- 1Traditional Chinese Medicine (ZHONG JING) School, Henan University of Traditional Chinese Medicine, Zhengzhou, Henan, China
- 2School of Foreign Languages, Henan University of Traditional Chinese Medicine, Zhengzhou, Henan, China
- 3Queen Mary College, Nanchang University, Nanchang, Jiangxi, China
- 4Reproductive Medicine Department, Henan Province Hospital of Traditional Chinese Medicine, Zhengzhou, Henan, China
- 5The Second Clinical Medical School, Henan University of Traditional Chinese Medicine, Zhengzhou, Henan, China
Although numerous Mendelian randomization studies on risk factors have been conducted in male medicine, a systematic synthesis of these findings is still lacking. This review searched relevant literature in PubMed and the Web of Science published before May 2024; systematically summarized the progress in the application of Mendelian randomization in male infertility, erectile dysfunction, prostate cancer, and prostatitis; summarized and classified the risk factors affecting men’s health, such as the gut microbiota, modifiable risk factors and related diseases; and presented some problems and solutions that were presented in these studies. This information offers valuable insights into the etiology and pathogenesis of male-specific diseases.
1 Introduction
Numerous medical statistics show that the incidence of male-specific diseases is increasing, and men’s health problems need urgent attention (1). Currently, the knowledge of risk factors associated with male-specific diseases needs to be further deepened. Mendelian randomization (MR) employs genetic variants highly correlated with exposure factors as instrumental variables (IVs) to ascertain the causal link between exposure and study outcomes. MR effectively reduces the impact of reverse causality and confounding factors. It also addresses the limitations of traditional medical statistics and epidemiological studies, offering a stronger foundation for identifying causal links between risk factors and disease risk (2). In recent years, many risk factor MR studies have been conducted in the field of male medicine, but there is a lack of systematic collation and summarization. In addition, a summary of the problems in the published literature is lacking. This article provides a systematic review of previous MR studies on risk factors for male-specific diseases, with the aim of providing ideas for the etiologic study and scientific prevention of male-specific diseases.
2 Methods
2.1 Fundamentals
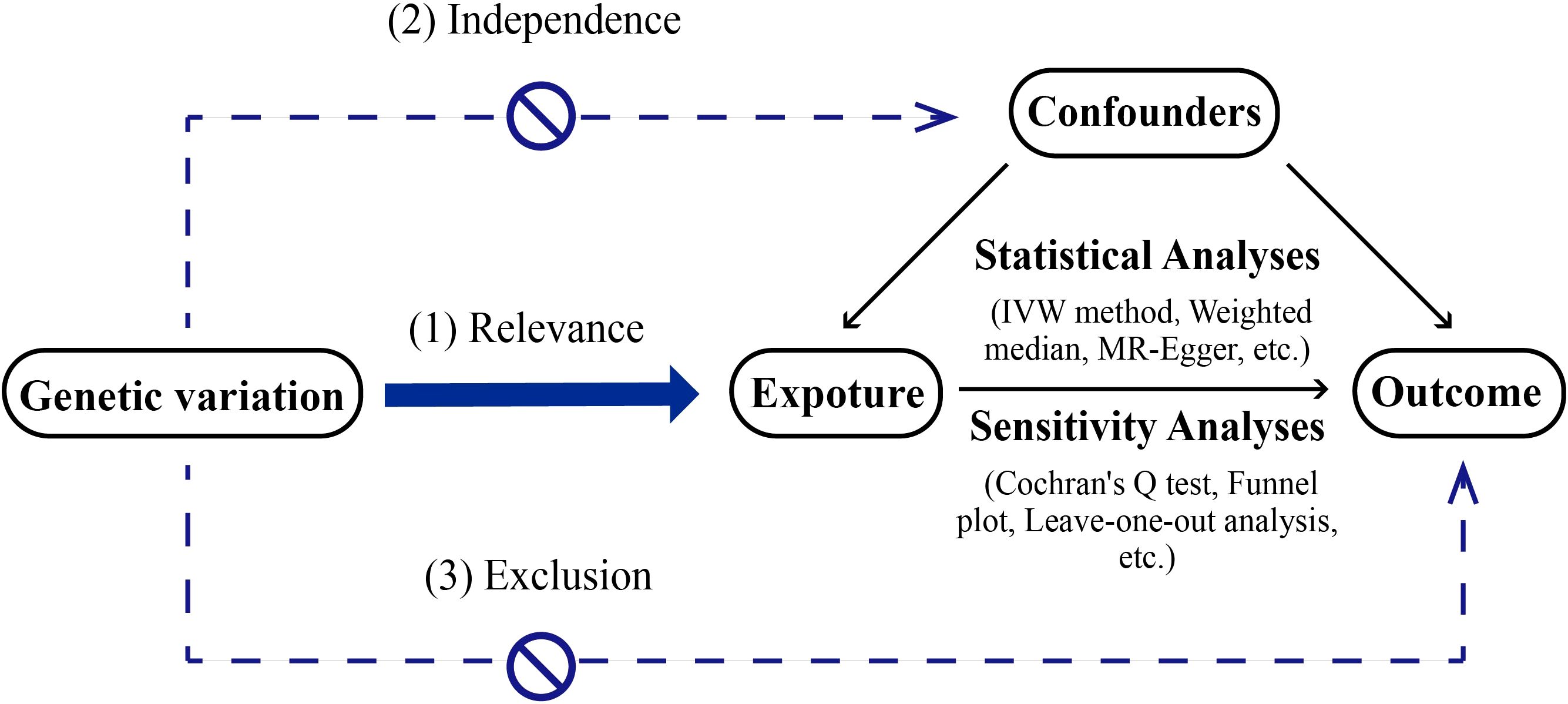
MR serves as a methodological tool in scientific inquiry aimed at elucidating causal connections between exposure factors and outcomes. It operates by leveraging genetic variants that are strongly associated with exposure factors as IVs. Unlike conventional observational epidemiological studies, MR draws on the principles of Mendelian inheritance. This approach can be likened to a naturally occurring randomized controlled trial (RCT), albeit conducted within the framework of genetic inheritance. This method reduces the impact of confounding factors found in observational studies and offers strong evidence. MR studies need to follow 3 core assumptions (2, 3): (1) the assumption of association, meaning the instrumental variable is strongly linked to the exposure factor; (2) the assumption of independence, meaning the instrumental variable is not related to confounding factors; (3) the assumption of exclusivity, meaning the instrumental variable affects the outcome only through the exposure factor The description of MR method is shown in Figure 1.
2.2 Common types of MR methods
The study of MR can be divided into single-sample Mendelian randomization, two-sample Mendelian randomization (TSMR), multivariate Mendelian randomization (MVMR), two-step Mendelian randomization, bidirectional Mendelian randomization, etc.
Single-sample Mendelian randomization means that the association between genetic variations and exposure, as well as the correlation between genetic variations and outcomes, is obtained in the same sample. In this research method, a correlation exists between the regression coefficients of the numerator and denominator due to confounders between exposure and outcome, and weak instrumental bias can lead to overestimation of the exposure–outcome association (4).
Two-sample Mendelian randomization (TSMR) refers to obtaining two types of data, namely, relationships between genetic variations and exposure as well as relationships between genetic variations and outcomes, from two nonoverlapping datasets. This method mitigates the effects of weak instrumental bias and has greatly expanded the application scope of MR studies (5).
Multivariate Mendelian randomization (MVMR) considers the causal effects of multiple exposures on one or more outcome variables. It enables the simultaneous evaluation of various causal pathways and helps to resolve confounding among these factors (6).
Two-step Mendelian Randomization investigates potential mediating mechanisms linking risk factors to outcomes (7).
Bidirectional Mendelian Randomization is employed to validate causal directionality when the direction of causal association between a risk factor and outcome remains ambiguous (8).
2.3 Common statistical methods
Commonly used MR statistical analyses include inverse variance weighting (IVW), Weighted median, MR-Egger regression, MR-PRESSO, etc (9).
Inverse variance weighting (IVW) is the standard method used to aggregate MR data, which integrates summary data from multiple genetic variants, weighting individual causal effect estimates by inverse variances to provide consistent and efficient causal inference under valid IVs without linkage disequilibrium (10). The weighted median approach calculates a weighted median estimate of causal effects derived from multiple IVs, with weights assigned based on the inverse of each estimate’s sampling variance to prioritize precision. This method operates under the assumption that valid instruments collectively contribute over 50% of the total weighting scheme, ensuring robustness even in the presence of invalid IVs (11).
The MR-Egger method employs weighted regression to analyze the influence of associations between IVs and exposures on the associations between IVs and outcomes. This method incorporates an intercept term to quantify the average direct effect of IVs on the outcome, with its core assumption being the absence of correlated horizontal pleiotropy. To enhance the model’s adaptability, the method can be extended by including a random effects term, which is used to analyze the over-dispersion of causal effects across different IVs, thereby modeling pleiotropic variation (12).MR-PRESSO conducts a global assessment to detect potential outliers within an IVW framework, followed by a localized analysis to pinpoint specific outliers. The method further quantifies their impact through a distortion test evaluating systematic bias in causal effect estimates (13).
2.4 Disease selection strategy
Reference to the International Classification of Diseases (ICD-11) published by the World Health Organization (WHO) and authoritative urological literature (Campbell-Walsh-Wein Urology, volume 6) (14, 15), male diseases can be classified into the following categories: 1. Sexual health-related disorders: Including male infertility (MI), erectile dysfunction (ED), ejaculatory dysfunction (e.g., premature ejaculation [PE]), and sexually transmitted infections, etc. 2. Neoplasms of male genital organs: Such as prostate cancer (PCa), testicular tumors, and penile tumors. 3. Prostate diseases: Encompassing prostatitis, other prostatic disorders, and benign prostatic hyperplasia. 4. Structural abnormalities and congenital disorders of genitalia: Including hydrocele, testicular torsion, phimosis, and cryptorchidism, etc. 5. Inflammatory and infectious diseases: Such as orchitis and genital herpes. The selected 4 diseases in this article hold priority within the aforementioned disease categories:
1. For sexual health-related disorders, MI exhibits a high incidence rate. Globally, approximately 8-12% of couples suffer from infertility (16), with 50% of fertility issues attributable to male factors (17). Additionally, ED and PE are prevalent male sexual disorders in the general population. Population-based research indicates that 5% to 20% of males experience clinically significant ED (18), while PE occurs in about 30% of men aged 40–80 years (19). Despite PE being more prevalent than ED under the category of ejaculatory dysfunction, significant disparities in global incidence statistics and low healthcare-seeking rates among patients have resulted in far fewer research resources on PE compared to ED. Moreover, MR methodology-related studies on PE remain nearly absent. Therefore, this article selects ED as a research focus.
2. Among male genital organ tumors, PCa accounts for 29% of male cancers (20), ranking as the most common cancer among males in developed countries today (21). Given its representativeness in male genital tumors and abundant research data, PCa is emphasized in this article.
3. In prostate diseases, prostatitis is the most common urinary system disorder in males under 50 years old (15), while benign prostatic hyperplasia (BPH) represents the most prevalent benign tumor in elderly males (22). Comparatively, against the backdrop of population aging, research on BPH primarily stems from public health urgency, whereas prostatitis requires intensified mechanistic exploration due to its younger onset trends and chronic disease management challenges. MR methodology is particularly suitable for investigating the pathogenesis of prostatitis. Between these two conditions, this article prioritizes prostatitis as the study subject.
4. For structural/genital abnormalities and congenital diseases (e.g., hydrocele, testicular torsion, phimosis, and cryptorchidism), their relatively low incidence precludes their selection in this study. Among inflammatory and infectious diseases, prostatitis is chosen as a representative condition.
In summary, considering the article’s scope limitations, this review focuses on 4 diseases characterized by high incidence rates, broad societal impact, significant impairment of male patients’ quality of life, complex etiological mechanisms, and substantial existing literature.
2.5 Risk factor selection and classification strategy
This study employed a three-tiered criteria for risk factor selection: First, integration of authoritative guidelines and consensus statements, including the World Health Organization (WHO) framework for noncommunicable disease risks, the European Association of Urology (EAU) Guidelines on Sexual and Reproductive Health, and etiological evidence from classical urological literature (15). Second, prioritization of factors with high evidence strength validated by large-scale cohort studies (e.g., smoking, sedentary behavior) to ensure conclusion reliability. Third, focus on clinically actionable risk indicators, particularly lifestyle-related factors (e.g., dietary patterns, exercise habits), as these can be modulated through public health policies or individual behavioral adjustments. This strategy balances scientific rigor with practical translational value, providing multidimensional evidence for male reproductive health management.
Based on these criteria, the risk factors included in this study are categorized into 6 types: Gut microbiota, circulatory substance (cytokines), related diseases, modifiable risk factors, drug targets, other risk factors (limited studies or irrelevant factors).
2.6 Search strategy and selection criteria of references
Original studies were identified by searching relevant articles in the PubMed and Web of Science databases through May 2024.The following terms were used to search: “mendelian randomization” or “genetic instrumental variable” or “genetic instrument”, “male infertility” or “male sterility”, “prostate cancer” or “prostatic carcinoma” or “prostatic cancer”, “erectile dysfunction” or “impotence”, “prostatitis” or “prostate inflammation”, etc. Inclusion criteria: (1) Mendelian Randomization (MR) study design; (2) Genetic variants or Genetic Risk Scores (GRS) were used as Instrumental Variables (IVs) to analyze the relationship between exposure and outcome. Articles were excluded for the following reasons: reviews, non-original articles, non-human studies, study protocols, letters, conference abstracts, and articles for which the full text is not available. We finally included 122 articles and categorized them according to disease type, as shown in Tables 1–4.
