroup evaluation for before mentioned variables, especially sample size, age, sex, vaccination history, follow-up duration, and study design. Uncovered biases remain, including accessibility to healthcare, medication availability, and therapeutic approaches to viral treatment, which technically can affect the outcomes of the studies.
Strategy for data interpretation
We prepared a table to show the results of selected studies, and then discussed the differences in the results of studies without running statistical analysis on detailed data. Meta-analysis was not performed because of the heterogeneity among selected articles and the lack of a tool to overcome the effects of uncovered biases on outcomes. There was no limit to manuscript selection.
Results
A total of thirty-two studies were carefully chosen during the selection process (14–45). The eligible manuscripts include 13 prospective and 19 retrospective or cross-sectional studies. An observational study by Fallahi et al. from Italy during the first phase of the pandemic in 2020, reported that patients with autoimmune thyroid disease have higher prevalence of SARS-CoV-2 infection (46). The prevalence of thyroid dysfunction was reported as 15% in a systematic review that evaluated 30 cohort studies and included 9,707 COVID-19 cases. Noticeably, thyroid dysfunction prevalence correlated with severity of COVID-19 (6.2% among mild to moderate cases and 20.8% among sever cases) (47). Another meta-analysis by Ashrafi in January 2024, reported 26% prevalence of non-thyroidal illness syndrome (NTIS) and 10% prevalence of thyrotoxicosis. The aforementioned study selected 8 out of 1,256 studies and included 1,654 participants. The prevalence of hypothyroidism (3%), isolated elevated FT4 (2%), and isolated low FT4 (1%) were unremarkable (48). NTIS is the most common abnormality seen in the literature following acute SARS-CoV-2 infection. Typical findings in NTIS include reduced triiodothyronine (T3) with normal or decreased thyroid stimulating hormone (TSH). The prevalence of NTIS in patients with COVID-19 ranges from 5 to 58 percent in the literature (21, 27). NTIS is also referred to as isolated low T3 syndrome and sick euthyroid syndrome. NTIS is reported from various areas, including Bangladesh (14), China (18–22), Hong Kong (24, 25), India (27, 28), Qatar (35), Saudi Arabia (37), and the United Kingdom (40–42).
Thyroiditis is another complication of SARS-CoV-2 infection, and includes SAT, painless thyroiditis, autoimmune thyroiditis and atypical thyroiditis (21, 49, 50). There is generally an increased risk of thyroiditis, thyroid autoimmunity, and autoimmune thyroiditis following acute SARS-CoV-2 infection, which is unlikely associated with persistent thyroid dysfunction, but may result in new-onset thyrotoxicosis or hypothyroidism (25–27, 31, 35, 36, 39, 51, 52). The reported incidence of thyroiditis and outcomes following acute SARS-CoV-2 infection varies by country, which could be due to the differences in population characteristics, severity of infection, and therapeutic interventions.
SAT typically present with fever, neck pain and symptoms and signs of thyrotoxicosis. The clinical manifestations of SAT following SARS-CoV-2 infection appear similar to the typical SAT (53). If acute SARS-CoV-2 infection is considered as a risk factor for SAT, then the incidence of SAT would be expected to rise after the pandemic. A nationwide study from South Korea found an increased incidence of SAT during the early phase of COVID-19 in 2020 (38). However, retrospective single-center studies reported that the incidence of SAT was not increased in Italy (32, 54) and Turkey (55, 56) during the COVID-19 pandemic. In addition, a multicenter nationwide study reported no differences in clinical characteristics or outcomes of SAT in Turkey (39) and no significant increase in thyroiditis in Hong Kong (25) after the pandemic. Finally, it seems that SARS-CoV-2 infection does not affect the onset, progression, and outcome of SAT as demonstrated by a multicenter prospective study from Italy that included 52 patients (7 COVID+ and 45 COVID-) (44).
Painless thyroiditis and autoimmune thyroiditis have also been reported following SARS-CoV-2 infection. In Hungary, rates of autoimmune thyroiditis were higher in the pediatric population with a history of SARS-CoV-2 infection (26). Data from Italy described an increased, almost doubled, rate of autoimmune thyroid disease prevalence in COVID-19 survivors, but a minority demonstrate thyroid function test abnormalities (31). SARS-CoV-2 infection can generally trigger autoimmunity as reported from Bulgaria (16), Columbia (23), Italy (31), Qatar (35), and Romania (36).
Atypical thyroiditis was reported by Muller et al. in 2020. It was reported in ICU patients with COVID-19 who presented with low TSH and T3 accompanied by normal or elevated thyroxine (T4) (50). This presentation is a combination of NTIS and thyrotoxicosis, which has been reported as T4 thyrotoxicosis previously (57).
