- 1School of Medicine, Nankai University, Tianjin, China
- 2Clinical Medical College of Tianjin Medical University, Tianjin, China
- 3Department of Cardiology, Tianjin First Central Hospital, Tianjin, China
Background: We aim to investigate the association between TyG(Triglyceride-Glucose index) and TyG-BMI(Triglyceride-Glucose-Body Mass Index) indices and the risk of non-alcoholic fatty liver disease (NAFLD) in patients undergoing percutaneous coronary intervention (PCI), an area where their predictive value is currently unclear, despite their established link to insulin resistance, metabolic syndrome, and cardiovascular disease.
Methods: In this cross-sectional study, 776 patients who underwent coronary angiography and PCI were categorized into NAFLD+PCI and PCI groups based on abdominal ultrasound. They were further classified by TyG and TyG-BMI indices. Continuous variables were compared using ANOVA, Wilcoxon-Mann-Whitney, or t-tests, while categorical variables were analyzed with χ² or Fisher exact tests. Logistic regression identified independent factors for NAFLD in PCI patients. ROC curves evaluated the predictive efficacy of TyG and TyG-BMI for NAFLD. Linear correlation and multiple linear regression assessed relationships among NAFLD fibrosis score (NFS), TyG, and TyG-BMI.
Results: Among 776 patients, NAFLD was detected in 305. After adjusting for age, smoking, hypertension, diabetes, sex, and cardiovascular disease, multivariate logistic regression showed the TyG index was a significant risk factor for NAFLD in PCI patients (OR = 2.04; 95% CI, 1.62-2.55; P < 0.001). Similarly, the TyG-BMI index, total cholesterol, triglycerides, LDL cholesterol, fasting blood glucose, and BMI were associated with increased NAFLD risk. Each unit increase in the TyG index raised the NAFLD risk by 2.63-fold (OR = 2.63; 95% CI, 1.78-3.8; P<0.001), and each unit increase in the TyG-BMI index by 3.80-fold (OR = 3.80; 95% CI, 2.55-5.68; P < 0.001). Multivariate linear regression indicated that in the PCI-NAFLD group, each unit increase in the TyG index increased the NFS value by 0.247 (β = 0.247; 95% CI, 0.19-0.45; P < 0.001), and each unit increase in the TyG-BMI index increased the NFS value by 0.344 (β = 0.344; 95% CI, 0.28-0.59; P < 0.001).
Conclusions: The TyG index and TyG-BMI were positively associated with the risk of NAFLD in patients treated with PCI, reflecting the severity of liver fibrosis.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD), a liver disease unrelated to alcohol consumption and characterized by an abnormal accumulation of fat in liver tissue, is a significant public health challenge worldwide (1). Current estimates indicate that the prevalence of NAFLD in adults approximately 20–30%; in certain populations, such as overweight or obese individuals and patients with type 2 diabetes, this proportion increases significantly and can reach 50—75%. The developmental mechanisms of NAFLD are extremely complex and include genetic predisposition, insulin resistance, chronic mild inflammatory responses and endoplasmic reticulum stress (2–4). Recent studies show a positive correlation between the TyG index and NAFLD severity. The TyG index, reflecting insulin sensitivity through triglyceride and fasting blood glucose levels, enhances our understanding of NAFLD’s pathophysiology and may provide new approaches for early detection and intervention (5–8).
Cardiovascular disease (CVD) is the leading global cause of mortality. Percutaneous coronary intervention (PCI) effectively treats coronary artery narrowing or blockage, reducing mortality and rehospitalization in acute coronary syndrome patients. Patients undergoing PCI often have metabolic disorders such as hypertension, elevated blood sugar, and lipid metabolism issues, which are closely linked to NAFLD development and progression (9, 10).
The triglyceride-glucose index (TyG), which combines fasting glucose and triglyceride levels, is a marker for insulin resistance (IR). The TyG-BMI extends this by incorporating body mass index (BMI), further clarifying the link between IR and overweight. Recent studies show that the TyG index is significantly associated with cardiovascular risks such as hypertension, hyperlipidemia, and type 2 diabetes (11–13). However, few studies have examined the predictive ability of IR for NAFLD risk in PCI patients. Thus, this study focuses on high-risk PCI patients to analyze the relationships between the TyG index and TyG-BMI and the risk of NAFLD. This is crucial for identifying high-risk patients, developing preventive strategies, and improving patient outcomes.
2 Data and methods
2.1 Subjects and study design
This cross-sectional study included 1329 patients hospitalized in the Department of Cardiology of Tianjin First Central Hospital between January and December 2022, who underwent coronary angiography and PCI. As shown in Figure 1, after excluding patients with key data loss and confounding data, 776 patients remained. The patients were divided into two groups based on abdominal ultrasonography results: NAFLD+PCI and PCI groups.
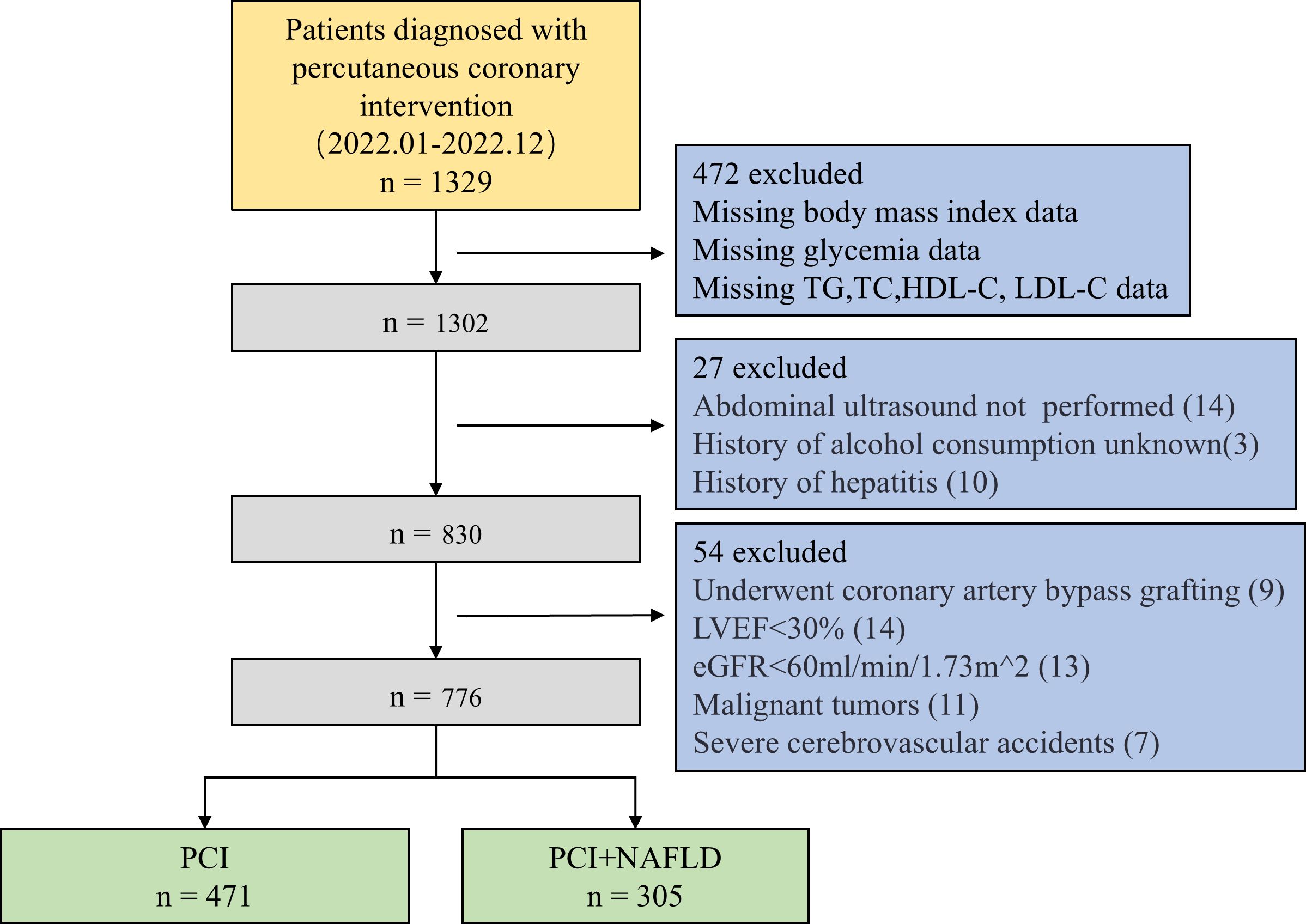
Figure 1. Subject selection flowchart. TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate.
The NAFLD Fibrosis Score (NFS) is a mathematical model constructed from six clinical parameters—age, body mass index (BMI), hyperglycemia (impaired fasting glucose), platelet count, albumin level, and the AST/ALT ratio—designed for the noninvasive quantification of liver fibrosis severity in patients with nonalcoholic fatty liver disease (NAFLD). An NFS > 0.676 indicates significant fibrosis, while an NFS < -1.455 suggests advanced liver fibrosis.
The NFS score was calculated via the formula: NFS = -1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m²) + 1.13 × TDM (yes = 1, no = 0) + 0.99 × AST/ALT - 0.013 × plt (×10^9/L) - 0.66 × Alb (g/dL).
NAFLD diagnostic criteria include: alcohol consumption <140 g/week for men and <70 g/week for women, exclusion of other chronic liver diseases (e.g., hepatitis B/C, autoimmune conditions, primary biliary cirrhosis, haemochromatosis, Wilson’s disease), and ultrasound confirmation of hepatic steatosis.
Patients with a history of coronary artery bypass grafting (CABG), other heart conditions, malignant tumors, autoimmune diseases, infections, severe cerebrovascular events, or severe kidney disease were excluded.
2.2 Data collection and measurements
Demographic characteristics, test results, and laboratory values were collected from electronic medical records. This included sex, age, height, weight, smoking and alcohol history, admission rhythm, and clinical information such as hypertension, diabetes, and cardiovascular disease.