3 Results
3.1 Male infertility
Infertility is defined as the inability to achieve pregnancy following 12 months of regular unprotected intercourse, 50% of infertility cases are attributable to male factors (23). The MR studies included in this article investigate the causal relationships between MI and risk factors such as gut microbiota, cytokines, related diseases, modifiable risk factors, and other factors.
3.1.1 Gut microbiota
Previous studies have demonstrated the association between gut microbes and MI but have not elucidated a causal relationship (24). The seven studies utilized various methods, including IVW, MR-Egger and maximum likelihood ratios, to evaluate the causal connection between the gut microbiota and MI risk.
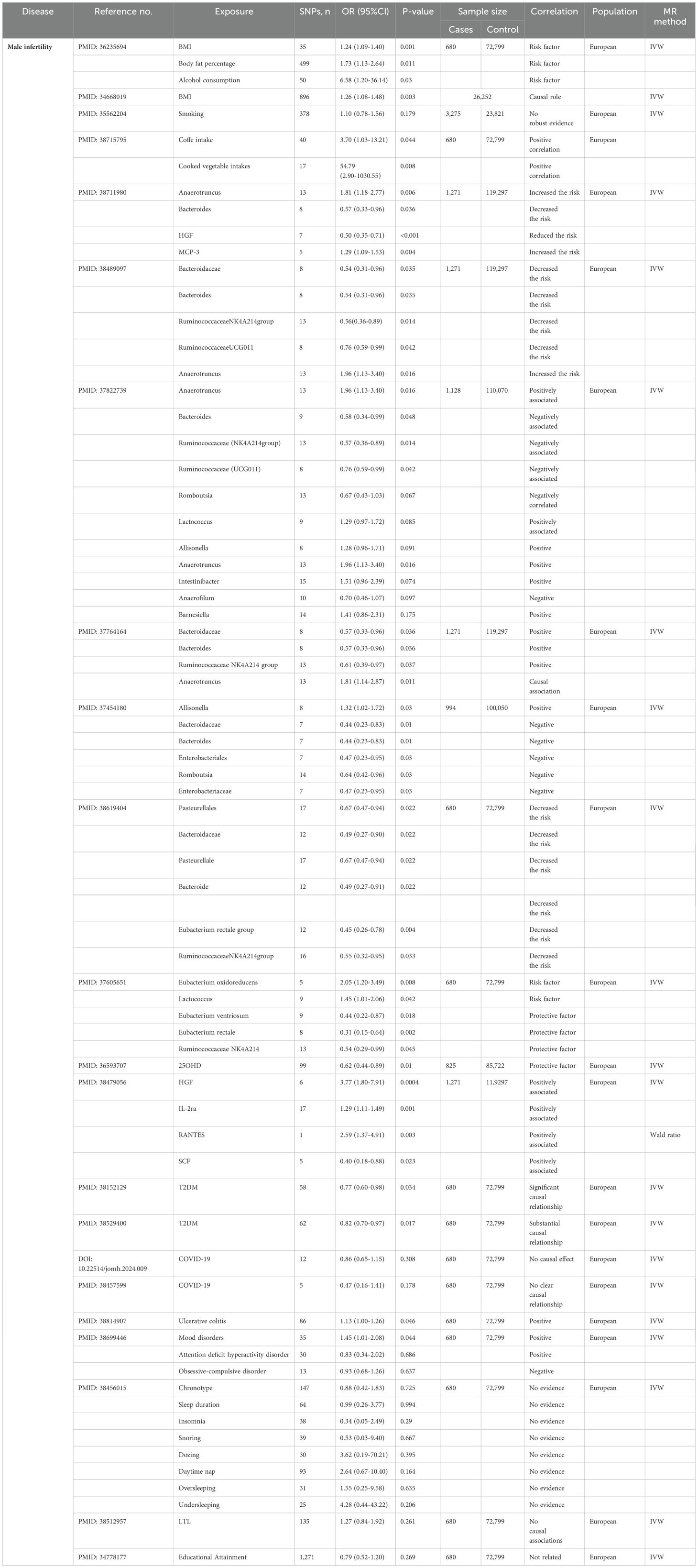
The MR analyses indicated that certain microbes, including Anaerotruncus (25–28), Allisonella (27, 29), Barnesiella, Intestinibacter and Lactococcus (27) are positively associated with MI risk. In contrast, Bacteroidaceae (26, 28–30), Bacteroides (25–30), Romboutsia (27, 29) and Ruminococcaceae (Ruminococcaceae, genus NK4A2140group, genus UCG011) (26–28, 30, 31) are protective against the development of MI.
Moreover, Li TZ et al. identified the family Enterobacteriaceae and the order Enterobacteriales as being linked to a low risk of MI (29). An MR study by Xi YJ et al. indicated that Eubacterium venereum and Eubacterium rectale have protective effects on MI, whereas Eubacterium oxidoreducens contribute to MI risk (31). Using TSMR analysis, Ma S-C et al. reported that Bacteroideae, Bacteriaceae, Pasteurella, Clostridium rectalis are associated with MI (30).
3.1.2 Cytokines
Zhang L et al. used MR methods such as IVW, MR-Egger and weighted median analyses to analyze the genetic association between cytokines and the risk of MI and concluded that the cytokines hepatocyte growth factor (HGF), IL-2ra, and RANTES potentially increase MI risk (32). Zou H et al. found that HGF reduced the risk of MI, and monocyte chemotactic protein 3 increased the risk of MI (25).
3.1.3 Related diseases
Using MR analysis, Zhu XB et al. reported that in type 2 diabetes mellitus (T2DM) can cause ED and MI a European population (33, 34). Two MR studies showed no significant association between COVID-19 and MI (35, 36). Wang X et al. proposed that ulcerative colitis may increase the risk of MI (37). Chen X et al. ‘s results found that mood disorders and attention deficit hyperactivity disorder were positively correlated with MI, whereas obsessive-compulsive disorder was negatively associated with MI (38).
3.1.4 Modifiable risk factors
Body mass index (BMI), body fat percentage, alcohol consumption and smoking are modifiable lifestyle factors linked to various health outcomes. Wentao et al. employed TSMR analyses to investigate the causal impacts of 22 diverse risk factors on MI and female infertility. Their findings indicated that BMI, body fat percentage, and alcohol consumption contribute to the risk of MI (39, 40). Greater smoking intensity was not strongly associated with MI according to MR analysis (41). The study of Chen X et al. found that coffee intake and cooked vegetable intakes increased the risk of MI (42). These insights underscore that the multifaceted interplay between lifestyle factors and health outcomes, moderate alcohol consumption, maintaining a healthy body weight and body fat, and practicing good lifestyle habits may help reduce MI risk and improve the quality of fertility.
3.1.5 Other factors
Yuan et al. observed that for every unit increase in genetically predicted 25 hydroxyvitamin D (25OHD) levels, there was a corresponding decrease in the risk of MI (43). This finding underscores the potential importance of vitamin D (VD) in mitigating the risk of MI. Therefore, the clinical use of VD supplements that increase serum 25OHD levels may have implications for the prevention of MI in the general population. In addition, current MR studies have shown no or weak associations between MI and several risk factors, such as sleep traits (44), leukocyte telomere length (LTL) (45), and educational attainment (46).
The application of MR in MI is shown in Table 1.
3.2 Erectile dysfunction
ED, characterized by persistent difficulties in attaining or maintaining erections adequate for sexual intercourse, often stems from multifactorial etiologies and may signal underlying comorbidities requiring clinical assessment (19, 47). The MR studies included in this article investigate the causal relationships between ED and risk factors such as gut microbiota, cytokine, related diseases, drug targets and other factors.
3.2.1 Gut microbiota
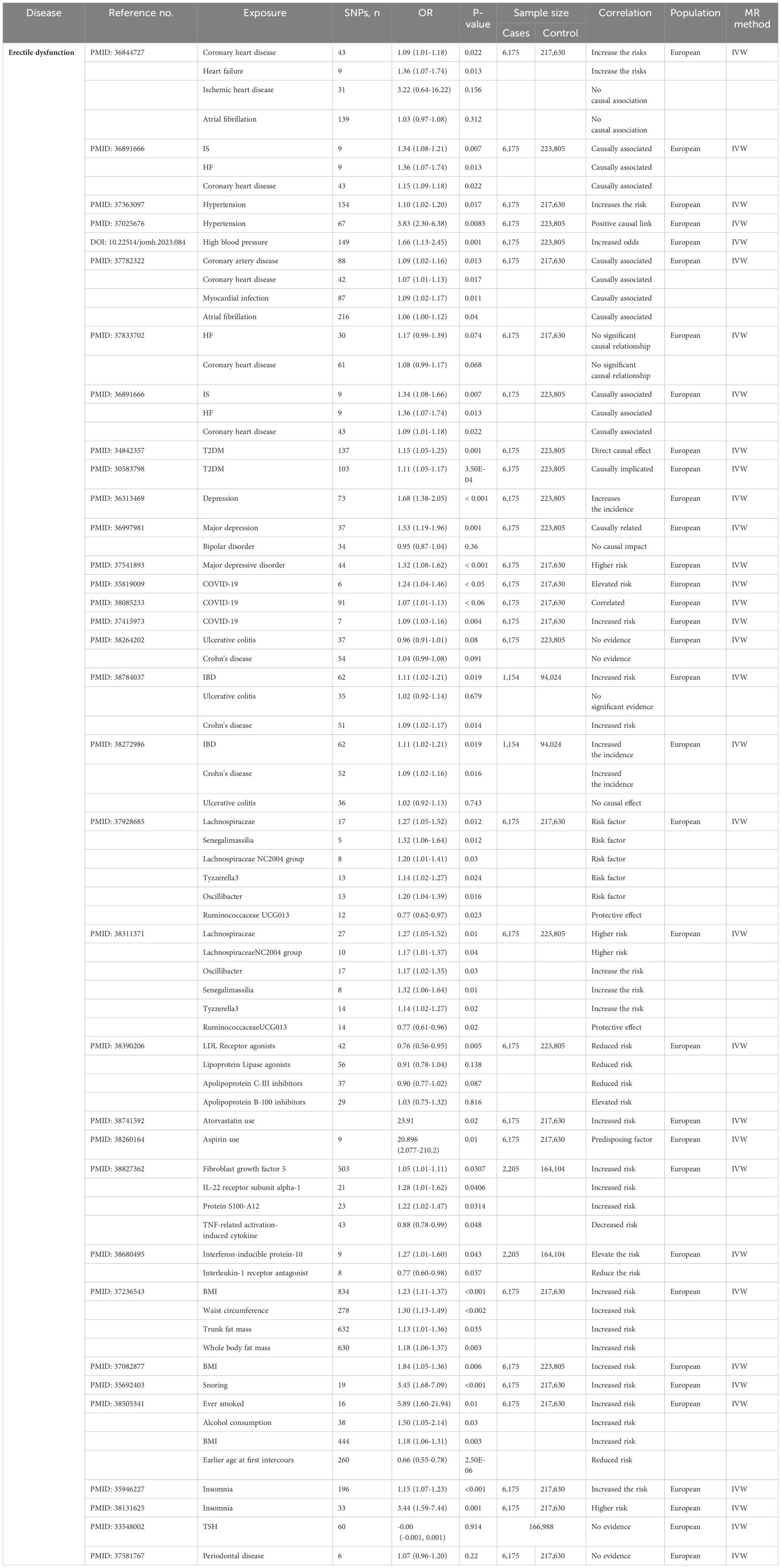
The gut microbiota may cause ED due to changes in endocrine sex hormone levels, the metabolic state of the organism and neurotransmitters (48). Using TSMR studies, Xu R et al. reported that the abundance of the genus Ruminococcaceae UCG-013 exhibited an inverse association with the risk of developing ED. Conversely, the genus Tyzzerella3, genus Erysipelotrichaceae UCG-003, genus LachnospiraceaeNC2004group, genus Oscillibacter, genus Senegalimassilia, and family Lachnospiraceae demonstrated positive associations with an increased risk of ED (49, 50). However, further research is needed to elucidate the pathogenic mechanism of the intestinal microbiota in ED.
3.2.2 Cytokine
The IVW analysis of Kang Z et al. indicates that fibroblast growth factor 5, IL-22 receptor subunit alpha-1, and protein S100-A12 are associated with increased risk of ED, TNF-related activation-induced cytokine is associated with decreased risk (51). According to the study by Liu D et al., elevated levels of interferon-inducible protein-10 were found to significantly elevate the risk of ED, while higher levels of interleukin-1 receptor antagonist (IL-1RA) were observed to markedly reduce the risk of ED (52).
3.2.3 Related diseases
3.2.3.1 Cardiovascular disease
Cardiovascular diseases include coronary heart disease (CHD), ischemic stroke (IS), myocardial infarction, heart failure (HF), ischemic heart disease, and atrial fibrillation, among others. Several studies have elucidated the causal relationship between CVD and ED using MR analyses. For example, genetically predicted CHD and HF increase the risk of ED (53). MR analysis by Miaoyong et al. revealed a causal link between genetic susceptibility to IS, HF, and CHD and ED. Additionally, bidirectional analyses indicated that a genetic predisposition to ED did not increase the risk of CVD (54). An MR study by Zhao C et al. indicated that hypertension increased the risk of ED (55–57). The causal connection between CVD and ED has been inconsistent across multiple MR studies, and further research is needed to confirm these causal claims (54, 58, 59). These findings may inform ED prevention and intervention strategies for patients with CVD.