Graves’ disease and Hashimoto’s thyroiditis are other possible complications of SARS-CoV-2 infection. There is a link between acute SARS-CoV-2 infection and Graves’ disease (17, 29). The first two cases of Graves’ disease after COVID-19 were reported in October 2020 (58). The epidemiologic studies about the prevalence of Graves’ disease during the COVID-19 pandemic are limited. An observational study from Spain described a significant increase in the incidence of Graves’ disease during the pandemic (17). In the United States reported an increased incidence of new-onset pediatric Graves’ disease during the first 2 years of COVID-19 (43). A retrospective cohort study from Taiwan that included 1,379,311 COVID-19 patients and 6,896,814 non-COVID-19 patients, reported an increased risk of thyroid dysfunction, including thyrotoxicosis and hypothyroidism, secondary to COVID-19. The risk of thyroid dysfunction, both thyrotoxicosis and hypothyroidism, following COVID-19 infection appears to be higher in older patients (aged 65 and above) and female (52). There is also a connection between SARS-CoV-2 infection and complications of Graves’ disease, such as orbitopathy (59, 60), thyrotoxic periodic paralysis (61–64) and thyroid storm (65–67). In terms of hypothyroidism, a bi-directional Mendelian randomization study supports a causal relationship between the host response to SARS-CoV-2 infection and increased risk of hypothyroidism (51). There are also studies from Bosnia and Herzegovina (15), Bulgaria (16), Hong Kong (25), India (27), Italy (30), Nepal (33), and Qatar (35) that reported development or increased risk of hypothyroidism after SARS-CoV-2 infection.
There are multiple studies investigating the correlation between pre-existing autoimmune thyroid diseases and the outcomes of COVID-19 (45, 68–73). Underlying thyroid disorders, especially hypothyroidism, are reported to be associated with a worse prognosis of COVID-19 in two systemic reviews from Indonesia in 2021 (74) and 2022 (75). Moreover, a large retrospective cohort from Turkey (total n=14,966; hypothyroidism n=8813; hyperthyroidism n=1822; normal thyroid function n=4331) showed a positive correlation between pre-existing hyperthyroidism or hypothyroidism and COVID-19 mortality (72). However, a large observational cohort (20,366 adult patients; pre-existing hypothyroidism in 1,616) from the USA showed that pre-existing hypothyroidism in hospitalized COVID-19 patients was not associated with worse outcomes of acute SARS-CoV-2 infection (45). In addition, two large population-based cohort studies are reassuring and suggested that hypothyroidism or hyperthyroidism were not associated with an increased risk of infection or worse outcomes in COVID-19 patients (69, 71).
Finally, it is important to mention that thyroid function testing has relatively good prognostic value for illness severity and predicting mortality risk in hospitalized moderate-to-severe COVID-19 patients (76).
Discussion
Although the mortality and morbidity seen during the height of the pandemic has diminished, the SARS-CoV-2 infection has both short and long-term complications. Further efforts to improve the outcomes of acute SARS-CoV-2 infection are necessary. Acute SARS-CoV-2 infection causes transient and permanent thyroid dysfunction. Transient thyroid dysfunction is associated with COVID-19 severity, hospitalization and even mortality (77, 78). Permanent thyroid dysfunction leads to long-term medication use and worsening of underlying medical comorbidities. COVID-19 also increases the risk of autoimmunity against the thyroid gland, which may lead to thyroiditis, hypothyroidism or hyperthyroidism (52).
The pathophysiologic and molecular mechanisms of thyroid dysfunction following acute SARS-CoV-2 infection have not been fully elucidated. SARS-CoV-2 enters into cells after attaching to cell receptor, predominantly ACE2. Subsequently, viral particle approximation, fusion and internalization are essential for viral RNA genome engulfment and viral replication in host cells. The processes of approximation and fusion are mediated by host proteases, such as TMPRSS2 and ADAM17 (11). There is evidence regarding the ability of SARS-CoV-2 to directly assault the thyroid gland including the presence of ACE2 and TMPRSS2 in the thyroid tissue (79), along with detection of SARS-CoV-2 infection in follicular thyroid cells (80) and upregulation of immune genes is the SARS-CoV-2-positive thyroid specimens (81). This evidence not only suggests the ability of SARS-CoV-2 to directly invade thyroid tissue, but also proposes another mechanism of tissue injury by heightening inflammation through pro-inflammatory cytokines (80–82). Therefore, SARS-CoV-2 may disrupt thyroid function by entering thyroid cells, replicating locally, and triggering autoimmunity (83). ACE2 and TMPRSS2 expression were also found in the hypothalamus and pituitary, which makes them susceptible to direct damage following acute SARS-CoV-2 infection (84). The presence of ACE2 on hypothalamus, pituitary and thyroid tissue could be associated with not only triggering autoimmunity against thyroid by SARS-CoV-2, but also affecting the hypothalamic-pituitary-thyroid (HPT) axis. Acute SARS-CoV-2 infection can also cause a hyper-inflammatory immune response or cytokine storm following pneumonia. The uncontrolled inflammatory response can damage host cells, which could potentially affect the function of thyroid gland or HPT axis too (82, 85). Moreover, it has been reported that pre-existing thyroid conditions may worsen COVID-19 outcomes. Therefore, a bidirectional relationship between acute SARS-CoV-2 infection and thyroid dysfunction may exist (Figure 2).