The following morning after overnight fasting, a 3 mL venous blood sample was drawn from the antecubital fossa and placed in an anticoagulation tube. An enzyme-linked immunosorbent assay (ELISA) was used to measure hematological parameters (white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin, and platelet count) and biochemical indicators (alanine transaminase (ALT), aspartate aminotransferase (AST), serum creatinine (Scr), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), C-reactive protein (CRP), and B-type natriuretic peptide (BNP)).
The body mass index (BMI), TyG index, and TyG-BMI index were calculated. BMI was calculated as weight (kg)/height² (m²). The TyG index was calculated using the formula: Ln[TG (mg/dL) × FBG (mg/dL)/2]. The TyG-BMI index was derived by multiplying the TyG index by the BMI.
2.3 Statistical analysis
Data were analyzed using SPSS 26.0, GraphPad Prism 8.0, and R 4.3.3. The Shapiro-Wilk test assessed the normal distribution of continuous variables. Normally distributed data are presented as mean ± standard deviation (x̅ ± s) and analyzed using the independent samples t-test. Non-normally distributed data are presented as median [interquartile range (Q1, Q3)] and analyzed using the Wilcoxon-Mann-Whitney test. Frequency data are presented as n (%) and analyzed using the chi-square test (χ²) or Fisher’s exact test. A p-value < 0.05 indicates statistical significance.
Univariate logistic regression was performed to identify potential predictive factors for NAFLD, followed by multivariate logistic regression to determine independent predictors and their effectiveness. The baseline characteristics of the TyG and TyG-BMI groups were compared using one-way ANOVA for continuous variables and the Kruskal-Wallis test for nonparametric data. Categorical variables were compared using the chi-square (χ²) or Fisher’s exact test. Univariate and multivariate logistic regressions assessed the impact of TyG and TyG-BMI levels on NAFLD risk. ROC curve analysis evaluated the predictive performance of TyG and TyG-BMI indices, based on AUC values. Linear correlation and multivariate linear regression were used to assess associations between the NFS score and the TyG and TyG-BMI indices.
3 Results
3.1 Demographic and clinical characteristics of patients who underwent PCI with and without NAFLD
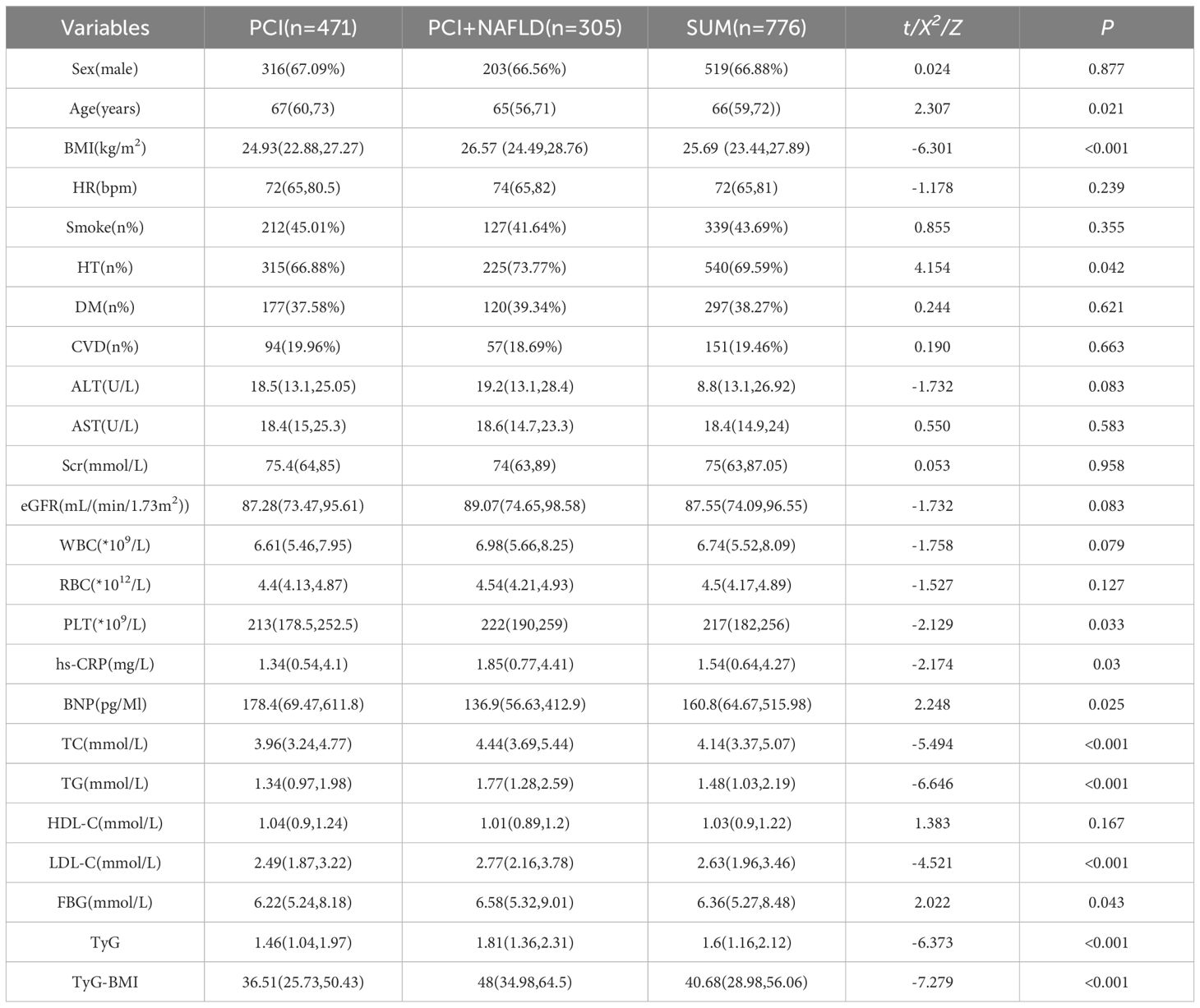