3.2.3.2 Type 2 diabetes mellitus
ED and systemic health conditions such as metabolic syndrome (e.g., CVD and diabetes) may share many common risk factors (60). Bovijn J et al. used MR analysis to demonstrate that T2DM directly causes ED, independent of obesity and dyslipidemia (61, 62).
Furthermore, CVD, DM, and their comorbid conditions demonstrate frequent comorbidity with ED (63), likely mediated by shared pathological mechanisms such as endothelial dysfunction and chronic inflammatory cascades (64, 65). Therefore, these comorbidities should be carefully accounted for as potential confounders in MR analyses.
3.2.3.3 Psychiatric disorders
The etiology of ED varies and can be organic, psychological or mixed (66). Consequently, ED is closely linked to neurological and mental health issues. Based on IVW analysis, Kai et al. suggested that psychiatric disorders, such as depression, significantly increase the incidence of ED, and genetically predicted depression plays a potential causal role in the development of ED (67–69).
3.2.3.4 COVID-19
Multiple MR analyses have revealed a causal relationship between genetic susceptibility to COVID-19 and an increased risk of ED (70–72).
3.2.3.5 Inflammatory bowel disease
MR analysis by Gao DW et al. did not reveal a causal connection between IBD and ED (73), but recent MR studies by Chen D et al. revealed that IBD can increase the risk of ED (74, 75).
3.2.4 Drug targets
Some drug targeting MR analysis showed that drugs such as LDL receptor, lipoprotein lipase agonists and apolipoprotein C-III inhibitors were associated with reduced ED risk, while apolipoprotein B-100 inhibitors (76), atorvastatin (77) and aspirin (78) were associated with increased ED risk.
3.2.5 Other factors
In addition to the above points, relevant MR studies have shown that numerous additional risk factors are associated with ED. For example, BMI, waist circumference, trunk fat mass, total body fat mass, poorer overall health scores, basal metabolic rate, stroke, smoking, snoring, insomnia, lipocalin and atorvastatin have been found to increase the risk of ED. A genetic predisposition to higher levels of sex hormone binding globulin reduces the risk of ED (79–84). In addition, there are many irrelevant factors, such as thyroid function (85), and periodontal disease (86) that are not associated with ED risk.
The application of MR in ED is shown in Table 2.
3.3 Prostate cancer
PCa remains the most prevalent malignancy in men (20), with mortality rates from metastatic PCa continuing to rise (87). Due to the complex mechanisms underlying the disease and the lack of a clearly defined optimal approach among diverse treatment options (88), exploring PCa-related risk factors is critical for refining clinical prevention and management strategies. The MR studies included in this article investigate the causal relationships between PCa and risk factors such as gut microbiota, circulatory substance, related diseases, modifiable risk factors, drug targets, leukocyte telomere length (LTL) and other factors.
3.3.1 Gut microbiota
A reverse MR analysis by Xu F et al. indicated that a greater risk of PCa was associated with a decrease in the abundance of Prevotella (89). Zixin W et al. confirmed that Alphaproteobacteria has a protective effect on PCa. MVMR analysis revealed that the protective effect of Alphaproteobacteria on PCa might be driven by BMI, smoking, and drinking behaviors (90, 91). Using the Wald ratio method, Mingdong W et al. reported that the abundance of Allisonella was negatively correlated with bladder cancer and PCa incidence (92). The IVW estimates of Xie Q et al. suggested that the relative abundance of Akkermansia muciniphila and Bacteroides salyersiae may decrease the odds of PCa, whereas that of Eubacterium biforme may increase the odds of PCa (93).
3.3.2 Circulatory substance
3.3.2.1 Plasma microgranulin-beta
Plasma microseminoprotein-beta (MSP) is a protein secreted by prostate epithelial cells that may protect against the development of PCa. A nested case-control study using a two-sample inverse variance method to calculate MR estimates showed that plasma MSP concentrations were negatively related to PCa risk after adjusting for the concentration of total prostate-specific antigen. This study suggested that men with high levels of circulating MSP concentrations are at a lower risk of developing PCa and that MSP may play a causal protective role in PCa (94).
3.3.2.2 Serum zinc, phosphorus and iron levels
The role of micronutrients in the development of urinary system tumors cannot be ignored. Using TSMR analysis, Marta et al. reported that an increase in serum zinc had a weak deleterious effect on PCa (95). Yi et al. conducted the TSMR study using pooled statistics from genome-wide association studies (GWAS) for four micronutrients and three major urologic cancer outcomes and demonstrated that each standard deviation (SD) increase in the serum zinc level increased the risk of PCa by 5.8% (96). The IVW analysis by Lin et al. indicated that for each SD increase in the serum phosphate concentration predicted by genetics, the risk of PCa increases by 19% (97). Using MR analysis, Jiacheng et al. reported that a genetically predicted increase in iron status was associated with a decrease in PCa risk and that iron has a protective effect on PCa risk. However, the mechanism by which micronutrients affect PCa needs further study (98, 99).
3.3.2.3 Blood lipids
Studies have shown an association between lipid levels and PCa risk (100, 101). MR analyses by Anna I et al. revealed that the genetically predicted lipoprotein A concentration is correlated with the risk of PCa (102). Bull CJ et al. reported that higher low-density lipoprotein (LDL) and triglyceride levels increase aggressive PCa risk, although the evidence is weak (103). Shiqiang F evaluated the relationship between genetically proxied inhibition of LDL-cholesterol-lowering drug targets and PCa risk using MR methods. Genetically proxied proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition may involve biological mechanisms that reduce the risk of overall and early-onset PCa through the regulation of Lp (a) (104, 105). Shusheng et al. found an association between the effect of triglycerides on PCa risk by applying IVW, suggesting that the odds of PCa increase with elevated triglyceride levels (106). MR analysis by Nabila K et al. revealed that monounsaturated fat levels were positively associated with overall PCa risk (99).
3.3.2.4 Amino acids
Cancer cells often exhibit abnormal growth and proliferation in which enhanced metabolism of amino acid substances is needed. Using TSMR, Yindan et al. demonstrated that serum aspartate levels may promote the development of PCa and breast cancer. An in-depth study of the underlying biochemical mechanisms would be valuable for the early assessment and diagnosis of these two cancers and for the development of clinical intervention strategies (107). MR analysis by Shaoxue Y et al. revealed that circulating alanine concentrations were positively associated with PCa risk and that genetically predicted alanine aminotransferase levels were inversely related to the risk of PCa (108).
3.3.2.5 Red blood cells and hemoglobin
An MR study by Pin et al. provided evidence that elevated mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration are potentially associated with reduced risks of developing PCa (109).
3.3.2.6 Circulating cytokines
Emma et al. performed analyses using methods such as TSMR and IVW and evaluated MR hypotheses in sensitivity and colocalization analyses, providing evidence of a positive correlation between the concentration of genetic proxies for macrophage inflammatory protein 1a (MIP1a) and overall PCa risk and a negative correlation between the concentration of genetic proxies for vascular endothelial growth factor and the risk of late-stage PCa (110). An MR study by Binghui L et al. suggested that long-term IL-6 levels may increase the risk of PCa, whereas long-term IL-1ra levels may reduce this risk (111).
3.3.2.7 Circulating free testosterone
Two MR analyses showed that circulating free testosterone levels were related to elevated PCa risk, whereas circulating total testosterone levels showed no association with PCa risk (112, 113).
3.3.3 Related diseases
Numerous studies have analyzed the potential association between other diseases and PCa risk using MR methods. For example, genetically predicted hyperthyroidism is related to a decreased risk of PCa occurrence (114). Patients with systemic lupus erythematosus have a lower risk of developing PCa (115, 116). Obstructive sleep apnea was significantly negatively associated with PCa susceptibility (117). There was a reverse causal relationship between PCa and pernicious anemia (118). The MR showed a significant association of PCa on erysipelas (119). Schizophrenia, depression and T2DM are not thought to be associated with PCa risk (120–123).
3.3.4 Modifiable risk factors
3.3.4.1 Obesity
The increasing prevalence of obesity globally poses a major threat to public health (124). However, current research suggests that the impact of obesity on PCa is complex. The precise pathophysiological mechanisms underlying the association between obesity and PCa incidence remain incompletely elucidated, with current scientific consensus yet to be definitively established (125, 126). A meta-analysis by Discacciati et al. demonstrated that obesity potentially reduces localized PCa risk and increases the risk of advanced PCa (127). Similarly, an MR analysis by Georgios et al. suggested that obesity increases the risk of advanced PCa (128). A meta-analysis of MR studies by Susanna et al. suggested that a genetically predicted higher adult BMI is related to a reduced risk of cancers such as PCa and breast cancer (129). Moreover, Nabila K et al. showed a negative correlation between BMI and overall PCa through TSMR (99, 130). There was no strong evidence that genetically determined metabolically unfavorable adiposity, favorable adiposity or BMI were correlated with overall PCa in the study by Aurora P-C et al. (131).
As for the conflicting results of the above studies, some believe that obesity may have different effects on PCa risk at different stages throughout the lifespan (132). The conclusion that a larger BMI and waist circumference are positively correlated with the risk of PCa mainly applies to the mid-to-late life, rather than early adulthood (133). Therefore, relevant MR studies should further clarify the effects of obesity at different time points on different developmental stages of PCa. In addition, current discrepancies in obesity-PCa associations across studies may stem from methodological limitations in adiposity assessment. The sole reliance on BMI as a clinical indicator of obesity may yield incomplete characterization of this relationship, as this metric fails to account for critical parameters such as metabolic health status and body composition metrics. Incorporating regional adiposity patterns (e.g., visceral vs. subcutaneous fat distribution) and functional adiposity biomarkers (e.g., leptin/adiponectin ratio) could better elucidate the heterogeneous biological pathways through which obesity may exert differential impacts on prostate carcinogenesis and disease progression (126). Moreover, the differences in the stages and classifications of PCa selected in different studies have led to varying results. Existing research has shown that obesity is associated with advanced or fatal PCa and reduces the risk of low-grade PCa (134), making the relationship with PCa incidence more complex.
In summary, the discrepancies among the research findings may stem from inappropriate assessment methods for obesity, variations in the stages and types of PCa selected across different studies, as well as the influence of obesity on PCa incidence being associated with distinct life stages.
3.3.4.2 Smoking
Cigarette smoking can have deleterious effects on humans and increase the risk of a number of diseases. However, a definitive causal relationship between smoking and PCa has not yet been established. The meta-analysis of MR studies by Susanna et al. concluded that smoking preference was negatively related to the risk of PCa (135, 136). A European pooled study showed that smokers had a lower risk of PCa, and this finding may be attributable to detection bias. In addition, smokers have a greater risk of dying from PCa, possibly due to the direct impact of smoking, which may lead to poor treatment outcomes (137). Using MVMR analysis, Yongle et al. proposed a possible explanation for these implausible findings and showed that each additional increase in the lifetime smoking index increases the risk of PCa by 95%, suggesting a definite causal relationship between smoking and PCa risk (138).
3.3.5 Drug targets
A MR study has shown that Sodium-glucose cotransporter 2 inhibitors inhibition is associated with an increased risk of PCa (139). Ding WJ et al. ‘s drug target MR study found that 3-hydroxy-3-methylglutaryl-assisted enzyme A reductase inhibitors (HMGCR) were associated with an elevated risk of PCa (140).Sun X et al., using a drug-targeted MR approach, found that genetically proxied metformin effects were associated with an increased risk of PCa (141).Sun L et al. ‘s MR study found that genetically proxied inhibition of PCSK9 was associated with reduced risk of PCa (105).The study by Yun Z. et al. provides strong evidence that the use of drugs that act on the renin-angiotensin system can reduce PCa risk (142). Ren F et al. proposed through MR analysis that genetically predicted KDEL containing 2, isoform CRA_a (KDELC2) is negatively associated with PCa. In addition, Kunitz-type protease inhibitor 2, Glutathione S-transferase P, and Cathepsin S may serve as potential therapeutic targets for PCa (143).
3.3.6 Leukocyte telomere length
Telomeres play a significant role in the development and progression of cancer. Cells with longer telomere lengths have greater proliferative potential and a greater cumulative probability of mutation (144). In addition, it has been proposed that telomere shortening can cause end-to-end chromosome fusions and attenuate the DNA damage response, thereby increasing genomic instability and causing carcinogenesis (145). In conclusion, telomeres play a dual role in cancer development, and the direction of action may depend on the type of cancer and other influencing factors. Based on the GRS and MR data, Yixin et al. concluded that a shorter LTL is inversely associated with the risk of cancers such as PCa (146). Junfeng et al. conducted a study to evaluate the relative LTL in PCa patients and its correlation with aggressive disease characteristics at diagnosis and biochemical recurrence (BCR) following aggressive treatment (radical prostatectomy and radiotherapy). Employing the MR method, they found a notable association between shorter LTL and higher Gleason scores in PCa patients. Furthermore, in localized patients undergoing prostatectomy or radiotherapy, shorter LTL and genetically predicted shorter LTL have significant positive correlations with BCR risk, i.e., patients with shorter LTL have a worse prognosis (147). A recent MR study demonstrated that a genetically determined longer LTL was associated with greater PCa risk (148).
3.3.7 Other factors
MR analysis revealed that many other factors, such as height (130, 149), circulating vitamin E levels (150), circulating vitamin C levels (151), circulating VD levels (152, 153), homocysteine levels (154), tryptophan (155), blood pressure (156), serum urea concentration (157), allergic diseases (158), Circulating Bilirubin Levels (159), processed meat, red meat (160), plasma phospholipid arachidonic acid concentrations (161), and circulating levels of C-reactive protein (162), are not associated with PCa risk or are weakly associated with PCa risk. Chen G et al. ‘s two-step MR analysis revealed that proinsulin functions as a suppressive factor in PCa, showing significant independence from insulin-like growth factor 1 (163).