Figure 2. The diagram demonstrates the cascade of events initiated by SARS-CoV-2 infection leading to thyroid dysfunction. ACE2 and TMPRSS2 are expressed at the level of the hypothalamic-pituitary-thyroid (HPT) axis, facilitating SARS-CoV-2 entry into thyroid tissue and the HPT axis, and subsequent tissue injury. Additionally, SARS-CoV-2 infects lungs triggering cytokine storm, which is associated with organ dysfunction including thyroid and HPT axis. Created with BioRender.com.
Furthermore, we must underscore the challenges in diagnosing thyroid complications after COVID-19, which are sometimes associated with overlapping symptoms and laboratory findings. Additionally, the increased risk of autoimmunity may precipitate development of long COVID syndrome (7). Long COVID is a multisystem condition that develops in almost 10% of infected patients, especially following a severe acute SARS-CoV-2 infection. Thyroid dysfunction appears unlikely, but has a possible correlation with long COVID. The heterogeneity of symptoms, and severity and duration of long COVID is sometimes associated with missed diagnosis and delayed treatment of potential preventable conditions such as thyroid dysfunction (7, 85). However, the data about correlation between thyroid dysfunction and long COVID are conflicting. It has been reported that there is a correlation between thyroid or pituitary dysfunction and long COVID (25, 86). However, multiple cohort studies reported that there is no meaningful correlation between long COVID and thyroid dysfunction (20, 22, 25, 41).
The current review has several limitations. Our review was limited to the studies written in the English language. The review excludes more recent publications completed after our initial review (March 31, 2024). We also reviewed information from different parts of the world, but there were still some missing areas, which limited the global applicability of the results. In addition, the differences in studies’ results may be caused by the evolution of treatment modalities for COVID-19 throughout the pandemic, the time of data collection in different studies and momentary incidence of the COVID-19 related complications.
The current recommendations for treating acute SARS-CoV-2 infection is primarily antivirals, such as remdesivir, molnupiravir and nirmatrelvir-ritonavir. Antivirals are designed to improve symptoms, reduce the duration of disease and prevent complications, such as hospitalization and post-acute sequelae of SARS-CoV-2 infection. However, SARS-CoV-2 continuously changes by altering the genetic codes, and routine usage of antivirals can induce new mutations too. For this reason, SARS-CoV-2 evolves gradually by accumulating mutations that can cause resistance to current antivirals and quicker spread of new variants with increased virulence (11, 87). Moreover, there are examples of hypothetical theories about the pathophysiology of post-acute sequelae of COVID-19, including sustained viral replication, presence of viral particles in organs, permanent inflammatory response, endothelial dysfunction, and altered immune function with a tendency toward autoimmunity (88, 89). Applying an alternative approach by targeting receptors, reducing virus engulfment and modulating immune response would be reasonable to improve COVID-19 outcomes.
Current literature supports the usefulness of dipeptidyl peptidase 4 inhibitors (DPP-4 inhibitors), metformin, spironolactone, and ursodeoxycholic acid (UDCA) in reducing virus entry into the cells and alleviating inflammatory responses (1). Each of the above medications has potential benefits to improve the clinical outcome of a patient with acute SARS-CoV-2 infection, and are not impacted by viral mutations. DPP-4 inhibitors are not only immunomodulators but also reduce SARS-CoV-2 interaction with receptors and diminish viral replication (11, 90). Spironolactone has anti-inflammatory and anti-thrombotic effects, and plays protective roles against SARS-CoV-2-mediated endothelial dysfunction by preventing damage to endothelial glycocalyx (91). Metformin reduces viral load, inflammation and thrombotic risks (92). Combining these medications can provide different valuable defenses against SARS-CoV-2 simultaneously, enhance the efficacy of treatment and further reduce complications of acute SARS-CoV-2 infection. In support of this statement, it has been shown that the combination of spironolactone and sitagliptin could reduce hospitalization of acute SARS-CoV-2 infection by almost 78 percent, which was superior to antivirals by some means (11, 93).
In conclusion, COVID-19 is associated with disruption of thyroid function, such as NTIS and thyroiditis. The preferred treatment options for COVID-19 may need to change as studies identify more promising medications that target the SARS-CoV-2 receptor. The use of antivirals seems inadvisable to prevent complications of acute SARS-CoV-2 infection. DPP-4 inhibitors, metformin, and spironolactone are relatively safe medications, which may be added to antivirals or used in combination for acute SARS-CoV-2 infection based on clinical judgment. It is crucial to evaluate these medications in clinical trials and produce more evidence to support their future use.
Author contributions
NA: Data curation, Writing – review & editing. SD: Data curation, Writing – review & editing. SM: Data curation, Writing – review & editing. MG: Data curation, Writing – review & editing. KK: Data curation, Writing – review & editing. LK: Data curation, Writing – review & editing. AM: Data curation, Writing – review & editing. KA: Investigation, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACE2, angiotensin-converting enzyme 2; ADAM17, A Disintegrin and Metalloproteinase domain-containing protein 17; COVID-19, coronavirus disease 2019; DPP-4, dipeptidyl peptidase 4; IRR, incidence rate ratio; NTIS, Non-thyroidal illness syndrome; OR, Odds ratio; RR, Relative Risk; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAT, subacute thyroiditis; TFT, thyroid function test; T3, triiodothyronine; T4, thyroxine; TSH, Thyroid Stimulating Hormone; TMPRSS2, Transmembrane protease, serine 2.