Data were obtained from 776 PCI patients (516 men, 260 women), including 305 with NAFLD and 471 without. The median ages (IQR) were 67 years (60-73) for the PCI group and 65 years (56-71) for the PCI plus NAFLD group. Table 1 shows the demographic and clinical characteristics of both groups. There were no significant differences in admission rhythm, smoking status, diabetes prevalence, cardiovascular disease incidence, ALT, AST, Scr, eGFR, WBC, RBC, PLT, hs-CRP, BNP, and HDL-C. However, the PCI plus NAFLD group had a lower proportion of males but a higher proportion with hypertension, a lower median age, and higher mean BMI, TC, TG, LDL-C, FBG, TyG index, and TyG-BMI.
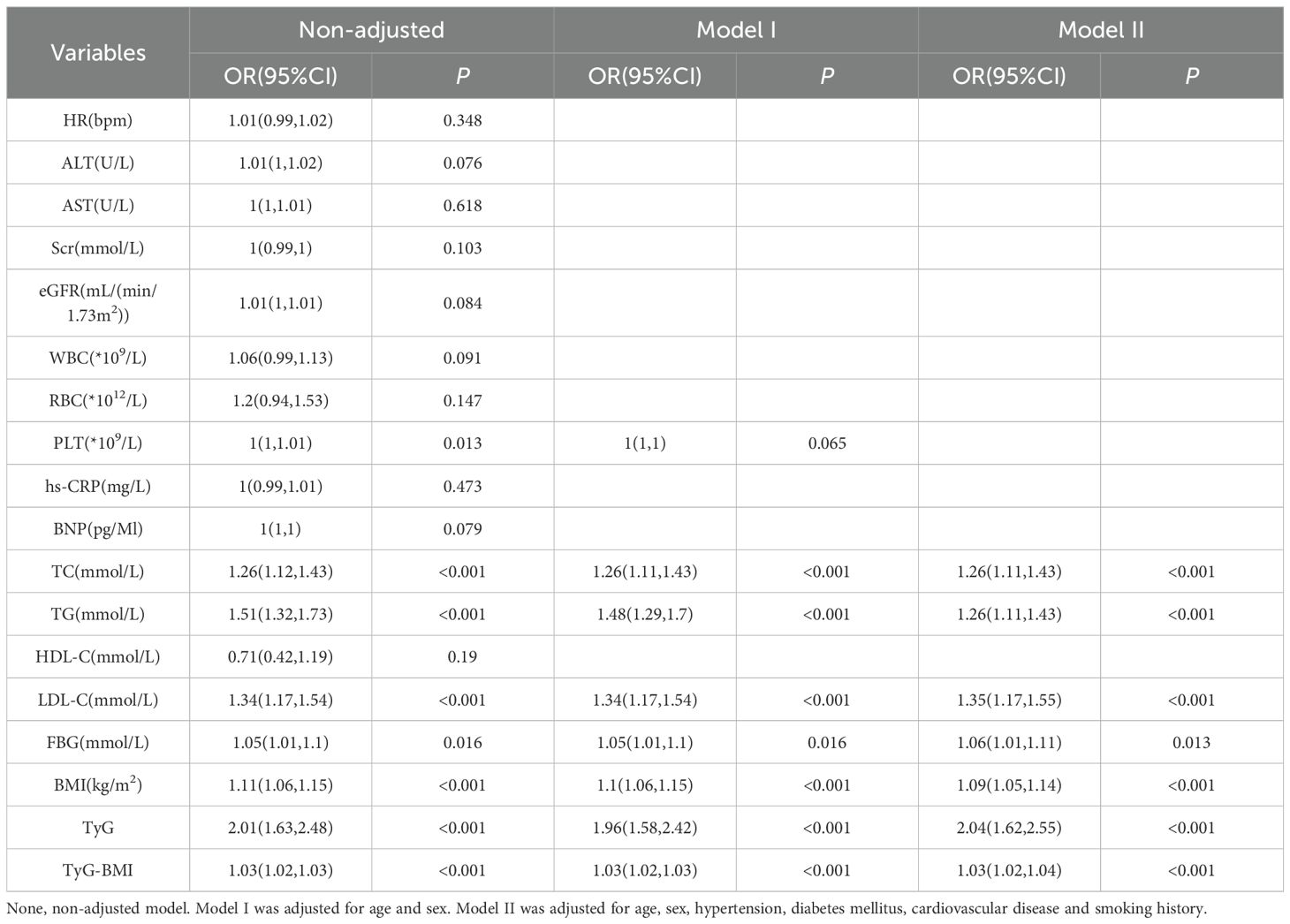
3.2 Univariate and multivariate analysis of factors associated with NAFLD in patients undergoing PCI
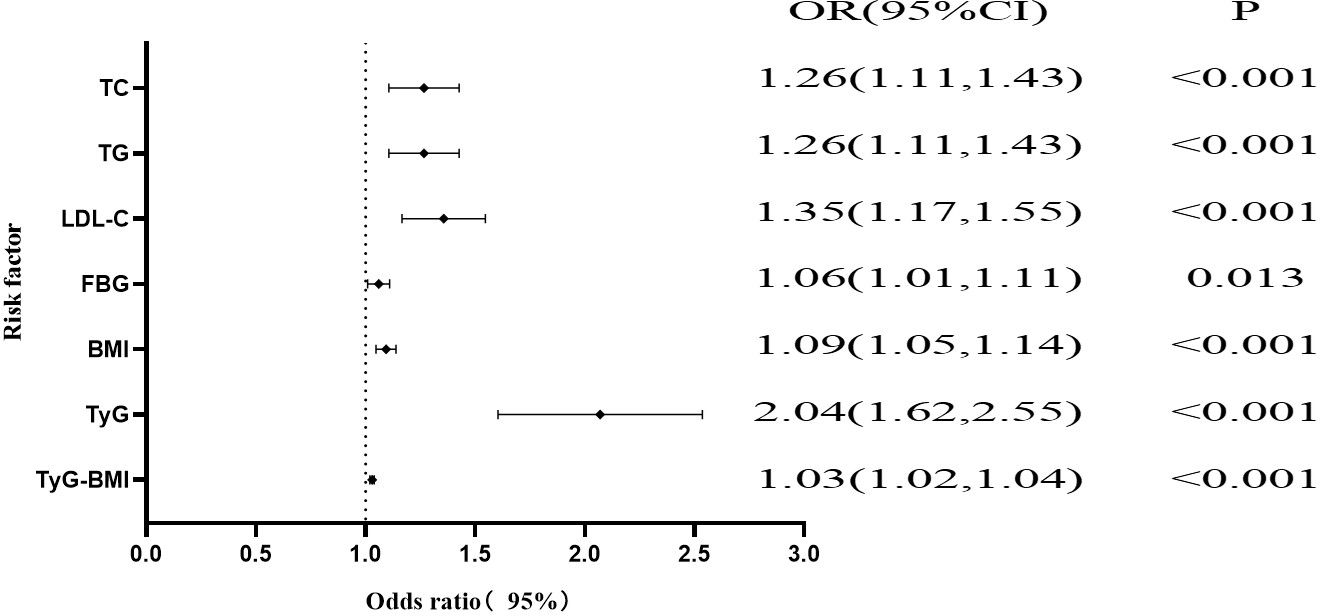
As shown in Figure 2; Table 2, univariate logistic regression revealed associations between PLT, TC, TG, LDL-C, FBG, BMI, TyG, and TyG-BMI and NAFLD in PCI patients. Multivariate logistic regression, adjusting for other variables, showed that PLT was not a risk factor for NAFLD. TC (OR, 1.26; 95% CI, 1.11-1.43; P < 0.001), TG (OR, 1.26; 95% CI, 1.11-1.43; P < 0.001), LDL-C (OR, 1.35; 95% CI, 1.17-1.55; P < 0.001), FBG (OR, 1.06; 95% CI, 1.01-1.11; P = 0.013), BMI (OR, 1.09; 95% CI, 1.05-1.14; P < 0.001), TyG (OR, 2.04; 95% CI, 1.62-2.55; P < 0.001), and TyG-BMI (OR, 1.03; 95% CI, 1.02-1.04; P < 0.001) remained significant risk factors for NAFLD.
3.3 Demographic and clinical characteristics associated with the TyG index
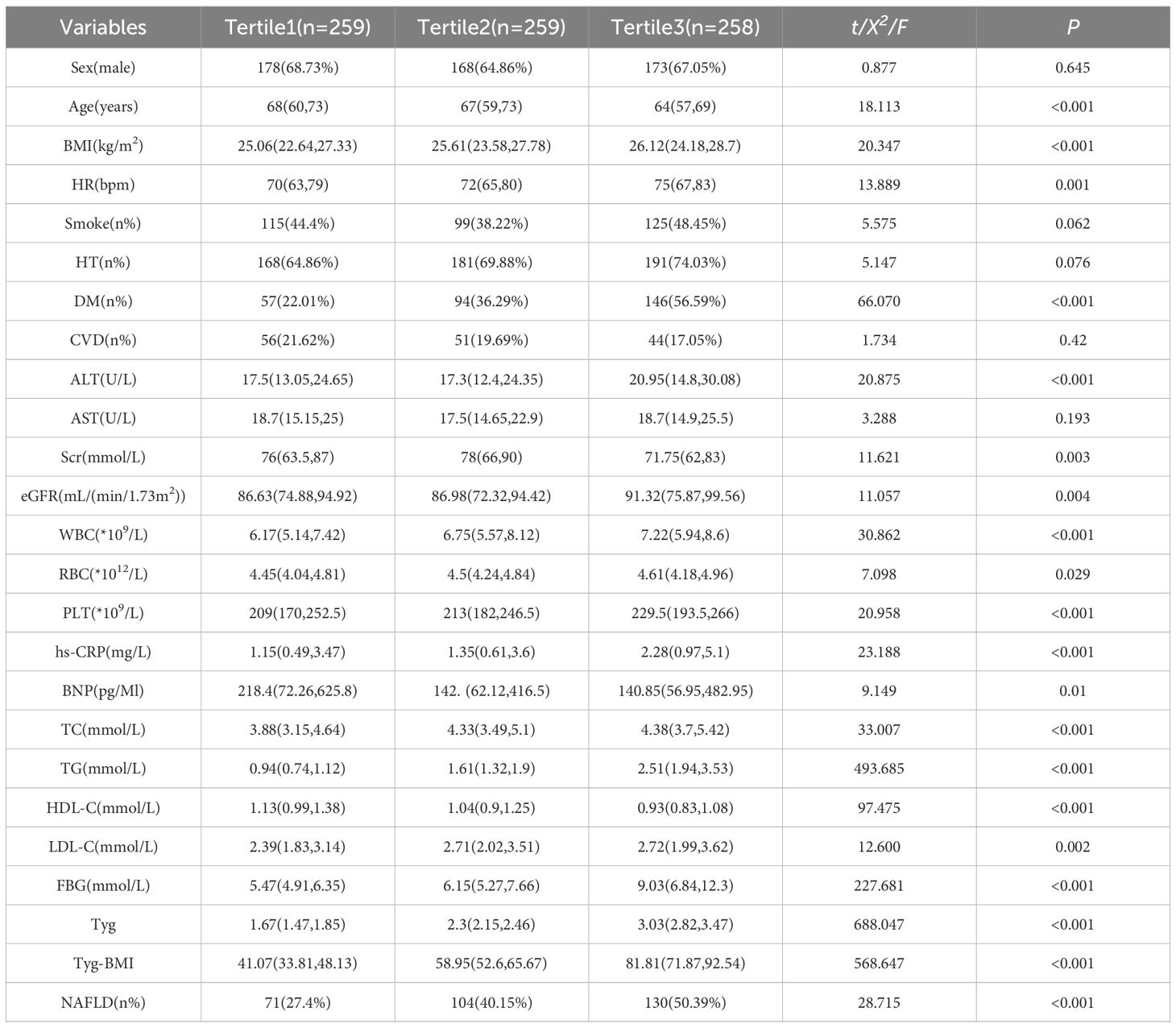
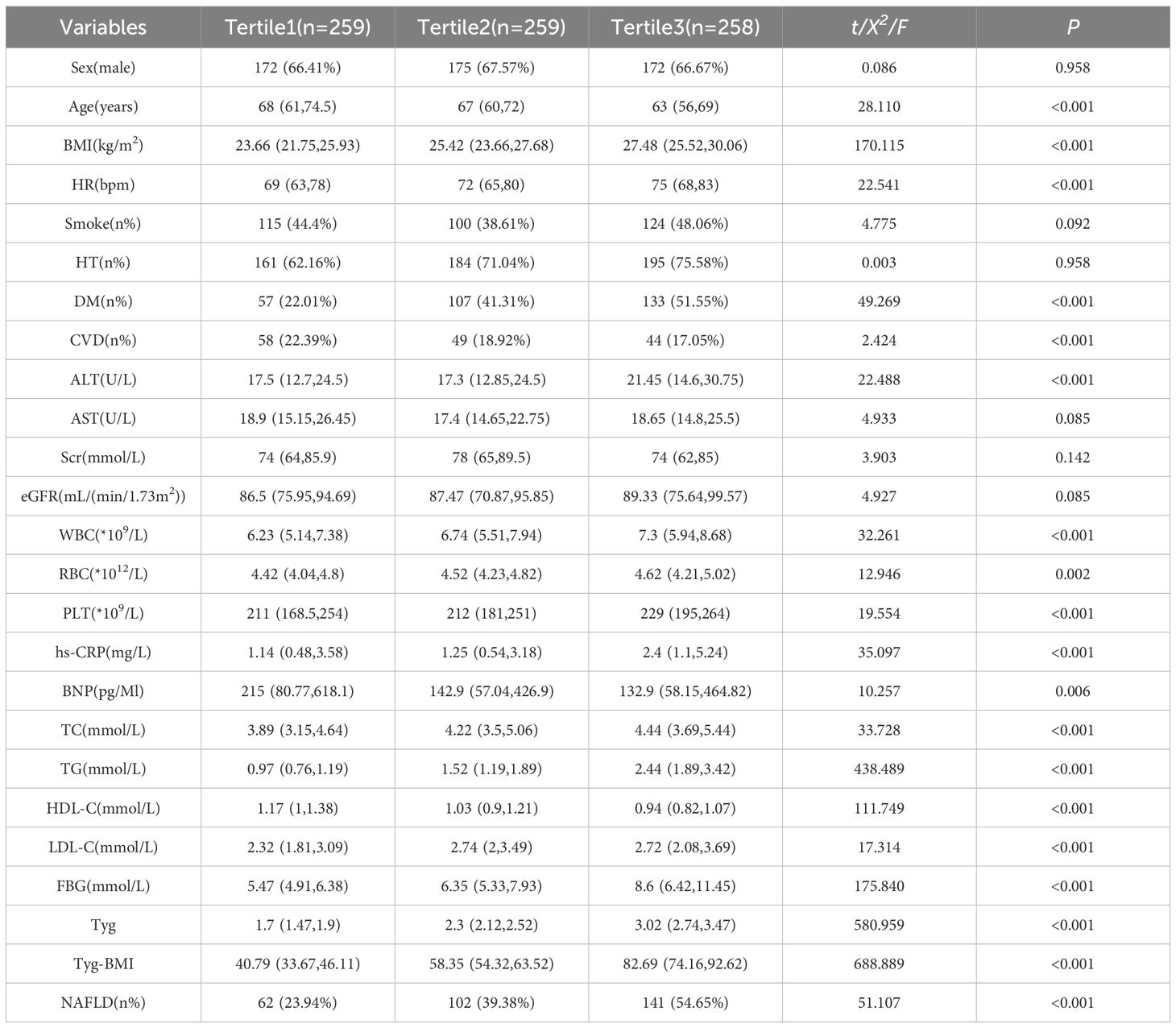
To investigate the associations between different levels of insulin resistance (according to the TyG index) and NAFLD in patients undergoing PCI, patients were categorized into three groups according to TyG index tertiles: Group 1 (n = 259) with TyG index ≤ 2.00; Group 2 (n = 259) with a 2.00 < TyG index ≤ 2.61; and Group 3 (n = 258) with a TyG index > 2.61. The mean TyG indices of these groups were 1.67 (1.47, 1.85), 2.30 (2.15, 2.46), and 3.03 (2.82, 3.47), respectively. As shown in Table 3, the statistical analysis revealed significant differences among the three groups in terms of the incidence of NAFLD, diabetes mellitus (DM), ALT, Scr, eGFR, WBC, RBC, PLT, hs-CRP, BNP, BMI, TC, TG, HDL-C, LDL-C, FBG, TyG index and TyG-BMI index (all P values < 0.05).