The application of MR in PCa is shown in Table 3.
3.4 Prostatitis
According to the National Institutes of Health (NIH) classification system, prostatitis is categorized into four types: Type I (acute bacterial prostatitis), Type II (chronic bacterial prostatitis), Type III (chronic prostatitis/chronic pelvic pain syndrome, CP/CPPS), and Type IV (asymptomatic inflammatory prostatitis). Given that Type III (chronic non-bacterial prostatitis) accounts for approximately 90% of clinical cases (164), this study focuses on Type III prostatitis. Chronic prostatitis (chronic pelvic pain syndrome) is defined as pelvic pain accompanied by variable urinary symptoms and sexual dysfunction persisting for at least three months (165). Accumulated evidence confirms significant correlations between prostate inflammation development and multiple biomarkers, encompassing immune-inflammatory indicators, hormonal profiles, tumor-associated proteins, and nutritional parameters (166).The MR studies included in this article investigate the causal relationships between prostatitis and risk factors such as gut microbiota, complement C4, immune cells and thyroid function.
3.4.1 Gut microbiota
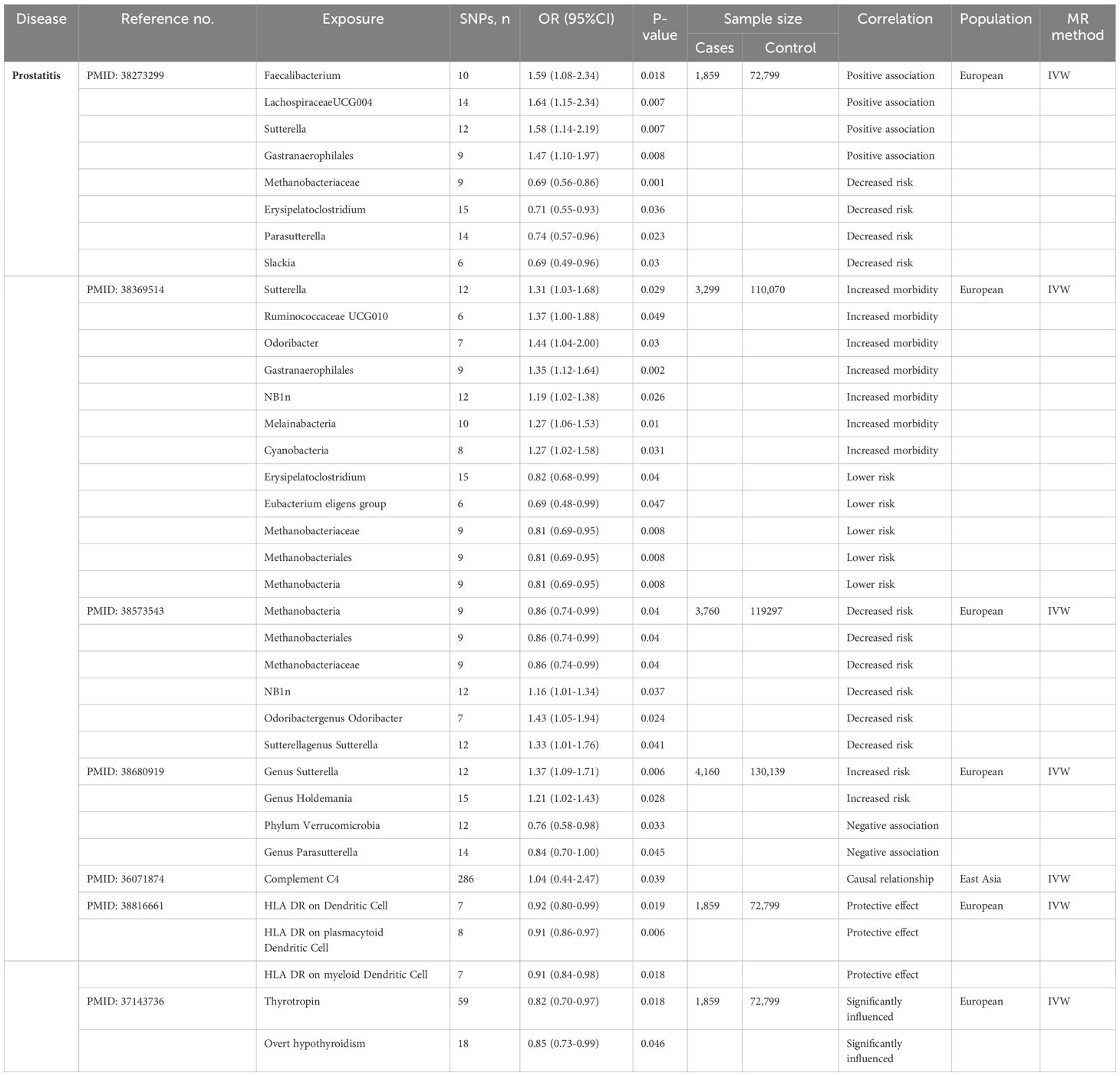
The physiological functions of the host organism can be modulated by gut microbiota through their regulatory effects on multiple biological pathways, encompassing immune regulation, oxidative stress response, inflammatory modulation, and the maintenance of anabolic-catabolic equilibrium (167, 168). While direct evidence linking gut microbiota to prostate pathophysiology remains elusive, emerging research suggests that prostate health may be compromised through indirect pathological pathways, with chronic inflammatory processes likely serving as the principal mediating mechanism (169–171). In 2016, Shoskes et al. pioneered the application of MiSeq sequencing technology to delineate significant gut microbial dysbiosis in chronic nonbacterial prostatitis (CNP) patients (172). More recently, MR analyses have further advanced mechanistic insights into the gut microbiota-PCa causal axis through rigorous causal inference frameworks. According to these MR studies, the risk of prostatitis may be decreased by the presence of Methanebacteria, Methanobacteriales, Methanobacteraceae, Erysipelatoclostridium, the Eubacterium eligens group, phylum Verrucomicrobia and Parasutterella. Faecalibacterium, LachnospiraceaeUCG004, Sutterellagenus Sutterella, NB1n, Gastranaerophilales, Odoribactergenus, Odoribacter, Ruminococcaceae UCG010, Melainabacteria, genus Holdemania and Cyanobacteria play causal roles in promoting the development of prostatitis (173–176).
3.4.2 Other factors
Few MR analyses have been conducted on prostatitis. However, certain risk factors, such as complement C4 (177), certain T cell subsets (178), and thyroid function (179) have been identified as having causal relationships with prostatitis. Complement C4, a pivotal component of the complement system, serves as a critical mediator in innate immunity by enabling rapid recognition and clearance of pathogenic microorganisms (180), while simultaneously reflecting systemic inflammatory activity (181). Importantly, a TSMR study recently validated a positive causal link between elevated complement C4 concentrations and chronic prostatitis pathogenesis (177).
While extensive research has established potential connections between immune cell activity and prostatitis (182, 183), the causal dynamics of specific immune populations in this inflammatory process remain undetermined. A recent investigation leveraging bidirectional MR systematically explored causal relationships between immunophenotypic characteristics and prostatitis pathogenesis. The analyses identified that particular T-cell subsets-notably CD3 + CD4 + T lymphocytes and CD3 + CD8 + T cells-demonstrated significant causal associations with elevated prostatitis risk (178).
Chronic prostatic inflammation may be modulated by endocrine hormone dysregulation or metabolic abnormalities (184). Although no studies have established direct associations between thyroid hormones and prostatitis risk, a large-scale observational investigation revealed prostate volume positively correlated with free thyroxine (FT4) levels (185). Given the potential overlap in pathogenic mechanisms underlying prostatic hypertrophy and prostatitis, Huang et al. employed MR to assess causal relationships between genetically predicted thyroid function alterations and benign prostatic disorders. Their findings demonstrated that elevated thyrotropin (TSH) concentrations and hypothyroidism development were inversely associated with risks of prostatic hypertrophy and inflammatory prostatic conditions (179).
The application of MR in prostatitis is shown in Table 4.
4 Discussion
4.1 Existing problems and solutions
While these MR studies advance our understanding of male reproductive disorders, several methodological limitations persist in the field. We examine these ongoing challenges specific to andrology research and propose solutions to enhance future studies.
4.1.1 Multi-methodology validation
Although MR studies can suggest causal associations between risk factors and male-specific diseases, they do not reveal the underlying mechanisms of their effects. Thus, the estimated magnitude of the effect of exposure on outcomes obtained from MR analysis is not equivalent to the actual causal effect (186). It is also necessary to compare MR analyses with findings from large cohort studies or RCTs to evaluate the consistency and robustness of the evidence. Example illustrations are provided for reference:
1. Validating consistency between MR results and large-scale cohort studies.
For instance, in the manuscript section exploring the causal relationship between PCa and obesity, MR studies have reported inconsistent findings. We identified relevant prospective studies indicating that obesity during mid-to-late adulthood (but not early adulthood) showed inverse associations with localized PCa. These studies also revealed dual associations between BMI and fatal PCa - reduced risk in men with obesity during early adulthood versus increased risk in those with obesity during mid-to-late adulthood (187).
Although the effects of obesity on PCa remain complex, such cohort studies can provide longitudinal associations between obesity and PCa to verify consistency with corresponding MR findings. Therefore, MR results aligning with cohort discoveries in the manuscript may be considered relatively conclusive regarding causal relationships (though higher-level evidence remains necessary). For MR results inconsistent with cohort findings or showing methodological limitations, rigorous evaluation should be conducted regarding analytical process integrity, methodological completeness, and disease staging comprehensiveness.
2. Assessing robustness of MR causal inference using RCT evidence.
As our MR analysis suggests close associations between genetically proxied LDL-cholesterol-lowering drug targets and reduced risks of overall PCa/early-onset PCa, we referenced statins-related RCT outcomes to validate MR robustness (188). The MR approach inherently avoids confounding factors, while combining both methodologies compensates for individual limitations. This multi-level evidence integration substantially enhances result credibility.
In conclusion, researchers should not limit themselves to MR methodology alone. Concurrent collection of regional patient data for broader cross-sectional/observational studies is crucial to validate findings. Result verification constitutes an authorial responsibility rather than readers’ obligation.
Additionally, MR authors must avoid selective result presentation. Objective, rigorous, and comprehensive selection of instrumental variables and datasets should be ensured. Disease-related datasets should be comprehensively incorporated, with multivariable analyses employed to guarantee result robustness.
4.1.2 European-dominant databases in prospective MR studies
Moreover, many prospective studies only use databases that include European populations, and there is a lack of relevant MR studies for Asian populations, which may lead to a lack of comprehensiveness and impact in the application of research results. Researchers can analyze large samples of data from different ethnic groups, taking into account population stratification, to achieve broader application of the research results. We humbly suggest some directions that might help resolve this difficulty:
1. Integration of population data.
We propose collaborating with Asian research institutions to conduct multicenter cohort studies, while advocating for government-supported transnational health data infrastructure development. This initiative should integrate educational, economic, and health datasets through standardized core variable definitions and establish a unified data collaboration platform with harmonized protocols (189).
To advance open science and data transparency, we recommend publicly sharing data preprocessing codes and statistical model parameters in research publications to enable reproducibility (190). This approach particularly encourages researchers to replicate and supplement findings with Asian population data. Furthermore, actively incorporating Asian-based studies (e.g., reports from China’s National Cancer Center (191)) would help counterbalance the current European-centric literature bias, thereby enhancing the reliability and generalizability of research conclusions.
2. Population stratification design.
Prospective studies should incorporate pre-stratification by race, region, and cultural background, with Asian populations further categorized into East, Southeast, and South Asian subgroups for distinct exposure-outcome analyses (192). Furthermore, sociocultural variables should be incorporated into analyses, particularly Asia-specific factors (e.g., family structure, healthcare accessibility) that may influence disease risk profiles.
These strategies will enhance the global representativeness of research findings, providing more generalizable evidence for precision medicine and public health policies.
4.1.3 Survivorship bias
In addition, in male-specific disease research, when the disease of interest is associated with a risk of death, there may be survivorship bias. For example, when a long-term study of a disease is conducted, the participants in the final analysis are not a random sample because some of the study participants died earlier, which may have had some impact on the results. Researchers can identify and adjust for this bias in a variety of ways, such as using data collected in the early stages of the disease or applying weighting methods to adjust for survivorship. Nonetheless, completely eliminating survivor bias is challenging, so this issue should be carefully considered when interpreting the results of MR studies.
This paper discusses possible solutions to address this problem.
1. Integration of early cohort data: Guided by Elston’s intention-to-treat principle (193), researchers should prioritize the incorporation of longitudinal cohorts with early disease phenotypes to capture participants prior to mortality-driven attrition, thereby minimizing attrition bias. When including early cohorts (such as UK Biobank baseline data (194)), focus should be placed on incident PCa cases to avoid reliance on prevalent cases that may overrepresent indolent cancers.
2. Consider implementing genetic risk stratification: Categorize subgroups through Genetic Risk Score (GRS) stratification to identify PCa cases with accelerated progression, as their shorter disease latency periods reduce survival-related attrition, thereby potentially mitigating survival bias (195, 196).