References
1. Kaashfi K, Anbardar N, Asadipooya A, and Asadipooya K. Type 1 diabetes and COVID-19: A literature review and possible management. Int J Endocrinol Metab. (2023) 21:e139768. doi: 10.5812/ijem-139768
2. Barreto Fernandes TF, Pilotto JH, Cezar PA, Côrtes FH, Morgado MG, Giacoia-Gripp CBW, et al. Modulation of RAAS receptors and miRNAs in COVID-19: implications for disease severity, immune response, and potential therapeutic targets. BMC Infect Dis. (2025) 25:399. doi: 10.1186/s12879-025-10803-y
3. Bakhtiari M and Asadipooya K. Metainflammation in COVID-19. Endocr Metab Immune Disord Drug Targets. (2022) 22(12):1154–66. doi: 10.2174/1871530322666220104103325
4. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, and Latrofa F. Subacute thyroiditis after sars-COV-2 infection. J Clin Endocrinol Metab. (2020) 105(7):1–4. doi: 10.1210/clinem/dgaa276
5. Sharma C and Bayry J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol. (2023) 19:399–400. doi: 10.1038/s41584-023-00964-y
6. Ziaka M and Exadaktylos A. Insights into SARS-CoV-2-associated subacute thyroiditis: from infection to vaccine. Virol J. (2023) 20:132. doi: 10.1186/s12985-023-02103-1
7. Davis HE, McCorkell L, Vogel JM, and Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiology. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
8. Panesar A, Gharanei P, Khovanova N, Young L, and Grammatopoulos D. Thyroid function during COVID-19 and post-COVID complications in adults: a systematic review. Front Endocrinol (Lausanne). (2024) 15:1477389. doi: 10.3389/fendo.2024.1477389
9. Upshaw TL, Brown C, Smith R, Perri M, Ziegler C, and Pinto AD. Social determinants of COVID-19 incidence and outcomes: A rapid review. PloS One. (2021) 16:e0248336. doi: 10.1371/journal.pone.0248336
10. Chavda VP, Sonak SS, Munshi NK, and Dhamade PN. Pseudoscience and fraudulent products for COVID-19 management. Environ Sci Pollut Res Int. (2022) 29:62887–912. doi: 10.1007/s11356-022-21967-4
11. Asadipooya K, Asadipooya A, and Adatorwovor R. Combination of spironolactone and DPP-4 inhibitors for treatment of SARS-CoV-2 infection: a literature review. Arch Virol. (2024) 169:122. doi: 10.1007/s00705-024-06043-1
12. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
13. Pieper D and Rombey T. Where to prospectively register a systematic review. Syst Rev. (2022) 11:8. doi: 10.1186/s13643-021-01877-1
14. Razu MH, Hossain MI, Ahmed ZB, Bhowmik M, Hasan MKE, Kibria MK, et al. Study of thyroid function among COVID-19-affected and non-affected people during pre and post-vaccination. BMC Endocr Disord. (2022) 22:309. doi: 10.1186/s12902-022-01187-0
15. Burekovic A, Halilovic D, and Sahbaz A. Hypothyroidism and subclinical hypothyroidism as a consequence of COVID-19 infection. Med Arch. (2022) 76:12–6. doi: 10.5455/medarh.2022.76.12-16
16. Yanachkova V, Stankova T, and Staynova R. Thyroid dysfunction as a long-term post-COVID-19 complication in mild-to-moderate COVID-19. Biotechnol Biotechnol Equipment. (2023) 37:194–202. doi: 10.1080/13102818.2023.2170829
17. Barajas Galindo DE, Ramos Bachiller B, González Roza L, García Ruiz de Morales JM, Sánchez Lasheras F, González Arnáiz E, et al. Increased incidence of Graves’ disease during the SARS-CoV2 pandemic. Clin Endocrinol (Oxf). (2023) 98:730–7. doi: 10.1111/cen.14860
18. Chen M, Zhou W, and Xu W. Thyroid function analysis in 50 patients with COVID-19: A retrospective study. Thyroid. (2021) 31:8–11. doi: 10.1089/thy.2020.0363
19. Wang W, Su X, Ding Y, Fan W, Zhou W, Su J, et al. Thyroid function abnormalities in COVID-19 patients. Front Endocrinol (Lausanne). (2020) 11:623792. doi: 10.3389/fendo.2020.623792
20. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Pang P, et al. Long COVID in patients with mild to moderate disease: do thyroid function and autoimmunity play a role? Endocr Pract. (2021) 27:894–902. doi: 10.1016/j.eprac.2021.06.016
21. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. (2021) 106:e926–35. doi: 10.1210/clinem/dgaa813