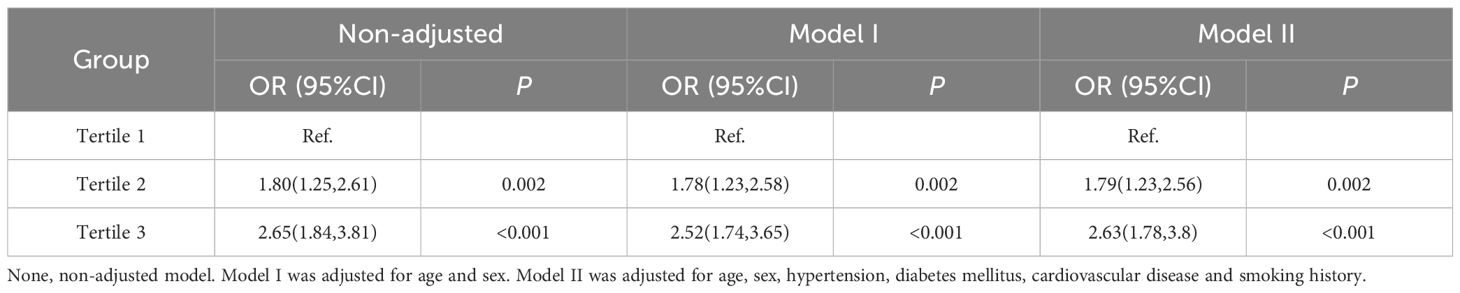
3.4 Univariate and multivariate logistic regression analysis of the TyG index groups for NAFLD
Logistic regression was performed to analyze the risk of NAFLD in the second and third groups compared with the first group as a control. After adjusting for age, sex, hypertension (HTN), DM, CVD and smoking, the results, shown in Table 4, indicate that a one-unit increase in the TyG index in the second group was associated with an approximately 1.79-fold increased risk of NAFLD (OR 1.79, 95% CI 1.23–2.56, P = 0.002). In the third group, a one-unit increase in the TyG index was associated with an approximately 2.63-fold increased risk of NAFLD (OR 2.63, 95% CI 1.78–3.80, P<0.001).
3.5 Demographic and clinical characteristics of the TyG-BMI index
To analyzed the relationship between different levels of insulin resistance (according to the TyG-BMI index) and NAFLD in patients undergoing PCI, patients were divided into three groups according to the terciles of the TyG-BMI index: Group 1 (n = 259) with a TyG-BMI index ≤ 50.22; Group 2 (n = 259) with a 50.22 < TyG-BMI index ≤ 68.41; and Group 3 (n = 258) with a TyG-BMI index > 68.41. As shown in Table 5, the mean TyG-BMI indices for these groups were 40.79 (33.67, 46.11), 58.35 (54.32, 63.52), and 82.69 (74.16, 92.62), respectively. Statistical analysis revealed significant differences between the three groups in terms of the incidence of NAFLD, HTN, DM, CVD, baseline rhythm at admission, ALT, WBC, RBC, PLT, hs-CRP, BMI, TC, TG, HDL-C, LDL-C, FBG, TyG index and TyG-BMI index (all P-values < 0.05).
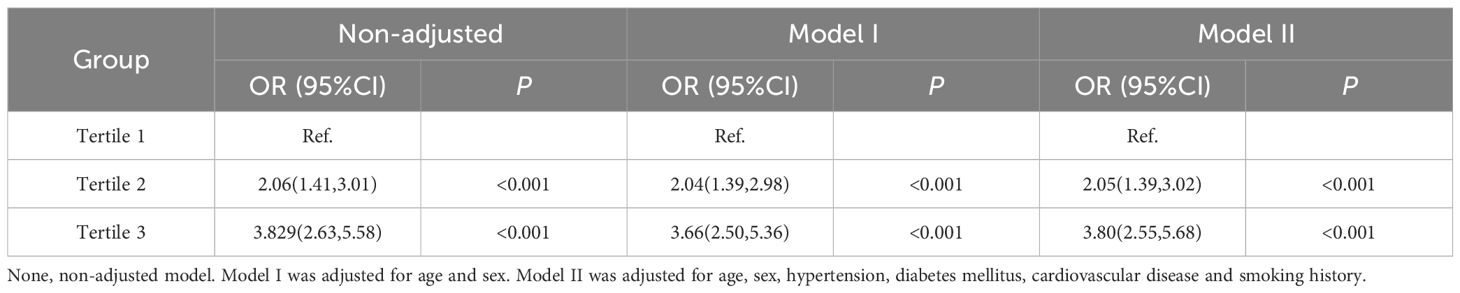
3.6 Univariate and multivariate logistic regression analysis of the TyG-BMI groups for NAFLD.
As shown in Table 6, logistic regression was performed to analyzed the risk of NAFLD in the second and third groups compared with the first group as a control. After adjusting for age, sex, HTN, DM, CVD and smoking, the results revealed that in the second group, a one-unit increase in the TyG-BMI index was associated with approximately a 2. 05-fold increased risk of NAFLD (OR 2.05, 95% CI 1.39 to 3.02, P<0.001); in the third group, a one-unit increase in the TyG-BMI index was associated with a 3.8-fold increased risk of NAFLD (OR 3.8, 95% CI 2.55 to 5.68, P<0.001).
3.7 ROC analysis of the TyG and TyG-BMI indices
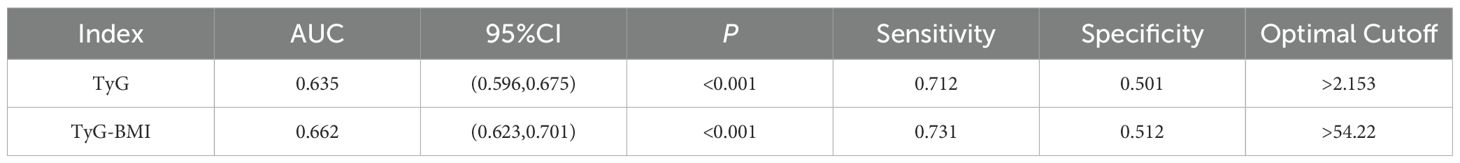
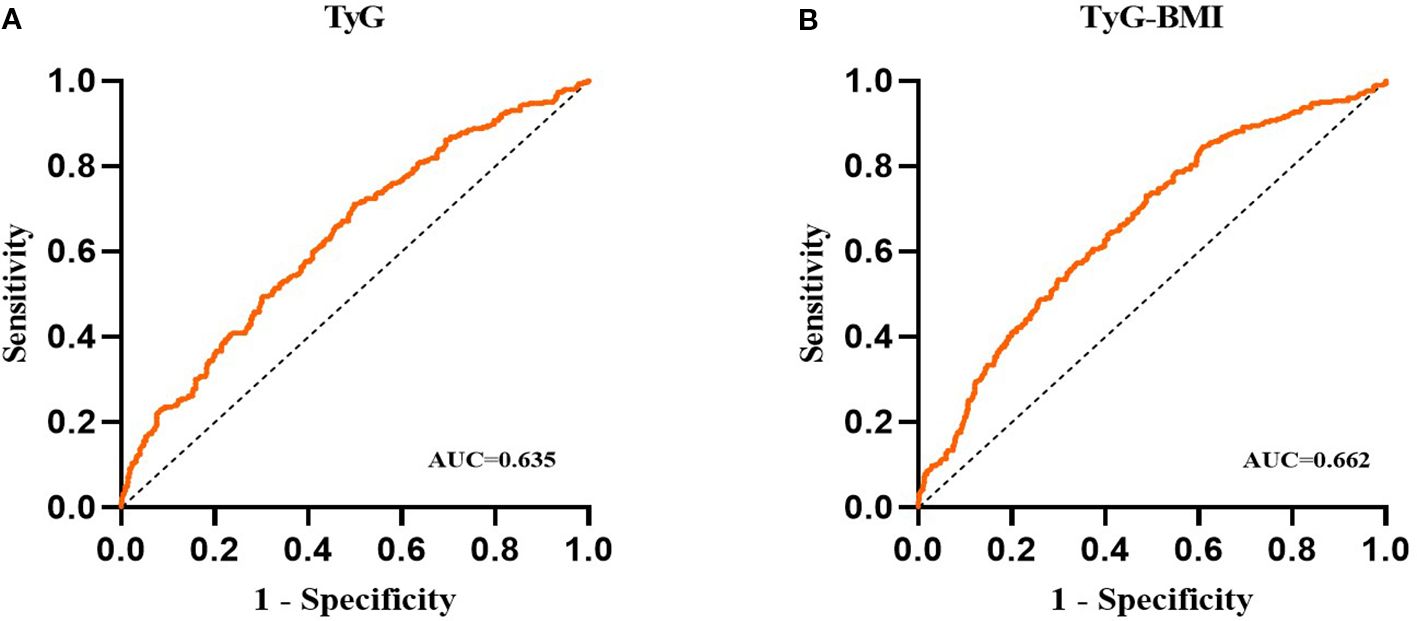
The results of the receiver operating characteristic (ROC) analysis and visualization of the TyG and TyG-BMI indices are summarized in Table 7; Figure 3. The ROC curve for the TyG index shows an area under the curve (AUC) of 0.635 (95% CI, 0.596 to 0.675; P < 0.001), with an optimal threshold value of 2.153 for identifying NAFLD (sensitivity of 0.712, specificity of 0.501). The ROC curve for the TyG-BMI index shows an AUC of 0.662 (95% CI, 0.623 to 0.701; P < 0.001), with an optimal threshold of 54.22 to identify NAFLD (sensitivity of 0.731, specificity of 0.512). These findings suggest that both the TyG index and the TyG-BMI index have some diagnostic efficacy in predicting NAFLD.