3. Methodological adjustments: To address survivor bias in mortality-related exposure effects, researchers could implement strategies under semi-parametric additive hazard models as proposed by Vansteelandt et al. (197). This approach enables dynamic adjustment for survival selection through lifetime modeling of genetic exposure effects, rather than relying solely on cross-sectional data snapshots.
4.1.4 Linkages between MR studies
The connections among several studies addressing similar research questions are weak, and some articles present contradictory views. For example, in studies exploring the correlation between LTL and PCa risk, one study showed that a shorter LTL was related to a reduced risk of PCa (146). However, other studies have suggested that a shorter LTL is detrimental to patient prognosis and that PCa patients with higher Gleason scores have shorter LTLs (147). The authors could review the relevant literature to discuss the plausibility of a potential causal connection between exposure and outcome, to interpret the results, or to suggest possible biological mechanisms (198). A lack of harmonization is observed among MR studies addressing similar research questions.
4.1.5 Standardization of reporting
Recent methodological advancements emphasize the critical need for standardized reporting frameworks in MR studies. The STROBE-MR checklist provides a 32-item guideline to enhance methodological transparency (199, 200). These guidelines provide actionable resources for researchers to refine methodological rigor and enhance the translational value of causal inference studies.
4.2 Summary and outlook
There has been significant progress in applying MR to male-specific diseases, offering novel insights into etiological mechanisms and paving the way for innovative preventive and therapeutic strategies. The clinical translation of MR findings can directly inform actionable approaches, including: (1) lifestyle interventions (BMI, smoking, and dietary optimization) for personalized prevention; (2) microbiome-targeted therapies (probiotics/nutritional modulation) in high-risk groups; (3) drug repurposing and development guided by genetic evidence; (4) early screening protocols based on genetic risk networks (For example, CVD and T2DM can serve as early screening recommendations for high-risk populations of ED); and (5) precision therapies (e.g., anti-inflammatory agents) leveraging causal biomarker profiles.
With the increasing abundance of genomics data, the number of IVs that can be used in MR studies is increasing, and the accuracy and resolution of the studies will continue to improve. In addition, with the increase in computational power and the continuous improvement of statistical methods, more complex genetic modeling problems are expected to be solved, and the potential of MR in the study of male-specific diseases is promising.
Current MR studies on male-specific diseases should further improve the quality of study design, pay attention to the standardization of reporting and linkage with previous large MR studies, rigorously validate the results, and make appropriate adjustments for possible bias. In addition, interdisciplinary cooperation should combine expertise in genetics, epidemiology and clinical medicine and make reasonable assumptions and comprehensive interpretations of potential causality by combining the biological mechanisms of diseases and evidence from observational cohort studies in an effort to provide a valuable basis for research on the etiology of male-specific diseases as well as for the formulation of preventive policies in public health.
Author contributions
QP: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. ZC: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. HL: Investigation, Methodology, Writing – review & editing. JC: Funding acquisition, Resources, Supervision, Writing – review & editing. SM: Methodology, Project administration, Supervision, Writing – review & editing. CZ: Funding acquisition, Resources, Supervision, Writing – review & editing. ZS: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the China Postdoctoral Science Foundation(2023T160201, 2022M721068), the Key Scientific Research Project of Higher Education Institutions in Henan Province(23A360005), the Key Research and Development and Promotion Project of Henan Province (232102311199), the Innovative Training Program for College Students in Henan Province (202410471025, 202410471027, 202410471036, 202410471046), the Henan Province Colleges and Universities Young Backbone Teacher Training Program(2024GGJS067), the Educational and Teaching Reform Research and Practice Project of Henan University of Chinese Medicine(2024JX89), and the Scientific Research Nursery Project of Henan University of Chinese Medicine(MP2024-54).
Acknowledgments
All the authors of the manuscript are immensely grateful to the foundations for their valuable support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MR, Mendelian randomization; RCT, randomized controlled trial; TSMR, two-sample Mendelian randomization; IVW, inverse variance weighting; IVs, instrumental variables; MVMR, multivariable Mendelian randomization; GRS, genetic risk scores; MI, male infertility; BMI, body mass index; SNP, single nucleotide polymorphisms; HGF, hepatocyte growth factor; T2DM, type 2 diabetes mellitus; 25OHD, 25 hydroxyvitamin D; VD, vitamin D; PCa, prostate cancer; LTL, leukocyte telomere length; BCR, biochemical recurrence; MSP, microseminoprotein-beta; GWAS, genome-wide association studies; SD, standard deviation; LDL, low-density lipoprotein; ED, erectile dysfunction; CVD, cardiovascular disease; IS, ischemic stroke; HF, heart failure; IBD, inflammatory bowel disease.
References
1. Niels ES, Niels J, Anna-Maria A, Anders J, Katharina MM, Tina Kold J, et al. Populations, decreasing fertility, and reproductive health. Lancet. (2019) 393:1500–1. doi: 10.1016/s0140-6736(19)30690-7
2. Connor AE, Amit VK, and Sekar K. Mendelian randomization. Jama. (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
3. Neil MD, Michael VH, and George Davey S. Reading mendelian randomization studies: A guide, glossary, and checklist for clinicians. Bmj. (2018) 362:k601. doi: 10.1136/bmj.k601
4. Stephen B, Neil MD, and Simon GT. Bias due to participant overlap in two-sample mendelian randomization. Genet Epidemiol. (2016) 40:59–608. doi: 10.1002/gepi.21998
5. Debbie AL. Commentary: two-sample mendelian randomization: opportunities and challenges. Int J Epidemiol. (2016) 45:908–15. doi: 10.1093/ije/dyw127
6. Stephen B and Simon GT. Interpreting findings from mendelian randomization using the mr-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
7. Caroline LR and George Davey S. Two-step epigenetic mendelian randomization: A strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. (2012) 41:161–76. doi: 10.1093/ije/dyr233
8. Sehoon P, Soo Jin L, Yaerim K, Yeonhee L, Min Woo K, Kwangsoo K, et al. Atrial fibrillation and kidney function: A bidirectional mendelian randomization study. Eur Heart J. (2021) 42:2816–23. doi: 10.1093/eurheartj/ehab291
9. Lane GC, Justin DT, Zipeng L, Thach TQ, and Pak CS. Mendelian randomization: causal inference leveraging genetic data. psychol Med. (2024) 54:1461–74. doi: 10.1017/s0033291724000321
10. Stephen B, Adam SB, and Simon GT. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
11. Jack B, George Davey S, Philip H, and Stephen B. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
12. Jack B, George Davey S, and Stephen B. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
13. Marie V, Chia-Yen C, Benjamin MN, and Ron D. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–98. doi: 10.1038/s41588-018-0099-7
16. The Lancet Diabetes E. Homing in on the causes of male infertility. Lancet Diabetes Endocrinol. (2022) 10:149. doi: 10.1016/s2213-8587(22)00049-3
17. Suks M, Carlo B, Luca B, Paolo C, Joana C, Nusret Can Ç, et al. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. (2021) 80:603–20. doi: 10.1016/j.eururo.2021.08.014
18. Konstantinos H, Edouard A, Ian E, François G, Dimitrios H, Francesco M, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. (2010) 57:804–14. doi: 10.1016/j.eururo.2010.02.020
19. Giovanni C. Erectile dysfunction and premature ejaculation: A continuum movens supporting couple sexual dysfunction. J Endocrinol Invest. (2022) 45:2029–41. doi: 10.1007/s40618-022-01793-8
20. Rebecca LS, Angela NG, and Ahmedin J. Cancer statistics, 2024. CA: A Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
21. Pejcic T. Prostate cancer: epidemiology, etiology, pathogenesis, and risk factors. Prostate Cancer. (2024) 3–19. doi: 10.1007/978-3-031-51712-9_1
22. Robert CL. Benign prostatic hyperplasia. Prim Care: Clinics Off Pract. (2019) 46:223–32. doi: 10.1016/j.pop.2019.02.003
23. Ashok A, Saradha B, Neel P, Chak-Lam C, Ralf H, Sarah CV, et al. Male infertility. Lancet. (2021) 397:319–33. doi: 10.1016/s0140-6736(20)32667-2
24. Yan W and Zuogang X. Exploring the role of gut microbiome in male reproduction. Int J Androl. (2022) 10:441–50. doi: 10.1111/andr.13143
25. Zou H, Xu N, Xu H, Xing X, Chen Y, and Wu S. Inflammatory cytokines may mediate the causal relationship between gut microbiota and male infertility: A bidirectional, mediating, multivariate mendelian randomization study. Front Endocrinol. (2024) 15:1368334. doi: 10.3389/fendo.2024.1368334
26. Deng R, Huang Y, Tian Z, and Zeng Q. Association between gut microbiota and male infertility: A two-sample mendelian randomization study. Int Microbiol. (2024) 27:1655–63. doi: 10.1007/s10123-024-00512-y
27. Fu Z-d, Wang Y, and Yan H-l. Male infertility risk and gut microbiota: A mendelian randomization study. Front Microbiol. (2023) 14:1228693. doi: 10.3389/fmicb.2023.1228693
28. Zhang F, Xiong Y, Wu K, Wang L, Ji Y, and Zhang B. Genetic insights into intestinal microbiota and risk of infertility: A mendelian randomization study. Microorganisms. (2023) 11(9):2319. doi: 10.3390/microorganisms11092319
29. Li T, Shao W, Wang Y, Zhou R, Yun Z, He Y, et al. A two-sample mendelian randomization analysis investigates associations between gut microbiota and infertility. Sci Rep. (2023) 13:11426. doi: 10.1038/s41598-023-38624-6
30. Ma S-C, Zhang C-M, Hao X-H, Ma R-M, Hu J, Yu W-L, et al. The causal relationship between gut microbiota and male reproductive and sexual health. Zhonghua Nan Ke Xue = Natl J Androl. (2023) 29:587–95. doi: 10.13263/j.cnki.nja.2023.07.002
31. Xi Y, Zhang C, Feng Y, Zhao S, Zhang Y, Duan G, et al. Genetically predicted the causal relationship between gut microbiota and infertility: bidirectional mendelian randomization analysis in the framework of predictive, preventive, and personalized medicine. Epma J. (2023) 14:405–16. doi: 10.1007/s13167-023-00332-6
32. Zhang L, Li H, Wu Z, Han L, and Zhang J. Associations between cytokines and the risk of female and male infertility: A two-sample mendelian randomization analysis. J Reprod Immunol. (2024) 163:104238. doi: 10.1016/j.jri.2024.104238
33. Zhu XB, Niu ZH, Fan WM, Sheng CS, and Chen Q. Type 2 diabetes mellitus and the risk of male infertility: A mendelian randomization study. Front Endocrinol. (2023) 14:1279058. doi: 10.3389/fendo.2023.1279058
34. Fan CH, Zhang JD, and Qiu DB. Causal relationship between genetically predicted type 2 diabetes mellitus and male infertility. Front Endocrinol. (2024) 15:1357279. doi: 10.3389/fendo.2024.1357279
35. Liu Q, Cui Y, Wang X, and Yang B. Genetic association between covid-19 and male infertility: A two-sample mendelian randomization analysis. J Mens Health. (2024) 20:62–72. doi: 10.22514/jomh.2024.009
36. Zhang M, Wen T, and Wang D. The association between covid-19 and infertility: mendelian randomization analysis. Medicine. (2024) 103(10):e37346. doi: 10.1097/md.0000000000037346
37. Wang X, Li T, and Chen Q. Causal relationship between ulcerative colitis and male infertility: A two-sample mendelian randomization study. PloS One. (2024) 19(5):e0303827. doi: 10.1371/journal.pone.0303827
38. Chen X, Hao X, Xie L, and Liu X. A bidirectional causal relationship study between mental disorders and male and female infertility. Front Psychiatry. (2024) 15:1378224. doi: 10.3389/fpsyt.2024.1378224