22. Lui DTW, Tsoi KH, Lee CH, Cheung CYY, Fong CHY, Lee ACH, et al. A prospective follow-up on thyroid function, thyroid autoimmunity and long COVID among 250 COVID-19 survivors. Endocrine. (2023) 80:380–91. doi: 10.1007/s12020-022-03281-8
23. Anaya JM, Monsalve DM, Rojas M, Rodríguez Y, Montoya-García N, Mancera-Navarro LM, et al. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J Transl Autoimmun. (2021) 4:100091. doi: 10.1016/j.jtauto.2021.100091
24. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Insights from a prospective follow-up of thyroid function and autoimmunity among COVID-19 survivors. Endocrinol Metab (Seoul). (2021) 36:582–9. doi: 10.3803/EnM.2021.983
25. Lui DTW, Xiong X, Cheung CL, Lai FTT, Li X, Wan EYF, et al. Risk of incident thyroid dysfunction in the post-acute phase of COVID-19: A population-based cohort study in hong kong. Endocr Pract. (2024) 30:528–36. doi: 10.1016/j.eprac.2024.03.389
26. Herczeg V, Garai R, Takács J, Kovács F, Luczay A, Hrapka E, et al. Thyroid disturbances after COVID-19 and the effect of vaccination in children: a prospective tri-center registry analysis. Eur J Pediatr. (2023) 182:4443–55. doi: 10.1007/s00431-023-05097-8
27. Arora S, Singh A, Kumar V, Mohan B, Mahajan R, Singh N, et al. Endocrine and metabolic manifestations of COVID-19 patients admitted to an intensive care unit. Cureus. (2022) 14:e24702. doi: 10.7759/cureus.24702
28. Mondal S, DasGupta R, Lodh M, and Ganguly A. Subacute thyroiditis following recovery from COVID-19 infection: novel clinical findings from an Eastern Indian cohort. Postgrad Med J. (2023) 99:558–65. doi: 10.1136/postgradmedj-2021-141429
29. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, and Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. (2020) 183:381–7. doi: 10.1530/EJE-20-0335
30. Pizzocaro A, Colombo P, Vena W, Ariano S, Magnoni P, Reggiani F, et al. Outcome of Sars-COV-2-related thyrotoxicosis in survivors of Covid-19: a prospective study. Endocrine. (2021) 73:255–60. doi: 10.1007/s12020-021-02758-2
31. Rossini A, Cassibba S, Perticone F, Benatti SV, Venturelli S, Carioli G, et al. Increased prevalence of autoimmune thyroid disease after COVID-19: A single-center, prospective study. Front Endocrinol (Lausanne). (2023) 14:1126683. doi: 10.3389/fendo.2023.1126683
32. Brancatella A, Viola N, Rutigliano G, Sgrò D, Santini F, and Latrofa F. Subacute thyroiditis during the SARS-coV-2 pandemic. J Endocr Soc. (2021) 5:bvab130. doi: 10.1210/jendso/bvab130
33. Adhikari P and Singh R. Subclinical hypothyroidism among patients with COVID-19 infection in a tertiary care centre: A descriptive cross-sectional study. JNMA J Nepal Med Assoc. (2023) 61:531–4. doi: 10.31729/jnma.8187
34. Malik J, Malik A, Javaid M, Zahid T, Ishaq U, and Shoaib M. Thyroid function analysis in COVID-19: A retrospective study from a single center. PloS One. (2021) 16:e0249421. doi: 10.1371/journal.pone.0249421
35. Elhadd T, Gul W, Dabbous Z, Beer S, and Bashir M. Bimodal distribution of thyroid dysfunction triggered by COVID-19 Infection: An experience from a single endocrine center-a case series and literature review. Qatar Med J. (2022) 2022:39. doi: 10.5339/qmj.2022.39
36. Ostapchuk VA and Ostapchuk VO. A Post COVID-19 changes in the structure and function of the thyroid in patients with autoimmune thyroiditis with hypothyroidism. Romanian J Diabetes Nutr Metab Diseases. (2023) 30:23–8. doi: 10.5339/qmj.2022.39
37. Mukhtar N, Bakhsh A, Alreshidi N, Aljomaiah A, Aljamei H, AlSudani N, et al. COVID-19 infection and thyroid function. Endocr Metab Sci. (2022) 7:100122. doi: 10.1016/j.endmts.2022.100122
38. Ahn HY, Choi HS, Ha S, and Cho SW. Incidence of subacute thyroiditis during the COVID-19 pandemic in South Korea using the national health insurance service database. Thyroid. (2022) 32:1299–306. doi: 10.1016/j.endmts.2022.100122
39. Batman A, Yazıcı D, Dikbaş O, Ağbaht K, Saygılı ES, Demirci İ, et al. Subacute THYROiditis related to SARS-coV-2 VAccine and covid-19 (THYROVAC study): A multicenter nationwide study. J Clin Endocrinol Metab. (2023) 108:e1013–26. doi: 10.1210/clinem/dgad235