3.8 Associations of the TyG and TyG-BMI Indices with NAFLD Fibrosis NFS
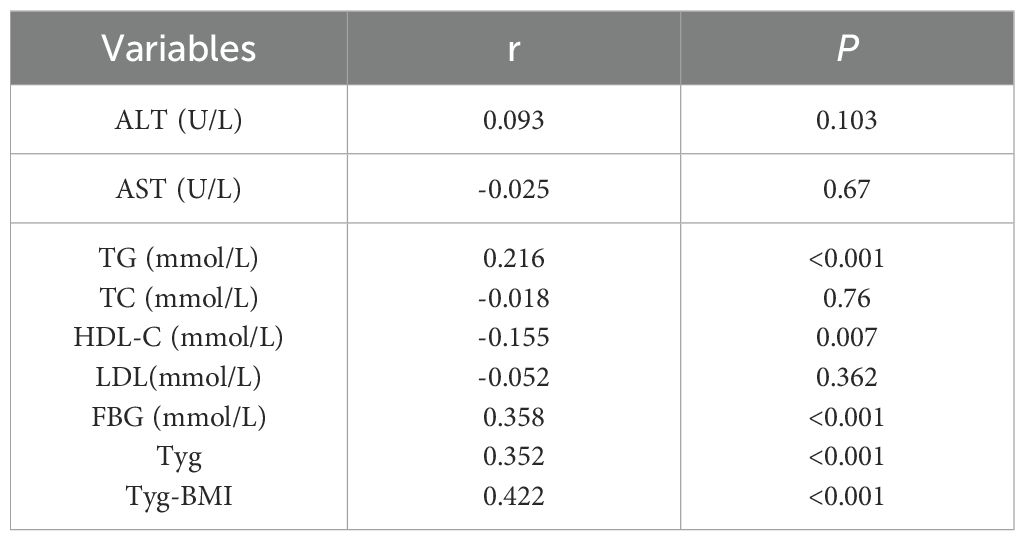
Spearman rank correlation analysis was used to explore the relationships between NFS scores and ALT, AST, TC, TG, HDL-C, LDL-C, FBG, the TyG index, and the TyG-BMI index. As shown in Table 8, NFS scores did not significantly correlate with ALT (r = 0.093, P = 0.103), AST (r = -0.025, P = 0.67), TC (r = -0.018, P = 0.76), or LDL-C (r = -0.052, P = 0.362). Weak correlations were observed with TG (r = 0.216, P < 0.001) and HDL-C (r = -0.155, P = 0.007). Moderate correlations were found with FBG (r = 0.358, P < 0.001), the TyG index (r = 0.352, P < 0.001), and the TyG-BMI index (r = 0.422, P < 0.001).
Figures 4, 5 respectively illustrate the NFSs of PCI patients with NAFLD grouped by TyG and TyG-BMI indices (n=102 per group), as well as the distributions of the TyG and TyG-BMI indices in PCI patients with NAFLD categorized by the NFS.
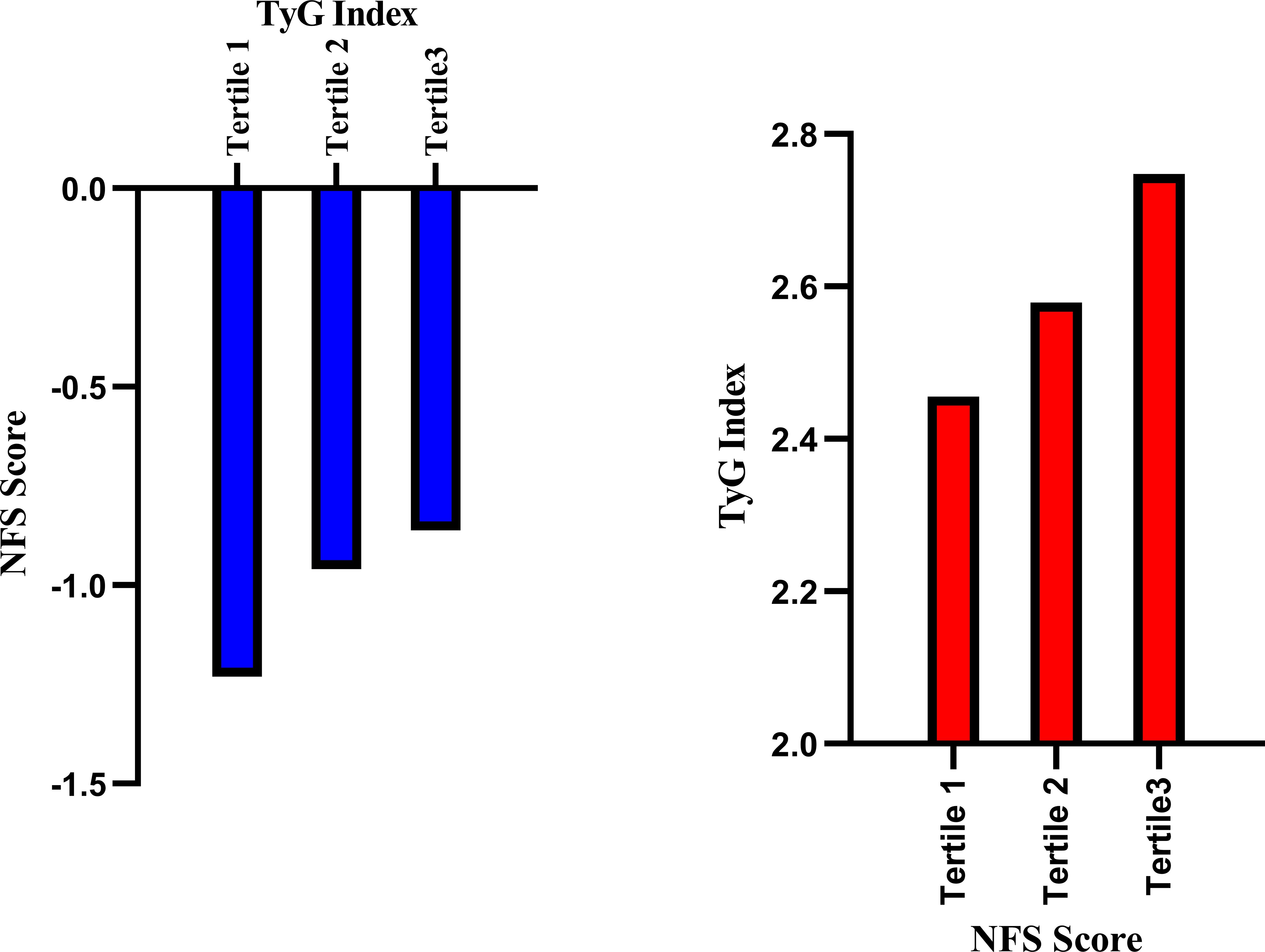
Figure 4. Relationships between the NFS and TyG index in PCI-NAFLD patients. The blue bar chart illustrates the NFS scores in the three TyG groups of PCI-NAFLD patients (Tertile 1 = 102, Tertile 2 = 102, Tertile 3 = 101), and the red bar chart illustrates the TyG index in the three NFS score groups of PCI-NAFLD patients (Tertile 1 = 102, Tertile 2 = 102.Tertile 3 = 101).