39. Wentao X, Yueyuan Y, Tianqi Y, and Jing L. Insights into modifiable risk factors of infertility: A mendelian randomization study. Nutrients. (2022) 14(19):4042. doi: 10.3390/nu14194042
40. Hernáez A, Rogne T, Skåra KH, Håberg SE, Page CM, Fraser A, et al. Body mass index and subfertility: multivariable regression and mendelian randomization analyses in the norwegian mother, father and child cohort study. Hum Reprod. (2021) 36:3141–51. doi: 10.1093/humrep/deab224
41. Hernáez A, Wootton RE, Page CM, Skåra KH, Fraser A, Rogne T, et al. Smoking and infertility: multivariable regression and mendelian randomization analyses in the norwegian mother, father and child cohort study. Fertil Steril. (2022) 118:180–90. doi: 10.1016/j.fertnstert.2022.04.001
42. Chen X, Ren C, Wu C, and Liu X. Mendelian randomization reveals the impact of diet on infertility in men and women. Front Endocrinol. (2024) 15:1376800. doi: 10.3389/fendo.2024.1376800
43. Yuan C, Liyuan X, Zhongyu J, and Banghua L. Vitamin D levels and risk of male factor infertility: A mendelian randomization study. World J Men’s Health. (2023) 41(3):640–48. doi: 10.5534/wjmh.220109
44. Shiqi L, Zhenqiang M, Wanzhen Z, Hao Z, Jun M, Hang D, et al. Association of sleep traits with male fertility: A two-sample mendelian randomization study. Front Genet. (2024) 15. doi: 10.3389/fgene.2024.1353438
45. An G, Zhao X, and Zhao C. Unraveling the causal association between leukocyte telomere length and infertility: A two-sample mendelian randomization study. PloS One. (2024) 19(3):e0298997. doi: 10.1371/journal.pone.0298997
46. Wang MH, Jian ZY, Gao XS, Yuan C, Jin X, Li H, et al. Causal associations between educational attainment and 14 urological and reproductive health outcomes: A mendelian randomization study. Front Public Health. (2021) 9:742952. doi: 10.3389/fpubh.2021.742952
47. Bobby BN and James AK. Erectile dysfunction. Jama. (2016) 316(17):1838. doi: 10.1001/jama.2016.12284
48. Giorgio Ivan R, Dafne B, Carmelo B, Nicolò M, Stefania S, Ioannis S, et al. The relationship between the gut microbiota, benign prostatic hyperplasia, and erectile dysfunction. Int J Impotence Res. (2022) 35:350–55. doi: 10.1038/s41443-022-00569-1
49. Wenkang C, Yijing Z, Hede Z, Bolin L, Hanfei L, Ruikun W, et al. Association between gut microbiota and erectile dysfunction: A two-sample mendelian randomization study. Res Square (Research Square). (2023). doi: 10.21203/rs.3.rs-2491164/v1
50. Tianle Z, Xi L, Peng Y, Yukuai M, Pan G, Jingjing G, et al. The association between the gut microbiota and erectile dysfunction. World J Men’s Health. (2024) 42(4):772–86. doi: 10.5534/wjmh.230181
51. Kang Z, Zhang Z-R, Feng Z-Y, Dong L-S, and Yang J. Inflammatory proteins mediate male erectile dysfunction via plasma metabolites. Sexual Med. (2024) 12:qfae02759. doi: 10.1093/sexmed/qfae027
52. Liu D, Qin Z, Yi B, Xie H, Liang Y, Zhu L, et al. Inflammatory cytokine profiles in erectile dysfunction: A bidirectional mendelian randomization. Front Immunol. (2024) 15:1342658. doi: 10.3389/fimmu.2024.1342658
53. Qingying L, Qiang L, Baoming R, and Sen B. Causal association between cardiovascular diseases and erectile dysfunction, a mendelian randomization study. Front Cardiovasc Med. (2023) 10. doi: 10.3389/fcvm.2023.1094330
54. Ye MY, Chen JX, Ma JX, Wang JW, Zhang CM, Chen BJ, et al. Causal association of cardiovascular disease with erectile dysfunction: A two-sample bidirectional mendelian randomization analysis. Andrology. (2023) 11:1368–76. doi: 10.1111/andr.13421
55. Zhao C, Feng JL, Deng S, Wang XP, Fu YJ, Wang B, et al. Genetically predicted hypertension, antihypertensive drugs, and risk of erectile dysfunction: A mendelian randomization study. Front Cardiovasc Med. (2023) 10:1157467. doi: 10.3389/fcvm.2023.1157467
56. Wang Z, Wang YY, Xiong JC, Gan XX, Bao YW, Jiang AM, et al. Causal effects of hypertension on risk of erectile dysfunction: A two-sample mendelian randomization study. Front Cardiovasc Med. (2023) 10:1121340. doi: 10.3389/fcvm.2023.1121340
57. Sun Z, Hu J, Luo YP, Shu H, Fan ZQ, Shuang ST, et al. Causal associations between erectile dysfunction and high blood pressure, negative psychology: A mendelian randomization study. J Mens Health. (2023) 19:36–58. doi: 10.22514/jomh.2023.084
58. Xi YJ, Yin XY, Zhou J, Shen RT, Qi LK, and Zhang SX. Genetically predicted cardiovascular diseases could increase the risk of erectile dysfunction: A bidirectional mendelian randomization. World J Urol. (2023) 41:3187–94. doi: 10.1007/s00345-023-04630-6
59. Shao KY, Chen WK, Li YL, Zheng HY, Hu RY, Zhang JQ, et al. Effects of heart failure and coronary artery disease on erectile dysfunction: A two-sample mendelian randomization study. BMC Urol. (2023) 23:163. doi: 10.1186/s12894-023-01335-1
60. Sanchez E, Pastuszak AW, and Khera M. Erectile dysfunction, metabolic syndrome, and cardiovascular risks: facts and controversies. Trans Androl Urol. (2017) 6:28–36. doi: 10.21037/tau.2016.10.01
61. Yuan C, Zhongyu J, Xiaoshuai G, Xi J, Menghua W, Liyuan X, et al. Type 2 diabetes mellitus increases risk of erectile dysfunction independent of obesity and dyslipidemia: A mendelian randomization study. Int J Androl. (2021) 10:518–24. doi: 10.1111/andr.13132
62. Bovijn J, Jackson L, Censin J, Chen CY, Laisk T, Laber S, et al. Gwas identifies risk locus for erectile dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am J Hum Genet. (2019) 104:157–63. doi: 10.1016/j.ajhg.2018.11.004
63. Elena C, Matteo M, Felice C, Biagio B, Fabio Massimo E, Davide A, et al. The relationship between obstructive sleep apnoea and erectile dysfunction: an underdiagnosed link? A prospective cross-sectional study. Andrologia. (2022) 54:e14504. doi: 10.1111/and.14504
64. Camilla MH, Kerri M, Craig LP, Ronald RG, and Yang L. To ed or not to ed – is erectile dysfunction in obstructive sleep apnea related to endothelial dysfunction? Sleep Med Rev. (2015) 20:5–14. doi: 10.1016/j.smrv.2014.03.004
65. Izolde B, Vaios P, Frank S, Charalampos M, Violeta M, NikoLaos MS, et al. Abnormal cytokine profile in patients with obstructive sleep apnea-hypopnea syndrome and erectile dysfunction. Mediators Inflammation. (2014) 2014. doi: 10.1155/2014/568951
66. Faysal AY, Lawrence CJ, Maarten A, Giovanni C, Andrea MI, Shari G, et al. Erectile dysfunction. Nat Rev Dis Primers. (2016) 2:16003. doi: 10.1038/nrdp.2016.3
67. Kai M, Peng S, Zhenghuan L, Lei Y, Linchun W, Jing Z, et al. Genetic evidence suggests that depression increases the risk of erectile dysfunction: A mendelian randomization study. Front Genet. (2022) 13. doi: 10.3389/fgene.2022.1026227
68. Weikang C, Tao Z, Daquan Y, Jingping L, Jing-Gen W, Lejun L, et al. Effects of major depression and bipolar disorder on erectile dysfunction: A two-sample mendelian randomization study. BMC Med Genomics. (2023) 16:66. doi: 10.1186/s12920-023-01498-8
69. Zhang FX, Xiong Y, Wu K, and Zhang B. Assessment of the causal link between depression and erectile dysfunction: A mendelian randomization study. Asian J Surg. (2023) 46:5533–4. doi: 10.1016/j.asjsur.2023.07.145
70. Zhang K, Gao HX, and Chen MW. Genetic susceptibility to covid-19 may increase the risk of erectile dysfunction: A two-sample mendelian randomization study. Andrologia. (2022) 54. doi: 10.1111/and.14527
71. Tao YM, Zhao R, Han J, and Li YS. Assessing the causal relationship between covid-19 and post-covid-19 syndrome: A mendelian randomization study. J Global Health. (2023) 13:06054. doi: 10.7189/jogh.13.06054
72. Chang ZL, An LY, Lei M, Song ZF, Deng J, Tang RZ, et al. The genetic associations of covid-19 on genitourinary symptoms. Front Immunol. (2023) 14:1216211. doi: 10.3389/fimmu.2023.1216211
73. Gao DW, Chen C, Wu ZL, Li HK, and Tang B. Relationship between inflammatory bowel disease and erectile dysfunction: A 2-sample mendelian randomization study. Sexual Med. (2023) 11:qfad067. doi: 10.1093/sexmed/qfad067
74. Pan R, Sun C, Zheng L, Liu J, and Xu W. Genetic liability to inflammatory bowel disease is causally associated with increased risk of erectile dysfunction: evidence from a bidirectional mendelian randomization study. Front Genet. (2024) 15:1334972. doi: 10.3389/fgene.2024.1334972
75. Chen D, Zhou C, Luo Q, Chen C, and Liu G. A mendelian randomization study on causal effects of inflammatory bowel disease on the risk of erectile dysfunction. Sci Rep. (2024) 14:2137. doi: 10.1038/s41598-024-52712-1
76. Su QX, Wang R, Luo YY, Tang QZ, and Wang KN. Genetic association of lipid-lowering drug target genes with erectile dysfunction and male reproductive health. Front Endocrinol. (2024) 15:1362499. doi: 10.3389/fendo.2024.1362499
77. Chen K, Huang H, Chen Y, and He W. Association between atorvastatin and erectile dysfunction: A comprehensive analysis incorporating real-world pharmacovigilance and mendelian randomization. Front Pharmacol. (2024) 15:1382924. doi: 10.3389/fphar.2024.1382924
78. Li R, Peng L, Deng D, Li G, and Wu S. Potential causal association between aspirin use and erectile dysfunction in european population: A mendelian randomization study. Front Endocrinol. (2024) 14:1329847. doi: 10.3389/fendo.2023.1329847
79. Yang X, Xianding W, Yangchang Z, Wei W, Yuxin R, Changjing W, et al. Insights into modifiable risk factors of erectile dysfunction, a wide-angled mendelian randomization study. J Adv Res. (2023) 58:149–61. doi: 10.1016/j.jare.2023.05.008
80. Bao BH, Guo JQ, Zhang L, Pan ZK, Huang HN, Qin ZJ, et al. Effects of obesity-related anthropometric indices and body composition on erectile dysfunction mediated by coronary artery disease: A mendelian randomization study. Andrology. (2024) 12:75–86. doi: 10.1111/andr.13443
81. Xiong Y, Zhong X, Zhang FX, Wang W, Zhang YC, Wu CJ, et al. Genetic evidence supporting a causal role of snoring in erectile dysfunction. Front Endocrinol. (2022) 13:896369. doi: 10.3389/fendo.2022.896369
82. Xi Y-J, Feng Y-G, Bai Y-Q, Wen R, Zhang H-Y, Su Q-Y, et al. Genetic prediction of modifiable lifestyle factors for erectile dysfunction. Sexual Med. (2024) 12:qfae010. doi: 10.1093/sexmed/qfae010
83. Xiong Y, Zhang FX, Zhang YC, Wu CJ, Qin F, and Yuan JH. Genetically predicted insomnia causally increases the risk of erectile dysfunction. Asian J Androl. (2023) 25:421–5. doi: 10.4103/aja202261
84. Zhang H, Wang SQ, Ma SC, Zhang CM, Wang ZL, and Yan PY. Causal relationship between worry, tension, insomnia, sensitivity to environmental stress and adversity, and erectile dysfunction: A study using mendelian randomization. Andrology. (2023) 12:1272–9. doi: 10.1111/andr.13574
85. Alisa DK, Eirini M, Areti P, Panos D, Aleksander K, Rosalie S, et al. Thyroid function, sex hormones and sexual function: A mendelian randomization study. Eur J Epidemiol. (2021) 36:335–44. doi: 10.1007/s10654-021-00721-z
86. Yu FY, Wang H, Wang QQ, Zhao BL, Zhao ZN, and Bian W. Evaluation of bi-directional causal association between periodontal disease and erectile dysfunction: A two-sample mendelian randomization study. Clin Investig. (2023) 27:5895–903. doi: 10.1007/s00784-023-05201-0
87. Shahneen S, Caroline MM, Edmund C, Himisha B, Robert GB, and Scott W. Prostate cancer. Lancet. (2021) 398:1075–90. doi: 10.1016/s0140-6736(21)00950-8
88. Philippe DV, Thomas A, Paul A, Jarno R, Henrikki S, Arnav A, et al. Decision aids for localized prostate cancer treatment choice: systematic review and meta-analysis. CA: A Cancer J Clin. (2015) 65:239–51. doi: 10.3322/caac.21272
89. Xu F, Fu Y, Sun T-y, Jiang Z, Miao Z, Shuai M, et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome. (2020) 8:145. doi: 10.1186/s40168-020-00923-9
90. Zixin W, Biying Y, Tiantian T, Zi-Jing X, Fengzhan Y, Xiaoyu L, et al. Gut microbiota and risk of five common cancers: A univariable and multivariable mendelian randomization study. Cancer Med. (2023) 12:10393–405. doi: 10.1002/cam4.5772
91. Xiaoyang L, Lei Y, Zhou P, Peng S, Zhenghuan L, Jing Z, et al. Causal associations between gut microbiota and three prostate diseases: A bidirectional two-sample mendelian randomization study. Res Square (Research Square). (2023). doi: 10.21203/rs.3.rs-3209956/v1
92. Mingdong W, Xiang G, Quan Y, Mingshuai W, and Ping H. Causal associations between gut microbiota and urological tumors: A two-sample mendelian randomization study. BMC Cancer. (2023) 23:854. doi: 10.1186/s12885-023-11383-3