40. Khoo B, Tan T, Clarke SA, Mills EG, Patel B, Modi M, et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab. (2021) 106:e803–11. doi: 10.1210/clinem/dgaa830
41. Clarke SA, Phylactou M, Patel B, Mills EG, Muzi B, Izzi-Engbeaya C, et al. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J Clin Endocrinol Metab. (2021) 106:2208–20. doi: 10.1210/clinem/dgab349
42. McCowan R, Wild E, Lucas-Herald AK, McNeilly J, Mason A, Wong SC, et al. The effect of COVID-19 on the presentation of thyroid disease in children. Front Endocrinol (Lausanne). (2022) 13:1014533. doi: 10.3389/fendo.2022.1014533
43. Donner JR, Has P, and Topor LS. Increased incidence and severity of new graves disease diagnoses in youth during the COVID-19 pandemic. Endocr Pract. (2023) 29:349–52. doi: 10.1016/j.eprac.2023.01.011
44. De Vincentis S, Loiacono S, Zanni E, Sueri R, Monzani ML, Santi D, et al. Subacute thyroiditis in the SARS-CoV-2 era: a multicentre prospective study. Eur Thyroid J. (2024) 13(3):e240083. doi: 10.1530/ETJ-24-0083
45. Bogojevic M, Bansal V, Pattan V, Singh R, Tekin A, Sharma M, et al. Association of hypothyroidism with outcomes in hospitalized adults with COVID-19: Results from the International SCCM Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry. Clin Endocrinol (Oxf). (2024) 101:85–93. doi: 10.1111/cen.v101.1
46. Fallahi P, Ferrari SM, Elia G, Paparo SR, Patrizio A, Balestri E, et al. Thyroid autoimmunity and SARS-CoV-2 infection: Report of a large Italian series. Autoimmun Rev. (2022) 21:103183. doi: 10.1016/j.autrev.2022.103183
47. Darvishi M, Nazer MR, Shahali H, and Nouri M. Association of thyroid dysfunction and COVID-19: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2022) 13:947594. doi: 10.3389/fendo.2022.947594
48. Ashrafi S, Hatami H, Bidhendi-Yarandi R, and Panahi MH. The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis. BMC Endocr Disord. (2024) 24:5. doi: 10.1186/s12902-023-01534-9
49. Viola N, Brancatella A, Sgrò D, Santini F, and Latrofa F. Clinical, biochemical features and functional outcome of patients with SARS-CoV-2-related subacute thyroiditis: a review. Endocrine. (2023) 79:448–54. doi: 10.1016/S2213-8587(20)30266-7
50. Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. (2020) 8:739–41. doi: 10.1016/S2213-8587(20)30266-7
51. Li GH, Tang CM, and Cheung CL. COVID-19 and thyroid function: A bi-directional two-sample mendelian randomization study. Thyroid. (2022) 32:1037–50. doi: 10.1089/thy.2023.0626
52. Huang LA, Lo SC, Yang YS, Huang CN, Wang CC, Wang YH, et al. Association of COVID-19 infection with subsequent thyroid dysfunction: an international population-based propensity score matched analysis. Thyroid. (2024) 34:442–9. doi: 10.1089/thy.2023.0626
53. Meftah E, Rahmati R, Zari Meidani F, Khodadadi S, Chitzan-Zadeh K, Esfahanian F, et al. Subacute thyroiditis following COVID-19: A systematic review. Front Endocrinol (Lausanne). (2023) 14:1126637. doi: 10.3389/fendo.2023.1126637
54. Pirola I, Gandossi E, Rotondi M, Marini F, Cristiano A, Chiovato L, et al. Incidence of De Quervain’s thyroiditis during the COVID-19 pandemic in an area heavily affected by Sars-CoV-2 infection. Endocrine. (2021) 74:215–8. doi: 10.1007/s12020-021-02841-8
55. Bahçecioğlu AB, Karahan ZC, Aydoğan BI, Kalkan IA, Azap A, and Erdoğan MF. Subacute thyroiditis during the COVID-19 pandemic: a prospective study. J Endocrinol Invest. (2022) 45:865–74. doi: 10.1007/s40618-021-01718-x
56. Bostan H, Sencar ME, Calapkulu M, Kayihan S, Hepsen S, Cimsir A, et al. Impact of the COVID-19 pandemic on the incidence, seasonal distribution, and characteristics of subacute thyroiditis. Endocrine. (2023) 79:323–30. doi: 10.1007/s12020-022-03197-3
57. Fliers E, Bianco AC, Langouche L, and Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. (2015) 3:816–25. doi: 10.1016/S2213-8587(15)00225-9
58. Mateu-Salat M, Urgell E, and Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J Endocrinol Invest. (2020) 43:1527–8. doi: 10.1007/s40618-020-01366-7
59. Allam MM, El-Zawawy HT, Ahmed SM, and Aly Abdelhamid M. Thyroid disease and covid-19 infection: Case series. Clin Case Rep. (2021) 9:e04225. doi: 10.1002/ccr3.4225