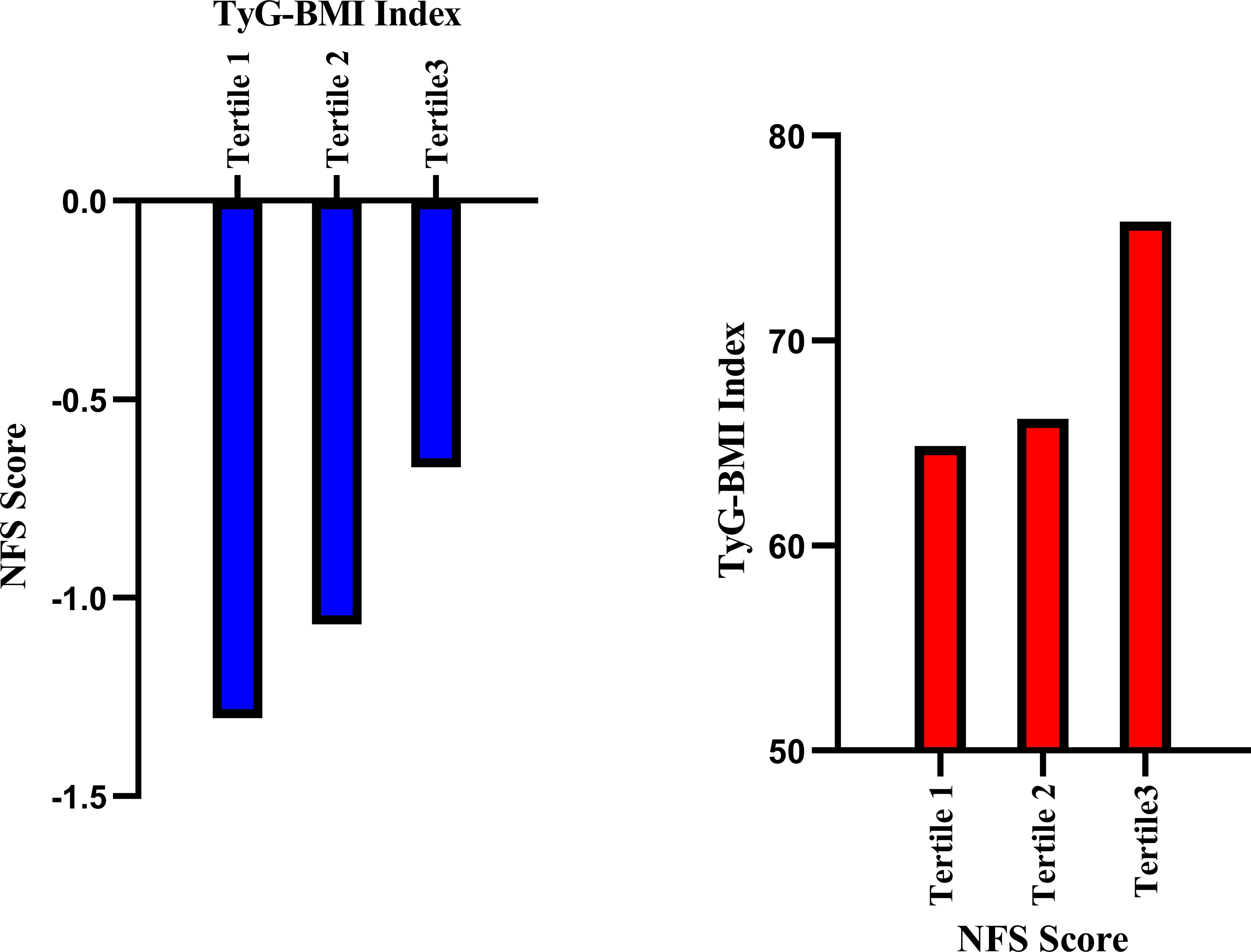
Figure 5. Relationships between the NFS score and the TyG-BMI in PCI-NAFLD patients. The blue bar chart illustrates the NFS score in the three-sectional TyG-BMI groups among the PCI-NAFLD patients (Tertile 1 = 102, Tertile 2 = 102, Tertile 3 = 101), and the red bar chart illustrates the TyG-BMI index in the three-sectional NFS score groups among the PCI-NAFLD patients (Tertile 1 = 102, Tertile 2 = 102, Tertile 3 = 101).
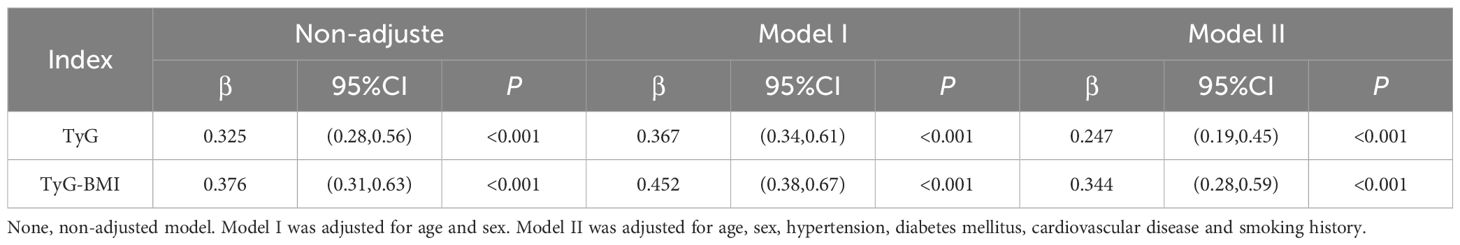
Figures 6A, B present the analysis graph showing the positive correlation between NFS scores and the TyG and TyG-BMI indices. The results of the multivariate linear regression analysis are provided in Table 9. The analysis revealed a significant positive correlation between NFS scores and both the TyG and TyG-BMI indices. In patients with PCI and NAFLD, an increase of one unit in the TyG index corresponds to a 0.325 increase in the NFS score (β = 0.325; 95% CI, 0.28 to 0.56; P<0.001); an increase of one unit in the TyG-BMI index corresponds to a 0.376 increase in the NFS score (β = 0.376; 95% CI, 0.31 to 0.63, P<0.001).
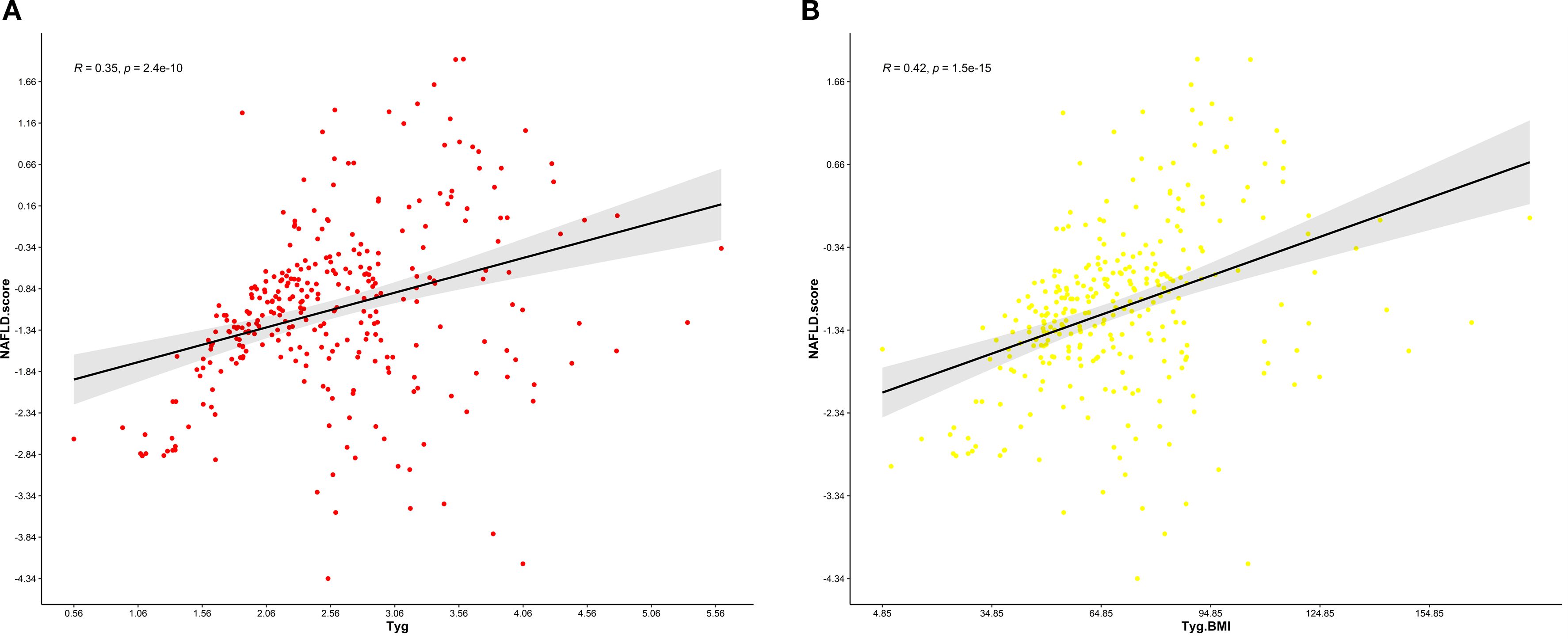
Figure 6. (A) Relationship between the NFS score and the TyG index.This scatter plot illustrates the correlation between the non-alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) and the triglyceride-glucose (TyG) index in a cohort of patients. The Pearson correlation coefficient (R) is 0.35, indicating a weak positive association between the NFS and the TyG index. (B) Relationship between the NFS score and the TyG-BMI index. This scatter plot illustrates the correlation between the non-alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) and the triglyceride-glucose-body mass index (TyG-BMI) index in a cohort of patients. The Pearson correlation coefficient (R) is 0.42, indicating a moderate positive association between the NFS and the TyG-BMI index.
4 Discussion
A growing number of studies have shown an association between the TyG index and the TyG-BMI index and NAFLD. A cross-sectional study with 1727 participants revealed that the TyG-BMI was a reliable predictor of NAFLD and moderate to severe fibrosis in U.S. adults (14). Another prospective cohort study involving 772 participants reported that the TyG index had moderate predictive value for cardiovascular events in patients with NAFLD (15). In addition, a further study with 10,390 participants revealed that the TyG-BMI was significantly associated with all-cause mortality, cardiovascular-related mortality, and diabetes-related mortality among adults with NAFLD in the United States; a more pronounced nonlinear positive association was observed among participants without progressive fibrosis (16). Taken together, these studies revealed an association between the TyG index and the TyG-BMI index with NAFLD, but it is worth noting that most of these studies recruited the general population rather than specific high-risk groups.
Coronary artery disease (CAD), the most common cardiovascular disease and a leading cause of death worldwide, is often treated with percutaneous coronary intervention (PCI). A retrospective cohort study involving 1,353 participants revealed that the TyG index can be used as a prognostic indicator of cardiovascular events after PCI (17). Another retrospective study involving 1,158 patients who had previously undergone coronary artery bypass grafting for acute coronary syndrome revealed that a higher TyG index was independently associated with an increased risk of serious adverse cardiac events (MACCEs), with a progressively stronger predictive effect of MACCEs (18). An analysis of 1,438 participants revealed a proportional association between higher TyG-BMI and an increased incidence of MACCEs in older or female patients (19).
Given the association between NAFLD and increased CVD risk, and the predictive value of the TyG index for both NAFLD and CVD, few studies have examined the predictive power of the TyG and TyG-BMI indices for NAFLD in PCI patients. The present study aims to address this gap.