93. Xie Q and Hu B. Effects of gut microbiota on prostatic cancer: A two-sample mendelian randomization study. Front Microbiol. (2023) 14:1250369. doi: 10.3389/fmicb.2023.1250369
94. Karl S-B, Paul NA, Timothy JK, Michael VH, Georgina KF, Antonio A, et al. The role of plasma microseminoprotein-beta in prostate cancer: an observational nested case–control and mendelian randomization study in the european prospective investigation into cancer and nutrition. Ann Oncol. (2019) 30:983–9. doi: 10.1093/annonc/mdz121
95. Marta RM, Ailin Falkmo H, Brooke NW, Laurent FT, Humaira R, Anica S, et al. New insights into the genetic etiology of 57 essential and non-essential trace elements in humans. medRxiv. (2023) 7:432. doi: 10.1101/2023.04.25.23289097
96. Yi L, Hao S, Yutao W, and Hongjun L. Micronutrients and risks of three main urologic cancers: A mendelian randomization study. Front Nutr. (2023) 10. doi: 10.3389/fnut.2023.1016243
97. Linshuoshuo L, Ye D, Jie C, Qian Y, Alan F, Jie S, et al. Circulating phosphorus concentration and risk of prostate cancer: A mendelian randomization study. Am J Clin Nutr. (2022) 115:534–43. doi: 10.1093/ajcn/nqab342
98. Ying J, Wang B, Han S, Song J, Liu K, Chen W, et al. Genetically predicted iron status was associated with the risk of prostate cancer. Front Oncol. (2022) 12:959892. doi: 10.3389/fonc.2022.959892
99. Nabila K, Philip H, Konstantinos KT, Brigid ML, Thérèse T, Richard MM, et al. Appraising causal relationships of dietary, nutritional and physical-activity exposures with overall and aggressive prostate cancer: two-sample mendelian-randomization study based on 79–148 prostate-cancer cases and 61–106 controls. Int J Epidemiol. (2019) 49(2):587–96. doi: 10.1093/ije/dyz235
100. Juzar J, Lauren EH, Emma HA, Adriana CV, Daniel M, Ramiro C-S, et al. Serum cholesterol and risk of high-grade prostate cancer: results from the reduce study. Prostate Cancer Prostatic Dis. (2017) 21:252–9. doi: 10.1038/s41391-017-0030-9
101. Teemu JM, Heimo S, Pasi P, Merja B, Tiina S, Timo Y, et al. The importance of ldl and cholesterol metabolism for prostate epithelial cell growth. PloS One. (2012) 7(6):e39445. doi: 10.1371/journal.pone.0039445
102. Anna I, Eleanor LW, Aurora P-C, Elizabeth AP, Ian GM, Timothy JK, et al. The relationship between lipoprotein a and other lipids with prostate cancer risk: A multivariable mendelian randomization study. PloS Med. (2022) 19(1):e1003859. doi: 10.1371/journal.pmed.1003859
103. Bull CJ, Bonilla C, Holly JMP, Perks CM, Davies N, Haycock P, et al. Blood lipids and prostate cancer: A mendelian randomization analysis. Cancer Med. (2016) 5:1125–36. doi: 10.1002/cam4.695
104. Shiqiang F, James Y, Dipender G, Caroline JB, Claire MP, George Davey S, et al. Association between genetically proxied pcsk9 inhibition and prostate cancer risk: A mendelian randomization study. PloS Med. (2023) 20(1):e1003988. doi: 10.1371/journal.pmed.1003988
105. Sun L, Ding H, Jia Y, Shi M, Guo D, Yang P, et al. Associations of genetically proxied inhibition of hmg-coa reductase, npc1l1, and pcsk9 with breast cancer and prostate cancer. Breast Cancer Res. (2022) 24:12. doi: 10.1186/s13058-022-01508-0
106. Shusheng Z, Xia H, and Yanpeng F. Association of triglyceride levels and prostate cancer: A mendelian randomization study. BMC Urol. (2022) 22:167. doi: 10.1186/s12894-022-01120-6
107. Yindan L, Zichen Y, Jingjia L, Yandi S, Xueyun Z, Zihao Q, et al. Effects of glutamate and aspartate on prostate cancer and breast cancer: A mendelian randomization study. BMC Genomics. (2022) 23:213. doi: 10.1186/s12864-022-08442-7
108. Shaoxue Y, Jie S, Hong Y, Wei L, Yuqing J, Xiaohui S, et al. Genetically predicted circulating concentrations of alanine and alanine aminotransferase were associated with prostate cancer risk. Clin Epidemiol. (2022) 2022(14):1255–64. doi: 10.2147/clep.s382116
109. Pin Z, Zhaowei Z, and Xuepei Xuepei Z. Hematological markers and prostate cancer risk: A mendelian randomization study. Res Square (Research Square). (2023). doi: 10.21203/rs.3.rs-2815251/v1
110. Bouras E, Karhunen V, Gill D, Huang J, Haycock PC, Gunter MJ, et al. Circulating inflammatory cytokines and risk of five cancers: A mendelian randomization analysis. BMC Med. (2022) 20:3. doi: 10.1186/s12916-021-02193-0
111. Binghui L, Siyu Y, Lisha L, Xian-Tao Z, Yongbo W, and Xinghuan W. Ten interleukins and risk of prostate cancer. Front Oncol. (2023) 13. doi: 10.3389/fonc.2023.1108633
112. Junke C, Yikang W, Sicheng Z, Ye T, Yan W, Jie T, et al. Genetically predicted testosterone and cancers risk in men: A two-sample mendelian randomization study. J Trans Med. (2022) 20:573. doi: 10.1186/s12967-022-03783-z
113. Watts EL, Perez-Cornago A, Fensom GK, Smith-Byrne K, Noor U, Andrews CD, et al. Circulating free testosterone and risk of aggressive prostate cancer: prospective and mendelian randomization analyses in international consortia. Int J Cancer. (2022) 151:1033–46. doi: 10.1002/ijc.34116
114. Feipeng X and Zhenxin C. Causal associations of hyperthyroidism with prostate cancer, colon cancer, and leukemia: A mendelian randomization study. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1162224
115. Jun O, Zhen K, Yaqian W, Zixuan X, Yangyi F, Qiming Z, et al. Systemic lupus erythematosus and prostate cancer risk: A pool of cohort studies and mendelian randomization analysis. J Cancer Res Clin Oncol. (2023) 149:9517–28. doi: 10.1007/s00432-023-04853-5
116. Deng X, Sun S, Yao W, Yue P, Guo F, Wang Y, et al. The association between three prevalent autoimmune disorders and the likelihood of developing prostate cancer: A mendelian randomization study. Sci Rep. (2024) 14:11755. doi: 10.1038/s41598-024-62716-6
117. Li W and Wang W. Revealing the causal impact of obstructive sleep apnea on cancer risk: insights from mendelian randomization analysis. Sleep Breathing. (2024) 28:1771–6. doi: 10.1007/s11325-024-03046-9
118. Che B, Yuan S, Zhang H, Zhai J, Zhang Y, Wu C, et al. Causal inference between pernicious anemia and cancers: A bidirectional two-sample mendelian randomization analysis. BMC Cancer. (2024) 24:586. doi: 10.1186/s12885-024-12354-y
119. Jin Y and Wang P. Association between prostate cancer and erysipelas: A mendelian randomization study. Asian J Surg. (2024) 47:2544–5. doi: 10.1016/j.asjsur.2024.02.058
120. Fan G, Zhenyu H, Ye-Ling L, Du X, Rui W, Weiyi L, et al. Association between schizophrenia and prostate cancer risk: results from a pool of cohort studies and mendelian randomization analysis. Compr Psychiatry. (2022) 115:152308. doi: 10.1016/j.comppsych.2022.152308
121. Chen X, Jianqiu K, Xiayao D, Jiahao C, Junjiong Z, Weibin X, et al. Depression and prostate cancer risk: A mendelian randomization study. Cancer Med. (2020). doi: 10.1002/cam4.3493
122. Shiu Lun Au Y and Schooling CM. Impact of glycemic traits, type 2 diabetes and metformin use on breast and prostate cancer risk: A mendelian randomization study. BMJ Open Diabetes Res Care. (2019). doi: 10.1136/bmjdrc-2019-000872
123. Shuai Y, Siddhartha K, Paul C, Mathew V, Amy MM, Stephen B, et al. Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample mendelian randomization study. Diabetes. (2020) 69(7):1588–96. doi: 10.2337/db20-0084
124. Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, and Lu Y. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. (2016) 387:1377–96. doi: 10.1016/s0140-6736(16)30054-x
125. Jillian LC, Philippa C, Robert S, and Bobby Chi-Hung L. Prostate cancer and obesity: current hypotheses and challenges. Int J Cancer Care Deliv. (2024). doi: 10.53876/001c.120988
126. Rebekah LW, Dennis RT, Robert UN, Nicholas H, Philippa LW, and Daniel AG. Obesity and prostate cancer: A narrative review. Crit Rev Oncol/Hematol. (2022) 169:103543. doi: 10.1016/j.critrevonc.2021.103543
127. Discacciati A, Orsini N, and Wolk A. Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. (2012) 23:1665–71. doi: 10.1093/annonc/mdr603
128. Georgios P, Dimitrios T, NikoLaos S, Natalia V, Irene K, Faidon M, et al. Obesity and main urologic cancers: current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin Cancer Biol. (2023) 91:70–98. doi: 10.1016/j.semcancer.2023.03.002
129. Susanna CL and Stephen B. Causal role of high body mass index in multiple chronic diseases: A systematic review and meta-analysis of mendelian randomization studies. BMC Med. (2021) 19:320. doi: 10.1186/s12916-021-02188-x
130. Neil MD, Tom RG, Sarah JL, Jeff MPH, Jenny D, Freddie CH, et al. The effects of height and bmi on prostate cancer incidence and mortality: A mendelian randomization study in 20,848 cases and 20,214 controls from the practical consortium. Cancer Causes Control. (2015) 26:1603–16. doi: 10.1007/s10552-015-0654-9
131. Aurora P-C, Karl S-B, Emma H, Cody ZW, Susan M, Timothy MF, et al. Genetic predisposition to metabolically unfavorable adiposity and prostate cancer risk: A mendelian randomization analysis. Cancer Med. (2023) 12:16482–489. doi: 10.1002/cam4.6220
132. Zhe F, Mingyang S, Dong Hoon L, and Edward G. The role of mendelian randomization studies in deciphering the effect of obesity on cancer. JNCI: J Natl Cancer Inst. (2021) 114:361–71. doi: 10.1093/jnci/djab102
133. Barbra AD and Lorelei AM. Obesity, height, and advanced prostate cancer: extending current evidence toward precision prevention. Ann Oncol. (2020) 31:7–8. doi: 10.1016/j.annonc.2019.10.011
134. Adriana CV, Lauren EH, Daniel MM, Ramiro CS, Gerald LA, and Stephen JF. Obesity increases the risk for high-grade prostate cancer: results from the reduce study. Cancer Epidemiol Biomarkers Prev. (2014) 23(12):2936–42. doi: 10.1158/1055-9965.epi-14-0795
135. Susanna CL and Stephen B. Appraising the causal role of smoking in multiple diseases: A systematic review and meta-analysis of mendelian randomization studies. EBioMedicine. (2022) 82:104154. doi: 10.1016/j.ebiom.2022.104154
136. Susanna CL, Paul C, Siddhartha K, Mathew V, Amy MM, Karl M, et al. Smoking, alcohol consumption, and cancer: A mendelian randomization study in uk biobank and international genetic consortia participants. PloS Med. (2020) 17(7):e1003178. doi: 10.1371/journal.pmed.1003178
137. Sylvia HJJ, Josef F, Christel H, Bengt J, Pär S, and Tanja S. Smoking and risk of prostate cancer and prostate cancer death: A pooled study. Eur Urol. (2023) 83:422–31. doi: 10.1016/j.eururo.2022.03.033
138. Zhan Y, Ruan X, Wang P, Huang D, Huang J, Huang J, et al. Causal effects of modifiable behaviors on prostate cancer in europeans and east asians: A comprehensive mendelian randomization study. Biology. (2023) 12:673. doi: 10.3390/biology12050673
139. Zhang L, Xue B, Yu F, Yin Y, and Jin S. Deciphering the causal relationship between sodium-glucose cotransporter 2 inhibition and cancer risks: A comprehensive mendelian randomization study. J Cancer. (2024) 15:3903–12. doi: 10.7150/jca.96435
140. Ding WJ, Chen LL, Xia JG, Pei B, Song B, and Li XJ. Causal association between lipid-lowering drugs and cancers: A drug target mendelian randomization study. Medicine. (2024) 103(18):e38010. doi: 10.1097/md.0000000000038010
141. Sun X, Ping J, Guo X, Long J, Cai Q, Shu X-o, et al. Drug-target mendelian randomization revealed a significant association of genetically proxied metformin effects with increased prostate cancer risk. Mol Carcinogene. (2024) 63:849–58. doi: 10.1002/mc.23692
142. Yun Z, Shen Y, Yan X, Tian S, Wang J, Teo CS, et al. Association between 19 medication use and risk of common cancers: A cross-sectional and mendelian randomization study. J Global Health. (2024) 14:04057. doi: 10.7189/jogh.14.04057
143. Ren F, Jin Q, Liu T, Ren X, and Zhan Y. Proteome-wide mendelian randomization study implicates therapeutic targets in common cancers. J Trans Med. (2023) 21:646. doi: 10.1186/s12967-023-04525-5
144. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
145. Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, et al. Telomere dysfunction: A potential cancer predisposition factor. J Natl Cancer Inst. (2003) 95:1211–8. doi: 10.1093/jnci/djg011
146. Yixin G, Yongyue W, Xiang Z, Shuiping H, Huashuo Z, and Ping Z. Assessing the relationship between leukocyte telomere length and cancer risk/mortality in uk biobank and tcga datasets with the genetic risk score and mendelian randomization approaches. Front Genet. (2020) 11. doi: 10.3389/fgene.2020.583106