60. Lanzolla G, Marcocci C, and Marinò M. Graves’ disease and Graves’ orbitopathy following COVID-19. J Endocrinol Invest. (2021) 44:2011–2. doi: 10.1007/s40618-021-01576-7
61. Tan SYT, Xiong J, Puar TH, Khoo J, Wong AJ, and Soh SB. Acute flaccid tetraparesis after COVID-19 infection: think of the thyroid. Case Rep Med. (2022) 2022:5827664. doi: 10.1155/2022/5827664
62. Fitriani F, Susanti VY, and Ikhsan MR. COVID-19 infection-related thyrotoxic hypokalemic periodic paralysis. Case Rep Endocrinol. (2022) 2022:1382270. doi: 10.1155/2022/1382270
63. Chinangkulpiwat S, Tantiprawan J, Amornvit J, Bunchaya-Anant P, and Snabboon T. Thyrotoxic hypokalemic periodic paralysis and COVID-19 infection. J Family Med Prim Care. (2022) 11:7416–8. doi: 10.4103/jfmpc.jfmpc_475_22
64. Lu A, Chen CC, Lin C, Wu TJ, and Lin SH. Artificial intelligence electrocardiography detecting thyrotoxic periodic paralysis following a SARS-coV-2 infection. Am J Med. (2024) 137:e91–3. doi: 10.1016/j.amjmed.2024.01.018
65. Sullivan K, Helgeson J, and McGowan A. COVID-19 associated thyroid storm: A case report. Clin Pract cases Emerg Med. (2021) 5:412–4. doi: 10.5811/cpcem
66. Das BB, Shakti D, Akam-Venkata J, Obi O, Weiland MD, and Moskowitz W. SARS-CoV-2 infection induced thyroid storm and heart failure in an adolescent girl. Cardiol Young. (2022) 32:988–92. doi: 10.1017/S1047951121004352
67. Pastor S, Molina Á, and De Celis E. Thyrotoxic crisis and COVID-19 infection: an extraordinary case and literature review. Cureus. (2020) 12:e11305. doi: 10.7759/cureus.11305
68. van Gerwen M, Alsen M, Little C, Barlow J, Naymagon L, Tremblay D, et al. Outcomes of patients with hypothyroidism and COVID-19: A retrospective cohort study. Front Endocrinol (Lausanne). (2020) 11:565. doi: 10.3389/fendo.2020.00565
69. Brix TH, Hegedüs L, Hallas J, and Lund LC. Risk and course of SARS-CoV-2 infection in patients treated for hypothyroidism and hyperthyroidism. Lancet Diabetes Endocrinol. (2021) 9:197–9. doi: 10.1016/S2213-8587(21)00028-0
70. Pereira DN, Silveira LFG, Guimarães MMM, Polanczyk CA, Nunes AGS, Costa ASM, et al. Hypothyroidism does not lead to worse prognosis in COVID-19: findings from the Brazilian COVID-19 registry. Int J Infect Dis. (2022) 116:319–27. doi: 10.1016/j.ijid.2022.01.016
71. Nguyen C, Yale K, Ghigi A, Zheng K, Mesinkovska NA, Wambier CG, et al. SARS-CoV-2 infection in patients with thyroid disease: a cross-sectional study. Ann Thyroid. (2022) 6:7. doi: 10.21037/aot-21-8
72. Sahin M, Demirci I, Haymana C, Tasci I, Emral R, Cakal E, et al. The clinical characteristics and outcomes of COVID-19 patients with pre-existing thyroid dysfunction: A nationwide study. Horm Metab Res. (2023) 55:25–30. doi: 10.1055/a-1971-8781
73. Bagalà V, Sala A, Trevisan C, Okoye C, Incalzi RA, Monzani F, et al. Clinical presentation and prognosis of COVID-19 in older adults with hypothyroidism: data from the GeroCovid observational study. J Endocrinol Invest. (2023) 46:1891–9. doi: 10.1007/s40618-023-02048-w
74. Damara FA, Muchamad GR, Ikhsani R, Hendro, Syafiyah AH, and Bashari MH. Thyroid disease and hypothyroidism are associated with poor COVID-19 outcomes: A systematic review, meta-analysis, and meta-regression. Diabetes Metab syndrome. (2021) 15:102312. doi: 10.1016/j.dsx.2021.102312
75. Permana H, Soeriadi EA, Damara FA, and Soetedjo NNM. The prognostic properties of thyroid disorders, hypothyroidism, and hyperthyroidism in predicting COVID-19 poor outcomes: A systematic review and diagnostic meta-analysis. Indian J Endocrinol Metab. (2022) 26:510–7. doi: 10.4103/ijem.ijem_20_22
76. Beltrão FEL, Beltrão DCA, Carvalhal G, Beltrão FEL, Brito ADS, Capistrano K, et al. Thyroid hormone levels during hospital admission inform disease severity and mortality in COVID-19 patients. Thyroid. (2021) 31:1639–49. doi: 10.1089/thy.2021.0225
77. Zhang Q, Zhang Z, Liu X, Wang Y, Chen H, Hao Y, et al. Thyroid dysfunction in the wake of Omicron: understanding its role in COVID-19 severity and mortality. Front Endocrinol (Lausanne). (2024) 15:1412320. doi: 10.3389/fendo.2024.1412320