The TyG and TyG-BMI indices, as markers of insulin resistance (IR), are strongly associated with the development of CHD and PCI. While the exact mechanisms are not fully understood, these indices are linked to insulin resistance, systemic inflammation, oxidative stress, lipid metabolism disorders, visceral fat accumulation, and endoplasmic reticulum stress. The TyG and TyG-BMI indices not only reflect IR but also correlate with these mechanisms, explaining the high prevalence of NAFLD in PCI patients.
Insulin resistance (IR) is defined as a reduced response to physiological insulin levels. IR is a key driver of several modern diseases, including metabolic syndrome, NAFLD, atherosclerosis, and type 2 diabetes mellitus (T2DM), playing a crucial role in their progression. IR leads to impaired glucose metabolism and organ dysfunction, particularly in the liver and heart (20). IR impairs the PI3K-NO pathway and enhances the MAPK-ET-1 pathway, leading to endothelial dysfunction, atherosclerotic plaque formation, exacerbated inflammation, and increased thrombosis risk, thereby worsening cardiovascular disease progression (21, 22). In NAFLD patients, impaired liver function fails to suppress excess glucose and fatty acid production, leading to local lipid and glucose accumulation that initiates and worsens IR. This results in systemic metabolic disorders and vascular endothelial damage, explaining the high risk of coronary heart disease in NAFLD patients.
Systemic inflammation plays a key role in the development of CAD and NAFLD. Haukeland’s analysis of high-quality serum samples from 47 NAFLD patients found that mild systemic inflammation, involving CCL2 and MCP-1, is prevalent and plays a key role in the disease process (23). Lipid accumulation in hepatocytes triggers local inflammation and immune cell activation. Activated immune cells release proinflammatory cytokines and chemokines, causing systemic inflammation. Signals from adipose tissue also activate and recruit hepatic immune cells, promoting inflammation that leads to cell damage and death, accelerating NAFLD progression (24). Therefore, systemic inflammation-induced IR increases the risk of NAFLD in CAD patients.
Dyslipidemia plays an important role in the development of coronary heart disease and non-alcoholic fatty liver disease. Atherogenic dyslipidemia, manifested by elevated plasma triglycerides, increased numbers of small, dense low-density lipoprotein (sdLDL) particles, and reduced levels of HDL-C, is prevalent in patients with varying degrees of NAFLD. These dysregulated fat stores in the liver or other tissues are often accompanied by cardiometabolic dysfunction, which increases the risk of cardiovascular events (25).
Visceral fat accumulation plays a key role in the development of CAD and NAFLD. The accumulation of visceral fat is closely associated with the chronic release of free fatty acid (FFA) into the bloodstream, which can trigger insulin resistance in the liver. Excessively produced triglycerides, sdLDL and LDL particles tend to deposit in peripheral blood vessels, contributing to the development of atherogenic dyslipidemia (26). Studies have shown that abdominal obesity is an independent risk factor for arterial disease in the upper and lower extremities (27). In addition, elevated FFA levels can directly or indirectly activate gluconeogenesis in the liver and reduce liver insulin sensitivity. A decrease in insulin sensitivity leads to an increase in the production of very low-density lipoprotein (VLDL) and a reduction in HDL cholesterol, which further accelerates the progression of atherogenic dyslipidemias (28). The accumulation of ectopic fat and the subsequent release of metabolites as well as the activation of inflammatory pathways trigger a series of pathophysiological changes both locally and systemically, which ultimately lead to the development of NAFLD and CVD (29). Visceral adipose tissue acts as an autonomous endocrine organ that secretes various adipokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10). These adipokines can not only trigger chronic inflammatory reactions, but also disrupt insulin signaling in liver cells, impair insulin sensitivity and thus promote the development of atherogenic dyslipidemia and the accumulation of free fatty acid (FFA) in liver cells (30, 31). Therefore, the accumulation of lipids in peripheral tissues and the liver leads to lipotoxic stress in mitochondria and the endoplasmic reticulum, which in turn leads to alterations in mitochondrial function and increased production of reactive oxygen species (ROS) (32). On the one hand, endoplasmic reticulum imbalance and oxidative stress have been shown to influence metabolic inflammatory responses by inducing endothelial dysfunction, increasing the susceptibility of patients with NAFLD to cardiovascular events (33); on the other hand, the resulting oxidative stress also promotes lipid peroxidation of polyunsaturated fatty acids, leading to the formation of toxic aldehyde products and causing lipid damage. These oxidatively modified lipids have an increased atherogenic effects (34).
Endoplasmic reticulum stress (ERS) plays a central role in the development of coronary artery disease (CHD) and NAFLD. ERS is profoundly pathophysiologically linked to NAFLD, CHD, and IR through the induction of apoptosis. In NAFLD, hepatocyte apoptosis exacerbates the inflammatory response and fibrosis in the liver (35). In addition, ERS disrupts insulin signaling and promotes the development of IR, which is not only a key factor in the progression of NAFLD but also an independent risk factor for CHD (36, 37). In CHD patients, cardiomyocytes undergo ERS-induced apoptosis, which further compromises myocardial function and structural integrity. Thus, ERS regulates a network of apoptotic and metabolic dysregulation that tightly links NAFLD, CHD, and IR, highlighting its central role in the development of these diseases and its potential as a therapeutic target.
In summary, IR, as a core pathophysiological process in patients with NAFLD, CHD, and those undergoing PCI (20, 38), contributes to the onset and progression of these conditions through a variety of mechanisms including systemic inflammation, oxidative stress, dyslipidemia, visceral obesity, and ERS. The TyG and TyG-BMI indices, as validated tools for assessing IR, not only reflect IR status, but also closely correlate with the aforementioned pathophysiological mechanisms, explaining the correlation between these indices and the high prevalence of NAFLD in PCI patients (5, 39, 40). Therefore, an in-depth understanding of the relationships between TyG and TyG-BMI indices and these diseases is essential for identifying at-risk individuals and optimizing preventive and therapeutic strategies.
Currently, conventional methods for assessing NAFLD risk in clinical practice have notable limitations: liver enzyme levels lack sufficient sensitivity; imaging modalities such as ultrasound and FibroScan require specialized equipment and entail relatively high costs; non-invasive fibrosis scores (e.g., FIB-4, NFS, APRI), although practical, were primarily developed for fibrosis risk assessment in the general population and their applicability and diagnostic performance in the specific context of PCI patients remain to be fully validated. Furthermore, liver biopsy, the gold standard for diagnosis, is invasive and thus unsuitable for large-scale screening. In contrast, the triglyceride-glucose (TyG) index and its derivative, TyG-BMI, offer significant clinical advantages. These indices are calculated from routinely available laboratory and anthropometric data—namely fasting triglycerides, glucose, and body mass index—making them highly feasible, cost-effective, and suitable for large-scale screening and longitudinal monitoring.
Patients undergoing percutaneous coronary intervention (PCI) are typically concerned about the risk of cardiac events, such as recurrent myocardial infarction or in-stent restenosis. However, this study highlights that this population is also at high risk for non-alcoholic fatty liver disease (NAFLD) and liver fibrosis. The TyG index is a reliable surrogate marker of insulin resistance, a central pathophysiological mechanism in the development and progression of NAFLD, thereby providing strong biological plausibility. The use of TyG-based indices could therefore facilitate large-scale screening and regular monitoring of liver disease in post-PCI patients, promoting a paradigm shift in clinical management from a “cardio-centric” approach to a more integrated “cardiometabolic and hepatic” care model.
There are several limitations to this study. First, this was a retrospective study with a limited sample size; the single-center nature and small sample size may have led to selection bias. Although a multivariable logistic regression was conducted to account for common confounding factors, the groups were not rigorously matched, and the differences between them were not adjusted for. Second, severe PCI patients requiring coronary vein bypass surgery or rheumatic heart disease were not included in this study, which may have led to an underestimation of the predictive power of the TyG and TyG-BMI indices for NAFLD. Third, the lack of liver enzyme data means that although ultrasonography is relatively accurate in detecting moderate to severe hepatic steatosis, it is less sensitive in detecting mild steatosis, which may lead to the underdiagnosis of mild NAFLD. Finally, the cross-sectional design of this study meant that although a positive correlation was found between the TyG and TyG-BMI indices and the prevalence of NAFLD in patients with PCI, no definitive conclusions could be drawn about their predictive value. Future large-scale, multicenter prospective studies are needed to further validate the predictive efficacy of the TyG and TyG-BMI indices for the risk of NAFLD in patients undergoing PCI.
5 Conclusions
In conclusion, this study demonstrated a significant association between the TyG index and the TyG-BMI and the risk of NAFLD in patients with PCI, and a positive correlation with the degree of liver fibrosis. As innovative markers of IR, the TyG index and TyG-BMI index have promising potential for wide application in primary health care facilities and community health services because of their simplicity, cost-effectiveness, and reliability. These results support the use of the TyG index and TyG-BMI index as valid tools for assessing the risk of NAFLD in patients undergoing PCI and provide a basis for follow-up studies. Future studies could validate these results with prospective cohort studies and explore additional biomarkers to improve their predictive accuracy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Tianjin First Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YC: Data curation, Formal analysis, Writing – original draft. CW: Data curation, Writing – original draft, Writing – review & editing. XD: Writing – original draft. XS: Writing – original draft. WS: Writing – review & editing. CL: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (81970303, 82470294), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-054B) and the Natural Science Foundation of Tianjin (21JCYBJC00250).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NAFLD, Non-alcoholic fatty liver disease; PCI, percutaneous coronary intervention; TyG, triglyceride-glucose; TyG-BMI, TyG-body mass index; NFS, NAFLD Fibrosis Score; CVD, cardiovascular disease; ELISA, enzyme-linked immunosorbent assay; WBC, white blood cell; RBC, red blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose; CRP, C-reactive protein; BNP, B-type natriuretic peptide; DM, diabetes mellitus; HTN, hypertension; ROC, receiver operating characteristic; AUC, area under the curve; FBG: fasting blood glucose.