147. Junfeng X, Wen Shin C, Chia Wen T, Da Tian B, Yifan X, John WD, et al. Leukocyte telomere length is associated with aggressive prostate cancer in localized prostate cancer patients. EBioMedicine. (2020) 52:102616. doi: 10.1016/j.ebiom.2019.102616
148. Bangbei W, Likui L, and Cai L. Mendelian randomization study on the causal relationship between leukocyte telomere length and prostate cancer. PloS One. (2023) 18(6):e0286219. doi: 10.1371/journal.pone.0286219
149. Nikhil KK, Xiao-Ou S, Wanqing W, Peter K, Sara L, Ulrike P, et al. Association between adult height and risk of colorectal, lung, and prostate cancer: results from meta-analyses of prospective studies and mendelian randomization analyses. PloS Med. (2016) 13(9):e1002118. doi: 10.1371/journal.pmed.1002118
150. Junyi X, Xia J, Shuai B, Qian Y, Su L, Zhengdong Z, et al. Association between circulating vitamin E and ten common cancers: evidence from large-scale mendelian randomization analysis and a longitudinal cohort study. BMC Med. (2022) 20:168. doi: 10.1186/s12916-022-02366-5
151. Yuanqing F, Fengzhe X, Longda J, Zelei M, Xiubo L, Jian Y, et al. Circulating vitamin C concentration and risk of cancers: A mendelian randomization study. BMC Med. (2021) 19:171. doi: 10.1186/s12916-021-02041-1
152. Thomas PL and Shaneda Warren A. Serum 25-hydroxyvitamin D and cancer risk: A systematic review of mendelian randomization studies. Nutrients. (2023) 15(2):422. doi: 10.3390/nu15020422
153. Xia J, Tian G, and Chia-Yen C. The causal role of circulating vitamin D concentrations in human complex traits and diseases: A large-scale mendelian randomization study. Sci Rep. (2021) 11:184. doi: 10.1038/s41598-020-80655-w
154. Qian H, Zichen Y, Yandi S, Zihao Q, Xueyao J, Jingjia L, et al. The impact of homocysteine on the risk of hormone-related cancers: A mendelian randomization study. Front Nutr. (2021) 8. doi: 10.3389/fnut.2021.645371
155. Ran L, Xuanyang W, Yuntao Z, Xiaoqing X, Lulu W, Chunbo W, et al. Analysis of tryptophan and its main metabolite kynurenine and the risk of multiple cancers based on the bidirectional mendelian randomization analysis. Front Oncol. (2022) 12:852718. doi: 10.3389/fonc.2022.852718
156. Nabila K, Elena VV, Khasanova GR, Sarah JL, and Denis P. Blood pressure, calcium channel blockers, and the risk of prostate cancer: A mendelian randomization study. Cancer Causes Control. (2023) 34:725–34. doi: 10.1007/s10552-023-01712-z
157. Yandi S, Jingjia L, Zihao Q, Zichen Y, Xueyao J, Yindan L, et al. Causal associations between serum urea and cancer: A mendelian randomization study. Genes. (2021) 12(4):498. doi: 10.3390/genes12040498
158. Jiang X, Dimou NL, Zhu Z, Bonilla C, Lewis SJ, Lindstrom S, et al. Allergy, asthma, and the risk of breast and prostate cancer: A mendelian randomization study. Cancer Causes Control. (2020) 31:273–82. doi: 10.1007/s10552-020-01271-7
159. Khoei NS, Carreras-Torres R, Murphy N, Gunter MJ, Brennan P, Smith-Byrne K, et al. Genetically raised circulating bilirubin levels and risk of ten cancers: A mendelian randomization study. Cells. (2021) 10(2):394. doi: 10.3390/cells10020394
160. Wu K, Liu L, Shu T, Li A, Xia D, and Sun X. The relationship between processed meat, red meat, and risk of types of cancer: A mendelian randomization study. Front Nutr. (2022) 9:942155. doi: 10.3389/fnut.2022.942155
161. Susanna CL, Paul C, Mathew V, Amy MM, Karl M, John AB, et al. Genetically predicted plasma phospholipid arachidonic acid concentrations and 10 site-specific cancers in uk biobank and genetic consortia participants: A mendelian randomization study. Clin Nutr. (2021) 40:3332–7. doi: 10.1016/j.clnu.2020.11.004
162. Chiyu H, Qian Y, Bin L, Shaoxue Y, Ye D, Xiaohui S, et al. Genetically predicted circulating level of C-reactive protein is not associated with prostate cancer risk. Front Oncol. (2020) 10. doi: 10.3389/fonc.2020.545603
163. Chen G, Wang Y, and Wang X. Insulin-related traits and prostate cancer: A mendelian randomization study. Comput Struct Biotechnol J. (2024) 23:2337–44. doi: 10.1016/j.csbj.2024.05.034
164. John NK. Nih consensus definition and classification of prostatitis. Jama. (1999) 282(3):236–7. doi: 10.1001/jama.282.3.236
165. Rhiannon DH, Charlotte T, and Aditya M. Chronic prostatitis (Chronic pelvic pain syndrome). BMJ. (2023) 383:e073908. doi: 10.1136/bmj-2023-073908
166. Yang C, Jie L, Yanling H, Haiying Z, Xiaobo Y, Yonghua J, et al. Multi-factors including inflammatory/immune, hormones, tumor-related proteins and nutrition associated with chronic prostatitis nih iiia+B and iv based on famhes project. Sci Rep. (2017) 7:9143. doi: 10.1038/s41598-017-09751-8
167. Jiayue Y, Yongshou Y, Manami I, Morio N, Wanping A, Nozomu O, et al. Does the gut microbiota modulate host physiology through polymicrobial biofilms? Microb Environ. (2020) 35(3):ME20037. doi: 10.1264/jsme2.me20037
168. Eric B, Jon C, and Ramnik JX. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe. (2023) 31:173–86. doi: 10.1016/j.chom.2023.01.009
169. Benedetta F, Giuseppe P, Annamaria M, Luciano A, and Mario M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Andrology. (2010) 33:475–88. doi: 10.1111/j.1365-2605.2009.00972.x
170. Priscilla NK. Inflammasomes and gut flora. Science. (2017) 357(6356):1109–10. doi: 10.1126/science.357.6356.1109-b
171. Jie C, Bo C, Bin L, Yin H, Jinze L, Jin L, et al. The role of gut microbiota in prostate inflammation and benign prostatic hyperplasia and its therapeutic implications. Heliyon. (2024) 10:e38302. doi: 10.1016/j.heliyon.2024.e38302
172. Daniel AS, Hannah W, Polackwich AS, Barbara T, Jessica A, and Charis E. Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. J Urol. (2016) 196:435–41. doi: 10.1016/j.juro.2016.02.2959
173. Shen C, Chen Z, Zhang W, Chen XF, Zheng B, and Shi CM. Preliminary study of the effect of gut microbiota on the development of prostatitis. BMC Med Genomics. (2024) 17:35. doi: 10.1186/s12920-024-01812-y
174. Liu X and Dong Q. Associations between gut microbiota and three prostate diseases: A bidirectional two-sample mendelian randomization study. Sci Rep. (2024) 14:4019. doi: 10.1038/s41598-024-54293-5
175. Liu D, Mei Y, Ji N, Zhang B, and Feng X. Causal effect of gut microbiota on the risk of prostatitis: A two-sample mendelian randomization study. Int Urol Nephrol. (2024) 56:2839–50. doi: 10.1007/s11255-024-04020-w
176. Qin PF, He YM, Shao H, and Jiang DW. Genetic insights into gut microbiota and risk of prostatitis: A mendelian randomization study. Front Microbiol. (2024) 15:1389715. doi: 10.3389/fmicb.2024.1389715
177. Shengfeng Z, Xing X, Lei Y, Nili J, Xi-Huan W, and Yanling H. Investigating causal relations between genetic-related intermediate endophenotype and risk of chronic prostatitis: mendelian randomization study. Oxid Med Cell Longevity. (2022) 2022. doi: 10.1155/2022/4560609
178. Genyi Q, Weimin J, Zhaohui L, Xing Z, Yijie W, Guang Y, et al. Assessing the causal relationship between immune cells and prostatitis: evidence from bidirectional mendelian randomization analysis. Mamm Genome. (2024) 35:474–83. doi: 10.1007/s00335-024-10044-5
179. Yan H, Cheng C, Wenyuan Z, Qian Z, Yong Z, Dehao H, et al. Genetically predicted alterations in thyroid function are associated with the risk of benign prostatic disease. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1163586
180. Hongbin W and Mengyao L. Complement C4, infections, and autoimmune diseases. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.694928
181. Melanie MC, Chack-Yung Y, and Robert RH. Complement components, C3 and C4, and the metabolic syndrome. Curr Diabetes Rev. (2018) 15:44–8. doi: 10.2174/1573399814666180417122030
182. Yuqian L, Reyaj M, Dianyou X, Junaid W, Sajan S, Rahat U, et al. Chronic prostatitis/chronic pelvic pain syndrome and prostate cancer: study of immune cells and cytokines. Fundam Clin Pharmacol. (2019) 34:160–72. doi: 10.1111/fcp.12517
183. Lei C, Meng Z, and Chaozhao L. Chronic prostatitis and pelvic pain syndrome: another autoimmune disease? Archivum Immunol Ther Experimentalis. (2021) 69:24. doi: 10.1007/s00005-021-00628-3
184. Sandro La V, Rosita AC, Giorgio Ivan R, Giuseppe M, and Aldo EC. Endocrine control of benign prostatic hyperplasia. Andrology. (2016) 4:404–11. doi: 10.1111/andr.12186
185. Jun Ho L, Yeon Won P, and Sung Won L. The relationships between thyroid hormone levels and lower urinary tract symptoms/benign prostatic hyperplasia. World J Men’s Health. (2019) 37(3):364–71. doi: 10.5534/wjmh.180084
186. Philip H, Stephen B, Kaitlin HW, Jack B, Caroline LR, and George Davey S. Best (but oft-forgotten) practices: the design, analysis, and interpretation of mendelian randomization studies. Am J Clin Nutr. (2016) 103:965–78. doi: 10.3945/ajcn.115.118216
187. Andrea D, Nicola O, Swen-Olof A, Ove A, Jan-Erik J, and Alicja W. Body mass index in early and middle-late adulthood and risk of localized, advanced and fatal prostate cancer: A population-based prospective study. Br J Cancer. (2011) 105:1061–8. doi: 10.1038/bjc.2011.319
188. Vettenranta A, Teemu JM, Jani R, Paavo R, Kirsi T, Kimmo T, et al. Outcomes of screening for prostate cancer among men who use statins. JAMA Oncol. (2022) 8(1):61–8. doi: 10.1001/jamaoncol.2021.5672
189. Hirotsugu U, Ye D, Ravindran K, Hao Z, Yeong-Shiau P, Edmund C, et al. United in fight against prostate cancer registry (Ufo): first results from a large, multi-center, prospective, longitudinal cohort study in asia. Ann Oncol. (2018) 29(Supplement 8):viii300–1. doi: 10.1093/annonc/mdy284.066
190. Maya BM and Matthew PF. Toward open and reproducible epidemiology. Am J Epidemiol. (2023) 192:658–64. doi: 10.1093/aje/kwad007
191. Hongmei Z, Yunning L, Lijun W, Peng Y, Baohua W, Ruiying F, et al. National cancer data linkage platform of China: design, methods, and application. China CDC Weekly. (2022) 4(13):271–5. doi: 10.46234/ccdcw2022.068
192. Nguyen PK, Zhao D, Okamura T, Chang Kim H, Wong ND, and Yang E. Atherosclerotic cardiovascular disease risk prediction models in China, Japan, and korea: implications for east asians? JACC Asia. (2025) 5:333–49. doi: 10.1016/j.jacasi.2025.01.006
194. Cathie S, John G, Naomi EA, Valerie B, Paul RB, John D, et al. Uk biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med. (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
195. Tyler MS, Isla PG, Anna P, Brandon AM, Veda NG, Michelle FJ, et al. Genetic risk prediction for prostate cancer: implications for early detection and prevention. Eur Urol. (2023) 83:241–8. doi: 10.1016/j.eururo.2022.12.021
196. Fredrick S, Ali Amin Al O, Sonja IB, Sara B, Mahbubl A, Ed S, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. (2018) 50:928–36. doi: 10.1038/s41588-018-0142-8
197. Stijn V, Oliver D, and Torben M. Survivor bias in mendelian randomization analysis. Biostatistics. (2017) 19:426–43. doi: 10.1093/biostatistics/kxx050
198. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the strobe-mr statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
199. Veronika S, Rebecca CR, Benjamin W, Neil MD, Sonja AS, Tyler JV, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization (Strobe-mr): explanation and elaboration. BMJ. (2021) 375:n2233. doi: 10.1136/bmj.n2233
Keywords: Mendelian randomization, male infertility, prostate cancer, erectile dysfunction, prostatitis
Citation: Pang Q, Chang Z, Liu H, Chen J, Ma S, Zhang C and Sun Z (2025) Review of Mendelian randomization studies on common male-specific diseases. Front. Endocrinol. 16:1541744. doi: 10.3389/fendo.2025.1541744
Received: 08 December 2024; Accepted: 22 April 2025;
Published: 16 May 2025.
Edited by:
María Laura Ribeiro, CONICET Centro de Estudios Farmacológicos y Botánicos (CEFYBO), ArgentinaReviewed by:
Biagio Barone, ASL Napoli 1 Centro, ItalyHongjun Li, Peking Union Medical College Hospital (CAMS), China
Copyright © 2025 Pang, Chang, Liu, Chen, Ma, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenming Zhang, emNtaG56eUAxNjMuY29t; Zixue Sun, c3VuaGh6eEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Qixin Pang
Qixin Pang Zhe Chang2†
Zhe Chang2† Sicheng Ma
Sicheng Ma Chenming Zhang
Chenming Zhang