78. Sror-Turkel O, El-Khatib N, Sharabi-Nov A, Avraham Y, and Merchavy S. Low TSH and low T3 hormone levels as a prognostic for mortality in COVID-19 intensive care patients. Front Endocrinol (Lausanne). (2024) 15:1322487. doi: 10.3389/fendo.2024.1322487
79. Park GC, Lee HW, Kim JM, Han JM, Kim HI, Shin SC, et al. ACE2 and TMPRSS2 immunolocalization and COVID-19-related thyroid disorder. Biol (Basel). (2022) 11(5):697. doi: 10.3390/biology11050697
80. Macedo S, Pestana A, Santos L, Neves C, Guimarães S, Duarte-Neto A, et al. Detection of SARS-CoV-2 infection in thyroid follicular cells from a COVID-19 autopsy series. Eur Thyroid J. (2022) 11(4):e220074. doi: 10.1530/ETJ-22-0074
81. Poma AM, Basolo A, Bonuccelli D, Proietti A, Macerola E, Ugolini C, et al. Activation of type I and type II interferon signaling in SARS-coV-2-positive thyroid tissue of patients dying from COVID-19. Thyroid. (2021) 31:1766–75. doi: 10.1089/thy.2021.0345
82. Attiq A, Afzal S, Wahab HA, Ahmad W, Kandeel M, Almofti YA, et al. Cytokine storm-induced thyroid dysfunction in COVID-19: insights into pathogenesis and therapeutic approaches. Drug Des Devel Ther. (2024) 18:4215–40. doi: 10.2147/DDDT.S475005
83. Fallahi P, Elia G, Ragusa F, Paparo SR, Patrizio A, Balestri E, et al. Thyroid autoimmunity and SARS-coV-2 infection. J Clin Med. (2023) 12(19):6365. doi: 10.3390/jcm12196365
84. Bellastella G, Cirillo P, Carbone C, Scappaticcio L, Maio A, Botta G, et al. Neuroimmunoendocrinology of SARS-coV 2 infection. Biomedicines. (2022) 10(11):2855. doi: 10.3390/biomedicines10112855
85. Lui DTW, Lee CH, Woo YC, Hung IFN, and Lam KSL. Thyroid dysfunction in COVID-19. Nat Rev Endocrinol. (2024) 20(6):336–48. doi: 10.1038/s41574-023-00946-w
86. Bray GA, Edelstein SL, Crandall JP, Aroda VR, Franks PW, Fujimoto W, et al. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. (2012) 35:731–7. doi: 10.2337/dc11-1299
87. Kosakovsky Pond SL and Martin D. Anti-COVID drug accelerates viral evolution. Nature. (2023) 623:486–7. doi: 10.1038/d41586-023-03248-3
88. Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, and Reeves WB. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). eLife. (2023) 12:e86002. doi: 10.7554/eLife.86002
89. Parotto M, Gyöngyösi M, Howe K, Myatra SN, Ranzani O, Shankar-Hari M, et al. Post-acute sequelae of COVID-19: understanding and addressing the burden of multisystem manifestations. Lancet Respir Med. (2023) 11:739–54. doi: 10.1016/S2213-2600(23)00239-4
90. Roy AN, Gupta AM, Banerjee D, Chakrabarti J, and Raghavendra PB. Unraveling DPP4 Receptor Interactions with SARS-CoV-2 Variants and MERS-CoV: Insights into Pulmonary Disorders via Immunoinformatics and Molecular Dynamics. Viruses. (2023) 15(10):2056. doi: 10.3390/v15102056
91. Fels B, Acharya S, Vahldieck C, Graf T, Käding N, Rupp J, et al. Mineralocorticoid receptor-antagonism prevents COVID-19-dependent glycocalyx damage. Pflugers Arch. (2022) 474(10):1069–76. doi: 10.1007/s00424-022-02726-3
92. Erickson SM, Fenno SL, Barzilai N, Kuchel G, Bartley JM, Justice JN, et al. Metformin for treatment of acute COVID-19: systematic review of clinical trial data against SARS-Cov-2. Diabetes Care. (2023) 46:1432–42. doi: 10.2337/dc22-2539
Keywords: ACE2, hyperthyroidism, hypothyroidism, SARS-CoV-2, thyroiditis
Citation: Anbardar N, Dixon SL, Munugoti S, Gaddam M, Kashfi K, Kasulis L, Messersmith AL and Asadipooya K (2025) Thyroid disorders and COVID-19: a comprehensive review of literature. Front. Endocrinol. 16:1535169. doi: 10.3389/fendo.2025.1535169
Received: 27 November 2024; Accepted: 16 April 2025;
Published: 19 May 2025.
Edited by:
Alessandro Antonelli, University of Pisa, ItalyCopyright © 2025 Anbardar, Dixon, Munugoti, Gaddam, Kashfi, Kasulis, Messersmith and Asadipooya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kamyar Asadipooya, a2FzMjI0QHVreS5lZHU=; a2FtaWFzYWRpcEB5YWhvby5jb20=
†ORCID: Kamyar Asadipooya, orcid.org/0000-0003-4484-1971
 Narges Anbardar1
Narges Anbardar1 Maneesh Gaddam
Maneesh Gaddam Kamyar Asadipooya
Kamyar Asadipooya