References
1. Huang X, Gan D, Fan Y, Fu Q, He C, Liu W, et al. The associations between healthy eating patterns and risk of metabolic dysfunction-associated steatotic liver disease: A case-control study. Nutrients. (2024) 16:1956. doi: 10.3390/nu16121956
2. Mehta M, Shah J, and Joshi U. Understanding insulin resistance in NAFLD: A systematic review and meta-analysis focused on HOMA-IR in south asians. Cureus. (2024) 16:e70768. doi: 10.7759/cureus.70768
3. Mohammadpour-Asl S, Roshan-Milani B, Roshan-Milani S, Saboory E, Ghobadian B, and Chodari L. Endoplasmic reticulum stress PERK-ATF4-CHOP pathway is involved in non-alcoholic fatty liver disease in type 1 diabetic rats: The rescue effect of treatment exercise and insulin-like growth factor I. Heliyon. (2024) 10:e27225. doi: 10.1016/j.heliyon.2024.e27225
4. Zhu M, Pu J, Zhang T, Shao H, Su R, and Tang C. Inhibiting TRIM8 alleviates adipocyte inflammation and insulin resistance by regulating the DUSP14/MAPKs pathway. Adipocyte. (2024) 13:2381262. doi: 10.1080/21623945.2024.2381262
5. Wang J, Yan S, Cui Y, Chen F, Piao M, and Cui W. The diagnostic and prognostic value of the triglyceride-glucose index in metabolic dysfunction-associated fatty liver disease (MAFLD): A systematic review and meta-analysis. Nutrients. (2022) 14:4969. doi: 10.3390/nu14234969
6. Dong W, Gong Y, Zhao J, Wang Y, Li B, and Yang Y. A combined analysis of TyG index, SII index, and SIRI index: positive association with CHD risk and coronary atherosclerosis severity in patients with NAFLD. Front Endocrinol. (2023) 14:1281839. doi: 10.3389/fendo.2023.1281839
7. Liu H, Chen J, Qin Q, Yan S, Wang Y, Li J, et al. Association between TyG index trajectory and new-onset lean NAFLD: a longitudinal study. Front Endocrinol. (2024) 15:1321922. doi: 10.3389/fendo.2024.1321922
8. Huang W, Wang H, Shen Z, Wang X, and Yu X. Association between TyG index and risk of carotid atherosclerosis in NAFLD patients: a retrospective cohort study. Front Endocrinol. (2024) 15:1448359. doi: 10.3389/fendo.2024.1448359
9. Yang S, Cao S-J, Li C-Y, Zhang Q, Zhang B-L, Qiu F, et al. Berberine directly targets AKR1B10 protein to modulate lipid and glucose metabolism disorders in NAFLD. J Ethnopharmacol. (2024) 332:118354. doi: 10.1016/j.jep.2024.118354
10. Yuan M, He J, Hu X, Yao L, Chen P, Wang Z, et al. Hypertension and NAFLD risk: Insights from the NHANES 2017–2018 and Mendelian randomization analyzeds. Chin Med J (Engl). (2024) 137:457–64. doi: 10.1097/CM9.0000000000002753
11. Xiang Q, Xu H, Zhan J, Lu S, Li S, Wang Y, et al. Association between the triglyceride-glucose index and vitamin D status in type 2 diabetes mellitus. Nutrients. (2023) 15:639. doi: 10.3390/nu15030639
12. Scott DA, Ponir C, Shapiro MD, and Chevli PA. Associations between insulin resistance indices and subclinical atherosclerosis: A contemporary review. Am J Prev Cardiol. (2024) 18:100676. doi: 10.1016/j.ajpc.2024.100676
13. Xu Z, Chen P, Wang L, Yan J, Yan X, and Li D. Relationship between TyG index and the degree of coronary artery lesions in patients with H-type hypertension. Cardiovasc Diabetol. (2024) 23:23. doi: 10.1186/s12933-023-02013-0
14. Xue Y, Xu J, Li M, and Gao Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index-related parameters. Front Endocrinol. (2022) 13:951689. doi: 10.3389/fendo.2022.951689
15. Colantoni A, Bucci T, Cocomello N, Angelico F, Ettorre E, Pastori D, et al. Lipid-based insulin-resistance markers predict cardiovascular events in metabolic dysfunction associated steatotic liver disease. Cardiovasc Diabetol. (2024) 23:175. doi: 10.1186/s12933-024-02263-6
16. Chen Q, Hu P, Hou X, Sun Y, Jiao M, Peng L, et al. Association between triglyceride-glucose related indices and mortality among individuals with non-alcoholic fatty liver disease or metabolic dysfunction-associated steatotic liver disease. Cardiovasc Diabetol. (2024) 23:232. doi: 10.1186/s12933-024-02343-7
17. Tao S, Yu L, Li J, Xie Z, Huang L, Yang D, et al. Prognostic value of triglyceride-glucose index in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2023) 22:322. doi: 10.1186/s12933-023-02060-7
18. Dong S, Zhao Z, Huang X, Ma M, Yang Z, Fan C, et al. Triglyceride-glucose index is associated with poor prognosis in acute coronary syndrome patients with prior coronary artery bypass grafting undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2023) 22:286. doi: 10.1186/s12933-023-02029-6
19. Cheng Y, Fang Z, Zhang X, Wen Y, Lu J, He S, et al. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. (2023) 22:75. doi: 10.1186/s12933-023-01794-8
20. Sh L, Sy P, and Cs C. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2022) 46(1):15–37. doi: 10.4093/dmj.2021.0280
21. Kim J, Montagnani M, Koh KK, and Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. (2006) 113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213
22. Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. (2005) 289:H813–822. doi: 10.1152/ajpheart.00092.2005
23. Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. (2006) 44:1167–74. doi: 10.1016/j.jhep.2006.02.011
24. Arrese M, Cabrera D, Kalergis AM, and Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. (2016) 61(5):1294–1303. doi: 10.1007/s10620-016-4049-x
25. Ghavidel F, Hashemy SI, Aliari M, Rajabian A, Tabrizi MH, Atkin SL, et al. The effects of resveratrol supplementation on the metabolism of lipids in metabolic disorders. Curr Med Chem. (2025) 32(11):2219–2234. doi: 10.2174/0109298673255218231005062112
26. Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. (2019) 71:1012–21. doi: 10.1016/j.jhep.2019.06.031
27. Wang Y, Guo X, Zhang Y, Zhang R, and Li J. Different associations of general and abdominal obesity with upper and lower extremity artery disease among a community population in China. Nutr Metab. (2023) 20:14. doi: 10.1186/s12986-023-00736-1
28. Sakurai Y, Kubota N, Yamauchi T, and Kadowaki T. Role of insulin resistance in MAFLD. Int J Mol Sci. (2021) 22:4156. doi: 10.3390/ijms22084156
29. Deprince A, Haas JT, and Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab. (2020) 42:101092. doi: 10.1016/j.molmet.2020.101092
30. Geng Y, Faber KN, de Meijer VE, Blokzijl H, and Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. (2021) 15:21–35. doi: 10.1007/s12072-020-10121-2
31. Badmus OO, Hillhouse SA, Anderson CD, Hinds TD, and Stec DE. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci Lond Engl 1979. (2022) 136:1347–66. doi: 10.1042/CS20220572
32. Dabravolski SA, Bezsonov EE, Baig MS, Popkova TV, and Orekhov AN. Mitochondrial lipid homeostasis at the crossroads of liver and heart diseases. Int J Mol Sci. (2021) 22:6949. doi: 10.3390/ijms22136949
33. Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, and Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. (2018) 100:1–19. doi: 10.1016/j.vph.2017.05.005
34. Hoebinger C, Rajcic D, and Hendrikx T. Oxidized lipids: common immunogenic drivers of non-alcoholic fatty liver disease and atherosclerosis. Front Cardiovasc Med. (2021) 8:824481. doi: 10.3389/fcvm.2021.824481
35. You K, Wang L, Chou C-H, Liu K, Nakata T, Jaiswal A, et al. QRICH1 dictates the outcome of ER stress through transcriptional control of proteostasis. Science. (2021) 371:eabb6896. doi: 10.1126/science.abb6896
36. Ajoolabady A, Kaplowitz N, Lebeaupin C, Kroemer G, Kaufman RJ, Malhi H, et al. Endoplasmic reticulum stress in liver diseases. Hepatol Baltim Md. (2023) 77:619–39. doi: 10.1002/hep.32562
37. Yang Z and Wang L. Current, emerging, and potential therapies for non-alcoholic steatohepatitis. Front Pharmacol. (2023) 14:1152042. doi: 10.3389/fphar.2023.1152042
38. Palma R, Pronio A, Romeo M, Scognamiglio F, Ventriglia L, Ormando VM, et al. The role of insulin resistance in fueling NAFLD pathogenesis: from molecular mechanisms to clinical implications. J Clin Med. (2022) 11:3649. doi: 10.3390/jcm11133649
39. Song J, Ma R, and Yin L. Associations between estimated glucose disposal rate and arterial stiffness and mortality among US adults with non-alcoholic fatty liver disease. Front Endocrinol. (2024) 15:1398265. doi: 10.3389/fendo.2024.1398265
40. Toro-Huamanchumo CJ, Urrunaga-Pastor D, Guarnizo-Poma M, Lazaro-Alcantara H, Paico-Palacios S, Pantoja-Torres B, et al. Insulin Resistance and Metabolic Syndrome Research Group. Triglycerides and glucose index as an insulin resistance marker in a sample of healthy adults. Diabetes Metab Syndr. (2019) 13:272–7. doi: 10.1016/j.dsx.2018.09.010
Keywords: non-alcoholic fatty liver disease, PCI, TyG, TyG-BMI, insulin resistance
Citation: Chen Y, Wang C, Du X, Sun X, Song W and Lu C (2025) Positive correlations between TyG and TyG-BMI indices and the risk of NAFLD and degree of liver fibrosis in patients undergoing PCI. Front. Endocrinol. 16:1541421. doi: 10.3389/fendo.2025.1541421
Received: 07 December 2024; Accepted: 17 September 2025;
Published: 29 September 2025.
Edited by:
Dimitrios S. Karagiannakis, Kapodistrian University of Athens, GreeceReviewed by:
Madhavi Annamanedi, West Virginia University, United StatesDragos Cretoiu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2025 Chen, Wang, Du, Sun, Song and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengzhi Lu, NTAyMDIwMDA3MkBuYW5rYWkuZWR1LmNu
 Yingxiang Chen
Yingxiang Chen Che Wang1
Che Wang1 Xiaoyu Du
Xiaoyu Du Chengzhi Lu
Chengzhi Lu